A Review on Gastroretentive Drug Delivery System
|
|
|
- Aleesha Pierce
- 5 years ago
- Views:
Transcription
1 A Review on Gastroretentive Drug Delivery System Pranav Joshi*, Priyank Patel,Hiren Modi, Dr.M.R. Patel, Dr.K.R.Patel, Dr.N.M.Patel Department of pharmaceutics, Shri B.M.Shah College of Pharmaceutical Education and Research, Modasa ABSTRACT: Gastroretentive drug delivery system comprised mainly of floating, bioadhesive, swelling, high density and magnetic systems have emerged as a current approaches of enhancing the bioavailability and controlled delivery of drugs that exhibit an absorption window. By prolonging the gastric emptying time of the dosage form, these systems not only provide controlled release of the drug for a prolonged period but also present the drug in an absorbable form at regions of optimal absorption. CR-GRDF provides a means to utilize all the pharmacokinetic (PK) and pharmacodynamic (PD) advantages of controlled release dosage forms for such drugs. Due to the complexity of pharmacokinetic and pharmacodynamic parameters, in vivo studies are required to establish the optimal dosage form for a specific drug. For a certain drug, interplay of its pharmacokinetic and pharmacodynamic parameters will determine the effectiveness and benefits of the CRGRDF compared to the other dosage forms. KEYWORDS: floating, bioadhesive, swelling, high density and magnetic systems INTRODUCTION: Article history: Received 18 Apr 2012 Accepted 05 May 2012 Available online 13 Jun 2012 For Correspondence: Mr. Pranav Joshi MAHADEVWADI OPP.SHAKHAGROUND GONDAL Telephone : joshipranavb@yahoo.com ( 1. GASTRORETENTIVE TECHNIQUES GRDDS prepared by using following methods: [1] By using gel forming hydrocolloids such as hydrophilic gums, gelatin, alginates, cellulose derivatives, etc. By using low density enteric materials such as methacrylic polymer, cellulose acetate phthalate. By reducing particle size and filling it in a capsule. By forming carbon dioxide gas and subsequent entrapment of it in the gel network. By preparing hollow micro-balloons of drug using acrylic polymer and filled in capsules. By incorporation of inflatable chamber which contained in a liquid e.g. solvent that gasifies at body temperature to cause the chambers to inflate in the stomach. Various techniques were used to encourage gastric retention of an oral dosage form as follow: 1.1 Floating drug delivery system, 1.2 Bio / Mucoadhesive systems, 1.3 Swelling/ Expanding Systems, 1.4 High-density systems (sedimentation/sinking system) 1.5 Magnetic systems Joshi P et al 123
2 1.1 Floating drug delivery system: [2-8] Intra Gastric Bilayer Floating Tablets: These are also compressed tablet as shown in Figure 3 and containing two layer i.e., Immediate release layer Sustained release layer. Figure 1: Classification of gastroretentive drug delivery system Based on the mechanism of buoyancy, two distinctly different technologies have been utilized in development of FDDS which are: Effervescent System, and Non-Effervescent System Effervescent System: Effervescent systems include use of gas generating agents, carbonates(ex. Sodium bicarbonate) and other organic acid(e.g. citric acid and tartaric acid) present in the formulation to produce carbon dioxide(co 2 ) gas, thus reducing the density of system and making it float on the gastric fluid. These effervescent systems further classified into two types. Figure 3: Intragastric floating bi-layer tablet Multiple Unit type floating pills: These systems consist of sustained release pills as seeds surrounded by double layers. The inner layer consists of effervescent agents while the outer layer is of swellable membrane layer. This lower density is due to generation and entrapment of CO 2 within the system A Gas generating systems B Volatile liquid/vacuum systems A Gas-generating Systems: Intra Gastric Single Layer Floating Tablets or Hydrodynamically Balanced System (HBS): These are as shown in Figure 2 and formulated by intimately mixing the CO 2 generating agents and the drug within the matrix tablet. These have a bulk density lower than gastric fluids and therefore remain floating in the stomach unflattering the gastric emptying rate for a prolonged period. This leads to an increase in the gastroretention time and a better control over fluctuation in plasma drug concentration. Figure 4: (a) multiple-unit oral floating dosage system. (b) Stages of floating Mechanism B Volatile Liquid / Vacuum Containing Systems: Intragastric Floating Gastrointestinal Drug Delivery System: Figure 2: Intragastric floating tablet These systems can be made to float in the stomach because of floatation chamber, which may be a vacuum or filled with air or a harmless gas, while drug reservoir is encapsulated inside a microporous compartment, as shown in Figure 5 Joshi P et al 124
