THE ROLE OF MELANOPSIN CONTAINING RETINAL GANGLION CELLS IN THE PUPILLARY RESPONSES OF HUMAN AND NON-HUMAN PRIMATES DAVID H.
|
|
|
- Hester Garrett
- 5 years ago
- Views:
Transcription
1 THE ROLE OF MELANOPSIN CONTAINING RETINAL GANGLION CELLS IN THE PUPILLARY RESPONSES OF HUMAN AND NON-HUMAN PRIMATES by DAVID H. MCDOUGAL KENT T. KEYSER, CHAIR PAUL D. GAMLIN TIMOTHY W. KRAFT JOEL POKORNY ROSALYN E. WELLER A DISSERTATION Submitted to the graduate faculty of The University of Alabama at Birmingham, in partial fulfillment of the requirements for the degree of Doctor of Philosophy BIRMINGHAM, ALABAMA 2008
2 THE ROLE OF MELANOPSIN CONTAINING RETINAL GANGLION CELLS IN THE PUPILLARY RESPONSES OF HUMAN AND NON-HUMAN PRIMATES DAVID MCDOUGAL VISION SCIENCE ABSTRACT Historically, the characteristics of the light-evoked neural signals driving the human pupillary light reflex (PLR) have been poorly understood. It was assumed that these light signals originated exclusively from rod and cone photoreceptors, although the measured spectral sensitivity and response dynamics of the PLR were difficult to reconcile with the known properties of rods and cones. Recently, a novel photoreceptive cell class has been discovered in the mammalian retina. These unique retinal ganglion cells not only receive rod and cone inputs, but also express the photopigment melanopsin, and exhibit intrinsic photoresponses independent of rod and cone inputs. These intrinsically photosensitive retinal ganglion cells (iprgcs) have been shown to project to the pretectum, the retinorecipient area of the brain responsible for the PLR. Initial reports have suggested that iprgcs contribute significantly to the PLR of rodents, but studies relating this unique cell class to primate PLR are lacking. We have therefore examined the role of the iprgcs in the primate PLR. Our results show that iprgcs contribute significantly to the human and non-human primate PLR. We demonstrate that the macaque PLR is still present after the pharmalogical blockade of rod and cone photoreceptors, and that the residual PLR is driven exclusively by the melanopsinmediated intrinsic response of iprgcs. We also report that the intrinsic response exclusively drives sustained post stimulus pupilloconstriction in both humans and non- ii
3 human primates. We extended our examination of the human PLR to determine the relative contribution of rod, cone and the intrinsic photoresponses to pupillary constriction during steady-state lighting. We established that the intrinsic response of iprgcs contributes significantly to the maintenance of half maximal pupilloconstriction in response to light stimuli of 30 seconds or longer, even at low photopic irradiances. Furthermore, we show that the intrinsic response contributes to three- quarter maximal pupilloconstriction in response to light stimuli as short as 2 seconds. We also demonstrate that, although cone photoresponses driving pupilloconstriction quickly adapt, rod photoresponses contribute to the maintenance of pupilloconstriction in response to steady-state light stimuli at both photopic and scotopic irradiances. iii
4 TABLE OF CONTENTS Page ABSTRACT... ii LIST OF TABLES... vi LIST OF FIGURES... vii LIST OF ABBREVIATIONS... ix INTRODUCTION...1 PUPILLARY CONTROL PATHWAYS...7 HUMAN AND MACAQUE PUPIL RESPONSES DRIVEN BY MELANOPSIN-CONTAINING RETINAL GANGLION CELLS...47 THE INFLUENCE OF INTRINSICALLY PHOTOSENSITIVE RETINAL GANGLION CELLS ON THE SPECTRAL SENSITIVIY AND RESPONSE DYNAMICS OF THE HUMAN PUPILLARY LIGHT REFLEX...73 Introduction...73 Intrinsically Photosensitive Retinal Ganglion Cells...74 The Role of iprgcs in the Mammalian Pupillary Light Reflex...75 Methods...77 Subjects...77 Apparatus...77 Stimulus Calibration...80 Experimental Procedure...80 Data Analysis...86 Curve Fitting...87 Results...93 Experiment Experiment Experiment Adaptation of photoresponses Combination of photoresponses Discussion iv
5 Light adaptation of the PLR and NIF visual system Relative photoreceptive contribution to the PLR and NIF visual system Relative contribution of rod, cone, and intrinsic responses to the dynamics of the PLR Concordance with previous studies of the spectral sensitivity ofthe human PLR SUMMARY REFERENCES APPENDIX: IACUC AND IRB APPROVALS v
6 LIST OF TABLES Tables Page 1 Relative Contribution of the Three Photoreceptive Mechanisms of Equation (8) to the Spectral Sensitivity Curve Fits in Experiment Relative Contribution of the Three Photoreceptive Mechanisms of Equation (8) to the Spectral Sensitivity Curve Fits in Experiment Relative Contribution of the Three Photoreceptive Mechanisms of Equation (8) to the Spectral Sensitivity Curve Fits in Experiment Curve Fitting Parameters Used to fit Equation (9) to the Photoreceptor Adaptation Data for Each Experimental Condition vi
7 LIST OF FIGURES Figures Page PUPILLARY CONTROL PATHWAYS 1 Anatomical Drawing Showing the Direct and Consensual Pupillary Light Reflex Pathways Low Power Photomicrograph of a Cross Section of the Macaque Iris Pupilloconstriction Elicited by a Ten Second Light Stimulus Luminance Neurons in the Pretectal Olivary Nucleus (PON) Drive the Pupillary Light Reflex Schematic of the Modified Dual Interaction Model Which Accounts for the Pupillary Near Response Component of the Near Response Triad Schematic Diagram of the Direct and Consensual Pupillary Light Pathways in Primates Including Humans...37 HUMAN AND MACAQUE PUPIL RESPONSES DRIVEN BY MELANOPSIN-CONTAINING RETINAL GANGLION CELLS 1 Pupillary and Ganglion Cell Responses Pupillary Responses During a Light Stimulus Under Normal Conditions and During Pharmacological Blockade Post-Stimulus, Sustained Pupillary Responses After Light OFF Under Normal Conditions and During Pharmacological Blockade Post-Stimulus, Sustained Pupillary Responses in Human After Light OFF...64 vii
8 THE INFLUENCE OF INTRINSICALLY PHOTOSENSITIVE RETINAL GANGLION CELLS ON THE SPECTRAL SENSITIVITY AND RESPONSE DYNAMICS OF THE HUMAN PUPILLARY LIGHT REFLEX 1 Experimental Apparatus Average Pupillary Behavior in Response to Three Different Monochromatic Light Stimuli Illustration of the Effect of Changing the Curve Fitting Parameters of Equation (4) on the Spectral Sensitivity of a Combination of Rod and Cone Signals Spectral Sensitivity of Half-Maximal Pupillary Constriction with a no Adapting Field Present Spectral Sensitivity of Half-Maximal Pupillary Constriction with a 50 td Adapting Field Present Spectral Sensitivity of Three Quarter-Maximal Pupillary Constriction with a 50 troland Adapting Field Present Relative Contribution of the Rod, Cone, and Intrinsic Photoresponse to the Spectral Sensitivity of the PLR Over Time Optimization of the Combination Parameters of Equation (8) for Inner and Outer Retinal Signals Comparison of the Steady-State Spectral Sensitivities of the Current Study With Previous Studies viii
9 LIST OF ABBREVIATIONS ANS CNQX DAG D-AP5 ERG EW IP3 iprgcs L-AP4 NIF NPC PAG PLC PLR PNR PON RAPD SCN SOA TEPRs autonomic nervous system 6-cyano-7-nitroquinoxaline-2,3-dione diacylglycerol D-2-amino-5-phosphonovaleric acid electroretinogram Edinger-Westphal nucleus inositol triphosphate intrinsically-photosensitive retinal ganglion cells L-2-amino-4-phosphobutyrate non-image forming nucleus of the posterior commissure periaqueductal grey phospholipase C pupillary light reflex pupillary near response pretectal olivary nucleus relative afferent pupillary defect suprachiasmatic nucleus supraoculomotor area task-evoked pupillary responses ix
10 1 INTRODUCTION At the turn of the twenty-first century, research investigating the pupillary light reflex (PLR) was limited. Only a few researchers continued to investigate the properties of this well known reflex, and most of these projects were not undertaken as the primary focus of the laboratories in which they were conducted. Studies of the PLR were not relegated to obscurity due to a complete understanding of the underlining mechanisms driving this response, but rather due to a long series of discrepancies in the literature and the drive towards reductionistic translational research. In 2000, the discovery of a novel photic pathway, which overturned over a century of vision science dogma, propelled the pupillary light reflex into the forefront of vision science research. This discovery not only provided the insight to reconcile many previous inconsistencies in PLR research, but also generated a renewed research interest which has significantly improved the state of knowledge regarding this important reflex. Historically, the greatest debates in the PLR literature revolved around the relative contribution of rod and cone photoreceptors to the response. Investigators used a number of different experimental paradigms to address this question, often with conflicting results. The first of the modern studies to address this topic used the Stiles-Crawford effect to suggest that both rod and cones were responsible for driving the PLR (Alpern & Benson, 1953). A subsequent study investigating the PLR in rod monochromats found that, even in subjects without cone photoreceptors, the pupil continued to react to photopic light stimuli well over that producing rod saturation in psychophysical investigations of vision (Alpern et al., 1960). This finding suggested the existence of a
11 2 strong rod mediated component to the PLR, even at intensities normally associated with cone vision. To further complicate matters, a study investigating the PLR of persons without any functional rod photoreceptors found that the PLR was also intact in these individuals (Ten Doesschate & Alpern, 1965), thus ending any suggestion of exclusive rod mediation of the response. Experiments investigating the spectral sensitivity of a visual response are generally seen as the most valid methods for determining the photoreceptive mechanisms responsible for the visual response in question. Therefore, several studies sought a definitive answer to the rod/cone debate by measuring the spectral sensitivity of the PLR at photopic light levels. Again, the results of these studies proved inadequate to resolve this question. Several studies measured spectral sensitivities that showed peak sensitivity at shorter wavelengths (Bouma, 1962, Laurens, 1923), again suggesting a rod or S-cone dominated response. Conversely, an additional study produced a spectral sensitivity which peaked at mid to long wavelengths, suggesting a much stronger influence from M and L-cones (Alpern & Campbell, 1962). Despite the theoretical strength of this approach, the results of the various investigators only added to a list of contradictory findings. Research investigating the PLR continued into the 1990s, but switched focus away from the photoreceptive inputs driving the reflex. During this time many studies focused on the response dynamics of the PLR (e.g. Kohn & Clynes, 1969; Sun et al., 1983; Young et al., 1993), which is made up of both a phasic and tonic component. Research on the PLR also expanded from investigations of retinal inputs, to investigations of the properties of the pretectum, the retino-recipient areas of the
12 3 midbrain responsible for the reflex (e.g. Clarke & Ikeda, 1985; Gamlin et al., 1995). Additionally, much research was focused on modulation of the PLR by spatial patterns and color (e.g. Barbur et al., 1992; Young & Kennish, 1993; Gamlin et al., 1998). Studies showed that these modulations of the PLR were cortical in origin, and likely the result of descending cortical inputs at the level of the Edinger-Westphal nucleus (Weber et al., 1981; Weiskrantz et al., 1999). Near the turn of the century, new advances in genetics allowed for the production of mice which lacked any functional rods and cones. Researchers investigating the PLR of these rodless/coneless (rd/rd cl) knockout mice reported an extraordinary finding. They found that these animals possessed a functional PLR in the absence of any rod or cone photoreceptors (Lucas et al., 2001; Lucas et al., 2003). About the same time, a series of studies demonstrated the existence of a novel photopigment, melanopsin, in the mammalian retina (Provencio et al., 1998; Provencio et al., 2000; Provencio et al., 2002). Subsequent studies investigating the response properties of the ganglion cells which expressed melanopsin showed that these neurons possessed intrinsic light-driven responses that were completely independent of rod and cone inputs (Berson et al., 2002; Warren et al., 2003). The discovery of the first new photoreceptor in the mammalian retina in over one hundred years, led to an explosion of research on this cell type. It was quickly determined that the two principal brain regions receiving inputs from these extraordinary cells were those areas responsible for driving the PLR (the pretectum) and entraining circadian rhythms to environmental day/night cycles (the suprachiasmatic nucleus) (Hattar et al., 2002; Dacey et al., 2005; Hattar et al., 2006). It was also determined that
13 4 based on environmental light levels, these cells regulate behavioral activity (Hattar et al., 2003; Mrosovsky & Hattar, 2003; Thompson et al., 2008) and neuroendocrine function (Brainard et al., 2001; Thapan et al., 2001). Given that the light-evoked neural signals driving these behaviors are quite unlike those which are responsible for conscious vision, the visual pathway responsible for these behaviors has been named the non-image forming (NIF) visual system. Due to its importance as a major output of the NIF visual system, the PLR has recently received renewed scientific interest. The research project presented in this document is a direct result of this renewed interest. The major goals of this project were: 1) to demonstrate the existence of a functional NIF pathway in human and non-human primates through investigations of the PLR, 2) to determine the relative influence of rods, cones, and melanopsin mediated photoresponse on the human PLR, and 3) to attempt to reconcile the inconsistencies of the human PLR literature based on these new findings regarding the NIF visual system. The products of this research effort are presented in the following chapters, the overall layout of which is as follows. Chapter 2 is composed of a book chapter that was written by myself and Dr. Paul Gamlin, entitled Pupillary Control Pathways. This chapter provides good background into the clinical and optical importance of the PLR, a detailed description of the neural circuitry involved in the PLR, as well as background on the non-photic influences on pupillary behavior, which must be adequately controlled in any study of the PLR. Chapter 3 consists of a study published in 2007 in the journal Vision Research. The findings of this study indicate a functional NIF visual pathway driving the PLR in
14 5 primates. Additionally, this study specifically addresses a well known phenomenon of human pupillary behavior, a sustained pupilloconstriction following light offset, which had previously proved very difficult to explain from the known dynamics of rod and cone photoreceptor signaling. Our findings showed conclusively that this pupillary behavior is driven by the melanopsin photoresponse. Thus, these results were able to rectify previously inconsistent findings regarding this behavior. Chapter 4 consists of the final phase of the current research project, which will be submitted for publication in the near future. Given that this research has yet to be published, certain latitude exists in how this material can be presented in the current manuscript. I have chosen to present this information is a very broad and detailed manner, quite unlike the format of current research articles. I felt this would allow for a more comprehensive review and evaluation of my current research project, as it makes up the bulk of the research conducted to meet the requirements for completion of my PhD. The research described in this chapter demonstrates how rod, cone, and melanopsin mediated photoresponses integrate to drive the PLR in humans. These finding provide valuable insights into the mechanism by which these responses come together to mediate not only the PLR, but also to influence the NIF visual system as a whole. This chapter also contains a detailed discussion of the historical findings related to the spectral sensitivity and response dynamics of the PLR, and attempts to reconcile these finding and with our improved understanding of the light signals responsible for these aspects of the PLR. Chapter 5 provides a summary of the findings of the research project as a whole. In this chapter, I also address how these findings have contributed to the advancement of
15 6 knowledge of the PLR and the NIF visual system. This section also contains a discussion of future research projects which have grown out of the current project, both those that are currently being conducted and those still in the proposal stage.
16 7 PUPILLARY CONTROL PATHWAYS by DAVID H. MCDOUGAL AND PAUL D. R. GAMLIN The Senses: A Comprehensive Reference. New York, Academic Press: Copyright 2008 by Elsevier B.V. Used by permission Format adapted and errata corrected for dissertation
17 8 Advantages of a Mobile Pupil The normal human pupil can change diameter from 8 mm to 1.5 mm, which corresponds to approximately a 28 fold change in area. Thus the movement of the iris can account for almost 1.5 log unit variation in retinal irradiance. Although the visual system can operate over a 10 log unit range of lighting levels through the process of adaptation, it can take several minutes for optimum sensitivity to return after an abrupt increase or decrease in retinal illumination. The rapid control of retinal irradiance by the iris allows the visual system to more quickly regain optimal sensitivity by dampening fast changes in ambient lighting levels and requiring less retinal adaptation to a given change in environmental lighting levels (Woodhouse and Campbell, 1975; Hood and Finkelstein, 1986). However, changes in pupil size affect not only retinal illumination, but also diffraction, optical aberrations, and depth of focus of the eye. These factors differentially affect visual performance and, given changing environmental lighting conditions and visual tasks, the nervous system continuously modulates pupil diameter for optimal visual performance. The diffraction of light rays by an aperture is a major limiting factor in the resolution of an image in any optical system. The amount of disruption in image quality caused by diffraction at a circular aperture decreases as the size of the opening increases. Therefore, as pupil diameter increases, there is decreased degradation in retinal image quality caused by diffraction (Campbell and Green, 1965; Fry, 1970; Charman, 1995). In contrast to diffraction, the image degrading effects of optical aberrations increase as aperture diameter increases. Therefore, as pupil diameter increases, the degradative effects of optical aberrations also increase, and offset the benefits gained by reduced
18 9 diffraction at larger pupil diameters (Liang and Williams, 1997; Schwiegerling, 2000). Over the normal range of pupillary diameter, diffraction impacts image quality less than optical aberration, and the optimal pupil diameter is therefore approximately between 2 mm and 4 mm (Jenkins, 1963; Westheimer, 1964; Campbell and Gubisch, 1966). Along with diffraction and optical aberrations, defocus is an important determinant of retinal image quality. Although the iris does not refract or focus light, it influences the depth of field of the eye. Depth of field is the range of distance in depth in which objects appear to be in focus. For example, when one reads a book, the power of the crystalline lens of the eyes changes in order to bring the text on the page into focus through a process called accommodation. With the eyes accommodated on the book, all objects within a range in front of and behind the book will also appear in focus. This range is called the depth of field and it is primarily dependent both on viewing distance and pupil diameter. When viewing distance is held constant, depth of field increases with decreases in pupil diameter, and therefore pupil diameter can affect the focus of the retinal image (Marcos et al., 1999; Wang and Ciuffreda, 2006). Clearly, a mobile pupil allows the nervous system to optimize retinal irradiance, diffraction, ocular aberrations, and depth of focus despite differing conditions and visual tasks. For example, across a range of daylight (photopic) luminances, pupil size corresponds to that required for the highest visual acuity (Campbell and Gregory, 1960), and the maximal information capacity of the retinal image (Laughlin, 1992; Hirata et al., 2003). On the other hand, under low light (scotopic) conditions in which poorer retinal image quality can be tolerated due to the lower resolution of rod photoreceptors, the pupil dilates sufficiently to maximize retinal illumination. Further evidence for the
19 10 optimization of pupil diameter for differing visual tasks is evident in the pupillary near response. When viewing distance changes from far to near, the pupils constrict to increase the field of view and reduce retinal image defocus. This compensates for the decrease in effective field of view that naturally occurs when viewing distance decreases (see pupillary near response section for more detail). Overview of the Pathways Controlling Pupil Diameter A summary diagram of the afferent, central, and efferent pathways controlling pupil diameter are shown in figure 1. This figure shows the iris musculature innervated by autonomic efferents from both the parasympathetic and sympathetic components of the autonomic nervous system. A detailed description of the autonomic nervous system is beyond the scope of this chapter, but is provided by Hockman (1987) and Robertson (2004). The parasympathetic component of the autonomic nervous system innervates the sphincter pupillae muscle of the iris. The preganglionic parasympathetic fibers controlling the sphincter pupillae originate from neurons in the Edinger-Westphal nucleus, the autonomic subdivision of the third cranial nerve nucleus, and travel via the third cranial nerve to the ciliary ganglion which is located within the orbit of the eye (see figure 1). Within the ciliary ganglion, the preganglionic pupilloconstriction neurons form cholinergic, nicotinic synapses with the postganglionic neurons. The axons of these postganglionic neurons leave the ciliary ganglion to enter the eye via the short ciliary nerves and travel to the iris. Here they
20 11 Figure 1. Anatomical drawing showing the direct and consensual pupillary light reflex pathways and the parasympathetic and sympathetic innervation of the iris in primates. The bilateral projection from the retina to the pretectum is also shown. The pretectal olivary nucleus (PON) receives input from the temporal retina of the ipsilateral eye and the nasal retina of the contralateral eye. The PON projects bilaterally to the Edinger Westphal nucleus, which contains preganglionic parasympathetic pupilloconstriction neurons. The axons of these preganglionic neurons travel in the third cranial nerve to synapse upon postganglionic pupilloconstriction neurons in the ciliary ganglion. The axons of these postganglionic neurons leave the ciliary ganglion and enter the eye via the short ciliary nerves, and then travel through the choroid to innervate the sphincter muscle of the iris. The preganglionic sympathetic pupillodilation neurons are found at the C8-T1
21 12 segmental levels of the spinal cord. The axons of these neurons project from the spinal cord via the dorsal roots and enter the sympathetic trunk, and then project rostrally to the superior cervical ganglion where they synapse with the postganglionic neurons. These postganglionic neurons project from the superior cervical ganglion through the neck and carotid plexus and into the orbit of the eye. These fibers enter the eye either by passing through the ciliary ganglion and entering in the short ciliary nerves or by bypassing the ciliary ganglion and entering via the long ciliary nerves (for clarity, only one of these alternative pathways is shown). Upon entering the eye, these axons travel through the choroid and innervate the dilator muscle of the iris. release acetylcholine which acts on the muscarinic receptors of the sphincter pupillae (see figure 2) (Hockman, 1987; Loewenfeld and Lowenstein, 1993; Oyster, 1999). The sympathetic component of the autonomic nervous system innervates the dilator pupillae muscle. The preganglionic sympathetic neurons which control pupillary dilation are located in the C8-T1 segments of the spinal cord, a region termed the ciliospinal center of Budge (and Waller). The axons of these preganglionic neurons project to the sympathetic chain and travel in the sympathetic trunk to the superior cervical ganglion (Hockman, 1987; Kardon, 2005). Within the superior cervical ganglion the preganglionic axons form nicotinic, cholinergic synapses with postganglionic pupillodilation neurons. The axons of these postganglionic neurons project from the superior cervical ganglion to the orbit, where they enter the eye via the short and long ciliary nerves and travel to the iris (see figure 1). Here they release norepinephrine which acts on the adrenoreceptors of the dilator muscle (see figure 2).
22 13 Iris Musculature In a cross section of the iris, the sphincter pupillae can be seen as an annular band of smooth muscle ( µm thick; mm wide) encircling the pupil (figure 2). The sphincter, which is located in the posterior iris immediately anterior to the pigmented epithelium, interdigitates with the surrounding stroma and connects Figure 2. Low power photomicrograph of a cross section of the macaque iris. Scale bar = 200 μm. to dilator muscle fibers (see below). The smooth muscle cells of the sphincter are clustered in small bundles and connected by gap junctions (Bron et al., 1997). These gap junctions ensure synchronized contraction of the sphincter muscle. The sphincter receives muscarinic, cholinergic innervation from the short ciliary nerves: parasympathetic, postganglionic fibers arising from the ciliary ganglion. The m3 subtype of muscarinic receptor is the predominant receptor subtype expressed by smooth muscle cells of the sphincter pupillae. In the human iris, the m3 receptor subtype comprises 60-75% of the total number of expressed muscarinic receptors, while other muscarinic
23 14 receptor subtypes (m1, m2, m4, m5), are expressed at lower levels (5-10%) (Gil et al., 1997). Binding of acetylcholine to m3 receptors initiates a series of events leading to the activation of phospholipase C (PLC) via G-proteins of the Gq family. Activated PLC generates inositol triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol biphosphate. The increase in IP3 elicits the release of Ca2+ ions from the endoplasmic reticulum and the influx of extracellular Ca2+ ions. The resultant increase in intracellular free Ca2+ concentration produces muscle contraction (for review see Eglen et al., 1996). Muscarinic receptor antagonists such as atropine, scopolamine, or tropicamide produce mydriasis, while agonists such as pilocarpine, bethanechol, metoclopramide, or oxotremorine produce miosis. The reversible cholinesterase inhibitor, physostigmine, also produces a marked miosis (for review see Thompson, 1992). The dilator pupillae is composed of radially oriented smooth muscle fibers that are myoepithelial in origin. Individual fibers are approximately 50 µm long and 5-7 µm wide. In the pupillary zone, dilator muscle processes fuse with the sphincter pupillae, while peripherally, their processes attach to the ciliary body. Contraction of the dilator muscle pulls the pupillary margin towards the ciliary body (Bron et al., 1997). The dilator receives adrenergic innervation from the long ciliary nerves: sympathetic, postganglionic fibers arising from the superior cervical ganglion. The alpha 1a adrenoreceptor appears to be the predominant receptor subtype expressed by the smooth muscle cells of the dilator pupillae (Nakamura et al., 1999). Binding of norepinephrine to the alpha 1a adrenoreceptor, a G protein-coupled receptor, produces muscle contraction through the same signaling cascade (PLC/ IP3) as that in the sphincter pupillae muscle.
24 15 Alpha-adrenoreceptor antagonists such as dapiprazole or thymoxamine produce miosis (Thompson, 1992), as does the more selective alpha 1a adrenoreceptor antagonist, tamsulosin (Parssinen et al., 2006). The non-specific adrenoreceptor agonist, phenylephrine, produces mydriasis. Mydriasis is also produced by hydroxyamphetamine and related drugs, which stimulate norepinephrine release from the postganglionic sympathetic nerve endings (Thompson, 1992). Pupillary Light Reflex Description The pupillary light reflex (PLR) is the constriction of the pupil that is elicited by an increase in illumination of the retina. The direct PLR, present in virtually all vertebrates, is the constriction of the pupil in the same eye as that stimulated with light. The consensual pupillary light reflex is the constriction of the pupil in the eye opposite to the eye stimulated with light. In mammals with laterally placed eyes, such as the rat and rabbit, the direct pupillary light reflex is more pronounced than the consensual PLR. However, in those mammalian species with frontally placed eyes such as humans and monkeys, the direct and consensual pupillary light reflex are essentially equal (Loewenfeld and Lowenstein, 1993). An example of a human consensual pupillary light reflex produced by two different wavelengths of light is shown in figure 3.
