BLOOD COMPONENTS are regulated as
|
|
|
- Byron Moore
- 6 years ago
- Views:
Transcription
1 Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More than 1 in 2,000 units were recalled in the late 1990s in the United States. The most common reason for these notices from donor centers is postdonation donor information. Most of these units had been transfused, and many present a risk of a risk (ie, a problem might have been present that might have affected the recipient). A few regulations and standards address recalls in general terms, but transfusion services generally have wide discretion in the management of specific common recall problems. The Food and Drug Administration (FDA) is now including posttransfusion evaluations in its guidelines for BLOOD COMPONENTS are regulated as drugs. However, because they are derived directly from humans, they will always be subject to biological variation. Their unit-by-unit production, testing, storage, distribution, and record keeping are also more complex than other medications, leading to further opportunities for deviations from the expected. The US Food and Drug Administration s (FDA) current good manufacturing practices extend to after manufacturing so that, if problems are found with the finished drug, measures must be taken to correct the product or prevent consequences to patients if possible. Over the past 10 to 15 years, the stricter application of these principles to blood components has led to a growing number of recalls and withdrawals by blood suppliers, as well as a concomitant increase in the numbers of notices sent to transfusion services about blood products they have received. Furthermore, because platelets and red blood cells have a short shelf life and because hospitals do not keep a large reserve of blood components in storage, most of these notices about nonconforming blood products are often received after the units had been transfused. In retrospect, many of these recalled units presented a risk of a risk (ie, that From the Department of Pathology, Feinberg School of Medicine, rthwestern University, Chicago, IL. Address reprint requests to Glenn Ramsey, MD, rthwestern University, Feinberg 7-301, 251 E. Huron St., Chicago, IL g-ramsey@northwestern.edu 2004 Elsevier Inc. All rights reserved /04/ $30.00/0 doi: /j.tmrv emerging infectious threats to the blood supply. We suggest that hospital transfusion services should have standard operating procedures for managing recalls and that the hospital transfusion committee and the quality management program should provide local input or oversight. Using the FDA s categories of donor center biological product deviations, we provide recommendations to consider for when to notify the recipient s physician, after postdonation information is received about a previously transfused blood component. More study of this important everyday issue in transfusion medicine is highly desirable Elsevier Inc. All rights reserved. the problem might have affected the patient if it had actually been present but whether it was truly present is often unknown). The lookback requirements for tracing units from donors later found to have human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections have been formalized by the FDA in rules and guidances. 1,2 (A revised rule for HIV and HCV lookbacks was proposed in 2000 but not finalized as of this writing. 3 ) HCV and HIV lookbacks have been discussed elsewhere 4,5 and will not be covered in depth here. In contrast to HIV and HCV lookbacks, other types of notices about blood products have few specific formal rules or guidelines as to how they should be handled by the transfusion service. The purpose of this review is to discuss the management of recalls and withdrawals of blood components. RECALLS, MARKET WITHDRAWALS, AND BIOLOGICAL PRODUCT DEVIATIONS Recall is an effective method of removing or correcting consumer products that are in violation of laws administered by the Food and Drug Administration (Title 21, Code of Federal Regulations (CFR), section 7.40 (21 CFR 7.40)). The FDA classifies recalls into 3 categories, from the most serious, Class I, to the least serious, Class III (Table 1). All recalls under FDA jurisdiction are published on line in the weekly FDA Enforcement Report. 6 There is a lag time of weeks to months from the recall to publication, and the Enforcement Report does not say when the original problem 36 Transfusion Medicine Reviews, Vol 18, 1 (January), 2004: pp 36-45
2 MANAGING BLOOD RECALLS 37 Table 1. FDA Definitions of Recalls and Market Withdrawals, 21 CFR 7.3 Recall: Removal or correction of a marketed product that the FDA considers to be in violation of the law it administers and against which the agency would initiate legal action, eg, seizure. Recall classification for use of, or exposure to, a violative product: Class I: Reasonable probability [of] serious adverse health consequences or death. Class II: May cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote. Class III: t likely to cause adverse health consequences. Market withdrawal: Removal or correction of a distributed product which involves a minor violation that would not be subject to legal action by the FDA or which involves no violation, eg, normal stock rotation practices, routine equipment adjustments and repairs. occurred. The FDA says recalls are voluntary by the manufacturer, although conducting recalls is required, and the FDA has the power to initiate recalls if the manufacturer does not act as required. Recalls can include public warnings in serious situations (21 CFR 7.42). In 1 blood component recall involving improper donor testing in a large centralized laboratory, several blood centers who had used that laboratory made community media announcements and advertisements notifying transfusion recipients to seek testing if desired. 7 Market withdrawals are also defined in Table 1. The FDA has stated that withdrawals because of problems beyond the control of the manufacturer may be classified as withdrawals. For example, the US nationwide removal of Tylenol (McNeil-PPC, Ft Washington, PA) from stores because of fatal cyanide tampering in Chicago in 1982 was classified as a market withdrawal not a recall. 8 Most notices about blood components involving postdonation donor information are now categorized as market withdrawals. Market withdrawals are not published by the FDA so their magnitude is unknown. Ramsey and Sherman 9 analyzed recalls of blood components published by the FDA from 1990 through During this 8-year period, recalls were announced for nearly a quarter-million blood components, comprising about 1 in 700 units available to hospitals. Seventy-six percent were in Class III recalls, 24% were of Class II, and only 12 units were designated in Class I (because of viral or bacterial infection). Three fourths of the recalled units involved incorrect or incomplete testing for syphilis or viral infection. The next largest categories were for donors with reactive or previously reactive infectious disease tests, which involved 10% of recalled units. Nearly 90% of recalled units were included in a small number (22) of large recalls of over 1,000 units each. By 1998, the final year examined, published blood component recalls had shifted away from incorrect or incomplete infectious-disease testing (down to 20% of units) and toward donors with previously reactive infectious-disease tests (51% of units). 10 Also in contrast to , two thirds of the recalled units were included in a growing number of smaller recalls of under 1,000 Table 2. FDA Publications Addressing tices From Blood Collection Facilities to Consignees Type of Publication Topic Date of Release Reference Rule HIV lookback Sep 9, Rule, proposed HCV lookback v 16, Guidance HTLV Aug 15, Guidance HCV lookback Oct 21, Guidance Anthrax Oct 17, Guidance CJD Jan 9, Guidance, draft Xenotransplantation Feb 1, Guidance Smallpox vaccination Dec 30, Guidance SARS Apr 17, Guidance West Nile virus May 1, Guidance, draft Syphilis Jun 25, Blood Memo Donor HBV, HTLV Jul 19, NOTE. The FDA web site lists rules, guidances, and blood memos separately; in reverse chronological order of release.
