Effects of Chronic Treatment with 9 -Tetrahydrocannabinol on Cannabinoid-Stimulated [ 35 S]GTP S Autoradiography in Rat Brain
|
|
|
- Tyler Lee
- 5 years ago
- Views:
Transcription
1 The Journal of Neuroscience, December 15, 1996, 16(24): Effects of Chronic Treatment with 9 -Tetrahydrocannabinol on Cannabinoid-Stimulated [ 35 S]GTP S Autoradiography in Rat Brain Laura J. Sim, Robert E. Hampson, Sam A. Deadwyler, and Steven R. Childers Department of Physiology and Pharmacology, Center for the Neurobiological Investigation of Drug Abuse, and Center for Investigative Neuroscience, Bowman Gray School of Medicine, Wake Forest University, Winston-Salem, North Carolina Chronic 9 -tetrahydrocannabinol ( 9 -THC) administration produces tolerance to cannabinoid effects, but alterations in signal transduction that mediate these changes are not yet known. The present study uses in vitro autoradiography of agoniststimulated [ 35 S]GTP S binding to localize cannabinoid receptor-activated G-proteins after chronic 9 -THC treatment. Cannabinoid (WIN )-stimulated [ 35 S]GTP S binding was performed in brain sections from rats treated chronically with 10 mg/kg 9 -THC for 21 d. Control animals received saline or an acute injection of 9 -THC. Acute 9 -THC treatment had no effect on basal or WIN stimulated [ 35 S]GTP S binding. After chronic 9 -THC treatment, net WIN stimulated [ 35 S]GTP S binding was reduced significantly (up to 70%) in most brain regions, including the hippocampus, caudate-putamen, perirhinal and entorhinal cortex, globus pallidus, substantia nigra, and cerebellum. In contrast, chronic 9 -THC treatment had no effect on GABA B -stimulated [ 35 S]GTP S binding. In membranes and brain sections, 9 -THC was a partial agonist, stimulating [ 35 S]GTP S by only 20% of the level stimulated by WIN and inhibiting WIN stimulated [ 35 S]GTP S at high concentrations. Because the EC 50 of WIN stimulated [ 35 S]GTP S binding and the K D of cannabinoid receptor binding were unchanged by chronic 9 -THC treatment, the partial agonist actions of 9 -THC did not produce the decrease in cannabinoid-stimulated [ 35 S]GTP S binding. These results suggest that profound desensitization of cannabinoid-activated signal transduction mechanisms occurs after chronic 9 -THC treatment. Key words: 9 -THC; [ 35 S]GTP S autoradiography; cannabinoid receptor; GABA B receptor; G-protein Marijuana produces behavioral effects via its biologically active constituent 9 -tetrahydrocannabinol ( 9 -THC) (Gaoni and Mechoulam, 1964; Deadwyler et al., 1995a). Studies using potent synthetic cannabinoid analogs demonstrated that this activity occurs at cannabinoid receptors (Devane et al., 1988). The cloned cannabinoid receptor exhibits seven transmembrane spanning regions characteristic of G-protein-coupled receptors (Matsuda et al., 1990). Cannabinoid receptors act via G i/o to inhibit adenylyl cyclase (Howlett, 1985; Howlett et al., 1986; Pacheco et al., 1991), alter potassium channel conductance (Hampson et al., 1995b), and decrease calcium channel conductance (Mackie and Hille, 1992). Cannabinoid receptors in the brain (CB1) are numerous compared with other G-protein-coupled receptors and are localized in most brain regions, including the hippocampus, cortex, caudate-putamen, globus pallidus, substantia nigra, and cerebellum (Herkenham et al., 1991b; Jansen et al., 1992). This anatomical distribution is consistent with behavioral effects of cannabinoids, including memory disruption, decreased motor activity, catalepsy, antinociception, and hypothermia (Dewey, 1986; Compton et al., 1993; Deadwyler et al., 1995a). Chronic 9 -THC treatment results in the development of behavioral tolerance (Carlini, 1968; Dewey, 1986; Abood et al., Received June 10, 1996; revised Sept. 30, 1996; accepted Oct. 2, These studies were supported by Public Health Service Grants DA and DA from the National Institute on Drug Abuse. Doug Byrd, Joanne Konstantopoulis, and Ruoyu Xiao provided excellent technical assistance. Dr. Dana E. Selley, Dr. Linda Porrino, and Christopher S. Breivogel provided helpful discussions. Correspondence should be addressed to Dr. Steven R. Childers, Department of Physiology and Pharmacology, Bowman Gray School of Medicine, Wake Forest University, Medical Center Boulevard, Winston-Salem, NC Copyright 1996 Society for Neuroscience /96/ $05.00/0 1993; Deadwyler et al., 1995b). Some laboratories have reported a decrease in the B max of cannabinoid receptors after chronic 9 -THC treatment (Oviedo et al., 1993; De Fonseca et al., 1994), whereas others reported no change in cannabinoid receptor density (Westlake et al., 1991; Abood et al., 1993). Moreover, changes in receptor binding may not reflect changes in receptor function, and a measurement of agonist efficacy is necessary to answer this question. In cultured neuroblastoma cells, chronic cannabinoid exposure desensitized cannabinoid-inhibited adenylyl cyclase (Dill and Howlett, 1988), indicating that cannabinoid tolerance may involve alterations in signal transduction. Changes in G-protein activity and G-protein levels after chronic drug treatment have been reported previously for other receptor systems, including opioid receptors, which also couple to G i/o (Nestler et al., 1994; Sim et al., 1996a; Selley et al., 1996a). Our laboratory has developed a technique for examining receptor-activated G-proteins in brain sections using [ 35 S]GTP S autoradiography (Sim et al., 1995). This technique is based on agonist-stimulated [ 35 S]GTP S binding in membranes (Hilf et al., 1989; Traynor and Nahorski, 1995). For [ 35 S]GTP S autoradiography, sections are first incubated with excess GDP to decrease basal [ 35 S]GTP S binding and then with [ 35 S]GTP S in the presence (stimulated) or absence (basal) of a specific agonist. The applicability of this method to chronic drug studies has been demonstrated in the opioid system, where regionally specific changes in opioid-stimulated [ 35 S]GTP S binding were identified after chronic morphine treatment (Sim et al., 1996a). The present study was performed to examine the effect of chronic treatment with 9 -THC on cannabinoid receptor-activated G-proteins in different brain regions. In addition to demonstrating
2 8058 J. Neurosci., December 15, 1996, 16(24): Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC that chronic 9 -THC treatment produced large decreases in G-protein activation throughout the brain, these studies also reveal that 9 -THC is a partial agonist in activating G-proteins in brain, which may have important implications in the mechanism of action of this drug. MATERIALS AND METHODS Materials. Male Sprague Dawley rats ( gm) were purchased from Zivic-Miller (Zelienople, PA). [ 35 S]GTP S (1228 Ci/mmol) was purchased from New England Nuclear (Boston, MA). Baclofen and WIN were obtained from Research Biochemicals International (Natick, MA). 9 -THC was provided by the National Institute on Drug Abuse. SR141716A was provided by Dr. F. Barth (Sanofi, Montpelier, France). GTP S and GDP for membrane assays were purchased from Boehringer Mannheim (New York, NY). GDP for autoradiography was obtained from Sigma (St. Louis, MO). Reflections autoradiography film was purchased from New England Nuclear. All other reagent grade chemicals were obtained from Sigma or Fisher Scientific (Houston, TX). 9 -THC treatment. 