Meeting report series. Report of the 5th Therapies Scientific Committee Meeting
|
|
|
- Opal Long
- 5 years ago
- Views:
Transcription
1 Meeting report series Report of the 5th Therapies Scientific Committee Meeting IRDiRC, Rare Disease Platform, Paris, France 19 th March 2014 Organization Organized by: Scientific Secretariat Hosted by: IRDiRC, Rare Disease Platform, Paris, France Participants Dr Seng Cheng, Framingham, USA Prof Gert-Jan Van Omen, Leiden, Netherlands Dr Karin Rademaker, Utrecht, Netherlands Dr Adam Heathfield, Sandwich, UK Mr Yann Le Cam, Paris, France (phone) Dr Fulvio Malvilio, Evry, France Dr Elizabeth Mc Neil, Bethesda, USA (GoToMeeting) Prof Josep Torrent i Farnell, Barcelona, Spain Dr Maria Mavris, Paris, France Dr Barbara Cagniard, Scientific Secretariat Dr Virginie Hivert, Scientific Secretariat Dr Sophie Höhn, Scientific Secretariat Apologies Dr Giles Campion, Leiden, the Netherlands Dr John McKew, Bethesda, USA Dr Luigi Naldini, Milan, Italy Dr Glen Nuckolls, Bethesda, USA Prof Asla Pitkänen, Joensuu, Finland Dr Robert Schaub, Waltham, USA Dr Marc Walton, Silver Spring, USA 3rd meeting of the TSC Public Version, 14 May
2 Dr Ellen Welsh, South Plainfield, USA Dr Anne Zajicek, Bethesda, USA Agenda 19th March 2014, 10:30 17:00 1. Introduction 2. Review and next steps for: WG on Biomarkers for disease progression and therapy response WG on Biotechnology-derived products including cell- and gene-based therapies WG on Chemically-derived products including repurposing WG on Orphan drug-development and regulatory processes 3. Feedback from the Scientific Secretariat on analysis requested by TSC and WGs 4. Recommendations of the TSC 5. Shenzhen conference 6. Next meeting date and location 3rd meeting of the TSC Public Version, 14 May
3 EXECUTIVE SUMMARY The third face-to-face meeting of the TSC took place on 19th March 2014 at IRDiRC, Rare Disease Platform, Paris, France. Seven members of the committee were present all day long and two members temporarily joined the meeting by phone or teleconference. The members of the TSC first reviewed the outcomes from the 4 WGs and discussed the next steps that should be considered in the setting up of their future work plan. The TSC then reviewed and agreed on the recommendations, in terms of policy and funding, from the TSC to be submitted to the Executive Committee. The main discussion concerned the targeting of funding to products which have been designated as orphan and went through scientific advice/guidance as a way to stimulate a high quality and faster orphan drug development. The TSC members also agreed on a common set of mandatory criteria and flexible shared criteria, addressing both non-clinical and clinical stages. The TSC member participating in the preparation of the scientific program of the IRDiRC conference to be held in Shenzhen gave an update on the advancement of the program. It was proposed to hold the next face-to-face meeting of the TSC in North America. 3rd meeting of the TSC Public Version, 14 May
4 3rd meeting of the TSC Public Version, 14 May
5 REPORT Josep Torrent i Farnell welcomed the participants and conveyed the apologies from the chair of the TSC, Yann Le Cam, who was unable to attend the meeting. Members of the TSC introduced themselves, as well as the members of the Scientific Secretariat. Review of the WGs outcomes and feedback on data analysis The morning session was devoted to the review of the outcomes from the 4 WGs and the discussion regarding the next steps of their work. For each of the WGs, the representative of the TSC in the WG or a member of the Scientific Secretariat, made a summary of the previous conference calls, questions and achievements. At the same time, the Scientific Secretariat gave a feedback from the data and analysis requested by the WGs and the TSC for each topic. WG on Biomarkers for disease progression and therapy response Feedback from WG The WG on Biomarkers has expressed the willingness to set up a survey in order to identify research projects of interest in the field, possible collaboration between those research projects and clusters of interest in terms of medical domains. Feedback from the TSC The TSC members agreed that this analysis is important, indeed, the data already compiled by Scientific Secretariat needs to be further evaluated and analyzed in a more detailed and in a deeply manner. The best way to finance it needs to be carefully considered. In this regard, it was discussed that it would be more appropriate that this funding could come directly from IRDiRC funding bodies, rather than from a call for proposals. The funding would serve to hire one information scientist to be attached to the organization of one of the WG members, as to deeply analyze the data that have already been provided from the Scientific Secretariat (all the files have been uploaded onto the private section of the IRDiRC website). A call for proposal could be beneficial but only once the data analysis has been performed in order to narrow down the scope of the call. The WG should propose a timeline in accordance with the analysis proposal. It was suggested that this phase of analysis should not last more than 6 months. The scope of the WG should focus on those orphan conditions for which biomarkers could really speed up the therapy development process but where there are currently no reliable clinical endpoints or outcome measures. 3rd meeting of the TSC Public Version, 14 May
6 As biomarkers could serve as therapeutic readout, but also for diagnosis purpose, a connection of this WG with the DSC is perceived as good. WG on Biotechnology-derived products including cell- and gene-based therapies Feedback from WG The WG on Biotechnology has expressed some concerns regarding the revision of the European Clinical Trials Regulation. They identified an issue with the wording of minimal risk and minimal burden and the use of a standard of treatment, which is not always available in the field of rare diseases. The WG would like the TSC to alert the Executive Committee of this problem and discuss possible actions. In terms of recommendations, the most important for the WG is to insist on a timely development of pharmacodynamics and biomarkers and on giving funding for natural history. Another important point to emphasize is to anticipate the re-analysis of data coming from clinical trials by statistical methods, for example by the pre-determination of subsection(s) of read out when setting up trials which will allow re-analysis afterwards. The WG on Biotechnology also received lists of projects/trials from the Scientific Secretariat (all the files have been uploaded onto the private section of the IRDiRC website), which they have found very useful to oversee the available information while quite difficult to review from the perspective of the WG. This WG has also agreed that in the field of interest, the COST action for Duchenne is a very good model for their future work plan, with its three main topics: Trial readiness, linked to biomarkers and natural history Cross-connection between different diseases Dialogue between scientists and regulators with continuous medical education and training from both sides WG on Chemically-derived products including repurposing Feedback from WG This WG identifies two main objectives related to its scope: to find candidates for therapeutic development and to improve the process in relation with these products. They agreed to focus on drugs already registered (as most of the repurposing comes from registered conventional drugs for non rare diseases). 3rd meeting of the TSC Public Version, 14 May
