Chapter 8.4, 8.5. Enzymes. AP Biology
|
|
|
- Hester Robbins
- 6 years ago
- Views:
Transcription
1 Chapter 8.4, 8.5 Enzymes
2 Activation energy Breaking down large molecules requires an initial input of energy activation energy large biomolecules are stable must absorb energy to break bonds cellulose energy CO 2 + H 2 O + heat
3 Too much activation energy for life Activation energy amount of energy needed to destabilize the bonds of a molecule moves the reaction over an energy hill glucose Not a match! That s too much energy to expose living cells to!
4 Reducing Activation energy Catalysts uncatalyzed reaction Pheeew that takes a lot less energy! catalyzed reaction NEW activation energy reactant product
5 Catalysts Reduce the amount of energy to start a reaction Cells use ENZYMES to reduce activation energy Call in the ENZYMES! ΔG
6 Enzymes Biological catalysts proteins (& RNA) facilitate chemical reactions increase rate of reaction without being consumed reduce activation energy don t change free energy (ΔG) released or required required for most biological reactions highly specific thousands of different enzymes in cells control reactions of life
7 Enzyme Vocabulary substrate reactant which binds to enzyme enzyme-substrate complex: temporary association product end result of reaction active site enzyme s catalytic site; substrate fits into active site substrate active site products enzyme transition state
8 Properties of Enzymes Reaction specific each enzyme works with a specific substrate chemical fit between active site & substrate forming H bonds, ionic bonds Not consumed in reaction single enzyme molecule can catalyze thousands or more reactions per second enzymes unaffected by the reaction (reusable) Affected by cellular conditions any condition that affects protein structure temperature, ph, salinity
9 Naming Conventions Enzymes named for reaction they catalyze sucrase breaks down sucrose proteases break down proteins lipases break down lipids DNA polymerase builds DNA adds nucleotides to DNA strand pepsin breaks down proteins (polypeptides)
10 Lock and Key model Simplistic model of enzyme action substrate fits into 3-D structure of enzyme active site H bonds between substrate & enzyme like key fits into lock In biology Size doesn t matter Shape matters!
11 Induced Fit model More accurate model of enzyme action 3-D structure of enzyme fits substrate substrate binding cause enzyme to change shape leading to a tighter fit conformational change bring chemical groups in position to catalyze reaction
12 How do enzymes lower E a? Variety of mechanisms to lower activation energy & speed up reaction 1) In synthesis, active site orients substrates in correct position for reaction enzyme brings substrate closer together 2) In digestion, active site puts stress on bonds that must be broken, making it easier to separate molecules 3) Providing a favorable microenvironment (ex: ph)
13 Got any Questions?!
14 Factors that Affect Enzymes
15 Factors Affecting Enzyme Function Enzyme concentration Substrate concentration Temperature ph Salinity Activators Inhibitors catalase
16 Enzyme concentration What s happening here?! reaction rate enzyme concentration
17 Factors affecting enzyme function Enzyme concentration as enzyme = reaction rate more enzymes = more frequently collide with substrate reaction rate levels off substrate becomes limiting factor not all enzyme molecules can find substrate reaction rate enzyme concentration
18 Substrate concentration What s happening here?! reaction rate substrate concentration
19 Factors affecting enzyme function Substrate concentration as substrate = reaction rate more substrate = more frequently collide with enzyme reaction rate levels off all enzymes have active site engaged enzyme is saturated maximum rate of reaction reaction rate substrate concentration
20 Temperature What s happening here?! reaction rate 37 temperature
21 Factors affecting enzyme function Temperature Optimum T greatest number of molecular collisions human enzymes = C (body temp = 37 C) Heat: increase beyond optimum T increased energy level of molecules disrupts bonds in enzyme & between enzyme & substrate H, ionic = weak bonds denaturation = protein loses 3D shape (3 structure) Cold: decrease below optimum T molecules move slower decreased collisions between enzyme & substrate
22 Enzymes and temperature Different enzymes function in different organisms in different environments human enzyme hot spring bacteria enzyme reaction rate 37 C temperature 70 C (158 F)
23 How do ectotherms do it?
24 ph What s happening here?! pepsin trypsin reaction rate trypsin pepsin ph
25 Factors affecting enzyme function ph changes in ph adds or remove H + disrupts bonds, disrupts 3D shape disrupts attractions between charged amino acids affects 2 & 3 structure denatures protein optimal ph? most human enzymes = ph 6-8 depends on localized conditions pepsin (stomach) = ph 2-3 trypsin (small intestines) = ph
26 Salinity What s happening here?! reaction rate salt concentration
27 Factors affecting enzyme function Salt concentration changes in salinity adds or removes cations (+) & anions ( ) disrupts bonds, disrupts 3D shape disrupts attractions between charged amino acids affect 2 & 3 structure denatures protein enzymes intolerant of extreme salinity Dead Sea is called dead for a reason!
