Table Of Contents. Page. Introduction...1. Possible Complications...5 Routine Maintenance
|
|
|
- Jordan Griffith
- 5 years ago
- Views:
Transcription
1 * * *
2
3 Table Of Contents Page Introduction...1 Description of Catheters Placement Indications for Use Contraindications Warnings Possible Complications...5 Routine Maintenance Hemodialysis and Plasmapheresis Catheter Irrigation Procedure...6 Routine Maintenance Supplies Recommended Heparin Protocol Catheter Removal Disposal Post Dialysis Dressing Change Procedure...9 Clearing Occluded Catheters with tpa...11 Clearing Occluded Catheters with other Fibrolytic Agents Troubleshooting Guide...16 I. Aspiration Difficulties II. Patient with Fever III. Insufficient Flow IV. Catheter Exchange V. Catheter Occlusion VI. Catheter Damage
4 I ntroduction Description of Catheters The HemoStar* and HemoSplit* catheters are made of radiopaque polyurethane. The catheters come with a Tissue Ingrowth Cuff for tissue anchoring. Placement The catheter is placed via one of the large central veins with the catheter tunnelled subcutaneously for several inches to the desired exit site. The Tissue Ingrowth Cuff, attached to the catheter, is positioned in the tunnel. The cuff helps secure the catheter through fibrous tissue ingrowth and creates a physical barrier to help reduce the potential for infection caused by the migration of bacteria through the subcutaneous tunnel. 1
5 Indications For Use The Long-Term Polyurethane Hemodialysis Catheters that Bard Access Systems distributes are indicated for use in attaining short-term or long-term vascular access for hemodialysis, hemoperfusion or apheresis therapy. Access is attained via the internal jugular, subclavian vein, or femoral vein. Catheters greater than 40cm are intended for femoral vein insertion. Contraindications 1. The device is contraindicated for use in patients with severe, uncontrolled thrombocytopenia or coagulopathy. 2. The optional antimicrobial cuff should not be used on patients with known sensitivites to silver ions or collagen. Warnings Signs of Pinch-off - Subclavian Placements Only Clinical: Difficulty with blood withdrawal Resistance to infusion of fluids Patient position changes required for infusion of fluids or blood withdrawal Radiologic: Grade 1 or 2 distortion on chest X-ray. Pinch-off should be evaluated for degree of severity prior to explantation. Patients indicating any degree of catheter distortion at the clavicle/first rib area should be followed diligently. There are grades of pinch-off that should be recognized with appropriate chest x-ray as follows: 1,2 Grade Severity Recommended Action Grade 0 No distortion No action. Grade 1 Distortion present without luminal narrowing Chest x-ray should be taken every one to three months to monitor progression of pinch off to grade 2 distortion. Shoulder positioning during chest x- rays should be noted as it can contribute to changes in distortion grades. Grade 2 Distortion present with luminal narrowing Removal of the catheter should be considered. Grade 3 Catheter transection or fracture Prompt removal of the catheter. 2
6 Use of ointments containing polyethylene glycol (PEG) with this catheter can cause failure of this device. A chlorhexidine patch or bacitracin zinc ointment in petrolatum is preferred 1. Prolonged or excessive use of alcohol should be avoided. Povidone iodine solution 2 or dilute aqueous sodium hypochloride solution 3 are the recommended antiseptic solutions to be used. Ensure the solution is completely dry before applying an occlusive dressing. Follow Universal Precautions when inserting and maintaining this device. Before dialysis begins, all connections to the extracorporeal circuit must be checked carefully. During all dialysis procedures frequent visual inspection must be conducted to detect leaks and prevent blood loss or entry of air into the extracorporeal circuit and check that the extensions are intact. Excess blood leakage may lead to patient shock. Close all clamps only in the center of the extension legs. Extensions may develop cuts or tears if subjected to excessive pulling or contact with rough edges. Repeated clamping near or on the luer lock connectors may cause tubing fatigue and possible disconnection. To avoid damage to vessels and viscus, infusion pressures should not exceed 25 psi. The use of a 10cc or larger syringe is recommended because smaller syringes generate more pressure than larger syringes. Note: A three pound force on the plunger of a 3cc syringe generates pressure in excess of 30 psi whereas the same three pound force on the plunger of a 10cc syringe generates less than 15psi of pressure. Accessories and components used in conjunction with this catheter must incorporate luer-lock adapters. The heparin solution must be aspirated out of both lumens immediately prior to using the catheter to prevent systemic heparinization of the patient. Failure to clamp extensions when not in use may lead to air embolism. 3
