Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
|
|
|
- Noah Lynch
- 5 years ago
- Views:
Transcription
1 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters
2 3 Implantable Ports with Open-Ended and Groshong Catheters
3 1 Description The BardPort, SlimPort, and X-Port implantable ports are implantable access devices designed to provide repeated access to the vascular system. Port access is performed by percutaneous needle insertion using a non-coring needle. The system consists of two primary components: an injection port with a self-sealing silicone septum and a radiopaque catheter. All materials are biocompatible and can be used with virtually all injectable solutions. For implantable ports with Groshong catheters, the Groshong catheter valve helps provide security against blood reflux and air embolism into the port/catheter system. The Groshong catheter may be flushed with normal saline, and it does not require heparin to maintain patency. Indication For Use The BardPort, SlimPort, and X-Port implantable ports are indicated for patient therapies requiring repeated access to the vascular system. The port system can be used for infusion of medications, I.V. fluids, parenteral nutrition solutions, blood products, and for the withdrawal of blood samples. Contraindications, Warnings, and Precautions Contraindications This device is contraindicated: When-the presence of device-related infection, bacteremia, or septicemia is known or suspected. When the patient s body size is insufficient for the size of the implanted device. When the patient is known or is suspected to be allergic to materials contained in the device. The device is primarily composed of silicone, polyacetal, and/or titanium. If severe chronic obstructive lung disease exists. If the prospective insertion site has been previously irradiated. If the prospective placement site has previously suffered episodes of venous thrombosis or vascular surgical procedures. If local tissue factors will prevent proper device stabilization and/or access.
4 2 Warnings During Placement: Intended for Single Use. DO NOT REUSE. Reuse and/or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patient. After use, this product may be a potential biohazard. Handle and discard in accordance with accepted medical practice and applicable local, state and federal laws and regulations. Place thumb over exposed opening of sheath or needle or attach syringe filled with sterile normal saline solution to minimize blood loss and prevent air embolism. The risk of air embolism is reduced by performing this part of the procedure with the patient performing the Valsalva maneuver and/or in Trendelenburg position. Do not suture catheter to port, port stem, or surrounding tissue. Any damage or constriction of catheter may compromise the catheter integrity. Bard Access Systems, Inc. does not recommend suturing around the catheter as doing so could compress, kink, or damage catheter, including catheter fragmenting and/or fracturing. Failure to completely advance the catheter on the dual lumen stem may result in subcutaneous leakage. Avoid vessel perforation. For Implantable ports with Groshong catheters, do not cut stylet. Withdraw stiffening stylet from catheter prior to cutting. Pinch-off Prevention: Catheters placed percutaneously or through a cutdown, into the subclavian vein, should be inserted at the junction of the outer and middle thirds of the clavicle, lateral to the thoracic outlet. The catheter should not be inserted into the subclavian vein medially, because such placement can lead to compression of the catheter between the first rib and the clavicle, which can cause damage and even sever the catheter. A radiographic confirmation of catheter placement should be made to ensure that the catheter is not being pinched by the first rib and clavicle. 1,2 Do not attempt to measure the patient s blood pressure on the arm in which the peripheral port system is located since catheter occlusion or other damage to the port system could occur. Do not access arm in which a peripheral port system is located, proximal to the port pocket as catheter puncture or other damage to the port system could occur. Alcohol should not be used to soak or declot polyurethane catheters because alcohol is known to degrade the polyurethane catheters.
5 3 Once needle is positioned in the septum do not tilt or rock needle as this may cause leakage or damage the port system. Do not manipulate catheter/port connection of pre-assembled or preconnected port system as the catheter could become disconnected from the port, or system damage could occur. Subclavian Vein Clavicle First Rib Vertebra Internal Jugular Vein Superior Vena Cava Axillary Vein Pinch-off Area Sternum Infraclavicular Fossa During Port Access: Do not use a syringe smaller than 10 ml. Flushing occluded catheters with small syringes can create excessive pressure within the port system. Exceeding maximum flow rate may result in port system failure and/or catheter tip displacement. Failure to ensure the patency of the catheter prior to infusion may result in port system failure. If local pain, swelling or signs of extravasation are noted during infusion, the injection should be stopped immediately. Signs of Pinch-Off Clinical: Difficulty with blood withdrawal Resistance to infusion of fluids Patient position changes required for infusion of fluids or blood withdrawal Radiologic: Grade 1 or 2 distortion on chest X-ray. Pinch-off should be evaluated for degree of severity prior to explantation. Patients indicating any degree of catheter distortion at the clavicle/first rib area should be followed diligently.