3 38 GLENN RAMSEY units each. About 10,000 blood components were recalled in the 1998 Enforcement Reports. When compared with the total annual blood components distributed in the United States, the risk of a unit being recalled after issue was about 1 in 2,000. Another gauge of the types of problems found with blood components comes from the recent requirement for reporting biological product deviations (BPDs) to the FDA. By definition, BPDs involve products that had been issued but that were later found to have safety, potency, or labeling problems. BPDs should include all problems that lead to recalls or market withdrawals. The FDA has summarized the first full fiscal year (FY 2002) of BPDs reported from licensed blood facilities. 11 Their statistics are presented as the number of reports, not the number of units involved, so the overall frequency of blood components involved is unknown. One BPD for incorrect testing could involve many units, whereas BPDs for donor suitability are often reported on a unit-by-unit basis. However, the categories and numbers of BPDs offer a useful FDA-defined framework for categorizing problems found in blood components currently. Nearly 22,000 BPDs were reported from licensed facilities (excluding plasma centers) in FY (Reports which actually did not need to be reported were excluded.) Seventy-six percent were for postdonation information concerning donor high-risk behavior and history. The most common categories here, in order, were travel to malaria and Creutzfeldt-Jacob risk areas, cancer, tattoos, disease or surgery, deferring medications, and intravenous drug use. The next largest type of BPD, in 10% of cases, was for quality control and distribution problems, such as clotted or hemolyzed units or segments, inappropriate product release (eg, unacceptable quality control, outdated), incorrect temperature, and failure to quarantine after another problem was found. The rest of the donor-center BPDs were in donor screening (6.0%), labeling (4.3%), routine testing (1.2%), component preparation (1.2%), collection (0.7%), miscellaneous (0.6%), donor deferral (0.2%), and viral testing (0.2%). The most common items within each of these areas were as follows: donor screening, deferring history, but not deferred; labeling, incorrect autologous donor tag; routine testing, incorrect Rh typing; component preparation, incorrect temperature; collection, bacterial contamination; miscellaneous, HCV lookback deviation; donor deferral, previous deferral for history; and infectious disease testing, incorrect syphilis testing. Incorrect infectious disease testing, previously the most common category of recalled units in the 1990s, was the least common category of BPDs in FY This may reflect the current widespread use of large, dedicated centralized testing laboratories for donors. REGULATORY REQUIREMENTS AND GUIDANCES The FDA has detailed regulations and support documents for the conduct of recalls by the manufacturer. 21 CFR 7.3 defines recalls, and 21 CFR describe the manufacturer s obligations and the FDA s processes for monitoring and assessing recalls. Two publications by the FDA s Office of Regulatory Affairs provide details for their inspectors about how to inspect recall operations: the Investigation Operations Manual, Chapter 8, and the Regulatory Procedures Manual, Chapter 7. 12,13 These 2 publications are on line at The FDA may conduct effectiveness checks, which are follow-up surveys of consignees such as transfusion services, to verify that recalls are carried out appropriately by the manufacturer. In contrast, the FDA provides much less general information about the response to recalls. One line in the CFR is addressed to consignees: Responsibility of recipient. Consignees that receive a recall communication should immediately carry out the instructions set forth by the recalling firm and, where necessary, extend the recall to its consignees in accordance with...this section (21 CFR 7.49 [d]). Therefore, if the hospital transfusion service has shipped the recalled component, or part of it, to another facility, it should conduct a recall to the second facility. For example, some hospital transfusion services send source plasma to a manufacturer so the source plasma buyer should be notified if the original unit is recalled. If the transfusion service is notified about an unsuitable product, but then issues it inadvertently, then a BPD report would be required. HIV and HCV lookbacks have been referred to previously (Table 2). 1-5 Donors with reactive tests for hepatitis B virus (HBV) and human T-cell lymphotropic viruses (HTLV) I and II were ad-
4 MANAGING BLOOD RECALLS 39 dressed by the FDA in a 1996 recommendation 14 (now listed under Blood Memos) and a 1997 guidance for HTLV. 15 The FDA recommended withdrawing in-dated components from donors with HBV and HTLV markers but stated that they were not recommending consignee notification for the purpose of recipient notification. In our own practice, we perform lookback on units from donors with confirmed hepatitis B surface antigen (HBsAg) or anti-htlv (see Management of Specific Problems), but this is not required by the FDA. Recent FDA guidances about emerging infection issues have included provisions about posttransfusion actions. In the January 2002 guidance 16 on Creutzfeldt-Jakob disease (CJD), the FDA stated that notifications about donors deferred for travel, bovine insulin, United Kingdom transfusion, or a single family member with CJD were intended only for product removal, and not for notification of recipients. For other more direct donor connections to CJD, including actual donor CJD subsequent to transfusion, consignee notification could enable the consignee to inform the physician...so that recipient tracing and medically appropriate notification and counseling may be performed at the discretion of health care providers. There is an ongoing study by the US Centers for Disease Control and Prevention (CDC) and the National Blood Data Resource Center to investigate transfusion recipients of blood from subsequently diagnosed CJD patients. 17 In this approved study, the recipients are not notified, but national deaths are monitored to see whether any of the recipients die of CJD. As of August 2002, 331 transfusion recipients with 1,325 person years of follow-up had been studied. A similar study is being conducted in Great Britain for recipients of blood from donors with later variant CJD (vcjd). 