Legacy WEBLink Test Updates
|
|
|
- Elijah Leo Stewart
- 5 years ago
- Views:
Transcription
1 ACE S ACH ACHR MOD ACHR R PAN ACHRBLO ANGIOTENSIN CONVERTING ENZYME ACETYLCHOLINE RECEPTOR BINDING ANTIBODY ACETYLCHOLINE RECEPTOR MODULATING ANTIBODY ACETYLCHOLINE RECEPTOR ANTIBODY REFLEX PANEL ACETYLCHOLINE RECEPTOR BLOCKING ANTIBODY 6/18/2014 Test name change Test name changed from ANGIOTENSIN CONVERTING ENZYME, BLOOD to ANGIOTENSIN CONVERTING ENZYME 6/18/2014 Test name change Test name changed from ACETYLCHOLINE RECEPTOR AB to ACETYLCHOLINE RECEPTOR BINDING ANTIBODY 6/18/2014 Test name change Test name changed from Acetylcholine Receptor Modulating Ab to ACETYLCHOLINE RECEPTOR MODULATING ANTIBODY 6/18/2014 Test name change Test name changed from Acetylcholine Receptor Ab Pan w/reflex to ACETYLCHOLINE RECEPTOR ANTIBODY REFLEX PANEL 6/18/2014 Test name change Test name changed from Acetyccholine Blocking Ab to ACETYLCHOLINE RECEPTOR BLOCKING ANTIBODY ACTH ACTH - ADRENOCORTICOTROPIC HORMONE 6/18/2014 Test name change Test name changed from ADRENOCORTICOTROPHIC HORMONE to ACTH - ADRENOCORTICOTROPIC HORMONE ACYLCARN ACYLCARNITINE PROFILE, QUANTITATIVE 6/18/2014 Test name change Test name changed from ACYLCARNITINES QUANTITATIVE to ACYLCARNITINE PROFILE, QUANTITATIVE For questions, please call Lab Outreach EMR Support at Page 1 of 15
2 AF ACHE AJ PAN ACETYLCHOLINESTERASE, AMNIOTIC FLUID ASHKENAZI JEWISH MUTATION ANALYSIS PANEL WITHOUT CYSTIC FIBROSIS (CF) 6/18/2014 Test name change Test name changed from AF ACETYLCHOLINESTERASE to ACETYLCHOLINESTERASE, AMNIOTIC FLUID 6/18/2014 Test name change Test name changed from ASHKENAZI JEWISH MUTATION PANEL W/O CF to ASHKENAZI JEWISH MUTATION ANALYSIS PANEL WITHOUT CYSTIC FIBROSIS (CF) AL S ALUMINUM, SERUM 6/18/2014 Test name change Test name changed from ALUMINUM, BLOOD to ALUMINUM, SERUM ALDOLASE ALDOLASE 6/18/2014 Test name change Test name changed from ALDOLASE, BLOOD to ALDOLASE AMIOD ANCA S AMIODARONE AND METABOLITE CYTOPLASMIC NEUTROPHIL ANTIBODIES, SERUM 6/18/2014 Test name change Test name changed from AMIODARONE WITH METABOLITE to AMIODARONE AND METABOLITE 6/18/2014 Test name change Test name changed from CYTOPLASMIC NEUTROPHILIC ANTIBODY S to CYTOPLASMIC NEUTROPHIL ANTIBODIES, SERUM For questions, please call Lab Outreach EMR Support at Page 2 of 15
3 APC RES ACTIVATED PROTEIN C RESISTANCE 6/18/2014 Test name change and test code change Test name changed from APC RESISTANCE PROFILE to ACTIVATED PROTEIN C RESISTANCE Test code changed from APC RST to APC RES APO E APOLIPOPROTEIN E (APOE) 2 MUTATIONS, CARDIOVASCULAR RISK 6/18/2014 Test name change Test name changed from Apolipoprotein E for Cardiovascular Risk to APOLIPOPROTEIN E (APOE) 2 MUTATIONS, CARDIOVASCULAR RISK ARS B ARSENIC, BLOOD 6/18/2014 Test name change Test name changed from ARSENIC, WHOLE BLOOD to ARSENIC, BLOOD AS PWS ASPER AG ASPER PRO ANGELMAN SYNDROME AND PRADER-WILLI SYNDROME BY METHYLATION ASPERGILLUS GALACTOMANNAN ANTIGEN BY EIA, SERUM ASPERGILLUS ANTIBODIES BY CF AND ID 6/18/2014 Test name change Test name changed from Angelman and Prader-Willi Syndromes to ANGELMAN SYNDROME AND PRADER-WILLI SYNDROME BY METHYLATION 6/18/2014 Test name change Test name changed from Aspergillus Galactomannan Antigen to ASPERGILLUS GALACTOMANNAN ANTIGEN BY EIA, SERUM 6/18/2014 Test name change Test name changed from ASPERGILLUS ANTIBODIES to ASPERGILLUS ANTIBODIES BY CF AND ID For questions, please call Lab Outreach EMR Support at Page 3 of 15
4 BARTPAN BCR RLFX BF CMVPCR BARTONELLA QUINTANA ANTIBODIES, IgG AND IgM BY IFA BCR-ABL1, QUALITATIVE WITH REFLEX TO BCR-ABL1 QUANTITATIVE CYTOMEGALOVIRUS (CMV) By PCR, BODY FLUID 6/18/2014 Test name change Test name changed from BARTONELLA QUINTANA AB IGG/IGM to BARTONELLA QUINTANA ANTIBODIES, IgG AND IgM BY IFA 6/18/2014 Test name change Test name changed from BCR-ABL1 Qualatative w/reflex to Quant to BCR-ABL1, QUALITATIVE WITH REFLEX to BCR- ABL1 QUANTITATIVE 6/18/2014 Test name change Test name changed from BODY FLUID CYTOMEGALOVIRUS (CMV) PCR MOLECULAR DETECTION to CYTOMEGALOVIRUS (CMV) By PCR, BODY FLUID CA-BREAST CANCER ANTIGEN-BREAST 6/18/2014 Test name change Test name changed from CA BREAST (CANCER ANTIGEN) to CANCER ANTIGEN-BREAST CARN FT CARNITINE FREE AND TOTAL 6/18/2014 Test name change Test name changed from CARNITINE FREE & TOTAL, PLASMA to CARNITINE FREE AND TOTAL CAT SCRTCH BARTONELLA HENSELAE (CAT SCRATCH) ANTIBODIES, IgG & IgM 6/18/2014 Test name change Test name changed from BARTONELLA HENSELAE AB IGG/IGM to BARTONELLA HENSELAE (CAT SCRATCH) ANTIBODIES, IgG & IgM For questions, please call Lab Outreach EMR Support at Page 4 of 15
