Ba*+ Ions Evoke Two Kinetically Distinct Patterns of Exocytosis in Chromaffin Cells, but Not in Neurohypophysial Nerve Terminals
|
|
|
- Pierce Owens
- 5 years ago
- Views:
Transcription
1 The Journal of Neuroscience, February 15, 1996, 76(4): Ba*+ Ions Evoke Two Kinetically Distinct Patterns of Exocytosis in Chromaffin Cells, but Not in Neurohypophysial Nerve Terminals Elizabeth P. Seward, Natalya I. Chernevskaya, and Martha C. Nowycky Department of Neurobiology and Anatomy, Medical College of Pennsylvania and Hahnemann University, Philadelphia, Pennsylvania The coupling between divalent cations and exocytosis of large dense-cored vesicles (LDCV) was studied with capacitancedetection techniques in nerve terminals of the rat neurohypophysis (NHP) and bovine chromaffin cells. Ba*+ substitution for Ca*+ produced kinetically distinct responses in the two preparations. In NHP terminals, Ba *+ ions behave as weak substitutes for Ca*+. Exocytotic events occur principally during depolarizing pulses, i.e., events are stimulus-coupled to Ba + entry through voltage-gated Ca*+ channels. Stimulus-coupled exocytosis apparently requires elevated submembrane cation concentrations that dissipate rapidly on hyperpolarization-induced Ca +-channel closure. Intracellular dialysis of NHP terminals with Ba + does not evoke exocytosis, nor does it interfere with depolarization-evoked Ca2+ influx and exocytosis. In chromaffin cells, Ba + ions evoke a small quantity of stimulus-coupled secretion, but the dominant response is an additional pronounced poststimulus capacitance increase that outlasts channel closures by sec. Stimulus-decoupled exocytosis is slow ( ff/sec) compared with Ca +evoked stimulus-coupled exocytosis (-1000 ff/sec). Decoupled secretion is not attributable to Ba2+ displacement of intracellular Ca*+ Ions, because it is insensitive to 10 mm EGTA or thapsigargin. Slow exocytosis is initiated by inclusion of Ba*+ ions in the recording pipette and continues steadily for 5-12 min, producing a total increase of several thousand ff, which ultimately doubles or triples the original cell-surface area. We propose that two pathways of regulated exocytosis with distinct kinetics and divalent cation sensitivity exist in chromaffin cells. Only a single kinetic pattern is detected in NHP terminals, suggesting that mechanisms for secretion are not universally distributed in excitable cells. Key words: calcium-secretion coupling; membranecapacitance detection; exocytosis; chromaffin cells; neurohypophysis; asynchronous release; large dense-cored vesicles Neurohypophysial (NHP) nerve endings and chromaffin cells are two classic systems for the investigation of Ca +-regulated secretion of large dense-cored vesicles (LDCV) (Douglas and Poisner, 1964; Douglas and Rubin, 1964). The two systems share many properties: (1) secretion is triggered by bursts of action potentials (APs) rather than single APs (Ozawa and Sand, 1986); (2) both respond to low concentrations of intracellular Cazt in permeabilized preparations (-1-10 PM) (Knight and Baker, 1982; Cazalis et al., 1987); and (3) each show s relatively rapid decay of the maximal secretory response despite maintained Cazt levels (Baker and Rink, 1975; Stuenkel and Nordmann, 1993). However, differences in Ca +-secretion coupling have also been noted: (1) in whole-cell recordings, secretion in NHP terminals is abolished within -15 set at elevated Cazt concentrations (Lindau et al., 1992) but in chromaffin cells continues for minutes, although at submaximal rates (Augustine and Neher, 1992); and (2) NHP terminals respond to trains of depolarizing pulses with a single kinetic mode termed threshold, whereas chromaffin cells con- Received Aug. 17, 1995; revised Nov. 6, 1995; accepted Nov. 29, This work was supported by Grants NS22281 from the National Institute of Neurological and Communicative Disorders and Stroke (M.C.N.) and a Canadian Medical Research Council Fellowship (E.P.S.). We thank Drs. K&hrin Engisch and Alla Fomina for critical comments on this manuscript. Corresoondcnce should be addressed to Dr. Martha C. Nowvckv. Deoartment of Neurobidlogy and Anatomy, Medical College of Pennsylvania and fiahncmann University, 3200 Henry Avenue, Philadelphia, PA Dr. Seward s present address: Department of Pharmacology, -. School of Medical Sciences, Unive&ty Walk, Bristol D&lTD, UK. Copyright Society for Neuroscience /96/ *$05,00/O tain an additional response termed docked secretion (Seward et al., 1995; Seward and Nowycky, 1996). In many excitable cells including both NHP terminals (Ishida, 1968; Cazalis et al., 1987) and chromaffin cells (Douglas and Rubin, 1964; Forsberg and Pollard, 1988; Heldman et al., 1989; TerBush and Holz, 1992), Bazt ions are potent secretagogues. Previous studies on Bazt -induced secretion in NHP terminals concluded that Ba2+ ions evoke vasopressin release, although they are only approximately one-third as effective as equimolar concentrations of Cazt ions (Cazalis et al., 1987). Studies on intact and permeabilized chromaffin cells are much more extensive. Although both Ca2+ and Bazt ions evoke catecholamine secretion, there are kinetic and other differences between the release produced by the two ions. Ba +-induced secretion is always slower than that evoked by Ca +, yet Ba2+ elicits much more total catecholamine release (Izumi et al., 1986; Heldman et al., 1989; Castillo et al., 1992; Michelena et al., 1993). Ba2+induced secretion does not self-limit as does Ca +-induced secretion (Heldman et al., 1989; Castillo et al., 1992; Michelena et al., 1993; Jankowski et al., 1994), which accounts for the difference in total release. Also, Ca +-induced secretion is blocked by trifluoperazine and imipramine, whereas Ba +-induced secretion is insensitive to both agents (Heldman et al., 1989). Here we examine Ba2+-induced secretion in single NHP terminals and chromaffin cells using the capacitance-detection technique, which has excellent temporal resolution and sensitivity. Although the technique only reports changes in surface membrane area, there is extensive earlier evidence that Ba2+ does
2 Seward et al. l Two Pathways for Regulated Exocytosis J. Neurosci., February 15, 1996, 76(4): A C G 25fF = B =o- ~ 50fF I 4Q- A A& A At! SE w 20- A.dAM o-k Figure 1. Ba*+ substitutes weakly for Ca*+ in triggering exocytosis from NHP terminals. A, Superimposed C, traces from two terminals with typical patterns of exocytosis observed with external Cazt and Ba +. In extracellular Ca +, the stimulus train consisted of 5 pulses, 46 msec in duration, and in extracellular Ba*+ of 20 pulses, 72 msec in duration. Depolarizing pulses are indicated by vertical bars below the C, traces. AC, jumps evoked by Ca2+ pulses were nonuniform (4-17 ff), whereas those triggered by Ba*+ influx were fairly uniform and small (O-4 ff). B, Data from A plotted as cumulative Ca + (open triangles) and Ba*+ (filled triangles) influx versus cumulative C, increases. In 30 terminals, the mean Ca2+ influx required to evoke a C, increase of ff was 154? 14 x lo6 ions, whereas in Ba2+ an equivalent C, increase (49? 6 ff) required 535 i 119 X lo6 ions. Thus, BaZf ions are approximately one-fifth as potent as Ca2+ ions in evoking stimuluscoupled secretion. C, Ba*+-evoked secretion is proportional to the amount of Bazt influx and persists in the presence of 10 mm EGTA,. Superimposed C, traces are from a single terminal stimulated with 3 trains of 30 pulses. The first BaZt current evoked by each train is shown below. In a, pulses were of 200 msec duration, TP = - 10 mv; in b, 200 msec, +20 mv; and in c, 400 msec, 120 mv. In 10 mm EGTA, a 49 I 6 ff C, increase was achieved by 4600? 