6 4 There are grades of pinch-off that should be recognized with appropriate chest x-ray as follows: 3,4 Grade Severity Recommended Action Grade 0 No distortion No action. Grade 1 Grade 2 Distortion present without luminal narrowing Distorion present with luminal narrowing Chest x-ray should be taken every one to three months to monitor progression of pinch off to grade 2 distortion. Shoulder positioning during chest x-rays should be noted as it can contribute to changes in distortion grades. Removal of the catheter should be considered. Grade 3 Catheter transection or fracture Prompt removal of the catheter. Precautions Carefully read and follow all instructions in these instructions for use (IFU). Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. Only qualified healthcare practitioners should insert, manipulate, and remove these devices. When utilizing the Arm Placement via Brachia/Basilic approach, the port should not be placed in the axillary cavity. Avoid inadvertent puncture of the skin or fascia with the tip of the tunneler. If the guidewire must be withdrawn while the needle is inserted, remove both the needle and the wire as a unit to prevent the needled from damaging or shearing the guidewire. Use only non-coring needless with the port. Prior to advancing the catheter lock, ensure that the catheter is properly positioned. A catheter not advanced to the proper region may not seat securely and lead to dislodgment and extravasation. The catheter must be straight with no sign of kinking. A slight pull on the catheter is sufficient to straighten it. Advancing the catheter lock over a kinked catheter may damage the catheter. Do not hold the catheter or cathlock with any instruments that could potentially damage either piece (e.g. hemostats). Follow Universal Precautions when inserting and maintaining the catheter. Follow all contraindications, warnings, precautions and instructions for all infusates as specified by their manufacturers.
7 5 Precautions are intended to help avoid catheter damage and/or patient injury. Prior to placement: Examine package carefully before opening to confirm its integrity and that the expiration date has not passed. The device is supplied in a double sterile package and is non-pyrogenic. Do not use if package is damaged, opened, or the expiration date has passed. Sterilized by ethylene oxide. Do not resterilize. Inspect kit for presence of all components. Check patient s records, and ask patient, whether they have any known allergies to chemicals or materials that will be used during the placement procedure. Fill (prime) the device with sterile normal saline solution to help avoid air embolism. When using an introducer kit, verify that the catheter fits easily through the introducer sheath. When utilizing port for arm placement, the port should not be placed in the axillary cavity. C. R. Bard, Inc. recommends the use of components provided in the kit. If additional items are to be used, check for proper fit prior to utilization. Note: Port body, catheter, and catheter-lock cannot be replaced with components outside the provided kit. If vessel has been used for previous port placement evaluate for patency prior to placing port. During Placement: Do not allow accidental device contact with sharp instruments. Mechanical damage may occur. Use only smooth edged, atraumatic clamps or forceps. Take care not to perforate, tear, or fracture the catheter during placement. After assembling catheter to port, check assembly for leaks or damage. Do not use the catheter if there is any evidence of mechanical damage or leaking. Do not bend catheter at sharp angles during implantation. This can compromise catheter patency. Carefully follow the connection technique given in these instructions to ensure proper catheter connection and to avoid catheter damage. Do not use sutures to secure catheter to the port stem as it could collapse or damage the catheter.
