Chilli II. Cooled Ablation Catheter ONLY < en >
|
|
|
- Dale Parrish
- 5 years ago
- Views:
Transcription
1 Chilli II Cooled Ablation Catheter TABLE OF CONTENTS < en > WARNING...1 DEVICE DESCRIPTION...1 Figure 1. The Chilli II Catheter...1 Figure 2. The Distal Segment of the Chilli II Catheter...1 INDICATIONS FOR USE...1 CONTRAINDICATIONS...1 WARNINGS...2 PRECAUTIONS...2 HOW SUPPLIED...2 Handling and Storage...2 Operating Environment...2 Transport Environment...2 Storage Environment...2 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions prior to use; including the Boston Scientific Corporation (BSC) Operators Manuals for the Model 8005 Pump and associated compatible, BSC radiofrequency (RF) Generator/Controller. Observe all contraindications, warnings and precautions noted in these directions. Failure to do so may result in patient complications. WARNING Contents supplied STERILE using an ethylene oxide (EO) process. Do not use if sterile barrier is damaged. If damage is found, call your Boston Scientific representative. For single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient. After use, dispose of product and packaging in accordance with hospital, administrative and/or local government policy. DEVICE DESCRIPTION The Chilli II Cooled Ablation System includes the Chilli II Cooled Ablation Catheter (Chilli II Catheter) (Figure 1) and the Model 8005 Pump (CircuCool Pump) with a compatible BSC RF Cardiac Ablation Controller (henceforth, referred to as the Controller). The appropriate accompanying Operator s Manuals must be consulted and reviewed for all equipment in use. The Chilli II Catheter has a distal electrode segment (Figure 2) and a proximal handle that are connected by a torquable catheter shaft. The electrode segment is comprised of a tip electrode and ring electrodes. The tip electrode has an embedded temperature sensor and delivers RF energy for cardiac ablation. The ring electrodes record ECG signals for mapping and deliver stimulus for pacing. The handle includes the electrical connector for the cable connection to the RF Cardiac Ablation Pod (henceforth, referred to as the Pod) and two luer fittings used to connect the catheter to the fluid pump. The steering assembly, electrode lead wires, and two lumens carrying cooling fluid reside within the catheter shaft. The Chilli II Catheter incorporates a closed-loop cooling mechanism capable of removing heat from the tip/tissue interface. The Pump circulates cooling fluid through the two lumens without infusing it into the heart. Two luer connections at the proximal end of the handle provide the connection for the cooling fluid to flow to and from the catheter tip via the pump and corresponding tubing kit. Fluid flow through the tip during ablation provides cooling of the tip. The catheter is shown in Figure 1 (below). Figure 5 (page 5) provides a connectivity diagram for the BSC Cardiac Ablation System with 8005 system. The Chilli II Catheter has high torque characteristics. The degree of tip deflection of the Chilli II Catheter is controlled by the Steering Knob on the catheter handle. Rotating the entire handle to the right or left rotates the catheter tip clockwise or counter-clockwise, respectively (as viewed from the proximal end of the catheter shaft). Once the catheter is positioned, the tension control knob on the handle of the catheter locks the curve into place. ADVERSE EVENTS...2 OBSERVED ADVERSE EVENTS...2 Table 1. Adverse Events That Occurred or Began Within Seven Days After Ablation...2 Potential Adverse Events...2 CLINICAL STUDIES...3 Table 2. Chronic (6 month) Success in the Randomized Trial...3 Figure 3. Freedom from VT Recurrence by Treatment* All patients in the Randomized Trial, N = Table 3. Patient Cohorts and Ablations Success...3 CHILLI VT POST APPROVAL STUDY (CHILLI VT PAS)...3 Table 4. Baseline Demographics For Chilli VT Pas...4 Table 5. Procedure-Related Major Adverse Event Rate (N=182)...4 Table 6. Patient Death Summary...4 Table 7. VT Recurrence at Six Months...4 Figure 4. Kaplan-Meier Event Free Survival Estimates...4 Table 8. Freedom From VT Recurrence At 6 Months...4 Table 9. Summary Of VT Episode Density...4 PATIENT INFORMATION...4 Patient Selection and Treatment...4 Individualization of Treatment...4 SPECIFIC PATIENT POPULATIONS...5 PATIENT COUNSELING INFORMATION...5 PATIENT INFORMATION...5 MATERIALS REQUIRED...5 PHYSICIAN TRAINING...5 CHILLI II CATHETER SETUP AND OPERATION...5 WARRANTY...5 Figure 5. The Chilli II Cooled Ablation System with BSC Cardiac Ablation System...5 Figure 1. The Chilli II Catheter Handle Connector Cooling Extension Tubing Luer Fitting Figure 2. The Distal Segment of the Chilli II Catheter 1.27mm Strain Relief Steering Knob Tension Control Knob 2.5mm 1.27mm Proximal Shaft 2.5mm Distal Segment 2.5mm 1.27mm Ring Electrodes Tip Electrode INDICATIONS FOR USE The Chilli II Cooled Ablation System is indicated for: cardiac electrophysiological mapping delivering diagnostic pacing stimuli radiofrequency ablation of mappable ventricular tachycardias attributable to ischemic heart disease or cardiomyopathy in patients who have failed drug therapy CONTRAINDICATIONS Do not use this device in the following patients: patients with active systemic infection; patients with a mechanical prosthetic heart valve through which the catheter must pass; patients with left ventricular thrombus; or with left atrial thrombus or myxoma via the transseptal approach; patients unable to receive heparin or an acceptable alternative to achieve adequate anticoagulation. 4mm
