The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
|
|
|
- Alan Houston
- 5 years ago
- Views:
Transcription
1 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov. Identification number NCT September 21 st,
2 Table of Contents Introduction....3 Study Contacts...3 Pharmacy Web Access Signature Log...4 Randomization and Web log-in...5 Study Supplements...7 Inventory of Study Supplements...8 Monthly Site Inventory Log...9 Storage of Study Supplements...10 Temperature Log...11 Dispensing of Study Supplements...12 Enteral Product Label Log...13 Enteral Study Supplement Dispensing Log...14 Parenteral Study Supplement Dispensing Log...17 Dosages According to Height...18 Nutrient Accountability Logs...19 Enteral AOX log...20 Enteral GLN log...21 Enteral AOX + GLN log...22 Enteral Placebo log...23 Dipeptiven log...24 Selenium Log...25 Keeping Records...26 Appendices Appendix A. Pharmacy Web Access Signature Log...27 Appendix B. Randomization Process on Web...28 Appendix C. Enteral Study Supplement Label Template...29 Appendix D. Parenteral Study Supplements Worksheets...30 Appendix E. Parenteral Study Supplement Label Template...35 Appendix F. Height and Dose of Dipeptiven...36 September 21 st,
3 Introduction The Pharmacy Manual provides details on the randomization process, the storage, dispensing of study supplements and the logs to be kept by the pharmacist(s) involved in the REducing Deaths due to OXidative Stress Study (The REDOXS Study). Principal Investigator Dr Daren Heyland Kingston General Hospital Angada 4, 76 Stuart Street Kingston ON K7L 2V7 Tel: ext Fax: Study Contacts Project Leader Rupinder Dhaliwal, RD Kingston General Hospital Angada 4, 76 Stuart Street Kingston ON K7L 2V7 Phone ext 3830 Cell Phone: Fax: September 21 st,
4 Pharmacy Web Access Signature Log Any Pharmacist that will be involved in the REDOXS Study by either dispensing or verifying dispensing/records must sign the Pharmacy Web Access Signature Log (Appendix A) provided by CERU (Clinical Evaluation Research Unit). The purpose of this log is to keep track of all the pharmacy staff members involved in the study and also to identify signatures. The site Pharmacist will be responsible for notifying the CERU Project Leaders with any changes in personnel accessing the web. Completed signature logs are to be faxed to the Project Leader at CERU at before a username and password to the web based system can be assigned. September 21 st,
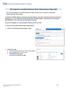
5 Randomization and Web Log in The Study Coordinator will enroll a patient and this is followed by the randomization process that occurs automatically online. The Study coordinator, who is blinded to the treatment arm, will contact the Pharmacist with the following patient details: patient s name initials DOB enrollment # height (in cms) Refer to the Randomization Process on Web, Appendix B for more details. The Pharmacist, who will be unblinded, will check the treatment arm the patient is allocated to by following these steps: 1. Log on to the website for the REDOXS study go to REDOXS Study tab on the left hand side of the page and click on REGISTER/LOGIN or directly to 2. Enter Username 3. Enter Password (please change this password after the first time you log in). Webshot of Log in Page September 21 st,
6 4. Click the Login button. If the login information is correct, you will automatically be brought to the Welcome Page that shows the treatment allocation for every patient enrolled at your site. Webshot of Welcome Page showing Treatment Assignment Note: this is not the randomization list that will be used for the study. This page will show the Pharmacist with 1 of 4 treatment assignments: o Antioxidants (AOX) o Glutamine (GLN) o Antioxidants and Glutamine (AOX+GLN) o Placebo Only these patient identifiers will be shown on the web page: Site ID, Screening #, Enrolment #, Age and Height. The Pharmacist will obtain other identifiers from the Study Coordinator (initials, DOB, CR#, etc). The Pharmacist must print off a copy of the randomization list (showing patient s treatment assignment) and have it signed by two pharmacists to ensure that the correct treatment arm has been noted. Please keep this signed randomization list in the pharmacy study binder. Click on Logout button on the top left hand of the screen. September 21 st,
7 Study Supplements THE DURATION OF THE STUDY SUPPLEMENTS SHOULD NOT EXCEED A TOTAL OF 28 DAYS. Study supplements will be discontinued in the event that the patient is discharged from ICU or dies before 28 days (exception: patients with ICU stay < 5 days and transferred to ward; duration of study supplements should be 5 days in total). The enteral study supplements (EN REDOXS formula) and the parenteral glutamine supplement (Dipeptiven) will be provided by Fresenius Kabi. The parenteral selenium (Micro-Selenium) will be supplied by BAXTER. Dipeptiven Micro-Selenium Enteral REDOXS formulas For details on the Study Supplements, refer to the Administration of Study Supplements Manual. September 21 st,
8 Inventory of Study Supplements An adequate amount of both the enteral and parenteral supplements, including the parenteral selenium, will be shipped to each pharmacy by Calea, Mississauga (Supplier for Fresenius Kabi) before the start of the trial. To ensure uninterrupted enrolment to the study, the Pharmacist at each site will complete a Monthly Site Inventory Log (see next page) to determine the amount of supplies needed at the end of every month. When determining the amount of supplies needed, the Pharmacist is to consider the minimum supply needed (as specified on the Log) and the projected need for the products based on patient enrollment. The Pharmacist will then fax this form to the Project Leader at the Clinical Evaluation Research Unit who will arrange for ordering the supplies. The original Monthly Site Inventory Log must be kept by the pharmacist. September 21 st,
9 September 21 st,
10 Storage of Study Supplements The study supplements are to be stored in the pharmacy and should be kept separate from regular stock. Both the enteral and parenteral products are to be stored at room temperature between C. It is imperative that the temperature does not drop below 15 C. Dipeptiven: once opened, is to be mixed into the other parenteral components immediately. Selenium: once opened, is to be used within 24 hrs if refrigerated. Parenteral mixtures: once the parenteral products are mixed, they are stable at room temperature (15-25 C) for 96 hours (as per stability and compatibility studies performed on the various mixtures). Enteral products: once opened, they are to be used within 24 hours. The enteral products may show precipitation over time. Please shake these bottles vigorously before use. Contact the Project Leader if the precipitation remains or if you have any concerns about the products. The pharmacist MUST keep a temperature log (see attached) to show that the products have been stored at the specified temperature ranges. The log is to be completed monthly and faxed to the Project Leader. These logs will be checked by the study monitor. You may use the temperature log that is currently being used at your pharmacy as long as it identifies that the products checked are related to the REDOXS Study. September 21 st,
11 September 21 st,
