Liver Investigation: Testing Marker Utility in Steatohepatitis
|
|
|
- Nickolas Fox
- 5 years ago
- Views:
Transcription
1 Collaboration in Action: The Investigative Medicines Initiative (IMI2) Liver Investigation: Testing Marker Utility in Steatohepatitis 2nd International Workshop on NASH Biomarkers, Washington DC, USA, May 2017 Prof Quentin M. Anstee PhD, FRCP Project Coordinator (Newcastle University, UK) Dr Julia Brosnan PhD Project Lead (Pfizer, USA)
2 Disclosure Slide Quentin M. Anstee Research Grant Funding: Abbvie, GlaxoSmithKline, Allergan/Tobira, Vertex. Consultancy/Speaker: Abbott Laboratories, Acuitas Medical, Allergan/Tobira, Eli Lilly, Falk, Galmed, Genfit, Gilead, Grunthal, Intercept, Inventiva, Janssen, Kenes, MedImmune, NewGene, Novartis, Pfizer, Raptor.
3 The Investigative Medicines Initiative (IMI2) Scheme Focus on unmet needs Non-competitive collaborative research Competitive Calls for proposals Pooling expertise, knowledge and resources, cross-fertilisation Developing incentives to address major unmet medical needs Providing a neutral trusted platform to align public and private interests
4 Evaluation Evaluation Typical IMI2 Two-Stage Call Process Topic definition Stage 1 Stage 2 Grant award Industry Assoc partners Academics Mid-size enterprises SMEs Hospitals Regulators Patients organisations Applicant consortium Industry APs Identification of topics and willingness to collaborate Applicant consortia submit short proposals Full consortium submits full proposal Project Agreement Grant Agreement Call launch Merger: applicants & industry Finalisation Project launch!
5 The Imperative for Biomarkers in NAFLD An important paradox exists: a significant proportion of the population have NAFLD but only a minority progress to advanced liver disease or morbidity/mortality Steatosis Steatosis + Lobular Inflam m ation NASH +/- Portal Inflam m ation Rapid Progressors Approx. 5-6 years Slow Progressors Approx. 7 years Advanced [F3-4] fibrosis (Cirrhosis)? Death or Transplantation Early [F1-2] fibrosis Dynamic steatotic/steatohepatitic phase Patients cycle between periods of steatosis and steatohepatitis. Some individuals exhibit a predominantly steatohepatitic disease process whilst others have generally less active disease. Those that spend proportionately more time in active steatohepatitis may subsequently exhibit greater fibrosis. Fibrotic phase Up to 10% of patients ultimately develop some hepatic fibrosis. The majority exhibit only slow fibrosis progression and are unlikely to progress beyond mild [F0-2] fibrosis whilst a minority, exhibit rapid fibrosis progression and develop advanced fibrosis and cirrhosis [F3-4].
6 The Imperative for Biomarkers in NAFLD An important paradox exists: a significant proportion of the population have NAFLD but only a minority progress to advanced liver disease or morbidity/mortality A lack of tractable non-invasive biomarkers has impeded the diagnosis, risk stratification and monitoring of patients and so many cases remain undiagnosed and present with advanced disease. The lack of biomarkers has also hampered drug development and the conduct of clinical trials, which still depend on histological effect as an endpoint.
7 LITMUS Concept A goal-oriented, tri-partite collaboration is best placed to deliver a definitive and impartial evaluation of available and new biomarkers. End-users of biomarker technologies Practicing clinicians with expertise in NAFLD Pharmaceutical industry (EFPIA partners & Partners in Research); Independent academics with expertise in the evaluation of medical test/biomarker performance Biomarker researchers and developers Academic Commercial
8 LITMUS Concept A goal-oriented, tri-partite collaboration is best placed to deliver a definitive and impartial evaluation of available and new biomarkers. LITMUS will implement a robust technology-unbiased platform and conduct the systematic study and validation of a broad range of noninvasive biomarkers and technologies relevant to NAFLD with reference to fully-adjudicated liver biopsy data. LITMUS will align with the EMA/FDA accord for Qualification of Biomarkers & Clinical Outcome Assessments, in compliance with: EMA CHMP Guidance on Qualification of Novel Methodologies for Drug Development; FDA 510(k)/PMA pathway or Drug Development Tools (DDT) Qualification Program; Generate the requisite level of high-quality data to support biomarker validation and the evidence needed for regulatory qualification.