18 secondary cases of CJD or vcjd have been reported to date. If a notice were received in the United States about a blood component from a donor with later CJD, the CDC or the National Blood Data Resource Center should be contacted. In the May 2003 West Nile virus (WNV) guidance, 19 if a donor has a medical diagnosis of WNV, then other units from 14 days before to 28 days after the onset of illness should be traced for consideration of notifying the recipients physicians. If the donor is the suspected likely source of another WNV transfusion case, then other units from that donor collected from 28 days to 28 days from the infectious donation should also be traced and the recipients physicians notified. However, in cases when a donor is potentially associated with a case of transmission of WNV, but the epidemiological investigation has not established the specific donor as a likely source of transmission of WNV, we are not recommending notification of the transfusion service. This is slightly misworded because units from recent donors associated with a transfused WNV patient should be sought for precautionary quarantine and retrieval from the transfusion service, but the implication is that the recipients physicians need not be notified if the donor is not the likely source. WNV nucleic acid testing (NAT) began in the US and Canada in the summer of When a donor tests reactive by WNV NAT, the FDA has not specified at this writing whether to use a 14-day lookback period (as per donor WNV illness) or a 28-day period (as per donor transmission of WNV) for recent donations. The December 2002 guidance on smallpox vaccination and blood donation 20 addressed postvaccination blood collections. If a donor should have been ineligible, but his/her units already have been transfused, then we recommend that medical directors consider the need for prompt record tracing and, as appropriate, notification of the treating physicians or notification of prior recipients of the affected blood and blood components previously collected from that donor. The April 2003 FDA guidance 21 on severe acute respiratory syndrome (SARS) gave recommendations for lookback investigation. If a blood product has been transfused from a donor who should have been ineligible at the time of donation, then we recommend that the establishments consider notifying the treating physician of those recipients about the post donation information, including whether the donor developed suspected SARS. Donors are deferred for 28 days after recovering from suspected SARS or for 14 days after exposure to a person with SARS or travel to SARS-risk areas. However, the guidance states that if the donor is symptom free for more than 14 days after exposure, then product retrieval and quarantine (and thus presumably notification of the treating physician) are not necessary. In the June 2003 draft guidance on donor syph-
5 40 GLENN RAMSEY ilis testing, 22 lookback, quarantine, and consignee notification are not recommended for previous units from donors with later syphilis or a confirmed syphilis test. The recent FDA draft guidance on xenotransplantation 23 and on anthrax 24 call for withdrawal of blood components inadvertently collected from donors with these unusual exposures. The anthrax guidance has recommendations on when to notify the recipient s physician after transfusion of a suspect unit. ACCREDITATION REQUIREMENTS In the 22nd edition of the Standards of the American Association of Blood Banks (AABB), effective vember 2003, chapter 7 is on deviations, nonconformances, and complications. 25 Standard 7.0 requires policies, processes, procedures and defined responsibility for detecting, investigating, and reviewing deviations. Standard 7.1 and its subsections call for nonconforming products to be evaluated, traced, segregated if still present, and prevented from unintended use. Blood banks and transfusion services must have processes for identification, quarantine, retrieval, and recall. nconforming products that already have been released must be evaluated for quality, and when quality may have been affected, the nonconformance shall be reported to the customer. (The customer is defined elsewhere as the receiver of a product or service, either another organization or another department within the same organization. In this context of nonconforming products, the customer does not refer to the patient who received the product.) Records of product nonconformances and actions about them must be maintained for 5 years (reference standards 6.2A and 6.2C). The transfusion medicine checklist of the Laboratory Accreditation Program of the College of American Pathologists (December 2002 edition) 26 has 2 questions touching on some aspects of recalls and notifications. TRM asks if there is a procedure to identify and quarantine all previous components from donors who now test repeatedly reactive in viral marker screening tests. TRM asks for a procedure consistent with [Medicare] and FDA regulations/guidelines for notification and counseling of recipients who have been transfused with a potentially infectious blood component. The commentary for this question refers to federal requirements for notifying recipients about subsequent confirmed positive infections in their donors. The main intent of the question is to require HIV and HCV lookback. However, the mention of guidelines in the question may be construed to include other recent FDA guidelines as discussed earlier. Some hospitals collect blood products, and some blood collection centers have transfusion services. The previously mentioned regulations and standards apply to those facilities as well (ie, notices should be transmitted from the collection arm to the transfusion arm of the same establishment when necessary). GENERAL RECOMMENDATIONS Within the extant regulations and standards summarized previously, the transfusion service has broad latitude on how to manage recalls and notifications from blood suppliers, other than HIV and HCV lookback. The remainder of this article offers recommendations based on our experience. However, these are only recommendations, and others may choose to use different approaches. Given the paucity of literature about this important everyday area of activity in transfusion medicine, we hope the following will be helpful in providing a framework for others to organize their programs as suited for their hospitals. Future study, analysis, and discourse on the management and outcomes of recalls and notifications will be very helpful. In particular, the yield of problem investigations and the medical benefit of these notices for transfusion recipients deserve more examination. At a minimum, we would suggest that recall management processes include the following key elements: 1. Have a standard operating procedure. However one chooses to manage recalls and notifications, and in however much detail desired, a procedure is a prerequisite for instructing staff in these key elements. 2. Act immediately to quarantine and discard, or return, blood components as instructed by the supplier. Time is of the essence when a notice is received. Immediate action should be taken to quarantine the unit and prevent release. Is a recalled unit of plasma being thawed in the waterbath? In case a large number of units is involved, the transfusion service staff should have round-the-clock ac-