12 Dispensing of Study Supplements The Pharmacist at each site will be responsible for dispensing the study supplements. Hence he/she will be unblinded to the treatment assignment. To maintain blinding and to avoid a potential for bias, every attempt should be made to ensure that the Pharmacist dispensing the drugs is NOT the Pharmacist that participates in ICU rounds. Enteral Products 1. After checking the treatment allocation online and verifying this with a second pharmacist, the primary pharmacist will obtain the appropriate enteral study supplement (as indicated on the company label AOX+GLN, AOX, GLN or Placebo). 2. The Pharmacist will then generate two labels for the enteral product as per template Enteral Study Supplement Template (Appendix C) (may be adapted to each site s needs but MUST contain all the elements shown). 3. He/she will remove the company label from the enteral bottle and place this on the Enteral Product Label Log, daily, and complete this form, including the date. He/she will place one of the pharmacy-generated label on the bottle. CR# refers to the unique ID# that is assigned to each patient that is hospitalized. 4. The other label will be sent with the enteral study supplement to the ICU. The label and the enteral study supplement bottle must be placed together in the same bag to avoid confusion with the supplements for other patients. The bedside nurse will be instructed to place this label on the feeding bag. 5. For each patient, the Pharmacist will then record the batch # of the bottle, the expiry date and their signature on the Enteral Study Supplement Dispensing Log. This will be verified by a second Pharmacist. This is to be done for every patient daily. For Study Day 1, the rate of the enteral and parenteral study supplements will likely be running at double rate to make up for the hours missed. Depending upon the timing of the scheduled preparation for the next day, you may need to prepare an additional bag of the parenteral solution and send an additional enteral formula bottle. Please ensure that there is enough volume prepared so that there is no interruption in the infusion of the supplements. September 21 st,
13 September 21 st,
14 September 21 st,
15 For Parenteral Products The Pharmacist will need to prepare the parenteral solutions according to the study group the patient has been assigned to. The parenteral products will be mixed using aseptic techniques in a laminar hood flow. (See below for the study groups). Study Groups for REDOXS Group Enteral Supplement Parenteral Supplement AOX AOX only Placebo (Normal Saline) + Selenium GLN Glutamine only Dipeptiven + Placebo (Normal Saline) GLN + AOX Glutamine + AOX Dipeptiven + Selenium + Placebo (Normal Saline) Placebo Placebo Placebo (Normal Saline) Both the parenteral supplements (Dipeptiven and Selenium) will be mixed in normal saline according to the group allocation. The final parenteral solution will be 250 ml and will run at 10 ml/hour in most cases. Exception: the patient is 196 cms tall (6 feet 5 inches) in which case the final infusion rate will need to be calculated by the Pharmacist. This rate will need to appear on the pharmacy-generated label. (See Dosages according to Height section and Worksheets, Appendix D) Instructions for preparing the Parenteral Study Supplement mixture (see Parenteral Study Supplement Worksheets for each group, Appendix D) 1) Obtain patient s height (in cms) from Study Coordinator. 2) Determine the patient s Normal Body Weight using Broca Formula as follows: Normal Weight (kgm) = Patients height cm minus 100 cms. 3) Determine the Volume of Dipeptiven to be given (for patients in the GLN +AOX and GLN groups). This equals: Glutamine 0.35g/ kg/day = L-alanyl-L-glutamine 0.5gm/kg/day = Dipeptiven 2.5 ml/kg/day. For patients in the AOX or Placebo group, this volume is zero. Example: 1) Patient s height =170 cms and is assigned to GLN+AOX group. 2) Normal body weight = = 70 kgms. 3) Dose of Glutamine = 0.35 gm x 70 = 24.5 gms glutamine or Dose of L-alanyl-L-glutamine = 0.5 gm x 70 = 35 gms L-alanyl-Lglutamine or Dose of Dipeptiven = 2.5 ml x 70 = 175 ml. September 21 st,
16 4) Dosage of Selenium (only for patients in the GLN +AOX and AOX groups) = 500 micrograms/day = 12.5 ml/day. For patients in the GLN or Placebo groups this volume is zero. 5) Combine the volume of Dipeptiven and Selenium together. This is the volume of normal saline that will need to be removed from a 250 ml bag of normal saline. Ignore the overfill in the normal saline bag. 6) Replace the normal saline with the Dipeptiven and Selenium. The final volume of the Parenteral Study Supplement is the combination of the volumes of Dipeptiven, Selenium and normal saline and will be 250 mls in most cases. 7) Record the volume of each component dispensed (Selenium, Dipeptiven and Normal Saline) on the Parenteral Study Supplement Dispensing Log. The batch # and the expiry dates of all the components will be recorded daily and will be signed by the Pharmacist and co-signed by a second Pharmacist. 8) Any unused product(s) returned to pharmacy or expired products should be recorded on the appropriate Nutrient Accountability Log and should be disposed of according to your pharmacy s drug destruction policy. 9) The Pharmacist will then generate a label for the final parenteral product as per template titled Parenteral Study Supplement Label (Appendix E) (may be adapted to each site s needs but must contain the shown elements) and attach this to a normal saline bag. If the volume of the parenteral supplement is to exceed 250 mls, the correct rate will need to be calculated and should appear on the label. 10) Partially used vials of selenium should be refrigerated and should be used within 24 hrs for subsequent doses. 11) The Dipeptiven bottles can be shared between multiple patients if needed. For e.g. if one patient needs 150 mls Dipeptiven and the next patient needs 130 mls, please use 3 bottles (100 mls each) for both instead of using 4 bottles (100 mls each) for both patients. Once opened, the Dipeptiven is to be mixed into the other parenteral components immediately. Weekend Hint: To help reduce workload on weekends, the parenteral study supplements can be dispensed ahead of time in batches of a three-day supply for each patient. For example, three parenteral bags can be prepared on Friday and given the expiry of 96 hours, these can be used for Saturday, Sunday and Monday (will expire Tuesday). The parenteral study supplements are to be mixed with saline but in the event of concerns of hypernatremia, the supplements can be mixed with D5W instead of saline. Compatibility studies of Dipeptiven and Selenium show that they are compatibility with saline and D5W. September 21 st,
17 September 21 st,
18 Dosages According to Height If the patient s height is 196 cms tall (6 feet 5 inches), the volume of the parenteral supplements increases and hence the volume of normal saline to be added decreases. Refer to Height and Dose of Dipeptiven (Appendix F) for volumes according to height. The final volume of the parenteral solution will be between 250 ml-300 mls. The 250 ml bag of normal saline can still be used, as it is able to hold up to 300 mls of fluid. In the event that there is no normal saline needed, you may use an empty sterile bag and add the supplements to this bag (instead of emptying an entire 250 ml bag of normal saline). The final rate of infusion will exceed 10 ml/hour and will need to be calculated by the Pharmacist and to appear on the pharmacy-generated label. Every attempt should be made to infuse the parenteral study supplements via a central line. Peripheral infusion is only recommended (for a maximum of 72 hours) in the event that there is no access to a central line. September 21 st,
