The mossy fiber bouton: the common or the unique synapse?
|
|
|
- Doreen Hampton
- 5 years ago
- Views:
Transcription
1 SYNAPTIC NEUROSCIENCE REVIEW ARTICLE published: 15 March 2010 doi: /fnsyn The mossy fiber bouton: the common or the unique synapse? Astrid Rollenhagen 1 and Joachim H. R. Lübke 1,2 * 1 Institute of Neuroscience and Medicine INM-2, Research Centre Jülich, Jülich, Germany 2 Department of Psychiatry and Psychotherapy, Medical Faculty, RWTH Aachen University, Jülich-Aachen Research Alliance Translational Brain Medicine, Aachen, Germany Edited by: Laszlo Acsady, Institute of Experimental Medicine, Hungary Reviewed by: Janos Szabadics, University of Szeged, Hungary David Amaral, University of California at Davis, USA Gianmaria Maccaferri, Northwestern University, USA *Correspondence: Joachim H. R. Lübke, Institute of Neuroscience and Medicine INM-2, Research Centre Jülich, D Jülich, Germany. j.luebke@fz-juelich.de Synapses are the key elements for signal processing and plasticity in the brain. They are composed of nearly the same structural subelements, an apposition zone including a pre- and postsynaptic density, a cleft and a pool of vesicles. It is, however, their actual composition that determines their different behavior in synaptic transmission and plasticity. Here, we describe and discuss the structural factors underlying the unique functional properties of the hippocampal mossy fiber (MF) synapse. Two membrane specializations, active zones (AZs; transmitter release sites), and puncta adherentia (PA), putative adhesion comples were found. On average, individual boutons had 20 AZs with a mean surface area of 0.1 µm 2 and a short distance of 0.45 µm between individual AZs. Mossy fiber boutons (MFBs) and their target structures were isolated from each other by astrocytes, but fine glial processes never reached the AZs. Therefore, two structural factors are likely to promote synaptic cross-talk: the short distance and the absence of fine glial processes between individual AZs. Thus, synaptic crosstalk may contribute to the high efficacy of hippocampal MF synapses. On average, an adult bouton contained 16,000 synaptic vesicles; 600 vesicles were located within 60 nm from the AZ, 4000 between 60 nm and 200 nm, and the remaining beyond 200 nm, suggesting large readily releasable, recycling, and reserve pools. Thus, the size of the three pools together with the number and distribution of AZs underlie the unique tent of synaptic efficacy and plasticity of the hippocampal MF synapse. Keywords: mossy fiber-ca3 pyramidal cell synapse, electron microscopy, 3D-reconstructions, quantitative analysis, actives zones, different pools of synaptic vesicles, synaptic transmission and plasticity, synaptic crosstalk INTRODUCTION Over the last 50 years synapses have been looked at from different viewpoints resulting in numerous original publications and summarized in several reviews and ttbooks. Nevertheless, a detailed description of their structural geometry, in particular their quantitative morphology, is still limited to a relatively small number of synapses. Such detailed descriptions are, however, required to understand and link structural and functional components of the signal cascades underlying synaptic transmission and plasticity. Using high end electron microscopy and related techniques that allow subcellular and even molecular resolution it became possible to unravel and quantify the structural subelements of synapses providing important constrains that can be used for realistic numerical simulations of those parameters of synaptic transmission that are still inaccessible to periment. Only recently the structural geometry of a synapse embedded in a higher cortical microcircuit, the hippocampal mossy fiber bouton (MFB)-CA3 pyramidal cell synapse, has been described in great detail (Rollenhagen et al., 2007a). In particular, those structural subelements relevant for synaptic transmission and plasticity at the MFB-CA3 pyramidal cell synapse have been investigated using computer-assisted 3D-reconstructions of serial electron microscopic images and subsequent quantitative analysis based on serial ultrathin sections through individual MFBs. It is widely accepted that the hippocampus is involved in learning and memory function, in particular that mossy fiber (MF) synapses play a key role in processing, storage and recall of spatial information in the hippocampal network (Lisman, 1999; for review see Nicoll and Schmitz, 2005; Bischofberger et al., 2006). Furthermore, the MF system may play a critical role in pattern completion, pattern separation and storage of sequences of events (for review see Bischofberger et al., 2006). Within the hippocampal formation the MF system is a structurally highly compl connecting the entorhinal cort via the MF axons of the dentate gyrus granule cells with CA3 pyramidal cells and GABAergic interneurons in the hilar region and the stratum lucidum of the CA3 subregion (for review see Frotscher et al., 2006). It is known that MF axons hibit three morphologically distinct presynaptic specializations: large giant boutons that are thought to represent the main bodies of MFs (Galimberti et al., 2006), small en passant boutons, and filopodial tensions emerging from the MFBs (Amaral and Dent, 1981). Large MFBs innervate spiny crescences of CA3 pyramidal neurons (Blackstad and Kjaerheim, 1961; Hamlyn, 1962; Rollenhagen et al., 2007a) and hilar mossy cells (Frotscher et al., 1994). In contrast, the filopodial tensions are thought to contact clusively the dendritic domain of different GABAergic interneurons in the hilus and stratum lucidum (Acsády et al., 1998; Szabadics and Soltesz, 2009), although large MFBs or Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 1
2 Rollenhagen and Lübke Thus, the high synaptic efficacy and plasticity at the MFB-CA3 pyramidal neuron synapses may be partially a result of the organization of the pool of synaptic vesicles in the large MFBs. Here, we summarize and discuss these findings that underlie the unique functional properties of the hippocampal MF-CA3 pyramidal cell synapse. We demonstrate that several structural features of hippocampal MFBs differ fundamentally from the calyx of Heldprincipal neuron synapse in the medial nucleus of the trapezoid body (MNTB, Rowland et al., 2000; Sätzler et al., 2002; Wimmer et al., 2006) and other previously investigated central presynaptic terminals (climbing and parallel fiber synapse: Xu-Friedman et al., 2001; endbulb of Held: Nicol and Walmsley, 2002; cerebellar mossy fiber synapse: Xu-Friedman and Regehr, 2003; retinal ribbon synapse: Sikora et al., 2005; tom Dieck and Brandstätter, 2006; Midorikawa et al., 2007; GABAergic nigrothalamic terminals: Bodor et al., 2008; input synapses from the nucleus reticularis and anterior pretectal nucleus to the posterior thalamic nucleus: Wanaverbecq et al., 2008; for review see Rollenhagen and Lübke, 2006). Thus, the MFB, with respect to its structural features, can be regarded as a highly specialized, and therefore unique synapse perfectly suited for its function within the hippocampal network. main bodies also directly contact the dendritic shafts of GABAergic interneurons (for review see Frotscher et al., 2006). A single granule cell axon gives rise to 15 large MFBs in the CA3 region, and a single CA3 pyramidal neuron receives input from 50 MF axons (Amaral et al., 1990). Thus, the morphological properties of the MF synapse are consistent with the view that it forms a powerful, but sparse synaptic connection. Hippocampal MF synapses show several unique transmission and plasticity properties when compared with other CNS synapses. MF synapses are powerful, generating large postsynaptic currents and potentials in both CA3 pyramidal neurons and GABAergic interneurons (Maccaferri et al., 1998; Henze et al., 2002a; Lawrence et al., 2004). Synaptic plasticity at the MF-CA3 pyramidal cell synapses is unique for several reasons, including a low basal release probability due to the tonic activation of A1 adenosine receptors (Moore et al., 2003). Furthermore, MF synapses can undergo substantial changes in synaptic strength and efficacy, hibiting marked paired pulse- and frequency facilitation, post-tetanic- and NMDA-receptor independent long-term potentiation (LTP; Salin et al., 1996; Kobayashi and Poo, 2004; Engel and Jonas, 2005; Alle and Geiger, 2006; Alle et al., 2009; for review see Nicoll and Schmitz, 2005). Finally, the properties of MF transmission are highly compartmentalized. At MFB-GABAergic interneuron synapses, the tent of synaptic plasticity is markedly smaller when compared with the MFB-CA3 pyramidal neuron synapses (Tóth et al., 2000; Szabadics and Soltesz, 2009). MF inputs to GABAergic interneurons in the stratum lucidum via the filopodial tensions undergo severe