7 This WG has listed 4 strategies to identify candidates: Rescuing drugs that have failed Buying library of drugs Asking feedback from patient support groups Exploring gene expression profiles An analysis of the outcomes from the already funded research projects (FP6, FP7, E-Rare, NIH, FDA) could also help to find candidates. The next step could be to release a list of couples (molecules & disease); the organization interested in moving on would contact the Orphan designated sponsor to harvest the data available and discuss IP whenever required. Feedback from TSC According to the TSC members, there are new possibilities in the rare disease field, mainly thanks to the NGS techniques. In particular, mechanistic approaches are of interest not only for repurposing or drugs that have failed, but also for drugs that have never been explored. Regarding these mechanistic approaches, NCATS and FDA have a more long-term overall experience. Repurposing in itself is often difficult to apply because even if data on efficacy are available, there is often a lack of information regarding other characteristics of the drug, i.e., dose, etc. Pharmaceuticals companies are often willing to help screening libraries if an assay is available. IRDiRC could serve as an international platform to release a list of proposed molecules and diseases and try to facilitate connection between companies willing to develop drugs and people that own them (academics, patient organizations, etc.), having in mind that intellectual property is also a major issue. As for the WG on Biomarkers, it was suggested to adopt a dynamic process and to allocate some funding to further analyze the outcomes from the already funded research projects (FP6, FP7, E- Rare, NIH, FDA). It has also been emphasized that the analysis of reports submitted to the regulatory Agencies would help to better understand the delay observed between designation and approval in some cases. In terms of organization, a link between this WG and the WG on Model systems should be created. It was considered beneficial to include a new industry member with direct experience in this area (i.e. an expert from Pfizer is going to be proposed). WG on Orphan drug-development and regulatory processes Feedback from WG 3rd meeting of the TSC Public Version, 14 May
8 This WG focused on several topics, particularly early dialogue, convergence/alignment of therapeutic guidelines, and adaptive clinical trials design. The WG has also evoked the usefulness of analysis of the report submitted to the regulatory Agencies, as mentioned before. Feedback from TSC The TSC member participating involved in the WG has the impression that although the representatives of the Agencies are really willing to be involved in the discussions of this WG, they do not always feel comfortable expressing themselves due to their position. The TSC stated that their role is to identify the bottlenecks that come up and propose appropriate ways to solve theses hurdles. During the meeting, the concept of Orphanage was also mentioned, which has been raised up at the joint workshop between the EMA, FDA and PMDA (Japan) on 10 th of March in London, in order to facilitate the development of molecules that have been left aside for different reasons. In particular it was considered adequate that a specific IRDiRC call in this point could be very effective in increasing the transfer of dormant/silent designated orphan products to more active sponsors wishing to further develop these compounds. Review of the document Recommendations of the TSC, Shenzhen conference and next meeting Recommendations of the TSC The document Policy & Funding Recommendations of the IRDiRC Therapies Scientific Committee (TSC) was presented by Yann Le Cam (Chair of TSC) who joined the meeting by phone. The document is based on 6 months of work by the TSC and its 4 WGs. It was prepared by a drafting group composed of Yann Le Cam, Josep Torrent i Farnell (former chair and member of the TSC), Maria Mavris (member of the TSC), Valentina Bottarelli (EURORDIS) and Virginie Hivert (Scientific Secretariat). The document and comments collected during the first rounds of review were inserted and have all been reviewed by the TSC members as to ensure that content and wording are in line with the outcomes of the TSC and WGs discussions and the maximal leverage effect that is awaited from these recommendations. The main discussion concerned the targeting of funding to products which have been designated as orphan and went through scientific advice/guidance at EMA or FDA, or ideally at both Agencies, as a way to stimulate better quality development. The TSC members finally agreed on a common set of mandatory criteria and flexible shared criteria, addressing both non-clinical and clinical stages. 3rd meeting of the TSC Public Version, 14 May
9 The document has then been reviewed once again by the drafting group after the TSC meeting in order to incorporate all the modifications that have been agreed during the day as to be presented to the Executive Committee on 10 th of April. The recommendations from the 3 Scientific Committees will then be merged in one single road map to be adopted by the Executive Committee on 7 th May in Berlin. Shenzhen conference The Shenzhen conference will take place in November 7-9, There will be four parallel tracks: Diagnostics / Therapies / Interdisciplinary-Technologies / Educational track. Some speakers have already been chosen. An will be sent to the TSC members in order for them to suggest some speakers within a week. Next meeting of the TSC It was suggested that the next meeting of the TSC will take place in North America, Boston for example, and that the TSC will adopt a recurrent timeline with two face-to-face meeting per year in March and October. 3rd meeting of the TSC Public Version, 14 May
Meeting report series. Report of the 6th Therapies Scientific Committee Meeting
 Meeting report series Report of the 6th Therapies Scientific Committee Meeting Paris, France February 6, 2017 Participants Dr Diego Ardigò, Parma, Italy Chair Prof Annemieke Aartsma-Rus, Leiden, the Netherlands
Meeting report series Report of the 6th Therapies Scientific Committee Meeting Paris, France February 6, 2017 Participants Dr Diego Ardigò, Parma, Italy Chair Prof Annemieke Aartsma-Rus, Leiden, the Netherlands
Global Pediatric Development: We Are Making Progress. PMDA Perspective. Junko Sato, PhD PMDA
 Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
EURORDIS SUMMER SCHOOL EXPRESS YOURSELF!