19 Nutrient Accountability Log The Pharmacist will keep track of the amount of each product dispensed (or destroyed) on the Nutrient Accountability Log. This log will be used by the study monitor to ensure that the patient actually received the right product. Please note that there are a total of 6 logs, one for each of the products used (i.e. 1 each for the 4 enteral products and 1 each for the Dipeptiven and Selenium). This will be done by cross-checking the patient enrollment #, product name and the batch # on Nutrient Accountability Log with the patient s initials/cr# and enrollment # on the Dispensing Logs. Record the Product Name on the Log. Use one separate log for each product i.e. Enteral products, Dipeptiven and Selenium. Record the date and the amount of product received (enteral products, Dipeptiven and Selenium) along with batch numbers and expiry dates. Record the amount of product dispensed, the patient(s) enrollment # and the balance of the product remaining DAILY. Record your signature. September 21 st,
20 September 21 st,
21 September 21 st,
22 September 21 st,
23 September 21 st,
24 September 21 st,
25 September 21 st,
26 Keeping Records The Pharmacist will keep the following signed records in addition to all other logs described in the manual above and others specified by the pharmacy. o Randomization record showing treatment assignment o Study Orders o Temperature Logs o All worksheets for each patient enrolled in the study. These will be reviewed by the study monitors for quality assurance purposes during site visits. September 21 st,
27 Appendix A. September 21 st,
28 Appendix B. Randomization Process on Web September 21 st,
29 Appendix C. Enteral Study Supplement Label Template September 21 st,
30 Appendix D. Parenteral Study Supplement Worksheets Use the appropriate worksheet according to the group the patient has been randomized to. These worksheets will assist in calculating the volumes of the parenteral study supplements and normal saline needed. Worksheet for AOX (Antioxidant) group Worksheet for GLN (Glutamine) group Worksheet for GLN +AOX (Glutamine + Antioxidant) group Worksheet for Placebo group September 21 st,
31 Parenteral Supplements Worksheet AOX (Antioxidant + Placebo) Patient will receive Antioxidants (Selenium) and Placebo (Normal Saline) Patient CR #: Patient Initials: Enrollment #: 1. Patient s height = Not needed for calculating AOX dose cms 2. Normal Body Weight = (#1) minus 100 cms = Not needed kgms 3. Dose of Dipeptiven* to be added = 0 ml 4. Dose of Selenium to be added = 12.5 ml 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements = 12.5_mL (add #3 and #4) 6. Add (#3) +(#4) + normal saline = 250 ml 7. Record the volumes of Selenium and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 8. Generate a label and attach to bag. To maintain blinding, if patient is 6 5 tall (196 cms) the total volume will be > 250 mls and the hourly rate should be higher than10 mls/hr. Refer to below: If 6 5 (196 cms), final volume should be 253 mls, hourly rate should be 10.5 ml/hr If 6 6 (198 cms), final volume should be 258 mls, hourly rate should be 10.8 mls/hr If 6 7 (201 cms), final volume should be 265 mls, hourly rate should be 11.0 mls/hr If 6 8 (203 cms), final volume should be 271 mls, hourly rate should be 11.3 mls/hr If 6 9 (206 cms), final volume should be 278 mls, hourly rate should be 11.6 mls/hr If 6 10 (208 cms), final volume should be 283 mls, hourly rate should be 11.8 mls/hr If 6 11 (211 cms), final volume should be 290 mls, hourly rate should be 12.1 mls/hr If 7 (213 cms), final volume should be 296 mls, hourly rate should be 12.3 mls/hr September 21 st,
32 Parenteral Supplements Worksheet GLN (Glutamine +Placebo) Patient will receive Glutamine (Dipeptiven) and Placebo (Normal Saline) Patient CR #: Patient Initials: Enrollment #: 1. Patient s height = cms 2. Normal Body Weight = (#1) minus 100 cms = kgms 3. Dose of Dipeptiven* to be added = (#2) x 2.5 ml ml 4. Dose of Selenium to be added = 0 ml 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements = ml (add #3 and #4) 6. Add (#3) +(#4) + normal saline = 250 ml 7. Record the volumes of Dipeptiven and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 8. Generate a label and attach to bag. *Dipeptiven 2.5 ml/kg/day =Glutamine 0.35 g/kg/day = L-alanyl-L-glutamine 0.5 g/kg/day To maintain blinding, if patient is 6 5 tall (196 cms) the total volume will be > 250 mls and the hourly rate should be higher than10 mls/hr. Refer to below: If 6 5 (196 cms), final volume should be 253 mls, hourly rate should be 10.5 ml/hr If 6 6 (198 cms), final volume should be 258 mls, hourly rate should be 10.8 mls/hr If 6 7 (201 cms), final volume should be 265 mls, hourly rate should be 11.0 mls/hr If 6 8 (203 cms), final volume should be 271 mls, hourly rate should be 11.3 mls/hr If 6 9 (206 cms), final volume should be 278 mls, hourly rate should be 11.6 mls/hr If 6 10 (208 cms), final volume should be 283 mls, hourly rate should be 11.8 mls/hr If 6 11 (211 cms), final volume should be 290 mls, hourly rate should be 12.1 mls/hr If 7 (213 cms), final volume should be 296 mls, hourly rate should be 12.3 mls/hr September 21 st,
33 Parenteral Supplements Worksheet GLN +AOX (Glutamine +Antioxidant) Patient will receive Glutamine (Dipeptiven) and Antioxidants (Selenium) Patient CR #: Patient Initials: Enrollment #: 1. Patient s height = cms 2. Normal Body Weight = (#1) minus 100 cms = kgms 3. Dose of Dipeptiven* to be added = (#2) x 2.5 ml ml 4. Dose of Selenium to be added = 12.5 ml ml 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements = ml (add #3 and #4) 6. Add (#3) +(#4) + normal saline = 250 ml 7. Record the volumes of Dipeptiven, Selenium and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 8. Generate a label and attach to bag. *Dipeptiven 2.5 ml/kg/day =Glutamine 0.35 g/kg/day = L-alanyl-L-glutamine 0.5 g/kg/day To maintain blinding, if patient is 6 5 tall (196 cms) the total volume will be > 250 mls and the hourly rate should be higher than10 mls/hr. Refer to below: If 6 5 (196 cms), final volume should be 253 mls, hourly rate should be 10.5 ml/hr If 6 6 (198 cms), final volume should be 258 mls, hourly rate should be 10.8 mls/hr If 6 7 (201 cms), final volume should be 265 mls, hourly rate should be 11.0 mls/hr If 6 8 (203 cms), final volume should be 271 mls, hourly rate should be 11.3 mls/hr If 6 9 (206 cms), final volume should be 278 mls, hourly rate should be 11.6 mls/hr If 6 10 (208 cms), final volume should be 283 mls, hourly rate should be 11.8 mls/hr If 6 11 (211 cms), final volume should be 290 mls, hourly rate should be 12.1 mls/hr If 7 (213 cms), final volume should be 296 mls, hourly rate should be 12.3 mls/hr September 21 st,
34 Parenteral Supplements Worksheet Placebo Group: Placebo Patient will only receive Placebo (Normal Saline) Patient CR #: Patient Initials: Enrollment #: 1. Patient s height = Not needed for calculating dose cms 2. Normal Body Weight = (#1) minus 100 cms = Not needed kgms 3. Dose of Dipeptiven* to be added = 0 ml 4. Dose of Selenium to be added = 0 ml 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements = 0 ml (add #3 and #4) 6. No mixing needed. The patient will receive a 250 ml bag of normal saline. 7. Record the volume of Normal Saline (=250 mls) on the Parenteral Study Supplement Log, for this patient daily. 8. Generate a label and attach to bag. To maintain blinding, if patient is 6 5 tall (196 cms) the total volume will be > 250 mls and the hourly rate should be higher than10 mls/hr. Refer to below: If 6 5 (196 cms), final volume should be 253 mls, hourly rate should be 10.5 ml/hr If 6 6 (198 cms), final volume should be 258 mls, hourly rate should be 10.8 mls/hr If 6 7 (201 cms), final volume should be 265 mls, hourly rate should be 11.0 mls/hr If 6 8 (203 cms), final volume should be 271 mls, hourly rate should be 11.3 mls/hr If 6 9 (206 cms), final volume should be 278 mls, hourly rate should be 11.6 mls/hr If 6 10 (208 cms), final volume should be 283 mls, hourly rate should be 11.8 mls/hr If 6 11 (211 cms), final volume should be 290 mls, hourly rate should be 12.1 mls/hr If 7 (213 cms), final volume should be 296 mls, hourly rate should be 12.3 mls/hr September 21 st,
35 Appendix E. Parenteral Study Supplement Label Template September 21 st,