8 6 When using peel-apart introducers: o Carefully insert the introducer and catheter to avoid inadvertent penetration to vital structures in the thorax. o Avoid blood vessel damage by maintaining a catheter or dilator as internal support when using a peel-apart introducer. o Avoid sheath damage by simultaneously advancing the sheath and dilator as a single unit using a rotational motion. Never use a catheter lock that appears cracked or otherwise damaged. Care should be taken to avoid excessive contact between the needle and port base. After Placement: Encourage patient to keep patient ID card and present it to clinicians accessing their port. Do not use the device if there is any evidence of mechanical damage or leaking. Damage to the catheter may lead to rupture, fragmentation, possible embolism, and surgical removal. Accessories and components with Luer Lock connections should be used with this device. If signs of extravasation exist, discontinue injections. Begin appropriate medical intervention immediately. DO NOT USE A SYRINGE SMALLER THAN 10 ml! Infusion pressure greater than 25 psi (172 kpa) may damage blood vessels and viscus and is not recommended. Use only non-coring needles with the port. Choose a needle length based on reservoir depth, tissue thickness, and the thickness of any dressing beneath the bend of the needle. Confirm correct positioning of the needle within the port reservoir by aspiration of blood before infusion of any substance. If there is doubt regarding proper needle placement, perform a radiographic dye procedure to confirm placement. Possible Complications The use of a subcutaneous port provides an important means of venous access for critically ill patients. However, the potential exists for serious complications, including the following : Air Embolism Allergic Reaction Bleeding Brachial Plexus Injury
9 7 Cardiac Arrhythmia Cardiac Puncture Cardiac Tamponade Catheter or Port Erosion Through the Skin Catheter Embolism Catheter Occlusion Damage or Breakage due to Compression between the Clavicle and First Rib Catheter or port-related Sepsis Device Rotation or Extrusion Endocarditis Extravasation Fibrin Sheath Formation Guidewire Fragment Embolism Hematoma Hemothorax Hydrothorax Infection, including but not limited to pocket, catheter tunnel and/or blood stream Inflammation, Necrosis, or Scarring of Skin Over Implant Area Intolerance or Reaction to Implanted Device Laceration of Vessels or Viscus Pain at or around port pocket site Perforation of Vessels or Viscus Pneumothorax Risks Normally Associated with Local or General Anesthesia, Surgery, and Post-Operative Recovery Spontaneous Catheter Tip Malposition or Retraction Thoracic Duct Injury Thromboembolism Vascular Thrombosis Vessel Erosion These and other complications are well documented in medical literature and should be carefully considered before placing the port. Implantation Instructions Please read through complete implantation instructions before implanting port, noting Contraindications, Warnings, and Precautions and Possible Complications sections of this manual before beginning procedure.
10 8 Preventing Pinch-Off The risk of pinch-off syndrome can be avoided by inserting the catheter via the internal jugular vein (IJ). Subclavian insertion of the catheter medial to the border of the first rib may cause catheter pinch-off, which in turn results in occlusion. If you choose to insert the catheter into the subclavian vein, it should be inserted lateral to the border of the first rib or at the junction with the axillary vein because such insertion will avoid compression of the catheter, which can cause damage and even sever the catheter. The use of image guidance upon insertion is strongly recommended. A radiographic confirmation of catheter insertion should be made to ensure that the catheter is not being pinched. Implantation Preparation 1. Select implantation procedure to be used. 2. Select the site for port placement. Note: Port pocket site selection should allow for port placement in an anatomic area that provides good port stability, does not interfere with patient mobility, does not create pressure points, has not previously been irradiated, does not show signs of infection, and does not interfere with clothing. For arm port placement, port site should be distal to the desired vein insertion site. Patient s arm movement should be considered when determining the length of the catheter and the final tip location. Consider the amount of cutaneous tissue over the port septum, as excessive tissue will make access difficult. Conversely, too thin a tissue layer over the port may lead to tissue erosion. A tissue thickness of 0.5 cm to 2 cm is appropriate. 3. Complete patient implant record, including length of catheter implanted, product reorder number and lot number. 4. Perform adequate anesthesia. 5. Create sterile field and open tray. Note: The catheter and port may be soaked in sterile normal saline prior to placement. 6. Surgically prep and drape the implantation site. 7a. For Attachable Catheters : Flush each lumen of open-ended catheters with sterile normal saline, through flushing connector and clamp the catheter closed several centimeters from the distal (port) end. Note: Clamped catheter segments will be cut off prior to attachment. 7b. For Pre-Attached Catheters: Use a non-coring needle to flush the port and catheter system with sterile normal saline.