5 CDG SERUM CARBOHYDRATE DEFICIENT TRANSFERRIN FOR CONGENITAL DISORDERS OF GLYCOSYLATION, SERUM 6/18/2014 Test name change Test name changed from CARBOHYDRATE DEFICIENT TRANSFERRIN SERUM to CARBOHYDRATE DEFICIENT TRANSFERRIN FOR CONGENITAL DISORDERS OF GLYCOSYLATION, SERUM CELIACGENE CELIAC GENETICS 6/18/2014 Test name change Test name changed from Prometheus Celiac Genetics to CELIAC GENETICS CELIACPLUS CELIAC PLUS 6/18/2014 Test name change Test name changed from Prometheus Celiac PLUS to CELIAC PLUS CENTROMERE CENTROMERE ANTIBODY 6/18/2014 Test name change Test name changed from CENTROMERE IgG AB to CENTROMERE ANTIBODY CLOMIP CLOMIPRAMINE AND METABOLITE 6/18/2014 Test name change Test name changed from CLOMIPRAMINE WITH METABOLITE to CLOMIPRAMINE AND METABOLITE CLONAZEPAM CLONAZEPAM 6/18/2014 Test name change Test name changed from Clonazepam Level ARUP to CLONAZEPAM CLOZAPINE CLOZAPINE 6/18/2014 Test name change Test name changed from CLOZAPINE LEVEL to CLOZAPINE For questions, please call Lab Outreach EMR Support at Page 5 of 15
6 CMV IGG CMV PAN CMV QUANT CMV-IGM CYTOMEGALOVIRUS IgG ANTIBODY CYTOMEGALOVIRUS ANTIBODIES, IgG and IgM CYTOMEGALOVIRUS QUANTITATIVE BY PCR CYTOMEGALOVIRUS IgM ANTIBODY 6/18/2014 Test name change Test name changed from CYTOMEGALOVIRUS IGG AB CMV to CYTOMEGALOVIRUS IgG ANTIBODY 6/18/2014 Test name change Test name changed from Cytomegalovirus (CMV) Antibodies IgG, IgM to CYTOMEGALOVIRUS ANTIBODIES, IgG and IgM 6/18/2014 Test name change Test name changed from Cytomegalovirus DNA Quant PCR to CYTOMEGALOVIRUS QUANTITATIVE BY PCR 6/18/2014 Test name change Test name changed from CYTOMEGALOVIRUS IGM AB CMV to CYTOMEGALOVIRUS QUANTITATIVE BY PCR CROHNS CROHN S PROGNOSTIC 6/18/2014 Test name change Test name changed from Prometheus Crohns Prognostic to CROHN S PROGNOSTIC CTDC CONNECTIVE TISSUE DISEASES CASCADE, SERUM 6/18/2014 Test name change Test name changed from CONNECTIVE TISSUE DISEASES to CONNECTIVE TISSUE DISEASES CASCADE, SERUM DHEA TMS DEHYDROEPIANDROSTERONE 6/18/2014 Test name change Test name changed from DHEA BLOOD to DEHYDROEPIANDROSTERONE For questions, please call Lab Outreach EMR Support at Page 6 of 15
7 DHT TMS 5-a-DIHYDROTESTOSTERONE BY TANDEM MASS SPECTROMETRY, SERUM 6/18/2014 Test name change Test name changed from Dihydrotestosterone TMS to 5-a- DIHYDROTESTOSTERONE BY TANDEM MASS SPECTROMETRY, SERUM DNA-SS ssdna ANTIBODY, IgG 6/18/2014 Test name change Test name changed from DNA - SINGLE STRANDED IGG AB to ssdna ANTIBODY, IgG ENAE ESTRNE TMS ESTRO FR TMS FA PROFILE EXTRACTABLE NUCLEAR ANTIGEN ANTIBODIES ESTRONE, BY TANDEM MASS SPECTROMETRY ESTROGENS, FRACTIONATED BY TANDEM MASS SPECTROMETRY FATTY ACID PROFILE, PEROXISOMAL (C22-C26), SERUM 6/18/2014 Test name change Test name changed from EXTRACTABLE NUCLEAR ANTIGEN AB (ENA) to EXTRACTABLE NUCLEAR ANTIGEN ANTIBODIES 6/18/2014 Test name change Test name changed from ESTRONE to ESTRONE, BY TANDEM MASS SPECTROMETRY 6/18/2014 Test name change Test name changed from ESTROGENS, FRACTIONATED to ESTROGENS, FRACTIONATED BY TANDEM MASS SPECTROMETRY 6/18/2014 Test name change Test name changed from Fatty Acid Profile Peroxisomal to FATTY ACID PROFILE, PEROXISOMAL (C22-C26), SERUM FIBROSPECT FIBROSPECT II 6/18/2014 Test name change Test name changed from Prometheus FibroSpect II to FIBROSPECT II For questions, please call Lab Outreach EMR Support at Page 7 of 15
8 HIV 1 GENO HUMAN IMMUNODEFICIENCY VIRUS-1, GENOTYPING 6/18/2014 Test name change Test name changed from HIV-1 Genotyping ARUP to HUMAN IMMUNODEFICIENCY VIRUS-1, GENOTYPING IBD SGI IBD SGI DIAGNOSTIC 6/18/2014 Test name change Test name changed from Prometheus IBD sgi Diagnostic to IBD SGI DIAGNOSTIC INFLIXIMAB PROMETHEUS ANSER IFX 6/18/2014 Test name change Test name changed from Prometheus Infliximab to PROMETHEUS ANSER IFX NIACIN B3 NIACIN 6/18/2014 Test name change Test name changed from Niacin - Vitamin B3 to NIACIN OSMO FRAG OSMOTIC FRAGILITY, ERYTHROCYTE 6/18/2014 Test name change Test name changed from Osmotic Fragility RBC to OSMOTIC FRAGILITY, ERYTHROCYTE OXCARB OXCARBAZEPINE 6/18/2014 Test name change Test name changed from Oxcarbazepine Metabolite Level to OXCARBAZEPINE PARVO G PARVO GM PARVOVIRUS B19 ANTIBODY, IgG PARVOVIRUS B19 ANTIBODIES, IgG and IgM 6/18/2014 Test name change Test name changed from Parvovirus B19 IgG Ab to PARVOVIRUS B19 ANTIBODY, IgG 6/18/2014 Test name change Test name changed from Parvovirus B19 IgG and IgM Abs to PARVOVIRUS B19 ANTIBODIES, IgG and IgM For questions, please call Lab Outreach EMR Support at Page 8 of 15
