Human and nonhuman primates tend to live in large social
|
|
|
- Elmer Benson
- 5 years ago
- Views:
Transcription
1 Articles Shared neural coding for social hierarchy and reward value in primate amygdala Jérôme Munuera *, Mattia Rigotti 2 and C. Daniel Salzman,3,4,5,6 * The social brain hypothesis posits that dedicated neural systems process social information. In support of this, neurophysiological data have shown that some brain regions are specialized for representing faces. It remains unknown, however, whether distinct anatomical substrates also represent more complex social variables, such as the hierarchical rank of individuals within a social group. Here we show that the primate amygdala encodes the hierarchical rank of individuals in the same neuronal ensembles that encode the rewards associated with nonsocial stimuli. By contrast, orbitofrontal and anterior cingulate cortices lack strong representations of hierarchical rank while still representing reward values. These results challenge the conventional view that dedicated neural systems process social information. Instead, information about hierarchical rank which contributes to the assessment of the social value of individuals within a group is linked in the amygdala to representations of rewards associated with nonsocial stimuli. Human and nonhuman primates tend to live in large social groups that possess dominant and subordinate members. In many settings, such as interviewing for a job or attending a family reunion, appropriate social interactions and emotional behavior depend upon an assessment of the hierarchical ranks of both oneself and the other people present. The hierarchical ranks of individuals and the accurate evaluation of the hierarchical ranks of others affect the ability to thrive for example, by influencing fluid and food access, mating priority and defensive behavior 2 5. The assessment of hierarchical rank requires accurate identification of social group members. Primates are masters at recognizing identity based on faces 6,7. Brain areas engaged during face processing include the occipital, fusiform and superior temporal sulcus face areas, as well as the amygdala and orbitofrontal cortex 8 5. The superior temporal sulcus is anatomically connected to brain areas implicated in social processing, including the anterior cingulate and orbitofrontal cortices (ACC and OFC) and the amygdala 6 8. These connections may link representations of facial identity to neural circuits processing motivational and emotional information about nonsocial stimuli. For example, the amygdala, OFC and ACC all provide neural representations of the value of conditioned stimuli acquired through reinforcement learning 9 2. How and where neural representations of social variables more complex than facial identity such as hierarchical rank emerge in the brain is an enduring mystery. The social brain hypothesis posits that the evolution of large brains in mammals reflects increasingly complex social demands 22, a proposal consistent with the discovery in primates of brain areas specialized for representing faces. In further support of this hypothesis, gray matter brain volume in human and nonhuman primates varies as a function of social status and/or social experiences However, the extent to which more complex social variables such as hierarchical rank are processed in specialized neural circuits remains unclear. Human neuroimaging studies indicate that the amygdala helps establish artificial hierarchies acquired during performance of a task in the laboratory 28 32, but the amygdala s role in representing naturally established hierarchies has not been demonstrated. It is possible that some social variables, such as the propensity to share rewards with a partner, may engage distinct neuronal ensembles 4,33 36, but other social variables may not engage neuronal circuits dedicated to processing social information alone. Indeed, rewarding stimuli and stimuli that possess hierarchical information have been observed to modulate the same neuronal ensembles in brain areas such as OFC, striatum and the lateral intraparietal area 2,4,35,37. In these experiments, however, neural responses to stimuli spanning a full social hierarchy were not examined, leaving unclear whether the studied neural ensembles in fact represent hierarchical rank per se. Monkeys (and other primates) almost certainly learn the social rank of other group members through experience. This learning process may entail assigning group members different values through experience, a process bearing resemblance to learning about the value of nonsocial stimuli. Here we provide evidence that the primate amygdala encodes the hierarchical rank of individuals in the same neuronal ensembles that also encode the rewards associated with nonsocial stimuli. By contrast, despite representing the rewards associated with nonsocial stimuli, OFC and ACC lack strong representations of hierarchical rank. These results challenge strong versions of the social brain hypothesis 22, the traditional view that the processing of social stimuli occurs exclusively in dedicated neural systems. Instead, although some aspects of social processing may occur in distinct neural circuits, some complex social variables, such as hierarchical rank, may be represented by neuronal ensembles that also process nonsocial stimuli. Results Two rhesus monkeys who had lived in the same stable group of ten monkeys for many years performed a task in which they viewed, in different blocks of trials, images of the faces of other members of their group or fractal images predicting different reward amounts (Fig. a,b). The face images were neutral in expression Department of Neuroscience, Columbia University, New York, NY, USA. 2 IBM T.J. Watson Research Center, Yorktown Heights, NY, USA. 3 Kavli Institute for Brain Sciences, Columbia University, New York, NY, USA. 4 Department of Psychiatry, Columbia University, New York, NY, USA. 5 New York State Psychiatric Institute, New York, NY, USA. 6 Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY, USA. * jerome.munuera@gmail.com; cds25@columbia.edu Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
2 Articles NaTure NeuroscIence and did not depict emotionally relevant expressions (see Methods). Viewer monkeys fixated for the first 4 ms of image presentation for all trials and then were permitted free viewing of the images for an additional, ms. Successful fixation resulted in delivery of one drop of liquid reward on face image trials or zero, one or two drops of liquid reward on fractal trials (depending upon the image). Anticipatory licking demonstrated that monkeys learned the different reward associations of the fractal images (Kruskal-Wallis test, P < 5, Supplementary Fig. ). a Social Fractal Fixation (4 ms) Fixation with image (4 ms) Free viewing (, ms) Trace (75 ms) The assessment of social hierarchy. Human observers scored hierarchical rank using established methodology that determined the winners of agonistic interactions between monkeys (Table and Supplementary Table ) 38. We also used the oculomotor behavior of viewer monkeys during electrophysiological recording sessions to construct an index of the monkeys assessment of hierarchical rank of the face images. Several measures of viewer monkeys oculomotor behavior varied depending upon the face image being displayed (Fig. c and Methods) 39. These measures capitalized on the fact that monkeys tended to look at the face images of dominant monkeys, that viewing dominant monkeys can be more arousing, and that the propensity to look at the eyes of an individual is modulated by hierarchical rank (Methods and Supplementary Fig. 2) 4. The social status indices for each viewer monkey were significantly correlated (r =.72, P =.43; Fig. e), perhaps reflecting that the two viewer monkeys both were in the middle of the hierarchy (Table ). Furthermore, the index constructed from oculomotor measures was correlated with the human scoring of hierarchical rank (r =.9, P =.2; Fig. d) and was not significantly correlated with monkey age or weight (r =.4, P =.35 and r =.7, P =.867, respectively). A behavioral experiment showed that the oculomotor measures obtained from viewer monkeys did not distinguish dominant from submissive monkeys if the faces displayed on the screen were unfamiliar to the viewer monkeys (Supplementary Fig. 3). The correlation of the social status index with a traditional scoring method of hierarchical organization by human observers provides strong evidence that during the electrophysiological recordings the viewer monkeys were actively assessing the hierarchical ranks of the monkeys depicted in the facial images. Neural representations of social hierarchy and nonsocial value. While monkeys performed the fixation task, we recorded the responses of individual neurons in the amygdala (96 neurons), OFC (34 neurons) and ACC (87 neurons) to face images (Fig. 2a c, left, and Supplementary Fig. 4). Across the populations of neurons, the proportion of neurons responsive to faces during fixation was higher in the amygdala than in OFC (z-test, z = 3.39, P =.2) or in ACC (z-test, z = 4.3, P < 4 ) (Fig. 2d, top row). OFC and ACC were statistically indistinguishable in this measure (z-test, z =.64, P =.539). However, responses to faces were usually not restricted to a single face image: 85.7% of face-responsive neurons in the amygdala responded to more than one face (8.2% of neurons in OFC and 59.4% of neurons in ACC exhibited the same property; Supplementary Fig. 5a). Only a minority of neurons responded to only a single face image (for example, Supplementary Fig. 5b). The proportion of neurons exhibiting a significant response to each face image did not differ across the face images for any brain area (chi-squared test, χ 2 = 2.42, P >.9 in amygdala; χ 2 = 3.93, P >.5 in OFC; χ 2 = 2.964, P >.5 in ACC; Fig. 2e). Although neurons often responded to more than one face image, an analysis using a linear decoder demonstrated that the population of amygdala neurons could be used to selectively discriminate which facial image was being viewed shortly after image onset (Fig. 2f and Methods) 4. Decoding performance for face image identity was markedly superior in the amygdala relative to OFC or ACC (Fig. 2f, left), suggesting that on average the amygdala b c d Social index score Social index score.8.6 Fractal block Social block Fractal block M6 M3 M M7 M2 Time (trials) M M2 M3 M4 M5 M6 M7 Viewed monkeys e.8.4 r =.9.2 P = Human scoring of social status * Social index viewer monkey R.6 M8 Reward.4 r =.72 P = Social index viewer monkey F Fig. Task and behavioral measures. a, Sequence of events in behavioral task. Monkeys view images of the faces of other monkeys who reside in their housing room (left) or fractal images (right). Successful fixation results in reward delivery. b, Behavioral task interleaving social and nonsocial (fractal) trial blocks. In fractal blocks, different images are associated with different reward amounts, unlike in trials for the social block. c, Social index from behavioral measures of hierarchical assessment observed during the recording sessions plotted for each viewed monkey, M through M8, where M is the alpha monkey and M8 the most submissive one. Kruskal-Wallis test (χ 2 (7,535) = 47., P < 7 ), Dunn s post hoc: M different from M3 (P =.23), M4 (P < 3 ), M5 (P < 3 ), M6 (P < 4 ), M7 (P < 4 ), M8 (P < 4 ); M2 versus M8, P =.74). Data represent the average of the four behavioral measures used to compute the social index across the sessions (n = 7) (see Methods and Supplementary Fig. 2). Red diamonds represent mean, white lines represent median, blue bars and whiskers represent 75th and 85th percentiles respectively, blue circles represent data points beyond the whiskers limits. d, Social index score plotted as a function of the scoring of the colony hierarchy by a human observer (see Methods, Pearson s correlation coefficient (two-sided), r =.9, P =.2). e, Index score for one viewer monkey plotted against the other (Pearson s correlation coefficient (two-sided), r =.72, P =.43). 46 Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