25 16 Figure 3. Pupilloconstriction elicited by a 10-s light stimulus of 493nm wavelength light at 14.0 log quantacm -2 s -1 irradiance (blue trace), and 613nm wavelength light at 14.1 log quantacm -2 s -1 irradiance (red trace). Note that a 473-nm stimulus, which effectively activates the intrinsic photoresponse of intrinsically photosensitive retinal ganglion cells (iprgcs), drives a larger pupillary response than the 613-nm stimulus (red trace), which does not effectively activate the intrinsic photoresponse of iprgcs at this irradiance level. Also note that the pupilloconstriction induced by the 473-nm light is maintained following stimulus offset. The pupillary light reflex has traditionally been divided into two separate pathways based on the clinical manifestations of the defects in this reflex. The afferent pathway is composed of both the retinal cells that project to the pretectum as well as their recipient neurons, which project bilaterally to the Edinger-Westphal nucleus (EW) (Figure 1). The efferent pathway is composed of the preganglionic pupilloconstriction
26 17 fibers of the EW and their postganglionic recipient neurons in the ciliary ganglion, which project to the sphincter muscle of the iris (Figure 1). Afferent Pathway The first neurons in the afferent pathway of the pupillary light reflex are retinal ganglion cells. It has recently been recognized that this reflex in rodents and primates is driven predominantly by a unique subset of intrinsically-photosensitive retinal ganglion cells (iprgcs) which project to the pretectal olivary nucleus, a small nucleus in the pretectum; the pretectum is located in the dorsal lateral aspect of the midbrain at the level of the superior colliculus (Gamlin, 2005) (see Figure 1). Early anatomical studies did not concentrate on the pretectal olivary nucleus specifically, but instead examined all retinal projections to the pretectum, which contains five retinorecipient nuclei. Several of these early studies utilized tracers injected into the vitreous of the eye to anterogradely label all retinal ganglion cell projections to the pretectum. These studies found that the retinal projections to the pretectum were densest to the nucleus of the optic tract and pretectal olivary nucleus and, in primates, were bilateral, with only a slightly denser contralateral component. In contrast, the retinal projection to the pretectum of rodents is predominantly contralateral, and only exhibits a moderate ipsilateral component in cats. The ratio of crossed to uncrossed projections of the retinopretectal projections appears to correlate with the ratio of the direct versus consensual pupillary light reflex in mammals. Other early anatomical studies used retrograde tracing to label pretectallyprojecting retinal ganglion cells by injecting the tracers into the pretectum. These studies are hard to interpret because fibers projecting to the superior colliculus were also often
27 18 labeled. Retrograde labeling studies across several different species have found that pretectally projecting cells represented only a small percentage of the total population of ganglion cells (1 6%), and that these cells generally possess small or medium-sized cell bodies and can be classified morphologically as being gamma or W-like. Very few studies were able to successfully target injections exclusively to the PON and therefore it is unclear to what extent the retinal cells labeled in these studies actually participate in the PLR. However, an injection centered on the PON in macaques gave rise to mediumsized labeled neurons, with a few coarse dendrites and extensive dendritic arbors (Perry and Cowey, 1984). The morphology of these labeled cells is consistent with a newly described retinal cell type which has recently been shown to contribute significantly to the PLR. Intrinsically photosensitive retinal ganglion cells (iprgcs). Prior to 2000, it was assumed that the pupillary light reflex was driven by retinal ganglion cells which received light signals exclusively from rod and cone photoreceptors, which up to that time were the only known photoreceptive cells in the retina. Recent findings suggest that the pupillary light reflex is driven by iprgcs which, unlike any other retinal ganglion cell class, are intrinsically photosensitive. The intrinsic photoresponse of iprgcs is mediated by the photopigment melanopsin, and has been shown to be well fit by a single pigment absorbance spectrum center at 482 nm. In addition to their intrinsic signal, it is clear that iprgcs receive rod and cone inputs. In response to a pulse of light, intracellular recordings from this cell type show a characteristic transient burst of firing at stimulus onset, which rapidly decays to a plateau of sustained firing that often extends
28 19 well past stimulus offset. The initial burst of firing is mediated by a rapidly adapting cone mediated photoresponse and the sustained firing which follows is driven by the intrinsic response of these cells (Berson, 2003; Fu et al., 2005). iprgcs project to the pretectum of rodents and primates, and it is clear that the confluence of photoreceptive signals impinging on this cell type has a significant impact on pupillary behavior. Several studies have examined the contribution of the separate photoresponses of iprgcs to the pupillary light reflex directly. These studies have demonstrated that the intrinsic photoresponse of iprgcs is necessary but not sufficient to produce a normal pupillary light reflex in both rodents and primates. Studies investigating the pupillary light reflex of melanopsin deficient knockout mice (Opn4-/-) have determined that these animals display a pupillary light reflex, but the pupil fails to constrict maximally in bright lights. Rodents lacking both rod and cone photoreceptors due to retinal degeneration or transgenic manipulation also still display a pupillary light reflex; however, the reflex has a higher irradiance threshold than normal. When rodless/coneless mice (rd/rd cl) are crossed with Opn4-/- mice to eliminate all potential photoreceptive inputs to iprgcs, the pupillary light reflex is completely absent (Berson, 2003; Fu et al., 2005). Recent studies in primates have also shown that the primate pupillary light reflex is present in absence of rod and cone input; however, the reflex has a higher retinal irradiance threshold than normal (Gamlin et al., 2006). Taken together, these results show that both the intrinsic photoresponse of iprgcs and classical photoreceptor inputs provide signals of retinal irradiance that drive the pupillary light reflex. There is evidence that the intrinsic photoresponse compensates for the rapid adaptation of cones,
29 20 and maintains pupilloconstriction during steady state exposure at all photopic (daylight) illuminance levels. It is still unclear whether the influence of traditional photoreceptors on the pupillary light reflex is mediated exclusively by the rod and cone inputs to iprgcs or by other classes of retinal ganglion cells that may to project to the PON. The intrinsic response of iprgcs also includes an accumulative irradiance history signal, which can affect pupillary behavior in the absence of overt light stimulation. As noted above, recordings from iprgcs have shown that these cells possess an ability to encode stimulus irradiance through an elevation of firing rate that extends beyond stimulus offset (Berson, 2003; Fu et al., 2005). This sustained firing after stimulus offset appears to be a mechanism for encoding stimulus intensity, as the magnitude and duration of this sustained response varies linearly with stimulus intensity (Dacey et al., 2005). This cellular response also appears to influence pupillary responses. Studies of the human pupillary light reflex have shown that bright light stimuli can produce a prolonged pupillary constriction that persists for up to 20 minutes, even when the subject is kept in complete darkness following stimulus presentation (Loewenfeld and Lowenstein, 1993) (see figure 3). It has been determined that this phenomenon is mediated primarily by the intrinsic photoresponse of iprgcs in primates including humans (Gamlin et al., 2006). Pretectal olivary nucleus. The second neurons in the afferent pathway of the pupillary light reflex are luminance neurons within the pretectal olivary nucleus (PON). PON luminance neurons are characterized by tonic firing rates that increase with increases in retinal illuminance. In primates, these neurons exhibit a transient burst of activity followed by sustained tonic activity in response to increases in retinal illuminance (Figure
30 21 4). In addition, the tonic firing rate of these cells is proportional to retinal illuminance over at least a three log unit range of stimulus intensities in primates (Gamlin et al., 1995) and in rats (Clarke and Ikeda, 1985b). Although it is clear that these luminance neurons receive inputs from iprgcs and that the response characteristic of PON luminance neurons to increases in retinal illuminance is reminiscent of that of iprgcs, it is possible these neurons may receive input from other types of retinal ganglion cells. In addition to these retinal afferents, the PON also receives significant cortical, ventral thalamic, and midbrain inputs which may also have a direct influence on the pupillary light reflex or other pupillary movements. Owing to its importance in the PLR, the best described efferent projection of the PON is to the EW. However, the PON has also been shown to have a variety of efferent connections that may influence pupillary behavior such as the hypothalamus, pons, and medulla (Gamlin, 2005). Electrical microstimulation of the Figure 4. Luminance neurons in the pretectal olivary nucleus (PON) drive the pupillary light reflex. (a) The response of a single neuron is the PON in response to a 100-troland light stimulus. The pupillary response to the same light stimulus is shown in the trace above. (b) Electrical microstimulation at the level of the PON produces pupillary constriction, even in the absence of a light stimulus.
31 22 PON in rats and monkeys elicits pupilloconstriction at short latencies (see figure 4b), and lesions of the PON in rats produce deficits in pupillomotor function (Gamlin, 2005). These results provide strong support for models in which luminance neurons within the PON mediate the pupillary light reflex. Recently, the characteristics of PON neurons have been more closely examined in the alert primate. This study found that there were three classes of luminance cells in the PON that could be distinguished by their receptive field extent and location. Approximately 40% of the primate PON luminance neurons responded well to stimuli, whether they were presented in either the contralateral or ipsilateral visual field. These neurons were classified as bilateral neurons. Approximately 30% of PON neurons responded only to stimuli presented in the contralateral visual field. These neurons were classified as contralateral neurons. Finally, 30% of PON neurons responded primarily to stimuli presented at or near the animal s fixation point. These neurons were classified as macular neurons. The mean firing rates of all classes of neurons increased with increases in stimulus size and luminance within their receptive fields. PON luminance neurons of the bilateral and contralateral classes possess very large receptive fields, which exceeded the sizes reported in cat and rat. In addition, while 84% of PON neurons are binocular in the primate, only 22% are reported to be binocular in cats. Thus, it is likely that PON neurons with such extensive, binocular receptive fields are unique to primates, and the existence of such neurons may also explain why the direct and consensual pupillary responses are of comparable magnitudes in primates (Gamlin, 2005).
32 23 Although it has been firmly established that both the PON and the EW (see next section for details) play a critical role in the PLR, there is disagreement as to the route of connectivity between these two nuclei. Some studies propose both a direct connection between PON and EW and an indirect connection via the nucleus of the posterior commissure (NPC) in the rat and in primate, while other studies have reported only the indirect connection via NPC in the cat and tree shrew. A number of studies in primates, using several different tracing techniques, have reported only the direct connection between the PON and the EW (Gamlin, 2005). Further study will be required to definitively resolve this issue. Efferent Pathway The efferent leg of the pupillary light reflex begins with preganglionic pupilloconstriction neurons of the Edinger Westphal nucleus (EW) that travel via the third cranial nerve to the ciliary ganglion (see figure 1). Within the ciliary ganglion, the preganglionic pupilloconstriction neurons synapse with the postganglionic pupilloconstriction neurons, and the axons of these postganglionic neurons leave the ciliary ganglion to enter the eye via the short ciliary nerves and travel to the iris. The Edinger-Westphal nucleus. EW is a distinct nucleus of the midbrain, lying immediately dorsal to the oculomotor complex. It is located just slightly ventral and lateral to the cerebral aqueduct at the level of the superior colliculus (see figure 1). This nucleus was first described in a developmental study of human neuroanatomical material by Edinger (1885) and, a short time afterward, in a neuropathological study by Westphal
33 24 (1887). Other early studies involving EW showed that pupilloconstriction could be evoked through the electrical stimulation of EW (Ranson and Magoun, 1933), and determined the precise location of the preganglionic neurons within EW (Warwick, 1954). Since these pioneering studies, additional studies have clearly shown in many vertebrate classes that the EW contains the preganglionic neurons that synapse with the postganglionic fibers which innervate the iris sphincter. Further support for the course of the efferent parasympathetic pupillary pathway and the importance of the EW in pupilloconstriction come from electrical stimulation studies in the vicinity of EW that elicited pupilloconstriction in a variety of animal models (Gamlin, 2000). The neurophysiology of EW pupilloconstriction neurons has not been extensively studied. This is due to the small size of EW and to the small number of pupilloconstriction neurons present, reported to be as few as 10% of the total number of cells in EW (Gamlin, 2000). A study in cats found that pupilloconstrictor EW neurons displayed firing rates of approximately 8 spikes/second during maximal pupilloconstriction, but these neurones also displayed transient firing rates with light on of up to 28 spikes/second. A similar finding has been reported in primates, with a pupilloconstrictor neuron displaying a firing rate that ranged from 10 spikes/second to 25 spikes/second with a burst-tonic response pattern (Gamlin, 2000). This firing pattern is similar to both iprgcs and PON luminance neurons, and it has been postulated that the initial burst at stimulus onset in the pupilloconstriction cells of EW serves to overcome the sluggish nature of the iris musculature.
34 25 The ciliary ganglion. The ciliary ganglion is approximately 3 mm in size, and located 2-3 mm posterior to the globe and lateral to the optic nerve. This ganglion contains the cell bodies of the postganglionic pupilloconstrictor neurons which innervate the sphincter muscle of the iris. Although these neurons receive input primarily from the preganglionic pupilloconstrictor neurons of EW, there is evidence for addition neuronal inputs which may act to modulate this signal (May and Warren, 1993). Therefore, the ciliary ganglion should not be considered only as a simple relay of preganglionic inputs from EW to the iris, but also as a site of potential neural integration (Gamlin, 2000). Sympathetic Influences on the PLR Although it is generally agreed that the parasympathetic pathway discussed above is the primary route of pupillary constriction associated with the pupillary light reflex (Thompson, 1992; Loewenfeld and Lowenstein, 1993; Kardon, 1995), there is evidence that light may also cause a reduction in the tone of the dilator muscle of the iris via the sympathetic pathway outlined in figure 1, and thus enhance the pupillary light reflex. Studies in cats have shown a light induced inhibition of postganglionic pupillodilation fibers at the level of the long ciliary nerves (Nisida et al., 1960), and preganglionic pupillodilation fibers at the level of the cervical sympathetic nerve (Passatore and Pettorossi, 1976). These studies found that the pupillodilation fibers were inhibited by light in an intensity dependent manner; i.e., a more intense light brought about a greater inhibition in firing rate. These findings have not been replicated in primates, in which there is evidence that the sympathetic system plays a tonic role and does not contribute to the dynamics of the PLR (Clarke et al., 2003).
35 26 Other studies were undertaken to determine the route of the inhibitory light signal to the pupillodilation centers of the spinal cord. By determining the effects of selective lesions at various levels of the CNS on the light induced inhibition of pupillodilation in the long ciliary nerves of cats, Okada and colleagues (1960) found that this light signal originated at the level of the pretectum, presumably from one of the retinorecipient nuclei contained within the pretectum. Further lesions in more caudal portions of the CNS suggested that the inhibitory light signal descended from the pretectum bilaterally to the pupillodilation centers of the spinal cord. A more recent series of anterograde labeling studies in rat support these findings by demonstrating an anatomical connection between the pretectal olivary nucleus and the pupillodilation center of the spinal cord (Klooster and Vrensen, 1998). It is not known if the light induced inhibitory signal is carried by pretectal neurons responding to an increase in light intensity, similar to the cells driving the parasympathetic pathway, although this would require an intervening sign inverting synapse somewhere in the descending pathway. It is possible that the inhibition is a direct result of cells which decrease their firing in response to light. The existence of so called darkness detector cells have been reported in the pretectum of rats. These cells show a reduction in firing rate in response to increases in retinal luminance (Clarke and Ikeda, 1985a). Taken together, these findings suggest that the pupillary light reflex is potentially augmented by a light induced reduction in tone of the dilator muscle that occurs in conjunction with the increased tone of the sphincter muscle brought about by the parasympathetic pathway.
36 27 The Pupillary Near Response Description The pupillary near response (PNR) is a pupillary constriction associated with a change in viewing distance from far to near that occurs in primates including humans. When the eyes move from viewing an object at distance to viewing one at near (less than 20 ft away), three oculomotor responses occur. The eyes converge to bring the image of the object onto similar regions of each retina, the refractive power of the crystalline lens is adjusted to bring the image of the object into focus on the retina, and the iris constricts thus reducing the diameter of the pupil. These collective processes are classically referred to as the near response or the near triad. Efferent Pathway of the Pupillary Near Response The PNR is thought to be driven solely by an increased drive to the sphincter muscle of the iris via the parasympathetic efferent pathway (Kasthurirangan and Glasser, 2005). Therefore, the neural control pathway of the pupillary near response shares a common efferent pathway with the pupillary light reflex, although the afferent inputs responsible for the pupillary near response are more complex. These two reflexes appear to converge at the Edinger-Westphal nucleus, since the activity of PON luminance neurons is not correlated with the pupil constriction that occurs during near viewing (Zhang et al., 1996). Further, certain clinical neurological conditions are characterized by an intact pupillary near response with the absence of the pupillary light reflex (light-near dissociation) (Lowenstein, 1956). It is generally accepted that preganglionic neurons in EW drive the PNR as well as the PLR. However, it has not been determined if separate
37 28 subpopulations of neurons exist in EW devoted exclusively to either the pupillary light reflex or the pupillary near response, or whether the same population of neurons drives pupillary constriction in both reflexes, although the latter seems most likely. Afferent Influences on the Pupillary Near Response Modern investigations into the afferent influences driving the PNR began in the late 1940s with the advent of infrared photographic recordings of the pupil during near viewing. This technique allowed researchers to measure the magnitude of the PNR in near darkness, thus eliminating any artifactual change in pupil diameter induced by decreased retinal illuminance originating from the constriction of the pupil during the response. The aim of these original investigations was to determine whether the PNR was driven primarily by ocular convergence or accommodation, the other two components of the near triad. Early studies found that the PNR was more closely associated with accommodation than with convergence (Fry, 1945; Knoll, 1949; Marg and Morgan, 1949; Marg and Morgan, 1950; Alpern et al., 1961). Later studies found a greater association with convergence (Backer and Ogle, 1964; Jones, 1989), and even showed that the PNR could be totally absence during certain blur driven accommodative responses (Stakenburg, 1991; Phillips et al., 1992). These conflicting results are likely a product of an incomplete disassociation between the convergence and accommodation systems during these experiments, as these two systems have been shown to be highly interdependent. A more modern view of the afferent influences of the PNR has recently emerged, in which the PNR is not seen as resulting from either accommodation or convergence
38 29 alone, but as a separate output of the neural pathways which drive both accommodation and convergence. This view has followed an increase in the knowledge of the neural circuitry involved in all three oculomotor processes of the near triad. Experiments investigating the interaction between convergence and accommodation lead to the introduction of the dual interaction model of convergence and accommodation (Semmlow and Heerema, 1979; Hung and Semmlow, 1980; Schor and Narayan, 1982) (Figure 5). This model proposes that two neural controllers operate in the near triad, one which integrates stimuli for accommodation such as blur, and one which integrates stimuli driving vergence, such as disparity between the images on the retinal of each eye (Mays and Gamlin, 1995; Busettini et al., 2007). It is now clear that the PNR is not driven exclusively by either the accommodation controller or the convergence controller, but actually by an interaction of the two controllers (Myers and Stark, 1990; Takagi et al., 1993). These finding support the view that the outputs of the dual-interaction model drive the PNR in addition to accommodation and vergence (see figure 5). These findings also suggest that the three oculomotor components of the near triad share common afferent influences. A number of brain areas play a role in controlling the near triad. These include cortical areas such as extrastriate cortex, parietal cortex, frontal eye fields, as well as the cerebellum and the midbrain. Of particular interest to the pupillary near response is the supraoculomotor area of the midbrain, which lies just dorsal and lateral to the oculomotor nucleus. The supraoculomotor area contains near response cells which are modulated by both vergence and accommodation, and which receive input from both the accommodation and vergence controllers. These cells project to medial rectus
39 30 Figure 5. Schematic of the modified dual-interaction model that accounts for the pupillary near response component of the near response triad. This model indicates that the combined output of the accommodation controller and the convergence controller drives the pupillary near response. motorneurons, and thus contribute to vergence eye movements. It seems likely that these cells also project to EW and are responsible for carrying the signal from the accommodation and convergence controller to the preganglionic, pupilloconstriction neurons (Mays and Gamlin, 1995; Gamlin, 2002; Busettini et al., 2007). Additional Cortical Influences on Pupillary Responses In addition to cortical afferents mediating the pupillary near response, the pupil is also influenced by both visual and non-visual cortical regions. These afferents manifest themselves in small changes in pupil diameter measured during presentation of visual
40 31 stimuli such as colored stimuli and gratings, as well as non-visual stimuli such auditory tones, and even during higher order cortical functions such as problem solving. These observations provide clear evidence that cortex exerts an influence on pupillary behavior, which cannot be thought of as entirely reflexive in nature. Visually Mediated Cortical Influences on Pupillary Behavior Recent studies have shown that the pupil responds to complex aspects of visual stimuli such as color, motion, and texture. Slight pupillary constrictions have been shown to occur in both human and macaque with the presentation of complex visual stimuli, even when the stimuli do not involve a change in viewing distance or retinal illuminance (Barbur, 1995; Gamlin et al., 1998). The three best described stimulus attributes which produce this effect are color, spatial frequency, and apparent motion. It is clear from physiological and functional imaging studies that these stimulus characteristics are encoded in areas of visual cortex. Furthermore, when these cortically driven pupillary responses are assayed in human subjects with well documented lesions to cortical areas involved in processing one or more of these stimulus characteristics, a deficit in the concurrent pupillary response is always observed (Barbur, 1995). In addition, Heywood and colleagues (1998) have demonstrated in macaques that lesions of rostral inferior temporal cortex but not V4 abolish pupillary responses to chromatically modulated gratings. These findings offer conclusive evidence of an influence of visual cortex on pupil diameter. Studies have investigated the neural pathways by which visual cortex influences pupil diameter. Research utilizing patients with a well defined neurological lesion of the
41 32 midbrain have helped to elucidate one possible neural pathways involved in these responses. The neurological syndrome to which the patients were affected selectively affects the pretectum while sparing the Edinger-Westphal nucleus. Therefore the patient's pupils were un-reactive to light but maintained a near response. These patients also maintained the pupillary responses to stimulus color and function and therefore it seem likely that this cortical influence in mediated by direct cortical inputs to EW (Wilhelm et al., 2002). Task-Evoked Pupillary Responses In the early 1960s, Hess and colleagues (1960; 1964; 1965) published a series of papers which reported modulations in human pupillary diameter associated with complex cognitive processes such as subjective attitudes or mental activity. Later studies failed to produce reliable replication of the findings relating pupillary dynamics to subjective attitudes, although the findings related to mental activity have been replicated and extensively studied. The small pupillary dilations associated with increased mental activity, or task-evoked pupillary responses (TEPRs), have now become a well established tool of cognitive psychology. These pupillary responses are generally reported to vary in magnitude from.2 mm to.7 mm, and have been shown to be an accurate reporter of cognitive load across such diverse functions as sensory perception, memory, language, and attention. TEPRs have been repeatedly shown to monotonically vary with the degree of mental activity required by a task as measured by other objective criterion such as reaction time and the extent of cortical activation indicated by PET scan,
42 33 and this has allowed TEPRs to be utilized successfully to empirically test theories of language processing and intelligence (Beatty and Lucero-Wagoner, 2000). Although the behavioral phenomenon of TEPRs has been extensively studied and quantified, much remains to be elucidated about the precise physiology that drives these responses. Very few, if any empirically driven theories have been developed to explain the neural pathways involved in these responses. It has been suggested that these pupillary responses may be driven by a neuromodulatory effect on pupillary control pathways mediated by noradrenergic projections from the locus coeruleus. The firing rate of neurons in this midbrain nucleus has been show to correlate with both pupil diameter and task related events (Aston-Jones and Cohen, 2005) Influence of Alertness on Pupillary Behavior Since the muscles of the iris are controlled by the autonomic nervous system (ANS), environmental or physiological conditions which cause changes in overall autonomic function can have a significant effect on pupillary behavior. Even though the environment or physiological conditions which produce the change in autonomic tone may not have a direct influence on the visual system, they may still manifest themselves by affecting pupil diameter. Arousal Situations or stimuli which produce an emotional or startle response often produce a profound pupillary dilation. This effect is mediated via the hypothalamus, the brain area responsible for the integration of autonomic function. This integration allows
43 34 for the coordination of the various functions of the autonomic nervous system and often leads to global changes in the balance between the sympathetic and parasympathetic branches of the autonomic nervous system. For example, an unexpected loud noise may produce a startle response which is characterized by increases in heart rate, respiratory rate, and pupil diameter; it is caused by a systemic increase in sympathetic tone mediated thorough the hypothalamus. This global increase in sympathetic tone can affect pupil diameter via activation of the pupillodilation centers of the spinal cord and inhibition of the pupilloconstriction neurons of EW. Neurons within the hypothalamus project to the sympathetic preganglionic pupillodilation neurons of the thoracic spinal cord. This direct effect of hypothalamic activation on pupil diameter can be shown through microstimulation of the posterior hypothalamus, which often causes rapid pupil dilation. Increase in sympathetic tone can also produce inhibition of pupilloconstriction neurons of EW via the influence of ascending neuromodulatory pathways (Yu and Koss, 2004) (see below for more detail). The hypothalamus is also the site at which autonomic function is regulated by the central nervous system via connections with the limbic system and cortical structures. The limbic system of the brain, which is responsible for emotions and short term memory, has a direct connection to the hypothalamus and therefore can have significant effects on autonomic balance. Situations or stimuli which produce an intense emotional response are often accompanied by pupillary dilation, which is certainly mediated through limbic connections to the hypothalamus. In addition, cortical influences on the hypothalamus allow a wide variety of stimuli to effect autonomic tone and thus pupil diameter (Hockman, 1987; Loewenfeld and Lowenstein, 1993).
44 35 Sleep Sleep has a pronounced effect on the autonomic nervous system, specifically a reduction in sympathetic outflow and an increase in parasympathetic outflow. Given this overall trend it is not surprising that pupillary behavior during sleep in characterized by prolonged constriction of the pupil. It has been shown that sleep induced pupillary constriction persists in animals with lesions of the preganglionic sympathetic pupillodilation fibers. This suggests that the sleep induced pupillary changes are mediated by an activation of the preganglionic parasympathetic pupilloconstriction fibers of the Edinger-Westphal nucleus. It should be noted that during some phases of sleep, particularly REM sleep, the pupils tonically dilate at random intervals. This dilation mirrors the reversal of parasympathetic dominance with sympathetic dominance during the same intervals (Parmeggiani, 1984). Ascending Neuromodulatory Systems The ascending neuromodulatory systems of the midbrain and brainstem can have a variety of effects on pupillary behavior. These nuclei are the origin of neuromodulatory fibers which release dopamine, norepinephrine, histamine, and serotonin at a number of brain areas implicated in pupillary control. These neuromodulatory systems appear to be critical in the regulation of sleep and arousal (Saper et al., 2005), as well as autonomic regulation (Boehm and Kubista, 2002) and cortical plasticity (Gu, 2002). In addition to these global neuromodulatory effects, all or some of which could have a profound influence on pupillary behavior, there is evidence for a direct inhibition of pupilloconstriction neurons in the Edinger-Westphal nucleus by adrenergic neurons
45 36 originating from the locus coeruleus in a number of animal models (Koss, 1986). This appears to be an additional mechanism for mydriasis produced by global sympathetic activation. Later studies in humans (Larson and Talke, 2001) and rabbits (Yu and Koss, 2004) have failed to find this direct noradrenergic inhibition of pupilloconstriction, neurons and it has been suggested that this effect is mediated by the dopaminergic neurons in these species (Yu and Koss, 2004). Drugs which agonize or antagonize these neuromodulatory neurotransmitters have been found to differentially affect pupillary behavior in a wide range of animal models and human studies. These differential effects are most likely due to the both interspecies variability in the projections of these neuromodulatory fibers (Gu, 2002), as well as the differential activation of multiple brain areas implicated in pupillary behavior due to the extensive projections of these neuromodulators. Clinical Significance of Pupillary Abnormalities The pupillary light response (PLR) and pupillary near response (PNR) are commonly used clinical measures of visual and neurological function. Due to the ease with which these responses can be elicited in a clinical setting, their assessment provides a rapid evaluation of certain critical brain functions. The varied nature of the brain areas these reflexes traverse allows the clinician to assess retinal, midbrain, and autonomic function concurrently with a few straightforward measurements of pupillary functions. This section will provide a broad overview of common pupillary dysfunctions and some of the clinical conditions indicated by each (see Kawasaki, 2005 for a more comprehensive review).
46 37 Dysfunctions in the Light Reflex Pathway As previously stated, the pupillary light reflex is driven by a simple, four neuron reflex arc which passes out of the eye, via the optic nerve, to a series of mid-brain nuclei, and back to the iris of the eye via the oculomotor nerve (Figure 1 and 6). For diagnostic purposes, abnormalities of the pupil are traditionally classified as afferent or efferent defects. These divisions are based on whether the lesion involved occurs in the afferent Figure 6. Schematic diagram of the direct and consensual pupillary light pathways in primates including humans. AQ, aqueduct; EW, Edinger Westphal nucleus; F, fovea; OC, optic chiasm; PC, posterior commissure; PON, pretectal olivary nucleus; SOA, supraoculomotor area.
47 38 or efferent leg of the PLR. Afferent defects are indicative of conditions affecting the retina or optic nerve, while efferent defects are indicative of autonomic dysfunction or conditions affecting the oculomotor nerve. Afferent defects in the pupillary light reflex are characterized by an asymmetry in the pupillary response between the two eyes in response to a brief flash of light (Thompson, 1992; Digre, 2005). This relative afferent pupillary defect (RAPD) is caused by a difference in the overall light sensitivity of one retina versus the other (figure 6 pathway #1). When a light is presented to one eye, the neural impulses generated travel to each PON via the optic nerve and therefore generate both a direct and consensual light response (figure 6 pathway #2). If the magnitude of the pupillary response differs when the same light in shown in the other eye, this indicates a dysfunction in the visual pathway at the level of the retina or optic nerve in the eye with the lesser response, as the light is less efficient at generating a response in that eye. The presence of an RAPD is a primary symptom of acute disorders of the retina and optic tract, such as optic neuritis, retinal detachment, and retinal vein occlusion. A RAPD is also observed in many of the major diseases of the retina such as glaucoma and macular degeneration, although it is not the primary indicator of these conditions (Thompson and Miller, 1998). As stated previously, a significant portion of the neural pathway which drives the pupillary light reflex is located in the midbrain, e.g., EW and PON. This midbrain segment of the reflex allows the pupillary light reflex to be used as a clinical assessment of overall midbrain function (figure 6 pathway #3). Various midbrain lesions can produce abnormalities in the PLR, which are characterized by an absence or attenuation of the light reflex in one or both eyes, even when a constant, bright stimulus is used.
48 39 Unless the lesion is small and localized to the pretectum or EW, the pupillary light reflex deficits will present secondarily to other, more prominent neurological defects. This component of the reflex also allows the pupillary light reflex to be used to evaluate overall brain function in unconscious or semi-conscious patients. Not only does the pupillary light reflex give a static measure of brain function in these patients, but it is also an important clinical tool used to assess the prognosis and progression of comatose patients and victims of severe head trauma (Thompson and Miller, 1998). Efferent defects of the PLR are most often characterized by a dissymmetry in the size of the two pupils under steady state illumination, which is termed anisocoria. Since pupil diameter at any given time is determined by the relative activation of the iris sphincter and dilator muscles, if the relative drive on these two muscles is different in each eye, then anisocoria is produced. This unequal drive can be caused by a specific lesion which blocks the outflow of neural impulses from EW to the sphincter, or from the superior cervical ganglion to the dilator muscle of one eye (figure 6 pathway #4). More commonly, anisocoria is caused by a unilateral global dysfunction in either the sympathetic or parasympathetic branches of the autonomic nervous system. Therefore, the presence of anisocoria is indicative of disorders of the autonomic nervous system such as familial dysautonomia and Horner s Syndrome, as well as acute disturbances of ocular parasympathetic input such as those caused by basal meningitis and oculomotor nerve palsies (Thompson and Miller, 1998).