9 -THC was dissolved in ethanol and prepared for injection as described previously (Heyser et al., 1993). The ethanol solution was suspended in a 1:4:1 ratio with Pluronic F68 detergent in ethanol and saline, and the ethanol was evaporated under a stream of nitrogen gas. The 9 -THC was diluted to 10 mg/ml in saline for injection. Chronically treated animals received a single daily intraperitoneal injection of 10 mg/kg 9 -THC for 21 d. Control animals received an equal volume of vehicle. Animals were killed 24 after the last injection. A separate group of animals received a single acute intraperitoneal injection of 10 mg/kg 9 -THC or vehicle 24 hr before they were killed. Agonist-stimulated [ 35 S]GTP S autoradiography. Animals were killed by rapid decapitation. Brains were removed and immediately immersed in isopentane at 35 C. Twenty micrometer horizontal sections were cut on a cryostat maintained at 20 C and mounted onto gelatin-subbed slides. Slides were incubated in assay buffer (50 mm Tris-HCl, 3 mm MgCl 2, 0.2 mm EGTA, 100 mm NaCl, 0.5% BSA, ph 7.4) at 25 C for 10 min, and then in 2 mm GDP in assay buffer for 15 min at 25 C. Slides were then transferred into assay buffer containing 2 mm GDP and 0.04 nm [ 35 S]GTP S, with (stimulated) or without (basal) 10 M WIN , and incubated at 25 C for 2 hr. Adjacent sections were incubated with 300 M baclofen and 0.04 nm [ 35 S]GTP S to evaluate GABA B receptor activation of G-proteins. Sections from control animals were also processed using 1 or 10 M WIN and 3 or 10 M 9 -THC alone and in combination. Slides were rinsed twice for 2 min each in 50 mm Tris-HCl buffer, ph 7.4, at 4 C, and once in deionized water, dried, and exposed to film for 48 hr. Films were digitized with a Sony XC-77 video camera and analyzed using the National Institutes of Health IMAGE program for Macintosh computers. Images were quantified by densitometric analysis with [ 14 C] standards, and values were corrected to nanocuries/gram [ 35 S] based on a correction factor determined with brain paste standards (Sim et al., 1996a). Data are mean values SE of duplicate sections of brains from five animals. Statistical significance was determined by the nonpaired two tailed Student s t test using JMP (SAS Institute, Cary, NC). Agonist-stimulated [ 35 S]GTP S binding in membranes. Cannabinoidstimulated [ 35 S]GTP S binding was determined as described previously (Selley et al., 1996b), using membranes from rat cerebellum (15 g protein). Membranes were incubated at 30 C for 1 hr in assay buffer (50 mm Tris-HCl, 3 mm MgCl 2, 0.2 mm EGTA, 100 mm NaCl, 0.1 mg/ml BSA, ph 7.4), with the appropriate concentrations of WIN or 9 -THC, in the presence of 20 M GDP and 0.05 nm [ 35 S]GTP S ina1mltotal volume. Basal binding was measured in the absence of agonist, and nonspecific binding was measured with 10 M GTP S. The reaction was terminated by rapid filtration under vacuum through Whatman GF/B filters, followed by three washes with cold Tris buffer. Bound radioactivity was determined by liquid scintillation spectrophotometry, at 95% efficiency for [ 35 S], after overnight extraction in 5 ml Ecolite scintillation fluid. Data are reported as mean SE values of three experiments that were performed in triplicate. Nonlinear iterative regression analyses of agonist concentration effect curves were performed with JMP (SAS, Cary, NC). RESULTS Effect of 9 -THC on cannabinoid-stimulated [ 35 S]GTP S binding in cerebellar membranes and brain sections Previous studies (Sim et al., 1995; Selley et al., 1996b; Sim et al., 1996b) have established that cannabinoid agonists stimulate [ 35 S]GTP S binding in both isolated membranes and brain sections, with a distribution and pharmacology that parallels that of cannabinoid receptor binding. To compare the effect of 9 -THC and a more potent cannabinoid agonist in this assay system, concentration effect curves of 9 -THC- and WIN stimulated [ 35 S]GTP S binding were generated in rat cerebellar membranes (Fig. 1A). Both 9 -THC- and WIN stimulated [ 35 S]GTP S binding were concentration-dependent. The maximal stimulation of [ 35 S]GTP S binding by WIN under these conditions was 270% over basal, with an EC 50 value of 0.12 M. The effect of 9 -THC was considerably less than that of WIN , with an apparent maximal stimulation of only 20% compared with that of WIN If 9 -THC were a true partial agonist in this system, then high concentrations of 9 -THC should antagonize the effect of a full agonist like WIN when the two drugs are added together, and the presence of residual 9 -THC in sections and membranes from chronic 9 - THC-treated rats could artificially reduce WIN stimulated [ 35 S]GTP S binding. This inhibitory effect of 9 -THC was indeed observed in cerebellar membranes (Fig. 1 A) when various concentrations of 9 -THC were added with either 1 M or 10 M WIN In the presence of 1 M WIN , 0.3 M 9 -THC began to produce significant inhibition of WIN stimulated [ 35 S]GTP S binding, and 0.8 M 9 -THC inhibited 50% of WIN stimulated [ 35 S]GTP S binding. As would be predicted for a partial agonist, in the presence of a higher concentration (10 M) of WIN , 9 -THC was less potent, and significant inhibition of WIN stimulated [ 35 S]GTP S required at least 10 M 9 -THC. From these data, it was estimated that 200 M 9 -THC would be required to inhibit 50% of WIN stimulated [ 35 S]GTP S binding in the presence of 10 M WIN The inhibitory effect of 9 -THC on WIN stimulated [ 35 S]GTP S binding was not attributable to a vehicle effect from a combination of the two drugs, because the same concentrations of vehicle (ethanol) had no effect on cannabinoid-stimulated [ 35 S]GTP S binding (data not shown). Similar experiments were performed in brain sections to determine the appropriate concentration of WIN to use in [ 35 S]GTP S autoradiography in 9 -THC-treated animals (Fig. 1B). The addition of 10 M WIN produced high levels of stimulated [ 35 S]GTP S binding in the substantia nigra, entopeduncular nucleus, and globus pallidus, with moderate levels of activation in hippocampus and cortex. A maximally effective concentration of 9 -THC alone (10 M) produced little stimulation of [ 35 S]GTP S binding. This concentration of 9 -THC had no significant effect on 10 M WIN stimulated [ 35 S]GTP S binding in the substantia nigra ( 5% decrease by densitometric analysis) (Fig. 1 B). In agreement with the membrane assays, however, this concentration of 9 -THC visibly inhibited [ 35 S]GTP S binding stimulated by 1 M WIN (data not shown). From these data, a concentration of 10 M WIN was used in autoradiographic experiments to minimize potential effects of residual 9 -THC on the cannabinoid-stimulated [ 35 S]GTP S autoradiographic signal.
3 Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC J. Neurosci., December 15, 1996, 16(24): Figure 1. Effect of 9 -THC and WIN on [ 35 S]GTP S binding in rat cerebellar membranes (A) and rat brain sections (B). Membranes (A) were incubated with 0.05 nm [ 35 S]GTP S and 20 M GDP, as described in Materials and Methods, with various concentrations of either 9 -THC or WIN alone (closed symbols) or with various concentrations of 9 -THC in the presence of either 1 M or 10 M WIN (open symbols). Data are expressed as percentage basal [ 35 S]GTP S binding and represent mean values SE from three separate experiments. Rat brain sections (B) were incubated with 0.04 nm [ 35 S]GTP S and2mmgdp, as described in Materials and Methods, and represent basal [ 35 S]GTP S binding, 10 M 9 -THC alone, 10 M WIN alone, and 10 M WIN M 9 -THC.
