The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs
|
|
|
- Sybil Malone
- 5 years ago
- Views:
Transcription
1 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs January Beth Pulliam University of Tennessee - Knoxville Recommended Citation Pulliam, January Beth, "The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs. " PhD diss., University of Tennessee, This Dissertation is brought to you for free and open access by the Graduate School at Trace: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of Trace: Tennessee Research and Creative Exchange. For more information, please contact trace@utk.edu.
2 To the Graduate Council: I am submitting herewith a dissertation written by January Beth Pulliam entitled "The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Animal Science. We have read this dissertation and recommend its acceptance: Dr. Kelly Robbins, Dr. Arnold Saxton, Dr. Robert Henry, Dr. Gina Pighetti (Original signatures are on file with official student records.) Dr. Alan Mathew, Major Professor Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School
3 To the Graduate Council: I am submitting herewith a dissertation written by January Beth Pulliam entitled The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs. I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Animal Science. Dr. Alan Mathew Major Professor We have read this dissertation and recommend its acceptance: Dr. Kelly Robbins Dr. Arnold Saxton Dr. Robert Henry Dr. Gina Pighetti Accepted for the Council: Dr. Anne Mayhew Vice Provost and Dean of Graduate Studies (Original signatures are on file with official student records.)
4 The Effects of Dietary Additives on the Growth Performance and Occurrance of Resistant Bacteria in Weanling Pigs. A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville January Beth Pulliam December 2003
5 ACKNOWLEDGEMENTS The author would like to thank Alltech, Inc. for the funding and support that made this research possible. The author would also like to thank her advisor, Dr. Alan Mathew for his patience and support. Appreciation is expressed to my committee members, Dr. Arnold Saxton, Dr. Kelly Robbins, Dr. Robert Henry and Dr. Gina Pighetti for their assistance with my research and course work. Special mention is given to Ms. Rose Cliff for her assistance in the laboratory and to Mr. Brent Pugh and Mr. Danny Sutton for their valuable advice and assistance with my pigs. I would also like to thank the faculty who encouraged and guided me. I have learned many valuable lessons from you, admire your dedication, and am truly grateful for the opportunity to work with such outstanding individuals. Thank you to Dr. J.C. McConnell for all of his valuable knowledge, advice and for believing in me enough to give me the chance to pursue my dreams. Thanks to Greg for his patience and understanding through all of the hard times, you have truly been a good friend and you will forever be in my heart. Most of all, thank you to my parents for standing behind me through all of these years in school and to God for giving me the faith, strength and courage to persevere. ii
6 ABSTRACT Three replicate trials, with a total of 36 ileal cannulated pigs, were conducted to determine the effects of including carbadox or mannanoligosaccharides in weanling pig diets. Individual crossbred (Yorkshire X Landrace X Duroc) pigs were used as the experimental units and were weaned at approximately 21 days of age, balanced by gender, genetics and weight, and allotted to pens in groups of three. Pens were randomly assigned to one of four treatments including: AB) 55 mg carbadox/kg, BM) 0.2% phosphorylated mannanoligosaccharide, RT) a rotation of the above two treatments, or CT) NRC based control treatment with no additives. Pigs were allowed ad libitum access to water and the assigned treatment as a single-phase diet for a 35-day period. Ileal digesta were collected on days 14, 21, 28, and 35 of the trial. Pigs were sacrificed on day 35 and digesta and tissue samples were collected from the duodenum, jejunum, ileum, cecum, and spiral colon. Digesta were analyzed for ph, dry matter, total aerobes, total anaerobes, lactobacilli, streptococci, E.coli and VFA including: acetate, propionate, butyrate, valerate, isovalerate, and isobutyrate. Tissue samples were fixed and stained with hematoxylin and eosin and examined for villi height and crypt depth measurements. Intestinal ph of the BM treatment was more alkaline and the CT treatment was more acidic when treatments were compared (P=0.0017). A site effect (P=0.0001) was also observed where the ileum was more alkaline and the cecum more acidic. Ileal dry matter increased (P=0.0007) for all treatment groups through day 28 postweaning and was lowest in the ileum and highest in the spiral colon (P=0.0001). Lactobacilli concentrations were lowest for the AB treatment and highest for the CT (P=0.0400). Time effects were observed where total anaerobe concentrations decreased (P=0.0034) 28 iii
7 and 35 postweaning for all treatments and ileal E.coli concentrations decreased (P=0.0004) on day 21 postweaning. A site effect (P=0.0001) was observed for all microbial concentrations. Treatment effects were noted for ileal butyrate (P=0.0070) and acetate (P=0.0174) where acetate and butyrate concentrations were greater in the ileum for the AB and RT treatments compared to the BM and CT treatments. Time affected ileal isovalerate (P=0.0113), butyrate (P=0.0001), and acetate (P=0.0001) concentrations. A site effect (P=0.0001) was observed for all VFA concentrations. Treatment X site interactions were observed (P=0.0297) for valerate concentrations. Villi length was significantly shorter in the ileum and crypt depths were deepest in the cecum and spiral colon (P=0.0001). In a subsequent study to compare effects of carbadox and mannanoligosaccharides on performance, a total of 48 crossbred (Yorkshire X Landrace X Duroc) pigs were weaned at approximately 21 days of age and balanced by gender, genetics, and weight. Pens were randomly assigned to one of the four previously mentioned treatments with each treatment consisting of 3 pens and 4 pigs per pen. Each group was allowed ad libitum access to water and the assigned treatment as a singlephase diet. Pens were used as the experimental units. Performance parameters, including average daily gain, average feed intake, and feed conversion ratio, were measured for a 28-day period and compared across treatments. Feed intake and feed conversion ratio were measured for individual pens. No treatment effects (P>0.05) were noted for any of the growth parameters measured. Twelve pigs were sacrificed on day 7 and 28 and digesta and tissue samples were collected from the duodenum, jejunum, ileum, cecum, and spiral colon. Digesta were iv
8 analyzed for ph, dry matter, lactobacilli, E.coli and VFA. Tissue samples were fixed and stained with hematoxylin and eosin and examined for villi height and crypt depth measurements. Individual pigs were used as the experimental units and data were analyzed over time. Treatment effects (P=0.0109) were observed on day 28 postweaning where ph was highest for the AB and BM treatments and lowest for the CT. Treatment X site interactions (P=0.0333) were observed for percentage dry matter. Site effects (P=0.0001) were observed for both ph and percentage dry matter. Treatment effects were observed for E.coli concentrations on day 28 (P=0.0063) and over all days (P=0.0282). Concentrations were lowest for the AB and BM treatments and highest for the CT treatment. Treatment X site interactions (P=0.0313) were observed for E.coli concentrations on day 28 postweaning. No treatment or site effects (P>0.05) were observed for E.coli resistance to carbadox. Resistance increased (P=0.0032) from day 7 to 28 postweaning for all treatments and sites. Treatment X site interactions (P=0.0500) were observed for E.coli resistance overall. Site effects (P<0.05) were observed for lactobacilli and E.coli concentrations. Treatment X site interactions were observed on day 28 for isobutyrate (P=0.0200), and on day 28 and overall (P=0.0001) for acetate concentrations. Treatment effects (P<0.05) were observed for valerate concentrations on day 7, 28 and over all days. Treatment effects (P=0.0002) were observed on day 28 for acetate concentrations. Site effects (P<0.05) were observed for isovalerate, valerate, propionate, acetate, and butyrate concentrations. Site effects (P<0.05) were observed for villi height and crypt depth. v
9 Results from this research indicate that the intestinal microbial environment is affected by the inclusion of carbadox or mannanoligosaccharides in weanling pig diets. vi
10 Table of Contents CHAPTER I. REVIEW OF LITERATURE 1 Development of Intestinal Digestion 1 Intestinal Morphology 2 Enzymatic Development 3 ph 4 Intestinal Permeability 5 Microbial Establishment 6 Factors Affecting Microbial Composition 7 Affects of Weaning on the Intestinal Tract 7 Morphology 8 Enzymatic Changes 9 Intestinal Microflora Establishment 9 Volatile Fatty Acids 11 ph 12 Pathogen Exposure 13 Adherence and Colonization of E.coli 14 E.coli Surface Antigens 14 Invasive E.coli 15 Non-Invasive E.coli 17 Intestinal Activity Against Antigens 19 Antibody Activity 19 Development and Growth Performance 21 Factors Contributing to the Lag Phase 22 Antibiotic Use in Animal Feeds 23 Antimicrobial Mechanisms of Antibiotics 24 Antibiotic Mechanisms for Growth Promotion 25 Effects of Digesta Flow and Feed Intake 26 Intestinal Mass and Absorption 26 Antibiotic Resistance 28 Mechanisms of Resistance 28 Patterns of Resistance 30 Affects of Feeding Regimens 31 Increasing Resistance 31 Alternatives to Antibiotics 32 Probiotics 32 Yeast Cultures 33 Prebiotics 34 Objectives of This Research 37 Page vii
11 Table of Contents (Continued) Page II. EXPERIMENT ONE 38 Materials and Methods 38 Experimental Design 38 Surgical Procedure 41 Sample Collection and Analysis 43 Microbial Analysis 44 Minimum Inhibitory Concentration Analysis (MIC) 45 Short-Chain Fatty Acid Analysis 46 Collection of Gastrointestinal Contents and Tissues 47 Tissue Analysis 48 Statistical Analysis 48 Results-Ileal Analyses 49 ph and Dry Matter 49 Microflora 49 Resistance Levels 56 Short-Chain Fatty Acids 56 Results-Gastrointestinal Locations 60 ph and Dry Matter 60 Microflora 65 Resistance Levels 74 Short-Chain Fatty Acids 74 Tissue 77 Discussion-Ileal Analyses 77 Discussion-Gastrointestinal Locations 83 III. EXPERIMENT TWO 86 Materials and Methods 86 Experimental Design 86 Sample Collection and Analysis 89 Microbial Analysis 90 Minimum Inhibitory Concentration Analysis (MIC) 91 Short-Chain Fatty Acid Analysis 92 Tissue Analysis 92 Statistical Analysis 93 Results 93 Growth Performance 93 ph and Dry Matter 97 Microflora 100 Resistance Levels 104 Short-Chain Fatty Acids 104 Tissue 114 viii