36 Appendix F. Height and Dose of Dipeptiven September 21 st,
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
Pharmacy Logs and Worksheets
 Pharmacy Logs and Worksheets 1 Monthly Site Inventory Log Month Year To be filled out by Site Pharmacy monthly and faxed to Clinical Evaluation Research Unit (CERU). Name of Site: Pharmacist: Phone: Product
Pharmacy Logs and Worksheets 1 Monthly Site Inventory Log Month Year To be filled out by Site Pharmacy monthly and faxed to Clinical Evaluation Research Unit (CERU). Name of Site: Pharmacist: Phone: Product
Investigational Product Procedures Manual
 A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Investigational Product Procedures Manual Intended Audience: Research Cooridnators/Pharmacists
A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Investigational Product Procedures Manual Intended Audience: Research Cooridnators/Pharmacists
IMPAACT MOCHA More Options for Children and Adolescents
 IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
DRUG ORDERING & DISPENSING:
 STUDY DRUG: List the study drugs that will be used at your site based on your hospital formulary 1. Insulin Humulin R 2. Insulin Humalog 3. Insulin Lantus DRUG SUPPLY & STORAGE: Since study drugs are not
STUDY DRUG: List the study drugs that will be used at your site based on your hospital formulary 1. Insulin Humulin R 2. Insulin Humalog 3. Insulin Lantus DRUG SUPPLY & STORAGE: Since study drugs are not
MOST Clinical Performing Site Study Drug Procedures
 Abbreviations and Definitions MOST Clinical Performing Site Study Drug Procedures ACS AIS Arm Acute Coronary Syndrome Acute Ischemic Stroke Argatroban (100µg/kg bolus and a 12-hour infusion at 3µg/kg/min)
Abbreviations and Definitions MOST Clinical Performing Site Study Drug Procedures ACS AIS Arm Acute Coronary Syndrome Acute Ischemic Stroke Argatroban (100µg/kg bolus and a 12-hour infusion at 3µg/kg/min)
Healthy Volunteer Ultrasound Test Manual
 A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Healthy Volunteer Ultrasound Test Manual Intended Audience: Site Investigators
A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Healthy Volunteer Ultrasound Test Manual Intended Audience: Site Investigators
REducing Deaths from OXidative Stress
 C a n adian Critic a The REDOXS Study REducing Deaths due to OXidative Stress The REDOXS Study REducing Deaths from OXidative Stress Study Chair Dr. Daren Heyland, MD, FRCPC Project Leaders Rupinder Dhaliwal,
C a n adian Critic a The REDOXS Study REducing Deaths due to OXidative Stress The REDOXS Study REducing Deaths from OXidative Stress Study Chair Dr. Daren Heyland, MD, FRCPC Project Leaders Rupinder Dhaliwal,
Pharmacy Manual. Cambridge University Hospitals NHS Foundation Trust & University of Cambridge
 Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
UKALL14 DRUG SUPPLY GUIDELINES
 CONTACT DETAILS UKALL14 DRUG SUPPLY GUIDELINES For further information on trial drugs, trial protocol, dosing, supply drug and distribution queries please contact: UKALL14 Trial Team Phone: 0207 679 9860
CONTACT DETAILS UKALL14 DRUG SUPPLY GUIDELINES For further information on trial drugs, trial protocol, dosing, supply drug and distribution queries please contact: UKALL14 Trial Team Phone: 0207 679 9860
EVERY SHOT COUNTS C O R O N A D O R O O M A U G U S T 1 6,
 EVERY SHOT COUNTS 2016-2 0 1 7 S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M A U G U S T 1 6, 2 0 1 6
EVERY SHOT COUNTS 2016-2 0 1 7 S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M A U G U S T 1 6, 2 0 1 6
VACCINE REMINDER SERVICE A GUIDE FOR SURGERIES
 VACCINE REMINDER SERVICE A GUIDE FOR SURGERIES Sign up to the free text and voicemail service to automatically remind patients eligible for flu vaccination to book their appointment. This guide shows how
VACCINE REMINDER SERVICE A GUIDE FOR SURGERIES Sign up to the free text and voicemail service to automatically remind patients eligible for flu vaccination to book their appointment. This guide shows how
Pharmacy Plan Guidance
 Pharmacy Plan Guidance The pharmacy plan is a tool used during the site readiness process to develop and document the site-specific procedures for study drug ordering, labeling and dispensing for the SHINE
Pharmacy Plan Guidance The pharmacy plan is a tool used during the site readiness process to develop and document the site-specific procedures for study drug ordering, labeling and dispensing for the SHINE
Center for Family Health Policy
 Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT
 HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT Protocol Code: ISRCTN11225767 How to manage and administer the trial treatment version 2.0 date 23/07/2017 What is the trial treatment? Random allocation
HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT Protocol Code: ISRCTN11225767 How to manage and administer the trial treatment version 2.0 date 23/07/2017 What is the trial treatment? Random allocation
Welcome to the New and Improved Member Website: Now Available to All Diocese Employees!
 Welcome to the New and Improved Member Website: Now Available to All Diocese Employees! www.ourhealthylives.org Jeff L. Soileau, MS Health & Wellness Program Manager Key Features Enhanced security features
Welcome to the New and Improved Member Website: Now Available to All Diocese Employees! www.ourhealthylives.org Jeff L. Soileau, MS Health & Wellness Program Manager Key Features Enhanced security features
ARCADIA Study Coordinator Training Investigator Meeting
 ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
2. Receive the Guidelines for the Allergist letter which is to be given to the prescribing allergist physician.
 POLICY: Name of Policy Allergy Immunotherapy Section Clinical Records and Health Information Date Effective January 20, 2005 Date Last Reviewed June 21, 2017 Responsible for Review UCF SHS Director Approved
POLICY: Name of Policy Allergy Immunotherapy Section Clinical Records and Health Information Date Effective January 20, 2005 Date Last Reviewed June 21, 2017 Responsible for Review UCF SHS Director Approved
The RE-ENERGIZE Study. A RandomizEd trial of ENtERal Glutamine to minimize thermal injury. Lab Study Manual
 The RE-ENERGIZE Study A RandomizEd trial of ENtERal Glutamine to minimize thermal injury Lab Study Manual Human Research Protection Office (HRPO) Log Number A-15774.0 HRPO Proposal Number 09155001 Clinical
The RE-ENERGIZE Study A RandomizEd trial of ENtERal Glutamine to minimize thermal injury Lab Study Manual Human Research Protection Office (HRPO) Log Number A-15774.0 HRPO Proposal Number 09155001 Clinical
PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS
 PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS The benefits of prefilled syringes for palliative care from the hospital pharmacy service In
PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS The benefits of prefilled syringes for palliative care from the hospital pharmacy service In
BASE 24-HOUR URINE COLLECTION LITHOLINK CORE LAB
 CHAPTER 34. BASE 24-HOUR URINE COLLECTION LITHOLINK CORE LAB 34.1 Background Participants who are enrolled in the CKD BASE Study will collect urine over a 24-hour period at B0, W12 and W28. A 50 ml tube
CHAPTER 34. BASE 24-HOUR URINE COLLECTION LITHOLINK CORE LAB 34.1 Background Participants who are enrolled in the CKD BASE Study will collect urine over a 24-hour period at B0, W12 and W28. A 50 ml tube
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration
 Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
Ascribe Rx Online Resource Center User Guide
 Ascribe Rx Online Resource Center User Guide Contents Introduction... 1... 1 Wildcard functionality... 2 Canister Reports... 3 Canister aging... 3 Canister issues... 3 Current inventory... 4 Dispense Reports...