7 In the rare event of a leak, the catheter must be clamped immediately. Necessary remedial action must be taken prior to resuming dialysis or infusion procedure. When catheter damage or connector separation occurs, the catheter should be immediately clamped or kinked closed to prevent any possibility of air embolism or loss of blood. The risk of infection is increased with femoral vein insertion. Do not resterilize the catheter or components by any method. The manufacturer will not be liable for any damages caused by reuse of the catheter or accessories. Enzymes in blood and heparin may cause temporary sticking of the extensions when clamped for extended periods of time. To release, open clamp and slide away, gently rotating the tube between fingers and thumb until the tubing separates. Repeated over tightening of blood lines, syringes and caps may reduce connector life and may lead to potential connector failure. In case of damage, clamp the catheter between the patient and the damaged area with a smooth-edged, atraumatic clamp. Soaking any part of the catheter in surgical scrubs such as Dynahex*, Betasept*, or maxicleanse is not recommended. Footnotes: 1. Catheter compatibility testing performed using Johnson & Johnson s BioPatch device. 2. Catheter compatibility testing performed using The Purdue Frederick s Co. Betadine Solution.1999 formulation. 3. Catheter compatibility testing performed using Alcavis solution formulation. 4
8 Possible Complications The use of an indwelling central venous catheter provides an important means of venous access for critically ill patients; however, the potential exists for serious complications including the following: Air Embolism Bleeding Brachial Plexus Injury Cardiac Arrhythmia Cardiac Tamponade Catheter or Cuff Erosion Through Skin Catheter Embolism Catheter or Cuff Occlusion Catheter Occlusion, Damage or Breakage due to Compression Between the Clavicle and First Rib Catheter-related Sepsis Endocarditis Exit Site Infection Exit Site Necrosis Extravasation Fibrin Sheath Formation Hematoma Hemothorax Hydrothorax Inflammation, Necrosis or Scarring of the Skin Over Implant Area Intolerance Reaction to Implanted Device Laceration of Vessels or Viscus Perforation of Vessels or Viscus Pneumothorax Spontaneous Catheter Tip Malposition or Retraction Thoracic Duct Injury Thromboembolism Venous Thrombosis Ventricular Thrombosis Vessel Erosion Risks Normally Associated with Local and General Anesthesia, Surgery, and Post-Operative Recovery These and other complications are well documented in medical literature and should be carefully considered before placing the catheter. References: 1. Hinke, D.H.; Zandt-Stastny, D.A.; Goodman, L.R.; et al. Pinch-off syndrome: A complication of implantable subclavian venous access devices. Radiology 177: , Ingle, Rebecca,; Nace, Corinne, Venous Access Devices: Catheter Pinch-off and Fracture, 1993, Bard Access Systems 3. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Warning of Impending Problems with Permanent Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp
9 Hemodialysis and Apheresis Catheter Irrigation Procedure Purpose To maintain catheter patency. Routine Maintenance Prior to initiation of therapy or reinstallation of fresh heparinized saline, the indwelling heparin should be aspirated from the catheter and the lumen(s) flushed with 10cc sterile normal saline. Flush each lumen with 10cc sterile normal saline and then heparin lock as per protocol ensuring correct volume for the catheter. Supplies Two 10cc syringes with attached 1 in. needle, filled with 10cc normal saline Two 10cc syringes filled with catheter priming volume of heparinized saline NOTE: The appropriate heparin concentration and flushing frequency should be based on the duration of the interdialytic period, patient s medical condition, laboratory tests, and prior experience. Heparin concentrations of USP units/ml have been found to be effective for maintaining the patency of hemodialysis and apheresis catheters. 1,2 6