12 Table of Contents (Continued) Page Discussion 117 IV. CONCLUSIONS 124 V. IMPLICATIONS 126 LITERATURE CITED 127 VITA 142 ix
13 LIST OF TABLES Table Page 1. Experiment one treatments applied to pig groups Experiment one percentage composition of treatment diets Effects of dietary additives on ileal ph and percentage dry 50 matter in weanling pigs. 4. Effects of dietary additives on ileal anaerobe concentrations 52 in weanling pigs. 5. Effects of dietary additives on ileal lactobacilli concentrations 54 in weanling pigs. 6. Effects of dietary additives on ileal E.coli concentrations 55 in weanling pigs. 7. Effects of dietary additives on ileal acetate concentrations 58 in weanling pigs. 8. Effects of dietary additives on ileal butyrate concentrations 59 in weanling pigs. 9. Effects of dietary additives on ileal isovalerate concentrations 61 in weanling pigs. 10. Effects of dietary additives on duodenal, jejunal, ileal, cecal, 66 and spiral colon ph in weanling pigs. 11. Effect of dietary additives on streptococci (Log 10 cfu/g) 70 concentrations in various locations of the gastrointestinal tracts in weanling pigs. 12. Effects of dietary additives on VFA concentrations 76 (mmoles/liter) in various gastrointestinal locations in weanling pigs. 13. Experiment two treatments applied to pig groups Experiment two percentage composition of treatment diets. 87 x
14 List of Tables (Continued) Table Page 15. Effects of dietary additives on ph in various locations in 98 the gastrointestinal tracts in weanling pigs on day 7 postweaning. 16. Effects of dietary additives on ph in various locations in 98 the gastrointestinal tracts in weanling pigs on day 28 postweaning. 17. Effects of dietary additives on ph in various locations in 98 the gastrointestinal tracts in weanling pigs over all days. 18. Effects of dietary additives on dry matter percentages in 99 various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 19. Effects of dietary additives on dry matter percentages in 99 various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 20. Effects of dietary additives on dry matter percentages in 99 various locations in the gastrointestinal tracts in weanling pigs over all days. 21. Effects of dietary additives on lactobacilli concentrations 101 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 22. Effects of dietary additives on lactobacilli concentrations 101 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 23. Effects of dietary additives on lactobacilli concentrations 101 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs over all days. 24. Effects of dietary additives on E.coli concentrations 103 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 25. Effects of dietary additives on E.coli concentrations 103 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. xi
15 List of Tables (Continued) Table Page 26. Effects of dietary additives on E.coli concentrations 103 (Log 10 cfu/g) in various locations in the gastrointestinal tracts in weanling pigs over all days. 27. Effects of dietary additives on E.coli resistance to carbadox 105 (µg/ml) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 28. Effects of dietary additives on E.coli resistance to carbadox 105 (µg/ml) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 29. Effects of dietary additives on E.coli resistance to carbadox 105 (µg/ml) in various locations in the gastrointestinal tracts in weanling pigs over all days. 30. Effects of dietary additives on isovalerate concentrations 107 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 31. Effects of dietary additives on isovalerate concentrations 107 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 32. Effects of dietary additives on isovalerate concentrations 107 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. 33. Effects of dietary additives on isobutyrate concentrations 109 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 34. Effects of dietary additives on isobutyrate concentrations 109 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 35. Effects of dietary additives on isobutyrate concentrations 109 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. xii
16 List of Tables (Continued) Table Page 36. Effects of dietary additives on valerate concentrations 110 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 37. Effects of dietary additives on valerate concentrations 110 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 38. Effects of dietary additives on valerate concentrations 110 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. 39. Effects of dietary additives on propionate concentrations 112 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 40. Effects of dietary additives on propionate concentrations 112 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 41. Effects of dietary additives on propionate concentrations 112 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. 42. Effects of dietary additives on acetate concentrations 113 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. 43. Effects of dietary additives on acetate concentrations 113 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 44. Effects of dietary additives on acetate concentrations 113 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. 45. Effects of dietary additives on butyrate concentrations 115 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 7 postweaning. xiii
17 List of Tables (Continued) Table Page 46. Effects of dietary additives on butyrate concentrations 115 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs on day 28 postweaning. 47. Effects of dietary additives on butyrate concentrations 115 (mmoles/liter) in various locations in the gastrointestinal tracts in weanling pigs over all days. 48. Effects of dietary additives on villi height (um) in the duodenum, 116 jejunum, and ileum in weanling pigs on day 7 postweaning. 49. Effects of dietary additives on villi height (um) in the duodenum, 116 jejunum, and ileum in weanling pigs on day 28 postweaning. 50. Effects of dietary additives on villi height (um) in the duodenum, 116 jejunum, and ileum in weanling pigs over all days. 51. Effects of dietary additives on crypt depth (um) in various 118 gastrointestinal locations in weanling pigs on day 7 postweaning. 52. Effects of dietary additives on crypt depth (um) in various 118 gastrointestinal locations in weanling pigs on day 28 postweaning. 53. Effects of dietary additives on crypt depth (um) in various 118 gastrointestinal locations in weanling pigs over all days. xiv
18 LIST OF FIGURES Figure Page 1. Effects of dietary additives on ileal aerobe concentrations 51 in weanling pigs. 2. Effects of dietary additives on ileal streptococci concentrations 53 in weanling pigs. 3. Effects of dietary additives on resistance levels of E.coli to 57 carbadox in ileal digesta of weanling pigs. 4. Effects of dietary additives on ileal isobutyrate concentrations 62 in weanling pigs. 5. Effects of dietary additives on ileal valerate concentrations 63 in weanling pigs. 6. Effects of dietary additives on ileal propionate concentrations 64 in weanling pigs. 7. Effects of dietary additives on percentage dry matter in the 67 duodenum, jejunum, ileum, cecum, and spiral colon in weanling pigs. 8. Effects of dietary additives on aerobe concentrations in 68 various locations of the gastrointestinal tracts in weanling pigs. 9. Effects of dietary additives on anaerobe concentrations in 69 various locations of the gastrointestinal tracts in weanling pigs. 10. Effects of dietary additives on lactobacilli concentrations in 72 various locations of the gastrointestinal tracts of weanling pigs. 11. Effects of dietary additives on E.coli concentrations in 73 various locations of the gastrointestinal tracts in weanling pigs. 12. Effects of dietary additives on resistance levels of E.coli to 75 carbadox in various locations of the gastrointestinal tracts in weanling pigs. 13. Effects of dietary additives on villi length in various locations 78 of the gastrointestinal tracts in weanling pigs. xv
19 List of Figures (Continued) Figure Page 14. Effects of dietary additives on crypt depths in various locations 79 of the gastrointestinal tracts in weanling pigs. 15. Effects of dietary additives on average daily gain of weanling 94 pigs from day 7 to day 28 postweaning. 16. Effects of dietary additives on average feed intake of weanling 95 pigs from day 7 to day 28 postweaning. 17. Effects of dietary additives on feed conversion ratio of weanling 96 pigs from day 7 to day 28 postweaning. 18. Effects of dietary additives on E.coli resistance levels to carbadox 106 in weanling pigs from day 7 to day 28 postweaning. xvi
20 I. REVIEW OF THE LITERATURE Development of Intestinal Digestion To formulate diets optimally suited to the digestive capacity of the piglet, it is necessary to know as much as possible about the efficiency of the piglet s digestive system and of any changes in the digestive capacity which occur as it gets older (Manners, 1976). There is a close relationship between the degree of maturation and absorptive functions of the intestines. Low (1976) defines digestion to be the process of reducing the size of organic molecules by hydrolysis while absorption is the process of uptake of molecules and ions by the epithelial cells lining the gut. Low (1976) also discusses that digestibility is not only the measure of susceptibility of the physical and chemical structure of the nutrient to hydrolysis; but also the capacity of the animal to secrete sufficient enzymes of the appropriate type to effect the hydrolysis, in the presence of a suitable mineral solution, and of the ability of the absorptive system to move the nutrient from the gut lumen. There is a need to examine the many facets of the digestive development in growing pigs for both absorption and digestibility answers. Some developmental changes are specific, such as when the transport of one or several solutes is changed, or nonspecific when the transport of many different solutes is altered. Nonspecific changes may reflect changes in the mucosal surface area, changes in proliferation and migration of enterocytes, changes in phospholipid composition and fluidity of the plasma membrane, and changes in para-cellular permeability. Specific changes may be related to the changes in cell turnover rate, affinity, and the density and expression of transporters. 1