3 NaTure NeuroscIence Table Hierarchical rank determined by human observers Monkey Number of won agonistic interactions observed Number of agonistic interactions Viewed: M Viewed: M Viewed: M Viewer: MF Viewed: M Viewer: MR Viewed: M Viewed: M Viewed: M Viewed: M Relative dominance status Summary of the overall observed social interactions used in the computation of the social status scored by human observer. MF and MR are the two viewer monkeys, monkeys F and R. communicated more facial identity information per neuron than the prefrontal areas. We next explored whether a neural representation of hierarchical rank was present in each brain area. A regression analysis was used to quantify the relationship between neural responses during fixation of the face images ( 4 ms after image onset) and the social position ( 8) of the viewed face, where position corresponds to the most dominant monkey (M). The regression coefficient β soc characterized neuronal tuning to the hierarchical rank of face images. For the amygdala neuron depicted in Fig. 2a (left), β soc was significantly less than (P < 4 ), indicating that the neuron tended to fire more strongly to face images from dominant monkeys. Overall, 3.6% of amygdala neurons, corresponding to 36% of faceresponsive neurons, exhibited statistically significant selectivity for hierarchical rank (Supplementary Table 2). Out of those 3.6% neurons, 63.6% responded more strongly to dominant face images and 36.4% more strongly to submissive face images (Supplementary Table 2). Neurons in OFC and ACC exhibited selectivity for hierarchical rank much less frequently (6.7% of OFC neurons, representing 23% of face-responsive OFC neurons, and 6.3% of ACC neurons, representing 9% of face-responsive ACC neurons). We confirmed the presence of a representation of hierarchical rank across the population of amygdala cells by performing an outof-sample test: we analyzed separately the neurons that responded more strongly to M (most dominant monkey) than to M8 (most submissive monkey) and those that responded more strongly to M8 than M (see Methods). We then assessed whether neural responses to faces from the remaining out-of-sample viewed monkeys (M2 M7) were correlated with hierarchical rank. For the amygdala, responses to monkey face images correlated significantly with the social position of viewed monkeys (P =. and P =.46 for dominant- and submissive-monkey-preferring neurons, respectively; Fig. 2g, left). These results indicate that neurons classified according to preferential responding to M or M8 exhibit correlated neural activity with hierarchical ranking when only considering responses to M2 M7. The relationship between neural activity and hierarchical rank is therefore not merely driven by responses to the most dominant (M) and most submissive (M8) monkeys. Unlike the amygdala, OFC and ACC did not provide a compelling representation of social hierarchy (Fig. 2g, middle and right). Moreover, only the amygdala exhibited regressions with oppositely signed slopes for dominant- and submissive-preferring neurons. We used a Fisher s r to z transformation test to assess the statistical significance of the difference between the correlation coefficients in Articles each brain area. This test revealed a statistically significant difference between the amygdala and ACC for dominant-preferring neurons and between the amygdala and OFC for submissive-preferring neurons (difference between correlations for dominant-preferring neurons: amygdala (r =.92) vs. OFC (r =.88), z =.26, P =.398; amygdala (r =.92) vs. ACC (r =.9), z = 2.8, P =.5; OFC (r =.88) vs. ACC (r =.9), z =.92; P =.28; for submissive-preferring neurons: amygdala (r =.82) vs. OFC (r =.32), z =.82; P =.34; amygdala (r =.82) vs. ACC (r =.87), z =.22, P =.43; OFC (r =.32) vs. ACC (r =.87), z = 2.4, P =.2). We also combined the data from dominant-preferring and submissive-preferring neurons by sign-flipping the data from the submissive-preferring neurons and then averaging these responses with those of dominant-preferring neurons. A correlation coefficient of these combined data was computed for each area. The amygdala was the only area significantly correlated with the social position of the viewed monkeys (r =.9, P =.3 for amygdala; r =.6, P =.2 for OFC; and r =.6, P =.2 for ACC). Our electrophysiological recordings were obtained from the same parts of the amygdala, OFC and ACC shown previously to contain individual neurons that respond differentially to a conditioned stimulus (CS) depending upon its reward association 9,2,4, a finding we also observed in this study (Fig. 2a c, right). We examined the encoding of reward magnitudes associated with fractal images by using a regression analysis to estimate a coefficient (β frac ) quantifying the relationship between neural activity and the reward associated with fractals (see Methods). β frac was significantly different from in 38.9%, 25.8% and 39.2% of neurons in amygdala, OFC and ACC, respectively, with different neurons exhibiting positive or negative valence preference (Supplementary Table 2). The proportion of neurons responsive to one or more fractal images was not statistically distinguishable across the three brain structures (Fig. 2d, middle row, amygdala vs. OFC, z =.57, P =.29; amygdala vs. ACC, z =.855, P =.393; OFC vs. ACC, z =.723, P =.47; z-test for all comparisons), but the proportion of neurons responsive to one or more face and one or more fractal images was significantly greater in the amygdala than OFC and ACC, which were indistinguishable from each other (Fig. 2d, bottom row; z-test: amygdala vs. OFC, z = 3.26, P =.2, amygdala vs. ACC, z = 3.4, P < 3 ; OFC vs. ACC, z =.8, P =.994). A linear decoder analysis revealed that neuronal populations in all three brain areas could be used to decode fractals associated with different rewards beginning shortly after image onset, consistent with prior observations (Fig. 2f, right) 4. The same neuronal ensembles in the amygdala represent social hierarchy and reward value. We next hypothesized that neural representations of hierarchical rank and of the rewards associated with nonsocial (fractal) images might be encoded by overlapping neuronal ensembles. To test this hypothesis, we trained a linear decoder to distinguish between trials when fractals were associated with large or no reward. The training procedure sets the weight of each neuron as well as a global threshold for distinguishing between the two conditions used for training (Fig. 3a). The decoder was then tested on held-out trials of the same types, as well as on trials in which monkeys viewed two different selected monkey face images (Fig. 3b). This decoding analysis was performed in a 3-ms sliding window (see Methods). As expected, decoding performance was high in all three brain areas when testing occurred on heldout fractal trials (Fig. 3c e), consistent with previously described neural representations of expected reward in all three brain areas 4. When the decoder was trained to distinguish between trials with fractals associated with large or no reward and was tested to distinguish between trials in which two monkey faces were presented that differed greatly in hierarchical rank (M vs. M8), decoding performance in the amygdala was significantly above chance shortly after Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
4 Articles NaTure NeuroscIence a Firing rate (Hz) b Firing rate (Hz) c Firing rate (Hz) Social.5.5 Amygdala neuron: MF729-7a M M2 M3 M4 M5 M6 M7 M Fractal.5.5 OFC MR3-3a 4 CS CS + CS ACC MR2-48a Time from image onset (s) Time from image onset (s) d e Percentage of neurons Percentage of responsive neurons Monkey: Fractal: Both: M M2M3 M4 M5 Amygdala 86% 76% 7% Amygdala M8 M6 M7 Viewed monkeys OFC *** ** 72% 68% *** ** 53% OFC M M2M3 M4 M8 M5 M6 M7 Viewed monkeys M M2M3 ACC 68% 72% 53% ACC M4 M8 M5 M6 M7 Viewed monkeys f Monkey Fractal Amygdala OFC.8 ACC.6 Dedcoding performance g Time from image onset (s) Amygdala 2.5 OFC 2.5 ACC 2. r =.88 P =.2 r =.32 P =.538 Time from image onset (s) 2. r =.9 P =.72 r =.87 P =.23 Mean firing rate (z-score) r =.92 r =.82 P =. P = Social position Social position Social position Fig. 2 The amygdala represents the hierarchical status of individuals. a c. Example neurons from the amygdala (a), OFC (b) and ACC (c) that respond to both face (left) and fractal images (right). The amygdala neuron responds most strongly to the dominant monkey face image and to the most highly rewarded fractal image, with responses decreasing monotonically with decreases in the hierarchical status of face images or the reward associated with fractals. d, The percentage of neurons in the three brain structures that respond to at least one monkey, one fractal or one of both types of image. **P <. and ***P <. (two-tailed z-test, top row: amygdala versus OFC, z = 3.39, P =.2; amygdala vs. ACC, z = 4.3, P < 4 ; OFC vs. ACC, z =.64, P =.539; middle row: amygdala vs. OFC, z =.57, P =.29; amygdala vs. ACC, z =.855, P =.393; OFC vs. ACC, z =.723, P =.47; bottom row: amygdala vs. OFC, z = 3.26, P =.2, amygdala vs. ACC, z = 3.4, P < 3 ; OFC vs. ACC, z =.8, P =.994. Analyses have been performed on the entire neuronal population for each brain area: amygdala, n = 96; OFC, n = 34; ACC, n = 87; see Methods). e, The proportion of neurons in each brain area that exhibit a significant response to each of the eight viewed faces. f, Average decoding performance for each brain area for classifying monkey image (left) or fractal (right) identity (n =, iterations). Left, curves show the decoding performance for an eight-way classification of monkey face images (equalized number of neurons for the three brain areas, n = neurons). Right, average decoding performance for each brain structure of discriminating between the three fractal images (three-way classifier; equalized number of neurons for the three brain areas, n = 3 neurons). Shading, 95% confidence intervals (bootstrap). Dotted lines, chance decoding level. g, Average z-scored firing rate plotted as a function of hierarchical status for neurons that respond more strongly (black line and dots) or weakly (gray line and dots) to a more highly ranked monkey (M) than to a lower ranked monkey (M8) in amygdala (left), OFC (center) and ACC (right). Since neural responses to M and M8 are used to classify the cells, these data are excluded from the regression analyses. Pearson s correlation coefficient (two-sided): amygdala, r =.92, P =., n = 2 neurons (black), r =.82, P =.46, n = 83 (gray); OFC, r =.88, P =.2, n = 79 (black), r =.32, P =.538, n = 49 (gray); ACC, r =.9, P =.72, n = 97 (black), r =.87, P =.23, n = 87 (gray). Error bars, s.e.m. Neural responses were analyzed during the time epoch extending from 4 ms after image onset for all panels in this figure; fixation was required during this interval. 48 Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