10 Recommended Heparin Protocol 1. Aspirate heparin lock pre-dialysis. 2. Flush both lumens pre and post dialysis with a minimum 10cc saline. 3. The most effective and commonly used heparin concentration is 5,000 units/ml/lumen. 4. Total volume of each heparin solution must be equal to the internal volume of each lumen. 5. Inject heparin quickly to ensure the heparin completely fills the lumens of the catheter. 6. Maintain positive pressure while clamping extensions and attach sterile injection caps. 7. Re-heparinize with the recommended heparin concentration post dialysis. References: 1. Paul, Jan; Conrad, Diann; Dunagan, Colleen. Management of Dialysis Catheters; Journal of Intravenous Nursing, Vol. 20 No. 5, September/October Haire, William D., Edney, James A; Landmark, James D., Kessinger, Anne. Thrombotic Complications of Subclavian Apheresis Catheters in Cancer Patients: Prevention with Heparin Infusion, Journal of Clinical Apheresis (1990) 7
11 Catheter Removal The white retention cuff facilitates tissue in-growth. The catheter must be surgically removed. Free the cuff from the tissue and pull the catheter gently and smoothly. Disposal After use, the catheter and accessories may be a potential biohazard. Handle and all dispose of in accordance with accepted medical practice and applicable laws and regulations. Post Dialysis 1. To maintain patency between treatments a heparin lock must be created in each lumen of the catheter. 2. Inject 5000 units of heparin per ml. of saline (or a concentration approved by your institution) into each lumen in amounts equal to the priming volume of each lumen. To ensure that each lumen is totally filled, inject vigorously and clamp extension while under positive pressure. Attach a sterile injection cap to each clamping extension. Warning: The heparin solution must be aspirated out of both lumens immediately prior to using the catheter to prevent systemic heparinization of the patient. 8
12 D ressing Change Procedure Purpose To prevent infection of the hemodialysis catheter. Frequency Change sterile dressing over exit site at each treatment. Care and Maintenance The care and maintenance of the catheter requires well trained, skilled personnel following a detailed protocol. The protocol should include a directive that the catheter is not to be used for any purpose other than the prescribed therapy. *Aseptic technique, including face masks for the nurse and patient, handwashing and gloves must be used for procedures. Procedure 1. Follow aseptic technique throughout catheter care. 2. Wash hands thoroughly. 3. Carefully remove old dressing and discard. Avoid tugging on the catheter, or the use of scissors, or other sharp objects near the catheter. 4. The catheter hub caps or bloodline connectors should be soaked for 3 to 5 minutes in povidone iodine and then allowed to dry prior to separation. 1 Soaking any part of the catheter in surgical scrubs such as Dynahex*, Betasept*, or maxicleanse is not recommended. 5. To advoid contamination, touch the threaded portion of the connectors or allow them to come in contact with a non-sterile object. 6. The catheter exit site must be checked daily. Inspect catheter exit site for swelling, inflammation, or exudate. Notify physician if signs of infection are present. 7. Wash hands thoroughly. 8. Put on sterile gloves. 9. Clean catheter exit site with a povidone iodine solution or aqueous sodium hypochloride solution following your institution s protocol. References: 1. National Kidney Foundation, Inc. K/DOQI Update Guideline 5 9
13 Dress the Catheter Warning: Use of PEG ointments with this catheter can cause failure of this device. A chlorhexidine patch is preferred Secure the catheter to the skin using one or two sterile tape strips. 12cm Optional: Place a pre-cut gauze dressing over the exit site, fitting it snugly around the catheter. Place a 2 in. x 2 in. (5 cm x 5 cm) gauze over the pre-cut gauze and catheter. 2a. 2. Apply a cover dressing, leaving the extension legs exposed. If using an occlusive or film-style dressing, the following is recommended: 2a. Cut a 1-2 inch (3-5 cm) slit in the short side of an occlusive dressing using sterile scissors. Remove the backing sheet. 