21 Intestinal Morphology The small intestine is usually divided into three regions with microscopic similarities, the duodenum, jejunum and the ileum. The mucosa of these sections consists of fingerlike villi and between the bases of the villi are simple tubular glands known as the crypts of Lieberkuhn (Dukes, 1957). Secretory cells that produce intestinal secretions line the crypts. Dramatic changes in the intestinal morphology and in the rate of cell proliferation and migration are two important mechanisms that determine the changes in mucosal weight and surface area and thus of the total transport capacity (Pacha, 2000). Intestinal development is associated with the growth of the intestines and also the changes in the intestinal villi in the small intestine. By birth, the stratified endotherm that is formed of undifferentiated cells has undergone a transition into a simple columnar epithelium with villi. Cellular proliferation is detectable along the villi, crypts have developed and intestine-specific genes can be detected (Rings et al., 1992). Crypt cell proliferation in the small intestine is low in the suckling pig until weaning. Early development of enterocytes is associated with amplification of the surface of microvilli and of the basement membrane. Developmental changes of surface area are influenced by rapid growth of intestinal mucosa that is predetermined by decreased cell turnover (Klein, 1989). This decrease in cell turnover rate increases the lifetime of the enterocytes and slower development influences the presence of different phenotypes in the immature intestines. These morphological changes relate to the rate of cell turnover in the intestinal epithelium because cell replacement is slow at birth. Immature crypts contain polyclonal cells whereas mature crypts are composed of cells of one phenotype (Dukes, 1972). 2
22 These immature crypts contain only a small group of proliferating, undifferentiated stem cells that give rise to several cell phenotypes that migrate onto adjacent villi. Early prenatal crypt development is typical for species with longer gestation periods such as the pig (Moxey and Trier, 1978). Cells that express one type of transporter are slowly replaced by cells exhibiting other transport properties. There are also differences in the distribution of transporters along the crypt to villi axis in the immature intestine. Nutrient transport in the immature intestine is localized in the upper villus and then develops along the whole villus down into the crypts. Along with the formation of villus and crypts there is development of various enterocytes (absorptive cells), mucus secreting goblet cells, and enzyme secreting cells. Enzymatic Development Insufficient enzymatic development provides a rational explanation for the inability of the young pig to digest certain carbohydrates in artificial diets and shows the importance of relating the diet composition to the enzymatic capabilities (Hudman et al., 1957). Gastric glands of the newborn pig contain peptic and parietal cells but proteolytic activity is low for the first two weeks of life. Acid secretion by the parietal cells is minimal during early life and trypsin is the main protein splitting enzyme (Dukes, 1972). The stomach increases in volume and acidity as the pig ages, which in turn increases the amount of pepsin, which is more fully activated due to the increase in secretion of hydrochloric acid (Manner, 1976). By three weeks of age, trypsin concentrations in the pancreas have increased and pepsin secretions have increased resulting in a stomach 3
23 acidity that is suitable for gastric proteolytic digestion. Smith (1988) concluded that negligible peptic activity occurs until 3 weeks of age. A rapid increase in pancreatic amylase occurs from birth to around 5 weeks of age, resulting in an increase in the capability of starch digestion as the pig matures. Corring et al. (1978) found that pancreatic amylase was low at birth and did not increase until after 21 days of age, whereas pancreatic lipase remained low until 35 days of age. This research also suggested that pancreatic function was stimulated from the third to fourth week of age and seemed to coincide with creep feeding. Corring et al. (1978) hypothesized that the creep feeding might have helped pancreatic adaptation of enzyme activity to the diet prior to weaning. Lactase is high at birth through two to three weeks of age and then rapidly declines, whereas sucrase and maltase activity is low at birth and rises gradually to a maximum after three weeks of age. Research by Aumaitre and Corring (1978) indicated that intestinal lactase activity decreased even in pigs prevented from eating solid feed. Walker (1959) found little bile in the gallbladder of newborn pigs, however, that product increased slowly during the first three weeks of life. ph The ph in the stomach of the nursing pig is close to that of its diet, differing considerably from that of the adult, which has a ph of approximately 2 (Manners, 1976). In a study conducted by Manners (1976) examining the development of the digestive tract in growing pigs, no low ph values were noted in the stomach of nursing pigs less than 7 weeks of age. Lower acidity in the digestive tract of the young pig allows bacteria to multiply more freely. This would potentially make the pigs more susceptible to gastrointestinal disease during this period. The study concluded that there seemed to be a 4
24 long period in which the stomach contents are insufficiently acidic (greater than ph of 3) to inhibit bacterial multiplication and to activate pepsinogen. Intestinal Permeability The regulation of intestinal transport function is largely dependent on the junctional complex connecting enterocytes. Regulation of transport determines the extent to which solutes and water are absorbed or secreted. Tight junctions form a barrier between the lumen and basolateral compartments and selectively control the passive diffusion of ions and other small solutes through the paracellular pathway. This influences any gradient created by activities of pathways associated with the transcellular route (Pacha, 2000). The presence of paracellular pathways with high permeability permits rapid diffusion and creates large transepithelial electrical potentials and concentration gradient across the epithelium. These paracellular routes enable the absorption of some solutes coupled with fluid absorption via solute drag, without any additional expenditure of energy. Routes may also indirectly influence transcellular movement by influencing Na+ dependent absorption of nutrients due to the paracellular back flux of Na+ (Madara, 1989). These observations are of considerable importance for understanding the physiology of absorption of nutrients, but questions regarding changes during development are still unanswered. Indirect evidence suggests that the permeability of the immature intestine in the newborn pig is higher than in adult animals and the response of the intestine to hypertonic perfusion is age dependent (Wisser and Horster, 1978). 5
25 Microbial Establishment The tract of the newborn pig is relatively sterile but is exposed to a variety of microorganisms shortly after parturition and rapidly acquires an intestinal bacterial population (Kenworthy and Crabb, 1963; Savage 1970). Various microorganisms colonize specific regions of the intestinal tract and are well adapted to that particular environments. The entire length of the intestinal tract of a healthy pig is occupied by bacteria with concentrations of 10 6 to 10 9 colony forming units (CFU)/g in the small intestine and 10 9 to CFU/g in the cecum and colon (Kenworthy and Crabb, 1963; Smith and Jones, 1963). Studies conducted by Savage (1970, 1977) determined that the first microorganisms to appear in the intestinal tract were lactobacilli and streptococci, both of which are lactic acid producing bacteria. Lactobacilli and streptococci colonize the nonglandular portion of the stomach and can be cultured from all areas of the intestinal tract by hours after birth. Briggs et al. (1954) also established that lactobacilli and streptococci were the major intestinal microflora. Subsequent work by Smith and Crabb (1961), Kenworthy and Crabb (1963), and Smith and Jones (1963) established that normal microflora of the pig were lactobacilli, streptococci, Bacteriodes spp., and Escherichia coli. The intestinal tract is inhabited by a complex and dynamic microbial ecosystem and must develop a means of keeping intestinal bacteria in check along with the ability to discriminate between resident microflora and enteric pathogens (Lu and Walker, 2001). 6
26 Factors Affecting Microbial Composition Factors generated by the animal can influence population levels and types of microorganisms that inhabit particular areas of the intestinal tract (Savage, 1977). Hydrogen ion concentration is a major factor that dictates the type of microorganisms that colonize specific areas. Peristalsis also imparts a strong influence that prevents microbial communities from developing in the lumen of the upper and middle regions of the small intestines. The intestinal temperature of 37 C is optimal for the growth of microorganisms and is an asset to their colonization (Savage, 1972). Anaerobic microbes have the ability to colonize in any location of the intestinal tract because of the low oxygen content (Walden and Hentges, 1975). Affects of Weaning on the Intestinal Tract Weaning is a critical period for the physiological development of the baby pig s digestive tract. Pigs are subjected to many stresses during weaning that can contribute to poor growth rate, either directly or indirectly. Weaning is a time of dramatic changes that can be important modulators of the structure and function of the gastrointestinal tract. The stimuli that regulate intestinal adaptation to the absorption of diets at weaning are incompletely understood. Alterations in the small intestinal structure have been described by numerous researchers (Pacha, 2000; Gay, 1976; Kenworthy, 1976; Hampson, 1986; and Hetty et al., 1998). Smith (1988) concluded that weaning induced problems in intestinal function appear to be caused more by changes in digestive function and intestinal structure than by any direct inhibition of transport properties of the small intestine. 7
