The induction and regulation of CD4 T cells following respiratory syncytial virus infection
|
|
|
- Mavis Wilcox
- 6 years ago
- Views:
Transcription
1 University of Iowa Iowa Research Online Theses and Dissertations Spring 214 The induction and regulation of CD4 T cells following respiratory syncytial virus infection Kayla Ann Weiss University of Iowa Copyright 214 Kayla Ann Weiss This dissertation is available at Iowa Research Online: Recommended Citation Weiss, Kayla Ann. "The induction and regulation of CD4 T cells following respiratory syncytial virus infection." PhD (Doctor of Philosophy) thesis, University of Iowa, Follow this and additional works at: Part of the Immunology of Infectious Disease Commons
2 THE INDUCTION AND REGULATION OF CD4 T CELLS FOLLOWING RESPIRATORY SYNCYTIAL VIRUS INFECTION by Kayla Ann Weiss A thesis submitted in partial fulfillment of the requirements for the Doctor of Philosophy degree in Immunology in the Graduate College of The University of Iowa May 214 Thesis Supervisor: Associate Professor Steven M. Varga
3 Graduate College The University of Iowa Iowa City, Iowa CERTIFICATE OF APPROVAL PH.D. THESIS This is to certify that the Ph.D. thesis of Kayla Ann Weiss has been approved by the Examining Committee for the thesis requirement for the Doctor of Philosophy degree in Immunology at the May 214 graduation. Thesis Committee: Steven M. Varga, Thesis Supervisor John T. Harty Stanley Perlman Kevin L. Legge John D. Colgan
4 ACKNOWLEDGMENTS It is my belief that completion of your Ph.D. is best accomplished through the extraordinary effort to keep your professional and personal lives fulfilled and content. This belief leaves me with many people to acknowledge for their guidance and patience through my graduate training. Professionally, I would like to thank my mentor Dr. Steven Varga and my lab mates Stacey Hartwig, Ross Fulton, Daniel McDermott, Cory Knudson, Paola Boggiatto and Allison Christiaansen, who together were the main influence in my scientific development and training. In addition, I would like to thank my thesis committee members and the countless number of other lab members who have provided insight for my research in meetings and collaborative projects. Personally, I would like to thank my husband Charles Schallhorn, and family and friends, who were willing to listen to rants about experimental failings and never took offense to any unreturned phone calls. ii
5 ABSTRACT Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in young children. RSV induces variable disease severities in infected children. Severe cases of RSV-induced disease result in bronchiolitis, with a subset of children going onto develop long-term airway morbidities. The host antiviral T cell response is believed to contribute to the severity of pulmonary disease following acute RSV infection. However recent work has questioned the relative proportion of T cells that migrate into the lung tissue following a respiratory virus infection. Using in vivo intravascular antibody labeling, >8% of antigen-specific effector T cells were found to remain in the pulmonary vasculature following an intratracheal infection with the systemic viral pathogen lymphocytic choriomeningitis virus (LCMV). Therefore, I determined the proportion of RSV-specific CD4 T cells located within the lung tissue following infection. In contrast to recent reports with LCMV-specific CD8 T cells, I found approximately 85% of RSV-specific CD4 T cells were located within the lung tissue, indicating that the vast majority of virus-specific effector CD4 T cells are located within the lung tissue and not in the pulmonary vasculature following an acute RSV infection. Genetic variations can occur in the circulating RSV strains both within and between infectious seasons. Therefore, I questioned if different RSV strains could induce differential CD4 T cell responses. I demonstrate that RSV strains induce various CD4 T helper responses, which are associated with the differential activation of the innate immune response. The RSV line 19 strain induced the early production of the proinflammatory cytokines IL-1β and IL-6, resulting in an increased Th17 response as compared to the RSV strains A2 and 2-2. Blockade and/or neutralization of IL-1β and IL-6 inhibited the ability of RSV line 19 to induce a Th17 response. These results demonstrate that RSV strains can differentially activate innate immunity that iii
6 subsequently influences the type of adaptive immune response. This in part may contribute to differential RSV pathogenesis and the development of long-term airway morbidities observed in humans. IL-1 is a pleotropic cytokine able to suppress the adaptive immune response. Because the host adaptive immune response is believed to contribute to RSV-induced pulmonary disease, I evaluated the role of IL-1 in modulating the RSV-specific immune response. I found that IL-1 protein levels in the lung were increased following acute RSV infection with maximum production corresponding to the peak of the virus-specific T cell response. Multiple populations of CD4 T cells accounted for the majority of IL-1 produced in the lung including Foxp3 + Tregs, Foxp3 - CD4 T cells that co-produce IFN-γ, and Foxp3 - CD4 T cells that do not co-produce IFN-γ. Furthermore, RSV-induced disease severity was increased in both the absence of IL-1 and following IL-1 receptor blockade as compared to control mice. I also observed an increase in the magnitude of the RSV-induced CD8 and CD4 T cell response that correlated with increased disease severity following IL-1 receptor blockade. IL-1 receptor blockade during acute RSV infection altered CD4 T cell subset distribution, resulting in a significant increase in IL- 17A-producing CD4 T cells and a concomitant decrease in Foxp3 + regulatory T cells. These results demonstrate that IL-1 plays a critical role in modulating the adaptive immune response to RSV by limiting T-cell-mediated pulmonary inflammation and injury. Overall, my data demonstrate that RSV-specific CD4 T cells migrate into the lung tissue with their differentiation influenced by the strain-specific activation of the innate immune response. IL-1 is then produced by CD4 T cells to regulate the RSVspecific T cell responses and inhibit virus-induced immunopathology. My data indicate that there are multiple targets for immunotherapy for individuals with severe RSVinduced disease. iv
7 TABLE OF CONTENTS LIST OF TABLES... viii LIST OF FIGURES... ix LIST OF ABBREVIATIONS... xi CHAPTER I. GENERAL INTRODUCTION...1 Immune regulation and lung homeostasis...1 Respiratory syncytial virus...3 RSV pathogenesis...5 The pro-inflammatory response following RSV infection...7 Inflammasomes...9 Inflammasome activation following RSV infection...1 CD4 T cells and RSV pathogenesis...11 Mechanisms of Treg regulation...13 Regulation of T cell responses following RSV infection...13 Immune suppression by IL Thesis objectives...15 CHAPTER II. LOCALIZATION OF CD4 T CELLS FOLLOWING RESPIRATORY SYNCYTIAL VIRUS INFECTION...21 Abstract...21 Introduction...22 Materials and Methods...23 Mice and infection...23 Intravascular antibody labeling and tissue processing...23 Tetramer and antibody staining...24 Statistical analysis...24 Results...25 Intravascular antibody labeling can distinguish the location of CD4 T cells following RSV infection...25 Majority of CD4 T cells are protected from intravascular antibody labeling following localized respiratory infections...25 The proportion of antigen-specific CD4 T cells in the lung remains constant into the memory phase...26 Conventional CD4 T cells protected from intravascular labeling exhibit an increased effector phenotype...27 Regulatory CD4 T cells in the lung have increased expression of activation-associated proteins...28 Discussion...28 CHAPTER III. RESPIRATORY SYNCYTIAL VIRUS STRAINS INDUCE DIFFERENTIAL INNATE IMMUNE AND CD4 T CELL RESPONSES...46 Abstract...46 Introduction...46 Materials and Methods...48 Mice, infection and treatments...48 v
8 Intravascular antibody labeling and tissue processing...49 Extracellular staining...49 Ex vivo cell stimulation...5 Intracellular cytokine and Foxp3 staining...5 Real-time PCR...51 In vitro RSV infection...52 ELISA...52 Statistical analysis...53 Results...53 No difference in total numbers of activated CD8 and CD4 T cells following infection with different strains of RSV...53 Differential T helper 17 response following infection with various RSV strains...54 Increased peak viral replication following RSV A2 infection as compared to RSV line 19 and Increased pro-inflammatory cytokines in the lungs following RSV line 19 infection...55 Increased responding and cytokine-producing innate cell populations following RSV line 19 infection...55 Increased IL-1β secretion following RSV line 19 infection is caspase 1-dependent...56 Pro-inflammatory cytokines induce the Th17 response following RSV line 19 infection...57 Discussion...57 CHAPTER IV. MULTIPLE CD4 T CELL SUBSETS PRODUCE IMMUNOMODULATORY INTERLEUKIN-1 DURING RESPIRATORY SYNCYTIAL VIRUS INFECTION...8 Abstract...8 Introduction...8 Materials and Methods...82 Mice, infection, and antibody treatment...82 Disease measurements...83 Tissue processing...83 Extracellular staining...84 Ex vivo cell stimulation...84 Intracellular cytokine staining...85 Foxp3 staining...85 Plaque assays...85 ELISA...86 Multiplex bead assay...86 Histology...87 Statistical analysis...88 Results...88 CD4 T cells account for the majority of IL-1-producing cells during the peak of the virus-specific T cell response...88 Multiple CD4 T cell subsets produce IL-1 in response to RSV infection...89 Increased RSV disease severity in the absence of IL-1 or following IL-1R blockade...9 Increased inflammatory environment in the absence of IL IL-1R blockade results in an increase in the magnitude of the RSV-specific CD8 T cell response...91 vi
9 IL-1R blockade results in an increased Th1 CD4 T cell response following acute RSV infection...92 Increased lung pathology following IL-1R blockade...93 Alteration of additional CD4 T cell subsets following IL-1R blockade...94 Discussion...94 CHAPTER V. GENERAL DISSCUSSION RSV strains induce differential immune responses Predisposing the RSV-specific T helper response Mechanisms to regulate RSV-specific immunity Production of IL CD4 T cells mediating long-term airway morbidities SUMMARY OF RESULTS Chapter II Chapter III Chapter IV REFERENCES vii
10 LIST OF TABLES Table 1. Frequencies of cytokine-producing CD4 T cells on day 6 following RSV...19 viii
11 LIST OF FIGURES Figure 1. Key components of the pulmonary immune response following RSV infection...17 Figure 2. Priming and activation of the inflammasome for IL-1β secretion...19 Figure 3. i.v. antibody labeling distinguishes CD4 T cell populations harvested from the lung...32 Figure 4. CD4 T cells preferentially localize to the lung following a local pulmonary infection...34 Figure 5. RSV-specific CD4 T cells remain in the lung tissue into memory...36 Figure 6. Activated conventional Foxp3 - CD4 T cells migrate into the lung following RSV infection...38 Figure 7. Increased proliferation of conventional Foxp3 - CD4 T cells that migrate into the lung...4 Figure 8. Activated regulatory Foxp3 + CD4 T cells migrate into the lung following RSV infection...42 Figure 9. Increased proliferation of Tregs that migrate into the lung following RSV infection following RSV infection...44 Figure 1. RSV strains induce an equivalent type 1 T cell response...62 Figure 11. Differential Th17 responses following infection with various strains of RSV...64 Figure 12. Increased expression of mucus-associated transcripts does not correlate with the increased RSV line 19-induced Th17 response...66 Figure 13. The increased RSV line 19-induced Th17 response does not correlate with an increase in virus replication...68 Figure 14. Increased levels of pro-inflammatory cytokines in the lung following RSV line 19 infection...7 Figure 15. No difference in DC populations and NK cells between RSV strains...72 Figure 16. Increased innate immune cell populations following RSV line 19 infection...74 Figure 17. Increased caspase 1-dependent IL-1β secretion following RSV line 19 infection...76 Figure 18. Innate-induced IL-1β and IL-6 differentiate the Th17 response following RSV line 19 infection...78 Figure 19. The kinetics of IL-1-producing cells during acute RSV infection...1 ix
12 Figure 2. CD4 T cells represent the majority of IL-1-producing cells at the peak of the RSV-specific adaptive immune response...12 Figure 21. CD4 T cells are the primary in vivo source of IL-1 during acute RSV infection Figure 22. Multiple CD4 T cell subsets produce IL-1 during acute RSV infection...16 Figure 23. IL-1 and IFN-γ co-producing Tregs express T-bet...18 Figure 24. IL-1 influences RSV-induced disease severity Figure 25. IL-1 does not affect RSV replication and clearance kinetics Figure 26. Increase in pro-inflammatory cytokines and chemokines in the absence of IL-1 during RSV infection Figure 27. RSV-induced cytokine and chemokine expression in the absence of IL Figure 28. The magnitude of the CD8 T cell response is increased following IL- 1R blockade. Mice were treated with anti-il-1r mab as described in the materials and methods Figure 29. Increased Th1 response following IL-1R blockade Figure 3. Increased lung pathology following IL-1R blockade Figure 31. Increased Th17 response and decreased T regulatory cell response following IL-1R blockade Figure 32. RSV strains induced differential CD4 T cell responses regulated by IL Figure 33. Pro-inflammatory cytokines and Th17 cells may mediate RSV pathogenesis observed in infants with severe disease x
13 LIST OF ABBREVIATIONS ADP Adenosine diphosphate AEC Airway epithelial cell AIM2 Absent in melanoma 2 AMP Adenosine monophosphate AM Alveolar macrophage APC Antigen presenting cell ASC Apoptosis-associated speck-like protein containing a CARD ATP Adenosine triphosphate BAL Bronchoalveolar lavage BCG Bacillus Calmette-Guérin cdc Conventional dendritic cell CTLA-4 Cytotoxic T lymphocyte-associated protein 4 DC Dendritic cell DNA Deoxyribonucleic acid DT Diphtheria toxin dln Draining lymph node ELISA Enzyme linked immunosorbent assay EMCV Encephalomyocarditis virus ERK Extracellular signal-related kinase F RSV fusion protein FCS Fetal calf serum FDA Food and Drug Administration G RSV attachment glycoprotein gmfi Geometric mean fluorescent intensitiy H&E Hematoxylin and eosin i.n. Intranasal i.t. Intratracheal i.v. Intravenous IAV Influenza A virus ICU Intensive care unit ID Interstitial disease IDO Indoleamine 2,3-dioxygenase IFN Interferon Ig Immunoglobulin IL Interleukin IL-1R IL-1 receptor IRF Interferon regulatory factor ITIM Immunoreceptor tyrosine-based inhibition motif itreg Inducible Treg JNK Jun N-terminal kinase L RSV large polymerase protein LAG-3 Lymphocyte activated gene-3 LCMV Lymphocytic choriomeningitis virus LFA-1 Lymphocyte function-associated antigen-1 xi
14 LRT Lower respiratory tract M RSV matrix protein MAPK p38 mitogen-activated protein kinase MOI Multiplicity of infection MCP-1 Monocyte chemotatic protein-1 medln Mediastinal lymph node MHC Major histocompatibility complex MIP-1α Macrophage inflammatory protein-1 alpha N RSV nucleocapsid protein n.d. None detected NF-κB Nuclear factor-kappa B NK Natural killer NLR Nod-like receptor NLRP1 NOD-like receptor family, pyrin domain containing 1 NLRP3 NOD-ike receptor family, pyrin domain containing 3 NOD nucleotide-binding oligomerization domain NP Nasopharyngeal NS RSV non-structural protein ntreg Natural Treg P RSV phosphoprotein p.i. Post-infection PAS Periodic acid-schiff stain PBS Phosphate buffered saline PCR Polymerase chain reaction pdc Plasmacytoid dendritic cell Penh Enhanced pause PFU Plaque forming unit PMA Phorbol 12-myristate 13-acetate PRDM1 PR domain containing 1 PRR Pattern recognition receptor PVA Perivascular aggregates of leukocytes RA Retinoic acid RANTES Regulated on activation, normal T cell expressed and secreted RAR Retinoic acid receptor ROR RAR-related orphan receptor ROS Reactive oxygen species RSV Respiratory syncytial virus SARS Severe acute respiratory syndrome SEM Standard error of the mean SH RSV small hydrophobic protein SNP Single nucleotide polymorphism SOCS3 Suppressor of cytokine signaling 3 STAT Signal transducer and activator of transcription SV5 Simian virus 5 TCIU Tissue culture infectious unit TCR T cell receptor xii
15 TIGIT TGF-β Th TLR TNF-α Tr1 Treg URT VACV T cell immunoreceptor with Ig and ITIM domains Transforming growth factor-beta T helper Toll-like receptor Tumor necrosis factor-alpha Type I regulatory cell Regulatory T cell Upper respiratory tract Vaccinia virus xiii
16 1 CHAPTER I. GENERAL INTRODUCTION Immune regulation and lung homeostasis The respiratory tract, with a total surface area exceeding 12 m 2, is frequently exposed to potential pathogens due to its direct interface between the host and environment (1). The lung is responsible for gas exchange with an average of 1, liters of air containing innocuous and potentially pathogenic material inhaled daily (2). A complex system of regulation has evolved to prevent aberrant immune activation while simultaneously protecting the host from constant pathogenic insults. This system includes both physical and chemical barriers, such as ciliated airway epithelial cells, mucus, surfactants and opsonins. In addition, a collection of host immune cells collaborate to create an immunological barrier in the lung that is critical in maintaining tissue homeostasis. The host immune cells primarily involved in this process include alveolar macrophages, respiratory dendritic cells (DCs) and regulatory CD4 T cells (Tregs; Figure 1). The lung mucosa utilizes multiple mechanisms to maintain a tolerogenic immune state while still providing protection against a pathogen insult. Alveolar macrophages (AMs) and airway epithelial cells (AECs) help maintain lung tissue homeostasis by establishing and promoting an anti-inflammatory environment (3). AMs comprise over 9 percent of the cells in the lung airways and are crucial in sustaining the suppressive environment (3, 4). AMs induce the expression of the integrin α v β 6 in AECs via a combination of direct cell contact and the production of transforming growth factor-beta (TGF-β) (4). In turn α v β 6 associates with TGF-β, inducing a conformational change to allow ligand interaction with the TGF-β receptor and the initiation of SMAD-dependent and -independent regulatory signaling pathways in AMs (4). AMs also produce interleukin (IL)-1 and express the IL-1 receptor (IL-1R), where binding of its ligand
17 2 IL-1 suppresses the production of pro-inflammatory cytokines in a suppressor of cytokine signaling 3 (SOCS3)-dependent manner (5, 6). In addition, the CD2 receptor expressed on AMs interacts with CD2 expressed on AECs to maintain lung homeostasis. This ligand-receptor interaction inhibits multiple pro-inflammatory signaling cascades, including the extracellular signal-related kinase (ERK), p38 mitogenactivated protein kinase (MAPK) and Jun N-terminal kinase (JNK) signaling pathways (7, 8). These mechanisms highlight the crucial role AMs play in maintaining lung homeostasis (4). However, AMs can overcome these suppressive mechanisms in the presence of sufficiently strong activation signals, such as ligation of pattern recognition receptors (PRRs) (4). In this context, AMs become potent producers of pro-inflammatory cytokines and chemokines, playing an active role in the immune response following infection. DCs in the lung mucosa favor the induction of either immunological tolerance or a low inflammatory Th2 response (3). Pulmonary DCs exhibit high expression of major histocompatibility complex (MHC) class II and CD25 with low expression of CD4, CD8 and CD86, making these cells capable of readily processing antigen, but less able to induce the activation of naïve T cells (3, 9, 1). CD11b + conventional DCs (cdcs) are the main DC subset located in the lamina propria of the lung and play a major role in cytokine and chemokine production (11-14). In contrast, CD13 + DCs are primarily associated with the epithelial cell layer through their expression of CD13, which interacts with E-cadherin expressed on epithelial cells. CD13 + DCs contribute to mucosal tolerance through their production of retinoic acid (RA), indoleamine 2,3- dioxygenase (IDO) and TGF-β (15). Furthermore, the production of these soluble factors promotes the induction and function of Tregs in the mucosa (15, 16). CD13 + DCs continuously sample antigen in the airways by extending dendrites into the airway lumen (11). Following activation via PRRs, CD13 + DCs undergo maturation and migrate into the lung draining lymph node (dln) to present antigen to and activate naïve T cells (11,
18 3 17-2). Pulmonary DCs also require activation signals through PRRs to fully mature and present antigen to activate naïve T cells (3). Tregs are CD4 T cells that play a major role in regulating immune responses dependent on their expression of the transcription factor Foxp3 (21). Tregs can be derived from either the thymus, termed natural Tregs (ntregs) or induced in the periphery, termed inducible Tregs (itregs) (21). Tregs also contribute to maintaining tolerance in the respiratory tract (22). Tregs primarily maintain lung homeostasis through their production of the suppressive cytokines IL-1 and TGF-β. Transgenic mice with Tregs lacking the ability to produce IL-1 demonstrate the crucial importance of this cytokine in maintaining lung homeostasis. In the absence of IL-1-producing Tregs, the lung airways exhibit an increased basal level of inflammation and cellular infiltration (23). In addition, Tregs prevent immune responses against innocuous antigens by inhibiting the sensitization process to allergens through their suppression of DCs and the production of suppressive cytokines (24-26). Maintaining homeostasis in the lung tissue is dependent on each of these cell populations contributing to the complex lung microenvironment, which requires even more elaborate coordination upon pathogenic insult. Respiratory syncytial virus Respiratory syncytial virus (RSV) is a negative sense, single-stranded RNA virus in the Paramyoviridae family. The genome is approximately 15 kilobases and encodes 11 known proteins (27). Similar to other RNA viruses, RSV exhibits extensive strain variation within and between infectious seasons (27). Furthermore, circulating RSV strains exhibit geographical clustering (28, 29). Although RSV initially replicates in the nasopharynx, it can disseminate to infect the lower respiratory tract. The virus has a tropism for both type I and II AECs, which are responsible for mediating gas exchange and producing surfactants, respectively (3). Although RSV primarily targets AECs, the
19 4 virus can replicate in human macrophages as well (31). During AEC infection, RSV enters and is released from the apical surface resulting in a localized infection within the respiratory tract. Virus entry is dependent on the RSV fusion (F) protein mediating fusion between the virus envelope and the plasma membrane of the target host cell (32). During replication, newly produced F protein expressed on the surface of the infected host cell mediates fusion with neighboring cells. The RSV glycoprotein (G) is an attachment protein that enhances virus entry. Recombinant virus lacking the G protein exhibits slight attenuation in vitro due to reduced virus entry and complete attenuation in vivo (27, 33, 34). Additionally, the G protein is able to modulate the host immune response due to its ability to mimic the chemokine fractalkine and suppress nuclear factor-kappa B (NF-κB) activation (35, 36). Moreover, the G protein is expressed as both a transmembrane and secreted protein. The secreted G protein is thought to aid in virus escape from neutralizing antibodies (37). Lastly, the small hydrophobic (SH) protein is expressed on the virus envelope. However the role of SH protein in aiding virus replication is not currently known. Recombinant RSV lacking the SH protein exhibits slight attenuation in murine and non-human primate pathogenesis models (38, 39). RSV replication is dependent on the nucleocapsid (N), phosphoprotein (P) and large (L) viral proteins (4). Although these proteins are sufficient for RSV replication, the matrix (M)2-1 protein increases transcription efficiency (4-42). Furthermore, virus replication is enhanced by the non-structural (NS)1 and NS2 proteins inhibiting the host immune response. NS1 and NS2 inhibit the phosphorylation of interferon regulatory factor (IRF)3, decreasing the transcription of interferon (IFN)-α/β (43, 44). Additionally, type I interferon signaling is suppressed due to decreased expression of signal transducer and activator of transcription (STAT)2 mediated by NS1- and NS2-induced protein degradation (45). RSV replication in AECs induces their loss of cilia and sloughing into the luminal space of the airways (27). This in part contributes to the disease associated
20 5 with RSV infection by occluding the airways and decreasing the efficiency of gas exchange (27). RSV pathogenesis RSV results in upper respiratory tract (URT) infections in all populations and is the leading cause of lower respiratory tract (LRT) disease resulting in bronchiolitis and pneumonia in young children (27). Although primarily a pediatric pathogen, RSV also induces respiratory disease in the elderly and immunocompromised individuals (27). RSV is a ubiquitous pathogen with approximately two million young children receiving medical care each year due to RSV infection in the United States and nearly all children infected by two years of age (46). For children that develop severe RSV-induced disease from a LRT infection, there are well-documented risk factors, including prematurity, age, pre-existing pulmonary diseases, genetic disorders and polymorphisms, socioeconomic status, race and sex (47-52). In addition, RSV strains are divided into two antigenic subgroups designated A and B. This designation is based on the ability of a monoclonal antibody to neutralize the virus by binding to the RSV G protein (53). During an infectious season, viruses from both subgroups can co-circulate as well as one group predominate (53). Infections by RSV group A strains have been reported to induce more severe disease as compared to group B strains (54-56). In contrast, others have reported no difference in disease severity between RSV strain groups as well as increased disease severity following infection with RSV group B strains (57-59). Thus, the role virus group A and B strains play in RSV-induced disease severity remains unclear. There is approximately 5 percent amino acid sequence variability in the G protein between the antigenic subgroups (53). Over time the G protein exhibits anti-genetic shift to escape neutralizing anti-g antibodies generated following natural RSV infection (53). Given the G protein defines the antigenic subgroups and is the most variable RSV protein, it has been the focus of
21 6 most studies (53). However other RSV proteins tolerate variability that does not necessarily associate with the defined antigenic subgroups (53). For example the SH and NS1 proteins exhibit approximately 25 and 15 percent amino acid sequence variability between antigenic subgroups, respectively (53). However the role of virus strains, without the context of antigenic subgroups, in severe disease is largely uninvestigated following RSV infection. The relative contribution of virus-induced cell death versus inflammation in the severity of RSV-induced pulmonary disease is also currently unclear. RSV infection causes increased mortality in severely immunocompromised individuals, such as hematopoietic stem cell transplant patients (6). The increased susceptibility of immunocompromised individuals to RSV-induced disease suggests that uncontrolled virus replication is responsible for disease. In contrast, a study by Wright et al. found no positive correlation between viral titers and disease severity in infants hospitalized due to RSV infection (61). Wright et al. did report a positive correlation between disease severity and host factors, such as age, congenital heart disease and bronchopulmonary dysplasia (61). Furthermore, minimal virus was detected in the lungs by post-mortem analysis of infants who developed fatal RSV-induced bronchiolitis (62, 63). Lastly, a number of studies have reported positive correlations between either host factors or genetic polymorphisms with both a heightened immune response and disease severity following RSV infection [reviewed in reference (64)]. These data would suggest that severe disease in infants following RSV infection might be the result of a complex combination of host factors, such as underlying conditions, age and genetic factors that modulate the host immune response. Protection from RSV-induced disease in humans positively correlates with the presence of either neutralizing maternal or naturally acquired antibodies (27). However, a primary RSV infection fails to elicit stable, long-lasting immunity. An individual can become reinfected with either the same or a genetically similar RSV strain within months
22 7 (65, 66). In addition, there is currently no Food and Drug Administration (FDA)- approved vaccine for RSV. Prophylactic administration of palivizumab is the most effective treatment available in preventing RSV-induced disease (67). Palivizumab is a humanized monoclonal antibody specific for the RSV F protein (67). For infants and young children considered high-risk for severe RSV-induced disease, palivizumab is administered as monthly intramuscular injections throughout the RSV season (67). Given the F protein is relatively conserved between RSV strains, Palivizumab treatments result in significantly decreased hospitalization rates due to RSV infection (53, 67). Although palivizumab is the most successful treatment in preventing RSV-induced disease, it is only administered to high-risk infants and young children due to the high costs associated with the treatment (67). Given that the current treatment for children with severe disease only includes supportive care, it is crucial to develop alternative strategies to prevent RSV infection and ameliorate RSV-induced disease. Over the years intense supportive care, including mechanical removal of secretions, oxygen administration and mechanical ventilation for severe cases, have combined to reduce RSV-associated mortality in infants (68). However, the high cost of supportive care contributes significantly to the high financial burden associated with RSV infections in children in the United States. In 2 it was estimated that 392 million dollars were spent on hospitalizations due to RSV infections in the United States, with a total of 652 million dollars including outpatient medical appointments (69). This high financial burden highlights the need for additional therapies for infants with severe RSV-induced disease. The pro-inflammatory response following RSV infection RSV activates toll-like receptors (TLRs) 2, 3, 4 and 7 (7-74). Following TLR ligation a number of pro-inflammatory cytokines and chemokines are expressed, giving RSV a distinct cytokine profile following infection (Figure 1). RSV-induced disease in
23 8 experimentally infected adults positively correlates with the cytokines and chemokines, IL-6, IL-8 (CXCL8), tumor necrosis factor-alpha (TNF-α), regulated on activation, normal T cell expressed and secreted (RANTES; CCL5), and macrophage inflammatory protein-1 alpha (MIP-1α; CCL3) (75). The chemokines present in the nasopharyngeal (NP) washes of infants with severe RSV-induced disease support the observations in adult volunteers. Increased levels of IL-8, RANTES and MIP-1α as well as monocyte chemotactic protein-1 (MCP-1; CCL2) are observed in infants (55, 76, 77). However, Brand and colleagues recently reported that RANTES is significantly decreased in children with severe disease as compared to children with mild and moderate disease (78). Thus there may be a more complex relationship than previously thought between RANTES and the severity of RSV-induced disease. The levels of both IL-6 and TNF-α are significantly increased in the NP washes of children with severe RSV-induced disease, again confirming observations from experimentally infected adults (55). In addition, there is a positive correlation between disease severity and the level of cytokine in the NP wash. Infants that are hospitalized in the intensive care unit (ICU) exhibit a 2.5-fold increased level of IL-6 in their NP wash as compared to infants hospitalized due to severe RSV infection but not admitted into the ICU (55). Recently, increased IL-1β has also been positively correlated with severe RSV-induced disease in infants (55). Tabarani and colleagues report a significant increase in the total IL-1β protein level in the NP washes of infants who develop severe RSV-induced disease in the ICU as compared to those hospitalized but not in the ICU (55). Following NF-κB activation, transcripts for pro-il-1β, IL-6 and TNF-α are expressed (79). However, once pro-il-1β is translated it requires further processing prior to secretion as mature IL-1β, a process which is mediated by the inflammasome (8).