9 LITMUS Concept A goal-oriented, tri-partite collaboration is best placed to deliver a definitive and impartial evaluation of available and new biomarkers. LITMUS will implement a robust technology-unbiased platform and conduct the systematic study and validation of a broad range of noninvasive biomarkers and technologies relevant to NAFLD with reference to fully-adjudicated liver biopsy data. Our ultimate goal is to establish a defined set of biomarkers that, singly or in combination, enable detection and monitoring of disease progression to and/or regression from NAFL through NASH to fibrosis and cirrhosis. To assist drug development and the conduct of clinical trials To enable the cost-effective management of NAFLD in clinical practice.
10 Strong Collaborative Foundations in Discovery Science EU FP7 6 million ( ) Discovery Science EU H million ( ) Clinical Application EU IMI2 34 million ( )
11 The EPoS European NAFLD Registry Aithal Ekstedt Cobbold Geier Boursier Miele
12 Clinical recruitment to use a hub and spoke model National hub centre(s) with feeding spoke sites Performance management for recruitment Quentin Anstee (UNEW) UK Vlad Ratziu (ICAN) France Jorn Schattenberg (UMC) Germany Andreas Geier (UHW) Germany Jean-Francois Dufour (UBE) Switzerland Michael Trauner (MUV) - Austria Sven Franque (UZA) - Belgium Elisabetta Bugianesi (UNITO) Italy Manuel Romero-Gomez (SAS) Spain Helena Cortez-Pinto (FML) Portugal Mattias Ekstedt (LIU) Sweden Hannele Yki-Jarvinen (UHEL) Finland Van Mil (UMCU) Netherlands George Papatheodoridis (NKUoA) Greece LITMUS Central Biobank Additional Links to initiate a Global NAFLD Network with USA (Harrison and Sanyal)
13 Clinical Data Anthropometrics Medical History Medication Hematology & Biochemistry Diet/Lifestyle Histopathology Digital Imagery of Histology Slides Central Reading by Expert Pathologists NIDDK NAS Score FLIP SAF Score Longitudinal Follow-up Annual Reviews Hard Endpoints Death/OLT HCC Biobank Resource Serum & Plasma Frozen Liver Tissue FFPE Liver Tissue Urine Faeces Integrated Omics Dataset SNP variation DNA methylation Transcriptomics Metabolomics/Lipidomics
14 The Registry is configured for both cross-sectional and longitudinal data collection. Standardised data include anthropometric, lifestyle/diet, co-morbidity, pharmacotherapy, clinical biochemistry, histological indices and incident disease/events
15 Enrolment + Urine & Faeces
16
17 Work Package Leaders & Key Partners
18 Conclusions LITMUS is a focused, pragmatic and goal-oriented programme, founded on a strong track-record of NAFLD research, that addresses the pressing need for validated non-invasive biomarkers. The LITMUS ambition is to make a fundamental difference to the way NAFLD/NASH is diagnosed, clinical trials are conducted and the way patients are managed. LITMUS offers the promise of a decisive advance in NAFLD biomarker development and regulatory qualification and will thus facilitate therapeutics discovery and support the targeting of medical care to those at greatest risk. LITMUS has the demonstrable capacity to provide much needed clarity on biomarker validity at scale and pace and thus deliver a step change in drug development and the care of patients with NAFLD
19
20
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
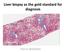 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD)
 Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
EVALUATION REPORT
 International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
International Workshop on NASH Biomarkers 2017 5-6 May 2017 Washington, D.C., EVALUATION REPORT www.expertmedicalevents.com/nashbiomarkers EXECUTIVE SUMMARY INTRODUCTION The 2 nd International Workshop
14 December :00 CET
 Webinar IMI2 Call 13 Assessment of the uniqueness of diabetic cardiomyopathy relative to other forms of heart failure using unbiased pheno-mapping approaches 14 December 2017 15:00 CET Agenda How to use
Webinar IMI2 Call 13 Assessment of the uniqueness of diabetic cardiomyopathy relative to other forms of heart failure using unbiased pheno-mapping approaches 14 December 2017 15:00 CET Agenda How to use
DIAGNOSIS OF NASH LIVER BIOPSY. PHC
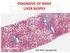 DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