27 Morphology The redifferentiation of the intestine during weaning is associated with accelerated proliferation of enterocytes that contributes to increased crypt depth and villus height. Pacha (2000) noted rapid reduction of villus height and an increase of crypt depth during the weaning period of species with longer gestation periods such as the pig. Research conducted by Hampson (1986) observed the difference in small intestinal morphology between groups of weaned and unweaned pigs. This research found that villi height of the weaned pigs was reduced to around 75 percent of the unweaned pigs within 24 hours after weaning. Villi height continued to decrease until approximately five days post weaning when the villi were approximately half their initial height. He observed only small alterations in the unweaned groups of pigs. Weaned pigs also demonstrated a significantly greater increase in crypt depth, especially in the distal half of the small intestine. Crypt cells migrate upwards, becoming villous enterocytes. The migration cycle from crypt base to extrusion at the villus apex takes two to four days (Moon, 1971). Hampson (1986) hypothesized that the reduction in enterocyte numbers in weaned pigs could be the result of an increased rate of cell loss or a brief reduction in crypt cell production rate and concluded that weaning appeared to be a point of transition from neonatal-type to adult-type intestines. This is supported by Hetty et al. (1998) who conducted experiments to observe differences between groups of pigs that were unweaned, weaned to a conventional weanling diet, and weaned but still provided a diet of sows milk. This study showed villi were significantly shorter and crypt were significantly deeper by day four in the pigs weaned to the conventional diet compared to any of the other groups. They concluded that villi height in the small intestines was 8
28 primarily influenced by the level of feed intake and not the composition of the diet. They also concluded that villous atrophy was partially caused by the stress of separating the pigs from the sow and moving them to other pens. This study also observed higher concentrations of short chain fatty acids (SCFA) and lower dry matter in the large intestines of the conventional pigs. Conventionally weaned pig s intestinal tracts weighed two or more times that of the other groups on both days four and seven post weaning and were abruptly changed from milk to solid. Enzymatic Changes The diet should be prepared on the basis that it is adapted to the digestive potential and nutritional tolerance of the young pig with attention being drawn to the possibility that total enzyme activities of the pancreas and small intestine are increasing during early life (Aumaitre and Corring, 1978). Smith (1988) found that there was a loss of dissaccharidase activity during the weaning period of pigs. Corring et al. (1978) concluded that in order to improve the productivity of the pig at weaning it was important to define the point at which the pancreatic enzyme function is sufficient for the hydrolysis of a diet that replaces milk. It was also concluded that weaning should be based on development of the digestive function rather than the age of the pig. Intestinal Microflora Establishment The gastrointestinal microflora (1) affect growth and development of the host, (2) influence nutritional requirements, (3) affect morphogenesis of the gastrointestinal tract, (4) modify by metabolic activity endogenous and exogenous substances introduced into the gastrointestinal lumen and (5) play an active role in preventing foreign microorganisms from becoming established (Visek, 1978). The newly weaned pig has 9
29 not developed the proper commensalate bacteria barrier to protect the intestine from invasion by harmful bacteria. Certain bacterial populations regulate other bacteria in the intestinal flora and it is important to maintain a balance between populations. The Lactobacillus genus appears to be a key participant in maintaining a balance in microbial number and types in the intestinal tract (Hutcheson, 1994). Oligosaccharides are readily fermented in the cecum and large intestine and promote the growth of lactic acid bacteria such as Bifidobacterium and Lactobacillus species (Gabert et al., 1994). These bacteria obtain energy for reproduction and life by fermenting organic compounds ingested or produced by the host. This fermentation yields organic products (volatile fatty acids) that are excreted by the bacteria and can be used by host tissues (Savage, 1994). Lactobacilli have also been shown to produce VFA in the intestinal tract that lower the ph and suppress the growth of pathogenic bacteria (Gabert, 1994; Hutcheson, 1994). The change in diet following weaning may lead to shifts in indigenous microflora and their metabolites. The presence of enteric bacteria may amplify the changes that take place during the weaning development period of the pig (Smith, 1988). Mathew et al. (1991) found that by two days post weaning, ileal lactobacilli concentrations decreased by nearly 1000 fold along with an increase in E. coli concentrations and an increase in ph. These values all returned to near preweaning levels over the next seven days. Similar observations were seen in research conducted by McAllister et al. (1979) and Hampson et al. (1985). Risley et al. (1993) observed a decrease in jejunal lactobacilli concentrations from 5 to 7 days post weaning and studies by Miller et al. (1984) concluded that weaning itself and the weaning procedure had a significant effect upon 10
30 both the proliferation of haemolytic E. coli and on whether that proliferation was associated with diarrhea. Miller et al. (1984) also suggests that postweaning gut damage in the pig may be due to a delayed hypersensitivity reaction to dietary antigens. The experiments compared groups of pigs that were fed creep feed prior to weaning, pigs that were primed (fed limited amounts of creep feed prior to weaning), and pigs that were abruptly weaned to conventional diets with no access to solid food prior to weaning. The abruptly weaned and primed groups exhibited greater proliferation after exposure to haemolytic E. coli, which was associated with a higher incidence of diarrhea than the other groups. They hypothesized that continual exposure to the solid food of the creep fed group led to a tolerance until maturity of the intestinal tract postweaning. It was concluded that dietary manipulation could influence the proliferation of bacteria and diet associated damage to the intestinal tract. Volatile Fatty Acids Production and absorption of volatile fatty acids (VFA) are important to the nutrition and normal secretory and absorptive functions of the large intestines of most mammals. VFA could originate from indigestible polysaccharides in the meat or grain of the diet, those present in the wall of desquamated gastrointestinal cells, and the polysaccharide of mucous provide a substantial source of approximately 80% of the total VFA concentration (Stevens, 1978). Volatile fatty acids are also the end products of microbial digestion of all forms of carbohydrates. According to Bergman (1990) the concentrations of VFA at different sites of the gastrointestinal tract are a direct function of the bacterial population and thus are proportional to the time or extent to which digesta 11
31 are retained. This research opens the door for at least two interesting topics involving VFA, bacterial population and digesta retention time. There is a more rapid flow of digesta through the upper portion of the small intestines that decreases along the tract. This supports the findings of Bergman (1990) and Friend et al. (1963) that show VFA concentrations in pigs were lowest in the stomach and small intestines while highest in the cecum and colon. Prohaszka and Lukas (1984) found that the VFA concentrations in the large intestines had an antibacterial effect due to lower ph and suggested that some E. coli infections could be controlled dietarily by VFA modifications. The type of microbial metabolism and the VFA produced can greatly affect the specific microbial populations that predominate. Research conducted by Mathew (1991) suggested that the abrupt change in VFA concentrations that occurred at weaning might have an effect on some species of enteric bacteria. ph Enterotoxigenic bacteria such as some types of E. coli tend to favor an environment that is more alkaline in ph, whereas commensalate bacteria such as lactobacilli prefer a more acidic ph. de Alwis (1970) noted that the survival rate of potentially pathogenic E. coli was reduced at a ph of 3.6 and below. Mathew (1991) showed that the highest concentrations of VFA occurred in the cecum and spiral colon the lowest concentration in the duodenum. The higher VFA concentrations coincided with the lowest ph in these areas and indicated that the cecum and colon should exhibit much greater fermentation activity than the upper small intestine. The lower concentration in the small intestine may be due to the rapid absorption of VFA in this area. VFA concentration and ph is especially important in the pig during the weaning 12
32 period. The abrupt change in diet and environment can lead to a reduced feed intake and flow of digesta along with an increase in the ph of the small intestine, creating a more favorable environment for enterotoxigenic bacteria (Mathew, 1991). Pathogen Exposure Since the small intestine is the major site for nutrient absorption as well as for pathogen invasion, changes in ph, VFA, enzymatic function and morphology can be responsible for many of the intestinal disruptions that lead to the post weaning lag phase. Such changes can produce a suitable environment for potentially pathogenic E.coli but not for the commensalate types of bacteria such as lactobacilli. A significant decrease in lactobacilli concentrations can occur within two days after weaning as well as an increase in ph, which may lead to the proliferation of pathogenic E. coli (Mathew et al., 1991). These results support earlier reports by Thomlinson and Lawrence (1981) who observed that the increase in E.coli at weaning could be delayed by lowering gastric ph. They also concluded that the increase in E. coli that is associated with weaning may be potentiated by the induction of ad libitum feeding. Gaastra and De Graaf (1982) suggested that adhesive filaments (fimbria) of certain strains of E.coli might be affected by the physiological environment of the gut such as decrease in ph and reduced receptor cites as a result of microbial populations and morphology. The increased production of specific VFA by the establishment of non-pathogenic bacteria may alter the intestinal environment to the point where it is not suitable for the proliferation or attachment of pathogenic bacteria. 13