2 WARNINGS Before using, inspect for physical damage, including electrical insulation on the cables and the catheter shaft. Replace damaged equipment. Care must be taken to ensure that any equipment used in connection with the BSC catheters be type CF, be defibrillation proof, and meet IEC electrical safety requirements, and comply with all local regulatory requirements for specified intended use. No modification of this equipment is allowed. Maximum Catheter Rated Voltage: 178 Vrms (251 Vpk) Patients with severe hemodynamic instability or cardiogenic shock are at increased risk for life threatening adverse events and ablation must be done with extreme caution. Do not ablate arrhythmias in patients with unablatable ventricular tachycardia and/or ventricular fibrillation without additional standard therapy such as an implantable cardioverter/defibrillator (ICD). Precautions in patients with implantable pacemakers and implantable cardioverter/defibrillators (ICDs): Deactivate ICDs as they could discharge and injure the patient or be damaged by the ablation procedure. Have temporary external sources of pacing and defibrillation available. Do not apply RF energy directly to a lead or to tissue immediately in contact with a lead because it could potentially damage the lead or lead function. Perform a complete analysis of the implanted device function after ablation. Do not ablate from the coronary artery as the resulting myocardial injury can be fatal. Adequate fluoroscopic visualization is necessary during the transaortic approach to avoid placement of the ablation catheter in the coronary vasculature. The Chilli II Catheter should be used only by physicians fully trained in cardiac electrophysiology. Ablation in contact with any other electrodes alters the function of the catheter and can lead to thrombus, coagulum, or char formation. At no time should a Chilli II Catheter be advanced or withdrawn when resistance is felt, without determining the cause. Vascular perforation is a risk with any intracardiac catheter. Closely monitor patients following left-sided ablation procedures until they are fully conscious and have been evaluated for embolic stroke or myocardial infarction. Significant x-ray exposure can result in acute radiation injury as well as dose-related risk for somatic and genetic effects. Take all appropriate measures to minimize x-ray exposure to both patients and clinical staff. Careful consideration should be given to the use of this device in pregnant women because of the risk of significant exposure to x-rays. The long-term risk of protracted fluoroscopy has not been established. Therefore, careful consideration must be given for the use of the device in prepubescent children. The long-term risks of lesions created by RF ablation have not been established. In particular, any long-term effects of lesions in proximity to the specialized conduction system or coronary vasculature are unknown. PRECAUTIONS Do not wipe this catheter with organic solvents such as alcohol, or immerse the handle cable connector in fluids. Excessive curves or kinking of the catheter may damage internal components and affect steering performance. The Chilli II Catheter is not intended to be used with a RF generator output setting exceeding 50 watts or 500 Volts peak. Do not allow the patient to contact grounded metal surface. The RF Generator must only be used in Power Control Mode. Electrical recording or stimulation equipment must be isolated and in compliance with relevant EN requirements for intended use. Current leakage from any electrical equipment that is connected to the patient must not exceed 10 microamps for intracardiac electrodes under any circumstances. Do not use the Chilli II Cooled Ablation System in the proximity of magnetic resonance imaging (MRI) equipment because the MRI equipment may adversely impact the function of a RF generator and the ablation system may adversely impact the image quality. Use only dispersive electrodes that meet or exceed EN requirements and follow the dispersive (grounding) electrode manufacturer s instructions for use. Do not increase power before checking for lead connection and appropriate dispersive electrode application. Verify effective contact between the patient and the dispersive electrode whenever the patient is repositioned. Do not use impedance cut-off setting greater than 200 Ohms or temperature cut-off settings of 100 C or greater because those settings have not been studied. Use both fluoroscopy and electrograms to monitor the advancement of the catheter to the area of the endocardium under investigation to avoid vascular damage, cardiac perforation or tamponade. The displayed temperature is not the temperature of the tissue. It is the temperature of the cooled electrode only and does not represent tissue temperature. Do not deliver RF energy with the catheter outside the target site. RF Generators can deliver significant electrical energy and may cause patient or operator injury. Avoid use of electrodes and probes of monitoring and stimulating devices that could provide paths for high frequency current. Reduce the burn hazard by placing the electrodes and probes as far away as possible from the ablation site and the dispersive electrode. In the event of a generator cut-off (impedance or temperature), the catheter must be withdrawn and the tip electrode cleaned of coagulum before RF energy is re-applied. Use only sterile saline and gauze pad to clean the tip. Do not scrub or twist the tip electrode as damage may cause catheter failure or patient injury. The Chilli II Catheter is highly torqueable. Avoid overtorquing. Over-rotating the handle and catheter shaft may cause damage to the distal tip or catheter assembly. Do not rotate the handle and catheter shaft more than 1 1/2 full rotations (540 ). If the desired catheter tip position is not achieved, adjust the catheter s curve to disengage the catheter tip from the heart wall, before resuming rotation of the handle and catheter shaft. Do not insert or withdraw the catheter without straightening the catheter tip (returning the steering lever to neutral position). In the event of a suspected failure of the integrity of fluid flow through the catheter, the procedure should be stopped, and both the catheter and the tubing kit should be replaced, primed, and then reinserted. If there is any abnormality of the integrity of fluid flow through the catheter, the catheter should be replaced by a different catheter. Do not use the Chilli II Catheter after the expiration date because the device performance may no longer be acceptable and/or the device may no longer be sterile. HOW SUPPLIED Do not use if package is opened or damaged. Do not use if labeling is incomplete or illegible. Handling and Storage Operating Environment Ambient