24 9 Inflammasomes For IL-1β to be secreted from a cell in its mature form two signals are required (Figure 2). The first signal induces the expression of pro-il-1β and inflammasome complex proteins. Most commonly this occurs following the activation of NF-κB downstream of TLR, IL-1 receptor or TNF receptor ligation and is referred to as inflammasome priming (Figure 2) (8). The second step is inflammasome activation, which is triggered by a variety of signals. Inflammasome activation results in the formation of a multiprotein complex responsible for inducing the activation of caspase 1, an enzyme that subsequently mediates the maturation of IL-1β (Figure 2). Four inflammasome complexes have been described to date including the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family, pyrin domain-containing 1 (NLRP1), NLR family, pyrin domain-containing 3 (NLRP3), IPAF and absent in melanoma 2 (AIM2) inflammasomes (8). The NLRP1 inflammasome is activated by both the Bacillus anthracis toxin and muramyl dipeptide found in bacterial cell walls (8). Numerous molecules (i.e. silica, extracellular ATP, etc.) that result in cellular stress inducing Ca 2+ flux, K + efflux and loss of mitochondrial membrane potential activate the NLRP3 inflammasome (8). Recent data suggest the NLRP3 inflammasome is activated by NLRP3 directly binding to cardiolipin following mitochondria destabilization (81). The IPAF inflammasome is activated in the presence of gram-negative bacteria with either type III or IV secretion systems (8). Lastly the only non-nlr inflammasome, AIM2, is activated by directly sensing cytosolic double stranded deoxyribonucleic acid (DNA) (8). Additionally inflammasome activation can result in a specialized, highly inflammatory cell death termed pyroptosis. Pyropotosis is defined by increased plasma membrane permeability allowing water influx and the secretion of highly proinflammatory proteins, leading to osmotic lysis; this most commonly occurs following intracellular infection of myeloid cells (82). Therefore this two-step process allows for
25 1 the tight regulation of the highly pro-inflammatory state following inflammasome priming and activation. Inflammasome activation following RSV infection RSV replication activates the NLRP3 inflammasome (83, 84). The RSV A2 strain activates TLR2 inducing the MyD88/NF-κB-dependent expression of pro-il-1β (83). Subsequently, cellular stress induced by RSV replication leads to NLRP3 inflammasome activation, which is dependent on the efflux of K + ions and the production of reactive oxygen species (ROS) (83). Upon NLRP3 inflammasome activation, NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase 1 oligomerize leading to the cleavage and activation of caspase 1. Active caspase 1 subsequently cleaves pro-il-1β into its mature form for cellular secretion [reviewed in reference (85)]. Triantafilou et al. have recently demonstrated that the RSV SH protein mediates NLRP3 inflammasome activation through its function as a viroporin (84). The SH protein is suggested to be a channel for the monovalent cations Na + and K + (84). This is in agreement with K + efflux being required for NLRP3 inflammasome activation following RSV infection. Furthermore, the viroporins of other viruses have recently been reported to activate the NLRP3 inflammasome as well, including both the M2 ion channel of influenza A virus (IAV) and 2B protein of encephalomyocarditis virus (EMCV) (86, 87). However the role of the SH protein in benefiting virus replication to outweigh the repercussions of inflammasome activation is currently unclear. Taken together, it is clear that RSV induces a robust pro-inflammatory response that is positively correlated with disease severity. However determining the balance of inflammation necessary for RSV clearance without leading to excessive immunopathology still needs to be delineated.
26 11 CD4 T cells and RSV pathogenesis T cells are observed in the lungs of children with untreated fatal RSV-induced disease (3). Histological analysis of the lung tissue indicates that CD3 + T cells are located between the pulmonary arteries and distal airway bronchioles (3). Furthermore, following antibody depletion of either CD4 or CD8 T cells RSV-induced disease is ameliorated, with disease completely abolished if mice are depleted of both CD4 and CD8 T cells (88). These findings indicate both CD4 and CD8 T cells may contribute to RSV pathogenesis in humans. CD4 T cells can differentiate into many phenotypes including, but not limited to, T helper (Th)1, Th2 and Th17 [reviewed in reference (89)]. The relative balance between the Th1 and Th2 response is thought to mediate acute RSV-induced pulmonary pathology in humans (9, 91). Th1 cells are induced by the IL-12-mediated activation of STAT4 leading to the sustained expression of the Th1 master transcription factor T-bet (89). Th1 cells are thus defined by the expression of T-bet and the production of the proinflammatory cytokines IFN-γ and TNF-α. Following a viral infection, a Th1 response is critical for optimal pathogen clearance. Conversely, a Th2 response is important for parasitic immunity and mediates allergic asthma. GATA-3 expression following IL-4 and IL-2 activation of STAT5 is required to induce Th2 cell differentiation (89). The production of IL-4, IL-5 and IL-13 as well as the expression of GATA-3 is used to specifically identify Th2 cells. Given the known role for Th2 cells in some forms of allergic asthma and the similarities between RSV pathogenesis and asthma, these cells have been implicated in contributing to the increased severity of RSV-induced disease. Infants hospitalized due to severe RSV infection have been reported to exhibit a Th2 bias as compared to controls (9, 91). In support of these observations, RSV pathogenesis in the murine model is mediated by the Th2-associated cytokine IL-13 (92, 93). Mucus production and airway hyperreactivity were ameliorated following IL-13 neutralization in
27 12 RSV-infected mice (93). However, neutralization of the Th2-associated cytokine IL-4 had no effect on airway hyperreactivity (93). Other observations do not support a Th2-biased immune response associated with severe RSV-induced disease and instead implicate Th17 cells in RSV pathogenesis. IL- 1β, IL-6, IL-21 and TGF-β induce Th17 cell differentiation (89, 94). Th17 cells express the transcription factor retinoic acid receptor (RAR)-related orphan receptor (ROR)γt following the phosphorylation of STAT3 leading to the phenotype-defining production of IL-17, IL-21 and IL-22 (89). Th17 cells are important in the immune response to extracellular bacterial infections, but are highly pro-inflammatory and mediate a number of autoimmune diseases (89). RSV-induced disease severity positively associates with a number of pro-inflammatory cytokines and chemokines including IL-1β and IL-6, which is supportive of Th17-mediated pathology (55, 76, 77, 95-97). Furthermore, the Th17- associated cytokine IL-17A has been positively associated with severe RSV-induced disease in the NP washes of infants (97). The role of IL-17A in RSV pathogenesis has since been delineated in the murine RSV model. RSV-induced IL-17A production mediates mucus secretion in the lung airways following infection (73, 97, 98). Mucus production is ameliorated following IL-17A neutralization in RSV-infected mice (97). IL-17A-induced mucus production may be indirect through inducing the expression of IL-13 (98). Due to ethical limitations, the role of CD4 T cells in contributing to severe RSV-induced pathology is only definitively demonstrated in the murine model and still requires additional experimental support from human studies. Given severe RSVinduced disease is associated with increased pro-inflammatory cytokines, I hypothesize that different RSV strains induce differential cytokine production influencing the differentiation of CD4 T cells and subsequent disease severity following infection.
28 13 Mechanisms of Treg regulation Tregs modulate immune responses through their expression of cell-surface molecules and the production of soluble mediators. Tregs can express high levels of CD25, a subunit of the high affinity IL-2 receptor (99). CD25 expression has been proposed to allow Tregs to deplete the environment of IL-2 by outcompeting effector T cells for this critical cytokine and therefore inhibiting their proliferation (99). The proliferation of effector T cells is also inhibited by the expression of CD39 and CD73 on Tregs. CD39 and CD73 are ectonucleotidases involved in hydrolyzing and converting adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP) into adenosine, which directly inhibits T cell proliferation (1-13). ATP depletion from the environment also prevents the ATP-driven maturation of DCs (12). In addition, Tregs inhibit DCs through their expression of cytotoxic T lymphocyte-associated protein 4 (CTLA-4), which downregulates the expression of costimulatory molecules CD8 and CD86 on DCs (14, 15). Similarly, lymphocyte activation gene 3 (LAG-3) expression on Tregs negatively regulates the ability of DCs to undergo maturation and express costimulatory molecules (16). Tregs not only inhibit the ability of DCs to mature and activate T cells through direct interaction, but also induce the production of the suppressive cytokines IL-1 and TGF-β by DCs through their expression of T cell immunoreceptor with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibition motif (ITIM) domains (TIGIT) (17). Moreover, Tregs enhance the suppressive environment through their own production of IL-1, IL-35 and TGF-β (18-11). Regulation of T cell responses following RSV infection It is evident that Tregs respond to RSV infection, since they increase in total number, exhibit an increased frequency of proliferation, and upregulate expression of activation and trafficking molecules in the lung, airways and dln following infection
29 14 (111). Furthermore, Tregs play a critical role in limiting immunopathology following RSV infection in the murine model (Figure 1) ( ). Following Treg depletion by administration of either anti-cd25 antibodies or diphtheria toxin (DT) to transgenic mice expressing the DT receptor under the control of the Foxp3 promoter, RSV-infected mice exhibit increased weight loss and airway resistance as compared to control mice (111, 114). Additionally, Treg depletion increases perivascular aggregation and mucus production in the airways, which positively correlates with an increased RSV-specific T cell response in the lungs as compared to non-depleted mice (111). Interestingly, RSVspecific immunodominant M CD8 T cells accumulate in the LN and are initially delayed in trafficking to the lung, correlating with a slight delay in viral clearance following Treg depletion (111, 113). However, the mechanism Tregs utilize to regulate RSV-specific CD8 T cell trafficking remains unknown. Lastly, Treg depletion results in a significant increase in the pulmonary level of the pro-inflammatory cytokines and chemokines MIP-1α/β, MCP-1, TNF-α, IFN-γ and IL-6 (112, 113). These proinflammatory cytokines and chemokines are similar to the cytokine profile observed in RSV-infected individuals with severe disease (55, 75-78). In addition, Treg depletion biases the CD4 T cell response towards a Th2 phenotype in the lung following RSV infection (114). These findings implicate that RSV-induced disease in humans may be the result of excessive immunopathology. However the precise mechanism that Tregs regulate the T cell response following RSV infection remains undetermined. Immune suppression by IL-1 Many immune cells including mast cells, NK cells, neutrophils, eosinophils, macrophages, DCs, CD4 T cells, CD8 T cells and B cells produce the inhibitory cytokine IL-1 (115). With nearly all immune cells expressing both subunits of the IL-1R, they are susceptible to the broad suppressive effects of IL-1 ( ). IL-1 directly inhibits the expression of pro-inflammatory cytokines, chemokines, nitrous oxide and
30 15 prostaglandins produced by macrophages ( ). IL-1 also suppresses the ability of antigen presenting cells (APCs) to present and activate T cells by inhibiting the surface expression of MHC class II and costimulatory molecules ( ). DCs treated with IL-1 are thus able to induce and maintain a state of anergy in activated T cells ( ). Furthermore, T cell activation by IL-1-producing DCs differentiates the naïve T cell into either a Th2 cell or IL-1-producing type I regulatory (Tr1) cell ( ). The suppressive effect of IL-1 on T cells is primarily indirect through its effects on APCs (123, 138). However, IL-1 is also able to directly suppress the capacity of T cells to produce the cytokines IL-2, IL-5 and TNF-α (12-122). Moreover, IL-1 is primarily produced by T cells and is important in regulating the effector T cell response following IAV infection (139, 14). Following a low dose IAV infection, IL-1 is necessary to regulate virus-induced inflammation while still permitting virus clearance (14). In contrast, following a high dose IAV infection, IL-1 inhibits the Th17 response resulting in increased mortality (139). The discrepancy between these data may be explained by the ability of these mice from different animal vendors to mount differential Th17 responses, even though they are the same strain of mice with the same infectious IAV dose. Given the fine balance between lung homeostasis while permitting virus clearance, determining the role of IL-1 in limiting RSV-induced immunopathology would be beneficial in the development of immunotherapies. Thesis objectives RSV is the leading cause of LRT infection in young children. Although RSVinduced mortality in children is low in the United States, virus-induced morbidity is high, resulting in a high financial burden associated with infection. Therefore, immunotherapies to alleviate RSV-induced morbidity and decrease the need for supportive care in hospitalized infants are needed. However, a better understanding of
31 16 the factors that modulate the induction and regulation of the RSV-specific CD4 T cell response is necessary for the development of targeted immunotherapies. Thus, my thesis addresses the following objectives: 1. To determine the localization of RSV-specific CD4 T cells following infection. 2. To determine if RSV strains differentially activate the innate immune response, therefore affecting the differentiation of the subsequent virusspecific CD4 T cell response. 3. To delineate if IL-1 is able to regulate the RSV-specific CD4 T cell response and inhibit viral pathogenesis.
32 17 Figure 1. Key components of the pulmonary immune response following RSV infection. The major immunological components involved during (top) homeostasis, (middle) inflammation following infection and (bottom) the resolution of RSV-induced inflammation are depicted. Lung tissue (top) homeostasis depends on the suppressive effects of AMs and their expression of both CD2R and IL-1R, and as well as the production of the immunosuppressive cytokines IL-1 and TGF-β by both AMs and Tregs. Respiratory DCs also contribute to lung homeostasis through the production of RA, IDO and TGF-β. Following RSV infection (middle), virus-induced inflammation results in a distinct cytokine and chemokine profile in humans. In the murine model, RSV-induced activation of TLRs has been demonstrated to induce the expression of these inflammatory cytokines. Furthermore, T cells are present in the lung following RSV infection in humans and have been demonstrated to produce IL-17A and TNF-α in the murine model of pathogenesis. Following virus clearance (bottom), TLRs cease to be activated reducing the amount of pro-inflammatory cytokines. Tregs are also important in suppressing the functions of effector T cells and virus-induced inflammation in the murine model of RSV pathogenesis.
33 18 HOMEOSTASIS IL-1 TGF-β Treg IL-1R AM Airway lumen CD2R mucus Epithelial cells Lamina propria RA, IDO, TGF-β DC INFLAMMATION IL-1β IL-8 MCP-1 AM T cells IL-6 MIP-1α RANTES IL-17A TNF-α TLR activation mucus Virus replication DC migration to LN RESOLUTION T cells Treg INFLAMMATION TLR activation mucus Virus clearance
34 19 Figure 2. Priming and activation of the inflammasome for IL-1β secretion. Secretion of mature IL-1β requires two signals. The first is referred to as inflammasome priming and occurs following the ligation of TLRs, the IL-1R or TNFR. Downstream signaling activates NF-κB inducing the transcription of IL-1β and inflammasome complex protein transcripts. The second signal required results in inflammasome activation. This occurs following various intracellular or extracellular signals inducing the complex formation of the ligand binding protein (NLRP1, NLRP3, IPAH or AIM2) with ASC and pro-caspase 1. Following protein oligermerization, pro-caspase 1 is cleaved into active caspase 1, which in turn cleaves pro-il-1β into mature IL-1β for cellular secretion.
35 2 Signal 1: Priming TLRs, IL-1R or TNFR Signal 2: Activation IL-1β Activation Signal NLRP1, NLRP3, IPAF, or AIM2 NF-κB activation pro-caspase 1 ASC caspase 1 Il1b pro-il-1β IL-1β IL-18 is similarly processed by the inflammasome
36 21 CHAPTER II. LOCALIZATION OF CD4 T CELLS FOLLOWING RESPIRATORY SYNCYTIAL VIRUS INFECTION Abstract The migration of pathogen-specific T cells into nonlymphoid tissues, such as the lung, is critical to control peripheral infections. The use of in vivo intravascular labeling of leukocytes has allowed for improved discrimination between cells located in the blood from cells present within peripheral tissues such as the lung. This is particularly important in the lung, which is comprised of an intricate network of blood vessels that harbor a large proportion of the total blood volume at any given time. Recent work has demonstrated that >8% of lung antigen-specific effector CD8 T cells remain in the pulmonary vasculature following an intratracheal infection with a systemic viral pathogen. However, it remains unclear what proportion of CD4 T cells are located within the lung tissue following a localized respiratory viral infection. Similar to effector and memory CD8 T cells, I observed that the majority of lung effector CD4 T cells are found in the vasculature after an intranasal infection with the systemic pathogens lymphocytic choriomeningitis virus (LCMV) and vaccinia virus (VACV). In contrast, following pulmonary viral infections with either RSV or IAV, 7-85% of the antigen-specific and the majority of regulatory CD4 T cells were located within the lung tissue. Furthermore, the conventional and regulatory CD4 T cells in the lung were enriched in increased expression of activation-associated proteins and cells that had undergone proliferation following RSV infection. My results indicate that CD4 T cells exhibit significantly altered distribution patterns dependent upon the tissue tropism of the infection and activation status of the cell.