28 Compounds which help enzymes Activators cofactors non-protein, small inorganic compounds & ions Mg, K, Ca, Zn, Fe, Cu bound within enzyme molecule coenzymes non-protein, organic molecules bind temporarily or permanently to enzyme near active site many vitamins NAD (niacin; B3) FAD (riboflavin; B2) Coenzyme A Fe in hemoglobin Mg in chlorophyll
29 Compounds which regulate enzymes Inhibitors molecules that reduce enzyme activity competitive inhibition noncompetitive inhibition (both could be irreversible) feedback inhibition
30 Competitive Inhibitor Inhibitor & substrate compete for active site penicillin blocks enzyme bacteria use to build cell walls disulfiram (Antabuse) treats chronic alcoholism blocks enzyme that breaks down alcohol severe hangover & vomiting 5-10 minutes after drinking Overcome by increasing substrate concentration saturate solution with substrate so it out-competes inhibitor for active site on enzyme
31 Non-Competitive Inhibitor Inhibitor binds to site other than active site allosteric inhibitor binds to allosteric site causes enzyme to change shape conformational change active site is no longer functional binding site keeps enzyme inactive some anti-cancer drugs inhibit enzymes involved in DNA synthesis stop DNA production stop division of more cancer cells cyanide poisoning irreversible inhibitor of Cytochrome C, an enzyme in cellular respiration stops production of ATP
32 Irreversible Inhibition Inhibitor (either competitive or noncompetitive) permanently binds to enzyme competitor permanently binds to active site allosteric (non-competitor) permanently binds to allosteric site permanently changes shape of enzyme nerve gas, sarin, many insecticides (malathion, parathion ) cholinesterase inhibitors - doesn t breakdown the neurotransmitter, acetylcholine
33 Allosteric regulation Conformational changes by regulatory molecules inhibitors keeps enzyme in inactive form activators keeps enzyme in active form Conformational changes Allosteric regulation
34 Metabolic pathways A B C D E F G enzyme 1 enzyme 2 enzyme enzyme enzyme 3 Chemical reactions of life are organized in pathways divide chemical reaction into many small steps artifact of evolution efficiency intermediate branching points control = regulation 4 enzyme 5 enzyme 6
35 Efficiency Organized groups of enzymes enzymes are embedded in membrane and arranged sequentially Link endergonic & exergonic reactions Whoa! All that going on in those little mitochondria!
36 Feedback Inhibition Regulation & coordination of production product is used by next step in pathway final product is inhibitor of earlier step allosteric inhibitor of earlier enzyme feedback inhibition no unnecessary accumulation of product A B C D E F G X enzyme 1 enzyme 2 enzyme 3 enzyme 4 enzyme 5 enzyme 6 allosteric inhibitor of enzyme 1
37 Feedback inhibition Example synthesis of amino acid, isoleucine from amino acid, threonine isoleucine becomes the allosteric inhibitor of the first step in the pathway as product accumulates it collides with enzyme more often than substrate does threonine isoleucine