6 MANAGING BLOOD RECALLS 41 cess to facsimile or secure electronic mail to facilitate transfer of information and prevent transcription errors from verbal messages. For laboratories with blood bank computer systems, both computer and physical quarantine must be done, although computer quarantine may be done first to expedite prompt blockage of issue. As noted previously, if a unit is erroneously released after a notice is received to quarantine it, then a biological product deviation report must be sent to the FDA. 3. Review and determine the medical implications of units already transfused. This is further discussed later. 4. Keep records of all notices and actions as required (eg, 5 years in AABB standards). 25 Telephone instructions should be logged and obeyed, but also documented pending written follow-up. Record systems should include the capability to track investigations in progress. Transfusion services may want to consider means to search recall records by unit number or patient or the ability to tag the unit or patient laboratory record that a recall has occurred. Large hospitals may find a confidential database useful. Transfusion services may wish to review their general strategies for oversight, management, and record keeping with the hospital s risk management and/or legal counsel. Although most of these problems occur before the hospital receives the units, there could be potential medicolegal implications for the hospital and the physician. For example, it may be advisable to consider these investigations as a subcategory of the hospital s overall incident management program for quality improvement or at least to bring serious problems to this forum. The transfusion committee or its local equivalent may wish to provide general oversight for the recall management process. There are several advantages to performing recall investigations under the aegis of the transfusion committee. This educates key physicians in the range of problems found with blood components after transfusion. It provides the medical staff s representatives a forum to review and approve the general and specific features of the procedure. The transfusion committee also provides a logical venue to bring all or selected recalls into the hospital quality improvement program. Some other resources of expertise in the hospital may be helpful in certain problems. The infection control office can provide advice on transfusions, which may have posed a serious infection risk in hindsight. Potentially, this consultation could include immediate measures such as baseline patient infectious disease testing (eg, a donor calls back to report recent previously unsuspected exposure to HIV) or antimicrobial therapy (eg, a platelet culture becomes positive after the product was already transfused). For perplexing conundrums in posttransfusion problems, the hospital ethics committee might provide a useful forum for discussion. The hospital public relations office should be informed when a problem could be of concern to the news media and the public, such as a large recall of blood products in the community. MANAGEMENT OF SPECIFIC PROBLEMS Many transfusion recipients have died of their underlying illness by the time a notice arrives about one of their blood components. In an HIV lookback, their next of kin must be notified. However, for other problems, no further investigation is needed. Table 3 gives suggestions for whether to inform a recipient s physician about a problem transfusion. The list of problems is adapted from the FDA s categories of donor-center BPDs, with some additions. Our general approach is that if a blood component might have posed a tangible infectious or other risk, then the patient s physician should evaluate whether the patient may have been affected. On the other hand, if the problem with the product did not pose a tangible risk to the patient, then the transfusion service physician, with the oversight of the transfusion committee if desired, can exercise informed medical judgment to not notify the recipient s physician. Blood suppliers should provide adequate information for the transfusion service to evaluate the problem and counsel the physician if needed. Without violating the donor s confidentiality, the transfusion service may seek further information from the blood supplier if the first notice is insufficient for a decision. Many physicians are not familiar with the details of when and why blood donors should be deferred. When the patient s physician is informed about a
7 42 GLENN RAMSEY Table 3. Suggested Approaches for Follow-up of Blood Components Discovered After Transfusion to Have Been in nconformance (Biological Product Deviations) Type of Deviation tify Patient s Physician? Postdonation information At donation of unit in question, donor should have been deferred for: Malaria-risk travel vcjd-risk travel, bovine insulin, one CJD relative, or United Kingdom transfusion Other vcjd risks Tattoo or ear/body piercing Cancer Disease/surgery Intravenous drug use Antibiotics or other medications Yes, if RBCs, granulocytes, or platelets (FDA guidance 16 ) (see post-donation CJD below) Yes, if sterility uncertain* Assess details for medical impact Yes* Yes, if teratogenic medication and pregnant recipient. If possible bacterial contamination, did a transfusion reaction occur? Smallpox vaccination See FDA guidance 20 Previously transmitting transfusion-related infection Yes* Seeking testing or asking for blood to be discarded Yes* Risk factors for HIV or hepatitis exposure Yes* Severe acute respiratory syndrome (SARS), or exposure Yes, unless donor was well for 14 days after exposure (FDA guidance 21 ) After unit in question, donor later developed: HIV infection Yes* (HIV lookback 1,3 ) Clinical hepatitis, or confirmed anti-hcv, HCV RNA, or HBsAg Yes* (lookback if HCV 2,3 ) (te: FDA does not require HBV lookback 14 ) Confirmed anti-htlv-i or II Yes*, if cellular product (although transmission unlikely after 10 days of refrigeration) (te: FDA does not require HTLV lookback 14,15 ) Confirmed syphilis antibody (FDA draft guidance 22 ) West Nile virus illness or positive WNV NAT Yes, if within dates of FDA guidance 19 SARS Yes, if within 14 days after donation 21 CJD Contact National Blood Data Resource Center 17 (see text) Indeterminate anti-hiv, anti-hcv, or anti-htlv Reactive screening test, but negative supplemental testing Reactive anti-hbc or elevated ALT (Anti-HBc, FDA memorandum 14 ) Donor screening and deferral Vital signs unacceptable or not documented Did recipient have septic transfusion reaction? Hematocrit unacceptable Screening incomplete (history, arm check, donor signature) Incorrect re-entry after reactive screening test Assess details of timing and results of testing Quality control and distribution Clotted or hemolyzed unit or segment Did recipient have transfusion reaction? Outdated product Did recipient have transfusion reaction? Shipped or stored at incorrect temperature Did recipient have transfusion reaction? Unacceptable RBC, platelet, or clotting factor