5 NaTure NeuroscIence image onset and extending through the fixation interval (Fig. 3f). During the beginning of free viewing, however, decoding performance was significantly below chance, indicating that the opposite classification was made in this time interval, but that a relationship still existed between the encoding of fractals associated with different rewards and hierarchical rank. By contrast, when the same analysis was applied to neural responses to monkeys who had similar hierarchical rank (M4 and M5), decoding performance was barely different from chance (Fig. 3i). The same analysis performed on OFC and ACC neurons failed to classify hierarchical status when training the decoder on fractal image trials (Fig. 3g,h,j,k). We used the analysis in Fig. 3f to define two time epochs: decoder above chance, which occupied 4 ms after image onset and occurred during fixation; and decoder below chance, which occupied 4 7 ms after image onset ( 3 ms after fixation point extinction). We then trained the decoder on large-reward and noreward fractal image trials separately during each epoch and tested decoding for all pairs of face images during the corresponding time intervals, using the neuronal populations recorded from each brain area. In general, for the amygdala, decoding performance became increasingly different from chance with increasing distance between the social positions of two monkeys (Fig. 4a). Moreover, consistent with the analysis in which the decoder was tested on M vs. M8 (Fig. 3f), there was an opposite sign in decoding accuracy for the two time epochs. The same analysis applied to OFC and ACC demonstrated markedly less robust decoding of hierarchical status (Fig. 4b,c). These data indicate that the ability to decode the reward magnitude associated with an image does not necessarily result in the capacity to decode hierarchical rank, although it does so in the amygdala. Decoder performance was quantitatively related to the social status index calculated from oculomotor behavior (see Fig. c). For each face image, we computed the average decoding performance for that image compared to each of the other faces when the decoder was trained to classify fractal images associated with large or no reward. In the amygdala, this average decoding performance was correlated with the computed social status index for that face image (P =.6 and P =.3, Fig. 4d and Fig. 4g, respectively). The regression slopes were opposite in the two time epochs, consistent with a change in sign in relationship between reward value and hierarchical status encoding at the ensemble level. A significant relationship between average decoding performance and social status index was not observed for OFC and ACC (P =.86 for OFC, Fig. 4e; P =.298 for ACC, Fig. 4f; P =.234 for ACC, Fig. 4i), except for during the second time epoch in OFC (P =.42, Fig. 4h). In addition, decoding performance between monkey face images was linearly correlated with the social distance between monkeys in the amygdala for the first and second time epochs, again with opposite signs for the two time epochs. (This analysis resampled the identities of the monkeys in the data points; Supplementary Fig. 6, r =.84, P =.8 and r =.92, P =.3.) We considered two possible explanations for the intriguing change in sign of the relationship between the decoding of trials with different reward magnitudes and the decoding of hierarchical rank in the amygdala that occurred as a function of time (during and after required fixation). One possibility was that individual neurons may change their response preferences over time with respect to either hierarchical rank or expected reward magnitude. Alternatively, response preferences might be preserved, but instead different subensembles of neurons might contribute to the relationship between hierarchical rank and reward expectation during the two time intervals. The evidence supports this latter possibility. First, the preferred response selectivity of neurons tended to be preserved across time intervals for both reward value and hierarchical rank preference. Each of the regression coefficients β frac and β soc, which quantify the relationship between firing rate and either Articles the reward associated with fractals or the hierarchical status of face images, were correlated across the two time intervals on a cell-bycell basis (r =.49, P < 6 for β frac, Supplementary Fig. 7a, and r =.79, P < 5 for β soc, Supplementary Fig. 7b; regression analyses). A change in sign in the relationship between decoding reward magnitude and hierarchical rank is therefore not likely to be accounted for by a change in the underlying response preferences of individual neurons. Second, in the first time epoch (during fixation), for the dominant-preferring amygdala neurons (β soc < ), we observed a significant correlation between the two regression coefficients β frac and β soc, such that neurons that fired more for larger expected rewards fired more for dominant face images (P =.32, r =.2). This was not observed in OFC and ACC for the subpopulations of neurons exhibiting a weak correlation with rank (Fig. 2g) (P =.94, r =.7 for dominant-preferring neurons in OFC and P =.337, r =. for submissive-preferring neurons in ACC, see Supplementary Fig. 8a d). Consistent with this, dominant-preferring amygdala neurons responded significantly more to the large-reward fractal than to the fractal predicting no reward (P =.45, paired t-test, Supplementary Fig. 9a), unlike submissive-preferring amygdala neurons (P =.22, paired t-test in all cases, Supplementary Fig. 9b) or the subpopulations of OFC and ACC neurons that exhibited a weak correlation with hierarchical rank (Fig. 2g) (P =.226 for OFC and P =.452 for ACC, paired t-test, Supplementary Fig. 9c,d). Finally, in the second time epoch (after the fixation requirement), a significant relationship was observed between β frac and β soc when considering all amygdala neurons (r =.5, P =.39, Supplementary Fig. a). This relationship seemed to be driven predominantly by neurons responding most strongly to submissive monkeys (r =.2, P =.66, Supplementary Fig. a) as opposed to neurons responding most strongly to dominant monkeys (r =.4, P =.694, Supplementary Fig. a), which were the subensemble of neurons correlated with reward selectivity during the first time epoch (see above). Furthermore, neurons that responded more strongly to M8 than M (submissive-preferring neurons) during the second time epoch responded significantly more strongly to the large-reward fractal than to the no-reward fractal (P =.3, paired t-test, Supplementary Fig. c), a relationship not observed for the dominant-preferring neurons (P =.434, paired t-test, Supplementary Fig. b). Overall, therefore, the change in sign in decoding performance in the amygdala for hierarchical rank during the second compared to the first time epoch appears to be accounted for by a change in the relative contribution of dominant- and submissive-preferring subensembles of neurons to decoding performance in the two time epochs. These results can also be observed at a qualitative level by examining average neural responses to dominant and submissive face images separately for populations of neurons that prefer large or no reward (Supplementary Fig. ). We considered the possibility that the differential engagement of subpopulations of neurons during the two time epochs was related to differences in oculomotor behavior that could emerge as a result of the initiation of a free viewing interval. However, we did not observe any differences in oculomotor metrics that were related to social hierarchy (Supplementary Fig. 2) or reward amount of the fractal images. Even during the time interval extending from 7, ms after image onset, significant differences in oculomotor behavior that were related to hierarchical rank were not observed (Supplementary Fig. 3). The time window used to compute the social index, where oculomotor differences emerged, was at the very end of the free viewing epoch, more than, ms after image onset. Despite the fact that the observed patterns of neural activity could not be accounted for by oculomotor behavior, covert changes in cognitive processes, such as a desire to shift gaze or attention depending upon the hierarchical rank of a viewed face, may occur upon fixation point offset that could cause differential engagement of amygdala neuronal subensembles. Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
6 Articles NaTure NeuroscIence a Training Fractal (CS ++/large reward) Fractal 3 (CS /no reward) b Testing Fractal or Monkey X Fractal 3 or Monkey Y c Decoding performance (fractals large vs. no reward) f Decoding performance (M vs. M8) i Decoding performance (M4 vs. M5) Neuron 2 firing rate Cond. Cond. 2 Neuron firing rate Neuron 2 firing rate Cond. Cond. 2 Neuron firing rate Amygdala OFC ACC Training and testing on fractal images d e st epoch ( to 4 ms after image onset).2 2nd epoch (4 to 7 ms after image onset) Training on fractal images and testing on social images g h j k Time from image onset (s) Time from image onset (s) Time from image onset (s) Fig. 3 The same neuronal ensemble in the amygdala can decode reward value and differences in hierarchical status. a,b. Procedure for determining whether same neural ensemble represents the reward associated with fractals and the hierarchical status of viewed face images. A linear decoder is trained to discriminate between fractals associated with large and no reward on a subset of trials (a). Decoding performance (b) was tested on held-out fractal trials or on trials with any two monkey face images (for example, monkey X and monkey Y). c e, Decoding performance plotted as a function of time for each brain area when testing the decoder on held-out large- and no-reward trials. f h, Decoding performance for each brain area when testing the decoder on high- vs. low-ranked monkey image trials (M vs. M8; i.e., the most distant monkeys in terms of social status). i k, Decoding performance when testing the decoder on two adjacent middle-ranked monkeys (M4 vs. M5). Shading, 95% confidence intervals (bootstrap). Dotted lines, chance decoding level. For all these analyses the decoder has been trained and tested on the entire neuronal population (i.e., without downsampling, n =, iterations; see Methods). Discussion This study took advantage of the fact that ten monkeys had lived together in the same social group for many years, enabling us to investigate the neurophysiological encoding of social hierarchy. Human observers and a social status index computed from measures of monkeys oculomotor behavior as they viewed face images of their social cohort indexed the social hierarchy within this group in a consistent manner. Electrophysiological data revealed that the primate amygdala provided a neural representation of the hierarchical rank of individuals. As in prior studies, neurons in the amygdala also represented the reward magnitudes associated with different nonsocial (fractal) images. The representation of hierarchical rank was apparent in the same neuronal ensemble that represent rewards associated with nonsocial stimuli. Training a linear decoder to discriminate between trials in which different nonsocial stimuli predict large or no reward permitted decoding of the hierarchical relationship between individuals in the monkeys social group. These data provide strong evidence that the same neuronal ensembles that encode expected reward magnitudes may mediate aspects of social behavior by representing a complex social variable, hierarchical rank 42. The data therefore argue against strong versions of the social brain hypothesis by demonstrating that, at least for the encoding of hierarchical rank, the same neuronal substrate in the amygdala appears to process social and nonsocial information. Our results indicate that neuronal ensembles that represent reward magnitudes associated with nonsocial stimuli may also be used to represent the hierarchical ranks of individuals. Stimuli that depict individuals with known hierarchical rank convey motivational meaning to a subject, just as reward-predictive stimuli convey motivational meaning. Both social and nonsocial stimuli can influence subsequent decisions, actions, and emotional responses more broadly. However, two lines of evidence indicate that representations of the reward magnitudes associated with nonsocial stimuli do not inherently represent hierarchical rank in all brain 42 Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
