Monday - Friday Monday - Friday Referred out weekdays to the Public Health Laboratory
|
|
|
- Naomi Thornton
- 5 years ago
- Views:
Transcription
1 H. pylori (see Helicobacter Serology) H. Pylori Breath Test test only available to Grey Bruce, Owen Sound and St. Mary's, Kitchener C14 Breath test PY Test C-Urea Breath Test Toxicology/Speci al Chemistry PY Test Kit PY Test Kit Monday - Friday See report H63D (see Hemochromatosis HFE gene) Haldol (see Haloperidol) Haloperidol Haldol Toxicology/Speci al Chemistry 6 ml Red top or 4.5 ml Green (Lithium Heparin) top Monday - Friday nmol/l Hantavirus Microbiology (VH) 5 ml Gold or 6 ml Red top PUBLIC HEALTH TEST Referred out weekdays to the Public Health Laboratory See report
2 Haptoglobin, Plasma HPT Core Adult: 4.5 ml Green (Lithium Heparin) top Vacutainer or 5 ml Gold top Pediatric: 0-2 years: 0.5 ml Light Green or Gold top (Li- Heparin) Microtainer 2-10 years: 3 ml Light Green or Gold top (Li- Heparin) Daily 0-<15 days: g/l 15 days-<1 year: g/l 1-<12 years: g/l 12-<19 years: g/l >19 years: g/l Turbidimetric assays not suitable for measurement of highly lipemic or hemolytic samples or samples containing high levels of circulating immune complexes. May be decreased in intravascular hemolysis and in hepatocellular damage, haptoglobin is an acute phase protein. HbA1c (see Glycated Hemoglobin) Hbe (see Hepatitis Be Antigen) HBe Ag (see Hepatitis Be Antigen) HBsAg (see Hepatitis B Surface Antigen) HBV Viral Load (see Hepatitis B DNA Viral Load)
3 hcg (see Chorionic Gonadotropin (Quantitative), Plasma/Serum, Chorionic Gonadotropin, Fluid) HCR (see Hemochromatosis HFE gene) HCV (see Hepatitis C Antibody) HCV Viral Load - pre-treatment (see Hepatitis C RNA - Quantitative) Heart Biopsy Endomyocardial biopsy Pathology - UH Heart muscle for diagnosis or post-transplant. Fresh or in saline. PowerChart: E- order choosing appropriate specimen. See Identification of Clinical Specimens Weekdays Testing not available after hours or weekends Helicobacter Serology H. pylori Microbiology (VH) 5 ml Gold or 6 ml Red top tube PUBLIC HEALTH TEST Referred out weekdays to Public Health Laboratory Hematocrit (HCT) (see Complete Blood Count)
4 Hemochromatosis HFE gene HFE C282Y H63D HCR Molecular Diagnostics Whole blood-1 x 4 ml Lavender EDTA top Vacutainer tube Tissue: 100mg or cm3 frozen immediately after collection Formalin-fixed imbedded tissue MOLECULAR DIAGNOSTIC As required Monday - Friday h See report For more information click on: Molecular Diagnostic Laboratory N/A Hemoglobin (Hb) (see Complete Blood Count) Hemoglobin A1c (see Glycated Hemoglobin) Hemoglobin A 2 (see Hemoglobinopathy Screen) Hemoglobin Electrophoresis (see Hemoglobinopathy Screen) Hemoglobin F (Fetal) (see Hemoglobinopathy Screen) Hemoglobin H (see Hemoglobinopathy Screen)
5 Hemoglobinopathy Screen Hemoglobin F (Fetal) Hemoglobin A 2 Hemoglobin Electrophoresis Hemoglobin H Ferritin Level Clinical Immunology Peripheral Blood 4 ml K 2 or K 3 EDTA Lavender top Vacutainer tube 5 ml Red top Pediatric: 0-2 yrs: Lavender 1.0 pk., Red 0.5 pk 2-10 yrs: 2 ml Lavender top, 2 ml Red Weekly Hb A: Newborn: < month: months: months: Adults: Hb F: Newborn: > month: months: months: Adults: Hb A 2 : Hb H: (more...)
6 Hemosiderin,Urine Investigational Hematology (VH) Random urine Monday-Friday Negative (reported as Positive or Negative) Hepadsorb,Plasma Core 2.7 ml Blue (3.2% Sodium Citrate) Pediatric: 0-10 yrs: 1.8 ml Blue (3.2% Sodium Citrate) As required See report Hepadsorbed APTT's will not be performed on any patients receiving heparin therapy. Done at Laboratory's discretion to confirm heparin contamination in investigation of prolonged APTT. Can be done off an INR/PTT tube.
7 Heparin Assay Anti Xa Assay Hemostasis and Thrombosis Laboratory (Victoria Hospital) 1 x 2.7 ml Blue (3.2% Na Citrate) Pediatric: 0-2 yrs: (HMMS # 85609) Mini Collect 1 ml Sodium Citrate Coagulation tube: Contact HAT lab ext for number of tubes required prior to sampling As authorized, Monday to Friday , unless otherwise requested by a Hematologist. See report Please direct any questions or concerns to: Michael Keeney Coordinator- Hematology & Flow Cytometry x Pager: All test requests, regardless of whether the patient is an adult or pediatric, must be authorized by a Hematologist (names and pagers listed below): Dr. Michael Kovacs Pager: Dr. Alejandro Lazo- Langner Pager: Authorization of pediatric testing may be provided by: (more...)
8 Heparin Induced Thrombocytopenia HIT Hemostasis and Thrombosis Laboratory (Victoria Hospital) 6 ml Red top Referred out Monday- Thursdays as required Negative Heparin Sulfamidase, Fibroblasts MPSIIIA Sanfilippo A Syndrome Biochemical Genetics Fibroblasts REGIONAL CYTOGENETI CS As required nmol/mg protein/17 hr Hepatitis A Antibody IgG Anti HAV IgG Hepatitis Anti HAV IgG Core/Microbiolog y Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Indicates immunity either from past infection or immunization.
9 Hepatitis A Antibody IgM anti HAV IgM Hepatitis anti-hav IgM Core/Microbiolog y Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Sample will be tested for Hepatitis A IgM antibody. Negative result indicates serology is not compatible with acute Hepatitis A infection. Positive result indicates serology is compatible with recent Hepatitis A infection. Hepatitis Anti HAV IgG (see Hepatitis A Antibody IgG) Hepatitis anti-hav IgM (see Hepatitis A Antibody IgM)
10 Hepatitis B Antiviral Resistance - HEPBAR Microbiology (VH) 5 ml Gold top (A minimum 1 ml of serum) Please completely fill out this Hepatits B Antiviral Resistance Requisition Test referred out to National Microbiology Lab once per week. See report Hepatitis B Core Antibody CORE Anti HBc (IgG + IgM) Core/Microbiolog y Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Testing detects both IgG and IgM antibodies (total). This test can be a life-long Hepatitis marker that represents past infection with Hepatitis B.
11 Hepatitis B Core IgM Antibody Core Adult: 5 ml Gold top Vacutainer Referred out Monday-Friday See report Pediatric: 0-2 yrs: Gold 0.5pk. 2-10yrs: 2 ml Gold top PUBLIC HEALTH Hepatitis B Diagnostic Panel Core/Microbiolog y Adult: 5 ml Gold top Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Panel includes Hepatitis B surface antigen, Hepatitis B surface antibody, Hepatitis B core antibody (IgG and IgM)
12 Hepatitis B DNA Viral Load HBV Viral Load Microbiology (VH) 2 x 5 ml Gold top Vacutainer tube HEPATITIS PCR Referred out to Toronto Public Health Laboratory Hepatitis B Surface Antibody AUSAB Australian Antibody Immune Status for Hepatitis B Core/Microbiolog y Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h Negative = 0 Indeterminate = 1-10 Immune = >10 If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Indicator of clinical recovery and subsequent immunity to Hepatitis B virus. Appearance is generally 1-4 months following onset of symptoms, but may be delayed much longer. A positive Hepatitis B surface antibody following Engerix indicates successful immunization.