content t irradiated, leukoreduced, or CMV-safe as ordered Yes, if patient did not receive required product Labeling Recipient ID incorrect (including autologous) Did wrong patient receive unit? Expiration extended erroneously If unit was given after true expiration, did recipient have transfusion reaction? ABO, Rh, or RBC antigen label incorrect Did recipient have transfusion reaction or receive Rh-incompatible RBC-containing product? Irradiation, leukoreduction, or CMV status incorrect Yes, if recipient did not receive required need Donor number incorrect, but fix patient and lab record with correct unit number Product type incorrect Assess medical impact Anticoagulant incorrect Testing (of the unit in question) Incorrect infectious disease testing Yes* Reactive infectious disease testing Yes, unless supplemental testing is negative Confirmed bacterial detection in product or co-component Yes Reactive indirect bacterial screen (e.g., ph, glucose), not confirmed Incorrect ABO, Rh, or RBC antigen testing Did recipient have transfusion reaction or receive Rh-incompatible RBC-containing product? Incorrect RBC antibody testing Component preparation Incorrect irradiation or leukoreduction Yes, if recipient did not receive required need Sterility compromised Did patient have transfusion reaction or infection? Incorrect temperature Did patient have transfusion reaction? Additive solution not added, or added incorrectly Was unit actually outdated when given? Collection Sterility compromised Did patient have transfusion reaction or infection? Outdated collection bag Did patient have transfusion reaction or infection? Phlebotomy time or volume incorrect *Unless donor later tested negative for marker(s) in question, after the appropriate seroconversion period (Table 4 and text). HIV or HCV nucleic acid tests (NAT) have short seroconversion windows, but postdonation NATs have not been incorporated yet into FDA rules and guidances on HIV or HCV lookbacks. 1-3
8 MANAGING BLOOD RECALLS 43 Table 4. Seroconversion (Window) Periods for Donor Viral Tests After Infection Test Mean (days) Range (days) Anti-HIV HIV NAT NA 7-12 Anti-HTLV Anti-HCV HCV NAT NA HBsAg NOTE. See text for CDC and FDA recommendations for testing in occupational exposure and HIV and HCV lookback. Abbreviation: NA, not available. Data from Schreiber et al 27 and Interorganizational Task Force on Nucleic Acid Testing (NAT) of Blood Donors. 28 problem with a nonconforming blood component, some background information is often useful. For recurring notices such as malaria-area travel, a form letter may be convenient. For most routine notices, we have not required follow-up information from the physician. However, for sensitive issues, the transfusion service physician may offer assistance in patient counseling if desired. When there is concern about the possibility of infection risk from the donor, testing of the donor and/or patient may be indicated. When a donor is deferred for a risk factor before donation, no testing is done at that time. From the collection facility s standpoint, this may discourage test seeking by ineligible donors. Unfortunately for the transfusion services, which have previously received blood components from that donor, the current infection status of the donor is thus left unknown. If the donor has been tested since the donation in question, the last date of testing should be included in the notification or be sought by the transfusion service. In serious donor risks, such as known HIV exposure, the transfusion service may ask the collection facility to seek donor testing if feasible. Seroconversion window information is helpful for counseling and donor testing after exposure or for recipients after transfusion. If the donor has tested negative after the seroconversion period of the test in question has elapsed, then donor infection at the time of the donation can be ruled out. Table 4 shows seroconversion window periods for viral tests required in blood donors. The CDC recommendation for HIV antibody testing after needlestick exposure is 6 months, 29 that is more conservative than the table figures. In the 1996 HIV lookback rule and the 2000 proposed lookback rule for HIV and HCV, 1,3 the FDA required 12 months before a negative antibody test to rule out the need for lookback in prior donations. NAT for HIV and HCV has much shorter window periods than antibody testing. The CDC recommends 4 to 6 weeks of follow-up testing for HCV RNA after needlestick exposure. 29 NAT has not yet been factored into FDA lookback rules and guidances. REDUCING RECALLS AND WITHDRAWALS Blood centers have greatly reduced infectious disease testing errors and problems that predominated in recalls of the early and mid-1990s. Today s challenge is the donor who does not reveal a deferring risk factor or condition. An anonymous survey of blood donors for risk behaviors revealed that 1.9% had a deferrable risk at the time of their donation (1.7% after testing and confidential unit exclusion). 30 Efforts have been made to reshape screening questions to improve their comprehension by donors. 31 Computer-assisted interviews may offer donors a more comfortable way to reveal risk factors. 32 Because many current donor risk factors are based on international travel, another area for consideration is making information more widely accessible for travelers and their physicians about when not to donate blood. For example, the CDC s key international travel health publication, the Yellow Book, does not tell physicians and travelers about when to avoid blood donation, and for how long. 33 Likewise, when blood donor deferrals began for SARS-area travel, this was not included in CDC information pages for travelers. 34 More publicity about the consequences of travel on blood donation might reduce recall rates for geographic donor risks. CONCLUSIONS In today s regulatory climate, hospital transfusion services receive numerous recalls and market withdrawals from their blood suppliers. Hospitals should have procedures for managing the quarantine, medical evaluation, and records of these recalls. The transfusion committee may provide oversight for local preferences about when to inform the recipient s physician. More study of the medical importance of recalls for transfused patients is needed. Because the predominant reason for notices