37 22 Introduction An intricate network of blood vessels is associated with the bronchial tree and alveolar sacs of the lung (141). The vascular network is necessary for respiratory function as well as for the trafficking of leukocytes into the lung during infection (142). Leukocytes that remain in the vasculature can be detected by intravenous (i.v.) administration of a specific antibody prior to perfusion and tissue isolation ( ). Recent work has shown that 8-95% of T cell receptor (TCR) transgenic effector and memory CD8 T cells are confined to the pulmonary vasculature following an intratracheal (i.t.) LCMV infection despite extensive lung perfusion (144). Importantly, LCMV disseminates systemically even after an i.t. or intranasal (i.n.) infection. Therefore it remains unclear what proportion of CD4 T cells isolated from a perfused lung are within the lung tissue versus the pulmonary vasculature following an i.n. infection with a localized respiratory infection. The relative balance of Th1 and Th2 cells, as well as the Th17 response has been implicated in RSV pathogenesis (73, 9, 91, 97, 98). In addition, Tregs play a vital role in limiting the immunopathology mediated by T cells following RSV infection ( ). Given the important role CD4 T cells play in RSV pathogenesis, understanding the localization patterns of these cells following RSV infection is critical. The proportion of RSV-specific CD4 T cells that migrate into the lung tissue is likely to impact disease severity. Furthermore, if a specific CD4 T cell subset preferentially migrates into the lung tissue, this would allow for a more directed target for immunotherapy. Using the i.v. antibody labeling technique, I observed that the majority of antigen-specific CD4 T cells following either an i.n. LCMV or VACV infection, both of which disseminate systemically, remain in the perivascular vessels and are not in the lung tissue. Conversely, the majority of regulatory and 7-85% of antigen-specific CD4 T cells have migrated into the lung parenchyma with the localized respiratory virus infections RSV and IAV. Furthermore, antigen-specific and Tregs that have migrated into the lung tissue
38 23 express increased cell surface levels of activation markers as well as undergone proliferation following RSV infection. These data demonstrate the restricted migration of activated conventional and regulatory CD4 T cells following a localized RSV infection. Materials and Methods Mice and infection BALB/cAnNCr mice (6-8 wks old) were obtained from the National Cancer Institute (Frederick, MD) and infected i.n. with 5 x 1 5 plaque forming units (PFUs) of LCMV Armstrong, 5 x 1 3 PFU of VACV strain Western Reserve, tissue culture infectious unit (TCIU) 5 of IAV strain A/PR8/34 or 1.6 x 1 6 of RSV strain A2. All experimental procedures involving mice were approved by the University of Iowa Animal Care and Use Committee. Intravascular antibody labeling and tissue processing 1 µg of CD9.2-PE (clone ) antibody was injected via the tail vein i.v. 3 min prior to sacrifice and cells from various tissues were subsequently harvested within 12 min (144). Bronchoalveolar lavage (BAL) cells were isolated by cannulation and three successive 1 ml washes with RPMI supplemented with 1% fetal calf serum (FCS) (Atlanta Biologicals, Lawrenceville, GA), 5 nm 2-mercaptoethanol (Sigma), 2 mm L- glutamine, 1 U/ml penicillin, 1 µg/ml streptomycin sulfate, 1 mm HEPES, 1mM Sodium Pyruvate, and.1 mm MEM non-essential amino acids (all obtained from Gibco) (146). Lungs were subsequently perfused with 1 ml of phosphate buffered saline (PBS) and then digested in 4 ml of HBSS with CaCl 2 and MgCL 2 (Gibco, Grand Island, NY) supplemented with 6 U/ml DNase I (Sigma, St. Louis, MO) and 125 U/ml collagenase (Invitrogen, Carlsbad, CA) for 3 minutes at 37 C. After digestion, lungs were pressed through a wire mesh screen (Cellector; Bellco Glass, Inc., Vineland, NJ) to create a single-cell suspension.
39 24 Tetramer and antibody staining Cells were incubated with tetramer for 3 min at 4 C LCMV NP , VACV F , IAV HA and RSV M (obtained from the NIH Tetramer Facility) and subsequently stained for extracellular expression of CD4 (clone RM4-5), CD11a (clone M17/4), CD25 (clone PC61), CD44 (clone IM7), CD49d (clone R1-2), CD9.2 (clone ), CD13 (clone 2E7) and ICOS (clone 7E.17G9) for 3 min at 4 C. Cells were subsequently washed three times with FACS buffer at 4 C, fixed with FACS lysing solution (BD Biosciences, San Diego, CA) for 15 mins at room temperature, washed three times with FACS buffer at 4 C and resuspended in FACS buffer for analysis. Samples were collected on a BD FACSCanto flow cytometer and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Intracellular expression of CTLA- 4 (clone UC1-4B9), Ki-67 (clone B56) and Foxp3 (clone FJK-16s; all antibodies obtained from BioLegend, San Diego, CA) was performed for 3 min at 4 C using the Mouse Regulatory T cell Staining Buffer Kit according to the manufacturer s instructions (ebioscience) (147). Cells were resuspended in FACS buffer, collected on a BD LSRFortessa (BD Biosciences) and analyzed using FlowJo software (Treestar, Inc., Ashland, OR). Statistical analysis Data were compiled and statistical analysis was calculated by performing a oneway or two-way ANOVA with a Tukey s post test in Prism software (Graphpad Software, San Diego, CA). *, p<.5; **, p<.1; ***, p<.1.
40 25 Results Intravascular antibody labeling can distinguish the location of CD4 T cells following RSV infection Intravascular staining with antibody has been utilized in several recent studies to discriminate between leukocytes in the lung tissue versus the pulmonary vasculature and has been previously shown not to stain perivascular cells ( ). To determine the proportion of endogenous T cells that have entered the lung tissue following a localized pulmonary viral infection, RSV-infected mice were injected intravenously with anti- CD9.2 antibody. The use of anti-cd9.2 antibody allowed us to specifically label all T cells in the peripheral blood. Following i.v. antibody labeling, I determined the frequency of CD4 T cells in the lung tissue on day 8 following RSV infection (Figure 3). In addition, CD4 T cells in the peripheral blood were nearly all labeled with i.v. antibody, whereas cells localized within the BAL were protected from i.v. labeling with antibody (Figure 3). Majority of CD4 T cells are protected from intravascular antibody labeling following localized respiratory infections Given the recent observation that the majority of LCMV-specific CD8 T cells remain in the perivascular vessels following an i.t. infection (144), I wanted to determine the distribution of CD4 T cells following systemic and localized pulmonary infections in mice utilizing i.v. labeling. I found that conventional Foxp3 -, regulatory Foxp3 + and antigen-specific CD11a hi CD49d + CD4 T cells were preferentially localized within the lung tissue following either IAV or RSV infection as compared to either i.n. LCMV or VACV infection at day 8 post-infection (p.i.) (Figure 4A-C). However, there was no difference in the frequency of unlabeled CD11a lo CD49d - CD4 T cells between infections (Figure 4D), indicating that naïve CD4 T cells were not preferentially recruited to the site of infection. These data suggest that antigen-specific CD4 T cells preferentially migrate
41 26 into the lung following a localized pulmonary infection as compared to a respiratory infection that disseminates systemically. The proportion of antigen-specific CD4 T cells in the lung remains constant into the memory phase Recent work by Turner et al. indicates that tissue-resident memory CD4 T cells primarily reside at locations determined by the initial site of infection (148), with the majority of memory CD4 T cells localized within the lung tissue following IAV infection. Given the majority of RSV-specific CD4 T cells are in the lung tissue at the peak of the T cell response, I wanted to determine the localization kinetics following RSV infection. The frequency of total CD4 T cells that were unlabeled within the lung tissue increased following infection and peaked at day 8 p.i, but decreased over time in correlation with contraction of the T cell response (Figure 5A). However, approximately 7% of antigen-specific CD11a hi CD49d + CD4 T cells (149) remained localized to the lung tissue up to 3 days following RSV infection. Foxp3 + Tregs shared the same localization pattern within the lung parenchyma as antigen-specific CD4 T cells following RSV infection (Figure 5A). However, the frequency of unlabeled naïve CD11a lo CD49d - CD4 T cells was unchanged following RSV infection. These data suggest that Tregs localize within the lung tissue coincident with the effector T cell response to suppress the adaptive immune response. To assess the total composition of the cellularity in the lung, I determined the total number of CD4 T cells in the lung tissue protected from i.v. labeling. The total number of antigen-specific CD11a hi CD49d + CD4 T cells protected from i.v. labeling peaked at days 6 and 8 p.i., similar to the frequency of unlabeled cells (Figure 5B). In addition, antigen-specific cells accounted for the majority of CD4 T cells in the lung during the acute phase of RSV infection and maintained a stable population up to day 3 p.i. (Figure 5B). The kinetics of the total number of Foxp3 + Tregs protected from i.v. labeling was
42 27 similar to the frequency of Tregs, however this population was decreased relative to the antigen-specific CD4 T cells (Figure 5B). Lastly, there were a constant total number of naïve CD4 T cells in the lung protected from i.v. labeling, indicating there is a basal level of naïve CD4 T cells either localized within or trafficking through the lung tissue. Together these data demonstrate that antigen-specific CD4 T cells account for the majority of CD4 T cells migrating into the lung tissue following RSV infection. Conventional CD4 T cells protected from intravascular labeling exhibit an increased effector phenotype Effector CD4 T cells can be identified based on the expression of several cell surface molecules that are upregulated following activation (15, 151). I next wanted to determine if there was differential expression of these activation markers between cells in the perivascular vessels and the lung tissue. CD11a, CD44 and CD49d are proteins involved in the trafficking of activated T cells to sites of inflammation. Each of these markers exhibited increased cell surface expression on unlabeled conventional CD4 T cells in the lung tissue and lung airways (BAL), as compared to cells labeled with the i.v. antibody harvested from the lung and peripheral blood on day 8 following RSV infection (Figure 6A). The costimulatory molecule ICOS exhibited increased expression on conventional CD4 T cells in the lung and lung airways as compared to the cells in the perivascular vessels on day 8 p.i. (Figure 6A). However, there was no difference in the expression of CD25 and CD13 between cells in the lung and perivascular vessels (Figure 6). There was a small population of CD25 high CD4 T cells protected from labeling resulting in a significant (p<.1) increase in the geometric mean fluorescent intensity (gmfi) as compared to the i.v. labeled cell population (Figure 6B). Furthermore, cells undergoing proliferation, as indicated by Ki-67 expression, were enriched in the lung tissue (Figure 7A). There was an approximate three-fold increase (p<.1) in proliferating conventional CD4 T cells protected from i.v. labeling in the
43 28 lung as compared to i.v. labeled cells on day 8 following RSV infection (Figure 7B). However, the frequency of proliferating conventional CD4 T cells was similar between cells in the perivascular vessels and lung tissue in naïve mice (Figure 7B). Together these data indicate that activated conventional CD4 T cells are enriched in the lung tissue based on expression of surface markers and a protein associated with proliferation. Regulatory CD4 T cells in the lung have increased expression of activation-associated proteins Tregs exhibit heterogeneous expression of activation markers following RSV infection (111). To determine if Tregs in the lung tissue exhibit a specific expression profile as compared to the Tregs remaining in the vasculature, I assessed the expression of various activation-associated proteins following RSV infection (111). Tregs in the perivascular vessels and the lung exhibited similar expression of CD44 (Figure 8). However unlabeled Tregs in the lung parenchyma exhibited an increased expression, as determined by gmfi, of CD11a, CD25, CD49d, CD13, ICOS and CTLA-4 (Figure 8). Like conventional CD4 T cells, there was also a significantly (p<.1) increased frequency of Tregs undergoing proliferation in the lung as compared to cells in the perivascular vessels on day 8 following RSV infection (Figure 9). Conversely, there was a significantly (p<.1) increased frequency of proliferating Tregs in the perivascular vessels as compared to cells in the lungs of naïve mice (Figure 9B). These data indicate that Tregs expressing increased levels of activation-associated proteins and undergoing proliferation are enriched in the lung tissue similar to conventional CD4 T cells following RSV infection. Discussion Previous work had demonstrated that 8-95% of effector and memory TCR transgenic CD8 T cells are confined to the pulmonary vasculature following an i.t. challenge with the systemic pathogen LCMV (144). These findings call into question the
44 29 actual location of T cells following a respiratory virus infection. However, the majority of antigen-specific CD4 T cells were located within the lung tissue following a localized respiratory infection with either RSV or IAV as compared to a significantly reduced frequency following infection with systemic pathogens LCMV and VACV (Figure 4). These results are in agreement with recent work by Turner et. al which shows that most of the effector CD4 T cells are located within the lung tissue following IAV infection (148). In addition, lung niches permit the formation and maintenance of IAV-specific, tissue-resident memory CD4 T cells. I also found that following RSV infection, the majority of antigen-specific CD4 T cells remained in the lung tissue for up to 3 days p.i. (Figure 5). Furthermore, lung resident IAV-specific memory CD4 T cells provide superior protection by decreasing the mortality, morbidity and virus load following a subsequent IAV infection as compared to splenic IAV-specific memory CD4 T cells (143). Similar to IAV-specific CD4 T cells, the RSV-specific memory CD4 T cells that are protected from i.v. antibody labeling may be important for immune protection following a subsequent RSV infection. CD4 T cells responding to RSV infection that migrate into the lung exhibit increased expression of CD11a, a subunit of lymphocyte function-associated antigen-1 (LFA-1) (Figure 6). I observe high CD11a expression on conventional CD4 T cells that have migrated into the lung, similar to the CD11a expression of cells in the lung airways on day 8 p.i. (Figure 6). In contrast, downregulated CD11a expression on CD8 T cells in the lung airways has been previously described on day 11 following Sendai virus infection (152). However, it remains to be determined if CD4 T cells downregulate CD11a expression similar to CD8 T cells following the resolution of inflammation at a later time point. Furthermore, LFA-1 has been demonstrated to mediate effector IAVspecific CD8 T cell migration into the lung tissue (145). Using i.v. antibody labeling, Galkina and colleagues demonstrate that approximately half of IAV-specific effector CD8 T cells have migrated into the lung tissue 6 hours post-adoptive transfer into naïve
45 3 recipient mice (145). I observed that a higher frequency of endogenous virus-specific CD4 T cells migrated into the lung tissue on day 8 following either RSV or IAV infection as compared to the systemic infections LCMV and VACV (Figure 4). I would anticipate that >5 percent of either transgenic or endogenous IAV-specific CD8 T cells would migrate into the lung tissue on day 8 following IAV infection. Furthermore, both conventional CD4 T cells and Tregs in the lung exhibit increased expression of effector molecules and the proliferation marker Ki-67 as compared to cells in the vasculature following RSV infection (Figures 6-9). These data demonstrate that highly activated RSV-specific CD4 T cells are localized within the lung tissue. Following RSV infection approximately 6 percent of Tregs express high levels of CD25 by conventional harvesting and profusion methods of the lung tissue (111). Therefore I hypothesisized the CD25 high Tregs would be located exclusively in the lung tissue and be protected from i.v. labeling. I did observe that CD25 high Tregs were enriched within the lung tissue. However this increased expression was also observed on a fraction of Tregs located in the vasculature, which did not support my initial hypothesis. CD13 expression had the most distinct differential expression between Tregs in the lung versus the vasculature. I observed a subset of CD13 + Tregs protected from i.v. labeling in the lung that was not present in the vasculature. Given CD13 interacts with E- cadherin expressed on epithelial cells, it would be interesting to determine if this is marking either a tissue resident memory Treg population required for maintaining lung homeostasis or signifies a mechanism for transiently retaining Tregs at the infection site as previously described (153, 154). Overall this study indicates that i.v. labeling of T cells using a CD9-specific antibody is a straightforward method to distinguish CD4 T cells in the peripheral blood from cells in the lung. My findings also indicate there are distinct localization patterns of effector CD4 T cells within the lung tissue following systemic versus localized respiratory viral infections. My results highlight the need to accurately assess the total
46 31 number of antigen-specific T cells within the lung tissue following a pulmonary infection with a pathogen capable of replicating systemically. In contrast, the majority of antigenspecific T cells are found within the lung tissue following infection with a pathogen that replicates primarily in the respiratory tract. This lessens the need to take specific measures in discriminating the location of antigen-specific T cells following a localized respiratory viral infection.
47 32 Figure 3. i.v. antibody labeling distinguishes CD4 T cell populations harvested from the lung. On day 8 post-rsv infection, mice were administered anti-cd9.2 antibody i.v. and harvested as described in the material and methods. The percentage of CD4 T cells either labeled or unlabeled with i.v. antibodies in the (left) peripheral blood (PBL), (center) lung and (right) BAL is displayed. The concatenated plots depict equal representation of four mice from one of two independent experiments (n=8).
48 33 PBL Lung BAL CD9.2 (i.v. label) CD4
49 34 Figure 4. CD4 T cells preferentially localize to the lung following a local pulmonary infection. Lymphocytes were isolated from the lung on day 8 p.i. following i.v. labeling in mice. The frequency of unlabeled (A) conventional Foxp3 -, (B) regulatory Foxp3 +, (C) CD11a hi CD49d + and (D) CD11a lo CD49d - CD4 T cells following LCMV, VACV, IAV and RSV infection. A one-way ANOVA with a Tukey s post test was performed. *p<.5, **p<.1, ***p<.1. Combined results from two independent experiments are shown (n=8).
50 35 A % unlabeled of Foxp3 - CD4 + T cells * ** B % unlabeled of Foxp3 + CD4 + T cells *** ** C D % unlabeled of CD11a hi CD49d + Foxp3 - CD4 + T cells ** *** % unlabeled of CD11a lo CD49d - Foxp3 - CD4 + T cells LCMV VACV IAV RSV
51 36 Figure 5. RSV-specific CD4 T cells remain in the lung tissue into memory. On days 4, 6, 8, 15 and 3 p.i., mice were i.v. labeled and the (A) frequency and (B) total number of antigen-specific CD11a hi CD49d + Foxp3 -, naïve CD11a lo CD49d - Foxp3 - and Foxp3 + CD4 T cells was determined. Combined results from two independent experiments are shown (n=8).