3 Single Layer Floating Tablets: Figure 5: Intragastric floating drug delivery device Inflatable Gastrointestinal Delivery Systems: In these systems an inflatable chamber is incorporated, which contains liquid ether that gasifies at body temperature to cause the chamber to inflate in the stomach. After oral administration, the capsule dissolves to release the drug reservoir together with the inflatable chamber. The system is shown in Figure 6 They are formulated by intimate mixing of drug wit gelforming hydrocolloid, which swells in contact with gastric fluid and maintain bulk density of less than unity. The air trapped by the swollen polymer confers buoyancy to these dosage forms. Bilayer Floating Tablets: A bilayer tablet contain two layer immediate release layer which release initial dose from system while the another sustained release layer absorbs gastric fluid, forming an impermeable colloidal gel barrier on its surface, and maintain a bulk density of less than unity and thereby it remains buoyant in the stomach. Raft systems incorporate alginate gels: Raft forming systems have received much attention for the drug delivery for gastrointestinal infections and disorders. Usually, the system ingredients includes a gel forming agent and alkaline bicarbonates or carbonates responsible for the formation of CO 2 to make the system less dense and float on the gastric fluids. Figure 6: Gastro-inflatable drug delivery device Intragastric Osmotically Controlled Drug Delivery System: It is comprised of an osmotic pressure controlled drug delivery device.in the stomach, the capsule quickly disintegrates to release the intragastric osmotically controlled drug delivery device contains a liquid that gasifies at body temperature to inflate the bag, given in figure 7 Hollow Microspheres: Hollow microspheres (microballons), loaded with drug in their outer polymer shells were prepared by a novel emulsion solvent diffusion method. The ethanol:dichloromethane solution of drug and enteric acrylic polymer was poured into an agitated aqueous solution of PVA that was thermally controlled at 400 C. The microballons floated continuously over the surface of acidic dissolution media containing surfactant for more than 12 hours in vitro. 1.2 Bio / Mucoadhesive systems: [9] Bio/mucoadhesive systems bind to the gastric epithelial cell surface, or mucin, and increase the GRT by increasing the intimacy and duration of contact between the dosage form and the biological membrane. Figure 7: Intragastric osmotic controlled drug delivery system Non-Effervescent System: The non effervescent FDDS based on mechanism of swelling of polymer or bioadhesion to mucosal layer in GI tract. The most commonly used excipients in non effervescent FDDS are gel forming or highly swellable cellulose type hydrocolloids, polysaccharides and matrix forming material such as polycarbonate, polyacrylate, polymethacrylate, polystyrene as well as bioadhesive polymer such as chitosan and carbopol. The various types of this system are as follows: Mucus is a viscoelastic, gel-like, stringy slime comprised mainly of glycoproteins. The primary function of mucus is to protect the surface mucosal cells from acid and peptidases The epithelial adhesive properties of mucin are well known and have been applied to the development of GRDDS through the use of bio/mucoadhesive polymers. The adherence of the delivery system to the gastric wall increases residence time at a particular site, thereby improving bioavailibility. A bio/mucoadhesive substance is a natural or synthetic polymer capable of adhering to a biological membrane (bioadhesive polymer) or the mucus lining of the GIT (mucoadhesive polymer). The characteristics of these polymers are molecular flexibility, hydrophilic functional groups, and specific molecular weight, chain length, and Joshi P et al 125
4 conformation.the binding of polymers to the mucin-epithelial surface can be subdivided into three broad categories: Hydration-mediated adhesion Bonding-mediated adhesion Receptor-mediated adhesion Hydration-mediated adhesion: Certain hydrophilic polymers tend to imbibe large amount of water and become sticky, thereby acquiring bioadhesive properties Bonding-mediated adhesion: The adhesion of polymers to a mucus or epithelial cell surface involves various bonding mechanisms, including physicalmechanical bonding and chemical bonding. Physicalmechanical bonds can result from the insertion of the adhesive material into the crevices or folds of the mucosa. Chemical bonds may be either covalent (primary) or ionic (secondary) in nature. Secondary chemical bonds consist of dispersive interactions (i.e., van der waals interactions) and stronger specific interactions such as hydrogen bonds. The hydrophilic functional groups responsible for forming hydrogen bonds are the hydroxyl and carboxylic groups Receptor-mediated adhesion: Certain polymers can bind to specific receptor sites on the surface of cells, thereby enhancing the gastric retention of dosage forms. Certain plant lectins such as tomato lectins interact specifically with the sugar groups present in mucus or on the glycocalyx. 