Temperature: 10 C to 40 C Relative Humidity: 30% to 75% Atmospheric Pressure: 70 kpa to 106 kpa Transport Environment Temperature: -29 C to 60 C Relative Humidity: 30% to 85% Atmospheric Pressure: Uncontrolled Storage Environment Ambient Temperature: 20 C to 30 C Relative Humidity: Uncontrolled Atmospheric Pressure: Uncontrolled ADVERSE EVENTS The Chilli Cooled Ablation System was used in the treatment of patients undergoing electrophysiologic (EP) mapping and RF catheter ablation for the treatment of ventricular tachycardia attributable to ischemic heart disease or cardiomyopathy. The clinical studies reported here were conducted using the Chilli Cooled Ablation System (The Chilli I Cooled Ablation Catheter and Model 8004 RF Generator and Pump System). Although 188 patients were enrolled in the clinical studies, only 150 patients received ablation therapy using the Chilli Cooled Ablation System. The assessment of adverse events is based on all 150 patients. Patients were followed for 8 ± 5 months (mean ± s.d.); the longest follow-up was 24 months. OBSERVED ADVERSE EVENTS Table 1. Adverse Events That Occurred or Began Within Seven Days After Ablation Adverse Event Category Description 2 Total No. of Pts Experiencing Adverse Events (N = 150) No. of Adverse Events Resulting in Death (N = 150) % of Pts Experiencing Adverse Events (95% C.I.) Major % [6.2, 16.7] Cerebrovascular accident/transient ischemic attack % [0.7, 6.7] Cardiac perforation % [0.7, 6.7] Acute myocardial infarction % [0.0, 3.7] Post-operative cardiogenic shock % [0.2, 4.7] Post-operative cardiogenic shock, & aortic valve injury % [0.0, 3.7] Cardiac insufficiency % [0.0, 3.7] Electromechanical dissociation % [0.0, 3.7] Third-degree heart block % [0.2, 4.7] Pneumonia % [0.0, 3.7] Minor* % [1.9, 9.4] All patients treated with Chilli Catheters, N = 150 * Patients with both minor and major adverse events were counted only as major adverse events. IDMC classified these events and deaths as possibly procedure-related. Two of the four instances of complete heart block were anticipated prior to the procedure and not classified as adverse events by the IDMC. [95% C.I.] = 95% confidence intervals by the exact method Major adverse events: Major adverse events were defined as any complication requiring an invasive intervention or prolonging or requiring a new hospitalization. Table 1 presents the observed adverse events for the 150 patients. An Independent Data Monitoring Committee (IDMC) also reviewed and classified events and deaths. Major adverse events were reported in 28 of 150 patients (19%), 16 of which occurred or began with the first seven days after ablation. Of the 26 total deaths, six occurred or may have been related to adverse events that began within seven days of the ablation procedure (Table 1), and 20 occurred later. Of the 20 late deaths, end-stage congestive heart failure was the cause in eight patients, end-stage ischemic heart disease and arrhythmias in six patients, ischemic heart disease in two patients, and non-cardiac causes in three patients. Minor adverse events: Minor adverse events were defined as those in which an observation was made or a medication was prescribed, but hospitalization was not required or prolonged. Seven minor adverse events occurred within seven days of ablation. The adverse events included transient loss of a lower extremity pulse, distal femoral artery dissection with pseudoaneurysm, defibrillation skin burns, dysarthria and diplopia attributed to sedation, transient lower extremity weakness attributed to sedation, chronic arterio-venous fistula, and left arm pain. Potential Adverse Events Adverse events (in alphabetical order), which may be associated with catheterization and ablation include: Catherization/Ablation Procedure Related air embolism arrhythmias AV fistula cardiac perforation cardiac/respiratory arrest hemorrhage
3 hemothorax hypotension nerve palsy or weakness pleuritis pulmonary edema pneumothorax pseudoaneurysm sinus or AV node injury tamponade thrombi thromboembolism thrombosis valvular damage vascular bleeding/local hematoma vasovagal reactions visual blurring RF Ablation Related cardiac perforation/tamponade cardiac thromboembolism cerebrovascular accident (CVA) chest pain/discomfort complete heart block coronary artery spasm coronary artery thrombosis defibrillation skin burn distal aortic/coronary artery dissection pericarditis transient ischemic attack (TIA) valvular damage ventricular tachyarrhythmia CLINICAL STUDIES The clinical studies reported here were conducted using the Chilli Cooled Ablation System before the introduction of the Chilli II Catheter model. A total of 188 patients were involved in clinical studies of the Chilli Cooled Ablation System. All patients had ischemic heart disease or cardiomyopathy and had experienced a minimum of two episodes of spontaneous ventricular tachycardia (VT) in the two months prior to enrollment. Of the 188 patients, 107 were enrolled in the Randomized Trial and 81 in other studies. Of the 107 patients enrolled in the Randomized Trial, 75 were assigned to receive RF ablation and 32 to optimized antiarrhythmic drug therapy. Acute success was defined as an inability to induce VT following the ablation Procedure. Chronic success was defined as freedom from spontaneous recurrence of any VT for six months following ablation. Results: The Randomized Trial was analyzed by intention-to-treat. Table 2 compares chronic success for patients randomized to RF ablation or antiarrhythmic drugs (control). Table 2. Chronic (6 month) Success in the Randomized Trial Chronic Success No recurrence of any VT at 6 months Ablation Patients 55% [43%, 66%] (41/75 ) Control Patients 19% [5.4%, 33%] (6/31 ) Difference 35% [17%, 53%] Percent [95% Confidence Interval], (Numerator/Denominator) All patients enrolled (N = 107) 95% confidence intervals by normal approximation Intention-to-treat includes 10 patients randomized to ablation who did not receive ablation treatment One patient was lost to follow-up prior to six months and was excluded from analysis. * Difference was statistically significant (p<0.005) by Fisher s Exact Test *Intention-to-treat