52 37 A % of unlabeled CD4 T cells Days post-infection B # of unlabeled CD4 T cells (1 5 ) CD11a hi CD49d + Foxp3 - CD11a lo CD49d - Foxp3 - Foxp Days post-infection
53 38 Figure 6. Activated conventional Foxp3 - CD4 T cells migrate into the lung following RSV infection. On day 8 p.i., the (A) expression and (B) gmfi of the indicated activation marker between the labeled (Label + ) and unlabeled (Label - ) conventional Foxp3 - CD4 T cells isolated from the lung as compared to the conventional Foxp3 - CD4 T cells harvested from the peripheral blood (PBL) and BAL are displayed. The gmfi graphs and concatenated histograms depict equal representation of three mice from one of two independent experiments (n=6). A two-tailed student s t test was calculated to determine statistical significance. **p<.1
54 39 A Label+ PBL Label- BAL CD44 CD13 CD25 Label+ PBL Label- BAL B 4 ICOS CD11a CD49d CD44 CD13 CD25 ICOS CD11a CD49d ** ** 4 gmfi Label + Label -
55 4 Figure 7. Increased proliferation of conventional Foxp3 - CD4 T cells that migrate into the lung following RSV infection. On day 8 p.i., (A) the expression of proliferationassociated protein Ki-67 between the labeled (Label + ) and unlabeled (Label - ) conventional Foxp3 - CD4 T cells isolated from the lung as compared to the conventional Foxp3 - CD4 T cells harvested from the peripheral blood (PBL) and BAL are displayed. The concatenated histograms depict equal representation of three mice from one of two independent experiments. (B) The frequency of conventional Foxp3 - CD4 T cells expressing Ki-67 from naïve and day 8 post-rsv infected lungs. Data are cumulative from two independent experiments (n=6). A two-way ANOVA with a Tukey s post test was calculated to determine statistical significance. ***p<.1
56 41 A B Label + PBL % of Ki-67 + Foxp3 - CD4 + T cells Naive *** Day 8 p.i. Label + Label - Label- BAL Ki-67
57 42 Figure 8. Activated regulatory Foxp3 + CD4 T cells migrate into the lung following RSV infection. On day 8 p.i., the (A) expression and (B) gmfi of activation-associated proteins between the labeled (Label + ) and unlabeled (Label - ) regulatory Foxp3 + CD4 T cells isolated from the lung as compared to the regulatory CD4 T cells harvested from the peripheral blood (PBL) and BAL are displayed. The gmfi graphs and concatenated histograms depict equal representation of three mice from one of two independent experiments (n=6). A two-tailed student s t test was calculated to determine statistical significance. *p<.5, **p<.1, ***p<.1
58 43 A Label + PBL Label- BAL CD44 CD13 CD25 Label + PBL Label- BAL B gmfi CTLA-4 ICOS CD11a CD49d CD44 CD13 CD25 CTLA-4 ICOS CD11a CD49d * 25 *** * ** * Label + Label -
59 44 Figure 9. Increased proliferation of Tregs that migrate into the lung following RSV infection. On day 8 p.i., (A) the expression of proliferation-associated protein Ki-67 between the labeled (Label + ) and unlabeled (Label - ) Tregs isolated from the lung as compared to the Tregs harvested from the peripheral blood (PBL) and BAL are displayed. The concatenated histograms depict equal representation of three mice from one of two independent experiments. (B) The frequency of Tregs expressing Ki-67 from naïve and day 8 post-rsv infected lungs. Data are cumulative from two independent experiments (n=6). A two-way ANOVA with a Tukey s post test was calculated to determine statistical significance. ***p<.1
60 45 A Label + PBL B % of Ki-67 + Foxp3 + CD4 + T cells *** Naive *** Day 8 p.i. Label + Label - Label- BAL Ki-67
61 46 CHAPTER III. RESPIRATORY SYNCYTIAL VIRUS STRAINS INDUCE DIFFERENTIAL INNATE IMMUNE AND CD4 T CELL RESPONSES Abstract RSV is the leading cause of lower respiratory tract infections in young children. RSV induces variable disease severities in young children, with the development of longterm airway morbidities associated with bronchiolitis. Virus strains inducing differential disease severities have been demonstrated in the murine model of RSV pathogenesis. Furthermore, both the relative balance of the Th1 and Th2 response, and a Th17 response has been associated with human RSV pathogenesis. Thus I sought to determine if different RSV strains could induce differential CD4 T cell responses. I found that RSV strains induced differential innate immune responses. In addition, the differential activation of the innate immune response was associated with altered induction of T helper subsets. The RSV line 19 strain induced the innate-associated production of the pro-inflammatory cytokines IL-1β and IL-6, which correlated with an increased subsequent Th17 response as compared to RSV strains A2 and 2-2. The blockade and/or neutralization of these cytokines ameliorated the ability of RSV line 19 to induce an increased Th17 response. These results demonstrate that RSV strains can differentially activate and polarize innate and adaptive immune responses, which could in part contribute to the differential RSV pathogenesis and development of long-term airway morbidities observed in humans. Introduction RSV is the leading cause of LRT infections in young children (27). However, still only a small proportion of children are either hospitalized due to severe RSV infection or develop long-term airway morbidities, such as asthma and chronic wheezing ( ). Although this is in part due to the genetic and environmental differences of
62 47 these individuals, the contribution of virus strain variability remains largely undetermined. Recently Stokes et al. compared the disease severity of RSV clinical isolates as well as laboratory strains in the murine RSV model (92). Differential disease severities, based on weight loss and mucus production, were observed following infection with the RSV clinical isolates and laboratory strains. Furthermore, they determined that increased mucus production was IL-13-dependent. IL-4, IL-5 and IL-13 are all Th2-associated cytokines that are produced in the mucosal lung environment. The relative balance between the Th1 and Th2 response is thought to mediate RSV-induced pathology in humans. Infants hospitalized due to severe RSV infection have been reported to exhibit a Th2-biased immune response as compared to uninfected controls (9, 91). However, others have reported results more supportive of a Th17 response being positively associated with severe RSV-induced disease. Recently Tabarani et al. reported RSVinduced disease severity to be positively correlated with a number of previously described as well as novel pro-inflammatory cytokines and chemokines including IL-1β and IL-6, which both promote Th17 differentiation (55, 76, 77, 94-97). In addition, the level of the Th17-associated cytokine IL-17A in the NP washes of infants has been positively associated with severe RSV-induced disease (97). The role of IL-17A has since been further investigated in the murine model of RSV pathogenesis. Mucus production and airway hyperreactivity have been reported to be IL-17A-dependent following RSV infection (73, 97, 98). Genetic variation resulting in different RSV strains occurs within and between infectious seasons (27). In addition, RSV proteins and virus replication can activate a number of PRRs, and thus RSV strains may differentially activate the immune system. Given these findings and that RSV-induced disease severity has been associated with Th2 and Th17 cytokine responses in humans, I hypothesized that RSV strains induce differential CD4 T cell responses. To test this hypothesis, I infected BALB/c mice with
63 48 various strains of RSV and quantified parameters associated with the innate immune response and CD4 T cell differentiation. RSV A2 represents the most common laboratory strain in the murine model of RSV pathogenesis. RSV line 19 is also a laboratory strain utilized, although it is not as extensively utilized as RSV A2. Compared to RSV A2, RSV line 19 has been reported to induce more mucus production correlating with decreased IL-1 in infected lungs (167). The RSV line 19 strain is genetically more similar to the RSV Long strain with several amino acid differences in the F protein (168). The RSV line 19 F protein correlates with increased IL-13 and mucus production, and airway hyperreactivity as compared to the RSV A2 and Long F proteins following infection with recombinant RSV A2 viruses expressing the various F proteins (168). Furthermore, the RSV 2-2 strain was isolated from a patient during the 21 RSV season and has been reported to induce more IL-13 and mucus production than RSV line 19 following infection (92). Using these strains of RSV, I determined that differential CD4 T cell responses are induced following infection of BALB/c mice. An increased Th17 response was observed following RSV line 19 infection as compared to RSV strains A2 and 2-2, which coincided with an increased early pro-inflammatory response. Following blocking and/or neutralization of proinflammatory cytokines generated by the innate immune response, the RSV line 19- induced Th17 response was ameliorated following infection. My results demonstrate that RSV strains differentially activate the innate immune response and subsequently induce a strain-specific CD4 T helper response. Materials and Methods Mice, infection and treatments Six to eight week-old female BALB/cAnNCr mice were purchased from the National Cancer Institute (Frederick, MD). The A2 strain of RSV was a gift from Dr. Barney Graham (National Institues of Health, Bethesda, MD) and was propagated on
64 49 HEp-2 cells (American Type Culture Collections; ATCC, Manassas, VA). The line 19 strain of RSV was a gift from Dr. Nicholas Lukacs (University of Michigan, Ann Arbor, MI). The 2-2 strain of RSV was a gift from Dr. Martin Moore (Emory University, Atlanta, GA). For all infections, mice were anesthetized with isoflurane and infected i.n. with 1 x 1 6 PFU of RSV. Mice were treated with.1 mg of Anakinra (University of Iowa Pharmacy, Iowa City, IA),.2 mg of anti-il-6 (clone MP5-2F3; Bio X Cell, West Lebanon, NH) and/or.2 mg rigg (MP Biomedicals, Santa Ana, CA) i.n. on days -1, 1 and 3 p.i. All experimental procedures involving mice were approved by the University of Iowa Animal Care and Use Committee. Intravascular antibody labeling and tissue processing 3 µg of CD45-PE (clone 3-F11) antibody was injected via the tail vein i.v. 3 min prior to sacrifice and cells from various tissues were subsequently harvested within 12 min (144). BAL cells were isolated by cannulation and three successive 1 ml washes with RPMI supplemented with 1% FCS (Atlanta Biologicals, Lawrenceville, GA), 5 nm 2-mercaptoethanol (Sigma), 2 mm L- glutamine, 1 U/ml penicillin, 1 µg/ml streptomycin sulfate, 1 mm HEPES, 1mM Sodium Pyruvate, and.1 mm MEM nonessential amino acids (all obtained from Gibco) (146). Lungs were perfused with 1 ml of PBS and subsequently digested in 4 ml of HBSS with CaCl 2 and MgCL 2 (Gibco, Grand Island, NY) supplemented with 6 U/ml DNase I (Sigma, St. Louis, MO) and 125 U/ml collagenase (Invitrogen, Carlsbad, CA) for 3 minutes at 37 C. After digestion, lungs where pressed through a wire mesh screen (Cellector; Bellco Glass, Inc., Vineland, NJ) to create a single-cell suspension. Extracellular staining 1-2 x 1 6 cells were placed in a 96-well round-bottom plate (Corning Inc., Corning, NY). Cells were incubated with mabs for extracellular proteins CD3 (clone 145-2C11; ebioscience, San Diego, CA), CD4 (clone RM4-5), CD8 (clone ),
65 5 CD11a (clone M17/4), CD11b (clone M1/7), CD11c (clone N418), CD19 (clone 6D5), CD44 (clone IM7; ebioscience), CD45R (B22; clone RA3-6B2), CD49b (clone DX5; ebioscience), CD49d (clone R1-2), CD9.2 (Thy1.2; clone ), CD13 (clone 2E7), F4/8 (clone BM8), I-A/I-E (clone M5/ ), Ly-6C (clone HK1.4), Ly-6G (clone 1A8), Siglec-F (clone E5-244; BD Biosciences, San Diego, CA) (unless otherwise noted all mabs were obtained from BioLegend, San Diego, CA) and simultaneously blocked with anti-fcγrii/iii mab (clone 93; isolated from 24G.2 cells in house) in FACS buffer (PBS, 2% FCS,.2% sodium azide) for 3 mins at 4 C. Cells were subsequently washed three times with FACS buffer at 4 C, fixed with FACS lysing solution (BD Biosciences) for 15 mins at room temperature, washed three times with FACS buffer at 4 C and resuspended in FACS buffer for analysis. Samples were collected on a BD LSRFortessa flow cytometer and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Ex vivo cell stimulation Cells were incubated at 1-2 x 1 6 cells/well in a 96-well round-bottom plate (Corning Inc.). For ex vivo stimulations, cells were incubated either with or without 5 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma) and 5 ng/ml ionomycin (Sigma) in the presence of 1 µg/ml brefeldin A (Biosynthesis, Inc., Lewisville, TX) in RPMI 164 supplemented with 1% FCS (Atlanta Biologicals, Lawrenceville, GA), 5 nm 2- mercaptoethanol (Sigma), 2 mm L- glutamine, 1 U/ml penicillin, 1 µg/ml streptomycin sulfate, 1 mm HEPES, 1mM Sodium Pyruvate, and.1 mm MEM non-essential amino acids (all obtained from Gibco) for 4 h at 37 o C (146). Intracellular cytokine and Foxp3 staining After ex vivo stimulation, cells were stained for extracellular proteins and fixed in FACS lysing solution as described above. Cells were subsequently incubated in FACS buffer containing.5% saponin (Sigma) and intracellular cytokine antibodies IFN-γ
66 51 (clone XMG1.2), pro-il-1β (clone NJTEN3 ; ebioscience), IL-6 (clone MP5-2F3; ebioscience), IL-17A (clone TC11-18H1.1), IL-17F (clone 9D3.1C8; unless otherwise noted all mabs were obtained from BioLegend) for 3 min at 4 C. Cells were washed twice with FACS buffer containing saponin, once with FACS buffer, and resuspended in FACS buffer. Alternatively for transcription factor analysis, cells were stained for Foxp3 (clone FJK-16s; ebioscience) and RORγt (clone B2D; ebioscience) using the mouse regulatory T cell staining buffer kit (ebioscience) according to the manufacturer s instructions. After staining, cells were resuspended in FACS buffer prior to analysis. Real-time PCR Lungs were harvested on days, 2, 4, 6, 8, 1 and 12 p.i. and homogenized in 1 ml of TRIzol (Invitrogen Life Technologies). RNA was purified by chloroform (2 µl/lung; Fisher Scientific) and isopropyl alcohol (5 µl/lung; Fisher Scientific) extraction. RNA pellets were subsequently washed in 7% ethanol, air dried and resuspended in distilled water. cdna for real-time polymerase chain reaction (PCR) was generated using the SuperScript First-Strand Synthesis kit with random hexamers following manufacturer s instructions (Applied Biosystems). Real-time PCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems) using an ABI 73 instrument to determine the total copies of the RSV N gene (169). For N gene primers 5 GCTCTTAGCAAAGTCAAGTTGAATGA and 5 TGCTCCGTTGGATGGTGTATT and probe 5 FAM-ACACTCAACAAAGATC AACTTCTGTCATCCAGC-3 TAMARA, for Gob-5 primers 5 GAGTGGGCTCA CTTCCGATG and 5 GCTGAACACCTCACTG CTTGG and probe 5 FAM-CAACAA CGACGAGAAGTTCTACTTATCAAAGGAAA-3 TAMARA, and for Muc-5ac primers 5 CCAGCACCATCTCTACAACCC and 5 GCAAAGCTCCTGTTTGCACTC and probe 5 FAM-CCCAAACTATCTCAACCTCA GGGTCCACC-3 TAMARA sequences were used for real-time PCR (17, 171).
67 52 In vitro RSV infection THP-1 cells obtained from Dr. Fayaz Sutterwala s laboratory (University of Iowa, IA) or primary human alveolar macrophages obtained from Dr. Martha Monic s laboratory (University of Iowa) were infected with RSV strains at a multiplicity of infection (MOI) of 5. Virus was UV-inactivated by exposing it to 312 nm UV light for 2 min on ice at 4 C. Alternatively cells were treated with 1 µm Z-YVAD-FMK (Millapore, Billerica, MA), the caspase 1 inhibitor, or dimethyl sulfide (DMSO) control throughout the course of infection. Supernatants were subsequently harvested at 24 or 36 hours p.i. and the levels of IL-1β present in the cell-free supernatent was quantified by ELISA. ELISA RSV-infected lungs from mice were processed using a tissue homogenizer (Ultra- Turrax T25; IKA Works, Inc., Wilmington, NC) in RPMI 164 containing 1% FCS and a 1/2 dilution of a protease inhibitor cocktail (Sigma). Lung homogenates were centrifuged at 2 rpm for 1 mins. Levels of murine IL-1β (R & D Systems, Minneapolis, MN) and IL-6 (ebioscience) protein in the lung supernatants were determined by enzyme linked immunosorbent assay (ELISA) (169). For supernatants from human cell RSV infections, human IL-1β and IL-6 (ebioscience) protein levels were also quantified by ELISA. Briefly, samples were plated in wells previously coated with 2 µg/ml of capture antibody and incubated overnight at 4 C. Biotinylated secondary antibody was subsequently added and incubated at room temperature for 2 hrs followed by avidin-peroxidase incubation for 3 mins at room temperature. Plates were developed with 3,3,5,5 -tetramethylbenzidine dihydrocholride (Sigma-Aldrich) and the reaction was stopped with 2 M H 2 SO 4 (Fisher Scientific). Plates were read using an ELx8 absorbance reader (Bio-tek, Winooski, VT) at 45 nm and analyzed using Gen5. software (Bio-tek).
68 53 Statistical analysis Data were graphically compiled using Prism software (Graphpad Software Inc., San Diego, CA) with bars representing the standard error of the mean (SEM). Statistical analysis was completed using Prism software (Graphpad Software Inc.); one-way and two-way ANOVAs with a Tukey s post test were calculated to determine statistical significance at a level of α=.5. Results No difference in total numbers of activated CD8 and CD4 T cells following infection with different strains of RSV Given the role of T cells in RSV pathogenesis (88), I determined the overall CD4 and CD8 T cell response following infection with different RSV strains. Between the three strains of RSV analyzed, I observed no difference in the total number of CD4 and CD8 T cells in the lung on day 8 p.i. (Figure 1A-B). In addition, there was no significant difference in the total number of activated CD11a hi CD49d hi CD4 T cells in the lung on day 8 p.i. between the RSV strains (Figure 1C) (149). Analysis of activated CD11a hi CD8 T cells yielded no significant difference in the total number of activated T cells in the lung on day 8 p.i. between the RSV strains (Figure 1D) (172). Furthermore, assessment of effector cytokine-producing T cells following PMA and ionomycin stimulation yielded no difference in the total number of IFN-γ + CD4 and CD8 T cells between the RSV strains (Figure 1E-F). These data demonstrate there is no difference in the total magnitude and Th1 effector response of T cells following infection with various strains of RSV.
69 54 Differential T helper 17 response following infection with various RSV strains Although I did not observe a difference in the Th1 response between the RSV strains, CD4 T cells can differentiate into a number of phenotypes including Th17 cells. Since mucus production following RSV line 19 infection is IL-17A-dependent (97), I next examined the Th17 response following infection with each of the RSV strains. The Th17 response was significantly increased (p<.1) in the lungs of RSV line 19-infected mice on day 8 p.i. (Figure 11A-B). Furthermore, there was an increased frequency and total number of CD4 T cells producing both IL-17A and co-producing IL-17F in the lungs (Figure 11A-B). Like the lung parenchyma, I observed a significant increase (p<.1) in the total number of IL-17A + IL-17F - CD4 T cells in the BAL following RSV line 19 infection as compared to RSV A2 and 2-2 (Figure 11C). IL-17 has been reported to induce mucus production following RSV line 19 infection (97). Therefore I quantified the expression of the mucus-associated transcripts Muc5a and Gob5 following infection with the RSV strains (Figure 12). However, I did not observe a positive correlation between increased Muc5a and Gob5 with the increased Th17 response following RSV line 19 infection (Figure 12). Together these data demonstrate there is a differential Th17 response induced between the RSV strains, which does not positively correlate with an increased expression of mucus-associated transcripts in the lung. Increased peak viral replication following RSV A2 infection as compared to RSV line 19 and 2-2 Next, I wanted to ensure the increased Th17 response observed following RSV line 19 infection was not due to increased virus replication. To determine the replication of the various RSV strains, lungs were harvested at day, 2, 4, 6, 8, 1 and 12 p.i. for real-time PCR to quantify the total number of the RSV N gene copies (Figure 13). RSV N copies were not detected at day p.i. and had a similar total copy number at day 2 p.i..
70 55 However on day 4 p.i., there was a significant increase (p<.5) in RSV N copies in the lung following RSV A2 infection as compared to RSV line 19 and 2-2 (Figure 13). By day 6, the total number of RSV N copies was similar between the RSV strains and no longer detectable in the lungs by day 8 p.i. following infection with any RSV strain. These data demonstrate that there is increased replication of RSV A2 on day 4 p.i. as compared to RSV line 19 and 2-2, which has been previously reported (92). However, these data do not correlate with the increased RSV line 19-induced Th17 response on day 8 p.i., indicating that the increased Th17 response following RSV line 19 infection is not the result of enhanced virus replication. Increased pro-inflammatory cytokines in the lungs following RSV line 19 infection Th17 cells are induced in mucosal tissues in the presence of the pro-inflammatory cytokines IL-1β and IL-6 (94). Therefore, I next determined the level of IL-1β and IL-6 protein in the lungs following infection with various strains of RSV. I observed a significant increase (p<.5) in the total level of IL-1β protein in the lungs on day 2 following RSV line 19 infection as compared to RSV A2 and 2-2 (Figure 14A). Additionally, the total amount of IL-6 protein on day 2 p.i. was significantly increased (p<.1) in RSV line 19-infected lungs as compared to RSV A2 and 2-2 (Figure 14B). However, there was no significant difference in the total levels of either IL-1β or IL-6 in the lungs at days 4 and 6 p.i. between the RSV strains (Figure 14). These data suggest that RSV line 19 induces the early production of these pro-inflammatory cytokines by innate immune cells, which subsequently induce the increased Th17 response. Increased responding and cytokine-producing innate cell populations following RSV line 19 infection Since the increased pro-inflammatory cytokine response induced by RSV line 19 occurs early after infection, I next wanted to determine if there are differences in the
71 56 responding innate cell populations following RSV line 19 infection. On day 2 p.i., there was no significant difference in DC populations assessed, including CD11b + cdcs (CD11c + CD11b + CD19 - DX5 - CD3 - ), CD13 + DCs (CD11c + CD13 + CD11b - CD19 - DX5 - CD3 - ) and plasmacytoid DCs (pdcs; CD11c int CD11b - B22 + CD19 - DX5 - CD3 - ) between the different strains of RSV (Figure 15A-C). In addition, I observed no difference in the total number of natural killer (NK) cells (CD3 - DX5 + ) between the RSV strains (Figure 15D). However, I observed a significant (p<.5) increase in the total number of responding macrophages (F4/8 + CD11b + CD11c + B22 - ) and neutrophils (Ly-6C int Ly- 6G hi CD11c - F48 - ) in the lung on day 2 following RSV line 19 infection as compared to RSV strains A2 and 2-2 (Figure 16A-B). Given the capacity of macrophages to produce pro-inflammatory cytokines, I assessed the ex vivo expression of pro-il-1β and IL-6 on day 2 p.i. (Figure 16C). Macrophages in the lung protected from i.v. anti-cd45 labeling exhibited increased expression of pro-il-1β and IL-6 following RSV line 19 infection as compared to RSV strains A2 and 2-2, and naïve mice (Figure 16C). These data demonstrate that the increased number of macrophages following RSV line 19 infection contribute to the increased pro-inflammatory cytokine environment. Increased IL-1β secretion following RSV line 19 infection is caspase 1-dependent IL-1β secretion following RSV A2 infection is dependent on the NLRP3 inflammasome activation and caspase 1 (83, 84). To determine if IL-1β levels were due to differential inflammasome activation, human monocyte THP-1 cells were infected with either purified RSV A2 or line 19 (MOI 5) and IL-1β was quantified in the supernatants 36 hours p.i. (Figure 17A-B). RSV line 19 induced significantly (p<.1) increased IL-1β secretion, which was dependent on virus replication, as compared to RSV A2 (Figure 17A). Furthermore, IL-1β secretion by THP-1 cells following purified
72 57 RSV line 19 infection was significantly (p<.1) decreased in the presence of the caspase 1 inhibitor YVAD (Figure 17B). Similar results were observed following RSV A2 and line 19 infection of primary human alveolar macrophages (Figure 17C). At 24 hours p.i., RSV line 19-infected alveolar macrophages had significantly (p<.5) increased levels of IL-1β in the supernatants as compared to RSV A2-infected and uninfected cells (Figure 17C). Together, these data suggest that RSV line 19 is able to induce increased inflammasome activation following infection of human monocytes as compared to RSV A2. Pro-inflammatory cytokines induce the Th17 response following RSV line 19 infection The pro-inflammatory cytokines IL-1β and IL-6 are required to differentiate Th17 cells in mucosal sites (94). To determine if the increased IL-1β and IL-6 cytokine levels during the innate immune response induced the differentiation of the Th17 response, I treated RSV line 19-infected mice with the IL-1R antagonist Anakinra and/or anti-il-6 neutralizing antibodies i.n. at days -1, 1 and 3 p.i.. On day 6 p.i., there was a significant decrease (p<.5) in the total number of RORγt-expressing CD4 T cells in the lung airways of Anakinra- and/or anti-il-6-treated mice as compared to rigg-treated controls (Figure 18A). Concordant with this observation, there was a significant decrease (p<.1) in the total number of IL-17A-producing CD4 T cells in the BAL following treatment with Anakinra and/or anti-il-6 (Figure 18B). These data indicate the innateinduced pro-inflammatory cytokines contribute to the differentiation of the subsequent CD4 T cell response. Discussion My data indicate that RSV strains induce differential innate and CD4 T cell responses. RSV line 19 induces macrophages to produce pro-il-1β and IL-6, resulting in increased levels of IL-1β and IL-6 in the lung as compared to RSV strains A2 and 2-2
73 58 (Figures 14 and 16). These pro-inflammatory cytokines as well as IL-17A are positively associated with severe RSV-induced disease in the NP washes of infected infants (55, 97). An increased production of pro-il-1β and IL-6 could be explained by increased NFκB activation (79) inducing increased transcription following RSV line 19 infection. RSV activates TLR2, 3, 4 and 7 (7-74), which results in the downstream activation of NF-κB. TLR2 and 4 are expressed on the cell surface. The RSV F protein has been reported to serve as a ligand for TLR4, however the RSV ligand for TLR2 is currently unknown (71). Therefore, RSV line 19 proteins may increase TLR2 and/or 4 activation, resulting in increased production of pro-inflammatory cytokines. However, I would not predict increased TLR3 or 7 activation to be responsible for an increase in NF-κB activation following infection with these strains of RSV, since differential TLR3 and 7 activation would likely be accompanied by differences in virus replication. However, the difference in in vivo replication observed in the lungs of RSV A2 infected mice does not correlate with the increased pro-inflammatory cytokine expression (Figure 13). Increased TLR ligation would induce increased NF-κB activation and transcription of pro-il-1β following RSV line 19 infection. However, inflammasome activation would subsequently still be required to process pro-il-1β into biologically active mature IL-1β. The inflammasome is a multimeric complex of proteins leading to the activation of caspase 1. To date, four inflammasome complexes have been described including the AIM2, NLRP1, NLRP3 and IPAF inflammasomes [reviewed in reference (8)]. RSV A2 has been previously reported to activate the NLRP3 inflammasome (83, 84). Segovia and colleagues demonstrated that RSV A2 activates TLR2 leading to the activation of NF-κB and transcription of pro-il-1β, IL-6 and NLRP3 (83). They further showed that NLRP3 inflammasome activation is dependent on the RSV-induced K + efflux and production of ROS leading to the cleavage and activation of caspase 1. Active caspase 1 in turn cleaves pro-il-1β leading to the secretion of mature IL-1β. In contrast, Triantafilou et al. observed a role for TLR4 in priming the inflammasome and not TLR2 as determined by
74 59 IL-1β secretion following sirna knockdown of TLRs in primary human lung epithelial cells (84). Segovia et al. identified a role for TLR2 in priming the inflammasome following RSV infection of bone marrow derived macrophages from TLR2- and TLR4- deficient mice (83). This discrepancy may be due to species-specific activation of TLRs, since the human RSV A2 strain was used in both experiments. Alternatively, the different cell lines used to propagate the infecting virus stock may account for the discrepancy. Triantafilou et al. propagated their virus stock in Vero cells, which results in virus expressing a truncated G protein (173). Although, the TLR2 RSV ligand is currently undetermined, a virus expressing a truncated G protein could be responsible for either directly or indirectly modulating TLR activation. Additionally, Triantafilou et al. have recently reported that the RSV SH protein plays a role in NLRP3 inflammasome activation following RSV A2 infection (84). Using a recombinant RSV virus lacking the expression of the SH protein, the authors demonstrate that IL-1β secretion is ameliorated whereas pro-il-1β and IL-6 expression are not altered. These data suggest the SH protein is required to activate the inflammasome, but does not affect the activation of NF-κB and inflammasome priming. Furthermore cytoplasmic NLRP3 was observed to be co-localized with the Golgi appatatus following RSV infection (84). Similarly, this atypical localization of NLRP3 was observed with IAV and ECMV dependent on the viroporin proteins M2 and 2B, respectively (86, 87). Following ECMV infection, Ca 2+ flux from the Golgi apparatus is mediated by the viral 2B protein and induces inflammasome activation (87). Given viroporins from multiple viruses have been described to activate the inflammasome, it will be of interest to determine the relative benefit of the viroporin to virus replication at the expense of activating the immune response. From these data a model is constructed where RSV primes the inflammasome through the activation of an extracellular TLR followed by inflammasome activation through the SH-mediated membrane permeability
75 6 facilitating ion transport. Therefore, I hypothesize that RSV line 19 induces increased inflammasome activation due to differential expression and/or function of its SH protein. Subsequent to the pro-inflammatory cytokine response, RSV line 19 induces an increased Th17 response as compared to RSV strains A2 and 2-2 (Figure 11). My data demonstrate that both IL-1β and IL-6 produced during the innate immune response are required for inducing the increased Th17 response following RSV line 19 infection (Figure 18). Either IL-1R- or IL-6-deficient hosts are not able to differentiate naïve CD4 T cells into a Th17 phenotype following a mucosal infection (94). In contrast, an infection in a non-mucosal site does not require IL-6 to be present in order to prime a Th17 response. These data demonstrate that both IL-1β and IL-6 are required to overcome the suppressive effects of RA produced by CD13 + DCs and prime a Th17 response at mucosal sites (94). Together these data support a model where early production of pro-inflammatory cytokines inhibit the suppressive bias of the lung environment following RSV line 19 infection by altering the functions of the APCs and thereby permitting the priming of a Th17 response. IL-17A induces mucus production following RSV infection (73, 97, 98). However, my data demonstrate there is not a positive correlation between the magnitude of the Th17 response and expression of the mucus-associated transcripts Muc5a and Gob5 (Figure 12). Furthermore my data do not support the observations previously reported that RSV line 19 and 2-2 induce more mucus production as compared to RSV A2 (92, 167, 168). This discrepancy may be due to the differences in experimental details, such as different animal vendors, infectious doses and virus stocks. However, histological analysis of mucus production would be required for me to definitively conclude there is not a direct correlation between the RSV-induced Th17 response and mucus production in the lung airways. If the histology data supported my Muc5a and Gob5 transcript data (Figure 12), then I would hypothesize that mucus is induced by IL- 13 and not directly by IL-17. IL-17-induced mucus production following RSV infection
76 61 has been suggested to be indirect by inducing the expression of IL-13 in the murine pathogenesis model (98). Furthermore, IL-13 is reported to induce mucus expression following RSV infection, but does not necessarily have to be dependent on IL-17 (92, 93, 167, 168, 174). However in primary human bronchial epithelial cells, IL-17A as well as IL-1β and IL-6 directly induce mucin expression via activation of NF-κB (175, 176). IL- 17A can also directly induce airway smooth muscle contraction (177). Furthermore, IL- 17A in the respiratory tract is independently positively associated with both asthma severity and severe RSV-induced disease in humans (97, 178, 179). Severe RSV-induced disease is also associated with the development of long-term airway morbidities like asthma and chronic wheezing ( ). Pre-term infants that do not receive the RSV prophylactic treatment palvizumab, are twice as likely to develop chronic wheezing at 3.5 years of age as compared to pre-term infants who do receive prophylactic treatment (18). Given these observations, it would be of interest to investigate whether specific virus strains positively correlate with an increased priming and activation of the inflammasome, a subsequent Th17 response and a risk of developing asthma in human RSV pathogenesis.
