Indications for Use Targis System Description Contraindications for Targis Treatment Warnings Precautions
|
|
|
- Shana Shields
- 6 years ago
- Views:
Transcription
1 Instructions for Use Targis System Control Unit, Model 4000 and Model 5000 Series Targis Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician trained and/or experienced in the use of this device as outlined in the required training program. CAREFULLY READ AND UNDERSTAND ALL INSTRUCTIONS, INDICATIONS, WARNINGS, PRECAUTIONS, AND DIRECTIONS FOR USE PRIOR TO USING ANY TARGIS COMPONENT OR THE CONTROL UNIT. FAILURE TO DO SO COULD RESULT IN COMPROMISED PATIENT SAFETY, PATIENT COMPLICATIONS AND/OR INSUFFICIENT TREATMENT. Indications for Use The Urologix Targis System is a non-surgical device intended to relieve symptoms and obstruction associated with Benign Prostatic Hyperplasia (BPH) and is indicated for men with prostatic urethra lengths of 3 to 5 cm (model number TA1121D) or 2.5 to 3.5 cm (model number TA1321D). Targis System Description The Targis System is comprised of a Control Unit which produces the microwave energy and monitors all aspects of the Targis procedure, an MDS (treatment catheter) which delivers the microwave energy to the targeted prostatic tissue, an RTU which measures rectal temperatures during the Targis procedure and a Coolant Bag which provides a reservoir of sterile coolant water to the MDS. The Targis MDS is used as part of the Targis System to treat men with prostatic urethra lengths as indicated on the MDS label and as measured from the bladder neck to the verumontanum. Contraindications for Targis Treatment Patients with a prostatic urethra<2.5 cm in length, measured from the bladder neck to the verumontanum Patients with urinary sphincter or any implant (metallic or non-metallic) which is within 1.5 inches (38 mm) of the prostatic urethra Patients with urethral stricture (unable to pass 22 F urethroscope) Patients with peripheral arterial disease with intermittent claudication or Leriches Syndrome (i.e. claudication of the buttocks or perineum) Patients who have undergone pelvic radiation therapy Patients with implanted active devices, including pacemakers or defibrillators, within 2.6 inches (6.5 cm) of the prostatic urethra. Warnings The Targis procedure has inherent associated risks of complications (refer to Adverse Events and Complications). The Targis System and components should not be used in any way other than the intended and indicated use and according to the Instructions For Use. Precautions Only those physicians who have been thoroughly trained on the operation of the Targis System and the Targis procedure should deliver the Targis procedure. The Targis procedure must not be initiated without assurance that the MDS is properly positioned in the patient. The correct positioning of the MDS must always be checked by ultrasound imaging prior to commencing treatment. Improper placement or orientation of the MDS may lead to procedure failures or heating damage of non-target tissues such as the bladder neck, external sphincter or penile urethra. The Targis procedure must not be initiated until an enema has been given and the RTU is properly placed into the patient s rectum and inflated. For patients with active implanted devices located greater than 2.6 inches (6.5 cm) from the prostatic urethra, it is recommended that non-cardiac devices be turned OFF during treatment with the Targis system, if possible (e.g. active implanted devices used in the treatment of pain or incontinence), to lessen the likelihood of adverse interaction caused by electromagnetic interference. If possible, active implanted devices that must remain ON should be programmed to the bipolar configuration. Implanted cardioverter defibrillators should be set to monitor only mode during therapy. Regardless of whether active implanted devices > 2.6 inches (6.5 cm) from the prostatic urethra can be turned OFF, the patient should be monitored during Targis treatment for possible interactions. In the unlikely event of an active implanted device programming change, the device should be interrogated following treatment with the Targis system. It is important that the patient not be over sedated. This may compromise his ability to communicate pain. All components of the Targis System must be used in a manner consistent with the instructions set forth in their respective Instructions For Use insert and the Targis System User Manual. Failure to do so may result in insufficient treatment or increased risk of injury or infection to the patient.