33 Adherence and Colonization of E. coli Pathogenic intestinal bacteria have been classified by Takeuchi (1967) into three groups according to where they colonize in the intestines: (1) pathogens that invade the intestinal mucosal barrier; (2) pathogens that attach themselves to intestinal epithelium but are not invasive; and (3) pathogens that neither penetrate nor attach themselves to intestinal epithelium, but none-the-less elicit symptoms. E. coli is a normal inhabitant of the intestinal tract of animals, only specific strains being an important cause of morbidity and mortality in young farm animals (Hinton and Linton, 1987). The surface of E. coli cells is covered with surface antigens, or adhesions, capable of provoking immunological reactions, which are commonly used for the serological classification of pathogenic and non-pathogenic strains. These surface antigens, the most common antigens being O, K, and H, include substances referred to as adhesions, which are responsible for the attachment of the cells to the epithelial mucosa (Gaastra and De Graaf, 1982). E. coli Surface Antigens The O antigens are lipopolysaccharide complexes and constitute part of the outer membrane. They are thermo-stable and are not inactivated by temperatures of 100 or 121 C. The H antigens are flagellar and protein in nature and are inactivated at 100 C. The K antigens are usually acidic polysaccharides that form an envelope or capsule around the cell wall. Porcine diarrhea can be characterized by the proliferation of certain serotypes of E. coli in the small intestine, many of which bear K antigens on their surface. The ability of these E. coli strains to proliferate and cause disease has been attributed to their ability to adhere to the piglets intestinal epithelium (Gaastra and De Graaf, 1982). 14
34 Invasive E. coli For some microorganisms to cause disease, they must first gain a foothold within the intestine in order to grow in sufficient numbers to produce clinical signs of disease. The ability of some E. coli strains to proliferate in the small intestines of pigs is dependent on the production of adhesins that specifically bind to sites on host cell surfaces. Several studies have demonstrated that the ability of E. coli to exhibit pathogenic characteristics is due to the ability to adhere to the mucosal epithelial layer of the intestines (Duguid et al., 1955; Arbuckle, 1970; Sellwood, 1980; Hinton and Linton, 1987). If attachment does not occur, the bacteria are expelled by the host s physiological mechanical defense mechanisms such as peristalsis and mucous secretion. Adhesive structures on the surface of bacteria bind to complementary adhesive structures on the surface of host cells known as receptors. Adhesins are carried on surface protein filaments referred to as fimbria. Some pathogenic and non-pathogenic E. coli produce non-specific, type I fimbria. Type I fimbria are capable of binding to a wide variety of animal and plant cell surfaces and their binding ability is known to be inhibited by free mannose units. This type of fimbria is the most universal adhesin of E. coli and their adhesive properties contribute to the pathogenicity of enteropathogenic strains (Gaastra and De Graaf, 1982). Originally, Duguid et al. (1955) correlated the haemagglutination of erythrocytes by E. coli with the presence of fimbria on the bacteria, and regarded the fimbria as organs of attachment. From this they hypothesized that these fimbria may also be effective in the in vivo adhesion of E. coli to intestinal epithelium. This hypothesis was confirmed by Arbuckle (1970), who presented evidence that in both natural and experimental cases of E. coli 15
35 diarrhea in pigs, enteropathogenic strains of E. coli attach themselves to small intestinal villi. E.coli was observed on the free border of the villous epithelium and between adjoining villi but was not considered invasive because the bacteria was rarely observed within the epithelial cells. E.coli was also observed within the apical region of the small intestinal crypts and was associated with the exfoliation of the microvilli prior to attachment of bacteria to the apical membrane. Adhesion commenced at the basal region of the villi and then progressed upwards. This region was thought to provide an environment more sheltered against peristaltic flow and render the site more suitable for the development of bacterial adhesion. Arbuckle also observed non-pathogenic E. coli on the villi but to a much lesser degree and concluded that the degree of adherence was greater for the enteropathogenic versus the non-enteropathogenic strains. Sellwood (1980) observed that the haemagglutination of E. coli could be inhibited by some glycoproteins and suggested that sugar residues are involved in the intestinal receptors. Studies on the attachment of E. coli to mouse macrophages have shown that attachment can be inhibited by D-mannose and substances with mannose residues. Mannose-sensitive attachment of E. coli has also been shown to occur to erythrocytes (Duguid, 1957) and brush borders of pigs (Sellwood, 1980), but this type of attachment was usually mediated by type I fimbria. Urinary tract infection by E. coli in mice has been shown to involve mannose-sensitive attachment of bacteria to the epithelial cells, which has been inhibited by methyl α-d-mannopyranoside (Aronson et al., 1979). Oyofo (1989) observed that the binding of E. coli to epithelial cells of the mucosa was mediated by a mannose-specific lectin-like substance present on the surface of the bacterium. This site on the bacteria may bind to a mannose-like residue site on the mucosal surface 16
INTESTINAL MICROBIOTA EXAMPLES OF INDIVIDUAL ANALYSES
 EXAMPLES OF INDIVIDUAL ANALYSES INTESTINAL MICROBIOTA Microbiota in the animal or human intestine has evolved together with the host. Consequently, the gastrointestinal tract could be considered a metacommunity,
EXAMPLES OF INDIVIDUAL ANALYSES INTESTINAL MICROBIOTA Microbiota in the animal or human intestine has evolved together with the host. Consequently, the gastrointestinal tract could be considered a metacommunity,
Biacid: A EU approved natural growth promoter for Broilers
 Biacid is a blend of calcium salts of organic acids and essential oils. Through the optimal combination of calcium salts of organic acids and essential oils, it enhances broiler microflora within the gut
Biacid is a blend of calcium salts of organic acids and essential oils. Through the optimal combination of calcium salts of organic acids and essential oils, it enhances broiler microflora within the gut
Digestion and Absorption
 Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
ROLE OF THE GUT BACTERIA
 ROLE OF THE GUT BACTERIA Our Good Bacteria In a perfect world, we would all have a proper ratio of good bacteria And what could this proper ratio do for us? The knowledge of the connections between our
ROLE OF THE GUT BACTERIA Our Good Bacteria In a perfect world, we would all have a proper ratio of good bacteria And what could this proper ratio do for us? The knowledge of the connections between our
The use of medium chain fatty acids as alternatives to antibiotic use in pigs
 3/5/2018 The use of medium chain fatty acids as alternatives to antibiotic use in pigs - VIV Online News The use of medium chain fatty acids as alternatives to antibiotic use in pigs Written by Product
3/5/2018 The use of medium chain fatty acids as alternatives to antibiotic use in pigs - VIV Online News The use of medium chain fatty acids as alternatives to antibiotic use in pigs Written by Product
Digestive Care Advisor Training #1. Digestion 101 & H.O.P.E.
 Digestive Care Advisor Training #1 & H.O.P.E. The Digestive System in Brief The Process of Digestion The human digestive system is a complex series of organs and glands that process food and excrete waste.
Digestive Care Advisor Training #1 & H.O.P.E. The Digestive System in Brief The Process of Digestion The human digestive system is a complex series of organs and glands that process food and excrete waste.
Digestive Lecture Test Questions Set 4
 Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
The Effect of Prebiotic and Probiotic Supplementation on Intestinal Maturity in Turkey Poults. Honors Research Thesis.
 The Effect of Prebiotic and Probiotic Supplementation on Intestinal Maturity in Turkey Poults Honors Research Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Honors Research
The Effect of Prebiotic and Probiotic Supplementation on Intestinal Maturity in Turkey Poults Honors Research Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Honors Research
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
Understandings, Applications & Skills
 D.2 Digestion Understandings, Applications & Skills Statement D.2.U1 Nervous and hormonal mechanisms control the secretion of digestive juices. D.2.U2 Exocrine glands secrete to the surface of the body
D.2 Digestion Understandings, Applications & Skills Statement D.2.U1 Nervous and hormonal mechanisms control the secretion of digestive juices. D.2.U2 Exocrine glands secrete to the surface of the body
General Structure of Digestive Tract
 Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Dr. Nabil Khouri General Structure of Digestive Tract Common Characteristics: Hollow tube composed of a lumen whose diameter varies. Surrounded by a wall made up of 4 principal layers: Mucosa Epithelial
Bacteriology. Mycology. Patient: SAMPLE PATIENT DOB: Sex: MRN: Rare. Rare. Positive. Brown. Negative *NG. Negative
 Patient: SAMPLE PATIENT DOB: Sex: MRN: 3.2 0.9-26.8 U/g 1.2 0.2-3.3 mg/g 2.2 1.3-8.6 micromol/g 1.1 1.3-23.7 mg/g 1.1 0.2-3.5 mg/g Rare 1.0 0.2-8.8 mg/g Rare 4.4 2.6-32.4 mg/g 64.6 >= 13.6 micromol/g Bacteriology
Patient: SAMPLE PATIENT DOB: Sex: MRN: 3.2 0.9-26.8 U/g 1.2 0.2-3.3 mg/g 2.2 1.3-8.6 micromol/g 1.1 1.3-23.7 mg/g 1.1 0.2-3.5 mg/g Rare 1.0 0.2-8.8 mg/g Rare 4.4 2.6-32.4 mg/g 64.6 >= 13.6 micromol/g Bacteriology
Pig digest: Bacteriology Manakorn Sukmak
 Pig digest: Bacteriology 24th International Pig Veterinary Society Congress 8th European Symposium of Porcine Health Management June 7th - 10th 2016Dublin, Ireland Manakorn Sukmak, DVM, MSc, PhD Dept.