13 Hepatitis B Surface Antigen HBsAg HpBsAg Core/Microbiolog y Adult: 5 ml Gold top Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH). Earliest serum indicator of acute Hepatitis B infection. Persistent elevation may indicate Hepatitis B carrier state.
14 Hepatitis Be Antibody Core Adult: 5 ml Gold top Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top or PUBLIC HEALTH TEST Referred weekdays to Public Health Laboratory, Toronto. See report
15 Hepatitis Be Antigen HBe Ag Hbe Core (UH) Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report Tested once per week in UH Core Laboratory. Early indicator of acute Hepatitis B representing the most infectious period. Usually short-lived (3-6 weeks). Persistence of e antigen in the acute stage beyond 10 weeks is indicative of progression to chronic carrier state and probable liver disease. Hepatitis C Antibody HCV HpCab Anti HCV Core/Microbiolog y Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Monday - Friday h See report If test is ordered at UH, testing will be performed at UH Core Lab. If test is ordered at VH, then testing will be performed in Microbiology (VH).
16 Hepatitis C NS3 Q80K Core 4 ml EDTA Lavender top HEPATITIS C NS3 Q80K Referred out Monday-Friday between to Public Health Laboratory See report f previous sample for HCV Viral Load or genotyping has been performed at PHL within six months, a sample does not have to be redrawn. Please fill out the requisition (see link above) and send to the Core Lab. Hepatitis C PCR (see Hepatitis C RNA - Qualitative **NO LONGER AVAILABLE**) Hepatitis C RNA - Qualitative **NO LONGER AVAILABLE** Hepatitis C PCR Core STAT requests must be prearranged with the Medical Microbiologist.
17 Hepatitis C RNA - Quantitative Quantitative RNA HCV Viral Load - pretreatment PCR Quantitative Microbiology Serum: 2 x 5 ml Gold or 6 ml Red Vacutainer tubes Plasma is also acceptable Plasma: 2 x 4.5 ml Lavender or 6 ml Pink top EDTA tubes HEPATITIS C (HCV) RNA Referred out to the Toronto Public Health Laboratory Hepatitis D Antibody Core Adult:5 ml Gold top Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top Referred out Monday-Friday See report
18 Hepatitis Donor Transplant Screen Donor Screen Core (UH) Adult: 5 ml Gold top Vacutainer Pediatric: 0-2 yrs: Gold 0.5pk yrs: 2 ml Gold top As required See individual tests Tested only at UH Core Lab. ALL POSITIVES phoned to Attending physician Sample will be tested for Hepatitis B surface antigen, Hepatitis B core antibody (total), Hepatitis C antibody, HIV, Cytomegalovirus, RPR (screening test for syphillis), HTLV 1/2 and West Nile Virus. Hepatitis/HIV testing on exposed individual of needle stick injury or exposure to blood/body fluids. (see Needle Stick Injury - Victim) Hepatitis/HIV testing on source of needlestick injury or blood/body fluid exposure. (see Needle Stick Injury - Source) Heptacarboxylic Acid (see Porphyrins, 24-Hour Urine, Porphyrins, Urine, Random)
19 Hereditary Sensory Neuropathy HSN1 Molecular Diagnostics Whole blood-2 x 4 ml Lavender EDTA top Vacutainer tube MOLECULAR DIAGNOSTIC As Required Monday - Friday h See report For more information click on: Molecular Diagnostic Laboratory N/A Hereditary sensory neuropathy type I (HSN1) is the most common hereditary disorder of peripheral sensory neurons, and is an autosomal dominant condition resulting in progressive degeneration of dorsal root ganglia and motor neurons with onset in the second or third decades. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1 have been shown to be responsible for this condition1,2. Early reports in the literature suggest that mutations both in exon 5 (more...)
20 Hereditary Spherocytosis Screening Test Investigational Hematology (VH) 4 ml K 2 or K 3 EDTA Lavender top Vacutainer tube Pediatric: 0-2 years: 0.5 ml Lavender pk years: 3 ml Lavender top Monday - Thursday Friday Negative for Hereditary Spherocytosis Herpes simplex IgG Total Antibody (HSVG) Herpes simplex IgG Total Antibody (HSVG) Clinical Immunology 5 ml Gold or 6 ml Red top PUBLIC HEALTH TEST See report
21 Herpes Simplex Virus PCR Herpes simplex Type 1/Type 2 HSVPCR Virology Laboratory CSF Plasma - EDTA (lavender top tube) Swabs - lesions Tissue Fluids VIROLOGY CSF samples are tested once daily Monday to Friday. Blood and lesions are tested twice weekly on Tuesday and Friday Tissues and Fluids are sent to Public Health Laboratory for testing See report Heterophile Antibodies (see Heterophile Antibody Screen) Heterophile Antibody Screen Infectious Mononucleosis Heterophile Antibodies Paul-Bunnell Core 4 ml K 2 or K 3 EDTA Lavender top Pediatric: 0-2 yrs: 0.5 ml Lavender pk yrs: 3 ml Lavender top As required Negative
22 Hexacarbolic Acid (see Porphyrins, 24-Hour Urine, Porphyrins, Urine, Random) Hexosaminidase (see Beta-N-Acetylhexosaminidase %A, A, A+B, Leukocyte/Plasma/Fibroblasts) HFE (see Hemochromatosis HFE gene) hgh (see Growth Hormone, Serum/Plasma) HHV6 (see Human Herpes Virus Type 6) High Density Lipoprotein Cholesterol (see Cholesterol-HDL,Plasma) High Resolution Banding (see Chromosome Analysis, Blood) High Sensitivity C- Reactive Protein High sensitivity CRP Assay hscrp Core 5 ml Gold top Referred out as required hscrp is considered a valuable risk stratification measurement in females > 60 years and males > 50 years. Values > 2.0 mg/l in these patients warrant further investigation. Refer to the CCS 2012 guideline for revised hscrp CVD risk criteria This test is useful for cardiac risk assessment only. A different CRP test ("C- Reactive Protein (CRP)") should be used to monitor or assess inflammatory disorders. This test will be used for cardiac risk assessment only. High sensitivity CRP Assay (see High Sensitivity C-Reactive Protein)
23 High Sensitivity Troponin T hs-tnt Troponin T - High sensitivity TNT - High sensitivity Core 4.5 ml Green (Lithium Heparin) top Vacutainer tube Pediatric: 2-10 yrs: 3 ml green top tube As required 14 ng/l Please note that the hs-tnt reporting units are ng/l. Order hs-tnt on patients with symptoms of myocardial ischemia to diagnose AMI. Reference limit is based on 99th percentile value in healthy population. hs- TnT may be significantly elevated in non-acs patients. Evaluate hs-tnt results in the clinical context and not in isolation. HIST (see Anti Histone Group,Serum) Histoplasma Culture (see Fungus Culture-Dimorphic)
24 Histoplasmosis Serology Microbiology (VH) 5 ml Gold or 6 ml Red top PUBLIC HEALTH TEST Referred out weekdays to Public Health Laboratory For Histoplasmosis Antigen detection, please consult Medical Microbiologist. HIT (see Heparin Induced Thrombocytopenia) HIV - High Risk IV Drug User (see HIV - High Risk Obstetrical and High Risk IV Drug User)
25 HIV - High Risk Obstetrical and High Risk IV Drug User HIV - High Risk Obstetrical Patients HIV - High Risk IV Drug User Core (UH) 5 ml Gold top HIV HIGH RISK TESTING (OBSTETRICS AND IV DRUG USERS) As needed Tested only at UH Core Lab. High Risk Obstetrics: This test is for specimens from the mother only. Any other specimen type from an obstetrical case (e.g. cord blood) must be authorized by the Microbiologist on call before testing can proceed. Note: This test is not available in PowerChart. To request this test, please fill out a High Risk Obstetrics and IV Drug User Requisition and send to the Core Lab. HIV - High Risk Obstetrical Patients (see HIV - High Risk Obstetrical and High Risk IV Drug User)
26 HIV - Viral Load Human Immunodeficiency Virus - Viral Load Quantitative HIV detection Microbiology 2 x 5 ml Lavender (EDTA) top Vacutainer tubes HIV Viral Load Requisition Referred STAT weekdays to Public Health Laboratory See report This is not a diagnostic test. Test is only available on patients known to be positive. Results are provided for prognostic purposes only and will be reported directly to physician. Sample must be spun and separated within 4 hours of collection. Ship to Microbiology immediately. Off hours, specimen receiving staff must spin sample immediately and aliquot and freeze plasma. Document time sample is separated on the requisition.