1 ST EUROPEAN. NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT.
 1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
1 ST EUROPEAN NASH Workshop BARCELONA, SPAIN 8-9 MARCH 2019 REQUEST FOR SUPPORT NEEDS ASSESSMENT NEEDS ASSESSMENT Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease affecting 25 % of
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Regulatory Updates Veronica Miller, PhD
 Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM
 IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM Dr Anke M Schulte, Head of Islet Biology Department Sanofi-Diabetes Frankfurt, Germany 11th of July, 2014 General
IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM Dr Anke M Schulte, Head of Islet Biology Department Sanofi-Diabetes Frankfurt, Germany 11th of July, 2014 General
The Innovative Medicines Initiative: an engine for therapeutic innovation
 The Innovative Medicines Initiative: an engine for therapeutic innovation Michel Goldman 26.11.2014 Brussels Bringing health-related life science and technology sectors into IMI2 The pillars of innovation
The Innovative Medicines Initiative: an engine for therapeutic innovation Michel Goldman 26.11.2014 Brussels Bringing health-related life science and technology sectors into IMI2 The pillars of innovation
From IMI to IMI2. Hugh Laverty Senior Scientific Project Manager
 From IMI to IMI2 Hugh Laverty Senior Scientific Project Manager FitForHealth June 2014 Innovative Medicines Initiative: Joining forces in the healthcare sector The biggest public/private partnership in
From IMI to IMI2 Hugh Laverty Senior Scientific Project Manager FitForHealth June 2014 Innovative Medicines Initiative: Joining forces in the healthcare sector The biggest public/private partnership in
Discovery and validation of novel. Webinar IMI2 - Call 9 Joint influenza vaccine effectiveness studies
 Discovery and validation of novel Webinar IMI2 - Call 9 Joint influenza vaccine effectiveness studies Dr. Angela Wittelsberger, IMI IMI webinar 29.04.2016 Today s webinar Will cover all aspects of the
Discovery and validation of novel Webinar IMI2 - Call 9 Joint influenza vaccine effectiveness studies Dr. Angela Wittelsberger, IMI IMI webinar 29.04.2016 Today s webinar Will cover all aspects of the
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
July 5 & 6, 2018, Paris SCIENTIFIC PROGRAM
 www.paris-nash.org Dear Colleagues, We are delighted to announce the 4th Paris NASH Meeting to be held on July 5-6 th 2018. This international academic meeting is aimed to do a deep-dive into many aspects
www.paris-nash.org Dear Colleagues, We are delighted to announce the 4th Paris NASH Meeting to be held on July 5-6 th 2018. This international academic meeting is aimed to do a deep-dive into many aspects
Organized by. Scientific comittee
 July 11 & 12, 2019 Institut Pasteur Organized by Arun Sanyal Virginia Commonwealth University School of Medicine, Richmond, Virginia, USA Lawrence Serfaty Hôpital Hautepierre Hôpitaux Universitaires de
July 11 & 12, 2019 Institut Pasteur Organized by Arun Sanyal Virginia Commonwealth University School of Medicine, Richmond, Virginia, USA Lawrence Serfaty Hôpital Hautepierre Hôpitaux Universitaires de
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
What s s on the Menu in Europe? - overview and challenges in the first pan- European food consumption survey
 What s s on the Menu in Europe? - overview and challenges in the first pan- European food consumption survey Liisa Valsta Data Collection and Exposure Unit What s s on the menu in Europe? Background Attempts
What s s on the Menu in Europe? - overview and challenges in the first pan- European food consumption survey Liisa Valsta Data Collection and Exposure Unit What s s on the menu in Europe? Background Attempts
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Newcastle NIHR BRC Liver Theme
 Newcastle NIHR BRC Liver Theme Professor Quentin M. Anstee PhD, FRCP Professor of Experimental Hepatology & Honorary Consultant Hepatologist, Institute of Cellular Medicine, Newcastle University, UK. Why
Newcastle NIHR BRC Liver Theme Professor Quentin M. Anstee PhD, FRCP Professor of Experimental Hepatology & Honorary Consultant Hepatologist, Institute of Cellular Medicine, Newcastle University, UK. Why
Future of Diabetes Research in Europe JDRF Perspective
 Future of Diabetes Research in Europe JDRF Perspective IMI-JDRF Diabetes Patient Meeting 20 May 2014 Brussels, Belgium Olivier ARNAUD, Pharm D JDRF European Research Director oarnaud@jdrf.org Future of
Future of Diabetes Research in Europe JDRF Perspective IMI-JDRF Diabetes Patient Meeting 20 May 2014 Brussels, Belgium Olivier ARNAUD, Pharm D JDRF European Research Director oarnaud@jdrf.org Future of