9 PARVO M PARVPCR PARVOVIRUS B19 ANTIBODY, IgM PARVOVIRUS B 19, BY PCR, SERUM OR PLASMA 6/18/2014 Test name change Test name changed from Parvovirus B19 IgM Ab to PARVOVIRUS B19 ANTIBODY, IgM 6/18/2014 Test name change Test name changed from PARVOVIRUS B19 BY PCR to PARVOVIRUS B 19, BY PCR, SERUM OR PLASMA PHYT AC PHYTANIC ACID 6/18/2014 Test name change Test name changed from PHYTANIC ACID QUANT, PLASMA to PHYTANIC ACID PLT HAT AB PNEUMO AB PNH PAN HEPARIN-INDUCED THROMBOCYTOPENIA (HIT) ANTIBODIES, PF4 IGG/IGM/IGA BY ELISA STREPTOCOCCUS PNEUMONIAE ANTIBODIES PAROXYSMAL NOCTURNAL HEMOGLOBINURIA PANEL, RBC AND WBC 6/18/2014 Test name change Test name changed from HEPARIN-INDUCED ANTIBODIES (HIT) to HEPARIN-INDUCED THROMBOCYTOPENIA (HIT) ANTIBODIES, PF4 IGG/IGM/IGA BY ELISA 6/18/2014 Test name change Test name changed from PNEUMOCOCCAL ABS to STREPTOCOCCUS PNEUMONIAE ANTIBODIES 6/18/2014 Test name change Test name changed from Paroxysmal Nocturnal Hemoglobinuria Panel to PAROXYSMAL NOCTURNAL HEMOGLOBINURIA PANEL, RBC AND WBC For questions, please call Lab Outreach EMR Support at Page 9 of 15
10 PNH RBC PNPABR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA, HIGH SENSITIVITY, RBC PARANEOPLASTIC ANTIBODIES (PCCA/ANNA) BY IFA WITH REFLEX TO TITER AND WESTERN BLOT 6/18/2014 Test name change Test name changed from Paroxysmal Nocturnal Hemoglobinuria RBC to PAROXYSMAL NOCTURNAL HEMOGLOBINURIA, HIGH SENSITIVITY, RBC 6/18/2014 Test name change Test name changed from PARANEOPLASTIC ABS RFLX SCN to PARANEOPLASTIC ANTIBODIES (PCCA/ANNA) BY IFA WITH REFLEX TO TITER AND WESTERN BLOT PORURINE PORPHYRINS, URINE 6/18/2014 Test name change Test name changed from U Porphyrins Fractionated Quant to PORPHYRINS, URINE PREGNLONE PTHRP PREGNENOLONE BY LC- MS/MS, SERUM OR PLASMA PARATHYROID HORMONE- RELATED PEPTIDE (PTHrP), PLASMA 6/18/2014 Test name change Test name changed from Pregnenolone Serum to PREGNENOLONE BY LC-MS/MS, SERUM OR PLASMA 6/18/2014 Test name change Test name changed from PTH-RELATED PEPTIDE HORMONE to PARATHYROID HORMONE-RELATED PEPTIDE (PTHrP), PLASMA QUETIAP QUETIAPINE 6/18/2014 Test name change Test name changed from Quetiapine Level to QUETIAPINE QUINIDINE QUINIDINE 6/18/2014 Test name change Test name changed from QUINIDINE LEVEL SERUM to QUINIDINE For questions, please call Lab Outreach EMR Support at Page 10 of 15
11 RABIES RABIES ANTIBODY, IgG (VACCINE RESPONSE) 6/18/2014 Test name change Test name changed from Rabies IgG Antibody to RABIES ANTIBODY, IgG (VACCINE RESPONSE) RBC MG MAGNESIUM, RBC 6/18/2014 Test name change Test name changed from RBC Magnesium to MAGNESIUM, RBC RIB RIBOSOME P ANTIBODY IgG 6/18/2014 Test name change Test name changed from RIBOSOME P AB IgG to RIBOSOME P ANTIBODY IgG RNP RIBONUCLEIC PROTEIN (ENA) ANTIBODY, IgG 6/18/2014 Test name change Test name changed from RNP ANTIBODY IgG to RIBONUCLEIC PROTEIN (ENA) ANTIBODY, IgG SELENIUM SELENIUM, SERUM 6/18/2014 Test name change Test name changed from SELENIUM, BLOOD to SELENIUM, SERUM SEROT-SER SEROTONIN, SERUM 6/18/2014 Test name change Test name changed from SEROTONIN, BLOOD to SEROTONIN, SERUM SEROT-WB SEROTONIN, WHOLE BLOOD 6/18/2014 Test name change Test name changed from Serotonin Level Whole Blood to SEROTONIN, WHOLE BLOOD SERTRALIN SERTRALINE 6/18/2014 Test name change Test name changed from SERTRALINE, BLOOD to SERTRALINE For questions, please call Lab Outreach EMR Support at Page 11 of 15
12 SRA UFH SEROTONIN RELEASE ASSAY (HEPARIN DEPENDENT PLATELET ANTIBODY), UNFRACTIONATED HEPARIN 6/18/2014 Test name change Test name changed from SEROTONIN RELEASE ASSAY, UFH to SEROTONIN RELEASE ASSAY (HEPARIN DEPENDENT PLATELET ANTIBODY), UNFRACTIONATED HEPARIN SSA SSA (Ro) (ENA) ANTIBODY, IgG 6/18/2014 Test name change Test name changed from SSA (RO) ANTIBODY IgG to SSA (Ro) (ENA) ANTIBODY, IgG SSB SSB (La) (ENA) ANTIBODY, IgG 6/18/2014 Test name change Test name changed from SSB (LA) ANTIBODY IgG to SSB (La) (ENA) ANTIBODY, IgG STZR SCN TEG/10 11 TESTOS FT STREPTOCOCCUS PYOGENES, GROUP A ANTIBODY (Stretozyme) WITH REFLEX TO TITER CARBAMAZEPINE AND METABOLITE, BLOOD TESTOSTERONE FREE AND TOTAL, FEMALES OR CHILDREN 6/18/2014 Test name change Test name changed from STREPTOCOCCAL AB SCREEN to STREPTOCOCCUS PYOGENES, GROUP A ANTIBODY (Stretozyme) WITH REFLEX TO TITER 6/18/2014 Test name change Test name changed from CARBAMAZEPINE EPOXIDE to CARBAMAZEPINE AND METABOLITE, BLOOD 6/18/2014 Test name change Test name changed from TESTOSTERONE FREE AND TOTAL FEMALE/CHILD to TESTOSTERONE FREE AND TOTAL, FEMALES OR CHILDREN For questions, please call Lab Outreach EMR Support at Page 12 of 15
13 TETANUS TETANUS ANTIBODY, IgG 6/18/2014 Test name change Test name changed from TETANUS AB IGG to TETANUS ANTIBODY, IgG THYROGLO R THYROGLOBULIN, SERUM OR PLASMA WITH REFLEX TO LC- MS/MS OR CIA 6/18/2014 Test name change Test name changed from THYROGLOBULIN SERUM W/REFLEX ARUP to THYROGLOBULIN, SERUM OR PLASMA WITH REFLEX TO LC-MS/MS OR CIA THYROGLO S THYROGLOBULIN, SERUM - ESOTERIX 6/18/2014 Test name change Test name changed from THYROGLOBULIN S to THYROGLOBULIN, SERUM - ESOTERIX TIAGABINE TIAGABINE, SERUM OR PLASMA 6/18/2014 Test name change Test name changed from TIAGABINE LEVEL to TIAGABINE, SERUM OR PLASMA TORCH M TORCH ANTIBODIES, IgM 6/18/2014 Test name change Test name changed from TORCH ANTIBODIES IGM to TORCH ANTIBODIES, IgM TOXOIGM TOXOPLASMA IgM ANTIBODY 6/18/2014 Test name change Test name changed from TOXOPLASMA ANTIBODY IGM to TOXOPLASMA IgM ANTIBODY TPMT ENZ TPMT ENZYME 6/18/2014 Test name change Test name changed from Prometheus TPMT Enzymatic to TPMT ENZYME For questions, please call Lab Outreach EMR Support at Page 13 of 15