2b. 2b. Viewing catheter site through the dressing, place the dressing on the skin so that the slit is over the catheter hub. Press one side of dressing into place while holding the other side off the skin. 12cm 2c. 2c. Partially remove the frame portion of the dressing near the catheter hub that has already been secured to the skin. 12cm 2d. 2d. Overlap the unsecured side of the dressing slightly over the secured side to seal dressing under the catheter hub. Carefully remove the frame from the dressing while firmly smoothing down the edges. Smooth down the entire dressing. Do not use any ointments containing polyethylene glycol (PEG) in either the exit site care or in the catheter extension leg dressing. Chlorhexidine patches are recommended for this use. 12cm Before using any ointment, please confirm with the ointment manufacturer that the ointment does not contain polyethylene glycol or propylene glycol and is appropriate and effective for catheter exit site care prior to use. Ointments containing alcohol should also be avoided. 10
14 Footnotes: 1. Catheter compatibility testing performed using Johnson & Johnson s BioPatch devices. 2. Catheter compatibility testing performed using Fougera s Triple Antiobiotic Ointment, Warner-Lambert s Neosporin and Warner-Lambert s Polysporin formulation. References: Howard, M. Patricia, Eisenberg, Patti G. Gianino, M. Stephanie, Dressing a Centeral Venous Catheter a Better Way, Nursing, March 1992, pgs Core Curriculum for Nephrology Nursing, Third Edition: American Nephrology Nurses Association, 1995 Clearing Occluded Catheters With tpa Purpose To restore patency to an occluded catheter. Supplies 1 Sterile injection cap Alteplase (t-pa) Sterile water 2 10cc syringe 1 10cc sterile normal saline-filled syringe Sterile 3-way stopcock Povidone-iodine wipes Procedure 1. Wash hands. 2. Position patient, preferably supine, with arm below level of the heart. 3. Don gloves. 4. Prepare the Alteplase 1mg/cc (either provided in syringe form, or prepared by mixing with sterile water per package directions.) 5. Clamp catheter. 11
15 6. Remove injection cap, attach an empty 10cc syringe, release clamp, and attempt to aspirate. If aspiration is successful, withdraw clots, clamp catheter, and attach salinefilled syringe. Release clamp and flush catheter with 10ml normal saline. Clamp catheter. Replace cap per injection cap change procedure. If aspiration is unsuccessful, proceed to step Obtain physician s order for the use of Alteplase to declot the catheter. 8. Aseptically attach the sterile stopcock to catheter hub, turned off to catheter. 9. Draw up enough Alteplase into a 10cc syringe to equal the internal volume of the catheter. 10. With the catheter clamped, attach the Alteplase 10cc syringe and a second, empty, syringe into stopcock. 11. Turn stopcock off to Alteplase syringe. Stopcock should be open to empty syringe and catheter. 12. Aspirate empty syringe back 5-9cc and hold, thereby creating negative pressure in catheter. Unclamp catheter. If blood return is achieved, proceed to step 20. Avoid forceful or excessive aspiration of the syringe! 13. Turn off stopcock to empty syringe, allowing negative pressure to pull the Alteplase into the catheter. 14. Clamp catheter, then turn off stopcock to patient. 15. Wait 15 minutes. 16. Turn stopcock off to Alteplase syringe so stopcock is open to empty syringe and catheter. Keeping empty syringe drawn back so as to retain negative pressure in catheter, unclamp catheter. If blood return is achieved, proceed to step If there is still no blood return, turn off stopcock to empty syringe, allowing negative pressure to pull more Alteplase into the catheter, then clamp catheter and turn off stopcock to patient. Wait 30 minutes. 18. Turn stopcock off to Alteplase syringe so stopcock is open to empty syringe and catheter. Keeping empty syringe drawn back to retain negative pressure, unclamp catheter. If blood return is achieved, proceed to step 20. Avoid forceful or excessive aspiration of the syringe! 12
16 19. In cases where the line still does not clear, do a third instillation and leave the Alteplase in the catheter 2 hours before aspirating. Avoid forceful or excessive aspiration of the syringe! 20. When patency is restored, aspirate 5cc of blood to assure removal of all drug and clots. 21. Clamp catheter, remove blood-filled syringe, and replace it with a 10cc syringe filled with normal saline. Open clamp and flush catheter to verify patency. 22. Clamp catheter and remove syringe. 23. Attach sterile heparin-filled injection cap and flush catheter with heparin per Catheter Irrigation Procedure. References: 1. Soft-Cell* and Hemodialysis Apheresis Catheters Nursing Procedure Manual SCOF ResourceNurse.Com, Protocol for Catheter Clearance with TPA Competency Assessment 3. Haire, WD, Herbst, SF, Concensus Conference on the Use of Alteplase (t-pa) for the Management of Thrombotic Catheter Dysfunction, Journal of Vascular Access Devices, Summer 2000 Highlights Bulletin, pp