NONALCOHOLIC STEATOHEPATITIS (NASH) - OPPORTUNITY ANALYSIS AND FORECASTS TO EVENT-DRIVEN UPDATE
 REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
REFERENCE CODE GDHC034POA PUBLICAT ION DATE MARCH 2014 NONALCOHOLIC STEATOHEPATITIS (NASH) - - EVENT-DRIVEN UPDATE Executive Summary NASH: Key Metrics in Six Major Pharmaceutical Markets 2012 Epidemiology
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
BBMRI-ERIC and BBMRI.fi
 BBMRI-ERIC and BBMRI.fi Connecting Biobanks at the European and National level to proviede hiqh quality samples and associated data for health 29.05.2018 Anu Jalanko Terveystalo Biobank Finland 31/05/2018
BBMRI-ERIC and BBMRI.fi Connecting Biobanks at the European and National level to proviede hiqh quality samples and associated data for health 29.05.2018 Anu Jalanko Terveystalo Biobank Finland 31/05/2018
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
A pathologist, a radiologist and a hepatologist walked into a bar
 A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs
 Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, june 28, 200 A European collaboration between pediatric rheumatology centers regarding
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, june 28, 200 A European collaboration between pediatric rheumatology centers regarding
ITCC-P4 International Workshop
 ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
Real world Outcomes across the AD spectrum for better care: Multi-modal data Access Platform
 Real world Outcomes across the AD spectrum for better care: Multi-modal data Access Platform Catherine Reed, Lilly on behalf of ROADMAP consortium 3 rd Nordic conference on Real World Data, 28-29 November
Real world Outcomes across the AD spectrum for better care: Multi-modal data Access Platform Catherine Reed, Lilly on behalf of ROADMAP consortium 3 rd Nordic conference on Real World Data, 28-29 November
European Patients Academy on Therapeutic Innovation
 European Patients Academy on Therapeutic Innovation http://www.patientsacademy.eu info@patientsacademy.eu The project is receiving support from the Innovative Medicines Initiative Joint Undertaking under
European Patients Academy on Therapeutic Innovation http://www.patientsacademy.eu info@patientsacademy.eu The project is receiving support from the Innovative Medicines Initiative Joint Undertaking under
Agenda 2030: One Nation Labour s Plan for science Response from Alzheimer s Research UK
 Agenda 2030: One Nation Labour s Plan for science Response from Alzheimer s Research UK 1. Introduction 1.1. Alzheimer s Research UK is the UK s leading dementia research charity. As research experts,
Agenda 2030: One Nation Labour s Plan for science Response from Alzheimer s Research UK 1. Introduction 1.1. Alzheimer s Research UK is the UK s leading dementia research charity. As research experts,
Good Laboratory Practice. EU-Serbia screening meeting Brussels, 19 June 2014
 Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
GLP in the European Union Ecolabel detergents, GLP and accreditation
 GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs
 Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, december 4, 2009 A European collaboration between pediatric rheumatology centers
Long-term PHARMacovigilance for Adverse effects in Childhood arthritis focusing on Immune modulatory drugs EMEA meeting, december 4, 2009 A European collaboration between pediatric rheumatology centers
Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia
 Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia On 27 June Alzheimer Europe held a successful lunch debate in the European Parliament
Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia On 27 June Alzheimer Europe held a successful lunch debate in the European Parliament
A precision medicine approach to comprehensive NAFLD diagnosis via metabolomics-based liquid biopsy
 A precision medicine approach to comprehensive NAFLD diagnosis via metabolomics-based liquid biopsy International Workshop on NASH Biomarkers May 5-6 2017, Washington D.C. Pablo Ortiz, MD, PhD (OWL CEO)
A precision medicine approach to comprehensive NAFLD diagnosis via metabolomics-based liquid biopsy International Workshop on NASH Biomarkers May 5-6 2017, Washington D.C. Pablo Ortiz, MD, PhD (OWL CEO)
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
NIHR Supporting collaboration in life sciences research
 NIHR Supporting collaboration in life sciences research Dr Matt Hallsworth - Head of External Relations, NIHR Office for Clinical Research Infrastructure (NOCRI) Research funders build capabilities and