500 X lo6 Ba2+ ions (n = 3), or -8-9 times as many as needed in the standard solution with 0.5 mm EGTA. release peptide hormones from NHP and catecholamines from chromaffin cells (see above). We report that, in both preparations, Bazt ions trigger exocytosis by substituting weakly for Cazt through a conventional, stimulus-coupled pathway. However, in chromaffin cells, but not in NHP terminals, the bulk of Bazt activated secretion takes place through a kinetically distinct, slow, stimulus-decoupled pathway. Some of these results have been presented in abstract form (Seward and Nowycky, 1991; Chernevskaya et al., 1995). MATERIALS AND METHODS Preparations. NHP terminals were prepared as described previously (Lim et al., 1990; Seward et al., 1995). The pipette solution consisted of (in mm): Cs-D-glutamate 115, HEPES 40, MgCl, 1, CsCl 10, Mg-ATP 2.5, and EGTA 0.5, adjusted to ph 7.2 with CsOH. Terminals were continuously superfused with external solution consisting of (in mm): NaCl 145, KC1 5, MgCl, 1, CaCl, or BaCl, 10, HEPES 10, glucose 10, and 1 /LM tetrodotoxin, adjusted to ph 7.2 with NaOH. Adult bovine chromaffin cells were cultured for 2-4 d as described in Vitale et al. (1991). The internal solution consisted of (in mm): CS-Dglutamate 145, HEPES 10, NaCl 9.5, Mg-ATP 2, and bis(2- aminophenoxy)ethane-n,n,n,n -tetra-acetic acid (BAPTA) 0.3, adjusted to ph 7.2 with CsOH. The external solution consisted of (in mm): NaCl 130, KC1 2, MgCl, 1, CaCl, or BaCl, 5, HEPES 10, glucose 10, and 1 PM tetrodotoxin, adjusted to ph 7.2 with NaOH. For the experiments shown in Figure 6, thapsigargin (1 PM) was added to the external solution after establishing whole-cell recording. Calculation of free intracellular BaZt concentrations. In some experiments, 1 mm Ba + was added to the pipette solution. To chelate Cazi present in distilled water and to control cytosolic Ca*+ that might be released from intracellular stores or displaced from proteins, 1 mm EGTA was used instead of BAPTA because it is much more selective for CaZt than Ba*+ (Fabiato and Fabiato, 1979) Free BaZt concentration was calculated using the program Maxchelator (version 6.72, C. Patton, Hopkins Marine Station, Pacific Grove, CA), using binding values estimated by Fabiato and Fabiato (1979). The final concentration of free Bazt concentration in the intracellular solution was estimated as 0.17 mm for NHP terminals and 0.15 mm for chromaffin cells. It is important to note that these are calculations based on available information on Ba*+-EGTA interactions that may not be exact for the specific solutions used. Recording conditions. Whole-cell Ca +-channel currents and capacitance measurements were performed as described previously (Lim et al., 1990), using a computer-based, phase-tracking method (Neher and Marty, 1982; Joshi and Fernandez, 1988; Fidler and Fernandez, 1989). All voltage-pulse stimulations are from a holding potential of -90 mv to a test potential (TP) of +20 mv. Capacitance data are sampled at msec intervals. C, jumps are calculated by averaging four to five points before and after a depolarizing pulse. Slow C, (Cmmalow) increases were calculated by subtracting the sum of C, jumps from the total C, increase for a given trace. Mean trace duration is 18 sec. Pipette diameters. Patch pipettes used for experiments on NHP terminals had -1 km diameters and resistances of 3-5 MO. Pipettes for chromaffin cell experiments had wrn diameters and resistances of MO. Statistics. In Figure 6C, statistical comparisons were made using unpaired Student s t test for groups in which the SDS were equal and Mann-Whitney test for unequal SDS. RESULTS NHP terminals Ba2+ weakly elicits Cm responses coupled to depolarizingpulses The capacitance-detection technique was used to monitor exocytotic events in response to trains of depolarizing pulses. Figure 1A contains traces from two NHP terminals that are representative of
3 1372 J. Neurosci., February 15, 1996, 16(4): Seward et al. l Two Pathways for Regulated Exocytosis w 8o 5 60 $ 40 d 20 z 0 80 OY E 60 g 40 (j 20 z 0 Ca*+ 0 IO 20 >25 * C,,, W) Figure 2. C, jump amplitudes elicited by trains of pulses recorded in Ca2+ and Ba*+. Histogram of individual C, jump amplitudes obtained in C&+ and Ba (250 events each). Individual AC, events in Ca*+ ranged up to 79 ff, with 27% of the events >lo ff. In Ba*+, maximal events were 13 ff, and only 2% of the events were >lo ff. those elicited in extracellular solutions containing Cazt or Bazt and that are selected to illustrate similar total amounts of evoked AC,,,. With Ca2+ as the divalent cation, C, increases occur mostly as jumps during periods of Ca*+ influx (Seward et al., 1995). The C, jump size is often larger on the second or third depolarizing pulse (Fig. L4), as described previously (Lim et al., 1990; Seward et al., 1995). Bazf acts as a weak substitute for Ca*+, because all Ba* -induced jumps are smaller and much more Ba2+ entry is required to elicit equivalent total C, increases (note 5 vs 20 pulses indicated below traces and longer pulse durations in Ba*+). A plot of cumulative AC,,, versus cumulative divalent cation influx for these traces (Fig. 1B) indicates that approximately fivefold greater Ba*+ than Ca2+ entry is needed to induce similar total C, changes. The magnitude of the total C, response is proportional to the amount of Ba2+ -ion influx during a train. In Figure lc, three capacitance traces are illustrated along with the Bazi current evoked by the first pulse of each stimulus train. Minimal Ba*+ entry has no effect on C,,, whereas progressively larger currents elicit proportionally larger C, changes. The C, traces have the typical appearance of threshold secretion in which the earliest pulses of the train do not evoke C, increases, whereas later pulses trigger C, jumps once a threshold amount of divalent cation entry is achieved (Seward et al., 1995). Even when Bazt influx per pulse is very high as in this terminal with large Ca2+-channel currents (-1 na), all C, jumps are small and C, increases remain tightly coupled to depolarizations. Individual C, jump amplitudes recorded from 35 terminals in extracellular Ba2+ versus extracellular Ca*+ are plotted in Figure 2. There is a prominent difference in C, jump amplitude distributions for the two ions. Both Ba*+ and Ca*+ evoke many small events (~10 ff). However, the large jumps seen in response to Ca2+ are not evoked by Bazc, even though the Ba*+ influx per pulse was usually greater than for Ca*+. Ba2+ acts independently of Ca2+ To determine whether Ba2+ causes nonspecific damage, we tested mixtures of Ca2+/Ba2+ (5:5 mm>, which is predicted to cause significant influx of Ba2+ (H ess and Tsien, 1984). Responses to depolarizing pulses resemble those elicited by Cazt alone (i.e., larger-amplitude C,, jumps; n = 3; data not shown), indicating that there is no damage and that Ba*+ ions do not block access to critical release sites. To determine whether Ba*+ acts directly or through release of Cazt from internal stores, we used a high concentration of intracellular EGTA, a chelator with much greater affinity for Ca2+ versus Ba*+ (Fabiato and Fabiato, 1979). Ten millimolar EGTA abolished Ca*+-evoked response in most terminals (Lim et al., 1990; Seward et al., 1995), even when cumulative Ca*+ influx was 20-fold greater than required with 0.5 mm EGTA. In contrast, Ba2+ still elicited C, increases with 10 mm EGTA, although the total amount of Ba*+ entry had to be increased by approximately eight- to ninefold (compare legends of Fig. lb,c). Because Ca2+- and Ba* --evoked secretion occurs mostly during depolarizations, that is, periods of active cation entry, it appears that the fusion machinery of NHP terminals responds only to elevated submembrane divalent cation concentrations, i.e., exocytosis is stimulus-coupled. To test this further, we dialyzed NHP terminals with Ba2+ (1 mm total, estimated at 0.17 mm free). Figure 3 illustrates that intracellular dialysis with Ba*+ has no significant effect on membrane capacitance, as expected if vesicle fusion requires elevated divalent cation concentrations near the membrane. A depolarizing train elicits normal exocytotic response if the terminal is simultaneously bathed in extracellular Ca*+, providing further evidence that the weak responsiveness to Ba2+ IS. not attributable to nonspecific damage or occlusion of critical receptors. Bovine chromaffin cells Ba2 weakly elicits stimulus-coupled secretion and stimulates an additional slow response Although Ca*+ -evoked secretion in chromaffin cells shares many similarities with the response in NHP terminals (Seward and Nowycky, 1996), Ba2+ -evoked secretion is markedly different in the two preparations. Typical chromaffin-cell responses to trains of depolarizing pulses in Ca2+ and Ba*+ are illustrated by two superimposed traces in Figure 4. In Ca2+ (top truce), most of the C,,, increase occurs as jumps during depolarizing pulses when Ca2+ concentration is at its highest in the vicinity of the plasma membrane. After the last pulse, C, increases terminate almost immediately. This secretory pattern is similar to what is observed in NHP terminals and is termed stimulus-coupled secretion. When Ba*+ is substituted for extracellular Ca2, C, increases begin later in the train and jumps are much smaller, although divalent cation influx is increased approximately fivefold per pulse (Fig. 4, bottom trace; compare current traces). During the train, C, increases occur both as slow drifts between pulses and as C, jumps. However, the bulk of the C, response occurs as a prolonged slow rise that outlasts the end of the depolarizing train (awow) by tens of seconds. This. response is termed stimulusdecoupled secretion to emphasize the temporal dissociation between the stimulus train and most of the C,, increase. Table 1 contains a summary of some of the properties of Ba2+ induced stimulus-decoupled secretion. The total amount of C, increase (both coupled and decoupled) reflects the fusion of several hundred vesicles. The highest secretory rate for decoupled secretion is obtained immediately after the end of a stimulus train and averages ff set- (*SD; n. = 9). In most cells, slow C,, increases continue for set after the train. The rate of decay of poststimulus secretion could be fit by a single exponential that falls with a time constant of sec. Stimulus-decoupled secretion is not attributable to toxic effects