77 62 Figure 1. RSV strains induce an equivalent type 1 T cell response. On day 8 p.i., lungs were harvested from RSV A2-, line 19-, or 2-2-infected mice and stained for extracellular surface markers to determine the total number of (A) CD4 T cells and (B) CD8 T cells, (C) CD11a hi CD49d + CD4 T cells and (D) CD11a hi CD8 lo T cells. Harvested cells were stimulated with PMA and ionomycin to determine the total number of IFN-γ + (E) CD4 and (F) CD8 T cells. Cumulative data from two-three independent experiments are presented (n=8-12). For statistical analysis a one-way ANOVA with a Tukey s post test was performed.
78 63 A C # of CD4 + T cells (1 5 ) # of CD11a hi CD49d hi CD4 + T cells (1 5 ) B D # of CD8 + T cells (1 6 ) # of CD11a hi CD8 lo T cells (1 5 ) E 5 F 15 # of IFN-γ + CD4 + T cells (1 5 ) # of IFNγ + CD8 + T cells (1 5 ) 1 5 A2 Line
79 64 Figure 11. Differential Th17 responses following infection with various strains of RSV. On day 8 p.i., lung and BAL were harvested from RSV A2-, line 19-, or 2-2-infected mice and stimulated in vitro with PMA and ionomycin. The (A) frequency ± SEM of IL- 17A- and IL-17F-producing CD4 T cells is depicted in the concatenated dot plots showing equal representation of four mice from one of two independent experiments. The total number of IL-17A + IL-17F - and IL-17A + IL-17F + CD4 T cells was determined in the (B) lung and (C) BAL. (B-C) Cumulative data from two independent experiments are presented (n=8). For statistical analysis a two-way ANOVA with a Tukey s post test was performed. ***p<.1
80 65 A A2 Line ±.5.3 ±.2.3 ±.5.1 ±.8.5 ±.3.2 ±.2 B 1 5 # of CD4 + T cells (1 4 )15 IL-17F *** ***.9 ±.1.3 ±.6.3 ±.7 IL-17A.5 ±.5 *** *** IL-17F - IL-17A + IL-17F + IL-17A +.3 ±.8.6 ± ±.7 C 2.5 ±.5 # of CD4 + T cells (1 3 ) ±.3.3 ±.5.3 ±.2.4 ±.1 No stim. PMA/Iono. *** *** A2 Line IL-17F - IL-17A + IL-17F + IL-17A +
81 66 Figure 12. Increased expression of mucus-associated transcripts does not correlate with the increased RSV line 19-induced Th17 response. RNA was isolated at multiple days p.i. for real-time PCR quantification of the mucus-associated transcript expression of Muc5a (left) and Gob5 (right) relative to expression in naïve lungs. Data presented are from two independent experiments (top and bottom) with n=4 per group. For statistical analysis a two-way ANOVA with a Tukey s post test was performed. *p<.5, **p<.1, ***p<.1
82 67 Muc5a Gob5 Experiment 1 2 (-ΔΔct) * *** *** Experiment 2 2 -ΔΔct * ** ** A2 Line Days post-infection Days post-infection
83 68 Figure 13. The increased RSV line 19-induced Th17 response does not correlate with an increase in virus replication. On days, 2, 4, 6, 8, 1 and 12 p.i. RNA was isolated from RSV A2-, line 19- and 2-2-infected lungs. Real-time PCR was performed to determine the total number of RSV N gene copies in the lung. Cumulative data from two independent experiments are presented (n=8). For statistical analysis a two-way ANOVA with a Tukey s post test was performed. L.O.D.=limit of detection. n.d.=none detected *p<.5
84 69 RSV N copies/lung * n.d. n.d. n.d Days post-infection A2 Line L.O.D.=118
85 7 Figure 14. Increased levels of pro-inflammatory cytokines in the lung following RSV line 19 infection. On days 2, 4 and 6 p.i. lungs were harvested and homogenized to determine the total level of (A) IL-1β and (B) IL-6 following RSV A2, line 19 and 2-2 infection by ELISA. Data from two independent experiments are presented (n=8). For statistical analysis a two-way ANOVA with a Tukey s post test was performed. n.d.=none detected *p<.5, ***p<.1
86 71 A 5 *** * IL-1β (ng/lung) B IL-6 (ng/lung) *** n.d. Days post-infection Days post-infection A2 Line
87 72 Figure 15. No difference in DC populations and NK cells between RSV strains. On day 2 p.i. lungs were harvested from RSV A2-, line 19-, or 2-2-infected mice. Cells were stained for extracellular surface markers to determine the total number of (A) CD11b + cdcs, (B) CD13 + DCs, (C) pdcs and (D) NK cells. Cumulative data from two independent experiments are presented (n=8). For statistical analysis a one-way ANOVA with a Tukey s was performed.
88 73 A C # of CD11b + CD11c + cells (1 6 ) # of CD11c int CD11b - B22 + cells (1 5 ) CD11b + cdcs pdcs B D # of CD11b - CD11c + CD13 + cells (1 4 ) # of CD3 - DX5 + cells (1 6 ) A2 Line CD13 + DCs NK cells
89 74 Figure 16. Increased innate immune cell populations following RSV line 19 infection. On day 2 p.i., lungs were harvested from RSV A2-, line 19-, or 2-2-infected mice and stained for extracellular surface markers to determine the total number of (A) macrophages and (B) neutrophils. Cumulative data from two independent experiments are presented (n=8). For statistical analysis a one-way ANOVA with a Tukey s post test was performed. *p<.5, ***p<.1. (C) Cells were intracellularly stained ex vivo for (left) pro-il-1β and (right) IL-6 expression by macrophages protected from i.v. anti- CD45 labeling from the lungs of naïve (grey), A2- (red), line 19- (blue) and 2-2-infected (green) mice. Plots shown are concatenated displaying equal representation of four mice from one of two independent experiments.
90 75 A 15 *** * B 1 *** *** # of CD11c + F4/8 + cells (1 6 ) 1 5 # of Ly-6C int Ly-6G hi cells (1 6 ) C A2 Line % of Max pro-il-1β IL-6 Naive A2 Line
91 76 Figure 17. Increased caspase 1-dependent IL-1β secretion following RSV line 19 infection. IL-1β quantified from the supernatants of (A) THP-1 cells infected with live or UV-inactivated purified RSV A2 or line 19 (MOI 5) and (B) THP-1 cells treated with either DMSO or the caspase-1 inhibitor YVAD and infected with purified RSV strains (MOI 5) as compared to uninfected and silica-induced NLRP3 activated controls at 36 hours p.i.. (A) Data presented are performed in triplicate and individually repeated twice displaying the average of the mean from each experiment and statistical analysis was determined by performing a one-way ANOVA with Tukey s post test. (B) Data presented was performed once in triplicate, displaying the triplicate average and statistically analyzed by performing a one-way ANOVA with a Tukey s post test. (C) IL-1β secretion by primary human alveolar macrophages infected with RSV strains A2 and line 19 (MOI 5) as compared to the uninfected control at 24 hours p.i. for six individual donors (n=6). For statistical analysis a Friedman test with a Dunn s post test was performed. *p<.5, **p<.1, ***p<.1.
92 77 A IL-1β (pg/ml) ** B IL-1β (pg/ml) ** *** DMSO YVAD Media Silica Live virus pa2 pln19 pa2 pln19 UV inactivated Media Silica pa2 pln19 C IL-1β (pg/ml) * ** Media A2 Line 19
93 78 Figure 18. Innate-induced IL-1β and IL-6 differentiate the Th17 response following RSV line 19 infection. Mice were treated intranasally with Anakinra and/or anti-il-6 antibody on days -1, 1 and 3 following RSV line 19 infection. On day 6 p.i., BAL cells were harvested for PMA and ionomycin stimulation. The Th17 response was quantified by determining the total number of conventional CD4 T cells (A) expressing RORγt and (B) producing IL-17A. Cumulative data from three independent experiments are presented (n=11-13). For statistical analysis a one-way ANOVA with a Tukey s post test was performed. *p<.5, **p<.1, ***p<.1
94 79 A 12 # of RORγt + Foxp3 - CD4 + T cells (1 3 ) * ** ** B # of IL-17A + Foxp3 - CD4 + T cells (1 3 ) ** *** *** rigg Anakinra+rIgG α-il-6 Anakinra+α-IL-6
95 8 CHAPTER IV. MULTIPLE CD4 T CELL SUBSETS PRODUCE IMMUNOMODULATORY INTERLEUKIN-1 DURING RESPIRATORY SYNCYTIAL VIRUS INFECTION Abstract The host immune response is believed to contribute to the severity of pulmonary disease induced by acute RSV infection. Because RSV-induced pulmonary disease is associated with immunopathology, I evaluated the role of IL-1 in modulating the RSVspecific immune response. I found that IL-1 protein levels in the lung were increased following acute RSV infection with maximum production corresponding to the peak of the virus-specific T cell response. The majority of IL-1 producing cells in the lung during acute RSV infection were CD4 T cells. The IL-1-producing CD4 T cells included Foxp3 + Tregs, Foxp3 - CD4 T cells that co-produce IFN-γ, and Foxp3 - CD4 T cells that do not co-produce IFN-γ. RSV infection of IL-1-deficient mice resulted in more severe disease, as measured by increased weight loss and airway resistance, as compared to control mice. I also observed an increase in the magnitude of the RSVinduced CD8 and CD4 T cell response that correlated with increased disease severity in either the absence of IL-1 or following IL-1R blockade. Interestingly, IL-1R blockade during acute RSV infection altered CD4 T cell subset distribution, resulting in a significant increase in IL-17A-producing CD4 T cells and a concomitant decrease in Foxp3 + Tregs. These results demonstrate that IL-1 plays a critical role in modulating the adaptive immune response to RSV by limiting T-cell-mediated pulmonary inflammation and injury. Introduction The lung environment, with its constant exposure to inert foreign antigens, must preserve a fine balance between tolerance and inflammation to effectively clear
96 81 respiratory pathogens while at the same time maintaining tissue homeostasis. Improper regulation of a pathogen-induced immune response can result in excessive immunemediated injury to the lung tissue, termed immunopathology. RSV is a leading cause of lower respiratory tract infections in young children, the elderly and immunocompromised individuals ( ). The host immune response is thought to contribute to the severity of RSV-induced respiratory disease (187). In addition to RSV, other respiratory viral pathogens, such as pandemic influenza strains and human coronaviruses that cause severe acute respiratory syndrome (SARS), are believed to induce a poorly regulated immune response resulting in immune-mediated disease (188, 189). The lung environment contains a number of mechanisms that regulate the immune response (3), including the production of immunosuppressive cytokines such as IL-1. Both innate and adaptive immune cells have the capacity to produce IL-1, including mast cells, NK cells, neutrophils, eosinophils, macrophages, DCs, CD4 T cells, CD8 T cells and B cells (115). In addition, almost all immune cells express the IL-1R1 and IL- 1R2 subunits ( ), which together comprise the IL-1R, and are thus capable of responding to IL-1. Due to the broad expression of IL-1 and the potential responsiveness of most immune cells, it is clear that IL-1 can influence both innate and adaptive immunity. IL-1 mediates pleiotropic effects on the immune response, including suppressing the activation and/or effector functions of immune cells. In macrophages, IL-1-induced signaling results in inhibition of pro-inflammatory cytokines and reduced production of chemoattractants required for the recruitment of T cells, monocytes, DCs and neutrophils to the site of infection (119). In addition, IL-1 suppresses the surface expression of MHC class II and costimulatory molecules on macrophages and immature DCs, resulting in the decreased activation and proliferation of T cells ( ). IL-1 can also directly inhibit T cell cytokine production (e.g. IL-2, IL-5, and TNF-α) and induce T cell anergy (12-122).
97 82 The critical role of IL-1 during persistent infections has been well established (119, 19), however its capacity to modulate the immune response during an acute viral infection resulting in immunopathology remains unknown. In the murine model of RSV infection, it is evident that RSV-induced disease is immune-mediated since depletion of T cells ameliorates disease even in the presence of prolonged viral replication (88, 191). This makes RSV an ideal model for studying the dysregulation of an immune response in the lung mucosal environment. To dissect the role of IL-1 during a respiratory viral infection resulting in immunopathology, I first identified the IL-1-producing cells during acute RSV infection and examined the effects of IL-1 deficiency on the adaptive immune response. I demonstrate that CD4 T cells account for the majority of IL-1 production during the peak of the virus-specific T cell response. Furthermore, I show that multiple CD4 T cell subsets produce IL-1. In either the absence of IL-1 or following IL-1R blockade I observed a significant increase in RSV-induced disease severity, which correlated with the kinetics of the RSV-specific adaptive immune response. Finally, I show that IL-1R blockade results in an increase in the number of Th1 and Th17 cells with a concomitant decrease in the number of Tregs. Taken together, my data suggest that IL-1 plays a critical role in modulating the adaptive immune response to acute RSV infection and in the prevention of immune-mediated pulmonary injury. Materials and Methods Mice, infection, and antibody treatment Six to eight week-old female BALB/cAnNCr mice were purchased from the National Cancer Institute (Frederick, MD). BALB/c IL-1-deficient mice (IL-1 KO) were obtained from Dr. Daniel Berg (University of Iowa, Iowa City, IA) (192, 193). VERT-x mice on the C57BL/6 background, expressing an IL-1-eGFP reporter, were obtained from Dr. Christopher Karp (Cincinnati Children s Hospital Research Foundation
98 83 and University of Cincinnati College of Medicine, Cincinnati, OH) (194). The A2 strain of RSV was a gift from Dr. Barney Graham (National Institues of Health, Bethesda, MD) and was propagated on HEp-2 cells (American Type Culture Collections; ATCC, Manassas, VA). For infections, mice were anesthetized with isoflurane and infected i.n. with of 1-3 x 1 6 PFU of RSV strain A2. In some experiments, mice were administered either.5 mg rat IgG (MP Biomedicals, Inc., Aurora, OH) or.5 mg anti-il-1r mab (clone 1B1.3) via i.p. injections starting one day prior to infection and every other day thereafter. CD4 and/or CD8 T cells were depleted via i.p. injection of.25 mg of anti- CD4 (clone GK1.5) and/or anti-cd8 (clone 2.43) antibody at days -2 and +2 relative to infection. All experimental procedures involving mice were approved by the University of Iowa Animal Care and Use Committee. Disease measurements Disease was assessed daily by monitoring weight loss as well as pulmonary function using an unrestrained whole body plethysmograph (Buxco Electronics, Wilmington, NC) (111, 195). Pressure and volume differences in the chamber caused by respiration were monitored for 5 mins and recorded to obtain a daily average in order to calculate the dimensionless parameter enhanced pause (Penh) (196). Penh positively correlates to airway hyper-reactivity (196), which is narrowing of the small airways in response to stimuli. Tissue processing BAL cells were isolated by tracheal cannulation and three successive 1 ml washes with RPMI supplemented with 1% FCS (Atlanta Biologicals, Lawrenceville, GA), 5 nm 2-mercaptoethanol (Sigma), 2 mm L- glutamine, 1 U/ml penicillin, 1 µg/ml streptomycin sulfate, 1 mm HEPES, 1mM Sodium Pyruvate, and.1 mm MEM nonessential amino acids (all obtained from Gibco) (146). Lungs were subsequently perfused with 1 ml of PBS. Lungs and lymph nodes were then digested in 4 ml or 1 ml,
99 84 respectively, of HBSS with CaCl 2 and MgCl 2 (Gibco, Grand Island, NY) supplemented with 6 U/ml DNase I (Sigma, St. Louis, MO) and 125 U/ml collagenase (Invitrogen, Carlsbad, CA) for 3 minutes at 37 C. After digestion, lungs where pressed through a wire mesh screen (Cellector; Bellco Glass, Inc., Vineland, NJ) to create a single-cell suspension. Mediastinal lymph nodes (medln) were disrupted by gentle pressing between the ends of frosted glass slides (Surgipath, Richmond, IL). Extracellular staining 1-2 x 1 6 cells were placed in a 96-well round-bottom plate (Corning Inc., Corning, NY). Cells were incubated with mabs for extracellular proteins CD9.2 (Thy1.2; clone ), CD4 (clone RM4.5), CD8 (clone ), CD11a (clone M17/4), and CD44 (clone IM7) and simultaneously blocked with anti-fcγrii/iii mab (clone 93) (all mabs obtained from ebioscience, San Diego, CA) in FACS buffer (PBS, 2% FCS,.2% sodium azide) for 3 mins at 4 C. Cells were subsequently washed three times with FACS buffer at 4 C, fixed with FACS lysing solution (BD Biosciences, San Diego, CA) for 15 mins at room temperature, washed three times with FACS buffer at 4 C and resuspended in FACS buffer for analysis. Samples were collected on a BD FACSCanto flow cytometer and data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Ex vivo cell stimulation Cells were incubated at 1-2 x 1 6 cells/well in a 96-well round-bottom plate (Corning Inc.). For peptide stimulations, cells were incubated with or without 1 µm of M peptide (Biosynthesis, Inc., Lewisville, TX) and 1 µg/ml brefeldin A (Sigma) in RPMI 164 supplemented with 1% FCS (Atlanta Biologicals, Lawrenceville, GA), 5 nm 2-mercaptoethanol (Sigma), 2 mm L- glutamine, 1 U/ml penicillin, 1 µg/ml streptomycin sulfate, 1 mm HEPES, 1mM Sodium Pyruvate, and.1 mm MEM nonessential amino acids (all obtained from Gibco) for 5 h at 37 o C (146). Alternatively, cells
100 85 were stimulated in the presence or absence of 5 ng/ml PMA (Sigma) and 5 ng/ml ionomycin (Sigma) in the presence of 1 µg/ml brefeldin A in 1% FCS complemented RPMI 164 for 5 hrs at 37 C. Intracellular cytokine staining Briefly after ex vivo stimulation, cells were stained for extracellular proteins and fixed in FACS lysing solution as described above. Cells were subsequently incubated in FACS buffer containing.5% saponin (Sigma) and intracellular cytokine antibodies IL- 1 (clone JES5-16E3), IFN-γ (clone XMG1.2 ), TNF-α (clone MP6-XT22), IL-17A (clone TC11-18H1.1) (all mabs obtained from ebioscience) for 3 mins at 4 C. Cells were washed twice with FACS buffer containing saponin, once with FACS buffer, and resuspended in FACS buffer prior to analysis on a BD FACSCanto. Foxp3 staining After staining extracellular proteins as described above, cells were stained for Foxp3 using the mouse regulatory T cell staining buffer kit (ebioscience) according to the manufacturer s instructions. Cells were stained with mabs against Foxp3 (clone FJK-16s), Helios (clone 22F6), IL-1 (clone JES5-16E3), and IFN-γ (clone XMG1.2) (all mabs obtained from ebioscience). After staining, cells were resuspended in FACS buffer prior to analysis. Plaque assays Lungs were harvested on days 4 and 7 post-infection in 1 ml of serum-free RPMI and tissue was subsequently homogenized (Ultra-Turrax T25; IKA Works) (197). Tissue homogenates were centrifuged at 2 rpm for 1 mins to collect supernatants for freezing in liquid nitrogen. Samples were thawed for a 1.5-hr infection with rocking of Vero cells (ATCC) in 6-well plates. Cells were subsequently overlaid with 4 ml of a 1:1 ratio of 1% agar and Eagle s MEM supplemented with 1% FCS (Atlanta Biologicals), 2
101 86 mm L- glutamine and 1 U/ml penicillin and incubated at 37 C. 5 days later cells were stained with 2 ml of a 1:1 ratio of 1% agar and supplemented Eagle s MEM with neutral red at a final concentration of.1% and incubated at 37 C 24 hours before counting the number of plaques. ELISA Lungs from RSV-infected mice were disrupted using a tissue homogenizer (Ultra- Turrax T25; IKA Works, Inc., Wilmington, NC) in RPMI 164 containing 1% FCS and a 1/2 dilution of a protease inhibitor cocktail (Sigma). Lung homogenates were centrifuged at 2 rpm for 1 min. Cytokine protein levels in the supernatant were determined for IL-1, IFN-γ, and IL-17 by ELISA (169) (IL-1 and IFN-γ Abs were obtained from ebioscience, IL-17 Abs were obtained from BioLegend, San Diego, CA). Briefly, samples were plated in wells previously coated with 2 µg/ml of capture antibody and incubated overnight at 4 C. Biotinylated secondary antibody was subsequently added and incubated at room temperature for 2 hrs followed by avidin-peroxidase incubation for 3 mins at room temperature. Plates were developed with 3,3,5,5 - tetramethylbenzidine dihydrocholride (Sigma-Aldrich) and the reaction was stopped with 2 M H 2 SO 4 (Fischer Scientific). Plates were read using an ELx8 absorbance reader (Bio-tek, Winooski, VT) at 45 nm and analyzed using Gen5. software (Bio-tek). Detection limits for cytokine protein levels were 62.5 pg/ml for IL-1 and 125 pg/ml for IFN-γ. Multiplex bead assay Lungs from RSV infected IL-1 KO and WT mice were harvested and supernatants were collected as described above. A Milliplex kit was used to determine the protein levels of 27 different cytokines and chemokines by following the
102 87 manufacture s instructions (Millipore, Billerica, MA). The assay was run on a BioPlex instrument (Bio-Rad, Hercules, CA). Histology Whole lungs from naïve and RSV infected rigg and anti-il-1r mab treated mice on day 6 p.i. were fixed in 1 percent formalin (Thermo Fisher Scientific, Waltham, MA). Fixed lungs were embedded in paraffin, sectioned at 4 µm thickness and stained by the University of Iowa Comparative Pathology Laboratory. Slides were either hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) stained and randomized and blinded for analysis by a board-certified veterinary pathologist (Dr. D. Meyerholz, University of Iowa). Sectioned slides were scored for perivascular aggregates of leukocytes (PVA) (1, within normal parameters; 2, small numbers of solitary cells with uncommon aggregates; 3, multifocal small to moderate aggregates; and 4, moderate to high cellularity with multifocal large cellular aggregates that may be expansive into adjacent tissues), interstitial disease (ID) (1, within normal parameters; 2, mild, detectable focal to multifocal congestion with uncommon to small numbers of leukocytes and some atelectasis; 3, moderate, multifocal to coalescing congestion, leukocyte cellularity, and atelectasis with rare luminal leakage of cellular and fluid debris; and 4, severe, coalescing interstitial congestion, leukocytes, and atelectasis with admixed extensive loss of airspace and luminal accumulation of cellular and fluid debris), edema (1, none; 2, rare with alveoli with wispy to partial pools of eosinophilic seroproteinaceous fluid; 3, focal microscopic fields with contiguous alveoli partially filled by pools of fluid; and 4, multiple fields having coalescing alveoli filled by pools of fluid), and mucus (1, none; 2, epithelial mucinous hyperplasia with none to rare luminal mucus; 3, epithelial mucinous hyperplasia with luminal mucus accumulation in airways; and 4, severe mucinous change with some airways completely obstructed by mucus). The total score is the sum of the PVA, ID, edema, and mucus scores.