4 8060 J. Neurosci., December 15, 1996, 16(24): Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC Table 1. Effect of chronic 9 -THC treatment on basal and WIN stimulated [ 35 S]GTP S binding in the rat brain Basal Stimulated Region Control Chronic 9 -THC Control Chronic 9 -THC Hippocampus (D) 100 6% 92 4% 181 9% 125 7%** Hippocampus (M) 100 8% 101 6% % %** Hippocampus (V) 100 7% 99 4% % 129 8%*** Perirhinal cortex (M) 100 6% 94 3% % 127 6%* Perirhinal cortex (V) % 83 2% % 117 6%** Entorhinal cortex (D) 100 5% 96 8% 156 7% 133 7%* Entorhinal cortex (M) 100 6% 100 2% 174 9% 132 3%*** Entorhinal cortex (V) % 108 4% % 144 5%* Septum 100 7% 104 6% % 144 2% Caudate-putamen (D) 100 6% 81 9% % 134 4%** Caudate-putamen (M) 100 5% 85 2%* % 118 7%*** Globus pallidus 100 8% 73 4%* % %*** Substantia nigra 100 6% 85 8% % %*** Cerebellum 100 5% 123 4%* % % PAG 100 8% 92 10% 122 4% 114 9% Sections were incubated with 2 mm GDP, and then with [ 35 S]GTP S (0.04 nm) and2mmgdp, with and without 10 M WIN Data are expressed as percentage of control basal binding and represent mean values SE of duplicate sections from five animals. The level of the sections measured are dorsal (D), Figure 2 (top); midlevel (M), Figure 2 (middle); and ventral (V), Figure 2 (bottom). *p 0.05; **p 0.01; ***p Effects of chronic and acute 9 -THC administration on WIN stimulated [ 35 S]GTP S binding To compare the effect of acute and chronic 9 -THC administration on cannabinoid receptor activation of G-proteins, rats were treated with either a single acute dose of 10 mg/kg 9 -THC or were administered daily injections of 10 mg/kg 9 -THC for 21 d. The effect of 9 -THC administration on cannabinoid-stimulated [ 35 S]GTP S binding was examined at four brain levels in horizontal sections from acute and chronic 9 -THC-treated and control animals. Sections were analyzed at the level of (1) cerebellum, (2) caudate-putamen/septum, (3) globus pallidus, and (4) substantia nigra. At the most dorsal level, cannabinoid-stimulated [ 35 S]GTP S binding in control sections was observed in the cerebellum, hippocampus, and cortex. Labeling in all of these areas was visibly reduced in sections from chronic 9 -THC-treated rats. At a more ventral level (Fig. 2, top), cannabinoid-stimulated [ 35 S]GTP S binding was most evident in the cortex, hippocampus, caudate-putamen, septum, and periaqueductal gray (PAG), and was significantly reduced in sections from chronic 9 -THCtreated rats. At the next level (Fig. 2, middle), the reduction in cannabinoid-stimulated [ 35 S]GTP S binding in the sections from chronic 9 -THC-treated rats was most evident throughout the cortex, hippocampus, caudate-putamen, and globus pallidus. In the most ventral sections (Fig. 2, bottom), the dense cannabinoidstimulated [ 35 S]GTP S labeling in substantia nigra was significantly reduced in sections from chronic 9 -THC-treated animals. Thus, visual inspection of autoradiograms demonstrated clear reductions in WIN stimulated [ 35 S]GTP S binding in virtually every region where significant cannabinoid stimulation of [ 35 S]GTP S binding was observed. To quantify these effects, autoradiograms from all four groups of animals (acute and chronic 9 -THC treated, and controls) were analyzed densitometrically. Figure 3 shows data from a number of brain regions in both acute and chronic groups, expressed as net nanocuries [ 35 S] per gram tissue (obtained by subtracting basal binding from WIN stimulated [ 35 S]GTP S binding values in each brain section). The results from the chronic 9 -THCtreated rats are shown in Figure 3A. These results demonstrated significant reductions in net binding in sections from chronic 9 -THC-treated rats compared with controls, with 50% reduction in a number of regions, including the perirhinal cortex, entorhinal cortex, hippocampus, and cerebellum. Smaller but significant reductions in net agonist-stimulated [ 35 S]GTP S binding were observed in the septum and caudate-putamen. The only region in which chronic 9 -THC failed to significantly reduce net stimulated [ 35 S]GTP S binding was the PAG; in this region, the level of net binding was reduced but failed to reach statistical significance. Figure 3B, shows net WIN stimulated [ 35 S]GTP S binding data from the same regions of acute 9 - THC-treated animals, along with their respective controls. In contrast to the results observed with the chronic treatment group, acute 9 -THC administration had no significant effect on net stimulated [ 35 S]GTP S binding in any region measured. Because the levels of net stimulated [ 35 S]GTP S binding in the globus pallidus and substantia nigra were much greater than those of the other regions, data from these regions are presented separately (Fig. 4). As observed for other regions, net agoniststimulated [ 35 S]GTP S binding was significantly reduced in chronic 9 -THC-treated animals compared with controls, with 45% and 20% reductions in the globus pallidus and substantia nigra, respectively. On the other hand, no significant differences were found comparing net [ 35 S]GTP S binding in these regions from acute 9 -THC-treated and control rats (Fig. 4). To determine the effect of acute and chronic 9 -THC treatment on basal [ 35 S]GTP S binding, Tables 1 and 2 present the densitometric data as percentages of the control basal values for each group. Table 1 shows the results comparing control and chronic 9 -THC-treated animals, whereas Table 2 shows the results from the acute 9 -THC-treated and control groups. In most regions studied, there was no significant effect of either chronic or acute 9 -THC treatment on basal [ 35 S]GTP S binding. In the chronic group (Table 1), the only exceptions were small decreases in basal binding in ventral caudate-putamen (15%) and globus pallidus (27%), and a small increase in basal binding in cerebellum (20%). As seen previously in Figures 3 and 4, however, there were significant decreases in WIN stimulated [ 35 S]GTP S
5 Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC J. Neurosci., December 15, 1996, 16(24): Figure 2. Autoradiograms of brain sections comparing basal and cannabinoid-stimulated [ 35 S]GTP S binding in control and chronic 9 -THC-treated rats. Sections were incubated with 2 mm GDP and then with [ 35 S]GTP S (0.04 nm) and2mmgdp in the presence and absence of 10 M WIN Basal binding (assessed in the absence of agonist) is shown on the left column. Sections from control (middle column) and chronic 9 -THC-treated (right column) rats are shown at the appropriate levels to show (1) caudate-putamen and PAG (top row), (2) caudate-putamen and globus pallidus (middle row), and (3) substantia nigra (bottom row). Cannabinoid-stimulated [ 35 S]GTP S binding in the cortex and hippocampus is seen in sections at all three levels.
6 8062 J. Neurosci., December 15, 1996, 16(24): Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC Figure 3. Net cannabinoid-stimulated [ 35 S]GTP S binding in brain regions from chronic (A) and acute (B) 9 -THC-treated and control rats. Sections were incubated with 2 mm GDP, and then with [ 35 S]GTP S (0.04 nm)and2mmgdp in the presence and absence of 10 M WIN Net stimulated [ 35 S]GTP S binding was determined by subtracting basal [ 35 S]GTP S binding from WIN stimulated [ 35 S]GTP S binding. The levels of sections correspond to the images shown in Figure 2: dorsal, Figure 2, top; middle, Figure 2, middle; and ventral, Figure 2, bottom. *p 0.005, **p CBLM, Cerebellum; CPU, caudate-putamen; ENT, entorhinal cortex; HIP, hippocampus; PAG, periaqueductal gray; PRH, perirhinal cortex; SEP, septum. binding in most brain regions in sections from chronic 9 -THCtreated animals (Table 1). For the acute group (Table 2), no significant changes in either basal or WIN stimulated [ 35 S]GTP S binding were observed compared with controls. Several independent methods were used to determine whether residual 9 -THC in sections was potentially affecting the results of the study (data not shown). First, another set of brain sections from these animals was incubated with 1 M SR141716A, the cannabinoid antagonist. Results revealed that SR141716A blocked WIN stimulated binding in sections from both control and chronic 9 -THC-treated rats but had no effect on basal [ 35 S]GTP S binding in sections from either group. Therefore, there was not sufficient residual 9 -THC acting as an agonist in these sections to affect the results. Another experiment assayed cannabinoid receptor binding in cerebellar membranes from both control and chronic 9 -THC-treated rats, using the specific antagonist [ 3 H]SR141716A as a radioligand. Results (not shown) revealed that chronic 9 -THC treatment had no effect on the K D of [ 3 H]SR141716A binding (0.50 nm in control vs 0.42 nm in chronic) but did decrease B max values (11.1 pmol/mg in control vs 8.5 pmol/mg in chronic) of [ 3 H]SR141716A binding sites in cerebellar membranes. These results argue against the presence of residual 9 -THC, because it would increase the K D value of [ 3 H]SR141716A binding. Finally, in the same cerebellar mem-
7 Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC J. Neurosci., December 15, 1996, 16(24): Figure 4. Net cannabinoid-stimulated [ 35 S]GTP S binding in the globus pallidus and substantia nigra. Sections were incubated with 2 mm GDP, and then with [ 35 S]GTP S (0.04 nm) and 2 mm GDP in the presence and absence of 10 M WIN Net stimulated binding was determined by subtracting basal [ 35 S]GTP S binding from WIN stimulated [ 35 S]GTP S binding. *p branes, the E max of WIN stimulated [ 35 S]GTP S binding was decreased by 32%, whereas the potency of WIN was not affected at all in membranes from chronic 9 -THC-treated rats (IC 50 values of 0.2 M WIN in control vs 0.25 M WIN in chronic). If residual 9 -THC were present in these membranes and acted as a partial agonist, it would shift the WIN concentration effect curve to the right and increase the EC 50 value of the full agonist (see Fig. 1). All of these results indicate that residual 9 -THC was not present at the receptor in levels high enough to produce changes in receptor-stimulated [ 35 S]GTP S binding. Effects of 9 -THC administration on GABA B -stimulated [ 35 S]GTP S autoradiography Our previous studies (Sim et al., 1995) showed that GABA B - stimulated [ 35 S]GTP S binding could be localized autoradiographically with use of baclofen as an agonist. Because cannabi- Table 2. Effect of acute 9 -THC treatment on basal and WIN stimulated [ 35 S]GTP S binding in the rat brain Basal Stimulated Region Control Acute 9 -THC Control Acute 9 -THC Hippocampus (D) 100 4% 92 2% % 140 6% Hippocampus (M) 100 5% 116 5% % 176 5% Hippocampus (V) % 123 2% % 194 8% Perirhinal cortex (M) 100 5% 112 6% % 166 8% Perirhinal cortex (V) 100 1% 101 3% 145 5% 147 8% Entorhinal cortex (D) 100 6% 97 1% % 148 4% Entorhinal cortex (M) 100 7% 120 6% % 176 6% Entorhinal cortex (V) % 112 4% % 164 7% Septum 100 7% 97 10% 141 6% 127 5% Caudate-putamen (D) % 100 3% % 181 2% Caudate-putamen (M) % 112 2% % 201 4% Globus pallidus 100 7% 109 6% % % Substantia nigra 100 8% 116 1% % % Cerebellum 100 6% 115 5% % 189 6% PAG 100 6% 102 7% 122 6% 124 5% Sections were incubated with 2 mm GDP, and then with [ 35 S]GTP S (0.04 nm) and2mmgdp, in the presence and absence of 10 M WIN Data are expressed as percentage of control basal binding and represent mean values SE of duplicate sections from three animals. The level of the sections measured are dorsal (D), midlevel (M), and ventral (V).