7 NaTure NeuroscIence Articles a Amygdala b OFC c ACC Social position Social position Social position d Amygdala e OFC f ACC r =.86.3 r =..3 r =.42 P =.6 P =.86 P = g h i r =.89 r =.72 r =.47.3 P =.3.3 P =.42.3 P = Viewed monkey social index Viewed monkey social index Viewed monkey social index Social position st epoch AVERAGE DECODING PERF. 2nd epoch AVERAGE DECODING PERF. First epoch Second epoch First epoch Second epoch First epoch Second epoch Fig. 4 The relationship between the encoding of reward value and hierarchical status. a c, Peak decoding performance in the amygdala, OFC and ACC tested after training the decoder to classify large- vs. no-reward trials and testing the decoder for all pairs of monkey face images (for example, M vs. M8, M vs. M7, etc.; see Methods). Color scale indicates decoding performance minus.5, so a value of corresponds to chance decoding. Warm and cool colors above and below the identity diagonal reflect decoding above and below chance in the two time intervals, respectively. Decoding performance during fixation ( 4 ms after image onset), upper left of each plot; decoding performance during free viewing (4 7 ms after image onset), lower right of each plot. d i, Average decoding performance for each monkey face image (across all comparisons) plotted as a function of that monkey s social status index for the first (d f) and second (g i) time epochs in the three brain areas. Note the inversion of sign in the amygdala between the first (d) and second (g) time epochs. For each panel n = 8, Pearson s correlation coefficient (two-sided): amygdala, r =.86, P =.6 (first epoch), r =.89, P =.3 (second epoch); OFC, r =., P =.86 (first epoch), r =.72, P =.42 (second epoch); ACC, r =.42, P =.298 (first epoch), r =.47, P =.234 (second epoch). Normalized decoding performance (peak) structures. First, the OFC and ACC both represent expected reward magnitudes strongly, but neither structure provided a compelling representation of hierarchical rank; training a linear decoder to discriminate between large and no reward fractal image trials did not allow us to decode hierarchical rank in those structures (Figs. 2 4). Second, we observed that the relationship between representations of reward magnitude and hierarchical rank in the amygdala changed as a function of time during a trial (Figs. 3 and 4). This observation argues strongly that the results cannot be easily accounted for by neural signals that merely encode variables such as arousal, attention, valuation or other related processes. It is very unlikely, for example, that an arousing and attention-grabbing alpha monkey becomes not arousing or attention-grabbing within a few hundred milliseconds, which would be required for signals encoding arousal or attention to account for our data. Instead, cell-by-cell regression analyses suggested that separate populations of amygdala neurons may be engaged at different times during a trial to represent hierarchical rank, and these subpopulations have differential relationships with the encoding of expected reward magnitudes (Supplementary Figs. 7 ). Our data are also unlikely to be accounted for by a low-level visual feature of face images that somehow conveys hierarchical rank. Training a decoder on neural responses to fractal images that predict different reward amounts should not be expected to allow decoding of hierarchical rank based on a low-level visual feature. In short, it is difficult to imagine that a particular sensory feature of face images that conveys hierarchical rank information has a consistent relationship with reward magnitudes associated with fractal images. However, the shared encoding in the amygdala of hierarchical rank and the rewards associated with nonsocial stimuli could reflect the provision of a common currency for stimuli of many different types that is present transiently in the amygdala (as the representation of social rank was not sustained across the entire trial, unlike reward value; Fig. 3c,f) 43, but further experiments are needed. To wit, the neuronal ensembles we have characterized may also represent other social variables, such as body gestures or complex facial expressions that convey emotional and motivational meaning, and it is unknown whether the representations of these social variables are related to encoding of the reward values of nonsocial stimuli. It remains possible that some aspects of primate social behavior Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
8 Articles share the same neuronal encoding as nonsocial processes, with other aspects of social behavior being mediated by different neural representations 42,44. Monkeys almost certainly learn the hierarchical rank of other group members through experience, so the acquisition of a neural representation of hierarchical rank may occur in a similar manner as when a subject learns the relationship between nonsocial stimuli and rewards through reinforcement learning 45. Of course, learning about hierarchical rank may also be facilitated through observational learning 46. The sharing of a neuronal substrate for learning about nonsocial rewards and about social hierarchy information has mechanistic implications for understanding the pathophysiology and potential treatments for some patients with psychiatric disorders. For example, patients with autism spectrum disorder or schizophrenia often exhibit deficits in social interactions, sometimes struggling to appropriately register social situations or cues or to respond to such situations adaptively 47,48. Some of these patients may also possess deficits in learning about the significance (for example, reward value) of nonsocial stimuli 49. Indeed, applied behavior analysis treatments based on reinforcing correct behavior with different types of reward have been shown to benefit many patients with autism spectrum disorder 5, suggesting that focused behavioral manipulations may compensate for deficits in learning mechanisms in these patients. However, the neural underpinnings of deficits in social processing that also requires learning has remained unclear, in large part because we have understood relatively little about how the brain represents and utilizes social information. The discovery of neural representations of hierarchical rank in the amygdala observed in the same neural ensembles that represent the rewards associated with nonsocial stimuli may thereby provide a significant step toward describing the neural networks critical for understanding psychiatric pathology and treatment. Methods Methods, including statements of data availability and any associated accession codes and references, are available at org/.38/s Received: 3 July 27; Accepted: 2 December 27; Published online: 9 February 28 References. Thierry, B. Covariation of conflict management patterns across macaque species. in Natural Conflict Resolution (ed. Aureli, F. & de Waal, F.B.M.) 6 28 (University of California Press, Berkeley, CA, USA, 2). 2. Bercovitch, F. B. Social-stratification, social strategies, and reproductive success in primates. Ethol. Sociobiol. 2, (99). 3. Cowlishaw, G. & Dunbar, R. Dominance rank and mating success in male primates. Anim. Behav. 4, (99). 4. Sapolsky, R. M. The influence of social hierarchy on primate health. Science 38, (25). 5. Byrne, R. & Whiten, A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes and Humans. (Oxford Science Publications, Oxford, 989). 6. Schell, A., Rieck, K., Schell, K., Hammerschmidt, K. & Fischer, J. Adult but not juvenile Barbary macaques spontaneously recognize group members from pictures. Anim. Cogn. 4, (2). 7. Pfefferle, D., Kazem, A. J., Brockhausen, R. R., Ruiz-Lambides, A. V. & Widdig, A. Monkeys spontaneously discriminate their unfamiliar paternal kin under natural conditions using facial cues. Curr. Biol. 24, 86 8 (24). 8. Tsao, D. Y., Freiwald, W. A., Tootell, R. B. H. & Livingstone, M. S. A cortical region consisting entirely of face-selective cells. Science 3, (26). 9. Gothard, K. M., Battaglia, F. P., Erickson, C. A., Spitler, K. M. & Amaral, D. G. Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol. 97, (27).. Hoffman, K. L., Gothard, K. M., Schmid, M. C. & Logothetis, N. K. Facial-expression and gaze-selective responses in the monkey amygdala. Curr. Biol. 7, (27).. Rutishauser, U. et al. Single-unit responses selective for whole faces in the human amygdala. Curr. Biol. 2, (2). NaTure NeuroscIence 2. Azzi, J. C., Sirigu, A. & Duhamel, J. R. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. USA 9, (22). 3. Tsao, D. Y., Schweers, N., Moeller, S. & Freiwald, W. A. Patches of faceselective cortex in the macaque frontal lobe. Nat. Neurosci., (28). 4. Watson, K. K. & Platt, M. L. Social signals in primate orbitofrontal cortex. Curr. Biol. 22, (22). 5. Chang, L. & Tsao, D. Y. The code for facial identity in the primate brain. Cell 69, 3 28.e4 (27). 6. Amaral, D., Price, J., Pitkanen, A. & Carmichael, S. Anatomical organization of the primate amygdaloid complex. in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction (ed. Aggleton, J.) 66 (Wiley-Liss, New York, 992). 7. Ghashghaei, H. T., Hilgetag, C. C. & Barbas, H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34, (27). 8. Stefanacci, L. & Amaral, D. G. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J. Comp. Neurol. 45, (22). 9. Paton, J. J., Belova, M. A., Morrison, S. E. & Salzman, C. D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, (26). 2. Morrison, S. E., Saez, A., Lau, B. & Salzman, C. D. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron 7, 27 4 (2). 2. Cai, X. & Padoa-Schioppa, C. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J. Neurosci. 32, (22). 22. Dunbar, R. I. M. The social brain hypothesis. Evol. Anthropol. 6, 78 9 (998). 23. Sallet, J. et al. Social network size affects neural circuits in macaques. Science 334, (2). 24. Noonan, M. P. et al. A neural circuit covarying with social hierarchy in macaques. PLoS Biol. 2, e94 (24). 25. Bickart, K. C., Wright, C. I., Dautoff, R. J., Dickerson, B. C. & Barrett, L. F. Amygdala volume and social network size in humans. Nat. Neurosci. 4, (2). 26. Powell, J. L., Lewis, P. A., Dunbar, R. I. M., García-Fiñana, M. & Roberts, N. Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia 48, (2). 27. Kanai, R., Bahrami, B., Roylance, R. & Rees, G. Online social network size is reflected in human brain structure. Proc. Biol. Sci. 279, (22). 28. Watanabe, N. & Yamamoto, M. Neural mechanisms of social dominance. Front. Neurosci. 9, 54 (25). 29. Kumaran, D., Melo, H. L. & Duzel, E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron 76, (22). 3. Zink, C. F. et al. Know your place: neural processing of social hierarchy in humans. Neuron 58, (28). 3. Ligneul, R., Obeso, I., Ruff, C. C. & Dreher, J. C. Dynamical representation of dominance relationships in the human rostromedial prefrontal cortex. Curr. Biol. 26, (26). 32. Kumaran, D., Banino, A., Blundell, C., Hassabis, D. & Dayan, P. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron 92, (26). 33. Chang, S. W. et al. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl. Acad. Sci. USA 2, (25). 34. Chang, S. W., Gariépy, J. F. & Platt, M. L. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 6, (23). 35. Klein, J. T. & Platt, M. L. Social information signaling by neurons in primate striatum. Curr. Biol. 23, (23). 36. Yoshida, K., Saito, N., Iriki, A. & Isoda, M. Social error monitoring in macaque frontal cortex. Nat. Neurosci. 5, (22). 37. Klein, J. T., Deaner, R. O. & Platt, M. L. Neural correlates of social target value in macaque parietal cortex. Curr. Biol. 8, (28). 38. Zumpe, D. & Michael, R. P. Dominance index a simple measure of relative dominance status in primates. Am. J. Primatol., 29 3 (986). 39. Shepherd, S. V. & Platt, M. L. Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta). Anim. Cogn., 3 2 (28). 4. Deaner, R. O., Khera, A. V. & Platt, M. L. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 5, (25). 4. Saez, A., Rigotti, M., Ostojic, S., Fusi, S. & Salzman, C. D. Abstract context representations in primate amygdala and prefrontal cortex. Neuron 87, (25). 422 Nature Neuroscience VOL 2 MARCH Nature America Inc., part of Springer Nature. All rights reserved.