49 40 Dysfunctions in the Near Response Pathway The pupillary near response (PNR) is also regularly clinically utilized as a diagnostic tool. The pupillary light reflex and PNR share a common efferent path from the midbrain to the iris, but due to differing afferent pathways, it is possible to retain one functional reflex in the absence of the other. This condition is called light-near dissociation and it is a classic symptom of neurosyphilis (Lowenstein, 1956), although a variety of other conditions can also cause this phenomenon, e.g., multiple sclerosis (Loewenfeld, 1969), neurosarcoidosis (Poole, 1984), and diabetes mellitus (Dacso and Bortz, 1989). The PNR is often assessed in conjunction with the PLR, as it provides insight into the source of any observed defects in the pupillary light reflex (Digre, 2005). Modern neuroimaging techniques have shown the probable site of lesion which leads to the phenomenon of light-near dissociation can be localized to the periaqueductal grey (PAG) matter of the midbrain (Poole, 1984; Dacso and Bortz, 1989). The PAG lies dorsal and lateral to EW, although the posterior to anterior extent of PAG is much more extensive than that of EW. It is likely that a lesion in the PAG interrupts fibers of passage from the PON to EW (figure 6 pathway #3), while sparing the connection between SOA and EW (figure 6 pathway #5). This would interrupt the afferent pathway of the PLR, while maintaining the PNR afferents. Conclusion The neural regulation of pupil diameter allows for the adaptation of the optics of the eye to a variety of visual task and environmental conditions. This regulation is mediated by a variety of brain areas that have a variety of sensory inputs and functions.
50 41 Although the control of pupil diameter is generally thought of as a simple reflexive process, the number and complexity of pupillary control pathways involved in the regulation of pupil diameter imparts an unexpected complexity that is only beginning to be appreciated and fully understood. Acknowledgements The authors thank Dr. Thomas Norton for comments on the manuscript. This work was supported by NIH grant EY09380 and the EyeSight Foundation of Alabama. References Alpern, M., Mason, G. L., and Jardinico, R. E Vergence and accommodation. V. Pupil size changes associated with changes in accommodative vergence. Am. J. Ophthalmol. 52, Aston-Jones, G. and Cohen, J. D An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, Backer, W. D. and Ogle, K. N Pupillary response to fusional eye movements. Am. J. Ophthalmol. 58, Barbur, J. L A Study of Pupil Response Components in Human Vision. In: Basic and Clinical Perspectives in Vision Research: A Celebration of the Career of Hisako Ikeda. (eds. J. G. Robbins et al.), pp Plenum Press. Beatty, J. and Lucero-Wagoner, B The Pupillary System. In: Handbook of Psychophysiology, AU10 2nd ed. (ed.), pp Berson, D. M Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 26, Boehm, S. and Kubista, H Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol. Rev. 54, Bron, A. J., Tripathi, R. C., Tripathi, B. J., and Wolff, E Wolff s Anatomy of the Eye and Orbit. Chapman & Hall Medical.
51 42 Busettini, C., Davison, R. C., and Gamlin, P. D. R The Near Triad: Vergence, Accommodation, and Pupilloconstriction. In: New Encyclopedia of Neuroscience (ed. L. Squire). Elsevier. Campbell, F. W. and Green, D. G Optical and retinal factors affecting visual resolution. J. Physiol. 181, Campbell, F. W. and Gregory, A. H Effect of size of pupil on visual acuity. Nature 187, Campbell, F. W. and Gubisch, R. W Optical quality of the human eye. J. Physiol. 186, Charman, W. N Optics of the Eye. In: Handbook of Optics (ed. M. Bass), pp McGraw-Hill. Clarke, R. J. and Ikeda, H. 1985a. Luminance and darkness detectors in the olivary and posterior pretectal nuclei and their relationship to the pupillary light reflex in the rat. I. Studies with steady luminance levels. Exp. Brain Res. 57, Clarke, R. J. and Ikeda, H. 1985b. Luminance detectors in the olivary pretectal nucleus and their relationship to the pupillary light reflex in the rat. II. Studies using sinusoidal light. Exp. Brain Res. 59, Clarke, R. J., Zhang, H., and Gamlin, P. D Characteristics of the pupillary light reflex in the alert rhesus monkey. J. Neurophysiol. 89, Dacso, C. C. and Bortz, D. L Significance of the Argyll Robertson pupil in clinical medicine. Am. J. Med. 86, Dacey, D. M., Liao, H. W., Peterson, B. B., Robinson, F. R., Smith, V. C., Pokorny, J., Yau, K. W., and Gamlin, P. D Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, Digre, K. B Principles and Techniques of Examination of the Pupil, Accommodation, and the Lacrimal System. Lippincott Williams & Wilkins. Edinger, L Ueber den verlauf der centralen hirnnervenbahnen mit demonstration von pra paraten. Arch. Psychiatr. Nervenkr. 16, Eglen, R. M., Hegde, S. S., and Watson, N Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 48, Fry, G. A The relation of pupil size to accommodation and convergence. Am. J. Optom. Arch. Am. Acad. Optom. 22, Fry, G. A The optical performance of the human eye. Prog. Optics 8, Fu, Y., Liao, H. W., Do, M. T. H., and Yau, K. W Nonimage-forming ocular photoreception in vertebrates. Curr. Opin. Neurobiol. 15,
52 43 Gamlin, P. D Functions of the Edinger-Westphal Nucleus. In: Nervous Control of the Eye (eds. G. Burnstock et al.), pp Harwood Academic. Gamlin, P. D. R Neural mechanisms for the control of vergence eye movements. Ann. NY Acad. Sci. 956, Gamlin, P. D The pretectum: connections and oculomotor-related roles. Prog. Brain Res. 151, Gamlin, P. D., McDougal, D. H., Pokomy, J., Smith, V. C., Yau, K. W., and Dacey, D. M Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. (in press). Gamlin, P. D., Zhang, H., and Clarke, R. J Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp. Brain Res. 106, Gamlin, P. D., Zhang, H., Harlow, A., and Barbur, J. L Pupil responses to stimulus color, structure and light flux increments in the rhesus monkey. Vision Res. 38, Gil, D. W., Krauss, H. A., Bogardus, A. M., and Woldemussie, E Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest. Ophthalmol. Vis. Sci. 38, Gu, Q Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, Hess, E. H Attitude and pupil size. Sci. Am. 212, Hess, E. H. and Polt, J. M Pupil size as related to interest value of visual stimuli. Science 132, Hess, E. H. and Polt, J. M Pupil size in relation to mental activity during simple problem solving. Science 143, Heywood, C. A., Nicholas, J. J., Lemare, C., and Cowey, A The effect of lesions to cortical areas V4 or AIT on pupillary responses to chromatic and achromatic stimuli in monkeys. Exp. Brain Res. 122, Hirata, Y., Yamaji, K., Sakai, H., and Usui, S Function of the pupil in vision and information capacity of retinal image. Syst. Comput. Jpn. 34, Hockman, C. H Essentials of Autonomic Function: The Autonomic Nervous System: Fundamental Concepts from Anatomy, Physiology, Pharmacology, and Neuroscience for Students and Professionals in the Health Sciences. Thomas. Hood, D. and Finkelstein, M Sensitivity to Light. In: Handbook of Perception and Human Performance (eds. K. R. Boff et al.), pp. 5/1 5/66. Wiley.
53 44 Hung, G. K. and Semmlow, J. L Static behavior of accommodation and vergence: computer simulation of an interactive dual-feedback system. IEEE Trans. Biomed. Eng. 27, Jenkins, T. C. A Aberrations of the eye and their effects on vision: Part 2. Br. J. Physiol. Opt. 20, Jones, R The effect of accommodation and convergence on the pupil. Invest. Ophthalmol. Vis. Sci. 30. Kardon, R Pupillary light reflex. Curr. Opin. Ophthalmol. 6, Kardon, R. H Anatomy and Physiology of the Autonomic Nervous System. In: Walsh and Hoyt s Clinical Neuroophthalmology, Vol. 3 (eds. N. R. Miller et al. ),. Lippincott Williams & Wilkins. Kasthurirangan, S. and Glasser, A Characteristics of pupil responses during far-tonear and near-to-far accommodation. Ophthalmic Physiol. Opt. 25, Kawasaki, A Disorders of Pupillary Function, Accommodation, and Lacrimation. In: Walsh and Hoyt s Clinical Neuro-Ophthalmology, Vol. 3 (eds. N. R. Miller et al.), Lippincott Williams & Wilkins. Klooster, J. and Vrensen, G. F. J. M New indirect pathways subserving the pupillary light reflex: projections of the accessory oculomotor nuclei and the periaqueductal gray to the Edinger-Westphal nucleus and the thoracic spinal cord in rats. Anat. Embryol. 198, Knoll, H. A Pupillary changes associated with accommodation and convergence. Am. J. Ophthalmol. 26, Koss, M. C Review article: pupillary dilation as an index of central nervous system alpha 2-adrenoceptor activation. J. Pharmacol. Methods 15, Larson, M. D. and Talke, P. O Effect of dexmedetomidine, an alpha-adrenoceptor agonist, on human pupillary reflexes during general anaesthesia. Br. J. Clin. Pharmacol. 51, Laughlin, S. B Retinal information capacity and the function of the pupil. Ophthalmic Physiol. Opt. 12, Liang, J. and Williams, D. R Aberrations and retinal image quality of the normal human eye. J. Opt. Soc. Am. A 14, Loewenfeld, I. E The Argyll Robertson pupil, : a critical survey of the literature. Surv. Ophthalmol. 14, Loewenfeld, I. E. and Lowenstein, O The Pupil: Anatomy, Physiology, and Clinical Applications. Iowa State University Press. Lowenstein, O The Argyll Robertson pupillary syndrome; mechanism and localization. Am. J. Ophthalmol. 42,
54 45 Marcos, S., Moreno, E., and Navarro, R The depth-of field of the human eye from objective and subjective measurements. Vision Res. 39, Marg, E. and Morgan, M. W The pupillary near reflex. Am. J. Optom. 26, Marg, E. and Morgan, W Further investigation of the pupillary near reflex. Am. J. Optom. 27, May, P. J. and Warren, S Ultrastructure of the macaque ciliary ganglion. J. Neurocytol. 22, Mays, L. E. and Gamlin, P. D. R Neuronal circuitry controlling the near response. Curr. Opin. Neurobiol. 5, Myers, G. A. and Stark, L Topology of the near response triad. Ophthalmic Physiol. Opt. 10, Nakamura, S., Taniguchi, T., Suzuki, F., Akagi, Y., and Muramatsu, I Evaluation of alpha1-adrenoceptors in the rabbit iris: pharmacological characterization and expression of mrna. Br. J. Pharmacol. 127, Nisida, I., Okada, H., and Nakano, O The activity of the ciliospinal centers and their inhibition in pupillary light reflex. Jpn. J. Physiol. 10, Okada, H., Nakano, O., Okamoto, K., Nakayama, K., and Nisida, I The central path of the light reflex via the sympathetic nerve in the cat. Jpn. J. Physiol. 10, Oyster, C. W The Iris and the Pupil. In: The Human Eye: Structure and Function (ed. C. W. Oyster), pp Sinauer Associates. Parmeggiani, P. L Autonomic Nervous System in Sleep. In: Sleep Mechanisms (eds. A. A. Borbe ly et al.), pp Springer-Verlag. Parssinen, O., Leppanen, E., Keski-Rahkonen, P., Mauriala, T., Dugue, B., and Lehtonen, M Influence of tamsulosin on the iris and its implications for cataract surgery. Invest. Ophthalmol. Vis. Sci. 47, Passatore, M. and Pettorossi, V. E Efferent fibers in the cervical sympathetic nerve influenced by light. Exp. Neurol.52, Perry, V. H. and Cowey, A Retinal ganglion cells thatproject to the superior colliculus and pretectum in the macaque monkey. Neuroscience 12, Phillips, N. J., Winn, B., and Gilmartin, B Absence of pupil response to blurdriven accommodation. Vision Res. 32, Poole, C. J Argyll Robertson pupils due to neurosarcoidosis: evidence for site of lesion. Br. Med. J. 289, 356.
55 46 Ranson, S. W. and Magoun, H. W The central path of the pupilloconstrictor reflex in response to light. Arch. Neurol. Psychiatry 30, Robertson, D Primer on the Autonomic Nervous System. Academic Press. Saper, C. B., Scammell, T. E., and Lu, J Hypothalamic regulation of sleep and circadian rhythms. Nature 437, Schor, C. M. and Narayan, V Graphical analysis of prism adaptation, convergence accommodation, and accommodative convergence. Am. J. Optom. Phys. Opt. 59, Schwiegerling, J Theoretical limits to visual performance. Surv. Ophthalmol. 45, Semmlow, J. and Heerema, D The synkinetic interaction of convergence accommodation and accommodative convergence. Vision Res. 19, Stakenburg, M Accommodation without pupillary constriction. Vision Res. 31, Takagi, M., Abe, H., Toda, H., and Usui, T Accommodative and pupillary responses to sinusoidal target depth movement. Ophthalmic Physiol. Opt. 13, Thompson, H. S The Pupil. In: Adler s Physiology of the Eye: Clinical Application (eds. F. H. Adler et al.), pp Mosby Year Book. Thompson, S. H. and Miller, N. R Disorders of Pupillary Function, Accommodation, and Lacrimation. In: Clinical Neuro-Ophthalmology (eds. N. R. Miller et al.), pp Williams & Wilkins. Wang, B. and Ciuffreda, K. J Depth-of-focus of the human eye: theory and clinical implications. Surv. Ophthalmol. 51, Warwick, R The ocular parasympathetic nerve supply and its mesencephalic sources. J. Anat. 88, Westheimer, G Pupil size and visual resolution. Vision Res. 4, Westphal, C Ueber einen Fall von chronischer progressiver La hmung der Augenmuskeln (ophthalmoplegia externa) nebst Beschreibung von Ganglienzellengruppen in Bereiche des Oculomotoriuskerns. Arch. Psychiat. Nervenkr. 18, Wilhelm, B. J., Wilhelm, H., Moro, S., and Barbur, J. L Pupil response components: studies in patients with Parinaud s syndrome. Brain 125, Woodhouse, J. M. and Campbell, F. W The role of the pupil light reflex in aiding adaptation to the dark. Vision Res. 15, Yu, Y. and Koss, M. C Alpha2-adrenoceptors do not mediate reflex mydriasis in rabbits. J. Ocul. Pharmacol. Ther. 20,
56 Zhang, H., Clarke, R. J., and Gamlin, P. D. R Behavior of luminance neurons in the pretectal olivary nucleus during the pupillary near response. Exp. Brain. Res. 112,
57 47 HUMAN AND MACAQUE PUPIL RESPONSE DRIVEN BY MELANOPSIN- CONTAINING RETINAL GANGLION CELLS by PAUL D. GAMLIN, DAVID H. MCDOUGAL, JOEL POKORNY, VIVIANNE C. SMITH, KING-WAI YAU, DENNIS M. DACEY Vision Research Volume 47, pp Copyright 2006 by Elsevier Ltd. Used by permission Format adapted and errata corrected for dissertation
58 48 Abstract Melanopsin, a novel photopigment, has recently been localized to a population of retinal ganglion cells that display inherent photosensitivity. During continuous light and following light offset, primates are known to exhibit sustained pupilloconstriction responses that resemble closely the photoresponses of intrinsically-photoreceptive ganglion cells. We report that, in the behaving macaque, following pharmacological blockade of conventional photoreceptor signals, significant pupillary responses persist during continuous light and following light offset. These pupil responses display the unique spectral tuning, slow kinetics, and irradiance coding of the sustained, melanopsinderived ganglion cell photoresponses. We extended our observations to humans by using the sustained pupil response following light offset to document the contribution of these novel ganglion cells to human pupillary responses. Our results indicate that the intrinsic photoresponses of intrinsically-photoreceptive retinal ganglion cells play an important role in the pupillary light reflex and are primarily responsible for the sustained pupilloconstriction that occurs following light offset. Introduction The pupillary light reflex controls the light intensity reaching the retina by a simple and well characterized pathway that links a sensory signal: irradiance, to a motor output: pupil constriction (Loewenfeld, 1993; Gamlin et al., 1995; Pong & Fuchs, 2000; Clarke et al., 2003). Pupil area decreases with increasing irradiance over a ~9 log intensity range. A key feature of the light reflex is its tonic nature in bright light: constriction is held steady under continuous illumination (Bouma, 1962; Loewenfeld,
59 ; Gamlin et al., 1995; Pong & Fuchs, 2000; Clarke et al., 2003). The light reflex is known, however, to be driven by pathways that originate in rod and cone photoreceptors, making it difficult to reconcile the sustained pupil constriction under photopic conditions with the rapid desensitization or light adaptation of the cone pathway (Shapley & Enroth-Cugell, 1984). Furthermore, at light cessation, primate pupil responses often exhibit a brief dilation followed by a post-stimulus, sustained reconstriction (Alpern & Campbell, 1962; Newsome, 1971; Alpern & Ohba, 1972; Hansen & Fulton, 1986). This post-stimulus, sustained pupil response has also never been satisfactorily explained, although it has been proposed to arise from bleached rhodopsin in rods (Alpern & Campbell, 1962; Alpern & Ohba, 1972; Hansen & Fulton, 1986). In rodents, melanopsin-containing, intrinsically photosensitive retinal ganglion cells have been described that influence non-image-forming functions, including the pupillary light reflex (Berson et al., 2002; Hattar et al., 2002, 2003; Gooley et al., 2003; Lucas et al., 2003; Panda et al., 2003). Specifically, mice lacking rod and cone photoreceptors still display pupil constriction in response to stimuli of high retinal irradiance (Lucas et al., 2001), mice lacking melanopsin show an abnormal pupillary light reflex in which the pupil fails to fully constrict at high retinal irradiance (Lucas et al., 2003), and mice lacking both melanopsin and functional rods and cones show no pupillary responses (Hattar et al., 2003; Panda et al., 2003). We have recently shown that a comparable population of retinal ganglion cells exists in primates, and these cells project to the pupillary control center in the pretectum (Dacey et al., 2005). When the melanopsin-associated response of these ganglion cells is isolated in vitro by pharmacological blockade of rod and cone transmission to the inner retina, they display a
60 50 slow, maintained depolarization in response to long-duration light pulses and repolarize only slowly upon light OFF (Dacey et al., 2005). These data suggest that the melanopsinbased response contributes significantly to the maintained pupil constriction in continuous light as well as the post-stimulus, sustained constriction seen after light OFF. The goal of this study was to test this hypothesis in the behaving macaque by measuring melanopsin-driven pupillary responses after pharmacological blockade of retinal rodcone pathways. Our results indicate that indeed intrinsically-photoreceptive retinal ganglion cells contribute significantly to the sustained component of the pupillary light reflex and entirely to the sustained, post-stimulus pupilloconstriction that occurs following light offset. We also provide evidence that this class of retinal ganglion cells plays the same functional roles in human pupillary responses. Methods In Vitro Preparation The in vitro whole mount retina preparation and recording methods have been described previously (Dacey et al., 2003; Dacey et al., 2005). Eyes were removed from deeply anesthetized animals and the retina dissected free of the vitreous and sclera in oxygenated Ames Medium (Sigma Chemical Co., St. Louis, MO). The retina-rpechoroid was placed flat, vitreal surface up, in a superfusion chamber mounted on the stage of a light microscope. Rhodamine-labeled cells were visualized as described above; autofluoresent granules were visualized with a blue filter block (excitation 490 nm; barrier 515 nm). Ganglion cells were targeted for intracellular recording using high impedance (~ MΩ) glass micropipettes filled with 2-3% Neurobiotin (Vector
61 51 Labs, Burlingame, CA) and 1-2% pyranine (Molecular Probes, Eugene, OR) in 1.0 M potassium acetate. Voltage responses were amplified (Axon instruments; Axoclamp) and digitized at 10 khz. Visual stimuli were delivered to the retina as previously described (Packer et al., 2001). Subjects Two juvenile, male Rhesus monkeys (Macaca mulatta) were used to investigate pupillary responses, and they gave similar results. All experimental procedures were approved by the UAB Institutional Animal Care and Use Committee, and complied with the USPHS Policy on Humane Care and Use of Laboratory Animals. All described surgical procedures were performed under sterile conditions using isoflurane anesthesia. Post-surgical animals received analgesics (Buprenex, 0.01 mg/kg) to minimize pain. Two male humans with normal corrected vision also participated in this study, and they gave similar results. All experimental procedures were approved by the UAB Institutional Review Board, and were undertaken with the understanding and written consent of each subject. Recording Procedures For eye-movement recording in macaques, scleral search coils were implanted under the conjunctiva of each eye (Judge et al., 1980), and the horizontal and vertical gains of each eye were calibrated at the beginning of each recording session. Human eye movements were monitored by video camera. Pupil diameters were measured in both eyes under infra-red illumination using video cameras and ISCAN RK406 pupillometer
62 52 systems. The positions of the right eye, left eye, and pupil diameters were sampled at 500 Hz. All samples were stored on computer disk for later analysis. Behavioral Task In order to measure their pupillary responses, subjects performed the following behavioral task. The right eye was dilated with 1.0% tropicamide/2.5% phenylephrine. At the beginning of a trial, the subject fixated with the left eye on a target (2 Maltese cross; 5 cd/m 2 ) presented on a computer monitor at a distance of 58 cm. Pupil diameter in the left eye was monitored continuously. After 5 seconds of fixation, a stimulus subtending 36 was presented in Maxwellian view to the right eye for 10 seconds. The stimulus ( log photons/cm 2 /sec) was presented at selected narrow-band wavelengths (8-10 nm full width at half maximum, Thermo-Oriel) between 430 nm and 613 nm. The stimulus was then extinguished, and the subject maintained fixation for another seconds. In all cases, stimulus generation was under computer control, including a stepper-controlled variable neutral-density filter, a 10-position filter wheel, and a mechanical shutter. An IL1700 Radiometer/ Photometer System was used to calibrate retinal irradiance at each wavelength. Intravitreal Injections and ERG Monitoring To study the influence of the intrinsically-photoreceptive retinal ganglion cells on the pupillary light reflex, we eliminated the reflex component normally mediated by rods and cones by blocking ON and OFF retinal channels with intravitreal injections of L-2- amino-4-phosphobutyrate (L-AP4) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)
63 53 (Dolan & Schiller, 1994; Sieving et al., 1994). In one animal, D-2-amino-5- phosphonovaleric acid (D-AP5) was also included in the cocktail to block any potential involvement of NMDA receptors. Prior to the intravitreal injection, the animal was anesthetized with a mixture of 4-5% isoflurane and 95-96% oxygen. Topical 0.5% Proparacaine HCl was instilled in the right eye. Then, using a 28-gauge needle inserted through the pars plana, we injected 100 µl of a cocktail of mm L-AP4, 2 mm CNQX, and 5 mm D-AP5 diluted in sterile saline. Assuming complete mixing in a vitreal volume of 2.0 ml, the retinal concentrations of these drugs would be mm L-AP4, 100 μm CNQX, and 250 μm D-AP5. To monitor the effectiveness of the intravitreal injection, we recorded the electroretinogram (ERG) (4 msec duration; 2.25 Cd/m 2 /sec, 6500K) with an Espion system (Diagnosys) equipped with a ColorBurst handheld mini-ganzfeld simulator. The right eye was dilated with 1.0% tropicamide/2.5% phenylephrine for ERG recording. Once the ERG confirmed that the injection had been effective, the animal was allowed to recover from the anesthesia for 1-2 hours, and visual testing began. Since the right eye was dilated for the pharmacological blockade sessions, it was maintained in a fully dilated state for all recording sessions. Previous studies have shown that such intravitreal pharmacological blockades remain effective for at least five hours, and weeks are required for full recovery (e.g., Sieving et al., 1994; Kondo & Sieving, 2001). Furthermore, we could confirm the continued effectiveness of the blockade from the measured pupillary responses.