8 8064 J. Neurosci., December 15, 1996, 16(24): Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC Figure 5. Net GABA B -stimulated [ 35 S]GTP S binding in brain regions from chronic 9 -THC-treated and control rats. Sections were incubated with 2 mm GDP, and then with [ 35 S]GTP S (0.04 nm) and2mmgdp in the presence and absence of 300 M baclofen. Net stimulated binding was determined by subtracting basal [ 35 S]GTP S binding from baclofen-stimulated [ 35 S]GTP S binding. CBLM, Cerebellum; ENT, entorhinal cortex; HIP, hippocampus; PRH, perirhinal cortex; PRL, prelimbic cortex. noid receptors have been colocalized with GABA B receptors on the same neurons in cerebellum (Pacheco et al., 1993), and because [ 35 S]GTP S autoradiography allows for the opportunity to assay multiple receptor systems on adjacent brain sections, it was of interest to determine the effect of chronic 9 -THC treatment on GABA B activation of G-proteins in sections from the same brains that demonstrated significant loss in WIN stimulated [ 35 S]GTP S binding. In these experiments, GABA B stimulation of [ 35 S]GTP S binding was performed using 300 M baclofen in sections adjacent to those used for WIN stimulated [ 35 S]GTP S autoradiography. The resulting autoradiograms (not shown) revealed that chronic 9 -THC treatment had no visible effects on baclofen-stimulated [ 35 S]GTP S binding in any region examined. When data from these autoradiograms were quantified (Fig. 5), no significant changes in baclofen-stimulated [ 35 S]GTP S binding were observed in the cortex, hippocampus, or cerebellum of chronic 9 -THC-treated animals compared with sections from control animals. DISCUSSION Chronic treatment of rats with 9 -THC resulted in decreased WIN stimulated [ 35 S]GTP S binding throughout the brain. This decrease in net WIN stimulated [ 35 S]GTP S binding was dramatic, with losses 50% in many regions. Any experiment administering chronic doses of 9 -THC must be interpreted with caution, however, because the lipophilic nature of the drug may cause residual 9 -THC to remain bound to sections and produce an artifactual result. The results of Figure 1, showing that 9 -THC behaved as a partial agonist/antagonist in this system, provide a possible rationale for such an artifact; i.e., the presence of high concentrations of residual 9 -THC could act as an antagonist to artificially reduce WIN stimulated [ 35 S]GTP S binding. This was not likely for several reasons. First, the concentration of 9 -THC required to inhibit 50% of 10 M WIN stimulated [ 35 S]GTP S binding was 200 M (Fig. 1), a level that is unlikely to be attained in these brain sections. Second, preliminary data from cannabinoid receptor binding studies, using the antagonist [ 3 H]SR141716A under the same conditions as the [ 35 S]GTP S binding assay, showed no change in K D value for [ 3 H]SR141716A in membranes from chronic 9 -THCtreated rats. Finally, Table 2 shows that acute 9 -THC injection had no effect on WIN stimulated [ 35 S]GTP S binding in any brain region examined. Therefore, the finding that 9 -THC is an agonist/antagonist in brain cannabinoid signal transduction systems may be important in regard to its mechanism of action, but this is unlikely to explain the large decrease in cannabinoid activation of G-proteins observed after chronic 9 -THC treatment. Because receptor activation of G-proteins is the critical step in the signal transduction pathway that determines agonist efficacy (Kenakin, 1993), the loss in agonist activity observed in this study is analogous to desensitization. Previous studies examining the effects of chronic 9 -THC treatment have reported both decreased (Oviedo et al., 1993; De Fonseca et al., 1994) and unchanged (Westlake et al., 1991; Abood et al., 1993) cannabinoid receptor binding after chronic 9 -THC treatment. Thus, effects on receptor function may occur without consistent detectable changes in the number of receptor binding sites. This is consistent with the established concept that desensitization and downregulation are separate processes, and that desensitization (uncoupling of G-proteins) precedes downregulation. In preliminary studies (C. Breivogel, L. Sim, and S. Childers, unpublished observations), a significant decrease in B max values of [ 3 H]SR141716A
9 Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC J. Neurosci., December 15, 1996, 16(24): binding was observed in cerebellar membranes from chronic 9 - THC-treated rats; thus, both desensitization and downregulation may be occurring after this chronic drug treatment. Detailed time course studies are now being performed to differentiate between these two processes. Another important consideration is the agonist used to develop cannabinoid tolerance. Chronic treatment with CP 55,940 (a full agonist) has been reported to decrease cannabinoid receptor number in mouse cerebellum (Fan et al., 1996), despite earlier reports that chronic 9 -THC treatment had no effect on cannabinoid receptor number in mouse brain (Abood et al., 1993). Chronic 9 -THC treatment also had no effect on cannabinoid receptor mrna (Abood et al., 1993), although chronic CP-55,940 treatment decreased cannabinoid receptor mrna in the caudate-putamen (Rubino et al., 1994) and increased it in the cerebellum (Fan et al., 1996). Interestingly, the effects of chronic 9 -THC treatment on cannabinoid-activated G-proteins contrast with the effects of chronic morphine treatment on opioid receptor function (Sim et al., 1996a). In that study, decreased opioid-stimulated [ 35 S]GTP S binding was found in only specific brainstem nuclei after chronic morphine treatment. Although both studies revealed that chronic agonist treatment decreased receptor-coupled G-protein activity, there are fundamental differences between the results of the two studies. The changes in opioid-stimulated [ 35 S]GTP S binding after chronic morphine treatment were anatomically discrete and relatively small in magnitude. In contrast, large, widespread decreases in cannabinoid-stimulated [ 35 S]GTP S binding were identified throughout the brain after chronic 9 -THC treatment. One explanation for this difference may be the catalytic efficiency of these two receptors; for example, in the striatum, each opioid receptor activates approximately seven times the number of G-proteins as each cannabinoid receptor (Sim et al., 1996b). This difference in catalytic amplification may have consequences during chronic agonist treatment, resulting in greater adaptive changes in receptor G-protein coupling in the less efficiently coupled system. The observation that chronic 9 -THC treatment produces a dramatic decrease in [ 35 S]GTP S binding throughout the brain, whereas chronic morphine administration has a much more subtle effect, also suggests that effector events downstream from the G-protein influence the development of tolerance. The effects in the cerebellum are of particular interest because of the known relationship between cannabinoid and GABA B receptors. Previous studies have shown that although cannabinoid and GABA B receptors are located on the same cerebellar neurons and act via the same pool of adenylyl cyclase, these receptors are coupled to distinct G-proteins (Pacheco et al., 1993). The finding that chronic 9 -THC treatment decreased cannabinoidstimulated [ 35 S]GTP S binding, but had no effect on GABA B - stimulated [ 35 S]GTP S binding in adjacent sections, suggests that this chronic effect of 9 -THC is best described as homologous desensitization. Furthermore, these results confirm that although these receptors may share common effectors, the receptors are coupled to different populations of G-proteins. The large decreases in cannabinoid-activated G-proteins in the nigrostriatal system may be relevant to the motor effects of cannabinoids. Cannabinoid receptors and cannabinoid-stimulated [ 35 S]GTP S binding are particularly high in the caudate-putamen, globus pallidus, and substantia nigra (Herkenham et al., 1991b; Jansen et al., 1992; Sim et al., 1995). Previous anatomical studies have shown that cannabinoid receptors are located on striatal neurons projecting to globus pallidus and substantia nigra (Herkenham et al., 1991a). Decreased cannabinoid-stimulated [ 35 S]GTP S binding in the caudate-putamen, globus pallidus, and substantia nigra indicates that all of the components of this system are affected by chronic 9 -THC treatment. Changes in these regions may be particularly relevant to the cannabinoid withdrawal syndrome elicited when tolerant animals are administered the antagonist SR A (Aceto et al., 1995; Tsou et al., 1995). This syndrome is characterized by patterns of motor behaviors that may be mediated via the nigrostriatal system. Perhaps the most dramatic effect of chronic 9 -THC treatment on cannabinoid-stimulated [ 35 S]GTP S binding was detected in hippocampus, consistent with behavioral effects of cannabinoids on short-term memory tasks (Heyser et al., 1993). In delayed-tomatch sample (DMS) tasks, acute exposure to 9 -THC disrupted performance in a manner analogous to that of a hippocampal lesion (Hampson et al., 1995a), except that the cannabinoid effect was fully reversible (Heyser et al., 1993). These acute effects were selectively associated with blockade of hippocampal cell activation during the information encoding phase in the DMS trial; hence, there was a delay-dependent deficit in performance under the influence of the drug (Heyser et al., 1993; Hampson et al., 1995a). The uncoupling of the receptor from the G-protein by chronic drug treatment would probably lead to a cessation of the disruptive acute effects of 9 -THC on DMS performance. The 21 d chronic treatment regimen and dose of 9 -THC used in the present study were chosen on the basis of a previous study (Deadwyler et al., 1995b) in which significant (75%) tolerance to 9 -THC effects was found at 21 d and complete tolerance was achieved after d. The results of the present study, showing profound reductions in hippocampal cannabinoid receptor Gprotein coupling during the same treatment period associated with marked recovery from the acute effects of 9 -THC on DMS performance, strongly suggest that the two phenomena are closely related. These findings also indicate an adaptive significance to the uncoupling of the receptor and G-protein after repeated drug exposure, especially in circumstances where acute cannabinoid receptor stimulation leads to a decrease in accuracy of performance. The mechanism by which cannabinoids disrupt hippocampal function has been examined in studies on the effects of cannabinoids on camp modulation of voltage-gated potassium A current in cultured hippocampal neurons (Deadwyler et al., 1993). The net effect of cannabinoid receptor stimulation is to decrease camp-mediated reductions in potassium A current at a given membrane potential. It has been hypothesized (Deadwyler et al., 1995a) that if cannabinoid receptor-linked potassium channels occupied a strategic position on the terminals of the perforant path projection from the entorhinal cortex to the molecular layer of the dentate gyrus [a region reported to have high densities of cannabinoid receptors (Herkenham et al., 1991b)], they could regulate transmitter release via changes in steady-state potassium conductances in perforant path terminals. The demonstrated receptor G-protein uncoupling after chronic 9 -THC treatment would therefore result in a relative increase (i.e., lack of cannabinoid-induced terminal hyperpolarization) in neurotransmitter release at the perforant path-to-dentate granule cell synapse. Because effects on potassium A channels (and indeed on all other effectors linked to these G i/o -coupled cannabinoid receptors) occur downstream from the receptor transducer coupling, the result of decreased activation of G-proteins after chronic agonist treatment should have profound implications at the effector level.