1.3 Swelling/ Expanding Systems: [9] After being swallowed, these dosage forms swell to a size that prevents their passage through the pylorus. As a result, the dosage form is retained in the stomach for a long period of time. These systems are sometimes referred to as plug type systems because they tend to remain lodged at the pyloric sphincter. The swollen system eventually will lose its integrity because of a loss of mechanical strength caused by abrasion or erosion or will burst into small fragments when the membrane ruptures because of continuous expansion. These systems also may erode in the presence of gastric juices so that after a predetermined time the device no longer can attain or retain the expanded configuration. 1.4 High-density systems (sedimentation/ sinking system): [9] Gastric contents have a density close to water. When high density pellets is given to the patient, it will sink to the bottom of the stomach and are entrapped in the folds of the antrum and withstand the peristaltic waves of the stomach wall. The only major drawbacks with this systems is that it is technically difficult to manufacture them with a large amount of drug (>50%) and to achieve the required density of g/cm Magnetic systems: [9] This system is based on a simple idea that the dosage form contains a small internal magnet and a magnet placed on the abdomen over the position of the stomach. Ito et al.used this technique in rabbits with bioadhesives granules containing ultrafine ferrite (g-fe 2 O 3 ). They guided them to the oesophagus with an external magnet (1700 G) for the initial 2 min and almost all the granules were retained in the region after 2 hr. 2. MATERIALS [1] Following types of the ingredients can be incorporated in to HBS dosage form: Hydrocolloids Suitable hydrocolloids are synthethics, anionic or non ionic like hydrophilic gums, modified cellulose derivatives. E.g. Acacia, pectin, agar, alginates, gelatin, casein, bentonite, veegum, MC, HPC, HEC, and Na CMC can be used. Release rate accelerant The release rate of the medicament from the formulation can be modified by including excipient like lactose and/or mannitol. These may be present from about 5-60% by weight. Buoyancy increasing agents Materials like ethyl cellulose, which has bulk density less than one, can be used for enhancing the buoyancy of the formulation. It may be adapted up to 80 % by weight. Excipients Excipients used most commonly in these systems include HPMC, polyacrylate polymers, polyvinyl acetate, Carbopol, agar, sodium alginate, calcium chloride, polyethylene oxide and polycarbonates. 3. Evaluation 3.1 Evaluation of powder blend: [10] Angle of repose: Lower the angle of repose, better the flow properties. The angle of repose may be calculated by measuring the height (h) of the pile and the radius of the base(r) with ruler Bulk density: Where, W = Weight of powder, BV = Bulk Volume Tan θ = h/r.. (1) Bulk Density = W/BV.. (2) Joshi P et al 126
5 3.1.3 Percentage porosity: Porosity provides information about hardness, disintegration, total porosity etc. % porosity = v.v / B.V * 100.(3) % porosity = ( B.V. T.V. / T.D. ) * 100..(4) Where,V.V. void volume, B.V.- bulk volume, T.V.- true volume, T.D.- true density. 3.2 Evaluation of granules: [11] Flow Properties of Granules: The flow properties of granules (before compression) were characterized in terms of angle of repose, Carr index and Hausner ratio HR= ρt/ρb IC = (ρt - ρb)/ρt..(5)..(6) Where ρb -Bulk density, ρt -tapped density, HR -Hausner ratio and IC -carr index. 3.3 Evaluation of floating tablets: [12,13,14] Measurement of buoyancy capabilities of the FDDS: The experiment is carried out in two different media, deionized water and simulated meal. The results showed that higher molecular weight polymers with slower rate of hydration had enhanced floating behavior and it was observed more in simulated meal medium compared to de-ionized water In Vitro floating and dissolution behavior: A small, loose piece of nonreactive material with not more than a few turns of a wire helix may be attached to the dosage units that would otherwise float Weight variation: The USP provides the weight variation test by weighing 20 tablets individually, calculating the average weight, and comparing the individual tablet weights to the average. The tablets meet the USP test if no more than 2 tablets are outside the percentage limit, and if no tablet differs by more than 2 times the percentage limit Hardness & Friability: Conventional compressed tablets that lose less than 0.5 to 1.0 % of their weight are generally considered acceptable Particle size analysis, surface characterization (for floating microspheres and beads): The external and cross-sectional morphology (surface characterization) is done by scanning electron microscope (SEM) X-Ray: It helps to locate dosage form in the gastrointestinal tract (GIT), by which one can predict and correlate the gastric emptying time and the passage of dosage form in the GIT. Here the inclusion of a radio-opaque material into a solid dosage form enables it to be visualized by X-rays Pharmacokinetic studies: Sawicki studied the pharmacokinetics of verapamil, from the floating pellets containing drug, filled into a capsule, and compared with the conventional verapamil tablets of similar dose (40 mg). 