2 Use of the Targis System results in the deposition of microwave energy within the patient s prostate and in adjacent regions of the body. Some animal studies in the literature suggest that there may be as yet unknown health effects from exposure to microwave radiation, including an increased incidence of tumors. Although it is not possible to extrapolate these studies to humans, they suggest that unnecessary microwave radiation exposure should be avoided. At least 20 cm of ventilation clearance must be provided around the base of the Control Unit. The Urologix Targis system emits a small amount of electromagnetic energy during a procedure. Urologix recommends that all electronic medical devices be kept at a minimum distance of 1.0 meter from the Targis System when performing a treatment. However, a 1.0 meter separation of electronic medical equipment from the Targis System does not guarantee that operation of other devices will not be impacted. The effect of this electromagnetic energy on all equipment can not be predicted due to age and quality of maintenance. The performance of each piece of equipment operated near the Targis system, during a procedure, must be evaluated for degradation. Since microwave energy can travel through walls, ceilings, and floors to affect other devices, it is important to understand that the 1 meter safety distance applies not only to the treatment room, but also to all adjacent rooms in the building, including the rooms above and below the treatment room. Do not operate the Targis system near equipment that emits electromagnetic energy, unless the effect on the Targis system has been evaluated and no degradation of performance was found. The national standard ANSI/IEEE C Edition (Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields) recommends a maximum stray field exposure level for whole body exposure of 3 mw/cm 2, as averaged for any six minute period. The maximum radiated field, at full power, from the Targis Control Unit patient cable and catheter, at five centimeters, is 2.1 mw/cm 2. Urologix recommends that the operator maintain a minimum distance to five centimeters from the patient cable and exposed portions of the catheter during the procedure. Operate the Control Unit and connected devices only in clinical environments where the electrical installation is in accordance with international standard DIN VDE 0107; and the national standard, ANSI/NFPA 70. The equipment must be connected to a fully tested, hospital grade power outlet with adequate grounding. The Control Unit must be plugged into the appropriate voltage outlet. The electrical equipment inside the Targis System uses voltages which are capable of causing serious injury or death from electric shock. To avoid this hazard, operators must never open the housing of the Control Unit. For further safety information, refer to the TARGIS SYSTEM USER MANUAL. The safety and effectiveness of the Targis procedure has not been established in patients with the following conditions: Clinical or histological evidence of prostatic cancer or bladder cancer Interest in preservation of future fertility Post Void Residual (PVR)>350 ml Previous pelvic surgery Previous rectal surgery (other than hemorrhoidectomy) Obstructing median lobe enlarged out of proportion to the rest of the prostate and protruding significantly into the bladder, sometimes referred to as a ball valve median lobe. Active urinary tract infection Prostatic urethra> 5 cm in length as measured from the bladder neck to the verumontanum Gross hematuria not due to BPH Prior prostatic surgery (excluding balloon dilation) Coexisting illness or specific obstructive symptoms found to be caused by any of the following conditions: Neurologic bladder disorders Bladder stones Prostate volume greater than 100 cc Evidence of bacterial prostatitis Bladder neck contracture Renal impairment Urinary sphincter abnormalities Coagulation disorders 2
3 A thorough physical exam should be performed on patients prior to initiation of the Targis procedure. Patients who have received treatment with the Targis System should be followed on an annual basis since the treatment does not result in complete destruction of the prostate. The prostate specific antigen (PSA) levels will increase significantly following treatment. This increase can be up to 10 times (1000 percent) higher at 1 week and will decrease back to approximate normal levels by 6 weeks following the Targis treatment. The use of PSA testing during this period will be unreliable. Physicians are cautioned to measure the serum PSA level before treatment for future comparisons. PSA levels should return to baseline by 3 months following Targis treatment and may once again be used as a diagnostic test. Attention by a qualified physician is required during the use of the Targis System. The Control Unit display must be monitored and controlled during the course of a treatment session to make sure that the MDS and rectal temperatures are within prescribed treatment parameters. Failure to monitor and deliver the Targis procedure per recommendations by Urologix may lead to decreased patient safety and/or reduced clinical effectiveness. The Targis MDS and coolant bag are intended for one time use only. DO NOT resterilize and/or reuse them as this will likely result in compromised device performance and increased risk of injury or infection to a patient. The Targis components must not be used with any other system. Do not use an MDS if it appears to be damaged. Use the MDS prior to the use before date specified on the package. Care should be taken in handling all components of the Targis System to avoid damage that may lead to subsequent failure of the component or procedure. Because the Targis procedure elevates intraprostatic tissue temperature causing tissue damage that may result in acute urinary retention, the patient may be catheterized for 2 to 5 days (median 3 days) following the procedure, and it is advisable that anti-inflammatory and antibiotic medications be prescribed for 3-5 days. As patient responses to the Targis procedure are variable, the patient should be evaluated by their physician following treatment. Failure to maintain the equipment may result in exposure of the patient and/or the operator to excessive microwave energy. Adverse Events and Complications Most complications occurred shortly after treatment and were transient. In the United States, multi-center prospective studies of the Targis procedure, 206 patients were treated and