Medical Device Conservation
|
|
|
- Geraldine Simpson
- 6 years ago
- Views:
Transcription
1 Medical Device Conservation The Science and Safety of Medical Device Reprocessing Think Green help save our planet!
2 Session Description The session describes the history, science and safety of medical device conservation (reprocessing). With strict FDA regulatory and manufacturing quality processes, the reprocessing of medical devices labeled for "single use" has been established as a standard practice in United States hospitals. And why it is imperative that every hospital associate embrace our reprocessing program and understand how their actions related to our reprocessing program impacts our ability to maintain safe, quality healthcare while conserving valuable resources.
3 Session Overview Reprocessing Definition Background Benefits Endorsements Widely accepted Government oversight and regulation FDA process validation Quality controlled process Vendor Disruption tactics Frequently asked questions Summary
4 Reprocessing Terminology OEM Original Equipment Manufacture SUD Stands for Single use device Hospitals recognized that the "single use" label was often motivated by economic objectives, rather than patient safety concerns. Reprocessing A FDA approved manufacturing process for SUD that upon completion the device remains substantially equivalent to its predicate device.
5 What is the definition of reprocessing Reprocessing is an FDA regulated manufacturing process by which medical devices labeled as single use, are cleaned, refurbished, tested and sterilized; making the device safe for reuse. Unlike sterilization which is the procedure of making some object free of live bacteria or other microorganisms (usually by heat or chemical means) Before a third party reprocessor can safely reprocess a medical device, the device must be cleared by the FDA through a scientific validation process called a 510K. A 510k is the compellation of comprehensive scientific and engineering test data that confirms the device is equivalent to a new device following the reprocessing process. The tests include: Functional and biocompatibility Mechanical, electrical and reliability testing Material and physical testing Sterilization
6 Reprocessing Background The reprocessing of medical devices labeled for "single use" has been standard practice in United States hospitals for over 20 years. Medical device reprocessing emerged when original equipment manufacturers (OEMs) began to change the labels on certain devices from "reusable" to "single use," without making significant structural changes in the devices. Historically, the majority of reprocessing had been conducted in the hospitals central sterile centers.
7 Reprocessing Back ground The decision to label a device as single use or reusable rests with the manufacturer, not the FDA. However, for a manufacturer to label a device as reusable, it must provide data demonstrating to the FDA s satisfaction that the device can be cleaned and sterilized without impairing its function. Therefore manufactures must label the device single use because the manufacture chooses not to conduct the studies needed to demonstrate that the device can be labeled reusable.
8 The benefits of Reprocessing There is something for everyone Reduces Supply Chain costs (on average 50% less than new) Allows physician s to use their preferred product/s without sacrificing clinical outcomes Prepares physicians for outcome/cost profiling Provides resources to other important competing priorities Indigent care New technology Community outreach programs Supporting charitable organizations
9 Reprocessing devices will divert more than 27 tons of waste from landfills over the next 3 years. A September 2008 article in Materials Management in Health Care magazine notes "for Banner Health, Phoenix, reprocessing medical devices isn t just a cost-saver, it's an Earth-saver." The article highlights the fact that "American health care facilities send 4 billion pounds of waste to landfills and commercial incinerators," and that health care is the second largest contributor to landfill space after the food service industry." According to the article, Banner officials predict that by using reprocessed devices they will divert more than 27 tons of waste from landfills over the next 3 years. AMDR - Medical Device Reprocessing in the News
10 Many leading professional organizations now endorse reprocessing AORN Reprocessing is recommended if the manufacture provides instructions, or if the reprocessor can demonstrate and document that the effectiveness and integrity of the medical device is not compromised American Hospital Association The safety record of many reprocessed SUD is as good or better than that of new devices American College Of Cardiology Reprocessing is a safe practice that hospitals and physicians have relied on for more than two decades. A significant body of peer reviewed literature demonstrates that certain devices labeled SUD can be safely and effectively reprocessed, including EP catheters JCAHO Joint Commission on Accreditation of Health Organizations removed the standard that prohibited reprocessing disposable medical devices in AAMI AAOS ACC ADDM AHA AHRMM AMDR AORN APIC ASGE ASHCSP CDC ECRI FDA HCFA (CMS) IAHCSMM JCAHO HASPE
11 Widely Accepted Over 70% of all hospitals currently reprocess 13 of 14 US News & World Report 2006 Top U.S. Hospitals All of US News & World Report Top 20 Teaching Hospitals 73% US News Top Orthopedic Hospitals 82% US News Top Heart Hospitals
12 FDA report concludes Reprocessing is safe! The GAO reported in 2000 and then again in Reprocessing does not pose an elevated health risk. In a post market surveillance of 350 hospitals conducted by the FDA on reprocessing; There were no reported increase in infections rates using reprocessed SUD s There were no reported concerns with mechanical related problems as compared to new dev ices In a report to the Committee on Government Reform the FDA concluded Reprocessing is Safe!