27 HIV PCR Microbiology Adult: 5 ml Lavender EDTA top Pediatric: 2 ml Lavender (EDTA) top HIV PCR TEST Referred STAT weekdays to the Public Health Laboratory See report Test detects HIV-1 only. Requests for HIV-2 PCR must be approved by the Microbiologist Sample is stable for 72 hours. Do not collect on Friday, Saturday or Sunday. Collect before 0800 hrs and transport to the Microbiology Laboratory at Victoria Hospital STAT. HIV Serology - Transplant Human Immunodeficiency Virus AIDS Core (UH) 5 ml Gold top Weekdays
28 HIV Serology, Routine - HIV 1 and 2 Human Immunodeficiency Virus AIDS Microbiology (VH) 6 ml Red top or 5 ml Gold top Vacutainer tube. PUBLIC HEALTH - HIV SEROLOGY TEST Referred weekdays to Public Health Laboratory The "HIV Ag/Ab Combo Screen" simultaneously detects both HIV p24 antigen (Ag) and antibodies (Ab) to HIV type 1 and type 2 (HIV-1/HIV-2) in human serum or plasma. Please note that the HIV Ag/Ab Combo result does not distinguish between the detection of HIVp24 antigen, HIV-1 antibodies, or HIV-2 antibodies. A specimen with a borderline or reactive result on an HIV screen test will proceed to confirmatory testing according to the HIV testing algorithm. For more information on HIV testing for Transplant pat (more...)
29 HIV/Hepatitis on exposed individual of needle stick injury or exposure to blood/body fluids. (see Needle Stick Injury - Victim) HIV/Hepatitis testing on source of needlestick injury or blood/body fluid exposure. (see Needle Stick Injury - Source)
30 HLA ABC Typing ABC Tissue Typing HLA Class I Typing Transplant (UH) 4 x 6 ml Green (Sodium Heparin) top Vacutainer tubes without gel separator This is the preferred specimen for patients with normal leukocytes or 2 x 4 ml Lavender (EDTA) top Vacutainer tubes -Use when leukocyte irregularities are possible (all potential bone marrow / stem cell recipients or HLA matched platele (more...) Monday to Friday See report Bone Marrow / Stem Cell donors- include the recipients name, relationship, physician and transplant centre. Testing is only performed for patients involved in the London Transplant Program. Unrelated marrow / stem cell donors are not normally tested in this laboratory. Please contact the laboratory ( ) if other situations apply to your patient. Disease Association HLA typings- Indicate the disease under investigation and the antigen of interest. This test is only appropriate for diseases associated with HLA ABC / Class I antigens. See HLA DR/DQ Tissue Typing for diseases a (more...)
31 HLA Antibody Screen (see HLA Antibody Testing) HLA Antibody Specificity Testing (see HLA Antibody Testing)
32 HLA Antibody Testing PRA (Panel Reactive Antibodies) HLA Monthly Serum HLA Antibody Screen HLA Antibody Specificity Testing Cytotoxic Antibodies (this is largely an obsolete term since most testing is now based on solid phase beads rather than cellular panels) Transplant (UH) 6 ml Red top - No additives or separator gel HLA Antibody Investigation Requisition The test is performed 3 times / week (MWF). Some samples will be archived for possible testing at a later date. The status of the patient on a transplant waiting list dictates when/if the sample will be tested. PRAs: 0-100% Specificity testing: usually the results will indicate if any antibodies were detected. In some cases, the detected antibodies may be listed This test should be ordered only for patients on a London solid organ transplant waiting list. Contact the Transplant Laboratory to discuss other situations. All patients that are active (or will soon be active) on the LHSC kidney waiting list should have this test ordered monthly since the sample will also be used for crossmatching potential donors. In addition, antibody specificities will be investigated. Testing intervals are determined by the transplant program. N/A Antibodies directed against HLA antigens indicate sensit (more...)
33 HLA B27 Tissue Typing (see HLA B27 Typing)
34 HLA B27 Typing HLA B27 Tissue Typing Transplant (UH) 4 x 6 ml Green (Sodium Heparin) top without gel separator This is the preferred specimen. -Order HLA B27 Serology (B27) As required Monday to Friday See report or 2 x 4mL Lavender (EDTA) top Vacutainer tubes -Use when leukocyte irregularities are possible or if the transit time is expected to exceed 24 hours -Order HLAB27 Special Order (SSP) (more...)
35 HLA CDC Crossmatch for Organ Transplant (Donor and Recipient Requirements for a Living Donor Situation) Transplant (UH) Donor Requirements- Initial Crossmatch*: 14 x 6 ml Sodium Heparin (dark green tops, no separator gel) 1 x 4 ml Lavender EDTA top *The blood requirements listed above include samples that will be used for the HLA Class I and HLA Class II typing of the donor plus the flow cytometry crossmatch, if required. Monday to Thursday Living donor transplant workups, including the crossmatching, are coordinated by the Living Donor Transplant Coordinator who can be contacted via the LHSC switchboard ( ). A positive crossmatch indicates the presence of anti-donor antibodies. Donor Requi (more...)
36 HLA CDC Crossmatch for Organ Transplant (Donor Requirements for a Deceased Donor Situation) Transplant (UH) Donor blood for a workup involving a kidney or kidney/pancre as (+/- other organs) transplant: 10 x 8.5 ml ACD (Solution A) (yellow tops)* 1 x 4 ml Lavender EDTA top Vacutainer tube Donor blood for a workup involving a heart transplant only: 8 x 8.5 ml. ACD (Solution A) (yellow tops)* 1 x 4 ml Lavender EDTA top (more...) As required See report A deceased donor situation must be coordinated by the TGLN and/or the local donor coordinators. TGLN: Local Donor Coordinators: contact LHSC N/A A positive crossmatch indicates the presence of anti-donor antibodies. Deceased donor crossmatch situations should be considered STAT and sample delivery should be handled accordingly. SRA staff: please page the donor coordinator on-call when donor blood arrives. The donor coordinator wil (more...)