long-term depression whereas MF-CA3 pyramidal cell synapses show long-term potentiation triggered by compartimentelized Ca2+ channels. This difference in synaptic plasticity indicate that neighboring, functionally divergent presynaptic elements along the same axon serve as autonomous computational units capable of modifying release independently (Pelkey and McBain, 2005; Pelkey et al., 2006). A MFB GENERAL DESCRIPTION OF THE GEOMETRY OF THE MFB The axons of granule cells in the dentate gyrus constitute a massive fiber bundle, the MFs that establish large, highly specialized en passant boutons or end terminals (Figure 1A) with proximal dendritic segments predominantly on spiny (thorny) crescences (Figures 1A and 2A) of citatory CA3 pyramidal cells and various GABAergic interneurons in the hilus and stratum lucidum (Acsády et al., 1998; Szabadics and Soltesz, 2009). Dendrites of CA3 pyramidal neurons receive dense synaptic input from multiple MFBs (Rollenhagen et al., 2007a); in turn, individual MFBs contact several dendritic segments of the same (Chicurel and Harris, 1992) or different pyramidal neurons (Galimberti et al., B MFB1 mi mi MFB mi mi MFB2 FIGURE 1 The hippocampal mossy fiber bouton. (A) Low power electron microscopic image of two adjacent MFBs (MFB1, MFB2) taken from an adult rat. Both MFBs outlined in yellow terminate on different dendritic segments, but preferentially on spiny crescences (blue contours). AZs are given in red and astrocytic profiles (dark diaminobenzidine reaction product) in green. Scale bar: 2.5 µm. (B) Glial coverage of an individual MFB showing the distribution of Frontiers in Synaptic Neuroscience astrocytic processes (highlighted in green) as revealed by glutamine synthetase pre-embedding immunohistochemistry. The surface of an individual MFB (in light yellow), its postsynaptic spiny crescences (; in light blue) was surrounded by several astrocytic processes (in green). Note, that the fine glial processes do not reach the AZs (in red) and the synaptic cleft. The mitochondria (mi) within the MFB are highlighted in white. Scale bar: 1 µm. March 2010 Volume 2 Article 2 2
3 2006). The density of MFBs terminating on dendritic segments of CA3 pyramidal cells in the stratum lucidum was found to be remarkably high. Mossy fiber boutons were highly variable in shape and size ranging from 2 to 5 µm in cross diameter, from 40 to 110 µm 2 in surface area and from 2 to 13 µm 3 in volume (Table 1). In addition, small caliber boutons ranging from 8.2 and 13.9 µm 2 in surface area and with a volume of less than 1 µm 3 were observed. However, these small boutons may either resemble those described as satellites (Galimberti et al., 2006) or could emerge from filopodia (Acsády et al., 1998). On average, two to four filopodial tensions of various shape and length (1 5 µm) emerged from the main body of the bouton, sometimes terminating abruptly or in an endbulb-like structure. These endbulbs often contained a single active zone (AZ). The main bodies or larger MFBs contained numerous mitochondria of various shape and size (33 57) that either formed cluster-like arrangements or constituted row-like assemblies (Figure 2C). Mitochondria contributed between 4% and 12% to the total volume of individual boutons and act not only as internal calcium stores (Rizzuto et al., 2000), but may also regulate internal Ca 2+ levels in the terminal. Furthermore, mitochondria may be involved in the mobilization of synaptic vesicles from the reserve pool (Verstreken et al., 2005). In MFBs, mitochondria were seen to be associated with the pool of synaptic vesicles (Figure 2C) suggesting a role for these structures in synaptic vesicle trafficking. However, they were not organized in mitochondria-associated comples (Rowland et al., 2000) or donut-like assemblies (Wimmer et al., 2006) as described for the calyx of Held nerve terminal. Interestingly, most of the MFBs investigated were penetrated and crossed by other axons of Table 1 Quantitative analysis of structural elements of adult MFBs. Bouton Active zones Cleft width Volume Surface No. Mean area Total area (%) Distance to AZ (nm ± SD) PA (nm ± SD) (µm 3 ) (µm 2 ) (µm 2 ) ± SD (µm 2 ) neighboring AZ (µm ± SD) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 0.44 Mean ± ± ± ± 1.21 CV Summary of structural parameters relevant for synaptic transmission that have been tracted from the detailed 3-D reconstructions of MFBs. The means and coefficient of variations (CVs) given in this table are averages per bouton (modified from Table 1 in Rollenhagen et al., 2007a). A B C FIGURE 2 Three-dimensional reconstructions of an adult MFB and its postsynaptic target dendrite. (A) Volume reconstructions of an en passant MFB (depicted in yellow) and its postsynaptic target dendrite (blue). Note, that the spiny crescences were almost entirely covered by the nerve terminal. (B) Distribution of the two membrane specializations, AZs (in red) and PAs (in orange) on the postsynaptic target dendrite (blue). Note, that AZs were mainly located on the spiny crescences, whereas PAs were clusively found at the dendritic shaft. (C) Organization of the pool of synaptic vesicles (green dots) at an individual MFB. Here, the pool was distributed throughout the entire nerve terminal. Mitochondria (in white) formed either cluster-like arrangements or bands associated with the pool of synaptic vesicles (green dots). Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 3
4 different caliber and of undefined origin (Rollenhagen et al., 2007a), a phenomenon that has not been reported yet for the MFB and other large central synapses. However, these axons never established either axo-axonic nor gap-junctional contacts with the MFBs. When compared with other central synapses MFBs have approximately the same size as cerebellar mossy terminals ( µm 2 ; Xu-Friedman and Regehr, 2003; µm 2 ; Hamori and Somogyi, 1983), but are significantly smaller than the giant calyx of Held endterminal (2500 µm 2 ; Sätzler et al., 2002) and significantly larger than thalamic (Bodor et al., 2008; Wanaverbecq et al., 2008) and neocortical synapses (7 17 µm 2 ; for review see Rollenhagen and Lübke, 2006). The size and shape of a synapse may influence in part the properties in synaptic transmission and plasticity and is also a very useful parameter for realistic numerical simulations of synaptic behavior. However, whether a direct relationship between the geometry of the terminal and the number, size and distribution of AZs and the pool of synaptic vesicles ists, remains to be investigated (see below). MEMBRANE SPECIALIZATIONS AT THE PRE- AND POSTSYNAPTIC APPOSITION ZONE As described for the calyx of Held-principal neuron synapses in the MNTB (Sätzler et al., 2002) and nigrothalamic terminals (Bodor et al., 2008), but not for other central synapses, two membrane specializations were found at the pre- and postsynaptic apposition zone: AZs, the sites of transmitter release and puncta adherentia (PA) that are thought to function as adhesion comples. ACTIVE ZONES The number, size, and distribution of AZs within a given synapse are one of the main structural determinants in synaptic transmission and long- and short-term plasticity. By definition, an AZ is characterized by the dense accumulation of synaptic vesicles located in close proximity to the presynaptic density, the symmetric and asymmetric pre- and postsynaptic densities and the characteristic widening of the synaptic cleft. At MFBs the pre- and postsynaptic densities formed bands of electron-dense fuzzy material. At individual MFBs two types of AZs were found: the majority ( 80%) had pre- and postsynaptic densities that were frequently interrupted, appearing as perforated structures, whereas the remaining AZs showed perforations in either the pre- or postsynaptic densities or were non-perforated (Rollenhagen et al., 2007a). The average cleft width, measured in the center under the pre- and postsynaptic densities was ± 1.87 nm. Interestingly, the cleft width was ± 2.93 when measured at the two edges (at the appearance and disappearance of the pre- and postsynaptic densities). This difference in the means between center and the edges of AZs was significantly different (P 0.001) which could be of importance for realistic simulations and models of transmitter diffusion via the synaptic cleft. The distribution of AZs and PAs is illustrated in Figure 2B. Here, AZs are depicted in red and PAs in orange. Active zones were mainly located on the spiny crescences, whereas PAs were clusively found on dendritic shafts. Two types of spiny crescences could be distinguished on the postsynaptic target dendrites: So-called simple spines were contacted only by a single, but often very large AZ (Figure 3A1) or at most two AZs. In contrast, compl spines were contacted by up to eight AZs that