17 Clearing Occluded Catheters with other Fibrolytic Agents Purpose To restore patency to an occluded catheter. Supplies 1 - Sterile injection cap 5,000 IU/cc fibrolytic agent (catheter priming volume) 1-10cc syringe with attached 1in. needle 1-10cc sterile normal saline-filled syringe with attached 1 in. needle Povidone-iodine wipes Procedure 1. Wash hands. 2. Clamp catheter. 3. Remove injection cap, attach an empty 10cc syringe, release clamp, and attempt to aspirate. If aspiration is successful, withdraw clots, clamp catheter, and attach salinefilled syringe. Release clamp and flush catheter with 10 ml. normal saline. Clamp catheter. Replace cap per Injection Cap Change Procedure. If aspiration is unsuccessful, proceed to step Obtain physician s order for the use of fibrolytic agent 5,000 IU/cc to declot the catheter. 5. Draw up enough fibrolytic agent 5,000 IU/cc into a 10cc syringe to equal the internal volume of the catheter 6. Aseptically attach the fibrolytic agent-filled syringe to the catheter hub. Release clamp and slowly and gently inject the fibrolytic agent solution into the catheter. To avoid catheter rupture, do not force entire amount into catheter. 14
18 7. Leave 10cc syringe attached to catheter. Do not attempt to aspirate for minutes. 8. After minutes, attempt to aspirate the drug and residual clot. If unsuccessful, repeat fibrolytic agent instillation. 9. When patency is restored, aspirate 5cc of blood to assure removal of all drug and clots. 10. Clamp catheter, remove blood-filled syringe, and replace it with a 10cc syringe filled with normal saline. Open clamp and flush catheter to verify patency. 11. Clamp catheter and remove syringe. 12. Attach sterile heparin-filled injection cap and flush catheter with heparin per Catheter Irrigation Procedure. References: 1. Bjeletich, J., Declotting Central Venous Catheters with Urokinase in the Home by Nurse Clinicians, NITA, Nov/Dec 1987, pp Faubion, WC, Bollish, SJ, Wesley, JR, Central Venous Catheter Occlusion Treated by Thrombolytic Agents, Nutritional Support Services, Vol. 3, No. 2, 1983, pp Pennington, CR, Pithic, AD, Ethanol Lock in the Management of Catheter Occlusion, Journal of Parenteral & Enteral Nutrition, Vol. 11, No. 5, 1987, pp Shulman, RJ, Reed, T., Pitre, D., Laine, L., Use of Hydrochloric Acid to Clear Obstructed Central Venous Catheters, Journal of Parenteral & Enteral Nutrition, Vol. 12, No. 6, 1988, pp Holcombe, BJ, Forloines-Lynn, S, Garmhausen, LW, Restoring Patency of Long-Term Central Venous Access Devices, Journal of Intravenous Nursing, Vol. 15, No. 1, January/February 1992, pp Northsea, Cynthia, Using Urokinase to Restore Patency in Double Lumen Catheters., ANNA Journal, August 1994, Vol. 21 No Bagnall-Reeb, Holly, Ryder, Marcia, Anglin, Mary Ann, Venous Access Device Occlusions, Independent Study Module, Oncology Nursing Society, 1993 Abbott Laboratories. 15
19 Troubleshooting Guide I. Aspiration Difficulties A. Possible Causes 1. Failure to flush according to Catheter Irrigation Procedure, resulting in lumen obstruction. 2. Catheter tip sucking up to vein wall with aspiration. 3. Blood clot, fibrin sheath, or particulate matter obstructing lumen when catheter is aspirated. A clot or other obstruction in the catheter lumen can produce a one-way valve effect. During infusion, the catheter wall expands slightly and allows fluid to flow around the plug. During aspiration, the catheter wall contracts slightly, tightening down around the obstruction and preventing aspiration. Fibrin sheaths usually begin to form within a few days after the insertion of a central venous catheter. If it has grown enough to extend to the tip of the catheter, it may be pulled into and obstruct the catheter opening when aspiration is attempted, but offer no resistance to infusion. 4. Compression or transection of the catheter between the clavicle and first rib ( pinch-off area ). 5. Kinked catheter outside or inside the body. Suture constriction at the catheter skin exit site, cuff, or vessel insertion site. Catheter may be pulled too tight through skin tunnel, causing kink at vessel insertion site, or where it curves into the subcutaneous tunnel. Catheter may be curled or kinked within the vessel, or under the dressing. Catheters with pre-curve design must be maintained in their pre-curved position. 16