Pig digest: Bacteriology 24th International Pig Veterinary Society Congress 8th European Symposium of Porcine Health Management June 7th - 10th 2016Dublin, Ireland Manakorn Sukmak, DVM, MSc, PhD Dept.
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
The number of microorganisms residing in our intestines is 10 times the number of our somatic and germ cells.
 The number of microorganisms residing in our intestines is 10 times the number of our somatic and germ cells. The number of microorganisms residing in our intestines is 10 times the number of our somatic
The number of microorganisms residing in our intestines is 10 times the number of our somatic and germ cells. The number of microorganisms residing in our intestines is 10 times the number of our somatic
Bacteriology. Mycology. Genova Diagnostics SAMPLE REPORT. Rare. Rare. Negative. Brown. Negative *NG. Negative
 Completed: November 2010 Genova Diagnostics eceived: October 2010 Collected: October 2010 oute Number:7 4.2 0.9-26.8 U/g 0.9 0.2-3.3 mg/g 0.8 1.3-8.6 micromol/g 42.7 1.3-23.7 mg/g 1.7 0.2-3.5 mg/g are
Completed: November 2010 Genova Diagnostics eceived: October 2010 Collected: October 2010 oute Number:7 4.2 0.9-26.8 U/g 0.9 0.2-3.3 mg/g 0.8 1.3-8.6 micromol/g 42.7 1.3-23.7 mg/g 1.7 0.2-3.5 mg/g are
Bacteriology. Mycology. Patient: REDOX Biomedicine Co., Ltd. Referring Laboratory Attn Alan Ou 5F, No. 369, Song Jiang Road Taipei, Taiwan
 ex: MN: Completed: eptember 23, 2011 eceived: eptember 15, 2011 Collected: eptember 14, 2011 EDOX Biomedicine Co., Ltd. eferring Laboratory Attn Alan Ou 5F, No. 369, ong Jiang oad Taipei, 10482 Taiwan
ex: MN: Completed: eptember 23, 2011 eceived: eptember 15, 2011 Collected: eptember 14, 2011 EDOX Biomedicine Co., Ltd. eferring Laboratory Attn Alan Ou 5F, No. 369, ong Jiang oad Taipei, 10482 Taiwan
Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption. Digestive Tract and Accessory Organs
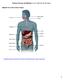 Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption Digestive Tract and Accessory Organs http://highered.mheducation.com/sites/0072495855/student_view0/chapter26/animation organs_of_digestion.html
Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption Digestive Tract and Accessory Organs http://highered.mheducation.com/sites/0072495855/student_view0/chapter26/animation organs_of_digestion.html
10/23/2013 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
10/18/2017 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
- Most nutrients are absorbed before reaching the ileum. - Colon is responsible for final removal of electrolytes and water.
 University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
INTRODUCING YOUR GUT BACTERIA
 INTRODUCING YOUR GUT BACTERIA Microflora Intestinal flora 1.5 kg We would die with 5 years of birth if we did not have them as we would not develop a proper immune system 1000 species and 5000 strains
INTRODUCING YOUR GUT BACTERIA Microflora Intestinal flora 1.5 kg We would die with 5 years of birth if we did not have them as we would not develop a proper immune system 1000 species and 5000 strains
Competing with antibiotic growth promoters the issues... Aoife Corrigan, Ph.D. Alltech Bioscience Centre Ireland November 2012
 Competing with antibiotic growth promoters the issues... Aoife Corrigan, Ph.D. Alltech Bioscience Centre Ireland November 2012 Are we ready for 9 billion??? The World needs to double the meat production
Competing with antibiotic growth promoters the issues... Aoife Corrigan, Ph.D. Alltech Bioscience Centre Ireland November 2012 Are we ready for 9 billion??? The World needs to double the meat production
CIE Biology GCSE 7: Human nutrition
 CIE Biology GCSE 7: Human nutrition Notes Humans need many different nutrients to survive. To receive these nutrients in the correct quantities, a balanced diet must be eaten. A balanced diet includes
CIE Biology GCSE 7: Human nutrition Notes Humans need many different nutrients to survive. To receive these nutrients in the correct quantities, a balanced diet must be eaten. A balanced diet includes
What location in the gastrointestinal (GI) tract has tight, or impermeable, junctions between the epithelial cells?
 CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
BPK 312 Nutrition for Fitness & Sport. Lecture 2. Digestion & Absorption of Food Nutrients
 BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
The Digestive System. Basic process of digestion. Mouth and Teeth 10/30/2016
 The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
Abdulrahman Alhanbali. Lojayn Salah. Mohammad Khatatbeh. 1 P a g e
 7 Abdulrahman Alhanbali Lojayn Salah Mohammad Khatatbeh 1 P a g e In this lecture we will talk about digestion and absorption of food in the alimentary tract. But first of all we have some important points
7 Abdulrahman Alhanbali Lojayn Salah Mohammad Khatatbeh 1 P a g e In this lecture we will talk about digestion and absorption of food in the alimentary tract. But first of all we have some important points
AN ANIMAL S DIET MUST SUPPLY CHEMICAL ENERGY, ORGANIC MOLECULES, AND ESSENTIAL NUTRIENTS
 1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
1) Four main feeding mechanisms of animals a) Suspension feeders i) (1) Humpback whales b) Substrate feeders i)
 1 AP Biology March 2008 Digestion Chapter 41 Homeostatic mechanisms manage an animal s energy budget. 1) Four main feeding mechanisms of animals Suspension feeders (1) Humpback whales Substrate feeders
1 AP Biology March 2008 Digestion Chapter 41 Homeostatic mechanisms manage an animal s energy budget. 1) Four main feeding mechanisms of animals Suspension feeders (1) Humpback whales Substrate feeders
Pathogenesis of Infectious Diseases. CLS 212: Medical Microbiology
 Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
Rumination or cud chewing consists of regurgitation, remastication, reinsalvation, and reswallowing.
 Nutrition 115 Midterm Exam 2 February 25, 2000 Name Please be sure to put your name at the top of each page. Any page without a name in the appropriate place will not be graded. Read each question carefully,
Nutrition 115 Midterm Exam 2 February 25, 2000 Name Please be sure to put your name at the top of each page. Any page without a name in the appropriate place will not be graded. Read each question carefully,
Gut Microbiota and IBD. Vahedi. H M.D Associate Professor of Medicine DDRI
 Gut Microbiota and IBD Vahedi. H M.D Associate Professor of Medicine DDRI 1393.3.1 2 GUT MICROBIOTA 100 Trillion Microbes - 10 times more than cells in our body Collective weight of about 1kg in human
Gut Microbiota and IBD Vahedi. H M.D Associate Professor of Medicine DDRI 1393.3.1 2 GUT MICROBIOTA 100 Trillion Microbes - 10 times more than cells in our body Collective weight of about 1kg in human
Digestive System. How your body obtains nutrients. Wednesday, March 2, 16
 Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Energy, Chemical Reactions and Enzymes
 Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Tissues and organs PART 1
 Tissues and organs PART 1 Animals and plants are multicellular (made of many cells). Cells become specialised according to their function Tissues: Many cells that perform one or several functions; they
Tissues and organs PART 1 Animals and plants are multicellular (made of many cells). Cells become specialised according to their function Tissues: Many cells that perform one or several functions; they
Gastrointestinal Anatomy and Physiology. Bio 219 Napa Valley College Dr. Adam Ross
 Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Ingestion Digestion- Absorption- Elimination
 DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
CLASS XI BIOLOGY. Digestion And Absorption. Finish Line & Beyond send your queries to
 CLASS XI BIOLOGY Digestion And Absorption 1. Choose the correct answer among the following : (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin, lipase and rennin (iii) trypsin, pepsin
CLASS XI BIOLOGY Digestion And Absorption 1. Choose the correct answer among the following : (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin, lipase and rennin (iii) trypsin, pepsin
1. Animals are heterotrophs that require food for fuel, carbon skeletons, and essential nutrients: an overview
 1. Animals are heterotrophs that require food for fuel, carbon skeletons, and essential nutrients: an overview A nutritionally adequate diet satisfies three needs: fuel (chemical energy) for all the cellular
1. Animals are heterotrophs that require food for fuel, carbon skeletons, and essential nutrients: an overview A nutritionally adequate diet satisfies three needs: fuel (chemical energy) for all the cellular
Chapter 14: The Digestive System
 Chapter 14: The Digestive System Digestive system consists of Muscular tube (digestive tract) alimentary canal Accessory organs teeth, tongue, glandular organs 6 essential activities 1. 2. 3. 4. 5. 6.
