Guidance for Industry
|
|
|
- Gabriel Gray
- 5 years ago
- Views:
Transcription
1 Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis Additional copies of this guidance are available from the Office of Communication, Outreach and Development (OCOD) New Hampshire Ave., Bldg. 71, Rm. 3128, Silver Spring, MD , or by calling or , or ocod@fda.hhs.gov, or from the Internet at nces/default.htm. For questions on the content of this guidance, contact OCOD at the phone numbers or address listed above. U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research September 2014 EAS Consulting Group, LLC 1700 Diagonal Road, Suite 750, Alexandria, Virginia (877) Toll Free (571) Local (703) Fax
2 Table of Contents I. INTRODUCTION... 1 II. BACKGROUND... 1 A. Transfusion-Transmission of Syphilis... 1 B. Testing of Blood and Blood Components for Syphilis... 3 III. CHARACTERISTICS OF SEROLOGIC ASSAYS FOR SYPHILIS... 4 IV. RECOMMENDATIONS FOR DONOR TESTING AND MANAGEMENT AND PRODUCT DISPOSITION WHEN USING TESTS FOR SYPHILIS... 5 A. Identification of Donors with a History of Syphilis... 5 B. Donor Testing and Management When Using a Nontreponemal Screening Test as the Test of Record for the Detection of Syphilis (See Figure 1)... 6 C. Donor Testing and Management When Using a Treponemal Screening Test as the Test of Record for the Detection of Syphilis (See Figure 2)... 9 V. REFERENCES i
3 Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis This guidance represents the Food and Drug Administration s (FDA s) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the appropriate FDA staff. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. I. INTRODUCTION We, FDA, are providing you, blood establishments that collect Whole Blood or blood components, including Source Plasma, with recommendations for screening and testing of donors and management of donations based on screening tests for syphilis. Licensed blood establishments must report the implementation of the recommendations contained in this guidance in accordance with 21 CFR For additional recommendations, see Guidance for Industry: Changes to an Approved Application: Biological Products: Human Blood and Blood Components Intended for Transfusion or for Further Manufacture, dated July This guidance finalizes the draft guidance of the same title, dated March 2013, and supersedes the memorandum of December 12, 1991, entitled Clarification of FDA Recommendations for Donor Deferral and Product Distribution Based on the Results of Syphilis Testing (Ref. 1). FDA s guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe FDA s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in FDA s guidances means that something is suggested or recommended, but not required. II. BACKGROUND A. Transfusion-Transmission of Syphilis Syphilis, caused by the spirochete Treponema pallidum (T. pallidum), is most often acquired after sexual contact with an infected individual. Syphilis can also be transmitted from mother to child or, rarely, transmitted by transfusion of blood or blood components from donors with active syphilis (Ref. 2). The last reported case of transfusiontransmitted syphilis in the United States (U.S.) occurred in 1966 (Ref. 3). Universal 1
4 testing of blood donors may have played a role in the disappearance of transfusiontransmitted syphilis. Other possible explanations for the decline in transfusiontransmitted syphilis include: that direct donor-to-recipient transfusions no longer take place; inactivation of T. pallidum (a cold-sensitive microorganism) in refrigerated blood components; the decline in rates of syphilis in the general population, which in turn is reflected in the donor population; self-deferral of blood donors who are ill during spirochetemia (presence of spirochetes the causative agent of syphilis infection in the circulating blood); deferral of potential donors who demonstrate high risk behavior for acquiring syphilis infection (e.g., persons who received money, drugs, or other payment for sex) through donor eligibility screening processes; wide use of antibiotics among transfusion recipients; and difficulties in diagnosing transfusion-transmitted syphilis in recipients (Ref. 4). However, none of these explanations has been quantified or adequately validated. Current testing requirements for T. pallidum are included in Title 21 of the Code of Federal Regulations (CFR) Part 610, Subpart E (Testing Requirements for Communicable Disease Agents), specifically under (i) (21 CFR (i)) and in (21 CFR ) for Source Plasma donors. FDA has sought public comments on the continued need for syphilis testing on several occasions, including in the proposed rule, entitled Requirements for Human Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use, November 8, 2007, (72 FR 63416) (Ref. 5). Information from the American Red Cross on serological testing of donors revealed that there were 324 cases of syphilis infections among American Red Cross repeat allogeneic donors in , a figure several times higher than the numbers of repeat allogeneic donors identified with human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and human T-cell lymphotropic virus (HTLV) infections, which were 92, 47, 127 and 9, respectively, during the same time period (Ref. 6). Several published studies (Refs. 7 through 12) that investigated the presence or absence of T. pallidum nucleic acid in blood samples from individuals with confirmed or possible syphilis detected spirochete nucleic acid in blood samples from persons with syphilis, some of whom had a latent infection with no symptoms. This suggests that some asymptomatic blood donors might have spirochetemia. Donations from such blood donors may have the potential to transmit syphilis to recipients. Although syphilis testing has a low sensitivity and positive predictive value as a surrogate marker for detecting known transfusion-transmitted viruses, in 2009, the American Red Cross published data (Ref. 13) indicating that there were significantly higher rates of HIV-, HCV-, HBV-, HBsAg (hepatitis B surface antigen)-, and HTLV-positive donations among donors with positive syphilis test results compared to donors with negative syphilis test results. 2
5 B. Testing of Blood and Blood Components for Syphilis Under (i), you must perform a serological screening test for syphilis on each donation of blood. 1 Donors who test reactive 2 with a screening test for syphilis must be deferred ( (a)) and notified of their deferral (21 CFR 630.6). Additional testing to requalify the donor in accordance with (b) is described below in sections IV.B and C of this document. In accordance with (h)(2)(vi), FDA allows use of blood and blood components, excluding Source Plasma, that test reactive by a screening test for syphilis, if the donation is further tested by an adequate and appropriate test which demonstrates that the reactive screening test is a biological false-positive, and the blood or blood component is labeled with both test results. Under (h)(2)(vii), you may use Source Plasma from a donor who tests reactive by a screening test for syphilis, if the donor meets the requirements of (b)(2). FDA requirements regarding syphilis testing specific to Source Plasma are as follows: 1. Current collection, testing and labeling requirements related to results of serologic tests are found in , , and (21 CFR ). 2. A sample of blood must be drawn from Source Plasma donors on the day of the first medical examination or plasmapheresis, whichever comes first, and at least every 4 months thereafter, and these samples must be tested for syphilis ( (b)(1)(i)). 3. A donor with a reactive test result for syphilis must not be plasmapheresed again until the donor tests nonreactive, except as stated in points 4 and 5, below ( (b)(2)(ii)). 4. A donor with a reactive biologic false-positive syphilis test result may be plasmapheresed, provided that the donor s file: (a) identifies the reactive serologic test and the results used to confirm the biologic false-positive results; and (b) indicates that the physician on the premises has determined the false-positive reaction is not the result of an underlying disorder that would disqualify the donor from participating in the plasmapheresis program ( (b)(2)(iii)). 5. A donor with a reactive syphilis test result may be plasmapheresed only to obtain plasma to be used for further manufacturing into control serum for the serologic test for syphilis, provided that: (a) the physician on the premises approves the donation; and (b) the donor s file contains a signed statement 1 Additional regulatory requirements related to syphilis are found under 21 CFR , , 640.5(a), , (a), (a), (a), (b)(1) and (b)(2), and The term reactive includes repeatedly reactive results. You should follow the instructions in the package insert of the assay you are using. 3