NIHR Supporting collaboration in life sciences research Dr Matt Hallsworth - Head of External Relations, NIHR Office for Clinical Research Infrastructure (NOCRI) Research funders build capabilities and
Cross Border Genetic Testing for Rare Diseases
 Cross Border Genetic Testing for Rare Diseases EUCERD Joint Action WP8 Helena Kääriäinen National Institute for Health an Welfare, Helsinki, Finland Starting point Possibilities and demand for genetic
Cross Border Genetic Testing for Rare Diseases EUCERD Joint Action WP8 Helena Kääriäinen National Institute for Health an Welfare, Helsinki, Finland Starting point Possibilities and demand for genetic
Joint Programming in Neurodegenerative Disease Research (JPND)
 Joint Programming in Neurodegenerative Disease Research (JPND) Building Alliances and Collaborations Prof. Philippe Amouyel, MD, PhD JPND Chair France Disclosure CEO of Fondation Plan Alzheimer Conference
Joint Programming in Neurodegenerative Disease Research (JPND) Building Alliances and Collaborations Prof. Philippe Amouyel, MD, PhD JPND Chair France Disclosure CEO of Fondation Plan Alzheimer Conference
Obesity, Inflammation and Liver Cancer
 Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Global Coverage. Regional Coverage. Country Coverage Company Coverage
 Global Fatty Liver Disease Drugs Market By Type (ALD, NAFLD), By Stages of ALD and NAFLD, By Region, By Country: Opportunities and Forecast (2017-2022) By Type- ALD and NAFLD ALD Stages - Alcoholic Steatosis
Global Fatty Liver Disease Drugs Market By Type (ALD, NAFLD), By Stages of ALD and NAFLD, By Region, By Country: Opportunities and Forecast (2017-2022) By Type- ALD and NAFLD ALD Stages - Alcoholic Steatosis
What EU research policy can do for conditions such as chronic pain?
 The EU Framework Programme for Research and Innovation HORIZON 2020 What EU research policy can do for conditions such as chronic pain? K Berkouk, PhD Deputy Head of Unit, Non-communicable diseases and
The EU Framework Programme for Research and Innovation HORIZON 2020 What EU research policy can do for conditions such as chronic pain? K Berkouk, PhD Deputy Head of Unit, Non-communicable diseases and
Angelos Hatzakis. 10th Paris Hepatology Conference
 Angelos Hatzakis Professor of Epidemiology and Preventive Medicine Faculty of Medicine National and Kapodistrian University of Athens Co-Chair, Hepatitis B and C Public Policy Association 10th Paris Hepatology
Angelos Hatzakis Professor of Epidemiology and Preventive Medicine Faculty of Medicine National and Kapodistrian University of Athens Co-Chair, Hepatitis B and C Public Policy Association 10th Paris Hepatology
Paris Hepatology Conference PROGNOSIS OF NASH
 Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
Paris Hepatology Conference PROGNOSIS OF NASH Palais des Congrès Monday 15th January 2018 14:45-15:00 PHC 2018 - www.aphc.info Driven to care Disclosures Advisory committees Speaking and teaching Research
Market surveillance of medical devices
 Market surveillance of medical devices A joint action on market surveillance of medical devices to reinforce public health protection Information for healthcare professionals Introduction The European
Market surveillance of medical devices A joint action on market surveillance of medical devices to reinforce public health protection Information for healthcare professionals Introduction The European
Development of novel inhaled antibiotic regimens in patients with cystic fibrosis (CF) and patients with non-cf bronchiectasis (BE)
 Development of novel inhaled antibiotic regimens in patients with cystic fibrosis (CF) and patients with non-cf bronchiectasis (BE) Dr. Juliane Bernholz, Novartis (Coordinator) Dr. Andrea Appenzeller,
Development of novel inhaled antibiotic regimens in patients with cystic fibrosis (CF) and patients with non-cf bronchiectasis (BE) Dr. Juliane Bernholz, Novartis (Coordinator) Dr. Andrea Appenzeller,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis
 Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
At Least 1 in 5 Patients in Your Practice Have Fatty Liver
 At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
At Least 1 in 5 Patients in Your Practice Have Fatty Liver What Can You Tell Your Patients Magnus McLeod MD FRCPC Assistant Professor Dalhousie University 30-NOV-2017 NAFLD Non-Alcoholic Fatty Liver Disease
Fatty Liver- how important is it? Jeremy F.L. Cobbold MA PhD MRCP Clinical Lecturer in Hepatology Imperial College London
 Fatty Liver- how important is it? Jeremy F.L. Cobbold MA PhD MRCP Clinical Lecturer in Hepatology Imperial College London Fatty liver- how important is it? Importance in terms of: Prevalence Pathogenesis
