MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE
|
|
|
- Crystal Crawford
- 5 years ago
- Views:
Transcription
1 MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE Guideline Title Medicinal Products in the Treatment of Cardiac Failure Legislative basis Directive 75/318/EEC as amended Date of first adoption November 1988 This version November 1995 Date of entry into June 1996 force Status Last revised November 1995 Previous titles/other CPMP/235/95 references Additional Notes This note for guidance is intended to provide guidance for the clinical investigation of medicinal products in the treatment of cardiac failure. It should be read in conjunction with Part 4 of the Annex of Directive 75/318/EEC as amended, and all other relevant guidelines as applicable and is intended to assist in the application of the Directive. This version replaces the previous 1988 guideline: Medicinal Products in the Treatment of Cardiac Failure. CONTENTS 1. INTRODUCTION 2. CLINICAL TRIALS 3. CRITERIA OF EFFICACY 4. METHODS TO ASSESS EFFICACY 5. SELECTION OF PATIENTS 6. STRATEGY - DESIGN 7. SAFETY ASPECTS ANNEX 263
2 MEDICINAL PRODUCTS IN THE TREATMENT OF CARDIAC FAILURE This note is intended to provide guidance for the clinical investigation of medicinal products in the treatment of cardiac failure. It should be read in conjunction with Part 4 of the Annex to Directive 75/318/EEC, as amended, and all other relevant guidelines as applicable and is intended to assist in the application of the Directive. 1. INTRODUCTION Cardiac failure (C.F.) is a clinical syndrome caused by an abnormality of the heart and manifest as symptoms and signs on exercise and ultimately at rest. Cardiac failure is a heterogeneous syndrome with a variety of causes. The abnormality of the heart may be related to primary impairment of myocardial function or to (chronic) pressure and/or volume overload. Cardiac failure can be acute or chronic and usually becomes progressively worse, the rate of this progression being dependent both on the primary pathology and the activity of the so called compensatory processes, the most important of these being neural, endocrine, renal and morphological. Although some of these processes (e.g. atrial natriuretic peptide) are beneficial, most (e.g. renin-angiotensin system) are detrimental and lead to peripheral or pulmonary vasoconstriction and create a vicious cycle of deteriorating circulatory function. The prognosis in C.F. is poor. Acute C.F. may lead to death in minutes. For patients with chronic CF in NYHA functional class IV (=symptomatic at rest), the 1-year mortality rate is at least 50%. Less than half of the patients survive five years after first diagnosis of C.F. Sudden death is common, ranging from 30% to 60% of the total number of deaths according to the series. High output heart failure is not considered in this guideline because the primary abnormality is not the heart and its the function is not reduced at rest. Neither does this guideline apply to asymptomatic left ventricular dysfunction. 2. CLINICAL TRIALS All parameters of scores or endpoints to assess efficacy in clinical trials should be defined prior to the start of the trial and included in the protocol. Parameters which are developed during the analysis are unlikely to be acceptable. Major efficacy endpoints in heart failure do not correlate with each other. This has important implications in the overall design of clinical trials. One parameter cannot necessarily serve as a reliable surrogate for others. To demonstrate that a particular medicinal product produces improvement in the major treatment endpoints in congestive heart failure, each endpoint must be studied as a n independent variable. Composite endpoints, specified a priori and when justified, may also be appropriate. As the therapeutic goals in established cardiac failure may be diverse, i.e. improvement i n symptoms and morbidity and reduction in mortality, attention should be paid to each of these 265
3 3CC18a aspects, even when no specific claims are made. Both benefits and risks should be clearly defined during the course of the development of a medicinal product in order to make a reasoned decision regarding its approval. 3. CRITERIA OF EFFICACY 3.1 Acute cardiac failure This condition presents as a medical emergency. It is usually linked to acute failure of the left ventricle and may occur in the course of chronic failure or as a primary event, e.g. i n myocardial infarction or following cardiac surgery. The main aim of treatment is to improve the patients' haemodynamic state within minutes to hours and equally importantly, relieve their symptoms and other manifestations of acute cardiac failure. Depending on the indications claimed, mortality is also an endpoint of importance in patients treated for acute cardiac failure. In addition to a positive effect on clinical status and haemodynamics, in-hospital mortality is a valid endpoint reflecting well the risk/benefit ratio of a new medicinal product for acute C.F. Data gained during studies in acute C.F. are very useful in planning trials in chronic C.F., but it has to be emphasised that these studies cannot be regarded as therapeutic pilot studies in chronic C.F. 3.2 Chronic cardiac failure Whatever the clinical features, the underlying cause (e.g. coronary artery disease, arterial hypertension, cardiomyopathy) or the precipitating cause (e.g. infection, arrhythmias, pulmonary embolism), treatment may improve the patient's condition in several ways. The most important objectives in the treatment of heart failure are improvement in symptoms and signs, morbidity, and survival. Endpoints of efficacy may be divided into those that are primary (survival, morbidity, clinical status, and functional capacity) and those that are supportive (quality of life, ejection fraction and neuroendocrine status). Depending on the positive endpoints of efficacy, a marketing authorisation may be granted for an indication in chronic cardiac failure in terms of its severity, the nature of therapeutic benefit anticipated and if appropriate, any restrictions (for example, specifying subsets of clinically identifiable patients or as add-on or second-line therapy) Primary endpoints of efficacy Clinical status Improvement of clinical status (symptoms and signs) such as dyspnoea or fatigue with limitation of effort and evidence of fluid retention (venous pressure and/or oedema) are important in evaluating efficacy. Symptomatic relief is more important than improvement in clinical signs. 266
4 Exercise Capacity Although exercise testing is less subjective than improvement of clinical status, it is not a surrogate variable of the clinical status. The methodology should be accurately and fully documented. The most commonly used method is measurement of total exercise duration using a bicycle or treadmill protocol. This parameter is objective, quantifiable and a useful tool for the evaluation of medicinal product efficacy in patients with cardiac failure. Alternatively, the 6-minute walk test appears to be reproducible and more closely correlated to daily activities Morbidity Cardiac failure has a high morbidity due to progression of the underlying disease, often requiring therapeutic intervention, a visit in the emergency department, or hospitalisation and changes in background therapy. A favourable influence on the natural course of the disease has become another objective of the treatment Survival This information is of the greatest interest since studies have demonstrated that certain medicinal products given to patients with symptomatic cardiac failure (NYHA Classes II - IV) are associated with a significant reduction in mortality. However, other medicinal product classes have been shown to increase mortality (despite an improvement in clinical status and/or exercise capacity) e.g. agents acting through an increase in the intracellular c-amp concentration such as phosphodiesterase inhibitors and the sympathomimetic agents. Survival studies are therefore required when specific claims are made and for safety reasons Supportive endpoints of efficacy Quality of life Prominent components of quality of life measures which require addressing are physical function, social and emotional function, intellectual function, symptoms and their consequences, occupational activities, job satisfaction, leisure activities, sexual adjustment, perceived health status, life satisfaction and interpersonal relationships. Various quality of life questionnaires have been used in the past and new ones devised. Until these have been fully validated, evidence of efficacy derived from quality of life questionnaires must be viewed as supportive only Haemodynamic state Although some haemodynamic parameters such as ejection fraction, cardiac index and wedge pressure are good predictors of prognosis, the correlation of other haemodynamic variables with prognosis is either poor or has not been established. Neither do they correlate with the quality of life. Thus haemodynamic data alone are insufficient to demonstrate benefit and their value as surrogate endpoints of benefit is highly questionable. Haemodynamic studies may be useful for determining the mode of action of a medicinal product, defining dose response relationships and in demonstrating changes in a patient's state over the study period relative to baseline rather than absolute measures reproducible from patient to patient. 267