includes 10 patients randomized to ablation who did not receive ablation treatment. The difference in VT recurrence was statistically significant by the Gehan test (p <0.001). Figure 3 shows the freedom from recurrence of any VT for both treatment groups in the Randomized Trial. Control patients who crossed over to ablation (N = 17) were censored (removed from the survival analysis). Freedom from any VT recurrence (%) Figure 3. Freedom from VT Recurrence by Treatment* All patients in the Randomized Trial, N = Ablation Control Days A total of 188 patients were enrolled in the studies; data from 150 patients with the Chilli Catheter inserted were analyzed, including 65 of the 75 patients randomized to ablation, 17 of the 32 patients initially randomly assigned to antiarrhythmic drug therapy (control) who crossed over to ablation, 15 of the 18 patients treated for emergency use, and 53 of the 63 patients treated after randomization was discontinued. Of these 150 patients in whom a Chilli Catheter was inserted, most (127) received ablation treatment on a single occasion, 18 were treated on two occasions, one patient on three occasions, and four received no ablation treatment because the VT could not be mapped. Only the first treatment was considered in the effectiveness analyses. Table 3 lists the number of patients enrolled, treated, and the acute and chronic (six-month) success. Table 3. Patient Cohorts and Ablations Success Patient Cohort Randomized to ablation Control cross-over No. of Patients Ablated 65 46/ /17 Emergency Use 15 7/10 Nonrandomized 53 42/53 Total /145 * [95% confidence intervals by exact measure] Acute success not assessed in five patients Six-month follow-up not available in 10 patients Acute Success Chronic Success Number Percent Number Percent 71% [60%, 82%]* 82% [57%, 96%] 70% [35%, 93%] 79% [68%, 90%] 75% [68%, 82%] 36/65 12/17 8/15 22/43 78/140 55% [43%, 68%]* 71% [44%, 90%] 53% [27%, 79%] 51% [36%, 66%] 56% [48%, 64%] CHILLI VT POST APPROVAL STUDY (CHILLI VT PAS) A post approval study (PAS) was conducted to further assess the safety and effectiveness of the Chilli Cooled Ablation System. The objective of this study was to confirm the safety and effectiveness of the Chilli Cooled Ablation System for the treatment of mappable VT in subjects with ischemic heart disease or non-ischemic cardiomyopathy who failed drug therapy. The Chilli VT PAS was conducted using the Chilli Cooled Ablation Catheter and Model 8004 RF Generator and Pump System or Model 8005 Pump. Study endpoints included Freedom from recurrence of any VT at six months, Freedom from Major Adverse Events and Reduction in VT episode density. Clinical data from the subjects were collected from the time of the ablation (Index) procedure and again at six months following the Index procedure. Clinical data were collected on 182 subjects. The analysis was performed on an Intent-To-Treat (ITT) basis so included the entire subject population (N = 182).
4 Baseline demographics for the Chilli VT PAS are presented below. Table 4. Baseline Demographics For Chilli VT Pas Baseline Demographic Number (%) Previous VT ablation 62/182 (34.1%) Previous ICD 142/182 (78.0%) Failed amiodarone 141/182 (77.5%) Failed other AADs 165/182 (90.7%) Ischemic Heart Disease 123/182 (67.6%) Non-Ischemic Cardiomyopathy Bundle branch reentry tachycardia MI 6 weeks before the procedure 42/182 (23.1%) 8/116 (6.9%) 10/172 (5.8%) NYHA Class IV heart failure 8/172 (4.7%) Heart transplant list candidate 15/172 (8.7%) Limited life expectancy (terminal illness) Ejection Fraction (%) Mean ± Std. Dev N Number of medications previously failed Mean ± Std. Dev Median Min-Max N 1/172 (0.6%) 31.7± ± Table 5 presents the procedure-related Major Adverse Event Rate. Major adverse events that occurred within seven days of the Index procedure, or the period of hospitalization if extended beyond seven days, were reported. It should be noted that a recurrence of VT was not counted nor reported as an AE since it was a primary study endpoint. Seventeen (17) subjects experienced a procedure-related Major Adverse Event. Freedom from Major Complications was observed in 90.7% of the subjects. Table 5. Procedure-Related Major Adverse Event Rate (N=182) Overall 17/182 (9.3%) One-Sided Exact 95% CI Upper Limit One-Sided Normal 95% CI Upper Limit Objective Performance Criteria (OPC) 13.7% 12.9% 13.8% OPC is the percentage of patients who experienced procedure related adverse events as prospectively established from the Investigational Device Exemption (IDE) clinical study. Table 6 presents a summary of the deaths experienced in Chilli VT PAS as compared to the Chilli IDE study. Eighteen (18) deaths occurred in the 182 subjects enrolled in the Chilli VT PAS. Table 6. Patient Death Summary Chilli VT PAS Chilli IDE Patient Deaths Number (%) Number (%) p-value Total 18/182 (9.9%) 11/75 (14.7%) Tachyarrhythmic 8/182 (4.4%) 3/75 (4.0%) Cardiac (non-tachyarrhythmic) 4/182 (2.2%) 7/75 (9.3%) Non-cardiac 4/182 (2.2%) 1/75 (1.3%) Unknown 2/182 (1.1%) Table 7 presents the observed VT recurrence rate at the Six Month followup visit. Eight (8) of the 182 enrolled subjects were excluded from this analysis: six (6) were lost to follow-up, two (2) subjects died prior to the Six Month follow-up visit; a total of 174 subjects were included in this analysis. The observed VT recurrence rate (57.5%) and the one-sided upper limits exceeded the 54.2% OPC. The differences between the PAS and the original IDE patient population contributed to the increase in the percentage of patients who experienced VT at six months post ablation procedure. The subject population changed significantly in the PAS Study from the original IDE Clinical Study. The enrolled subjects in the PAS Study encompassed a wider inclusion (ischemic or non-ischemic cardiomyopathy), and more pathologic population (terminal end stage treatment for VT) than the patient population of the original IDE study. The inclusion criteria were broader in the PAS study than in the IDE study and therefore the sicker patient was not excluded. Table 7. VT Recurrence at Six Months Observed VT Recurrence Rate 4 One-Sided Exact 95% CI Upper Limit One-Sided