 EXPERT PATIENTS AND RESEARCHERS EURORDIS SUMMER SCHOOL EXPRESS YOURSELF! CASTELLDEFELS, BARCELONA, SPAIN JUNE 11-15, 2018 A capacity building programme for patient representatives & researchers on medicines
EXPERT PATIENTS AND RESEARCHERS EURORDIS SUMMER SCHOOL EXPRESS YOURSELF! CASTELLDEFELS, BARCELONA, SPAIN JUNE 11-15, 2018 A capacity building programme for patient representatives & researchers on medicines
Economic and Social Council
 United Nations Economic and Social Council Distr.: General 13 September 2013 ECE/WG.1/2013/4 Original: English Economic Commission for Europe Working Group on Ageing Sixth meeting Geneva, 25-26 November
United Nations Economic and Social Council Distr.: General 13 September 2013 ECE/WG.1/2013/4 Original: English Economic Commission for Europe Working Group on Ageing Sixth meeting Geneva, 25-26 November
PHOTO. VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe
 www.eurordis.org VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe Lucca ( Italy ) March 13-14, 2008 PHOTO www.eurordis.org
www.eurordis.org VHPB/ELPA MEETING Prevention and Control of Viral Hepatitis The role and impact of patients and advocacy groups in and outside Europe Lucca ( Italy ) March 13-14, 2008 PHOTO www.eurordis.org
EPF s response to the European Commission s public consultation on the "Summary of Clinical Trial Results for Laypersons"
 EPF s response to the European Commission s public consultation on the "Summary of Clinical Trial Results for Laypersons" August 2016 This document received funding under an operating grant from the European
EPF s response to the European Commission s public consultation on the "Summary of Clinical Trial Results for Laypersons" August 2016 This document received funding under an operating grant from the European
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
U.S. Fund for UNICEF Campus Initiative LEADERSHIP TRANSITION HANDBOOK
 U.S. Fund for UNICEF Campus Initiative LEADERSHIP TRANSITION HANDBOOK Table of Contents Introduction and Club Organization 1 Transition Guidelines 3 Sample Outgoing Officer Questionnaire 5 Sample Position
U.S. Fund for UNICEF Campus Initiative LEADERSHIP TRANSITION HANDBOOK Table of Contents Introduction and Club Organization 1 Transition Guidelines 3 Sample Outgoing Officer Questionnaire 5 Sample Position
GLOBAL BIOANALYSIS CONSORTIUM
 GLOBAL BIOANALYSIS CONSORTIUM Regulated Bioanalysis - A Proposed Global Harmonization Process Presented by Peter van Amsterdam for GBC SoFAQ International Seminar "Quality Assurance and Electronic Data"
GLOBAL BIOANALYSIS CONSORTIUM Regulated Bioanalysis - A Proposed Global Harmonization Process Presented by Peter van Amsterdam for GBC SoFAQ International Seminar "Quality Assurance and Electronic Data"
Retina International General Assembly Auckland, New Zealand
 Retina International General Assembly 2018 - Auckland, New Zealand Thursday, February 8th, 2018 Location: University of Auckland Business School Owen G Glenn Building, 12 Grafton Road, Auckland, 1010 Room:
Retina International General Assembly 2018 - Auckland, New Zealand Thursday, February 8th, 2018 Location: University of Auckland Business School Owen G Glenn Building, 12 Grafton Road, Auckland, 1010 Room:
Update on public hearing
 Update on public hearing PCWP/HCPWP joint meeting Presented by Juan Garcia Burgos on 0 September 017 Head of Public Engagement Department An agency of the European Union A public hearing provides An opportunity
Update on public hearing PCWP/HCPWP joint meeting Presented by Juan Garcia Burgos on 0 September 017 Head of Public Engagement Department An agency of the European Union A public hearing provides An opportunity
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Towards a New Era in Dermocosmetics
 3 rd ISM Symposium on Skin Microbiota 2018 Towards a New Era in Dermocosmetics June 15, 2018 - Paris, France The International Society of Microbiota (ISM) organizes three meetings in 2018, one ISM annual
3 rd ISM Symposium on Skin Microbiota 2018 Towards a New Era in Dermocosmetics June 15, 2018 - Paris, France The International Society of Microbiota (ISM) organizes three meetings in 2018, one ISM annual
Annotations to the provisional agenda
 Technology Executive Committee 29 August 2017 Fifteenth meeting Bonn, Germany, 12 15 September 2017 Annotations to the provisional agenda 1. Opening of the meeting 1. The Chair of the Technology Executive
Technology Executive Committee 29 August 2017 Fifteenth meeting Bonn, Germany, 12 15 September 2017 Annotations to the provisional agenda 1. Opening of the meeting 1. The Chair of the Technology Executive
Safety Pharmacology Post Meeting Survey 2014
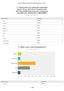 Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
Q1 Please enter your personal information below. (If you want to be included in the 2014 Annual Meeting survey for a chance to win $200, this information is REQUIRED) Answered: 132 Skipped: 14 Name: Company:
BDA IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS NOVEMBER 2013 WORKSHOP ADVANCE PROGRAMME
 BDA WORKSHOP IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS 18-19 NOVEMBER 2013 PARIS, FRANCE BIOTHERAPY DEVELOPMENT ASSOCIATION encca European Network for Cancer Research in Children
BDA WORKSHOP IMPROVING ONCOLOGY DRUG DEVELOPMENT FOR CHILDREN AND ADOLESCENTS 18-19 NOVEMBER 2013 PARIS, FRANCE BIOTHERAPY DEVELOPMENT ASSOCIATION encca European Network for Cancer Research in Children
The MOVE Trial Summary. Palovarotene FAQs. 1. What is the MOVE Trial?
 MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
 MEETING PROSPECTUS www.virology-education.com CONTENT Introduction... 3 Workshop description... 4 Background...4 Meeting Objectives...4 Learning Objectives...4 Format...4 Unique Meeting Features...4 Target
MEETING PROSPECTUS www.virology-education.com CONTENT Introduction... 3 Workshop description... 4 Background...4 Meeting Objectives...4 Learning Objectives...4 Format...4 Unique Meeting Features...4 Target
AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community)
 AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community) Faye Vazvaei, Roche Innovation Center New York The 8th JBF Meeting, 8-9 February 2017 Background
AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community) Faye Vazvaei, Roche Innovation Center New York The 8th JBF Meeting, 8-9 February 2017 Background
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Outreach and Communications
 Care for Elders July 2008 June 2009 Year End Accomplishments Outreach and Communications 2-1-1/Elder Care Experts Access Network Distributed information to 105 locations o Distributed 8,675 brochures,
Care for Elders July 2008 June 2009 Year End Accomplishments Outreach and Communications 2-1-1/Elder Care Experts Access Network Distributed information to 105 locations o Distributed 8,675 brochures,
Cancer prevention and control in the context of an integrated approach
 SEVENTIETH WORLD HEALTH ASSEMBLY A70/A/CONF./9 Agenda item 15.6 25 May 2017 Cancer prevention and control in the context of an integrated approach Draft resolution proposed by Brazil, Canada, Colombia,
SEVENTIETH WORLD HEALTH ASSEMBLY A70/A/CONF./9 Agenda item 15.6 25 May 2017 Cancer prevention and control in the context of an integrated approach Draft resolution proposed by Brazil, Canada, Colombia,
NICOLE s Shared vision on Sustainable Remediation
 NICOLE s Shared vision on Sustainable Remediation Green Remediation conference 09 November 2009 Copenhagen www.nicole.org 1 Agenda Methodology followed Work completed since Oct 2008 Main lessons learned
NICOLE s Shared vision on Sustainable Remediation Green Remediation conference 09 November 2009 Copenhagen www.nicole.org 1 Agenda Methodology followed Work completed since Oct 2008 Main lessons learned
EFPA BOARD of SCIENTIFIC AFFAIRS Board Meeting London, United Kingdom 12 th May 2018
 EFPA BOARD of SCIENTIFIC AFFAIRS Board Meeting London, United Kingdom 12 th May 2018 AGENDA Venue British Psychological Society, London Office, 30 Tabernacle St, London, EC2A 4UE Time 10.00 16:00 (GMT)
EFPA BOARD of SCIENTIFIC AFFAIRS Board Meeting London, United Kingdom 12 th May 2018 AGENDA Venue British Psychological Society, London Office, 30 Tabernacle St, London, EC2A 4UE Time 10.00 16:00 (GMT)
CONSULTATION DRAFT. Draft Council paper on GEF and civil society. A New Vision to Enhance Civil Society Engagement with the GEF.