4 Seward et al.. Two Pathways for Regulated Exocytosis J. Neurosci., February 15, 1996, 76(4): Ba2+ in the pipette iiz 150- Ca2+, ,*, 1. I - I Figure 3. Intracellular dialysis with 1 mm Ba + does not elicit C, increases in NHP terminals and does not block secretion. The standard intracellular solution was modified by addition of 1 mm Ba +. The chelator was increased to 3 mm EGTA, yielding an estimated free EGTA concentration of mm (see Materials and Methods). During the time indicated by the solid bar, the terminal was stimulated by a train of 20 depolarizing pulses, 40 msec in duration, +20 mv, 5 Hz. The first depolarization exceeded the threshold and induced a relatively large C, jump. Each C, Increase (indicated by breaks in the trace) was followed by very rapid C, Time (set) decreases, which are observed occasionally in these terminals and may reflect a rapid endocytotic process. of Bazt because prominent stimulus-coupled events are observed after reintroduction of Ca +. Figure 5A illustrates Ca +-induced C, increases before (i) and after (iii) stimulation in Ba2+ (ii). The second response to Ca 2+ is smaller because of secretory rundown (Seward and Nowycky, 1996), but it terminates within 2 set of the last pulse. Slow C, increases could be evoked several times by repeated stimulation with Ba2+. When three pulse trains are given in rapid succession (Fig. 5B; note continuous time scale), there is little depression of the secretory response to the third train and, instead, C, increases to the second and third stimulus begin after a shorter delay than is evident in the first trace of the series. The magnitude of AC,,, is correlated with the absolute amount of Baz+ entry and is saturable. The two traces in Figure 5C are from a single cell stimulated with two pulse durations. In the trace with short pulses (i), there is less total AC, and it proceeds at a slower rate than for greater Ba2+ entry (ii), but is similarly prolonged. The relationship between total amount of Bazt entry and C, increases for both stimulus-coupled (open squares) and stimulus-decoupled (solid squares) responses for all recordings with 0.3 mm intracellular BAPTA is plotted in Figure 5D. When the total Bazt entry is <OS X 10 ions, no stimulus-decoupled secretion is observed (data not shown). The rate of stimulus: decoupled secretion never exceeded 50 ff/sec, even at the largest values of Ba2+ entry. Ba2+ evokes stimulus-decoupled secretion independently of Ca Chromaffin cells contain large-capacity, active intracellular Cazt stores that can support exocytosis in the absence of APs and voltage-gated Cazt entry (Cheek and Thastrup, 1989; O Sullivan et al., 1989; Mollard et al., 1995) and might contribute to the Ba2+-elicited C, increases. To test this, we used a combination of thapsigargin, a Ca +-ATPase inhibitor that depletes intracellular stores, and high concentrations of EGTA. A representative experiment is illustrated in Figure 6A. The recording was performed Table 1. Rates of slow, Ba*+-induced C,,, increases in single cells IOOfF -I 2s 50 ms Figure 4. Ca2+ and Ba2+ ions elicit exocytotic responses with different kinetics in chromaffin cells. Two superimposed traces from one cell. Stimulus trains consisted of 20 depolarizing pulses, 20 msec in duration for Ca + and 100 msec for Ba*+. The current traces evoked by the first and last depolarizing pulse of each train are plotted below. The timing of the last depolarizing stimulus is indicated by arrows. Other pulses are indicated as breaks in the C, trace. Asterisk marks 100 ff calibration deflections. 1 na Initial ZC, poststimulus Experimental ZBaZt influx increase rate ~decay conditions (X lo- ions) (ff) (ff secc ) (set) 0.3 rnm BAPTAi rnm BAPTA, CLM Thp, mm EGTA, mm EGTA, GM TM,
5 1374 J. Neurosci., February 15, 1996, 76(4): Seward et al. l Two Pathways for Regulated Exocytosis i ii 400- XlJlc,; i 200-, : I 600 B 1 r Time (WC),/ / Time (set) /I C 600 i ii 400 g oe a 200 I tqi w 1 F! i 4 Et, 1, <, I. I I I. I Time (set) I: Ba2+ ions (x 1 Og Figure 5. Ba*+-evoked stimulus-decoupled secretion is reversible, can be evoked multiple times, and depends on amount of Ba*+ entry. A, Ba +-evoked slow secretion is reversed in Cazt. Three C,, traces from a single chromaffin cell (0.3 mm BAPTA,): i, recorded 3.5 min after break in with 5 mm extracellular Ca*+ and stimulated with 20 pulses, 20 msec in duration (C, calibration of 100 ff); ii, 8 min later with 5 mm extracellular Ba*+, stimulated with 20 pulses, 100 msec in duration; iii, 14 min later, after reintroduction of 5 mm extracellular Ca +. Note appearance of slow endocytosis and a 100 ff poststimulus drift, but which terminates within 2 set, in contrast to Ba2+ -evoked secretion. B, Multiple Ba*+ -evoked slow C,, increases may be evoked in a single cell. Consecutive C, traces in response to three stimuli each consisting of 20 pulses, 100 msec in duration (0.3 mm BAPTA,, 5 mm Ba*+, and 1 FM thapsigargin). Previous exposure to Ba*+ decreases the delay in the onset of exocytosis in the second and third stimuli. C, Ba*+ dependence of C, increase. Two traces from a cell stimulated with (i) 20 pulses of 20 msec duration, with a cumulative Ba + influx of 9 x 10s ions and C, increase of 174 ff, (ii) 20 pulses of 100 msec duration, with a cumulative Ba + influx of 25 X 10s ions and 566 ff C, increase (0.3 mm BAPTA,, 5 mm Ba +, and 1 pm thapsigargin). D, Mean 2 SEM C, increases evoked by trains of depolarizations plotted against cumulative Ba z+ influx/train: filled symbols, poststimulus C, increases; open symbols, C, jumps coupled to depolarizations. Results are from 13 cells with 0.3 mm BAPTA,.
6 Seward et al. l Two Pathways for Regulated Exocytosis J. Neurosci., February 15, 1996, 16(4): g A 200 a ge I, M+@----,,,,+, -;, a2+ ; L+ '"- *,,,,, ;,, Con TM EGTA 80 ~,, d. f 0,~~ 0 1 I I I I I I I t Time (set) 20 0 t EdTA Figure 6. Bazf influx triggers slow, stimulus-decoupled exocytosis via a Ca*+ -independent mechanism. A, Three C, traces from a single chromaffin cell recorded with 10 mm EGTA in the internal solution. a, C,, increases evoked by 20 depolarizations, 100 mscc duration in Ca ~. High concentrations of EGTA greatly attenuated the secretory response of cells to Ca *+ influx (Seward and Nowycky, 1996). h, Addition of 1 pm thapsigargin to the superfusate had no effect on resting C, and minimal effect on Ca + -evoked C,,, increases in the presence of 10 mm EGTA. c, C,, response to 20 depolarizations of 200 msec duration after substitution of external Ca + with Ba ~ and in the continued presence of thapsigargin. The kinetics of the slow poststimulus C, increase is indistinguishable from recordings without high EGTA or thapsigargin pretreatment. B, C, Stimulus-coupled and -decoupled secretions were cstimatcd by dividing C, traces into changes occurring during depolarizations (see Materials and Methods) and those occurring during the interpulse interval and after the last depolarizing pulse. The fraction of stimulus-decoupled secretion is given directly in C. In L?, the remaining stimulus-coupled secretion is expressed in terms of AC,,,/divalent cation, which allows direct comparisons between the efficacy of Ca2+ versus Ba. B, Ba (hatched bars) is less efficient than Ca (solid b US ) m evoking stimulus-coupled secretion. The mean C,, increase during jumps per Ca or Ba ion is plotted in control conditions (Con; 0.3 mm intracellular BAPTA), after application of 1 FM thapsigargin (Thp; 0.3 mm intracellular BAPTA), and with 10 mm extracellular EGTA, for the number of experiments shown in purentheses. This normalization makes no assumptions about the relationship between Ca influx and amount of C, increase. C, The Bazt -Induced slow secretion is insensitive to thapsigargin trcatmcnt and 10 tim EGTA. Same data set as in B, but measuring only C,,, increases that occurred between pulses or after stimulus termination. Ten micromolar EGTA complctcly abolished stimulusdecoupled secretion evoked by Ca +. Both thapsigargin (Thp) and high EGTA had significant effects (p < 0.01) on Cazf-induced slow secretion (solid bars), whereas effects on Bazt -induced slow secretion (hatched bars) are not significantly different 0, > 0.05). Con, Control. with 10 mm intracellular EGTA in the pipette. The first two traces are elicited in external Cazt before (a) and 4 min after (b) application of 1 PM thapsigargin. Because of the high internal chelator concentration, there is a ~50 ff C, increase for a stimulus protocol that typically would elicit >300 ff AC,, at lower chelator concentrations (see Fig. 6B in Seward and Nowycky, 1996). The extracellular solution was then exchanged for one containing Ba + and thapsigargin. In the continued presence of 10 mm EGTA and 1 WM thapsigargin, Ba2+ entry induces the characteristic slow, prolonged C,, increase [Fig. 6A(c)]. Thus, Ba2+evoked secretion is not caused by displacement of Ca2 ~ ions from intracellular stores or other sources.