Normal 95% CI Upper Limit Objective Performance Criteria (OPC) 100/174 (57.5%) 63.8% 63.6% 54.2% OPC is the percentage of patients who experienced procedure related VT as prospectively established from the Investigational Device Exemption (IDE) clinical study. Figure 4 presents the Kaplan-Meier Event Free Survival Estimates of the freedom from VT rate and the associated 95% confidence intervals. The event-free survival estimates are reported at interval end and the standard errors are calculated by the Greenwood formula. % Cumulative Event Free 100 Figure 4. Kaplan-Meier Event Free Survival Estimates Table 8. Freedom From VT Recurrence At 6 Months Days Since Index Procedure Legend Lower CI Survival Upper CI Interval Ending (Days Post Procedure) Entered Censored Events At Risk Events/month Event-Free 90.8% 74.6% 71.2% 66.5% 56.0% 43.1% 43.1% Std Error 2.2% 3.3% 3.4% 3.6% 3.8% 3.8% 3.8% Pre-and post-procedure episode density was analyzed as a measure of clinically meaningful ablation success (Table 9). One hundred and forty one subjects (141) had VT episode density data collected both pre-procedure and at Six Months post-procedure, where the VT episode collection methodology that was at least as comprehensive post-procedure as is was pre-procedure. Table 9. Summary Of VT Episode Density Statistic Pre Procedure Post-Procedure Difference Mean Percentage Change Mean Std. Dev % CI - - [-43.3,-15.4] Min Max N N (%) of patients with 75% reduction (2) : 80.7% (113/140) [73.2%, 86.9%] 95% CI was estimated by normal approximation (2) 95% CI was estimated by exact method Paired-t Test P-value Wilcoxon Signed Rank test 86.34% < < The mean percent change can be affected by outliers so the data was examined to determine how many subjects experienced at least a 75% reduction in episodes to determine if subjects were benefiting from the therapy. This analysis found that 81% of the subjects enrolled experienced at least a 75% reduction in episodes post-procedure compared to episodes pre-procedure. PATIENT INFORMATION Patient Selection and Treatment Individualization of Treatment To screen patients for left ventricular or atrial thrombus or myxoma,it is recommended that patients undergo surface or transesophageal echocardiography or a comparable cardiac imaging study prior to the ablation procedure. To avoid thromboemboli, intravenous heparin or an acceptable alternative must be used when entering the left heart during ablation. During the trial, monitoring of activated clotting time (ACT) was performed as follows: The patient s baseline ACT was measured. An initial intravenous heparin bolus of 5,000 to 10,000 units was given prior to ablation. Heparin was given to prolong the ACT to 2 to 2 1/2 times the baseline value throughout the time the Chilli Catheter remained in the left heart. ACT was measured every 30 to 60 minutes. Aspirin, and less often warfarin, was given to most patients after ablation. No consensus yet exists about the need for continued anticoagulation or antiplatelet therapy after ablation. In the clinical studies, the antiplatelet and/or anticoagulation therapy was continued for approximately 3 months following ablation.
5 SPECIFIC PATIENT POPULATIONS The safety and effectiveness of cardiac ablation has not been adequately studied in: patients who have not failed antiarrhythmic drug therapy patients with idiopathic VT or bundle-branch reentrant tachycardia patients with only unmappable or hemodynamically unstable VT patients who are pregnant asymptomatic patients with ventricular tachycardia PATIENT COUNSELING INFORMATION Physicians should consider the following points in counseling the patient about this device: Alternative treatments for ventricular tachycardia include antiarrhythmic medications, surgical implantation of an implantable cardioverter/defibrillator (ICD), or surgical intervention to remove abnormal heart tissue or disconnect the abnormal pathway. Risks of the ablation procedure include bleeding at the catheter insertion site, catheter damage to the heart and blood vessels, blood clots, infection, myocardial infarction, cerebrovascular accident and death. A potential benefit of the Chilli Cooled Ablation System procedure in the revention of the recurrence of ventricular tachycardia. PATIENT INFORMATION A patient information brochure titled Understanding Arrhythmias is available through your Boston Scientific representative. MATERIALS REQUIRED Boston Scientific s Chilli II Catheter Commercially available disposable dispersive pads which are in compliance with IEC /IEC Sterile dextrose 5% in water (commercially available) 8F (2.67 mm) Venous Introducer Sheath The following compatible Boston Scientific RF Generator/Controllers can be used in conjunction with the Chilli II Catheter: BSC Maestro 4000 Cardiac Ablation Controller and: RF Ablation Pod 681 Cable 8005 Pump (CircuCool Pump) Tubing Kit 2104 Or BSC Maestro 3000 Cardiac Ablation Controller and RF Ablation Pod 681 Cable 8005 Pump (CircuCool Pump) Tubing Kit 2104 PHYSICIAN TRAINING Physicians must be familiar with the techniques and appropriately trained for cardiac mapping and ablation procedures. Physicians must have received an in-service from qualified Boston Scientific personnel regarding the Chilli Cooled Ablation system prior to performing the initial case with the system. Boston Scientific personnel must be present during the first two procedures performed with the system to answer any technical questions regarding the system. All mapping and ablation procedures must be performed in a fully-equipped electrophysiology laboratory. CHILLI II CATHETER SETUP AND OPERATION Note: Operation of the Model 8005 RF Pump is completely described in the Model 8005 Operator s Manual. The operator should read the Operator s Manual prior to using the system. Note: The operator should read the appropriate operator s manual prior to use. Consult the Operator s Manual for use of the RF Generator in Power Control Mode. Before use, inspect the packaging for any violation of the sterile barrier and inspect the catheter for any defects. Do not use potentially contaminated or defective equipment. 