38 Don t be inhibited! Ask Questions!
39 Cooperativity Substrate acts as an activator substrate causes conformational change in enzyme induced fit favors binding of substrate at 2 nd site makes enzyme more active & effective hemoglobin Hemoglobin 4 polypeptide chains can bind 4 O 2 ; 1 st O 2 binds now easier for other 3 O 2 to bind
AP Biology. Metabolism & Enzymes
 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
GRU3L1 Metabolism & Enzymes. AP Biology
 GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
Metabolism & Enzymes. From food webs to the life of a cell. Flow of energy through life. Life is built on chemical reactions
 Metabolism & Enzymes 2007-2008 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic
Metabolism & Enzymes 2007-2008 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic
Enzymes. Ms. Paxson. From food webs to the life of a cell. Enzymes. Metabolism. Flow of energy through life. Examples. Examples
 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions sun transforming energy from one form to another solar energy ATP & organic molecules
From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions sun transforming energy from one form to another solar energy ATP & organic molecules
increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
 Enzymes Enzymes Biological catalysts proteins (& RNA) facilitate chemical reactions increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
Enzymes Enzymes Biological catalysts proteins (& RNA) facilitate chemical reactions increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
Chapter 6. Metabolism & Enzymes. AP Biology
 Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Examples. Chapter 8. Metabolism & Enzymes. Flow of energy through life. Examples. Chemical reactions of life. Chemical reactions & energy
 WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
Chapter 6. Flow of energy through life. Chemical reactions of life. Examples. Examples. Chemical reactions & energy 9/7/2012. Enzymes & Metabolism
 Flow of energy through life Chapter 6 Life is built on chemical reactions Enzymes & Metabolism Chemical reactions of life Examples Metabolism Forming bonds between molecules Dehydration synthesis Anabolic
Flow of energy through life Chapter 6 Life is built on chemical reactions Enzymes & Metabolism Chemical reactions of life Examples Metabolism Forming bonds between molecules Dehydration synthesis Anabolic
Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions
 Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does three main kinds of work: Chemical Transport Mechanical To do work, cells manage energy resources
Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does three main kinds of work: Chemical Transport Mechanical To do work, cells manage energy resources
Unit 7 Part I: Introductions to Biochemistry
 Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19 March 2014 Averett 1 Reaction Graphs Every chemical reaction involves bond breaking and bond forming. In order for bonds
Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19 March 2014 Averett 1 Reaction Graphs Every chemical reaction involves bond breaking and bond forming. In order for bonds
A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell.
 Enzymes Metabolism Metabolism A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell. Energy is the capacity to do work. Metabolism
Enzymes Metabolism Metabolism A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell. Energy is the capacity to do work. Metabolism
Review of Energetics Intro
 Review of Energetics Intro Learning Check The First Law of Thermodynamics states that energy can be Created Destroyed Converted All of the above Learning Check The second law of thermodynamics essentially
Review of Energetics Intro Learning Check The First Law of Thermodynamics states that energy can be Created Destroyed Converted All of the above Learning Check The second law of thermodynamics essentially
Chapter 5 Metabolism: Energy and Enzymes
 Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
Part 1: Energy and Metabolism
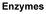 Part 1: Energy and Metabolism Life is highly organized rganisms need free energy to survive, grow, and reproduce In each system, the arrow is pointing in the direction of spontaneous change. Why? 1 2 More
Part 1: Energy and Metabolism Life is highly organized rganisms need free energy to survive, grow, and reproduce In each system, the arrow is pointing in the direction of spontaneous change. Why? 1 2 More
6.5 Enzymes. Enzyme Active Site and Substrate Specificity
 180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!!
 Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!! Key Words Enzyme Substrate Product Active Site Catalyst Activation Energy Denature Enzyme-Substrate Complex Lock & Key model Induced fit model
Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!! Key Words Enzyme Substrate Product Active Site Catalyst Activation Energy Denature Enzyme-Substrate Complex Lock & Key model Induced fit model
Chapter 5- Enzymes. State Standard Standard 1.b.
 Chapter 5- Enzymes State Standard Standard 1.b. Enzymes Speed Up Chemical Reactions Most of the essential chemical reactions in cells must occur quickly and precisely for the cell to survive For a chemical
Chapter 5- Enzymes State Standard Standard 1.b. Enzymes Speed Up Chemical Reactions Most of the essential chemical reactions in cells must occur quickly and precisely for the cell to survive For a chemical
Slide 1. Slide 2. Slide 3. Chapter 5- Enzymes. State Standard. Enzymes Speed Up Chemical Reactions. Standard 1.b.
 Slide 1 Chapter 5- Enzymes Slide 2 State Standard Standard 1.b. Slide 3 Enzymes Speed Up Chemical Reactions Most of the essential chemical reactions in cells must occur quickly and precisely for the cell
Slide 1 Chapter 5- Enzymes Slide 2 State Standard Standard 1.b. Slide 3 Enzymes Speed Up Chemical Reactions Most of the essential chemical reactions in cells must occur quickly and precisely for the cell
Enzymes. Enzyme Structure. How do enzymes work?