37 HLA Celiac Investigation Transplant 2 x 4 ml Lavender (EDTA) top Vacutainer tubes TRANSPLANT Monday-Friday HLA Class I Typing (see HLA ABC Typing) HLA Class II Tissue Typing (see HLA DR/DQ Typing)
38 HLA Donor Specific Antibody (DSA testing) DSA testing Transplant (UH) 6 ml Red top HLA Antibody Investigation (NS5920) Testing is performed Mondays, Wednesdays and Fridays except on Statutory holidays This test should only be ordered at the request of the medical team when following a transplanted patient in a London solid organ transplant program. Contact the Transplant Laboratory to discuss other situations. ( ) Antibodies directed against donor HLA antigens may predict risk of rejection. Draw by venipuncture and pre-dialysis (if applicable) to avoid contamination with anticoagulants.
39 HLA DR/DQ Typing HLA Class II Tissue Typing Transplant (UH) 2 x 4 ml Lavender (EDTA) top Vacutainer tubes except for potential bone marrow/stem cell transplant patients or patients being investigated for HLA matched platelets. For these patients 4 x 4mL Lavender (EDTA) top tubes are required. Increase collection volumes if leukocyte count is low. Smaller volumes apply for pediatric patients. (more...) Monday to Friday See report Bone Marrow / Stem Cell donors- include the recipients name, relationship, physician and transplant centre. Testing is only performed for patients involved in the London Transplant Program. Unrelated marrow / stem cell donors are not normally tested in this laboratory. Please contact the laboratory ( ) if other situations apply to your patient. Disease Association HLA typings- Indicate the disease under investigation and the antigen of interest. This test is only appropriate for diseases associated with HLA DR/DQ or Class II antigens. See HLA ABC Tissue Typing for dis (more...)
40 HLA Flow Cytometry Crossmatch for Organ Transplant (Donor and Recipient Requirements for a Living Donor Situation) Transplant (UH) Donor Requirements: There are no additional blood requirements if 14 dark green tops are already being drawn for the CDC crossmatch. Draw 7 x 6 ml Sodium Heparin (dark green tops, no separator gel) in those rare occasions when the CDC crossmatch is not being performed. Contact the Living Donor Coordinator or the Transplant Laboratory for clarification. (more...) Monday to Friday Consult the Tissue Transplant Laboratory Living donor transplant workups, including the crossmatching, are coordinated by the Living Donor Transplant Coordinator who can be contacted via the LHSC switchboard ( ). Contact the Transplant Laboratory (x 33320) if a flow cytometry crossmatch involving a deceased donor is required. A positive crossmatch indicates the presence of anti-donor antibodies
41 HLA Monthly Serum (see HLA Antibody Testing) Homocysteine Total Homocysteine Core (UH) 4 ml K 2 or K 3 EDTA Lavender top Note: Specimens not placed on ice immediately may exhibit a 10-20% increase in concentration. Thursdays Male: umol/l Female: umol/l Drugs that interfere with homocysteine metabolism, e.g. nitric oxide, methotrexate, isoniazid, penicillamine and various antiepileptic drugs: carbamazepine, phenytoin, may give elevated levels of total homocysteine. Do not store samples at room temperature. Keep vacutainer on ice until centrifugation then freeze.
42 Homovanillic Acid, Urine HVA Toxicology/Speci al Chemistry 24-hour urine or random urine. Random urine testing available for pediatric patients only. Monday Friday Random Urine (mol/mmol creatinine) 0-2 years: years: years: years: 7.9 > 19 years: hour Urine (mol/day) 0-2 years: years: years: years: 39.5 > 19 years: VHHU24 (for 24-hour urine; includes VMA and HVA) or HVAR (for random urine; includes VMA and HVA) Orders for VMA and HVA are coupled so that the levels of both analytes are measured and reported for each sample. For STAT requests, please call the toxicology lab at ext to make arrangements. Please note: Testing method changed May 22, For 24-hour urine collections, transfer an aliquot of the measured urine to a 5-mL Greiner tube.
43 HpBsAg (see Hepatitis B Surface Antigen) HpCab (see Hepatitis C Antibody) HPT (see Haptoglobin, Plasma) HPV testing, for Gynaecological Liquid Based PAP test (Cytology), Liquid based PAP test (Cytology) PAP test LBP Cytopathology- UH hs-tnt (see High Sensitivity Troponin T) Cervical, endocervical, vaginal samples CYTOLOGY AND HPV TESTING Specimens are sent to Life Labs weekly for testing (Wednesday morning) Cytopathology Laboratory UH Room A3-242 UH (519) x 36391/36392 Clinical history is an important component for diagnostic interpretation The specimen is ThinPrep processed and must be collected in specific Gyn collection containers (PreservCyt) hscrp (see High Sensitivity C-Reactive Protein) HSN1 (see Hereditary Sensory Neuropathy)
44 HTLV Serology (HTLV 1 / 2) - Bone Bank, Eye Bank, partial tissue transplant, Tissue Bank, Routine Human T-cell Leukemia/Lymphoma Virus Microbiology (VH) 5 ml Gold top or 6 ml Red top PUBLIC HEALTH HIV SEROLOGY TEST Referred weekdays to the Public Health Laboratory Human Cytomegalovirus (HCMV) Anti-viral Resistance Genotyping Virology Laboratory HCMV positive plasma 5 ml Lavender EDTA Requisition for Viral STI, Polyoma and Herpesvirus Testing Referred to National Microbiology Lab HCMV Anti-viral Resistance Genotyping is available on HCMV positive patients only Human Growth Hormone (see Growth Hormone, Serum/Plasma)
45 Human Herpes Virus Type 6 HHV6 Exanthum subitum Roseola infantum Microbiology (VH) Blood - 5 ml Lavender top (EDTA) (A minimum 1 ml of plasma is required) or CSF - Encephalitis cases only (minimum 500uL CSF) must be accompanied by one 5 ml Lavender top tube) HHV6 Requisition Referred out weekly to the National Microbiology Laboratory See report Should you need more information on HHV6 testing, please contact the Viral Exanthemata Lab or fax Human Immunodeficiency Virus (see HIV Serology - Transplant, HIV Serology, Routine - HIV 1 and 2) Human Immunodeficiency Virus - Viral Load (see HIV - Viral Load) Human T-cell Leukemia/Lymphoma Virus (see HTLV Serology (HTLV 1 / 2) - Bone Bank, Eye Bank, partial tissue transplant, Tissue Bank, Routine) Hunter Syndrome (see Iduronate-2-Sulfate Sulfatase, Leukocytes/Plasma/Fibroblasts) Hurler Syndrome (see Alpha-Iduronidase, Leukocyte/Plasma/Fibroblasts) HVA (see Homovanillic Acid, Urine)
46 Hydroxyproline,Urine Test not available (Various) 24 Hour Urine with 20 ml of 6 mol/l (6N) HCL preservative. TEST NO LONGER AVAILABLE April 17, 2012 Hydroxyproline is no longer available. For bone density testing, please order C- Telopeptide (serum/plasma)
47 Hypercoagulable Screen Thrombophilia Screen Hemostasis and Thrombosis Laboratory (Victoria Hospital) Adult: 6 x 2.7 ml Blue (3.2% Sodium Citrate) top Vacutainer tubes and 2 x 4 ml Lavender (EDTA) top Vacutainer tubes Weekly See individual tests Pediatric: 0-2 yrs: (HMMS # 85609) Mini Collect 1 ml Sodium Citrate Coagulation tube: Contact HAT lab ext for number of tubes required prior to sampling and one 4 ml Lavender (EDTA) top GENE (more...)
