ULTRA ICE PLUS ULTRASOUND IMAGING CATHETER. Know where you are. See what you want to avoid.
|
|
|
- Benedict Harmon
- 5 years ago
- Views:
Transcription
1 ULTRA ICE PLUS ULTRASOUND IMAGING CATHETER Know where you are. See what you want to avoid.
2 ULTRA ICE PLUS for Transseptal Puncture Know where you are. See what you want to avoid. Fluoroscopy alone provides limited visualization of Intracardiac anatomy. Puncture of the aorta and left atrial free wall (LAFW) are serious complications that may occur more frequently in procedures guided by fluoro alone. Transseptal Puncture Know where you are. The Boston Scientific ULTRA ICE PLUS Catheter is designed to provide the visualization of both: Intracardiac anatomy Devices positioned within the heart with precise details in real time. Not only does it help you in identifying anatomical structures, it also helps you in visualizing where your devices are relative to those structures. The images above show an ULTRA ICE PLUS Catheter positioned in the right atrium, adjacent to the fossa ovalis, visualizing the structures critical to successful transseptal puncture: the septum, aorta, needle position, tenting, and the LAFW.* See what you want to avoid. Performing a successful transseptal puncture involves not only making sure the needle passes through the fossa, but also assuring the needle AVOIDS structures such as the aorta and the left atrial free wall. Being able to visualize those structures can provide an added measure of confidence. In the image to the right, notice the patient s reduced Left Atrium, the tenting of the septum and its relationship to the LAFW. The corresponding fluoroscopic image may suggest that puncture has already occurred. However, the ULTRA ICE PLUS image shows that this is not the case and guides the physician to redirect the needle in a puncture angle away from the LAFW. * Results of one case study are not predictive of results in other cases. Results in other cases may vary.
3 By using ULTRA ICE PLUS to visualize soft tissue in real-time, the user can observe catheter movement, monitor the interactions between devices and the tissue (e.g., tenting and puncture of the fossa ovalis), and guide devices into position relative to the anatomy. Advanced Applications: Crossing to the left side ULTRA ICE PLUS provides the combination of real-time imaging and soft tissue visualization that cannot be duplicated by fluoroscopy, pre-operative imaging (CT or MR) or electroanatomic mapping. Thus, ULTRA ICE PLUS can bring valuable clinical information, either when used by itself or in conjunction with these other imaging modalities. A key application for the ULTRA ICE PLUS Catheter involves crossing the septum and then monitoring and helping to guide left-sided procedures. In this setting, ULTRA ICE PLUS Catheter is designed to allow the user to: Visualize left atrial anatomy Confirm catheter location relative to the anatomy Verify tip-to-tissue contact Identify location of the esophagus relative to the ablation catheter Characterize acute lesion morphology: swelling, dimpling, and crater formation Monitor for any early signs of thrombus formation, stenosis, or pericardial effusion ULTRA ICE PLUS
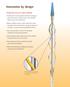
4 The ULTRA ICE PLUS Ultrasound Imaging Catheter Excellent near-field detail. Stable positioning. Intuitive interpretation. Unique Vision: 360 view of the world Rotating Drive Shaft Clear Acoustic Window Radiopaque Tip Single Large Aperture 9 MHz Transducer The radiopaque tip of the ULTRA ICE PLUS Catheter facilitates placement and serves as a fluoroscopic marker during the procedure. The area of interest is the central point in the ultrasound image, providing a clear panorama of what you want to visualize.
5 VS. ULTRA ICE PLUS Catheter 360 view Phased Array Catheter Pie-Shaped Wedge View Stable Positioning The radiopaque tip of the ULTRA ICE PLUS Catheter can be positioned directly adjacen to the area of interest (in this case the fossa) under fluoroscopic guidance. A phased array catheter must be positioned at a distance from the area of interest, then steered to bring the area into view. This requires coordination of (a) catheter advancement within the atrium, (b) distance from the septum, (c) array rotation around the catheter s axis, and (d) angle of the array relative to the septum. Detailed Near-Field Resolution; Wide Field of View The ULTRA ICE PLUS Catheter generates a panoramic 360 image perpendicular to the catheter, with the tip as a central reference point. This allows the user to visualize structures (such as the fossa) directly adjacent to the catheter tip and still see a detailed cross-section of the entire septum. Near-field detail is dependant on the position of the catheter head relative to the area of interest and the orientation of the imaging plane. The far-field image is constrained to a narrow, pie-shaped wedge view. Intuitive Interpretation The close proximity between the catheter tip and area of interest (such as the fossa) is designed to allow intuitive interpretation of the ULTRA ICE PLUS image. The tip also serves as a fluoroscopic marker to guide the placement of the transseptal dilator tip at the fossa. The distance required between the tip and the area of interest may effect the correlation between the catheter tip and anatomic structures. It may also prohibit the tip from serving as a marker for placement of other catheters.