6 from a physician or clinic establishing that treatment for syphilis has commenced and that continuance in the plasmapheresis program will not interfere with or jeopardize the syphilitic donor s treatment ( (b)(2)(iv)). 6. Plasma collected from a donor with a reactive test result for syphilis must be appropriately labeled, as stated in (e)(5)(iv). III. CHARACTERISTICS OF SEROLOGIC ASSAYS FOR SYPHILIS There are two different types of serologic assays for syphilis: (A) nontreponemal assays; and (B) treponemal assays. (A) Nontreponemal assays, such as the rapid plasma reagin (RPR) test, the venereal disease research laboratory (VDRL) test, and the automated reagin test (ART), are nonspecific tests that detect reagin antibodies directed against an antigen called cardiolipin that is present in a variety of tissues. Antibodies to cardiolipin appear in the serum of persons with active syphilis and with some other conditions. However, some individuals who were previously infected with syphilis but successfully treated maintain low levels of antibody to cardiolipin for a long time; such persons are classified as being serofast. Sera and plasma from individuals previously infected with syphilis who were successfully treated do not generally remain reactive in nontreponemal tests for more than one to two years after successful treatment. Therefore, persons with active or recently treated syphilis infections generally have reactive results in nontreponemal tests, while uninfected persons or persons successfully treated years earlier usually have nonreactive nontreponemal test results. Nontreponemal assays are useful in identifying recent syphilis infection, and to monitor the progression of syphilis and response to antibiotic therapy. (B) Treponemal assays include enzyme immunoassays (EIA), fluorescent treponemal antibody absorbed assays (FTA-ABS), Treponema pallidum microhemagglutination assays (MHA-TPA) and Treponema pallidum particle agglutination assays (TP-PA). Treponemal assays test for antibodies to antigens that are specific to treponemes. Treponemal assays are most useful in identifying recent and historically remote syphilis infections. They are not generally useful in monitoring the response to antibiotic therapy. With some exceptions, positive results of tests for specific treponemal antibodies remain positive throughout an individual s life regardless of whether the individual is currently infected or has been cured following successful treatment (Ref. 14). Retesting sera that are reactive in nontreponemal assays using a specific treponemal test is valuable in distinguishing true-positive results that indicate active syphilis infection from biological false-positive results due to other conditions. Since both the nontreponemal and treponemal assays detect antibodies rather than the infectious treponemes themselves, neither assay reliably identifies patients in the window period of very 4
7 early syphilis, after infection has been acquired but before antibodies to either treponemal antigens or to cardiolipin have appeared. Before 1990, blood establishments typically used nontreponemal tests to screen donors for syphilis and then performed a treponemal test on the reactive samples to determine the specificity of nontreponemal test results. In 1990, FDA cleared a 510(k) pre-market notification for an automated modified microhemagglutination test that detects specific antibodies to T. pallidum. After this new treponemal assay was implemented for screening donors, some repeat blood donors who had formerly been found suitable based on nonreactive nontreponemal test results were deferred because they were found to have specific antibodies to T. pallidum. By largely replacing donor screening with nontreponemal assays, this test eliminated most biological false-positive nontreponemal screening test results, and its use caused no overall increase in the total number of donors deferred (Refs. 15 and 16). Many blood establishments now use automated treponemal assays with high throughput that have been cleared by FDA for purposes of screening donors for syphilis, such as the treponemal assays EIA, MHA-TPA and TP-PA. However, some blood establishments might be using a nontreponemal assay as a screening test for syphilis. Therefore, we are providing recommendations for management of donors and blood and blood components for situations in which a blood establishment would use either a nontreponemal screening test or a treponemal screening test. To distinguish the results of screening tests for syphilis from the results of additional syphilis tests, we use the terms reactive 2 and nonreactive for results of screening tests (both nontreponemal and treponemal) and reserve the terms positive and negative for results of subsequent treponemal tests (used for donor counseling and requalification) on samples of the same blood specimen previously used for screening. A test result that is read as indeterminate, questionable, or equivocal according to the package insert should be considered as equivalent to a reactive test result (for screening tests) or positive test result (for subsequent treponemal tests) for purposes of this guidance document. Establishments that screen donors using a nontreponemal assay as the test of record may use a treponemal diagnostic test for the purposes of donor counseling, donor reentry, and also for demonstrating that a reactive screening test result is a biological false-positive ( (h)(2)(vi)). However, we recommend that in all instances serologic tests used to screen donors for syphilis be cleared by FDA for such intended use (see recommendations in sections IV.B and C of this document.) IV. RECOMMENDATIONS FOR DONOR TESTING AND MANAGEMENT AND PRODUCT DISPOSITION WHEN USING TESTS FOR SYPHILIS A. Identification of Donors with a History of Syphilis 1. To assess the eligibility of the donor as required in 640.3, we recommend that you question prospective donors to determine if they have had syphilis or gonorrhea in the past 12 months or if they were treated for either of these diseases during that time. 5