Fatty Liver- how important is it? Jeremy F.L. Cobbold MA PhD MRCP Clinical Lecturer in Hepatology Imperial College London Fatty liver- how important is it? Importance in terms of: Prevalence Pathogenesis
IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM
 IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM Anke M. Schulte, Sanofi-Diabetes 30.09.2014 IMI Open Info Day 2014 Brussels, Belgium General T1DM disease facts
IMI2 T1DM Call Topic Text: Translational approaches to disease modifying therapy of T1DM Anke M. Schulte, Sanofi-Diabetes 30.09.2014 IMI Open Info Day 2014 Brussels, Belgium General T1DM disease facts
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017
 WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
Update from IMI PREFER: Patient preferences in benefitrisk assessments during the medical product lifecycle
 1 1 Update from IMI PREFER: Patient preferences in benefitrisk assessments during the medical product lifecycle Presenter: Chiara Whichello on behalf of the PREFER consortium June 5, 2018 The statements
1 1 Update from IMI PREFER: Patient preferences in benefitrisk assessments during the medical product lifecycle Presenter: Chiara Whichello on behalf of the PREFER consortium June 5, 2018 The statements
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES
 DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
Data Collection on Adverse Events of Anti-HIV Drugs. The D:A:D Study. Signe W. Worm MD, PhD, D:A:D Study Coordinator >
 Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
2008 Public Status Report on the Implementation of the European Risk Management Strategy. Executive Summary
 European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
THE MICHAEL J. FOX FOUNDATION FOR PARKINSON S RESEARCH. Funding Opportunities
 THE MICHAEL J. FOX FOUNDATION FOR PARKINSON S RESEARCH Funding Opportunities AGENDA 1. Brief Overview of MJFF Philosophy 2. MJFF Funding Programs 3. How to apply 4. What happens next? 5. Q&A Please feel
THE MICHAEL J. FOX FOUNDATION FOR PARKINSON S RESEARCH Funding Opportunities AGENDA 1. Brief Overview of MJFF Philosophy 2. MJFF Funding Programs 3. How to apply 4. What happens next? 5. Q&A Please feel
Thomas Karlsson & Esa Österberg National Research and Development Centre for Welfare and Health Alcohol and Drug Research Group P.O.
 European Comparative Alcohol Study Europe and Alcohol Policy Thomas Karlsson & Esa Österberg National Research and Development Centre for Welfare and Health Alcohol and Drug Research Group P.O.BO 220 FIN-00531
European Comparative Alcohol Study Europe and Alcohol Policy Thomas Karlsson & Esa Österberg National Research and Development Centre for Welfare and Health Alcohol and Drug Research Group P.O.BO 220 FIN-00531
Addressing the challenges of diabetes and its complications: The IMI Diabetes Platform
 Addressing the challenges of diabetes and its complications: The IMI Diabetes Platform EACPT 2013 Congress 29 August CICG Geneva Bernd Jablonka Sanofi Challenges of Diabetes Treatment Novel targets / therapeutic
Addressing the challenges of diabetes and its complications: The IMI Diabetes Platform EACPT 2013 Congress 29 August CICG Geneva Bernd Jablonka Sanofi Challenges of Diabetes Treatment Novel targets / therapeutic
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop
 Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
From Medical Treatment to Liver Transplantation PROGRAM NAFLD/NASH CONSENSUS CONFERENCE 2018
 www.ilts.org From Medical Treatment to Liver Transplantation PROGRAM NAFLD/NASH Course Directors Patrizia Burra, MD, PhD University of Padova Padova, Italy Marina Berenguer, MD University and Polytechnic
www.ilts.org From Medical Treatment to Liver Transplantation PROGRAM NAFLD/NASH Course Directors Patrizia Burra, MD, PhD University of Padova Padova, Italy Marina Berenguer, MD University and Polytechnic
Involving research participants in research: The example of the EPAD (European Prevention of Alzheimer s Dementia) project
 Involving research participants in research: The example of the EPAD (European Prevention of Alzheimer s Dementia) project Stina Saunders Alzheimer Europe Annual Conference Barcelona 2018 @stina_saunders
Involving research participants in research: The example of the EPAD (European Prevention of Alzheimer s Dementia) project Stina Saunders Alzheimer Europe Annual Conference Barcelona 2018 @stina_saunders
Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic
 NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
Improving the care of patients suffering from acute or chronic pain