9 44 GLENN RAMSEY about blood products is postdonation information about the donor, this is an important area for quality improvement by blood suppliers. New measures to improve donor understanding and communication about deferring information could help reduce blood component recalls and withdrawals. 1. Current good manufacturing practices for blood and blood components: notification of consignees receiving blood and blood components at increased risk for transmitting HIV infection; final rule. Rockville, MD, Food and Drug Administration, September 9, Guidance for industry: current good manufacturing practice for blood and blood components: (1) quarantine and disposition of units from prior collections from donors with repeatedly reactive screening test for antibody to hepatitis C virus (anti-hcv); (2) supplemental testing, and the notification of consignees and blood recipients of donor test results for anti- HCV. Rockville, MD, Food and Drug Administration, October 21, Current good manufacturing practice for blood and blood components; notification of consignees and transfusion recipients receiving blood and blood components at increased risk of transmitting HCV infection ( lookback ); proposed rule. Rockville, MD, Food and Drug Administration, vember 16, Goldman M, Juodvalkis S, Gill P, et al: Hepatitis C lookback. Transfusion Med Rev 12:84-93, Busch MP, Young MJ, Samson SM, et al: Risk of human immunodeficiency virus (HIV) transmission by blood products before the implementation of HIV antibody screening. Transfusion 31:4-11, FDA Enforcement Report. Rockville, MD, Food and Drug Administration (weekly). Available at: (internet). Accessed April 18, Steinhauer J: Blood center ads, meant to be calming, prove alarming. New York Times, October 21, 1998, p B3 8. rdenberg T: Recalls: FDA, industry cooperate to protect consumers. FDA Consumer Magazine 29(8), October Ramsey G, Sherman LA: Blood component recalls in the United States. Transfusion 39: , Ramsey G, Sherman LA: Blood component recalls in the United States, Transfusion 40: , 2000 (letter) 11. Biological product deviation reports-fy02 annual summary. Rockville, MD, Food and Drug Administration, April 23, 2003, Available at: Accessed April 24, Recall activities, in Investigations Operation Manual Rockville, MD, Food and Drug Administration, 2002, pp , Available at: Accessed April 18, Recall and emergency procedures, in Regulatory Procedures Manual. Rockville, MD, Food and Drug Administration, 2001, chapter 7. Available at: Accessed April 18, Recommendations for the quarantine and disposition of units from prior collections from donors with repeatedly reactive screening tests for hepatitis B virus (HBV), hepatitis C virus (HCV), and human T-lymphotropic virus type I (HTLV- I). Rockville, MD, Food and Drug Administration, July 19, Guidance for industry: Donor screening for antibodies to REFERENCES HTLV-II. Rockville, MD, Food and Drug Administration, August 15, Guidance for industry: revised preventive measures to reduce the possible risk of transmission of Creutzfeldt-Jakob disease (CJD) and variant Creutzfeldt-Jakob disease (vcjd) by blood and blood products. Rockville, MD, Food and Drug Administration, January 9, Creutzfeldt-Jakob disease investigational lookback study updated 08/26/02. Bethesda, MD, National Blood Data Resource Center. Available at: cjd.htm. Accessed April 17, Hewitt P, Llewelyn C, Will R: Follow up of donations from patients with vcjd. Vox Sang 83:1, 2002 (Suppl 2, abstr) 19. Guidance for industry: Revised recommendations for the assessment of donor suitability and blood and blood product safety in cases of known or suspected West Nile virus infection. Rockville, MD, Food and Drug Administration, May 1, Guidance for industry: Recommendations for deferral of donors and quarantine and retrieval of blood and blood products in recent recipients of smallpox vaccine (vaccinia virus) and certain contacts of smallpox vaccine recipients. Rockville, MD, Food and Drug Administration, December 30, Guidance for industry: Recommendations for the assessment of donor suitability and blood product safety in cases of suspected severe acute respiratory syndrome (SARS) or exposure to SARS. Rockville, MD, Food and Drug Adminstration, April 17, Draft guidance for industry: Revised recommendations for donor and product management based on screening tests for syphilis. Rockville, MD, Food and Drug Administration, June 25, Draft guidance for industry: precautionary measures to reduce the possible risk of transmission of zoonoses by blood and blood products from xenotransplantation product recipients and their intimate contacts. Rockville, MD, Food and Drug Administration, February 1, Guidance for industry: recommendations for assessment of donor suitability and blood and blood product safety in cases of possible exposure to anthrax. Rockville, MD, Food and Drug Administration, October 17, Fridey JL: Standards for Blood Banks and Transfusion Services (ed 22) Bethesda, MD, American Association of Blood Banks, Transfusion medicine checklist, in Laboratory Accreditation Program. rthfield, IL, College of American Pathologists, December Available at: Accessed April 18, Schreiber GB, Busch MP, Kleinman SH, et al: The risk of transfusion-transmitted viral infections. N Engl J Med 334: , Report of the interorganizational task force on nucleic acid amplification testing of blood donors: Nucleic acid amplification testing of blood donors for transfusion-transmitted infectious diseases. Transfusion 40: , 2000
10 MANAGING BLOOD RECALLS Centers for Disease Control and Prevention. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR 50(. RR-11):21-27, Williams AE, Thomson RA, Schreiber GB, et al: Estimates of infectious disease risk factors in US blood donors. JAMA 277: , Orton SL, Virvos VJ, Williams AE: Validation of selected donor-screening questions: structure, content, and comprehension. Transfusion 40: , Sanchez AM, Schreiber GB, Glynn SA, et al: Blooddonor perceptions of health history screening with a computerassisted self-administered interview. Transfusion 43: , Health Information for International Travel. Atlanta, Centers for Disease Control and Prevention. Available at: www. cdc.gov/travel/yb/index.htm. Accessed April 22, Interim guidelines about severe acute respiratory syndrome (SARS) for persons traveling to SARS-affected areas. Atlanta, Centers for Disease Control and Prevention, April 22, Available at: htm. Accessed April 22, 2003
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Process or performance improvement is often discussed
 Process Improvement in Transfusion Medicine Ongoing efforts to decrease costs in the clinical laboratory make continuous process improvement especially important in difficult economic times. Process improvement
Process Improvement in Transfusion Medicine Ongoing efforts to decrease costs in the clinical laboratory make continuous process improvement especially important in difficult economic times. Process improvement
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
CERTIFIED MAIL RETURN RECEIPT REQUESTED. Esther Hernandez President United States Blood Bank, Inc NW 95th Avenue Miami, Florida
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
Guidance for Industry
 Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Department of Health and Human Services
 Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
15. Procuring, processing and transporting gametes and
 15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
SOP 17 BLOOD BANK. 3. Overall Responsibility: Blood Bank In-Change/Pathologist.