13 Science based cleaning and testing approach All reprocessed devices must meet these requirements: Original functionality specifications FDA s cleaning requirements FDA s sterility requirements Reprocessors must comply with: FDA 510(k) clearances and Quality System Requirements ISO quality assurance system; same as OEM s The FDA believes that reprocessed SUDs are as safe and effective as a new devices A number of the new requirements for SUD reprocessors exceed the requirements for original manufacturers. - Dr. Daniel Schultz, Director, Center for Devices and Radiological Health, FDA
14 Point of Use Collection Options Device are collected at the facility and then transported to an approved FDA third party reprocessor Several different types of point of use collections containers will be used to collect devices for reprocessing. Your manager will direct you to the location of the collection container for your area. Always place devices into the collection container. Do not inhibit the collection process by leaving any item on top of the collection container.
15 Reprocessing Cleaning Approach Devices are cleaned in consistent stages: Microblasting Manual cleaning Ultrasonic cleansing Disinfection Specialized cleaning Lumen cleaners, etc Similar to the quality approach used by pharmaceutical companies, SterilMed validates its cleaning process by measuring the level of bioburden at the molecular level These processes far exceed typical hospital practices for cleaning re-usable instruments.
16 Many devices are refurbished Sharps: computer-controlled equipment resharpens shavers and blades. Eliminates variability of manual sharpening Only reliable way to sharpen complex edges Trocars: sharpen bladed models Pulse oximeters: replace all patient contact materials Catheters: improve tip joint bond integrity on some models
17 100% of reprocessed devices are inspected Confirms that devices: Meet cleaning requirements Are free of visual defects Conforms to specifications Original equipment manufacturers test only a fraction of new devices in each individual lot Microscopic examination
18 Devices are functionally tested Specialized processes used to confirm original specs are met Mechanical Tests Sharpness Bladed devices Spring actuation Clip appliers, trocars Pressure test of seals Trocars, sleeves Electrical Sensor function All catheters, pulse ox Insulation All electrical devices Image AcuNav catheter Diagnostics Pulse ox
19 Packaging for Ease of Use Similar packaging to the manufacturer is used when possible Store the same as new devices Pull reprocessed devices first Easy to manage inventory Specifically designed for individual products, including: EP catheters - boxed tray packs and hanging peel pouches Ultrasonic scalpels - Custom designed tray packs Pulse oximeter sensors - easy to select model with color-coded packages
20 Final Step - Sterilization 100% EO sterilization Devices are sterilized using Ethylene Oxide (EO) system Biological indicators are included in every load and checked to confirm sterilization. No devices are released until BI s indicate proper kill. Scan package into tracking system to verify order completion Inspect packaging for integrity
21 Quality Monitoring and Reporting Quality Reporting Adverse event free rate (MAUDE) better than % Zero patient injury claims Superior FDA audit record I wish we had this kind of technology in our sterile processing department - SPD Manager after visiting a reprocessing facility
22 The number of device categories cleared for reprocessing will continually increase. The device categories currently approved include: DVT Sleeves Pulse Oximeters Biopsy forceps Laparoscopic instruments Trocars Harmonic scalpels Staplers and clip appliers Tourniquet cuffs Arthroscopic shavers Arthroscopic probes Burrs, bits and blades External fixators Phaco tips Open/Unused devices
23 FAQ s How are reprocessed devices marked for tracking purposes? (A) A Reprocessor tracks the number of times a device has been reprocessed by placing a permanent mark on the device each time it has been reprocessed. On catheters, a bar code may be applied to the device, which will provide data for each reprocessing. In addition, a Reprocessor must place their initials on every device. How many times can a device be reprocessed? (A) The actual number of times that a device can be reprocessed is dependent upon a number of factors: device construction, material composition, handling by the clinician and the FDA s 510K clearance. Do I need patient consent to use reprocessed SUDs? (A) No additional patient consent is necessary when using reprocessed devices. Unlike investigational devices that require special patient consent, FDA considers reprocessed devices as substantially equivalent to a new device. Therefore, no special consent form is required. At the local level, hospitals drive the decision to use any approved/cleared products including reprocessed devices as part of their best practices. General patient consent for a procedure covers the hospital s prerogative to select devices as they see fit.