 Improving the care of patients suffering from acute or chronic pain Petra Bloms-Funke, Hiltrud Liedgens, Keith Phillips, Jens Nagel 7 th December 2016 IMI webinar Pain Topics The continued high need for
Improving the care of patients suffering from acute or chronic pain Petra Bloms-Funke, Hiltrud Liedgens, Keith Phillips, Jens Nagel 7 th December 2016 IMI webinar Pain Topics The continued high need for
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Venue: EIZ, Zagreb. Maja Jokić (IDIZ) and Andrea Mervar (EIZ) This project is co-funded by the European Union
 I3U Open Access to Research Results and Research Information Services (commitment #20) Project Steering Meeting and Stakeholders Meeting 29-30 September 2016 Venue: EIZ, Zagreb Maja Jokić (IDIZ) and Andrea
I3U Open Access to Research Results and Research Information Services (commitment #20) Project Steering Meeting and Stakeholders Meeting 29-30 September 2016 Venue: EIZ, Zagreb Maja Jokić (IDIZ) and Andrea
V8 NEXUS. Glyco Liver Profile NA2 NA3 NA2FB NGA2F. The next generation in non-invasive liver diagnostics INFLAMMATION FIBROSIS CIRRHOSIS HCC
 V8 NEXUS Glyco Liver Profile The next generation in non-invasive liver diagnostics INFLAMMATION FIBROSIS CIRRHOSIS HCC The Glyco Liver Profile is a unique, non-invasive test that provides the clinician
V8 NEXUS Glyco Liver Profile The next generation in non-invasive liver diagnostics INFLAMMATION FIBROSIS CIRRHOSIS HCC The Glyco Liver Profile is a unique, non-invasive test that provides the clinician
WORLDWIDE EPIDEMIOLOGY OF NASH
 WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
Hiding in Plain Sight Finding the NASH Patient
 Hiding in Plain Sight Finding the NASH Patient The Silent Disease Nonalcoholic fatty liver disease (NAFLD) and its advanced form, nonalcoholic steatohepatitis (NASH), are increasingly prevalent today and
Hiding in Plain Sight Finding the NASH Patient The Silent Disease Nonalcoholic fatty liver disease (NAFLD) and its advanced form, nonalcoholic steatohepatitis (NASH), are increasingly prevalent today and
Corporate Presentation
 Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
EPAG UPDATE. Lenja Wiehe. European Patient Advocacy Groups Manager, EURORDIS
 EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
2 nd International Workshop on NASH Biomarkers, Washington DC, May 5-6, 2017
 Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Hepatic Proton Density Fat Fraction Correlates With Histologic Measures of Steatosis and Is Responsive to Changes in Those Measures in a Multi-center Nonalcoholic Steatohepatitis Clinical Trial Michael
Smokefree Policies in Europe: Are we there yet?
 Smokefree Policies in Europe: Are we there yet? 14 April 2015, 9:00 10:30am Rue de l Industrie 24, 1040 Brussels Permanent Partners: Temporary Partners: The research for the SFP Smokefree Map was partially
Smokefree Policies in Europe: Are we there yet? 14 April 2015, 9:00 10:30am Rue de l Industrie 24, 1040 Brussels Permanent Partners: Temporary Partners: The research for the SFP Smokefree Map was partially
Horizon Introduction. Alex Harris Medical Research Council
 Horizon 2020 - Introduction Alex Harris Medical Research Council Agenda MRC and Horizon 2020 UK Success Rate (FP7 Health) What is Horizon 2020? The Horizon 2020 Health Challenge NCP support MRC and Horizon
Horizon 2020 - Introduction Alex Harris Medical Research Council Agenda MRC and Horizon 2020 UK Success Rate (FP7 Health) What is Horizon 2020? The Horizon 2020 Health Challenge NCP support MRC and Horizon
EPIDEMIOLOGY. Accurate, in-depth information for understanding and assessing targeted markets
 EPIDEMIOLOGY Accurate, in-depth information for understanding and assessing targeted markets WHY ACCURATE MARKET FORECASTS Start with accurate understanding Epidemiology is the study of disease patterns
EPIDEMIOLOGY Accurate, in-depth information for understanding and assessing targeted markets WHY ACCURATE MARKET FORECASTS Start with accurate understanding Epidemiology is the study of disease patterns
Welcome and introducing ESHRE
 ESHRE European Society of Human Reproduction and Embryology Welcome and introducing ESHRE János Rigó Semmelweis University Faculty of Medicine 1st Department of Obstetrics and Gynaecology ESHRE guidelines
ESHRE European Society of Human Reproduction and Embryology Welcome and introducing ESHRE János Rigó Semmelweis University Faculty of Medicine 1st Department of Obstetrics and Gynaecology ESHRE guidelines
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019
 GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database
 Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
Building a Premier Oncology Biotech