 SOP 17 BLOOD BANK 1. Purpose: To ensure the availability of safe blood unit with facility for compatibility testing, storage and issue of blood in an aseptic environment on 24*7 basis trough trained professionals.
SOP 17 BLOOD BANK 1. Purpose: To ensure the availability of safe blood unit with facility for compatibility testing, storage and issue of blood in an aseptic environment on 24*7 basis trough trained professionals.
[Submitted Electronically]
![[Submitted Electronically] [Submitted Electronically]](/thumbs/82/85246007.jpg) [Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
[Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
Blood Donor Counselling
 Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION*
 11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
Compliance Program Guidance Manual. Chapter 42 - Blood and Blood Products
 Compliance Program Guidance Manual Chapter 42 - Blood and Blood Products Inspection of Licensed and Unlicensed Blood Banks, Brokers, Reference Laboratories, and Contractors- 7342.001 Implementation Date:
Compliance Program Guidance Manual Chapter 42 - Blood and Blood Products Inspection of Licensed and Unlicensed Blood Banks, Brokers, Reference Laboratories, and Contractors- 7342.001 Implementation Date:
Database on Blood Safety 2009 in ECO Member States
 Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
The establishment of well-organized, nationally-coordinated blood transfusion services with quality systems in all areas
 Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Draft Agreed by Biologics Working Party December Draft Agreed by Blood Products Working party December 2010
 15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
The testing of Donated Blood and Components at NHSBT
 The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
N.J.A.C. 8:8-1 et seq.
 N.J.A.C. 8:8-1 et seq. COLLECTION, PROCESSING, STORAGE AND DISTRIBUTION OF BLOOD BLOOD BANK INSPECTION CHECKLIST REGULATIONS N.J.A.C. 8:8-1.3 (a) N.J.A.C. 8:8-1.3 (b) N.J.A.C. 8:8-1.3 (c) N.J.A.C. 8:8-1.3
N.J.A.C. 8:8-1 et seq. COLLECTION, PROCESSING, STORAGE AND DISTRIBUTION OF BLOOD BLOOD BANK INSPECTION CHECKLIST REGULATIONS N.J.A.C. 8:8-1.3 (a) N.J.A.C. 8:8-1.3 (b) N.J.A.C. 8:8-1.3 (c) N.J.A.C. 8:8-1.3
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
Blood Supply and Wastage
 Blood Supply and Wastage Inga Willett Lab Matters, Oake Manor, 21 st June 2017 Blood supply UK supplied by 4 blood services: SNBTS NIBTS NHSBT WBS http://commons.wikimedia.org/wiki/file:uk_map_home_nations.png
Blood Supply and Wastage Inga Willett Lab Matters, Oake Manor, 21 st June 2017 Blood supply UK supplied by 4 blood services: SNBTS NIBTS NHSBT WBS http://commons.wikimedia.org/wiki/file:uk_map_home_nations.png
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
Guidance for Industry
 Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282
 Note to Surveyors: INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282 This form consists of portions of the FDA-2609. These portions are the only parts of the FDA-2609 that have been approved
Note to Surveyors: INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282 This form consists of portions of the FDA-2609. These portions are the only parts of the FDA-2609 that have been approved
For information only: all participants in the graduated plan procedure. 7 January 2013
 Paul-Ehrlich-Institut P.O. Box 63207 Langen, Germany To: All marketing authorisation holders of cellular blood products and therapeutic single plasma as well as holders of an authorisation for stem cells
Paul-Ehrlich-Institut P.O. Box 63207 Langen, Germany To: All marketing authorisation holders of cellular blood products and therapeutic single plasma as well as holders of an authorisation for stem cells
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM
 THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies
 2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
Guide to the preparation, use and quality assurance of blood components
 Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
2006 HIV Diagnostics Survey
 Public Health Laboratory Issues in Brief: 2006 HIV Diagnostics Survey Association of Public Health Laboratories November 2007 Background Nearly 20 years ago, the Association of Public Health Laboratories
Public Health Laboratory Issues in Brief: 2006 HIV Diagnostics Survey Association of Public Health Laboratories November 2007 Background Nearly 20 years ago, the Association of Public Health Laboratories
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Source of effectiveness data Effectiveness data were derived from a single study combined with a review of previously completed studies.