3.4 Evaluation of bioadhesive system: [15] The bioadhesive strength of a polymer can be determined by measuring the force required to separate the polymer specimen sandwiched between the layers of either an artificial (e.g., cellophane) or biological (e.g., rabbit stomach tissue) membrane. This force can be measured by using a modified precision balance or an automated texture analyzer. 3.5 Evaluation of swelling systems: [16] Weight gain and water uptake (WU): The study is done by immersing the dosage form in simulated gastric fluid at 37 0 C and determining these factors at regular intervals. The dimensional changes can be measured in terms of the increase in tablet diameter and/or thickness over time. WU is measured in terms of percent weight gain, as given by the equation 7 WU = ([W.sub.t] - [W.sub.0]) x 100/[W.sub.0].(7) Where [W.sub.t] and [W.sub.0] are the weights of the dosage form at time t and initially Gastroretention: The inclusion of a radio-opaque material into a solid dosage form enables it to be visualized by X-rays. The use of X-rays involves exposing a patient to an X-ray beam, thus permitting the visualization of the GI transit of the dosage form Dissolution/drug release: The major requirement for the dissolution test is to allow a dosage form to sink to the bottom of the vessel before the rotation of the paddle. 4. REFERENCES 1. delivery-system-innovative-approach-prolong-gastricretention accessed on 29/10/ Arora S, Ali J, Ahuja A, Khar R K, Baboota S, Floating drug delivery systems:a Review, AAPS Pharmaceutical science technology, 47, pp. no , Moes A J, Gastroretentive Dosage forms, Crit Rev Ther Drug Carrier Syst., 10(2), pp. no , Joshi P et al 127
6 4. Singh B N, Kim K H, Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention, Journal of Controlled Release, 63, pp. no , Klausner E A, Lavy E, Friedman M, Hoffman A, Expandable gastroretentive dosage forms, J. Control. Rel., 90, pp. no , Timmermans J, Moes A J, Factors controlling the buoyancy and gastric retention capabilities of floating capsules: new data for reconsidering the controversy, Journal of Pharmaceutical Science, 83, pp. no , Burns S J, Corness D, Hay G, Higginbottom S, Whelan I., Development and validation of an in vitro dissolution method for a floating dosage form with biphasic release characteristics, Int. J.Pharm, 121, pp. no , Atyabi F, Sharma H L, Mohammad H A, Fell J T, Controlled drug release from coated floating ion exchange resin beads, J. Control. Release, 42, pp. no.25 28, Anand S, Surana K, Rakhee K, An overview on various approaches to oral controlled drug delivery system via gastroretention, 2(2), 14, pp no , Mathur P, Saroha K, Syan N, Verma S V, Floating drug delivery system: An innovative acceptable approach in gastroretentive drug delivery, Archives of Applied Science Research, 2(2), pp. no , Subrahmanyam CVS, Setty J T, Laboratory manual of physical pharmaceutics, Jain MK for vallabh prakashan, pp. no , Sharma S, Pawar A, Low density multiparticulate system for pulsatile release of meloxicam, International Journal of Pharmaceutical Science, 313, pp. no , Shah S H, Patel J K, Patel N V, Stomach specific floating drug delivery system: A Review, International Journal of Pharmaceutical Science, coden USA, 3, pp. no , Shinde A K, Gastroretentive Drug Delivery System: An Overview, 13, pp. no , Koner P, Saudagar R B, Daharwal S J, Gastro-Retentive Drugs: A Novel Approach Towards Floating Therapy, 5 (1), pp. no , Vinod K R, Padma Sri A, Banji D, Anbazhagan S, Vasa S, Sandhya S, Formulation and in vitro characterization of lansoprazole floating gastroretentive microspheres by modified non aqueous solvent evaporation method, Der Pharma Chemica, 2(5), pp. no ,2010. Joshi P et al 128
Gastro Retentive Drug Delivery System
 Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
DISSOLUTION RATE LIMITED ABSORPTION:
 INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
Int. J. Pharm. Sci. Rev. Res., 27(2), July August 2014; Article No. 15, Pages: Review on Gastro Retentive Drug Delivery System (GRDDS)
 Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE
 INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
Available Online through Research Article
 ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
SHRI JAGDISHPRASAD JHABARMAL TIBREWALA UNIVERSITY 1
 INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
LIST OF TABLES. Page No. No List of US Patents for FDDS Gastroretentive products available in the market
 LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
Gastroretentive Drug Delivery Systems: A Review
 J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
EVALUATION OF EFFERVESCENT FLOATING TABLETS. 6.7 Mathematical model fitting of obtained drug release data
 EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
 Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
Volume 8, Issue 2, May June 2011; Article-029
 Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
Formulation and Evaluation of Atenolol Floating Tablets Using Different Polymers: Guargum, Sodium Alginate, Hpmc100cps and Carbopol940
 ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
FLOATING DRUG DELIVERY OF RANITIDINE HYDROCHLORIDE
 Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