48 Hyperkalemic Periodic Paralysis Type 1 (see Paramyotonia Congenita Hyperkalemic Periodic Paralysis) Hypersensitivity Pneumonitis Screen (see Allergic Alveolitis Group,Serum) Hypoxanthine- Guanine Phosphoribosyl Transferase, Whole blood Lesch-Nyhan Syndrome Biochemical Genetics 6 ml Green (Sodium Heparinized) top Vacutainer tube Test must be prearranged before collection by calling the lab at Specimen Receiving ext See report issued by lab
H. PYLORI AB, IGG HPYL
 H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
H. PYLORI AB, IGG HPYL Specimen Required: 3 ml blood, Serum gel tube Analytical Time: 1 day CPT Code: 86677 HAPTOGLOBIN HAP Specimen Required: 1.5 ml blood, gel tube Reference Range: 30-200 mg/dl CPT Code:
Specimen Collection Requirements. Test Name Specimen Type Storage Time Storage Conditions
 1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
Test no longer available effective February 1, 2001.
 Lactate Dehydrogenase Isoenzymes (serum) LD Isoenzymes Test not available Test no longer available effective February 1, 2001. Lactate Dehydrogenase,Fluid Core Fluid As required See report 2010-05-17 Hemolysis
Lactate Dehydrogenase Isoenzymes (serum) LD Isoenzymes Test not available Test no longer available effective February 1, 2001. Lactate Dehydrogenase,Fluid Core Fluid As required See report 2010-05-17 Hemolysis
Schedule of Accreditation
 Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
Specimen Collection Requirements
 The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
Transfusion Medicine (Comprehensive, Limited) J, J1
 www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
COLLECTION TUBES FOR PHLEBOTOMY
 COLLECTION TUBES FOR PHLEBOTOMY Red Top None Blood clots, and the serum is separated by centrifugation Chemistries, Immunology and Serology, Blood Bank (Crossmatch) Gold Top None Serum separator tube (SST)
COLLECTION TUBES FOR PHLEBOTOMY Red Top None Blood clots, and the serum is separated by centrifugation Chemistries, Immunology and Serology, Blood Bank (Crossmatch) Gold Top None Serum separator tube (SST)
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
Monday-Friday: tested as required
 Occult Blood (UH) Fresh random stool applied to Hemoccult card by ward As required Negative 2009-08-27 Olanzapine, Urine Qualitative Zyprexa Toxicology/Speci al Chemistry Minimum 10 ml random urine collected
Occult Blood (UH) Fresh random stool applied to Hemoccult card by ward As required Negative 2009-08-27 Olanzapine, Urine Qualitative Zyprexa Toxicology/Speci al Chemistry Minimum 10 ml random urine collected
Specimen Collection Requirements
 The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
The following is a job aid listing the specimen collection requirements for laboratory testing at Colchester East Hants Health Center. Specimens must be accompanied by the Patient Information Form G09.
Ward Manual. PHC Diagnostic Virology & Reference Laboratory
 PHC Diagnostic Virology & Reference Laboratory I. General Information The SPH Virology Laboratory is located at St. Paul s Hospital 1081 Burrard Street, Room 625, Burrard Building, Vancouver, B.C. V6Z
PHC Diagnostic Virology & Reference Laboratory I. General Information The SPH Virology Laboratory is located at St. Paul s Hospital 1081 Burrard Street, Room 625, Burrard Building, Vancouver, B.C. V6Z
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
October Courier Service on Holidays There will be no courier service Thursday, Dec. 24th, 2011.
 University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
University of Washington October 2011 TEST CHANGE ACTH Aldosterone BCR/ABL by PCR, Qualitative Chlamydia trachomatis & N.gonorrhoeae, Amp. NA Creutzfeldt-Jakob, CSF Desipramine Doxepine and Nordoxepine,
Test Name Laboratory Specimen Type Test Schedule Reference Range. Effective Date. Comments. F8 (see Factor VIII) F9 (see Factor IX)
 F8 (see Factor VIII) F9 (see Factor IX) Fabry's Disease (see Alpha-Galactosidase, Leukocyte, Alpha-Galactosidase, Plasma) Factor IX F9 Hemostasis and Thrombosis Laboratory (Victoria Hospital) 2 x 2.7 ml
F8 (see Factor VIII) F9 (see Factor IX) Fabry's Disease (see Alpha-Galactosidase, Leukocyte, Alpha-Galactosidase, Plasma) Factor IX F9 Hemostasis and Thrombosis Laboratory (Victoria Hospital) 2 x 2.7 ml
History of the Vacutainer Tube. Coagulant Blood Tests
 History of the Vacutainer Tube The different tubes are known as a vacutainer and were developed by Joseph Kleiner in 1947. The vacutainer is currently being manufactured by Becton, Dickinson and Company
History of the Vacutainer Tube The different tubes are known as a vacutainer and were developed by Joseph Kleiner in 1947. The vacutainer is currently being manufactured by Becton, Dickinson and Company
TEST REQUEST INFORMATION- VIROLOGY
 Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
Chlamydia/ Gonorrhea Nucleic Acid Amplification VC75 Qualitative Nucleic Acid Amplification SPECIMEN: Genital swab or first catch urine CONTAINER:GEN-PROBE APTIMA 2 Combo swab transport tube or urine transport
March Immunoglobulins, Total (IgA, IgE, IgG, IgM) Interleukin-6 (IL6) Protein Electrophoresis, CSF. Thyrotropin Receptor Antibody
 University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
University of Washington March 2012 TEST CHANGE Alpha 1-Antitrypsin Anti-IgE Receptor Antibody Anti-Mullerian Hormone Aquaporin-4 Receptor Antibody ColoSeq - Polyposis Complement testing, C3, C4 Everolimus
Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
 Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
PATHOLOGY SPECIMEN TUBES QUICK REFERENCE GUIDE : Edition Expires July 2013
 PATHOLOGY SPECIMEN TUBES QUICK REFERENCE GUIDE : 2012 2013 Edition Expires July 2013 FOR THE MOST UP TO DATE INFORMATION AND FOR TESTS THAT ARE NOT LISTED HERE PLEASE CHECK THE INTRANET SITE AT: http://rhhlas26/path_tests/lab_default.asp
PATHOLOGY SPECIMEN TUBES QUICK REFERENCE GUIDE : 2012 2013 Edition Expires July 2013 FOR THE MOST UP TO DATE INFORMATION AND FOR TESTS THAT ARE NOT LISTED HERE PLEASE CHECK THE INTRANET SITE AT: http://rhhlas26/path_tests/lab_default.asp
Alaska Native Medical Center Anchorage, AK
 ANMC Lab Test Requirements Key: Room Temp (20-25C), Refrigerated (2-8C), (-15 to -25C), Hr (Hours), D (Days), W (Weeks), Mo (Months), Yr (Years). Basic Processing Instructions: Centrifuge all blood specimens
ANMC Lab Test Requirements Key: Room Temp (20-25C), Refrigerated (2-8C), (-15 to -25C), Hr (Hours), D (Days), W (Weeks), Mo (Months), Yr (Years). Basic Processing Instructions: Centrifuge all blood specimens
Patient Preparation Unique patient preparation requirements are listed under each test in the Test Directory.