followed for complications, complaints and observations. The following table shows the clinical adverse events and complications that were possibly, probably and definitely related to the Targis procedure. An asterisk (*) denotes events which were temporary or minor, requiring minimal or no medical intervention. Study investigators were required to notify Urologix of any complications which may have developed as a direct result of the Targis procedure. The following table is for the patients in the 60 minute vs. the 28.5 minute study. In the case of pain or discomfort during sexual activity, urgency, nocturia and urethral strictures, the rates for the 28.5 minute treatment are very similar to the original clinical study data. After excluding post treatment conditions that are associated with BPH itself (i.e., dysuria, interrupted flow, sensation of not emptying bladder, frequency, hesitancy, urgency, post void dribbling, weak stream and nocturia), the complication rates for most categories are lower for the 28.5 minute treatment. These lower rates include: temporary or partial loss of ejaculate; hemospermia; prostatic urethral damage; temporary acute incontinence; bladder trabeculation; severe pain during treatment; blood pressure changes during treatment; hospitalization in general related to treatment; complete loss of ejaculate; persistent erectile 3
4 dysfunction; and epididymitis. The categories in which adverse events were found to be higher are rectal irritation, pain or discomfort during sexual activity, urinary tract infection, transient erectile dysfunction, and urethral stricture. 60 min 28.5 min Post-treatment catheterization 91.1%* 89.6%* 0 8.9%* 10.4%* 1-4 days 62.2%* 62.5%* 5-7 days 15.6%* 12.5%* 8-27 days 13.3%* 14.6%* Mild hematuria 65.2%* 58.3%* Dysuria 47.8%* 52.1%* Clots in urine 47.8%* 43.8%* Temporary or partial ejaculate loss 45.7%* 20.8%* Pain or irritation in the groin or pelvis 41.3%* 31.3%* Bladder spasms 39.1%* 33.3%* Hemospermia 32.6%* 22.9%* Prostatic urethra damage 30.4%* 20.8%* Rectal irritation 23.9%* 39.6%* Temporary acute incontinence 21.7%* 14.6%* Interrupted flow 13.0%* 18.8%* Sensation of not emptying bladder 13.0%* 16.7%* Bladder trabeculation 10.9%* 6.3%* Severe pain during treatment 8.9%* 2.1%* Pain or discomfort during sexual activity 8.7%* 14.6%* Frequency 8.7%* 4.2%* Hesitancy 6.5%* 18.8%* Urgency 6.5%* 10.4%* Post-void dribbling 6.5%* 10.4%* Blood pressure changes during treatment 6.5%* 2.1%* Hospitalization in general related to the treatment 6.5% 0 Transient erectile dysfunction 4.3%* 12.5%* Urinary tract infection 4.3% 8.3% Complete loss of ejaculate 4.3%* 2.1%* Bladder diverticali 2.2%* 2.1%* Weak stream 2.2%* 2.1%* Erectile dysfunction 2.2% 0 Epididymitis 2.2%* 0 Prostatitis 2.2% 0 Nocturia 0 6.3%* Urethral stricture 0 2.1%* Rectal Fistula Although not observered in clinical studies, isolated incidences of rectal fistula have been reported to Urologix, Inc. following product commercialization. It is believed that the incidence of this serious complication can be minimized through careful patient selection and strict adherence to the treatment instructions described in these instructions for use. Clinical Summary Original Data Follow-up on patients treated with the Targis System demonstrated that this device is associated with significant improvements in BPH symptoms and urine flow rates. The multiple U.S. clinical studies, which include 152 patients at 6 month follow-up, show that 79% of the Targis System treated patients experienced symptom improvement greater than 25% and that 59% experienced improvement greater than 50%. The mean improvement in AUA Symptom Score was 51%. With regard to uroflometry, 66% of the patients demonstrated improvements in peak flow rate of greater than 25%. The mean change in peak flow rate was 4.1 ml/s, a 53% increase over baseline. 4
5 In a randomized and blinded study, patients who received an active treatment exhibited significantly greater improvements in symptom score than sham treated patients (p=0.011). Patients who received an active treatment also exhibited significantly greater improvements in peak flow than sham treated patients (p=0.002). One hundred seven patients have matched data on AUA Symptom Score from baseline to one year follow-up. The mean symptom improvement in this patient group was 10.2 points (51%). Eighty-nine patients have matched data on peak flow from baseline to one year follow-up. Mean peak flow improvement in this patient group was 3.8 ml/s (49%). Significant improvements were seen in additional measured parameters of Quality of Life, Symptom Problem Index (SPI) and the BPH Impact Index (BII). All improvements remained stable through one year follow-up. These improvements are consistent with additional data collected from international studies on over 111 patients at one year follow-up minute vs. 60 minute study data A randomized study comparing a 28.5 minute treatment to a 60 minute treatment was conducted, enrolling 48 and 46 patients respectively. The following table summarizes the 6-month results of 44 patients followed under the Express protocol (28.5 min.) and 39 patients followed under the standard protocol (60 min.). Follow-up at 6 months indicate that both of the treatment groups demonstrated significant improvement from baseline (p-value in all cases) in their AUA Symptom Score, Peak Flow Rate and Quality of Life. The results are shown as mean values. At six months, the difference in the change in values for the AUA Symptom Score and the QoL scores between the 60 minute and 28.5 minute treatments were statistically significant (p-value <.002). Both the 28.5 and 60 minute treatments are effective and clinically relevant treatment options. Outcome Measurement 60 minute results 28.5 minute results Pre-treatment AUA Symptom Score AUA Symptom Score at 6 months Pre-treatment Peak Flow Rate 8.7 ml./sec 8.7 ml./sec Peak Flow Rate at 6 months 13.7 ml./sec 11.1 ml./sec Pre-treatment Quality of Life Quality of Life at 6 months Alternative Surgical Treatment at 6 months 2.2% 0% The long term (12 month) effectiveness of the 28.5 minute treatment has not yet been established. This data will be provided when available. From the current data available for the Targis clinical investigations, it is possible to identify any characteristics that are associated with a more favorable response. The physician should give consideration to both the difference in effectiveness and adverse events in determining the most appropriate treatment