6 The ilab Ultrasound Imaging System
7 Total lab integration. Always there, always available. ilab Ultrasound Imaging System transformed visualization during EP procedures as the first intracardiac ultrasound system customized for the EP lab. It offers an easy user -interface and automatic enhancement of ICE images. The Modular hardware design comes either installed or on a cart allowing flexibility to upgrade. The ilab system is compatible with all Boston Scientific ultrasound catheters: Intracardiac (ICE), Intravascular (IVUS) and Peripheral (PI). New, intuitive user interface Large high definition monitor Convenient touchpad interface Automatic enhancement of ULTRA ICE PLUS images Modular hardware design easy to upgrade Ordering Information Description UPN ULTRA ICE PLUS Catheter M Fluid Dock Filling Device M MDU5 PLUS Motor Drive H749MDU5PLUS0 MDU5 PLUS Sterile Bag H749MDU5PLUSBAG0 ilab Ultrasound Imaging System (Integrated) H749ILAB120N2710 ilab Ultrasound Imaging System (Cart) H749ILAB120C2710
8 ULTRA ICE PLUS Ultrasound Imaging Catheter from Boston Scientific INDICATIONS FOR USE Ultra ICE Plus Catheter: This rounded tip catheter is indicated for enhanced ultrasonic visualization of intracardiac structures. MDU5 PLUS Sterile Bag: The MDU5 PLUS Sterile Bag is intended to cover the motordrive during intravascular ultrasound procedures to maintain the sterile field and prevent transfer of microorganisms, body fluids and particulate material to the patient and healthcare worker. CONTRAINDICATIONS This product is contraindicated in the presence of conditions which create unacceptable risk during catheterization. This device is not intended to be used in the coronary arteries. This device is not intended for fetal use. WARNINGS DO NOT advance the catheter if resistance is encountered. The catheter should never be forcibly inserted into lumens narrower than the catheter body or forced through a tight stenosis. If resistance is met upon withdrawal of the catheter, verify resistance using fluoroscopy, then remove the entire system simultaneously. When utilizing a steerable guide sheath, it is not recommended to articulate the sheath tip beyond 55 degrees. Utilizing a fixed curve guide sheath with an angle greater than 55 degrees is not recommended. A guide sheath with an inner diameter less than 2.84 mm must never be utilized. When utilizing the ICE catheter, it is not recommended to place the transducer assembly within the curve of the guide sheath while imaging. PRECAUTIONS Contents supplied STERILE using a gamma radiation (Cobalt 60) process. Do not use if sterile barrier is damaged. For single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. This device should be used by physicians thoroughly trained in the techniques of invasive cardiology and in the specific approach to be used. After the procedure, inspect the catheter carefully for any damage which may have occurred during use. The catheter has no user serviceable parts. Do not attempt to repair or to alter any component of the catheter assembly as provided. Do not attempt to connect the catheter to electronic equipment other than the designated systems. Never attempt to attach or detach the catheter while the motor is running. Throughout the procedure anticoagulant therapy is recommended for patients undergoing left-sided and transseptal cardiac procedures and should be considered for selected patients undergoing right-sided procedures. Avoid any sharp bends, pinching or crushing of the catheter. Do not kink or sharply bend the catheter at any time. An insertion angle greater than 45 is considered excessive. Turn the MDU5 PLUS OFF before withdrawing the imaging catheter, or when advancing the catheter in the body. Prior to utilizing the ICE catheter, verify there are not kinks in either the ICE catheter or guide sheath. COMPLICATIONS The risks and discomforts involved in imaging cardiac structures include those associated with similar types of diagnostic procedures in the heart. However, any of these risks or discomforts may occur with greater frequency or severity than previously reported. Additionally, these complications may necessitate additional medical treatment including surgical intervention. Abnormal heart rhythms, cardiac wall injury including perforation, damage to cardiac valvular structures, death, endocarditis, hematoma, hypotension/hypertension, infection/discomfort, myocardial infarction, stroke/embolism, thrombosis, vascular wall injury including perforation. As with all procedures that utilize the Seldinger Technique for introducing a catheter into an artery, the following complications have been reported: infection and pain in the region of the insertion site, hemorrhage, arteriovenous fistula (Rev AA) ilab Ultrasound Imaging System from Boston Scientific INDICATIONS AND CONTRAINDICATIONS FOR