10 8066 J. Neurosci., December 15, 1996, 16(24): Sim et al. [ 35 S]GTP S Binding after Chronic 9 -THC REFERENCES Abood ME, Sauss C, Fan F, Tilton CL, Martin BR (1993) Development of behavioral tolerance to 9 -THC without alteration of cannabinoid receptor binding or mrna levels in whole brain. Pharmacol Biochem Behav 46: Aceto MD, Scates SM, Lowe JA, Martin BR (1995) Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR A. Eur J Pharmacol 282:R1 R2. Carlini EA (1968) Tolerance to chronic administration of cannabis sativa (marijuana) in rats. Pharmacology 1: Compton DR, Rice KC, DeCosta BR, Razdan RK, Melvin LS, Johnson MR, Martin BR (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265: De Fonseca FR, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA (1994) Downregulation of rat brain cannabinoid binding sites after chronic 9 -tetrahydrocannabinol treatment. Pharmacol Biochem Behav 47: Deadwyler SA, Hampson RE, Bennett BA, Edwards TA, Mu J, Pacheco MA, Ward SJ, Childers SR (1993) Cannabinoids modulate potassium current in cultured hippocampal neurons. Receptors Channels 1: Deadwyler SA, Hampson RE, Childers SR (1995a) Functional significance of cannabinoid receptors in brain. In: Cannabinoid receptors (Pertwee RG, ed), pp New York: Academic. Deadwyler SA, Heyser CJ, Hampson RE (1995b) Complete adaptation to the memory disruptive effects of delta-9-thc following 35 days of exposure. Neurosci Res Commun 17:9 18. Devane WA, Dysarz FAI, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: Dewey WL (1986) Cannabinoid pharmacology. Pharmacol Rev 48: Dill JA, Howlett AC (1988) Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. J Pharmacol Exp Ther 244: Fan F, Tao Q, Abood ME, Martin BR (1996) Cannabinoid receptor down-regulation without alteration of the inhibitory effect of CP 55,940 on adenylyl cyclase in the cerebellum of CP 55,940-tolerant mice. Brain Res 706: Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: Hampson RE, Byrd D, Konstantopolous J, Bunn T, Jarrard LE, Deadwyler SA (1995a) Proactive interference and short term memory during performance of a DNMS task in normal rats and rats with hippocampus removed. Soc Neurosci Abstr 21:1215. Hampson RE, Evans GJO, Mu J, Zhuang S, King VC, Childers SR, Deadwyler SA (1995b) Role of cyclic AMP dependent protein kinase in cannabinoid receptor modulation of potassium A-current in cultured rat hippocampal neurons. Life Sci 56: Herkenham M, Lynn AB, de Costa BR, Richfield EK (1991a) Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547: Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991b) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: Heyser CJ, Hampson RE, Deadwyler SA (1993) Effects of delta-9- tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264: Hilf G, Gierschik P, Jakobs KH (1989) Muscarinic acetylcholine receptor-stimulated binding of guanosine 5 -O-(3-thiotriphosphate) to guanine-nucleotide-binding proteins in cardiac membranes. Eur J Biochem 186: Howlett AC (1985) Cannabinoid inhibition of adenylate cyclase: biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol 27: Howlett AC, Qualy JM, Khachatrian LL (1986) Involvement of G i in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29: Jansen EM, Haycock DA, Ward SJ, Seybold VS (1992) Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res 575: Kenakin T (1993) Efficacy. In: Pharmacologic analysis of drug-receptor interaction (Kenakin T, ed), pp New York: Raven. Mackie K, Hille B (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89: Matsuda LA, Lolait SJ, Brownstein MJ, Young AL, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cdna. Nature 346: Nestler EJ, Alreja M, Aghajanian GK (1994) Molecular and cellular mechanisms of opiate action: studies in the locus coeruleus. Brain Res Bull 35: Oviedo A, Glowa J, Herkenham M (1993) Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res 616: Pacheco M, Childers SR, Arnold R, Casiano F, Ward SJ (1991) Aminoalkylindoles: actions on specific G-protein-linked receptors. J Pharmacol Exp Ther 257: Pacheco M, Ward SJ, Childers SR (1993) Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Res 603: Rubino T, Massi P, Patrini G, Venier I, Giagnoni G, Parolaro D (1994) Chronic CP-55,940 alters cannabinoid receptor mrna in the rat brain: an in situ hybridization study. NeuroReport 5: Selley DE, Nestler EJ, Breivogel CS, Childers SR (1996a) Opioid receptor-coupled G-proteins in rat locus coeruleus membranes: decrease in activity after chronic morphine treatment. Brain Res, in press. Selley DE, Stark S, Sim LJ, Childers SR (1996b) Cannabinoid receptor stimulation of guanosine-5 -O-(3-[ 35 S]thio)triphosphate binding in rat brain membranes. Life Sci 59: Sim LJ, Selley DE, Childers SR (1995) In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5 -[ -[ 35 S]thio]-triphosphate binding. Proc Natl Acad Sci USA 92: Sim LJ, Selley DE, Dworkin SI, Childers SR (1996a) Effects of chronic morphine administration on mu opioid receptor-stimulated [ 35 S]GTP S autoradiography in rat brain. J Neurosci 16: Sim LJ, Selley DE, Xiao R, Childers SR (1996b) Differences in G-protein activation by mu and delta opioid, and cannabinoid, receptors in rat striatum. Eur J Pharmacol 307: Traynor JR, Nahorski SR (1995) Modulation by -opioid agonists of guanosine-5 -O-(3-[ 35 S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47: Tsou K, Patrick SL, Walker JM (1995) Physical withdrawal in rats tolerant to 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol 280:R13 R15. Westlake TM, Howlett AC, Ali SF, Paule MG, Scallet AC, Slikker W (1991) Chronic exposure to 9 -tetrahydrocannabinol fails to irreversibly alter brain cannabinoid receptors. Brain Res 544:
CHRISTOPHER S. BREIVOGEL and STEVEN R. CHILDERS
 0022-3565/00/2951-0328$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 1 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics 2718/851330
0022-3565/00/2951-0328$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 1 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics 2718/851330
Evidence for a New G Protein-Coupled Cannabinoid Receptor in Mouse Brain
 0026-895X/01/6001-155 163$3.00 MOLECULAR PHARMACOLOGY Vol. 60, No. 1 Copyright 2001 The American Society for Pharmacology and Experimental Therapeutics 669/911440 Mol Pharmacol 60:155 163, 2001 Printed
0026-895X/01/6001-155 163$3.00 MOLECULAR PHARMACOLOGY Vol. 60, No. 1 Copyright 2001 The American Society for Pharmacology and Experimental Therapeutics 669/911440 Mol Pharmacol 60:155 163, 2001 Printed
INTRODUCTION. Neuropsychopharmacology (2010) 35, & 2010 Nature Publishing Group All rights reserved X/10 $32.00
 (2010) 35, 1775 1787 & 2010 Nature Publishing Group All rights reserved 0893-133X/10 $32.00 www.neuropsychopharmacology.org FAAH / Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor
(2010) 35, 1775 1787 & 2010 Nature Publishing Group All rights reserved 0893-133X/10 $32.00 www.neuropsychopharmacology.org FAAH / Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor
MOL Laura J. Sim-Selley, Nicole S. Schechter, W. Kirk Rorrer, George D. Dalton, Jerry Hernandez, Billy R. Martin and Dana E.