time for each patient based on the patient s age, medical condition, and ability to tolerate the procedure. Information for Patients Physicians should inform patients that an inherent risk of complications is associated with the Targis procedure (refer to Adverse Events and Complications). Physicians should inform patients that loss of ejaculation may occur as a result of the Targis procedure and thus should be considered by men who may wish to have further offspring. Physicians should inform patients that the volume of ejaculate may be decreased in some men who undergo the Targis procedure. Temporary acute urinary retention, temporary incontinence, minimal bleeding, pain or urination and intercourse and urinary tract infection may be associated with the Targis procedure. The patient should be informed that a small risk of urethral stricture may result from the Targis procedure requiring further intervention (refer to Adverse Events and Complications). Patients may likely be catheterized for a 2 to 5 day (median 3 day) period following the Targis procedure. Patients may be prescribed anti-inflammatory and antibiotic medications for a period following the Targis procedure. Patient should be informed that they may experience discomfort during the procedure that may require the use of analgesics or sedatives to deliver an effective treatment. Product Description The Targis System utilizes proprietary microwave technology to deliver thermal energy into the anterior and lateral portions of the prostate preferentially and within the axis of the MDS antenna. This allows the Targis System to deliver energy continuously, with minimal concern of treatment-limiting shutdowns from rectal and urethral alarms. Thus, the Targis System can selectively generate and maintain peak intraprostatic tissue temperature levels of 65ºC and mean intraprostatic tissue temperature levels of 50ºC with 5
6 minimal risk of damage to adjacent structures. This allows the Targis System to overcome the heat sink effect of the prostatic blood flow resulting in necrosis of a large volume of diseased hyperplastic tissues. The Procedure Kit is composed of the following individual components: Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag The procedure kit is designed to be used as a system with the control unit and is intended for the treatment of symptomatic and obstructive Benign Prostatic Hyperplasia (BPH) and its associated voiding difficulities. All components must be in place with the Control Unit to begin a Targis procedure. The Targis System is a nonsurgical device intended to relieve symptoms associated with BPH and is indicated for men with prostatic urethra lengths of 3.0 to 5.0 cm, measured from the bladder neck to the verumontanum. For complete and detailed instructions, refer to the Targis System User Manual. MDS The MDS is a catheter-based system that includes a fiber optic temperature sensor, microwave antenna and cable, coolant channels and connectors, urine drainage channel and a distal balloon for placement. The MDS is designed to deliver microwave energy to the target prostatic tissue, while at the same time cooling and protecting the urethra. The fiber optic sensor in the MDS monitors the urethral temperature and is used to determine the amount of energy delivered during a procedure. In the event that MDS temperature rises above 44.5ºC, the Control Unit will discontinue microwave energy delivery to the MDS. For the Targis procedure, the MDS is placed in the patient s urethra with the tip and balloon in the bladder and it is anchored into place with the 10 cc balloon. The MDS is oriented with the coolant tubing in the anterior direction. Microwave energy is transmitted from the Control Unit to the MDS antenna and directed into the prostate. The microwave energy generates heat in the prostate that causes tissue necrosis, which, when resolved can improve the patient s symptoms, flow rate and quality of life associated with BPH. RTU The RTU is a catheter-based system that incorporates a compliant balloon and temperature sensors used to monitor the patient s rectal temperature during a Targis procedure. The RTU is placed in the patient s rectum prior to a Targis procedure and left in place for the duration of the procedure. In the event that rectal temperatures are raised above 42.5ºC, the RTU will signal the Control Unit to discontinue microwave energy delivery to the MDS. The RTU can be provided as a single use disposable device or provided in a configuration that will facilitate the cleaning and disinfection for multiple (up to 30) treatments. Refer to the Targis System User Manual for more detailed information for the use of the Targis System RTU. Extension Tubing 6
7 Coolant Bag The Coolant Bag is used as a reservoir for sterile water, which, during treatment is chilled and circulated through the MDS by the Control Unit to protect the urethra from heat generated by the MDS. The Coolant Bag is comprised of three (3) main subassemblies: coolant reservoir, sensor module and tubing with connectors. The coolant reservoir is a polymer bag that is filled with sterile water. The coolant reservoir mates with the chill plate of the Control Unit to remove heat generated by the MDS during the Targis procedure. The sensor module pressure and temperature windows mate with the corresponding measurement devices on the Control Unit. The sensor module works in communication with the Control Unit to allow pressure and temperature to be monitored during the Targis procedure. The Control Unit uses this information to monitor these parameters during the entire Targis procedure. The inlet and outlet tubing connectors mate with the connectors of the MDS to establish a closed loop coolant system involving the Coolant Bag and the MDS. Control Unit The Control Unit is used to provide and control energy generation and safety for the Targis procedure. The Control Unit includes a computer, microwave energy source, coolant system, fiber optic temperature monitoring system and software. These systems are integrated to precisely and continuously monitor all aspects of a Targis procedure includeing energy generation and delivery, coolant temperature, and the use interface to provide safety shutdowns when procedural and patient safety issues are detected. The Control Unit also maintains a procedural file on the parameters of treatment for an individual patient and procedure. Accessories Several accessories accompany the Targis System. They include: Targis System Users Manual Control Unit Dust Cover Patient Comfort Kit containing 2 Knee Cushions and an MDS Holder Patient Cable Holder An optional Transport Kit containing a Trolley, Protective Cover, and Electrical Safety Tester 7