were predominantly located opposite to spine heads (Figure 3A2). The AZs varied substantially in shape and size, forming patches, bands or ring-like structures and covered on average 10.6% of the total surface area of the spiny crescences and therefore occupy 50% more of the total surface area than their counterparts at the calyx of Held endterminal. On average, 20 AZs (mean 18.30, range 7 34) with a mean surface area of 0.11 ± 0.07 µm 2 were found in adult rats (see also Table 1). They varied substantially in shape and size as indicated by the large CV (0.64). Beside very large (0.17 µm 2 ) also very small (0.07 µm 2 ) AZs were observed (Figure 3B) that may in part contribute and plain the huge differences in synaptic efficacy and plasticity. It is noteworthy to mention that there is a dynamic change in both the number and size of AZs from development through adulthood (Rollenhagen et al., 2007a). A1 B 14 C A2 Az s of no. 8 6 of AZs no surface area (µm 2 ) distance (µm) FIGURE 3 Number, shape and location of AZs. Two representative amples of AZs (red areas) found on simple (A1) and compl (A2) spiny crescences (blue) taken from an adult rat. Simple spines had only a single or at most two AZs whereas compl spines had up to eight AZs. Note, also the different shape and size of individual AZs that formed ring- or patch-like arrangements. Scale bar: 1 µm. (B) Histogram showing the distribution of surface areas of AZs for adult MFBs. (C) Histogram of the distribution of nearest-neighbor distances between centers of gravity of all AZs for adult MFBs (modified from Rollenhagen et al., 2007a). Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 4
5 The size (surface area) of the AZ in MFBs was comparable with those in the calyx of Held in young rats (0.10 µm 2 ; Sätzler et al., 2002), adult deaf cats (0.14 µm 2 ; Ryugo et al., 1997), to that in climbing fiber synapses (0.14 µm 2 ; Xu-Friedman et al., 2001) and parallel fiber synapses of rat cerebellum (0.13 µm 2 ; Xu-Friedman et al., 2001). However, AZs in MFBs were larger than those in the endbulb of Held in rats (0.06 µm 2 ; Nicol and Walmsley, 2002), the calyx of Held in cats (0.07 µm 2 ; Rowland et al., 2000), GABAergic nigrothalamic terminals (0.029 µm 2 ; Bodor et al., 2008), input synapses from the nucleus reticularis thalami (0.05 µm 2 ) and anterior pretectal nucleus (0.04 µm 2 ) to the posterior thalamic nucleus (Wanaverbecq et al., 2008) and glutamatergic synapses in the hippocampal CA1 region (0.06 µm 2 ; Harris and Stevens, 1989; Schikorski and Stevens, 2001; Marrone et al., 2005), but significantly smaller than input synapses terminating on layer 5 pyramidal neurons (0.20 µm 2 ; Rollenhagen et al., 2007b). Whereas the size of the AZs in MFBs was comparable to that in other central synapses, the number and density of AZs were substantially different when compared with other central synapses investigated so far (cerebellar climbing fiber synapse: 67; cerebellar mossy fiber bouton: range ; nigrothalamic terminal: range 2 26; cortical synapses: range 1 3). The average number of AZs at individual MFBs was 20, and the largest number of AZs found was 45. Thus, the MFBs contained a relatively large number of AZs (see also Chicurel and Harris, 1992) which covered on average 10% of the total surface area where the postsynaptic target apposed the presynaptic terminal. In contrast, in the calyx of Held 550 AZs contributed only 5% to the apposition area between the pre- and postsynaptic membranes (Sätzler et al., 2002). Thus, AZs in MFBs covered a much larger fraction of the presynaptic surface area than in the calyx of Held. The density of AZs per bouton area in MFBs is important for both, synaptic strength of individual MFBs and input of multiple MFBs on the same postsynaptic CA3 pyramidal neuron, allowing convergence of 50 MFBs on a single CA3 pyramidal neuron (Amaral and Dent, 1981). Another functionally relevant structural factor is the spacing (nearest-neighbor relation) of individual AZs, which is one possible determinant for spillover of transmitter and hence synaptic crosstalk (Barbour and Häusser, 1997). Release sites that are close to each other suggest that glutamate spillover or synaptic crosstalk is most likely to occur at these synapses. In addition, spillover of transmitter may as a consequence desensitize receptors at neighboring postsynaptic densities, possibly resulting in a reduced synaptic efficacy (Silver et al., 1996; Ishikawa et al., 2002; for review see Xu- Friedman and Regehr, 2004). On the other hand, nearest neighbor AZs at tremely short distances may lead to an increase in synaptic efficacy if receptors are simultaneously activated and not saturated by the neurotransmitter (Nusser et al., 1998). In MFBs, the nearest neighbor distance was on average 0.48 ± 0.19 µm, comparable with values estimated for the cerebellar MF synapse (0.46 µm; Xu-Friedman and Regehr, 2003), but different from the endbulb of Held (0.15 µm; Nicol and Walmsley, 2002), the calyx of Held (0.59 µm; Sätzler et al., 2002), nigrothalamic synapses (rat: 0.17 µm, monkey: 0.18 µm; Bodor et al., 2008) and ribbon-associated (1.66 µm) and ribbon-free (1.53 µm) goldfish bipolar synapses (Midorikawa et al., 2007). The nearest-neighbor distances varied widely ( µm), as also indicated by the large standard deviation and CV. However, as 80% of the distances between neighboring AZs at individual MFBs were in the range between 0.32 and 0.60 µm (Figure 3C), indicates that crosstalk may occur both presynaptically via diffusion of Ca 2+ and postsynaptically via spillover of glutamate from the synaptic cleft, as proposed for the cerebellar MF synapses (DiGregorio et al., 2002). The differences in nearest-neighbor relationships of individual AZs over a wide range of distribution suggest that glutamate spillover and as a consequence synaptic crosstalk is differentially regulated at central synapses. PUNCTA ADHERENTIA The second type of membrane specializations were PAs (Figure 2B, orange structures; see Hamlyn, 1962; Honda et al., 2006). These were characterized by two parallel bands of electron dense material of approximately the same width at the pre- and postsynaptic membranes, but lacking synaptic vesicles and the characteristic broadening of the cleft typical for AZs. For PAs, the average cleft width was ± 1.21 nm in the center and ± 0.97 nm at the edges of the two densities in adult rats (see also Table 1) and are in good agreement with quantitative data reported earlier (Hamlyn, 1962). At individual MFBs the majority of PAs were organized in rows of up to 15 PAs that were clusively found at the apposition zone of the presynaptic terminal and the dendritic shaft. The surface area of individual PAs was, on average, 0.07 µm 2, approximately half of the size of individual AZs, however, their size distribution showed a similar degree of variability (Rollenhagen et al., 2007a). An important structural difference between MFBs, the calyx of Held and nigrothalamic terminals in rat and monkey was the arrangement of the two membrane specializations. In the MFB both membrane specializations were spatially separated from each other; PAs were clusively found at the apposition zone between the bouton and the dendritic shaft whereas AZs were located on the spiny crescences. In contrast, in the calyx of Held PAs formed cluster-like arrangements between individual AZs that may isolate individual AZs from each other, thereby presumably preventing glutamate spillover and synaptic crosstalk. In addition, nigrothalamic terminals in rat and monkey show a centrally located network of PAs surrounded by laterally spaced AZs (Bodor et al., 2008). GLIAL COVERAGE OF MFBs Glial cells, in particular astrocytes and their fine processes, play an important role in synaptic function, acting as physical barriers to glutamate diffusion and mediating transmitter uptake by glutamate transporters (for review see Danbolt, 2001; Oliet et al., 2004). Strikingly, nearly all MFBs and their target structures were surrounded by astrocytic processes (Figure 1B). Thus, the synaptic compl consisting of an MFB and its postsynaptic target dendrite or spiny crescences were physically isolated not only from the surrounding neuropil but also from neighboring synaptic comples. Glial fingers were never observed to reach as far as to individual AZs located at the spiny crescences or even the synaptic cleft; instead, spiny crescences were almost completely covered by the boutons (Figure 2A). In contrast, at other central synapses, fine glial processes were observed at the pre- and postsynaptic apposition zone (Xu-Friedman and Regehr, 2003; Bodor et al., 2008), or even reached the synaptic cleft (Xu-Friedman et al., 2001; Rollenhagen et al., 2007b). As astrocytes act as physical barriers to glutamate Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 5