 Specimen Collection Lab results are only as good as the specimen provided. Patient preparation, venipuncture technique, specimen handling, and transportation can all affect the quantity of results. Health
Specimen Collection Lab results are only as good as the specimen provided. Patient preparation, venipuncture technique, specimen handling, and transportation can all affect the quantity of results. Health
UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE
 UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE HISTOCOMPATIBILITY AND DNA LABORATORY LABORATORY CATALOG P a g e 2 Introduction The
UNIVERSITY OF PUERTO RICO MEDICAL SCIENCES CAMPUS - SCHOOL OF MEDICINE DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE HISTOCOMPATIBILITY AND DNA LABORATORY LABORATORY CATALOG P a g e 2 Introduction The
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK National Infection Service, Public Health England Colindale 61 Colindale Avenue London NW9 5EQ Contact: Dr Sanjiv
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Controls & Calibrators. Disease Quality Controls
 Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091608 Age 26 Years Gender Female 30/8/2017 92900AM 30/8/2017 94717AM 31/8/2017 90216AM Ref By Final HEATITIS ACUTE VIRUS CONFIRMATION HEATITIS A ANTIBODY (ANTI HAV),
LL - LL-ROHINI (NATIONAL REFERENCE 135091608 Age 26 Years Gender Female 30/8/2017 92900AM 30/8/2017 94717AM 31/8/2017 90216AM Ref By Final HEATITIS ACUTE VIRUS CONFIRMATION HEATITIS A ANTIBODY (ANTI HAV),
TEST LIST SAMPLE REQUIREMENT. 1 ml serum None
 ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
Recommendations for Celiac Disease Testing. IgA & ttg IgA
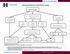 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Results that require action
 Following is a list of Allina Health Laboratory Results That Require Action (formerly Critical Values). Results That Require Action describes a lab test result that may indicate a lifethreatening situation.
Following is a list of Allina Health Laboratory Results That Require Action (formerly Critical Values). Results That Require Action describes a lab test result that may indicate a lifethreatening situation.
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
LL - LL-ROHINI (NATIONAL REFERENCE 135091606 Age 24 Years Gender Male 30/8/2017 92800AM 30/8/2017 94631AM 31/8/2017 90306AM Ref By Final HEATITIS A & B VIRUS EVALUATION HEATITIS A ANTIBODY (ANTI HAV),
Batched analysis SI Units: nmol/g
 UIBC (see Transferrin Saturation, Unsaturated Iron Binding Capacity) Umbilical Cord Blood Testing (see Cord Blood Testing) Unsaturated Iron Binding Capacity Iron Binding Capacity Saturation UIBC TIBC Iron
UIBC (see Transferrin Saturation, Unsaturated Iron Binding Capacity) Umbilical Cord Blood Testing (see Cord Blood Testing) Unsaturated Iron Binding Capacity Iron Binding Capacity Saturation UIBC TIBC Iron
Providence Medford Medical Center Pathology Department
 Providence Medford Medical Center Pathology Department Anatomic pathology services including histology, cytology and autopsies are offered through Providence Medford Medical Center Pathology Department.
Providence Medford Medical Center Pathology Department Anatomic pathology services including histology, cytology and autopsies are offered through Providence Medford Medical Center Pathology Department.
TRANSFUSION REACTION EVALUATION
 Lab Dept: Test Name: Transfusion Services TRANSFUSION REACTION EVALUATION General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: TRXR Transfusion Complication Workup; Hemolytic reaction
Lab Dept: Test Name: Transfusion Services TRANSFUSION REACTION EVALUATION General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: TRXR Transfusion Complication Workup; Hemolytic reaction
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Reference Range: Age/sex dependent; appropriate normals provided with result Analytical Time: 4 hours Day(s) Test Set Up: Daily CPT Code: 84100
 Parathyroid Hormone See PTH PARIETAL CELL ANTIBODY APCAB; PARIETAL; PCA Specimen Required: 2.5 ml blood, gel tube Analytical Time: 3-5 days CPT Code: 86255 PCP SCREEN URINE PCPQU Specimen Required: 50
Parathyroid Hormone See PTH PARIETAL CELL ANTIBODY APCAB; PARIETAL; PCA Specimen Required: 2.5 ml blood, gel tube Analytical Time: 3-5 days CPT Code: 86255 PCP SCREEN URINE PCPQU Specimen Required: 50
1. Purpose 1.1. To define testing locations, schedule and order priority for each test performed in the core laboratory.
 Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Specimen Collection Guide
 Specimen Collection Guide 1 Blood Collection Tubes SODIUM CITRATE TUBE - PALE BLUE TOP 2.7 Coagulation studies: Prothrombin time Factor assays etc. APTT Fibrinogen Thrombophilia tests INR D-Dimer SERUM
Specimen Collection Guide 1 Blood Collection Tubes SODIUM CITRATE TUBE - PALE BLUE TOP 2.7 Coagulation studies: Prothrombin time Factor assays etc. APTT Fibrinogen Thrombophilia tests INR D-Dimer SERUM
REFERENCE LABORATORY. Regular Hours - Monday through Friday 8:00 AM to 4:00 PM. On-Call Staff - Evenings, Nights, Weekend and Holidays.
 I. REFERENCE LABORATORY HOURS OF OPERATION: Regular Hours - Monday through Friday 8:00 AM to 4:00 PM. On-Call Staff - Evenings, Nights, Weekend and Holidays. All Reference Lab procedures are subject to
I. REFERENCE LABORATORY HOURS OF OPERATION: Regular Hours - Monday through Friday 8:00 AM to 4:00 PM. On-Call Staff - Evenings, Nights, Weekend and Holidays. All Reference Lab procedures are subject to
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids
 Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Management of Needlestick and Mucous Membrane Exposures To Blood/Body Fluids August 2004 Table Of Contents Protocol - Management of Needlestick and Mucous Membrane Exposures to Blood/Body Fluids Assessment
Activated Partial Thromboplastin Time (aptt)
 Activated Partial Thromboplastin Time (aptt) Order Name: PTT Test Number: 1500050 REV DATE:12/26/2008 Activated Partial Thromboplastin Time (aptt) CLOT Preferred 2.7 ml Whole Blood Sodium Citrate 3.2%
Activated Partial Thromboplastin Time (aptt) Order Name: PTT Test Number: 1500050 REV DATE:12/26/2008 Activated Partial Thromboplastin Time (aptt) CLOT Preferred 2.7 ml Whole Blood Sodium Citrate 3.2%
HANDLING GENERAL INFORMATION TEST REQUESTS TYPES OF TESTS TEST REQUEST FORM
 SECTION 3: SAMPLE COLLECTION & HANDLING GENERAL INFORMATION SAMPLE INTRODUCTION COLLECTION & HANDLING GENERAL results. INFORMATION The quality of laboratory test results depends greatly on the quality
SECTION 3: SAMPLE COLLECTION & HANDLING GENERAL INFORMATION SAMPLE INTRODUCTION COLLECTION & HANDLING GENERAL results. INFORMATION The quality of laboratory test results depends greatly on the quality
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates September 25, 2009 Dear Colleague: Specialty Laboratories is pleased to announce a new assay, PT and PTT-LA Mixing
IU Health Pathology Laboratory. DOS Updates
 IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
Viral hepatitis. Supervised by: Dr.Gaith. presented by: Shaima a & Anas & Ala a
 Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Primary Sample Manual Infectious Serology Issue No Effective Date: 20/09/17 Page 1 of 15 EUROFINS BIOMNIS
 Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
Issue No. 2.02 Effective Date: 20/09/17 Page 1 of 15 Written / Revised By: Dr. Mike Louw, Medical Director Date: Reviewed By: Date: Dr. Sinead Kelly, Infectious Serology Consultant Authorised By: Jean-Sébastien