SYSTEM USE The ilab Ultrasound Imaging System is intended for ultrasound examinations of intravascular pathology. Intravascular ultrasound is indicated in patients who are candidates for transluminal interventional procedures such as angioplasty and atherectomy. Refer to the Catheter Directions for Use provided with all Boston Scientific Ultrasound Imaging Catheters to determine compatibility with the ilab System. All Ultrasound Imaging Catheters will be referred to as Imaging Catheters throughout the remainder of this user s guide. The Imaging Catheters generate ultrasound images and are intended for ultrasound examination of vascular and cardiac pathology. Boston Scientific manufactures a wide variety of catheters for different applications. The recommended use of each of these catheters may vary depending on the size and type of the catheter. Please refer to Imaging Catheter Directions for Use, packaged with each catheter. CONTRAINDICATIONS FOR SYSTEM USE Use of the Imaging Catheter is contraindicated where introduction of any catheter would constitute a threat to patient safety. This instrument is contraindicated for fetal imaging. The contraindications include the following patient characteristics: General Bacteremia or sepsis Major coagulation system abnormalities Unsuitability for coronary artery bypass surgery Unsuitability for balloon angioplasty (PTCA) Total occlusion Severe hemodynamic instability or shock Coronary artery spasm Myocardial infarction Intra-arterial or intra-ventricle thrombosis Life-threatening rhythmic disorders Mechanical heart valves that would be crossed by the imaging catheter INDICATIONS FOR AUTO PULLBACK USE Automatic Pullback is indicated when the following occurs: The physician/operator wants to standardize the method in which intravascular ultrasound images are obtained and documented: procedure-toprocedure, operator-to-operator. The physician/operator wants to make linear distance determinations post-procedurally, which requires the imaging core of a catheter to be pulled back at a known uniform speed. Two-dimensional, longitudinal reconstruction of the anatomy is desired. CONTRAINDICATIONS FOR AUTO PULLBACK USE Use of the Automatic Pullback is contraindicated where introduction of any catheter would constitute a threat to patient safety. For further information, please consult the Imaging Catheter Directions for Use packaged with each Imaging Catheter. WARNINGS, CAUTIONS, AND PRECAUTIONS LISTS Inappropriate use of the ilab System may lead to patient illness, injury, or death. Please read this User s Guide and the package inserts for the Imaging Catheters carefully and completely before attempting to use the System..To avoid the risk of electrical shock, this equipment must only be connected to a supply mains with protective earth. Other than the fuses on the AC Power Isolation Transformer, the ilab System enclosures contain no operator-serviceable components. Refer servicing to Boston Scientific-authorized personnel only. Possible explosion hazard if used in the presence of flammable anesthetics. Use only Imaging Catheters that are specifically approved for the ilab System. For instructions on proper disposal methods for the following consumable items, please refer to the Directions for Use packaged with the item: Disposable Sled Motordrive Sterile Bag Imaging Catheter (and packaged accessories) Tableside Controller Drape Care should also be exercised in adjusting all settings to avoid obscuring low-level signals that may have diagnostic value. Improper settings can seriously degrade image quality. Do not attempt to autoclave, immerse, or sterilize the Motordrive Unit. The user has the ultimate responsibility for any use of these measurements in the direction of interventions. CAUTIONS LIST Ensure that the Imaging Catheter is carefully inserted through the opening in the Motordrive Sterile Bag, without catching any part of the Bag between the Imaging Catheter and the Motordrive. Do not attempt to manually move the Motordrive in the Sled once it is installed in a Sled without first depressing the Release Lever. The AC Power Isolation Transformer is intended to be used only with ilab System equipment. Always begin powering off the system by first using the Control Panel and then turning off the main AC power switch. For more information, refer to Powering Down the ilab System in the chapter, Using the ilab System in the User s Guide. PRECAUTIONS LIST Verify that both latches on the Sled are fully engaged with the Motordrive. Observe the precautions provided from the manufacturer of the media regarding handling, labeling, and storage. If the ilab System does not produce a usable image when connected to a Catheter Simulator, please contact your Boston Scientific representative for technical assistance. NOTE: Medical electrical equipment requires