6 diffusion and mediate transmitter uptake, e.g. by the glutamate transporters EAAT1 and EAAT2 the remote location of fine glial processes in the MFB may further enhance postsynaptic crosstalk at hippocampal MF synapses (Barbour and Häusser, 1997) whereas at the other synapses glutamate spillover and as a consequence synaptic crosstalk is not likely to occur. Thus, two structural factors are likely to promote crosstalk at the MFB-CA3 pyramidal neuron synapse and also other synapses: (1) the short distance between adjacent AZs at spiny crescences and (2) the absence of fine glial processes at and between AZs. Synaptic crosstalk may prolong and amplify the synaptic current, contributing to the efficacy of hippocampal MF synapses. SIZE AND ORGANIZATION OF THE POOL OF SYNAPTIC VESICLES The other important structural key determinant for synaptic efficacy and plasticity is the organization of the pool of synaptic vesicles, in particular, the size of the readily releasable (RRP), the recycling (RP), and the reserve pool as measured by imaging, capacitance measurements and quantal analysis studies (Rosenmund and Stevens, 1996; Schneggenburger et al., 1999; Sahara and Takahashi, 2001; Sun and Wu, 2001; Hallermann et al., 2003; Saviane and Silver, 2006; for review see Harata et al., 2001; Schneggenburger et al., 2002). However, structural data upon the composition of the three functionally defined pools of synaptic vesicles are only available for a very small number of synapses. Among the most commonly studied synapses, the striking feature is the widely different total number of synaptic vesicles in each nerve terminal, for ample goldfish retinal bipolar nerve terminal: 700,000 vesicles; frog neuromuscular junction: 500,000 vesicles; calyx of Held: 700,00 180,000 vesicles; drosophila larvae neuromuscular junction: 84,000 vesicles; cerebellar mossy fiber synapses: 200,000 vesicles (Rizzoli and Betz, 2004; for review see Rizzoli and Betz, 2005). Synaptic vesicles in individual MFBs were distributed throughout the entire terminal (Figures 1A, 2C and 4A,B) and comprised an average volume of 0.26 µm 3, corresponding to 4.2% of the total volume of the MFB. However, it is noteworthy to mention that a marked difference in the organization of the pool of synaptic vesicles was found between MFBs in postnatal day 28 (P28) and adult rats. Synaptic vesicles in young rats were evenly distributed throughout the terminal thereby completely surrounding the spiny crescences. In adult MFBs a structural re-organization of the pool of synaptic vesicles towards a more cluster-like arrangement at individual AZs was observed (Figure 4B). These clusters are of various sizes at individual AZs and/or a row of several AZs. The distribution pattern at P28 may correspond to the functionally described maxipool of synaptic vesicles described by capacitance measurements of individual MFBs (Hallermann et al., 2003). Interestingly, in both ages three different types of vesicles were found: (1) Small clear synaptic vesicles with a mean diameter of ± 5.20 nm for adult rats, ranging between nm and nm for individual boutons, (2) large clear vesicles (Henze et al., 2002b; 70 nm) that constituted 1% of the total pool and were intermingled with the population of smaller synaptic vesicles and (3) large densecore vesicles. Dense-core vesicles are not observed in all central synapses investigated so far. Interestingly, at input synapses in layer 5 input dense core vesicles contribute with 10% to the total vesicular pool (Rollenhagen et al., 2007b) and were also seen to fuse with the AZ. It may therefore be speculated that these vesicles not only belong to the o- and endocytosis machinery and contain various peptides and hormones (for review see Harata et al., 2006; Salio et al., 2006), but are also involved in the build-up of AZs thereby influencing the release properties and neurotransmitter trafficking at the synapse (for review see Schoch and Gundelfinger, 2006). A B C mean no. of synaptic vesicles adult MFB P28 MFB L5 synapses Calyx of Held RP RRP distance from AZ (in nm) FIGURE 4 Organization of the pool of synaptic vesicles. (A, B) Two representative amples with a large ((A) 17,138 vesicles) and smaller ((B) 7559 vesicles) total pool of synaptic vesicles (green dots) are given. (C) The graph shows the correlation for the mean number of synaptic vesicles as a function of distance from an AZ (averaged over all AZs) for P28 MFBs (light blue line), adult MFBs (dark blue line), the calyx of Held (green line) and layer 5 input synapses (red line). The light yellow boxes indicate the borders between the RRP, the RP and the reserve pool of synaptic vesicles. Note, that the slope of the curves is markedly higher in the MFBs and layer 5 input synapses when compared with the calyx of Held synapse, even though the spacing between individual AZs is comparable at these synapses. Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 6
7 In order to identify subsets of synaptic vesicles as anatomical correlates for the three functionally defined pools of releasable quanta of transmitter postulated from measurements of release rates (for criteria see also Rizzoli and Betz, 2004, 2005) the RRP, the RP and the reserve pool were determined as the number of synaptic vesicles within a perimeter of 60 nm ( 2 vesicle diameters), 200 nm ( 5 vesicle diameters) and 500 nm ( 15 vesicle diameters) (Figure 4C). The number of synaptic vesicles in each of the pools was clearly correlated with the size of individual AZs (Rollenhagen et al., 2007a). Assuming a linear relationship between both measures, it was possible to define a mean vesicle density for a given perimeter. Thus, the density of synaptic vesicles at AZs in both P28 and adult MFBs (light and dark blue curves) was about two-fold higher than in the calyx of Held (green curve), but similar to values quantified for layer 5 input synapses (red curve) in the barrel field of the rat somatosensory cort (Rollenhagen et al., 2007b). The total pool of synaptic vesicles within individual large MFBs was on average 25,000 (range 11,000 48,000) at P28 and 16,000 (range ,000) in adult rats (see Table 2). In contrast, the number of synaptic vesicles in small filopodia was , two orders of magnitude smaller than in the large boutons. If the RRP is defined by 2 vesicle diameters (60 nm) then the total RRP per large bouton was 1200 vesicles (range ) in P28 and 600 vesicles (range ) in adult rats, corresponding to 4% of the total pool of synaptic vesicles (see Table 2). This corresponds to ± (P28) and ± (adult) vesicles per single AZ. If the RP is defined by 5 vesicle diameters, then the mean of the RP at individual MFBs is 5700 (range ,100) vesicles ( 21% of the total pool) in P28 and 3700 (range ) vesicles ( 20% of the total pool) in the adult. The relative size of the RP in the MFB is in good agreement with RP estimates reported for other central synapses (Rizzoli and Betz, 2004, 2005). However, it has to be mentioned that the RRP Table 2 Synaptic vesicle pools in the adult MFB. Bouton Synaptic Size of the three different pools vesicles of synaptic vesicles Total Putative RRP Putative RP Putative number 60 nm 60 nm > reserve distance 200 pool >200 No. No. No./AZ No. No./AZ No. 1 13, , , , , , Mean 15, ,963 CV Summary of structural parameters relevant for release that have been tracted from the detailed 3D-reconstructions. For the RRP and RP, two values are given: the total number of synaptic vesicles per MFB and per individual AZ. For the reserve pool, the number of synaptic vesicles per bouton is given (modified from table 1 in Rollenhagen et al., 2007a). and the RP in P28 and adult MFBs showed a large variability, as indicated by the large standard deviation and a consistently large CV of about 1.0. Capacitance measurements at hippocampal MFBs revealed a large size of the releasable pool ( maxipool ) with 1400 vesicles released during a 30-ms pulse (Hallermann et al., 2003). However, the structural correlate of the releasable pool, although large, comprises only a small fraction of the total pool. The total pool of synaptic vesicles was 25,000 for P28 and 16,000 vesicles for adult MFBs, distributed over 30 and 20 AZs, respectively. Thus, the total pool size of synaptic vesicles per AZ is 850 in both ages. A very different scenario was found in the calyx of Held, where 70,000 vesicles were distributed over 550 release sites with 125 synaptic vesicles per AZ. In cerebellar MF synapses, fluctuation analysis of synaptic currents suggested a total pool size of 200,000 synaptic vesicles distributed over 330 release sites (Saviane and Silver, 2006). Thus, the number of synaptic vesicles per AZ would be 600 at cerebellar MF synapses. Interestingly, the total pool per AZ in the large MFB ( 900) appears to be larger than the pool in