Supplementary materials for: Executive control processes underlying multi- item working memory
 Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Double dissociation of value computations in orbitofrontal and anterior cingulate neurons
 Supplementary Information for: Double dissociation of value computations in orbitofrontal and anterior cingulate neurons Steven W. Kennerley, Timothy E. J. Behrens & Jonathan D. Wallis Content list: Supplementary
Supplementary Information for: Double dissociation of value computations in orbitofrontal and anterior cingulate neurons Steven W. Kennerley, Timothy E. J. Behrens & Jonathan D. Wallis Content list: Supplementary
Behavioral generalization
 Supplementary Figure 1 Behavioral generalization. a. Behavioral generalization curves in four Individual sessions. Shown is the conditioned response (CR, mean ± SEM), as a function of absolute (main) or
Supplementary Figure 1 Behavioral generalization. a. Behavioral generalization curves in four Individual sessions. Shown is the conditioned response (CR, mean ± SEM), as a function of absolute (main) or
Nature Neuroscience: doi: /nn Supplementary Figure 1. Behavioral training.
 Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
The primate amygdala combines information about space and value
 The primate amygdala combines information about space and Christopher J Peck 1,8, Brian Lau 1,7,8 & C Daniel Salzman 1 6 npg 213 Nature America, Inc. All rights reserved. A stimulus predicting reinforcement
The primate amygdala combines information about space and Christopher J Peck 1,8, Brian Lau 1,7,8 & C Daniel Salzman 1 6 npg 213 Nature America, Inc. All rights reserved. A stimulus predicting reinforcement
The individual animals, the basic design of the experiments and the electrophysiological
 SUPPORTING ONLINE MATERIAL Material and Methods The individual animals, the basic design of the experiments and the electrophysiological techniques for extracellularly recording from dopamine neurons were
SUPPORTING ONLINE MATERIAL Material and Methods The individual animals, the basic design of the experiments and the electrophysiological techniques for extracellularly recording from dopamine neurons were
Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more
 1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature14066 Supplementary discussion Gradual accumulation of evidence for or against different choices has been implicated in many types of decision-making, including value-based decisions
doi:10.1038/nature14066 Supplementary discussion Gradual accumulation of evidence for or against different choices has been implicated in many types of decision-making, including value-based decisions
Sum of Neurally Distinct Stimulus- and Task-Related Components.
 SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
Decision neuroscience seeks neural models for how we identify, evaluate and choose
 VmPFC function: The value proposition Lesley K Fellows and Scott A Huettel Decision neuroscience seeks neural models for how we identify, evaluate and choose options, goals, and actions. These processes
VmPFC function: The value proposition Lesley K Fellows and Scott A Huettel Decision neuroscience seeks neural models for how we identify, evaluate and choose options, goals, and actions. These processes
Supplementary Material for
 Supplementary Material for Selective neuronal lapses precede human cognitive lapses following sleep deprivation Supplementary Table 1. Data acquisition details Session Patient Brain regions monitored Time
Supplementary Material for Selective neuronal lapses precede human cognitive lapses following sleep deprivation Supplementary Table 1. Data acquisition details Session Patient Brain regions monitored Time
Methods to examine brain activity associated with emotional states and traits
 Methods to examine brain activity associated with emotional states and traits Brain electrical activity methods description and explanation of method state effects trait effects Positron emission tomography
Methods to examine brain activity associated with emotional states and traits Brain electrical activity methods description and explanation of method state effects trait effects Positron emission tomography
Supplementary Information
 Supplementary Information The neural correlates of subjective value during intertemporal choice Joseph W. Kable and Paul W. Glimcher a 10 0 b 10 0 10 1 10 1 Discount rate k 10 2 Discount rate k 10 2 10
Supplementary Information The neural correlates of subjective value during intertemporal choice Joseph W. Kable and Paul W. Glimcher a 10 0 b 10 0 10 1 10 1 Discount rate k 10 2 Discount rate k 10 2 10
Decoding a Perceptual Decision Process across Cortex
 Article Decoding a Perceptual Decision Process across Cortex Adrián Hernández, 1 Verónica Nácher, 1 Rogelio Luna, 1 Antonio Zainos, 1 Luis Lemus, 1 Manuel Alvarez, 1 Yuriria Vázquez, 1 Liliana Camarillo,
Article Decoding a Perceptual Decision Process across Cortex Adrián Hernández, 1 Verónica Nácher, 1 Rogelio Luna, 1 Antonio Zainos, 1 Luis Lemus, 1 Manuel Alvarez, 1 Yuriria Vázquez, 1 Liliana Camarillo,
Supplementary Information. Gauge size. midline. arcuate 10 < n < 15 5 < n < 10 1 < n < < n < 15 5 < n < 10 1 < n < 5. principal principal
 Supplementary Information set set = Reward = Reward Gauge size Gauge size 3 Numer of correct trials 3 Numer of correct trials Supplementary Fig.. Principle of the Gauge increase. The gauge size (y axis)
Supplementary Information set set = Reward = Reward Gauge size Gauge size 3 Numer of correct trials 3 Numer of correct trials Supplementary Fig.. Principle of the Gauge increase. The gauge size (y axis)
CS/NEUR125 Brains, Minds, and Machines. Due: Friday, April 14
 CS/NEUR125 Brains, Minds, and Machines Assignment 5: Neural mechanisms of object-based attention Due: Friday, April 14 This Assignment is a guided reading of the 2014 paper, Neural Mechanisms of Object-Based
CS/NEUR125 Brains, Minds, and Machines Assignment 5: Neural mechanisms of object-based attention Due: Friday, April 14 This Assignment is a guided reading of the 2014 paper, Neural Mechanisms of Object-Based
Face processing in different brain areas and face recognition
 F Face processing in different brain areas and face recognition Edmund T Rolls Oxford Centre for Computational Neuroscience, Oxford, UK Department of Computer Science, University of Warwick, Coventry,
F Face processing in different brain areas and face recognition Edmund T Rolls Oxford Centre for Computational Neuroscience, Oxford, UK Department of Computer Science, University of Warwick, Coventry,
Resistance to forgetting associated with hippocampus-mediated. reactivation during new learning
 Resistance to Forgetting 1 Resistance to forgetting associated with hippocampus-mediated reactivation during new learning Brice A. Kuhl, Arpeet T. Shah, Sarah DuBrow, & Anthony D. Wagner Resistance to
Resistance to Forgetting 1 Resistance to forgetting associated with hippocampus-mediated reactivation during new learning Brice A. Kuhl, Arpeet T. Shah, Sarah DuBrow, & Anthony D. Wagner Resistance to
Delay activity dynamics: task dependent time encoding and low dimensional trajectories
 Delay activity dynamics: task dependent time encoding and low dimensional trajectories Christopher J. Cueva 1 4, Encarni Marcos 5, Alex Saez 1, Aldo Genovesio 5, Mehrdad Jazayeri 6,7, Ranulfo Romo 8,9,
Delay activity dynamics: task dependent time encoding and low dimensional trajectories Christopher J. Cueva 1 4, Encarni Marcos 5, Alex Saez 1, Aldo Genovesio 5, Mehrdad Jazayeri 6,7, Ranulfo Romo 8,9,
Supplementary Figure 1. Recording sites.