 Molecular Pharmacology This article has not Fast been Forward. copyedited and Published formatted. The on final June version 7, 2006 may differ as doi:10.1124/mol.105.019612 from this version. Prolonged
Molecular Pharmacology This article has not Fast been Forward. copyedited and Published formatted. The on final June version 7, 2006 may differ as doi:10.1124/mol.105.019612 from this version. Prolonged
Agonist ef cacy and receptor ef ciency in heterozygous CB1 knockout mice: relationship of reduced CB1 receptor density to G-protein activation
 Journal of Neurochemistry, 2001, 77, 1±11 Agonist ef cacy and receptor ef ciency in heterozygous CB1 knockout mice: relationship of reduced CB1 receptor density to G-protein activation Dana E. Selley,*
Journal of Neurochemistry, 2001, 77, 1±11 Agonist ef cacy and receptor ef ciency in heterozygous CB1 knockout mice: relationship of reduced CB1 receptor density to G-protein activation Dana E. Selley,*
GRAEME GRIFFIN, PETER J. ATKINSON, VINCENT M. SHOWALTER, BILLY R. MARTIN and MARY E. ABOOD
 0022-3565/98/2852-0553$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2852-0553$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Neurotransmitter Systems II Receptors. Reading: BCP Chapter 6
 Neurotransmitter Systems II Receptors Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important chemical
Neurotransmitter Systems II Receptors Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important chemical
Localization of mrna expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development
 Development 125, 3179-3188 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1298 3179 Localization of mrna expression and activation of signal transduction mechanisms for cannabinoid
Development 125, 3179-3188 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1298 3179 Localization of mrna expression and activation of signal transduction mechanisms for cannabinoid
A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing
 Psychopharmacology (1998) 137:147 156 Springer-Verlag 1998 ORIGINAL INVESTIGATION selor&:d. Carriero J. Aberman S.Y. Lin A. Hill A. Makriyannis J.D. Salamone A detailed characterization of the effects
Psychopharmacology (1998) 137:147 156 Springer-Verlag 1998 ORIGINAL INVESTIGATION selor&:d. Carriero J. Aberman S.Y. Lin A. Hill A. Makriyannis J.D. Salamone A detailed characterization of the effects
Marijuana and cannabinoids
 Psych 181: Dr. Anagnostaras Lec 10: Marijuana Marijuana and cannabinoids Cannabis sativa, hemp One of earliest non-food plants cultivated fiber for rope, seeds for oil and birdseed 1st archaeological evidence
Psych 181: Dr. Anagnostaras Lec 10: Marijuana Marijuana and cannabinoids Cannabis sativa, hemp One of earliest non-food plants cultivated fiber for rope, seeds for oil and birdseed 1st archaeological evidence
Synthetic cannabinoids: Flying high under the RADAR Brett Ginsburg, Ph.D.
 Synthetic cannabinoids: Flying high under the RADAR Brett Ginsburg, Ph.D. Introduction Brett Ginsburg, Ph.D. Associate Professor Department of Psychiatry Introduction What are synthetic cannabinoids? Manufacture
Synthetic cannabinoids: Flying high under the RADAR Brett Ginsburg, Ph.D. Introduction Brett Ginsburg, Ph.D. Associate Professor Department of Psychiatry Introduction What are synthetic cannabinoids? Manufacture
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Drug Receptor Interactions and Pharmacodynamics
 Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Large Receptor Reserve for Cannabinoid Actions in the Central Nervous System 1
 0022-3565/99/2882-0478$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 288, No. 2 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2882-0478$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 288, No. 2 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Neurophysiology and Neurochemistry in PsychoGeriatrics
 Tel Aviv University Sackler Faculty of Medicine CME in Psychiatry Neurophysiology and Neurochemistry in PsychoGeriatrics Nicola Maggio, MD, PhD Sackler Faculty of Medicine Tel Aviv University Department
Tel Aviv University Sackler Faculty of Medicine CME in Psychiatry Neurophysiology and Neurochemistry in PsychoGeriatrics Nicola Maggio, MD, PhD Sackler Faculty of Medicine Tel Aviv University Department
CB1 Receptor Antagonist Precipitates Withdrawal in Mice Exposed to 9 -Tetrahydrocannabinol 1
 0022-3565/98/2853-1150$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2853-1150$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Supporting Information
 Supporting Information Burford et al. 1.173/pnas.1339311 SI Materials and Methods β-arrestin Recruitment Assay. PathHunter human osteosarcoma cells (U2OS) expressing either μ-opioid receptors (U2OS- OPRM1)
Supporting Information Burford et al. 1.173/pnas.1339311 SI Materials and Methods β-arrestin Recruitment Assay. PathHunter human osteosarcoma cells (U2OS) expressing either μ-opioid receptors (U2OS- OPRM1)
Course Calendar - Neuroscience
 2006-2007 Course Calendar - Neuroscience Meeting Hours for entire semester: Monday - Friday 1:00-2:20 p.m. Room 1200, COM August 28 August 29 August 30 August 31 September 1 Course introduction, Neurocytology:
2006-2007 Course Calendar - Neuroscience Meeting Hours for entire semester: Monday - Friday 1:00-2:20 p.m. Room 1200, COM August 28 August 29 August 30 August 31 September 1 Course introduction, Neurocytology:
Low dose combination of morphine and Δ 9 -tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors
 European Journal of Pharmacology 571 (2007) 129 137 www.elsevier.com/locate/ejphar Low dose combination of morphine and Δ 9 -tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization
European Journal of Pharmacology 571 (2007) 129 137 www.elsevier.com/locate/ejphar Low dose combination of morphine and Δ 9 -tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization
Classes of Neurotransmitters. Neurotransmitters
 1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
MOLECULAR BIOLOGY OF DRUG ADDICTION. Sylvane Desrivières, SGDP Centre
 1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
1 MOLECULAR BIOLOGY OF DRUG ADDICTION Sylvane Desrivières, SGDP Centre Reward 2 Humans, as well as other organisms engage in behaviours that are rewarding The pleasurable feelings provide positive reinforcement
Chapter 2: Studies of Human Learning and Memory. From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D.
 Chapter 2: Studies of Human Learning and Memory From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Medium Spiny Neuron A Current Conception of the major memory systems in the brain Figure
Chapter 2: Studies of Human Learning and Memory From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Medium Spiny Neuron A Current Conception of the major memory systems in the brain Figure
Receptors and Drug Action. Dr. Subasini Pharmacology Department Ishik University, Erbil
 Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Effects of Intrastriatal Cannabinoids on Rotational Behavior in Rats: Interactions With the Dopaminergic System
 SYNAPSE 30:221 226 (1998) Effects of Intrastriatal Cannabinoids on Rotational Behavior in Rats: Interactions With the Dopaminergic System M. CLARA SAÑUDO-PEÑA,* MICHELLE FORCE, KANG TSOU, ADRIENNE S. MILLER,
SYNAPSE 30:221 226 (1998) Effects of Intrastriatal Cannabinoids on Rotational Behavior in Rats: Interactions With the Dopaminergic System M. CLARA SAÑUDO-PEÑA,* MICHELLE FORCE, KANG TSOU, ADRIENNE S. MILLER,
Developmental regulation of Medium Spiny Neuron dendritic arborization. Lorene M. Lanier Department of Neuroscience
 Developmental regulation of Medium Spiny Neuron dendritic arborization Lorene M. Lanier Department of Neuroscience Diversity in dendritic arbors Pyramidal Purkinje Medium Spiny http://youtu.be/_tqpca6wx84
Developmental regulation of Medium Spiny Neuron dendritic arborization Lorene M. Lanier Department of Neuroscience Diversity in dendritic arbors Pyramidal Purkinje Medium Spiny http://youtu.be/_tqpca6wx84
Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action
 Psychopharmacology (1999) 143:322 326 Springer-Verlag 1999 RAPID COMMUNICATION Mei-Chuan Ko James H. Woods Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception
Psychopharmacology (1999) 143:322 326 Springer-Verlag 1999 RAPID COMMUNICATION Mei-Chuan Ko James H. Woods Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception
Cogs 107b Systems Neuroscience lec9_ neuromodulators and drugs of abuse principle of the week: functional anatomy
 Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
D. Nishizawa 1, N. Gajya 2 and K. Ikeda 1, * Global Research & Development, Nagoya Laboratories, Pfizer Japan Inc, Nagoya, Japan
 Current Neuropharmacology, 2011, 9, 113-117 113 Identification of Selective Agonists and Antagonists to G Protein-Activated Inwardly Rectifying Potassium Channels: Candidate Medicines for Drug Dependence
Current Neuropharmacology, 2011, 9, 113-117 113 Identification of Selective Agonists and Antagonists to G Protein-Activated Inwardly Rectifying Potassium Channels: Candidate Medicines for Drug Dependence
C81ADD Psychology of Addiction. Alcohol. Ethyl alcohol (ethanol) School of Psychology. Tobias Bast.