the endbulbs emerging from filopodial tensions of the same terminal ( vesicles per AZ). This may contribute to target-cell specific differences in synaptic dynamics (Maccaferri et al., 1998; Tóth et al., 2000; Pelkey and McBain, 2005; Pelkey et al., 2006). At the calyx of Held, taking the 60 nm criterion, the total RRP was 10,000 distributed over 550 AZs, equivalent to 18 synaptic vesicles per AZ. Similarly, the putative RP was 5700 vesicles for P28 MFBs, 3700 vesicles for adult MFBs and 25,000 for the calyx of Held. When estimated for individual AZs, the RP was fourfold larger in MFBs than in the calyx of Held (Figure 4C). Finally, the putative reserve pool was 18,000 vesicles for P28 MFBs, 12,000 for adult MFBs and 25,000 for the calyx of Held.When estimated for individual AZs the reserve pool was 10-fold larger in MFBs than in the calyx of Held (Figure 4C). The large size of the RRP, RP, and reserve pool at each AZ may be a key structural correlate of presynaptic efficacy and plasticity at hippocampal MFBs (Salin et al., 1996; Geiger and Jonas, 2000). As already mentioned above a huge variability not only in the total pool of synaptic vesicles ists, but also for the size of the readily releasable, recycling and reserve pool at different synaptic systems. In particular, the size of the RRP differs substantially between species (drosophila larvae neuromuscular junction: 300, 0.4% of the total pool; frog neuromuscular junction: 10,000, 2% of the total pool; hippocampal cultured neurons: 10, 5% of the total pool; goldfish retina: 1200, 0.17% of the total pool. These differences are also observed for the RP ranging from 0.6% to 20% of the total pool size. Only the size of the reserve pool with 80% of the total seems to be a constant factor between different synapses. It has to be mentioned though that the comparison of pool sizes may be inaccurate due to different perimental procedures and methods used, the state of the synapse and also the different age of the animals among species investigated. FUNCTIONAL SIGNIFICANCE OF THE STRUCTURAL COMPONENTS FOR MFB SYNAPTIC TRANSMISSION Both the relatively large number and distribution of release sites together with the large size of the RRP, RP, and reserve pool will have important functional implications. The large number of AZs Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 7
8 together with the large RRP and RP will contribute to the high efficacy of the hippocampal MF synapse in vitro (Geiger and Jonas, 2000) and in vivo (Henze et al., 2002a; reviewed by Bischofberger et al., 2006). The high density of AZs at the pre- and postsynaptic apposition zone at the spiny crescences is important to accommodate convergent input of multiple MFBs on the same postsynaptic CA3 pyramidal neuron (Amaral et al., 1990). The large RRP may prevent depletion during repetitive stimulation, e.g. if the animal is located in the center of the place field of a granule cell or during odor-guided behavioral tasks (reviewed by Bischofberger et al., 2006). However, even more importantly, the large size of RP and reserve pool could be used to rapidly refill the releasable pool if it has been depleted by long-lasting repetitive stimulation. If the refilling rates in MFBs were activity dependent, the large size of the RP could plain MF plasticity, e.g. the nearly 10-fold increase in synaptic strength during frequency facilitation and post-tetanic potentiation (Salin et al., 1996). Thus, multiple structural determinants converge on the generation of a conditional detonator synapse that makes the MF-CA3 pyramidal cell synapse ceptional, unique, and therefore perfectly suited for its role in the hippocampal network. PERSPECTIVE The quantitative structural data on the MFB provide a basis for numerical simulations and realistic models of MF transmission, which may help to constrain functional parameters that are, at present, inaccessible perimentally. However, there are still a lot of open questions to be answered in the future. At the molecular level, it has been shown using a wide spectrum of perimental methods that the basal release is regulated by presynaptic adenosine A 1 receptors, that specific subtypes of metabotropic glutamate receptors strongly depress neurotransmitter release, that kainate receptors on the other hand are involved in the increase in citability (Kwon and Castillo, 2008), but that LTP is NMDA- receptor independent and crucially depends on the activation of P/Q and N-type Ca 2+ channels. However, it is still rather unknown how these and other receptors and channels are located at the MF synapse (pre- versus postsynaptic, central versus lateral organization of receptors and their subunits at the AZ, synaptic, peri- and trasynaptic localization) and in which density and distribution they occur. This question could be addressed using freeze fracture replica preparations combined with single- or multiple postembedding immunohistochemistry against different receptors and their subunits, and quantitative analysis in form of receptor fingerprints at individual synapses (Shigemoto et al., 1997; Masugi-Tokita et al., 2007; for review see Masugi-Tokita and Shigemoto, 2007). Furthermore, the molecular components that distinguish vesicles belonging to one of the three vesicular pools remain to be identified and which mechanisms drive the transfer from one pool to the other. Finally, it has to be pointed out that the structural composition of synapses and the arrangement of their subelements determine the functional properties of synapses embedded in different neuronal microcircuits. Synapses are highly specialized structures that are perfectly adapted both structurally and functionally to their job within a given network. Therefore, the dream of a model synapse that describes the structure and function of all synapses in the brain seems to become more and more unrealistic. Thus, much more tensive work is needed to unravel the unique behavior of synapses embedded in different microcircuits of the brain. ACKNOWLEDGMENT The ongoing financial support by the Deutsche Forschungsgemeinschaft (grants to J.H.R.L) is gratefully acknowledged. REFERENCES Acsády, L., Kamondi, A., Sik, A., Freund, T., and Buzsaki, G. (1998). GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 18, Alle, H., and Geiger, J. R. (2006). Combined analog and action potential coding in hippocampal mossy fibers. Science 311, Alle, H., Roth, A., and Geiger, J. R. (2009). Energy-efficient action potentials in hippocampal mossy fibers. Science 325, Amaral, D. G., and Dent, J. A. (1981). Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their pansions. J. Comp. Neurol. 195, Amaral, D. G., Ishizuka, N., and Claiborne, B. (1990). Neurons, numbers and the hippocampal network. Prog. Brain Res. 83, Barbour, B., and Häusser, M. (1997). Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 20, Bischofberger, J., Engel, D., Frotscher, M., and Jonas, P. (2006). Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflügers Arch. 453, Blackstad, T. W., and Kjaerheim, A. (1961). Special axo-dendritic synapses in the hippocampal cort: electron and light microscopic studies on the layer of mossy fibers. J. Comp. Neurol. 117, Bodor, A. L., Giber, K., Rovó, Z., Ulbert, I., and Acsády, L. (2008). Structural correlates of efficient GABAergic transmission in the basal gangliathalamus pathway. J. Neurosci. 28, Chicurel, M. E., and Harris, K. M. (1992). Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J. Comp. Neurol. 325, Danbolt, N. C. (2001). Glutamate uptake. Prog. Neurobiol. 65, DiGregorio, D. A., Nusser, Z., and Silver, R. A. (2002). Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron 35, Engel, D., and Jonas, P. (2005). Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45, Frotscher, M., Jonas, P., and Sloviter, R. S. (2006). Synapses formed by normal and abnormal hippocampal mossy fibers. Cell Tissue Res. 326, Frotscher, M., Soriano, E., and Misgeld, U. (1994). Divergence of hippocampal mossy fibers. Synapse 16, Galimberti, I., Gogolla, N., Alberi, S., Santos, A. F., Muller, D., and Caroni, P. (2006). Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by perience. Neuron 50, Geiger, J. R., and Jonas, P. (2000). Dynamic control of presynaptic Ca 2+ inflow by fast-inactivating K + channels in hippocampal mossy fiber boutons. Neuron 28, Hallermann, S., Pawlu, C., Jonas, P., and Heckmann, M. (2003). A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl. Acad. Sci. U.S.A. 100, Hamlyn, L. H. (1962). The fine structure of the mossy fibre endings in the hippocampus of the rabbit. J. Anat. 96, Hamori, J., and Somogyi, J. (1983). Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. J. Comp. Neurol. 220, Harata, N. C., Aravanis, A. M., and Tsien, R. W. (2006). Kiss-and-run and full-collapse fusion as modes of o-endocytosis in neurosecretion. J. Neurochem. 97, Harata, N. C., Pyle, J. L., Aravanis, A. M., Mozhayeva, M., Kavalali, E. T., and Tsien, R. W. (2001). Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 24, Harris, K. M., and Stevens, J. K. (1989). Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 9, Henze, D. A., Wittner, L., and Buzsaki, G. (2002a). Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 5, Henze, D. A., McMahon, D. B., Harris, K. M., and Barrionuevo, G. (2002b). Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 8