 Vision Statement CONSULTATION DRAFT Draft Council paper on GEF and civil society A New Vision to Enhance Civil Society Engagement with the GEF Executive Summary A. The overarching objective of engagement
Vision Statement CONSULTATION DRAFT Draft Council paper on GEF and civil society A New Vision to Enhance Civil Society Engagement with the GEF Executive Summary A. The overarching objective of engagement
Held on , Parma. (Agreed on )
 AHAW UNIT SCIENTIFIC PANEL ON ANIMAL HEALTH AND WELFARE Minutes of the 10 th meeting of the Working Group on possible risks posed by the Influenza A (H3N2v) virus for animal health and its potential spread
AHAW UNIT SCIENTIFIC PANEL ON ANIMAL HEALTH AND WELFARE Minutes of the 10 th meeting of the Working Group on possible risks posed by the Influenza A (H3N2v) virus for animal health and its potential spread
Request for Proposals
 Background The U.S. Preventive Services Task Force (USPSTF or Task Force) is an independent, volunteer panel of national experts in prevention and evidence-based medicine. Since its inception in 1984,
Background The U.S. Preventive Services Task Force (USPSTF or Task Force) is an independent, volunteer panel of national experts in prevention and evidence-based medicine. Since its inception in 1984,
EPAG UPDATE. Lenja Wiehe. European Patient Advocacy Groups Manager, EURORDIS
 EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
EPAG UPDATE Lenja Wiehe European Patient Advocacy Groups Manager, EURORDIS Patient Centre & Empowerment European Reference Networks (ERNs) created on founding principles of patient-centred care, patient
The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report
 INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report Introduction The ICH
INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE The ICHS1 Regulatory Testing Paradigm of Carcinogenicity in rats - Status Report Introduction The ICH
Duchenne Therapy Reviews on the Horizon. The FDA Advisory Committee Meetings. September 2, Host: Pat Furlong, PPMD President & Founder
 Duchenne Therapy Reviews on the Horizon The FDA Advisory Committee Meetings September 2, 2015 Host: Pat Furlong, PPMD President & Founder Guest presenters: FDA Office of Health & Constituent Affairs, and
Duchenne Therapy Reviews on the Horizon The FDA Advisory Committee Meetings September 2, 2015 Host: Pat Furlong, PPMD President & Founder Guest presenters: FDA Office of Health & Constituent Affairs, and
The EU PIP - a step in Pediatric Drug Development. Thomas Severin Bonn,
 The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
The EU PIP - a step in Pediatric Drug Development Thomas Severin Bonn, 13.01.2009 Agenda Implications for Industry Company Preparation Time of PIP Submission Content of the PIP The PIP Process and first
METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE
 METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE Mission The mission of the MISTEC Subcommittee is to develop methodologies for establishing appropriate dose-metrics to
METHODOLOGIES FOR INTERMITTENT AND SHORT-TERM EXPOSURE TO CARCINOGENS SUBCOMMITTEE Mission The mission of the MISTEC Subcommittee is to develop methodologies for establishing appropriate dose-metrics to
Dear Dr. Kloiber, Our comments on paragraphs 15, 22, and 34 of the April 2013 draft proposal follow. Sincerely,
 June 15, 2013 Dr. Otmar Kloiber Secretary General of the World Medical Association By email to: doh@wma.net 13, ch. du Levant CIB - Bâtiment A 01210 Ferney-Voltaire France Dear Dr. Kloiber, Please accept
June 15, 2013 Dr. Otmar Kloiber Secretary General of the World Medical Association By email to: doh@wma.net 13, ch. du Levant CIB - Bâtiment A 01210 Ferney-Voltaire France Dear Dr. Kloiber, Please accept
Process for appraising orphan and ultra-orphan medicines and medicines developed specifically for rare diseases Effective from September 2015
 Introduction Process for appraising orphan and ultra-orphan medicines and medicines developed specifically for rare diseases Effective from September 2015 From September 2015 the All Wales Medicines Strategy
Introduction Process for appraising orphan and ultra-orphan medicines and medicines developed specifically for rare diseases Effective from September 2015 From September 2015 the All Wales Medicines Strategy
Study protocol. Version 1 (06 April 2011) Ethics ref: R&D ref: UK CRC portfolio ID:
 Identifying and prioritising important research questions for the treatment of eczema a collaborative partnership between patients, carers, clinicians and researchers Study protocol Version 1 (06 April
Identifying and prioritising important research questions for the treatment of eczema a collaborative partnership between patients, carers, clinicians and researchers Study protocol Version 1 (06 April
Alexis Howerton On Starting Up From New Science
 Magazine Article August 2, 2018 Alexis Howerton On Starting Up From New Science Source: Life Science Leader By Wayne Koberstein, Executive Editor, Life Science Leader magazine Follow Me On Twitter @WayneKoberstein
Magazine Article August 2, 2018 Alexis Howerton On Starting Up From New Science Source: Life Science Leader By Wayne Koberstein, Executive Editor, Life Science Leader magazine Follow Me On Twitter @WayneKoberstein
IAPP KnowledgeNet Chapter Chair Manual 2018
 IAPP KnowledgeNet Chapter Chair Manual 2018 CHAIR ROLES & EXPECTATIONS IAPP RESPONSIBILITIES PLANNING A CHAPTER MEETING INFORMATION & TIPS FOR HOST VENUE INFORMATION & TIPS FOR SPEAKERS OTHER CHAPTER VOLUNTEERS
IAPP KnowledgeNet Chapter Chair Manual 2018 CHAIR ROLES & EXPECTATIONS IAPP RESPONSIBILITIES PLANNING A CHAPTER MEETING INFORMATION & TIPS FOR HOST VENUE INFORMATION & TIPS FOR SPEAKERS OTHER CHAPTER VOLUNTEERS
European Patients Academy on Therapeutic Innovation
 European Patients Academy on Therapeutic Innovation http://www.patientsacademy.eu info@patientsacademy.eu The project is receiving support from the Innovative Medicines Initiative Joint Undertaking under
European Patients Academy on Therapeutic Innovation http://www.patientsacademy.eu info@patientsacademy.eu The project is receiving support from the Innovative Medicines Initiative Joint Undertaking under
Panel on Dietetic products, Nutrition and Allergies 18 August 2008
 Panel on Dietetic products, Nutrition and Allergies 18 August 2008 MINUTES OF THE 21ST PLENARY MEETING OF THE SCIENTIFIC PANEL ON DIETETIC PRODUCTS, NUTRITION AND ALLERGIES HELD ON 9-11 JULY 2008 PARTICIPANTS
Panel on Dietetic products, Nutrition and Allergies 18 August 2008 MINUTES OF THE 21ST PLENARY MEETING OF THE SCIENTIFIC PANEL ON DIETETIC PRODUCTS, NUTRITION AND ALLERGIES HELD ON 9-11 JULY 2008 PARTICIPANTS
Introduction. About the Lupus Academy
 Content Introduction Past Activities Barcelona 2012 Buenos Aires 2013 Further Development of the Lupus Academy Berlin 2014 Philippines (APLAR) 2014 Educational support programme Budgets 2 Introduction