8 If you are administering an abbreviated donor history questionnaire, we recommend that you ask these donors if they have had any new medical problems or diagnoses and any new medical treatments since their last donation. 2. We recommend that for donors who state that in the past 12 months they have had or have been treated for syphilis or gonorrhea, you defer such donors for 12 months after being told they had syphilis or gonorrhea or after completion of treatment. After this 12-month period, the donor may be eligible to donate again, provided that the donor satisfies all applicable donor eligibility criteria. B. Donor Testing and Management When Using a Nontreponemal Screening Test as the Test of Record for the Detection of Syphilis (See Figure 1) You must perform a serological test for syphilis on each donation of blood ( (i)). 1 You must test a donor of Source Plasma for syphilis at least every 4 months ( (b)(1)(i)). We recommend that all serological tests used to screen donations for syphilis be cleared by FDA for such intended use. 1. If the nontreponemal screening test is nonreactive, the donor is considered to be negative for syphilis infection. You may release the donation, provided it meets all other requirements, and retain the donor. 2. If the nontreponemal screening test is reactive 2, you must defer the donor indefinitely ( (a)) and you must not ship or use the donation, unless an exception for shipment or use is applicable ( (h)). However, Source Plasma donors may be allowed to donate plasma for further manufacture into noninjectable products ( (b)(2)(ii) through (iv)). Absent an exception under (h)(2) being applicable, donations of blood and blood components must not be used ( (h)) unless a negative treponemal test result is obtained using the method described below. The donor may be eligible for reentry following the method described below. Product Management and Reentry under (b) of Deferred Donors 3. If the nontreponemal test is reactive 2, you may perform a treponemal test 3 using either a sample from the index donation or a follow-up sample from the donor collected at a later date. a. If the treponemal test result is negative (suggesting that the reactive nontreponemal test result was a biological false-positive result), you may 3 Establishments may use a treponrmal diagnostic test for donor counseling, donor reentry or for demonstrating that the reactive screening test is a biological false-positive ( (h)(2)(vii)). 6
9 reenter the donor under (b). If the sample tested was from the index donation, the donation, excluding Source Plasma, may be released ( (h)(2)(vi)), provided the donation meets all other criteria, but must be appropriately labeled under (h)(2)(vi) and Under (h)(2)(vii), you may use Source Plasma from a donor who tests reactive by a screening test for syphilis, if the donor meets the requirements of (b)(2). You may consider counseling the donor about the potential medical significance of a biological false-positive screening test. b. If the treponemal test result is positive, you must continue to defer the donor indefinitely ( (a)) and discard the donation unless an exception for shipment or use is applicable ( (h)). You may reenter the donor under (b), if the donor subsequently: i. Presents written evidence from a physician or public health clinic of completion of a known, effective treatment for syphilis that was completed at least 12 months before the next donation 4 ; and ii. Meets all other donor eligibility criteria. You may use either an FDA-cleared nontreponemal screening test or an FDA-cleared treponemal screening test to test the reentered donor s next donation and subsequent donations. 4 If your medical director determines that an individual donor never had a syphilis infection, you may submit a request to FDA to reenter the individual under (b). 7
10 Figure 1: Donor Testing and Management When Using a Nontreponemal Screening Test as the Test of Record for the Detection of Syphilis Perform FDA-cleared nontreponemal screening test Nonreactive Reactive 1 Defer donor indefinitely. Release donation. Donation may be suitable for use. 2 Donor may be eligible for reentry. Perform treponemal test 3 for purposes of product management and donor reentry. Negative Donor may be reentered. 4 Donation may be released but must be appropriately labeled. 5 Positive Donor remains deferred indefinitely. Discard donation. The donor may be reentered 12 months after completion of treatment for syphilis with written evidence from a physician or public health clinic of completion of known effective treatment, provided all other donor eligibility criteria are met. Test the reentered donor s next donation and subsequent donations using an FDA-cleared nontreponemal or treponemal screening test. 1 The term reactive includes repeatedly reactive results. You should follow the instructions in the package insert of the assay you are using. 2 Source Plasma donations with these test results may be used under some circumstances, but the donor is deferred unless certain conditions apply ( (h)(2)(vii) and (b)(2)(ii) through (iv)). Absent the applicability of an exception under (h)(2), donations of blood and blood components must not be used unless a negative treponemal test result is obtained ( (h)). 3 For donor counseling, donor reentry or demonstrating that the reactive screening test is a biological false-positive ( (h)(2)(vi), you may use a treponemal diagnostic test for donor counseling, donor reentry or for demonstrating that the reactive screening test is a biological false-positive 4 Consider counseling the donor about the potential medical significance of a biological false-positive screening test. 5 Sample tested must be from index donation. Excluding Source Plasma, you must label such donations as reactive by a screening test for syphilis and negative by a treponemal test ( (h)(2)(vi)). Under (h)(2)(vii), you may use Source Plasma from a donor who tests reactive by a screening test for syphilis, if the donor meets the requirements of (b)(2). 8
11 C. Donor Testing and Management When Using a Treponemal Screening Test as the Test of Record for the Detection of Syphilis (See Figure 2) You must perform a serological test for syphilis on each donation of blood ( (i)). You must test a donor of Source Plasma for syphilis at least every 4 months ( (b)(1)(i)). We recommend that all serological tests used to screen donations for syphilis be cleared by FDA for such intended use. 1. If the treponemal screening test is nonreactive, the donor is considered to be negative for syphilis infection. You may release the donation, provided it meets all other requirements and retain the donor. 2. If the treponemal screening test is reactive 2, you must defer the donor indefinitely and you must not ship or use the donation, unless an exception for shipment or use is applicable ( (h)). However, Source Plasma donors may be allowed to donate plasma for manufacture into non-injectable products ( (b)(2)(iv)). The donor may be eligible for reentry following the method described below. Reentry under (b) of Deferred Donors 3. Because the possibility exists that a treponemal test might be false-positive for reasons unrelated to the analyte (e.g., failure to remove excess conjugate during the performance of the test), the donor may be eligible for reentry. You may perform another treponemal screening test that is different from the initial treponemal screening test used as the test of record, using either a sample from the index donation or a follow-up sample from the donor collected at a later date. 5 a. If the additional treponemal screening test result is negative, you may reenter the donor. b. If the additional treponemal screening test result is positive, the donor remains deferred indefinitely ( (a)). The donor may be eligible for reentry, as described immediately below. 5 When treponemal screening test results are reactive, we do not consider negative results on an additional treponemal test to be indicative of biological false-positives on the screening test under (h)(2)(vi). Regardless of the result from the additional treponemal screening test, under (h) you must not release the index donation unless an exception applies. Source Plasma donations with these test results may be used under some circumstances, provided that the requirements under (h)(2)(vii) and (b)(2)(ii) through (iv) are met. 9