 Page 1 of 6 Enzymes Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
Page 1 of 6 Enzymes Enzymes are biological catalysts. There are about 40,000 different enzymes in human cells, each controlling a different chemical reaction. They increase the rate of reactions by a factor
3/1/2011. Enzymes. Enzymes and Activation Energy. Enzymes Enzyme Structure and Action. Chapter 4 Outline. Enzymes
 Free content 3/1/2011 Chapter 4 Outline Enzymes as catalysts Control of enzyme activity Bioenergetics Enzymes 4-2 4-3 Enzymes Enzymes - function as biological catalysts permit reactions to occur rapidly
Free content 3/1/2011 Chapter 4 Outline Enzymes as catalysts Control of enzyme activity Bioenergetics Enzymes 4-2 4-3 Enzymes Enzymes - function as biological catalysts permit reactions to occur rapidly
Enzymes: Helper Protein molecules
 Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enzymes: The Catalysts of Life
 Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Life and the Flow of Energy. Chapter 6. The Flow of Energy
 Life and the Flow of Energy Chapter 6 Metabolism: Energy and Enzymes Energy is the ability to do work Cells (and organisms) need a constant supply of Life on Earth is dependent on solar Solar The Flow
Life and the Flow of Energy Chapter 6 Metabolism: Energy and Enzymes Energy is the ability to do work Cells (and organisms) need a constant supply of Life on Earth is dependent on solar Solar The Flow
CHAPTER 2- ENZYMES PROTEINS B. AMINO ACID- 10/4/2016
 CHAPTER 2- ENZYMES BIOL. 1 AB KENNEDY PROTEINS A. DEFINITION- LARGE MACROMOLECULES MADE OF CARBON, HYDROGEN, NITROGEN, OXYGEN, AND SULFUR THEIR PRIMARY BUILDING BLOCK IS THE AMINO ACID THEY FUNCTION AS
CHAPTER 2- ENZYMES BIOL. 1 AB KENNEDY PROTEINS A. DEFINITION- LARGE MACROMOLECULES MADE OF CARBON, HYDROGEN, NITROGEN, OXYGEN, AND SULFUR THEIR PRIMARY BUILDING BLOCK IS THE AMINO ACID THEY FUNCTION AS
AP Biology Protein Structure and Enzymes
 AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
Microbial Metabolism (Chapter 5) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus
 Microbial Metabolism (Chapter 5) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology An Introduction
Microbial Metabolism (Chapter 5) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Source for figures and content: Tortora, G.J. Microbiology An Introduction
CHEM121. Unit 6: Enzymes. Lecture 10. At the end of the lecture, students should be able to:
 CHEM121 Unit 6: Enzymes Lecture 10 At the end of the lecture, students should be able to: Define the term enzyme Name and classify enzymes according to the: type of reaction catalyzed type of specificity
CHEM121 Unit 6: Enzymes Lecture 10 At the end of the lecture, students should be able to: Define the term enzyme Name and classify enzymes according to the: type of reaction catalyzed type of specificity
Enzymes Biological Catalysts Review
 Enzymes Biological Catalysts Review Catalyst a substance that speeds up a reaction but is not actually a part of the reaction nor changes because of the reaction Catalysis the process of speeding a chemical
Enzymes Biological Catalysts Review Catalyst a substance that speeds up a reaction but is not actually a part of the reaction nor changes because of the reaction Catalysis the process of speeding a chemical
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Biological Science 101 General Biology
 Lecture Seven: Cellular Respiration Ch. 9, Pgs. 163-181 Figs. 9.2-9.20 Biological Science 101 General Biology Cellular Respiration: - A series of processes that is involved in converting food to energy
Lecture Seven: Cellular Respiration Ch. 9, Pgs. 163-181 Figs. 9.2-9.20 Biological Science 101 General Biology Cellular Respiration: - A series of processes that is involved in converting food to energy
Enzymes. Ch 3: Macromolecules
 Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Notes 2-4. Chemical Reactions and Enzymes
 Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
Terminology-Amino Acids
 Enzymes 1 2 Terminology-Amino Acids Primary Structure: is a polypeptide (large number of aminoacid residues bonded together in a chain) chain of amino acids linked with peptide bonds. Secondary Structure-
Enzymes 1 2 Terminology-Amino Acids Primary Structure: is a polypeptide (large number of aminoacid residues bonded together in a chain) chain of amino acids linked with peptide bonds. Secondary Structure-
Chapter 6. Ground Rules of Metabolism
 Chapter 6 Ground Rules of Metabolism Alcohol dehydrogenase removes ethanol molecules from the liver Binge Drinking by State (Source Centers for Disease Control and Prevention (CDC). Rank States Amount
Chapter 6 Ground Rules of Metabolism Alcohol dehydrogenase removes ethanol molecules from the liver Binge Drinking by State (Source Centers for Disease Control and Prevention (CDC). Rank States Amount
DNA and Protein Synthesis Practice
 Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
BACKGROUND INFORMATION:
 BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
Microbial Metabolism
 PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 5 Microbial Metabolism Microbial Metabolism The sum total of chemical reactions that take place within cells (of an organism) Metabolic
PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 5 Microbial Metabolism Microbial Metabolism The sum total of chemical reactions that take place within cells (of an organism) Metabolic
Digestion and Human Health
 Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
The building blocks of life.