8 REFER TO THE UROLOGIX USER MANUAL FOR SPECIFIC AND COMPLETE INSTRUCTIONS FOR THE OPERATION STEPS AND PROTOCOL. ENSURE PATIENT HAS RECEIVED AN EFFECTIVE ENEMA WITHIN 2 HOURS PRIOR TO INITIATION OF TREATMENT References Paul D. Miller, Keith Parsons, Ernest Ramsey, Transurethral Microwave Thermoablation (TUMT) for Benign Prostatic Hyperplasia Using a New Device (T3), Presented at the 90th Annual Meeting of the AUA, Las Vegas, NV April, Ramsey E.W., Miler P.D., Parsons K., Transurethral Microwave Thermotherapy in the Treatment of Benign Prostatic Hyperplasia: Results obtained With the Urologix T3 Device. World Journal of Urology (1998) Larson T.R., Bostwick D.G., Corica A., Temperature Correlated Histopathologic Changes Following Microwave Thermoablation of Obstructive Tissue in Patients with Benighn Prostatic Hyperplasia. Urology. 1996:47 (4): Larson T.R., Blute M.L., Tri J.L., Whitlock S.V. Contrasting Heating Patterns and efficacy of the Prostatron and Targis Microwave Antennae for Thermal Treatment of Benign Prostatic Hyperplasia. Urology 51 (6), Djavan B., Larson T.R., Blute M.L., Marberger M. Transurethral Microwave Thermotherapy: What Role Should it Play Versus Medical Management in the Treatment of Benign Prostatic Hyperplasia? Urology 52 (6), The Targis System was formerly known as the T3 System during its development and clinical trails. How Supplied All components of the Procedure Kit are intended for one time use only. DO NOT resterilize and/or reuse them as this will likely result in compromised device performance and increased risk of injury or infection to a patient and treating staff. The Procedure Kit components are supplied in sterile double barrier packaging that has been exposed to ethylene oxide. The Procedure Kit and components are placed in shelf boxes with tamper proof labels. DO NOT USE COMPONENTS THAT HAVE EVIDENCE OF A COMPROMISED PACKAGE OR DAMAGE. Store in ambient conditions between 59-95ºF (15-35ºC). Restricted Devices The Procedure Kit, its components and the Control Unit are intended for urological use only in the treatment of patients suffering voiding difficulties secondary to BPH. Candidates for Targis procedure require careful consideration. The Procedure Kit components must not be used with any other system. Only those physicians who have been thoroughly trained on the operation of the Targis System and the Targis procedure should deliver the Targis procedure. Warranty and Limitations Urologix, Inc. warrants that each component of this device has been manufactured, packaged and tested with reasonable care and will be free from defects in workmansip and material. Urologix will not be liable for any incidental, special, or consequential loss, damage or expense direct or indirect, from the use of its product. Urologix sole obligation shall be to repair, replace, at its option, any device that we feel was defective at time of shipment if notice thereof is received within one year of shipment. Buyer assumes all liability, whether arising on warranty, contract, negligence, compliance with all local laws and regulations which govern the use and sale of this device, or otherwise for damage resulting from the handling, possession, use, or misuse of the product. Because Urologix has no control over the operation, inspection, maintenance, or use of its products after sale and has no control over the selection of patients, THIS WARRANTY IS EXPRESSLY IN LIEU OF ANY OTHER EXPRESSED OR IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE, AND OF ANY OTHER OBLIGATION ON THE PART OF THE SELLER. If the user is located outside the United States, Urologix invokes all oral or written expressed or implied warranty, limitations legally permissible in that jurisdiction. The remedies set forth in the above Warranty and Limitations shall be the exclusive remedy available to any person. No agent, employee or representative of Urologix has any authority to change any of the foregoing or assume or bind Urologix to any additional liability or responsibility in connection with this device. Urologix, Inc. Emergo Europe st Avenue North Molenstraat 15 Minneapolis, Minnesota, USA 2513 BH The Hague Tel: The Netherlands Fax: Tel: (31) (0) Toll Free: Fax: (31) (0) Urologix, Inc. All rights reserved. Printed in U.S.A. Targis System IFU Rev. K 07/10 8
P ROLIEVE. Thermodilatation System. The Prolieve System Patient Information is Directed to You, the Patient. A Transurethral Microwave Therapy Device
 P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
Transurethral microwave thermotherapy
 Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Cooled ThermoTherapy TM
 Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Part # Rev L
 Part #00-00 Rev L Patient Safety Summary This patient safety summary is an overview of the key patient safety information provided in the CoolWave Control Unit User Manual. Please refer to the user manual
Part #00-00 Rev L Patient Safety Summary This patient safety summary is an overview of the key patient safety information provided in the CoolWave Control Unit User Manual. Please refer to the user manual
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Rezūm procedure for the Prostate
 Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Name of Policy: Transurethral Microwave Thermotherapy
 Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
MODULE 3: BENIGN PROSTATIC HYPERTROPHY
 MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL. Introduction. Warnings
 VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL Introduction This protocol is provided to you to be used in conjunction with normal irritant smoke fit test procedures
VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL Introduction This protocol is provided to you to be used in conjunction with normal irritant smoke fit test procedures
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA. A Minimally Invasive Innovative Treatment
 PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE
 493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Overview. Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia. Iain McAuley September 15, 2014
 Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Prostate surgery. What is the prostate? What is a TURP? Why is a TURP operation necessary? Deciding to have a TURP operation.