Chapter 14: The Digestive System Digestive system consists of Muscular tube (digestive tract) alimentary canal Accessory organs teeth, tongue, glandular organs 6 essential activities 1. 2. 3. 4. 5. 6.
NOTES: The Digestive System (Ch 14, part 2)
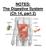 NOTES: The Digestive System (Ch 14, part 2) PANCREAS Structure of the pancreas: The pancreas produces PANCREATIC JUICE that is then secreted into a pancreatic duct. The PANCREATIC DUCT leads to the The
NOTES: The Digestive System (Ch 14, part 2) PANCREAS Structure of the pancreas: The pancreas produces PANCREATIC JUICE that is then secreted into a pancreatic duct. The PANCREATIC DUCT leads to the The
Ever wonder what s really happening on the inside?
 For Practitioners Ever wonder what s really happening on the inside? Are your patients suffering from diarrhea, constipation, bloating, gas or indigestion? Rocky Mountain Analytical is now offering Gut-Well
For Practitioners Ever wonder what s really happening on the inside? Are your patients suffering from diarrhea, constipation, bloating, gas or indigestion? Rocky Mountain Analytical is now offering Gut-Well
Lavanya Nutankalva,MD Consultant: Infectious Diseases
 Lavanya Nutankalva,MD Consultant: Infectious Diseases Introduction The word Probiotic was derived from the Greek phrase meaning for life." was first coined in the 1960s by Lilly and Stillwell. Probiotics
Lavanya Nutankalva,MD Consultant: Infectious Diseases Introduction The word Probiotic was derived from the Greek phrase meaning for life." was first coined in the 1960s by Lilly and Stillwell. Probiotics
Chapter 22 The Digestive System. Yoon Seo Orite, Jack Kohm, And Jackson Masuda
 Chapter 22 The Digestive System Yoon Seo Orite, Jack Kohm, And Jackson Masuda Main Idea The digestive system breaks down ingested food and provides necessary nutrients to the body through various processes.
Chapter 22 The Digestive System Yoon Seo Orite, Jack Kohm, And Jackson Masuda Main Idea The digestive system breaks down ingested food and provides necessary nutrients to the body through various processes.
220 SUBJECT INDEX. D Diarrhea and sodium balance, 74 weanling, 161,179,208,212; see also Infection
 Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
Two main groups Alimentary canal continuous coiled hollow tube Accessory digestive organs
 Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
4. ABSORPTION. Transport mechanisms. Absorption ABSORPTION MECHANISMS. Active transport. Active transport uses metabolic energy
 4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
4. ABSORPTION ABSORPTION MECHANISMS Once the digestive process is completed, the nutrients have to be transferred across the digestive tract epithelium into the intracellular space and eventually into
Outline. Nutritional Strategies to Improve the Health & Performance of Dairy Calves. Gastrointestinal Maturation. Why do so many calves get sick?
 Outline Nutritional Strategies to Improve the Health & Performance of Dairy Calves Why do pre-weaned calves get sick? Development of gastrointestinal immunity Nutrition and immunity of calves Reducing
Outline Nutritional Strategies to Improve the Health & Performance of Dairy Calves Why do pre-weaned calves get sick? Development of gastrointestinal immunity Nutrition and immunity of calves Reducing
The Digestive System. What is the advantage of a one-way gut? If you swallow something, is it really inside you?
 The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
Colonization of the Porcine Gastrointestinal Tract by Lactobacilli
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 1989, p. 279-283 0099-2240/89/020279-05$02.00/0 Copyright C) 1989, American Society for Microbiology Vol. 55, No. 2 Colonization of the Porcine Gastrointestinal
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 1989, p. 279-283 0099-2240/89/020279-05$02.00/0 Copyright C) 1989, American Society for Microbiology Vol. 55, No. 2 Colonization of the Porcine Gastrointestinal
DIGESTIVE SYSTEM CLASS NOTES. tube along with several
 DIGESTIVE SYSTEM CLASS NOTES Digestion Breakdown of food and the of nutrients in the bloodstream. Metabolism Production of for and cellular activities. The digestive system is composed of the canal which
DIGESTIVE SYSTEM CLASS NOTES Digestion Breakdown of food and the of nutrients in the bloodstream. Metabolism Production of for and cellular activities. The digestive system is composed of the canal which
Swine. Health. Nutrition. Line. since SILO patented 1-Monoglycerides from C1 to C7 for treating animals. Patent n. EP B1.
 Line Program Health Swine H The production area is about 30,000 square metres. The production N capacity is 120,000 tons of Monoglycerides per year. SILO is the leader in the development and production
Line Program Health Swine H The production area is about 30,000 square metres. The production N capacity is 120,000 tons of Monoglycerides per year. SILO is the leader in the development and production
The Digestive System. Chapter 25
 The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
The Digestive System Chapter 25 Introduction Structure of the digestive system A tube that extends from mouth to anus Accessory organs are attached Functions include Ingestion Movement Digestion Absorption
Digestive System. Part 3
 Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
Small intestine. Small intestine
 General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
General features Tubular organ longest part; 5-6 m most of chemical digestion absorption of nutrients reabsorption of H2O occurs. Two structural features; maximize the lumenal surface area villi microvilli
Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, ,
 IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Digestive System. Why do we need to eat? Growth Maintenance (repair tissue) Energy
 Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
Laboratory report. Test: Leaky gut test. Sample material: stool. John Doe Main St 1 Anytown
 1 / 5 Verisana LAB John Doe Main St 1 Anytown Surname, First name Doe, John DOB 02/13/1980 Sex male Laboratory # 20020181 Date collected 01/25/2018 Date received 02/01/2018 Report date 02/13/2018 Laboratory
1 / 5 Verisana LAB John Doe Main St 1 Anytown Surname, First name Doe, John DOB 02/13/1980 Sex male Laboratory # 20020181 Date collected 01/25/2018 Date received 02/01/2018 Report date 02/13/2018 Laboratory
Digestion and Nutrition. Chapter 40
 Digestion and Nutrition Chapter 40 Impacts, Issues Hormones and Hunger Fat cells secrete leptin, which reduces appetite; an empty stomach secretes ghrelin, which makes you hungry the goal is healthy nutrition
Digestion and Nutrition Chapter 40 Impacts, Issues Hormones and Hunger Fat cells secrete leptin, which reduces appetite; an empty stomach secretes ghrelin, which makes you hungry the goal is healthy nutrition
ORGANS OF THE DIGESTIVE SYSTEM
 ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
Summary of Product Characteristics
 Brand Name: OXALO [Pre Probiotic] Capsules Therapeutic Category: Prevention of Stone Formation Urinary tract stone disease has been a part of the human condition for millennia; in fact, bladder and kidney
Brand Name: OXALO [Pre Probiotic] Capsules Therapeutic Category: Prevention of Stone Formation Urinary tract stone disease has been a part of the human condition for millennia; in fact, bladder and kidney
Food is taken in, taken apart, and taken up in the process of animal nutrition. Omnivores regularly consume animals as well as plants or algae
 Ch 41 Animal Nutrition Need to Feed Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae
Ch 41 Animal Nutrition Need to Feed Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae
AF 1201 Digestive System. Dr. A.M.J.B. Adikari Dept. of Animal and Food Sciences
 AF 1201 Digestive System Dr. A.M.J.B. Adikari Dept. of Animal and Food Sciences Complex / Compound Stomach Large structure, located on the left side 4 parts Rumen, Reticulum, Omasum Abomasum Fore stomach
AF 1201 Digestive System Dr. A.M.J.B. Adikari Dept. of Animal and Food Sciences Complex / Compound Stomach Large structure, located on the left side 4 parts Rumen, Reticulum, Omasum Abomasum Fore stomach
Class XI Chapter 16 Digestion and Absorption Biology
 Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Soft palate elevates, closing off the nasopharynx. Hard palate Tongue Bolus Epiglottis. Glottis Larynx moves up and forward.