12 You may test the sample from the donor which was positive on the additional treponemal screening test using a nontreponemal screening test to assess whether the donor has an active infection. 6 i) If the nontreponemal screening test result is negative you may reenter the donor under (b) if the donor: A) Presents written evidence from a physician or public health clinic of completion of a known effective treatment for syphilis completed at least 12 months before the next donation 4 ; and B) Meets all other donor eligibility criteria. You may use either a nontreponemal screening test or a treponemal screening test that has been cleared by FDA for such intended use to test the reentered donor s next donation and subsequent donations. ii) If the nontreponemal screening test result is positive, the donor remains deferred indefinitely ( (a)). The donor may be eligible for reentry 12 months after completion of treatment for syphilis. You may reenter the donor ( (b)), if the donor: A) Presents written evidence from a physician or public health clinic of completion of a known, effective treatment for syphilis that was completed at least 12 months before the next donation 4 ; and B) Meets all other donor eligibility criteria. 6 Performing a nontreponemal test after positive treponemal test results are obtained can assist in determining a donor s status in regard to syphilis infection. A positive nontreponemal test result, with positive treponemal test results, indicates an active or a recently treated syphilis infection. A negative nontreponemal test result, with positive treponemal test results, indicates recovery or cure from a previous syphilis infection. Thus, knowledge of the nontreponemal test result, with positive treponemal test results, can assist in counseling the donor and in determining future eligibility of the donor. 10
13 Figure 2: Donor Testing and Management When Using a Treponemal Screening Test as the Test of Record for the Detection of Syphilis Perform FDA-cleared treponemal screening test Nonreactive Reactive 1 Release donation. Defer donor indefinitely. Discard donation 2 Donor may be eligible for reentry. Donor Reentry Perform FDA-cleared treponemal screening test different from initial treponemal screening test on index donation or follow-up sample. Negative Donor may be reentered. Positive Donor remains deferred indefinitely. Donor may be eligible for reentry. Perform FDA-cleared nontreponemal screening test. 3 Negative The donor may be reentered with written evidence from a physician or public health clinic of completion of a known, effective treatment for syphilis at least 12 months before the next donation, provided all other donor eligibility criteria are met. Positive The donor may be eligible for reentry 12 months after completion of treatment for syphilis. The donor may be reentered with written evidence from a physician or public health clinic of completion of a known, effective treatment for syphilis at least 12 months before next donation, provided all other donor eligibility criteria are met. Test reentered donor s next donation and subsequent donations using an FDA-cleared nontreponemal or treponemal screening test. 1 The term reactive includes repeatedly reactive results. You should follow the instructions in the package insert of the assay you are using.. 2 Source Plasma donations with reactive test results for syphilis may be used under some circumstances, but the donor is deferred, unless certain conditions apply ( (h)(2)(vii) and (b)(2)(ii) through (iv)). Donations of blood and blood components must be discarded, unless an exception is applicable (( (h)(2)). Note that all applicable labeling requirements must be met, should you be able to use the donations ( and (h)(2)). 3 Performing a nontreponemal test after positive treponemal test results are obtained can assist in determining a donor s status in regard to syphilis infection. A positive nontreponemal test result, with positive treponemal test results, indicates an active or a recently treated syphilis infection. A negative nontreponemal test result, with positive treponemal test results, indicates recovery or cure from a previous syphilis infection. Thus, knowledge of the nontreponemal test result, with positive treponemal test results, can assist in counseling the donor and in determining future eligibility of the donor. 11
14 V. REFERENCES 1. FDA Memorandum to Registered Blood Establishments, Clarification of FDA Recommendations for Donor Deferral and Product Distribution Based on the Results of Syphilis Testing, December 12, Turner TB and Diseker TH. Duration of infectivity of Treponema pallidum in citrated blood stored under conditions obtaining in blood banks. Bull Johns Hopkins Hosp 1941; 68: Chambers, RW, Foley, HT, Schmidt, PJ. Transmission of syphilis by fresh blood components. Transfusion 1969; 9: Katz, LM. A test that won t die: the serological test for syphilis. Transfusion 2009; 49: FDA Proposed Rule: Requirements for Human Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use, November 8, 2007, 72 FR Zou, S, Stramer, SL, Dodd, RY. Donor testing and Risk: current prevalence, incidence, and residual risk of transfusion-transmitted agents in US allogeneic donations. Transfusion Medicine Reviews 2012; 26(2): Marfin, AA, et al. Amplification of DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilis. Diagnostic Microbiology and Infectious Disease 2001; 40: Orton, SL. Prevalence of Treponema pallidum DNA and RNA in blood donors with confirmed-positive syphilis tests. Transfusion 2002; 42: Kouznetzov, AV, et al. Detection of the 47-kilodalton membrane immunogen gene of Treponema pallidum in various tissue sources of patients with syphilis. Diagnostic Microbiology and Infectious Disease 2005; 51: Castro, R, et al. Detection of Treponema pallidum sp pallidum DNA in latent syphilis. International Journal of STD & AIDS 2007; 18: Flasarova, M, et al. (Abstract) Molecular detection and subtyping of Treponema pallidum subsp. Pallidum in clinical specimens. Epidemiol. Mikrobiol. Immunol. 2006; 3: Rodionova, EN, et al. Detection of Treponema pallidum DNA and RNA in clinical material from patients with syphilis at different stages. Zh. Mikrobiol Epidemiol. Immunobiol. 2003; 3: Zou, S, Notari, EP, Fang, CT, Stramer, SL, Dodd, RK. Current value of serologic test for syphilis as a surrogate marker for blood-borne viral infections among blood donors in the United States. Transfusion 2009; 49: Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening. MMWR 2011 (February); 60(5): Aberle-Grasse J, Orton SL, Notari E IV, Layug LP, Cable RG, Badon S, Popovsky MA, Grindon AJ, Lenes BA, Williams AE. Predictive value of past and current screening tests for syphilis in blood donors: changing from a rapid plasma reagin test to an automated specific treponemal test for screening. Transfusion 1999 (February); 39(2): Blood Products Advisory Committee meeting transcripts on Review and Discussion of Recommendations for Revised Donor Testing and Suitability Criteria Related to Using Treponemal Screening Tests for Syphilis, October 20-21,
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
Reprinted from FDA s website by Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening Tests for Syphilis DRAFT GUIDANCE
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry
 Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Serological screening for syphilis in HIV-infected individuals: is a non-treponemal test adequate in the era of increasing of new syphilis infections?