 Supplementary Figure 1 Recording sites. (a, b) Schematic of recording locations for mice used in the variable-reward task (a, n = 5) and the variable-expectation task (b, n = 5). RN, red nucleus. SNc,
Supplementary Figure 1 Recording sites. (a, b) Schematic of recording locations for mice used in the variable-reward task (a, n = 5) and the variable-expectation task (b, n = 5). RN, red nucleus. SNc,
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load
 Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
There are, in total, four free parameters. The learning rate a controls how sharply the model
 Supplemental esults The full model equations are: Initialization: V i (0) = 1 (for all actions i) c i (0) = 0 (for all actions i) earning: V i (t) = V i (t - 1) + a * (r(t) - V i (t 1)) ((for chosen action
Supplemental esults The full model equations are: Initialization: V i (0) = 1 (for all actions i) c i (0) = 0 (for all actions i) earning: V i (t) = V i (t - 1) + a * (r(t) - V i (t 1)) ((for chosen action
EDGE DETECTION. Edge Detectors. ICS 280: Visual Perception
 EDGE DETECTION Edge Detectors Slide 2 Convolution & Feature Detection Slide 3 Finds the slope First derivative Direction dependent Need many edge detectors for all orientation Second order derivatives
EDGE DETECTION Edge Detectors Slide 2 Convolution & Feature Detection Slide 3 Finds the slope First derivative Direction dependent Need many edge detectors for all orientation Second order derivatives
Nature Medicine: doi: /nm.4084
 Supplementary Figure 1: Sample IEDs. (a) Sample hippocampal IEDs from different kindled rats (scale bar = 200 µv, 100 ms). (b) Sample temporal lobe IEDs from different subjects with epilepsy (scale bar
Supplementary Figure 1: Sample IEDs. (a) Sample hippocampal IEDs from different kindled rats (scale bar = 200 µv, 100 ms). (b) Sample temporal lobe IEDs from different subjects with epilepsy (scale bar
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Chapter 5. Summary and Conclusions! 131
 ! Chapter 5 Summary and Conclusions! 131 Chapter 5!!!! Summary of the main findings The present thesis investigated the sensory representation of natural sounds in the human auditory cortex. Specifically,
! Chapter 5 Summary and Conclusions! 131 Chapter 5!!!! Summary of the main findings The present thesis investigated the sensory representation of natural sounds in the human auditory cortex. Specifically,
A CONVERSATION ABOUT NEURODEVELOPMENT: LOST IN TRANSLATION
 A CONVERSATION ABOUT NEURODEVELOPMENT: LOST IN TRANSLATION Roberto Tuchman, M.D. Chief, Department of Neurology Nicklaus Children s Hospital Miami Children s Health System 1 1 in 6 children with developmental
A CONVERSATION ABOUT NEURODEVELOPMENT: LOST IN TRANSLATION Roberto Tuchman, M.D. Chief, Department of Neurology Nicklaus Children s Hospital Miami Children s Health System 1 1 in 6 children with developmental
Supplementary Figure 1. Localization of face patches (a) Sagittal slice showing the location of fmri-identified face patches in one monkey targeted
 Supplementary Figure 1. Localization of face patches (a) Sagittal slice showing the location of fmri-identified face patches in one monkey targeted for recording; dark black line indicates electrode. Stereotactic
Supplementary Figure 1. Localization of face patches (a) Sagittal slice showing the location of fmri-identified face patches in one monkey targeted for recording; dark black line indicates electrode. Stereotactic
NIH Public Access Author Manuscript Nat Neurosci. Author manuscript; available in PMC 2012 November 25.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Neurosci. ; 14(10): 1247 1249. doi:10.1038/nn.2899. A category-specific response to animals in the right human amygdala Florian
NIH Public Access Author Manuscript Published in final edited form as: Nat Neurosci. ; 14(10): 1247 1249. doi:10.1038/nn.2899. A category-specific response to animals in the right human amygdala Florian
Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex
 The Journal of Neuroscience, March 14, 2012 32(11):3791 3808 3791 Behavioral/Systems/Cognitive Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex Xinying Cai 1 and Camillo
The Journal of Neuroscience, March 14, 2012 32(11):3791 3808 3791 Behavioral/Systems/Cognitive Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex Xinying Cai 1 and Camillo
Neurons in the human amygdala selective for perceived emotion. Significance
 Neurons in the human amygdala selective for perceived emotion Shuo Wang a, Oana Tudusciuc b, Adam N. Mamelak c, Ian B. Ross d, Ralph Adolphs a,b,e, and Ueli Rutishauser a,c,e,f, a Computation and Neural
Neurons in the human amygdala selective for perceived emotion Shuo Wang a, Oana Tudusciuc b, Adam N. Mamelak c, Ian B. Ross d, Ralph Adolphs a,b,e, and Ueli Rutishauser a,c,e,f, a Computation and Neural
Reorganization between preparatory and movement population responses in motor cortex
 Received 27 Jun 26 Accepted 4 Sep 26 Published 27 Oct 26 DOI:.38/ncomms3239 Reorganization between preparatory and movement population responses in motor cortex Gamaleldin F. Elsayed,2, *, Antonio H. Lara
Received 27 Jun 26 Accepted 4 Sep 26 Published 27 Oct 26 DOI:.38/ncomms3239 Reorganization between preparatory and movement population responses in motor cortex Gamaleldin F. Elsayed,2, *, Antonio H. Lara
The Integration of Features in Visual Awareness : The Binding Problem. By Andrew Laguna, S.J.
 The Integration of Features in Visual Awareness : The Binding Problem By Andrew Laguna, S.J. Outline I. Introduction II. The Visual System III. What is the Binding Problem? IV. Possible Theoretical Solutions
The Integration of Features in Visual Awareness : The Binding Problem By Andrew Laguna, S.J. Outline I. Introduction II. The Visual System III. What is the Binding Problem? IV. Possible Theoretical Solutions
 Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 11: Attention & Decision making Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis
Introduction to Computational Neuroscience Lecture 11: Attention & Decision making Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature21682 Supplementary Table 1 Summary of statistical results from analyses. Mean quantity ± s.e.m. Test Days P-value Deg. Error deg. Total deg. χ 2 152 ± 14 active cells per day per mouse
doi:10.1038/nature21682 Supplementary Table 1 Summary of statistical results from analyses. Mean quantity ± s.e.m. Test Days P-value Deg. Error deg. Total deg. χ 2 152 ± 14 active cells per day per mouse
The Frontal Lobes. Anatomy of the Frontal Lobes. Anatomy of the Frontal Lobes 3/2/2011. Portrait: Losing Frontal-Lobe Functions. Readings: KW Ch.
 The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
correlates with social context behavioral adaptation.
 REVIEW OF FRONTAL LOBE STRUCTURES Main organization of frontal cortex: 1. Motor area (precentral gyrus). 2. Premotor & supplementary motor areas (immediately anterior to motor area). Includes premotor,
REVIEW OF FRONTAL LOBE STRUCTURES Main organization of frontal cortex: 1. Motor area (precentral gyrus). 2. Premotor & supplementary motor areas (immediately anterior to motor area). Includes premotor,
TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab
 TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab This report details my summer research project for the REU Theoretical and Computational Neuroscience program as
TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab This report details my summer research project for the REU Theoretical and Computational Neuroscience program as
Effects of Amygdala Lesions on Reward-Value Coding in Orbital and Medial Prefrontal Cortex
 Article Effects of Amygdala Lesions on Reward-Value Coding in Orbital and Medial Prefrontal Cortex Peter H. Rudebeck, 1, * Andrew R. Mitz, 1 Ravi V. Chacko, 1 and Elisabeth A. Murray 1 1 Section on the
Article Effects of Amygdala Lesions on Reward-Value Coding in Orbital and Medial Prefrontal Cortex Peter H. Rudebeck, 1, * Andrew R. Mitz, 1 Ravi V. Chacko, 1 and Elisabeth A. Murray 1 1 Section on the
Supplemental Information: Adaptation can explain evidence for encoding of probabilistic. information in macaque inferior temporal cortex
 Supplemental Information: Adaptation can explain evidence for encoding of probabilistic information in macaque inferior temporal cortex Kasper Vinken and Rufin Vogels Supplemental Experimental Procedures
Supplemental Information: Adaptation can explain evidence for encoding of probabilistic information in macaque inferior temporal cortex Kasper Vinken and Rufin Vogels Supplemental Experimental Procedures
Toward a Mechanistic Understanding of Human Decision Making Contributions of Functional Neuroimaging
 CURRENT DIRECTIONS IN PSYCHOLOGICAL SCIENCE Toward a Mechanistic Understanding of Human Decision Making Contributions of Functional Neuroimaging John P. O Doherty and Peter Bossaerts Computation and Neural
CURRENT DIRECTIONS IN PSYCHOLOGICAL SCIENCE Toward a Mechanistic Understanding of Human Decision Making Contributions of Functional Neuroimaging John P. O Doherty and Peter Bossaerts Computation and Neural
A model to explain the emergence of reward expectancy neurons using reinforcement learning and neural network $
 Neurocomputing 69 (26) 1327 1331 www.elsevier.com/locate/neucom A model to explain the emergence of reward expectancy neurons using reinforcement learning and neural network $ Shinya Ishii a,1, Munetaka
Neurocomputing 69 (26) 1327 1331 www.elsevier.com/locate/neucom A model to explain the emergence of reward expectancy neurons using reinforcement learning and neural network $ Shinya Ishii a,1, Munetaka
Neuronal representations of cognitive state: reward or attention?
 Opinion TRENDS in Cognitive Sciences Vol.8 No.6 June 2004 representations of cognitive state: reward or attention? John H.R. Maunsell Howard Hughes Medical Institute & Baylor College of Medicine, Division
Opinion TRENDS in Cognitive Sciences Vol.8 No.6 June 2004 representations of cognitive state: reward or attention? John H.R. Maunsell Howard Hughes Medical Institute & Baylor College of Medicine, Division
Orbitofrontal cortical activity during repeated free choice
 J Neurophysiol 107: 3246 3255, 2012. First published March 14, 2012; doi:10.1152/jn.00690.2010. Orbitofrontal cortical activity during repeated free choice Michael Campos, Kari Koppitch, Richard A. Andersen,
J Neurophysiol 107: 3246 3255, 2012. First published March 14, 2012; doi:10.1152/jn.00690.2010. Orbitofrontal cortical activity during repeated free choice Michael Campos, Kari Koppitch, Richard A. Andersen,
Distinct Value Signals in Anterior and Posterior Ventromedial Prefrontal Cortex
 Supplementary Information Distinct Value Signals in Anterior and Posterior Ventromedial Prefrontal Cortex David V. Smith 1-3, Benjamin Y. Hayden 1,4, Trong-Kha Truong 2,5, Allen W. Song 2,5, Michael L.
Supplementary Information Distinct Value Signals in Anterior and Posterior Ventromedial Prefrontal Cortex David V. Smith 1-3, Benjamin Y. Hayden 1,4, Trong-Kha Truong 2,5, Allen W. Song 2,5, Michael L.
Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B
 Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B Cortical Analysis of Visual Context Moshe Bar, Elissa Aminoff. 2003. Neuron, Volume 38, Issue 2, Pages 347 358. Visual objects in context Moshe Bar.
Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B Cortical Analysis of Visual Context Moshe Bar, Elissa Aminoff. 2003. Neuron, Volume 38, Issue 2, Pages 347 358. Visual objects in context Moshe Bar.
Neural correlates of multisensory cue integration in macaque MSTd
 Neural correlates of multisensory cue integration in macaque MSTd Yong Gu, Dora E Angelaki,3 & Gregory C DeAngelis 3 Human observers combine multiple sensory cues synergistically to achieve greater perceptual
Neural correlates of multisensory cue integration in macaque MSTd Yong Gu, Dora E Angelaki,3 & Gregory C DeAngelis 3 Human observers combine multiple sensory cues synergistically to achieve greater perceptual
HHS Public Access Author manuscript Neuron. Author manuscript; available in PMC 2017 June 15.