 Corporate Deck Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO November 2018 Forward-Looking Statements All of the statements in this presentation that are not statements of historical
Corporate Deck Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO November 2018 Forward-Looking Statements All of the statements in this presentation that are not statements of historical
Solutions for Language Services in Healthcare
 Solutions for Language Services in Healthcare We improve communication between patients and providers to enhance patient care and reduce cost, while improving patient outcomes. InDemand partners with today
Solutions for Language Services in Healthcare We improve communication between patients and providers to enhance patient care and reduce cost, while improving patient outcomes. InDemand partners with today
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health
 CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
Liver disease in 2017: challenges and opportunities
 Liver disease in 2017: challenges and opportunities Dr Matthew Cowan Consultant Gastroenterologist and Hepatologist Surrey and Sussex Healthcare NHS Trust Faculty of Physician Associates 2 nd National
Liver disease in 2017: challenges and opportunities Dr Matthew Cowan Consultant Gastroenterologist and Hepatologist Surrey and Sussex Healthcare NHS Trust Faculty of Physician Associates 2 nd National
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives. Oncology Therapeutic Area Janssen Research & Development, LLC
 Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
European Collaboration on Dementia. Luxembourg, 13 December 2006
 European Collaboration on Dementia Luxembourg, 13 December 2006 2005 Call for projects Special attention has also to be given to information and definition of indicators on neurodegenerative, neurodevelopment,
European Collaboration on Dementia Luxembourg, 13 December 2006 2005 Call for projects Special attention has also to be given to information and definition of indicators on neurodegenerative, neurodevelopment,
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Keeping pace. Progress in dementia research capacity. March 2017
 Keeping pace Progress in dementia research capacity March 2017 Contents Executive Summary 3 Recommendations 4 1. Introduction 5 2. Methodology 6 3. Progress 7 3.1 Research output 7 3.2 Estimate of number
Keeping pace Progress in dementia research capacity March 2017 Contents Executive Summary 3 Recommendations 4 1. Introduction 5 2. Methodology 6 3. Progress 7 3.1 Research output 7 3.2 Estimate of number
IARC A UNIQUE AGENCY. Cancer research for cancer prevention
 IARC A UNIQUE AGENCY Cancer research for cancer prevention Director s message IARC is a unique organization. For the past 50 years, the Agency has been making important contributions to the global fight
IARC A UNIQUE AGENCY Cancer research for cancer prevention Director s message IARC is a unique organization. For the past 50 years, the Agency has been making important contributions to the global fight
EUROPEAN AEROSOL PRODUCTION
 Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
GLOBAL INVESTMENT IN HIV CURE RESEARCH AND DEVELOPMENT IN 2017 AFTER YEARS OF RAPID GROWTH FUNDING INCREASES SLOW
 GLOBAL INVESTMENT IN HIV CURE RESEARCH AND DEVELOPMENT IN 2017 AFTER YEARS OF RAPID GROWTH FUNDING INCREASES SLOW TOWARDS AN CURE PEOPLE FOCUSED SCIENCE DRIVEN JULY 2018 A GLOBAL STRATEGIC APPROACH TOWARDS
GLOBAL INVESTMENT IN HIV CURE RESEARCH AND DEVELOPMENT IN 2017 AFTER YEARS OF RAPID GROWTH FUNDING INCREASES SLOW TOWARDS AN CURE PEOPLE FOCUSED SCIENCE DRIVEN JULY 2018 A GLOBAL STRATEGIC APPROACH TOWARDS
Liver Pathology and the Clinician in 2015: At the Crossroads. Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York
 Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Rodman & Renshaw 17 th Annual Global Investment Conference
 Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Type 1 Diabetes Australian Research Impact Analysis
 Type 1 Diabetes Australian Research Impact Analysis Executive Overview Summary Type 1 diabetes research in Australia Australia is making a significant contribution to the quantity and quality of the global
Type 1 Diabetes Australian Research Impact Analysis Executive Overview Summary Type 1 diabetes research in Australia Australia is making a significant contribution to the quantity and quality of the global
Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018
 Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018 Summary: This document follows on from the findings of the CM-Path The
Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018 Summary: This document follows on from the findings of the CM-Path The