1. Connect the patient to an ECG recording system to facilitate arrhythmia monitoring per the standard operating procedure of the electrophysiology lab or manufacturer s operator s manual. Note: This should be done prior to introducing any intracardiac catheters. 2. Attach the dispersive pad per the manufacturer s directions for use. 3. Place an 8F (2.67 mm) venous introducer sheath percutaneously into the vein or artery using the Seldinger technique. 4. Open the Chilli II Catheter package and the Tubing Kit package. Carefully transfer the package contents into the sterile field, maintaining sterile technique. 5. Connect the cooling fluid extension tubes of the Tubing Kit to the respective Pump according to Figure 5 (page 5). Refer to the Instructions provided with the Tubing Kit. 6. Connect the Chilli II Catheter to the Tubing Kit. Care must be taken to ensure all luer fittings are secure to prevent leaking. For the Model 8005 Pump, use ONLY sterile, dextrose 5% in water (D5W). For the Model 8005 Pump, 40 ml of sterile D5W are needed to prime the tubing and catheter. No additional fluid is required for the procedure. 7. Before placing the Chilli II Catheter in the sheath, start the pump according to the Operator s Manual provided with the Model 8005 Pump. Check for proper connections, circulation of fluid and lumen patency. Ensure the pump section of tubing is properly seated on the rollers. Completely prime the pump, tube set and catheter for approximately 5 minutes. Check for leaks at the tip of the catheter, at the handle, and at the luer connections. Do not use a Chilli II Catheter that leaks. Assure that fluid flows completely through the catheter to the cooling fluid outlet lumen. Leave the pump on. 8. Under fluoroscopic guidance, insert the catheter into the sheath and advance through the vasculature into the heart. 9. Attach the appropriate EGM cable by pushing the cable connector into the catheter handle. The connector is keyed to ensure that appropriate connections are made between the handle and the cable. Ensure that the cable/catheter connection remains dry throughout the procedure. 10. Connect the opposite end of the cable to the Controller according to their respective Operator s Manuals. Refer to Figure 5 (page 5). 11. The degree of tip deflection of the Chilli II Catheter is controlled by the Steering Knob on the catheter handle. If the Steering Knob is turned in a clockwise direction from its neutral position, the tip will curve proportionately depending upon the curve option selected. Turning the Steering Knob in the counter clockwise direction will cause the tip to deflect in the opposite direction. To prevent overstressing the tip, the Steering Knob movement is limited by the handle design. The tension adjust knob may be used when the desired catheter placement is achieved. 12. Determine the area of interest for ablation. Refer to the Operator s Manual for initial cooling system operation. 13. Refer to the Controller Operator s manual for setting ablation parameters. 14. Perform the ablation procedure in accordance with standard medical procedure. Ensure that cooling fluid is circulating throughout the application of RF energy. 15. Fluid should be continuously circulated through the tip. The 8005 Pump will automatically determine the fluid flow rate. Do not attempt to modify the fluid flow rate or any part of the tubing kit. 16. In the event of a suspected failure of the integrity of fluid flow through the catheter, the procedure should be stopped, and both the catheter and the tubing kit should be replaced, primed and then reinserted. If the parameters do not appear normal or if there is any abnormality of the integrity of fluid flow through the catheter, the catheter should be replaced by a different catheter. 17. Withdraw the Chilli II Catheter when the procedure is finished. 18. Dispose of the Chilli II Catheter per hospital procedures. 19. Carefully monitor patient while in recovery to ensure hemostasis is achieved and any complications are immediately treated. WARRANTY Boston Scientific Corporation (BSC) warrants that reasonable care has been used in the design and manufacture of this instrument. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation of law or otherwise, including, but not limited to, any implied warranties of merchantability or fitness for a particular purpose. Handling, storage, cleaning and sterilization of this instrument as well as other factors relating to the patient, diagnosis, treatment, surgical procedures and other matters beyond BSC s control directly affect the instrument and the results obtained from its use. BSC s obligation under this warranty is limited to the repair or replacement of this instrument and BSC shall not be liable for any incidental or consequential loss, damage or expense directly or indirectly arising from the use of this instrument. BSC neither assumes, nor authorizes any other person to assume for it, any other or additional liability or responsibility in connection with this instrument. BSC assumes no liability with respect to instruments reused, reprocessed or resterilized and makes no warranties, express or implied, including but not limited to merchantability or fitness for a particular purpose, with respect to such instruments. Dispersive Pad (Commercially Available) Pod BSC Cardiac Ablation Controller Figure 5. The Chilli II Cooled Ablation System with BSC Cardiac Ablation System Model 653S EGM Cable 5 Chilli II Catheter Syringe For Filling 5% Sterile Dextrose (Commercially Available) Power Cord Model 8005 CircuCool Pump Power Cord Model 2104 Tubing Kit Model 681 Chilli II Catheter Cable
6 EC REP Boston Scientific Limited Ballybrit Business Park Galway IRELAND EU Authorized Representative Australian AUS Sponsor Address Boston Scientific (Australia) Pty Ltd PO Box 332 BOTANY NSW 1455 Australia Free Phone Free Fax Argentina ARG Local Contact Para obtener información de contacto de Boston Scientific Argentina SA, por favor, acceda al link Legal Manufacturer Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA USA USA Customer Service Do not use if package is damaged. Recyclable Package 2015 Boston Scientific Corporation or its affiliates. All rights reserved.