Biswajit Biswal IRJP 2 (7)
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DESIGN DEVELOPMENT AND EVALUATION OF TRIMETAZIDINE DIHYDROCHLORIDE FLOATING BILAYER
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DESIGN DEVELOPMENT AND EVALUATION OF TRIMETAZIDINE DIHYDROCHLORIDE FLOATING BILAYER
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW
 MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist
 Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium
 Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED RELEASE FLOATING MATRIX TABLETS OF METFORMIN HYDROCHLORIDE
 ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE
 FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN
 FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System
 Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING
 SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: Journal homepage:
 Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: 2320-9267 Indian Journal of Pharmaceutical and Biological Research (IJPBR) Journal homepage: www.ijpbr.in Review Article A REVIEW ON
Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: 2320-9267 Indian Journal of Pharmaceutical and Biological Research (IJPBR) Journal homepage: www.ijpbr.in Review Article A REVIEW ON
Factors responsible for the impaired bioavailability and instability rifampicin FDC
 Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride
 Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Int J Pharm Bio Sci. Harshal P. Gahiwade *et al Available Online through IJPBS Volume 2 Issue 1 JAN-MARCH
 Page166 IJPBS Volume 2 Issue 1 JAN-MARCH 2012 166-172 FORMULATION AND IN-VITRO EVALUATION OF TRIFLUOPERAZINE HYDROCHLORIDE BILAYER FLOATING TABLET Harshal P. Gahiwade *, Manohar V. Patil, Bharat W. Tekade,
Page166 IJPBS Volume 2 Issue 1 JAN-MARCH 2012 166-172 FORMULATION AND IN-VITRO EVALUATION OF TRIFLUOPERAZINE HYDROCHLORIDE BILAYER FLOATING TABLET Harshal P. Gahiwade *, Manohar V. Patil, Bharat W. Tekade,
A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES
 Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Design and Evaluation of Atenolol Floating Drug Delivery System
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
Formulation and evaluation of modified release oral solid dosage forms. prof. dr. Saša Baumgartner University of Ljubljana Faculty of Pharmacy
 Formulation and evaluation of modified release oral solid dosage forms prof. dr. Saša Baumgartner University of Ljubljana Faculty of Pharmacy Content Terminology Why modified release? How to select appropriate
Formulation and evaluation of modified release oral solid dosage forms prof. dr. Saša Baumgartner University of Ljubljana Faculty of Pharmacy Content Terminology Why modified release? How to select appropriate
International Journal of Medicine and Pharmaceutical Research
 International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 5.210 Volume 4, Issue 11, 1313-1332 Review Article ISSN 2278 4357 A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS Sayyed Sarfaraz
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 5.210 Volume 4, Issue 11, 1313-1332 Review Article ISSN 2278 4357 A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS Sayyed Sarfaraz
This PDF is available for free download
 Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
Formulation and evaluation of sustained release atenolol
 9 Formulation, optimization and evaluation of sustained release layer of atenolol Atenolol is a cardioselective β-blocker widely prescribed for asymptomatic condition such as hypertension. It is poorly
9 Formulation, optimization and evaluation of sustained release layer of atenolol Atenolol is a cardioselective β-blocker widely prescribed for asymptomatic condition such as hypertension. It is poorly
A NOVEL APPROACH FOR FLOATING DRUG DELIVERY SYSTEM
 ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate
 Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Asian Journal of Biochemical and Pharmaceutical Research
 ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
Design and In-vitro Evaluation of Silymarin Bilayer Tablets
 CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
REVISION OF MONOGRAPH ON TABLETS. Tablets
 March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Venkateswara Rao et.al Indian Journal of Research in Pharmacy and Biotechnology ISSN: (Print) ISSN: (Online)
 Design and development of Metformin hydrochloride Tried sustained release tablets Venkateswara Rao T 1 *, Bhadramma N 1, Raghukiran CVS 2 and Madubabu K 3 Bapatla College of Pharmacy, Bapatla, Guntur-522101
Design and development of Metformin hydrochloride Tried sustained release tablets Venkateswara Rao T 1 *, Bhadramma N 1, Raghukiran CVS 2 and Madubabu K 3 Bapatla College of Pharmacy, Bapatla, Guntur-522101
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN
 Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION SPRAY DRYING TECHNIQUE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION
Volume: I: Issue-2: Aug-Oct ISSN APPROACHES FOR GASTROTENTIVE DRUG DELIVERY SYSTEMS. Nalanda College of Pharmacy, Nalgonda, A.P.