7 1376 J. Neurosci., February 15, 1996, 76(4): Seward et al. l Two Pathways for Regulated Exocytosis io- Y f Figure 7. Intracellular dialysis with Ba*+ elicits massive C, increases in chromaffin cells. The slow capacitance rise began set after establishing whole-cell recording mode. Each line represents an -18 set capacitance recording interval. Gaps between traces represent interruptions during which the C,,,, compensation circuitry of the EPC-7 patch clamp was adjusted because of the magnitude of the exocytotic response. To estimate the amount of exocytosis that occurred during the interruptions, secretory rates were calculated at the end of one trace and the beginning of the next available capacitance trace, and the estimated exocytosis is represented by the position of the second trace. The small upward dejlections on several of the traces are 100 fp calibration pulses. Pipette solution contained 1 mm Ba + and 1 mm EGTA, instead of the usual 0.3 mm BAPTA, because EGTA is more selective for CaZt over BaZi than is BAPTA and, therefore, was included at higher concentration to control cytosolic Ca +. The initial cell capacitance was -5 pf. Inset, Immediately after the last trace shown, the cell was stimulated with a train of 20 depolarizations, 40 msec in duration, 4 Hz c- Q OE Q 4-2- o- / J J J / J / / Time (WC) I I I I I Time (set) In the experiment illustrated in Figure 6A, a combination of thapsigargin and high EGTA is used to eliminate any participation of Ca2+ tons. Thapsigargin or high EGTA alone were also used as tools to study the divalent cation dependency of stimuluscoupled and -decoupled secretion. For this, we divided the changes in C, into the fraction of the response occurring during the depolarizing pulses (i.e., stimulus-coupled secretion; Fig. 6B) and the fraction that occurred between pulses and after the last stimulus of the train (stimulus-decoupled secretion, Cm.slow; Fig. 6C). Stimulus-coupled secretion is expressed as AC,,, per amount of divalent cation entry (Fig. 6B). Ba2+ ions appear to substitute -16fold less potently than Cazt ions in stimulus-coupled secretion (Fig. 6B). Thapsigargin treatment does not influence the coupled response to either divalent cation significantly. Ten millimolar EGTA dramatically reduces the efficacy of Cazt (9-fold) and has a significant, although smaller, effect on the efficacy of Bazt (3.4-fold), as expected from the lower affinity of the chelator for Bazt (F a b la t o and Fabiato, 1979). Thus, the efficacy of both Ca2+ and Bazt in evoking stimulus-coupled secretion is reduced by Cazt chelators (Seward and Nowycky, 1996), but is insensitive to block of divalent cation uptake into intracellular stores. Thapsigargin and 10 mm EGTA have qualitatively different effects on slow secretion. In control Cazt conditions, -16% of total changes in C,n occur as slow interpulse drifts and post-train increases (Cm.s,ow). Thapsigargin increases the amount of Ca +induced stimulus-decoupled drift approximately twofold, whereas 10 mm EGTA completely abolishes it. These results are consistent with a model in which residual Ca2+ promotes a modest additional amount of secretion between and after periods of active Cazt entry. The amount of residual Ca2+ is prolonged by preventing Ca2+ uptake into intracellular stores and is completed eliminated by higher concentrations of Cazt chelator. In Ba2+, the majority of the secretory response occurs as c mms,ow (65%; Fig. 6C). Thapsigargin has no significant effect on the fraction (Fig. 6C) or the total amount of C&iow (Table 1). Ten millimolar EGTA slightly diminishes the average amount of C m.s,ow and increases the number of Bazt ions necessary to elicit stimulus-decoupled secretion. For cells in which total Ba2+ influx exceeded 5 X 10 ions, the mean Cm.s,ow was 176 t 36 ff (n = 13) compared with 312 i 59 ff in 0.3 mm BAPTA (n = 17). High EGTA had no significant effect on the kinetics of the poststimulus response (Table l), nor on the relative contribution of C,.,,,, to the overall secretory response (Fig. 6C). The results with thapsigargin are expected and are consistent with the inability of native Ca2+-ATPases to remove Bazt (Kwan and Putney, 1990). The relatively weak effect of 10 mm EGTA on the decoupled response is the opposite of what is expected for a residual Cazt model. Intracellular dialysis with Ba2+ elicits slow secretion in chromafin cells von Ruden et al. (1993) reported that intracellular dialysis with Ba2+ induced slow C, increases in bovine chromaffin that did not exceed an average maximum of 30 ff/sec over the range of 0.1-l mm Bazt (recordings with 0.1 mm Fura-2). We performed similar experiments with an internal solution that contained 1 mm Ba2+, with 1 mm EGTA substituted for BAPTA to control for Ca2+ ions in the cytosol (see Materials and Methods). A representative result is illustrated in Figure 7. Within seconds of establishing the whole-cell dialysis mode, the cell capacitance begins to increase at a rate that never exceeds 50 ff/sec and is typically -20 ff/sec or slower. The slow C, increase continues for 6-12 min (n = 13); during this time, the original cell-surface area doubles or triples. This massive exocytosis is visible under the microscope as an increase in cell diameter and transition to a bumpy and irregular surface (data not shown). The massive exocytosis induced by intracellular Ba2+ does not interfere with Ca +-stimulated coupled exocytosis. The example illustrated in Figure 7 (inset) shows, on an expanded time and capacitance scale, Ca + -coupled exocytosis triggered -10 min after the initiation of dialysis with Ba +. Once the cell is stimulated with a train of 20 pulses (timing indicated by bar), discrete C, jumps are observed. The secretory response has threshold-like kinetics, because later pulses in the train are more effective than early ones in eliciting C, jumps (Seward and Nowycky, 1996). The presence of a qualitatively typical secretory response to Ca2+ entry through voltage-gated channels indicates that massive Baztinduced exocytosis does not obliterate or damage the stimulus-
8 Seward et al. l Two Pathways for Regulated Exocytosis J. Neurosci., February 15, 1996, 76(4): coupled mechanism and that there is no particular priming of vesicles by Ba +. Robust coupled exocytosis is seen when cells are stimulated with depolarizing trains both within l-2 min of whole-cell dialysis with Ba2+ (n = 515) an d as much as 8-10 min later (n = 414). Secretory responsiveness eventually fails: in two chromaffin cells first stimulated after prolonged dialysis (1.5 and 24 min), there was no stimulus-coupled secretion; however, Cazf currents were markedly reduced at these late times. At earlier time points, Ca2+-induced, stimulus-coupled secretion could be elicited up to three or four times before the secretory responsiveness was exhausted. Thus, as for NHP terminals, dialysis of chromaffin cells with Ba2+ does not interfere with the stimulus-coupled exocytosis induced by elevated submembrane Ca2+ ions. In addition, in chromaffin cells the massive exocytosis induced by the stimulusdecoupled pathway does not obliterate or damage sites for stimulus-coupled exocytosis. Finally, dialysis with Ba2+ ions in both cell types does not potentiate or otherwise influence the stimulus-coupled response. DISCUSSION Ba*+ is a weak agonist for stimulus-coupled secretion Ba2+ ions appear to be an adequate although weak substitute for Ca2+ m conventional stimulus-coupled secretion in both NHP terminals and chromaffin cells. In NHP terminals, the kinetics of Ba2+- and Ca +-induced exocytosis are similar, in that capacitance increases do not begin until after several depolarizing pulses (i.e., after achieving threshold; Seward et al., 1995) then occur mostly during depolarizations and terminate rapidly after the last pulse of a stimulus train. The observation that stimulus-coupled secretion occurs mostly during periods of active cation influx implies that the final step(s) of threshold secretion requires elevated levels of divalent cation concentration in submembrane sites and that the concentration falls below critical levels within milliseconds of channel closure (Sala and Hernandez-Cruz, 1990; Nowycky and Pinter, 1993). Ba2+ ions are weak agonists for conventional stimulus-coupled exocytosis in a number of different preparations. A small amount of stimulus-coupled secretion is observed in chromaffin cells (this paper) and in pancreatic /3 cells (Proks and Ashcroft, 1995). Similarly, at fast synapses Ba2+ ions either weakly evoke APcoupled EPSPs of low quanta1 content (Blioch et al., 1968; Silinsky, 1978; Zengel and Magleby, 1981; Augustine and Eckert, 1984) or are completely ineffective (McLachlan, 1977; Stanley, 1993). Proteins such as synaptotagmins with different affinities for both Ca2+ and Ba may participate in stimulus-coupled secretion in a wide variety of neuronal and endocrine cells (Davletov and Sudhof, 1993; Li et al., 1995). Ba*+ triggers stimulus-decoupled secretion in chromaffin cells In chromaffin cells, the major response to Ba2+ entry consists of slow exocytosis that outlasts a stimulus train by tens of seconds. Slow exocytosis begins only after a large amount of Ba2+ entry and then proceeds at characteristic rates. Remarkably, the rates are similar under different experimental conditions, including in the presence of high concentrations of divalent cation chelators (10 mm EGTA) or when triggered by continuous Ba2+ dialysis. von Ruden et al. (1993) demonstrated that over a lo-fold range of Bazc concentrations (0.1-l mm), the secretory rate averaged 25 ff/sec. This suggests that both in dialysis experiments and in experiments with depolarization-induced Ba2+ entry, the slow secretory rate may be an intrinsic property of the stimulusdecoupled