103 88 Statistical analysis Data were graphically compiled using Prism software (Graphpad Software Inc., San Diego, CA) with bars representing the SEM. Statistical analysis was completed using InStat software (Graphpad Software Inc.); unpaired, two-tailed Student t tests and analysis of variances with a Dunnett s post test were calculated to determine statistical significance at a level of α=.5. Results CD4 T cells account for the majority of IL-1-producing cells during the peak of the virus-specific T cell response To determine the role of IL-1 during acute RSV infection I first examined the kinetics and cellular source of IL-1 production. Cells from the lung, medln, and lung airways (i.e. BAL) were stimulated in vitro with PMA and ionomycin and stained for intracellular IL-1. The total number of IL-1-producing cells peaked on day 6 p.i. in the lung and medln and on day 8 p.i. in the BAL (Figure 19A). CD4 T cells comprised the majority of IL-1-producing cells (Figure 19B). In contrast, IL-1-producing CD8 T cells did not accumulate in large numbers in any of the tissues analyzed (Figure 19C). To verify my results that were obtained using in vitro stimulation, I utilized mice that express an IL-1-eGFP reporter to examine IL-1-producing cells directly ex vivo during an acute RSV infection. In agreement with my in vitro restimulation data discussed above, the frequency of IL-1-eGFP + cells peaked on day 8 p.i. in the lung and BAL (Figure 2). Approximately 8 to 9 percent of IL-1-eGFP + cells on day 6 and 8 p.i. were T cells as identified by CD9.2 staining in the lung and BAL (Figure 2). Within the CD9.2 + population, CD4 T cells represented the majority of IL-1-eGFP + T cells in lungs and BAL at all time points examined. Taken together, these data demonstrate CD4 T cells account for the majority of IL-1-producing cells during the peak of the virus-specific T cell response.
104 89 To confirm that CD4 T cells produce the majority of IL-1 in vivo during RSV infection, I quantified IL-1 protein levels in the lungs of mice depleted of CD4 and/or CD8 T cells. My results indicate that CD4 T cells were responsible for the majority of IL-1 protein produced in the lung during an acute RSV infection (Figure 21A). Elimination of CD8 T cells resulted in increased IL-1 production suggesting that CD8 T cells may suppress IL-1 production by CD4 T cells. IFN-γ production was also measured using the above approach and as expected CD8 T cells produced significantly more IFN-γ than the CD4 T cells in response to RSV infection (Figure 21B). Taken together, these data demonstrate that CD4 T cells are the major producers of IL-1 in vivo during an acute RSV infection. The capacity of CD4 T cells to produce the antiinflammatory cytokine IL-1, suggests their potential to significantly modulate the immune response. Multiple CD4 T cell subsets produce IL-1 in response to RSV infection Since CD4 T cells accounted for the majority of RSV-induced IL-1-producing cells, I next determined the CD4 T cell subsets responsible for the IL-1 production. On day 6 p.i., lung, medln, and BAL cells were stimulated in vitro with PMA and ionomycin and stained for intracellular IL-1, IFN-γ, and Foxp3. In all tissues analyzed, I observed equal proportions of IL-1 + CD4 T cells that only produced IL-1, co-produced IFN-γ (i.e. Th1 cells) or co-expressed Foxp3 (i.e. Tregs) (Figure 22). Interestingly, in the BAL I observed a small population of Foxp3 + Tregs that co-produced IL-1 and IFN-γ. This population of cytokine co-producing Tregs exhibited increased expression of T-bet as compared to Tregs only producing IL-1 (Figure 23). These data demonstrate that multiple CD4 T cell subsets contribute to IL-1 production during an acute RSV infection.
105 9 In addition, I determined the frequency of IL-1 producing cells within these CD4 T cell subsets (Table 1). On day 6 p.i., I observed the highest frequency of IL-1-only producing conventional and regulatory CD4 T cells (5.1 ± 1.6 and 36.7 ± 6. percent, respectively) in the BAL. Furthermore, in the BAL I observed the highest proportion of conventional and regulatory CD4 T cells that co-produce IL-1 and IFN-γ (11.1 ± 4.4 and 8.8 ± 4.1 percent, respectively) when compared to other tissues. In all tissues analyzed IL-1 producing conventional and regulatory CD4 T cells were observed as summarized in Table 1. These data demonstrate the distribution of IL-1 producing cells in the conventional and regulatory CD4 T cell compartments following acute RSV infection. Increased RSV disease severity in the absence of IL-1 or following IL-1R blockade To examine the role of IL-1 during acute RSV infection, I utilized either IL-1 deficient (IL-1 KO) mice or anti-il-1r mab to determine disease severity in the absence of IL-1 or following IL-1R blockade. IL-1 KO mice exhibited greater weight loss and increased Penh compared to control mice following acute RSV infection (Figure 24A). Similar results were obtained from mice treated with anti-il-1r mab prior to and during RSV infection as compared to IgG-treated controls (Figure 24B). These data indicate that IL-1 plays a critical role in limiting disease severity at the peak of the adaptive immune response during RSV infection. To determine if IL-1 had an effect on viral titers, I performed plaque assays using whole lung homogenates to measure viral load at the peak of RSV replication (i.e. day 4 p.i.) and viral clearance (i.e. day 7 p.i.). There were no significant differences in viral titer at either time point in IL-1 KO mice (Figure 25A) or anti-il-1r mab treated mice (Figure 25B). These results indicate that the effect of IL-1 deficiency on disease severity is not due to either altered viral replication or delayed viral clearance, but instead may be the result of alterations of the RSV-specific immune response.
106 91 Increased inflammatory environment in the absence of IL-1 Given the increased disease severity observed during RSV infection in the absence of IL-1, I next examined the overall inflammatory environment in the lung following acute RSV infection. Using a multiplex bead assay, I quantified cytokine and chemokine protein levels from whole lung homogenates at days, 4, 6, 8, and 1 p.i. from control and IL-1 KO mice infected with RSV. In the absence of IL-1, I observed a marked increase in the levels of pro-inflammatory cytokines and chemokines following RSV infection (Figure 26). In the lungs of RSV-infected IL-1 KO mice, I observed a significant increase in IL-6 (p <.1) and TNF-α (p <.1) on day 6 p.i., and a significant increase in IFN-γ on days 6 (p <.1) and 8 p.i. (p <.5) as compared to control mice. In addition, CXCL1 was significantly increased in the lungs of IL-1 KO mice at days 4 (p <.5) and 6 p.i. (p <.1) and slightly increased on day 8 p.i. when compared to control mice. CXCL9 was increased and CCL2 was significantly increased (p <.1) on day 6 p.i. in the absence of IL-1 following RSV infection. Additionally, other cytokines and chemokines do not have increased expression in the absence of IL-1 (Figure 27). Overall, in the absence of IL-1 I observed increased levels of proinflammatory cytokines and chemokines that can enhance inflammation and the infiltration of immune cells responsible for mediating RSV-induced immunopathology. Taken together, these data support the hypothesis that IL-1 plays a critical role in regulating the severity of RSV-induced pulmonary disease by regulating inflammation and limiting immunopathology. IL-1R blockade results in an increase in the magnitude of the RSV-specific CD8 T cell response Since the increase in RSV-induced disease severity (days 6-8 p.i.) in the absence of IL-1 or following IL-1R blockade occurred during the rise of the RSV-specific
107 92 adaptive immune response, I next investigated the impact of IL-1 deficiency on the magnitude of the RSV-specific CD8 T cell response. In mice treated with anti-il-1r mab, there is a significant increase (p <.1) in the total number of CD8 T cells in the medln on day 6 p.i. (Figure 28A). Furthermore, there was a significant increase (p <.1) in the total number of CD11a hi CD8 lo T cells in the medln of mice treated with anti-il-1r mab on day 6 p.i. (Figure 28B), indicating the presence of antigenexperienced cells (172). Using the M peptide to stimulate RSV-specific CD8 T cells ex vivo, I observed a significant increase in the magnitude of the M RSV-specific CD8 T cell response in the medln at day 6 p.i. (p <.1) and in the lung on day 8 p.i. (p <.1) (Figure 28C-D). In the medln of mice treated with anti-il-1r, I observed a significant increase in the total number of M specific CD8 T cells producing IFN-γ (p <.1) or co-producing IFN-γ and TNF-α (p <.1) on day 6 p.i. In the lungs of anti-il-1r mab treated mice, there was a significant increase in the total number of M specific CD8 T cells that produce IFN-γ (p <.1) or co-produce IFN-γ and TNF-α (p <.1) on day 8 p.i. These data demonstrate an increase in both the magnitude and effector function of RSV-specific CD8 T cells following IL-1R blockade, demonstrating a role for IL-1 in limiting the RSV-specific CD8 T cell response. IL-1R blockade results in an increased Th1 CD4 T cell response following acute RSV infection Since the RSV-specific CD8 T cell response was increased following IL-1R blockade, I next evaluated the impact of IL-1 on the magnitude of the effector CD4 T cell response during acute RSV infection. IL-1R blockade resulted in a significant increase in the total number of CD4 T cells in the lung (p <.1) and BAL (p <.5) on day 8 p.i. (Figure 29A). Intracellular cytokine staining for IFN-γ and TNF-α production by CD4 T cells from the lung and medln stimulated with PMA and ionomycin revealed
108 93 a significant increase (p <.1) in the total number of effector CD4 T cells in the lung on day 8 p.i. in mice administered anti-il-1r mab (Figure 29B). Furthermore, on day 8 p.i. in the lung, mice treated with anti-il-1r mab exhibited a significant increase in the number of CD4 T cells that produce IFN-γ (p <.1) or co-produce IFN-γ and TNFα (p <.5). Thus, similar to the increase in the number of CD8 T cells described above, I observed an increase in the magnitude of the Th1 CD4 T cell response in the lungs of mice administered anti-il-1r mab during acute RSV infection. This increase in the number of virus-specific IFN-γ-producing CD8 and CD4 T cells following IL-1R blockade during a RSV infection suggests a critical role for IL-1 in limiting the magnitude of the RSV-specific adaptive immune response. Increased lung pathology following IL-1R blockade To determine if the increased disease severity and effector CD8 and CD4 T cell response results in increased lung pathology following IL-1R blockade, histological analysis of sections from whole lungs were stained with either H&E or PAS and analyzed. On day 6 p.i., a significant increase (p <.1) was observed in lung pathology when scoring the perivascular aggregation of leukocytes (PVA), interstitial disease (ID), eosinophilic seroproteinaceous fluid in the airspaces (i.e. edema), and luminal mucus accumulation in the airways (Figure 3A). Histology of rigg-treated mice following RSV infection revealed tissue inflammation with minimal mucus in the luminal space of the lung airways (Figure 3B). The histological sections from mice treated with anti-il- 1R mab demonstrate increased pathology with increased areas of edema, tissue inflammation and increased mucus in the luminal space of the lung airways (Figure 3C). Together these data indicate that IL-1R blockade results in increased lung pathology that is associated with increased disease severity following RSV infection.
109 94 Alteration of additional CD4 T cell subsets following IL-1R blockade With the increased Th1 CD4 T cell response during RSV infection in the absence of IL-1, I wanted to investigate the effect of IL-1 deficiency on additional CD4 T cell subsets. IL-1R blockade resulted in alterations in the Treg and Th17 CD4 T cell subsets following acute RSV infection (Figure 31). Although the expression of the transcription factor Helios is no longer thought to identify ntregs from itregs, it can be utilized to determine Treg activation (198-21). On day 8 p.i., there was a significant decrease in Foxp3 + Helios + CD4 T cells in the lung (p <.1), medln (p <.1), and BAL (p <.5) in mice administered anti-il-1r mab (Figure 31A). However there was no difference in the total number of Foxp3 + Helios - CD4 T cells between mice treated with the anti-il-1r mab and controls. These data suggest that IL-1, either directly or indirectly, supports the activation of Tregs during an acute RSV infection. Coincident with the decrease in the total number of activated Tregs, I observed a significant increase in the total number of IL-17A-producing CD4 T cells in mice treated with anti-il-1r mab (Figure 31B). On day 8 p.i., IL-17A + CD4 T cells were significantly increased following IL-1R blockade in the lung (p <.1) and BAL (p <.5) during acute RSV infection. Thus, IL-1R blockade leads to decreased numbers of Tregs in the lung with a concomitant increase in the number of Th17 cells. Discussion Here I examined the kinetics of IL-1 production during acute RSV infection and found that the highest frequency of IL-1-producing cells occurred between days 6 through 1 p.i., a time frame that overlaps with the virus-specific adaptive immune response (Figures 19-2) (22, 23). I found that three major subsets of CD4 T cells were responsible for the majority of IL-1 production at the peak of the immune response following RSV infection (Figure 21). Approximately one third of the IL-1-producing
110 95 CD4 T cells expressed Foxp3, representing a portion of the Treg population, whereas the remainder were conventional Foxp3 - CD4 T cells that produced either IL-1 alone or coproduced IFN-γ. In addition, I observed a small frequency of IL-1 + IFN-γ + regulatory (Foxp3 + ) CD4 T cells only in the lung airways following RSV infection. I believe that the environment is unique in the lung airways since it s the primary site of virus replication, causing these CD4 T cells to alter their phenotype. Previously, Tregs in the presence of IFN-γ in vivo have been described to express T-bet, which allows them to specifically regulate type I immune responses (24). Therefore, I hypothesize that more concentrated levels of IFN-γ in the lung airways induces T-bet expression in Tregs, which results in a small fraction of these cells expressing IFN-γ following in vitro stimulation (Figure 22). Th1 cells have been previously reported to be the major producers of IL-1 in other pathogenic models including IAV, Leishmania species, mycobacterial and Borrelia burgdorferi infections (139, 25-28). The observation that other CD4 T cell subsets are capable of producing IL-1 and that IL-1 can regulate T cell function, has led to the hypothesis that IL-1 acts in either an autocrine manner to directly suppress T cell activation or a paracrine manner through DCs to control immunopathology (115, ). My results support the conclusion that in the absence of IL-1 and its regulation of T cells, increased RSV-induced disease severity is a consequence of increased immunopathology. However, the molecular mechanism that induces IL-1 production by effector CD4 T cells during RSV infection remains to be determined. I demonstrate that CD4 T cells produce the majority of IL-1 in vivo during an acute RSV infection. In contrast, I observe only a small number of IL-1-producing CD8 T cells (Figure 19). Although I do observe up to 3 percent of IL-1-producing T cells are CD8 + following infection of IL-1-eGPF reporter mice (Figure 2). However, I believe this discrepancy is due to the different mouse strains used and that RSV replication is more permissive in BALB/c mice (212). IL-1 production by T cells has
111 96 been examined in a number of other respiratory virus infections. Consistent with my results, McKinstry et al. recently reported that CD4 T cells make the majority of IL-1 following a high dose IAV infection (139). Virus-specific CD8 T cells that co-produce IL-1 and IFN-γ in the lung have been reported during acute Simian virus 5 (SV5) infection (213). However these studies did not assess IL-1 production by CD4 T cells during SV5 infection. Therefore it is unclear if either CD4 or CD8 T cells represent the dominant cell source of IL-1 during acute SV5 infection. In contrast to my findings with RSV, Sun et al. report that CD8 T cells are responsible for the majority of IL-1 production during an acute low dose IAV infection (14). It is currently unclear what accounts for the reported discrepancies in the proportion of CD4 and CD8 T cells that make IL-1 during respiratory viral infections. A more recent study by Sun et al. indicates that IL-1 production by CD8 T cells during an acute IAV infection requires the expression of PR domain zinc finger protein 1 (PRDM1; also known as Blimp-1) and is synergistically promoted by IL-2 produced by helper CD4 T cells as well as IL-27 produced by innate immune cells (214). It remains to be determined if the reduced number of IL-1-producing CD8 T cells during an acute RSV infection is the result of a lack of CD4 T cell help and/or decreased IL-27 production and if IL-1-producing CD4 T cells are induced in a similar manner following RSV infection. Exacerbated disease severity and increased levels of pro-inflammatory cytokines and chemokines were observed in the absence of IL-1 following RSV infection (Figures 24 and 26). However, I did not observe any changes in peak viral titers (day 4 p.i.) or viral clearance (day 7 p.i.) between IL-1 KO or anti-il-1r mab treated mice compared to their controls (Figure 25). These data support the idea that increased disease severity is independent of RSV replication and clearance kinetics (88). Instead, alterations in the RSV immune response (i.e. increased inflammatory conditions) result in increased immunopathology. Similarly, Haeberle et al. reported that treatment with recombinant IL-1 significantly decreased the production of chemokines, such as CCL2,
112 97 CCL3 and CCL5 in the lung airways when analyzed 24 hrs after RSV infection (215). CCL5 has been positively associated with RSV-induced disease severity and blocking CCL5 results in the increased production of IL-12 and decreased airway hyperreactivity (216). Consistent with its effect on chemokine production, treatment with recombinant IL-1 significantly reduced the number of cellular infiltrating lymphocytes and decreased alveolar inflammation following acute RSV infection (215). Exogenous addition of IL- 1 did not affect viral replication (215). I demonstrate that IL-1 deficiency significantly increases weight loss and airway resistance following RSV infection (Figure 24). The above results combined with my findings suggest that IL-1 acts by inhibiting proinflammatory cytokine production in the lung, which in turn reduces RSV-induced pulmonary inflammation and immunopathology. Similar to these observations, findings from the avian H5N1 outbreaks in Hong Kong revealed that patients with severe disease exhibited high serum cytokine levels of IL-6, TNF-α and IFN-γ and increased serum chemokine levels of CCL2, CXCL9 and CXCL1 when compared to less severely ill controls (188, ). The levels of IL-6, IFN-α, IFN-γ, CCL2 and CXCL1 were also significantly elevated in the serum of SARS patients with more severe disease ( ). Overall, in the absence of IL-1 my data support the notion that a strong pro-inflammatory response contributes to increased RSV disease severity. Thus therapeutic approaches aimed at increasing IL-1 production may offer a means to decrease RSV-induced immunopathology without affecting viral clearance. IL-1R blockade resulted in an increase in the magnitude and effector function of RSV-specific CD8 T cells and CD4 T cells (Figures 28 and 3). A similar observation was reported by Sun et al. where acute IAV infection resulted in an increased total number of IFN-γ producing CD8 and CD4 T cells in mice treated with anti-il-1r mab (14). In addition, I observed a significant increase in the number of Th17 CD4 T cells during an acute RSV infection in the absence of IL-1 (Figure 31). In agreement with
113 98 my findings, McKinstry et al. recently reported a significant increase in IL-17 production and the accumulation of Th17 cells in the lungs of IL-1 KO mice following an acute high dose IAV infection (139). In contrast to the increased number of Th17 cells discussed above, I observed a decrease in the total number of Helios + Foxp3 + Tregs in the lung following IL-1R blockade in RSV infected mice. It has been suggested that Helios expression may selectively identify ntregs (198, 199). However, recent work has delineated that Helios does not specifically identify ntregs, but is expressed following Treg activation (2, 21). Thus, the decreased number of activated Helios + Tregs may directly contribute to increased disease severity. In a model of colitis, IL-1 was reported to be necessary to maintain Foxp3 expression of CD4 T cells under inflammatory conditions (224). Murai et al. demonstrated that the suppressive function of Tregs was maintained by IL-1R expression and the capacity to directly respond to IL-1. However, the environment in the intestinal mucosa is unique with different regulatory factors than the lung, and the findings of Murai et al. may not necessarily translate to lung mucosal tissue. Therefore, the role of IL-1 in the regulation of Foxp3 expression will need to be further evaluated in the lung environment. IL-1 polymorphisms have been associated with RSV-induced disease severity in humans (225, 226). Here I demonstrate that IL-1 plays a critical role in regulating the immune response and preventing exacerbated disease due to immune-mediated pulmonary injury during acute RSV infection. Multiple respiratory viruses, such as pandemic IAV strains and the human coronavirus that causes SARS, are believed to induce immunopathology resulting in severe morbidity and mortality (188, 189, ). Therefore, identifying novel approaches to modulate virus-induced immunopathology would be beneficial in treating acute respiratory viral infections. Exogenous IL-1 treatment has been shown to be safe and well tolerated in clinical trials for several chronic inflammatory diseases including Crohn s disease, psoriasis, and
114 99 chronic hepatitis C ( ). In patients with chronic hepatitis C who were nonresponsive to IFN-based therapy, IL-1 treatment did not affect viral titers but reduced chronic liver inflammation and fibrosis (23). Using IL-1 as an immunosuppressive treatment during a respiratory viral infection resulting in immunopathology may be feasible to prevent severe morbidity and mortality. My data indicate that IL-1 is an attractive target based on its ability to regulate RSV-induced innate and adaptive immune responses without affecting viral clearance.