64 54 Data Analysis Individual trials showing relevant pupil traces were displayed and analyzed offline by computer. For measurements of the light-evoked pupilloconstriction, the average pupilloconstriction was measured over the 5-second period between 5 and 10 seconds after light onset. For measurements of the post-stimulus, sustained pupillary constriction after termination of the light stimulus, the average pupilloconstriction was measured over the 10-second period between 6 and 16 seconds after light offset in macaques. In humans, for measurements of the post-stimulus, sustained pupillary constriction after termination of the light stimulus, the average pupilloconstriction was measured over the 15-second period between 15 and 30 seconds after light offset. The relation between irradiance and pupilloconstriction was fit with the Hill equation: Pupilloconstriction = P max * [I B / (I B + C B )], where I is irradiance, P max is maximal pupilloconstriction, B is a constant, and C is the irradiance at which half-maximal pupilloconstriction is produced. For measurements of light-evoked pupillary responses during pharmacological blockade and for poststimulus, sustained pupilloconstriction following light termination, P max and B were determined at 493 nm and kept constant. Only C was varied to obtain best fits at other wavelengths. For measurements of light-evoked pupillary responses under normal conditions, P max, B and C were varied to obtain best fits. We then generated plots of relative quantal sensitivity as a function of wavelength. In humans, we estimated the absorbance of the lens of each subject using D&H Color Rule settings (Coren, 1987) and an algorithm to convert to lens density functions (Pokorny et al., 1987), and corrected quantal sensitivity appropriately. These resultant spectral sensitivity functions were analyzed by optimizing fits of the data to a single pigment absorbance template for a
65 55 retinal 1 -based pigment as described by Kraft and colleagues (Kraft et al., 1998). To determine λ max, we fit a sixth order polynomial to a plot of log sensitivity versus wavelength as described by Baylor and colleagues (Baylor et al., 1984; Baylor et al., 1987). Each term of that polynomial has the form a n [log(λ max /λ 561)] n. Results We first examined the correspondence between the response characteristics of the intrinsically photoreceptive ganglion cells recorded in vitro with those of pupillary responses recorded in vivo under equivalent conditions. Figure 1A shows the response of the primate pupil to a 10-second pulse of light (493 nm) under photopic conditions. At light ON, the pupil rapidly constricted and the constriction was maintained for the duration of the stimulus. At light OFF, the pupil dilated transiently, but then reconstricted and displayed a post-stimulus, sustained constriction in darkness. Figure 1B shows the response of an intrinsically-photoreceptive retinal ganglion cell recorded from the in vitro macaque retina under similar conditions to those in Figure 1A. Overall, the retinal ganglion cell activity closely matched the observed pupillary responses in time course. At light ON, the ganglion cell depolarized rapidly (latency to first spike ~35 msec) and exhibited sustained firing for the stimulus duration. (Dacey et al., 2005). At light OFF, the cell hyperpolarized transiently to briefly cancel the sustained intrinsic response; the cell then repolarized to give rise to the post-stimulus, sustained discharge in
66 56 Figure 1. Pupillary and ganglion cell responses in macaques. (a) Averaged pupillary responses (n = 5) showing sustained pupilloconstriction to a 10 sec pulse of light (493 nm, 13.3 log quanta/cm 2 /s). At light OFF, there is a transient pupil dilation followed by a sustained pupilloconstriction. The transient pupil dilation is not always evident, since it depends on prior stimulus conditions and the magntitude of the sustained, post-stimulus pupil constriction. (b) In vitro, intracellular recording from an intrinsicallyphotoreceptive retinal ganglion cell. For further details see Dacey et al. (2005). A 10 sec pulse of light (470 nm, 13.5 log quanta/cm 2 /s) was presented. Note the similarity between
67 57 the retinal ganglion cell response and the pupillary response. (c) In vivo, flash electroretinogram under normal conditions (upper trace) and following intravitreal injection of L-AP4/CNQX/D-AP5 (lower trace). The b wave is virtually eliminated following this pharmacological blockade. (d) Normal condition. Averaged responses (n = 3) of the pupil to 493 nm light of 14.0 log quanta/cm 2 /s irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm 2 /s irradiance (red trace). Following light extinction, there is a pronounced sustained pupilloconstriction that, in this case, masks the transient pupil dilation often seen at light OFF. (e) Pharmacological blockade condition. Averaged responses (n = 3) of the pupil to 493 nm light of 14.0 log quanta/cm 2 /s irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm 2 /s irradiance (red trace). Note the robust and sustained pupillary responses elicited by light of 493 nm but not of 613 nm. (f) Retinal irradiance-pupillary response plots for 532 nm irradiance for the normal condition ( ) and during pharmacological blockade ( ) (SEM error bars). darkness before slowly returning to the resting potential in darkness (Dacey et al., 2005). The complex dynamics of the ganglion cell response derives from an interaction between cone input, which provides the short-latency responses at light onset and offset, and the inherent photoresponse, which maintains depolarization and firing for the duration of the stimulus followed by an extremely slow decay (shown by the red line drawn through the voltage response) at light OFF (Dacey et al., 2005). The hypothesis that combined cone and melanopsin-derived light responses account respectively for transient and sustained pupil behavior, we examined the spectral sensitivity of these responses. The slow and sustained intrinsic photoresponse of the
68 58 retinal ganglion cells is characterized by an absorbance template corresponding to a single retinal 1 -based pigment with a peak at 482 nm, whereas the fast and relatively transient cone-mediated response is complex and broadband, with significant sensitivity to longer wavelengths derived from L and M cone inputs (Dacey et al., 2005). Under normal photopic conditions, substantial and prompt pupilloconstriction was elicited by retinal illumination at both 493 nm (activating both cones and the intrinsic photoresponse) and 613 nm (activating predominantly cones) (Fig. 1D). However, during pharmacological blockade of rod and cone inputs (confirmed by the substantial reduction in the b-wave of the electroretinogram; see Fig. 1C), the pupillary response was delayed by approximately one second and was more sluggish than normal (Fig. 1E); at the same time, substantial pupilloconstriction was elicited only by retinal illumination at 493 nm, suggesting that the intrinsic photoresponse of ganglion cells drove this pupillary response. Similarly, sustained pupilloconstriction after light cessation was present only after retinal illumination at 493 nm (Fig. 1 D,E). To more completely characterize the spectral sensitivity of these macaque pupillary responses, the irradiance-response relations for pupil constriction were measured at ten wavelengths between 430 nm and 613 nm, and fitted with the Hill equation. As an example, the filled circles in Fig. 1F show the normal relation during the light stimulus at 532 nm. During pharmacological blockade (open squares), the response was absent at irradiance levels below ~11 log quanta/cm 2 /sec, presumably due to the elimination of rod-driven input. At higher irradiance levels, substantial pupilloconstriction occurred, despite the elimination of cone-driven responses. Overall,
69 59 at 532 nm, the irradiance-response relation was shifted by ~1.5 log units on the irradiance axis after pharmacological blockade of rod and cone inputs. Figure 2A shows Hill equation fits to the irradiance-response relations of the sustained response during illumination for all ten wavelengths under normal conditions. Pupil response-irradiance functions obtained for stimuli between 452 nm and 552 nm were comparable, with a light-evoked pupil constriction of ~3.2 mm at a retinal irradiance of 14.0 log quanta/cm 2 /sec. However, at longer wavelengths, pupil constriction was reduced. The collective spectral responsivity data could not be well fit by a single pigment absorbance template for a retinal 1 -based pigment (Fig. 2C, triangles), indicating the presence of more than one component. Figure 2B shows Hill equation fits to the irradiance-response relations during pharmacological blockade to isolate the intrinsic photoresponse. The pupillary responses for a given retinal irradiance were largest for wavelengths between 453 and 510 nm, with light-evoked pupil constriction of ~2.1 mm at a retinal irradiance of 14.0 log quanta/cm 2 /sec. At longer wavelengths, light-evoked pupillary responses were significantly reduced, and were essentially absent at 613 nm (Fig 2B). The collective spectral responsivity data were well fit by the melanopsin spectrum ( max 482 nm) (Fig. 2C, circles). Corresponding experiments on the sustained pupillary constriction after light OFF showed that this response was driven exclusively by the intrinsic photoresponse. More specifically, a large pupilloconstriction was produced by prior exposure to 10-second light pulses between 432 nm and 510 nm (Fig. 3A, blue and green traces), whereas little response was observed after 10-second light pulses at 613 nm, a wavelength that does not readily evoke the intrinsic response (Fig. 3A, red trace). At all wavelengths, the
70 60 Figure 2. Pupillary responses of macaques during a light stimulus under normal conditions and during pharmacological blockade. (a) Retinal irradiance-pupillary response plots under normal conditions. (b) Retinal irradiance-pupillary response plots after pharmacological blockade. The white dotted line indicates the retinal irradiance at 470 nm required to produce half-maximal pupilloconstriction. In (a) and (b), line color
71 61 represents an approximation of stimulus wavelength. (c) Spectral sensitivity data derived from (a) and (b). The data in the normal condition ( ) is poorly fit (R 2 = 0.77) by a best fit, vitamin A1 pigment nomogram (peak sensitivity at 522 nm). The data obtained during pharmacological blockade ( ) is well fit (R 2 = 0.99) by a vitamin A1 pigment nomogram with peak sensitivity at 482 nm. magnitude of the post-stimulus, sustained pupilloconstriction after light pulses of a given retinal irradiance in the absence of pharmacological blockers was comparable to that of the sustained light-evoked pupillary response evoked by light of the same irradiance in the presence of blockers (cf. Figs 2B and 3A). Furthermore, a comparable retinal irradiance was required in both cases to produce half-maximal pupilloconstriction at 493 nm. With or without pharmacological blockade, the irradiance-response relation for poststimulus, sustained pupilloconstriction was the same at all wavelengths (Fig. 3A, B), and had the same spectral responsivity, being well fit by the melanopsin spectrum ( max 482 nm) (Fig. 3C). A melanopsin-associated photosensitive pathway appears to exist in humans (Dacey et al., 2005; Rollag et al., 2003; Hannibal et al., 2004), but definitive evidence linking it to a functional role is still lacking. Because the post-stimulus, sustained pupil response at light OFF appears to arise solely from the melanopsin pathway in macaque, we sought to investigate the same phenomenon in normal human subjects. We found that pupilloconstriction in human subjects persisted after exposure to a 10-second light at 493 nm (Fig. 4A, blue trace), but not at 613 nm for the same irradiance (Fig. 4A, red trace). The irradiance-response relation at 493 nm (Fig. 4B) showed a half-maximal retinal
72 62 Figure 3. Post-stimulus, sustained pupillary responses of macaques under normal conditions and during pharmacological blockade. (a) Retinal irradiance-pupillary response plots under normal conditions. (b) Retinal irradiance-pupillary response plots after pharmacological blockade. In both (a) and (b), the white dotted line indicates the retinal irradiance at 470 nm required to produce half-maximal pupilloconstriction, line
73 63 color represents an approximation of stimulus wavelength, and P max = 3.0 mm was used for fitting the Hill equations. (c) Spectral sensitivity data derived from (a) and (b). The solid curve, a vitamin A 1 pigment nomogram with peak sensitivity at 482 nm, closely matches the data obtained both under normal conditions (, R 2 = 0.98) (best fit k max 483 nm) and during pharmacological blockade (, R 2 = 0.97) (best fit: k max 476 nm). irradiance (13.6 log quanta/cm 2 /sec) very similar to that in macaque (cf. Fig. 3A). Next, for eight wavelengths between 452 nm nm, we calculated the retinal irradiance required to produce a given criterion pupil response if mediated by a Vitamin A 1 pigment nomogram with a peak sensitivity at 482 nm (pilot data suggested a spectral sensitivity peaking between 480 and 485 nm). Repeated measures were obtained for the sustained pupil response at each wavelength and retinal irradiance. We found that the actual data obtained in this experiment departed only slightly from the predicted values (Fig. 4C). Indeed, these data, following correction for minor departures from the predicted values as described in the legend to figure 4, were well fit (R 2 = 0.99) by a Vitamin-A 1 pigment nomogram with a peak sensitivity at 482 nm (Fig. 4D), closely matching our results in macaques. Discussion Our results clearly demonstrate in macaques that the melanopsin signal contributes significantly to sustained light-evoked pupillary responses; they also show in primates, including humans, that the melanopsin signal is the sole source of the sustained, poststimulus, sustained pupilloconstriction observed following light offset.
74 64 Figure 4. Post-stimulus, sustained pupillary responses in humans. (a) Averaged responses (n = 3) of the pupil to 493 nm light of 14.1 log quanta/cm 2 /s irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm 2 /s irradiance (red trace). (b) Retinal irradiance-pupillary response plot for 493 nm irradiance. The white dotted line indicates the retinal irradiance required to produce half-maximal pupilloconstriction. (c) Criterion pupil response. Magnitude of the sustained post-stimulus pupillary response after light OFF at eight different wavelengths (SEM error bars). The retinal irradiance (log quanta/cm 2 /s) used at each wavelength is indicated above the data point. Each stimulus irradiance was calculated to produce the same sustained pupil constriction if these responses were mediated solely by a vitamin A 1 pigment nomogram with a peak
75 65 sensitivity at 482 nm. (d) Spectral sensitivity data derived from the results shown in (c). For each wavelength, the difference between the measured and criterion pupilloconstriction in (c) was converted into an irradiance difference value based on the irradiance-pupilloconstriction response curve in (b). This difference value was then combined with the stimulus irradiance value to determine the retinal irradiance required to produce the criterion pupil response at that wavelength. The solid curve, a vitamin A 1 pigment nomogram with peak sensitivity at 482 nm, closely matches the data (, R 2 = 0.99). Melanopsin-containing RGCs Contribute Significantly to the Primate Pupillary Light Reflex Melanopsin-containing RGCs provide the major retinal input to the pretectal olivary nucleus in primates (Dacey et al., 2003) and rodents (Morin et al., 2003; Hattar et al., 2006). Consistent with this projection, substantial light-evoked pupillary responses occur despite pharmacological inactivation of conventional photoreceptor signals. For example, during blockade, a stimulus with a retinal irradiance of approximately 14.5 log quanta/cm 2 /sec at 493 nm elicits a pupil constriction of 2.7 mm (Fig. 2b), while half maximal pupil constriction is elicited by a retinal irradiance of approximately 13.5 log quanta/cm 2 /sec (Fig. 2b). The action spectrum of these pupillary responses is well fit by the melanopsin spectrum, and the irradiance sensitivities and kinetics of these responses are also comparable to the intrinsic photoresponses of melanopsin-containing RGCs in vitro (Dacey et al., 2005). These results clearly indicate that melanopsin-containing RGCs contribute significantly to the primate pupillary light reflex. Furthermore, these results suggest that in primates the role of the melanopsin signal is to combine with the
76 66 cone signal over the photopic range, serving to maintain pupil constriction during continuous daylight illumination. Indeed, data obtained in humans prior to the discovery of intrinsically-photoreceptive ganglion cells supports the importance of the intrinsic photoresponse for maintaining pupil constriction during continuous daylight illumination. Bouma (1962) reported on the size of the static pupil as a function of wavelength and retinal irradiance. He obtained a spectral sensitivity curve with a λ max at 490 nm, which he interpreted as resulting from S-cone and rod inputs driving the sustained pupil response during maintained illumination. Since this interpretation could not be reconciled with the known properties of these photoreceptors, it did not gain standing. It is now clear that Bouma's data approximate the action spectrum of intrinsicallyphotoreceptive retinal ganglion cells in primates. Melanopsin-containing RGCs Drive Post-stimulus, Sustained Pupil Constriction Our data show that there is a sustained, post-stimulus pupil constriction following light offset in primates. The threshold for this response is high in both macaques and humans. For example, at 30 seconds poststimulus, a retinal irradiance of 12 log quanta/cm 2 /sec (492 nm) is required to produce any sustained pupil constriction in darkness. However, as the retinal irradiance of the stimulus increases, substantial sustained pupil constriction is produced 30 seconds following light offset. For example, a stimulus with a retinal irradiance of 13.5 log quanta/cm 2 /sec produces a sustained pupil constriction of > 1.5 mm in macaques and humans (Figs 3a,b; Fig. 4b); while in humans, a stimulus with a retinal irradiance of 15 log quanta/cm 2 /sec produces a sustained pupil constriction of 2.9 mm (Fig. 4b). The action spectrum of these responses is very well fit
77 67 by the melanopsin spectrum, and the kinetics and sensitivity of these responses closely match the intrinsic photoresponses of melanopsin-containing RGCs in vitro (Dacey et al., 2005). These results help to resolve a long-standing debate regarding the underlying process responsible for the post-stimulus pupillary response (Alpern & Campbell, 1962; Alpern & Ohba, 1972; Hansen & Fulton, 1986; Loewenfeld, 1993). Our results clearly demonstrate that such post-stimulus, sustained pupil constriction is mediated entirely by the melanopsin-driven, intrinsic photoresponse and not by sustained rod activity resulting from bleached rhodopsin as had previously been suggested (Alpern & Campbell, 1962; Alpern & Ohba, 1972; Hansen & Fulton, 1986). Comparison to Rodent Studies Rodents possess melanopsin-containing, intrinsically photosensitive retinal ganglion cells that influence non-image-forming functions, including the pupillary light reflex (Berson et al., 2002; Hattar et al., 2002, 2003; Gooley et al., 2003; Lucas et al., 2003; Panda et al., 2003). It has been previously reported that mice lacking rod and cone photoreceptors, but presumably with intact melanopsin-driven intrinsic photoresponses, display substantial pupil constriction in response to stimuli of high retinal irradiance (Lucas et al., 2001). In these animals, pupil constriction is driven by a single opsin/vitamin A-based photopigment with peak sensitivity around 479 nm (Lucas et al., 2001). These results closely match our results in primates, including humans, which demonstrate that the intrinsic photoresponse driving pupillary responses can be well fit by a single pigment nomogram with a λ max of 482 nm. Furthermore, mice lacking melanopsin show an abnormal pupillary light reflex in which the pupil fails to fully
78 68 constrict at high retinal irradiance (Lucas et al., 2003), while mice lacking both melanopsin and functional rods and cones show no pupillary responses (Hattar et al., 2003; Panda et al., 2003). These results in rodents are consistent with our suggestion that the melanopsin signal contributes significantly to the sustained pupilloconstriction component of the pupillary light reflex in primates. To our knowledge, no study in rodents has examined the sustained, post-stimulus pupil constriction that occurs following light offset. Role of Melanopsin in Intrinsically Photosensitive Retinal Ganglion Cells Much of our discussion assumes that melanopsin is the photopigment eliciting the photoresponse in intrinsically photosensitive retinal ganglion cells. But is this a valid assumption? Until recently, this point had not been definitively demonstrated, and the possibility remained that melanopsin was merely a photoisomerase while the photopigment had still to be identified (Fu et al., 2005). However, several studies have recently provided substantial evidence that melanopsin is indeed the photopigment responsible for the photoresponse of intrinsically photoreceptive ganglion cells (Qiu et al., 2005; Panda et al., 2005; Melyan et al., 2005; Fu et al., 2005). Evidence for a Functional, Melanopsin-driven Inner Retinal Visual Pathway in Humans Previous studies in humans have shown that a novel photopigment is responsible for melatonin regulation (Thapan et al., 2001; Brainard et al., 2001) and cone adaptation (Hankins & Lucas, 2002), but could not identify the photopigment involved. More recently, melanopsin has been shown to be present in human retina and retinohypothalamic tract (Dacey et al., 2005; Rollag et al., 2003; Hannibal et al., 2004),
79 69 but these studies did not directly address the physiological role played by this photopigment in humans. Our experiments extend these previous studies by demonstrating the influence of melanopsin-containing retinal ganglion cells on human pupillary responses, and hence providing further evidence for the existence of a functional, melanopsin-driven inner retinal visual pathway in humans. Acknowledgements The authors thank Dr. Timothy Kraft for help with ERG recording, Drs. Michael Loop and Thomas Norton for comments on the manuscript, and Sam Hayley and Jill Woods for technical assistance. This work was supported by NIH grant EY09380 and EyeSight Foundation of Alabama (PDG), EY06678 and EY09625 (DMD), Kayser Award, EY00901 (JP), and EY06837 and EY14596 (K-WY). References Alpern, M., & Campbell, F. W. (1962). The behaviour of the pupil during darkadaptation. Journal of Physiology-London, 165, 5 7. Alpern, M., & Ohba, N. (1972). The effect of bleaching and backgrounds on pupil size. Vision Research, 12, Baylor, D. A., Nunn, B. J., & Schnapf, J. L. (1984). The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. Journal of Physiology, 357, Baylor, D. A., Nunn, B. J., & Schnapf, J. L. (1987). Spectral sensitivity of cones of the monkey Macaca fascicularis. Journal of Physiology, 390, Berson, D. M., Dunn, F. A., & Takao, M. (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295, Bouma, H. (1962). Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature, 193,
80 70 Brainard, G. C., Hanifin, J. P., Greeson, J. M., Byrne, B., Glickman, G., Gerner, E., et al. (2001). Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience, 21, Clarke, R. J., Zhang, H. Y., & Gamlin, P. D. R. (2003). The primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. Journal of Neurophysiology, 89, Coren, S. (1987). A rapid method to assess crystalline lens pigment density in vivo. Acta Ophthalmologica, 65, Dacey, D. M., Peterson, B. B., Robinson, F. R., & Gamlin, P. D. (2003). Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron, 37, Dacey, D. M., Liao, H., Peterson, B., Robinson, F., Smith, V., Pokorny, J., et al. (2005). Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature, 433, Dolan, R. P., & Schiller, P. H. (1994). Effects of ON channel blockade with 2-amino-4- phosphonobutyrate (L-AP4) on brightness and contrast perception in monkeys. Visual Neuroscience, 11, Fu, Y., Liao, H. W., Do, M. T., & Yau, K. W. (2005). Non-image-forming ocular photoreception in vertebrates. Current Opinion in Neurobiology, 15, Gamlin, P. D. R., Zhang, H., & Clarke, R. J. (1995). Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Experimental Brain Research, 106, Gooley, J. J., Lu, J., Fischer, D., & Saper, C. B. (2003). A broad role for melanopsin in nonvisual photoreception. Journal of Neuroscience, 23, Hannibal, J., Hindersson, P., Ostergaard, J., Georg, B., Heegaard, S., Larsen, P. J., et al. (2004). Melanopsin is expressed in PACAP containing retinal ganglion cells of the human retinohypothalamic tract. Investigative Ophthalmology and Visual Science, 45, Hankins, M. W., & Lucas, R. J. (2002). The primary visual pathway in humans is regulated according to long-term light exposure through theaction of a nonclassical photopigment. Current Biology, 12, Hansen, R. M., & Fulton, A. B. (1986). Pupillary changes during dark adaptation in human infants. Investigative Ophthalmology and Visual Science, 27, Hattar, S., Kumar, M., Park, A., Tong, P., Tung, J., Yau, K. W., et al. (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology, 497,
81 71 Hattar, S., Liao, H. W., Takao, M., Berson, D. M., & Yau, K. W. (2002). Melanopsincontaining retinal ganglion cells:architecture, projections, and intrinsic photosensitivity. Science, 295, Hattar, S., Lucas, R. J., Mrosovsky, N., Thompson, S., Douglas, R. H., Hankins, M. W., et al. (2003). Melanopsin and rod cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424, Judge, S. J., Richmond, B. S., & Chu, F. C. (1980). Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Research, 20, Kondo, M., & Sieving, P. A. (2001). Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Investigative Ophthalmology and Visual Science, 42, Kraft, T. W., Neitz, J., & Neitz, M. (1998). Spectra of human L cones. Vision Research, 38, Loewenfeld, I. E. (1993). The pupil. Anatomy, physiology, and clinical applications (Vol.1). Iowa State University Press, Wayne State University Press: Ames, Detroit. Lucas, R. J., Douglas, R. H., & Foster, R. G. (2001). Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience, 4, Lucas, R. J., Hattar, S., Takao, M., Berson, D. M., Foster, R. G., & Yau, K. W. (2003). Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science, 299, Melyan, Z., Tarttelin, E. E., Bellingham, J., Lucas, R. J., & Hankins, M. W. (2005). Addition of human melanopsin renders mammalian cells photoresponsive. Nature, 433, Morin, L. P., Blanchard, J. H., & Provencio, I. (2003). Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. Journal of Comparative Neurology, 465, Newsome, D. A. (1971). Afterimage and pupillary activity following strong light exposure. Vision Research, 11, Nygaard, R. W., & Frumkes, T. E. (1982). Calibration of the retinal illuminance provided by maxwellian views. Vision Research, 22, Packer, O., Diller, L. C., Verweij, J., Lee, B. B., Pokorny, J., Williams, D. R., et al. (2001). Characterization and use of a digital light projector for vision research. Vision Research, 41,
82 72 Panda, S., Provencio, I., Tu, D. C., Pires, S. S., Rollag, M. D., Castrucci, A. M., et al. (2003). Melanopsin is required for non-image-forming photic responses in blind mice. Science, 301, Panda, S., Nayak, S. K., Campo, B., Walker, J. R., Hogenesch, J. B., & Jegla, T. (2005). Illumination of the melanopsin signaling pathway. Science, 307, Pokorny, J., Smith, V. C., & Lutze, M. (1987). Aging of the human lens. Applied Optics, 26, Pong, M., & Fuchs, A. F. (2000). Characteristics of the pupillary light reflex in the macaque monkey: metrics. Journal of Neurophysiology, 84, Qiu, X., Kumbalasiri, T., Carlson, S. M., Wong, K. Y., Krishna, V., Provencio, I., et al. (2005). Induction of photosensitivity by heterologous expression of melanopsin. Nature, 433, Rollag, M. D., Berson, D. M., & Provencio, I. (2003). Melanopsin, ganglion-cell photoreceptors, and mammalian entrainment. Journal of Biological Rhythms, 18, Shapley, R., & Enroth-Cugell, C. (1984). Visual adaptation and retinal gain controls. In N. Osborne & G. Chader (Eds.). Progress in retinal research (Vol. 3, pp ). London: Pergamon. Sieving, P. A., Murayama, K., & Naarendorp, F. (1994). Push pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Visual Neuroscience, 11, Thapan, K., Arendt, J., & Skene, D. J. (2001). An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. Journal of Physiology, 535,
83 73 THE INFLUENCE OF INTRINSICALLY PHOTOSENSITIVE RETINAL GANGLION CELLS OF THE SPECTRAL SENSITIVIY AND RESPONSE DYNAMICS OF THE HUMAN PUPILLARY LIGHT REFLEX Introduction The pupillary light reflex (PLR) is a well studied neurological reflex characterized by a reduction in pupil diameter in response to an increase in retinal illumination. The PLR is an important clinical metric of retinal, midbrain and autonomic function (Girkin, 2003; Kawasaki, 2005) as well as being a major determinate of retinal image quality (Campbell & Gregory, 1960; Hirata et al., 2003; Gamlin et al., 2007). Although it is well accepted that the major afferent influence on pupil diameter is environmental light levels, the nature of the light signal and the receptors responsible for the origin of this important reflex have historically been the subject of much disagreement (e.g. Alpern & Campbell, 1962; Bouma, 1962; Ten Doesschate & Alpern, 1965; Loewenfeld & Lowenstein, 1993). Such disagreements are made more understandable given the recent discovery of a new retinal photopigment, melanopsin (Provencio et al., 2000), which has been shown to contribute to the human PLR (Gamlin et al., 2007; Young & Kimura, 2008). Prior to 2004, all studies investigating the properties of the light signal driving the human PLR were conducted without the knowledge of melanopsin. Hence there is little detailed information on the influence of this photopigment on human pupillary behavior. Therefore, the purpose of the current study is to determine the influence of melanopsin on the sensitivity and response dynamics of the human PLR.
84 74 Intrinsically Photosensitive Retinal Ganglion Cells Recently, in mice and non-human primates, a class of retinal ganglion cells has been reported that express melanopsin (Gooley et al., 2001; Dacey et al., 2005), and are intrinsically photosensitive (Berson et al., 2002; Dacey et al., 2005). In addition to their intrinsic photosignal, these cells receive classical rod and cone inputs (Dacey et al., 2005; Jusuf et al., 2007). These cells have been termed intrinsically-photosensitive retinal ganglion cells (iprgcs). The two primary projections of iprgcs are the pretectum, the midbrain nuclei associated with the PLR, and the suprachiasmatic nucleus (SCN), the area of the brain responsible for circadian rhythms (Hattar et al., 2002; Dacey et al., 2005; Hattar et al., 2006). Although iprgcs receive classical rod and cone inputs, their unique intrinsic photosensitivity ensures that they code photic information differently from all other retinal ganglion cell types. In response to a pulse of light, these cells show a characteristic transient burst of firing at stimulus onset, which rapidly decays to a plateau of sustained firing that often extends well past stimulus offset (Berson et al., 2002; Dacey et al., 2005; Tu et al., 2005; Wong et al., 2007). It has been suggested that the initial burst of firing at stimulus onset is mediated by photoreceptors of the outer retina, while the sustained firing is driven by the melanopsin mediated intrinsic response (Dacey et al., 2005). More recent studies have also provided evidence that outer retinal photoreceptors also contribute to sustained firing during long duration light stimuli (Drouyer et al., 2007; Wong et al., 2007).
85 75 The Role of iprgcs in the Mammalian Pupillary Light Reflex Initial studies investigating the influence of iprgcs on the PLR utilized the mouse model, which allowed for the genetic manipulation of the different photoresponses involved in the reflex. It was shown that the PLR was present in rodless/coneless (rd/rd cl) mice, although the latency to maximal constriction was increased, and the irradiance needed to produce an equivalent constriction was higher than in wild type mice (Lucas et al., 2001). A subsequent study investigating the PLR in melanopsin knockout mice (opn -/-) found the PLR to be aberrant at high irradiances (Lucas et al., 2003). Studies involving human and non-human primates have suggest a role for iprgcs in the human and non-human primate PLR. Gamlin and colleagues (2007) found that when outer retinal photoreceptive signals were blocked pharmacologically, the PLR persisted in macaques, and that the spectral sensitivity of the residual response was closely matched by the spectral sensitivity of melanopsin, which is maximally sensitive to 483 nm light. In addition, this study found that in both humans and macaques, the intrinsic response of iprgcs is responsible for the previously reported sustained pupillary constriction following light offset induced by high intensity light stimuli (i.e., Newsome, 1971; Alpern & Ohba, 1972). Although conducted prior to the discovery of melanopsin, historical studies also suggest that iprgcs contribute to the human PLR. It has been shown that the pupils of rod achromats continues to respond to light increments well over levels commonly accepted to saturate rod photoreceptors (Alpern et al., 1960), thus implying an additional photopigment is involved in the pupillary responses of these individuals. Additionally, spectral sensitivity measurements of pupillary constriction to steady state illumination
86 76 have shown short wavelength sensitivity that is not well matched by either rod or S-cone contributions (Bouma, 1962). Historical investigations of the response dynamics of the PLR are also suggestive of a role for iprgcs in the behavior of the pupil in response to light increments. Several investigators have proposed models of pupillary dynamics which utilize both a transient and sustained component to the PLR (Kohn & Clynes, 1969; Young et al., 1993; Privitera & Stark, 2006). These transient/sustained dynamics are very similar to the cellular response of iprgcs, which display a transient response mediated by outer retinal photoreceptors, followed by a sustained response mediated the inner retinal photointrinsic response. A recent, brief report by Young and Kimura (2008), which reanalyzed previous data, reported the relative contribution of short and long wavelength light to the sustained component of the PLR, and suggested that melanopsin plays a role in the response. However, this study did not examine the complete spectral sensitivity of the PLR, so a more rigorous investigation is required to determine the complete extent of the influence of melanopsin on the human PLR. In addition, this study (Young & Kimura, 2008) only examined light-driven pupillary responses for 10 seconds or less. Therefore, the present study was undertaken to more fully describe the relative contributions of rod, cone, and intrinsic light responses to the spectral sensitivity and response dynamics of the pupil during long light stimuli, and to compare these responses to those obtained with briefer stimuli.