24 FAQ s How do you sterilize SUDs at the end of reprocessing? (A) Most medical device manufactures and Rporcessors partner with Steris-Isomedix for all sterilization services. Steris delivers state-of-the-art services as part of a complete program that emphasizes exceptional process quality control. Steris-Isomedix employs a 100% ethylene oxide (EtO) sterilization process for the essential destruction of bacteria, viruses, spores, etc. Steris-Isomedix is regulated by the FDA and differs in intensity from EtO sterilization performed in hospitals. Does a Reprocessor guarantee its reprocessed devices and services? (A) A Reprocessor warrants that it will comply with all laws and regulations applicable to its services, including but not limited to FDA and HIPAA, and that reprocessed products will meet all FDA requirements and be fit for their intended purpose. How does a reprocessor handle a recall issued by the original equipment manufacturer (OEM) recalls? (A) A Reprocessor must be vigilant in tracking product recalls by the original equipment manufacturers (OEMs). The FDA automatically s us to alert us to recalls and our Director of Quality Assurance/Regulatory Affairs receives a bi-monthly newsletter listing current OEM recalls. Customers also alert us to OEM product recalls. It is important to note that many recalls by original equipment manufacturers are due to compromised packaging or issues associated with sterilization. A reprocessor performs both packaging and sterilization so in those recall scenarios, it may be deemed unnecessary to retrieve the product. However, since a Reprocessor odes not track OEM lot numbers, any lot number specific recall by the OEM would require SterilMed to retrieve all reprocessed devices of that type to ensure compliance.
25 FAQ s Will reprocessed devices get reimbursed inpatient? (A) For inpatient procedures, Medicare like most insurance companies uses a diagnosisbased payment system, providing a fixed reimbursement amount for the hospitals according to patient diagnosis. The hospitals then utilize procedures and equipment they deem as best practices within that reimbursement framework including approved reprocessed medical devices. For private pay/private insurance patients (roughly 10% of total hospital patients), there may be an adjustment of line item charges. In other words, in order to reflect the lower acquisition cost associated with reprocessed devices, the hospital would bill the patient (and insurance company, if applicable) for the actual cost of the item. Will reprocessed devices get reimbursed outpatient? (A) The Centers for Medicare and Medicaid Services (CMS), formerly known as the Health Care Financing Administration (HCFA) states that, since the FDA sees a reprocessed device as a substantially equivalent device, Medicare will provide payment for devices used in outpatient procedures. For private pay/private insurance patients (roughly 10% of total hospital patients), there may be an adjustment of line item charges. In other words, in order to reflect the lower acquisition cost associated with reprocessed devices, the hospital would bill the patient (and insurance company, if applicable) for the actual cost of the item.
26 FAQ s What kind of product liability insurance does a Reprocessor have? (A) A physician is not subject to increased risk of liability if a reprocessed device causes patient injury. The reasons are as follows: Physicians contractually protected A Reprocessor contractually agrees to indemnify and hold harmless a medical facility, physicians and clinicians against claims and liabilities arising out of a Reprocessors negligence or breach of warranty. A Reprocessor warrants that the reprocessed device is fit for its intended purpose. This indemnity and hold harmless obligation shall not apply to damages arising out of misuse of medical devices (including off-label use) reprocessed by a Reprocessor. A Reprocessor, not the hospital, is liable for patient injuries due to medical device failure - If a patient injury occurs due to the failure of a medical device, the device manufacturer and not the physician is held liable unless physician negligence is involved. Once a device is reprocessed, a Reprocessor is considered by the FDA and our insurance carriers to be the device manufacturer for its next use.
27 Summary Reprocessing is FDA regulated The FDA has concluded that reprocessed device are save as new devices. Post market surveillance by the FDA of over 300 accounts found: No evidence of an increase in infection rate with reprocessed products No evidence of a decline in clinical outcomes Reprocessed devices do not fail more often than similar new devices Reprocessing is better for the environment savings million s of pounds of medical waste from our landfills. Reprocessing is supported by AHA, AORN, CMS, JCAHO, and multiple other professional and regulatory organizations Reprocessing is widely accepted 70% of all hospitals reprocess and 93% of US News Best Hospitals Reprocessing is an important part of our facilities approach toward conserving valuable resources and reducing our impact on the environment.