 Abstract no. WEPE 494 Serological screening for syphilis in HIV-infected individuals: is a non-treponemal test adequate in the era of increasing of new syphilis infections? G.Chrysos 1, D.Karageorgopoulos
Abstract no. WEPE 494 Serological screening for syphilis in HIV-infected individuals: is a non-treponemal test adequate in the era of increasing of new syphilis infections? G.Chrysos 1, D.Karageorgopoulos
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Guidance for Industry
 Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Department of Health and Human Services
 Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
Friday, August 24, 2007 Part III Department of Health and Human Services Food and Drug Administration 2 CFR Parts 606 and 60 Current Good Manufacturing Practice for Blood and Blood Components; Notification
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Clinical Application of New Treponemal Antibody Test in Blood Donors
 Clinical Application of New Treponemal Antibody Test in Blood Donors Parichart Permpikul, MD Department of Transfusion Medicine Faculty of Medicine Siriraj Hospital Mahidol University Bangkok, Thailand
Clinical Application of New Treponemal Antibody Test in Blood Donors Parichart Permpikul, MD Department of Transfusion Medicine Faculty of Medicine Siriraj Hospital Mahidol University Bangkok, Thailand
Replaces: 04/13/17. / Formulated: 7/05 SYPHLIS
 Effective Date: 81017 Replaces: 041317 Page 1 of 7 POLICY: The Texas Department of Criminal Justice (TDCJ) will identify, test, and manage all offenders with suspected or confirmed syphilis with a uniform
Effective Date: 81017 Replaces: 041317 Page 1 of 7 POLICY: The Texas Department of Criminal Justice (TDCJ) will identify, test, and manage all offenders with suspected or confirmed syphilis with a uniform
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis
 Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis Guidance for Medical Providers and Laboratories in California These guidelines were developed by the California Department of Public
Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis Guidance for Medical Providers and Laboratories in California These guidelines were developed by the California Department of Public
Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States
 545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
10/19/2012. Serologic Testing for Syphilis. Disclosures. Comparison of the Traditional and Reverse Screening Algorithms. Outline.
 Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Susanne Norris Zanto, MPH, MLS (ASCP) CM, SM Montana Public Health Laboratory
 Susanne Norris Zanto, MPH, MLS (ASCP) CM, SM Montana Public Health Laboratory Describe the challenges in syphilis diagnostics Present two testing algorithms Non-treponemal test as initial screen Treponemal
Susanne Norris Zanto, MPH, MLS (ASCP) CM, SM Montana Public Health Laboratory Describe the challenges in syphilis diagnostics Present two testing algorithms Non-treponemal test as initial screen Treponemal
FDA Reentry Guidance
 FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
Direct Comparison of the Traditional and Reverse Syphilis Screening Algorithms
 JCM Accepts, published online ahead of print on 16 November 2011 J. Clin. Microbiol. doi:10.1128/jcm.05636-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
JCM Accepts, published online ahead of print on 16 November 2011 J. Clin. Microbiol. doi:10.1128/jcm.05636-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
Screening Donated Blood for Transfusion Transmissible Infections in Bangladesh
 Screening Donated Blood for Transfusion Transmissible Infections in Bangladesh National Guidelines 2013 Foreword Mandatory screening of donated blood for transfusion in Bangladesh began in 2000 after implementation
Screening Donated Blood for Transfusion Transmissible Infections in Bangladesh National Guidelines 2013 Foreword Mandatory screening of donated blood for transfusion in Bangladesh began in 2000 after implementation
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
CERTIFIED MAIL RETURN RECEIPT REQUESTED. Esther Hernandez President United States Blood Bank, Inc NW 95th Avenue Miami, Florida
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 CERTIFIED MAIL RETURN RECEIPT REQUESTED Esther Hernandez President United States Blood Bank, Inc. 2400 NW 95th
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
INFECTIOUS SYPHILIS NOTIFICATION FORM
 INFECTIOUS SYPHILIS NOTIFICATION FORM This is a Schedule 1, Section C disease notifiable to the Medical Officer of Health under Sections 74 and 74AA of the Health Act 1956 using non-identifiable data.
INFECTIOUS SYPHILIS NOTIFICATION FORM This is a Schedule 1, Section C disease notifiable to the Medical Officer of Health under Sections 74 and 74AA of the Health Act 1956 using non-identifiable data.
4/18/2018. Syphilis Testing. Disclosure. Learner Objectives. Outline. Employee and stockholder of Bio-Rad Laboratories, Inc.