Content Introduction Past Activities Barcelona 2012 Buenos Aires 2013 Further Development of the Lupus Academy Berlin 2014 Philippines (APLAR) 2014 Educational support programme Budgets 2 Introduction
September 26-27, 2016 Austin, Texas DoubleTree by Hilton Austin
 Sponsor and Exhibitor Information September 26-27, 2016 Austin, Texas DoubleTree by Hilton Austin The 2016 Congenital CMV Public Health and Policy Conference partners invite you to become a supporter and/or
Sponsor and Exhibitor Information September 26-27, 2016 Austin, Texas DoubleTree by Hilton Austin The 2016 Congenital CMV Public Health and Policy Conference partners invite you to become a supporter and/or
Patient-Focused Drug Development Public Meeting on Chagas Disease
 This document is scheduled to be published in the Federal Register on 12/10/2014 and available online at http://federalregister.gov/a/2014-28828, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/10/2014 and available online at http://federalregister.gov/a/2014-28828, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
The President s Emergency Plan for AIDS Relief Next Generation Indicators
 The President s Emergency Plan for AIDS Relief Next Generation Indicators PEPFAR Next Generation Working in partnership with host nations, PEPFAR will support the following legislative goals: Treatment
The President s Emergency Plan for AIDS Relief Next Generation Indicators PEPFAR Next Generation Working in partnership with host nations, PEPFAR will support the following legislative goals: Treatment
Microbiota & Food 2018
 Microbiota & Food 2018 Towards a New Era in Agro-Food Industry Food and Gut Microbiota Interaction: Implication for Human Health June 14, 2018 - Paris, France The International Society of Microbiota (ISM)
Microbiota & Food 2018 Towards a New Era in Agro-Food Industry Food and Gut Microbiota Interaction: Implication for Human Health June 14, 2018 - Paris, France The International Society of Microbiota (ISM)
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives. Oncology Therapeutic Area Janssen Research & Development, LLC
 Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
STRATEGIC APPROACH & WORKPLAN 2007
 Rare Diseases Europe STRATEGIC APPROACH 2007-2009 & WORKPLAN 2007 Paris, March 2007 www.eurordis.org Contents Our strategic approach 2007 2009 o Eurordis in 2010 o Strategic orientations o Priorities 2007-2009
Rare Diseases Europe STRATEGIC APPROACH 2007-2009 & WORKPLAN 2007 Paris, March 2007 www.eurordis.org Contents Our strategic approach 2007 2009 o Eurordis in 2010 o Strategic orientations o Priorities 2007-2009
Annex H - Pandemic or Disease Outbreak
 or Disease Outbreak Version: 1.0 Effective: 10/01/2015 Revision Date: 10/01/2015 Approved By: John Pitcher Purpose A pandemic is a worldwide epidemic of an infectious disease. It occurs when a new organism
or Disease Outbreak Version: 1.0 Effective: 10/01/2015 Revision Date: 10/01/2015 Approved By: John Pitcher Purpose A pandemic is a worldwide epidemic of an infectious disease. It occurs when a new organism
confirmatory clinical trials - The PMDA Perspective -
 EMA workshop on the investigation of subgroups in confirmatory clinical trials The investigation of subgroups in confirmatory clinical trials - The PMDA Perspective - Yuki Ando Senior Scientist for Biostatistics
EMA workshop on the investigation of subgroups in confirmatory clinical trials The investigation of subgroups in confirmatory clinical trials - The PMDA Perspective - Yuki Ando Senior Scientist for Biostatistics
Table Of Content. European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs...
 Table Of Content European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs... 7 D04 - Electronic Newsletter (EN)... 7 D01 - Activity Report EURORDIS
Table Of Content European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs... 7 D04 - Electronic Newsletter (EN)... 7 D01 - Activity Report EURORDIS
Capricor Therapeutics
 Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
Hope Farm Medical Centre Patient Participation Group Meeting Date of Meeting: 17 th January 2017
 Meeting: Hope Farm Medical Centre Patient Participation Group Meeting Date of Meeting: 17 th January 2017 Delegates Present & apologies: Present: Ken Salter (chair) Carol Williams Sue MacDonald Keith Anderton
Meeting: Hope Farm Medical Centre Patient Participation Group Meeting Date of Meeting: 17 th January 2017 Delegates Present & apologies: Present: Ken Salter (chair) Carol Williams Sue MacDonald Keith Anderton
Annotations to the provisional agenda
 Technology Executive Committee 11 September 2018 Seventeenth meeting Bonn, Germany, 25 28 September 2018 Annotations to the provisional agenda 1. Opening of the meeting 1. The Chair of the Technology Executive
Technology Executive Committee 11 September 2018 Seventeenth meeting Bonn, Germany, 25 28 September 2018 Annotations to the provisional agenda 1. Opening of the meeting 1. The Chair of the Technology Executive
Patient-Centered Endpoints in Oncology
 Patient-Centered Endpoints in Oncology SIXTH ANNUAL PATIENT-REPORTED OUTCOME CONSORTIUM WORKSHOP April 29-30, 2015 Silver Spring, MD Disclaimer The views and opinions expressed in the following slides
Patient-Centered Endpoints in Oncology SIXTH ANNUAL PATIENT-REPORTED OUTCOME CONSORTIUM WORKSHOP April 29-30, 2015 Silver Spring, MD Disclaimer The views and opinions expressed in the following slides
Family Violence Integration Project. Eastern Community Legal Centre
 Family Violence Integration Project Eastern Community Legal Centre Mid Term Report February 2012 Prepared by Clare Keating, Effective Change Pty Ltd Introduction Commencing in February 2011, the Family
Family Violence Integration Project Eastern Community Legal Centre Mid Term Report February 2012 Prepared by Clare Keating, Effective Change Pty Ltd Introduction Commencing in February 2011, the Family
President Patricia Herbst Annual Report
 President Patricia Herbst Chaired Board meetings Created and mailed out Agendas and President Reports for all meetings Worked with fellow Board Members in developing Fiscal 2015-16 Plan of Work for the
President Patricia Herbst Chaired Board meetings Created and mailed out Agendas and President Reports for all meetings Worked with fellow Board Members in developing Fiscal 2015-16 Plan of Work for the
Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia
 Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia On 27 June Alzheimer Europe held a successful lunch debate in the European Parliament
Alzheimer Europe s European Parliament lunch debate focuses on current and future treatment of Alzheimer s dementia On 27 June Alzheimer Europe held a successful lunch debate in the European Parliament
Ms Nadia Isler, representing the PCB Chair, welcomed participants to the Bureau meeting before the 39th PCB meeting on 6-8 December 2016.