5 3CC18a Neuroendocrine status Current understanding of the pathophysiology of heart failure emphasises the role of other organs particularly the autonomic nervous system and various peptide hormones. Therefore information on changes in neuroendocrine parameters may be included as supportive data only. 4. METHODS TO ASSESS EFFICACY There are many diverse methods available for studying patients with heart failure when investigating the efficacy and the safety of new therapeutic interventions. A number of endpoints of efficacy are subject to placebo effects. Changes in the efficacy variables may also be brought about by changes in concomitant medications. Their influences on efficacy endpoints should be carefully considered and critically scrutinised. Methods which can be used to evaluate efficacy are the following: 4.1 Clinical status Several systems have been proposed to assess the clinical status of the patient. The one most commonly used is the classification system of the New York Heart Association. However, its use is limited by subjectivity, relative insensitivity and poor reproducibility. Another classification developed more recently is the Specific Activity Scale. Changes in signs of fluid retention can be assessed by physical examination, body weight, measurement of fluid balance and the detection of pulmonary congestion by chest radiography. These changes are particularly helpful in evaluating changes following acute diuresis. The evaluation of fluid retention by physical and radiologic examination remains highly subjective and is not easily quantified. Tests of renal function and measurement of electrolytes also provide valuable information on fluid retention and tissue perfusion. 4.2 Exercise testing Exercise testing should be carried out using appropriate (maximal and/or submaximal) protocols. These protocols should be designed to reflect the capabilities of patients with cardiac failure by starting at a low workload and with small (rather than large) increments in energy requirements. Protocols should specify a priori the symptoms that will terminate the tests. Other symptoms during the exercise test are also important when evaluating the results of these tests. However, it is highly dependent on the motivation of both the patient and the physician and therefore, the patient should first be made familiar with the technique before the patient is included in the trial and variability between two tests should be less than 15% and kept to a minimum. Maximal exercise testing may also provide complementary information. The value of laboratory-based treadmill or bicycle exercise tests may be enhanced by the measurement of respiratory variables of gaseous exchange in a small but adequate number of patients during Phase II studies. 268
6 4.3 Morbidity As heart failure has a progressive nature, subjective and objective evidence of worsening heart failure severe enough to require a therapeutic intervention may be used as endpoint for efficacy. This may be indicated by a change in the background therapy, a visit to the emergency department, or hospitalisation. A decrease in frequency of hospitalisations may also be an endpoint of interest if there is no increase in mortality due to the investigational medicinal product. In multicentre studies, any effects due to large inter-centre differences in the need for changes in therapy or hospitalisations should be carefully and critically considered, if this is to serve as a valid efficacy endpoint. 4.4 Survival As surrogate end points for survival are not available, the effect of any therapeutic intervention on mortality can be assessed in the context of a randomised placebo-controlled trial in a large number of patients. Survival studies using positive control medicinal product(s) may be acceptable Mortality Data Even if survival is not the end point of the study, it is mandatory to report all mortality data. These data should be specified with regard to underlying cause and a distinction should be made between instantaneous death which was unexpected (almost certainly due to arrhythmias), death due to acute deterioration of clinical status (e.g. due to a myocardial infarction) and death due to chronic progression of heart failure. Deaths due to any other intercurrent events (e.g. stroke or pulmonary embolism) should also be distinguished from cardiac deaths Mortality Endpoint When a reduction in mortality is claimed as an indication, long-term controlled studies will be necessary to confirm the therapeutic benefit. Data should be gathered so as to enable evaluation of the clinical causes of reduction in mortality (such as arrhythmias, stroke, pulmonary emboli). 4.5 Quality of life A broadly based assessment of the quality of life scales is recommended in heart failure studies because almost all the components of the life quality may be influenced by an intervention for heart failure. It is particularly important to consider whether (a) the scale is linear over the range of measurements, (b) is sensitive to the changes anticipated, (c) it is valid and useful to adjust results using the baseline scores, (d) there is any correlation between the score and the objective responses, (e) the observer and the patient should be blinded and (f) training of both the observer and the patient is necessary. Rating scales to assess quality of life should also be considered and should have been validated beforehand in the context of the proposed trial and its aims. The effects of therapy on daily activity and self-care, sleep, recreational and pleasure activities, in performing social roles, intellectual and cognitive functions, life satisfaction and expectations from the 269
7 3CC18a therapy require particular assessment. The Minnesota Living With Heart Failure Questionnaire is one of the many systems used in cardiac failure. Translations of questionnaires used should also have been well validated beforehand. Nevertheless, at present these data must be viewed as supportive only. 4.6 Haemodynamic effects Ventricular dysfunction is the hallmark of cardiac failure. A variety of techniques are available for both non-invasive and invasive measurements of ventricular function. Confounding factors e.g. increased pulmonary and/or systemic vascular resistance especially during the first invasive study should be carefully taken into account. Changes in various invasive and non-invasive measures of left ventricular performance have not been shown to correlate closely with each other and many of them do not correlate with clinical status or functional capacity. Although haemodynamic data are valuable i n defining dose response relationships, the value of these measures in evaluating the efficacy of the medicinal product in patients with cardiac failure during long-term treatment is very limited, if at all. Therefore, haemodynamic data which may include ventricular dimensions, ejection fraction and indice of systolic and diastolic functions (e.g. LVedp) should be regarded as supportive only Acute C.F. Invasive studies (e.g. cardiac output, cardiac index, contractility index, dp/dt, filling pressures) are desirable for trials dealing with acute C.F. Whilst these studies may be useful for some pilot studies in chronic C.F., to define a dose response relation, their value i n establishing the dose for pivotal studies now appears questionable Chronic C.F. The use of these newer techniques used to study medicinal product action and efficacy and safety in heart failure must be validated beforehand and justified. Non-invasive techniques including echocardiography, Doppler studies and systolic time intervals have been proven to be objective and quantifiable. They have particular appeal i n evaluating systolic ventricular dysfunction but some of these techniques show inter-operator variability. Measurement of ejection fraction by an isotopic method and/or by echocardiography is desirable to quantify the degree of systolic ventricular dysfunction and its response to treatment. They are also useful in defining patient subgroups (e.g. systolic versus diastolic dysfunctions). In view of the inter-centre variability of norms, the investigators from each centre should specify the norms for their laboratory and the criteria adopted for recruitment of patients and evaluation of efficacy. 4.7 Neuroendocrine status Because of the importance of the neuroendocrine systems in patients with cardiac failure and their modulation by therapeutic interventions, measurement of effects on neurohumoral compensatory mechanisms is highly desirable in the evaluation of a new therapeutic agent. 270