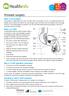 What is the prostate? The prostate is a gland about the size of a walnut that is only present in men. It is located just below the bladder and surrounds the urethra, the tube through which urine flows
What is the prostate? The prostate is a gland about the size of a walnut that is only present in men. It is located just below the bladder and surrounds the urethra, the tube through which urine flows
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Activated Transponder & Activator Instruction Manual
 Activated Transponder & Activator Instruction Manual Westhold Corporation General Warranty Modules and other equipment ("Goods") purchased from Westhold Corporation are warranted against defects in materials
Activated Transponder & Activator Instruction Manual Westhold Corporation General Warranty Modules and other equipment ("Goods") purchased from Westhold Corporation are warranted against defects in materials
Dr. Aso Urinary Symptoms
 Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
THE UROLOGY GROUP
 THE UROLOGY GROUP www.urologygroupvirginia.com 1860 Town Center Drive Suite 150/160 Reston, VA 20190 703-480-0220 19415 Deerfield Avenue Suite 112 Leesburg, VA 20176 703-724-1195 224-D Cornwall Street,
THE UROLOGY GROUP www.urologygroupvirginia.com 1860 Town Center Drive Suite 150/160 Reston, VA 20190 703-480-0220 19415 Deerfield Avenue Suite 112 Leesburg, VA 20176 703-724-1195 224-D Cornwall Street,
Allium Round Posterior Urethral Stent System (RPS) Instructions For Use
 Allium Round Posterior Urethral Stent System (RPS) Instructions For Use Manufactured by Allium Ltd. 2 Ha-Eshel St. Caesarea Industrial Park 38900 Israel Device Name: Allium Round Posterior Urethral Stent
Allium Round Posterior Urethral Stent System (RPS) Instructions For Use Manufactured by Allium Ltd. 2 Ha-Eshel St. Caesarea Industrial Park 38900 Israel Device Name: Allium Round Posterior Urethral Stent
Canada Medtronic of Canada Ltd Kitimat Road Mississauga, Ontario L5N 1W3 Canada Tel Fax
 What PATIENTS About TUNA Say Therapy TUNA THERAPY I would consider it to be a complete success. I have essentially no residual problems of any kind. I m back to the way I was before I ever had any symptoms
What PATIENTS About TUNA Say Therapy TUNA THERAPY I would consider it to be a complete success. I have essentially no residual problems of any kind. I m back to the way I was before I ever had any symptoms
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
The Enlarged Prostate Symptoms, Diagnosis and Treatment
 The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
NOTE: This policy is not effective until April 1, Transurethral Water Vapor Thermal Therapy of the Prostate
 NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
WallFlex Biliary RX Partially Covered Stent System Prescriptive Information
 Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Pruitt Irrigation Occlusion Catheter. English Instructions for Use. Pruitt Irrigation Occlusion Catheter
 English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
LeMaitre Embolectomy Catheter
 Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Benign Prostatic Hyperplasia (BPH)
 Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
REF PERINEOMETER (BIOFEEDBACK MONITOR) FOR FECAL INCONTINENCE AND KEGEL EXERCISES SEE INSTRUCTIONS FOR USE INSTRUCTION MANUAL
 REF 18-1005 PERINEOMETER (BIOFEEDBACK MONITOR) FOR FECAL INCONTINENCE AND KEGEL EXERCISES SEE INSTRUCTIONS FOR USE INSTRUCTION MANUAL TABLE OF CONTENTS Introduction...................... 1 Monitor 5 Device
REF 18-1005 PERINEOMETER (BIOFEEDBACK MONITOR) FOR FECAL INCONTINENCE AND KEGEL EXERCISES SEE INSTRUCTIONS FOR USE INSTRUCTION MANUAL TABLE OF CONTENTS Introduction...................... 1 Monitor 5 Device
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1
 PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Sacral Nerve Stimulation (SNS) System Implantation
 Patient & Family Guide 2016 Sacral Nerve Stimulation (SNS) System Implantation www.nshealth.ca Sacral Nerve Stimulation (SNS) System Implantation SNS system implantation involves placing a lead ( leed,
Patient & Family Guide 2016 Sacral Nerve Stimulation (SNS) System Implantation www.nshealth.ca Sacral Nerve Stimulation (SNS) System Implantation SNS system implantation involves placing a lead ( leed,
INSTALLATION MANUAL. VIDEO Camera, Probe and Lightsource OTOSCOPES.
 INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
Model 380B DNA Synthesizer Version 1.1
 Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
3. Urinary Catheters. Indications. Methods of Bladder Catheterization. Hashim Hashim
 3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
PATIENT SELECTION GUIDE. finding the right fit
 PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
What is Benign Prostatic Hyperplasia (BPH)?
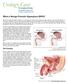 What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Executive Summary. Non-drug local procedures for treatment of benign prostatic hyperplasia 1. IQWiG Reports - Commission No.
 IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T
 Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
MP A Prospective Evaluation of the Catheter Science M3 Mini Catheter for Patients with Prostatic Obstruction. Gaines W. Hammond Jr.
 MP73-06 - A Prospective Evaluation of the Catheter Science M3 Mini Catheter for Patients with Prostatic Obstruction Gaines W. Hammond Jr. MD FACS M3 Mini Catheter M3 Segmented M3 Plus Dynamic Wings M3
MP73-06 - A Prospective Evaluation of the Catheter Science M3 Mini Catheter for Patients with Prostatic Obstruction Gaines W. Hammond Jr. MD FACS M3 Mini Catheter M3 Segmented M3 Plus Dynamic Wings M3
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Management of LUTS after TURP and MIT
 Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
Subject: Temporary Prostatic Stent and Prostatic Urethral Lift
 02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
You have been booked for a. Flexible Cystoscopy. Under Local Anaesthetic
 You have been booked for a Flexible Cystoscopy Under Local Anaesthetic 1 WHAT IS A FLEXIBLE CYSTOSCOPY A flexible cystoscopy is a test to examine the uretha (waterpipe) and bladder using a thin, lighted
You have been booked for a Flexible Cystoscopy Under Local Anaesthetic 1 WHAT IS A FLEXIBLE CYSTOSCOPY A flexible cystoscopy is a test to examine the uretha (waterpipe) and bladder using a thin, lighted
Electrodes (for TransAeris System)
 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Neurogenic bladder. Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder.