 C81ADD Psychology of Addiction Alcohol Ethyl alcohol (ethanol) Tobias Bast School of Psychology tobias.bast@nottingham.ac.uk 1 Selected aspects of the psychopharmacology of alcohol (ethanol) Primary neuropharmacological
C81ADD Psychology of Addiction Alcohol Ethyl alcohol (ethanol) Tobias Bast School of Psychology tobias.bast@nottingham.ac.uk 1 Selected aspects of the psychopharmacology of alcohol (ethanol) Primary neuropharmacological
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Receptors Families. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
2013 W. H. Freeman and Company. 12 Signal Transduction
 2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
Cannabinoid physiology and pharmacology: 30 years of progress
 Neuropharmacology 47 (2004) 345 358 www.elsevier.com/locate/neuropharm Cannabinoid physiology and pharmacology: 30 years of progress Allyn C. Howlett a,c,, Christopher S. Breivogel b, Steven R. Childers
Neuropharmacology 47 (2004) 345 358 www.elsevier.com/locate/neuropharm Cannabinoid physiology and pharmacology: 30 years of progress Allyn C. Howlett a,c,, Christopher S. Breivogel b, Steven R. Childers
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Pharmacodynamics. OUTLINE Definition. Mechanisms of drug action. Receptors. Agonists. Types. Types Locations Effects. Definition
 Pharmacodynamics OUTLINE Definition. Mechanisms of drug action. Receptors Types Locations Effects Agonists Definition Types Outlines of Pharmacodynamics Antagonists Definition Types Therapeutic Index Definition
Pharmacodynamics OUTLINE Definition. Mechanisms of drug action. Receptors Types Locations Effects Agonists Definition Types Outlines of Pharmacodynamics Antagonists Definition Types Therapeutic Index Definition
Cannabinoids Reveal the Necessity of Hippocampal Neural Encoding for Short-Term Memory in Rats
 The Journal of Neuroscience, December 1, 2000, 20(23):8932 8942 Cannabinoids Reveal the Necessity of Hippocampal Neural Encoding for Short-Term Memory in Rats Robert E. Hampson and Sam A. Deadwyler Department
The Journal of Neuroscience, December 1, 2000, 20(23):8932 8942 Cannabinoids Reveal the Necessity of Hippocampal Neural Encoding for Short-Term Memory in Rats Robert E. Hampson and Sam A. Deadwyler Department
Differential Blockade of the Antinociceptive Effects of Centrally Administered Cannabinoids by SR141716A 1
 0022-3565/98/2863-1301$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 286, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2863-1301$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 286, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
NS219: Basal Ganglia Anatomy
 NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
Exam 2 PSYC Fall (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
 Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
CASE 49. What type of memory is available for conscious retrieval? Which part of the brain stores semantic (factual) memories?
 CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
SR141716A Antagonizes the Disruptive Effects of Cannabinoid Ligands on Learning in Rats 1
 0022-3565/97/2823-1526$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 282, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/97/2823-1526$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 282, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Making Things Happen 2: Motor Disorders
 Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Basics of Pharmacology
 Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Neurobiological and functional consequences of chronic partial sleep deprivation Roman, Viktor
 University of Groningen Neurobiological and functional consequences of chronic partial sleep deprivation Roman, Viktor IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
University of Groningen Neurobiological and functional consequences of chronic partial sleep deprivation Roman, Viktor IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
Comparative Receptor Binding Analyses of Cannabinoid Agonists and Antagonists
 0022-3565/98/2851-0285$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2851-0285$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Cannabis. Member of the Cannabaceae family of flowering plants (along with hops) Cannabis sativa (v. sativa, indica, afghanica, ruderalis)
 Member of the Cannabaceae family of flowering plants (along with hops) sativa (v. sativa, indica, afghanica, ruderalis) Only females flowers contain high concentrations of psychoactive oils (cannabinoids)
Member of the Cannabaceae family of flowering plants (along with hops) sativa (v. sativa, indica, afghanica, ruderalis) Only females flowers contain high concentrations of psychoactive oils (cannabinoids)
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Course Calendar
 Clinical Neuroscience BMS 6706C Charles, Ph.D., Course Director charles.ouimet@med.fsu.edu (850) 644-2271 2004 2005 Course Calendar Click here to return to the syllabus Meeting Hours for entire semester:
Clinical Neuroscience BMS 6706C Charles, Ph.D., Course Director charles.ouimet@med.fsu.edu (850) 644-2271 2004 2005 Course Calendar Click here to return to the syllabus Meeting Hours for entire semester:
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
LY320135, a Novel Cannabinoid CB1 Receptor Antagonist, Unmasks Coupling of the CB1 Receptor to Stimulation of camp Accumulation 1
 0022-3565/98/2841-0291$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 284, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2841-0291$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 284, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Leen Osama, Lujain Hamdan, Osama Mohd, Razi Kittaneh... Faisal Mohammad
 23 Leen Osama, Lujain Hamdan, Osama Mohd, Razi Kittaneh... Faisal Mohammad Revision of previous lectures G-proteins coupled receptors mechanism: When a hormone binds to G-protein coupled receptor, GTP
23 Leen Osama, Lujain Hamdan, Osama Mohd, Razi Kittaneh... Faisal Mohammad Revision of previous lectures G-proteins coupled receptors mechanism: When a hormone binds to G-protein coupled receptor, GTP
Differentiation of delta and mu opiate receptor localizations by light
 Proc. Nati. Acad. Sci. USA Vol. 77, No. 10, pp. 6239-6243, October 1980 Neurobiology Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography (multiple receptors/enkephalin)
Proc. Nati. Acad. Sci. USA Vol. 77, No. 10, pp. 6239-6243, October 1980 Neurobiology Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography (multiple receptors/enkephalin)
Regional differences in mu and kappa opioid receptor G-protein activation in brain in male and female prairie voles
 Regional differences in mu and kappa opioid receptor G-protein activation in brain in male and female prairie voles Thomas J. Martin, Wake Forest University Tammy Sexton, Wake Forest University Susy A.
Regional differences in mu and kappa opioid receptor G-protein activation in brain in male and female prairie voles Thomas J. Martin, Wake Forest University Tammy Sexton, Wake Forest University Susy A.