 Orbitofrontal cortex value signals depend on fixation location during free viewing Vincent B. McGinty 1, Antonio Rangel 2, and William T. Newsome 1,3 1 Department of Neurobiology, Stanford University,
Orbitofrontal cortex value signals depend on fixation location during free viewing Vincent B. McGinty 1, Antonio Rangel 2, and William T. Newsome 1,3 1 Department of Neurobiology, Stanford University,
Choosing the Greater of Two Goods: Neural Currencies for Valuation and Decision Making
 Choosing the Greater of Two Goods: Neural Currencies for Valuation and Decision Making Leo P. Surgre, Gres S. Corrado and William T. Newsome Presenter: He Crane Huang 04/20/2010 Outline Studies on neural
Choosing the Greater of Two Goods: Neural Currencies for Valuation and Decision Making Leo P. Surgre, Gres S. Corrado and William T. Newsome Presenter: He Crane Huang 04/20/2010 Outline Studies on neural
Emotion I: General concepts, fear and anxiety
 C82NAB Neuroscience and Behaviour Emotion I: General concepts, fear and anxiety Tobias Bast, School of Psychology, University of Nottingham 1 Outline Emotion I (first part) Studying brain substrates of
C82NAB Neuroscience and Behaviour Emotion I: General concepts, fear and anxiety Tobias Bast, School of Psychology, University of Nottingham 1 Outline Emotion I (first part) Studying brain substrates of
Memory-Guided Sensory Comparisons in the Prefrontal Cortex: Contribution of Putative Pyramidal Cells and Interneurons
 The Journal of Neuroscience, February 22, 2012 32(8):2747 2761 2747 Behavioral/Systems/Cognitive Memory-Guided Sensory Comparisons in the Prefrontal Cortex: Contribution of Putative Pyramidal Cells and
The Journal of Neuroscience, February 22, 2012 32(8):2747 2761 2747 Behavioral/Systems/Cognitive Memory-Guided Sensory Comparisons in the Prefrontal Cortex: Contribution of Putative Pyramidal Cells and
Hebbian Plasticity for Improving Perceptual Decisions
 Hebbian Plasticity for Improving Perceptual Decisions Tsung-Ren Huang Department of Psychology, National Taiwan University trhuang@ntu.edu.tw Abstract Shibata et al. reported that humans could learn to
Hebbian Plasticity for Improving Perceptual Decisions Tsung-Ren Huang Department of Psychology, National Taiwan University trhuang@ntu.edu.tw Abstract Shibata et al. reported that humans could learn to
Neurobiological Foundations of Reward and Risk
 Neurobiological Foundations of Reward and Risk... and corresponding risk prediction errors Peter Bossaerts 1 Contents 1. Reward Encoding And The Dopaminergic System 2. Reward Prediction Errors And TD (Temporal
Neurobiological Foundations of Reward and Risk... and corresponding risk prediction errors Peter Bossaerts 1 Contents 1. Reward Encoding And The Dopaminergic System 2. Reward Prediction Errors And TD (Temporal
brain valuation & behavior
 brain valuation & behavior 9 Rangel, A, et al. (2008) Nature Neuroscience Reviews Vol 9 Stages in decision making process Problem is represented in the brain Brain evaluates the options Action is selected
brain valuation & behavior 9 Rangel, A, et al. (2008) Nature Neuroscience Reviews Vol 9 Stages in decision making process Problem is represented in the brain Brain evaluates the options Action is selected
Selective bias in temporal bisection task by number exposition
 Selective bias in temporal bisection task by number exposition Carmelo M. Vicario¹ ¹ Dipartimento di Psicologia, Università Roma la Sapienza, via dei Marsi 78, Roma, Italy Key words: number- time- spatial
Selective bias in temporal bisection task by number exposition Carmelo M. Vicario¹ ¹ Dipartimento di Psicologia, Università Roma la Sapienza, via dei Marsi 78, Roma, Italy Key words: number- time- spatial
Representation of negative motivational value in the primate
 Representation of negative motivational value in the primate lateral habenula Masayuki Matsumoto & Okihide Hikosaka Supplementary Figure 1 Anticipatory licking and blinking. (a, b) The average normalized
Representation of negative motivational value in the primate lateral habenula Masayuki Matsumoto & Okihide Hikosaka Supplementary Figure 1 Anticipatory licking and blinking. (a, b) The average normalized
Spectro-temporal response fields in the inferior colliculus of awake monkey
 3.6.QH Spectro-temporal response fields in the inferior colliculus of awake monkey Versnel, Huib; Zwiers, Marcel; Van Opstal, John Department of Biophysics University of Nijmegen Geert Grooteplein 655
3.6.QH Spectro-temporal response fields in the inferior colliculus of awake monkey Versnel, Huib; Zwiers, Marcel; Van Opstal, John Department of Biophysics University of Nijmegen Geert Grooteplein 655
Lateral view of human brain! Cortical processing of touch!
 Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Encoding of Reward and Space During a Working Memory Task in the Orbitofrontal Cortex and Anterior Cingulate Sulcus
 J Neurophysiol 12: 3352 3364, 29. First published September 23, 29; doi:1.1152/jn.273.29. Encoding of Reward and Space During a Working Memory Task in the Orbitofrontal Cortex and Anterior Cingulate Sulcus
J Neurophysiol 12: 3352 3364, 29. First published September 23, 29; doi:1.1152/jn.273.29. Encoding of Reward and Space During a Working Memory Task in the Orbitofrontal Cortex and Anterior Cingulate Sulcus
Supplemental Information. Triangulating the Neural, Psychological, and Economic Bases of Guilt Aversion
 Neuron, Volume 70 Supplemental Information Triangulating the Neural, Psychological, and Economic Bases of Guilt Aversion Luke J. Chang, Alec Smith, Martin Dufwenberg, and Alan G. Sanfey Supplemental Information
Neuron, Volume 70 Supplemental Information Triangulating the Neural, Psychological, and Economic Bases of Guilt Aversion Luke J. Chang, Alec Smith, Martin Dufwenberg, and Alan G. Sanfey Supplemental Information
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Neuronal reference frames for social decisions in primate frontal cortex
 ARTICLES Neuronal reference frames for social decisions in primate frontal cortex Steve W C Chang 1,, Jean-François Gariépy & Michael L Platt 1 3 npg 13 Nature America, Inc. All rights reserved. Social
ARTICLES Neuronal reference frames for social decisions in primate frontal cortex Steve W C Chang 1,, Jean-François Gariépy & Michael L Platt 1 3 npg 13 Nature America, Inc. All rights reserved. Social
Supporting Information
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 Supporting Information Variances and biases of absolute distributions were larger in the 2-line
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 Supporting Information Variances and biases of absolute distributions were larger in the 2-line
Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis
 Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis (OA). All subjects provided informed consent to procedures
Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis (OA). All subjects provided informed consent to procedures
5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng
 5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng 13:30-13:35 Opening 13:30 17:30 13:35-14:00 Metacognition in Value-based Decision-making Dr. Xiaohong Wan (Beijing
5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng 13:30-13:35 Opening 13:30 17:30 13:35-14:00 Metacognition in Value-based Decision-making Dr. Xiaohong Wan (Beijing
Key questions about attention
 Key questions about attention How does attention affect behavioral performance? Can attention affect the appearance of things? How does spatial and feature-based attention affect neuronal responses in
Key questions about attention How does attention affect behavioral performance? Can attention affect the appearance of things? How does spatial and feature-based attention affect neuronal responses in
Neuronal Representations of Value
 C H A P T E R 29 c00029 Neuronal Representations of Value Michael Platt and Camillo Padoa-Schioppa O U T L I N E s0020 s0030 s0040 s0050 s0060 s0070 s0080 s0090 s0100 s0110 Introduction 440 Value and Decision-making
C H A P T E R 29 c00029 Neuronal Representations of Value Michael Platt and Camillo Padoa-Schioppa O U T L I N E s0020 s0030 s0040 s0050 s0060 s0070 s0080 s0090 s0100 s0110 Introduction 440 Value and Decision-making
Neural Coding. Computing and the Brain. How Is Information Coded in Networks of Spiking Neurons?
 Neural Coding Computing and the Brain How Is Information Coded in Networks of Spiking Neurons? Coding in spike (AP) sequences from individual neurons Coding in activity of a population of neurons Spring
Neural Coding Computing and the Brain How Is Information Coded in Networks of Spiking Neurons? Coding in spike (AP) sequences from individual neurons Coding in activity of a population of neurons Spring
Planning activity for internally generated reward goals in monkey amygdala neurons
 Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 March ; 18(3): 461 469. doi:10.1038/nn.3925. Planning activity for internally generated reward goals in
Europe PMC Funders Group Author Manuscript Published in final edited form as: Nat Neurosci. 2015 March ; 18(3): 461 469. doi:10.1038/nn.3925. Planning activity for internally generated reward goals in
The Function and Organization of Lateral Prefrontal Cortex: A Test of Competing Hypotheses
 The Function and Organization of Lateral Prefrontal Cortex: A Test of Competing Hypotheses Jeremy R. Reynolds 1 *, Randall C. O Reilly 2, Jonathan D. Cohen 3, Todd S. Braver 4 1 Department of Psychology,
The Function and Organization of Lateral Prefrontal Cortex: A Test of Competing Hypotheses Jeremy R. Reynolds 1 *, Randall C. O Reilly 2, Jonathan D. Cohen 3, Todd S. Braver 4 1 Department of Psychology,
Supplemental Information. Direct Electrical Stimulation in the Human Brain. Disrupts Melody Processing
 Current Biology, Volume 27 Supplemental Information Direct Electrical Stimulation in the Human Brain Disrupts Melody Processing Frank E. Garcea, Benjamin L. Chernoff, Bram Diamond, Wesley Lewis, Maxwell
Current Biology, Volume 27 Supplemental Information Direct Electrical Stimulation in the Human Brain Disrupts Melody Processing Frank E. Garcea, Benjamin L. Chernoff, Bram Diamond, Wesley Lewis, Maxwell
Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia
 1 Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia Christine I. Hooker, Taylor L. Benson, Anett Gyurak, Hong Yin,
1 Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia Christine I. Hooker, Taylor L. Benson, Anett Gyurak, Hong Yin,
Visual Categorization: How the Monkey Brain Does It
 Visual Categorization: How the Monkey Brain Does It Ulf Knoblich 1, Maximilian Riesenhuber 1, David J. Freedman 2, Earl K. Miller 2, and Tomaso Poggio 1 1 Center for Biological and Computational Learning,
Visual Categorization: How the Monkey Brain Does It Ulf Knoblich 1, Maximilian Riesenhuber 1, David J. Freedman 2, Earl K. Miller 2, and Tomaso Poggio 1 1 Center for Biological and Computational Learning,
2 INSERM U280, Lyon, France
 Natural movies evoke responses in the primary visual cortex of anesthetized cat that are not well modeled by Poisson processes Jonathan L. Baker 1, Shih-Cheng Yen 1, Jean-Philippe Lachaux 2, Charles M.