 Declining value of alanine aminotransferase in screening of blood donors to prevent posttransfusion hepatitis B and C virus infection Busch M P, Korelitz J J, Kleinman S H, Lee S R, Aubuchon J P, Schreiber
Declining value of alanine aminotransferase in screening of blood donors to prevent posttransfusion hepatitis B and C virus infection Busch M P, Korelitz J J, Kleinman S H, Lee S R, Aubuchon J P, Schreiber
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019
![[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019 [Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019](/thumbs/95/123651537.jpg) SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
Changing eligibility criteria for MSM: The Canadian Perspective
 Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Report on the Transplantation Transmission Sentinel Network (TTSN)
 Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview
 IPFA Workshop Session 7: REGULATORY CONSIDERATIONS TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview Mary Lavithis, CSL Behring Australia Presented at: IPFA Asia
IPFA Workshop Session 7: REGULATORY CONSIDERATIONS TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview Mary Lavithis, CSL Behring Australia Presented at: IPFA Asia
Revisions to Labeling Requirements for Blood and Blood Components, Including Source Plasma
 This document is scheduled to be published in the Federal Register on 01/03/2012 and available online at http://federalregister.gov/a/2011-33554, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/03/2012 and available online at http://federalregister.gov/a/2011-33554, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Executive Summary for Executive Licensing Panel 20 May Dr Kamal Ahuja. Follow-up inspection 14 April 2010
 Summary for Licensing Panel 20 May 2010 Centre number 0011 Centre name The London Sperm Bank Person Responsible Dr Kamal Ahuja Background Follow-up inspection 14 April 2010 1. The London Sperm Bank underwent
Summary for Licensing Panel 20 May 2010 Centre number 0011 Centre name The London Sperm Bank Person Responsible Dr Kamal Ahuja Background Follow-up inspection 14 April 2010 1. The London Sperm Bank underwent
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Armed Services Blood Program
 Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
Armed Services Blood Program Defense Health Board Concerns Regarding the Collection and Transfusion of Non-FDA Compliant Blood Products in Theater Information Brief Defense Health Board 17 August 2009
LIVE INTERACTIVE YOUR DESKTOP. Food Recall Process. Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement
 LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
AFDO Conference June 9, Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide
 AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
Center for Biologics Evaluation and Research
 Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
CORD BLOOD TRANSPLANTATION STUDY CBU EXCLUSION AND QUARANTINE RELEASE FORM
 (Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
(Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
Guidance for Industry
 Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH IDENTIFIED DONOR SPERM
 THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH IDENTIFIED DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH IDENTIFIED DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
8. Quality Assurance Guidelines (Includes Appendices A-G)
 8. Quality Assurance Guidelines (Includes Appendices A-G) Quality Assurance Guidelines for Testing Using the OraQuick HCV Rapid Antibody Test This document has been modified by OraSure Technologies from
8. Quality Assurance Guidelines (Includes Appendices A-G) Quality Assurance Guidelines for Testing Using the OraQuick HCV Rapid Antibody Test This document has been modified by OraSure Technologies from
Il ruolo delle VEQ per la sicurezza trasfusionale
 Il ruolo delle VEQ per la sicurezza trasfusionale The role of EQA for transfusion safety Giulio Pisani CNCF Ist. Sup. Sanità Global Blood Product Safety, Roma 10 aprile 2019 Blood transfusion is an essential
Il ruolo delle VEQ per la sicurezza trasfusionale The role of EQA for transfusion safety Giulio Pisani CNCF Ist. Sup. Sanità Global Blood Product Safety, Roma 10 aprile 2019 Blood transfusion is an essential
Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital
 Transfusion Reactions/Complications Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital Acute Transfusion Reactions Can be seen with
Transfusion Reactions/Complications Mary Berg, M.D. Medical Director, Transfusion Services Associate Professor of Pathology University of Colorado Hospital Acute Transfusion Reactions Can be seen with
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL
 A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL This promotional educational activity is brought to you by Ortho
A Transfusion Reaction What Do I Do Now? Judith A. Sullivan, MS, MT(ASCP)SBB, CQA(ASQ) ASCLS Region III Triennial Meeting Birmingham AL This promotional educational activity is brought to you by Ortho
Question: 1. Are you currently taking an antibiotic?
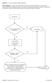 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Emerging TTIs How Singapore secure its blood supply
 Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
Exhibit 2 RFQ Engagement Letter
 Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public Hearing;
 4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
Hepatitis Case Investigation
 * indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
* indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
17. Storage of gametes and embryos
 17. Storage of gametes and embryos This guidance note contains: Mandatory requirements Extracts from the HFE Act 1990 (as amended) Extracts from licence conditions Reference to relevant HFEA Directions
17. Storage of gametes and embryos This guidance note contains: Mandatory requirements Extracts from the HFE Act 1990 (as amended) Extracts from licence conditions Reference to relevant HFEA Directions
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID
 UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil,
 236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
The TTSN - A Collaborative Biovigilance System
 The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Original article J Bas Res Med Sci 2017; 4(2):4-8.
 Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY
 Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2002D0364 EN 01.07.2012 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COMMISSION DECISION of 7 May 2002 on common technical
2002D0364 EN 01.07.2012 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COMMISSION DECISION of 7 May 2002 on common technical
Question: 1. Are you currently taking an antibiotic?
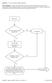 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
The pages that follow contain information critical to protecting the health of your patients and the citizens of Colorado.
 Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Richard L. Vogt, M.D. Executive Director The pages that follow
Health Alert Network Tri-County Health Department Serving Adams, Arapahoe and Douglas Counties Phone 303/220-9200 Fax 303/741-4173 www.tchd.org Richard L. Vogt, M.D. Executive Director The pages that follow
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
The New Regulations - Special IVD Issues
 The New Regulations - Special IVD Issues Dirk Stynen, Ph. D. President - Principal Consultant Qarad Geel, Belgium RMD Brussels October 2018 The IVD Regulation 2017/746 October 29, 2018 www.qarad.com 2
The New Regulations - Special IVD Issues Dirk Stynen, Ph. D. President - Principal Consultant Qarad Geel, Belgium RMD Brussels October 2018 The IVD Regulation 2017/746 October 29, 2018 www.qarad.com 2
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL?
 BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
19/08/2014. What is Injection Safety?
 Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Infection Prevention and Control A Foundation Course SAFE INJECTION PRACTICES AND SHARPS MANAGEMENT Fiona Barry IPCN Mercy University Hospital, Cork 2014 Links to CDC Materials http://www.youtube.com/watch%3fv%3d6d0stmoz80k
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Hepatitis C January 26, 2018
 Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Impact of Testing Strategies to Reduce Transmission Risk for HBV. Ravi Reddy, M Vermeulen South African National Blood Service (SANBS) 29 July 2013
 Impact of Testing Strategies to Reduce Transmission Risk for HBV Ravi Reddy, M Vermeulen South African National Blood Service (SANBS) 29 July 2013 Overview of SANBS SANBS is a private not for profit company
Impact of Testing Strategies to Reduce Transmission Risk for HBV Ravi Reddy, M Vermeulen South African National Blood Service (SANBS) 29 July 2013 Overview of SANBS SANBS is a private not for profit company