11 9 7c. For Groshong catheters: Flush catheter with sterile normal saline through the pre-loaded stylet connector. 8. Place patient in the Trendelenberg position with head turned away from the intended venipuncture site. For arm port placement, position the arm in an abducted, externally rotated position. Note: Recommended veins for arm placement are cephalic, basilic, or medial cubital basilic. Note: Recommended veins for chest placement are internal jugular or lateral subclavian. Refer to the Warnings section covering catheter pinch-off if inserting the catheter via the subclavian vein. Percutaneous Procedure 1. Locate and access vessel with introducer needle attached to a syringe. 2. Aspirate gently as the insertion is made. If the artery is entered, withdraw the needle and apply manual pressure for several minutes. If the pleural space is entered, withdraw the needle and evaluate patient for possible pneumothorax. 3. When the vein has been entered, remove the syringe leaving the needle in place. Warning: Place thumb over exposed opening of the needle to minimize blood loss and prevent air embolism. The risk of air embolism is reduced by performing this part of the procedure with the patient performing the Valsalva maneuver. If using a micropuncture set, insert the flexible end of the micropuncture guidewire into the introducer needle. Advance the guidewire as far as appropriate. Verify correct positioning, using fluoroscopy or appropriate technology. Gently withdraw and remove the needle, while holding the micropuncture guidewire in position. Advance the small sheath and dilator together as a unit over the micropuncture guidewire, using a slight rotational motion. Withdraw the dilator and guidewire, leaving the microintroducer sheath in place. Caution: If the guidewire must be withdrawn while the needle is inserted, remove both needle and wire as a unit to prevent the needle from damaging or shearing the guidewire. Warning: Place thumb over opening of sheath or needle, or attach syringe filled with sterile normal saline solution to minimize blood loss and prevent air embolism. The risk of air embolism is reduced by performing this part of the procedure with the patient performing the Valsalva maneuver.
12 Straighten J tip of standard guidewire with tip straightener and insert tapered end of tip straightener into the needle (or microintroducer sheath if using a micropuncture set). Note: Do not advance guidewire if obstruction is encountered. 5. Remove the tip straightener and advance the guidewire into the superior vena cava. Advance the guidewire as far as appropriate for the procedure. Verify correct positioning using fluoroscopy or appropriate technology. 6. Gently withdraw and remove needle (or microintroducer sheath if using micropuncture set). Caution: If the guidewire must be withdrawn while the needle is inserted, remove both the needle and wire as a unit to help prevent the needle from damaging or shearing the guidewire Peel-Apart Sheath Introducer Instructions 1. Advance the vessel dilator and sheath introducer as a unit over the exposed wire using a rotational motion. Advance it into the vein as a unit, leaving at least 2 cm of sheath exposed. Note: Placement may be facilitated by making a small incision to ease introduction of vessel dilator and sheath introducer. Warning: Avoid vessel perforation. 2. Release the locking mechanism and gently withdraw the vessel dilator and J wire, leaving the sheath in place. Warning: Place thumb over exposed opening of sheath or needle or attach syringe filled with sterile normal saline solution to minimize blood loss and prevent air embolism. The risk of air embolism is reduced by performing this part of the procedure with the patient performing the Valsalva maneuver and/ or in Trendelenburg position. 3. Insert catheter into the sheath. Advance the catheter through the sheath into the vessel to the desired infusion site. Catheters should be positioned with the catheter tip at the junction of the superior vena cava and the right atrium. 4. Verify correct catheter tip position using fluoroscopy or appropriate technology. 5. Grasp the two handles of the peel-apart sheath and pull outward and upward at the same time. Peel the sheath away from the catheter completely. Make sure the catheter is not dislodged from vessel.