14 TPMT GEN TPMT GENETICS 6/18/2014 Test name change Test name changed from Prometheus TPMT Genetics to TPMT GENETICS TRAB S THYROID STIMULATING HORMONE RECEPTOR ANTIBODY (TRAb) 6/18/2014 Test name change Test name changed from TSH Receptor Antibody TRAB to THYROID STIMULATING HORMONE RECEPTOR ANTIBODY (TRAb) TRYPTASE TRYPTASE 6/18/2014 Test name change Test name changed from TRYPTASE LEVEL to TRYPTASE URFISH REQ UROVYSION FISH 6/18/2014 Test name change Test name changed from Urovysion Fish Request to UROVYSION FISH VASC ANTINEUTROPHIL CYTOPLASMIC ANTIBODIES VASCULITIS PANEL, SERUM 6/18/2014 Test name change Test name changed from ANCA Panel for Vasculitis w/reflex to ANTINEUTROPHIL CYTOPLASMIC ANTIBODIES VASCULITIS PANEL, SERUM VIT B1 VITAMIN B1, WHOLE BLOOD 6/18/2014 Test name change Test name changed from VITAMIN B1-THIAMINE-WHOLE BLD to VITAMIN B1, WHOLE BLOOD VIT B1 P VITAMIN B1, PLASMA 6/18/2014 Test name change Test name changed from Vitamin B1-Thiamine Plasma to VITAMIN B1, PLASMA For questions, please call Lab Outreach EMR Support at Page 14 of 15
15 VIT B6 VITAMIN B6 (PYRIDOXAL 5- PHOSPHATE) 6/18/2014 Test name change Test name changed from VITAMIN B6 TOTAL, PLASMA to VITAMIN B6 (PYRIDOXAL 5-PHOSPHATE) VIT C LVL VITAMIN C LEVEL 6/18/2014 Test name change Test name changed from Vitamin C Level - Ascorbic Acid to VITAMIN C LEVEL WNILE-SER YER PAN WEST NILE VIRUS ANTIBODIES, IgG & IgM, SERUM YERSINIA ANTIBODIES, IgA AND IgG BY IMMUNOBLOT 6/18/2014 Test name change Test name changed from WEST NILE VIRUS ANTIBODY PANEL BLOOD to WEST NILE VIRUS ANTIBODIES, IgG & IgM, SERUM 6/18/2014 Test name change Test name changed from YERSINIA IGA & IGG ANTIBODIES to YERSINIA ANTIBODIES, IgA AND IgG BY IMMUNOBLOT For questions, please call Lab Outreach EMR Support at Page 15 of 15
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
Reflex Test Protocols
 University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
University of Colorado Hospital Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Saskatchewan ND Price List
 Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
Saskatchewan ND Price List Note: There is a $55 per requisition documentation fee. Prices valid only in Saskatchewan. PANEL Healthy Living Assessment Panel $115.00 Enhanced Healthy Living Assessment Panel
ONTARIO ND PRICE LIST
 ONTARIO ND PRICE LIST Note: There is a $7.76 per requisition documentation fee. Prices valid only in Ontario. PANEL Healthy Living Assessment Panel $100.00 Enhanced Healthy Living Assessment Panel $180.00
ONTARIO ND PRICE LIST Note: There is a $7.76 per requisition documentation fee. Prices valid only in Ontario. PANEL Healthy Living Assessment Panel $100.00 Enhanced Healthy Living Assessment Panel $180.00
TECHNICAL NOTICE. The CPT coding in this notice is effective January 1, 2013 and replaces the coding currently in use for these assays
 TECHNICAL NOTICE The CPT coding in this notice is effective January 1, 2013 and replaces the coding currently in use for these assays December 2012 The notation (MAAA) indicates "Multianalyte Assay with
TECHNICAL NOTICE The CPT coding in this notice is effective January 1, 2013 and replaces the coding currently in use for these assays December 2012 The notation (MAAA) indicates "Multianalyte Assay with
ICL Integrative Laboratory Services Test Menu Contact ICL Client Care x300
 Alletess Food Sensitivity Fingerstick 96 Foods IgG with or without Wellness Program 184 Foods IgG with or without Wellness Program Alletess Food Allergy/Sensitivity Serum 96 Foods IgG with or without Wellness
Alletess Food Sensitivity Fingerstick 96 Foods IgG with or without Wellness Program 184 Foods IgG with or without Wellness Program Alletess Food Allergy/Sensitivity Serum 96 Foods IgG with or without Wellness
Current September 2017
 1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
1 Lab Service PRICE 87077 PATHOGEN ID 6 87088 PRESUMP ID, URINE CULTUR 6 87140 HSV CULTURE TYPING 13 87186 MIC (MIN INHIB CONC) 7 AMYLASE, SERUM 3 ANA SCREEN/RFX TITER AND DSDNA 4 ANA SCREEN/RFX TITER/COMPREHEN
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
28. Bilirubin (total, total conjugated, unconjugated, direct and indirect).
 1. 17-OH-Progesterone. 2. 5α-dihydrotestosterone (DHT). 3. Adrenocorticotropic Hormone ACTH. 4. Alanine Transaminase (ALT, SGPT). 5. Albumin, qualitative. 6. Aldosterone. 7. Allergen Specific IgG Antibody.