Ascribe Rx Online Resource Center User Guide Contents Introduction... 1... 1 Wildcard functionality... 2 Canister Reports... 3 Canister aging... 3 Canister issues... 3 Current inventory... 4 Dispense Reports...
mehealth for ADHD Parent Manual
 mehealth for ADHD adhd.mehealthom.com mehealth for ADHD Parent Manual al Version 1.0 Revised 11/05/2008 mehealth for ADHD is a team-oriented approach where parents and teachers assist healthcare providers
mehealth for ADHD adhd.mehealthom.com mehealth for ADHD Parent Manual al Version 1.0 Revised 11/05/2008 mehealth for ADHD is a team-oriented approach where parents and teachers assist healthcare providers
Template Standard Operating Procedure For: Handling of Midazolam and other controlled drugs in Dental Practices
 Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
VFC Inventory Reporting through GRITS Changes. Effective Friday, September 7, 2012
 VFC Inventory Reporting through GRITS Changes Effective Friday, September 7, 2012 Summary of Changes Monthly Comprehensive Report and Vaccine Accountability Statement are available in one click. Both reports
VFC Inventory Reporting through GRITS Changes Effective Friday, September 7, 2012 Summary of Changes Monthly Comprehensive Report and Vaccine Accountability Statement are available in one click. Both reports
Feidhmeannacht na Seirbhíse Sláinte
 Feidhmeannacht na Seirbhíse Sláinte Health Service Executive TITLE FORENAME SURNAME ADDRESS 1 ADDRESS 2 ADDRESS 3 COUNTY Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíoca Príomhchúraim Bealach amach
Feidhmeannacht na Seirbhíse Sláinte Health Service Executive TITLE FORENAME SURNAME ADDRESS 1 ADDRESS 2 ADDRESS 3 COUNTY Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíoca Príomhchúraim Bealach amach
November 9, Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085
 November 9, 2017 Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085 A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1
November 9, 2017 Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085 A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1
Texas Vendor Drug Program Specialty Drug List Process. February 2019
 Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document)
 INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
My Fitness Pal Health & Fitness Tracker A User s Guide
 My Fitness Pal Health & Fitness Tracker A User s Guide By: Angela McCall Introduction My Fitness Pal is an online diet, health, and fitness tracker that allows you to track your nutrition and fitness goals
My Fitness Pal Health & Fitness Tracker A User s Guide By: Angela McCall Introduction My Fitness Pal is an online diet, health, and fitness tracker that allows you to track your nutrition and fitness goals
Asthma Please complete packet and return to nurse at child s school
 Health forms for students with Asthma Please complete packet and return to nurse at child s school What s in this packet? 1) Asthma Questionnaire to describe student s asthma 2) Release of Information
Health forms for students with Asthma Please complete packet and return to nurse at child s school What s in this packet? 1) Asthma Questionnaire to describe student s asthma 2) Release of Information
CYCLE Vanguard: Flow Sheet for Protocol Schedule of Forms + Procedures. Binder Title and Cover Pages. Required Materials Study Schema RC flowsheet
 Binder Title and Cover Pages Required Materials Study Schema flowsheet 1 1. Screening a. Screen patient for eligibility. ASAP after ICU admission (within 4 days of mechanical ventilation and 7 days of
Binder Title and Cover Pages Required Materials Study Schema flowsheet 1 1. Screening a. Screen patient for eligibility. ASAP after ICU admission (within 4 days of mechanical ventilation and 7 days of
Getting Started.
 Getting Started www.scientificbraintrainingpro.com Summary 1. First steps... 2 2. Log in... 2 3. Create an account for a patient... 3 4. Access an exercise with this patient... 4 5. Viewing the results
Getting Started www.scientificbraintrainingpro.com Summary 1. First steps... 2 2. Log in... 2 3. Create an account for a patient... 3 4. Access an exercise with this patient... 4 5. Viewing the results
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Introduction. Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients)
 Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
EasyComp. The TPN Compatibility Software. Clinical Nutrition
 EasyComp The TPN Compatibility Software Clinical Nutrition Content 1. General Information 5 1.1 What is EasyComp? 4 1.2 Features and functions of EasyComp 5 1.3 IT requirements 5 t2. Starting EasyComp
EasyComp The TPN Compatibility Software Clinical Nutrition Content 1. General Information 5 1.1 What is EasyComp? 4 1.2 Features and functions of EasyComp 5 1.3 IT requirements 5 t2. Starting EasyComp
Guidelines for ordering the My Care Kit
 Guidelines for ordering the My Care Kit Breast Cancer Network Australia is delighted to work in partnership with Berlei, a Pacific Brands company, to provide this important service to women in Australia
Guidelines for ordering the My Care Kit Breast Cancer Network Australia is delighted to work in partnership with Berlei, a Pacific Brands company, to provide this important service to women in Australia
Reporting Nonviable MDH Vaccine to MIIC
 Reporting Nonviable MDH Vaccine to MIIC This guide describes how to use the Minnesota Immunization Information Connection (MIIC) to report nonviable vaccine for the Minnesota Vaccines for Children (MnVFC)
Reporting Nonviable MDH Vaccine to MIIC This guide describes how to use the Minnesota Immunization Information Connection (MIIC) to report nonviable vaccine for the Minnesota Vaccines for Children (MnVFC)
Intravenous Immunoglobulin (IVIg) prescribing guidance
 Intravenous Immunoglobulin (IVIg) prescribing guidance Kejal Mehta (Specialist Pharmacist) [December 2016] Review Date: December 2017 Contents Introduction:... 2 Prior to Intravenous immunoglobulin (IVIg)
Intravenous Immunoglobulin (IVIg) prescribing guidance Kejal Mehta (Specialist Pharmacist) [December 2016] Review Date: December 2017 Contents Introduction:... 2 Prior to Intravenous immunoglobulin (IVIg)
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN
 Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
*Note - If associate is unsuccessful in their attempt to complete the dosage calculation test
 Competency Statement: Demonstrates ability to use basic mathematic concepts related to the correct administration of medications. Instructional Strategies: Review of basic math concepts, completion of
Competency Statement: Demonstrates ability to use basic mathematic concepts related to the correct administration of medications. Instructional Strategies: Review of basic math concepts, completion of
Glossary 3189 Report - Discontinued 2007 see Supplemental Vaccine Certification Form
 Glossary 3189 Report - Discontinued 2007 see Supplemental Vaccine Certification Form 3231 Report see Certificate of Immunization ACIP Advisory Committee on Immunization Practices. Along with the Centers
Glossary 3189 Report - Discontinued 2007 see Supplemental Vaccine Certification Form 3231 Report see Certificate of Immunization ACIP Advisory Committee on Immunization Practices. Along with the Centers
Nonparenteral medications
 Nonparenteral medications Capsules and unscored tablets are rounded to the nearest whole tablet. Scored tablets are rounded to the nearest 1/2 tablet. Liquid medications are rounded to one decimal place
Nonparenteral medications Capsules and unscored tablets are rounded to the nearest whole tablet. Scored tablets are rounded to the nearest 1/2 tablet. Liquid medications are rounded to one decimal place