9 Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J. Neurophysiol. 87, Honda, T., Sakisaka, T., Yamada, T., Kumazawa, N., Hoshino, T., Kajita, M., Kayahara, T., Ishizaki, H., Tanaka- Okamoto, M., Mizoguchi, A., Manabe, T., Miyoshi, J., and Takai, Y. (2006). Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol. Cell Neurosci. 31, Ishikawa, T., Sahara, Y., and Takahashi, T. (2002). A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron 34, Kobayashi, K., and Poo, M. M. (2004). Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron 41, Kwon, H. B., and Castillo, P. E. (2008). Long-term potentiation selectively pressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron 57, Lawrence, J. J., Grinspan, Z. M., and McBain, C. J. (2004). Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J. Physiol. (Lond.) 554, Lisman, J. E. (1999). Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-ca3 interactions. Neuron 22, Maccaferri, G., Toth, K., and McBain, C. J. (1998). Target-specific pression of presynaptic mossy fiber plasticity. Science 279, Marrone, D. F., LeBoutillier, J. C., and Petit, T. L. (2005). Ultrastructural correlates of vesicular docking in the rat dentate gyrus. Neurosci. Lett. 378, Masugi-Tokita, M., and Shigemoto, R. (2007). High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr. Opin. Neurobiol. 17, Masugi-Tokita, M., Tarusawa, E., Watanabe, M., Molnar, E., Fujimoto, K., and Shigemoto, R. (2007). Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freezefracture replica labeling. J. Neurosci. 27, Midorikawa, M., Tsukamoto, Y., Berglund, K., Ishii, M., and Tachibana, M. (2007). Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cell. Nat. Neurosci. 10, Moore, K. A., Nicoll, R. A., and Schmitz, D. (2003). Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. U.S.A. 100, Nicol, M. J., and Walmsley, B. (2002). Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. J. Physiol. (Lond.) 539, Nicoll, R. A., and Schmitz, D. (2005). Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, Nusser, Z., Lujan, R., Laube, G., Roberts, J. D., Molnar, E., and Somogyi, P. (1998). Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21, Oliet, S. H., Piet, R., Poulain, D. A., and Theodosis, D. T. (2004). Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia 47, Pelkey, K. A., and McBain, C. J. (2005). How to dismantle a detonator synapse. Neuron 45, Pelkey, K. A., Topolnik, L., Lacaille, J. C., and McBain, C. J. (2006). Compartmentalized Ca 2+ channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron 52, Rizzoli, S. O., and Betz, W. J. (2004). The structural organization of the readily releasable pool of synaptic vesicles. Science 303, Rizzoli, S. O., and Betz, W. J. (2005). Synaptic vesicle pools. Nat. Rev. Neurosci. 6, Rizzuto, R., Bernardi, P., and Pozzan, T. (2000). Mitochondria as all-round players of the calcium game. J. Physiol. (Lond.) 529, Rollenhagen, A., and Lübke, J. H. R. (2006). The morphology of citatory central synapses: from structure to function. Cell Tissue Res. 326, Rollenhagen, A., Sätzler, K., Rodriquez, E. P., Jonas, P., Frotscher, M., and Lübke, J. H. R. (2007a). Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, Rollenhagen, A., Sätzler, K., Roth, A., and Lübke, J. H. R. (2007b). Structural determinants of transmission and plasticity of input synapses on layer 5 pyramidal neurons. Program No Neuroscience Meeting Planer. San Diego, CA, Society for Neuroscience, Online. Rosenmund, C., and Stevens, C. F. (1996). Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, Rowland, K. C., Irby, N. K., and Spirou, G. A. (2000). Specialized synapse-associated structures within the calyx of Held. J. Neurosci. 20, Ryugo, D. K., Pongstaporn, T., Huchton, D. M., and Niparko, J. K. (1997). Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J. Comp. Neurol. 385, Sahara, Y., and Takahashi, T. (2001). Quantal components of the citatory postsynaptic currents at a rat central auditory synapse. J. Physiol. (Lond.) 536, Salin, P. A., Scanziani, M., Malenka, R. C., and Nicoll, R. A. (1996). Distinct shortterm plasticity at two citatory synapses in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 93, Salio, C., Lossi, L., Ferrini, F., and Merighi, A. (2006). Neuropeptides as synaptic transmitters. Cell Tissue Res. 326, Sätzler, K., Söhl, L. F., Bollmann, J. H., Borst, J. G., Frotscher, M., Sakmann, B., and Lübke, J. H. R. (2002). Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J. Neurosci. 22, Saviane, C., and Silver, R. A. (2006). Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature 439, Schikorski, T., and Stevens, C. F. (2001). Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 4, Schneggenburger, R., Meyer, A. C., and Neher, E. (1999). Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 23, Schneggenburger, R., Sakaba, T., and Neher, E. (2002). Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 25, Schoch, S., and Gundelfinger, E. D. (2006). Molecular organization of the presynaptic active zone. Cell Tissue Res. 326, Shigemoto, R., Kinoshita, A., Wada, E., Nomura, S., Ohishi, H., Takada, M., Flor, P. J., Neki, A., Abe, T., Nakanishi, S., and Mizunom, N. (1997). Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, Sikora, M. A., Gottesman, J., and Miller, R. F. (2005). A computational model of the ribbon synapse. J. Neurosci. Methods 145, Silver, R. A., Colquhoun, D., Cull-Candy, S. G., and Edmonds, B. (1996). Deactivation and desensitazation of non-nmda receptors in patches and the time course of EPSCs in rat cerebellar granule cells. J. Physiol. (Lond.) 493, Sun, J. Y., and Wu, L. G. (2001). Fast kinetics of ocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron 30, Szabadics, J., and Soltesz, I. (2009). Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J. Neurosci. 29, tom Dieck, S., and Brandstätter, J. H. (2006). Ribbon synapses of the retina. Cell Tissue Res. 326, Tóth, K., Suares, G., Lawrence, J. J., Philips- Tansey, E., and McBain, C. J. (2000). Differential mechanisms of transmission at three types of mossy fiber synapse. J. Neurosci. 20, Verstreken, P., Ly, C. V., Venken, K. J., Koh, T. W., Zhou, Y., and Bellen, H. J. (2005). Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, Wanaverbecq, N., Bodor, A. L., Bokor, H., Slézia, A., Lüthi, A., and Acsády, L. (2008). Constrasting the functional properties of GABAergic axon terminals with single and multiple release synapses in the thalamus. J. Neurosci. 28, Wimmer, V. C., Horstmann, H., Groh, A., and Kuner, T. (2006). Donut-like topology of synaptic vesicles with a central cluster of mitochondria wrapped into membrane protrusions: a novel structure-function module of the adult calyx of Held. J. Neurosci. 26, Xu-Friedman, M. A., Harris, K. M., and Regehr, W. G. (2001). Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J. Neurosci. 21, Xu-Friedman, M. A., and Regehr, W. G. (2003). Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J. Neurosci. 23, Xu-Friedman, M. A., and Regehr, W. G. (2004). Structural contributions to short-term synaptic plasticity. Physiol. Rev. 84, Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Received: 23 December 2009; paper pending published: 11 January 2010; accepted: 12 February 2010; published online: 15 March Citation: Rollenhagen A and Lübke JHR (2010) The mossy fiber bouton: the common or the unique synapse? Front. Syn. Neurosci. 2:2. doi: /fnsyn Copyright 2010 Rollenhagen and Lübke. This is an open-access article subject to an clusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited. Frontiers in Synaptic Neuroscience March 2010 Volume 2 Article 2 9
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse
 Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Abstract. 1 Introduction
 Biophysical model of a single synaptic connection: transmission properties are determined by the cooperation of pre- and postsynaptic mechanisms Julia Trommershäuser and Annette Zippelius Institut für
Biophysical model of a single synaptic connection: transmission properties are determined by the cooperation of pre- and postsynaptic mechanisms Julia Trommershäuser and Annette Zippelius Institut für
Three-Dimensional Reconstruction of a Calyx of Held and Its Postsynaptic Principal Neuron in the Medial Nucleus of the Trapezoid Body