13 11 1 2a 2b 3 5 Cut-Down Procedure 1. Use a cut-down incision to expose the entry vein of choice. 2. Perform vessel incision after vessel is isolated and stabilized to prevent bleeding and air embolism. 3. If using a vein pick, insert its tapered end through the incision and advance the pick. 4. Advance the catheter tip into the vessel. 5. Withdraw the vein pick, if used. 6. Advance the catheter into the vessel to the desired infusion site. Note: Catheters should be positioned with the catheter tip at the junction of the superior vena cava and the right atrium. Verify correct catheter tip position using fluoroscopy or appropriated technology. Catheter Vein pick Vessel Catheter Tunneling Procedure 1. Create a subcutaneous pocket using blunt dissection. Note: Do a trial placement to verify that the pocket is large enough to accommodate the port and that the port does not lie beneath the incision. Attachable Catheters Create a subcutaneous tunnel from the venous site to the port pocket site using tunneler or long forceps per the following: a. Make a small incision at the venous entry site. b. Insert tip of tunneler into the small incision.
14 12 c. Form tunnel by advancing tip of tunneler from the venous entry site to the port pocket site. Caution: Avoid inadvertent puncture of the skin or fascia with the tip of the tunneler. d. Remove catheter lock from the catheter. For implanted ports with Groshong catheters, remove the catheter lock and stiffener stylet from the catheter prior to cutting catheter to appropriate length. Warning: Do not cut stiffening stylet. Withdraw stiffening stylet from catheter prior to cutting. Caution: Never use a catheter lock that appears cracked or otherwise damaged. e. Attach end of catheter onto the tunneler barb with a twisting motion. Note: Barb threads must be completely covered by the catheter to adequately secure the catheter as it is pulled through the tunnel. A suture may be tied around the catheter between the tunneler body and the large barb to hold it more securely. f. Pull the tunneler through to the port pocket site while gently holding the catheter. Note: The catheter must not be forced. g. Cut off end of the catheter attached to tunneler. Pre-Attached Catheters Create subcutaneous tunnel from the port pocket site to the venous entrance site per the following: a. Form tunnel by advancing the tip of the tunneler from the port pocket site to the venous entry site. Caution: Avoid inadvertent puncture of the skin or fascia with the tip of the tunneler. b. Connect the catheter tip onto the end of the tunneler. c. Pull the tunneler through to the venous entry site while gently holding the catheter. Note: The catheter must not be forced. d. Cut off end of the catheter attached to tunneler. e. Estimate the catheter length required for the tip placement at the junction of the superior vena cava and right atrium by placing the catheter on the chest along the venous path to the right atrium. Cut catheter to length at a 90 angle.
15 13 Connect Catheter To Port For Attachable Catheters 1. Flush all air from each lumen of the port body using a 10 ml syringe with a non-coring needle filled with sterile normal saline. Insert the needle through the septum and inject the fluid while pointing the stem up. 2. Cleanse all system components with irrigation solution. Caution: Prior to advancing the catheter lock, ensure that the catheter is properly positioned. A catheter not advanced to the proper region may not seat securely and lead to dislodgement and extravasation. The catheter must be straight with no sign of kinking. A slight pull on the catheter is sufficient to straighten it. Advancing the catheter lock over a kinked catheter may damage the catheter. Do not hold the catheter or cathlock with any instruments that could potentially damage either piece (e.g. hemostats). 3. Connect catheter to port: a. Place catheter lock back onto catheter, ensuring the black radiopaque ring on the catheter lock faces away from the port body. b. Cut the catheter to the proper length at a 90 angle, allowing sufficient slack for body movement and port connection. Check catheter for any damage. If any damage is noted, cut damaged section off before connecting catheter to port. Note: Ensure that no guidewires or stiffening wires remain in the catheter lumen prior to cutting and adjusting catheter to desired length. c. For single lumen ports, align port stem with catheter. When placing dual lumen ports, align the port stem with both lumens. Note: If the catheter and catheter lock are connected and then disconnected, the catheter end must be re-trimmed to ensure a secure reconnection. Note: When using the catheter lock, be sure the end containing a colored radiopaque ring faces away from the port. The catheter lock should be sufficient to secure catheter to port. Note: Sterile gauze may be used to facilitate stem to catheter connection. d. For single lumen ports: Advance catheter over port stem to midway point. Note: Advancing catheter too far along port stem could lead to mushrooming of tubing when the catheter lock is advanced. Should this occur, it is advisable to stop advancing the catheter lock, pull the catheter back along the stem away from the port, and re-assemble the connection. e. For dual lumen ports: Advance catheter completely onto the stem prior to advancing catheter lock. Warning: Failure to completely advance the catheter on the dual lumen stem may result in subcutaneous leakage.