Scheduling a Course as a Professional Development Event
 Scheduling a Course as a Professional Development Event Scheduling an approved course as a professional development event is the second step you will need to complete in order to have your trainings reviewed
Scheduling a Course as a Professional Development Event Scheduling an approved course as a professional development event is the second step you will need to complete in order to have your trainings reviewed
Brown-Lupton Health Service Texas Christian University Campus P.O. Box Fort Worth, TX Dear Student,
 Brown-Lupton Health Service Texas Christian University Campus P.O. Box 297400 Fort Worth, TX 76129 817-257-7940 Dear Student, Enclosed you will find our policies, procedures and student consent form for
Brown-Lupton Health Service Texas Christian University Campus P.O. Box 297400 Fort Worth, TX 76129 817-257-7940 Dear Student, Enclosed you will find our policies, procedures and student consent form for
Review calculation procedures for the following:
 Review calculation procedures for the following: Convert in the Metric System 1000 mg = 1 gram 1000 ml = 1 liter To convert from grams to mg, move the decimal point 3 places to the right (add zeros to
Review calculation procedures for the following: Convert in the Metric System 1000 mg = 1 gram 1000 ml = 1 liter To convert from grams to mg, move the decimal point 3 places to the right (add zeros to
Holme Valley Primary School Asthma Policy
 Date adopted: March 2015 Review date: March 2018 Date of next review : March 2021 Holme Valley Primary School Asthma Policy This policy has been written using guidance from the Department of Health (September
Date adopted: March 2015 Review date: March 2018 Date of next review : March 2021 Holme Valley Primary School Asthma Policy This policy has been written using guidance from the Department of Health (September
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
Roanoke College Wellness Program for 2018 MaroonsRWell
 Roanoke College Wellness Program for 2018 MaroonsRWell Welcome to bebetter Health, your partner in wellness! All Roanoke College benefit-eligible employees and spouses covered on the health plan are eligible
Roanoke College Wellness Program for 2018 MaroonsRWell Welcome to bebetter Health, your partner in wellness! All Roanoke College benefit-eligible employees and spouses covered on the health plan are eligible
BEECHFIELD SCHOOL. Asthma Policy
 BEECHFIELD SCHOOL Asthma Policy Author: James Roach Date: April 2017 Approved by: Governing Body Date: June 2017 To be reviewed by: Governing Body Date: June 2019 Beechfield School recognises that asthma
BEECHFIELD SCHOOL Asthma Policy Author: James Roach Date: April 2017 Approved by: Governing Body Date: June 2017 To be reviewed by: Governing Body Date: June 2019 Beechfield School recognises that asthma
C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S
 2017-2 0 1 8 C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine
2017-2 0 1 8 C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine
Student Orientation 2010, Centricity Enterprise EMR Training, and netlearning.parkview.com Instructions for Nursing Students
 Updated 11/29/2011 Welcome to Parkview Health. We are glad you are coming to learn with us, and we thank you in advance for your participation in providing the best care for the patients in our region.
Updated 11/29/2011 Welcome to Parkview Health. We are glad you are coming to learn with us, and we thank you in advance for your participation in providing the best care for the patients in our region.
P R O V I D E R T R A I N I N G P R E S E N T E D B Y
 Understanding and Utilizing Your Portal P R O V I D E R T R A I N I N G P R E S E N T E D B Y Discussion Topics HCA Website Accessing the Portal Requesting Interpreters Cancelling and/or Updating Appointments
Understanding and Utilizing Your Portal P R O V I D E R T R A I N I N G P R E S E N T E D B Y Discussion Topics HCA Website Accessing the Portal Requesting Interpreters Cancelling and/or Updating Appointments
SATURDAY, APRIL 14, AM MALL OF AMERICA FRASER.ORG/WALK
 Toolkit SATURDAY, APRIL 14, 2018 7AM Dear Friend of Fraser, Thank you for your interest in supporting Fraser through the 2018 Fraser Walk for Autism. Fraser serves children and adults with more than 60
Toolkit SATURDAY, APRIL 14, 2018 7AM Dear Friend of Fraser, Thank you for your interest in supporting Fraser through the 2018 Fraser Walk for Autism. Fraser serves children and adults with more than 60
STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION
 2014-2015 STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION Nancy Knickerbocker Quality Assurance Specialist County of San Diego Immunization Program 1 DOCUMENTING UNACCOUNTED STATE INFLUENZA
2014-2015 STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION Nancy Knickerbocker Quality Assurance Specialist County of San Diego Immunization Program 1 DOCUMENTING UNACCOUNTED STATE INFLUENZA
AMERICAN CANCER SOCIETY FUNDRAISING APP FAQS
 AMERICAN CANCER SOCIETY FUNDRAISING APP FAQS We're here to answer any questions you might have about the American Cancer Society Fundraising App. Below are answers to some of the most frequently asked
AMERICAN CANCER SOCIETY FUNDRAISING APP FAQS We're here to answer any questions you might have about the American Cancer Society Fundraising App. Below are answers to some of the most frequently asked
NICU Nutrition Pathway
 NICU Nutrition Pathway Safely Infusing NICU TPN Starter and Custom TPN April17 th 2018 Pharmacists: Paul Kasprzak RPH BCPS Kelly Kopec PharmD Major Practice Changes in the Preparation and Administration
NICU Nutrition Pathway Safely Infusing NICU TPN Starter and Custom TPN April17 th 2018 Pharmacists: Paul Kasprzak RPH BCPS Kelly Kopec PharmD Major Practice Changes in the Preparation and Administration
Version: Date. Version 2: 02-September Feb-2016
 A RandomizEd Trial of ENtERal Glutamine to minimize Thermal Injury Pharmacy Manual Intended Audience: Pharmacists, Pharmacy Technicians This study is registered at Clinicaltrials.gov. Identification number
A RandomizEd Trial of ENtERal Glutamine to minimize Thermal Injury Pharmacy Manual Intended Audience: Pharmacists, Pharmacy Technicians This study is registered at Clinicaltrials.gov. Identification number
Hazardous Materials Medication Box and Exchange Procedure
 September 2012 Page 1 of 6 Hazardous Materials Medication Box and Exchange Procedure EMS Service Stock 1. Each EMS Agency will be responsible for the security and storage of the supply of hazardous material
September 2012 Page 1 of 6 Hazardous Materials Medication Box and Exchange Procedure EMS Service Stock 1. Each EMS Agency will be responsible for the security and storage of the supply of hazardous material
Hazardous Materials Medication Box and Exchange Procedure
 May 2017 Page 1 of 5 Hazardous Materials Medication Box and Exchange Procedure EMS Service Stock 1. Each EMS Agency will be responsible for the security and storage of the supply of hazardous material
May 2017 Page 1 of 5 Hazardous Materials Medication Box and Exchange Procedure EMS Service Stock 1. Each EMS Agency will be responsible for the security and storage of the supply of hazardous material
Tool kit SATURDAY, APRIL 13, AM MALL OF AMERICA FRASER.ORG/WALK SATURDAY, APRIL 13, AM MALL OF AMERICA FRASER.ORG/WALK
 Tool kit 1 Dear Friend of Fraser, Thank you for your interest in supporting Fraser through the 2019 Fraser Walk for Autism. Fraser is Minnesota s largest and most experienced provider of autism services.
Tool kit 1 Dear Friend of Fraser, Thank you for your interest in supporting Fraser through the 2019 Fraser Walk for Autism. Fraser is Minnesota s largest and most experienced provider of autism services.