8 This applies specially to effects on the renin-angiotensin system, atrial natriuretic factor and sympathetic nervous system. The data, however, must be regarded as supportive only. 5. SELECTION OF PATIENTS The criteria used for the diagnosis of cardiac failure in patients recruited into the clinical trials must be clearly defined. Criteria for patient selection and stratification should be stated clearly with reference to the normal values for each criterion at each centre. 5.1 Acute cardiac failure Patients hospitalised with acute left ventricular failure should be defined according to underlying disease, severity and concomitant treatment. 5.2 Chronic cardiac failure Patients should suffer from dyspnoea and/or fatigue and should exhibit some criteria of systolic and/or diastolic left ventricular dysfunction. Patients with all grades of cardiac failure should be studied. Separate studies should be designed for ambulatory patients and for hospitalised patients. Treatment groups should be balanced in respect of patient demography, underlying cause, systolic or diastolic dysfunction, severity of disease and duration of symptoms. The question of aetiology must be carefully examined and the proportion of patients with ischaemic and non-ischaemic cardiomyopathy must be specified. Since the response to treatment may vary depending on the severity of the C.F., it is essential that the patients chosen for study should exhibit a wide range of severity of C.F. Alternatively, the same information may be obtained by restricting clinical trials to specific severity of disease for example, one study to patients with mild heart failure and the other to patients with severe heart failure. Only with this approach is it possible to formulate the most appropriate indications and contraindications. It is not unusual for a medicinal product effective in mild heart failure to be detrimental i n severe heart failure. Stability of the patients recruited into the trials deserves special attention Patients The natural history of chronic C.F. is of relapses and remissions, the long-term course being downhill. The rate of progression varies from patient to patient. Patients who have had an acute episode or a period in hospital within the preceding 6 weeks (3 months in the case of acute myocardial infarction) should be excluded because their cardiac status may be unstable. In patients with severe cardiac failure, a shorter period, for example 2 weeks, might be sufficient. It is advisable to exclude patients with a short history of disease (e.g. less than 3 months) Background treatment When the medicinal product under evaluation is added to previous treatment it is mandatory to avoid any change and/or adjustment in medication during the 2 weeks preceding the study. 271
9 3CC18a 6. STRATEGY - DESIGN 6.1 Initial Tolerance Studies Studies involving the first administration of medicinal products for C.F. to man do not essentially differ from those dealing with other cardioactive medicinal products Pharmacodynamics These studies will include data on haemodynamic parameters, effects on the (intra-cardiac) impulse formation and conduction, neurohumoral parameters, renal and pulmonary effects, and tolerance. Varying severities of heart failure, ranging from the mild to the severe, need to be studied. The pharmacodynamic activity of the substance needs to be defined as much as possible with regard to cardiac contractility, arterial and venous tone, myocardial oxygen consumption, and diastolic/systolic function and the absence of a proarrhythmic effect of the investigational medicinal product Pharmacokinetics The pharmacokinetic information required is stated in detail in the appropriate guidelines on Pharmacokinetic Studies in Man. In this context it is important to bear in mind that medicinal product absorption, distribution, metabolism and excretion as well as its delivery to various tissues may be altered substantially during the treatment of congestive heart failure. Apart from the pharmacokinetic studies in healthy volunteers, studies should be performed in the elderly, in patients with varying degrees of congestive heart failure and i n patients with varying degrees of renal dysfunction and/or hepatic dysfunction. The pharmacological activity of the main metabolites should be quantified and studied i n detail if they are likely to contribute substantially to the therapeutic or toxic effects. 6.2 Initial therapeutic studies The objectives will be to identify patients who may benefit from the medicinal product and to determine the appropriate therapeutic range including dose-concentration-response relationship. Although small controlled pilot studies are by no means impossible, trials which are not blinded and without a control group are acceptable early in this phase of investigation. When sufficient data are gained controlled studies are necessary. Dose ranging studies i n congestive heart failure of patients should thoroughly assess the lower end of the effective dose range. A parallel, fixed dose, double-blind placebo controlled design has proved useful in evaluating new medicinal products. At least 3 dosages should be studied (low, medium, high) with a total therapy phase of at least 12 weeks. Such studies should assess clinical status as well as haemodynamic responses (invasively and/or non-invasively if well validated). The dose schedule selected for pivotal studies must be justified on the basis of pharmacokinetic and pharmacodynamic data in the target population. Based on the information from dose-concentration and concentration-response relationships, dose schedules should be clearly defined for patients with varying degrees of congestive heart failure, renal dysfunction and/or hepatic dysfunction. 272
10 6.3 Main therapeutic studies Acute C.F. Randomisation is mandatory but double-blinding may not be possible. The trial should continue not only until the haemodynamic and clinical status of the patient is normal, or what is regarded as normal for that patient (such an endpoint should be described in the report) but until the patient is discharged from the hospital. It is appreciated that although the patient may not be in receipt of the investigational medicinal product for the whole of this period, an adequate in-hospital follow up is essential for evaluation of post-therapeutic risk/benefit of the investigational medicinal product Chronic C.F. A run-in period of not less than two weeks is recommended during which the investigator must carry out full clinical and laboratory assessment and verify the stability of patient's clinical status and performance in exercise testing. Controlled double-blind randomised studies are required. A control group on active comparator is preferable. It is always useful to include a placebo group and when it is proposed to indicate the investigational medicinal product as an add-on to an existing treatment, a placebo group is mandatory. The decision to include a placebo group in other situations should only be made after careful scientific and ethical considerations. Groups should be as similar as possible in respect of age, sex, pathology, state of disease, severity of disease and duration of symptoms. Stratified allocation may be desirable. Background treatment should be identical. Controlled studies of at least three months' duration are mandatory to demonstrate efficacy in relation to symptomatic benefit or morbidity. Long-term controlled studies (at least one year in duration) will certainly be required in order to confirm a specific claim of reduction in mortality. Although in principle, one large well controlled trial of adequate statistical power may be sufficient to confirm the efficacy of a new medicinal product provided it is soundly based and well designed, executed and reported in practice these ideals are difficult to achieve in the field of heart failure. Hence, for medicinal products tested for the treatment of chronic cardiac failure, it is prudent to plan at least two such trials (see guideline on Biostatistical Methodology in Clinical Trials, section 2.2). Sample sizes should be chosen so that a predefined clinically relevant difference between the treatment groups can be recognised as being statistically significant. The applicant must indicate clearly the degree(s) of severity for which the medicinal product is appropriate and also the criteria by which suitable patients could be identified for medicinal product administration clinically. 7. SAFETY ASPECTS As treatment in C.F. is usually prolonged, long-term data on adverse effects and interactions should be provided. If the investigational medicinal product belongs to a new pharmacological class or when agents in the same class have been associated with detrimental effects, a prospective, randomised, controlled survival study will be required i n order to establish safety over a minimum period of 12 months in patients with chronic heart failure. 273