115 1 Figure 19. The kinetics of IL-1-producing cells during acute RSV infection. At the indicated time points, cells from the lung, medln, and BAL of RSV-infected mice were isolated and stimulated with PMA and ionomycin followed by intracellular cytokine staining for IL-1. The total number of (A) IL-1 + cells, (B) IL-1 + CD4 + T cells, and (C) IL-1 + CD8 T cells in the lung, medln, and BAL. To determine statistical significance, an analysis of variances was performed with a Dunnett s post test making comparisons between each time point (days 4, 6, 8, and 1) and baseline (day ). Cumulative data from two independent experiments are shown (n = 8). *p<.5, **p<.1.
116 11 A Lung medln BAL B # of IL-1 + cells (1 4 ) # of IL-1 + CD4 + T cells (1 4 ) C ** ** # of IL-1 + cells (1 3 ) # of IL-1 + CD4 + T cells (1 3 ) ** ** # of IL-1 + cells (1 3 ) # of IL-1 + CD4 + T cells (1 3 ) * ** # of IL-1 + CD8 + T cells (1 4 ) 1 5 ** * # of IL-1 + CD8 + T cells (1 3 ) # of IL-1 + CD8 + T cells (1 3 ) Days post-infection
117 12 Figure 2. CD4 T cells represent the majority of IL-1-producing cells at the peak of the RSV-specific adaptive immune response. IL-1-eGFP C57BL/6 reporter mice were used to assess in vivo IL-1 production at the indicated time points following RSV infection. Lung and BAL cells were collected for analysis of IL-1-eGFP expression by flow cytometry (left panels). Anti-CD9.2 Ab staining was used to determine the frequency of T cells within the IL-1-producing cell population (middle panels). IL-1-producing T cells were further gated on CD4 and CD8 to determine the distribution of IL-1- producing T cells (right panels). Numbers indicate the frequency of the gated cells. Plots are concatenated using Flowjo software showing equal representation of three individual mice at each time point. Data are representative of three independent experiments (n=9).
118 13 Lung BAL Day Day Day Day Day IL-1 SSC-A CD9.2 SSC-A CD4 CD8 IL-1 SSC-A CD9.2 SSC-A CD4 CD8
119 14 Figure 21. CD4 T cells are the primary in vivo source of IL-1 during acute RSV infection. Wild-type mice were treated with depleting anti-cd4 and/or anti-cd8 mabs or control rat IgG and subsequently infected with RSV. On day 6 p.i., lungs were analyzed for (A) IL-1 and (B) IFN-γ protein via ELISA. Cumulative data from two independent experiments are shown (n = 8). Limit of detection is indicated by dotted line. **p<.1, ***p<.1, n.d. = none detected.
120 15 A pg/lung ** IL-1 ** *** *** *** B IFN-γ pg/lung ** ** rigg α-cd4 α-cd8 α-cd4 + α-cd8 n.d.
121 16 Figure 22. Multiple CD4 T cell subsets produce IL-1 during acute RSV infection. At day 6 p.i., cells from the lung, medln, and BAL were stimulated with PMA and ionomycin. Cells were subsequently stained for CD4, CD9.2, IL-1, Foxp3, and IFN-γ. Gated IL-1 + T cells depicting (A) Isotype control staining or (B) Foxp3 expression (xaxis) and IFN-γ production (y-axis) is shown. Plots are concatenated using Flowjo software from one of two independent experiments displaying equal representation from four individual mice (n = 4).
122 17 A Gated on IL-1 + CD4 + T cells Lung medln BAL B Isotype Isotype IFN-γ 36.7 Foxp
123 18 Figure 23. IL-1 and IFN-γ co-producing Tregs express T-bet. At day 6 p.i., cells from the BAL were harvested and stimulated with PMA and ionomycin. IL-1-producing Foxp3 + regulatory CD4 T cells are gated exhibiting the T-bet (x-axis) expression of IFNγ - (red) and IFN-γ + (blue) cells compared to the isotype control (gray). Plot was concatenated using Flowjo software from one of two independent experiments displaying equal representation from four individual mice (n = 4).
124 19 Gated on IL-1 + Foxp3 + CD4 + T cells T-bet Isotype IFN-γ - IFN-γ+
125 11 Table 1. Frequencies of cytokine-producing CD4 T cells on day 6 following RSV CD4 T cell IFN-γ + IL-1 - IFN-γ + IL-1 + IFN-γ - IL-1 + Conventional (Foxp3 - ) Lung 1.1 ± 3.2 a 2.8 ± ±.4 MedLN 5.4 ±.9.6 ±.2.5 ±.2 BAL 26.9 ± ± ± 1.6 Regulatory (Foxp3 + ) Lung 1.6 ± ± ± 1.4 MedLN 1.9 ± 1..6 ± ± 1.1 BAL 3.1± ± ± 6. a Percent ± standard deviation
126 111 Figure 24. IL-1 influences RSV-induced disease severity. Weight loss depicted as the percent of initial weight and airway function (Penh) were assessed daily following RSV infection of (A) IL-1-deficient or (B) anti-il-1r mab treated mice. Cumulative data from two independent experiments are shown (n=8). *p<.5, **p<.1, ***p<.1.
127 112 A B % of initial weight 1 9 ** ** ** * * ** * Penh ** * WT IL-1 KO % of initial weight 1 9 ** ** *** Penh * *** *** ** rigg α-il-1r Days post-infection
128 113 Figure 25. IL-1 does not affect RSV replication and clearance kinetics. Lungs were harvested on day 4 and 7 p.i. from RSV-infected (A) IL-1 KO or (B) anti-il-1r mab treated mice. RSV titers were determined by plaque assay. The limit of detection (L.O.D.) is indicated as a horizontal dotted line. Cumulative data from two independent experiments are shown (n=8).
129 114 A 1 6 WT PFU/g of lung IL-1 KO 1 Day 4 Day 7 B 1 6 rigg PFU/g of lung α-il-1r 1 Day 4 Day 7
130 115 Figure 26. Increase in pro-inflammatory cytokines and chemokines in the absence of IL- 1 during RSV infection. At days, 4, 6, 8, and 1 p.i., lungs from IL-1 KO mice were harvested and cytokine and chemokine protein levels within the lung supernatants were determined by multiplex cytokine array analysis. Pro-inflammatory (A) cytokine and (B) chemokine amounts in the lung. Cumulative data from two independent experiments are shown (n=8). *p<.5, **p<.1, ***p<.1.
131 116 A IL-6 IFN-γ TNF-α pg/lung 6 ** 4 2 P = *** * 6 *** B CXCL1 CXCL9 CCL2 pg/lung * ** P = P = *** WT IL-1 KO Days post-infection
132 117 Figure 27. RSV-induced cytokine and chemokine expression in the absence of IL-1. At days, 4, 6, 8, and 1 p.i., lungs from IL-1 KO mice were harvested and cytokine and chemokine protein levels within the lung supernatants were determined by multiplex cytokine array analysis. Cumulative data from two independent experiments are shown (n=4-8). *p<.5, **p<.1.
133 118 pg/lung IL-1α IL-1β IL-2 WT IL-1KO IL-3 IL-4 1 ** 8 IL-5 pg/lung *** pg/lung IL IL * IL-15 P =.66 * pg/lung MIP-1α ** MIP-1β MIP-2 ** * Eotaxin GM-CSF 2 P = RANTES pg/lung Days post-infection Days post-infection Days post-infection
134 119 Figure 28. The magnitude of the CD8 T cell response is increased following IL-1R blockade. Mice were treated with anti-il-1r mab as described in the materials and methods. (A) The total number of CD8 T cells at day 6 (left) and 8 (right) p.i. in the lung and medln. (B) The total number of CD11a + CD8 T cells cells at day 6 (left) and 8 (right) p.i. in the lung and medln. (C) The total number of M specific CD8 T cells producing only IFN-γ (closed bars) or co-producing IFN-γ and TNF-α (open bars) in the medln at day (left) and 8 (right) p.i. (D) The total number of M specific CD8 T cells producing only IFN-γ (closed bars) or co-producing IFN-γ and TNF-α (open bars) in the lung at day (left) and 8 (right) p.i. Data in C and D are displayed as stacked bar graphs and the significant difference are marked for total responding cells. Cumulative data from two independent experiments are shown (n=8). Unpaired, two-tailed student s t test was used to compare groups. **p<.1, ***p<.1.
135 12 A Day 6 p.i. Day 8 p.i. B # of CD8 + T cells (1 5 ) # of CD11a hi CD8 lo T cells (1 5 ) Lung ** medln *** 6 rigg α-il-1r Lung medln rigg α-il-1r Lung medln Lung medln C D # of CD8 + T cells (1 4 ) # of CD8 + T cells (1 5 ) *** rigg α-il-1r rigg α-il-1r rigg α-il-1r ** rigg α-il-1r IFN-γ + TNF-α - IFN-γ + TNF-α +
136 121 Figure 29. Increased Th1 response following IL-1R blockade. (A) The total number of T cells in the lung, medln, and BAL at day 6 (left) and 8 (right) p.i. (B) The total number of PMA and ionomycin stimulated T cells producing only IFN-γ (closed bars) or co-producing of IFN-γ and TNF-α (open bars) in the lung at day 6 (left) and 8 (right) p.i. Data are displayed as stacked bar graphs and significant difference are marked for total responding cells. Cumulative data from two independent experiments are shown (n=8). Unpaired, two-tailed student s t test was used to compare groups. *p<.5, **p<.1.
137 122 A Day 6 p.i. Day 8 p.i. B # of CD4 + T cells (1 5 ) # of CD4 + T cells (1 4 ) Lung medln BAL rigg α-il-1r ** * Lung medln BAL ** rigg α-il-1r rigg α-il-1r IFN-γ + TNF-α - IFN-γ + TNF-α +
138 123 Figure 3. Increased lung pathology following IL-1R blockade. (A) Cumulative pathological scoring analysis of sections from whole lungs of rigg and anti-il-1r mab treated mice following RSV infection. Representative hematoxylin and eosin (H&E) stained images with Periodic acid Schiff stain (PAS; inset, bar = 11 µm) from (B) rigg and (C) anti-il-1r mab treated lungs, bar = 75 µm. Edema (asterisks), inflammation (arrows) and mucous cell proliferation with lumen obstruction (inset, arrowhead). Cumulative data from two independent experiments are shown (n=8). One sample and two sample t test were used to compare groups. ***p<.1.
139 124 A Pathology Score *** *** *** *** *** rigg α-il-1r B PVA ID Edema Mucus Total C * * * *
140 125 Figure 31. Increased Th17 response and decreased T regulatory cell response following IL-1R blockade. (A) At day 8 p.i., Helios expression by Tregs was quantified in the lung, medln, and BAL of RSV-infected mice administered anti-il-1r mab. (B) At day 8 p.i., Th17 cells were quantified by intracellular staining of IL-17A in the lung and BAL of anti-il-1r mab treated mice infected with RSV. Cumulative data from two independent experiments are shown (n=8). Unpaired, two-tailed student s t test was used to compare groups. *p<.5, **p<.1, ***p<.1.
141 126 A # of CD4 + T cells (1 5 ) ** Lung Foxp3 + Helios + Foxp3 + Helios - # of CD4 + T cells (1 5 ) 5 *** medln Foxp3 + Helios + Foxp3 + Helios - # of CD4+ T cells (1 3 ) BAL 1 * Foxp3 + Helios + Foxp3 + Helios - rigg α-il-1r B 25 ** # of IL-17A + CD4 + T cells (1 3 ) * Lung BAL
142 127 CHAPTER V. GENERAL DISSCUSSION RSV strains induce differential immune responses RSV strains are categorized into two antigenic subgroups designated A and B based on the protein variability of the G protein (27). There are conflicting reports concluding that either subgroup A or B strains are more pathogenic, or that both A and B strains have equal pathogenicity (54-59). However, using the murine model of RSV pathogenesis, it is evident that strains induce differential disease severities (92). Therefore I hypothesize that RSV strain variation contributes to human pathogenesis through the induction of differential host immune responses. In Chapter III I demonstrate RSV strains can induce differential activation of the innate immune response and subsequent CD4 T cell differentiation (Figure 32). Induction of an increased Th17 response following RSV line 19 infection was mediated by the early production of IL-1β and IL-6 (Figure 18). Pro-IL-1β and IL-6 transcription are induced following the activation of TLRs and NF-κB (79). Given that RSV activates TLRs 2, 3, 4 and 7 and IL-6 levels are increased in the lung following RSV line 19 infection, I hypothesize that RSV strains induce differential activation of TLRs. The RSV F protein is known to stimulate TLR4 (71). However, the F protein amino acid sequence is relatively conserved in the genome due to its critical function during fusion with the host cellular membrane (53). Thus I hypothesize another protein on the virion surface, such as the G protein, mediates differential TLR2 activation. To hypothesize that the increased IL-6 following RSV line 19 infection is mediated by increased activation of either TLR3 or 7 would imply there are differences in total virus replication in the lung. However, I do not observe increased virus replication in the lung following RSV line 19 infection as compared to RSV strains A2 and 2-2 (Figure 13).
143 128 The maturation of IL-1β for cellular release is mediated by the NLRP3 inflammasome following RSV A2 infection (83, 84). Additionally, the RSV SH proteindependent disruption of the ionic balance in the host cell is required for inflammasome activation (84). SH expression co-localizes NLRP3 to the Golgi apparatus membrane and mediates the transport of K + and Na + monovalent cations (84). Therefore, I also hypothesize that RSV strains can differentially activate the inflammasome through the function/expression of the SH protein. If a RSV strain expressed a SH protein that was more stable in the host cellular membrane, then the ionic balance of the host cell could be further disrupted, inducing increased inflammasome activation. Thus, if a RSV strain induced increased TLR and inflammasome activation leading to increased levels of IL-6 and mature IL-1β, respectively, than the microenvironment would be more conducive to priming a Th17 response. Given that IL-1β and IL-6, as well as the Th17 response, are associated with severe RSV-induced disease in infants, I believe further investigation of RSV strain variation in inducing disease severity without relying solely on antigenic subgroups is warranted. Predisposing the RSV-specific T helper response A Th2-biased as well as Th17 response is associated with severe RSV-induced disease in infants. However, neonates are impaired in their ability to induce a Th1 response (233). This is primarily because neonatal DCs are inhibited in their transcription of the p35 subunit of IL-12, which is essential for Th1 differentiation (234, 235). However this can be overcome by the presence of IFN-γ, which restores p35 transcription in neonatal DCs to a similar level observed in adult DCs (235). Therefore, the neonatal immune system is more prone to differentiate CD4 T cells into a Th2 phenotype over a Th1 phenotype. However IL-1β, IL-6 and IL-17A in the NP washes are associated with severe RSV-induced disease in infants (55, 97). I demonstrated in Chapter III that RSV strains induce differential Th17 responses due to the production of
144 129 IL-1β and IL-6 by the innate immune response. Thus, mechanisms to promote a protective Th1 response while avoiding induction of Th2 and Th17 responses would be beneficial for children with severe RSV-induced disease. One mechanism to promote a Th1 response is to target IL-12. In Chapter II, I demonstrated that the approximately 85% of the RSV-specific CD4 T cells are located in the lung tissue at the peak of the T cell response (Figure 4). Thus, either IL-12 could be exogenously added or cells could be induced to produce IL-12 in the lung tissue to polarize CD4 T cells to a Th1 phenotype. In the murine model of RSV-induced vaccine enhanced disease, treatment with exogenous IL-12 increased the number of Th1 cells while decreasing the number of Th2 cells in the lung (236, 237). Moreover, given the role of IL-12 in inducing Th1 responses and IFN-γ production by CD8 T cells and NK cells, it has previously been targeted as a cancer immunotherapy in humans (238). Although due to its toxicity when delivered systemically and minimal efficacy in most clinical trials, IL-12 is not a current FDA-approved treatment used in humans. However, its role in combinatorial therapies and for specific diseases is still under investigation. Alternatively, the addition of exogenous IFN-γ to the respiratory tract by nebulization could promote the in vivo DC production of IL-12, as suggested by the in vitro observations with neonatal DCs (235). Targeting IFN-γ for immunotherapies via recombinant IFN-γ1b (Actimmune) has been utilized longer in humans with more success than IL-12 (239). However these approaches are still likely to be too high of a risk to pursue for infants with severe RSV-induced disease. A more feasible way to bias RSV-specific CD4 T cells towards a Th1 phenotype may be through vaccination. The Bacillus Calmette-Guérin (BCG) and whole cell pertussis vaccines are the only vaccines reported to induce a Th1 response in infants (24-243). Furthermore, recombinant BCG strains expressing either the RSV N or M2 protein provide mice with protection and ameliorate disease against a subsequent RSV infection (244). Inducing a balanced memory CD4 and CD8 T cell response with
145 13 vaccination would be crucial to avoid vaccine-enhanced disease following a natural infection, since data in the murine model indicates that priming a strong memory CD4 T cell response in the absence of a CD8 T cell response promotes vaccine enhanced disease (197, 245, 246). Both recombinant BCG strains expressing a RSV protein induce protective Th1 and CD8 T cell memory responses (244). Given the known efficacy and safety of BCG vaccines in infants, the use of recombinant BCG strains would be a good way to potentially induce RSV-specific Th1 cells in infants. However I think it would be of interest to assess the humoral immune response following recombinant BCG vaccination. If anti-rsv antibodies were present after vaccination, I believe using a recombinant BCG strain expressing the F protein may provide better protection for infants, given the success of palivizumab as a RSV prophylactic treatment. However, the location of the memory CD4 T cells is important for subsequent protection following infection. Teijaro and colleagues have demonstrated that IAV-specific lung resident memory CD4 T cells are crucial for optimal protection following a subsequent IAV infection as compared to systemic memory CD4 T cells (143). Furthermore, as I demonstrated in Chapter II, RSV-specific CD4 T cells remain in the lung tissue at least 3 days p.i. and may be necessary for providing the host with protection from a subsequent RSV infection while avoiding the Th2- and Th17-associated pathology with severe RSV-induced disease. Although the BCG vaccine is a mucosal vaccine administered intradermally, its ability to prime cells comparable to lung resident CD4 T cells following a natural RSV infection would need to be investigated. However, live enterically coated adenovirus vaccines administered orally, reduced adenovirus-induced respiratory disease 95-99% in military training camps ( ). These results indicate that priming T cells at another mucosal site can provide superior protection from virusinduced respiratory disease. Alternative to promoting a Th1 response, the pro-inflammatory response as well as the Th17 response can be targeted for inhibition in infants with severe RSV-induced
146 131 disease. Blocking and/or neutralizing IL-1β and/or IL-6, respectively, ameliorates the increased Th17 response induced following RSV line 19 infection in the lungs of mice (Figure 18). Given these data and the increase of IL-1β and IL-6 in infants with severe RSV-induced disease as compared to mild disease, targeting these cytokines as an immunotherapy for severe disease should be investigated further (55). Anakinra is an IL- 1 receptor antagonist and tocilizumab is a humanized anti-il-6 receptor monoclonal antibody that are both FDA-approved immunotherapies for inflammatory diseases such as rheumatoid arthritis. However, the systemic long-term use of these immunotherapies can have adverse effects (25, 251). Thus, I propose investigating the transient, localized use of these immunotherapies at a low dose in cases of severe RSV-induced disease. This treatment could be nebulized and inhaled by individuals with an extremely high RSV-induced pro-inflammatory cytokine and chemokine response. Additionally, monoclonal antibodies directly targeting either IL-17 or its receptor have been developed and their use in treating psoriasis has shown promising results in clinical trials (252, 253). Inhibition of the Th17 response by similar mechanisms in infants with severe RSVinduced disease could alternatively be used as an immunotherapy, which may have less adverse effects than the use of either anakinra or tocilizumab. Mechanisms to regulate RSV-specific immunity Tregs are critical in limiting the immunopathology following RSV infection as discussed in Chapter I ( ). Furthermore, I demonstrated in Chapter II that Tregs with increased expression of activation markers migrate into the lung tissue. Thus, mechanisms to promote the suppression of the RSV-specific T cell response by Tregs may have the potential to limit RSV pathogenesis. RA induces the induction of Tregs (254). Theoretically exogenous RA treatment could induce the differentiation of itregs. However, it is likely that additional signals are required from the respiratory DC for itreg differentiation, which may be absent. Additionally, IL-2 and anti-il-2 JES6-1 antibody
147 132 complexes can induce the transient expansion of Tregs (255). Tregs that proliferate in response to the IL-2/anti-IL-2 complexes exhibited increased expression of activationassociated proteins, such as ICOS and CTLA-4. Importantly this treatment preferentially induces Treg proliferation over non-regulatory T cells. Given my data demonstrating that Tregs in the lung tissue exhibit increased expression of ICOS and CTLA-4 as compared to Tregs in the periphery (Figure 8), I hypothesize that localized respiratory IL-2/anti-IL- 2 complex treatment would induce the proliferation of respiratory Tregs with a similar phenotype as compared to RSV-induced Tregs. If IL-2/anti-IL-2 complex treatment for children with severe RSV-induced disease selectively increases the Tregs in the lung, then I would expect RSV pathogenesis to be decreased. However, the IL-2/anti-IL-2 complex treatment induces systemic proliferation of Tregs when administered i.p. in an animal disease model (255). Moreover, IL-2/anti-IL-2 complex treatment has been shown to reduce asthma and allergic inflammation in a manner dependent on the expansion of IL-1-producing Tregs (256). Thus, alternative mechanisms to therapeutically induce IL-1 production may decrease the severity of RSV-induced disease. Production of IL-1 IL-1 has been variably detected in the NP washes of infants with severe RSVinduced disease (55, 9, ). Thus, there is currently no clear correlation between IL-1 protein levels and RSV-induced disease severity in humans. Therefore, studies with larger numbers of infants with correlation to the adaptive immune response may further elucidate the role of IL-1 in RSV pathogenesis. However, there are multiple single nucleotide polymorphisms (SNPs) in the human IL-1 gene that have been correlated to RSV-induced disease in infants (225, 226). Hoebee et al. demonstrated an association between the IL1-592C SNP and RSV-induced bronchiolitis in infants less than six months of age (226). In contrast, Wilson et al. did not report finding the same
148 133 association between the -592C (referred to as -627C in their manuscript) and RSVinduced bronchiolitis (225). Instead, they determined there are significant independent associations between both the IL1-1117G and -3585A SNPs, and the need for mechanical ventilation in infants less than 12 months of age hospitalized for RSV infection (225). However, a relatively small number of RSV-infected infants were analyzed in both of these studies, highlighting the need for an analysis of a larger population size to clearly assess the relationship between IL1 SNPs and RSV pathogenesis. Moreover, a relationship between IL1 SNPs and RSV-induced disease may in part contribute to race being an identified risk factor for severe disease in humans, since certain IL1 SNPs are associated with specific races (26). In recent years, the role of IL-1 in the murine model of RSV pathogenesis has been under investigation. In agreement with the data in Chapter IV, additional investigators have demonstrated that IL-1 is produced and regulates the adaptive immune response by limiting immunopathology following RSV infection (Figure 32) (261, 262). Utilization of the IL-1-eGFP reporter mouse strain demonstrates that T cells are the majority of IL-1-producing cells in vivo following RSV infection (Figure 2) (261). Similarly, RSV infection of Rag2-deficient mice decreases the level of IL-1 protein in the lungs (261). I observed CD4 T cells to be the T cell population responsible for producing IL-1 ex vivo and following in vitro stimulation (Figures 19-2). Furthermore, using antibody-mediated depletion, I observed that CD4 T cells account for the majority of IL-1 protein produced in the lung following RSV infection (Figure 21). However, others have reported that CD8 T cells as well as CD4 T cells produce IL-1 following RSV infection (261, 262). This discrepancy could be accounted for by the amount of either viral antigen or cytokines present in the lung. High levels of antigen in the presence of IL-12 as well as the presence of either IL-21 or IL-27 induces T cells to produce IL-1 (115, 214, ).