 The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
AP Biology Review: Theme 3- Energy
 AP Biology Review: Theme 3- Energy 3.1: All living systems require constant input of free energy. 3.2: Interactions between molecules affect their structure and function. 3.3: Organisms capture and store
AP Biology Review: Theme 3- Energy 3.1: All living systems require constant input of free energy. 3.2: Interactions between molecules affect their structure and function. 3.3: Organisms capture and store
Amino acids. Side chain. -Carbon atom. Carboxyl group. Amino group
 PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Ch 07. Microbial Metabolism
 Ch 07 Microbial Metabolism SLOs Differentiate between metabolism, catabolism, and anabolism. Fully describe the structure and function of enzymes. Differentiate between constitutive and regulated enzymes.
Ch 07 Microbial Metabolism SLOs Differentiate between metabolism, catabolism, and anabolism. Fully describe the structure and function of enzymes. Differentiate between constitutive and regulated enzymes.
c. Reaction will drive Reaction in a reaction. d. Which statement (A or B) has more energy in products than reactants?
 Energy and Enzymes (32 questions) 1. Chemical reactions involve a. Formation of chemical bonds b. Breakage of chemical bonds c. Both formation and breakage of chemical bonds d. Neither formation and breakage
Energy and Enzymes (32 questions) 1. Chemical reactions involve a. Formation of chemical bonds b. Breakage of chemical bonds c. Both formation and breakage of chemical bonds d. Neither formation and breakage
Section 2.1: Enzymes and Digestion
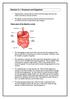 Section 2.1: Enzymes and Digestion Glands produce enzymes that are used to break down large molecules into smaller ones that are ready for abortion. The digestive system provides an interface between the
Section 2.1: Enzymes and Digestion Glands produce enzymes that are used to break down large molecules into smaller ones that are ready for abortion. The digestive system provides an interface between the
Enzymes - Exercise 3 - Germantown
 Enzymes - Exercise 3 - Germantown Objectives -Understand the function of an enzyme. -Know where catechol oxidase (enzyme) used in today s experiment came from. -Understand why enzymes require a cofactor.
Enzymes - Exercise 3 - Germantown Objectives -Understand the function of an enzyme. -Know where catechol oxidase (enzyme) used in today s experiment came from. -Understand why enzymes require a cofactor.
ENZYMES. A protein with catalytic properties due to its power of specific activation Paul Billiet ODWS
 ENZYMES A protein with catalytic properties due to its power of specific activation Chemical reactions Chemical reactions need an initial input of energy = THE ACTIVATION ENERGY During this part of the
ENZYMES A protein with catalytic properties due to its power of specific activation Chemical reactions Chemical reactions need an initial input of energy = THE ACTIVATION ENERGY During this part of the
ENZYMES. A protein with catalytic properties due to its power of specific activation Paul Billiet ODWS
 ENZYMES A protein with catalytic properties due to its power of specific activation HISTORY of Enzymes As early as the late 1700s and early 1800s, the digestion of meat by stomach secretions and the conversion
ENZYMES A protein with catalytic properties due to its power of specific activation HISTORY of Enzymes As early as the late 1700s and early 1800s, the digestion of meat by stomach secretions and the conversion
Microbial Metabolism. PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
a. What is the stimulus? Consuming a large pumpkin spice muffin and caramel macchiato.
 : Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
: Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
Enzyme Action. Intermediate 2 Biology Unit 1: Living Cells
 Enzyme Action Intermediate 2 Biology Unit 1: Living Cells Learning Objectives Describe 2 ways in which chemical reactions can be speeded up. Name the products of the breakdown of hydrogen peroxide. State
Enzyme Action Intermediate 2 Biology Unit 1: Living Cells Learning Objectives Describe 2 ways in which chemical reactions can be speeded up. Name the products of the breakdown of hydrogen peroxide. State
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy
 Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy ATP Cycle - Enzymes Speed Up Reactions Enzyme Function, Factors
Key Concepts - All Cells Use Energy Energy Conversions - Reactions Absorb or Release Energy Endergonic, Exergonic - ATP is Cellular Energy ATP Cycle - Enzymes Speed Up Reactions Enzyme Function, Factors
Proteins have many structures, resulting in a wide range of functions
 Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells. They are instrumental in almost everything an organism does. Protein
Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells. They are instrumental in almost everything an organism does. Protein
Human Biochemistry. Enzymes
 Human Biochemistry Enzymes Characteristics of Enzymes Enzymes are proteins which catalyze biological chemical reactions In enzymatic reactions, the molecules at the beginning of the process are called
Human Biochemistry Enzymes Characteristics of Enzymes Enzymes are proteins which catalyze biological chemical reactions In enzymatic reactions, the molecules at the beginning of the process are called
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Energy. Energy is the ability to do work or bring about a change. Energy transactions must follow the laws of Thermodynamics
 Chapter 6 Energy Energy is the ability to do work or bring about a change Kinetic Energy: Energy of motion Potential Energy: Stored energy Energy transactions must follow the laws of Thermodynamics 1 st
Chapter 6 Energy Energy is the ability to do work or bring about a change Kinetic Energy: Energy of motion Potential Energy: Stored energy Energy transactions must follow the laws of Thermodynamics 1 st
Biochemistry Macromolecules and Enzymes. Unit 02
 Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
An Introduction to Enzyme Structure and Function
 An Introduction to Enzyme Structure and Function Enzymes Many reactions in living systems are similar to laboratory reactions. 1. Reactions in living systems often occur with the aid of enzymes. 2. Enzymes
An Introduction to Enzyme Structure and Function Enzymes Many reactions in living systems are similar to laboratory reactions. 1. Reactions in living systems often occur with the aid of enzymes. 2. Enzymes
Chapter 11 Enzymes and Metabolic Pathways
 Chapter 11 Enzymes and Metabolic Pathways 11.1. Metabolism Metabolism comes from the Greek metabole, meaning "change". It is an emergent property of life. It includes all the chemical processes needed
Chapter 11 Enzymes and Metabolic Pathways 11.1. Metabolism Metabolism comes from the Greek metabole, meaning "change". It is an emergent property of life. It includes all the chemical processes needed
Cofactors and coenzymes. Reversible, irreversible, competitive, and noncompetitive inhibitors. Allosteric enzymes. Feedback inhibition.
 Enzyme regulation Cofactors and coenzymes. Reversible, irreversible, competitive, and noncompetitive inhibitors. Allosteric enzymes. Feedback inhibition. Introduction The genome of a typical organism,
Enzyme regulation Cofactors and coenzymes. Reversible, irreversible, competitive, and noncompetitive inhibitors. Allosteric enzymes. Feedback inhibition. Introduction The genome of a typical organism,
Unit 1: Biochemistry
 Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy
 Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Chapter 8. Metabolism. Topics in lectures 15 and 16. Chemical foundations Catabolism Biosynthesis
 Chapter 8 Topics in lectures 15 and 16 Metabolism Chemical foundations Catabolism Biosynthesis 1 Metabolism Chemical Foundations Enzymes REDOX Catabolism Pathways Anabolism Principles and pathways 2 Chemical
Chapter 8 Topics in lectures 15 and 16 Metabolism Chemical foundations Catabolism Biosynthesis 1 Metabolism Chemical Foundations Enzymes REDOX Catabolism Pathways Anabolism Principles and pathways 2 Chemical
UNIT #3: Enzymes. What is an enzyme? How do enzymes work?