Natural movies evoke responses in the primary visual cortex of anesthetized cat that are not well modeled by Poisson processes Jonathan L. Baker 1, Shih-Cheng Yen 1, Jean-Philippe Lachaux 2, Charles M.
Nature Neuroscience: doi: /nn Supplementary Figure 1. Large-scale calcium imaging in vivo.
 Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Basic definition and Classification of Anhedonia. Preclinical and Clinical assessment of anhedonia.
 Basic definition and Classification of Anhedonia. Preclinical and Clinical assessment of anhedonia. Neurobiological basis and pathways involved in anhedonia. Objective characterization and computational
Basic definition and Classification of Anhedonia. Preclinical and Clinical assessment of anhedonia. Neurobiological basis and pathways involved in anhedonia. Objective characterization and computational
Memory, Attention, and Decision-Making
 Memory, Attention, and Decision-Making A Unifying Computational Neuroscience Approach Edmund T. Rolls University of Oxford Department of Experimental Psychology Oxford England OXFORD UNIVERSITY PRESS Contents
Memory, Attention, and Decision-Making A Unifying Computational Neuroscience Approach Edmund T. Rolls University of Oxford Department of Experimental Psychology Oxford England OXFORD UNIVERSITY PRESS Contents
Supplementary Materials for
 Supplementary Materials for Folk Explanations of Behavior: A Specialized Use of a Domain-General Mechanism Robert P. Spunt & Ralph Adolphs California Institute of Technology Correspondence may be addressed
Supplementary Materials for Folk Explanations of Behavior: A Specialized Use of a Domain-General Mechanism Robert P. Spunt & Ralph Adolphs California Institute of Technology Correspondence may be addressed
Choice-related activity and correlated noise in subcortical vestibular neurons
 Choice-related activity and correlated noise in subcortical vestibular neurons Sheng Liu,2, Yong Gu, Gregory C DeAngelis 3 & Dora E Angelaki,2 npg 23 Nature America, Inc. All rights reserved. Functional
Choice-related activity and correlated noise in subcortical vestibular neurons Sheng Liu,2, Yong Gu, Gregory C DeAngelis 3 & Dora E Angelaki,2 npg 23 Nature America, Inc. All rights reserved. Functional
Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation
 Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation GREGG H. RECANZONE 1 AND ROBERT H. WURTZ 2 1 Center for Neuroscience and Section of Neurobiology, Physiology and Behavior,
Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation GREGG H. RECANZONE 1 AND ROBERT H. WURTZ 2 1 Center for Neuroscience and Section of Neurobiology, Physiology and Behavior,
Chapter 4. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories
 131 Chapter 4. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories 4.1 Introduction 4 Episodic memories allow us to remember not only whether we have seen something
131 Chapter 4. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories 4.1 Introduction 4 Episodic memories allow us to remember not only whether we have seen something
Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex
 The Journal of Neuroscience, March 11, 29 29(1):3259 327 3259 Behavioral/Systems/Cognitive Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex Steven W. Kennerley and Jonathan D.
The Journal of Neuroscience, March 11, 29 29(1):3259 327 3259 Behavioral/Systems/Cognitive Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex Steven W. Kennerley and Jonathan D.
Big brains may hold clues to origins of autism
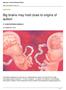 VIEWPOINT Big brains may hold clues to origins of autism BY KONSTANTINOS ZARBALIS 23 FEBRUARY 2016 A persistent challenge to improving our understanding of autism is the fact that no single neurological
VIEWPOINT Big brains may hold clues to origins of autism BY KONSTANTINOS ZARBALIS 23 FEBRUARY 2016 A persistent challenge to improving our understanding of autism is the fact that no single neurological
The perirhinal cortex and long-term familiarity memory
 THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY 2005, 58B (3/4), 234 245 The perirhinal cortex and long-term familiarity memory E. T. Rolls, L. Franco, and S. M. Stringer University of Oxford, UK To analyse
THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY 2005, 58B (3/4), 234 245 The perirhinal cortex and long-term familiarity memory E. T. Rolls, L. Franco, and S. M. Stringer University of Oxford, UK To analyse
Behavioral Neuroscience: Fear thou not. Rony Paz
 Behavioral Neuroscience: Fear thou not Rony Paz Rony.paz@weizmann.ac.il Thoughts What is a reward? Learning is best motivated by threats to survival? Threats are much better reinforcers? Fear is a prime
Behavioral Neuroscience: Fear thou not Rony Paz Rony.paz@weizmann.ac.il Thoughts What is a reward? Learning is best motivated by threats to survival? Threats are much better reinforcers? Fear is a prime
Repetition Suppression Accompanying Behavioral Priming. in Macaque Inferotemporal Cortex. David B.T. McMahon* and Carl R. Olson
 Repetition Suppression Accompanying Behavioral Priming in Macaque Inferotemporal Cortex David B.T. McMahon* and Carl R. Olson * To whom correspondence should be addressed: mcmahon@cnbc.cmu.edu Center for
Repetition Suppression Accompanying Behavioral Priming in Macaque Inferotemporal Cortex David B.T. McMahon* and Carl R. Olson * To whom correspondence should be addressed: mcmahon@cnbc.cmu.edu Center for
Geography of the Forehead
 5. Brain Areas Geography of the Forehead Everyone thinks the brain is so complicated, but let s look at the facts. The frontal lobe, for example, is located in the front! And the temporal lobe is where
5. Brain Areas Geography of the Forehead Everyone thinks the brain is so complicated, but let s look at the facts. The frontal lobe, for example, is located in the front! And the temporal lobe is where
Distinct valuation subsystems in the human brain for effort and delay
 Supplemental material for Distinct valuation subsystems in the human brain for effort and delay Charlotte Prévost, Mathias Pessiglione, Elise Météreau, Marie-Laure Cléry-Melin and Jean-Claude Dreher This
Supplemental material for Distinct valuation subsystems in the human brain for effort and delay Charlotte Prévost, Mathias Pessiglione, Elise Météreau, Marie-Laure Cléry-Melin and Jean-Claude Dreher This
Motor Systems I Cortex. Reading: BCP Chapter 14
 Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Reward prediction based on stimulus categorization in. primate lateral prefrontal cortex
 Reward prediction based on stimulus categorization in primate lateral prefrontal cortex Xiaochuan Pan, Kosuke Sawa, Ichiro Tsuda, Minoro Tsukada, Masamichi Sakagami Supplementary Information This PDF file
Reward prediction based on stimulus categorization in primate lateral prefrontal cortex Xiaochuan Pan, Kosuke Sawa, Ichiro Tsuda, Minoro Tsukada, Masamichi Sakagami Supplementary Information This PDF file
The role of amplitude, phase, and rhythmicity of neural oscillations in top-down control of cognition
 The role of amplitude, phase, and rhythmicity of neural oscillations in top-down control of cognition Chair: Jason Samaha, University of Wisconsin-Madison Co-Chair: Ali Mazaheri, University of Birmingham
The role of amplitude, phase, and rhythmicity of neural oscillations in top-down control of cognition Chair: Jason Samaha, University of Wisconsin-Madison Co-Chair: Ali Mazaheri, University of Birmingham
Models of Attention. Models of Attention
 Models of Models of predictive: can we predict eye movements (bottom up attention)? [L. Itti and coll] pop out and saliency? [Z. Li] Readings: Maunsell & Cook, the role of attention in visual processing,
Models of Models of predictive: can we predict eye movements (bottom up attention)? [L. Itti and coll] pop out and saliency? [Z. Li] Readings: Maunsell & Cook, the role of attention in visual processing,
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1139 a d Whisker angle (deg) Whisking repeatability Control Muscimol.4.3.2.1 -.1 8 4-4 1 2 3 4 Performance (d') Pole 8 4-4 1 2 3 4 5 Time (s) b Mean protraction angle (deg) e Hit rate (p
doi:1.138/nature1139 a d Whisker angle (deg) Whisking repeatability Control Muscimol.4.3.2.1 -.1 8 4-4 1 2 3 4 Performance (d') Pole 8 4-4 1 2 3 4 5 Time (s) b Mean protraction angle (deg) e Hit rate (p
Theta sequences are essential for internally generated hippocampal firing fields.
 Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Neural Correlates of Human Cognitive Function:
 Neural Correlates of Human Cognitive Function: A Comparison of Electrophysiological and Other Neuroimaging Approaches Leun J. Otten Institute of Cognitive Neuroscience & Department of Psychology University
Neural Correlates of Human Cognitive Function: A Comparison of Electrophysiological and Other Neuroimaging Approaches Leun J. Otten Institute of Cognitive Neuroscience & Department of Psychology University
Functional Elements and Networks in fmri
 Functional Elements and Networks in fmri Jarkko Ylipaavalniemi 1, Eerika Savia 1,2, Ricardo Vigário 1 and Samuel Kaski 1,2 1- Helsinki University of Technology - Adaptive Informatics Research Centre 2-
Functional Elements and Networks in fmri Jarkko Ylipaavalniemi 1, Eerika Savia 1,2, Ricardo Vigário 1 and Samuel Kaski 1,2 1- Helsinki University of Technology - Adaptive Informatics Research Centre 2-
Supplemental Material
 1 Supplemental Material Golomb, J.D, and Kanwisher, N. (2012). Higher-level visual cortex represents retinotopic, not spatiotopic, object location. Cerebral Cortex. Contents: - Supplemental Figures S1-S3
1 Supplemental Material Golomb, J.D, and Kanwisher, N. (2012). Higher-level visual cortex represents retinotopic, not spatiotopic, object location. Cerebral Cortex. Contents: - Supplemental Figures S1-S3
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