 UNAIDS PCB Bureau MEETING SUMMARY DATE: Monday, 31 October 2016 PARTICIPANTS Ms Nadia Isler (Switzerland: chairing); Ms Laila Heward-Mills (Ghana); Ms Monica Martinez (Ecuador), Ms Laurel Sprague and Mr
UNAIDS PCB Bureau MEETING SUMMARY DATE: Monday, 31 October 2016 PARTICIPANTS Ms Nadia Isler (Switzerland: chairing); Ms Laila Heward-Mills (Ghana); Ms Monica Martinez (Ecuador), Ms Laurel Sprague and Mr
Enhancement of the communication and outreach strategy of the
 Technology Executive Committee 27 February 2018 Sixteenth meeting Bonn, Germany, 13 16 March 2018 Enhancement of the communication and outreach strategy of the Technology Executive Committee Background
Technology Executive Committee 27 February 2018 Sixteenth meeting Bonn, Germany, 13 16 March 2018 Enhancement of the communication and outreach strategy of the Technology Executive Committee Background
Brief history of the development of the Framework on Sharing influenza viruses and access to vaccines and other benefits
 Brief history of the development of the Framework on Sharing influenza viruses and access to vaccines and other benefits The Intergovernmental (IGM) Process Since the late 1940's, WHO has coordinated a
Brief history of the development of the Framework on Sharing influenza viruses and access to vaccines and other benefits The Intergovernmental (IGM) Process Since the late 1940's, WHO has coordinated a
5 th PVRI Annual Drug Discovery & Development Symposium for Pulmonary Hypertension. The pathway to breakthrough therapies WELCOME TO BETHESDA
 Insights from leaders in academia, industry & regulatory... Promising targets ready for clinical development... PVRI Innovative drug discovery for faster approval... Pulmonary vascular diseases... Right
Insights from leaders in academia, industry & regulatory... Promising targets ready for clinical development... PVRI Innovative drug discovery for faster approval... Pulmonary vascular diseases... Right
Speaker Success Plan. Your message has the ability to transform lives.
 Speaker Success Plan Your message has the ability to transform lives. Imagine reaching millions of people who are eager to hear your message. By completing this plan and putting it into action, you ll
Speaker Success Plan Your message has the ability to transform lives. Imagine reaching millions of people who are eager to hear your message. By completing this plan and putting it into action, you ll
Policy: Client Involvement and Empowerment
 Policy: Client Involvement and Empowerment Updated January 2017 Contents: 1. Introduction 2. How do we involve and empower those to whom we provide housing and/or support? 3. How do we involve and empower
Policy: Client Involvement and Empowerment Updated January 2017 Contents: 1. Introduction 2. How do we involve and empower those to whom we provide housing and/or support? 3. How do we involve and empower
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH 2017 REQUEST FOR APPLICATION (RFA)
 LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
Motivational Interviewing Enhancing Motivation to Change Strategies
 Motivational Interviewing Enhancing Motivation to Change Strategies Learning Objectives At the end of the session, you will be able to 1. Describe the stages of change. 2. Demonstrate at least two methods
Motivational Interviewing Enhancing Motivation to Change Strategies Learning Objectives At the end of the session, you will be able to 1. Describe the stages of change. 2. Demonstrate at least two methods
REVISED DRAFT STRATEGY ON UNESCO s CONTRIBUTION TO THE PROMOTION OF OPEN ACCESS TO SCIENTIFIC INFORMATION AND RESEARCH OUTLINE
 36 C 36 C/62 20 October 2011 Original: English Item 5.26 of the provisional agenda REVISED DRAFT STRATEGY ON UNESCO s CONTRIBUTION TO THE PROMOTION OF OPEN ACCESS TO SCIENTIFIC INFORMATION AND RESEARCH
36 C 36 C/62 20 October 2011 Original: English Item 5.26 of the provisional agenda REVISED DRAFT STRATEGY ON UNESCO s CONTRIBUTION TO THE PROMOTION OF OPEN ACCESS TO SCIENTIFIC INFORMATION AND RESEARCH
NORDIC CONFERENCE ON RARE DISEASES
 www.eurordis.org NORDIC CONFERENCE ON RARE DISEASES 31 May 2012, Reykjavik, Iceland www.eurordis.org RARE DISEASES IN EUROPE Terkel ANDERSEN President of EURORDIS European Organisation for Rare Diseases
www.eurordis.org NORDIC CONFERENCE ON RARE DISEASES 31 May 2012, Reykjavik, Iceland www.eurordis.org RARE DISEASES IN EUROPE Terkel ANDERSEN President of EURORDIS European Organisation for Rare Diseases
SPONSORSHIP & EXHIBITION OPPORTUNITIES
 2019 American Association of Veterinary Immunologists INTERNATIONAL VETERINARY IMMUNOLOGY SYMPOSIUM 13-16 AUGUST SEATTLE, USA SPONSORSHIP & EXHIBITION OPPORTUNITIES International Veterinary Immunology
2019 American Association of Veterinary Immunologists INTERNATIONAL VETERINARY IMMUNOLOGY SYMPOSIUM 13-16 AUGUST SEATTLE, USA SPONSORSHIP & EXHIBITION OPPORTUNITIES International Veterinary Immunology
EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH)
 12 June 2017 EMA/848554/2016 Rev.1 Human Medicines Research and Development Support Division EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH) 12-13 June 2017, meeting
12 June 2017 EMA/848554/2016 Rev.1 Human Medicines Research and Development Support Division EMA/FDA/Health Canada workshop on paediatric pulmonary arterial hypertension (PAH) 12-13 June 2017, meeting
COMMITTEE ON WORLD FOOD SECURITY
 July 2013 CFS 2013/40/4 E COMMITTEE ON WORLD FOOD SECURITY Fortieth Session Rome, Italy, 7-11 October 2013 COMMUNICATION STRATEGY FOR THE COMMITTEE ON WORLD FOOD SECURITY Table of Contents Pages I. INTRODUCTION...