87 77 Methods Subjects Six subjects participated in at least one of the three different experimental conditions, while two subjects, A and B, participated in all experimental conditions. All subjects had normal corrected visual acuity and normal color vision as measured by the Nagel anomaloscope, Farnsworth D-15, and HRR plate test. Subjects A, B, D, and F were males ages 33, 29, 51, and 27 years respectively. Subjects C and E were females ages 33 and 40 years respectively. Subjects D and E required approximately +2 diopters of visual correction. All experimental procedures were approved by the UAB Institutional Review Board, and were undertaken with the understanding and written consent of each subject. Apparatus The same experimental apparatus (figure 1) was used in all three experimental conditions. The apparatus was specifically designed to measure consensual pupillary responses to light while minimizing confounding afferent influences on pupil diameter, i.e., changes in accommodation and convergence. The light from two different light sources, a tungsten halogen light source and a xenon projector, is presented to the right eye. The light from the xenon projector is used to produce an intermittent 36 degree stimulus presented in Maxwellian view to the right eye. The spectral character of the light from the xenon projector was determined by 11 narrow bandwidth interference filters (8-10 nm full width at half maximum, Andover), housed in a stepper motor driven
88 78 Monitired Stimulated Eye Eye Figure 1. Experimental apparatus. Abbreviations: AG Anti-reflective glass; BS 50/50 Beam splitter; BF Bandpass filter; CL Camera lens; CM Cold mirror; FW Filter wheel; HD Holographic diffuser; L1-L3 Lens doublets; LS Halogen light source; IR Infrared illuminators; M Mirror; ND Neutral density wedges; P Xenon light source
89 79 filter wheel. The intensity of the light which passed through the filter wheel was attenuated by a system of two counter rotating neutral density wedges (5 OD, Reynard) driven by a stepper motor. The stepper motors driving the filter wheel and neutral density filters were controlled using potentiometer feedback via computer. The halogen light source was used in two of the three experimental conditions to provide a diffuse 36 degree background adaptation field that was presented continuously throughout the experimental session. The spectral characteristics and intensity of the halogen light source were controlled by a narrow bandpass filter with peak transmission at 510 nm (Andover) and Kodak Wratten neutral density filter (Edmund Optics) respectively. A single pane of antireflective glass to which a 2 o black cross was affixed was placed in the optical path of both light sources, thus presenting a target to the subject's right eye when the light from either light source passes through the optical system. The position of the pane could be adjusted, to compensate for subject refractive error. A target generated on a computer monitor (2 o white cross) was presented to the subject's left eye via a badal lens system (see Bennett et al., 1998). The badal system allows the target to be viewed at optical infinity by each subject regardless of refractive error without producing any change in the angular size of the target. Both targets give accommodative feedback to each of the subject's eyes, while the perceived relative alignment of the black and the white crosses provides the subjects with vergence feedback. During the presentation of the monochromatic stimulus, the subjects could visualize both targets, and they were instructed to report any blurring or disassociation of the two targets which would indicate an undesirable change in accommodation or vergence angle. In this way, changes in a
90 80 subject's accommodation and vergence angle, which could act as confounding influences in these experiments, were minimized. Stimulus Calibration Spectral transmission through each of the interference filters used in the apparatus was shown to be reduced by at least 3 log units within a 15 nm deviation from the wavelength of peak transmission, as measured by a PR-680 spectroradiometer (Photo Research, Inc). Irradiance measurements were collected using an IL1700 Radiometer (International Light Technologies). These measurements showed a 1 log unit change in irradiance for every 140 steps turned by the stepper motor driving the neutral density wedges, thus yielding a theoretical irradiance control of better than one hundredth of a log unit per step. Spot checks of stimulus irradiance were performed prior to all experimental sessions to verify that there were no variations in stimulus intensity between sessions. All calibrations were measured after the light transversed all optical elements, i.e., near the location of the right eye in figure 1. Experimental Procedure Spectral sensitivity Measurements. Traditionally, the spectral sensitivities of pupillary responses were derived from complete irradiance response curves generated using monochromatic light. These curves were collected by stepping a dark adapted observer through increasing light increments at regular time intervals. The intensity of the light stimulus was thus varied from threshold levels to those producing a near maximal response. Once all the curves for each individual wavelength were collected, the
91 81 intensity of light necessary to produce a criterion response at each wavelength was then calculated and a spectral sensitivity produced. There are several problems with this approach, which we sought to eliminate with our experimental procedure. The primary problem is that the time involved in the collection of all the responses at any given wavelength prevents the collection of responses to all wavelengths in a single experimental session. It is well known that daily variations in pupillary responses and baselines occur within individual subjects (Loewenfeld & Lowenstein, 1993), and using the procedure described above allows these variations to have differential influences on each of the wavelength tested. Additionally, it is also well established that pupillary diameter can be affected by an individual's level of alertness (Lowenstein, Feinberg et al. 1963; Yoss, Moyer et al. 1970; Wilhelm, Giedke et al. 2001; McLaren, Hauri et al. 2002). The traditional procedure only provides an initial measure of the subject's baseline pupil diameter (no light stimulus present) and therefore no way of assessing whether a subject's state of arousal is changing and therefore adding or subtracting an influence on the subject's pupil diameter over and above than produced by the light stimulus. Additionally, these early studies were further hampered by the necessity to measure pupil diameter photographically, and therefore were unable to ascertain changes in pupil diameter in real time. Given modern advances in technology, it is now possible to record and measure pupil diameters in real time, allowing criterion responses to be estimated across all sampled wavelengths in a single experimental session. The spectral sensitivity data collected in the present study follows the general procedures set out by Webster and colleagues (1968), which allows for the more rapid
92 82 assessment of a predetermined criterion response, thus overcoming confounding influences of the previously described methods. In short, the real time measurement of the changes in pupil diameter allows for the use of a modified method of adjustment in which the irradiance necessary to produce a given criterion response at any wavelength of stimuli can quickly be estimated. Data is then collected only at a narrow range of irradiances (+/-.75 log quanta) above and below this estimate, and a more precise measure of the irradiance necessary to produce the criterion response at each wavelength is determined through post hoc analysis. An additional benefit of this approach is that the estimates of the irradiance required to produce the criterion response are generated from multiple repeats within a narrow range of irradiances instead of single repeats at a wide range of irradiances, thus further refining the precision of the irradiance response relationship at or near the criterion response. Data Collection. During experimental sessions, both of the subject s eyes were visualized under infrared illumination via video camera. The diameter of the left eye was measured at 50 Hz with an ISCAN RK406 pupillometer system, which was calibrated with apertures of known diameters placed at the plane of the subject's eye. Prior to each experimental session, the subject s right eye was dilated with topical 1% tropicamide in order keep their pupils in open loop conditions. The subjects' right eyes were precisely aligned with the optical system prior to each experimental trial, and any trials in which this alignment was not maintained were discarded. In general, the spectral sensitivity of each of the five stimulus duration conditions (1, 3.16, 10, 31.6, and 100 seconds) in each experimental condition was collected in separate experimental sessions. The exception
93 83 being, that the two shortest duration conditions were collected in the same session for each of the three experimental conditions. Therefore, the collection of all the spectral sensitivities for a single subject for all of the duration, response, and adapting conditions required 11 experimental sessions. The same general procedures were utilized in each experimental session, and are as follows. Given the findings that suggest the intrinsic response of iprgcs is mediated by a bistable photopigment Melyan et al., 2005; Mure et al., 2007), we were concerned that a pseudorandom presentation of the different wavelength stimuli could introduce a confounding potentiation of subsequent wavelength responses. Therefore, to control for any such confounds, we systematically moved though the different wavelength stimuli in an alternating sequence from short to long wavelengths and from long to short wavelengths. Each session started by measuring a subject s responses to three stimuli of either the longest or shortest wavelength used in a particular condition, followed by three repeats at the next wavelength, etc., until the subject had been tested at all wavelengths used in a particular condition. The subject was then offered a break, and subsequently this procedure was repeated, but the wavelengths were presented in reverse order, i.e., either from short to long wavelength or long to short wavelength based on the previous sequence. The same procedure was followed a third and final time following a brief break. In this way the subjects pupillary responses at each wavelength were assessed a total of nine times during an experimental session. There was no evidence of a sequence effect in the measured pupillary responses, which would be indicated by systematic differences in pupillary responses at a given wavelength based on the previous wavelengths tested. Intertrial intervals were approximately two minutes in duration, this
94 84 allowed for the repositioning of the neutral density wedges and filter wheels as necessary between trials. A slight deviation from this procedure was necessary for the 100 second duration condition, in which time permitted the measurement of only one trial per wavelength per sequence for a total three trials per wavelength total. Each trial during an experimental session was also of the same general design. The trials began with the presentation of a fixation target to the left eye for approximately 13 seconds, during which the subject's baseline pupillary diameter was measured. Following this fixation period, a monochromatic stimulus was presented to the right eye for approximately 4, 12, 34, or 110 seconds depending on the duration condition being assessed. Both the baseline and stimulus durations were randomly varied from trial to trial to prevent anticipation of either stimulus onset or offset by the subject, although the measurement interval was the same for each trial of a given duration condition. The change in pupil diameter produced by a given stimulus was determined by subtracting the baseline pupil diameter from the pupil diameter measured during a specific interval near the test stimulus offset. Baseline pupillary diameter was always measured during the 5 seconds immediately preceding the onset of the test stimulus. The test stimulus induced pupillary diameter was always measured for an interval centered at either 1, 3.16, 10, 31.6 or 100 seconds (0-2 log seconds) with the interval defined by 15% of the stimulus duration (figure 2). For example, the light induced change in pupillary diameter produced by a 10 second trial would be generated by measuring the average diameter of a subjects pupil during an interval from 9.25 seconds to seconds after stimulus onset (1.5 second interval centered at 10 seconds), which is then
95 85 Figure 2. Average pupillary behavior in response to three different monochromatic light stimuli, 450 nm (blue trace), 530 nm (green trace), and 610 nm (red trace) from experiment 2, with durations of (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 31.6 seconds, and (E) 100 seconds (n=6). The black bar in the upper right hand corner of each panel indicates the measurement interval utilized in each of the five duration conditions.
96 86 subtracted from the average pupil diameter measured during an interval from 0 to 5 seconds before stimulus onset. Data Analysis The pupil diameter of the subject s left eye was continuously recorded during each behavioural trial and these data were written to computer via analog to digital convertor board (National Instruments Corp.) for analysis offline. The procedures for that analysis are as follows. Initially, the average baseline pupil diameter, measured as previously described, was determined for an experimental session and used to determine the precise value of the criterion response, i.e., 1/2 or 3/4 maximal pupillary constriction, for that particular experimental condition. These values varied as much as 0.8 mm between subjects based on their typical resting pupil diameter, yet they were very consistent (+/- 0.1 mm) for the same subject over different experimental sessions. Any trials with baselines that deviated +/- 0.5 mm from the average baseline for the session were excluded from further analysis. The differences in pupil diameter produced by the monochromatic stimuli were measured as described above and plotted as a function of irradiance. Previous findings indicate a linear relationship between irradiance and pupillary constriction at half maximal constriction in primates (Gamlin et al., 2007), therefore a linear regression analysis was performed on the scatter plot and a regression function was generated for each individual wavelength used during the session. This function was then used to predict the irradiance necessary to produce the criterion response at that wavelength. An individual spectral sensitivity plot was then generated by determining the irradiance necessary to produce this criterion response at the most
97 87 sensitive wavelength, then subtracting the irradiances necessary to produce the criterion response at all the remaining wavelengths from that peak value, thus producing a log relative sensitivity for each individual subject for each experimental condition. Average spectral sensitivities across subjects were produced by aligning each of the subject s spectral sensitivity plots relative to each other in order to produce the least scatter between all the curves via the Excel solver routine. Once the scatter had been minimized, an average of the values at each wavelength was generated. Curve Fitting To produce a smooth function through the average spectral sensitivities, we sought to combine the known sensitivities of the photoreceptive processes influencing iprgcs. That is to say, that the sensitivity to any particular wavelength would be produced by the combination of the sensitivity of all the photoreceptive processes, both intrinsic and extrinsic, that are sensitive to that particular wavelength. Given that iprgcs receive outer retinal inputs from rod and cone photoreceptors (Dacey et al., 2005; Tu et al., 2005; Viney et al., 2007; Wong et al., 2007), the simplest estimation of the spectral sensitivity of iprgcs would have the form S(λ) = S inner (λ) + S outer (λ) (1) where S inner represents the spectral sensitivity of the inner retinal photoreceptive mechanisms of iprgcs, i.e., the melanopsin mediated intrinsic response, and S outer
98 88 represents the outer retinal inputs received by iprgcs. If we first concern ourselves with the S outer term, we can further define this as a combination of rod and cone inputs. S outer (λ) = S rods (λ) + S cones (λ) (2) To move from the theoretical model of equation (2) to a more specific mathematical prediction of the spectral sensitivity of the outer retinal photoreceptive signal impinging on iprgcs, one must address how the spectral sensitivity of each individual cell type is combined to produce the composite sensitivity. It has been proposed by Quick (1974) that the total sensitivity of an array of elements each having different individual sensitivities can be modeled with a function of the form S total =[ i (S i k ] 1/k (3) where S is sensitivity of the entire array and the parameter k defines how the individual sensitivities are combined. This model, often termed the Quick pooling model, has been successfully used to model the sensitivity of a variety of visual functions, such as contrast sensitivity (Robson & Graham, 1981), mesopic spectral sensitivity (Kurtenbach et al., 1999) and increment threshold spectral sensitivity (Miyahara et al., 1996). A more complete description of the Quick pooling model can be found in Graham (2001). To produce a more precise estimation of the spectral sensitivity of the entire array of outer retinal photoreceptive elements, we can convert equation (2) into the form of equation (3)
99 89 S outer (λ) = ({r[s rods (λ)]} k1 + {c[s cones (λ)]} k1 ) 1/k1 (4) For the purposes of the current study, we further defined the sensitivity of the rod and cone signals as S rods = S V lambda (λ) (5) and S cones (λ) = {p[s lws (λ)] + (p-1) [S mws (λ)]} (6) where S V'lambda is the CIE scotopic luminosity function, and where S lws and S mws are the LWS and MWS Stockman and Sharpe 10 o cone fundamentals respectively, (Stockman & Sharpe, 2000), and p defines the LWS/MWS cone ratio. In order to limit the free parameters involved in fitting the function to the data, the LWS/MWS ratio was fixed at (p = 0.62), which is the LWS/MWS ratio of the standard observer (Pokorny et al., 1993). The addition or subtraction of a SWS cone signal did not improve the fit of the function to that of any spectral sensitivity data collected in the present study, and therefore was omitted from the current model. This was expected, as it has been previously suggested that primate iprgcs receive only MWS and LWS ON inputs (Dacey et al., 2005). All spectral sensitivity templates were corrected for prereceptoral filtering in order to convert the corneal sensitivity functions to retinal sensitivity functions. The prereceptoral filtering estimates were produced by using the average age
100 90 of the subjects involved in studies on which the templates were generated (e.g., Wald, 1945; Crawford, 1949) and predicting the average prereceptoral filtering based on this average age using the method of Pokorny et al. (1987). Figure 3 illustrates the effect of changing the parameters k 1 in equation (4) on the composite spectral sensitivity of outer retinal photoreceptive responses. In the simplest case, k=1, the total sensitivity is defined by the linear sum of the rod and cone sensitivities (Panel A). As k increases to values slightly greater than 1, a nonlinear addition of the individual sensitivities occurs, which can be used to approximate a situation of probability summation, where the sensitivity of the array is slightly augmented at the points at which the sensitivities of individual elements in the array overlap over the parameter of interest. More specifically in the case of spectral sensitivity, probability summation would cause the sensitivity of the array to a given wavelength to be slightly enhanced at wavelengths to which more than one photoreceptor type is nearly equally sensitive (Panel C). Furthermore, as k is increased from a value slightly larger than 1 towards infinity, the sensitivity of the array approaches a situation of winner takes all, where the most sensitive element in an array at a particular wavelength defines the total sensitivity of the array at that wavelength (Panel E). Figure 3 also illustrates the effect of changing the relative sensitivities of the rod and cone signal, i.e., varying the relative values of r and c in equation (4), on the composite function while keeping k constant. For example, if a large mesopic stimulus is used to assess spectral sensitivity, it would be likely that the relative contribution of rod photoreceptor sensitivity to the composite sensitivity would be larger than that of cones,
101 91 Figure 3. Illustration of the effect of changing the curve fitting parameters of equation (4) on the spectral sensitivity of a combination of rod and cone signals. Panels A, C, and E demonstrate the effect of changing the value of the parameter k to (A) 1, (C) 2, and (E) 100. Panels B, D, and F demonstrate the effect of changing the relative contribution of the rod and cone signals on the spectral sensitivity of the overlying function, by changing the c parameter to (B) a value equivalent to r, (D) a value one tenth the value of r, and (F) three hundredths the value of r.
102 92 due to the extrafoveal nature of the stimulus. This could be modeled by reducing the value of the c parameter relative to r. If c is one half the value of r, this would yield a function produced by the combination of rod sensitivity with a cone sensitivity that was log units less sensitive relative to the rod signal (Panel B). Similarly, if c is reduced to one tenth the value of r (panel D) or roughly three hundredths the value of r (panel F), the composite function would be made up a cone sensitivity reduced by 1 and 1.5 log units respectively relative to the rod sensitivity. Following similar reasoning to our combination of outer photoreceptive mechanisms just discussed, equation 1 can be converted into the form of equation (3), S(λ) = {[S inner (λ)] k2 + [Souter(λ)] k2 } 1/k2 (7) Given the differing origins and physiology of the photoreceptive mechanisms responsible for the S inner and S outer terms, we allow for the possibility these two mechanisms may combine differently than the rod and the cone signals in the S outer term. This is reflected in the differentiation of the two k parameters between equation (4) and equation (7), where k 1 reflects the combination rule for the outer retinal sensitivities and k 2 reflects the combination of the outer retinal signals with the intrinsic response. Expanding equation 7 to reflect the specific spectral sensitivity estimates used in this study and combining it with equation (7) yields S(λ) = ({(m[s opn4 (λ)]) k2 + [{(c[s cones (λ)]) k1 + (c[s rods (λ)]) k1 } k1 ] 1/k1 } k2 ) 1/k2 (8)
103 93 where the parameters m, along with the previously discussed parameters r, and c, allow the relative weights of the intrinsic, cone, and rod photoreceptive influences on the total spectral sensitivity to be adjusted, and where S opn4 is a Baylor nomogram (Baylor et al., 1987) with a lambda max at 483 nm. The Microsoft Excel solver routine was used to fit equation 8 to each of the spectral sensitivity plots generated in the study. Specifically, the parameters m, c, and r were varied to minimize the sum of squares of the residuals of the function from the collected data. That is to say, that the relative gain of the intrinsic, cone, and rod photoresponses were systematically changed until the deviation of the function from the actual data points at each wavelength measured was minimized. The values of the combination parameters k 1 and k 2 were also optimized by comparing their influence on the goodness of fit of the function to the data. Once the optimal values were determined, these parameters were fixed at those values for all functional fits to the data (see section 3.5 for more details). Results The present study was conducted to determine the influence of the intrinsic photoresponse of iprgcs on the human pupillary light reflex. As with any photoreceptive process, the intrinsic response of iprgcs has a unique photic sensitivity that can be utilized to indicate its influence on visually driven behaviors. By determining the spectral sensitivity of a behavior and comparing it to known spectral sensitivities of the photoreceptive processes of the human eye, one can ascertain the degree to which each of these process drive the behavior in question.
104 94 Experiment 1 Given the known differences in the speed and magnitude of the light adaptation of the intrinsic and outer retinal photoresponses (Dacey et al., 2005; Wong et al., 2007) we first sought to determine if the intrinsic response of iprgcs would act to overcome the light adaptation of outer retinal photoresponses and drive the PLR in response to steadystate light increments. To test this hypothesis, we measured the spectral sensitivity of half-maximal pupillary constriction for light durations increasing from 1 second to 100 seconds at one-half log second intervals. If our hypothesis was correct, we would expect the spectral sensitivities of the short duration light stimuli to be indicative of outer retinal photoreceptive processes, while the spectral sensitivities of the long duration light responses would indicate a contribution by the intrinsic response. The spectral sensitivities of the intermediate durations would also show a transition between each of these two extremes. The average spectral sensitivity of three subjects are shown in figure 4. The spectral sensitivities of all the duration conditions show an enhanced sensitivity to short wavelength light, although the wavelength of peak sensitivity changes from 510 nm (panel A) to 470 nm (panel D and E) as the duration of the light stimuli increases. The spectral sensitivities for all the duration conditions also point to a reduced sensitivity to long wavelength light, with the sensitivity to long wavelengths relative to short wavelength decreasing from -1 log units for the shortest duration stimulus (panel A, 510 nm vs. 610 nm) to -2 log units for longest duration stimulus (panel E, 470 nm vs. 610 nm). It is important to note as well, that the absolute irradiance necessary to produce half-maximal pupillary constriction at the wavelength of peak sensitivity, plotted on the right y-axes in figure 4, increases from 10.5 log quanta/cm 2 for short duration stimuli
105 95 Figure 4. Spectral sensitivity of half-maximal pupillary constriction with no adapting field present. Mean spectral sensitivity measurements (n=3) at nine different wavelengths are represented by ( ) for five different stimulus duration conditions, (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 31.6 seconds, and (E) 100 seconds (SEM error bars). The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities (see text and table 1 for details).
106 96 (panels A and B) to ~11.5 log quanta/cm 2 for long duration stimuli (panels D and E). Taken together, these results suggest not only a shift in peak relative spectral sensitivity as the duration the light stimuli increase, but also a decrease in the absolute sensitivity of response. None of the spectral sensitivity plots in figure 4 are well fit by a single photoreceptive mechanism, and therefore must be the result of a combination of two or more different mechanisms. In order to ascertain the underlying mechanisms and their relative contributions to the response at each of the duration conditions, a smooth curve of the form of equation 8 was fitted to each spectral sensitivity plot (continuous line in panels A-E). Table 1 list the values of the parameters r, c, and m of equation 8 that produce the best fit to the data at each duration condition. These values can be thought of as the gain of each of the photoreceptive signals needed to fit the data, and they provide an insight into the relative contribution of either the intrinsic, rod, or cone photoresponses to the spectral sensitivity at each duration condition. It is clear from the data of table 1 Table1. Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 1 Photoreceptive 1 sec 3.16 sec 10 sec 31.6 sec 100 sec Mechanism Rods Cones Intrinsic Response
107 97 that the outer retinal photoreceptive mechanisms drive the response at 1 second, 3.16 seconds, and 10 seconds. Furthermore, by comparing the gain values of the rod and cone photoresponses at these duration conditions, it can be concluded that the rod response dominates the spectral sensitivities at these durations, and that the relative contribution of the cone photoresponse decreases systematically from 1 to 10 seconds. Additionally it is clear that the intrinsic photoresponse does contribute significantly to the spectral sensitivity of the response at 31.6 seconds and 100 seconds. It should be noted that the values in table 1 only reflect the relative contribution of the intrinsic, rod, and cone photoresponses at each time interval without regard to the decrease in overall absolute sensitivity seen as stimulus duration is increased. Experiment 2 The most unexpected result of experiment 1 was the dominance of the rod photoresponse in the outer retinal photoreceptor component of the spectral sensitivity plots at all duration conditions. Previous investigations of the spectral sensitivity of the human PLR to transient stimulation have reported a greater contribution by L and M- cone photoresponses (Alpern & Campbell, 1962; Kimura & Young, 1995) than that found in our experiment. These previous experiments were conducted under conditions that may have produced a saturation of the rod photoresponses, and therefore we sought to repeat our initial experiments using a steady-state 510 nm adapting field to selectively adapt the rod photoresponse. It is generally accepted that rod saturation of the conscious visual pathway occurs at an intensity of ~ 3 log scotopic trolands for steady-state adapting backgrounds (Adelson, 1982). The results of experiments conducted with
108 98 background intensity such as this, were unsatisfactory for the majority of subjects. In these subjects, either baseline pupil diameters decreased steadily to unacceptable levels within 15 minutes of the start of the experimental session, or baseline pupil diameters abruptly decreased following exposure to a monochromatic light stimulus and never returned to acceptable levels without removal of the adapting stimulus. This is consistent with either the slow integration of the adapting field by the intrinsic response, similar to results reported by Dacey et al. (2005), or a photopotentiation of the adapting field by the previous exposure to the monochromatic light stimuli, similar to that reported in the murine PLR (Zhu et al., 2007). In order to balance these confounding effects of the adapting background with the intended effect of light adaptation of the rod photoresponse, we utilized a 50 scotopic troland (1.7 log scotopic troland) adapting background during experiment 2. A further modification of experiment 2 from experiment 1 was the use of an additional long wavelength monochromatic light stimulus, a 650 nm stimulus for the three shorter duration conditions and a 630 nm stimulus for the two longer duration conditions. This long wavelength stimulus was added in order to better characterize the cone contribution of the response and to verify that the decrease in long wavelength sensitivity seen in experiment 1 was not due to an opponent mechanism similar to that responsible for the Sloan notch observed in increment threshold spectral sensitivities (e.g., Sperling & Harwerth, 1971). The use of the 630 nm stimulus was required at longer durations because the intensity of the 650 nm stimulus necessary to produce a half maximal pupillary constriction at these durations proved uncomfortably bright for some subjects.
109 99 The results of experiment 2 utilizing five subjects (figure 5) are remarkably similar to those of experiment 1. The spectral sensitivity plots at each duration condition show greatest sensitivity to short wavelength light and a marked insensitivity to long wavelength light. This long wavelength insensitivity is evident at both 630 nm and 650 nm, thus showing no evidence of chromatic opponency in the spectral sensitivity of the cone contribution to the PLR. If cone chromatic opponency were present, it would be indicated by an abrupt decrease in sensitivity at ~ 590 nm, followed by an increase in sensitivity at wavelengths greater that 600 nm peaking at ~610 nm (Sperling & Harwerth, 1971). As in experiment 1, there is again a shift in peak sensitivity from 510 nm for the shorter duration conditions (panels A-C) to 470 nm and 490 nm in the longer duration conditions (panels D and E), and, as stimulus duration increases, an increase in the absolute irradiance needed to produce a half maximal response. It is interesting to note that even with the addition of an adapting background, the intensity of these absolute irradiance values only deviated slightly from the values found in experiment 1. This would suggest that the overall light adaptation level of the photoreceptive responses driving the PLR in experiments 1 and 2 are not driven by the absence or presence of light between trials, but more likely by the overall influence of the monochromatic light stimuli themselves. A function of the form of equation 8 was again used to fit a smooth curve to the spectral sensitivity plots (smooth line in each panel of figure 5). The values of the parameters of r, c, and m necessary to produce the best fit to the data in experiment 2 are displayed in table 2. These parameters show the same general pattern as that found in experiment 1, i.e., a dominance of the rod photoresponse in the outer retinal signal, and
110 100 Figure 5. Spectral sensitivity of half-maximal pupillary constriction with a 50 troland adapting field present. Mean spectral sensitivity measurements (n=5) at ten different wavelengths are represented by ( ) for five different stimulus duration conditions, (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 31.6 seconds, and (E) 100 seconds (SEM error bars). The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities (see text and table 2 for details).
111 101 the emergence of the intrinsic photoresponse at the 31.6 and 100 second duration conditions. The addition of the 630 nm or 650 nm points did not affect the relative gain between the rod and cone photoresponses found in experiment 1, thus further demonstrating the dominance of the rod photoresponse in the outer retinal component of the spectral sensitivity of the half maximal PLR at all duration conditions. Table 2. Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 2 Photoreceptive 1 sec 3.16 sec 10 sec 31.6 sec 100 sec Mechanism Rods Cones Intrinsic Response Experiment 3 It has been demonstrated in numerous studies that a decrease in pupil diameter has a significant impact on retinal image quality by increasing depth of focus (Campbell, 1957; Tucker & Charman, 1975) and reducing the effects of optical aberrations (Campbell & Gubisch, 1966; Woodhouse, 1975; Williams & Chalupa, 1983). This improvement in retinal image quality leads to a subsequent improvement in visual acuity, and it has been found that a pupil diameter of ~ 3 mm is optimum for visual acuity
112 102 (Campbell & Gregory, 1960; Campbell & Gubisch, 1966; Tucker & Charman, 1975; Woodhouse, 1975). Additionally, it has been shown in the murine PLR that the intrinsic photoresponse of iprgcs is necessary for complete pupillary constriction (Lucas et al., 2003). Taken together, these finding suggest that the intrinsic photoresponse would dominate the spectral sensitivity of pupillary constrictions to diameters optimal for visual acuity, even for short duration stimuli. To investigate this possibility, we sought to use a paradigm similar to that of experiment 2, except the criterion response used would be a 3/4 maximal response rather than a 1/2 maximal response. This criterion would produce an average absolute pupil diameter at or just below that reported for maximal visual acuity, while remaining sufficiently below a maximal response to allow for accurate irradiance estimates using the methods of experiment 1 and 2. The results of experiment 3 using three subjects from experiment 1 and 2 are shown in figure 6. The 1 second duration condition (figure 4A and 5A) was replaced by a 1.78 second duration condition (figure 6A) in experiment 3, since the sluggish nature of the iris musculature prevented 3/4 maximal constriction within 1 second of light onset. The spectral sensitivity plots for all four stimulus duration conditions again showed an increased sensitivity to short wavelength light. The insensitivity to long wavelength light increases as the duration of the light stimulus increases, which parallels the trend seen in experiments 1 and 2. This would suggest that the outer retinal photoreceptive processes are again adapting, therefore becoming less sensitive at the longer duration time points. Two major differences between the results of experiment 3 and experiments 1 and 2 were observed. First, as expected the average absolute irradiance needed to produce the three-quarter maximal response increased to ~12.5 log quanta/cm 2 /sec from an average of
113 103 Figure 6. Spectral sensitivity of three quarter-maximal pupillary constriction with a 50 td adapting field present. Mean spectral sensitivity measurements (n=3) at ten different wavelengths are represented by ( ) for four different stimulus duration conditions, (A) 1.78 seconds, (B) 3.16 seconds, (C) 10 seconds, and (D) 31.6 seconds (SEM error bars). The left y-axis represents the log spectral sensitivity relative to the most sensitive wavelength at each duration condition. The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities (see text and table 3 for details).
114 104 ~11.0 log quanta/cm 2 /sec necessary to produce a half maximal constriction. Secondly, the wavelength of peak sensitivity is either 490 nm or 470 nm for all duration conditions, not just the longer duration conditions. This suggests that the intrinsic response is responsible for the peak sensitivity of the response at all duration conditions. This is borne out by the curve fitting parameters used to produce the smooth function through the plots (table 3), which indicate the dominance of the intrinsic response at all duration conditions. The relative values of the r and c parameters are also slightly different than Table 3. Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 3 Photoreceptive 1.78 sec 3.16 sec 10 sec 31.6 sec Mechanism Rods Cones Intrinsic Response those produced in experiments 1 and 2, where the value of r is much lower in experiment 3 and the value of c is very similar for all three experiments for the same duration condition. This would suggest a decrease in the relative contribution of the rod photoresponse to the outer retinal photoreceptive mechanisms in experiment 3 versus the previous two experiments and may be indicative of some saturation of the rod response at the irradiances used to drive the 3/4 maximal constriction.