 Definition: Neurogenic bladder Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder. Types: Nervous system diseases: Congenital: like myelodysplasia like meningocele.
Definition: Neurogenic bladder Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder. Types: Nervous system diseases: Congenital: like myelodysplasia like meningocele.
TRANSURETHRAL RESECTION OF THE PROSTATE
 TRANSURETHRAL RESECTION OF THE PROSTATE A discussion of the operation and the pre and post operative care You and your doctor have considered the possibility that you have a transurethral resection of
TRANSURETHRAL RESECTION OF THE PROSTATE A discussion of the operation and the pre and post operative care You and your doctor have considered the possibility that you have a transurethral resection of
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
OPERATING INSTRUCTIONS. Orascoptic DK
 User Manual Orascoptic DK is a versatile, 3-in-1 dental device that employs a handheld LED light source and three unique, interchangeable diagnostic instruments. Table of Contents 1. Orascoptic DK Kit
User Manual Orascoptic DK is a versatile, 3-in-1 dental device that employs a handheld LED light source and three unique, interchangeable diagnostic instruments. Table of Contents 1. Orascoptic DK Kit
COMPRESSOR NEBULIZER MODEL: JB USER S MANUAL
 COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
AquaJogg Hydro Treadmill
 AquaJogg Hydro Treadmill PART #: F-WXAQJG 330 LB. [150 kg] MAXIMUM WEIGHT CAPACITY MANDATORY LEAVE THIS MANUAL WITH TREADMILL OWNER To assemble your equipment read entire manual and follow all instructions.
AquaJogg Hydro Treadmill PART #: F-WXAQJG 330 LB. [150 kg] MAXIMUM WEIGHT CAPACITY MANDATORY LEAVE THIS MANUAL WITH TREADMILL OWNER To assemble your equipment read entire manual and follow all instructions.
PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA
 St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
Urinary Catheter or Urinary Tract Infection Critical Element Pathway
 Use this pathway for a resident who has a symptomatic urinary tract infection (UTI) and/or an indwelling urinary catheter. Review the Following in Advance to Guide Observations and Interviews: Review the
Use this pathway for a resident who has a symptomatic urinary tract infection (UTI) and/or an indwelling urinary catheter. Review the Following in Advance to Guide Observations and Interviews: Review the
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Transurethral Resection of Prostate (TURP)
 Transurethral Resection of Prostate (TURP) Department of Urology Patient Information What What and and where where is the is prostate? the prostate? The prostate is a small gland, about the size of a walnut,
Transurethral Resection of Prostate (TURP) Department of Urology Patient Information What What and and where where is the is prostate? the prostate? The prostate is a small gland, about the size of a walnut,
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH
 MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH
 Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH The more you know, the better you ll feel. You ve likely had a discussion with your doctor about BPH 1. What follows are some
Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH The more you know, the better you ll feel. You ve likely had a discussion with your doctor about BPH 1. What follows are some
Control & confidence. You deserve both.
 Learn more about BPH and Plasma therapy Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH Your doctor is always happy to offer all the guidance you need so that you feel completely
Learn more about BPH and Plasma therapy Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH Your doctor is always happy to offer all the guidance you need so that you feel completely
Experience the Innovative Therapy for Benign Prostate Enlargement
 Experience the Innovative Therapy for Benign Prostate Enlargement A Guide to Treatment of Benign Prostatic Hyperplasia 1. 2. The Prostate The prostate gland is a part of the male reproductive system. A
Experience the Innovative Therapy for Benign Prostate Enlargement A Guide to Treatment of Benign Prostatic Hyperplasia 1. 2. The Prostate The prostate gland is a part of the male reproductive system. A
PORTO 2 VENT CPAP OS. Operator s Manual. PORTO 2VENT CPAP OS System Operator s Manual Part Number Rev I
 PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
PATIENT INFORMATION 2017 NeoTract, Inc. All rights reserved. Printed in the USA. MAC Rev A
 PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
THE UROLOGY GROUP
 THE UROLOGY GROUP www.urologygroupvirginia.com 1860 Town Center Drive Suite 150/160 Reston, VA 20190 703-480-0220 19415 Deerfield Avenue Suite 112 Leesburg, VA 20176 703-724-1195 224-D Cornwall Street,
THE UROLOGY GROUP www.urologygroupvirginia.com 1860 Town Center Drive Suite 150/160 Reston, VA 20190 703-480-0220 19415 Deerfield Avenue Suite 112 Leesburg, VA 20176 703-724-1195 224-D Cornwall Street,
Transurethral Resection of the Prostate (TURP) Transurethral Incision of the Prostate (TUIP) PROCEDURE EDUCATION LITERATURE
 Transurethral Resection of the Prostate (TURP) Transurethral Incision of the Prostate (TUIP) PROCEDURE EDUCATION LITERATURE We recommend that you read this handout carefully in order to prepare yourself
Transurethral Resection of the Prostate (TURP) Transurethral Incision of the Prostate (TUIP) PROCEDURE EDUCATION LITERATURE We recommend that you read this handout carefully in order to prepare yourself
An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms
 Case Report INJ 2010;14:125-129 An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms Joo-Yong Lee, Dong-Hyuk Kang, Hee-Young Park, Jung-Soo Park, Young-Woo Son, Hong-Sang