 Disclosure Employee and stockholder of Bio-Rad Laboratories, Inc. Unraveling the Complexities of Syphilis Testing Maria Crisostomo, April 30 & May 1, 2018 2 Learner Objectives Syphilis Testing Upon completion
Disclosure Employee and stockholder of Bio-Rad Laboratories, Inc. Unraveling the Complexities of Syphilis Testing Maria Crisostomo, April 30 & May 1, 2018 2 Learner Objectives Syphilis Testing Upon completion
SYPHILIS (Treponema pallidum) IMMEDIATE NOTIFICATION STD PROGRAM
 SYPHILIS (Treponema pallidum) IMMEDIATE NOTIFICATION STD PROGRAM Event Name: Event Time Period: Clinical Description (CDC 2014) Syphilis 180 days Syphilis is a complex sexually transmitted disease that
SYPHILIS (Treponema pallidum) IMMEDIATE NOTIFICATION STD PROGRAM Event Name: Event Time Period: Clinical Description (CDC 2014) Syphilis 180 days Syphilis is a complex sexually transmitted disease that
Syphilis Treatment Protocol
 STD, HIV, AND TB SECTION Syphilis Treatment Protocol CLINICAL GUIDANCE FOR PRIMARY AND SECONDARY SYPHILIS AND LATENT SYPHILIS www.lekarzol.com (4/2016) Page 1 of 8 Table of Contents Description... 3 Stages
STD, HIV, AND TB SECTION Syphilis Treatment Protocol CLINICAL GUIDANCE FOR PRIMARY AND SECONDARY SYPHILIS AND LATENT SYPHILIS www.lekarzol.com (4/2016) Page 1 of 8 Table of Contents Description... 3 Stages
Documentation, Codebook, and Frequencies
 Documentation, Codebook, and Frequencies MEC Laboratory Component: Syphilis(IgG), Syphilis Rapid Plasma Reagin (RPR), and Treponema pallidum Particle Agglutination (TP- PA) Survey Years: 2003 to 2004 SAS
Documentation, Codebook, and Frequencies MEC Laboratory Component: Syphilis(IgG), Syphilis Rapid Plasma Reagin (RPR), and Treponema pallidum Particle Agglutination (TP- PA) Survey Years: 2003 to 2004 SAS
February 3, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 February 3, 2014 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 ATTN: Leslie Kux, Assistant Commissioner for Policy Ref: [Docket No.
February 3, 2014 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 ATTN: Leslie Kux, Assistant Commissioner for Policy Ref: [Docket No.
2019 HIV Diagnostics Conference March 27, 2019
 2019 HIV Diagnostics Conference March 27, 2019 Performance of the Syphilis Reverse Algorithm Using the Abbott Architect Syphilis TP (ASTP) and its Role in a Blended Diagnostic Application in Florida s
2019 HIV Diagnostics Conference March 27, 2019 Performance of the Syphilis Reverse Algorithm Using the Abbott Architect Syphilis TP (ASTP) and its Role in a Blended Diagnostic Application in Florida s
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry
 Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
The Use of a Rapid Syphilis Test with Specimens from an HIV Cluster Investigation in Rural West Virginia
 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention The Use of a Rapid Syphilis Test with Specimens from an HIV Cluster Investigation in Rural West Virginia Lara E. Pereira, Ph.D. Centers
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention The Use of a Rapid Syphilis Test with Specimens from an HIV Cluster Investigation in Rural West Virginia Lara E. Pereira, Ph.D. Centers
test. It appeared that the MHA-TP test could be
 JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 1983, p. 405-409 0095-1137/83/030405-05$02.00/0 Copyright 1983, American Society for Microbiology Vol. 17, No. 3 Reactivity of Microhemagglutination, Fluorescent
JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 1983, p. 405-409 0095-1137/83/030405-05$02.00/0 Copyright 1983, American Society for Microbiology Vol. 17, No. 3 Reactivity of Microhemagglutination, Fluorescent
Coding for Preventive Services A Guide for HIV Providers
 Coding for Preventive Services A Guide for HIV Providers Jessie Murphy, MPH and Michelle Cataldo, LCSW, April 2016 Implementation of the Patient Protection and Affordable Care Act and other regulatory
Coding for Preventive Services A Guide for HIV Providers Jessie Murphy, MPH and Michelle Cataldo, LCSW, April 2016 Implementation of the Patient Protection and Affordable Care Act and other regulatory
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
ALASKA NATIVE MEDICAL CENTER SEXUALLY TRANSMITTED DISEASE SCREENING AND TREATMENT GUIDELINES
 ALASKA NATIVE MEDICAL CENTER SEXUALLY TRANSMITTED DISEASE SCREENING AND TREATMENT GUIDELINES A. Screening Page Chlamydia and Gonorrhea 1 HIV 1 Syphilis 1 Genital Herpes 2 Hepatitis A 2 Hepatitis B 2 Hepatitis
ALASKA NATIVE MEDICAL CENTER SEXUALLY TRANSMITTED DISEASE SCREENING AND TREATMENT GUIDELINES A. Screening Page Chlamydia and Gonorrhea 1 HIV 1 Syphilis 1 Genital Herpes 2 Hepatitis A 2 Hepatitis B 2 Hepatitis
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Identifying false-positive syphilis antibody results using a semi-quantitative
 CVI Accepts, published online ahead of print on 20 April 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05066-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
CVI Accepts, published online ahead of print on 20 April 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05066-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
Antibodies to Treponema pallidum
 APPLIED MICROBIOLOGY, July 1972, p. 26- Copyright 0 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Evaluation of the Qualitative and Automated Quantitative Microhemagglutination
APPLIED MICROBIOLOGY, July 1972, p. 26- Copyright 0 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Evaluation of the Qualitative and Automated Quantitative Microhemagglutination
Background. Restricted Siemens Healthcare GmbH, >1 year Late latent syphilis. Restricted Siemens Healthcare GmbH, 2017
 Background Nonneutralizing The Evolution of Syphilis Testing: Clinical Benefits of a Reverse Screening Algorithm Katherine Soreng PhD Lafond RE, et al. Clin Microbiol Rev. 06;19(1):29 49. Disease course:
Background Nonneutralizing The Evolution of Syphilis Testing: Clinical Benefits of a Reverse Screening Algorithm Katherine Soreng PhD Lafond RE, et al. Clin Microbiol Rev. 06;19(1):29 49. Disease course:
Letter of Amendment # 3 to:
 Letter of Amendment # 3 to: HPTN 078: Enhancing Recruitment, Linkage to Care and Treatment for HIV-Infected Men Who Have Sex with Men (MSM) in the United States Version 1.0, dated 8 October 2015 DAIDS
Letter of Amendment # 3 to: HPTN 078: Enhancing Recruitment, Linkage to Care and Treatment for HIV-Infected Men Who Have Sex with Men (MSM) in the United States Version 1.0, dated 8 October 2015 DAIDS
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies
 2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
Primary Sample Manual Infectious Serology Issue No Effective Date: 20/09/17 Page 1 of 15 EUROFINS BIOMNIS
 Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Validation of a New Testing Algorithm for Syphilis in Trinidad & Tobago
 Validation of a New Testing Algorithm for Syphilis in Trinidad & Tobago R. Dass, A. Sebro, J. Edwards Ministry of Health, Trinidad and Tobago rianna.dass@hotmail.com, asebro@yahoo.com, jeffreye2000@gmail.com
Validation of a New Testing Algorithm for Syphilis in Trinidad & Tobago R. Dass, A. Sebro, J. Edwards Ministry of Health, Trinidad and Tobago rianna.dass@hotmail.com, asebro@yahoo.com, jeffreye2000@gmail.com
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
[Submitted Electronically]
![[Submitted Electronically] [Submitted Electronically]](/thumbs/82/85246007.jpg) [Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
[Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
GUIDE TO INSPECTIONS OF INFECTIOUS DISEASE MARKER TESTING FACILITIES 10/96
 Seite 1 von 15 GUIDE TO INSPECTIONS OF INFECTIOUS DISEASE MARKER TESTING FACILITIES 10/96 TABLE OF CONTENTS Introduction Pg 1 Background Pg 1 List of Memoranda Pg 2 Operations Pg 2 Facilities Pg 4 Equipment
Seite 1 von 15 GUIDE TO INSPECTIONS OF INFECTIOUS DISEASE MARKER TESTING FACILITIES 10/96 TABLE OF CONTENTS Introduction Pg 1 Background Pg 1 List of Memoranda Pg 2 Operations Pg 2 Facilities Pg 4 Equipment
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Sexually transmitted infections (in women)
 Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
Int.J.Curr.Microbiol.App.Sci (2018) 7(8):
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 08 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.708.085
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 08 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.708.085
Syphilis. syphilis. Sera from all stages of syphilis and the. to compare dilution titers with those obtained
 JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 1982, p. 238-242 0095-1137/82/020238-05$02.00/0 Vol. 15, No. 2 Unheated Serum Reagin Test as a Quantitative Test for Syphilis DEBORAH E. PETTIT, SANDRA A. LARSEN,*
JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 1982, p. 238-242 0095-1137/82/020238-05$02.00/0 Vol. 15, No. 2 Unheated Serum Reagin Test as a Quantitative Test for Syphilis DEBORAH E. PETTIT, SANDRA A. LARSEN,*
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry
 Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Microbiology Devices; Reclassification of Influenza Virus Antigen Detection Test Systems
 This document is scheduled to be published in the Federal Register on 01/12/2017 and available online at https://federalregister.gov/d/2017-00199, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/12/2017 and available online at https://federalregister.gov/d/2017-00199, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Syphilis. sera from the Venereal Disease Serology Laboratory. Serum Bank, Centers for Disease Control, Atlanta,
 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1981, p. 441-445 0095-1137/81/0441-05$02.00/0 Vol. 14, No. 4 Specificity, Sensitivity, and Reproducibility Among the Fluorescent Treponemal Antibody-Absorption Test,
JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1981, p. 441-445 0095-1137/81/0441-05$02.00/0 Vol. 14, No. 4 Specificity, Sensitivity, and Reproducibility Among the Fluorescent Treponemal Antibody-Absorption Test,
HIV Update in Laboratory Testing. Patricia Slev, PhD, D(ABCC)
 HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
VDRL v/s TPHA for diagnosis of syphilis among HIV sero-reactive patients in a tertiary care hospital
 ISSN: 2319-7706 Volume 3 Number 5 (2014) pp. 726-730 http://www.ijcmas.com Original Research Article VDRL v/s TPHA for diagnosis of syphilis among HIV sero-reactive patients in a tertiary care hospital
ISSN: 2319-7706 Volume 3 Number 5 (2014) pp. 726-730 http://www.ijcmas.com Original Research Article VDRL v/s TPHA for diagnosis of syphilis among HIV sero-reactive patients in a tertiary care hospital
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry
 Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
HIV Basics: Clinical Tests and Guidelines
 HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
Syphilis Technical Instructions for Civil Surgeons
 National Center for Emerging and Zoonotic Infectious Diseases Syphilis Technical Instructions for Civil Surgeons Joanna J. Regan, MD, MPH, FAAP Medical Officer Medical Assessment and Policy Team Immigrant,
National Center for Emerging and Zoonotic Infectious Diseases Syphilis Technical Instructions for Civil Surgeons Joanna J. Regan, MD, MPH, FAAP Medical Officer Medical Assessment and Policy Team Immigrant,
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
IQPP Qualified Donor Standard
 IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors
 Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
Structure and duties of Blood service
 NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
Annals of Internal Medicine. 1991;114:
 Serologic Response to Treatment of Infectious Syphilis Barbara Romanowski, MD; Ruth Sutherland, DPH, RN; Gordon H. Fick, PhD; Debbie Mooney, BSc; and Edgar J. Love, MD, PhD Objective: To evaluate the serologic
Serologic Response to Treatment of Infectious Syphilis Barbara Romanowski, MD; Ruth Sutherland, DPH, RN; Gordon H. Fick, PhD; Debbie Mooney, BSc; and Edgar J. Love, MD, PhD Objective: To evaluate the serologic
G-B 2016 Kit Instructions CAP 2016
 G-B 2016 Kit Instructions CAP 2016 Syphilis Serology Survey Table of Contents Kit Contents Kit Contents... 1 Important: Before You Begin... 1 Detailed Testing Instructions... 1 Reporting Your Results...