115 105 Adaptation of photoresponses The change in the relative gains of the outer retinal photoresponses over time was an important result of experiments 1 and 2, and much can also be elucidated about the adaptation of the intrinsic response, or lack thereof, in experiment 3. By incorporating the decrease in the absolute sensitivity of the response over time with the values of relative gains of each of the three photoresponses involved in the response (tables 1-3), it is possible to plot the absolute change in the gain of the photoresponses due to time of light exposure, or more simply their rate of light adaptation. To incorporate the two values in question into a single plot, we first took the log of the curve fitting parameters r, c, and m, thus allowing the comparison of the relative sensitivities of the responses on a log scale (see figure 3). This allowed for the addition of the decrease in log absolute sensitivity as stimulus duration was increased to the log of the gain components. This combination produced a plot which incorporated both changes in the relative gains of the photoresponses and the global changes in gain in all photoresponses over time (figure 7). In this plot, the change in the absolute gain of each photoresponse relative to the most sensitive response at the shortest time duration is plotted as a function of time for each of the three experiments. The presence of light adaptation would be indicated by an exponential decay of the photoresponses on a linear timescale. The integration of light over time, which has been reported in the intrinsic response of iprgcs (e.g. Dacey et al., 2005), would be indicated by the inverse behavior, with the gain of the intrinsic response increasing exponentially in the linear plots. The data from all three experiments show evidence for light adaptation of the outer retinal photoresponses of iprgcs. There is no convincing
116 106 Figure 7. Relative contribution of the rod, cone, and intrinsic photoresponse to the spectral sensitivity of the PLR over time
117 107 evidence of light integration or light adaptation of the intrinsic response in the data of experiments 1 and 2 (Panels A and B). On the contrary, the results of experiment 3 (Panel C) indicate a slow light adaptation of the intrinsic response. A three parameter single exponential function of the form, Adapt(t) = Adapt 0 + A(e -αt ) (9) was fit to the photoresponse data which showed evidence of adaptation (smooth lines in figure 7 A-C). The parameters of equation (9) give an indication of the speed and magnitude of the adaptation, where A estimates the magnitude of the total loss in log sensitivity from time 0, and the reciprocal of α is the time constant of the decay. Table 4 list the values of these two parameters plus the R 2 values of the curve fitting for each photoreceptive mechanism under each of the three experimental conditions. These results show that although the time constant of cone adaptation is greater than that of rods, the magnitude of the loss in sensitivity is approximately twice as large in cones as in rods. Additionally, the time constant of the decay, or the time is takes to reach a 66% loss in sensitivity, of the outer photoreceptor signals in each experimental condition are sufficiently short to suggest that complete adaptation of these photoresponses are seem within the longest stimulus duration condition in each experiment. Combination of photoresponses As mentioned previously, the parameters k 1 and k 2 of equation (8) represent the rules of combination for the individual outer retinal photoresponses (k 1 ), and the
118 108 combination of this composite outer retinal signal with that of the intrinsic photoresponse of iprgcs (k 2 ) Table 4. Curve fitting parameters used to fit equation (9) to the photoreceptor adaptation data for each experimental condition. Rods Cones Intrinsic Response Experiment 1 Experiment 2 Loss of sensitivity Time constant 11.5 sec 24.6 sec R Loss of sensitivity Time constant 6.0 sec 19.1 sec R Experiment 3 Loss of sensitivity Time constant 8.9 sec 14.6 sec 27.5 R
119 109 In order to limit the free parameters involved in the fitting of equation (8) to the individual spectral sensitivity plots, we sought to optimize these two parameters, thus allowing a fixed value to be used for each of our curve fitting procedures. To accomplish this optimization, we first sought to determine what k 1 value best represented the combination of rod and cone signals in the outer retinal component of the response. Given that the spectral sensitivities from the 1 and 3.16 second duration conditions of experiment 1 and 2 were clearly driven exclusively by outer retinal photoresponses, we systematically changed the value of k 1 used in our curve fitting of these data and determined its effect on the average goodness of fit to these data (figure 8a). We found that as the value of k 1 became increasingly small, the standard deviation of the data from the function at each wavelength tested was significantly reduced. This suggested that the outer retinal signal impinging on iprgcs and driving the PLR is the result of the linear summation of rod and cone photoresponses. Similarly, to determine the optimum value of k 2, we fixed the value of k 1 at 1, and calculated the effect of changing the value of k 2 on the average goodness of fit of our function to the data of duration conditions which clearly were influenced by the intrinsic response of iprgcs (figure 8b). We found that as k 2 became increasingly large, the standard deviation of the data from the function at each wavelength tested was significantly reduced. This suggests that the intrinsic response of iprgcs acts in a "winner takes all" manner, and is exclusively driving the PLR when the threshold for this response is exceeded. Given the results of this optimization, the values of k 1 and k 2 were fixed at 1 and 10, respectively, when fitting equation 8 to the data of each experiment in this study.
120 110 Figure 8. Optimization of the combination parameters of equation (8) for inner and outer retinal signals Discussion The major findings of this study are that the intrinsic photoresponse of iprgcs contributes not only to pupillary constriction at high irradiances, but also acts in conjunction with rods to maintain pupillary diameter in low photopic steady-state lighting conditions. We have also characterized how the photic signals of the inner and outer
121 111 retina dynamically combine to produce pupillary constriction. We found that in response to steady-state light steps, within 10 seconds of light onset, cones contribute only slightly to the maintenance of steady-state pupillary diameter at both low and high photopic irradiances. Furthermore, we have shown that rod contributions to the PLR also adapt, but ultimately reach a steady state which still contributes significantly to steady-state pupillary constriction at both low and high photopic irradiances. In addition, we provide evidence that the outer retinal photoresponses do not linearly combine with the inner retinal intrinsic response, but in fact the two signals act in a winner take all mechanism. The findings of the present study are distinctive in the fact that they have relevance to two fields of study. It is clear that iprgc physiology is the major determinant of pupillary light sensitivity and dynamics (Gamlin et al., 2007; Kawasaki & Kardon, 2007; Guler et al., 2008; Young & Kimura, 2008). Therefore, these findings not only address persistent questions regarding pupillary dynamics and spectral sensitivity, but also have implications in the understanding iprgc physiology and thus the neural signals driving non-image forming (NIF) visual behaviors. It should be noted that there is increasing evidence for different morphological (Provencio et al., 2002; Dacey et al., 2005; Hattar et al., 2006; Jusuf et al., 2007; Viney et al., 2007; Baver et al., 2008) and functional (Tu et al., 2005; Viney et al., 2007) classes of iprgcs, and for a differential projection of these cell classes to the pretectum and the hypothalamus (Baver et al., 2008). These findings may cast doubt on the ability to generalize the findings of the current study to iprgc physiology and other NIF visual functions, but despite the above studies, there is very little evidence for a differential light sensitivity between different types of NIF behaviors and in vitro recordings of iprgcs.
122 112 It has recently been reported that the irradiance sensitivity of circadian phase shifting and steady-state pupillary constriction differ by as much as 1.5 log units in the Syrian hamster (Hut et al., 2008). However, it is generally accepted that circadian phase shifting can be significantly influenced by the long term light integration of the intrinsic photoresponse, and shows temporal summation over hundreds of seconds (Nelson & Takahashi, 1991; 1999). Therefore, these findings can easily be explained by the author s use of stimulus durations which differed by 1.5 log seconds (900 sec vs. 30 sec) in generation of the two types of responses. Given the recent evidence that iprgcs provide all photic signals which drive the murine PLR (Guler et al., 2008), coupled with our previous studies demonstrating a close correspondence between iprgc physiology and the behavior of the human and non-human primate PLR (Gamlin et al., 2007), we believe that human pupillary responses to light provide a powerful model of the light sensitivity of human iprgcs as well as other NIF visual behaviors. Light adaptation of the PLR and NIF visual system One of the most significant findings of the present study is the effect of light adaptation on the relative contribution of the photoreceptive mechanisms which drive the PLR and thus the NIF visual system. In contrast to the currently held belief that the intrinsic response of iprgcs only drives NIF functions at high irradiances (Hattar et al., 2003; Mrosovsky & Hattar, 2003; Panda et al., 2003), we have shown that this response acts to maintain NIF functions following light adaptation of outer retinal photoresponses, even at low photopic luminances. Additionally we were able to determine the kinetics of the light adaptation of both the outer and inner retinal photoresponses at several different
123 113 light intensities and adaptation states. These decay kinetics not only serve to further elucidate the relative contributions of rod, cone, and intrinsic photoresponses to NIF functions, but also serve to validate the findings of in vitro studies in which the health of the cells involved in the responses might have been compromised. Light adaptation of the visual system has traditionally been measured using two experimental paradigms. One involves measuring the effect of adapting backgrounds on visual threshold, while the other involves following the kinetics of adaptation of a photoresponse in response to a long duration steady-state light step (see Perlman & Normann, 1998). Given that the current study is concerned with suprathreshold responses, more specifically half-maximal and three quarter maximal responses, comparisons to the later of the two experimental paradigms is more reasonable. A recent study evaluating the site of light adaption in primate retina found that in response to low intensity light stimuli, light adaptation occurs post-receptorally, i.e., in cells which fall between the photoreceptors and ganglion cells in the visual processing stream of the retina. Conversely, the same study found that light adaptation to high intensity light stimuli occurs primarily via mechanisms intrinsic to the photoreceptors themselves (Dunn et al., 2007). These finding suggest that the kinetics of the light adaptation of the outer retinal photoresponses measured in the current study should closely mirror the decay kinetics of rod and cone photocurrents measured in response to steady-state light steps. Studies investigating the slow decay of cone photocurrents in response to steadystate light steps in a variety of animal models show good agreement with our measured decay in cone contribution to the PLR under similar intensities. In recordings of turtle cones, Baylor and Hodgkin (1974) reported a decay time constant of 20 sec, whereas a
124 114 study recordings in monkey cones showed a time constant ranging from 11 seconds to 45 seconds when stimuli of similar duration and intensity to those of the present study were utilized (Schnapf et al., 1990). In this same study it was noted that the magnitude of the time constant decreased as stimulus intensity increased. Additional studies in salamander (Nakatani & Yau, 1988; Soo et al., 2008) and primate (Dunn et al., 2007) also show an intensity dependent modulation of the decay kinetics of cone photoresponses, although no time constants are explicitly reported. These findings not only support the values of the cone decay time constants measured in the present study, but also agree with the decrease in the duration of the time constants measured with the addition of an adapting background (experiment 2) and the increase in monochromatic light intensity (experiment 3). Additionally, it has been shown that the overall reduction in magnitude of the photocurrent is intensity dependent, with higher intensity stimuli producing a decrease in gain reduction of the measured photocurrents (Baylor et al., 1974; Schnapf Schnapf et al., 1990; Soo et al., 2008). These findings are very consistent with the finding of the current study as the magnitude of the total loss of sensitivity of the cone photoresponse decreased from ~ 3 log units to ~1.5 log units as intensity was increased (figure 7, table 4). It has been proposed that the kinetics of this slow adaptation in cone photocurrents in response to steady-state light steps is not necessarily a function of photopigment bleaching, but may also be due to a drop in intracellular Ca 2+ (Schnapf et al., 1990), or an inactivation of the phototransduction cascade (Soo et al., 2008). Studies addressing the light adaptation of rod photocurrents to steady-state light steps have produced conflicting results. Light adaptation of rod photocurrents has been found in a variety of non-primate animal models, such as salamander (Nakatani & Yau,
125 ), bullfrog (Calvert et al., 2002), guinea pig (Matthews, 1991), cat (Tamura et al., 1989) rabbit, cow, and rat (Nakatani et al., 1991). When explicitly stated, the time constant of this decay is consistently reported to be approximately 2 seconds (Nakatani et al., 1991; Calvert et al., 2002), and in studies in which the time constant is not reported, raw traces of the responses are consistent with this value. The existence of similar decay in photocurrents of primate rods is controversial. Studies by Baylor ang collegues (1984) in monkey and Kraft et al. (1993) in human rods found no evidence for photocurrent decay similar to that found in the above studies. Conversely Tamura et al. (1991) recorded photocurrents from primate rods and found response properties and decay time constants similar to the experiments in non-primate species. Our results show no evidence for decay in the rod photoresponse with a time constant of 2 sec. This is likely due to a saturation of this effect by the photopic light intensities utilized in the present study. This effect has been shown to be absent at irradiances greater than ~800 photons/μm 2 (Tamura et al., 1989; Tamura et al., 1991; Kraft et al., 1993; Calvert et al., 2002). The irradiances necessary to product the criterion response in this study exceeded this value for all stimulus durations greater than 1 sec, and thus it is unlikely that light adaptation on this timescale was allowed by our stimuli. Although few studies addressing the adaptation of rod photocurrents have utilized stimulus durations approaching those of the present study, a slower kinetic light adaptation has been reported in human (Kraft et al., 1993) and bullfrog (Calvert et al., 2002) rod photocurrents, and traces consistent with this type of adaptation are published in studies of primate rod photocurrents (Tamura et al., 1991). We report time constants of the decay in rod photoresponses of 11.5 sec in experiment one, 6 sec in experiments 2, and
126 sec in experiment 3. These values are consistent with a recent study of bullfrog rods which reports a slow phase of rod photocurrent adaptation measured in response to 60 sec steady-state light steps which has a time constant of 8-9 seconds (Calvert et al., 2002). This study also reports an intensity dependent decrease in the loss of sensitivity of the response that is consistent with the values reported in table 4. Calvert and colleagues (2002) propose that this slow adaptation may be mediated by a Ca 2+ dependent modulation of the phototransduction cascade. It is important to note that studies investigating the kinetics of light adaptation of both rod and cone photoresponses often report adaptation of the responses which occurs within milliseconds of light onset (e.g., Baylor et al., 1974; Schnapf et al., 1990; Kraft et al., 1993). Any light adaptation occurring on these time scales would be undetectable in our adaptation paradigm which utilized a half and three quarter maximal criterion response. For these response magnitudes, the sluggish movement of the iris would act as a low pass filter and prevent the measurement of light adaptation kinetics which occur within 500 msec of stimulus onset. Few studies have addressed the light adaptation of the intrinsic response of iprgcs; therefore relevant data generated in the current study are of more consequence. Prior to the discovery of iprgcs, a series of papers by Nelson and Takahashi (1991; 1999) suggested that circadian entrainment was driven by a short wavelength photopigment, which resisted light adaptation and was capable of integrating light signals over tens of minutes. Subsequently, many of these findings have been confirmed; namely, the spectral sensitivity of the photopigment involved (Lucas et al., 2001; Berson et al., 2002; Hattar et al., 2003; Dacey et al., 2005) as well as its ability to integrate light
127 117 signals over time (Dacey et al., 2005; Gamlin et al., 2007; Hut et al., 2008). Conversely, a study by Wong et al. (2005) found evidence for light adaptation in whole cell recordings of iprgcs, but due to the inherent cellular disruption of this technique, their results require further validation. The results of the current study in regards to the light adaptation of the intrinsic photoresponse of iprgcs are more consistent with the finding of Wong et al (2005). We found evidence for slight light adaptation of the intrinsic response to steady-state light steps, yet only at high irradiances (figure 7C). The results of experiment 1 and 2 show no evidence for light adaptation of the intrinsic response (figure 7A and B). This finding would suggest that the adaptation of the intrinsic response to steady-state light is intensity dependent. It seems unlikely that the intensity of the steady-state light pulses utilized by Nelson and Takahashi (1991) were low enough to prevent significant light adaptation, as the intensities reported were well above the intensities utilized in the present study. It should be noted that Wong and colleagues (2005) examination of the light adaptation of the intrinsic response was much more comprehensive than the present study, which can only speak to the steady-state adaptation of the response. Additionally, we found no evidence of the light integrating capacity of the intrinsic response during steady-state light exposure at the stimulus durations studied. This effect has been shown in a number of studies, but only following the cessation of light stimuli (Berson et al., 2002; Dacey et al., 2005; Gamlin et al., 2007; Wong et al., 2007). Our results suggest that the intrinsic response may act as a leaky integrator during steady-state light exposure. Alternatively, the integrative function of the intrinsic response may be intensity dependent as the irradiances of the stimuli in the current study
128 118 are less than that which produced a significant integration in previous studies of both the PLR (Gamlin et al., 2007), and in vitro cellular recordings (Berson et al., 2002; Dacey et al., 2005; Wong et al., 2007). On the contrary, studies investigating the integrating capacity of the intrinsic responses to light in phase shifting studies in hamsters have found convincing evidence of integration of light stimuli at irradiances utilized in all three experimental conditions of the present study (Nelson & Takahashi, 1991; 1999; Hut et al., 2008). The lack of light integration reported in the current study may represent a fundamental difference in the characteristics of the intrinsic response driving circadian versus pupillary behavior. Conversely, this difference could be due to the shorter stimulus durations used in the present study relative to those found to be most effective in producing integration of light signal driving circadian phase shifting. Further studies of the PLR utilizing longer duration light stimuli would resolve this controversy. These studies would have to be conducted under full field lighting conditions as subject fatigue and alignment would become a limiting factor if the present paradigm was used. Relative photoreceptive contribution to the PLR and NIF visual system Another significant finding of the current study is our results detailing the dynamic contribution of outer and inner retinal photoresponses to the PLR and iprgcs. The ability to do so in rodent in vitro electrophysiological studies was lacking in all but the most recent studies of the response properties of iprgcs, as these previous studies utilized fluorescent retrograde labeling of cells from either the SCN or OPN (Berson et al., 2002; Warren et al., 2003; Wong et al., 2005; Viney et al., 2007). This paradigm
129 119 requires the use of high intensity fluorescent light to visualize labeled cells, and as a consequence produces an unrecoverable bleach of the outer retinal photoreceptors. Relative rod contribution to the PLR and NIF visual system. Recent studies utilizing multi-electrode arrays have successfully indentified and recorded iprgcs based on their response properties alone, thus allowing the study of normal outer retinal influences on iprgc firing (Tu et al., 2005; Wong et al., 2007). In particular, Wong and colleagues (2007) reported that the firing of iprgcs in response to light stimuli well below the threshold of the melanopsin mediated intrinsic response was characterized by a tonic firing rate quite unlike the responses of conventional RGCs, as it was maintained throughout the entire duration of the light stimuli. The authors presumed this to be mediated by rods, and the findings of the present study support this assumption, although there is a slight difference in the rod/cone ratio between humans (20:1) (Curcio et al., 1990) and rodents (100:1) (Szel and Rohlich 1992). Our data shows that, following a short period of adaptation, iprgcs receive a tonic rod signal, even at high photopic irradiances (experiment 3). A study involving in vitro recordings of primate iprgcs (Dacey et al., 2005), which was conducted in the absence of the bleaching effects of fluorescent localization, also addressed the relative contribution of rods to the physiology of iprgcs. Contrary to our finding, Dacey et al (2005) reported a rod contribution only at scotopic irradiances. It is possible that the rod contribution at higher irradiances was overlooked due to 10 second stimulus durations utilized in this study. At this stimulus duration, our results would predict that cone inputs would still contribute significantly to the response of
130 120 iprgcs, thus possibly obscuring the contribution by rods. The use of stimulus durations greater than 10 seconds would allow for a more significant adaptation of the cone responses, possibly unmasking a rod contribution at photopic irradiances. It has been recently shown in the rat retina that rod bipolar cells synapse directly onto iprgcs (Østergaard et al., 2007), thus circumventing the conventional rod pathway through cone bipolar cell via AII amacrine cell gap junctions. This pathway could provide a conduit for a sustained rod signal which avoids the traditional shunting by cone responses which occurs in the conventional retinal circuitry at high irradiances. Addition evidence for this tonic rod signal can be found in a recent study investigating light responses of SCN neurons (Drouyer et al., 2007), which found a tonic outer retinal signal influencing the firing rate of these neurons. The authors postulated that the signal was not mediated by rods due to the long duration and high irradiance of the light stimuli used, yet given the present findings and the findings of Østergaard et al (2007), this assumption may not be valid. Although a similar direct connection between rod bipolar cells and iprgcs was not detected in the marmoset retina (Jusuf et al., 2007), evidence in the present study of a sustained rod signal at high irradiances supports the presence of a similar connection in primate retina. Relative cone contribution to the PLR and NIF visual system. Although it appears that rodent retina is becoming the preferred model for in vitro recording of iprgcs (Wong et al., 2007), the close overlap in spectral sensitivity between rodent rods and M- cones, 498 nm and 508 nm respectively (Aggelopoulos & Meissl, 2000; Lucas et al., 2001; Thompson et al., 2008) combined with the lower cone to rod ratio (100:1) of the
131 121 rodent retina (Szel & Rohlich, 1992), make it difficult to assess the relative contribution of cone photoresponses to iprgc physiology. Thus, previous rodent studies addressing the relative contribution of rod, cone, and intrinsic photoresponses to iprgc physiology and NIF visual function generally make no distinction between rod and cone photoresponse, and group them together as outer retinal inputs (Berson et al., 2002; Panda et al., 2002; Hattar et al., 2003; Lucas et al., 2003; Mrosovsky & Hattar, 2003; Panda et al., 2003; Guler et al., 2008). Given the larger spectral distinction between primate rods (498 nm) and M- and L-cones (533 nm and 564 nm respectively) (Dowling, 1987) as well as the higher cone to rod ratio in comparision to rodents, (20:1) in humans (Curcio et al., 1990) and non-human primates (Finlay et al., 2008), it appears that human and non-human primate studies may be better suited to address this important photoreceptive contribution to NIF visual functions. The previously mentioned in vitro recording of primate iprgcs by Dacey and colleagues (2005), was able to address the relative contribution of cone photoresponses to iprgc physiology. This study found a unique cone opponency driving iprgc firing, which is characterized by an L and M-cone mediated ON response and an S-cone mediated OFF response. In addition, this study provided evidence that the L and M-cone signals driving the ON response quickly adapted to steady-state light stimuli. The findings of the present study match well with these conclusions. For all three experimental conditions, we measured spectral sensitivities that were well matched with a rapidly adapting cone signal derived from a linear combination of L and M-cone signals. Similarly, we also found no evidence for S-cone involvement in the ON portion of the
132 122 human PLR, although there is evidence for involvement of UVS-cones in murine NIF behavior (Thompson et al., 2008). Relative contribution of the intrinsic response to the PLR and NIF visual system. Very few studies have examined the relative contribution of the intrinsic photoresponse to iprgc physiology and NIF behavior with outer photoreceptors signals still intact. It is becoming increasingly clear that the relative contribution of the intrinsic response to these behaviors is quite different when outer retinal influences remain viable (see discussion in Thompson et al., 2008). Given the tonic nature of the intrinsic response in the initial investigations of iprgc physiology, it was assumed that the intrinsic response was required for the maintenance of NIF behavior in steady-state lighting conditions. We have found that the intrinsic response indeed acts to maintain pupillary diameter in steady-state lighting conditions at a wide range of photopic irradiances, but our data also support the existence of a tonic rod photoresponse, which also sustains steady-state pupil diameter at photopic irradiances. This rod response appears to augment the sensitivity of iprgcs to steady-state long wavelength light. A recent study of the spectral sensitivity of negative masking of locomotor behavior in mice also suggests a role for outer photoreceptive inputs in augmentation of the intrinsic response under steady-state lighting conditions (Thompson et al., 2008). As previously noted, it has been shown that outer retinal photoreceptors also provide a tonic signal to iprgcs which drives sustained firing in iprgcs themselves (Wong et al., 2007) and also in their recipient neurons in the SCN (Drouyer et al., 2007). The findings of these studies, coupled with that of the present study, suggest that the intrinsic response is
133 123 not exclusively responsible for the maintenance of NIF functions under extended lighting conditions. As noted in Thompson et al. (2008), this would explain how significant functionality of the NIF behavior was maintained in studies of melanopsin knockout mice (Hattar et al., 2003; Lucas et al., 2003; Mrosovsky & Hattar, 2003; Panda et al., 2003). Relative contribution of rod, cone, and intrinsic responses to the dynamics of the PLR Historically, many models of human PLR dynamics have been proposed by various research groups. A common characteristic of all these models are the parallel input of tonic and phasic signals driving pupillary constriction in response to light stimulation. Two of these models postulated independent visual processes driving the phasic and tonic portions of the response (Clynes, 1961; Kohn & Clynes, 1969; Young et al., 1993; Kimura & Young, 1995). Other more biometric theories of PLR dynamics did not explicitly specify the visual processes driving the phasic and tonic signals driving the response (Sun et al., 1983; Privitera & Stark, 2006). These models of PLR dynamics were proposed to explain a fundamental feature of the behavioral dynamics of the PLR, which is characterized by an initial robust transient constriction at light onset followed by pupillary dilation to a larger pupil diameter which is then sustained for the remainder of the light stimulus. An example of this phenomenon can be seen in the red and green traces in panels C and D of figure 2. This behavior is often termed pupillary escape and until the discovery of the response properties of iprgc, the visual processes driving this behavior were poorly understood. The present study provides the first quantitative description of the relative influences of rod, cone, and intrinsic photoresponses on pupillary escape and other
134 124 aspects of human pupillary dynamics. Given the previous models describing the transient and sustained nature of pupillary behavior and the phasic and tonic response properties of iprgcs, it was initially assumed that the transient portion of the PLR was driven exclusively by rods or cones and the sustained portion was driven solely by the intrinsic response at photopic irradiances (Kawasaki & Kardon, 2007; Young & Kimura, 2008). The findings of the present study contradict this assumption on several levels. It is clear that at low photopic irradiances (experiments 1 and 2), pupillary escape is seen in the absence of any contribution of the intrinsic response (see figure 2B and 2C). At these irradiances and durations, pupillary escape is seen and can be explained exclusively by the exponential decay of the outer retinal photoreceptor signals. For longer duration stimuli at these same irradiances, a more extensive pupillary escape is seen, especially in response to long wavelength light (see figure 2D and 2E). Even for these long duration stimuli, the sustained portion of the response is not mediated solely by the intrinsic response, but is also augmented by outer receptor inputs in response to long wavelength lights. Additionally, at higher irradiances where the intrinsic response contributes to pupillary constriction at all duration conditions (experiment 3), the intrinsic response in not solely responsible for sustaining pupillary constriction during steady-state light exposure, as the measured spectral sensitivity of the response is still augmented by rod and cone photoresponses at long wavelengths. The behavioral phenomenon of pupillary escape was first described by Lowenstein and Loewenfeld (1959) and subsequently given the name pupillary escape in a subsequent work by the same authors (Lowenstein & Loewenfeld, 1969). Subsequent to these seminal works describing pupillary behavior, the dynamics of
135 125 pupillary escape began to be used clinically as a useful assessment of retinal disease states (see Bergamin & Kardon, 2002). With the discovery of iprgcs came a renewed interest in the clinical uses of pupillary escape as a clinical evaluation of retinal disease. As mentioned previously, it was incorrectly assumed that the transient nature of pupillary escape was produced by a fast adapting outer retinal input to iprgcs, and that the sustained component of the PLR was driven solely by the intrinsic response. Studies were conducted to determine if the absence of the transient component of pupillary escape could be used to localize retinal disease to the level of the outer retina (Grozdanic et al., 2007; Kawasaki & Kardon, 2007). These studies compared the pupillary responses of humans and dogs to long wavelength and short wavelength light stimuli. These two stimulus types were intended to isolate the pupillary component of the intrinsic response (short wavelength stimulus) and cone responses (long wavelength stimuli), and therefore presumably differentiate between defects involving the outer and inner retina. The findings of the present study show this paradigm to be too simplistic. It is conceivable that sustained pupillary constriction in response to short wavelength light could be maintained by rod photoresponses in the absence of the intrinsic response, although the magnitude of the response would be reduced. In a similar manner, the pupillary responses to the long wavelength stimulus could be utilized to determine the relative health of rod and cone photoreceptors, as our results suggest that both these receptor types would exclusively contribute to pupillary responses evoked by this stimulus. In addition, our result suggest the use of high photopic irradiance stimuli would be necessary for the short duration stimuli used in these types of clinical tests in order to activate the intrinsic response, and that the intensities of the two stimuli should be
136 126 calibrated on the basis of irradiance rather than illuminance, as illuminance measurements are biased toward the activation of L and M-cones. Concordance with previous studies of the spectral sensitivity of the human PLR Previous studies of the spectral sensitivity of the human PLR can be grouped into two categories based on stimulus duration and methodology. One type of experiment investigated the spectral sensitivity of the PLR to transient light stimulation and the other investigated the spectral sensitivity of the PLR to steady-state light stimuli. The former set of studies generated transient light responses either by the rapid exchange of monochromatic lights (Alpern & Campbell, 1962; Young & Alpern, 1980), the presentation of brief light stimuli (Krastel et al., 1985), or the measurement of the transient portion of the response to extended light stimuli (Kimura & Young, 1995; Kimura & Young, 1999). With the exception of Alpern and Campbell (1962), these studies presented their stimuli on either a bright white or monochromatic adapting field in a paradigm similar to that utilized in psychophysical increment threshold spectral sensitivity experiments (e.g. Sperling & Harwerth, 1971). In contrast to the spectral sensitivities reported in the present study, the spectral sensitivities generated in these experiments were reminiscent of the three lobed increment threshold spectral sensitivities of the parvocellular cortical visual pathway. It is generally agreed that these spectral sensitivities are a result of descending cortical influences on midbrain pupillary centers and not reflective of the spectral sensitivity of the direct retino-pretectal fibers (Young & Alpern, 1980; Barbur, 1995; Weiskrantz et al., 1999; Wilhelm et al., 2002). Furthermore, in contrast to the half maximal and three quarter maximal criterion response utilize in the
137 127 present study, the criterion pupillary responses used in the previous mentioned studies were generally at or near threshold (Alpern & Campbell, 1962; Young & Alpern, 1980; Krastel et al., 1985; Kimura & Young, 1995). Taken together, these results show that the spectral sensitivity of the PLR to very brief light stimuli using small criterion responses is not a result of influences of photoresponses originating in iprgcs, and thus explains the deviations of the spectral sensitivities of the present study from these previous results. Historical studies investigating the spectral sensitivity of the PLR in response to steady-state light stimuli were primarily focused on the determination of the relative contribution of rod and cones to the PLR. Very similar to the present study, the spectral sensitivity of the response was compared to the known spectral sensitivities of rod and cone driven visual responses, thus producing an estimate of the relative contribution of the two known human photoreceptor classes to the PLR. In an effort to refute earlier claims that the human PLR was driven exclusively by cones (Brown & Page, 1939), Wagman and Gullberg (1942) examined the spectral sensitivity of the human steady-state PLR for a 0.5 mm criterion response and generated a spectral sensitivity well matched by the scotopic luminosity function and therefore rod dominated. This result is not unreasonable given the small light evoked change in pupil diameter chosen as a criterion response, and it is consistent with the findings of previous and subsequent studies using the same criterion (Laurens, 1923; Alpern & Campbell, 1962). It is interesting to note that, although a 0.5 mm criterion response was chosen by the authors, data throughout the complete range of pupillary diameters was collected and published. Using these published irradiance response plots, it is possible to generate a spectral sensitivity of half maximal pupillary constriction to the steady-state light stimulation. When the data from
138 128 that study are analyzed in a manner similar to the current study and corrected for prereceptoral filtering ( figure 9A), the results are well matched by our data ( Figure 9A), with a slight deviation at longer wavelengths. Two subsequent studies of the spectral sensitivity of the steady-state PLR produced results that either closely matched our present results (Bouma, 1962) ( Figure 9A), or deviated significantly from our results (Alpern & Campbell, 1962) ( figure 9B). The deviations of the data of these previous studies from our current work may be explained by the different experimental methodologies utilized in these studies. Both the Wagman and Gullberg (1942), and Alpern and Campbell (1962) studies utilized an experimental design which collected the data on each wavelength during separate experimental sessions, which were often conducted days or weeks apart from each other. It is well known that pupillary responses to light stimuli of similar intensities can fluctuate from day to day in the same individual (Loewenfeld & Lowenstein, 1993), and given this experimental design, these fluctuations would likely differentially affect the results of each wavelength, thus introducing confounding influences. Additionally, only a single measure of baseline pupillary diameter was established for each wavelength tested. This measurement was taken prior to the onset of the first light stimulus which made up a series of light increments taking between 10 and 20 minutes to complete. It has been demonstrated that pupillary diameter can be significantly influenced by non photic processes such as changes in accommodative state (Marg & Morgan, 1949; Ishikawa et al., 2004; Kasthurirangan & Glasser, 2005; Busettini et al., 2007), changes in state of arousal
139 129 Figure 9. Comparison of the steady-state spectral sensitivities of the current study with previous studies.