MedStar Digital Task Management System User Guide for Stakeholders
 MedStar Digital Task Management System User Guide for Stakeholders Table of Contents Introduction to Task Management System Page 2 Why Task Management? Stakeholder Benefits Accessing the MedStar Digital
MedStar Digital Task Management System User Guide for Stakeholders Table of Contents Introduction to Task Management System Page 2 Why Task Management? Stakeholder Benefits Accessing the MedStar Digital
3.0 POSITION ON PRE-LOADING SYRINGES WITH VACCINE
 1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
Tech Lectures For the Pharmacy Technician
 1 Tech Lectures For the Pharmacy Technician P.O. Box 19357 Denver, CO 80219-0357 303-984-9877 Lecture 14 - Hospital Calculations It is respectfully requested by the Author that no part of this Tech Lecture
1 Tech Lectures For the Pharmacy Technician P.O. Box 19357 Denver, CO 80219-0357 303-984-9877 Lecture 14 - Hospital Calculations It is respectfully requested by the Author that no part of this Tech Lecture
Department of Pathology and Laboratory Medicine
 Department of Pathology and Laboratory Medicine Nova Scotia Health Authority Central Zone TITLE: QE BT BloodTrack Enquiry and HemoSafe Doc #: 24764 Section: Management System\PLM\Blood Transfusion Services\HemoSafe
Department of Pathology and Laboratory Medicine Nova Scotia Health Authority Central Zone TITLE: QE BT BloodTrack Enquiry and HemoSafe Doc #: 24764 Section: Management System\PLM\Blood Transfusion Services\HemoSafe
New regulatory requirements DPCS Regulations 2017
 New regulatory requirements DPCS Regulations 2017 Prescribers and pharmacists May 2017 Table of Contents Explanatory notes... 1 Clarifying the meaning of key terms... 1 Chart instructions and prescriptions...
New regulatory requirements DPCS Regulations 2017 Prescribers and pharmacists May 2017 Table of Contents Explanatory notes... 1 Clarifying the meaning of key terms... 1 Chart instructions and prescriptions...
Medical information Cook Children s
 Medical information Health care providers Primary care provider (PCP): City: State: ZIP code: Phone: Fax: Email: Preferred hospital: Phone: Fax: Email: Specialty hospital: Phone: Fax: Email: Lab: Phone:
Medical information Health care providers Primary care provider (PCP): City: State: ZIP code: Phone: Fax: Email: Preferred hospital: Phone: Fax: Email: Specialty hospital: Phone: Fax: Email: Lab: Phone:
RESULTS REPORTING MANUAL. Hospital Births Newborn Screening Program June 2016
 RESULTS REPORTING MANUAL Hospital Births Newborn Screening Program June 2016 CONTENTS GETTING STARTED... 1 Summary... 1 Logging In... 1 Access For New Hires... 2 Reporting Parental Refusals... 3 Adding
RESULTS REPORTING MANUAL Hospital Births Newborn Screening Program June 2016 CONTENTS GETTING STARTED... 1 Summary... 1 Logging In... 1 Access For New Hires... 2 Reporting Parental Refusals... 3 Adding
EHS Integrator Controlled Substance Waste Pickup Request Help Guide
 EHS Integrator Controlled Substance Waste Pickup Request Help Guide Use the EHS Integrator Controlled Substance Waste Pickup Form to request a Controlled Substance/Drug waste pickup. To request a controlled
EHS Integrator Controlled Substance Waste Pickup Request Help Guide Use the EHS Integrator Controlled Substance Waste Pickup Form to request a Controlled Substance/Drug waste pickup. To request a controlled
User Instruction Guide
 User Instruction Guide Table of Contents Logging In and Logging Out of MMSx 1 Creating a TPN (Terminal Profile Number) 2 Single Merchant 2 From Navigation Bar 2 From Home Page Link 4 Multiple Merchants
User Instruction Guide Table of Contents Logging In and Logging Out of MMSx 1 Creating a TPN (Terminal Profile Number) 2 Single Merchant 2 From Navigation Bar 2 From Home Page Link 4 Multiple Merchants
OneTouch Reveal Web Application. User Manual for Healthcare Professionals Instructions for Use
 OneTouch Reveal Web Application User Manual for Healthcare Professionals Instructions for Use Contents 2 Contents Chapter 1: Introduction...4 Product Overview...4 Intended Use...4 System Requirements...
OneTouch Reveal Web Application User Manual for Healthcare Professionals Instructions for Use Contents 2 Contents Chapter 1: Introduction...4 Product Overview...4 Intended Use...4 System Requirements...
ProScript User Guide. Pharmacy Access Medicines Manager
 User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
PYXIS MEDSTATION EXPANSION - IMPLEMENTATION SCHEDULE
 PHASE 1 - Planning for MedStation Conversion Review Narcotic/Cerner/AMS usage and determine mins and maxs for all areas (drawer configurations) 11/01/07 Map medstations (assign meds to drawers and pockets
PHASE 1 - Planning for MedStation Conversion Review Narcotic/Cerner/AMS usage and determine mins and maxs for all areas (drawer configurations) 11/01/07 Map medstations (assign meds to drawers and pockets
Immunization Toolkit for Immunization Providers
 Immunization Toolkit for Immunization Providers Communicable Disease Prevention and Control Division Public Health Branch Department of Health and Wellness 09/09/2014 1 Contents Introduction... 3 General
Immunization Toolkit for Immunization Providers Communicable Disease Prevention and Control Division Public Health Branch Department of Health and Wellness 09/09/2014 1 Contents Introduction... 3 General
Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface
 Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface Organization Name: University of Maryland Type: Acute Care Hospital Medical Center Contact Person: Belinda Todjo, PharmD
Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface Organization Name: University of Maryland Type: Acute Care Hospital Medical Center Contact Person: Belinda Todjo, PharmD
When/How to Update a ClinicalTrials.gov Record
 When/How to Update a ClinicalTrials.gov Record When to Update a ClinicalTrials.gov Record? 1) Within 30 days of a change in: Locations Recruitment Status at any participating site (Opened, Suspended, Closed)
When/How to Update a ClinicalTrials.gov Record When to Update a ClinicalTrials.gov Record? 1) Within 30 days of a change in: Locations Recruitment Status at any participating site (Opened, Suspended, Closed)
Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating Service PROCEDURE
 UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
Southern Trust Home IV Service. Guidelines for the administration of IV antibiotics
 Southern Trust Home IV Service Guidelines for the administration of IV antibiotics Title: Author: CLINICAL GUIDELINES ID TAG Antibiotic guidelines - Southern Trust Home IV service guidelines for the administration
Southern Trust Home IV Service Guidelines for the administration of IV antibiotics Title: Author: CLINICAL GUIDELINES ID TAG Antibiotic guidelines - Southern Trust Home IV service guidelines for the administration
Administration of Medication
 Administration of Medication Prescribed medications must arrive in a container with the original, unaltered prescription label attached. The label must display all legal information required for a pharmacist
Administration of Medication Prescribed medications must arrive in a container with the original, unaltered prescription label attached. The label must display all legal information required for a pharmacist
MEAT CONTENT CALCULATION
 MEAT CONTENT CALCULATION Introduction Amendments to the European Labelling Directive have resulted in the need to harmonise the definition of meat across Europe. This new definition attempts to ensure
MEAT CONTENT CALCULATION Introduction Amendments to the European Labelling Directive have resulted in the need to harmonise the definition of meat across Europe. This new definition attempts to ensure
Newfoundland and Labrador Pharmacy Board Standards of Practice
 Newfoundland and Labrador Pharmacy Board Standards of Practice Standards for the Safe and Effective Administration of Drug Therapy by Inhalation or Injection June 2015 Table of Contents 1) Introduction...