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15. Instructions for Use
 Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters
 Reprocessed by Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale
Reprocessed by Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
IntellaTip MiFi Open-Irrigated Ablation Catheter
 91110327-01 2016-11 < en > IntellaTip MiFi Open-Irrigated Ablation Catheter TABLE OF CONTENTS WARNING...1 DEVICE DESCRIPTION...1 Figure 1. IntellaTip MiFi OI Catheter...1 User Information...1 Contents...1
91110327-01 2016-11 < en > IntellaTip MiFi Open-Irrigated Ablation Catheter TABLE OF CONTENTS WARNING...1 DEVICE DESCRIPTION...1 Figure 1. IntellaTip MiFi OI Catheter...1 User Information...1 Contents...1
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Contents Ultra ICE Plus 9 MHz Imaging Catheter MDU5 PLUS Sterile Bag 26 gauge needle 10 ml (cc) syringe
 91040401-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions prior to use. Observe all contraindications, warnings and precautions
91040401-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions prior to use. Observe all contraindications, warnings and precautions
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Figure 1: Revolution TM Peripheral Atherectomy System Diagram. Table 1: Revolution TM Peripheral Atherectomy System Specifications Minimum Burr
 Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Contents Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter MDU5 PLUS Sterile Bag 26 gauge needle 10 ml (10 cc) syringe
 50677333-01 Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter ONLY 2018-05 < en > Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions
50677333-01 Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter ONLY 2018-05 < en > Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information
 Melbourne Heart Rhythm Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information What is Ventricular Tachycardia? Ventricular tachycardia (VT) is an abnormal rapid heart
Melbourne Heart Rhythm Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information What is Ventricular Tachycardia? Ventricular tachycardia (VT) is an abnormal rapid heart
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU)
 Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
NAVIGATE WITH CONFIDENCE
 NAVIGATE WITH CONFIDENCE Nav-Enabled Catheter Portfolio INTELLAMAP ORION MAPPING CATHETER INTELLANAV MIFI OI INTELLANAV OI INTELLANAV MIFI XP RHYTHMIA HDx Mapping System The Boston Scientific nav-enabled
NAVIGATE WITH CONFIDENCE Nav-Enabled Catheter Portfolio INTELLAMAP ORION MAPPING CATHETER INTELLANAV MIFI OI INTELLANAV OI INTELLANAV MIFI XP RHYTHMIA HDx Mapping System The Boston Scientific nav-enabled
Emerge. PTCA Dilatation Catheter ONLY. monorail. over-the-wire
 90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Electrodes (for TransAeris System)
 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
VT Ablation in Structural Heart Disease Patient Information
 Melbourne Heart Rhythm VT Ablation in Structural Heart Disease Patient Information Ventricular Tachycardia in Structural Heart Disease (VT-SHD) Ventricular tachycardia (VT) is an abnormal rapid heart rhythm
Melbourne Heart Rhythm VT Ablation in Structural Heart Disease Patient Information Ventricular Tachycardia in Structural Heart Disease (VT-SHD) Ventricular tachycardia (VT) is an abnormal rapid heart rhythm
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
Advanced Innovation for Exceptional Performance
 Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
System.
 System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
WallFlex Biliary RX Partially Covered Stent System Prescriptive Information
 Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
OptiCross MHz Peripheral Imaging Catheter
 91080532-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. WARNING Contents supplied STERILE using a Radiation process. Do not use if sterile barrier is
91080532-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. WARNING Contents supplied STERILE using a Radiation process. Do not use if sterile barrier is
ULTRA ICE PLUS ULTRASOUND IMAGING CATHETER. Know where you are. See what you want to avoid.