 The Journal of Neuroscience, December 15, 2002, 22(24):10567 10579 Three-Dimensional Reconstruction of a Calyx of Held and Its Postsynaptic Principal Neuron in the Medial Nucleus of the Trapezoid Body
The Journal of Neuroscience, December 15, 2002, 22(24):10567 10579 Three-Dimensional Reconstruction of a Calyx of Held and Its Postsynaptic Principal Neuron in the Medial Nucleus of the Trapezoid Body
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Neuroscience 201A (2016) - Problems in Synaptic Physiology
 Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Memory Systems II How Stored: Engram and LTP. Reading: BCP Chapter 25
 Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Differential Mechanisms of Transmission at Three Types of Mossy Fiber Synapse
 The Journal of Neuroscience, November 15, 2000, 20(22):8279 8289 Differential Mechanisms of Transmission at Three Types of Mossy Fiber Synapse Katalin Toth, Gregory Suares, J. Josh Lawrence, Emily Philips-Tansey,
The Journal of Neuroscience, November 15, 2000, 20(22):8279 8289 Differential Mechanisms of Transmission at Three Types of Mossy Fiber Synapse Katalin Toth, Gregory Suares, J. Josh Lawrence, Emily Philips-Tansey,
THE SYNAPTIC VESICLE CYCLE
 Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Synaptic plasticity. Activity-dependent changes in synaptic strength. Changes in innervation patterns. New synapses or deterioration of synapses.
 Synaptic plasticity Activity-dependent changes in synaptic strength. Changes in innervation patterns. New synapses or deterioration of synapses. Repair/changes in the nervous system after damage. MRC Centre
Synaptic plasticity Activity-dependent changes in synaptic strength. Changes in innervation patterns. New synapses or deterioration of synapses. Repair/changes in the nervous system after damage. MRC Centre
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki
 TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
TNS Journal Club: Interneurons of the Hippocampus, Freund and Buzsaki Rich Turner (turner@gatsby.ucl.ac.uk) Gatsby Unit, 22/04/2005 Rich T. Introduction Interneuron def = GABAergic non-principal cell Usually
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Biology 218 Human Anatomy
 Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Synaptic Communication. Steven McLoon Department of Neuroscience University of Minnesota
 Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus
 Article Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus Nadine Gogolla, 1,2 Ivan Galimberti, 1 Yuichi Deguchi, 1 and Pico
Article Wnt Signaling Mediates Experience-Related Regulation of Synapse Numbers and Mossy Fiber Connectivities in the Adult Hippocampus Nadine Gogolla, 1,2 Ivan Galimberti, 1 Yuichi Deguchi, 1 and Pico
Activity Dependent Changes At the Developing Neuromuscular Junction
 Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Activity Dependent Changes At the Developing Neuromuscular Junction (slides 16, 17 and 18 have been slightly modified for clarity) MCP Lecture 2-3 9.013/7.68 04 Neuromuscular Junction Development 1. Muscle
Cellular mechanisms of information transfer: neuronal and synaptic plasticity
 Cellular mechanisms of information transfer: neuronal and synaptic plasticity Ivan Pavlov (UCL Institute of Neurology, UK) Anton Chizhov (Ioffe Physical Technical Institute) Pavel Zykin (St.-Petersburg
Cellular mechanisms of information transfer: neuronal and synaptic plasticity Ivan Pavlov (UCL Institute of Neurology, UK) Anton Chizhov (Ioffe Physical Technical Institute) Pavel Zykin (St.-Petersburg
BIPN140 Lecture 12: Synaptic Plasticity (II)
 BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
The Neuron. Consists Of: - cell body. - Dendrites - axon - axon terminal - myelin. dendrites Axon terminal. Cell body. nucleus. axon.
 The Neuron Consists Of: - cell body - Dendrites - axon - axon terminal - myelin dendrites Axon terminal Cell body nucleus myelin axon THE SYNAPSE Definition: It is a point of contact between the axon of
The Neuron Consists Of: - cell body - Dendrites - axon - axon terminal - myelin dendrites Axon terminal Cell body nucleus myelin axon THE SYNAPSE Definition: It is a point of contact between the axon of
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Neurobiology. Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2010 1 The nervous system Central nervous system (CNS) Peripheral nervous system (PNS) 2 Enteric nervous system (digestive tract, gall bladder and
Neurobiology Cells of the nervous system Anthony Heape 2010 1 The nervous system Central nervous system (CNS) Peripheral nervous system (PNS) 2 Enteric nervous system (digestive tract, gall bladder and
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Supplementary Figure 1. Basic properties of compound EPSPs at
 Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
Supplementary Figure 1. Basic properties of compound EPSPs at hippocampal CA3 CA3 cell synapses. (a) EPSPs were evoked by extracellular stimulation of the recurrent collaterals and pharmacologically isolated
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale
 Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Dendritic Signal Integration
 Dendritic Signal Integration 445 Dendritic Signal Integration N Spruston, Northwestern University, Evanston, IL, USA ã 2009 Elsevier Ltd. All rights reserved. Overview: Questions Most neurons have elaborately
Dendritic Signal Integration 445 Dendritic Signal Integration N Spruston, Northwestern University, Evanston, IL, USA ã 2009 Elsevier Ltd. All rights reserved. Overview: Questions Most neurons have elaborately
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
Area CA3 Interneurons Receive Two Spatially Segregated Mossy Fiber Inputs
 HIPPOCAMPUS 00:000 000 (2009) RAPID COMMUNICATION Area CA3 Interneurons Receive Two Spatially Segregated Mossy Fiber Inputs Kathleen E. Cosgrove, Emilio J. Galván, Stephen D. Meriney, and Germán Barrionuevo*
HIPPOCAMPUS 00:000 000 (2009) RAPID COMMUNICATION Area CA3 Interneurons Receive Two Spatially Segregated Mossy Fiber Inputs Kathleen E. Cosgrove, Emilio J. Galván, Stephen D. Meriney, and Germán Barrionuevo*
COMMENTARY THE MULTIFARIOUS HIPPOCAMPAL MOSSY FIBER PATHWAY: A REVIEW
 Pergamon www.elsevier.com/locate/neuroscience Hippocampal mossy fibers Neuroscience Vol. 98, No. 3, pp. 407 427, 2000 407 2000 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights
Pergamon www.elsevier.com/locate/neuroscience Hippocampal mossy fibers Neuroscience Vol. 98, No. 3, pp. 407 427, 2000 407 2000 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights
Synaptic Plasticity and the NMDA Receptor
 Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
GABAA AND GABAB RECEPTORS
 FAST KINETIC MODELS FOR SIMULATING AMPA, NMDA, GABAA AND GABAB RECEPTORS Alain Destexhe, Zachary F. Mainen and Terrence J. Sejnowski* The Salk Institute for Biological Studies and The Howard Hughes Medical
FAST KINETIC MODELS FOR SIMULATING AMPA, NMDA, GABAA AND GABAB RECEPTORS Alain Destexhe, Zachary F. Mainen and Terrence J. Sejnowski* The Salk Institute for Biological Studies and The Howard Hughes Medical
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]](/thumbs/86/94924826.jpg) QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
How neurons talk. Nivedita Chatterjee Vision Research Foundation, Sankara Nethralaya, Chennai
 How neurons talk Nivedita Chatterjee Vision Research Foundation, Sankara Nethralaya, Chennai The Synaptic Bouton of a chemical synapse Chemical synapse with synaptic cleft The Electrical synapse works
How neurons talk Nivedita Chatterjee Vision Research Foundation, Sankara Nethralaya, Chennai The Synaptic Bouton of a chemical synapse Chemical synapse with synaptic cleft The Electrical synapse works
Resonant synchronization of heterogeneous inhibitory networks
 Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
Cerebellar oscillations: Anesthetized rats Transgenic animals Recurrent model Review of literature: γ Network resonance Life simulations Resonance frequency Conclusion Resonant synchronization of heterogeneous
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Structure of a Neuron:
 Structure of a Neuron: At the dendrite the incoming signals arrive (incoming currents) At the soma current are finally integrated. At the axon hillock action potential are generated if the potential crosses
Structure of a Neuron: At the dendrite the incoming signals arrive (incoming currents) At the soma current are finally integrated. At the axon hillock action potential are generated if the potential crosses
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström
 Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Presynaptic NMDA receptor control of spontaneous and evoked activity By: Sally Si Ying Li Supervisor: Jesper Sjöström NMDA receptors are traditionally known to function as post-synaptic coincidence detectors.