11 3CC18a All adverse effects occurring during the course of clinical trials should be fully documented. Any groups specially at-risk should be identified. Any information available concerning clinical features and therapeutic measures in accidental overdosage or deliberate selfpoisoning should be provided. Special efforts should be made to assess potential adverse effects that are characteristic of the class of medicinal product being investigated. Particular attention should be paid to the following specific adverse effects: 7.1 Hypotension This may be either symptomatic or asymptomatic. Special attention should be paid to firstdose phenomenon, hypotension following an increase in dose and postural hypotension. 7.2 End-organ consequences (kidney, heart, CNS) Effect of alterations in regional blood flow in other organ systems, especially the kidney, heart and brain, may be studied. Special emphasis should be put on renal function and electrolyte homeostasis. 7.3 Effect on cardiac rhythm It is essential to investigate the potential for proarrhythmic effects. These investigations should include electrocardiography and continuous ambulatory monitoring which may require to be supplemented by some electrophysiologic studies. 7.4 Pro-ischaemic effects Medicinal products in heart failure may increase myocardial oxygen consumption. Together with potential hypotensive effects, this may lead to angina pectoris and myocardial infarction. 7.5 Morbidity and mortality This has already been discussed under 4.3 and Interactions Considerable progress has been made recently on the rational investigation of medicinal product interactions. Apart from investigating the pharmacokinetic and the pharmacodynamic interactions with widely used agents in the target population, interactions with other substrates of the same isozyme should be investigated. It is recognised that medicinal product interactions may be predicted on the basis of isozymes involved in the metabolism of the new medicinal product. Interactions are most likely with other substrates and/or inhibitors of that isozyme. It has become evident that interactions predicted from enzyme kinetic considerations have later been shown to occur in vivo. Studies to exclude any interactions between anti-failure medicinal products of different modes of action or chemical classes are also essential. 274
12 ANNEX Although the note for guidance Cardiac Glycosides (Council Recommendation 87/176/EEC, Annex 111) contained valuable information with regard to bioavailability and problems arising from the low therapeutic/toxic dose ratio, it did not specify how cardiac glycosides should be evaluated clinically with regard to efficacy and safety. Ambiguity remained about the number of patients needed to be studied, the way studies should be carried out and the parameters which needed to be assessed to demonstrate efficacy especially in heart failure. Recent years have shown some major developments in this field, necessitating more detailed guidance. This note for guidance on Medicinal Products in the Treatment of Cardiac Failure addresses most of these items and is indispensable for the clinical evaluation of cardiac glycosides. The same applies for the other indications for cardiac glycosides, the treatment of supraventricular arrhythmias. The note for guidance on Medicinal Products for the Treatment of Arrhythmias provides detailed information about the evaluation of the antiarrhythmic effects, the selection of patients and safety, all of which are also essential i n the clinical investigation of cardiac glycosides. Therefore the combination of these two notes for guidance largely replaces the earlier note on cardiac glycosides. 275
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS
 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS
 MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
Heart Failure (HF) Treatment
 Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Dronedarone for the treatment of non-permanent atrial fibrillation
 Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *)
 DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER
 CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER Guideline Title Clinical Investigation of Medical Products
CLINICAL INVESTIGATION OF MEDICAL PRODUCTS IN THE TREATMENT OF GENERALISED ANXIETY DISORDER, PANIC DISORDER AND OBSESSIVE- COMPULSIVE DISORDER Guideline Title Clinical Investigation of Medical Products
DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS)
 DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS) Beta-blockers have been widely used in the management of angina, certain tachyarrhythmias and heart failure, as well as in hypertension. Examples
DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS) Beta-blockers have been widely used in the management of angina, certain tachyarrhythmias and heart failure, as well as in hypertension. Examples
Protocol Identifier Subject Identifier Visit Description. [Y] Yes [N] No. [Y] Yes [N] N. If Yes, admission date and time: Day Month Year
![Protocol Identifier Subject Identifier Visit Description. [Y] Yes [N] No. [Y] Yes [N] N. If Yes, admission date and time: Day Month Year Protocol Identifier Subject Identifier Visit Description. [Y] Yes [N] No. [Y] Yes [N] N. If Yes, admission date and time: Day Month Year](/thumbs/76/74263761.jpg) PAST MEDICAL HISTORY Has the subject had a prior episode of heart failure? o Does the subject have a prior history of exposure to cardiotoxins, such as anthracyclines? URGENT HEART FAILURE VISIT Did heart
PAST MEDICAL HISTORY Has the subject had a prior episode of heart failure? o Does the subject have a prior history of exposure to cardiotoxins, such as anthracyclines? URGENT HEART FAILURE VISIT Did heart
CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *)
 3CC9a CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *) Guideline Title Clinical Investigation of Medicinal Products in Geriatrics*) Legislative basis Directive 75/318/EEC, as amended Date
3CC9a CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN GERIATRICS *) Guideline Title Clinical Investigation of Medicinal Products in Geriatrics*) Legislative basis Directive 75/318/EEC, as amended Date
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES
 CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES Guideline Title Clinical Investigation of Oral Contraceptives Legislative basis Directive 75/318/EEC as amended Date of first adoption February 1987 Date of
CLINICAL INVESTIGATION OF ORAL CONTRACEPTIVES Guideline Title Clinical Investigation of Oral Contraceptives Legislative basis Directive 75/318/EEC as amended Date of first adoption February 1987 Date of
Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases Draft
 1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
Lnformation Coverage Guidance
 Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Reflection paper on assessment of cardiovascular safety profile of medicinal products
 25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
Heart Failure. Cardiac Anatomy. Functions of the Heart. Cardiac Cycle/Hemodynamics. Determinants of Cardiac Output. Cardiac Output
 Cardiac Anatomy Heart Failure Professor Qing ZHANG Department of Cardiology, West China Hospital www.blaufuss.org Cardiac Cycle/Hemodynamics Functions of the Heart Essential functions of the heart to cover
Cardiac Anatomy Heart Failure Professor Qing ZHANG Department of Cardiology, West China Hospital www.blaufuss.org Cardiac Cycle/Hemodynamics Functions of the Heart Essential functions of the heart to cover
Cardiac Output MCQ. Professor of Cardiovascular Physiology. Cairo University 2007
 Cardiac Output MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 90- Guided by Ohm's law when : a- Cardiac output = 5.6 L/min. b- Systolic and diastolic BP
Cardiac Output MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 90- Guided by Ohm's law when : a- Cardiac output = 5.6 L/min. b- Systolic and diastolic BP
Diastolic Heart Failure. Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012
 Diastolic Heart Failure Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012 Disclosures Have spoken for Merck, Sharpe and Dohme Sat on a physician advisory
Diastolic Heart Failure Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012 Disclosures Have spoken for Merck, Sharpe and Dohme Sat on a physician advisory
FAILURE IN PATIENTS WITH MYOCARDIAL INFARCTION
 Br. J. clin. Pharmac. (1982), 14, 187S-19lS BENEFICIAL EFFECTS OF CAPTOPRIL IN LEFT VENTRICULAR FAILURE IN PATIENTS WITH MYOCARDIAL INFARCTION J.P. BOUNHOURE, J.G. KAYANAKIS, J.M. FAUVEL & J. PUEL Departments
Br. J. clin. Pharmac. (1982), 14, 187S-19lS BENEFICIAL EFFECTS OF CAPTOPRIL IN LEFT VENTRICULAR FAILURE IN PATIENTS WITH MYOCARDIAL INFARCTION J.P. BOUNHOURE, J.G. KAYANAKIS, J.M. FAUVEL & J. PUEL Departments
Heart Failure. Subjective SOB (shortness of breath) Peripheral edema. Orthopnea (2-3 pillows) PND (paroxysmal nocturnal dyspnea)
 Pharmacology I. Definitions A. Heart Failure (HF) Heart Failure Ezra Levy, Pharm.D. HF Results when one or both ventricles are unable to pump sufficient blood to meet the body s needs There are 2 types
Pharmacology I. Definitions A. Heart Failure (HF) Heart Failure Ezra Levy, Pharm.D. HF Results when one or both ventricles are unable to pump sufficient blood to meet the body s needs There are 2 types
Heart Failure Update John Coyle, M.D.