20 6. Malposition of catheter tip (i.e. jugular vein, outside of vein). 7. Improper catheter length selection for patient size. B. Possible Solutions 1. Visually check catheter for any exterior kinks, or constricting sutures. Check operative report, or with placement physician, for placement of sutures. If sutures are present, their removal may release the constriction and allow aspiration, if sufficient time has elapsed for tissue ingrowth. 2. If no resistance to infusion is felt, attempt to flush with 10cc normal saline. Then pull back gently on syringe plunger 2-3cc, pause and proceed with aspiration. 3. If resistance to infusion is felt, check for signs of extravasation. If present, notify physician of possible catheter leakage or transection and embolization. If not present, see step Attempt to aspirate with a 20cc syringe (creates a greater vacuum). 5. Move patient s arm, shoulder and head to see if a change in position will allow aspiration. If aspiration can only be accomplished with the patient in a certain position, the patient should be examined to see if the catheter has been placed in the pinch-off area. See step Obtain physician s order and instill fibrolytic agent or alteplase per Clearing Occluded Catheters Procedure. 7. Obtain physician s order for a chest x-ray or dye study to determine the position of the catheter. If the catheter tip is not at the junction of the superior vena cava right atrium, the catheter may need to be repositioned. If the catheter has been placed through the pinch-off area, between the clavicle and the first rib, and is being compressed enough to interfere with infusion or aspiration, it is at risk for catheter transection and embolization. The physician should evaluate the patient for catheter replacement. 17
21 References: Aitken, Delmar R., and Minton, John P., The Pinch-Off Sign : A Warning of Impending Problems with Permanent Subclavian Catheters. American Journal of Surgery, Vol. 148, November 1984, pp Rubenstein, Richard B., et al, Hickman* Catheter Separation, Journal of Parenteral and Enteral Nutrition, Vol. 9, No. 6, Nov/Dec 1985, pp II. Patient with Fever Infection: Symptoms: Inflammation at exit site Fever Positive site culture/ or blood cultures. If signs of infection are present: Notify physician Culture site drainage, follow hospital protocol for central-line site infection. Cleanse area as per site maintenance care. 18
22 III. Insufficient Flow Excessive force must not be used to flush an obstructed lumen. Insufficient blood flow may be caused by occluded arterial holes resulting from a clot or by contacting the wall of the vein. If manipulation of the catheter or reversing the arterial and venous lines does not help, then the physician may attempt to dissolve the clot with a Fibrolytic agent. Physician discretion advised. IV. Catheter Exchange It may become neccessary to exchange the indwelling catheter over a guidewire due to infection or a persistent rise in pressures or decrease of flow rates which cannot be rectified through troubleshooting. V. Catheter Occlusion A. Possible Causes 1. Blood clot completely obstructing lumen. 2. May be kinked, coiled, damaged, or compressed between the clavicle and the first rib. 3. Catheter tip may be poorly positioned. 4. If sutures are wrapped tight around the catheter, they can tighten and restrict flow. 5. May be partially or completely transected. Transection can occur from the repeated pressure of the clavicle and the first rib on the catheter during normal movement if it is placed through the pinch-off area. For subclavian placements only. 6. Improper catheter length for patient size. 7. Venous lumens must be placed cephalad. 19