16 14 Warning: Do not suture catheter to port, port stem, or surrounding tissue. Any damage or constriction of catheter may compromise catheter integrity. Bard Access Systems, Inc. does not recommend suturing around the catheter as doing so could compress, kink, or damage catheter. Single Lumen Port Device: Shoulder Catheter Advance to midway point Stem Catheter Lock Stem Radiopaque Ring Dual Lumen Port Device: Shoulder Catheter Advance all the way Stem Catheter Lock Stem Radiopaque Ring Warning: Advance catheter completely on stem prior to advancing catheter lock Position Port and Close Incision Site 1. Place the port in the subcutaneous pocket away from the incision line. Secure the port to the underlying fascia using non-absorbable, monofilament sutures. Leave sufficient slack in the catheter to permit slight movement, and verify that the catheter is not kinked. This will reduce the risk of port migration and the possibility of it flipping over. 2. After suturing the port in the pocket, flush the wound with sterile normal saline or an appropriate antibiotic solution, per institutional protocol. 3. Conduct flow studies on each lumen of the catheter using a non-coring needle and 10 ml syringe to confirm that the flow is not obstructed, that no leak exists, and that the catheter is correctly positioned. 4. Aspirate to confirm the ability to draw blood. 5. Flush and lock each lumen of the port system as described under heparin lock procedure for open-ended catheters or saline lock procedures for implantable ports with Groshong catheters. Close clamp while injecting last 0.5 ml of flush solution.
17 15 Caution: Remember that some patients may be hyper-sensitive to heparin or suffer from heparin induced thrombocytopenia (HIT). These patients must not have their port locked with heparinized saline. 6. Close the incision site, so that the port does not lie beneath the incision. 7. Apply dressing according to hospital practice. Determining Port System Volumes For Port Lock Procedures To calculate a close approximation of port system volume for each lumen, you will need to determine the length of catheter used for each individual patient. (For future reference, it will be helpful to record this information on the patient s chart and/or patient ID card.) For BardPort, SlimPort and X-Port implantable port catheters, use the formula and tables below: Port System Volume = Catheter length: cm x catheter volume cm + reservoir volume. Catheter 6F ChronoFlex catheter Calculated Catheter Volumes Volume cm (per lumen) 6.6F Silicone catheter 7F Silicone (dual lumen) 0.01 ml/cm 7F Groshong catheter 9.5F Groshong (dual lumen) 10F Silicone (dual Lumen) 8F ChronoFlex catheter 9.6F Silicone catheter 0.02 ml/cm 8F Groshong catheter 9.5F ChronoFlex (dual lumen) 12F Silicone (dual lumen)
18 16 Port SlimPort Port Reservoir Volumes Per Reservoir Volume X-Port Port 0.2 ml Titanium Low Profile Port M.R.I. Low-Profile SlimPort Ultra Low Profile Dual SlimPort Rosenblatt Port 0.3 ml 0.27 Dist Prox. ml M.R.I. Port M.R.I. Hard Base Port X-Port isp Port 0.6 ml X-Port duo Port Titanium Port M.R.I. Dual-Port Dome Port 0.8 ml 1.2 ml Note: This calculated volume represents the port system volume for each port reservoir. Heparin Lock Procedure for Open-Ended Catheters To help prevent clot formation and catheter blockage, each lumen of the implanted ports with open-ended catheters should be filled with sterile heparinized saline after each use. If the port remains unused for long periods of time, the heparin lock should be changed at least once every 28 days. Caution: Remember that some patients may be hypersensitive to heparin or suffer from heparin induced thrombocytopenia (HIT) and these patients must not have their port locked with heparinized saline. If the port catheter length is not known, the following are recommended flushing volumes for open-ended catheters, otherwise follow institutional protocol.