 Heart Failure Update 2011 John Coyle, M.D. Causes of Heart Failure Anderson,B.Am Heart J 1993;126:632-40 It It is now well-established that at least one-half of the patients presenting with symptoms and
Heart Failure Update 2011 John Coyle, M.D. Causes of Heart Failure Anderson,B.Am Heart J 1993;126:632-40 It It is now well-established that at least one-half of the patients presenting with symptoms and
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE
 Press Release Issued on behalf of Servier Date: June 6, 2012 PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE The new ESC guidelines for the diagnosis and
Press Release Issued on behalf of Servier Date: June 6, 2012 PROCORALAN MAKING A STRONG ENTRY TO THE NEW ESC GUIDELINES FOR THE MANAGEMENT OF HEART FAILURE The new ESC guidelines for the diagnosis and
Chapter 21: Clinical Exercise Testing Procedures
 Publisher link: thepoint http://thepoint.lww.com/book/show/2930 Chapter 21: Clinical Exercise Testing Procedures American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise
Publisher link: thepoint http://thepoint.lww.com/book/show/2930 Chapter 21: Clinical Exercise Testing Procedures American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise
The Failing Heart in Primary Care
 The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
Guideline on clinical investigation of medicinal products for the treatment of acute heart failure
 1 2 3 20 September 2012 CHMP/EWP/2986/03 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Guideline on clinical investigation of medicinal products for the treatment of acute heart failure
1 2 3 20 September 2012 CHMP/EWP/2986/03 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Guideline on clinical investigation of medicinal products for the treatment of acute heart failure
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN THE TREATMENT OF HYPERTENSION
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 19 November 1998 CPMP/EWP/238/95 Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 19 November 1998 CPMP/EWP/238/95 Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR
Copyright 2011, 2007 by Mosby, Inc., an affiliate of Elsevier Inc. Normal Cardiac Anatomy
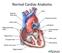 Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
COMPOSITION. A film coated tablet contains. Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar (Film coated tablets) Irbesartan
 Rotazar (Film coated tablets) Irbesartan Rotazar 75 mg, 150 mg, 300 mg COMPOSITION A film coated tablet contains Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar 75 mg, 150 mg, 300 mg PHARMACOLOGICAL
Rotazar (Film coated tablets) Irbesartan Rotazar 75 mg, 150 mg, 300 mg COMPOSITION A film coated tablet contains Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar 75 mg, 150 mg, 300 mg PHARMACOLOGICAL
Exercise Test: Practice and Interpretation. Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine
 Exercise Test: Practice and Interpretation Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine 2 Aerobic capacity and survival Circulation 117:614, 2008
Exercise Test: Practice and Interpretation Jidong Sung Division of Cardiology Samsung Medical Center Sungkyunkwan University School of Medicine 2 Aerobic capacity and survival Circulation 117:614, 2008
DOWNLOAD PDF ABC OF HEART FAILURE
 Chapter 1 : The ABCs of managing systolic heart failure: Past, present, and future Heart failure is a multisystem disorder which is characterised by abnormalities of cardiac, skeletal muscle, and renal
Chapter 1 : The ABCs of managing systolic heart failure: Past, present, and future Heart failure is a multisystem disorder which is characterised by abnormalities of cardiac, skeletal muscle, and renal
Updates in Congestive Heart Failure
 Updates in Congestive Heart Failure GREGORY YOST, DO JOHNSTOWN CARDIOVASCULAR ASSOCIATES 1/28/2018 Disclosures Edwards speaker on Sapien3 valves (TAVR) Stages A-D and NYHA Classes I-IV Stage A: High risk
Updates in Congestive Heart Failure GREGORY YOST, DO JOHNSTOWN CARDIOVASCULAR ASSOCIATES 1/28/2018 Disclosures Edwards speaker on Sapien3 valves (TAVR) Stages A-D and NYHA Classes I-IV Stage A: High risk
NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT OF SYSTEMIC EXPOSURE IN TOXICITY STUDIES S3A
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NOTE FOR GUIDANCE ON TOXICOKINETICS: THE ASSESSMENT
Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of pulmonary arterial hypertension
 5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
To Correlate Ejection Fraction with 6 Minute Walked Distance and Quality of Life in Patients with Left Ventricular Heart Failure
 To Correlate Ejection Fraction with 6 Minute Walked Distance and Quality of Life in Patients with Left Ventricular Heart Failure Pramila S Kudtarkar*, Mariya P Jiandani*, Ashish Nabar** Abstract Purpose
To Correlate Ejection Fraction with 6 Minute Walked Distance and Quality of Life in Patients with Left Ventricular Heart Failure Pramila S Kudtarkar*, Mariya P Jiandani*, Ashish Nabar** Abstract Purpose
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal. Serelaxin for treating acute decompensation of heart failure
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Serelaxin for treating acute decompensation of heart Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Serelaxin for treating acute decompensation of heart Draft scope (pre-referral) Draft remit/appraisal objective To
Supplementary material 1. Definitions of study endpoints (extracted from the Endpoint Validation Committee Charter) 1.
 Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Regulatory Experience in Reviewing CV Safety for Diabetes
 Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Topic Page: congestive heart failure
 Topic Page: congestive heart failure Definition: congestive heart f ailure from Merriam-Webster's Collegiate(R) Dictionary (1930) : heart failure in which the heart is unable to maintain an adequate circulation
Topic Page: congestive heart failure Definition: congestive heart f ailure from Merriam-Webster's Collegiate(R) Dictionary (1930) : heart failure in which the heart is unable to maintain an adequate circulation
In the name of GOD. Animal models of cardiovascular diseases: myocardial infarction & hypertension
 In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
Synopsis. Study title. Investigational Product Indication Design of clinical trial. Number of trial sites Duration of clinical trial / Timetable
 Synopsis Study title Investigational Product Indication Design of clinical trial Number of trial sites Duration of clinical trial / Timetable Repetitive levosimendan infusions for patients with advanced
Synopsis Study title Investigational Product Indication Design of clinical trial Number of trial sites Duration of clinical trial / Timetable Repetitive levosimendan infusions for patients with advanced
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
Type of intervention Treatment and secondary prevention. Economic study type Cost-effectiveness analysis.