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Chapter 11: Nervous System and Nervous Tissue
 Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Synaptic Integration
 Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Modeling of Hippocampal Behavior
 Modeling of Hippocampal Behavior Diana Ponce-Morado, Venmathi Gunasekaran and Varsha Vijayan Abstract The hippocampus is identified as an important structure in the cerebral cortex of mammals for forming
Modeling of Hippocampal Behavior Diana Ponce-Morado, Venmathi Gunasekaran and Varsha Vijayan Abstract The hippocampus is identified as an important structure in the cerebral cortex of mammals for forming
Dorsal Cochlear Nucleus. Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS
 Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
NERVOUS SYSTEM 1 CHAPTER 10 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1
 BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Innervation of the Cochlea. Reading: Yost Ch. 8
 Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
DO NOW: ANSWER ON PG 73
 DO NOW: ANSWER ON PG 73 1. Name 1 neurotransmitter that we have learned about. 2. Draw a basic graph of a neuron action potential. Label resting potential, threshold, depolarization, and repolarization
DO NOW: ANSWER ON PG 73 1. Name 1 neurotransmitter that we have learned about. 2. Draw a basic graph of a neuron action potential. Label resting potential, threshold, depolarization, and repolarization
Human Brain and Senses
 Human Brain and Senses Outline for today Levels of analysis Basic structure of neurons How neurons communicate Basic structure of the nervous system Levels of analysis Organism Brain Cell Synapses Membrane
Human Brain and Senses Outline for today Levels of analysis Basic structure of neurons How neurons communicate Basic structure of the nervous system Levels of analysis Organism Brain Cell Synapses Membrane
Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA
 Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
CYTOARCHITECTURE OF CEREBRAL CORTEX
 BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
Receptorarchitecture and Neural Systems
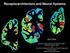 Receptorarchitecture and Neural Systems Karl Zilles Institute of Neuroscience and Medicine INM-1 Research Centre Jülich and University Hospital of Psychiatry, Psychotherapy and Psychosomatics RWTH Universität
Receptorarchitecture and Neural Systems Karl Zilles Institute of Neuroscience and Medicine INM-1 Research Centre Jülich and University Hospital of Psychiatry, Psychotherapy and Psychosomatics RWTH Universität
Parallel Driving and Modulatory Pathways Link the Prefrontal Cortex and Thalamus
 Boston University OpenBU Health Sciences http://open.bu.edu SAR: Health Sciences: Scholarly Papers 2007-9-5 Parallel Driving and Modulatory Pathways Link the Prefrontal Cortex and Thalamus Zikopoulos,
Boston University OpenBU Health Sciences http://open.bu.edu SAR: Health Sciences: Scholarly Papers 2007-9-5 Parallel Driving and Modulatory Pathways Link the Prefrontal Cortex and Thalamus Zikopoulos,
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Synapse Formation. Steven McLoon Department of Neuroscience University of Minnesota
 Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Synaptic communication
 Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 11: Functional Organization of Nervous Tissue
 Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Presynaptic control of e$cacy of GABAergic synapses in the hippocampus
 Neurocomputing 38}40 (2001) 99}104 Presynaptic control of e$cacy of GABAergic synapses in the hippocampus N. Axmacher *, M. Stemmler, D. Engel, A. Draguhn, R. Ritz Innovationskolleg Theoretische Biologie,
Neurocomputing 38}40 (2001) 99}104 Presynaptic control of e$cacy of GABAergic synapses in the hippocampus N. Axmacher *, M. Stemmler, D. Engel, A. Draguhn, R. Ritz Innovationskolleg Theoretische Biologie,
Serotonergic Control of the Developing Cerebellum M. Oostland
 Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Chapter 12 Nervous Tissue. Copyright 2009 John Wiley & Sons, Inc. 1
 Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
Outline. Animals: Nervous system. Neuron and connection of neurons. Key Concepts:
 Animals: Nervous system Neuron and connection of neurons Outline 1. Key concepts 2. An Overview and Evolution 3. Human Nervous System 4. The Neurons 5. The Electrical Signals 6. Communication between Neurons
Animals: Nervous system Neuron and connection of neurons Outline 1. Key concepts 2. An Overview and Evolution 3. Human Nervous System 4. The Neurons 5. The Electrical Signals 6. Communication between Neurons
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Спайковые и бионические нейронные сети, нейроморфные системы. Dmitry Nowicki
 Спайковые и бионические нейронные сети, нейроморфные системы Dmitry Nowicki 1 Биологический нейрон 2 Нейрон МакКаллока-Питтса (не похож!?) 3 Сеть прямого распространения 4 Сеть Хопфилда: простейшая ассоциативная
Спайковые и бионические нейронные сети, нейроморфные системы Dmitry Nowicki 1 Биологический нейрон 2 Нейрон МакКаллока-Питтса (не похож!?) 3 Сеть прямого распространения 4 Сеть Хопфилда: простейшая ассоциативная
Revisiting the Role of the Hippocampal Mossy Fiber Synapse
 HIPPOCAMPUS 11:408 417 (2001) Revisiting the Role of the Hippocampal Mossy Fiber Synapse Nathaniel N. Urban,* Darrell A. Henze, and German Barrionuevo Department of Neuroscience and Center for Neural Basis
HIPPOCAMPUS 11:408 417 (2001) Revisiting the Role of the Hippocampal Mossy Fiber Synapse Nathaniel N. Urban,* Darrell A. Henze, and German Barrionuevo Department of Neuroscience and Center for Neural Basis
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
浙江大学医学院基础医学整合课程 各论 III. The Nervous System. Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine
 The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine xiongzhang@zju.edu.cn http://10.202.77.12/ 1 Part 1. Summary of the nervous system 2 The Nervous System Central Nervous System
The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine xiongzhang@zju.edu.cn http://10.202.77.12/ 1 Part 1. Summary of the nervous system 2 The Nervous System Central Nervous System
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
Modeling synaptic facilitation and depression in thalamocortical relay cells
 College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Theme 2: Cellular mechanisms in the Cochlear Nucleus
 Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