July 2013 CFS 2013/40/4 E COMMITTEE ON WORLD FOOD SECURITY Fortieth Session Rome, Italy, 7-11 October 2013 COMMUNICATION STRATEGY FOR THE COMMITTEE ON WORLD FOOD SECURITY Table of Contents Pages I. INTRODUCTION...
JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON CONTAMINANTS IN FOODS 12 th Session Utrecht, The Netherlands, March 2018 PROPOSED
 E Agenda Item 9 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON CONTAMINANTS IN FOODS 12 th Session Utrecht, The Netherlands, 12-16 March 2018 CX/CF 18/12/9-Add.1 PROPOSED DRAFT CODE OF PRACTICE
E Agenda Item 9 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON CONTAMINANTS IN FOODS 12 th Session Utrecht, The Netherlands, 12-16 March 2018 CX/CF 18/12/9-Add.1 PROPOSED DRAFT CODE OF PRACTICE
Personnel. Women in the Secretariat. Report by the Director General
 Board of Governors General Conference GOV/2017/39-GC(61)/19 Date: 2 August 2017 General Distribution Original: English For official use only Item 8(b)(ii) of the Board s provisional agenda (GOV/2017/33)
Board of Governors General Conference GOV/2017/39-GC(61)/19 Date: 2 August 2017 General Distribution Original: English For official use only Item 8(b)(ii) of the Board s provisional agenda (GOV/2017/33)
Successful collaboration for research development between the NIH and patient organisations
 Successful collaboration for research development between the NIH and patient organisations Stephen C. Groft, Pharm.D. Office of Rare Diseases s of Health Department of Health and Human Services EURORDIS
Successful collaboration for research development between the NIH and patient organisations Stephen C. Groft, Pharm.D. Office of Rare Diseases s of Health Department of Health and Human Services EURORDIS
Minutes of Healthwatch Sefton Steering Group meeting.
 Page1 Minutes of Healthwatch Sefton Steering Group meeting. Held on 11 th December 2013. Board Room. 3 rd Floor, Suite 3B, North Wing, Burlington House Crosby Road North, Waterloo. L22 0LG 1. Welcome,
Page1 Minutes of Healthwatch Sefton Steering Group meeting. Held on 11 th December 2013. Board Room. 3 rd Floor, Suite 3B, North Wing, Burlington House Crosby Road North, Waterloo. L22 0LG 1. Welcome,
2010 Annual Meetings Assessment
 2010 Annual Meetings World Bank Group International Monetary Fund CIVIL SOCIETY POLICY FORUM OCTOBER 6 10, 2010 2010 Annual Meetings Assessment Feedback from CSO Representatives The World Bank and IMF
2010 Annual Meetings World Bank Group International Monetary Fund CIVIL SOCIETY POLICY FORUM OCTOBER 6 10, 2010 2010 Annual Meetings Assessment Feedback from CSO Representatives The World Bank and IMF
The Inclusion of Seasonal Influenza Viruses and Genetic Sequence Data (GSD) in the Context of the Pandemic Influenza Preparedness (PIP) Framework
 The Inclusion of Seasonal Influenza Viruses and Genetic Sequence Data (GSD) in the Context of the Pandemic Influenza Preparedness (PIP) Framework A position paper from the Directors of the GISRS WHO Collaborating
The Inclusion of Seasonal Influenza Viruses and Genetic Sequence Data (GSD) in the Context of the Pandemic Influenza Preparedness (PIP) Framework A position paper from the Directors of the GISRS WHO Collaborating
Article 1: MEDSTARTUP
 Article 1: MEDSTARTUP MedStartUp is a GALIEN FOUNDATION BUSINESS FRANCE program initiative which aim to allow, encourage and reward new international partnerships between innovative French and North American
Article 1: MEDSTARTUP MedStartUp is a GALIEN FOUNDATION BUSINESS FRANCE program initiative which aim to allow, encourage and reward new international partnerships between innovative French and North American
Re: Docket No. FDA D Presenting Risk Information in Prescription Drug and Medical Device Promotion
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
Clinical Chemistry and Clinical Toxicology Devices Panel of the Medical Devices Advisory
 This document is scheduled to be published in the Federal Register on 03/01/2018 and available online at https://federalregister.gov/d/2018-04167, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/01/2018 and available online at https://federalregister.gov/d/2018-04167, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Development of Paediatric Medicines: From Learning to Adapting
 Preliminary Programme Joint EFGCP / DIA / EMA Medicines for Children Conference on Development of Paediatric Medicines: From Learning to Adapting 26 & 27 September 2012 De Vere Venues Canary Wharf, London,
Preliminary Programme Joint EFGCP / DIA / EMA Medicines for Children Conference on Development of Paediatric Medicines: From Learning to Adapting 26 & 27 September 2012 De Vere Venues Canary Wharf, London,
MS Priority Setting Partnership. PROTOCOL August 2012
 MS Priority Setting Partnership PROTOCOL August 2012 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the MS Priority Setting Partnership (PSP) and the basic roles
MS Priority Setting Partnership PROTOCOL August 2012 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the MS Priority Setting Partnership (PSP) and the basic roles
Peer counselling A new element in the ET2020 toolbox
 shutterstock Peer counselling A new element in the ET2020 toolbox Information Note. Main characteristics of the peer counselling tool Peer learning in the context of the education cooperation at EU level
shutterstock Peer counselling A new element in the ET2020 toolbox Information Note. Main characteristics of the peer counselling tool Peer learning in the context of the education cooperation at EU level
Victims and Survivors Forum Consultation
 Victims and Survivors Forum Consultation Introduction The Victims and Survivors Forum was set up to be a self nominating network for victims and survivors to discuss the work of the Inquiry, and contribute
Victims and Survivors Forum Consultation Introduction The Victims and Survivors Forum was set up to be a self nominating network for victims and survivors to discuss the work of the Inquiry, and contribute
Spring 2016 Member Meeting Invitation to Register & Participate
 February 23, 2016 Memo To: Collaborative Members and Associates From: Lauren Katzman, Executive Director Subject: Spring 2016 Member Meeting Invitation to Register & Participate Registration is now open
February 23, 2016 Memo To: Collaborative Members and Associates From: Lauren Katzman, Executive Director Subject: Spring 2016 Member Meeting Invitation to Register & Participate Registration is now open
Welcome/Introductions: Jennifer Longdon, Committee Chair, welcomed all to this informational meeting, and introductions were completed.