The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012
 1 The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012 2 Topics to be Covered Introduction to AMDR Introduction to single-use medical
1 The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012 2 Topics to be Covered Introduction to AMDR Introduction to single-use medical
Topics To Be Covered. AMDR Member Companies. What is SUD reprocessing?
 1 Topics To Be Covered Dan Vukelich, Esq., CAE 18 October 2014 Introduction to AMDR Introduction to single use medical device reprocessing How regulated reprocessing works Safety, economics, and environmentalism
1 Topics To Be Covered Dan Vukelich, Esq., CAE 18 October 2014 Introduction to AMDR Introduction to single use medical device reprocessing How regulated reprocessing works Safety, economics, and environmentalism
Reprocessing at UCSF Wins for the environment and the Medical Center
 Reprocessing at UCSF Wins for the environment and the Medical Center Virginia Terra Hodge, Interventional Cardiology Chris Pollak, Performance Excellence Reprocessing is one of the best things we ve ever
Reprocessing at UCSF Wins for the environment and the Medical Center Virginia Terra Hodge, Interventional Cardiology Chris Pollak, Performance Excellence Reprocessing is one of the best things we ve ever
Maximizing Sustainability Through Reprocessing Single Use Devices
 Maximizing Sustainability Through Reprocessing Single Use Devices Maximizing our Investment in Healthcare 2011 CADTH Symposium Vancouver, BC April 4, 2011 Dianne Trudeau Operations Leader Medical Device
Maximizing Sustainability Through Reprocessing Single Use Devices Maximizing our Investment in Healthcare 2011 CADTH Symposium Vancouver, BC April 4, 2011 Dianne Trudeau Operations Leader Medical Device
SAMED Position: Reuse of SUDs
 SAMED Position: Reuse of SUDs What We Will Cover SAMED - who we are and what we do Definition of single-use device Overview of single-use device reprocessing Overview of SA and other countries regulatory
SAMED Position: Reuse of SUDs What We Will Cover SAMED - who we are and what we do Definition of single-use device Overview of single-use device reprocessing Overview of SA and other countries regulatory
2016 CADTH Symposium Dan Vukelich, Esq., CAE 11 April 2016 Ottawa
 2016 CADTH Symposium Dan Vukelich, Esq., CAE 11 April 2016 Ottawa 1 Disclosure I am employed by the Association of Medical Device Reprocessors (AMDR), the trade association representing the interests of
2016 CADTH Symposium Dan Vukelich, Esq., CAE 11 April 2016 Ottawa 1 Disclosure I am employed by the Association of Medical Device Reprocessors (AMDR), the trade association representing the interests of
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health. Preface
 U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health Preface Public Comment: The Food and Drug Administration (FDA) is announcing the availability
U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health Preface Public Comment: The Food and Drug Administration (FDA) is announcing the availability
OR Today Live! Surgical Conference Hilton Oak Brook Hills Resort August 29, 2016 Dan Vukelich, Esq., CAE
 OR Today Live! Surgical Conference Hilton Oak Brook Hills Resort August 29, 2016 Dan Vukelich, Esq., CAE 1 Topics To Be Covered Overview: Introduction to AMDR Commercial SUD reprocessing Savings Sustainability
OR Today Live! Surgical Conference Hilton Oak Brook Hills Resort August 29, 2016 Dan Vukelich, Esq., CAE 1 Topics To Be Covered Overview: Introduction to AMDR Commercial SUD reprocessing Savings Sustainability
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
(City, State, Zip Code)
 This Partner Agency Agreement, dated this day of, 2015, is between COMMUNITY FOOD SHARE, INC. (CFS), whose address is 650 South Taylor Avenue, Louisville, CO 80027, and (Partner Agency) whose address is
This Partner Agency Agreement, dated this day of, 2015, is between COMMUNITY FOOD SHARE, INC. (CFS), whose address is 650 South Taylor Avenue, Louisville, CO 80027, and (Partner Agency) whose address is
Workshop on the reprocessing of medical devices. 5. December Brussels. Nikou Ghassemieh Managing Director
 Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
Workshop on the reprocessing of medical devices 5. December 2008 - Brussels Nikou Ghassemieh Managing Director Established: 2003 Members from science, industry and medical practice Tasks and Goals: - Increasing
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Joint Commission In Dental Settings
 Joint Commission In Dental Settings Jay Afrow DMD, MHA Surveyor, Ambulatory Health Care The Joint Commission The Joint Commission Disclaimer These slides are current as of 1/19/2018. The Joint Commission