140 130 (Lowenstein et al., 1963; Yoss et al., 1970; Morad et al., 2000; Wilhelm et al., 2001; Mclaren et al., 2002; Aston-Jones & Cohen, 2005), or cognitive activity (Hess & Polt, 1960; 1964; Beatty & Wagoner, 1978; Beatty, 1982; Beatty & Lucero-Wagoner, 2000). Any non-photic induced change in baseline pupil diameter during the initial measurement period or during the subsequent light stimuli would produce a cofounding influence on the subject s pupil diameter that would be incorrectly attributed to the light stimulus. This is particularly apparent in the study by Alpern and Campbell (1962), in which the baseline pupillary diameter measured for the 502 nm and 480 nm stimuli were significantly elevated from that of the other seven wavelength utilized in the study, thus inducing a perceived reduction in the effectiveness of those wavelengths to produce an equivalent change in pupil diameter from baseline. It seems likely that this elevation in baseline pupillary diameter was only transient, thus skewing the measurement of the data collected later in the experiment. If one removes the effect of these likely erroneous baseline measurements by selecting a criterion response of an absolute pupillary diameter of 3.5 mm, thus assuming a similar average baseline for every experimental session, the spectral sensitivity plot produced ( figure 9B) more closely matched the data of the current study ( Figure 9A). A more recent study investigating the spectral sensitivity of the PLR to steadystate light stimuli was conducted as part of the previously mentioned study by Kimura and Young (1995). This study utilized a measurement paradigm similar to the present study in which baseline pupillary diameter was assessed prior to each trial and therefore any non-photic pupillary influences on baseline pupil diameter were appropriately controlled for. Their results for an ~ 0.1 mm criterion response at 3.7 seconds after
141 131 stimulus onset utilizing a 1000 td white background ( figure 9C) are in very good agreement with our results measured at a similar time interval ( figure 9C). In general, historical studies of the spectral sensitivity of the PLR are in close concordance with those generated in the present study, which serves to validate our analysis of the dynamic contribution of rod, cone, and intrinsic photoresponses to the PLR and the NIF visual system.
142 132 SUMMARY At the commencement of this research project, not only were there many outstanding questions regarding the human PLR, but also many questions regarding iprgc physiology and its effect on the NIF visual system. Like most visual psychophysics experiments, the overall goal of this project was to take findings related to retinal physiology and relate it directly to visually driven behavior. In addition, we sought to use characteristics of the same visually driven behaviors to make predictions about retinal physiology. At the conclusion of this project, we can state with satisfaction, that our goals have been met. We directed our initial experiments towards simply verifying the existence of a NIF visual pathway in human and non-human primates. Most of the ground breaking studies in this field were conducted in the rodent, and verification of a similar pathway in primates was required. We demonstrated the existence of this pathway by showing the persistence of the macaque PLR following the pharmalogical blockade of rod and cone photoreceptors. By measuring the spectral sensitivity of this residual response, we conclusively showed that the photopigment melanopsin was responsible for the response and therefore contributed to the primate PLR. Additionally, we made a very significant discovery regarding the sustained pupilloconstriction following light exposure. Our results showed that the spectral sensitivity of this well known pupillary phenomenon was well fit by the spectral sensitivity of melanopsin, even in the absence of pharmacological blockade of outer retinal influences. This finding provided the first evidence for a visual behavior driven exclusively by the intrinsic response of iprgcs. By reproducing these
143 133 results in human subjects, we were able to demonstrate a method for assessing the functionality of iprgcs in humans. This finding allowed the pursuit of a completely new research project, the details of which will be expounded on below. The response dynamics of the macaque PLR under blockade of the outer retina, along with in vitro records of primate iprgcs, suggested that the intrinsic response might be responsible for the maintenance of pupilloconstriction during steady-state light stimuli. These results suggested that the outer retinal signal impinging on iprgcs might rapidly adapt to steady-state light, leaving only the intrinsic response driving the PLR. The next phase of the research project sought to test the hypothesis that, following the adaptation of rod and cone photoresponses, the intrinsic response of iprgcs was the primary photoresponse driving pupillary constriction during steady-state light stimuli. We sought to test this hypothesis by determining the relative contribution of rods, cones, and melanopsin to the spectral sensitivity of the human PLR for stimulus durations varying from 1 second to 100 seconds in 0.5 log second intervals. Additionally, these experiments addressed a more global question regarding the NIF visual system: How do rod, cone, and intrinsic photoresponses combined to influence NIF behaviors? This research question is difficult to assess via in vitro retinal physiology due to the limitations inherent to the electrophysiological techniques utilized and the difficulty in spectrally isolating cone and rod spectral sensitivities in the rodent retina. We demonstrated that not only was the intrinsic photoresponse of iprgcs responsible for the persistence of half-maximal pupilloconstriction in response to steady-state light stimuli of 30 seconds or longer, but that rods also contribute significantly to this persistence. We also determined that cone inputs to iprgcs, and therefore to pupillary constriction, rapidly adapt, and
144 134 become ~ 2 log units less sensitive within 30 seconds of light onset. Furthermore, we showed that the intrinsic response of iprgcs contributed significantly to driving three quarter maximal pupillary constriction, which produces pupillary diameters optimal for visual acuity, at all stimulus durations We made two findings during this project that predict novel retinal physiology or behaviorally confirm anatomical evidence of novel retinal physiology. Through the analysis of the effect of the combination of inner and outer retinal spectral sensitivities on the overall spectral sensitivity of the human PLR, we predict that the signals from the inner and outer retina behave in a winner take all manner at the level of iprgcs. This suggests that activation of the intrinsic photoresponse acts to shunt the signals from the outer retina, possibly by changing the input impedance of the cell. These findings will need to be confirmed via intracellular recordings, and should inspire a more careful examination into the manner in which signals from the inner and outer retina combine to influence NIF behaviors. An additional finding which has implications for retinal physiology is our result suggesting that rods contribute significantly to the spectral sensitivity of the PLR at photopic irradiances. This is in contrast to the visual pathway responsible for conscious vision, in which rods only contribute to spectral sensitivity at scotopic irradiances. This finding confirms recent anatomical experiments which show a direct synaptic connection between rod bipolar cells and iprgcs, which is not present in the image forming visual pathway. Although it was assumed that the intrinsic response was exclusively responsible for driving NIF behaviors in response to steady-state illumination, we have shown, along with other researchers, that this is too simplistic a view. This leaves our demonstration
145 135 that the intrinsic response exclusively drives sustained pupilloconstriction following light offset as the sole behavioral correlate of intrinsic response function in humans. We are currently pursuing other research projects that will demonstrate this response in a diverse population of human subjects, as well as determine if this response could be used to assess the presence or progression of retinal diseases affecting retinal ganglion cells. Furthermore, this behavioral correlate of intrinsic response function could be used to diagnose clinical conditions which may arise in persons with deficits in melanopsin function, such as sleep disorders or seasonal affective disorder. The spectral sensitivities of NIF visual behavior measured in the current project are quite unlike those measured under similar irradiances in the image forming visual pathway. The spectral sensitivity of both the magno- and parvocellular pathways show a much greater contribution by L- and M- cones, and thus greater sensitivity to long wavelength light. Our spectral sensitivity measurements should lead to a better understanding of how indoor light sources, with emission spectra generally skewed toward long wavelengths, may be ineffective in driving critical NIF behaviors such as neuroendocrine modulation and pupillary constriction to diameters optimal for visual acuity. In addition, information from this project could be utilized in designing light sources which could be used to treat acute or chronic disorders of the human circadian systems, such as insomnia, seasonal effective disorder, and jetlag.
146 136 REFERENCES Adelson, E.H. (1982). Saturation and adaptation in the rod system. Vision Research, 22 (10), Aggelopoulos, N., & Meissl, H. (2000). Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. Journal of Physiology-London, 523 Pt 1, Alpern, M., & Benson, D.J. (1953). Directional sensitivity of the pupillomotor photoreceptors. American Journal of Optometry Archives American Academy Optometry, 30 (11), Alpern, M., & Campbell, F.W. (1962). The spectral sensitivity of the consensual light reflex. Journal of Physiology-London, 164, Alpern, M., Falls, H.F., & Lee, G.B. (1960). The enigma of typical total monochromacy. American Journal of Optometry, 50, Alpern, M., & Ohba, N. (1972). The effect of bleaching and backgrounds on pupil size. Vision Research, 12 (5), Aston-Jones, G., & Cohen, J.D. (2005). An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annual Reviews in Neuroscience, 28, Barbur, J.L. (1995). A Study of Pupil Response Components in Human Vision. In: J.G. Robbins, M.B.A. Djamgoz, & A. Taylor (Eds.), Basic and Clinical Perspectives in Vision Research : A Celebration of the Career of Hisako Ikeda (pp. 3-18). New York: Plenum Press. Barbur, J.L., Harlow, A.J., & Sahraie, A. (1992). Pupillary responses to stimulus structure, colour and movement. Ophthalmic and Physiological Optics, 12 (2), Barnard, A.R., Appleford, J.M., Sekaran, S., Chinthapalli, K., Jenkins, A., Seeliger, M., Biel, M., Humphries, P., Douglas, R.H., Wenzel, A., Foster, R.G., Hankins, M.W., & Lucas, R.J. (2004). Residual photosensitivity in mice lacking both rod opsin and cone photoreceptor cyclic nucleotide gated channel 3 alpha subunit. Visual Neuroscience, 21 (5), Baver, S.B., Pickard, G.E., Sollars, P.J., & Pickard, G.E. (2008). Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. European Journal of Neuroscience, 27 (7),
147 137 Baylor, D.A., & Hodgkin, A.L. (1974). Changes in time scale and sensitivity in turtle photoreceptors. Journal of Physiology-London, 242 (3), Baylor, D.A., Hodgkin, A.L., & Lamb, T.D. (1974). The electrical response of turtle cones to flashes and steps of light. Journal of Physiology-London, 242 (3), Baylor, D.A., Nunn, B.J., & Schnapf, J.L. (1984). The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. Journal of Physiology-London, 357, Baylor, D.A., Nunn, B.J., & Schnapf, J.L. (1987). Spectral sensitivity of cones of the monkey Macaca fascicularis. Journal of Physiology-London, 390, Beatty, J. (1982). Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological bulletin, 91 (2), Beatty, J., & Lucero-Wagoner, B. (2000). The Pupillary System. In: J.T. Cacioppo, L.G. Tassinary, & G.G. Berntson (Eds.), Handbook of Psychophysiology (2nd Ed.) (pp ): Cambridge University Press. Beatty, J., & Wagoner, B.L. (1978). Pupillometric signs of brain activation vary with level of cognitive processing. Science, 199 (4334), Belenky, M.A., Smeraski, C.A., Provencio, I., Sollars, P.J., & Pickard, G.E. (2003). Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. Journal of Comparative Neurology, 460 (3), Bennett, A.G., Rabbetts, R.B., & Bennett, A.G. (1998). Bennett and Rabbetts' clinical visual optics. (pp. viii, 451 p.). Oxford ; Boston: Butterworth-Heinemann. Bergamin, O., & Kardon, R.H. (2002). Greater pupillary escape differentiates central from peripheral visual field loss. Ophthalmology, 109 (4), Berson, D.M., Dunn, F.A., & Takao, M. (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295 (5557), Bouma, H. (1962). Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature, 193, Brainard, G.C., Hanifin, J.P., Greeson, J.M., Byrne, B., Glickman, G., Gerner, E., & Rollag, M.D. (2001). Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience, 21 (16), Brown, R.H., & Page, H.E. (1939). Pupil dilatation and dark adaptation. Journal of Experimental Psychology, 25 (4),
148 138 Busettini, C., Davison, R.C., & Gamlin, P.D.R. (2007). The Near Triad: Vergence, Accommodation, and Pupilloconstriction. In: L. Squire (Ed.) New Encyclopedia of Neuroscience (Oxford: Elsevier. Calvert, P., Govardovskii, V., Arshavsky, V., & Makino, C. (2002). Two temporal phases of light adaptation in retinal rods. Journal of General Physiology, 119 (2), Campbell, F.W. (1957). The depth of field of the human eye. Optica Acta, 4 (4), Campbell, F.W., & Gregory, A.H. (1960). Effect of size of pupil on visual acuity. Nature, 187, Campbell, F.W., & Gubisch, R.W. (1966). Optical quality of the human eye. J Physiol, 186 (3), Clarke, R.J., & Ikeda, H. (1985). Luminance detectors in the olivary pretectal nucleus and their relationship to the pupillary light reflex in the rat. II. Studies using sinusoidal light. Experimental Brain Research, 59 (1), Clynes, M. (1961). Unidirectional rate sensitivity: a biocybernetic law of reflex and humoral systems as physiologic channels of control and communication. Ann N Y Acad Sci, 92, Crawford, B.H. (1949). The Scotopic Visibility Function. Proc. Phys. Soc. B 62, 62 (5), Curcio, C.A., Sloan, K.R., Kalina, R.E., & Hendrickson, A.E. (1990). Human photoreceptor topography. J Comp Neurol, 292 (4), Dacey, D.M., Liao, H.W., Peterson, B.B., Robinson, F.R., Smith, V.C., Pokorny, J., Yau, K.W., & Gamlin, P.D. (2005). Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature, 433 (7027), Dowling, J.E. (1987). The retina : an approachable part of the brain. (pp. xii, 282 p., [284] p. of plates). Cambridge, Mass.: Belknap Press of Harvard University Press. Drouyer, E., Rieux, C., Hut, R.A., & Cooper, H.M. (2007). Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. Journal of Neuroscience, 27 (36), Dunn, F., Lankheet, M., & Rieke, F. (2007). Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature, 449 (7162), Enyedi, L.B., Dev, S., & Cox, T.A. (1998). A comparison of the Marcus Gunn and alternating light tests for afferent pupillary defects. Ophthalmology, 105 (5),
149 139 Finlay, B.L., Franco, E.C., Yamada, E.S., Crowley, J.C., Parsons, M., Muniz, J.A., & Silveira, L.C. (2008). Number and topography of cones, rods and optic nerve axons in New and Old World primates. Vis Neurosci, 25 (3), Gamlin, P.D., McDougal, D.H., Pokorny, J., Smith, V.C., Yau, K.W., & Dacey, D.M. (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res, 47 (7), Gamlin, P.D., Zhang, H., & Clarke, R.J. (1995). Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res, 106 (1), Gamlin, P.D., Zhang, H., Harlow, A., & Barbur, J.L. (1998). Pupil responses to stimulus color, structure and light flux increments in the rhesus monkey. Vision Res, 38 (21), Girkin, C.A. (2003). Evaluation of the pupillary light response as an objective measure of visual function. Ophthalmol Clin North Am, 16 (2), Gooley, J.J., Lu, J., Chou, T.C., Scammell, T.E., & Saper, C.B. (2001). Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci, 4 (12), Graham, N.V.S. (2001). Visual pattern analyzers. Oxford psychology series ; no. 16 (pp. xvi, 646 p.). New York: Oxford University Press. Grozdanic, S.D., Matic, M., Sakaguchi, D.S., & Kardon, R.H. (2007). Evaluation of retinal status using chromatic pupil light reflex activity in healthy and diseased canine eyes. Invest Ophthalmol Vis Sci, 48 (11), Guler, A.D., Ecker, J.L., Lall, G.S., Haq, S., Altimus, C.M., Liao, H.W., Barnard, A.R., Cahill, H., Badea, T.C., Zhao, H., Hankins, M.W., Berson, D.M., Lucas, R.J., Yau, K.W., & Hattar, S. (2008). Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature, 453 (7191), Hattar, S., Kumar, M., Park, A., Tong, P., Tung, J., Yau, K.W., & Berson, D.M. (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology, 497 (3), Hattar, S., Liao, H.W., Takao, M., Berson, D.M., & Yau, K.W. (2002). Melanopsincontaining retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science, 295 (5557), Hattar, S., Lucas, R.J., Mrosovsky, N., Thompson, S., Douglas, R.H., Hankins, M.W., Lem, J., Biel, M., Hofmann, F., Foster, R.G., & Yau, K.W. (2003). Melanopsin and rod-
150 140 cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424 (6944), Hess, E.H., & Polt, J.M. (1960). Pupil size as related to interest value of visual stimuli. Science, 132, Hess, E.H., & Polt, J.M. (1964). Pupil size in relation to mental activity during simple problem solving. Science, 143, Hirata, Y., Yamaji, K., Sakai, H., & Usui, S. (2003). Function of the pupil in vision and information capacity of retinal image. Syst Comput Jpn, 34 (9), Hut, R.A., Oklejewicz, M., Rieux, C., & Cooper, H.M. (2008). Photic sensitivity ranges of hamster pupillary and circadian phase responses do not overlap. J Biol Rhythms, 23 (1), Ishikawa, H., Asakawa, K., & Yoshitomi, T. (2004). Pupillary near reflex. Neuro- Ophthalmology Japan, 21 (3), Jusuf, P.R., Lee, S.C., Hannibal, J., & Grunert, U. (2007). Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. European Journal of Neuroscience, 26 (10), Kasthurirangan, S., & Glasser, A. (2005). Characteristics of pupil responses during farto-near and near-to-far accommodation. Ophthalmic and Physiological Optics, 25 (4), Kawasaki, A. (2005). Disorders of Pupillary Function, Accommodation, and Lacrimation. In: N.R. Miller, F.B. Walsh, & W.F. Hoyt (Eds.), Walsh and Hoyt's Clinical Neuro-Ophthalmology (pp ). Philadelphia: Lippincott Williams & Wilkins. Kawasaki, A., & Kardon, R.H. (2007). Intrinsically photosensitive retinal ganglion cells. Journal of Neuroophthalmology, 27 (3), Kimura, B., & Young, R.S.L. (1995). Nature of the pupillary responses evoked by chromatic flashes on a white background. Vision Research, 35 (7), Kimura, E., & Young, R.S. (1999). S-cone contribution to pupillary responses evoked by chromatic flash offset. Vision Research, 39 (6), Kohn, M., & Clynes, M. (1969). Color dynamics of the pupil. Annuals of the NY Academy of Science, 156 (2), Kraft, T.W., Schneeweis, D.M., & Schnapf, J.L. (1993). Visual Transduction in Human Rod Photoreceptors. Journal of Physiology-London, 464,
151 141 Krastel, H., Alexandridis, E., & Gertz, J. (1985). Pupil increment thresholds are influenced by color opponent mechanisms. Ophthalmologica, 191 (1), Kurtenbach, A., Meierkord, S., & Kremers, J. (1999). Spectral sensitivities in dichromats and trichromats at mesopic retinal illuminances. Journal of the Optical Society of America A: Optics and Image Science, and Vision, 16 (7), Laurens, H. (1923). Studies on the Relative Physiological Value of Spectral Lights: III. The Pupillomotor Effects of Wave-Lengths of Equal Energy Content. American Journal Physiology, 64 (1), Loewenfeld, I.E., & Lowenstein, O. (1993). The Pupil : Anatomy, Physiology, and Clinical Applications. ( Iowa State University Press.) Lowenstein, O., Feinberg, R., & Loewenfeld, I.E. (1963). Pupillary movements during acute and chronic fatigue. Investigative Ophthalmology, 2, Lowenstein, O., & Loewenfeld, I.E. (1959). Influence of retinal adaptation upon the pupillary reflex to light in normal man. Part I. Effect of adaptation to bright light on the pupillary threshold. American Journal of Ophthalmology, 48(5)Pt 2, Lowenstein, O., & Loewenfeld, I.E. (1969). The Pupil. In: H. Davson, & L.T. Graham (Eds.), The eye, 3 (pp ). New York,: Academic Press. Lucas, R.J., Douglas, R.H., & Foster, R.G. (2001). Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience, 4 (6), Lucas, R.J., Hattar, S., Takao, M., Berson, D.M., Foster, R.G., & Yau, K.W. (2003). Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science, 299 (5604), Marg, E., & Morgan, M.W. (1949). The pupillary near reflex. American Journal of Optometry, 26, Matthews, H.R. (1991). Incorporation of chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. Journal of Physiology-London, 436, McDougal, D.H., & Gamlin, P.D.R. (2008). Pupillary Control Pathways. In: I.B. Allan, K. Akimichi, M.S. Gordon, & W. Gerald (Eds.), The Senses: A Comprehensive Reference (pp ). New York: Academic Press. McLaren, J.W., Hauri, P.J., Lin, S.C., & Harris, C.D. (2002). Pupillometry in clinically sleepy patients. Sleep Medicine, 3 (4),
152 142 Melyan, Z., Tarttelin, E.E., Bellingham, J., Lucas, R.J., & Hankins, M.W. (2005). Addition of human melanopsin renders mammalian cells photoresponsive. Nature, 433 (7027), Miyahara, E., Pokorny, J., & Smith, V.C. (1996). Increment threshold and purity discrimination spectral sensitivities of X-chromosome-linked color-defective observers. Vision Research, 36 (11), Morad, Y., Lemberg, H., Yofe, N., & Dagan, Y. (2000). Pupillography as an objective indicator of fatigue. Current Eye Research, 21 (1), Mrosovsky, N., & Hattar, S. (2003). Impaired masking responses to light in melanopsinknockout mice. Chronobiology International, 20 (6), Mure, L.S., Rieux, C., Hattar, S., & Cooper, H.M. (2007). Melanopsin-dependent nonvisual responses: Evidence for photopigment bistability in vivo. Journal of Biological Rhythms, 22 (5), Nakatani, K., Tamura, T., & Yau, K.W. (1991). Light adaptation in retinal rods of the rabbit and 2 other nonprimate mammals. Journal of General Physiology, 97 (3), Nakatani, K., & Yau, K. (1988). Calcium and light adaptation in retinal rods and cones. Nature, 334 (6177), Nelson, D.E., & Takahashi, J.S. (1991). Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). Journal of Physiology, 439, Nelson, D.E., & Takahashi, J.S. (1999). Integration and saturation within the circadian photic entrainment pathway of hamsters. American Journal Physiology, 277 (5 Pt 2), R Newsome, D.A. (1971). Afterimage and pupillary activity following strong light exposure. Vision Research, 11 (3), Østergaard, J., Hannibal, J., & Fahrenkrug, J. (2007). Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Investigative Ophthalmology and Visual Science, 48 (8), Panda, S., Provencio, I., Tu, D.C., Pires, S.S., Rollag, M.D., Castrucci, A.M., Pletcher, M.T., Sato, T.K., Wiltshire, T., Andahazy, M., Kay, S.A., Van Gelder, R.N., & Hogenesch, J.B. (2003). Melanopsin is required for non-image-forming photic responses in blind mice. Science, 301 (5632),
153 143 Panda, S., Sato, T.K., Castrucci, A.M., Rollag, M.D., DeGrip, W.J., Hogenesch, J.B., Provencio, I., & Kay, S.A. (2002). Melanopsin (Opn4) requirement for normal lightinduced circadian phase shifting. Science, 298 (5601), Perlman, I., & Normann, R. (1998). Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Progress in Retinal and Eye research, 17 (4), Pokorny, J., Jin, Q., & Smith, V.C. (1993). Spectral-luminosity functions, scalar linearity, and chromatic adaptation. Journal of the Optical Society of America A: Optics and Image Science, and Vision, 10 (6), Pokorny, J., Smith, V.C., & Lutze, M. (1987). Aging of the human lens. Applied. Optics., 26 (8), Privitera, C.M., & Stark, L.W. (2006). A binocular pupil model for simulation of relative afferent pupil defects and the swinging flashlight test. Biolological Cybernetics, 94 (3), Provencio, I., Jiang, G., De Grip, W.J., Hayes, W.P., & Rollag, M.D. (1998). Melanopsin: An opsin in melanophores, brain, and eye. Proceedings of the National Academy of Science U S A, 95 (1), Provencio, I., Rodriguez, I.R., Jiang, G., Hayes, W.P., Moreira, E.F., & Rollag, M.D. (2000). A novel human opsin in the inner retina. Journal of Neuroscience, 20 (2), Provencio, I., Rollag, M.D., & Castrucci, A.M. (2002). Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature, 415 (6871), 493. Quick, R.F., Jr. (1974). A vector-magnitude model of contrast detection. Kybernetik, 16 (2), Robson, J.G., & Graham, N. (1981). Probability summation and regional variation in contrast sensitivity across the visual field. Vision Research, 21 (3), Schnapf, J., Nunn, B., Meister, M., & Baylor, D. (1990). Visual transduction in cones of the monkey Macaca fascicularis. Journal of Physiology-London, 427, Sekaran, S., Foster, R.G., Lucas, R.J., & Hankins, M.W. (2003). Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Current Biology, 13 (15),
154 144 Soo, F.S., Detwiler, P.B., & Rieke, F. (2008). Light adaptation in salamander L-cone photoreceptors. Journal of Neuroscience, 28 (6), Sperling, H.G., & Harwerth, R.S. (1971). Red-green cone interactions in the incrementthreshold spectral sensitivity of primates. Science, 172 (979), Stockman, A., & Sharpe, L.T. (2000). The spectral sensitivities of the middle- and longwavelength-sensitive cones derived from measurements in observers of known genotype. Vision Research, 40 (13), Sun, F., Krenz, W.C., & Stark, L.W. (1983). A systems model for the pupil size effect. I. Transient data. Biological Cybernetics, 48 (2), Sun, F., & Stark, L. (1983). Pupillary escape intensified by large pupillary size. Vision Research, 23 (6), Szel, A., & Rohlich, P. (1992). Two cone types of rat retina detected by anti-visual pigment antibodies. Experimental Eye Research, 55 (1), Tamura, T., Nakatani, K., & Yau, K.W. (1989). Light adaptation in cat retinal rods. Science, 245 (4919), Tamura, T., Nakatani, K., & Yau, K.W. (1991). Calcium feedback and sensitivity regulation in primate rods. Journal of General Physiology, 98 (1), ten Doesschate, J., & Alpern, M. (1965). Response of the pupil to steady-state retinal illumination: contribution by cones. Science, 149 (687), Thapan, K., Arendt, J., & Skene, D.J. (2001). An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. Journal of Physiology-London, 535 (Pt 1), Thompson, S., Foster, R.G., Stone, E.M., Sheffield, V.C., & Mrosovsky, N. (2008). Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. European Journal of Neuroscience, 27 (8), Tu, D.C., Zhang, D.Y., Demas, J., Slutsky, E.B., Provencio, I., Holy, T.E., & Van Gelder, R.N. (2005). Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron, 48 (6), Tucker, J., & Charman, W.N. (1975). The depth-of-focus of the human eye for Snellen letters. American Journal of Optometry and Physiological Optics, 52 (1), Viney, T., Balint, K., Hillier, D., Siegert, S., Boldogkoi, Z., Enquist, L., Meister, M., Cepko, C., & Roska, B. (2007). Local retinal circuits of melanopsin-containing ganglion
155 145 cells identified by transsynaptic viral tracing. Current Biology, 17 (11), Wagman, I.H., & Gullberg, J.E. (1942). The relationship between monochromatic light and pupil diameter the low internsity visibility curve as measured by pupillary measurements. American Journal Physiology, 137 (4), Wald, G. (1945). The spectal sensitivity of the human eye. Journal of the Optical Society of America., 35 (3), Warren, E.J., Allen, C.N., Brown, R.L., & Robinson, D.W. (2003). Intrinsic light responses of retinal ganglion cells projecting to the circadian system. European Journal of Neuroscience, 17 (9), Weber, J.T., Young, R., & Hutchins, B. (1981). Morphologic and autoradiographic evidence for a laminated pretectal olivary nucleus in the squirrel monkey. Brain Research, 224 (1), Webster, J.G., Cohen, G.H., & Boynton, R.M. (1968). Optimizing the use of the criterion response for the pupil light reflex. Journal of the Optical Society of America, 58 (3), Weiskrantz, L., Cowey, A., & Barbur, J.L. (1999). Differential pupillary constriction and awareness in the absence of striate cortex. Brain, 122 (8), Wilhelm, B., Giedke, H., Ludtke, H., Bittner, E., Hofmann, A., & Wilhelm, H. (2001). Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. Journal of Sleep Research, 10 (1), 1-7. Wilhelm, B.J., Wilhelm, H., Moro, S., & Barbur, J.L. (2002). Pupil response components: Studies in patients with Parinaud's syndrome. Brain, 125 (10), Williams, R.W., & Chalupa, L.M. (1983). Development of the retinal pathway to the pretectum of the cat. Neuroscience, 10 (4), Wong, K., Dunn, F., Graham, D., & Berson, D. (2007). Synaptic influences on rat ganglion-cell photoreceptors. Journal of Physiology-London, 582 (Pt 1), Wong, K.Y., Dunn, F.A., & Berson, D.M. (2005). Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron, 48 (6), Woodhouse, J.M. (1975). The effect of pupil size on grating detection at various contrast levels. Vision Res, 15 (6),
156 146 Yoss, R.E., Moyer, N.J., & Hollenhorst, R.W. (1970). Pupil size and spontaneous pupillary waves associated with alertness, drowsiness, and sleep. Neurology, 20 (6), Young, R.S., & Alpern, M. (1980). Pupil responses to foveal exchange of monochromatic lights. Journal of the Optical Society of America, 70 (6), Young, R.S., & Kimura, E. (2008). Pupillary correlates of light-evoked melanopsin activity in humans. Vision Research, 48 (7), Young, R.S.L., Han, B.C., & Wu, P.Y. (1993). Transient and sustained components of the pupillary responses evoked by luminance and color. Vision Research, 33 (4), Young, R.S.L., & Kennish, J. (1993). Transient and sustained components of the pupil response evoked by achromatic spatial patterns. Vision Research, 33 (16), Zhu, Y., Tu, D.C., Denner, D., Shane, T., Fitzgerald, C.M., & Van Gelder, R.N. (2007). Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Investigative Ophthalmology and Visual Science, 48 (3),
157 147 APPENDIX IACUC AND IRB APPROVALS
158 148
Dark and light adaptation: a job that is accomplished mainly in the retina
 Dark and light adaptation: a job that is accomplished mainly in the retina Dark adaptation: recovery in darkness (of sensitivity) and photoreceptor pigment. Light adaptation: The ability of the visual
Dark and light adaptation: a job that is accomplished mainly in the retina Dark adaptation: recovery in darkness (of sensitivity) and photoreceptor pigment. Light adaptation: The ability of the visual
let's continue talking about the eye,
 Eye is mainly composed of 3 layers: External layer, which called The Sclera which is a hard connective tissue that gives the eye its round shape. Extension of the sclera into the front is the cornea, which
Eye is mainly composed of 3 layers: External layer, which called The Sclera which is a hard connective tissue that gives the eye its round shape. Extension of the sclera into the front is the cornea, which
VISUAL REFLEXES. B. The oculomotor nucleus, Edinger-Westphal nucleus, and oculomotor nerve at level of the superior colliculus.
 Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15 HyperBrain: Ch 7 with quizzes and or Lab 7 videotape http://www-medlib.med.utah.edu/kw/hyperbrain/anim/reflex.html
Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15 HyperBrain: Ch 7 with quizzes and or Lab 7 videotape http://www-medlib.med.utah.edu/kw/hyperbrain/anim/reflex.html
Neuroanatomy, Text and Atlas (J. H. Martin), 3 rd Edition Chapter 7, The Visual System, pp ,
 Normal CNS, Special Senses, Head and Neck TOPIC: FACULTY: LECTURE: READING: RETINA and CENTRAL VISUAL PATHWAYS P. Hitchcock, Ph.D. Department Cell and Developmental Biology Kellogg Eye Center Friday, 20
Normal CNS, Special Senses, Head and Neck TOPIC: FACULTY: LECTURE: READING: RETINA and CENTRAL VISUAL PATHWAYS P. Hitchcock, Ph.D. Department Cell and Developmental Biology Kellogg Eye Center Friday, 20
2. METHODS. 2.1 Apparatus
 Pupillary light reflex associated with melanopsin and cone photorecetors Sei-ichi Tsujimura, 1 Katsunori Okajima, 2 1 Faculty of Sciences and Engineering, Kagoshima University, Japan 2 Faculty of Environment
Pupillary light reflex associated with melanopsin and cone photorecetors Sei-ichi Tsujimura, 1 Katsunori Okajima, 2 1 Faculty of Sciences and Engineering, Kagoshima University, Japan 2 Faculty of Environment
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
1. The responses of on-center and off-center retinal ganglion cells
 1. The responses of on-center and off-center retinal ganglion cells 2. Responses of an on-center ganglion cell to different light conditions 3. Responses of an on-center ganglion cells to different light
1. The responses of on-center and off-center retinal ganglion cells 2. Responses of an on-center ganglion cell to different light conditions 3. Responses of an on-center ganglion cells to different light
Drugs Affecting The Autonomic Nervous System(ANS)
 Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
CHAPTER 15 LECTURE OUTLINE
 CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
The Visual System. Retinal Anatomy Dr. Casagrande February 2, Phone: Office: T2302 MCN
 The Visual System Retinal Anatomy Dr. Casagrande February 2, 2004 Phone: 343-4538 Email: vivien.casagrande@mcmail.vanderbilt.edu Office: T2302 MCN Reading assignments and Good Web Sites Chapter 2 in Tovée,
The Visual System Retinal Anatomy Dr. Casagrande February 2, 2004 Phone: 343-4538 Email: vivien.casagrande@mcmail.vanderbilt.edu Office: T2302 MCN Reading assignments and Good Web Sites Chapter 2 in Tovée,
Neuroscience - Problem Drill 13: The Eye and Visual Processing
 Neuroscience - Problem Drill 13: The Eye and Visual Processing Question No. 1 of 10 needed, (3) Pick the answer, and (4) Review the core concept tutorial as needed. 1. Which of the following statements
Neuroscience - Problem Drill 13: The Eye and Visual Processing Question No. 1 of 10 needed, (3) Pick the answer, and (4) Review the core concept tutorial as needed. 1. Which of the following statements
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD
 AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
Nervous system Reflexes and Senses
 Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
Lujain Hamdan. Ayman Musleh & Yahya Salem. Mohammed khatatbeh
 12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
Normal Pupil. The normal pupil is 2 mm to 6 mm in diameter. In ordinary ambient light the pupils are usually 3 mm to 4 mm in diameter.
 Normal Pupil The normal pupil is 2 mm to 6 mm in diameter. In ordinary ambient light the pupils are usually 3 mm to 4 mm in diameter. Normal Pupil The pupils are small and poorly reactive at birth and
Normal Pupil The normal pupil is 2 mm to 6 mm in diameter. In ordinary ambient light the pupils are usually 3 mm to 4 mm in diameter. Normal Pupil The pupils are small and poorly reactive at birth and
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
ParasymPathetic Nervous system. Done by : Zaid Al-Ghnaneem
 ParasymPathetic Nervous system Done by : Zaid Al-Ghnaneem In this lecture we are going to discuss Parasympathetic, in the last lecture we took sympathetic and one of the objectives of last lecture was
ParasymPathetic Nervous system Done by : Zaid Al-Ghnaneem In this lecture we are going to discuss Parasympathetic, in the last lecture we took sympathetic and one of the objectives of last lecture was
The Visual System. Anatomical Overview Dr. Casagrande January 21, 2004
 The Visual System Anatomical Overview Dr. Casagrande January 21, 2004 Phone: 343-4538 Email: vivien.casagrande@mcmail.vanderbilt.edu Office: T2302 MCN How the Brain Works Useful Additional Reading: Adler,
The Visual System Anatomical Overview Dr. Casagrande January 21, 2004 Phone: 343-4538 Email: vivien.casagrande@mcmail.vanderbilt.edu Office: T2302 MCN How the Brain Works Useful Additional Reading: Adler,
What do we perceive?
 THE VISUAL SYSTEM Aditi Majumder What do we perceive? Example: Switch off the light in room What we perceive Not only the property of the scene But also that of the visual system Our perception is filtered
THE VISUAL SYSTEM Aditi Majumder What do we perceive? Example: Switch off the light in room What we perceive Not only the property of the scene But also that of the visual system Our perception is filtered
Arielle Bokhour, class of 2017
 Arielle Bokhour, class of 2017 Objectives 1. Understand the actions and innervation of the extrinsic and intrinsic eye muscles 2. Describe the pathways for pupillary constriction and dilation 3. Understand
Arielle Bokhour, class of 2017 Objectives 1. Understand the actions and innervation of the extrinsic and intrinsic eye muscles 2. Describe the pathways for pupillary constriction and dilation 3. Understand
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Test of visual pathway function
 The visual system Test of visual pathway function Suppose you have a patient who may have some damage to the visual pathways leading to visual cortex, for example from multiple sclerosis. How could you
The visual system Test of visual pathway function Suppose you have a patient who may have some damage to the visual pathways leading to visual cortex, for example from multiple sclerosis. How could you
Vision II. Steven McLoon Department of Neuroscience University of Minnesota
 Vision II Steven McLoon Department of Neuroscience University of Minnesota 1 Ganglion Cells The axons of the retinal ganglion cells form the optic nerve and carry visual information into the brain. 2 Optic
Vision II Steven McLoon Department of Neuroscience University of Minnesota 1 Ganglion Cells The axons of the retinal ganglion cells form the optic nerve and carry visual information into the brain. 2 Optic
Utility of colored-light pupil response in patients with age-related macular degeneration
 Original Contribution Kitasato Med J 2014; 44: 195-200 Utility of colored-light pupil response in patients with age-related macular degeneration Ken Asakawa, 1 Hitoshi Ishikawa, 1 Yoshiaki Ichibe, 2 Kimiya
Original Contribution Kitasato Med J 2014; 44: 195-200 Utility of colored-light pupil response in patients with age-related macular degeneration Ken Asakawa, 1 Hitoshi Ishikawa, 1 Yoshiaki Ichibe, 2 Kimiya
Composed by Natalia Leonidovna Svintsitskaya, Associate professor of the Chair of Human Anatomy, Candidate of Medicine
 Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
LISC-322 Neuroscience. Visual Field Representation. Visual Field Representation. Visual Field Representation. Visual Field Representation
 LISC-3 Neuroscience THE VISUAL SYSTEM Central Visual Pathways Each eye sees a part of the visual space that defines its visual field. The s of both eyes overlap extensively to create a binocular. eye both
LISC-3 Neuroscience THE VISUAL SYSTEM Central Visual Pathways Each eye sees a part of the visual space that defines its visual field. The s of both eyes overlap extensively to create a binocular. eye both
CNS 2 Physiology lab
 It should be noted that the doctor emphasized that this material is also considered as continuation of the theory material and is INCLUDED IN THE THEORY EXAM. Presbiopia: is decrease in accommodation of
It should be noted that the doctor emphasized that this material is also considered as continuation of the theory material and is INCLUDED IN THE THEORY EXAM. Presbiopia: is decrease in accommodation of
Carlson (7e) PowerPoint Lecture Outline Chapter 6: Vision
 Carlson (7e) PowerPoint Lecture Outline Chapter 6: Vision This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display,
Carlson (7e) PowerPoint Lecture Outline Chapter 6: Vision This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display,
Vision Seeing is in the mind
 1 Vision Seeing is in the mind Stimulus: Light 2 Light Characteristics 1. Wavelength (hue) 2. Intensity (brightness) 3. Saturation (purity) 3 4 Hue (color): dimension of color determined by wavelength
1 Vision Seeing is in the mind Stimulus: Light 2 Light Characteristics 1. Wavelength (hue) 2. Intensity (brightness) 3. Saturation (purity) 3 4 Hue (color): dimension of color determined by wavelength
AUTONOMIC NERVOUS SYSTEM (ANS):
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical 1 st year students, 2017/2018. ++++++++++++++++++++++++++++++++++++++++++++++++++ Textbook of Medical Physiology,
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical 1 st year students, 2017/2018. ++++++++++++++++++++++++++++++++++++++++++++++++++ Textbook of Medical Physiology,
THE VISUAL WORLD! Visual (Electromagnetic) Stimulus
 THE VISUAL WORLD! Visual (Electromagnetic) Stimulus Perceived color of light is determined by 3 characteristics (properties of electromagnetic energy): 1. Hue: the spectrum (wavelength) of light (color)
THE VISUAL WORLD! Visual (Electromagnetic) Stimulus Perceived color of light is determined by 3 characteristics (properties of electromagnetic energy): 1. Hue: the spectrum (wavelength) of light (color)
The Nervous System: Autonomic Nervous System Pearson Education, Inc.
 17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
Cranial Nerves. Steven McLoon Department of Neuroscience University of Minnesota
 Cranial Nerves Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Sensory and Motor Systems Sensory Systems:
Cranial Nerves Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Sensory and Motor Systems Sensory Systems:
The Nervous System: Autonomic Nervous System
 17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Chapter 16. APR Enhanced Lecture Slides
 Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Autonomic Nervous System
 Autonomic Nervous System Autonomic Nervous System Ref: Textbook of Medical Physiology, Guyton, 12th ed: 729-738, 11th ed. P748-760, and 10th ed. p697-708. Fig.17.02 General functions Control and Adaptation
Autonomic Nervous System Autonomic Nervous System Ref: Textbook of Medical Physiology, Guyton, 12th ed: 729-738, 11th ed. P748-760, and 10th ed. p697-708. Fig.17.02 General functions Control and Adaptation
Autonomic Nervous System. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Targeting of the attenuated diphtheria toxin (adta) into the melanopsin locus. a,
 doi: 1.138/nature6829 a DTA HSV- TK PGK-Neo Targeting construct b kb.85.65 L WT adta/+ adta/ adta Melanopsin (Opn 4) Genomic Locus 1 kb.4 Supplementary Figure 1: Targeting of the attenuated diphtheria
doi: 1.138/nature6829 a DTA HSV- TK PGK-Neo Targeting construct b kb.85.65 L WT adta/+ adta/ adta Melanopsin (Opn 4) Genomic Locus 1 kb.4 Supplementary Figure 1: Targeting of the attenuated diphtheria
Introduction to Physiological Psychology
 Introduction to Physiological Psychology Vision ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html This class n Sensation vs. Perception n How light is translated into what we see n Structure
Introduction to Physiological Psychology Vision ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html This class n Sensation vs. Perception n How light is translated into what we see n Structure
Derived copy of Divisions of the Autonomic Nervous System *
 OpenStax-CNX module: m56161 1 Derived copy of Divisions of the Autonomic Nervous System * Stephanie Fretham Based on Divisions of the Autonomic Nervous System by OpenStax This work is produced by OpenStax-CNX
OpenStax-CNX module: m56161 1 Derived copy of Divisions of the Autonomic Nervous System * Stephanie Fretham Based on Divisions of the Autonomic Nervous System by OpenStax This work is produced by OpenStax-CNX
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
THE VISUAL WORLD! Visual (Electromagnetic) Stimulus
 THE VISUAL WORLD! Visual (Electromagnetic) Stimulus Perceived color of light is determined by 3 characteristics (properties of electromagnetic energy): 1. : the spectrum (wavelength) of light (color) 2.
THE VISUAL WORLD! Visual (Electromagnetic) Stimulus Perceived color of light is determined by 3 characteristics (properties of electromagnetic energy): 1. : the spectrum (wavelength) of light (color) 2.
Autonomic Nervous System
 Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Biology 218 Human Anatomy
 Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
I. Neural Control of Involuntary Effectors. Chapter 9. Autonomic Motor Nerves. Autonomic Neurons. Autonomic Ganglia. Autonomic Neurons 9/19/11
 Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Ch 9. The Autonomic Nervous System
 Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota
 Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Monday, Nov 6, 9:00-10:00am Surdyk s Café in Northrop Auditorium
Autonomic Nervous System (the visceral motor system) Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Monday, Nov 6, 9:00-10:00am Surdyk s Café in Northrop Auditorium
Biological Bases of Behavior. 6: Vision
 Biological Bases of Behavior 6: Vision Sensory Systems The brain detects events in the external environment and directs the contractions of the muscles Afferent neurons carry sensory messages to brain
Biological Bases of Behavior 6: Vision Sensory Systems The brain detects events in the external environment and directs the contractions of the muscles Afferent neurons carry sensory messages to brain
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Autonomic Nervous System Dr. Ali Ebneshahidi
 Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
NEURONS ARE ORGANIZED INTO NERVOUS SYSTEMS 34.5
 NEURONS ARE ORGANIZED INTO NERVOUS SYSTEMS 34.5 INTRODUCTION The cnidarians have nerve nets, the most simple type of nervous system. The sea anemone has a nerve net that serves simple behaviours such as
NEURONS ARE ORGANIZED INTO NERVOUS SYSTEMS 34.5 INTRODUCTION The cnidarians have nerve nets, the most simple type of nervous system. The sea anemone has a nerve net that serves simple behaviours such as
Parallel streams of visual processing
 Parallel streams of visual processing RETINAL GANGLION CELL AXONS: OPTIC TRACT Optic nerve Optic tract Optic chiasm Lateral geniculate nucleus Hypothalamus: regulation of circadian rhythms Pretectum: reflex
Parallel streams of visual processing RETINAL GANGLION CELL AXONS: OPTIC TRACT Optic nerve Optic tract Optic chiasm Lateral geniculate nucleus Hypothalamus: regulation of circadian rhythms Pretectum: reflex
OPTO 5320 VISION SCIENCE I
 OPTO 5320 VISION SCIENCE I Monocular Sensory Processes of Vision: Color Vision Mechanisms of Color Processing . Neural Mechanisms of Color Processing A. Parallel processing - M- & P- pathways B. Second
OPTO 5320 VISION SCIENCE I Monocular Sensory Processes of Vision: Color Vision Mechanisms of Color Processing . Neural Mechanisms of Color Processing A. Parallel processing - M- & P- pathways B. Second
LOOKING AT BLINDNESS FROM NEUROLOGIST S PERSPECTIVE
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk LOOKING AT BLINDNESS FROM NEUROLOGIST S PERSPECTIVE Author : LAURENT S GAROSI Categories : Vets Date : June 23, 2008 LAURENT
Vet Times The website for the veterinary profession https://www.vettimes.co.uk LOOKING AT BLINDNESS FROM NEUROLOGIST S PERSPECTIVE Author : LAURENT S GAROSI Categories : Vets Date : June 23, 2008 LAURENT
Neuropsychiatry Block
 Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
The Nervous System. Autonomic Division. C h a p t e r. PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas
 C h a p t e r 17 The Nervous System Autonomic Division PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson
C h a p t e r 17 The Nervous System Autonomic Division PowerPoint Lecture Slides prepared by Jason LaPres North Harris College Houston, Texas Copyright 2009 Pearson Education, Inc., publishing as Pearson
Physiology Unit 2 SENSORY PHYSIOLOGY
 Physiology Unit 2 SENSORY PHYSIOLOGY In Physiology Today Sensory System Sensory information Conscious sensations Unconscious sensations Sensory processing Transferring stimulus energy into a graded potential
Physiology Unit 2 SENSORY PHYSIOLOGY In Physiology Today Sensory System Sensory information Conscious sensations Unconscious sensations Sensory processing Transferring stimulus energy into a graded potential
I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1)
 I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1) Acetylcholine - ACh 2. Ganglion (Ganglia) 3. Ganglionic Neuron
I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1) Acetylcholine - ACh 2. Ganglion (Ganglia) 3. Ganglionic Neuron
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Parallel pathways in the retina
 Retinal origins of parallel pathways in the primate visual system Wednesday, September 23, 2015 Sherry, 2002 1 Parallel pathways in the retina Several different images of the outside world are sent simultaneously
Retinal origins of parallel pathways in the primate visual system Wednesday, September 23, 2015 Sherry, 2002 1 Parallel pathways in the retina Several different images of the outside world are sent simultaneously
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution
 Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells
 Vision Research 47 (2007) 946 954 www.elsevier.com/locate/visres Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells Paul D.R. Gamlin a, *, David H. McDougal a, Joel
Vision Research 47 (2007) 946 954 www.elsevier.com/locate/visres Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells Paul D.R. Gamlin a, *, David H. McDougal a, Joel
General organization of central and peripheral components of the nervous system
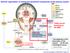 General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
The Autonomic Nervous System
 The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.)
 Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.) Peripheral nerves contain both motor and sensory neurons Among the motor neurons, some of these are somatic and innervate skeletal muscles while some
Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.) Peripheral nerves contain both motor and sensory neurons Among the motor neurons, some of these are somatic and innervate skeletal muscles while some
ANATOMY & PHYSIOLOGY - CLUTCH CH THE AUTONOMIC NERVOUS SYSTEM.
 !! www.clutchprep.com ANATOMY & PHYSIOLOGY - CLUTCH CONCEPT: THE AUTONOMIC NERVOUS SYSTEM: DIVISIONS AND STRUCTURE The Autonomic Nervous System and its Divisions: Autonomic Nervous System (ANS) controls
!! www.clutchprep.com ANATOMY & PHYSIOLOGY - CLUTCH CONCEPT: THE AUTONOMIC NERVOUS SYSTEM: DIVISIONS AND STRUCTURE The Autonomic Nervous System and its Divisions: Autonomic Nervous System (ANS) controls
Special Senses: The Eye
 Unit 4 Special Senses: The Eye ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY The Senses General senses of touch Temperature Pressure Pain Special senses Smell Taste Sight Hearing Equilibrium The Eye and Vision
Unit 4 Special Senses: The Eye ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY The Senses General senses of touch Temperature Pressure Pain Special senses Smell Taste Sight Hearing Equilibrium The Eye and Vision
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Chapter 15: The Autonomic Nervous System. Copyright 2009, John Wiley & Sons, Inc.
 Chapter 15: The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems Comparison of Somatic and Autonomic Nervous Systems Anatomy of Autonomic Motor Pathways Preganglionic neuron
Chapter 15: The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems Comparison of Somatic and Autonomic Nervous Systems Anatomy of Autonomic Motor Pathways Preganglionic neuron
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Lighta part of the spectrum of Electromagnetic Energy. (the part that s visible to us!)
 Introduction to Physiological Psychology Vision ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ /~ksweeney/psy260.html Lighta part of the spectrum of Electromagnetic Energy (the part that s visible to us!)
Introduction to Physiological Psychology Vision ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ /~ksweeney/psy260.html Lighta part of the spectrum of Electromagnetic Energy (the part that s visible to us!)
Vision I. Steven McLoon Department of Neuroscience University of Minnesota
 Vision I Steven McLoon Department of Neuroscience University of Minnesota 1 Eye Cornea Sclera Conjunctiva 2 Eye The conjunctiva lines the inner surface of the eyelids and outer surface of the sclera. 3
Vision I Steven McLoon Department of Neuroscience University of Minnesota 1 Eye Cornea Sclera Conjunctiva 2 Eye The conjunctiva lines the inner surface of the eyelids and outer surface of the sclera. 3
Biology. A Guide to the Natural World. Chapter 27 Lecture Outline Communication and Control 1: The Nervous System. Fifth Edition.
 Biology A Guide to the Natural World Chapter 27 Lecture Outline Communication and Control 1: The Nervous System Fifth Edition David Krogh The Nervous System Nervous tissue is composed of two kinds of cells:
Biology A Guide to the Natural World Chapter 27 Lecture Outline Communication and Control 1: The Nervous System Fifth Edition David Krogh The Nervous System Nervous tissue is composed of two kinds of cells:
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
Oculomotor System George R. Leichnetz, Ph.D.
 Oculomotor System George R. Leichnetz, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Define different types of eye movement and their underlying neural
Oculomotor System George R. Leichnetz, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Define different types of eye movement and their underlying neural
Autonomic Nervous System
 Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
Lab 16: PNS: Nerves and Autonomic NS Hamilton Answers to Pre- Lab Assignments
 Lab 16: PNS: Nerves and Autonomic NS Hamilton Answers to Pre- Lab Assignments Pre-Lab Activity 1: 1. a. olfactory nerve b. optic nerve c. oculomotor nerve d. abducens nerve e. trochlear nerve f. trigeminal
Lab 16: PNS: Nerves and Autonomic NS Hamilton Answers to Pre- Lab Assignments Pre-Lab Activity 1: 1. a. olfactory nerve b. optic nerve c. oculomotor nerve d. abducens nerve e. trochlear nerve f. trigeminal
ACTIVITY2.15 Text:Campbell,v.8,chapter48 DATE HOUR NERVOUS SYSTEMS NEURON
 AP BIOLOGY ACTIVITY2.15 Text:Campbell,v.8,chapter48 NAME DATE HOUR NERVOUS SYSTEMS NEURON SIMPLE REFLEX RESTING POTENTIAL ACTION POTENTIAL ACTION POTENTIAL GRAPH TRANSMISSION ACROSS A SYNAPSE QUESTIONS:
AP BIOLOGY ACTIVITY2.15 Text:Campbell,v.8,chapter48 NAME DATE HOUR NERVOUS SYSTEMS NEURON SIMPLE REFLEX RESTING POTENTIAL ACTION POTENTIAL ACTION POTENTIAL GRAPH TRANSMISSION ACROSS A SYNAPSE QUESTIONS:
The lowest level of stimulation that a person can detect. absolute threshold. Adapting one's current understandings to incorporate new information.
 absolute threshold The lowest level of stimulation that a person can detect accommodation Adapting one's current understandings to incorporate new information. acuity Sharp perception or vision audition
absolute threshold The lowest level of stimulation that a person can detect accommodation Adapting one's current understandings to incorporate new information. acuity Sharp perception or vision audition
Autonomic Nervous System
 Autonomic Nervous System Touqeer Ahmed PhD 3 rd March, 2017 Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System Divisions The peripheral nervous system
Autonomic Nervous System Touqeer Ahmed PhD 3 rd March, 2017 Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System Divisions The peripheral nervous system
Sensation and Perception. A. Sensation: awareness of simple characteristics B. Perception: making complex interpretations
 I. Overview Sensation and Perception A. Sensation: awareness of simple characteristics B. Perception: making complex interpretations C. Top-Down vs Bottom-up Processing D. Psychophysics -- thresholds 1.
I. Overview Sensation and Perception A. Sensation: awareness of simple characteristics B. Perception: making complex interpretations C. Top-Down vs Bottom-up Processing D. Psychophysics -- thresholds 1.
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Ch 5. Perception and Encoding
 Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
NERVOUS SYSTEM. Academic Resource Center. Forskellen mellem oscillator og krystal
 NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
NERVOUS SYSTEM Academic Resource Center Forskellen mellem oscillator og krystal Overview of the Nervous System Peripheral nervous system-pns cranial nerves spinal nerves ganglia peripheral nerves enteric
The Autonomic Nervous
 Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
Brain Stem. Nervous System (Part A-3) Module 8 -Chapter 14
 Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Systems Neuroscience November 21, 2017 The autonomic nervous system
 Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
REVIEW QUESTIONS AND SAMPLE MIDTERM QUESTIONS FOR THE MIDTERM EXAM
 REVIEW QUESTIONS AND SAMPLE MIDTERM QUESTIONS FOR THE MIDTERM EXAM REVIEW QUESTIONS Chapter 1 / Lecture 1 1. Diagram a neuron and label its components. In what ways are neurons specialized for communication?
REVIEW QUESTIONS AND SAMPLE MIDTERM QUESTIONS FOR THE MIDTERM EXAM REVIEW QUESTIONS Chapter 1 / Lecture 1 1. Diagram a neuron and label its components. In what ways are neurons specialized for communication?
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY
 Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
4/22/16. Eye. External Anatomy of Eye. Accessory Structures. Bio 40B Dr. Kandula
 Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands modified sweat glands l Small sebaceous glands l
Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands modified sweat glands l Small sebaceous glands l