 AZSILC SPIL Development Committee Meeting Notes of August 27, 2015 Participants: Jennifer Longdon, J. J. Rico, Paula Seanez, Nikki Jeffords, Cody Hallenbeck, Georgia McLaughlin, Larry Clausen, Laura Duval,
AZSILC SPIL Development Committee Meeting Notes of August 27, 2015 Participants: Jennifer Longdon, J. J. Rico, Paula Seanez, Nikki Jeffords, Cody Hallenbeck, Georgia McLaughlin, Larry Clausen, Laura Duval,
UPDATED VISION TO ENHANCE CIVIL SOCIETY ENGAGEMENT WITH THE GEF. (Prepared by the Ad-hoc Council Working Group on Civil Society)
 53 rd GEF Council Meeting November 28 30, 2017 Washington, D.C. GEF/C.53/10/Rev.01 November 30, 2017 Agenda Item 08 UPDATED VISION TO ENHANCE CIVIL SOCIETY ENGAGEMENT WITH THE GEF (Prepared by the Ad-hoc
53 rd GEF Council Meeting November 28 30, 2017 Washington, D.C. GEF/C.53/10/Rev.01 November 30, 2017 Agenda Item 08 UPDATED VISION TO ENHANCE CIVIL SOCIETY ENGAGEMENT WITH THE GEF (Prepared by the Ad-hoc
SCHOLARLY ACTIVITY STEERING COMMITTEE MEETING (SASC) Friday March 28, 2014 at 9:00 am Meeting Room B144 M I N U T E S
 SCHOLARLY ACTIVITY STEERING COMMITTEE MEETING (SASC) Friday March 28, 2014 at 9:00 am Meeting Room B144 M I N U T E S Present: Regrets: Leelah Dawson, Janet Douglas, John Falcus, Margaret Heldman (Chair),
SCHOLARLY ACTIVITY STEERING COMMITTEE MEETING (SASC) Friday March 28, 2014 at 9:00 am Meeting Room B144 M I N U T E S Present: Regrets: Leelah Dawson, Janet Douglas, John Falcus, Margaret Heldman (Chair),
A Strategy for Evaluating ICD-10 Implementation and Updating Process: 2005 Status Report
 WHO-FIC NETWORK MEETING Recommendations Marjorie S. Greenberg, Head, North American Collaborating Center National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville,
WHO-FIC NETWORK MEETING Recommendations Marjorie S. Greenberg, Head, North American Collaborating Center National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville,
 Preliminary Programme Version 9 September 2010 EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions,
Preliminary Programme Version 9 September 2010 EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions,
 Final Programme EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions, Daily Challenges, Ways Forward
Final Programme EFGCP Children s Medicines Working Party 6th Annual Conference & DIA Paediatric SIAC 4th Forum Current & Future Perspectives for Paediatric Medicines: Visions, Daily Challenges, Ways Forward
Data Collection on Adverse Events of Anti-HIV Drugs. The D:A:D Study. Signe W. Worm MD, PhD, D:A:D Study Coordinator >
 Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
Data Collection on Adverse Events of Anti-HIV Drugs The D:A:D Study Signe W. Worm MD, PhD, D:A:D Study Coordinator 2005 -> Background Combination ART (cart) widely introduced in the Europe in 1996 cart
European Food Safety Authority (EFSA)
 TECHNICAL REPORT APPROVED: 06 April 2017 doi:10.2903/sp.efsa.2017.en-1210 Outcome of the preliminary pesticides peer review meeting on the assessment of endocrine disrupting properties in mammalian toxicology
TECHNICAL REPORT APPROVED: 06 April 2017 doi:10.2903/sp.efsa.2017.en-1210 Outcome of the preliminary pesticides peer review meeting on the assessment of endocrine disrupting properties in mammalian toxicology
in the ICH Regions Table of Content Annexes to Guideline and 3. Why is Q4B necessary? Q4B Annexes? for Human Use
 Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
Reflections on Brighton & Hove s SEND Inspection May 2016
 Reflections on Brighton & Hove s SEND Inspection May 2016 If it s May it must be Ofsted time. Why us? Along with Bolton, we were the first local area to be inspected using the new framework We were an
Reflections on Brighton & Hove s SEND Inspection May 2016 If it s May it must be Ofsted time. Why us? Along with Bolton, we were the first local area to be inspected using the new framework We were an
2. The scope of the work of the Stocktaking Working Group according to the Bergen Communiqué
 BOLOGNA STOCKTAKING WORKING GROUP NOTES OF MEETING OF 9 DECEMBER 2005, RIGA, LATVIA Present Prof Andrejs Rauhvargers, Latvia (Chair) Ms Marie-Anne Persoons, Belgium (Flemish Community) Dr Heli Aru, Estonia
BOLOGNA STOCKTAKING WORKING GROUP NOTES OF MEETING OF 9 DECEMBER 2005, RIGA, LATVIA Present Prof Andrejs Rauhvargers, Latvia (Chair) Ms Marie-Anne Persoons, Belgium (Flemish Community) Dr Heli Aru, Estonia
Wellington is the capital of New Zealand and its activity is centred on government, as well as the creative industries.
 IABC Wellington: Membership Marketing work plan 2015 Context Wellington is the capital of New Zealand and its activity is centred on government, as well as the creative industries. IABC Wellington is a
IABC Wellington: Membership Marketing work plan 2015 Context Wellington is the capital of New Zealand and its activity is centred on government, as well as the creative industries. IABC Wellington is a
NIH StrokeNet Network Standard Operating Procedure
 1. PURPOSE The purpose of this SOP is to outline procedures for Institutional Review Board (IRB) approval and oversight of National Institutes of Health (NIH) StrokeNet affiliated protocols conducted by
1. PURPOSE The purpose of this SOP is to outline procedures for Institutional Review Board (IRB) approval and oversight of National Institutes of Health (NIH) StrokeNet affiliated protocols conducted by
EUROPLAN National Conferences and Post-NCs activities in support to Member States
 EUROPLAN National Conferences and Post-NCs activities in support to Member States Yann Le Cam, Ariane Weinman, Valentina Bottarelli EURORDIS Team Paris, France Brussels, Belgium EUROPLAN B Domenica Taruscio,
EUROPLAN National Conferences and Post-NCs activities in support to Member States Yann Le Cam, Ariane Weinman, Valentina Bottarelli EURORDIS Team Paris, France Brussels, Belgium EUROPLAN B Domenica Taruscio,
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Your Opportunity Begins Here.
 Your Opportunity Begins Here. ASN Scientific Councils and Research Interest Sections Share Your Scientific Expertise with Like Minds Through ASN ASN s Scientific Councils and Research Interest Sections
Your Opportunity Begins Here. ASN Scientific Councils and Research Interest Sections Share Your Scientific Expertise with Like Minds Through ASN ASN s Scientific Councils and Research Interest Sections