23 B. Possible Solutions 1. Attempt to aspirate blood clot. 2. Move patient s arm, shoulder and head to see if position change affects ability to infuse. If so, see step 5 (could be pinch-off). 3. Inspect patient and operative report for presence of sutures around the catheter. If sutures are too tight, they should be removed. 4. Obtain physician s order and instill fibrolytic agent or other solution per Clearing Occluded Catheters Procedure. 5. Obtain physician s order for a chest x-ray or dye study to determine the position of the catheter. If the catheter tip is not in the superior vena cava, the catheter should be repositioned. If the catheter tip is not in a vein, the catheter should be replaced. If the catheter has been placed through the pinch-off area, between the clavicle and the first rib, and is being compressed enough to interfere with infusion or aspiration, it is at risk for catheter transection and embolization. The physician should evaluate the patient for catheter replacement. References: See Aspiration Difficulties. VI. Catheter Damage In the event that a connector, clamp, or extension leg is damaged, the catheter may be repairable using Repair Kit Note that a minimum 4.5 cm of viable (undamage) or useable Repair Kit per IFU extension tubing is needed for such a repair. 20
24 Revised Date: January 2011 Please consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions, and instructions for use. Patents Pending. *Bard, Hemosplit, Hemostar, Hickman and Soft-Cell are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks are the property of their respective owners C. R. Bard, Inc. All rights reserved. MC R Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT Customer Service and Ordering: Clinical Information Hotline:
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Exceptional Performance and Ease of Placement
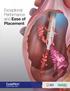 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Polyurethane Catheter
 References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
Groshong Central Venous Catheters
 Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Short-Term Dialysis Catheters
 Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
The Power of Purple* Polyurethane PICC. Patient Guide. Access Systems
 The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
40 35
 NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
The High-Flow Port Designed & Indicated for Apheresis
 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Infusion Skills Competency Checklist To be used at annual skills fair or at any other time for IV Competency
 Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
Overview of CVADs. Type of device commonly used. Dwell time Flushing requirement Associated complications. lumens
 Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS)
 Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Education for Self Administration of Intravenous Therapy HOME IV THERAPY PICC. Portacath
 HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
MANITOBA RENAL PROGRAM
 MANITOBA RENAL PROGRAM SUBJECT Use of Closed Needleless Access Device with Hemodialysis Central Venous Catheters (CVC) SECTION CODE 30.20.04 30.20 Vascular Access AUTHORIZATION Professional Advisory Committee,
MANITOBA RENAL PROGRAM SUBJECT Use of Closed Needleless Access Device with Hemodialysis Central Venous Catheters (CVC) SECTION CODE 30.20.04 30.20 Vascular Access AUTHORIZATION Professional Advisory Committee,
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices
 Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
IV Fluids. Nursing B23. Objectives. Serum Osmolality
 IV Fluids Nursing B23 Objectives Discuss the purpose of IV Discuss nursing interventions in IV therapy Identify complications of IV therapy Differentiate between peripheral line, central line, and PICC
IV Fluids Nursing B23 Objectives Discuss the purpose of IV Discuss nursing interventions in IV therapy Identify complications of IV therapy Differentiate between peripheral line, central line, and PICC
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Intravenous Catheter Complications
 Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Department Policy. Code: D:PC Entity: Fairview Pharmacy Services. Department: Fairview Home Infusion. Manual: Policy and Procedure Manual
 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
IV Drug Delivery Systems used in Cancer Care
 IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Totally indwelling venous access devices
 Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
St George Hospital Renal Department Internal Policy
 SUMMARY: TROUBLESHOOTING POOR BLOOD FLOW IN VASCATHS: Please see the flow chart at the end of the protocol describing possible causes to be considered and how to deal with these in a systematic fashion.
SUMMARY: TROUBLESHOOTING POOR BLOOD FLOW IN VASCATHS: Please see the flow chart at the end of the protocol describing possible causes to be considered and how to deal with these in a systematic fashion.