mechanism. Intracellular dialysis with Ba2+ and the resulting exocytosis neither interfere with nor potentiate stimulus-coupled secretion. As in NHP terminals, Ba2+ ions apparently do not occlude sites that are critical for stimulus-coupled exocytosis. Perhaps surprisingly, massive addition of vesicular membrane to the plasma membrane also does not destroy or block the stimulus-coupled secretory machinery. Finally, dialysis with Ba2+ does not potentiate stimulus-coupled secretion and, therefore, does not appear to participate in any divalent cation preparatory steps that have been postulated in recent models of stimulus-coupled LDCV secretion (Heinemann et al., 1994; Seward et al., 1995). The possibility that prolonged exocytosis elicited by Ba2+ entry through voltage-gated channels is attributable to retention of Ba2+ tons within restricted spaces or specialized Ba +-buffering mechanisms found in chromaffin cells, but not in NHP terminals, cannot be excluded absolutely. It seems unlikely because few Ca +-binding proteins are able to bind Ba2+ (Chao et al., 1984) and because measurements of Ba2+ diffusion in snail neurons showed slightly faster rates than for Cazf diffusion (Nasi and Tillotson, 1985). Nevertheless, further proof will require the discovery of a tool that selectively eliminates one but not the other kinetic mode. The relationship between stimulus-coupled and stimulus-decoupled secretion Ca +-ion entry through voltage-gated channels induces a small amount of slower capacitance increases between pulses and after the final depolarization (see Fig. 5C). The amount of slow capacitance increase is approximately doubled by blocking Ca2+ uptake into stores with thapsigargin and is abolished by 10 mm EGTA. Although there is no absolute way to distinguish whether these capacitance changes are attributable to induction of the decoupled mechanism or residual Ca2+ acting via coupled release, the observations are consistent with a residual Ca2+ mechanism. Why does Ca + influx during depolarizations not elicit much stimulus-decoupled secretion? We can only speculate that because Ca2+ ions activate many proteins that do not bind Ba2+, including proteins with the E-F hand motif such as calmodulin (Chao et al., 1984), or with C2 domains, such as protein kinase C (PKC), activation of a Ba2+- insensitive protein somehow actively inhibits the stimulus-decoupled pathway. For example, phosphorylation of annexins I and II by PKC abolishes their ability to induce chromaffin granule aggregation (Johnstone et al., 1993). Alternatively, because synaptotagmin isoforms have different affinities for Ca2+ and Bazt (Davletov and Sudhof, 1993; Li et al., 1995), and because multiple isoforms are present in chromaffin cells (Mizuta et al., 1994; Roth-and Burgoyne, 1994), the two ions may be selectively activating distinct molecular entities. Stimulus-decoupled secretion is observed in other cell types In a paper that appeared during the review process, slow Ba2+evoked secretion that persists long after stimulus trains was reported to occur in most pancreatic /? cells recorded in perforatedpatch mode (Proks and Ashcroft, 1995). The authors tentatively conclude that the slow second phase of secretion may result from retention of Ba2+ in the cytoplasm of essentially intact cells. However, the changes in capacitance appear to have a similar time course as in our whole-cell recordings (compare their Fig. 6 to our
9 1378 J. Neurosci., February 15, 1996, 76(4): Seward et al. l Two Pathways for Regulated Exocytosis Table 1). Extremely prolonged secretory responses to Bazt were seen when the ion was puffed onto intact bovine chromaffin cells that were not voltage-clamped (Jankowski et al., 1994). In that study, catecholamine release, measured with amperometric techniques, continued for several minutes after a 15 set puff of Ba +. This extended response appears to involve a combination of Cazt release from intracellular stores and possibly subsequent Ca2+ influx through the plasma membrane. Thus, although the physiological significance of the decoupled pathway is unknown at present, one possibility is that it may be activated by G-proteincoupled receptor mechanisms that produce lower intracellular Ca*+ concentrations than those apparently achieved during stimuluscoupled secretion (Cheek and Thastrup, 1989; O Sullivan et al., 1989). Bazt ions also evoke slow, stimulus-decoupled secretion at fast synapses. Despite their weak activation of AP-coupled EPSPs, Ba2+ ions are severalfold more potent than Cazt ions in eliciting an increased.frequency of miniature end-plate potentials (MEPPs) between individual stimuli and after stimulus trains, a form of release also known as tonic or asynchronous release (Blioch et al., 1968; McLachlan, 1977; Silinsky, 1978; Zengel and Magleby, 1981; Guan et al., 1988; Quastel and Saint, 1988). The increased MEPP frequency builds up slowly over several seconds during a train and then decays slowly as a prominent tail after the train, lasting as long as 30 set (McLachlan, 1977; Silinsky, 1978; Zengel and Magleby, 1981; Guan et al., 1988), kinetics that resembles that observed in chromaffin cells and pancreatic p cells (this paper) (see also Jankowski et al., 1994; Proks and Ashcroft, 1995). Recently, Geppert et al. (1994) reported that in mice carrying a mutation of synaptotagmin I, AP-coupled synchronous or phasic neurotransmitter release is impaired, whereas at least some asynchronous release processes are unaffected. Similarly, mutation of Drosophila synaptotagmin strongly decreases evoked transmitter release, but has no effect on spontaneous release (Littleton et al., 1993). Both groups suggest that the coexistence of multiple release mechanisms accounts for these results. Further work should establish whether synchronous and asynchronous release at fast synapses corresponds to the stimulus-coupled and -decoupled mechanisms described here. In this study, we describe a secretory pathway for release of LDCVs that is present in chromaffin cells, but not in NHP terminals. The lack of a stimulus-decoupled mechanism in NHP terminals is thus far unique and supports the hypothesis that stimuluscoupled and -decoupled pathways differ in at least one critical divalent cation-sensitive step, rather than reflect different kinetics of Ba*+ versus Ca binding to a single class of receptors. Although the similarity between secretory pathways has been stressed during recent years, there may also be diversity within a single cell type. In light of the many forms of endocytosis that utilize distinct proteins, it is not surprising to find diversity in exocytotic mechanisms as well. REFERENCES Augustine GJ, Eckert R (1984) Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol (Lond) 346: Augustine GJ, Neher E (1992) Calcium requirements for secretion in bovine chromaffin cells. J Physiol (Lond) 450: Baker PF, Rink TJ (1975) Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol (Lond) Blioch ZL, Glagoleva IM, Liberman EA, Nenashev VA (1968) A study of the mechanism of quanta1 transmitter release at a chemical synapse. J Physiol (Lond) 199: Castillo CJF, Moro MA, Del Valle M, Sillero A, Garcia AG, Sillero MAG (1992) Diadenosine tetraphosphate is co-released with ATP and catecholamines from bovine adrenal medulla. J Neurochem 59: Cazalis M, Dayanithi G, Nordmann JJ (1987) Requirements for hormone release from permcabilized nerve endings isolated from the rat neurohypophysis. J Physiol (Lond) 390: Chao S-H, Suzuki Y, Zysk JR, Cheung WY (1984) Activation by calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol 26: Cheek TR, Thastrup 0 (1989) Internal Ca2+ mobilization and secretion in bovine adrenal chromallin cells. Cell Calcium 10: Chernevskaya NI, Seward EP, Nowycky MC (1995) Divalent cation and Mg-ATP dependence of cxocytosis in bovine chromaffin cells (Abstr). Biophys J 68:395A. Davletov BA, Sudhof TC (1993) A single C2 domain from synaptotagmin I is sufficient for high ahinity Ca +/phospholipid binding. J Biol Chem 268:263X Douglas WW, Poisner AM (1964) Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from the neurohypophysis. J Physiol (Lond) 172:1-18. Douglas WW, Rubin RP (1964) Effects of alkaline earths and other divalent cations on adrenal mcdullary secretion. J Physiol (Lond) 175: Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: Fidler N, Fernandez JM (1989) Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J 56: Forsberg EJ, Pollard HB (1988) Ba +-induced ATP release from adrenal medullary chromaflin cells is mediated by Ba entry through both voltage- and receptor-gated Ca + channels. Neuroscience 27: Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79: Guan Y-Y, Quastel DMJ, Saint DA (1988) Single Ca entry and transmitter release systems at the neuromuscular synapse. Synapse 2: Heinemann C, Chow RH, Neher E, Zucker RS (1994) Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca. Biophys J 67: Heldman E, Levine M, Raveh L, Pollard HB (1989) Barium ions cntcr chromaffin cells via voltage-dependent calcium channels and induce secretion by a mechanism independent of calcium. J Biol Chem 264: Hess P, Tsien RW (1984) Mechanism of ion permeation through calcium channels. Nature 309: Ishida A (1968) Stimulus-secretion coupling of the oxytocin release from the isolated posterior pituitary lobe. Jpn J Physiol 18: Izumi F, Toyohira Y, Yanagihara N, Wada A, Kobayashi H (1986) Barium-evoked release of catecholamincs from digitonin-permeabilized adrenal medullary cells. Neurosci Lett 69: Jankowski JA, Finnegan JM, Wightman RM (1994) Extracellular ionic composition alters kinetics of vesicular release of catecholamines and quanta1 size during exocytosis at adrenal medullaty cells. J Neurochem 63: Johnstone SA, Hubaishy I, Waisman DM (1993) Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J Biol Chcm 267: Joshi C, Fernandez JM (1988) Capacitance measurements: an analysis of the phase detection technique used to study exocytosis and endocytosis. Biophys J 53: Knight PF, Baker DE (1982) Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol 68: Kwan C-Y, Putney Jr JW (1990) Uptake and intracellular sequestration of divalent cations in resting and metacholine-stimulated mouse lacrimal acinar cells: dissociation by Sr and Ba of agonist-stimulated cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem 265:
10 Seward et al. l Two Pathways for Regulated Exocytosis J. Neurosci., February 15, 1996, 76(4): I Li C, Ullrich B, Zhang JZ, Anderson RGW, Brose N, Sudhof TC (1995) Ca +-dependent and -independent activities of neural and non-neural synaptotagmins. Nature 375: Lim NF, Nowycky MC, Bookman RD (1990) Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature 344: Lindau M, Stuenkel EL, Nordmann JJ (1992) Depolarization, intracellular calcium and exocytosis in single vertebrate nerve endings. Biophys J 61: Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ (1993) Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+-activated neurotransmitter release. Cell 74: McLachlan EM (1977) The effects of strontium and barium ions at synapses in sympathetic ganglia. J Physiol (Lond) 267: Michelena P. Garcia-Perez L-E. Artaleio AJ. Garcia AG (1993) \ Seoara- 1 tion between cytosolic calcium a and secretion in chromaffin, cells superfused with calcium ramps. Proc Nat1 Acad Sci USA 90: Mizuta M, Inagaki N, Nemoto Y, Matsukara S, Takahashi M, Seino S (1994) Synaptotagmin III is a novel isoform of rat synaptotagmin expressed in endocrine and neuronal cells. J Biol Chem 269: Mollard P, Seward EP, Nowycky MC (1995) Activation of nicotinic receptors triggers exocytosis in the absence of membrane depolarization. Proc Nat1 Acad Sci USA 92: Nasi E. Tillotson D (1985) The rate of diffusion of Ca2+ and BaZi in a nerve cell body. Biophys J 47: Neher E, Marty A (1982) Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine chromaffin cells. Proc Nat1 Acad Sci USA 79: Nowycky MC, Pinter MJ (1993) Time courses of calcium and calciumbound buffers following calcium influx in a model cell. Biophys J O Sullivan AJ, Cheek TR, Moreton RB, Berridge MJ, Burgoyne RD (1989) Localization and heterogeneity of agonist induced changes in cytosolic calcium concentration in single bovine chromaffin cells from video imaging of fura-2. EMBO J 8: Ozawa S, Sand 0 (1986) Electrophysiology of excitable endocrine cells. Physiol Rev ProksP, Ashcroft FM (1995) Effects of divalent cations on exocytosis and endocytosis from single mouse pancreatic p-cells. J Physiol (Lond) Quastel DMJ, Saint DA (1988) Transmitter release at mouse motor nerve terminals mediated by temporary accumulation of intracellular barium. J Physiol (Lond) 406: Roth D, Burgoyne RD (1994) SNAP-25 is present in a SNARE complex in adrenal chromaffin cells. FEBS Lett 351: Sala F, Hernandez-Cruz A (1990) Calcium diffusion modeling in a spherical neuron: relevance of buffering properties. Biophys J 57: Seward EP, Nowycky MC (1991) Cazt and Bazf produce different patterns of exocytosis in chromaffin cells and neurohypophysial terminals as measured by membrane capacitance techniques. Sot Neurosci Abstr 65:l. Seward EP, Nowycky MC (1996) Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells: docked and threshold secretory modes. 3 Neurosci 16~ Seward EP, Chernevksaya NI, Nowycky MC (1995) Exocytosis in peptidergic nerve terminals exhibits two calcium sensitive phases during pulsatile calcium entry. J Neurosci 15: Silinsky EM (1978) On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol (Lond) 274: Stanley EF (1993) Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron 11: Stuenkel EL, Nordmann JJ (1993) Intracellular calcium and vasopressin release from rat isolated neurohypophysial nerve endings. J Physiol (Lond) 468: TerBush DR, Holz RW (1992) Barium and calcium stimulate secretion from bovine adrenal chromaffin cells by similar pathways. J Neurochem 58: Vitale ML, de1 Castillo AR, Tchakarov L, Trifaro J-M (1991) Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol 113: von Ruden L, Garcia AG, Lopez MG (1993) The mechanism of Ba +induced exocytosis from single chromaffin cells. FEBS Lett 336: Zengel JE, Magleby KL (1981) Changes in miniature endplate potential frequency during repetitive nerve stimulation in presence of Ca, Ba*+, and SrZt at the frog neuromuscular junction. J Gen Physiol 77:
Kinetics of Stimulus-Coupled Secretion in Dialyzed Bovine Chromaffin Cells in Response to Trains of Depolarizing Pulses
 The Journal of Neuroscience, January 15, 1996, 76(2):553-562 Kinetics of Stimulus-Coupled Secretion in Dialyzed Bovine Chromaffin Cells in Response to Trains of Depolarizing Pulses Elizabeth P. Seward
The Journal of Neuroscience, January 15, 1996, 76(2):553-562 Kinetics of Stimulus-Coupled Secretion in Dialyzed Bovine Chromaffin Cells in Response to Trains of Depolarizing Pulses Elizabeth P. Seward
Exocytosis in Peptidergic Nerve Terminals Exhibits Two Calcium- Sensitive Phases during Pulsatile Calcium Entry
 The Journal of Neuroscience, May 1995, 15(5): 3390-3399 Exocytosis in Peptidergic Nerve Terminals Exhibits Two Calcium- Sensitive Phases during Pulsatile Calcium Entry Elizabeth P. Seward,a Natalya I.
The Journal of Neuroscience, May 1995, 15(5): 3390-3399 Exocytosis in Peptidergic Nerve Terminals Exhibits Two Calcium- Sensitive Phases during Pulsatile Calcium Entry Elizabeth P. Seward,a Natalya I.
Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons.
 Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons. Roland G.W. Staal a, Eugene Mosharov a, Anthonia Hananiya a and David
Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons. Roland G.W. Staal a, Eugene Mosharov a, Anthonia Hananiya a and David
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
DOI: /jphysiol The Physiological Society Rapid Report
 (2002), 545.2, pp. 337 343 DOI: 10.1113/jphysiol.2002.032516 The Physiological Society 2002 www.jphysiol.org Rapid Report Regulation by Rab3A of an endogenous modulator of neurotransmitter release at mouse
(2002), 545.2, pp. 337 343 DOI: 10.1113/jphysiol.2002.032516 The Physiological Society 2002 www.jphysiol.org Rapid Report Regulation by Rab3A of an endogenous modulator of neurotransmitter release at mouse
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
The Relation of Exocytosis and Rapid Endocytosis to Calcium Entry Evoked by Short Repetitive Depolarizing Pulses in Rat Melanotropic Cells
 The Journal of Neuroscience, January 1, 1998, 18(1):81 92 The Relation of Exocytosis and Rapid Endocytosis to Calcium Entry Evoked by Short Repetitive Depolarizing Pulses in Rat Melanotropic Cells Huibert
The Journal of Neuroscience, January 1, 1998, 18(1):81 92 The Relation of Exocytosis and Rapid Endocytosis to Calcium Entry Evoked by Short Repetitive Depolarizing Pulses in Rat Melanotropic Cells Huibert
Quantal Analysis Problems
 Quantal Analysis Problems 1. Imagine you had performed an experiment on a muscle preparation from a Drosophila larva. In this experiment, intracellular recordings were made from an identified muscle fibre,
Quantal Analysis Problems 1. Imagine you had performed an experiment on a muscle preparation from a Drosophila larva. In this experiment, intracellular recordings were made from an identified muscle fibre,
Neuroscience 201A (2016) - Problems in Synaptic Physiology
 Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
MCB MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY
 MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit
 Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Objectives. Functions of smooth muscle. Smooth muscle. Smooth Muscle Contraction: Mechanism. Latch state. Smooth muscle contraction
 Objectives Functions of smooth muscle Sompol Tapechum,, M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj hospital อธ บายล กษณะการหดต วของกล ามเน อเร ยบได อธ บายกลไกและป จจ ยท ม ผลต อการหดต
Objectives Functions of smooth muscle Sompol Tapechum,, M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj hospital อธ บายล กษณะการหดต วของกล ามเน อเร ยบได อธ บายกลไกและป จจ ยท ม ผลต อการหดต
Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris
 ZOOLOGICAL SCIENCE 21: 131 138 (2004) 2004 Zoological Society of Japan Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris Kazunori Oami* Institute
ZOOLOGICAL SCIENCE 21: 131 138 (2004) 2004 Zoological Society of Japan Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris Kazunori Oami* Institute
Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis
 Gen. Physiol. Biophys. (1988), 7, 651-656 65! Short communication Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis M. HENČĽK, D. ZACHAROVÁ and J. ZACHAR
Gen. Physiol. Biophys. (1988), 7, 651-656 65! Short communication Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis M. HENČĽK, D. ZACHAROVÁ and J. ZACHAR
A Persistent Activity-Dependent Facilitation in Chromaffin Cells Is Caused by Ca 2 Activation of Protein Kinase C
 The Journal of Neuroscience, January 15, 1999, 19(2):589 598 A Persistent Activity-Dependent Facilitation in Chromaffin Cells Is Caused by Ca 2 Activation of Protein Kinase C Corey Smith Department of
The Journal of Neuroscience, January 15, 1999, 19(2):589 598 A Persistent Activity-Dependent Facilitation in Chromaffin Cells Is Caused by Ca 2 Activation of Protein Kinase C Corey Smith Department of
Ameen Alsaras. Ameen Alsaras. Mohd.Khatatbeh
 9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
THE SYNAPTIC VESICLE CYCLE
 Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Synaptic communication
 Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
What effect would an AChE inhibitor have at the neuromuscular junction?
 CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
-Opioid Receptor Activation Modulates Ca 2 Currents and Secretion in Isolated Neuroendocrine Nerve Terminals
 The Journal of Neuroscience, September 1, 1997, 17(17):6565 6574 -Opioid Receptor Activation Modulates Ca 2 Currents and Secretion in Isolated Neuroendocrine Nerve Terminals K. I. Rusin, D. R. Giovannucci,
The Journal of Neuroscience, September 1, 1997, 17(17):6565 6574 -Opioid Receptor Activation Modulates Ca 2 Currents and Secretion in Isolated Neuroendocrine Nerve Terminals K. I. Rusin, D. R. Giovannucci,
Autonomous Function of Synaptotagmin 1 in Triggering Synchronous Release Independent of Asynchronous Release
 Neuron, Vol. 48, 547 554, November 23, 2005, Copyright ª2005 by Elsevier Inc. DOI 10.1016/j.neuron.2005.09.006 Autonomous Function of Synaptotagmin 1 in Triggering Synchronous Release Independent of Asynchronous
Neuron, Vol. 48, 547 554, November 23, 2005, Copyright ª2005 by Elsevier Inc. DOI 10.1016/j.neuron.2005.09.006 Autonomous Function of Synaptotagmin 1 in Triggering Synchronous Release Independent of Asynchronous
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
The action potential travels down both branches because each branch is a typical axon with voltage dependent Na + and K+ channels.
 BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
BIO 360 - MIDTERM FALL 2018 This is an open book, open notes exam. PLEASE WRITE YOUR NAME ON EACH SHEET. Read each question carefully and answer as well as you can. Point values are shown at the beginning
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization
 Proc. Natl. Acad. Sci. USA Vol. 92, pp. 365-369, March 1995 Neurobiology Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization (stimulus-secretion
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 365-369, March 1995 Neurobiology Activation of nicotinic receptors triggers exocytosis from bovine chromaffin cells in the absence of membrane depolarization (stimulus-secretion
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Neurons. Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons.
 Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
At the synapse, rapid release of neurotransmitter occurs when
 Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells Guilherme Neves*, Ana Gomis*, and Leon Lagnado Medical Research Council Laboratory of Molecular Biology,
Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells Guilherme Neves*, Ana Gomis*, and Leon Lagnado Medical Research Council Laboratory of Molecular Biology,
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
The Readily Releasable Pool of Vesicles in Chromaffin Cells Is Replenished in a Temperature-Dependent Manner and Transiently Overfills at 37 C
 The Journal of Neuroscience, November 15, 2000, 20(22):8377 8383 The Readily Releasable Pool of Vesicles in Chromaffin Cells Is Replenished in a Temperature-Dependent Manner and Transiently Overfills at
The Journal of Neuroscience, November 15, 2000, 20(22):8377 8383 The Readily Releasable Pool of Vesicles in Chromaffin Cells Is Replenished in a Temperature-Dependent Manner and Transiently Overfills at
Skeletal Muscle Contraction 4/11/2018 Dr. Hiwa Shafiq
 Skeletal Muscle Contraction 4/11/2018 Dr. Hiwa Shafiq Skeletal Muscle Fiber About 40 per cent of the body is skeletal muscle, and 10 per cent is smooth and cardiac muscle. Skeletal muscles are composed
Skeletal Muscle Contraction 4/11/2018 Dr. Hiwa Shafiq Skeletal Muscle Fiber About 40 per cent of the body is skeletal muscle, and 10 per cent is smooth and cardiac muscle. Skeletal muscles are composed
NEURONS Chapter Neurons: specialized cells of the nervous system 2. Nerves: bundles of neuron axons 3. Nervous systems
 NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
Biol 219 Lec 12 Fall 2016
 Cell-to-Cell: Neurons Communicate at Synapses Electrical synapses pass electrical signals through gap junctions Signal can be bi-directional Synchronizes the activity of a network of cells Primarily in
Cell-to-Cell: Neurons Communicate at Synapses Electrical synapses pass electrical signals through gap junctions Signal can be bi-directional Synchronizes the activity of a network of cells Primarily in
Neurophysiology of Nerve Impulses
 M52_MARI0000_00_SE_EX03.qxd 8/22/11 2:47 PM Page 358 3 E X E R C I S E Neurophysiology of Nerve Impulses Advance Preparation/Comments Consider doing a short introductory presentation with the following
M52_MARI0000_00_SE_EX03.qxd 8/22/11 2:47 PM Page 358 3 E X E R C I S E Neurophysiology of Nerve Impulses Advance Preparation/Comments Consider doing a short introductory presentation with the following
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 16, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
Synaptic Transmission
 Synaptic Transmission Postsynaptic Mechanisms Synapses electrical and chemical Part I Neurotransmitters categories and life cycle Neurotransmitters examples and postsynaptic effects Pathology Part II Neurotransmitter
Synaptic Transmission Postsynaptic Mechanisms Synapses electrical and chemical Part I Neurotransmitters categories and life cycle Neurotransmitters examples and postsynaptic effects Pathology Part II Neurotransmitter
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Cellular Bioelectricity
 ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 22 Friday, February 28, 2014 10. THE NEUROMUSCULAR JUNCTION We will look at: Structure of the neuromuscular junction Evidence for the quantal nature
ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 22 Friday, February 28, 2014 10. THE NEUROMUSCULAR JUNCTION We will look at: Structure of the neuromuscular junction Evidence for the quantal nature
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles
 Pflugers Arch - Eur J Physiol (2004) 448: 347 362 DOI 10.1007/s00424-004-1247-8 INVITED REVIEW Jakob Balslev Sørensen Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles
Pflugers Arch - Eur J Physiol (2004) 448: 347 362 DOI 10.1007/s00424-004-1247-8 INVITED REVIEW Jakob Balslev Sørensen Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Activity Dependent Changes At the Developing Neuromuscular Junction
 Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
In the neuroendocrine chromaffin cells of the adrenal
 Published Online: 17 November, 1997 Supp Info: http://doi.org/10.1083/jcb.139.4.885 Downloaded from jcb.rupress.org on April 29, 2018 Multiple Forms of Endocytosis In Bovine Adrenal Chromaffin Cells Corey
Published Online: 17 November, 1997 Supp Info: http://doi.org/10.1083/jcb.139.4.885 Downloaded from jcb.rupress.org on April 29, 2018 Multiple Forms of Endocytosis In Bovine Adrenal Chromaffin Cells Corey
Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells
 Keywords: Endocytosis, Chromaffin cell, Calcineurin 6986 Journal of Physiology (1998), 506.3, pp. 591 608 591 Compensatory and excess retrieval: two types of endocytosis following single step depolarizations
Keywords: Endocytosis, Chromaffin cell, Calcineurin 6986 Journal of Physiology (1998), 506.3, pp. 591 608 591 Compensatory and excess retrieval: two types of endocytosis following single step depolarizations
Summary of Calcium Regulation inside the Cell
 Overview of Calcium Summary of Calcium Regulation inside the Cell Plasma membrane transport a. Influx via receptor & voltage-regulated channels b. Efflux via Ca-ATPase & Na-Ca antiporter ER/SR membrane
Overview of Calcium Summary of Calcium Regulation inside the Cell Plasma membrane transport a. Influx via receptor & voltage-regulated channels b. Efflux via Ca-ATPase & Na-Ca antiporter ER/SR membrane
Neuroscience 201A Problem Set #1, 27 September 2016
 Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1
 BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
Skeletal Muscle Contraction 5/11/2017 Dr. Hiwa Shafiq
 Skeletal Muscle Contraction 5/11/2017 Dr. Hiwa Shafiq Skeletal Muscle Fiber About 40 per cent of the body is skeletal muscle, and 10 per cent is smooth and cardiac muscle. Skeletal muscles are composed
Skeletal Muscle Contraction 5/11/2017 Dr. Hiwa Shafiq Skeletal Muscle Fiber About 40 per cent of the body is skeletal muscle, and 10 per cent is smooth and cardiac muscle. Skeletal muscles are composed
Electrical activity and exocytotic correlates of biphasic insulin secretion from β-cells of canine islets of Langerhans
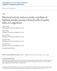 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Electrical activity and exocytotic correlates of biphasic insulin secretion from β-cells of canine islets of
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Electrical activity and exocytotic correlates of biphasic insulin secretion from β-cells of canine islets of
Medicine, University of Lund, Sweden
 336 J. Phy8iol. (1961), 156, pp. 336-343 With 6 text-ftgures Printed in Great Britain AN ELECTROPHYSIOLOGIC STUDY OF THE NEURO- MUSCULAR JUNCTION IN MYASTHENIA GRAVIS BY 0. DAHLBACK, D. ELMQVIST, T. R.