 UNIT #3: Enzymes What is an enzyme? How does an enzyme work? How is an enzyme structured? What are some factors that affect enzyma8c reac8ons? How are enzymes controlled in the body? What is an enzyme?
UNIT #3: Enzymes What is an enzyme? How does an enzyme work? How is an enzyme structured? What are some factors that affect enzyma8c reac8ons? How are enzymes controlled in the body? What is an enzyme?
A cell has enough ATP to last for about three seconds.
 Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Biochemistry. Definition-
 Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Practice Quiz 1 AP Bio Sept 2016 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The element present in all organic molecules is A) hydrogen.
Practice Quiz 1 AP Bio Sept 2016 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The element present in all organic molecules is A) hydrogen.
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
How Cells Work. Chapter 4
 How Cells Work Chapter 4 Energy Laws Energy is the capacity to do work The total amount of energy in the universe is constant-energy can t be created or destroyed..only transferred! Energy is flowing from
How Cells Work Chapter 4 Energy Laws Energy is the capacity to do work The total amount of energy in the universe is constant-energy can t be created or destroyed..only transferred! Energy is flowing from
UNIT 2 BIOLOGICAL CHEMISTRY. ORGANIC MOLECULES: Molecules composed of a carbon skeleton
 1 UNIT 2 BIOLOGICAL CHEMISTRY ORGANIC MOLECULES: Molecules composed of a carbon skeleton Monomers: single building units Polymers: (macromolecules) Very large molecules composed of many monomers put together.
1 UNIT 2 BIOLOGICAL CHEMISTRY ORGANIC MOLECULES: Molecules composed of a carbon skeleton Monomers: single building units Polymers: (macromolecules) Very large molecules composed of many monomers put together.
Unit 7 ~ Learning Guide
 Unit 7 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Unit 7 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Enzymes what are they?
 Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
Lab Activity 30. Digestive Enzymes. Portland Community College BI 233
 Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
7/5/2014. Microbial. Metabolism. Basic Chemical Reactions Underlying. Metabolism. Metabolism: Overview
 PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University Basic Chemical Reactions Underlying Metabolism Metabolism C H A P T E R 5 Microbial Metabolism Collection
PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University Basic Chemical Reactions Underlying Metabolism Metabolism C H A P T E R 5 Microbial Metabolism Collection
Student Handout. green B 1. foam pieces. ) into a single unit to model the substrate in this reaction.
 Student Handout Introduction Enzymes are specialized proteins that catalyze or speed up chemical reactions within cells. The substance upon which an enzyme acts is called a substrate. Substrates are small
Student Handout Introduction Enzymes are specialized proteins that catalyze or speed up chemical reactions within cells. The substance upon which an enzyme acts is called a substrate. Substrates are small
Chapter 23 Enzymes 1
 Chapter 23 Enzymes 1 Enzymes Ribbon diagram of cytochrome c oxidase, the enzyme that directly uses oxygen during respiration. 2 Enzyme Catalysis Enzyme: A biological catalyst. With the exception of some
Chapter 23 Enzymes 1 Enzymes Ribbon diagram of cytochrome c oxidase, the enzyme that directly uses oxygen during respiration. 2 Enzyme Catalysis Enzyme: A biological catalyst. With the exception of some
Chapter 5. The Working Cell. Lecture by Richard L. Myers
 Chapter 5 The Working Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers MEMBRANE STRUCTURE AND FUNCTION
Chapter 5 The Working Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers MEMBRANE STRUCTURE AND FUNCTION
Enzymes. Essential Question: Why are the actions of an enzymes important to us?