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Occlusion Management of Central Vascular Access Devices in Adult Patients
 Approved by: Vice President & Chief Medical Officer; and Vice President & Chief Operating Officer Occlusion Management of Central Vascular Access Devices in Corporate Policy & Procedures Number: Manual
Approved by: Vice President & Chief Medical Officer; and Vice President & Chief Operating Officer Occlusion Management of Central Vascular Access Devices in Corporate Policy & Procedures Number: Manual
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
IV Fluids Nursing B23 Objectives Serum Osmolality 275 to 295 Isotonic
 1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
Sterile Technique & IJ/Femoral Return Demonstration
 Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
The University of Toledo Medical Center and its Medical Staff
 Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Successful IV Starts Revised February 2014
 Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Central venous access devices for children with lysosomal storage disorders
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT
 PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Treatments POLICY NUMBER: 428. Effective Date: August 31, 2006
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Treatments POLICY NUMBER: 428 Effective Date: August 31, 2006 SUBJECT: CENTRAL VASCULAR ACCESS DEVICES (CVADs): CARE AND USE 1. PURPOSE:
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION Treatments POLICY NUMBER: 428 Effective Date: August 31, 2006 SUBJECT: CENTRAL VASCULAR ACCESS DEVICES (CVADs): CARE AND USE 1. PURPOSE:
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
1/22/2016. Disclaimer. Disclaimer
 Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
POWER INJECTABLE IMPLANTABLE INFUSION PORT
 POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
External Ref: Andres, D.A., et al. Catheter Pinch-Off Syndrome: Recognition and Management.
 Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear)
 CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Quattro Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC4593/
 Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Peripherally Inserted Central Catheter (PICC) Booklet
 Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS
 A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Emergency clamp should always be readily available in case of accidental catheter fracture
 Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
Drainage Frequency: PATIENT GUIDE. Dressing Frequency: Every Drainage Weekly Drainage. Physician Contact Information. Dr. Phone:
 Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections M A R C H 15, 2017
 The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Procedures/Risks:central venous catheter
 Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE)
 ESPEN Congress Lisbon 2015 HOW TO MAKE HOME PARENTERAL NUTRITION SAFER Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE) Choosing the right
ESPEN Congress Lisbon 2015 HOW TO MAKE HOME PARENTERAL NUTRITION SAFER Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE) Choosing the right
2. Need for serial arterial blood gas determinations. 2. Anticipation of the initiation of thrombolytic therapy
 I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
ICY Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC-3893A/
 Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
CENTRAL VENOUS ACCESS DEVICES. BETHANY COLTON
 CENTRAL VENOUS ACCESS DEVICES. BETHANY COLTON Aims and Objectives To know what central venous access devices (CVAD) are. Types of CVADS used in haematology. To understand why we use them To know the complications
CENTRAL VENOUS ACCESS DEVICES. BETHANY COLTON Aims and Objectives To know what central venous access devices (CVAD) are. Types of CVADS used in haematology. To understand why we use them To know the complications
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
DRAFT. Caring for Your Central Venous Catheter. For adult patients. Contents
 UW MEDICINE PATIENT EDUCATION Caring for Your Central Venous Catheter For adult patients Contents Page What is a central venous catheter?... 2 What can I expect when the catheter is placed?... 2 How is
UW MEDICINE PATIENT EDUCATION Caring for Your Central Venous Catheter For adult patients Contents Page What is a central venous catheter?... 2 What can I expect when the catheter is placed?... 2 How is
ENGLISH SHORT TERM HAEMODIALYSIS CATHETER
 SHORT TERM HAEMODIALYSIS CATHETER Guides & Instruction for use Intended use: Sterile single use device indicated for use in attaining short term access for Hemodialysis or aphaeresis. How supplied: The
SHORT TERM HAEMODIALYSIS CATHETER Guides & Instruction for use Intended use: Sterile single use device indicated for use in attaining short term access for Hemodialysis or aphaeresis. How supplied: The
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY. All GCC Countries
 TITLE/DESCRIPTION: MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL CENTRE
TITLE/DESCRIPTION: MANAGEMENT OF INTRAVASCULAR (IV) LINES AND THERAPY INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL CENTRE
Victoria Chapman BS, RN, HP (ASCP)
 Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Caring for Your Peripherally Inserted Central Catheter (PICC)
 PATIENT & CAREGIVER EDUCATON Caring for Your Peripherally Inserted Central Catheter (PICC) This information will help you care for your peripherally inserted central catheter (PICC) at home. A PICC is
PATIENT & CAREGIVER EDUCATON Caring for Your Peripherally Inserted Central Catheter (PICC) This information will help you care for your peripherally inserted central catheter (PICC) at home. A PICC is
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Fundamentals of Flushing and Locking
 Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