 Volume: I: Issue-2: Aug-Oct -2010 ISSN 0976-4550 Review article APPROACHES FOR GASTROTENTIVE DRUG DELIVERY SYSTEMS *Vinod K.R. 1, Santhosh Vasa 1, Anbuazaghan S 2, David Banji 1, Padmasri A 1, Sandhya
Volume: I: Issue-2: Aug-Oct -2010 ISSN 0976-4550 Review article APPROACHES FOR GASTROTENTIVE DRUG DELIVERY SYSTEMS *Vinod K.R. 1, Santhosh Vasa 1, Anbuazaghan S 2, David Banji 1, Padmasri A 1, Sandhya
MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY FLOATING APPROCHES
 Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Review Article REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEMS
 ISSN 2395-3411 Available online at www.ijpacr.com 73 Review Article REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEMS Ashwini T *, Shabaraya AR and Vineetha K Department of Pharmaceutics, Srinivas College
ISSN 2395-3411 Available online at www.ijpacr.com 73 Review Article REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEMS Ashwini T *, Shabaraya AR and Vineetha K Department of Pharmaceutics, Srinivas College
The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation
 METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery Tablet
 International Journal of Pharmacy and Medical Sciences (1): 01-06, 01 ISSN -5854 IDOSI Publications, 01 DOI: 10.589/idosi.ijpms.01..1.641 Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery
International Journal of Pharmacy and Medical Sciences (1): 01-06, 01 ISSN -5854 IDOSI Publications, 01 DOI: 10.589/idosi.ijpms.01..1.641 Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery
Journal of Pharmaceutical and Scientific Innovation Research Article
 Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Research Article FORMULATION AND IN VITRO EVALUATION OF GLIPIZIDE AS FLOATING DRUG DELIVERY SYSTEM WITH NATURAL POLYMER (GUAR-GUM)
Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Research Article FORMULATION AND IN VITRO EVALUATION OF GLIPIZIDE AS FLOATING DRUG DELIVERY SYSTEM WITH NATURAL POLYMER (GUAR-GUM)
Chapter 1 Introduction
 Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin
 Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin Amiya kumar Prusty, Archana P. Chand Institute of Pharmacy and Technology, salipur, Biju Patnaik University of technology,
Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin Amiya kumar Prusty, Archana P. Chand Institute of Pharmacy and Technology, salipur, Biju Patnaik University of technology,
Formulation Development and Evaluation of Sitagliptin Floating Tablets Containing Natural Polymer
 Human Journals Research Article May 2015 Vol.:3, Issue:2 All rights are reserved by Tanvi P Gunjal et al. Formulation Development and Evaluation of Sitagliptin Floating Tablets Containing Natural Polymer
Human Journals Research Article May 2015 Vol.:3, Issue:2 All rights are reserved by Tanvi P Gunjal et al. Formulation Development and Evaluation of Sitagliptin Floating Tablets Containing Natural Polymer
Preparation and Evaluation of Silymarin Controlled Release Tablets Prepared Using Natural Gums
 1368 International Journal of Pharmaceutical Sciences and Nanotechnology Volume 4 Issue 1 April-June 211 Research Paper International Journal of Pharmaceutical Sciences and Nanotechnology Volume 4 Issue
1368 International Journal of Pharmaceutical Sciences and Nanotechnology Volume 4 Issue 1 April-June 211 Research Paper International Journal of Pharmaceutical Sciences and Nanotechnology Volume 4 Issue
Figure 1.1: (a) Drug release mechanism and (b) Floating drug delivery systems
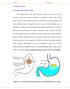 1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
Formulation and in vitro Evaluation of Oral Floating Matrix Tablets of Diclofenac Sodium
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.4, pp 2420-2428, Oct-Dec 2010 Formulation and in vitro Evaluation of Oral Floating Matrix Tablets of Diclofenac
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.4, pp 2420-2428, Oct-Dec 2010 Formulation and in vitro Evaluation of Oral Floating Matrix Tablets of Diclofenac
DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL COMPOSITE DESIGN
 Academic Sciences Asian Journal of Pharmaceutical and Clinical Research Vol, Suppl 1, 2012 ISSN - 0974-2441 Research Article DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL
Academic Sciences Asian Journal of Pharmaceutical and Clinical Research Vol, Suppl 1, 2012 ISSN - 0974-2441 Research Article DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2013, 4(4):141-150 ISSN: 0976-8688 CODEN (USA): PSHIBD Development and in-vitro evaluation of intra gastric cefadroxil monohydrate
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2013, 4(4):141-150 ISSN: 0976-8688 CODEN (USA): PSHIBD Development and in-vitro evaluation of intra gastric cefadroxil monohydrate
Principals and Dosage Forms in the Therapy Modified Drug Release. Institute of Pharmaceutical Technology and Biopharmacy
 Principals and Dosage Forms in the Therapy Modified Drug Release Institute of Pharmaceutical Technology and Biopharmacy Dosage forms Definition: Dosage forms are the means by which drug molecules are delivered
Principals and Dosage Forms in the Therapy Modified Drug Release Institute of Pharmaceutical Technology and Biopharmacy Dosage forms Definition: Dosage forms are the means by which drug molecules are delivered
CONTENTS PAGE. Please note: Preface Matrix system Selection of METOLOSE grades Specifications
 Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems
 Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Research Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND EVALUATION OF BILAYERED FLOATING TABLETS OF METFORMIN HYDROCHLORIDE R.Margret Chandira*, P.Palanisamy,
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND EVALUATION OF BILAYERED FLOATING TABLETS OF METFORMIN HYDROCHLORIDE R.Margret Chandira*, P.Palanisamy,
Formulation and Evaluation of Pulsatile Drug Delivery System of Rosuvastatin Calcium Using Different Swelling Polymers
 ISSN: 2277-769 CODEN Code: PIHNBQ ZDB-Number: 266338-2 IC Journal No: 772 Vol. 1 No. 7 212 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION Formulation and Evaluation of Pulsatile Drug
ISSN: 2277-769 CODEN Code: PIHNBQ ZDB-Number: 266338-2 IC Journal No: 772 Vol. 1 No. 7 212 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION Formulation and Evaluation of Pulsatile Drug
BUCCAL DRUG DELIVERY SYSTEM
 BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Research Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION DEVELOPMENT AND EVALUATION OF CLARITHROMYCIN ORAL DOSAGE FORM AGAINST HELICOBACTER PYLORI INFECTION
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION DEVELOPMENT AND EVALUATION OF CLARITHROMYCIN ORAL DOSAGE FORM AGAINST HELICOBACTER PYLORI INFECTION
1. LITERATURE REVIEW Jaimini et al.6 (2007), Chaudhari et al.7 (2011), Sivabalan M et al.8 (2011), Punitha et al.9 (2010), Patel et al.