 ULTRA ICE PLUS ULTRASOUND IMAGING CATHETER Know where you are. See what you want to avoid. ULTRA ICE PLUS for Transseptal Puncture Know where you are. See what you want to avoid. Fluoroscopy alone provides
ULTRA ICE PLUS ULTRASOUND IMAGING CATHETER Know where you are. See what you want to avoid. ULTRA ICE PLUS for Transseptal Puncture Know where you are. See what you want to avoid. Fluoroscopy alone provides
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
LeMaitre Embolectomy Catheter
 Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Cardiac Electrical Therapies. By Omar AL-Rawajfah, PhD, RN
 Cardiac Electrical Therapies By Omar AL-Rawajfah, PhD, RN Outlines What are cardiac electrical therapies Ablation Defibrillation Cardioversion What are the nursing considerations for each type of therapy
Cardiac Electrical Therapies By Omar AL-Rawajfah, PhD, RN Outlines What are cardiac electrical therapies Ablation Defibrillation Cardioversion What are the nursing considerations for each type of therapy
Kadlec Regional Medical Center Cardiac Electrophysiology
 Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
PORTO 2 VENT CPAP OS. Operator s Manual. PORTO 2VENT CPAP OS System Operator s Manual Part Number Rev I
 PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
Ventricular Arrhythmias
 Presenting your most challenging cases Venice Arrhythmias Ventricular Arrhythmias Gioia Turitto, MD Presenter Disclosure Information A questionable indication for CRT-D in a patient with VT after successful
Presenting your most challenging cases Venice Arrhythmias Ventricular Arrhythmias Gioia Turitto, MD Presenter Disclosure Information A questionable indication for CRT-D in a patient with VT after successful
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY
 SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
Ruby Coil. Large Volume Detachable Coils
 Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
ASAHI Corsair INSTRUCTIONS FOR USE
 ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
Single Pass MCA Revascularization with Trevo
 Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
Pacing Lead Implant Testing. Document Identifier
 Pacing Lead Implant Testing 1 Objectives Upon completion of this presentation, the participant should be able to: Name the two primary surgical options for implanting pacing leads Describe three significant
Pacing Lead Implant Testing 1 Objectives Upon completion of this presentation, the participant should be able to: Name the two primary surgical options for implanting pacing leads Describe three significant
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
INSTRUCTIONS FOR USE
 PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
Automatic External Defibrillators
 Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Wolverine Coronary Cutting Balloon
 50482216-01 2017-02 < en > Wolverine Coronary MONORAIL OVER-THE-WIRE Microsurgical Dilatation Device Table of Contents Warning...1 device description...1 Contents...1 Quantity Material...1 Intended use/indications
50482216-01 2017-02 < en > Wolverine Coronary MONORAIL OVER-THE-WIRE Microsurgical Dilatation Device Table of Contents Warning...1 device description...1 Contents...1 Quantity Material...1 Intended use/indications
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T
 Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM
 Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
WATCHMAN Left Atrial Appendage Closure Device
 WATCHMAN Left Atrial Appendage Closure Device Patient Information Guide WATCHMAN Left Atrial Appendage Closure Device PATIENT INFORMATION GUIDE Your doctor has recommended that you consider undergoing
WATCHMAN Left Atrial Appendage Closure Device Patient Information Guide WATCHMAN Left Atrial Appendage Closure Device PATIENT INFORMATION GUIDE Your doctor has recommended that you consider undergoing
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
CAPTIVATE SUMMARY CLINICAL SUMMARY. CAPTure Information Via Automatic Threshold Evaluation
 CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
Understanding aneurysms and flow diversion treatment
 Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Model 5392 EPG Temporary Pacer
 Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
THINK OUTSIDE THE PILLBOX
 THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
THINK OUTSIDE THE PILLBOX
 THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW
 JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
Invasive and Medical Treatments for Atrial Fibrillation. Thomas J Dresing, MD Section of Electrophysiology and Pacing Cleveland Clinic
 Invasive and Medical Treatments for Thomas J Dresing, MD Section of Electrophysiology and Pacing Cleveland Clinic Disclosures Fellow s advisory panel for St Jude Medical Speaking honoraria from: Boston
Invasive and Medical Treatments for Thomas J Dresing, MD Section of Electrophysiology and Pacing Cleveland Clinic Disclosures Fellow s advisory panel for St Jude Medical Speaking honoraria from: Boston
Non-Invasive Ablation of Ventricular Tachycardia
 Non-Invasive Ablation of Ventricular Tachycardia Dr Shaemala Anpalakhan Newcastle upon Tyne Hospitals NHS Foundation Trust Freeman Road, Newcastle Upon Tyne, NE7 7DN Contact: shaemala@doctors.org.uk Introduction
Non-Invasive Ablation of Ventricular Tachycardia Dr Shaemala Anpalakhan Newcastle upon Tyne Hospitals NHS Foundation Trust Freeman Road, Newcastle Upon Tyne, NE7 7DN Contact: shaemala@doctors.org.uk Introduction
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
M Series with Rectilinear Biphasic Waveform Defibrillator Option Indications for Use
 DEFIBRILLATOR OPTION General Information Introduction M Series products are available with an advanced electrical design that provides a unique rectilinear biphasic waveform for defibrillation and cardioversion.
DEFIBRILLATOR OPTION General Information Introduction M Series products are available with an advanced electrical design that provides a unique rectilinear biphasic waveform for defibrillation and cardioversion.
Pruitt Irrigation Occlusion Catheter. English Instructions for Use. Pruitt Irrigation Occlusion Catheter
 English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
Title: Automatic External Defibrillators Division: Medical Management Department: Utilization Management
 Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
ROTABLATORTM. Peripheral. Rotational Atherectomy System. Quick Reference Cards
 Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
Kadlec Regional Medical Center Cardiac Electrophysiology
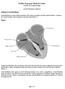 Definition of atrial fibrillation Kadlec Regional Medical Center Cardiac Electrophysiology Atrial Fibrillation Ablation Atrial fibrillation is a heart rhythm disturbance that causes an irregular (and often
Definition of atrial fibrillation Kadlec Regional Medical Center Cardiac Electrophysiology Atrial Fibrillation Ablation Atrial fibrillation is a heart rhythm disturbance that causes an irregular (and often
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
AXIOS Stent and Delivery System
 PRESCRIPTIVE INFORMATION AXIOS Stent and Delivery System Warning REFER TO THE DEVICE DIRECTIONS FOR USE FOR COMPLETE INSTRUCTIONS ON DEVICE USE. RX ONLY. CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE
PRESCRIPTIVE INFORMATION AXIOS Stent and Delivery System Warning REFER TO THE DEVICE DIRECTIONS FOR USE FOR COMPLETE INSTRUCTIONS ON DEVICE USE. RX ONLY. CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
ENABLE MRI CLINICAL STUDY SUMMARY
 CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Lotus transducer Instructions for use
 Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Part 1: General requirements
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