Pathology Specimen Handling Requirements
 CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
Specimen Collection Requirements for Neonates
 Neonatal Page 1 of 21 ABG-Arterial Blood Gases Hep. Syringe 0.3 ml Send on ice ACTH (Adrenocorticotrophic hormone) 15 days Lavender (Serum, heparin NOT suitable) 1 ml Pre chill tubes Mix gently after drawing
Neonatal Page 1 of 21 ABG-Arterial Blood Gases Hep. Syringe 0.3 ml Send on ice ACTH (Adrenocorticotrophic hormone) 15 days Lavender (Serum, heparin NOT suitable) 1 ml Pre chill tubes Mix gently after drawing
EXT ADDRESS CITY LAST NAME ID NUMBER PT. ADDRESS CITY INSURANCE PROVIDER. 0 Other. 0 IGH/B-cell cionaliry (PCR)
 THE UNIVERSITY OF TEXAS *Required Fields PHYSICIAN! FACILITY/ CLIENT INFORMATION MDAndersonefief &ter MaltgararEettcy 'REQUESTING PHYSICIAN Division of Pathology! Laboratory Medicine Services Test Requisition
THE UNIVERSITY OF TEXAS *Required Fields PHYSICIAN! FACILITY/ CLIENT INFORMATION MDAndersonefief &ter MaltgararEettcy 'REQUESTING PHYSICIAN Division of Pathology! Laboratory Medicine Services Test Requisition
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Specific Panels. Celiac disease panel. Pancreas Panel:
 Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Cytomegalovirus (CMV) End-Point PCR Kit Product# EP36300
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Cytomegalovirus (CMV) End-Point PCR Kit Product# EP36300 Product
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Cytomegalovirus (CMV) End-Point PCR Kit Product# EP36300 Product
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
Laboratory Values, Critical. ThedaCare. Date Last Reviewed: 4/5/2018 Reviewing Body(s): Quality; Laboratory Leadership
 Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
Laboratory Values, Critical ThedaCare Policy & Procedure Policy Title: Laboratory Values, Critical Policy Number: 694 Location(s): All ThedaCare Department(s): Laboratory Date Last Reviewed: 4/5/2018 Reviewing
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
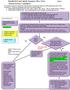 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
HIV Basics: Clinical Tests and Guidelines
 HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
HIV Basics: Clinical Tests and Guidelines ACTHIV 2010 Zelalem Temesgen MD Mayo Clinic Topics Baseline laboratory evaluation Laboratory monitoring through the continuum of care Patients not on antiretroviral
HIV Update in Laboratory Testing. Patricia Slev, PhD, D(ABCC)
 HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
In-Common Laboratories
 Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
Stability Information: Specimen stability information has been added or revised for the following tests: Vitamin B6, Dehydroepiandrosterone, Thyroid Antibodies (Thyroid Peroxidase Ab & Thyroglobulin Ab)
MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS
 MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS OVERVIEW (1) Background (2) Sample collection vials useful for different types of specimens (3) Stability of nucleic
MOLECULAR DIAGNOSTICS OF INFECTIOUS DISEASES: STABILITY OF SPECIMENS DURING PREANALYTICS OVERVIEW (1) Background (2) Sample collection vials useful for different types of specimens (3) Stability of nucleic
Physician Office Laboratory Tests
 Important Change Effective March 1, 2018 Physician Office Laboratory Tests Molina Healthcare of Michigan has updated its list of payable laboratory tests that may be performed in a physician s office.
Important Change Effective March 1, 2018 Physician Office Laboratory Tests Molina Healthcare of Michigan has updated its list of payable laboratory tests that may be performed in a physician s office.
Diagnostic Methods of HBV infection. Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO)
 Diagnostic Methods of HBV infection Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO) Hepatitis B-laboratory diagnosis Detection of HBV infection involves
Diagnostic Methods of HBV infection Zohreh Sharifi,ph.D of Virology Research center, Iranian Blood Transfusion Organization (IBTO) Hepatitis B-laboratory diagnosis Detection of HBV infection involves
The information contained here may be very important to your practice. Please take a moment to review this document.
 September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
September 2017 Dear Healthcare Provider, The information contained here may be very important to your practice. Please take a moment to review this document. TEST BULLETIN T-SPOT TB TESTING Please see
EC CERTIFICATE. National Institute for Biological Standards and Control (NIBSC) Blanche Lane South Mimms Potters Bar Hertfordshire EN6 3QG UK
 National Institute for Biological Standards and Control (NIBSC) EC Certificate - Full Quality Assurance System Approval Certificate Annex IV (excluding sections 4 and 6) of Council Directive 98/79/EC on
National Institute for Biological Standards and Control (NIBSC) EC Certificate - Full Quality Assurance System Approval Certificate Annex IV (excluding sections 4 and 6) of Council Directive 98/79/EC on
Learning Objectives: Hepatitis Update. Primary Causes of Chronic Liver Disease in the U.S. Hepatitis Definition. Hepatitis Viruses.
 Learning Objectives: Hepatitis Update ASCLS-Michigan March 31, 2016 Dr. Kathleen Hoag Upon attendance of this seminar and review of material provided, the attendees will be able to: 1. List hepatitis viruses
Learning Objectives: Hepatitis Update ASCLS-Michigan March 31, 2016 Dr. Kathleen Hoag Upon attendance of this seminar and review of material provided, the attendees will be able to: 1. List hepatitis viruses
Medicaid Family Planning Waiver Services CPT Codes and ICD-10 Diagnosis Codes
 CPT Code Description of Covered Codes Evaluation and Management 99384FP 99385FP Family planning new visit 99386FP 99394FP 99395FP Family planning established visit 99396FP 99401FP HIV counseling (pre-test)
CPT Code Description of Covered Codes Evaluation and Management 99384FP 99385FP Family planning new visit 99386FP 99394FP 99395FP Family planning established visit 99396FP 99401FP HIV counseling (pre-test)
Testing for Fabry Disease. What You Need to Know
 Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
TESTS, REF TESTS)
 ENGLISH Read the Package Insert completely before using the product. Follow the instructions carefully when performing testing. Failure to do so may result in inaccurate test results. For In Vitro Diagnostic
ENGLISH Read the Package Insert completely before using the product. Follow the instructions carefully when performing testing. Failure to do so may result in inaccurate test results. For In Vitro Diagnostic
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
Uses and Misuses of Viral Hepatitis Testing. Origins of Liver Science
 Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Uses and Misuses of Viral Hepatitis Testing Richard S Lang, MD, MPH, FACP Chairman, Preventive Medicine Vice-Chair, Wellness Institute Raul J Seballos, MD, FACP Vice-Chair, Preventive Medicine Wellness
Mary Keogan, on Mary behalf Keogan of all in NHISSOT On behalf of all in NHISSOT. 4th April 2014
 Solid Organ Transplantation How the Lab Contributes to Improved Patient Outcomes Mary Keogan, on Mary behalf Keogan of all in NHISSOT On behalf of all in NHISSOT 4th April 2014 Solid Organ Transplantation