19 17 Flushing Volumes, Open-Ended Catheters Procedure When port not in use Volume 5 ml heparinized saline every 28 days (100 U/mL) After each infusion of medication or TPN After blood withdrawl 10 ml sterile normal saline then 5 ml heparinized saline (100 U/mL) 20 ml sterile normal saline then 5 ml heparinized saline (100 U/mL) Equipment: Non-coring needle 10 ml syringe filled with sterile saline per lumen 10 ml syringe filled with 5 ml heparinized saline (100 U/mL) per lumen Note: Other concentrations of heparinized saline (10 to 1000 U/mL) have been found to be effective. Determination of proper concentration and volume should be based on patient s medical condition, laboratory tests, and prior experience. Procedure: 1. Explain procedure to patient and prepare injection site. 2. Attach a 10 ml syringe filled with sterile normal saline to needle. 3. Aseptically locate and access port. 4. After therapy completion, flush port per institutional protocol, then repeat with 5 ml 100 U/mL heparinized-saline, or with volume calculated above. Close clamp while injecting last 0.5 ml of flush solution. Warning: Alcohol should not be used to soak or declot polyurethane catheters because alcohol is known to degrade the polyurethane catheters over time with repeated and prolonged exposure.
20 18 Saline Lock Procedure for Groshong Catheters To help prevent clot formation and catheter blockage, implanted ports with Groshong catheters should be filled with sterile normal saline after each use. If the port remains unused for long periods of time, the saline lock should be changed by flushing at least once every 28 days. If the port catheter length is not known, the following chart outlines the recommended flushing volumes for Groshong catheters - otherwise follow institutional protocol. Flushing Volumes, Groshong Catheters Procedure Volume When port not in use After each infusion of medication or TPN After blood withdrawl 5 ml sterile normal saline every 28 days 10 ml sterile normal saline 20 ml sterile normal saline Equipment: Non-coring needle 10 ml syringe filled with sterile normal saline Procedure: 1. Explain procedure to patient and prepare injection site. 2. Attach a 10 ml syringe filled with sterile normal saline to needle. 3. Aseptically locate and access port. 4. After therapy completion, flush port per institutional protocol. Close clamp while injecting last 0.5 ml of flush solution.
21 19 References 1. Aitken, D.R.; Minton, J.P. The Pinch-Off Sign : A Warning of Impending Problems with Permanent Subclavian Catheters :, American Journal of Surgery, Vol. 148, Nov. 1984, pp Rubenstein, R.B.; Alberty, R.E.; Michels, L.E.; et al. Hickman Catheter Separation:, JPEN, Vol. 9, No. 6, Nov./Dec. 1985, pp Hinke, D.H.; Zandt-Stastny, D.A.; Goodman, L.R.; et al. Pinch-off syndrome: A complication of implantable subclavian venous access devices. Radiology 177: , Ingle, Rebecca; Nace, Corinne. Venous Access Devices: Catheter Pinch-off and Fracture. 1993, Bard Access Systems, Inc. Revised: March 2015 Bard, BardPort, Groshong, SlimPort, and X-Port are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks are the property of their respective owners C. R. Bard, Inc. All rights reserved.
22 20 Implantable ports are classified as MR Safe and MR Conditional. To determine whether a Bard port is MR Safe or MR Conditional, refer to the patient identification card, patient implant record and/or the device unit label for these images: MR MR Safe MR MR Conditional These classifications are described below. MR MR Safe Non-clinical testing has demonstrated that the device is MR Safe. A patient with this device can be scanned safely immediately after placement under any conditions. MR Safe implantable ports will not create displacement, torque or local heating, and will give minimal or no MR image artifact. MR MR Conditional Non-clinical testing has demonstrated that the device is MR Conditional. A patient with this device can be scanned safely immediately after placement under the following conditions: Static Magnetic Field - Static Magnetic Field of 3 Tesla or less - Maximum spatial gradient magnetic field of 3000 Gauss/cm or less MRI-Related Heating In non-clinical testing, the device produced a temperature rise of up to 1.9 C during MRI performed for 15 minutes of scanning (i.e., per pulse sequence) in the 3-Tesla (3-Tesla/128-MHz, Excite, HDx, Software 14X.M5, General Electric Healthcare, Milwaukee, WI) MR system. Therefore, the MRI-related heating experiments for the device at 3 Tesla using a transmit/receive RF body coil at an MR system reported whole body averaged specific absorption rate (SAR) of 2.9 W/kg (i.e., associated with a calorimetrymeasured whole body averaged value of 2.7 W/kg) indicated that the greatest amount of heating that occurred in association with these specific conditions was equal to or less than 1.9 C.