 Cost effectiveness in the treatment of heart failure with ramipril: a Swedish substudy of the AIRE study Erhardt L, Ball S, Andersson F, Bergentoft P, Martinez C. Record Status This is a critical abstract
Cost effectiveness in the treatment of heart failure with ramipril: a Swedish substudy of the AIRE study Erhardt L, Ball S, Andersson F, Bergentoft P, Martinez C. Record Status This is a critical abstract
Heart Failure Treatments
 Heart Failure Treatments Past & Present www.philippelefevre.com Background Background Chronic heart failure Drugs Mechanical Electrical Background Chronic heart failure Drugs Mechanical Electrical Sudden
Heart Failure Treatments Past & Present www.philippelefevre.com Background Background Chronic heart failure Drugs Mechanical Electrical Background Chronic heart failure Drugs Mechanical Electrical Sudden
Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris
 Br. J. clin. Pharmac. (1987), 23, 391-396 Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris J. V. SHERIDAN, P. THOMAS, P. A. ROUTLEDGE & D. J. SHERIDAN Departments
Br. J. clin. Pharmac. (1987), 23, 391-396 Effects of felodipine on haemodynamics and exercise capacity in patients with angina pectoris J. V. SHERIDAN, P. THOMAS, P. A. ROUTLEDGE & D. J. SHERIDAN Departments
Diagnosis & Management of Heart Failure. Abena A. Osei-Wusu, M.D. Medical Fiesta
 Diagnosis & Management of Heart Failure Abena A. Osei-Wusu, M.D. Medical Fiesta Learning Objectives: 1) Become familiar with pathogenesis of congestive heart failure. 2) Discuss clinical manifestations
Diagnosis & Management of Heart Failure Abena A. Osei-Wusu, M.D. Medical Fiesta Learning Objectives: 1) Become familiar with pathogenesis of congestive heart failure. 2) Discuss clinical manifestations
Technology appraisal guidance Published: 28 November 2012 nice.org.uk/guidance/ta267
 Ivabradine adine for treating chronic heart failure Technology appraisal guidance Published: 28 November 2012 nice.org.uk/guidance/ta267 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ivabradine adine for treating chronic heart failure Technology appraisal guidance Published: 28 November 2012 nice.org.uk/guidance/ta267 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
M2 TEACHING UNDERSTANDING PHARMACOLOGY
 M2 TEACHING UNDERSTANDING PHARMACOLOGY USING CVS SYSTEM AS AN EXAMPLE NIGEL FONG 2 JAN 2014 TODAY S OBJECTIVE Pharmacology often seems like an endless list of mechanisms and side effects to memorize. To
M2 TEACHING UNDERSTANDING PHARMACOLOGY USING CVS SYSTEM AS AN EXAMPLE NIGEL FONG 2 JAN 2014 TODAY S OBJECTIVE Pharmacology often seems like an endless list of mechanisms and side effects to memorize. To
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 26 June 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON THE CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 26 June 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON THE CLINICAL
Angina pectoris due to coronary atherosclerosis : Atenolol is indicated for the long term management of patients with angina pectoris.
 Lonet Tablet Description Lonet contains Atenolol, a synthetic β1 selective (cardioselective) adrenoreceptor blocking agent without membrane stabilising or intrinsic sympathomimetic (partial agonist) activity.
Lonet Tablet Description Lonet contains Atenolol, a synthetic β1 selective (cardioselective) adrenoreceptor blocking agent without membrane stabilising or intrinsic sympathomimetic (partial agonist) activity.
Listing Form: Heart or Cardiovascular Impairments. Medical Provider:
 Listing Form: Heart or Cardiovascular Impairments Medical Provider: Printed Name Signature Patient Name: Patient DOB: Patient SS#: Date: Dear Provider: Please indicate whether your patient s condition
Listing Form: Heart or Cardiovascular Impairments Medical Provider: Printed Name Signature Patient Name: Patient DOB: Patient SS#: Date: Dear Provider: Please indicate whether your patient s condition
Therapeutic Targets and Interventions
 Therapeutic Targets and Interventions Ali Valika, MD, FACC Advanced Heart Failure and Pulmonary Hypertension Advocate Medical Group Midwest Heart Foundation Disclosures: 1. Novartis: Speaker Honorarium
Therapeutic Targets and Interventions Ali Valika, MD, FACC Advanced Heart Failure and Pulmonary Hypertension Advocate Medical Group Midwest Heart Foundation Disclosures: 1. Novartis: Speaker Honorarium
Means failure of heart to pump enough blood to satisfy the need of the body.
 Means failure of heart to pump enough blood to satisfy the need of the body. Due to an impaired ability of the heart to adequately to fill or eject blood. HEART FAILURE Heart failure (HF) means decreased
Means failure of heart to pump enough blood to satisfy the need of the body. Due to an impaired ability of the heart to adequately to fill or eject blood. HEART FAILURE Heart failure (HF) means decreased
Heart Failure. Acute. Plasma [NE] (pg/ml) 24 Hours. Chronic
![Heart Failure. Acute. Plasma [NE] (pg/ml) 24 Hours. Chronic Heart Failure. Acute. Plasma [NE] (pg/ml) 24 Hours. Chronic](/thumbs/90/103241154.jpg) Heart Failure Heart failure is the inability of the heart to deliver sufficient blood to the tissues to ensure adequate oxygen supply. Clinically it is characterized by signs of volume overload or symptoms
Heart Failure Heart failure is the inability of the heart to deliver sufficient blood to the tissues to ensure adequate oxygen supply. Clinically it is characterized by signs of volume overload or symptoms
ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS.
 European Medicines Agency London, 25 May 2005 CHMP/ICH/2/04 ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS ICH Step 4 NOTE FOR
European Medicines Agency London, 25 May 2005 CHMP/ICH/2/04 ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS ICH Step 4 NOTE FOR
Cardiac Drugs: Chapter 9 Worksheet Cardiac Agents. 1. drugs affect the rate of the heart and can either increase its rate or decrease its rate.
 Complete the following. 1. drugs affect the rate of the heart and can either increase its rate or decrease its rate. 2. drugs affect the force of contraction and can be either positive or negative. 3.
Complete the following. 1. drugs affect the rate of the heart and can either increase its rate or decrease its rate. 2. drugs affect the force of contraction and can be either positive or negative. 3.