Solid Organ Transplantation How the Lab Contributes to Improved Patient Outcomes Mary Keogan, on Mary behalf Keogan of all in NHISSOT On behalf of all in NHISSOT 4th April 2014 Solid Organ Transplantation
Anti-FceR (CU Index) Antibody
 Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Anti-FceR (CU Index) Antibody Order Name: ANTI-FCER Test Number: 5587500 REV DATE:5/31/2007 Anti-FceR (CU Index) Antibody CU Index Preferred 2 ml (1mL) Serum Clot Activator SST (Red/Gray or Tiger Top)
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit
 Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection. Masoud Mardani M.D,FIDSA
 Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Memo No: Date: 25-Nov NEW Serology and Virology Testing
 Memo No: 2014-61 Date: 25-Nov-2014 www.hicl.on.ca Re: NEW Serology and Virology Testing In Common Laboratories has been working hard to expand our test menu. We are pleased to announce the availability
Memo No: 2014-61 Date: 25-Nov-2014 www.hicl.on.ca Re: NEW Serology and Virology Testing In Common Laboratories has been working hard to expand our test menu. We are pleased to announce the availability
HML Update. Summer 2009 Volume 15, No. 3. Keeping Our Clients Informed Questions or Comments: Call
 HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
Section 9. Laboratory Considerations
 Section 9. Laboratory Considerations 9.1 Overview and General Guidance This section contains information on the laboratory procedures performed in MTN-012/IPM 010. As transmission of HIV and other infectious
Section 9. Laboratory Considerations 9.1 Overview and General Guidance This section contains information on the laboratory procedures performed in MTN-012/IPM 010. As transmission of HIV and other infectious
COLLECTION GUIDELINE FOR LABORATORY DIAGNOSIS OF COMMON VIRAL INFECTIONS
 1 of 5 Policy #: Subject: 609 (PLH-609-07) Effective Date: 9/30/2004 Reviewed Date: 8/1/2016 COLLECTION GUIDELINE FOR LABORATORY DIAGNOSIS OF COMMON VIRAL INFECTIONS Approved by: Laboratory Director, Jerry
1 of 5 Policy #: Subject: 609 (PLH-609-07) Effective Date: 9/30/2004 Reviewed Date: 8/1/2016 COLLECTION GUIDELINE FOR LABORATORY DIAGNOSIS OF COMMON VIRAL INFECTIONS Approved by: Laboratory Director, Jerry
The ABCs of Viral Hepatitis Diagnosis. Ila Singh, M.D., Ph.D. P & S Viral Hepatitis. Hepatitis A, B, C, D, E and G viruses
 The ABCs of Viral Hepatitis Diagnosis Ila Singh, M.D., Ph.D. P & S 14-453 is132@columbia.edu Viral Hepatitis Hepatotropic viruses Hepatitis A, B, C, D, E and G viruses Generalized infection plus infection
The ABCs of Viral Hepatitis Diagnosis Ila Singh, M.D., Ph.D. P & S 14-453 is132@columbia.edu Viral Hepatitis Hepatotropic viruses Hepatitis A, B, C, D, E and G viruses Generalized infection plus infection
CLIA APPROVED PROFICIENCY TESTING PROGRAMS ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts (800)
 ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts 01886 (800) 665-2575 MICROBIOLOGY Bacteriology Aerobic Culture and Identification Antibiotic Susceptibility Testing Direct Antigen Detection Gram Stain
ACCUTEST, INC. P.O. Box 999 Westford, Massachusetts 01886 (800) 665-2575 MICROBIOLOGY Bacteriology Aerobic Culture and Identification Antibiotic Susceptibility Testing Direct Antigen Detection Gram Stain
Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September 20, 2001
 Policy # MI/SER/04/v01 Page 1 of 9 Section: Subject Title: Hepatitis Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September
Policy # MI/SER/04/v01 Page 1 of 9 Section: Subject Title: Hepatitis Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: September
Epidemiologists and Laboratory Science
 Epidemiologists and Laboratory Science Najib Aziz, M.D. Adjunct Associate Professor Department of Epidemiology UCLA, Fielding School of Public Health January 2014 Introduction: Advance in the laboratory
Epidemiologists and Laboratory Science Najib Aziz, M.D. Adjunct Associate Professor Department of Epidemiology UCLA, Fielding School of Public Health January 2014 Introduction: Advance in the laboratory
Sources for blood collection
 Blood Collection Purpose Hematological investigations Biochemical investigations Serological investigations For culture For transfusion Therapeutic measure in polycythemia Sources for blood collection
Blood Collection Purpose Hematological investigations Biochemical investigations Serological investigations For culture For transfusion Therapeutic measure in polycythemia Sources for blood collection
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 25, 2010 Dear Colleague: This client letter contains many changes to our reference ranges in the area of chemistry
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates November 25, 2010 Dear Colleague: This client letter contains many changes to our reference ranges in the area of chemistry
The ABC s (and D & E s) of the Viral Hepatitides Part 2 DIAGNOSTIC TESTS 3/7/2013
 The ABC s (and D & E s) of the Viral Hepatitides Part 2 Thomas Novicki PhD DABMM Clinical Microbiologist Division of Laboratory Medicine novicki.thomas@marshfieldclinic.org Objectives 2 1. Explain the
The ABC s (and D & E s) of the Viral Hepatitides Part 2 Thomas Novicki PhD DABMM Clinical Microbiologist Division of Laboratory Medicine novicki.thomas@marshfieldclinic.org Objectives 2 1. Explain the
BK Virus (BKV) Management Guideline: July 2017
 BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
BK Virus (BKV) Management Guideline: July 2017 BK virus has up to a 60-80% seroprevalence rate in adults due to a primary oral or respiratory exposure in childhood. In the immumocompromised renal transplant
First Probable West Nile Virus Case in the Simcoe-Muskoka Area
 Dr. Charles Gardner, Medical Officer of Health Dr. Colin Lee, Associate Medical Officer of Health Dr. Lisa Simon, Associate Medical Officer of Health First Probable West Nile Virus Case in the Simcoe-Muskoka
Dr. Charles Gardner, Medical Officer of Health Dr. Colin Lee, Associate Medical Officer of Health Dr. Lisa Simon, Associate Medical Officer of Health First Probable West Nile Virus Case in the Simcoe-Muskoka
SOLE-SOURCE DETERMINATION
 SOLE-SOURCE DETERMINATION The Purchasing Division has been requested to approve a sole source purchase for the commodity or service described below. Pursuant to West Virginia Code 5A-3-10c, the Purchasing
SOLE-SOURCE DETERMINATION The Purchasing Division has been requested to approve a sole source purchase for the commodity or service described below. Pursuant to West Virginia Code 5A-3-10c, the Purchasing
Epic Labs Orderable As STAT PRIORITY As of 06/22/2016
 ABG+HB(CORDARTERIAL) - BABY A ABG+HB(CORD ARTERIAL)- BABY B ABG+HB(CORD ARTERIAL)- BABY C ACETAMINOPHEN LEVEL ALANINE AMINOTRANSFERASE (ALT) ALBUMIN, FLUID ALBUMIN, PLEURAL FLUID ALBUMIN, SYNOVIAL FLUID
ABG+HB(CORDARTERIAL) - BABY A ABG+HB(CORD ARTERIAL)- BABY B ABG+HB(CORD ARTERIAL)- BABY C ACETAMINOPHEN LEVEL ALANINE AMINOTRANSFERASE (ALT) ALBUMIN, FLUID ALBUMIN, PLEURAL FLUID ALBUMIN, SYNOVIAL FLUID
OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST
 11/5/2018 OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept
11/5/2018 OHIOHEALTH Laboratory Services INPATIENT, ED AND OTHER HOSPITAL BASED PATIENT CRITICAL VALUE NOTIFICATION LIST Test Critical Low Critical High Alerting CHEMISTRY Amylase > 400 U/L Emergency Dept
Multiple Choice Questions - Paper 1
 Multiple Choice Questions - Paper 1 Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could
Multiple Choice Questions - Paper 1 Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could