336 J. Phy8iol. (1961), 156, pp. 336-343 With 6 text-ftgures Printed in Great Britain AN ELECTROPHYSIOLOGIC STUDY OF THE NEURO- MUSCULAR JUNCTION IN MYASTHENIA GRAVIS BY 0. DAHLBACK, D. ELMQVIST, T. R.
marked secretion ofcatecholamines and a subsequent inhibition ofsecretion although the basal secretion shows an initial rise.
 J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 18, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 18, 2016 -- PAGE 1 of 8 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 18, 2016 -- PAGE 1 of 8 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Calcium Dependencies of Regulated Exocytosis in Different Endocrine Cells
 Physiol. Res. 60 (Suppl. 1): S29-S38, 2011 REVIEW Calcium Dependencies of Regulated Exocytosis in Different Endocrine Cells J. DOLENŠEK 1, M. SKELIN 1, M. S. RUPNIK 1,2 1 Institute of Physiology, Faculty
Physiol. Res. 60 (Suppl. 1): S29-S38, 2011 REVIEW Calcium Dependencies of Regulated Exocytosis in Different Endocrine Cells J. DOLENŠEK 1, M. SKELIN 1, M. S. RUPNIK 1,2 1 Institute of Physiology, Faculty
and Delayed Release at Single Frog Neuromuscular
 The Journal of Neuroscience August 1986, 6(8): 2366-2370 Facilitation Junctions and Delayed Release at Single Frog Neuromuscular I. S. Cohen and W. Van der Kloot Department of Physiology and Biophysics,
The Journal of Neuroscience August 1986, 6(8): 2366-2370 Facilitation Junctions and Delayed Release at Single Frog Neuromuscular I. S. Cohen and W. Van der Kloot Department of Physiology and Biophysics,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
Ca 2+ and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus
 J Physiol 555.3 (2004) pp 699 711 699 Ca 2+ and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus Brandi L. Soldo 1, David R. Giovannucci
J Physiol 555.3 (2004) pp 699 711 699 Ca 2+ and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus Brandi L. Soldo 1, David R. Giovannucci
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
Supporting Information
 Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve
 Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve impulses - Impulse may be blocked in its transmission
Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve impulses - Impulse may be blocked in its transmission
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Calcium dependencies of regulated exocytosis in different
 Calcium dependencies of regulated exocytosis in different endocrine cells Jurij Dolenšek 1, Maša Skelin 1 and Marjan Rupnik 1,2 1 Institute of Physiology, Faculty of Medicine, University of Maribor, Slomškov
Calcium dependencies of regulated exocytosis in different endocrine cells Jurij Dolenšek 1, Maša Skelin 1 and Marjan Rupnik 1,2 1 Institute of Physiology, Faculty of Medicine, University of Maribor, Slomškov
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 15, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 15, 2018 -- PAGE 1 of 8 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 15, 2018 -- PAGE 1 of 8 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013
 BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
Outline. Neuron Structure. Week 4 - Nervous System. The Nervous System: Neurons and Synapses
 Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
3) Most of the organelles in a neuron are located in the A) dendritic region. B) axon hillock. C) axon. D) cell body. E) axon terminals.
 Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Neuromuscular Transmission Diomedes E. Logothetis, Ph.D. (Dr. DeSimone s lecture notes revised) Learning Objectives:
 Neuromuscular Transmission Diomedes E. Logothetis, Ph.D. (Dr. DeSimone s lecture notes revised) Learning Objectives: 1. Know the subunit composition of nicotinic ACh channels, general topology of the α
Neuromuscular Transmission Diomedes E. Logothetis, Ph.D. (Dr. DeSimone s lecture notes revised) Learning Objectives: 1. Know the subunit composition of nicotinic ACh channels, general topology of the α
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]](/thumbs/86/94924826.jpg) QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
Human TRPC6 Ion Channel Cell Line
 TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
Relation between Membrane Potential Changes and Tension in Barnacle Muscle Fibers
 Relation between Membrane Potential Changes and Tension in Barnacle Muscle Fibers CHARLES EDWARDS, SHIKO CHICHIBU, and SUSUMU HAGIWARA From the Department of Physiology, University of Minnesota, Minneapolis,
Relation between Membrane Potential Changes and Tension in Barnacle Muscle Fibers CHARLES EDWARDS, SHIKO CHICHIBU, and SUSUMU HAGIWARA From the Department of Physiology, University of Minnesota, Minneapolis,
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Lambert-Eaton Antibodies Inhibit Ca 2 Currents But Paradoxically Increase Exocytosis during Stimulus Trains in Bovine Adrenal Chromaffin Cells
 The Journal of Neuroscience, May 1, 1999, 19(9):3384 3395 Lambert-Eaton Antibodies Inhibit Ca 2 Currents But Paradoxically Increase Exocytosis during Stimulus Trains in Bovine Adrenal Chromaffin Cells
The Journal of Neuroscience, May 1, 1999, 19(9):3384 3395 Lambert-Eaton Antibodies Inhibit Ca 2 Currents But Paradoxically Increase Exocytosis during Stimulus Trains in Bovine Adrenal Chromaffin Cells
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
3.E.2 Continued. This is the essential knowledge statement from the curriculum framework. Detect---process--- response
 Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
* * 11. GnRH. Molecular Biology of the Cell 5th Ed. Alberts et al., Garland Science, 2008
 I e-mail: okay@bs.s.u-tokyo.ac.jp http://www.bs.s.u-tokyo.ac.jp/~naibunpi/lab.html Molecular Biology of the Cell 5th Ed. Alberts et al., Garland Science, 2008 Ion Channels of Excitable Membranes 3rd Ed.
I e-mail: okay@bs.s.u-tokyo.ac.jp http://www.bs.s.u-tokyo.ac.jp/~naibunpi/lab.html Molecular Biology of the Cell 5th Ed. Alberts et al., Garland Science, 2008 Ion Channels of Excitable Membranes 3rd Ed.
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Cellular Neurobiology / BIPN 140
 SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
SECOND MIDTERM EXAMINATION Fall, 2015 GENERAL INSTRUCTIONS 1. Please write your name on ALL 6 pages. 2. Please answer each question IN THE SPACE ALLOTTED. 1) /10 pts 2) /10 pts 3) /15 pts 4) /15 pts 5)
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Sample Lab Report 1 from 1. Measuring and Manipulating Passive Membrane Properties
 Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
In Vitro Analog of Operant Conditioning in Aplysia
 The Journal of Neuroscience, March 15, 1999, 19(6):2261 2272 In Vitro Analog of Operant Conditioning in Aplysia. II. Modifications of the Functional Dynamics of an Identified Neuron Contribute to Motor
The Journal of Neuroscience, March 15, 1999, 19(6):2261 2272 In Vitro Analog of Operant Conditioning in Aplysia. II. Modifications of the Functional Dynamics of an Identified Neuron Contribute to Motor
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
photometry on the extruded cytoplasm.
 Answers To Midterm 2011 Question 1. a) Isoproterenol. Used to dissect presynaptic and postsynaptic components of sympathetic modulation of neuromuscular junction (Orbelli effect). Specifically activates
Answers To Midterm 2011 Question 1. a) Isoproterenol. Used to dissect presynaptic and postsynaptic components of sympathetic modulation of neuromuscular junction (Orbelli effect). Specifically activates
BIOL Week 6. Nervous System. Transmission at Synapses
 Collin County Community College BIOL 2401 Week 6 Nervous System 1 Transmission at Synapses Synapses are the site of communication between 2 or more neurons. It mediates the transfer of information and
Collin County Community College BIOL 2401 Week 6 Nervous System 1 Transmission at Synapses Synapses are the site of communication between 2 or more neurons. It mediates the transfer of information and
Elizabeth Biopsychology (PSY 302) The Synapses 08/29/2017. The Synapses
 Elizabeth Biopsychology (PSY 302) The Synapses 08/29/2017 The Synapses Conduction of a Depolarization o In dendrites: passive propagation : There is attenuation of signal transmission -Further away they
Elizabeth Biopsychology (PSY 302) The Synapses 08/29/2017 The Synapses Conduction of a Depolarization o In dendrites: passive propagation : There is attenuation of signal transmission -Further away they
Exocytosis in Bovine Chromaffin Cells: Studies with Patch-Clamp Capacitance and FM1-43 Fluorescence
 Biophysical Journal Volume 83 August 2002 849 857 849 Exocytosis in Bovine Chromaffin Cells: Studies with Patch-Clamp Capacitance and FM1-43 Fluorescence Gordan Kilic Department of Medicine, University
Biophysical Journal Volume 83 August 2002 849 857 849 Exocytosis in Bovine Chromaffin Cells: Studies with Patch-Clamp Capacitance and FM1-43 Fluorescence Gordan Kilic Department of Medicine, University
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes
 A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