The right heart: the Cinderella of heart failure
 The right heart: the Cinderella of heart failure Piotr Ponikowski, MD, PhD, FESC Medical University, Centre for Heart Disease Clinical Military Hospital Wroclaw, Poland none Disclosure Look into the Heart
The right heart: the Cinderella of heart failure Piotr Ponikowski, MD, PhD, FESC Medical University, Centre for Heart Disease Clinical Military Hospital Wroclaw, Poland none Disclosure Look into the Heart
NCAP NATIONAL CARDIAC AUDIT PROGR AMME NATIONAL HEART FAILURE AUDIT 2016/17 SUMMARY REPORT
 NCAP NATIONAL CARDIAC AUDIT PROGR AMME NATIONAL HEART FAILURE AUDIT 2016/17 SUMMARY REPORT CONTENTS PATIENTS ADMITTED WITH HEART FAILURE...4 Demographics... 4 Trends in Symptoms... 4 Causes and Comorbidities
NCAP NATIONAL CARDIAC AUDIT PROGR AMME NATIONAL HEART FAILURE AUDIT 2016/17 SUMMARY REPORT CONTENTS PATIENTS ADMITTED WITH HEART FAILURE...4 Demographics... 4 Trends in Symptoms... 4 Causes and Comorbidities
For Discussion Purposes Only
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Step 1 Draft 2 (July 17, 2003) Draft 3 (November 12, 2003), Draft 4 (June 10, 2004)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Step 1 Draft 2 (July 17, 2003) Draft 3 (November 12, 2003), Draft 4 (June 10, 2004)
Index of subjects. effect on ventricular tachycardia 30 treatment with 101, 116 boosterpump 80 Brockenbrough phenomenon 55, 125
 145 Index of subjects A accessory pathways 3 amiodarone 4, 5, 6, 23, 30, 97, 102 angina pectoris 4, 24, 1l0, 137, 139, 140 angulation, of cavity 73, 74 aorta aortic flow velocity 2 aortic insufficiency
145 Index of subjects A accessory pathways 3 amiodarone 4, 5, 6, 23, 30, 97, 102 angina pectoris 4, 24, 1l0, 137, 139, 140 angulation, of cavity 73, 74 aorta aortic flow velocity 2 aortic insufficiency
Metoprolol Succinate SelokenZOC
 Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
Scientific conclusions and detailed explanation of the scientific grounds for the differences from the PRAC recommendation
 Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Going to high altitude with heart disease. Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern
 Going to high altitude with heart disease Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern stefano.rimoldi@insel.ch There are very few studies on patients with heart disease going
Going to high altitude with heart disease Prof. Dr. med. Stefano Rimoldi High Altitude Medicine Inselspital Bern stefano.rimoldi@insel.ch There are very few studies on patients with heart disease going
SECONDARY HYPERTENSION
 HYPERTENSION Hypertension is the clinical term used to describe a high blood pressure of 140/90 mmhg or higher (National Institute of Health 1997). It is such a health risk the World Health Organisation
HYPERTENSION Hypertension is the clinical term used to describe a high blood pressure of 140/90 mmhg or higher (National Institute of Health 1997). It is such a health risk the World Health Organisation
The ACC Heart Failure Guidelines
 The ACC Heart Failure Guidelines Fakhr Alayoubi, Msc,R Ph President of SCCP Cardiology Clinical Pharmacist Assistant Professor At King Saud University King Khalid University Hospital Riyadh-KSA 2017 ACC/AHA/HFSA
The ACC Heart Failure Guidelines Fakhr Alayoubi, Msc,R Ph President of SCCP Cardiology Clinical Pharmacist Assistant Professor At King Saud University King Khalid University Hospital Riyadh-KSA 2017 ACC/AHA/HFSA
Medical Treatment for acute Decompensated Heart Failure. Vlasis Ninios Cardiologist St. Luke s s Hospital Thessaloniki 2011
 Medical Treatment for acute Decompensated Heart Failure Vlasis Ninios Cardiologist St. Luke s s Hospital Thessaloniki 2011 2010 HFSA guidelines for ADHF 2009 focused update of the 2005 American College
Medical Treatment for acute Decompensated Heart Failure Vlasis Ninios Cardiologist St. Luke s s Hospital Thessaloniki 2011 2010 HFSA guidelines for ADHF 2009 focused update of the 2005 American College
Amlodipine plus Lisinopril Tablets AMLOPRES-L
 Amlodipine plus Lisinopril Tablets AMLOPRES-L COMPOSITION AMLOPRES-L Each uncoated tablet contains: Amlodipine besylate equivalent to Amlodipine 5 mg and Lisinopril USP equivalent to Lisinopril (anhydrous)
Amlodipine plus Lisinopril Tablets AMLOPRES-L COMPOSITION AMLOPRES-L Each uncoated tablet contains: Amlodipine besylate equivalent to Amlodipine 5 mg and Lisinopril USP equivalent to Lisinopril (anhydrous)
Treating the patient with acute heart failure. What do we really know? Principles of acute heart failure treatment
 ESC 2012 27Aug - 3Sep, 2012, Munich, Germany Treating the patient with acute heart failure. What do we really know? Principles of acute heart failure treatment Marco Metra, MD, FESC Cardiology University
ESC 2012 27Aug - 3Sep, 2012, Munich, Germany Treating the patient with acute heart failure. What do we really know? Principles of acute heart failure treatment Marco Metra, MD, FESC Cardiology University
Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November 2010
 Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November Clinical trials in Paediatric Heart Failure List of participants: Michael Burch, Hugo Devlieger, Angeles Garcia, Daphne
Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November Clinical trials in Paediatric Heart Failure List of participants: Michael Burch, Hugo Devlieger, Angeles Garcia, Daphne
Innovation therapy in Heart Failure
 Innovation therapy in Heart Failure P. Laothavorn September 2015 Topics of discussion Basic Knowledge about heart failure Standard therapy New emerging therapy References: standard Therapy in Heart Failure
Innovation therapy in Heart Failure P. Laothavorn September 2015 Topics of discussion Basic Knowledge about heart failure Standard therapy New emerging therapy References: standard Therapy in Heart Failure
How much atrial fibrillation causes symptoms of heart failure?
 ORIGINAL PAPER How much atrial fibrillation causes symptoms of heart failure? M. Guglin, R. Chen Linked Comment: Lip. Int J Clin Pract 2014; 68: 408 9. SUMMARY Introduction: Patients with atrial fibrillation
ORIGINAL PAPER How much atrial fibrillation causes symptoms of heart failure? M. Guglin, R. Chen Linked Comment: Lip. Int J Clin Pract 2014; 68: 408 9. SUMMARY Introduction: Patients with atrial fibrillation
À ². The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 19 September 2012
 À ² The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 PROCORALAN 5 mg film-coated tablets B/56 (CIP code: 371 676-2) B/100 (CIP code: 567 208-1) PROCORALAN
À ² The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 PROCORALAN 5 mg film-coated tablets B/56 (CIP code: 371 676-2) B/100 (CIP code: 567 208-1) PROCORALAN
HEART FAILURE SUMMARY. and is associated with significant morbidity and mortality. the cornerstone of heart failure treatment.