149 134 There is a clear role for IL-1 in suppressing RSV-induced immunopathology (147, 261, 262). Similar to my findings reported in Chapter IV, both additional reports demonstrate that RSV-induced disease severity is increased either in the absence of IL-1 or following IL-1R blockade (261, 262). Increased weight loss with decreased lung function is observed following RSV infection in both IL-1-deficient and anti-il-1r antibody treated mice as compared to controls (Figure 24). Furthermore, increased RSVinduced pathogenesis is associated with increased levels of pro-inflammatory cytokines and chemokines in the lungs (Figure 26) (261, 262). Interestingly, the increase in proinflammatory cytokines and chemokines observed following Treg depletion are very similar to the observations in IL-1-deficient mice following RSV infection with significantly increased IL-6, IFN-γ, TNF-α, MIP-1α and MCP-1 as compared to controls (Figure 26) (112, 113, 262). These data suggest that Tregs may either produce IL-1 or induce its production, and that IL-1 is critical for limiting immunopathology following RSV infection. Furthermore, the belief that human RSV pathogenesis is the result of immunopathology is supported by the heightened inflammatory response in the lungs of IL-1-deficient mice as compared to controls. The cytokines and chemokines in the NP washes of infants with severe RSV-induced disease are upregulated similar to IL-1- deficient mice as compared to infants with mild disease and wild-type mice, respectively (55). In conjunction with the increased pro-inflammatory environment, there is an increase in lung cellular infiltration comprised of primarily neutrophils as well as monocytes and lymphocytes either in the absence of IL-1 or following IL-1R blockade (261, 262). Given neutrophils as well as monocytes are the primary cell populations in the airways of infants with severe RSV-induced disease, the increase in these cell populations either in the absence of IL-1 or following IL-1R blockade further support the belief that human RSV-pathogenesis is immune-mediated ( ). In the absence of IL-1 or following IL-1R blockade, CD4 and CD8 T cells also have increased effector functions (Figures 28, 29 and 31) (261, 262). Moreover, T cell
150 135 production of IL-1 provides a mechanism for their self-regulation following RSV infection. Transgenic mice engineered to specifically lack the expression of the IL-1R on the surface of T cells, exhibit similar pathology following RSV infection as compared to both IL-1-deficient and anti-il-1r antibody treated mice (261). Together these data highlight the critical role T cells play in the production of IL-1 leading to their autocrine/paracrine regulation and amelioration of RSV-induced pathogenesis. Exogenous IL-1 administration was demonstrated to be safe and beneficial for several inflammatory diseases in clinical trials, including Crohn s disease, psoriasis and chronic hepatitis C ( ). Subcutaneous treatment with recombinant IL-1 reduced clinical disease in patients with psoriasis (227, 27). Psoriasis is an inflammatory disease mediated by type I cytokines as well as IL-1, IL-6, IL-8, IL-17 and TNF-α (227, 271). Importantly, IL-1 treatment did not affect hepatitis C virus titers, but reduced the chronic liver inflammation and fibrosis observed in patients (23). Given the success of IL-1 treatment with pro-inflammatory diseases in clinical trials, it would be worthwhile to investigate its efficacy in infants with severe RSV-induced disease who exhibit similar cytokine and chemokine profiles. Furthermore, Haeberle et al. demonstrated a decrease in chemokines and disease severity in RSV-infected mice administered recombinant IL- 1 i.n. with no alterations in virus clearance (215). However, IL-1 was administered at the time of RSV inoculation and the effect of recombinant IL-1 administration following infection still needs to be determined, which would be more relevant for the treatment of clinical disease. CD4 T cells mediating long-term airway morbidities Severe RSV-induced disease is associated with the development of long-term airway morbidities, such as asthma and chronic wheezing ( ). This increased risk of developing these long-term airway morbidities is thought to be the combination of predisposing genetic factors and experiencing severe disease following RSV infection. A
151 136 clinical study followed the development of chronic wheezing in pre-term infants who either were or were not treated prophylactically with palivizumab, the humanized anti-f monoclonal antibody. At 3.5 years of age, children that had not received the RSV prophylactic treatment as an infant were twice as likely to have long-term airway morbidities as compared to children that had received the prophylactic treatment (18). These findings demonstrate the positive relationship between severe RSV-induced disease and development of long-term airway morbidities. There is an emerging role for Th17 cells in asthma and wheezing. It is evident that there are different types of asthma including Th2-associated atopic asthma and Th17- associated neutrophilic asthma (272). IL-17A levels are increased in the serum and NP washes of asthmatic patients (178, 179). Furthermore, severe RSV-induced disease is associated with increased IL-17A and neutrophils in the lungs of infected infants (97, ). Severe RSV-induced disease is also associated with increased levels of IL-1β, IL-6 and TNF-α as compared to infants with mild disease (55). Moreover, the proinflammatory cytokines IL-1β, IL-6 and IL-17A directly induce primary human bronchiole epithelial cells to express mucins, whereas Th2-associated cytokines do not (175, 176). Moreover, IL-17A produced by Th17 cells mediates airway hyperreactivity by directly inducing airway smooth muscle contraction (177). IL-1β, IL-6 and IL-17A also mediate the chemotaxis of neutrophils as well as collagen deposition and fibrosis in the lungs, which is dependent on inflammasome activation ( ). In fatal cases of RSV infection, fibrin deposition is observed in the lungs of infants by histology (3). As demonstrated in Chapter III, the increased levels of IL-1β and IL-6 induced increased Th17 cell differentiation following RSV line 19 infection. Thus, I hypothesize that the increased risk of developing long-term airway morbidities, such as asthma and wheezing, following severe RSV-induced disease is dependent on the infecting virus strain inducing an increased pro-inflammatory cytokine and Th17 response (Figure 33).
152 137 Figure 32. RSV strains induced differential CD4 T cell responses regulated by IL-1. Infection with various strains of RSV (top) resulted in differential innate immune and pro-inflammatory cytokine responses influencing the induction of the Th17 response. The magnitude of the pro-inflammatory cytokines IL-1β and IL-6 positively associated with the magnitude of the Th17 response. The (bottom) T cells are then regulated by IL- 1 produced by Tregs, Tr1 cells and Th1 cells.
153 138 INFLAMMATION AM IL-1β IL-6 Th17 cells IL-17 Airway lumen TLR Virus replication Lamina propria RESOLUTION Tr1 IL-1 T cells Th1 Treg INFLAMMATION mucus Virus clearance
154 139 Figure 33. Pro-inflammatory cytokines and Th17 cells may mediate RSV pathogenesis observed in infants with severe disease. In the proposed model, RSV strains induce differential activation of TLRs on the extracellular surface of AMs, thus leading to the production of pro-il-1β and IL-6. RSV replication induces differential activation of the NLRP3 inflammasome through varied expression of the SH protein. With inflammasome activation, pro-caspase 1 becomes cleaved. Activated caspase 1 in turn cleaves pro-il-1β into mature IL-1β for cellular secretion. IL-1β and IL-6 subsequently induce the differentiation of CD4 T cells into the Th17 phenotype. IL-1β and IL-6 in conjunction with IL-17A produced by Th17 cells directly induces increased mucus production and collagen deposition, and mediates Th17-associated neutrophilic asthma.
155 14 Differential TLR activation IL-6 IL-1β Th17 SH Virus replication Inflammasome activation IL-1β IL-17A IL-1β/IL-6/IL-17A AM Airway lumen pro-il-1β Th17-associated neutrophilic Asthma Epithelial cells Increased mucus production and collagen deposition
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
The impact of robust memory T cell responses against respiratory syncytial virus
 University of Iowa Iowa Research Online Theses and Dissertations Spring 2015 The impact of robust memory T cell responses against respiratory syncytial virus Cory James Knudson University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations Spring 2015 The impact of robust memory T cell responses against respiratory syncytial virus Cory James Knudson University of Iowa Copyright
Newly Recognized Components of the Innate Immune System
 Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD.
 Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 13: Mechanisms of Immunity to Viral Disease Prepared by
Allergy and Immunology Review Corner: Chapter 13 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 13: Mechanisms of Immunity to Viral Disease Prepared by
Immunology for the Rheumatologist
 Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
DNA vaccine, peripheral T-cell tolerance modulation 185
 Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Effector T Cells and
 1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
The Adaptive Immune Response: T lymphocytes and Their Functional Types *
 OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Central tolerance. Mechanisms of Immune Tolerance. Regulation of the T cell response
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. Mechanisms of Immune Tolerance ACTIVATION (immunity) SUPPRESSION (tolerance)
Mechanisms of Immune Tolerance
 Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
Immunoregulation: A balance between activation and suppression that achieves an efficient immune response without damaging the host. ACTIVATION (immunity) SUPPRESSION (tolerance) Autoimmunity Immunodeficiency
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
The Immune System. These are classified as the Innate and Adaptive Immune Responses. Innate Immunity
 The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
Putting it Together. Stephen Canfield Secondary Lymphoid System. Tonsil Anterior Cervical LN s
 Putting it Together Stephen Canfield smc12@columbia.edu Secondary Lymphoid System Tonsil Anterior Cervical LN s Axillary LN s Mediastinal/Retroperitoneal LN s Thoracic Duct Appendix Spleen Inguinal LN
Putting it Together Stephen Canfield smc12@columbia.edu Secondary Lymphoid System Tonsil Anterior Cervical LN s Axillary LN s Mediastinal/Retroperitoneal LN s Thoracic Duct Appendix Spleen Inguinal LN
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine
 Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Chapter 13: Cytokines
 Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
Chapter 13: Cytokines Definition: secreted, low-molecular-weight proteins that regulate the nature, intensity and duration of the immune response by exerting a variety of effects on lymphocytes and/or
CELL BIOLOGY - CLUTCH CH THE IMMUNE SYSTEM.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
!! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
There are 2 major lines of defense: Non-specific (Innate Immunity) and. Specific. (Adaptive Immunity) Photo of macrophage cell
 There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Animal Models to Understand Immunity
 Animal Models to Understand Immunity Hussein El Saghire hesaghir@sckcen.be Innate Adaptive immunity Immunity MAPK and NF-kB TLR pathways receptors Fast Slow Non-specific Specific NOD-like receptors T-cell
Animal Models to Understand Immunity Hussein El Saghire hesaghir@sckcen.be Innate Adaptive immunity Immunity MAPK and NF-kB TLR pathways receptors Fast Slow Non-specific Specific NOD-like receptors T-cell
T cell regulation of acute and chronic viral infection
 University of Iowa Iowa Research Online Theses and Dissertations Spring 2016 T cell regulation of acute and chronic viral infection Allison Fae Christiaansen University of Iowa Copyright 2016 Allison Fae
University of Iowa Iowa Research Online Theses and Dissertations Spring 2016 T cell regulation of acute and chronic viral infection Allison Fae Christiaansen University of Iowa Copyright 2016 Allison Fae
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Time course of immune response
 Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D.
 Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Immune Regulation and Tolerance
 Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Immune Regulation and Tolerance Immunoregulation: A balance between activation and suppression of effector cells to achieve an efficient immune response without damaging the host. Activation (immunity)
Allergy and Immunology Review Corner: Chapter 19 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD.
 Allergy and Immunology Review Corner: Chapter 19 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 19: Tolerance, Autoimmunity, and Autoinflammation Prepared
Allergy and Immunology Review Corner: Chapter 19 of Immunology IV: Clinical Applications in Health and Disease, by Joseph A. Bellanti, MD. Chapter 19: Tolerance, Autoimmunity, and Autoinflammation Prepared
Darwinian selection and Newtonian physics wrapped up in systems biology
 Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Adaptive Immunity: Humoral Immune Responses
 MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
Basis of Immunology and
 Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
Immune response to infection
 Immune response to infection Dr. Sandra Nitsche (Sandra.Nitsche@rub.de ) 20.06.2018 1 Course of acute infection Typical acute infection that is cleared by an adaptive immune reaction 1. invasion of pathogen
Immune response to infection Dr. Sandra Nitsche (Sandra.Nitsche@rub.de ) 20.06.2018 1 Course of acute infection Typical acute infection that is cleared by an adaptive immune reaction 1. invasion of pathogen
Regulation of virus-specific T cells in the lung during respiratory virus infections
 University of Iowa Iowa Research Online Theses and Dissertations Fall 21 Regulation of virus-specific T cells in the lung during respiratory virus infections Ross Bane Fulton University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations Fall 21 Regulation of virus-specific T cells in the lung during respiratory virus infections Ross Bane Fulton University of Iowa Copyright
TCR, MHC and coreceptors
 Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Autoimmunity. Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens
 Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Innate Immunity. Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016
 Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016 Objectives: Explain how innate immune system recognizes foreign substances
Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016 Objectives: Explain how innate immune system recognizes foreign substances
Chapter 1. Chapter 1 Concepts. MCMP422 Immunology and Biologics Immunology is important personally and professionally!
 MCMP422 Immunology and Biologics Immunology is important personally and professionally! Learn the language - use the glossary and index RNR - Reading, Note taking, Reviewing All materials in Chapters 1-3
MCMP422 Immunology and Biologics Immunology is important personally and professionally! Learn the language - use the glossary and index RNR - Reading, Note taking, Reviewing All materials in Chapters 1-3
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Chapter 23 Immunity Exam Study Questions
 Chapter 23 Immunity Exam Study Questions 1. Define 1) Immunity 2) Neutrophils 3) Macrophage 4) Epitopes 5) Interferon 6) Complement system 7) Histamine 8) Mast cells 9) Antigen 10) Antigens receptors 11)
Chapter 23 Immunity Exam Study Questions 1. Define 1) Immunity 2) Neutrophils 3) Macrophage 4) Epitopes 5) Interferon 6) Complement system 7) Histamine 8) Mast cells 9) Antigen 10) Antigens receptors 11)
Chapter 10 (pages ): Differentiation and Functions of CD4+ Effector T Cells Prepared by Kristen Dazy, MD, Scripps Clinic Medical Group
 FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
T Lymphocyte Activation and Costimulation. FOCiS. Lecture outline
 1 T Lymphocyte Activation and Costimulation Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline T cell activation Costimulation, the B7:CD28 family Inhibitory receptors of T cells Targeting costimulators for
1 T Lymphocyte Activation and Costimulation Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline T cell activation Costimulation, the B7:CD28 family Inhibitory receptors of T cells Targeting costimulators for
IMMUNOTHERAPY FOR CANCER A NEW HORIZON. Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust
 IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
Immunological Tolerance
 Immunological Tolerance Introduction Definition: Unresponsiveness to an antigen that is induced by exposure to that antigen Tolerogen = tolerogenic antigen = antigen that induces tolerance Important for
Immunological Tolerance Introduction Definition: Unresponsiveness to an antigen that is induced by exposure to that antigen Tolerogen = tolerogenic antigen = antigen that induces tolerance Important for
T Cell Activation, Costimulation and Regulation
 1 T Cell Activation, Costimulation and Regulation Abul K. Abbas, MD University of California San Francisco 2 Lecture outline T cell antigen recognition and activation Costimulation, the B7:CD28 family
1 T Cell Activation, Costimulation and Regulation Abul K. Abbas, MD University of California San Francisco 2 Lecture outline T cell antigen recognition and activation Costimulation, the B7:CD28 family
T Cell Effector Mechanisms I: B cell Help & DTH
 T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
The Role of CC Chemokine Receptors 6 and 7 in the Immune Response. to Respiratory Syncytial Virus. Lara Elizabeth Kallal
 The Role of CC Chemokine Receptors 6 and 7 in the Immune Response to Respiratory Syncytial Virus by Lara Elizabeth Kallal A dissertation submitted in partial fulfillment of the requirements for the degree
The Role of CC Chemokine Receptors 6 and 7 in the Immune Response to Respiratory Syncytial Virus by Lara Elizabeth Kallal A dissertation submitted in partial fulfillment of the requirements for the degree
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1. The scavenger receptor, CD36, functions as a coreceptor for which TLR? a. TLR ½ b. TLR 3 c. TLR 4 d. TLR 2/6
 Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells
 Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells Andrew H. Lichtman, M.D. Ph.D. Department of Pathology Brigham and Women s Hospital and Harvard
Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells Andrew H. Lichtman, M.D. Ph.D. Department of Pathology Brigham and Women s Hospital and Harvard
Lecture on Innate Immunity and Inflammation
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
AGAINST VIRAL INFECTIONS. Identify the types of immunity involve in the mechanisms of protection against viral infections.
 LECTURE: 02 Title: THE IMMUNOLOGICAL PROTECTIVE MECHANISMS AGAINST VIRAL INFECTIONS LEARNING OBJECTIVES: The student should be able to: Identify the types of immunity involve in the mechanisms of protection
LECTURE: 02 Title: THE IMMUNOLOGICAL PROTECTIVE MECHANISMS AGAINST VIRAL INFECTIONS LEARNING OBJECTIVES: The student should be able to: Identify the types of immunity involve in the mechanisms of protection
chapter 17: specific/adaptable defenses of the host: the immune response
 chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
Chapter 24 The Immune System
 Chapter 24 The Immune System The Immune System Layered defense system The skin and chemical barriers The innate and adaptive immune systems Immunity The body s ability to recognize and destroy specific
Chapter 24 The Immune System The Immune System Layered defense system The skin and chemical barriers The innate and adaptive immune systems Immunity The body s ability to recognize and destroy specific
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
T cell-mediated immunity
 T cell-mediated immunity Overview For microbes within phagosomes in phagocytes.cd4+ T lymphocytes (TH1) Activate phagocyte by cytokines studies on Listeria monocytogenes For microbes infecting and replicating
T cell-mediated immunity Overview For microbes within phagosomes in phagocytes.cd4+ T lymphocytes (TH1) Activate phagocyte by cytokines studies on Listeria monocytogenes For microbes infecting and replicating
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals. Taniawati Supali. Department of Parasitology
 Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
I. Critical Vocabulary
 I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
Immunology. T-Lymphocytes. 16. Oktober 2014, Ruhr-Universität Bochum Karin Peters,
 Immunology T-Lymphocytes 16. Oktober 2014, Ruhr-Universität Bochum Karin Peters, karin.peters@rub.de The role of T-effector cells in the immune response against microbes cellular immunity humoral immunity
Immunology T-Lymphocytes 16. Oktober 2014, Ruhr-Universität Bochum Karin Peters, karin.peters@rub.de The role of T-effector cells in the immune response against microbes cellular immunity humoral immunity
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Allergic rhinitis (Hay fever) Asthma Anaphylaxis Urticaria Atopic dermatitis
 Hypersensitivity Disorders Hypersensitivity Disorders Immune Response IgE Disease Example Ragweed hay fever IgG Cytotoxic Immune complex T Cell Hemolytic anemia Serum sickness Poison ivy IgE-mediated Diseases
Hypersensitivity Disorders Hypersensitivity Disorders Immune Response IgE Disease Example Ragweed hay fever IgG Cytotoxic Immune complex T Cell Hemolytic anemia Serum sickness Poison ivy IgE-mediated Diseases
Fluid movement in capillaries. Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system
 Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Effector Mechanisms of Cell-Mediated Immunity
 Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases. Abul K. Abbas UCSF
 Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc.
 NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc. Cytokines Properties of Cytokines Cytokines are proteins with specific roles
NTD Vaccine Design Toolkit and Training Workshop Providence, RI January 05, 2011 Cytokines Leslie P. Cousens, PhD EpiVax, Inc. Cytokines Properties of Cytokines Cytokines are proteins with specific roles
Genetics. Environment. You Are Only 10% Human. Pathogenesis of IBD. Advances in the Pathogenesis of IBD: Genetics Leads to Function IBD
 Advances in the Pathogenesis of IBD: Genetics Leads to Function Pathogenesis of IBD Environmental Factors Microbes Scott Plevy, MD Associate Professor of Medicine, Microbiology & Immunology UNC School
Advances in the Pathogenesis of IBD: Genetics Leads to Function Pathogenesis of IBD Environmental Factors Microbes Scott Plevy, MD Associate Professor of Medicine, Microbiology & Immunology UNC School
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
Innate Immunity. Jan 8 th Prof. dr. sc. Ivana Novak Nakir 1
 Innate Immunity Jan 8 th 2018. Prof. dr. sc. Ivana Novak Nakir 1 Adaptive Innate 2 Immune system overview 1 st line of defense skin (2m 2 ) and mucosal membranes (~400m 2 ): physical barrier, lymphoid
Innate Immunity Jan 8 th 2018. Prof. dr. sc. Ivana Novak Nakir 1 Adaptive Innate 2 Immune system overview 1 st line of defense skin (2m 2 ) and mucosal membranes (~400m 2 ): physical barrier, lymphoid
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE
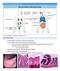 LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
LECTURE 12: MUCOSAL IMMUNITY GUT STRUCTURE - Small intestine in humans is around 3-4 metres long - Internal surface of the small intestines are lined by villi o Villi are composed of absorptive cells (epithelial/enterocytes)
11/25/2017. THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS BARRIER DEFENSES INNATE IMMUNITY OF VERTEBRATES
 THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
Cutaneous Immunology: Innate Immune Responses. Skin Biology Lecture Series
 Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
How the Innate Immune System Profiles Pathogens
 How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel:
 Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
Examples of questions for Cellular Immunology/Cellular Biology and Immunology
 Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Innate Immunity. Bởi: OpenStaxCollege
 Innate Immunity Bởi: OpenStaxCollege The vertebrate, including human, immune system is a complex multilayered system for defending against external and internal threats to the integrity of the body. The
Innate Immunity Bởi: OpenStaxCollege The vertebrate, including human, immune system is a complex multilayered system for defending against external and internal threats to the integrity of the body. The
FOCiS. Lecture outline. The immunological equilibrium: balancing lymphocyte activation and control. Immunological tolerance and immune regulation -- 1
 1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
Lecture on Innate Immunity and Inflammation. Innate Immunity: An Evolutionary View
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