G-B 2016 Kit Instructions CAP 2016 Syphilis Serology Survey Table of Contents Kit Contents Kit Contents... 1 Important: Before You Begin... 1 Detailed Testing Instructions... 1 Reporting Your Results...
The establishment of well-organized, nationally-coordinated blood transfusion services with quality systems in all areas
 Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
Evaluation of a New Rapid Plasma Reagin Card Test as a Screening Test for Syphilis
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 1982, p. 286-290 0095-1 137/82/080286-05$02.00/0 Vol. 16, No. 2 Evaluation of a New Rapid Plasma Reagin Card Test as a Screening Test for Syphilis MARY W. PERRYMAN,'*
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 1982, p. 286-290 0095-1 137/82/080286-05$02.00/0 Vol. 16, No. 2 Evaluation of a New Rapid Plasma Reagin Card Test as a Screening Test for Syphilis MARY W. PERRYMAN,'*
The Technical Aspects of Pathogen Testing in Canada. Nancy Angus Canadian Blood Services Director, Testing
 PERMISSON TO USE: Please note that, by making their presentations available on-line, primary authors have agreed to share their presentations. However, should you want to use some of the data or slides
PERMISSON TO USE: Please note that, by making their presentations available on-line, primary authors have agreed to share their presentations. However, should you want to use some of the data or slides
2/13/ Graphic photographs or cartoons used during this presentation might be offensive to some; for this I apologize in advance.
 Leon Bullard, MD, MA Medical Consultant, DHEC, DADE The 23 rd Annual APRN Conference Charleston, SC February 24, 2017 1. Provide a brief (very) review of the syphilis story. 2. Define and discuss the stages
Leon Bullard, MD, MA Medical Consultant, DHEC, DADE The 23 rd Annual APRN Conference Charleston, SC February 24, 2017 1. Provide a brief (very) review of the syphilis story. 2. Define and discuss the stages
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
Performance Characteristics of the Reverse Syphilis Screening Algorithm in a Population With a Moderately High Prevalence of Syphilis
 Performance Characteristics of the Reverse Syphilis Screening Algorithm in a Population With a Moderately High Prevalence of Syphilis Angela R. Rourk, Frederick S. Nolte, PhD, and Christine M. Litwin,
Performance Characteristics of the Reverse Syphilis Screening Algorithm in a Population With a Moderately High Prevalence of Syphilis Angela R. Rourk, Frederick S. Nolte, PhD, and Christine M. Litwin,
Transfusion-transmitted Diseases Part 1 D. Joe Chaffin, MD
 I. Outline: A. General philosophy B. Basic screening test descriptions C. Terminology (phases/periods) D. Specific organisms II. III. Transfusion-transmitted Diseases Part 1 D. Joe, MD www.bbguy.org General
I. Outline: A. General philosophy B. Basic screening test descriptions C. Terminology (phases/periods) D. Specific organisms II. III. Transfusion-transmitted Diseases Part 1 D. Joe, MD www.bbguy.org General
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
Lymphogranuloma Venereum (LGV) Surveillance Project
 Lymphogranuloma Venereum (LGV) Surveillance Project Lymphogranuloma venereum (LGV) is a systemic, sexually transmitted disease (STD) caused by a type of Chlamydia trachomatis (serovars L1, L2, L3) that
Lymphogranuloma Venereum (LGV) Surveillance Project Lymphogranuloma venereum (LGV) is a systemic, sexually transmitted disease (STD) caused by a type of Chlamydia trachomatis (serovars L1, L2, L3) that
Notice. Guidance for Manufacturers of Human Immunodeficiency Virus (HIV) Test Kits intended to be used in the Laboratory
 December 7, 2011 Notice Our file number: 11-121739-524 Re: Test Kits intended to be used in the Laboratory is pleased to announce the release of the final version of the Guidance for Manufacturers of Test
December 7, 2011 Notice Our file number: 11-121739-524 Re: Test Kits intended to be used in the Laboratory is pleased to announce the release of the final version of the Guidance for Manufacturers of Test
NEWS BK NEWS # 378 /MKT DATE : TITLE. Dear colleagues,
 1 NEWS BK NEWS # 378 /MKT DATE : 16022016 TITLE : New Bioelisa reagents Brochures Dear colleagues, We are pleased to present the new Bioelisa reagents Brochures, which are a very useful marketing and selling
1 NEWS BK NEWS # 378 /MKT DATE : 16022016 TITLE : New Bioelisa reagents Brochures Dear colleagues, We are pleased to present the new Bioelisa reagents Brochures, which are a very useful marketing and selling
[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019
![[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019 [Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019](/thumbs/95/123651537.jpg) SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282
 Note to Surveyors: INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282 This form consists of portions of the FDA-2609. These portions are the only parts of the FDA-2609 that have been approved
Note to Surveyors: INSTRUCTIONS FOR BLOOD BANK INSPECTION CHECKLIST AND REPORT CMS-282 This form consists of portions of the FDA-2609. These portions are the only parts of the FDA-2609 that have been approved
Sexually Transmitted Disease Treatment Tables
 Sexually Transmitted Disease Treatment Tables Federal Bureau of Prisons Clinical Practice Guidelines June 2011 Clinical guidelines are made available to the public for informational purposes only. The
Sexually Transmitted Disease Treatment Tables Federal Bureau of Prisons Clinical Practice Guidelines June 2011 Clinical guidelines are made available to the public for informational purposes only. The
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Sexually transmitted infections (in women)
 Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
DHQ Flowcharts v2.0 eff. February 2016
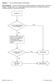 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
CTYOMEGALOVIRUS (CMV) - BACKGROUND
 CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