1. 17-OH-Progesterone. 2. 5α-dihydrotestosterone (DHT). 3. Adrenocorticotropic Hormone ACTH. 4. Alanine Transaminase (ALT, SGPT). 5. Albumin, qualitative. 6. Aldosterone. 7. Allergen Specific IgG Antibody.
Overseas Referred Tests 2014
 OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
OVERSEAS REFERRALS Listed below are some of the tests currently being offered by CML via Quest Diagnostics and Quest Nichols Institute The fees are quoted in US$ and a JA$ handling charge is also applicable.
Product List Autobio Diagnostics. Autobio. autobio diagnostics an autobio group company
 Product List Autobio Diagnostics Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of the largest
Product List Autobio Diagnostics Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of the largest
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
Clinical Laboratory. 14:41:00 Complement Component 3 50 mg/dl Oct-18
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Specific Panels. Celiac disease panel. Pancreas Panel:
 Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
June Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
June 2013 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test
Minimum Whole Blood Volumes for Microcollection Tubes for Neonates, Pediatrics, Patients less than 45 kg (100 lb) and Difficult Collections
 The following table outlines the minimum whole blood volume that must be drawn into a microcollection tube (unless otherwise stated) for an individual test on a neonate, pediatric, patient weighing less
The following table outlines the minimum whole blood volume that must be drawn into a microcollection tube (unless otherwise stated) for an individual test on a neonate, pediatric, patient weighing less
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
BID ANNUAL CONTRACT FOR LAB TESTING
 1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
1 ACID FAST BACILLISTAIN 100 10.48 1,048.00 8.00 800.00 2 AFP, TUMOR (CHIRON) 10 9.04 90.40 7.00 70.00 3 AMYLASE 160 3.13 500.80 2.75 440.00 4 BASIC METAB PNL 1,800 1.44 2,592.00 1.95 3,510.00 5 BZV IGGAB
ALPS Screen Order Set B-Cell Panel Order Set (% and absolute #) (% and Absolute #) ALPS (CD3+/CD4-/CD8-/TCR αβ+)
 All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
All test indicated in a Panel or are available individually. Version 1/1/2018 ALPS Screen B-Cell Panel (% and absolute #) (% and Absolute #) ALPS (/CD4-/CD8-/TCR αβ+) CD5+/ ANA Profile * Anti-Nuclear Ab*
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST (* updated:28/03/2017) Note: If any test(s) in esoteric list below is included in campaign profile, outlet/requester must notify processing lab at least 2-3
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST (* updated:28/03/2017) Note: If any test(s) in esoteric list below is included in campaign profile, outlet/requester must notify processing lab at least 2-3
No. Code TEST SPECIMEN TAT TO Aldolase 3 ml Plain Blood/Serum (10-12 hour fasting
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST (* updated:20/03/2018) Note: If any test(s) in esoteric list below is included in campaign profile, outlet/requester must notify processing lab at least 2-3
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST (* updated:20/03/2018) Note: If any test(s) in esoteric list below is included in campaign profile, outlet/requester must notify processing lab at least 2-3
PAML interface maintenance bulletin 5/25/2012 ATTN: INTERFACE DATA MANAGER
 PAML interface maintenance bulletin 5/25/2012 ATTN: INTERFACE DATA MANAGER 390 Please use in conjunction with PAML Test Change Alert 390 to update your database system as appropriate GA workpar decimals
PAML interface maintenance bulletin 5/25/2012 ATTN: INTERFACE DATA MANAGER 390 Please use in conjunction with PAML Test Change Alert 390 to update your database system as appropriate GA workpar decimals
Is it Autoimmune or NOT! Presented to AONP! October 2015!
 Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Business Unit: Quest Diagnostics Nichols Institute, Valencia. June 2011 Update Dear Colleague,
 Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Volume 2 Apr/2011 Business Unit: June 2011 Update Dear Colleague, This letter begins with a thank you for your very prompt response to our recent request to visit our website and update your contact information.
Thyroid Care & Diagnostic Centre
 Thyroid Care & Diagnostic Centre Laboratory Test List DEPARTMENT OF CLINICAL PATHOLOGY BLOOD : CBC HCT/ PCV Hb, TC, DC & ESR MCV CE MCH PC MCHC MP Reticulocyte count PBF LE Cell Hb-Electrophoresis Protin
Thyroid Care & Diagnostic Centre Laboratory Test List DEPARTMENT OF CLINICAL PATHOLOGY BLOOD : CBC HCT/ PCV Hb, TC, DC & ESR MCV CE MCH PC MCHC MP Reticulocyte count PBF LE Cell Hb-Electrophoresis Protin
PRICE LIST Effective Date: October 16, 2017
 Effective October 16, 2017 we are offering our new tests for Lyme IGXSpot, Lyme Borreliosis, and Tick-borne Relapsing Fever Borreliosis The new ImmunoBlot tests have replaced the original Western Blot
Effective October 16, 2017 we are offering our new tests for Lyme IGXSpot, Lyme Borreliosis, and Tick-borne Relapsing Fever Borreliosis The new ImmunoBlot tests have replaced the original Western Blot
1105 Aldolase 3 ml Plain Blood/Serum (10-12 hour fasting) 1 week NUH S'pore Alpha Thalassaemia Genotyping 3 ml EDTA blood 2-3 weeks NUH S'pore
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
 Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
Panel & Test Price List Effective Date: October 16, 2017
 -New tests now available: Bartonella IGXSpot, Bartonella Western Blot IgG & IgM -Tests Now available for New York residents: Lyme ImmunoBlots IgG & IgM *IGXSP IGXSpot Panel $442.50 Lyme IGXSpot 86352 Bartonella
-New tests now available: Bartonella IGXSpot, Bartonella Western Blot IgG & IgM -Tests Now available for New York residents: Lyme ImmunoBlots IgG & IgM *IGXSP IGXSpot Panel $442.50 Lyme IGXSpot 86352 Bartonella
Please contact the Client Services Team if you require further information.
 Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
Reference ranges are quoted on all reports where appropriate for the test carried out. The reference range and reporting units, including any interpretive information, is specific to the methodology used
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
University of Pretoria
 University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
Clinical Laboratory. [None
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Tests by age. specific inherited syndromes and inflammatory bowel disease
 Prevention Guidelines for Men 18 49 Tests by age Screening Who needs it Tests Colorectal cancer Men diagnosed with specific inherited syndromes and inflammatory bowel disease ANCA p+c ASCA IgA ASCA IgG
Prevention Guidelines for Men 18 49 Tests by age Screening Who needs it Tests Colorectal cancer Men diagnosed with specific inherited syndromes and inflammatory bowel disease ANCA p+c ASCA IgA ASCA IgG
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia
 Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
LIST OF INVESTIGATIONS
 Karyotyping: K001 K002 LIST OF INVESTIGATIONS SAMPLE CONTAINER TYPE cells For Karyotyping [Single] cells For Karyotyping [Couple] Vacutainer Vacutainer 7-8 7-8 K003 Fetal Blood Sample For Karyotyping Vacutainer
Karyotyping: K001 K002 LIST OF INVESTIGATIONS SAMPLE CONTAINER TYPE cells For Karyotyping [Single] cells For Karyotyping [Couple] Vacutainer Vacutainer 7-8 7-8 K003 Fetal Blood Sample For Karyotyping Vacutainer
CMS will not implement the new tier codes for Medicare/Medicaid claims for calendar year 2012.
 January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
January 1, 2012 Re: 2012 AMA CPT Code Changes Dear Valued Client: The American Medical Association (AMA) has made Current Procedural Terminology (CPT) code changes to the 2012 edition of the CPT coding
Acetylcholine Receptor Binding Antibody
 Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Acetylcholine Receptor Binding Antibody Order Name: ACETY BND Test Number: 5500010 REV DATE:9/17/2008 Acetylcholine Receptor Binding Antibody RIA Preferred 1 ml (0.5) Serum Clot Activator (Red Top, No-Gel)
Panel # Panel CPT Code Price 2010 AUTOIMMUNE PROFILE - BASIC 99.00
 2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
Tel: Tel: Web: CODE DESCRIPTION UNITS
 New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
Immunoassay. Product Portfolio. Part of the IDS group
 Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
ELISA. AccuDiag Autoimmune Diseases and Others. ANA Screen Qualitative ~135 min 90.4% 100%
 / AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
/ AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
MAGLUMI 1000 MAGLUMI 1000
 MAGLUMI 1000 Outstanding Technology Power of MAGLUMI Chemiluminescence Immunoassay (CLIA) System CLIA uses two important technologies, one is labeling technology which determines reaction mode; and the
MAGLUMI 1000 Outstanding Technology Power of MAGLUMI Chemiluminescence Immunoassay (CLIA) System CLIA uses two important technologies, one is labeling technology which determines reaction mode; and the
Tests that have had changes to the method/ CPT code, units of measurement, scope of analysis, reference comments, or specimen requirements.
 Updates In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below
Updates In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital
 Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Recommendations for Celiac Disease Testing. IgA & ttg IgA
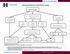 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
77th OREGON LEGISLATIVE ASSEMBLY Regular Session. Enrolled. House Bill 5202 CHAPTER... AN ACT
 77th OREGON LEGISLATIVE ASSEMBLY--2014 Regular Session Enrolled House Bill 5202 Sponsored by JOINT COMMITTEE ON WAYS AND MEANS CHAPTER... AN ACT Relating to state financial administration; and declaring
77th OREGON LEGISLATIVE ASSEMBLY--2014 Regular Session Enrolled House Bill 5202 Sponsored by JOINT COMMITTEE ON WAYS AND MEANS CHAPTER... AN ACT Relating to state financial administration; and declaring
1. Purpose 1.1. To define testing locations, schedule and order priority for each test performed in the core laboratory.
 Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
Department Of Pathology GEN.1017.03 Integrated Test Schedule Version# 5 Department Specimen Processing POLICY NO. 1938 PAGE NO. 1 OF 6 Printed copies are for reference only. Please refer to the electronic
OVERVIEW SALIVA, BLOOD SPOT & SERUM HORMONE PROFILES
 OVERVIEW SALIVA, BLOOD SPOT & SERUM HORMONE PROFILES Abbreviations as they appear on test requisitions and test reports *Tests available in New York **Saliva & Blood Spot Saliva I Saliva II Saliva III
OVERVIEW SALIVA, BLOOD SPOT & SERUM HORMONE PROFILES Abbreviations as they appear on test requisitions and test reports *Tests available in New York **Saliva & Blood Spot Saliva I Saliva II Saliva III
IMMEDIATE HOT LINE: Effective January 6, 2014
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
Volume 2 February 2011 LABORATORY UPDATE
 Volume 2 February 2011 LABORATORY UPDATE www.dlolab.com Routine Testing New Tests Bacterial Pneumonia Panel... 3 Gastrointestinal Panel... 4 Infectious Disease Panel... 5 Respiratory Infections Panel...
Volume 2 February 2011 LABORATORY UPDATE www.dlolab.com Routine Testing New Tests Bacterial Pneumonia Panel... 3 Gastrointestinal Panel... 4 Infectious Disease Panel... 5 Respiratory Infections Panel...
JAJ International, Inc./LuSys Laboratories, Inc.
 JAJ International, Inc./LuSys Laboratories, Inc. 10054 Mesa Ridge Court, Suite #120 San Diego, California 92121 USA Tel: 1(858) 866-0788, Fax: 1(858) 866-1688 Email: info@jajinternational.com Website:
JAJ International, Inc./LuSys Laboratories, Inc. 10054 Mesa Ridge Court, Suite #120 San Diego, California 92121 USA Tel: 1(858) 866-0788, Fax: 1(858) 866-1688 Email: info@jajinternational.com Website:
HML Update. Summer 2009 Volume 15, No. 3. Keeping Our Clients Informed Questions or Comments: Call
 HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
HML Update Summer 2009 Volume 15, No. 3 Keeping Our Clients Informed Questions or Comments: Call 651-232-3500 Inside: Panel Changes...1-2 New Panels...2-3 H1N1... 3 Note of Appreciation... 3 BD Vacutainer
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
In-OfficeLabTesting. Effective date: August 1, 2017
 Effective date: August 1, 2017 In-OfficeLabTesting The lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted
Effective date: August 1, 2017 In-OfficeLabTesting The lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted
Laboratory diagnosis of congenital infections
 Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
In Office Lab Testing
 Effective January 1, 201, the lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted laboratory providers.