 HEART FAILURE SUMMARY + Heart Failure is a condition affecting a large number of Irish people and is associated with significant morbidity and mortality. + ACE inhibitors, in combination with diuretics,
HEART FAILURE SUMMARY + Heart Failure is a condition affecting a large number of Irish people and is associated with significant morbidity and mortality. + ACE inhibitors, in combination with diuretics,
Pharmacological Treatment for Chronic Heart Failure. Dr Elaine Chau HK Sanatorium & Hospital, Hong Kong 3 August 2014
 Pharmacological Treatment for Chronic Heart Failure Dr Elaine Chau HK Sanatorium & Hospital, Hong Kong 3 August 2014 1 ACC/AHA 2005 guideline update for Diagnosis & management of CHF in the Adult -SA Hunt
Pharmacological Treatment for Chronic Heart Failure Dr Elaine Chau HK Sanatorium & Hospital, Hong Kong 3 August 2014 1 ACC/AHA 2005 guideline update for Diagnosis & management of CHF in the Adult -SA Hunt
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders
 C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
Summary Protocol ISRCTN / NCT REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6
 Summary Protocol REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6 Background: Epidemiology In 2002, it was estimated that approximately 900,000 individuals in the United Kingdom had a diagnosis
Summary Protocol REVIVED-BCIS2 Summary protocol version 4, May 2015 Page 1 of 6 Background: Epidemiology In 2002, it was estimated that approximately 900,000 individuals in the United Kingdom had a diagnosis
eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd
 eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd 08 June 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
eplerenone 25, 50mg film-coated tablets (Inspra ) SMC No. (793/12) Pfizer Ltd 08 June 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
Declaration of conflict of interest. None to declare
 Declaration of conflict of interest None to declare Risk management of coronary artery disease Arrhythmias and diabetes Hercules Mavrakis Cardiology Department Heraklion University Hospital Crete, Greece
Declaration of conflict of interest None to declare Risk management of coronary artery disease Arrhythmias and diabetes Hercules Mavrakis Cardiology Department Heraklion University Hospital Crete, Greece
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
National Horizon Scanning Centre. Irbesartan (Aprovel) for heart failure with preserved systolic function. August 2008
 Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
ARRHYTHMIAS AND DEVICE THERAPY
 Topic List A BASICS 1 History of Cardiology 2 Clinical Skills 2.1 History Taking 2.2 Physical Examination 2.3 Electrocardiography 2.99 Clinical Skills - Other B IMAGING 3 Imaging 3.1 Echocardiography 3.2
Topic List A BASICS 1 History of Cardiology 2 Clinical Skills 2.1 History Taking 2.2 Physical Examination 2.3 Electrocardiography 2.99 Clinical Skills - Other B IMAGING 3 Imaging 3.1 Echocardiography 3.2
Instruct patient and caregivers: Need for constant monitoring Potential complications of drug therapy
 Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency London, 18 December 2008 Doc. Ref. EMEA/CHMP/EWP/356954/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT CHMP GUIDELINE ON THE CLINICAL INVESTIGATIONS OF MEDICINAL
European Medicines Agency London, 18 December 2008 Doc. Ref. EMEA/CHMP/EWP/356954/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT CHMP GUIDELINE ON THE CLINICAL INVESTIGATIONS OF MEDICINAL
Arrhythmias and Heart Failure Dr Chris Lang Consultant Cardiologist and Electrophysiologist Royal Infirmary of Edinburgh
 Arrhythmias and Heart Failure Dr Chris Lang Consultant Cardiologist and Electrophysiologist Royal Infirmary of Edinburgh Arrhythmias and Heart Failure Ventricular Supraventricular VT/VF Primary prevention
Arrhythmias and Heart Failure Dr Chris Lang Consultant Cardiologist and Electrophysiologist Royal Infirmary of Edinburgh Arrhythmias and Heart Failure Ventricular Supraventricular VT/VF Primary prevention
Physiological Response to Hypovolemic Shock Dr Khwaja Mohammed Amir MD Assistant Professor(Physiology) Objectives At the end of the session the
 Physiological Response to Hypovolemic Shock Dr Khwaja Mohammed Amir MD Assistant Professor(Physiology) Objectives At the end of the session the students should be able to: List causes of shock including
Physiological Response to Hypovolemic Shock Dr Khwaja Mohammed Amir MD Assistant Professor(Physiology) Objectives At the end of the session the students should be able to: List causes of shock including
LCZ696 A First-in-Class Angiotensin Receptor Neprilysin Inhibitor
 The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine)
 EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
Δακτυλίτιδα και Ινότροπα Φάρμακα στην Καρδιακή Ανεπάρκεια. Ι.Κανονίδης
 Δακτυλίτιδα και Ινότροπα Φάρμακα στην Καρδιακή Ανεπάρκεια Ι.Κανονίδης Cardiac Glycosides Chronic Congestive Heart Failure DIGOXIN Na-K ATPase Na + K + Na-Ca Exchange Na + Ca ++ Ca ++ K + Na + Myofilaments
Δακτυλίτιδα και Ινότροπα Φάρμακα στην Καρδιακή Ανεπάρκεια Ι.Κανονίδης Cardiac Glycosides Chronic Congestive Heart Failure DIGOXIN Na-K ATPase Na + K + Na-Ca Exchange Na + Ca ++ Ca ++ K + Na + Myofilaments
Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to
 Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to 90 mmhg. These pressures are called Normal blood pressure
Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to 90 mmhg. These pressures are called Normal blood pressure
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Pharmacology. Drugs affecting the Cardiovascular system (Antianginal Drugs)
 Lecture 7 (year3) Dr Noor Al-Hasani Pharmacology University of Baghdad College of dentistry Drugs affecting the Cardiovascular system (Antianginal Drugs) Atherosclerotic disease of the coronary arteries,
Lecture 7 (year3) Dr Noor Al-Hasani Pharmacology University of Baghdad College of dentistry Drugs affecting the Cardiovascular system (Antianginal Drugs) Atherosclerotic disease of the coronary arteries,
CARDIOLOGY. 3 To develop in the trainees the humanistic, moral and ethical aspects of medicine.
 CARDIOLOGY (I) OBJECTIVES 1 To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism required of a specialist in Cardiology. 2 To
CARDIOLOGY (I) OBJECTIVES 1 To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism required of a specialist in Cardiology. 2 To
The ACC 50 th Annual Scientific Session
 Special Report The ACC 50 th Annual Scientific Session Part One From March 18 to 21, 2001, physicians from around the world gathered to learn, to teach and to discuss at the American College of Cardiology
Special Report The ACC 50 th Annual Scientific Session Part One From March 18 to 21, 2001, physicians from around the world gathered to learn, to teach and to discuss at the American College of Cardiology
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
Optimal Adrenergic Blockades in Heart Failure. Jae-Joong Kim MD, PhD Asan Medical Center, University of Ulsan, Seoul, Korea
 Optimal Adrenergic Blockades in Heart Failure Jae-Joong Kim MD, PhD Asan Medical Center, University of Ulsan, Seoul, Korea Contents Harmful effects of adrenergic system in heart failure Clinical studies
Optimal Adrenergic Blockades in Heart Failure Jae-Joong Kim MD, PhD Asan Medical Center, University of Ulsan, Seoul, Korea Contents Harmful effects of adrenergic system in heart failure Clinical studies
LAC-USC Cardiology Consult Service
 LAC-USC Cardiology Consult Service RESIDENT ORIENTATION First Day of Rotation: Report to 1 st day at LAC + USC Hospital 4 th floor Cardiology units Page Fellow day before rotation for more information
LAC-USC Cardiology Consult Service RESIDENT ORIENTATION First Day of Rotation: Report to 1 st day at LAC + USC Hospital 4 th floor Cardiology units Page Fellow day before rotation for more information