special precautions regarding EMC. This equipment needs to be installed and put into service according to the EMC information contained within the accompanying documents. Due to the unique nature of archived (DICOM) files on CD, DVD or Removable Hard Drive media should be labeled, handled, and stored according to individual manufacturer s recommendations to avoid data loss or corruption over time. POTENTIAL SYSTEM USAGE COMPLICATIONS The following complications may occur as a consequence of intravascular or intracardiac imaging: Abrupt closure Angina Cardiac arrhythmias including but not limited to: ventricular tachycardia, ventricular fibrillation, and complete heart block Catheter/Guidewire/Pressure wire entrapment Embolism Emergent Coronary Artery Bypass Graft (CABG) surgery Infection Myocardial infarction Myocardial ischemia Myocardial perforation Stent strut damage Stroke (including Cerebral Vascular Accident and Transient Ischemic Attack) Thrombus formation Total occlusion Valvular injury Vessel dissection, injury, or perforation Vessel spasm For further information, please consult the Catheter Directions for Use packaged with each Imaging Catheter. myocardial perforation, stent strut damage, stroke (including Cerebral Vascular Accident and Transient Ischemic Attack), thrombus formation, total occlusion, valvular injury, vessel dissection, injury, or perforation, vessel spasm. For further information, please consult the Catheter Directions for Use packaged with each Imaging Catheter (Rev AB) CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the complete Directions for Use for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator s Instructions. CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for the use only in countries with applicable health authority product registrations. Information not intended for use or distribution in France. Rhythm Management 300 Boston Scientific Way Marlborough, MA Medical Professionals: CARDIAC ( ) Customer Service: by Boston Scientific Corporation or its affiliates. All rights reserved. EP AD
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Contents Ultra ICE Plus 9 MHz Imaging Catheter MDU5 PLUS Sterile Bag 26 gauge needle 10 ml (cc) syringe
 91040401-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions prior to use. Observe all contraindications, warnings and precautions
91040401-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions prior to use. Observe all contraindications, warnings and precautions
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW
 JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Contents Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter MDU5 PLUS Sterile Bag 26 gauge needle 10 ml (10 cc) syringe
 50677333-01 Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter ONLY 2018-05 < en > Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions
50677333-01 Ultra ICE Plus - PI 9 MHz Peripheral Imaging Catheter ONLY 2018-05 < en > Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Carefully read all instructions
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Advanced Innovation for Exceptional Performance
 Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Figure 1: Revolution TM Peripheral Atherectomy System Diagram. Table 1: Revolution TM Peripheral Atherectomy System Specifications Minimum Burr
 Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Innovation by design. Technology that sets a new standard
 Innovation by design Technology that sets a new standard Flexible nitinol scoring element with three rectangular spiral struts works in tandem with a semi-compliant balloon to score the target lesion Balloon
Innovation by design Technology that sets a new standard Flexible nitinol scoring element with three rectangular spiral struts works in tandem with a semi-compliant balloon to score the target lesion Balloon
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
System.
 System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
Single Pass MCA Revascularization with Trevo
 Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
ROTABLATORTM. Peripheral. Rotational Atherectomy System. Quick Reference Cards
 Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ
 BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ 1 Case 1 78 yr. old female Rutherford Class II/III lesion 1 block claudicant 2 Pre Treatment Post Treatment Anterior Tibial Artery Occlusion
BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ 1 Case 1 78 yr. old female Rutherford Class II/III lesion 1 block claudicant 2 Pre Treatment Post Treatment Anterior Tibial Artery Occlusion
Talent Abdominal Stent Graft
 Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15. Instructions for Use
 Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM
 Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION?
 DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION? Do you have atrial fibrillation ()? Do you think you might have it? If so, the time to take control is now. There are three important things to do. 1. If you
DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION? Do you have atrial fibrillation ()? Do you think you might have it? If so, the time to take control is now. There are three important things to do. 1. If you
Ruby Coil. Large Volume Detachable Coils
 Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
OptiCross MHz Peripheral Imaging Catheter
 91080532-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. WARNING Contents supplied STERILE using a Radiation process. Do not use if sterile barrier is
91080532-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. WARNING Contents supplied STERILE using a Radiation process. Do not use if sterile barrier is
Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters
 Reprocessed by Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale
Reprocessed by Instructions for Use Reprocessed Diagnostic Electrophysiology Catheters Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale
Watchman. Left Atrial Appendage Closure Device. Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8
 TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING
 Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
ASAHI Corsair INSTRUCTIONS FOR USE
 ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
Every heartbeat is precious. Thanks to you, he ll have 100 million more.
 Every heartbeat is precious. Thanks to you, he ll have 100 million more. Master the Complex Thrombectomy Portfolio FETCH 2 Aspiration Catheter ANGIOJET Ultra Thrombectomy System EVERY HEARTBEAT IS PRECIOUS
Every heartbeat is precious. Thanks to you, he ll have 100 million more. Master the Complex Thrombectomy Portfolio FETCH 2 Aspiration Catheter ANGIOJET Ultra Thrombectomy System EVERY HEARTBEAT IS PRECIOUS
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY
 SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
SHARED DECISION MAKING: AN EVIDENCE-BASED CORNERSTONE OF LAAC THERAPY SHARED DECISION MAKING: AN EVIDENCE BASED CORNERSTONE OF LAAC THERAPY Shared decision making is a collaborative process that allows
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
Management of Acute DVT Extending From the Tibial Veins to the Common Iliac Vein Using the AngioJet Thrombectomy System
 FEATURED TECHNOLOGY: ANGIOJET THROMBECTOMY SYSTEM Sponsored by Boston Scientific Corporation Management of Acute DVT Extending From the Tibial Veins to the Common Iliac Vein Using the AngioJet Thrombectomy
FEATURED TECHNOLOGY: ANGIOJET THROMBECTOMY SYSTEM Sponsored by Boston Scientific Corporation Management of Acute DVT Extending From the Tibial Veins to the Common Iliac Vein Using the AngioJet Thrombectomy
CTO Product Portfolio
 CTO Product Portfolio 1 2 CTO Recanalization Via ATHERECTOMY Occluded anterior tibial artery Tip of Crosser Catheter 3 4 Central lumen vessel recanalization by the Crosser Catheter The Crosser Catheter
CTO Product Portfolio 1 2 CTO Recanalization Via ATHERECTOMY Occluded anterior tibial artery Tip of Crosser Catheter 3 4 Central lumen vessel recanalization by the Crosser Catheter The Crosser Catheter
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU)
 Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Trans-septal Catheterization. December 8, Jonathan Tobis, MD Professor of Medicine Interventional Cardiology, UCLA
 Trans-septal Catheterization December 8, 2015 Jonathan Tobis, MD Professor of Medicine Interventional Cardiology, UCLA No conflicts of interest for this talk BRK = Brockenbrough needle BRK may be easier
Trans-septal Catheterization December 8, 2015 Jonathan Tobis, MD Professor of Medicine Interventional Cardiology, UCLA No conflicts of interest for this talk BRK = Brockenbrough needle BRK may be easier
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES. Medtronic Further. Together
 DRUG-COATED BALL0ON TREATMENT FOR PATIENTS WITH INTERMITTENT CLAUDICATION: INSIGHTS FROM THE IN.PACT GLOBAL FULL CLINICAL COHORT MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES Medtronic Further. Together