Newfoundland and Labrador Pharmacy Board Standards of Practice Standards for the Safe and Effective Administration of Drug Therapy by Inhalation or Injection June 2015 Table of Contents 1) Introduction...
TRANSFUSION OF BLOOD COMPONENTS ADMINISTRATION. All blood components are administered according to BOP DHB Policy and NZBS Guidelines.
 STANDARDS All blood components are administered according to BOP DHB Policy and NZBS Guidelines. EQUIPMENT IV administration set with 260 micron filter either integrated blood filter; or add on blood filter
STANDARDS All blood components are administered according to BOP DHB Policy and NZBS Guidelines. EQUIPMENT IV administration set with 260 micron filter either integrated blood filter; or add on blood filter
Initiation of Clozapine Treatment Community Patients
 Initiation of Clozapine Treatment Community Patients Who Should Read This Policy Target Audience All clinical staff working in the community N/A N/A Initiation of Clozapine Treatment for Patients in the
Initiation of Clozapine Treatment Community Patients Who Should Read This Policy Target Audience All clinical staff working in the community N/A N/A Initiation of Clozapine Treatment for Patients in the
Guidance on Bulk Prescribing for Care Home Patients
 Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
Request for Diabetic Information
 Wylie ISD building our future Dear Parent, Request for Diabetic Information Our records indicate that your child has diabetes that may require treatment at school or a school related event. Attached to
Wylie ISD building our future Dear Parent, Request for Diabetic Information Our records indicate that your child has diabetes that may require treatment at school or a school related event. Attached to
A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients
 original article A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients Daren Heyland, M.D., John Muscedere, M.D., Paul E. Wischmeyer, M.D., Deborah Cook, M.D., Gwynne Jones, M.D.,
original article A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients Daren Heyland, M.D., John Muscedere, M.D., Paul E. Wischmeyer, M.D., Deborah Cook, M.D., Gwynne Jones, M.D.,
Pa. Immunization Education Program
 Pa. Immunization Education Program 2016 Maintaining the Cold Chain The cold chain is a temperature-controlled environment used to maintain and distribute vaccine in optimal condition. Provider responsibility
Pa. Immunization Education Program 2016 Maintaining the Cold Chain The cold chain is a temperature-controlled environment used to maintain and distribute vaccine in optimal condition. Provider responsibility
Therapy Activation Date: Review Date:
 MEDICAL DIRECTIVE Title: Nicotine Replacement Number: TCFHT-MD10 Therapy Activation Date: 14-06-2011 Review Date: 01-05-2016 Sponsoring/Contact Person(s) (name, position, contact particulars): Sherry Kennedy,
MEDICAL DIRECTIVE Title: Nicotine Replacement Number: TCFHT-MD10 Therapy Activation Date: 14-06-2011 Review Date: 01-05-2016 Sponsoring/Contact Person(s) (name, position, contact particulars): Sherry Kennedy,
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Homely Remedies Guidance for Care Homes
 Homely Remedies Guidance for Care Homes What is a homely remedy? Homely or household remedy is another name for a non-prescription medicine which is used in a care home for the short term management of
Homely Remedies Guidance for Care Homes What is a homely remedy? Homely or household remedy is another name for a non-prescription medicine which is used in a care home for the short term management of
INTRODUCTION: Welcome to the Saturday 10 AM Paths to Recovery Traditions Meeting, a one hour meeting, which uses the Paths to Recovery book.
 Saturday 10am - Technical Information For the Secretary/Chair to read before leading a phone bridge meeting (Click below for full version technical information)..\technical.htm The Meeting Format Starts
Saturday 10am - Technical Information For the Secretary/Chair to read before leading a phone bridge meeting (Click below for full version technical information)..\technical.htm The Meeting Format Starts
Feeding Protocols Enteral or Parenteral. AM Poleÿ 2012
 Practical aspects on Feeding Protocols Enteral or Parenteral AM Poleÿ 2012 Enteral Feeding Facts A reduction in mortality Prophylaxis for stress ulcers Full-strength Time to start enteral nutrition If
Practical aspects on Feeding Protocols Enteral or Parenteral AM Poleÿ 2012 Enteral Feeding Facts A reduction in mortality Prophylaxis for stress ulcers Full-strength Time to start enteral nutrition If
HOLY FAMILY CATHOLIC SCHOOL MEDICATION AND ASTHMA POLICY
 HOLY FAMILY CATHOLIC SCHOOL MEDICATION AND ASTHMA POLICY Policy Statement - We would ask parents to request that their doctor, wherever possible, prescribe medication, which can be taken outside the school
HOLY FAMILY CATHOLIC SCHOOL MEDICATION AND ASTHMA POLICY Policy Statement - We would ask parents to request that their doctor, wherever possible, prescribe medication, which can be taken outside the school
CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS
 CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS This chapter presents the examination schedule for AREDS participants during Phase II, highlighting the important
CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS This chapter presents the examination schedule for AREDS participants during Phase II, highlighting the important
University of Alaska Connected! FAQs
 University of Alaska Connected! FAQs 1. What is Connected? Connected! allows employees and spouses/fips to connect a fitness device or app to Healthyroads.com. This will allow additional tracking options
University of Alaska Connected! FAQs 1. What is Connected? Connected! allows employees and spouses/fips to connect a fitness device or app to Healthyroads.com. This will allow additional tracking options
2010 March of Dimes Foundation
 - 2010 March of Dimes Foundation Welcome to March for Babies 2011! We re so glad you decided to join us as we walk together for stronger, healthier babies. March for Babies is America s favorite walking
- 2010 March of Dimes Foundation Welcome to March for Babies 2011! We re so glad you decided to join us as we walk together for stronger, healthier babies. March for Babies is America s favorite walking
Feidhmeannacht na Seirbhíse Sláinte
 Feidhmeannacht na Seirbhíse Sláinte Health Service Executive Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíocaíochta Cúraim Phríomhúil Plás J5 Lárionad Gnó na Páirce Thuaidh Bealach Amach 5, M50 An
Feidhmeannacht na Seirbhíse Sláinte Health Service Executive Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíocaíochta Cúraim Phríomhúil Plás J5 Lárionad Gnó na Páirce Thuaidh Bealach Amach 5, M50 An
Parenteral Nutrition Recommendations for Pediatric Patients
 Fluid Dextrose Amino acid Lipid Parenteral Nutrition Recommendations for Pediatric Patients (Calculated for normal organ function and normal caloric requirements) PN orders are due by 11 AM daily Newborn
Fluid Dextrose Amino acid Lipid Parenteral Nutrition Recommendations for Pediatric Patients (Calculated for normal organ function and normal caloric requirements) PN orders are due by 11 AM daily Newborn
PN Compounding Practice: The Kuwaiti Experience. Ahmed Abo-Bakr Mahmoud, R.Ph,M.Sc.,BCNSP
 PN Compounding Practice: The Kuwaiti Experience Ahmed Abo-Bakr Mahmoud, R.Ph,M.Sc.,BCNSP Disclosure Information PN Compounding practice: The Kuwaiti Experience Ahmed Abo-Bakr Mahmoud I have no financial
PN Compounding Practice: The Kuwaiti Experience Ahmed Abo-Bakr Mahmoud, R.Ph,M.Sc.,BCNSP Disclosure Information PN Compounding practice: The Kuwaiti Experience Ahmed Abo-Bakr Mahmoud I have no financial