Case Report INJ 2010;14:125-129 An Anteriorly Positioned Midline Prostatic Cyst Resulting in Lower Urinary Tract Symptoms Joo-Yong Lee, Dong-Hyuk Kang, Hee-Young Park, Jung-Soo Park, Young-Woo Son, Hong-Sang
BULKAMID STANDARD OPERATING PROCEDURE
 BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
Chapter 16 URINARY, SEXUAL AND REPRODUCTIVE IMPAIRMENT
 Chapter 16 URINARY, SEXUAL AND REPRODUCTIVE IMPAIRMENT Introduction This chapter provides criteria for assessing permanent impairment from entitled urinary, sexual and reproductive conditions. The chapter
Chapter 16 URINARY, SEXUAL AND REPRODUCTIVE IMPAIRMENT Introduction This chapter provides criteria for assessing permanent impairment from entitled urinary, sexual and reproductive conditions. The chapter
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Flex-Point HEATED MUSCLE MASSAGER
 Flex-Point HEATED MUSCLE MASSAGER TABLE OF CONTENTS Cautions and Warnings...1 FCC Information...5 Location of Controls...6 Operation...6 Using the Node Covers...7 Care and Maintenance...7 Specifications...7
Flex-Point HEATED MUSCLE MASSAGER TABLE OF CONTENTS Cautions and Warnings...1 FCC Information...5 Location of Controls...6 Operation...6 Using the Node Covers...7 Care and Maintenance...7 Specifications...7
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide
 TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide TMNS is a therapeutic genital vibrator to treat sexual dysfunction or as adjunct to Kegel s exercise device. (Tightening of the muscles of
TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide TMNS is a therapeutic genital vibrator to treat sexual dysfunction or as adjunct to Kegel s exercise device. (Tightening of the muscles of
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Guide for Use. Apogee Plus Touch Free Intermittent Catheter System
 Apogee Plus Touch Free Intermittent Catheter System Guide for Use Touch free, sterile, and self-contained, Hollister Apogee Plus intermittent catheter empowers you to maintain your independence and live
Apogee Plus Touch Free Intermittent Catheter System Guide for Use Touch free, sterile, and self-contained, Hollister Apogee Plus intermittent catheter empowers you to maintain your independence and live
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
National Kidney and Urologic Diseases Information Clearinghouse
 Urodynamic Testing National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is the urinary tract? The urinary tract
Urodynamic Testing National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is the urinary tract? The urinary tract
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Guide for Use. Advance Plus Touch Free Intermittent Catheter System
 Advance Plus Touch Free Intermittent Catheter System Guide for Use Hollister Advance Plus intermittent catheter features Touch Free technique enabling users to catheterize with confidence virtually anytime,
Advance Plus Touch Free Intermittent Catheter System Guide for Use Hollister Advance Plus intermittent catheter features Touch Free technique enabling users to catheterize with confidence virtually anytime,
AMS 800. Urinary Control System. User s Guide. Table of Contents. The AMS 800 Urinary Control System...1. Using the AMS 800 Urinary Control System...
 AMS 800 Urinary Control System User s Guide Table of Contents The AMS 800 Urinary Control System...1 Using the AMS 800 Urinary Control System...2 Special precautions to take with your 800 before undergoing
AMS 800 Urinary Control System User s Guide Table of Contents The AMS 800 Urinary Control System...1 Using the AMS 800 Urinary Control System...2 Special precautions to take with your 800 before undergoing
Prostate Gland Disorders
 Prostate Gland Disorders THE PROSTATE GLAND A male s prostate gland is located in the floor of the pelvis surrounding the urethra between the bladder and the penis. The prostate is positioned immediately
Prostate Gland Disorders THE PROSTATE GLAND A male s prostate gland is located in the floor of the pelvis surrounding the urethra between the bladder and the penis. The prostate is positioned immediately
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
 P R E S E N T S Dr. Mufa T. Ghadiali is skilled in all aspects of General Surgery. His General Surgery Services include: General Surgery Advanced Laparoscopic Surgery Surgical Oncology Gastrointestinal
P R E S E N T S Dr. Mufa T. Ghadiali is skilled in all aspects of General Surgery. His General Surgery Services include: General Surgery Advanced Laparoscopic Surgery Surgical Oncology Gastrointestinal
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
The Biomet EBI Bone Healing System. Patient Manual
 The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
TYPES OF PROSTATITIS There are three types of prostatitis-type presentations:
 SPECIALIZED UROLOGIC CONSULTANTS, SC 10400 Southwest Hwy 16632 S. 107 th Ct. Chicago Ridge, IL 60453 Orland Park, IL 60467 Tel (708) 423-8706 Tel (708) 349-6350 PROSTATITIS - REVIEW Prostatitis is an inflammation
SPECIALIZED UROLOGIC CONSULTANTS, SC 10400 Southwest Hwy 16632 S. 107 th Ct. Chicago Ridge, IL 60453 Orland Park, IL 60467 Tel (708) 423-8706 Tel (708) 349-6350 PROSTATITIS - REVIEW Prostatitis is an inflammation
Suprapubic Catheter Insertion Clinic
 Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter A urinary catheter is a tube used to drain urine from the bladder. The commonest catheters are ones
Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter A urinary catheter is a tube used to drain urine from the bladder. The commonest catheters are ones