 The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
Chapter 9: Digestion Review Assignment
 _ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
_ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
PREMIUM QUALITY FEED-ENHANCER SPECIES GENEX SOW PIGS. Supporting the reproductive cycle
 PREMIUM QUALITY FEED-ENHANCER SPECIES PIGS Supporting the reproductive cycle Background Today s farming production systems are more intensive and much more is expected from farm animals. The modern sow
PREMIUM QUALITY FEED-ENHANCER SPECIES PIGS Supporting the reproductive cycle Background Today s farming production systems are more intensive and much more is expected from farm animals. The modern sow
Normal Flora. CLS 212: Medical Microbiology
 Normal Flora CLS 212: Medical Microbiology Relationships between Organisms Symbiosis Permanent association between two different organisms. Neutralism Two organisms living together, and neither is affected
Normal Flora CLS 212: Medical Microbiology Relationships between Organisms Symbiosis Permanent association between two different organisms. Neutralism Two organisms living together, and neither is affected
Nutrition and Digestion
 Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS. Mar 16 10:34 PM
 DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
DIGESTIVE SYSTEM ALIMENTARY CANAL / GI TRACT & ACCESSORY ORGANS Mar 16 10:34 PM 1 I. Digestive System Functions > Ingestion the taking in of food > Propulsion movement caused by force > Digestion breakdown
 Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Question 1: Choose the correct answer among the following: (a) Gastric juice contains (i) pepsin, lipase and rennin (ii) trypsin lipase and rennin (iii) trypsin, pepsin and lipase (iv) trypsin, pepsin
Series Editors: Daniel Kamin, MD and Christine Waasdorp Hurtado, MD
 NASPGHAN Physiology Lecture Series GI Physiology Module: Absorption of Water and Ions Jason Soden, MD Reviewers: George Fuchs MD: UAMS College of Medicine / Arkansas Children s Hospital Wayne Lencer MD:
NASPGHAN Physiology Lecture Series GI Physiology Module: Absorption of Water and Ions Jason Soden, MD Reviewers: George Fuchs MD: UAMS College of Medicine / Arkansas Children s Hospital Wayne Lencer MD:
(*) (*) Ingestion, digestion, absorption, and elimination. Uptake of nutrients by body cells (intestine)
 Human Digestive System Food is pushed along the digestive tract by peristalsis the rhythmic waves of contraction of smooth muscles in the wall of the canal Accessory glands. Main stages of food processing
Human Digestive System Food is pushed along the digestive tract by peristalsis the rhythmic waves of contraction of smooth muscles in the wall of the canal Accessory glands. Main stages of food processing
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
The process by which nutrient molecules pass through the wall of your digestive system into your blood. ABSORPTION AS RELATED TO DIGESTION
 ABSORPTION AS RELATED TO DIGESTION The process by which nutrient molecules pass through the wall of your digestive system into your blood. 3 FUNCTIONS OF DIGESTION Breaks down food into molecules the body
ABSORPTION AS RELATED TO DIGESTION The process by which nutrient molecules pass through the wall of your digestive system into your blood. 3 FUNCTIONS OF DIGESTION Breaks down food into molecules the body
Non-Invasive Assessment of Intestinal Function
 Overview Non-Invasive Assessment of Intestinal Function Introduction This paper will demonstrate that the 13 C-sucrose breath test ( 13 C-SBT) determines the health and function of the small intestine.
Overview Non-Invasive Assessment of Intestinal Function Introduction This paper will demonstrate that the 13 C-sucrose breath test ( 13 C-SBT) determines the health and function of the small intestine.
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System
 The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
COMPLETE DIGESTIVE STOOL ANALYSIS - (CDSA) Level 4
 Macroscopic Appearance Colour Brown Consistency Semi-formed Fibres 0-2 Food Remnants 0-2 2 3 Brown Formed Microscopic Appearance Starch Cells 0 0Ref 0 Fat Globules 0 0 0 Meat Fibres 0 Ref Vegetable Fibres
Macroscopic Appearance Colour Brown Consistency Semi-formed Fibres 0-2 Food Remnants 0-2 2 3 Brown Formed Microscopic Appearance Starch Cells 0 0Ref 0 Fat Globules 0 0 0 Meat Fibres 0 Ref Vegetable Fibres
Digestive System Processes *
 OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
Digestive System Practice Test
 Name: Class Period: Section 1: Digestive System Practice Test Directions: Match the items in Column B to the definitions or explanations offered in Column A. Write the matching letter, on the line provided
Name: Class Period: Section 1: Digestive System Practice Test Directions: Match the items in Column B to the definitions or explanations offered in Column A. Write the matching letter, on the line provided
Calf Notes.com. Calf Note #155 Day 2. Introduction
 Calf Notes.com Calf Note #155 Day 2 Introduction Calf nutrition and management seems to be divided into two distinct periods namely, the first day of life and everything after. We all know of the importance
Calf Notes.com Calf Note #155 Day 2 Introduction Calf nutrition and management seems to be divided into two distinct periods namely, the first day of life and everything after. We all know of the importance
Digestive Tract. Also called alimentary canal or gastrointestinal tract. stomach small intestine large intestine - anus
 Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
Digestive Tract Also called alimentary canal or gastrointestinal tract Mouth pharynxepiglottis- esophagus stomach small intestine large intestine - anus Digestive Tract Digestion: The mechanical and chemical
MCAT Biology Problem Drill 20: The Digestive System
 MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
e. Undigested material is compacted and stored until the colon is full. When the colon is full, a signal to empty it is sent by sensors in the walls
 Digestive System 1. General a. Animals obtain energy by breaking food molecules into smaller pieces. b. The basic fuel molecules are amino acids, lipids and sugars c. Digestion is the chemical breakdown
Digestive System 1. General a. Animals obtain energy by breaking food molecules into smaller pieces. b. The basic fuel molecules are amino acids, lipids and sugars c. Digestion is the chemical breakdown
Chapter 41: Animal Nutrition
 Metabolic Rate Animals are heterotrophs that require food for: 1) Fuel 2) Carbon Skeletons 3) Essential Nutrients Bioenergetics: Flow of energy through an organism Sets upper / lower limits Metabolic Rate:
Metabolic Rate Animals are heterotrophs that require food for: 1) Fuel 2) Carbon Skeletons 3) Essential Nutrients Bioenergetics: Flow of energy through an organism Sets upper / lower limits Metabolic Rate:
HUMAN NUTRITION: ABSORPTION & ASSIMILATION 14 MAY 2014
 HUMAN NUTRITION: ABSORPTION & ASSIMILATION 14 MAY 2014 In this lesson, we: Absorption Lesson Description Examine and understand absorption Define absorption and describe where it occurs Study the structure
HUMAN NUTRITION: ABSORPTION & ASSIMILATION 14 MAY 2014 In this lesson, we: Absorption Lesson Description Examine and understand absorption Define absorption and describe where it occurs Study the structure
Nutrition. Autotrophs. plants, some protists & bacteria producers
 Nutrition Autotrophs plants, some protists & bacteria producers Nutrition Heterotrophs animals, fungi, some protists & bacteria consumers Animal Nutrition Most obtain food by ingestion take in their food
Nutrition Autotrophs plants, some protists & bacteria producers Nutrition Heterotrophs animals, fungi, some protists & bacteria consumers Animal Nutrition Most obtain food by ingestion take in their food
Lesson Overview The Digestive System
 30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
Monosaccharides: Little amounts Don t need any digestion
 Slide 8 Digestion result in mono and disaccharides & alpha-dextrins (oligosaccharides) Alpha1-4 in sequences / alpha1-6 at branches Dietary carbohydrates: Polysaccharides: 1) Containing α(1,4)/ α(1,6)
Slide 8 Digestion result in mono and disaccharides & alpha-dextrins (oligosaccharides) Alpha1-4 in sequences / alpha1-6 at branches Dietary carbohydrates: Polysaccharides: 1) Containing α(1,4)/ α(1,6)
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE
 LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
Studies on probiotics effects on innate immune functions in the gastrointestinal tract of broiler chicks (SUMMARY)
 Doctoral Thesis Studies on probiotics effects on innate immune functions in the gastrointestinal tract of broiler chicks (SUMMARY) ELSAYED SEDDEK IBRAHEM MOHAMMED Department of Bioresource Science Graduate
Doctoral Thesis Studies on probiotics effects on innate immune functions in the gastrointestinal tract of broiler chicks (SUMMARY) ELSAYED SEDDEK IBRAHEM MOHAMMED Department of Bioresource Science Graduate
Digestive System Module 4: The Stomach *
 OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
Human Organ Systems. Circulatory, Respiratory, Digestive
 Human Organ Systems Circulatory, Respiratory, Digestive The Circulatory System The circulatory system picks up and transports nutrients and oxygen to all the cells in the body, and carries wastes to the
Human Organ Systems Circulatory, Respiratory, Digestive The Circulatory System The circulatory system picks up and transports nutrients and oxygen to all the cells in the body, and carries wastes to the
Sphincters heartburn diaphragm The Stomach gastric glands pepsin, chyme The Small Intestine 1-Digestion Is Completed in the Small Intestine duodenum
 Sphincters are muscles that encircle tubes and act as valves. The tubes close when the sphincters contract and they open when the sphincters relax. When food or saliva is swallowed, the sphincter relaxes
Sphincters are muscles that encircle tubes and act as valves. The tubes close when the sphincters contract and they open when the sphincters relax. When food or saliva is swallowed, the sphincter relaxes
PHYSIOLOGY OF THE DIGESTIVE SYSTEM
 Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Ch 7 Nutrition in humans
 Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
Ch 7 Nutrition in humans Think about (Ch 7, p.2) 1. The stomach churns food into smaller pieces physically. The stomach wall secretes proteases to chemically digest proteins. It also releases hydrochloric
GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA
 GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA Anatomy of the GI Tract The GI tract is essentially a hollow tube connecting the mouth to the anus.
GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA Anatomy of the GI Tract The GI tract is essentially a hollow tube connecting the mouth to the anus.
Digestion and Absorption
 Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