 1. LITERATURE REVIEW Jaimini et al. 6 (2007), prepared floating tablets of famotidine by using Methocel K 15 M and Methocel K 100 M as gel forming agent and combination of citric acid and sodium bicarbonate
1. LITERATURE REVIEW Jaimini et al. 6 (2007), prepared floating tablets of famotidine by using Methocel K 15 M and Methocel K 100 M as gel forming agent and combination of citric acid and sodium bicarbonate
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
Optimization of Valsartan SR Floating Tablet Formulation by 2 2 Factorial Design and Multiple Regression Technique
 Optimization of Valsartan SR Floating Tablet by 2 2 Factorial Design and Multiple Regression Technique K. Srinivasa Reddy 1, Surya Kanta Swain 2, K. P. R. Chowdary *3, S. V. U. M. Prasad 4 1 School of
Optimization of Valsartan SR Floating Tablet by 2 2 Factorial Design and Multiple Regression Technique K. Srinivasa Reddy 1, Surya Kanta Swain 2, K. P. R. Chowdary *3, S. V. U. M. Prasad 4 1 School of
Scholars Research Library. Formulation and evaluation of buccoadhesive tablet of Atenolol
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
7. SUMMARY, CONCLUSION AND RECOMMENDATIONS
 211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol
 Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol K.Viveksarathi, K.Kannan*, S.Selvamuthu Kumar, R.Manavalan Department of Pharmacy, Faculty of Engineering
Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol K.Viveksarathi, K.Kannan*, S.Selvamuthu Kumar, R.Manavalan Department of Pharmacy, Faculty of Engineering
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(3):159-164 Studies on formulation and in vitro evaluation
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(3):159-164 Studies on formulation and in vitro evaluation
FORMULATION AND EVALUATIONOF AMOXYCILLIN: THREE-LAYER GUAR GUM MATRIX TABLET
 Gupta and Singh, IJPSR, 2013; Vol. 4(7): 2683-2690. ISSN: 0975-8232 IJPSR (2013), Vol. 4, Issue 7 (Research Article) Received on 05 March, 2013; received in revised form, 29 April, 2013; accepted, 29 June,
Gupta and Singh, IJPSR, 2013; Vol. 4(7): 2683-2690. ISSN: 0975-8232 IJPSR (2013), Vol. 4, Issue 7 (Research Article) Received on 05 March, 2013; received in revised form, 29 April, 2013; accepted, 29 June,
A review on Gastroretentive Drug Delivery Systems
 29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
PREPARATION AND EVALUATION OF GASTRO RETENTIVE FLOATING TABLETS: OF QUETIAPINE FUMARATE
 PREPARATION AND EVALUATION OF GASTRO RETENTIVE FLOATING TABLETS: OF QUETIAPINE FUMARATE S. PRATHYUSHA 1, Y. MOHAN KUMAR 2, D. LAVANYA 3, M. KIRAN KUMAR 4 1 M.pharm, Department of pharmacognosy, Tirupathi,
PREPARATION AND EVALUATION OF GASTRO RETENTIVE FLOATING TABLETS: OF QUETIAPINE FUMARATE S. PRATHYUSHA 1, Y. MOHAN KUMAR 2, D. LAVANYA 3, M. KIRAN KUMAR 4 1 M.pharm, Department of pharmacognosy, Tirupathi,
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p.
 Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Formulation and Development of Sustained Release Tablets of Valsartan Sodium
 INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
FORMULATION AND EVALUATION OF SUSTAINED RELEASE FLOATING TABLETS OF LORATADINE
 IJPSR (2014), Vol. 5, Issue 10 (Research Article) Received on 28 March, 2014; received in revised form, 16 May, 2014; accepted, 13 July, 2014; published 01 October, 2014 FORMULATION AND EVALUATION OF SUSTAINED
IJPSR (2014), Vol. 5, Issue 10 (Research Article) Received on 28 March, 2014; received in revised form, 16 May, 2014; accepted, 13 July, 2014; published 01 October, 2014 FORMULATION AND EVALUATION OF SUSTAINED
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
Gellan Gum. Rm.1702, West Unit, No. 41, Donghai Xi Rd, Qingdao, China Post Code:
 Gellan Gum Gellan gum (E418) is a bacterial exopolysaccharide, prepared commercially by aerobic submerged fermentation from Sphingomonas elodea (previously called Pseudomonas elodea), in a manner similar
Gellan Gum Gellan gum (E418) is a bacterial exopolysaccharide, prepared commercially by aerobic submerged fermentation from Sphingomonas elodea (previously called Pseudomonas elodea), in a manner similar
Niacin or nicotinic acid (NA) is used in the treatment of hyperlipidemia. NA immediate release formulation shows
 RESEARCH ARTICLE Formulation and evaluation of floating tablets of niacin for sustained release Haripriya Puthoori, TEGK Murthy, Atul Kaushik 1, Murthy K 2 Department of Pharmaceutics, Bapatla College
RESEARCH ARTICLE Formulation and evaluation of floating tablets of niacin for sustained release Haripriya Puthoori, TEGK Murthy, Atul Kaushik 1, Murthy K 2 Department of Pharmaceutics, Bapatla College
DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS OF LEVETIRACETAM
 DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS OF LEVETIRACETAM Dissertation submitted to The Tamilnadu Dr. M.G.R Medical University, Chennai -32 In partial fulfillment of the requirements For the award
DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS OF LEVETIRACETAM Dissertation submitted to The Tamilnadu Dr. M.G.R Medical University, Chennai -32 In partial fulfillment of the requirements For the award
Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs
 Mansoura University Faculty of Pharmacy Department of Pharmaceutics Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs Thesis presented by Ali Saeed
Mansoura University Faculty of Pharmacy Department of Pharmaceutics Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs Thesis presented by Ali Saeed
Pharmaceutical Preparation For Internal Use
 Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Journal of Chemical and Pharmaceutical Research, 2015, 7(5): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(5):1225-1231 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Formulation and evaluation of raft forming sustained
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(5):1225-1231 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Formulation and evaluation of raft forming sustained
FORMULATION AND IN-VITRO EVALUATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEM FOR NEVIRAPINE
 FORMULATION AND IN-VITRO EVALUATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEM FOR NEVIRAPINE Mahajan Ashwini *, Dr. Sarode S. M., Prof. Sathe B.S., Dr. Vadnere G.P. Department Of Pharmaceutics. Smt. S.S.
FORMULATION AND IN-VITRO EVALUATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEM FOR NEVIRAPINE Mahajan Ashwini *, Dr. Sarode S. M., Prof. Sathe B.S., Dr. Vadnere G.P. Department Of Pharmaceutics. Smt. S.S.
JJT University, Rajasthan Page 62
 4. 4.1 Aim of the Present Work There are several types of gastro retentive system but among of all these system gastro retentive floating drug delivery system had major advantages. In these system the
4. 4.1 Aim of the Present Work There are several types of gastro retentive system but among of all these system gastro retentive floating drug delivery system had major advantages. In these system the
Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H.
 Pharmaceutical Research Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H. pylori Infection Shikha Jain, Vikas Jain, S.C. Mahajan
Pharmaceutical Research Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H. pylori Infection Shikha Jain, Vikas Jain, S.C. Mahajan
Critical material properties for the design of robust drug products : excipient functionality related characteristics
 Critical material properties for the design of robust drug products : excipient functionality related characteristics Dr Liz Meehan, Pharmaceutical Development, Macclesfield UK 1 Excipients Definition
Critical material properties for the design of robust drug products : excipient functionality related characteristics Dr Liz Meehan, Pharmaceutical Development, Macclesfield UK 1 Excipients Definition
FABRICATION AND EVALUATION OF GLIMEPIRIDE CORDIA DICHOTOMA G.FORST FRUIT MUCILAGE SUSTAINED RELEASE MATRIX TABLETS
 Int. J. Chem. Sci.: 7(4), 2009, 2555-2560 FABRICATION AND EVALUATION OF GLIMEPIRIDE CORDIA DICHOTOMA G.FORST FRUIT MUCILAGE SUSTAINED RELEASE MATRIX TABLETS HINDUSTAN ABDUL AHAD *, B. PRADEEP KUMAR, C.
Int. J. Chem. Sci.: 7(4), 2009, 2555-2560 FABRICATION AND EVALUATION OF GLIMEPIRIDE CORDIA DICHOTOMA G.FORST FRUIT MUCILAGE SUSTAINED RELEASE MATRIX TABLETS HINDUSTAN ABDUL AHAD *, B. PRADEEP KUMAR, C.
Formulation and Evaluation
 Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
Non-Food Uses of Polysaccharides
 Non-Food Uses of Polysaccharides John Mitchell John.Mitchell@biopolymersolutions.co.uk Acknowledgements Fundamentals of Hydrocolloid Technology Course (2003-2009) Rob Winwood Colin Melia Steve Harding
Non-Food Uses of Polysaccharides John Mitchell John.Mitchell@biopolymersolutions.co.uk Acknowledgements Fundamentals of Hydrocolloid Technology Course (2003-2009) Rob Winwood Colin Melia Steve Harding
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Formulation and Evaluation of Gastroretentive Dosage form of Ciprofloxacin Hydrochloride.
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