Effective January 1, 201, the lab services below can be performed and reimbursed in an office setting. All other office-based lab services must be submitted through our contracted laboratory providers.
Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18
![Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18 Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18](/thumbs/95/125595183.jpg) Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
February Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Code Test Name Effective Date
DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations
 Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Disorders Associated with the Immune System
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 19 Disorders Associated with the Immune System Disorders of the Immune System Disorders of the
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 19 Disorders Associated with the Immune System Disorders of the Immune System Disorders of the
ISO 15189:2012 Internationally-Recognized Accredited Laboratory. SPECTRA EAST, INC. Rockleigh, NJ
 ISO 15189:2012 Internationally-Recognized Accredited Laboratory A2LA has accredited SPECTRA EAST, INC. Rockleigh, NJ for technical competence in the field of Clinical Testing This laboratory is accredited
ISO 15189:2012 Internationally-Recognized Accredited Laboratory A2LA has accredited SPECTRA EAST, INC. Rockleigh, NJ for technical competence in the field of Clinical Testing This laboratory is accredited
Received 24 November 1997/Returned for modification 11 February 1998/Accepted 6 April 1998
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, July 1998, p. 486 490 Vol. 5, No. 4 1071-412X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Seroprevalence of Antibodies
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, July 1998, p. 486 490 Vol. 5, No. 4 1071-412X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Seroprevalence of Antibodies
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schuetz C, Huck K, Gudowius S, et al. An immunodeficiency disease
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schuetz C, Huck K, Gudowius S, et al. An immunodeficiency disease
Quarterly HOTLINE: Effective November 13, 2017
 MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
MEDICARE COVERAGE OF LABORATORY TESTING Please remember when ordering laboratory tests that are billed to Medicare/Medicaid or other federally funded programs, the following requirements apply: 1. Only
Table of Contents (continued)
 Emerging Molecular and Immunohematology Blood Typing, Grouping And Infectious Disease NAT Screening Assays And Companies Developing New Technologies and Products Table of Contents 1. Blood Typing and Grouping
Emerging Molecular and Immunohematology Blood Typing, Grouping And Infectious Disease NAT Screening Assays And Companies Developing New Technologies and Products Table of Contents 1. Blood Typing and Grouping
HIV Update in Laboratory Testing. Patricia Slev, PhD, D(ABCC)
 HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
PAML interface maintenance bulletin 8/12/2013 ATTN: INTERFACE DATA MANAGER
 PAML interface maintenance bulletin 8/12/2013 ATTN: INTERFACE DATA MANAGER 410 Please use in conjunction with PAML Test Change Alert 410 to update your database system as appropriate GA workpar decimals
PAML interface maintenance bulletin 8/12/2013 ATTN: INTERFACE DATA MANAGER 410 Please use in conjunction with PAML Test Change Alert 410 to update your database system as appropriate GA workpar decimals
HIV Diagnostic Testing
 In The name of God HIV Diagnostic Testing By : Dr. Shahzamani PhD of Medical virology Purpose of HIV Testing To identify asymptomatic individuals To diagnose HIV infection in those who practice high risk
In The name of God HIV Diagnostic Testing By : Dr. Shahzamani PhD of Medical virology Purpose of HIV Testing To identify asymptomatic individuals To diagnose HIV infection in those who practice high risk
The Study of Congenital Infections. A/Prof. William Rawlinson Dr. Sian Munro
 The Study of Congenital Infections A/Prof. William Rawlinson Dr. Sian Munro Current Studies SCIP Study of Cytomegalovirus (CMV) Infection in Pregnancy ASCI Amniotic Fluid Study of Congenital Infections
The Study of Congenital Infections A/Prof. William Rawlinson Dr. Sian Munro Current Studies SCIP Study of Cytomegalovirus (CMV) Infection in Pregnancy ASCI Amniotic Fluid Study of Congenital Infections
HUMAN IMMUNODEFICIENCY VIRUS (HIV) NON-IMMEDIATE NOTIFICATION STD PROGRAM. Version
 1 HUMAN IMMUNODEFICIENCY VIRUS (HIV) NON-IMMEDIATE NOTIFICATION STD PROGRAM Event Name: Event Time Period: ADULT HIV 900 (AIDS.gov 12/31/2015) HIV Lifelong HIV (human immunodeficiency virus) is a retrovirus
1 HUMAN IMMUNODEFICIENCY VIRUS (HIV) NON-IMMEDIATE NOTIFICATION STD PROGRAM Event Name: Event Time Period: ADULT HIV 900 (AIDS.gov 12/31/2015) HIV Lifelong HIV (human immunodeficiency virus) is a retrovirus
CORRIGENDUM NOTICE 1
 CORRIGENDUM NOTICE 1 Outsourcing of High-end Laboratory Services for 7 Government District Hospitals of Uttar Pradesh [Bid Ref No: UPHSSP/PATHLAB/2015-16/02] S.N. Bid Clause ref no 1. Section II, Bidding
CORRIGENDUM NOTICE 1 Outsourcing of High-end Laboratory Services for 7 Government District Hospitals of Uttar Pradesh [Bid Ref No: UPHSSP/PATHLAB/2015-16/02] S.N. Bid Clause ref no 1. Section II, Bidding
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Building 9 Contact: Dr Catherine Sturgeon BioQuarter Tel: +44 (0)131-2426885 Little France Road Fax: +44 (0)131-242-6882 E-Mail: c.sturgeon@ed.ac.uk Websites: http://edqas.org EH16 4UX Proficiency Testing
Building 9 Contact: Dr Catherine Sturgeon BioQuarter Tel: +44 (0)131-2426885 Little France Road Fax: +44 (0)131-242-6882 E-Mail: c.sturgeon@ed.ac.uk Websites: http://edqas.org EH16 4UX Proficiency Testing
SPECTRA EAST, INC. Rockleigh, NJ
 A2LA has accredited SPECTRA EAST, INC. Rockleigh, NJ for technical competence in the field of Medical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC
A2LA has accredited SPECTRA EAST, INC. Rockleigh, NJ for technical competence in the field of Medical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC
VASCULITIS PRODUCT HIGHLIGHTS
 VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
 27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
27027 Tourney Road Valencia, CA 91355 800 421 7110 www.specialtylabs.com Test Updates July 27, 2009 Dear Colleague: As we prepare for the upcoming flu season, Specialty Laboratories clients should be aware
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Autoimmune diagnostics. A comprehensive product line for the detection of autoantibodies
 Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Annex to the Accreditation Certificate D-EP according to DIN EN ISO/IEC 17043:2010
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-EP-19851-01-00 according to DIN EN ISO/IEC 17043:2010 Period of validity: 10.04.2017 to 21.01.2021 Holder of certificate: ESfEQA
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-EP-19851-01-00 according to DIN EN ISO/IEC 17043:2010 Period of validity: 10.04.2017 to 21.01.2021 Holder of certificate: ESfEQA
SAMPLE REPORT. Order Number: PATIENT. Age: 40 Sex: F MRN:
 Patient: Age: 40 Sex: F MRN: SAMPLE PATIENT Order Number: Completed: Received: Collected: SAMPLE REPORT Progesterone ng/ml 0.34 0.95 21.00 DHEA-S mcg/dl Testosterone ng/ml 48 35 0.10 0.54 0.80 430 Sex
Patient: Age: 40 Sex: F MRN: SAMPLE PATIENT Order Number: Completed: Received: Collected: SAMPLE REPORT Progesterone ng/ml 0.34 0.95 21.00 DHEA-S mcg/dl Testosterone ng/ml 48 35 0.10 0.54 0.80 430 Sex
IN-COMMON LABORATORIES
 FREEZING STRICTLY REQUIRED (Updated Feb 2016) Results will be significantly affected by delayed freezing or repeated thawing of specimens. If the specimen thaws it is unsuitable for analysis. Α-2-Antiplasmin
FREEZING STRICTLY REQUIRED (Updated Feb 2016) Results will be significantly affected by delayed freezing or repeated thawing of specimens. If the specimen thaws it is unsuitable for analysis. Α-2-Antiplasmin
July Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated. Test Name Effective Date Page #
DECLARATION OF CONFORMITY
 Page: 1 of 5 DECLARATION OF CONFORMITY 1) Manufacturer (Name, department): Calbiotech, Inc. Address: 10461 Austin Drive, SPRING VALLEY, CA 91978, USA. and 2) European authorized representative: CEpartner4U
Page: 1 of 5 DECLARATION OF CONFORMITY 1) Manufacturer (Name, department): Calbiotech, Inc. Address: 10461 Austin Drive, SPRING VALLEY, CA 91978, USA. and 2) European authorized representative: CEpartner4U
PRODUCT LIST 2018 Immunoassays RIA, IRMA, REA
 PRODUCT LIST 2018 Immunoassays RIA, IRMA, REA REPRODUCTIVE - FERTILITY REPRODUCTIVE ANDROGENS Androstanediol glucuronide DSL9200 RIA CT 100 185 Androstenedione IM0674 RIA CT 100 185 Androstenedione DSL3800
PRODUCT LIST 2018 Immunoassays RIA, IRMA, REA REPRODUCTIVE - FERTILITY REPRODUCTIVE ANDROGENS Androstanediol glucuronide DSL9200 RIA CT 100 185 Androstenedione IM0674 RIA CT 100 185 Androstenedione DSL3800
High Hemoglobin F in a Saudi Child Presenting with Pancytopenia
 Case Report imedpub Journals http://www.imedpub.com Journal of Pediatric Care ISSN 2471-805X DOI: 10.21767/2471-805X.100002 High Hemoglobin F in a Saudi Child Presenting with Pancytopenia Abstract Saudi
Case Report imedpub Journals http://www.imedpub.com Journal of Pediatric Care ISSN 2471-805X DOI: 10.21767/2471-805X.100002 High Hemoglobin F in a Saudi Child Presenting with Pancytopenia Abstract Saudi
Disclosures. Rheumatological Approaches to Differential Diagnosis, Physical Examination, and Interpretation of Studies. None
 Rheumatological Approaches to Differential Diagnosis, Physical Examination, and Interpretation of Studies Sarah Goglin MD Assistant Professor of Medicine Division of Rheumatology Disclosures None 1 [footer
Rheumatological Approaches to Differential Diagnosis, Physical Examination, and Interpretation of Studies Sarah Goglin MD Assistant Professor of Medicine Division of Rheumatology Disclosures None 1 [footer
Bead Chemiluminescense IMMULITE 2000, 2000 XPI & 2500
 Bead Chemiluminescense IMMULITE 2000, 2000 XPI & 2500 For internal use only / Copyright Siemens AG 2006. All rights reserved. المانھای مھم در روشھای کميلومينسانس: کميلومينسانسر مرحله جداسازی.1.2 Page 2
Bead Chemiluminescense IMMULITE 2000, 2000 XPI & 2500 For internal use only / Copyright Siemens AG 2006. All rights reserved. المانھای مھم در روشھای کميلومينسانس: کميلومينسانسر مرحله جداسازی.1.2 Page 2
Test Definition: FMCPS Meningoencephalitis Comprehensive Panel (Serum)
 Reporting Title: Meningoencephalitis Comp Panel, S Performing Location: Focus Diagnostics, Specimen Requirements: Draw blood in a plain, red-top tube(s). (Serum gel tube is acceptable.) Spin down and send
Reporting Title: Meningoencephalitis Comp Panel, S Performing Location: Focus Diagnostics, Specimen Requirements: Draw blood in a plain, red-top tube(s). (Serum gel tube is acceptable.) Spin down and send
Controls & Calibrators. Disease Quality Controls
 Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
ANA testing can now be ordered in several ways, depending on the clinical circumstances:
 LAB TEST CONNECT Multiplex ANA Screen Dr. Joseph Schappert, M.D.,Medical Director Chief Medical Offi cer ANA has been the primary screening test for connective tissue diseases (CTD s) for many years. While
LAB TEST CONNECT Multiplex ANA Screen Dr. Joseph Schappert, M.D.,Medical Director Chief Medical Offi cer ANA has been the primary screening test for connective tissue diseases (CTD s) for many years. While
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
The goal of teaching:
 The goal of teaching: 1. Principles of LDG of SFI: Hair Nail Skin Mucosal Eye Ear 3. Methods for fungal identification Test methods for fungal susceptibility testing Diffusion Dilution Etest 2. Principles
The goal of teaching: 1. Principles of LDG of SFI: Hair Nail Skin Mucosal Eye Ear 3. Methods for fungal identification Test methods for fungal susceptibility testing Diffusion Dilution Etest 2. Principles
Accredited entity according to ČSN EN ISO 15189:2013 Laboratoře Mikrochem a.s. Mikrochem Laboratories Nezvalova 984/2, Hodolany, Olomouc
 Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