23 Artifact Information MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the device (within 4 40 cm 2, depending on port size and materials). Therefore, optimization of MR imaging parameters to compensate for the presence of this device may be necessary. 21
24 Distributed by: Bard Peripheral Vascular, Inc West Third Street Tempe, AZ USA Clinical Information: Manufactured by: Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, Utah USA / Customer Service: Clinical Information: R
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT
 PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
POWER INJECTABLE IMPLANTABLE INFUSION PORT
 POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Polyurethane Catheter
 References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
The High-Flow Port Designed & Indicated for Apheresis
 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Groshong Central Venous Catheters
 Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Exceptional Performance and Ease of Placement
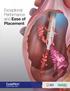 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS
 PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
40 35
 NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
ompanionport Speciality Medical Devices For The Veterinary Community Surgical Suggestions
 Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Totally indwelling venous access devices
 Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement
 Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Vaxcel Implantable Ports Valved and Non-Valved. A Patient s Guide
 Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports
 Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
ICY Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC-3893A/
 Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Overview of CVADs. Type of device commonly used. Dwell time Flushing requirement Associated complications. lumens
 Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Hospital of the University of Pennsylvania NURSING. Insertion of Peripherally Inserted Central Catheter (PICC) and Midline Catheter (MLC)
 Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Short-Term Dialysis Catheters
 Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
Quattro Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC4593/
 Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS)
 Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
BioFlo PICC. with ENDEXO Technology. Directions For Use A
 BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore
 CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
1/22/2016. Disclaimer. Disclaimer
 Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case Report
 Kosin Medical Journal 2016;31:167-172. https://doi.org/10.7180/kmj.2016.31.2.167 KMJ Case Report Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case
Kosin Medical Journal 2016;31:167-172. https://doi.org/10.7180/kmj.2016.31.2.167 KMJ Case Report Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case
Sterile Technique & IJ/Femoral Return Demonstration
 Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Procedures/Risks:central venous catheter
 Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
IV Drug Delivery Systems used in Cancer Care
 IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
The Power of Purple* Polyurethane PICC. Patient Guide. Access Systems
 The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices
 Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
for the ideal insertion of peritoneal dialysis catheter (patent pending)
 for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Advocate Christ Medical Center CVC Placement Certification Course
 Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Carry this card with you at all times. Show this card to any medical professional treating you. Patient Implant Card. Option ELITE Vena Cava Filter.
 Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Complications Associated With IV Therapy
 Occlusion is the partial or complete obstruction of a catheter, which obstructs the infusion of solutions or medications. Occlusions can result from the coagulation of blood (thrombotic) or from obstruction
Occlusion is the partial or complete obstruction of a catheter, which obstructs the infusion of solutions or medications. Occlusions can result from the coagulation of blood (thrombotic) or from obstruction
Successful IV Starts Revised February 2014
 Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
Table Of Contents. Page. Introduction...1. Possible Complications...5 Routine Maintenance
 * * * Table Of Contents Page Introduction...1 Description of Catheters Placement Indications for Use Contraindications Warnings Possible Complications...5 Routine Maintenance Hemodialysis and Plasmapheresis
* * * Table Of Contents Page Introduction...1 Description of Catheters Placement Indications for Use Contraindications Warnings Possible Complications...5 Routine Maintenance Hemodialysis and Plasmapheresis
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
REFILL KIT For use with Prometra Programmable Infusion Systems
 REFILL KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3 Warnings...
REFILL KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3 Warnings...
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
Central Venous Catheter Insertion: Assisting
 Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Pressure Injectable PICC Product. Venous Access Critical Care
 Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Vascular access device selection & placement. Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University
 Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Infusion Skills Competency Checklist To be used at annual skills fair or at any other time for IV Competency
 Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