DRUG-COATED BALL0ON TREATMENT FOR PATIENTS WITH INTERMITTENT CLAUDICATION: INSIGHTS FROM THE IN.PACT GLOBAL FULL CLINICAL COHORT MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES Medtronic Further. Together
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
Coronary angiography and PCI
 Coronary arteries Coronary angiography and PCI Samo Granda, Franjo Naji Department of Cardiology Clinical department of internal medicine University clinical centre Maribor Coronary arteries Atherosclerosis
Coronary arteries Coronary angiography and PCI Samo Granda, Franjo Naji Department of Cardiology Clinical department of internal medicine University clinical centre Maribor Coronary arteries Atherosclerosis
COIL SYSTEM ORDERING INFORMATION
 EMBOLIZATION SYSTEM RUBY COIL SYSTEM FRAME with Ruby Standard FILL with Ruby Soft Now with 3 & 40 mm sizes! SYSTEM ANCHOR with Now designed to embolize 3 4 mm vessels! PACK with Coil Now with WAVE Shape
EMBOLIZATION SYSTEM RUBY COIL SYSTEM FRAME with Ruby Standard FILL with Ruby Soft Now with 3 & 40 mm sizes! SYSTEM ANCHOR with Now designed to embolize 3 4 mm vessels! PACK with Coil Now with WAVE Shape
EP lab robotic support
 EP lab robotic support Philips and Hansen team to enhance complex EP procedures Who/where J. Michael Mangrum, MD Associate Professor of Medicine and Director of Atrial Fibrillation Center University of
EP lab robotic support Philips and Hansen team to enhance complex EP procedures Who/where J. Michael Mangrum, MD Associate Professor of Medicine and Director of Atrial Fibrillation Center University of
Intravascular Ultrasound
 Scan for mobile link. Intravascular Ultrasound Intravascular ultrasound (IVUS) uses a transducer or probe to generate sound waves and produce pictures of the coronary arteries. IVUS can show the entire
Scan for mobile link. Intravascular Ultrasound Intravascular ultrasound (IVUS) uses a transducer or probe to generate sound waves and produce pictures of the coronary arteries. IVUS can show the entire
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Lotus transducer Instructions for use
 Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
VT Ablation in Structural Heart Disease Patient Information
 Melbourne Heart Rhythm VT Ablation in Structural Heart Disease Patient Information Ventricular Tachycardia in Structural Heart Disease (VT-SHD) Ventricular tachycardia (VT) is an abnormal rapid heart rhythm
Melbourne Heart Rhythm VT Ablation in Structural Heart Disease Patient Information Ventricular Tachycardia in Structural Heart Disease (VT-SHD) Ventricular tachycardia (VT) is an abnormal rapid heart rhythm
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
INSTRUCTIONS FOR USE
 PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
eptfe Vascular Graft Instructions for Use
 TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
CPT Code Details
 CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
Understanding aneurysms and flow diversion treatment
 Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Cardiac-Interventional Radiography
 STRUCTURED EDUCATION REQUIREMENTS Cardiac-Interventional Radiography The purpose of structured education is to provide the opportunity for individuals to develop mastery of discipline-specific knowledge
STRUCTURED EDUCATION REQUIREMENTS Cardiac-Interventional Radiography The purpose of structured education is to provide the opportunity for individuals to develop mastery of discipline-specific knowledge
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
1 Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis. N Engl J Med 352;13, March 31, 2005
 The risk of ischemic stroke in patients with Intracranial Atherosclerotic Disease (ICAD) ranges from 7 to 24%. 1,2 Developed specifically for the treatment of ICAD, the Wingspan Stent System and Gateway
The risk of ischemic stroke in patients with Intracranial Atherosclerotic Disease (ICAD) ranges from 7 to 24%. 1,2 Developed specifically for the treatment of ICAD, the Wingspan Stent System and Gateway
Access More Patients. Customize Each Seal.
 Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Cardiac, Vascular and Transplant Surgery Quality Assessment. Intraoperative Ultrasound Imaging and TTFM
 Cardiac, Vascular and Transplant Surgery Quality Assessment Intraoperative Ultrasound Imaging and TTFM Reduce risk of early graft failure, stroke, myocardial infarction or recurrent angina and provide
Cardiac, Vascular and Transplant Surgery Quality Assessment Intraoperative Ultrasound Imaging and TTFM Reduce risk of early graft failure, stroke, myocardial infarction or recurrent angina and provide
8 Performing Medical Procedures
 8 The Terason usmart3200t Ultrasound System can aid in performing medical procedures such as biopsies. Depending on whether you purchased the additional equipment required for these procedures, you may
8 The Terason usmart3200t Ultrasound System can aid in performing medical procedures such as biopsies. Depending on whether you purchased the additional equipment required for these procedures, you may
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Emerge. PTCA Dilatation Catheter ONLY. monorail. over-the-wire
 90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
New Cardiac Technologies. Melva Meminger MSN, RN, CCRN
 New Cardiac Technologies Melva Meminger MSN, RN, CCRN What s New in Cardiac Care? Bigger is not always better: the new smaller Loop recorders First we burned and now we freeze (sometimes!): Cryoablation
New Cardiac Technologies Melva Meminger MSN, RN, CCRN What s New in Cardiac Care? Bigger is not always better: the new smaller Loop recorders First we burned and now we freeze (sometimes!): Cryoablation
Instructions for Use io-flex Probe
 DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT)
 Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT) Clare McLaren Great Ormond Street Hospital London Introduction IVUS and OCT supplementary techniques to angiography provide information
Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT) Clare McLaren Great Ormond Street Hospital London Introduction IVUS and OCT supplementary techniques to angiography provide information
THINK OUTSIDE THE PILLBOX
 THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
THINK OUTSIDE THE PILLBOX An innovative one-time procedure that reduces the risk of stroke in your non-valvular atrial fibrillation (NVAF) patients and the long-term risk of bleeding that comes with a
Intracardiac EchoCardiography (ICE) Common Views
 Intracardiac EchoCardiography (ICE) Common Views Introduction What is ICE? Catheter with microscopic ultrasound transducer tip and doppler capabilities inserted into the heart via the IVC (typically) or
Intracardiac EchoCardiography (ICE) Common Views Introduction What is ICE? Catheter with microscopic ultrasound transducer tip and doppler capabilities inserted into the heart via the IVC (typically) or
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Patient Information. Atrial Septal Defect (ASD) Repair
 Patient Information Atrial Septal Defect (ASD) Repair Table of Contents Overview................................. 4 Symptoms................................ 5 Causes..................................
Patient Information Atrial Septal Defect (ASD) Repair Table of Contents Overview................................. 4 Symptoms................................ 5 Causes..................................
Transjugular liver access and biopsy
 Transjugular liver access and biopsy An illustrated guide Illustrations by Lisa Clark LABS LIVER ACCESS AND BIOPSY SET MEDICAL Support your procedures by using products that are specifically designed for
Transjugular liver access and biopsy An illustrated guide Illustrations by Lisa Clark LABS LIVER ACCESS AND BIOPSY SET MEDICAL Support your procedures by using products that are specifically designed for
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
Indications. The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli
 Indications Straight Cannula or Cannula with 20 O Angle 17F Working Channel The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli Radiopaque
Indications Straight Cannula or Cannula with 20 O Angle 17F Working Channel The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli Radiopaque
Cardioplegia Circuit Products { ANTEGRADE}
 Cardioplegia Circuit Products { ANTEGRADE} Antegrade cannulae are designed to deliver cardioplegia solution to the heart via the coronary ostia in the normal direction of blood flow (antegrade perfusion).
Cardioplegia Circuit Products { ANTEGRADE} Antegrade cannulae are designed to deliver cardioplegia solution to the heart via the coronary ostia in the normal direction of blood flow (antegrade perfusion).
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
TREATING PAROXYSMAL ATRIAL FIBRILLATION WITH CRYOBALLOON ABLATION
 TREATING PAROXYSMAL ATRIAL FIBRILLATION WITH CRYOBALLOON ABLATION ABOUT YOUR AF Atrial fibrillation (AF or Afib) is an irregular heart rhythm that affects the upper chambers (atria) of the heart. This
TREATING PAROXYSMAL ATRIAL FIBRILLATION WITH CRYOBALLOON ABLATION ABOUT YOUR AF Atrial fibrillation (AF or Afib) is an irregular heart rhythm that affects the upper chambers (atria) of the heart. This
LeMaitre Embolectomy Catheter
 Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