 Enzymes 18.11- Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors such as ph and temperature, and their effect on enzyme activity. Enzymes
Enzymes 18.11- Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors such as ph and temperature, and their effect on enzyme activity. Enzymes
Organic molecules are the molecules in living things There are four types of organic (carbon-based) molecules: Carbohydrates Lipids (fats) Proteins
 Organic molecules are the molecules in living things There are four types of organic (carbon-based) molecules: Carbohydrates Lipids (fats) Proteins Nucleic Acids Protein Muscles are made of proteins Enzymes
Organic molecules are the molecules in living things There are four types of organic (carbon-based) molecules: Carbohydrates Lipids (fats) Proteins Nucleic Acids Protein Muscles are made of proteins Enzymes
Ch. 9 Cell Respiration. Title: Oct 15 3:24 PM (1 of 53)
 Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Chemical Energy. Valencia College
 9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
The Cell and Cellular transport
 Cell theory (1838): The Cell 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
Cell theory (1838): The Cell 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
Proteins. Biomolecules. Nucleic Acids. The Building Blocks of Life
 Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
Energy, Chemical Reactions and Enzymes
 Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
ENZYMES: CLASSIFICATION, STRUCTURE
 ENZYMES: CLASSIFICATION, STRUCTURE Enzymes - catalysts of biological reactions Accelerate reactions by a millions fold Common features for enzymes and inorganic catalysts: 1. Catalyze only thermodynamically
ENZYMES: CLASSIFICATION, STRUCTURE Enzymes - catalysts of biological reactions Accelerate reactions by a millions fold Common features for enzymes and inorganic catalysts: 1. Catalyze only thermodynamically
Metabolism. Metabolism. Energy. Metabolism. Energy. Energy 5/22/2016
 5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
LAB 5 - Enzymes BACKGROUND INFORMATION
 LAB 5 - Enzymes BACKGROUND INFORMATION Chemical Reactions The cells of organisms, from bacteria to plants to animals, carry out hundreds to thousands of chemical reactions that must be properly coordinated
LAB 5 - Enzymes BACKGROUND INFORMATION Chemical Reactions The cells of organisms, from bacteria to plants to animals, carry out hundreds to thousands of chemical reactions that must be properly coordinated
Metabolism. Chapter 8 Microbial Metabolism. Metabolic balancing act. Catabolism Anabolism Enzymes. Topics. Metabolism Energy Pathways Biosynthesis
 Chapter 8 Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis Catabolism Anabolism Enzymes Metabolism 1 2 Metabolic balancing act Catabolism and anabolism simple model Catabolism Enzymes
Chapter 8 Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis Catabolism Anabolism Enzymes Metabolism 1 2 Metabolic balancing act Catabolism and anabolism simple model Catabolism Enzymes
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration
 Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
BIOCHEMISTRY Unit 2 Part 4 ACTIVITY #7 (Chapter 8.4) Enzymes. A. Is this reaction dehydration synthesis or hydrolysis?
 AP BIOLOGY BIOCHEMISTRY Unit 2 Part 4 ACTIVITY #7 (Chapter 8.4) NAME DATE PERIOD Enzymes 8.4 1. Enzymes are an important type of protein. For now, use this sketch to review what you know about enzymes.
AP BIOLOGY BIOCHEMISTRY Unit 2 Part 4 ACTIVITY #7 (Chapter 8.4) NAME DATE PERIOD Enzymes 8.4 1. Enzymes are an important type of protein. For now, use this sketch to review what you know about enzymes.
Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life
 Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The Metabolism of Microbes metabolism all chemical
Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The Metabolism of Microbes metabolism all chemical
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Wake Acceleration Academy - Biology Note Guide Unit 2: The Chemistry of the Cell
 Wake Acceleration Academy - Biology Note Guide Unit 2: The Chemistry of the Cell Extra Resources Website: http://waa-science.weebly.com Module 1: Carbohydrates, Lipids, Proteins, and Nucleic Acids Vocabulary
Wake Acceleration Academy - Biology Note Guide Unit 2: The Chemistry of the Cell Extra Resources Website: http://waa-science.weebly.com Module 1: Carbohydrates, Lipids, Proteins, and Nucleic Acids Vocabulary
I. ROLE OF CARBON IN ORGANISMS:
 Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Chapter 5 Ground Rules Of Metabolism
 Chapter 5 Ground Rules Of Metabolism Energy and the World of Life Energy Capacity to do work Two Forms Of energy Kinetic Energy is the energy an object has because it is moving Potential Energy is the
Chapter 5 Ground Rules Of Metabolism Energy and the World of Life Energy Capacity to do work Two Forms Of energy Kinetic Energy is the energy an object has because it is moving Potential Energy is the
Foundations in Microbiology Seventh Edition
 Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 8 An Introduction to Microbial Metabolism Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Lecture PowerPoint to accompany Foundations in Microbiology Seventh Edition Talaro Chapter 8 An Introduction to Microbial Metabolism Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