Zoektocht naar innovatieve geneesmiddelen voor de toekomst - Verslaving en ander gedrag in moleculair perspectief
 Zoektocht naar innovatieve geneesmiddelen voor de toekomst - Verslaving en ander gedrag in moleculair perspectief Prof. dr. Chris Kruse Swammerdam Institute, UvA Solvay Pharmaceuticals Drug Discovery Assets
Zoektocht naar innovatieve geneesmiddelen voor de toekomst - Verslaving en ander gedrag in moleculair perspectief Prof. dr. Chris Kruse Swammerdam Institute, UvA Solvay Pharmaceuticals Drug Discovery Assets
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Neurotransmitters acting on G-protein coupled receptors
 Neurotransmitters acting on G-protein coupled receptors Part 1: Dopamine and Norepinephrine BIOGENIC AMINES Monoamines Diamine Overview of Neurotransmitters and Their Receptors Criteria for defining a
Neurotransmitters acting on G-protein coupled receptors Part 1: Dopamine and Norepinephrine BIOGENIC AMINES Monoamines Diamine Overview of Neurotransmitters and Their Receptors Criteria for defining a
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
AMPK Assay. Require: Sigma (1L, $18.30) A4206 Aluminum foil
 AMPK Assay Require: Acetone Sigma (1L, $18.30) A4206 Aluminum foil Ammonium sulfate Fisher BP212R-1 AMP Sigma A1752 ATP Sigma A6144 (alt. use A7699) Beta-mercaptoethanol Sigma M6250 (alt. use M7154) Bio-Rad
AMPK Assay Require: Acetone Sigma (1L, $18.30) A4206 Aluminum foil Ammonium sulfate Fisher BP212R-1 AMP Sigma A1752 ATP Sigma A6144 (alt. use A7699) Beta-mercaptoethanol Sigma M6250 (alt. use M7154) Bio-Rad
MOVEMENT OUTLINE. The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement
 MOVEMENT 2 Dr. Steinmetz 3 OUTLINE The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement Parkinson s Disease Huntington s Disease 1 4 TYPES
MOVEMENT 2 Dr. Steinmetz 3 OUTLINE The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement Parkinson s Disease Huntington s Disease 1 4 TYPES
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
QING TAO and MARY E. ABOOD
 0022-3565/98/2852-0651$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2852-0651$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
The Neuroscience of Addiction: A mini-review
 The Neuroscience of Addiction: A mini-review Jim Morrill, MD, PhD MGH Charlestown HealthCare Center Massachusetts General Hospital Disclosures Neither I nor my spouse/partner has a relevant financial relationship
The Neuroscience of Addiction: A mini-review Jim Morrill, MD, PhD MGH Charlestown HealthCare Center Massachusetts General Hospital Disclosures Neither I nor my spouse/partner has a relevant financial relationship
Lack of GPR88 enhances medium spiny neuron activity and alters. motor- and cue- dependent behaviors
 Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue- dependent behaviors Albert Quintana, Elisenda Sanz, Wengang Wang, Granville P. Storey, Ali D. Güler Matthew J. Wanat, Bryan
Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue- dependent behaviors Albert Quintana, Elisenda Sanz, Wengang Wang, Granville P. Storey, Ali D. Güler Matthew J. Wanat, Bryan
Pharmacodynamics. Dr. Alia Shatanawi
 Pharmacodynamics Dr. Alia Shatanawi Drug Receptor Interactions Sep-17 Dose response relationships Graduate dose-response relations As the dose administrated to single subject or isolated tissue is increased,
Pharmacodynamics Dr. Alia Shatanawi Drug Receptor Interactions Sep-17 Dose response relationships Graduate dose-response relations As the dose administrated to single subject or isolated tissue is increased,
School of Medical Sciences, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen, UK
 British Journal of Pharmacology (1), 159, 129 141 9 The Authors Journal compilation 9 The British Pharmacological Society All rights reserved 7-1188/9 www.brjpharmacol.org RESEARCH PAPER Evidence that
British Journal of Pharmacology (1), 159, 129 141 9 The Authors Journal compilation 9 The British Pharmacological Society All rights reserved 7-1188/9 www.brjpharmacol.org RESEARCH PAPER Evidence that
Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning
 1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
Understanding the Brain: What Drugs Can Tell Us
 LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Understanding the Brain: What Drugs Can Tell Us Presented by: Dr. Rochelle D. Schwartz-Bloom March 24, 2011 Understanding the Brain: What Drugs Can Tell Us Rochelle
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Understanding the Brain: What Drugs Can Tell Us Presented by: Dr. Rochelle D. Schwartz-Bloom March 24, 2011 Understanding the Brain: What Drugs Can Tell Us Rochelle
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Synthesis and Characterization of Potent and Selective Agonists of the Neuronal Cannabinoid Receptor (CB1) 1
 0022-3565/99/2893-1427$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 289, No. 3 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2893-1427$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 289, No. 3 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
11/8/16. Cell Signaling Mechanisms. Dr. Abercrombie 11/8/2016. Principal Parts of Neurons A Signal Processing Computer
 Cell Signaling Mechanisms Dr. Abercrombie 11/8/2016 Principal Parts of Neurons A Signal Processing Computer A Multitude of Synapses and Synaptic Actions Summation/Synaptic Integration 1 The Synapse Signal
Cell Signaling Mechanisms Dr. Abercrombie 11/8/2016 Principal Parts of Neurons A Signal Processing Computer A Multitude of Synapses and Synaptic Actions Summation/Synaptic Integration 1 The Synapse Signal
Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA
 Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Pharmacology. Biomedical Sciences. Dynamics Kinetics Genetics. School of. Dr Lindsey Ferrie
 Pharmacology Dynamics Kinetics Genetics Dr Lindsey Ferrie lindsey.ferrie@ncl.ac.uk MRCPsych Neuroscience and Psychopharmacology School of Biomedical Sciences Dynamics What the drug does to the body What
Pharmacology Dynamics Kinetics Genetics Dr Lindsey Ferrie lindsey.ferrie@ncl.ac.uk MRCPsych Neuroscience and Psychopharmacology School of Biomedical Sciences Dynamics What the drug does to the body What
Embryological origin of thalamus
 diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD
 marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
Biological Bases of Behavior. 8: Control of Movement
 Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD
 marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Journal of Natural Sciences Research ISSN (Paper) ISSN (Online) Vol.2, No.9, 2012
 Evaluation of Total Brain Acetylcholine in rats treated with inhaled Tetrahydrocannabinol. (A Bioassay Study) Meraiyebu Ajibola. B (Corresponding author) Department of Physiology, College of medicine Bingham
Evaluation of Total Brain Acetylcholine in rats treated with inhaled Tetrahydrocannabinol. (A Bioassay Study) Meraiyebu Ajibola. B (Corresponding author) Department of Physiology, College of medicine Bingham
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 16, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
AM281, Cannabinoid Antagonist/Inverse agonist, Ameliorates Scopolamine- Induced Cognitive Deficit
 Iranian Journal of Basic Medical Sciences Vol. 15, No. 5, Sep-Oct 2012, 1106-1110 Received: May 28, 2011; Accepted: Dec 24, 2011 www.mums.ac.ir Short Communication AM281, Cannabinoid Antagonist/Inverse
Iranian Journal of Basic Medical Sciences Vol. 15, No. 5, Sep-Oct 2012, 1106-1110 Received: May 28, 2011; Accepted: Dec 24, 2011 www.mums.ac.ir Short Communication AM281, Cannabinoid Antagonist/Inverse
- Neurotransmitters Of The Brain -
 - Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
- Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD
 marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
marijuana and the teen brain MARY ET BOYLE, PH. D. DEPARTMENT OF COGNITIVE SCIENCE UCSD in this talk what is marijuana? the brain on marijuana is the teen brain special? current research what is marijuana?
The Pharmacology of SR A: A Review
 CNS Drug Reviews Vol. 5, No. 1, pp. 43 58 1999 Neva Press, Branford, Connecticut The Pharmacology of SR 141716A: A Review E. M. Nakamura-Palacios, J. M. Moerschbaecher and L. A. Barker Post-Graduation
CNS Drug Reviews Vol. 5, No. 1, pp. 43 58 1999 Neva Press, Branford, Connecticut The Pharmacology of SR 141716A: A Review E. M. Nakamura-Palacios, J. M. Moerschbaecher and L. A. Barker Post-Graduation
Plasma membranes. Plasmodesmata between plant cells. Gap junctions between animal cells Cell junctions. Cell-cell recognition
 Cell Communication Cell Signaling Cell-to-cell communication is essential for multicellular organisms Communicate by chemical messengers Animal and plant cells have cell junctions that directly connect
Cell Communication Cell Signaling Cell-to-cell communication is essential for multicellular organisms Communicate by chemical messengers Animal and plant cells have cell junctions that directly connect
The cannabinoid CB 1 receptor antagonist SR141716A attenuates the memory impairment produced by 9 -tetrahydrocannabinol or anandamide
 Psychopharmacology (1998) 140:11 19 Springer-Verlag 1998 ORIGINAL INVESTIGATION Paul E. Mallet Richard J. Beninger The cannabinoid CB 1 receptor antagonist SR141716A attenuates the memory impairment produced
Psychopharmacology (1998) 140:11 19 Springer-Verlag 1998 ORIGINAL INVESTIGATION Paul E. Mallet Richard J. Beninger The cannabinoid CB 1 receptor antagonist SR141716A attenuates the memory impairment produced
Receptorarchitecture and Neural Systems
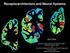 Receptorarchitecture and Neural Systems Karl Zilles Institute of Neuroscience and Medicine INM-1 Research Centre Jülich and University Hospital of Psychiatry, Psychotherapy and Psychosomatics RWTH Universität
Receptorarchitecture and Neural Systems Karl Zilles Institute of Neuroscience and Medicine INM-1 Research Centre Jülich and University Hospital of Psychiatry, Psychotherapy and Psychosomatics RWTH Universität
2. Name and give the neurotransmitter for two of the three shown (Fig. 26.8) brainstem nuclei that control sleep and wakefulness.
 Put your name here-> BL A-415 Nerve cell mechanisms in behavior - Prof. Stark BL A-615 Neural bases of behavior Final examination - Tuesday, Dec. 12, 2000 12 noon - 1:50 p.m. Keep "essays" brief. Pay close
Put your name here-> BL A-415 Nerve cell mechanisms in behavior - Prof. Stark BL A-615 Neural bases of behavior Final examination - Tuesday, Dec. 12, 2000 12 noon - 1:50 p.m. Keep "essays" brief. Pay close
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
Damage on one side.. (Notes) Just remember: Unilateral damage to basal ganglia causes contralateral symptoms.
 Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
PHRM20001: Pharmacology - How Drugs Work!
 PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
Study Guide Unit 2 Psych 2022, Fall 2003
 Study Guide Unit 2 Psych 2022, Fall 2003 Subcortical Anatomy 1. Be able to locate the following structures and be able to indicate whether they are located in the forebrain, diencephalon, midbrain, pons,
Study Guide Unit 2 Psych 2022, Fall 2003 Subcortical Anatomy 1. Be able to locate the following structures and be able to indicate whether they are located in the forebrain, diencephalon, midbrain, pons,