Joint Commission In Dental Settings Jay Afrow DMD, MHA Surveyor, Ambulatory Health Care The Joint Commission The Joint Commission Disclaimer These slides are current as of 1/19/2018. The Joint Commission
21 March JCI Reuse of Single-Use Devices White Paper. Dear and :
 21 March 2018 RE: JCI Reuse of Single-Use Devices White Paper Dear and : I write regarding the Joint Commission International White Paper, Reuse of Single-Use Devices: Understanding Risks and Strategies
21 March 2018 RE: JCI Reuse of Single-Use Devices White Paper Dear and : I write regarding the Joint Commission International White Paper, Reuse of Single-Use Devices: Understanding Risks and Strategies
Hospitals could save two millions - if someone acted
 Hospitals could save two millions - if someone acted How to achieve that goal? By reprocessing of some of the expensive medical single-use devices, which for example - safe for the patients has already
Hospitals could save two millions - if someone acted How to achieve that goal? By reprocessing of some of the expensive medical single-use devices, which for example - safe for the patients has already
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
June 6, Re: File Code CMS-1345-NC2. Dear Dr. Berwick:
 June 6, 2011 Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1345-NC2 Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: File Code CMS-1345-NC2
June 6, 2011 Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1345-NC2 Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: File Code CMS-1345-NC2
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Larry! 28/06/2016. House Keeping. Welcome! From the GoToWebinar page:
 A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
Section Processing
 Section 10000 Processing Table of Contents 10100 Introduction 10200 Participation 10300 Submit Requests in Pounds, Not Cases 10400 Approved Processors 10500 TDA Processing Contracts 10600 Value Pass-Through
Section 10000 Processing Table of Contents 10100 Introduction 10200 Participation 10300 Submit Requests in Pounds, Not Cases 10400 Approved Processors 10500 TDA Processing Contracts 10600 Value Pass-Through
The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led
 The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led expert panel meeting earlier this year. Hospitals
The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led expert panel meeting earlier this year. Hospitals
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL
 EN EN EN EUROPEAN COMMISSION Brussels, 27.8.2010 COM(2010) 443 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Report on the issue of the reprocessing of medical devices in
EN EN EN EUROPEAN COMMISSION Brussels, 27.8.2010 COM(2010) 443 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL Report on the issue of the reprocessing of medical devices in
PPG Single Use Medical Devices
 Area Section Subsection Document Type Infection Prevention and Control General N/A Policy Scope Approved By All Health Care Providers Original Effective Date Revised Effective Date Reviewed Date Glenda
Area Section Subsection Document Type Infection Prevention and Control General N/A Policy Scope Approved By All Health Care Providers Original Effective Date Revised Effective Date Reviewed Date Glenda
PRE-STERILIZED SPINE IMPLANTS
 PRE-STERILIZED SPINE IMPLANTS Surgical Technical Technique Report Hip Knee Spine Navigation PRE-STERILIZED SPINE IMPLANTS Technical Report Hip Knee Spine Navigation 1PRE-STERILIZED INTRODUCTION SPINE IMPLANTS
PRE-STERILIZED SPINE IMPLANTS Surgical Technical Technique Report Hip Knee Spine Navigation PRE-STERILIZED SPINE IMPLANTS Technical Report Hip Knee Spine Navigation 1PRE-STERILIZED INTRODUCTION SPINE IMPLANTS
Approval Signature: Original signed by B. Postl. June 2007
 POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
Standards, Education, Verification. Patient Focused Certification
 Standards, Education, Verification Patient Focused Certification PFC Training helps you achieve quality standards. Staff Certification is now available online www.pfctraining.org jahan@safeaccessnow.org
Standards, Education, Verification Patient Focused Certification PFC Training helps you achieve quality standards. Staff Certification is now available online www.pfctraining.org jahan@safeaccessnow.org
AST Guidelines for Best Practices in Monitoring Sterility
 1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
A high-level overview of the requirements of medical packaging standards
 Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
NEW PROVIDER ENROLLMENT FOR ADULT SITE
 New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
Darwin Marine Supply Base HSEQ Quality Management Plan
 Darwin Marine Supply Base HSEQ Quality Management Plan REVISION SUMMARY Revision Date Comment Authorised 0 29.9.13 Initial input JC 1 12.1.15 General Review JC 2 3 4 5 6 7 8 9 Revision Log Revision No
Darwin Marine Supply Base HSEQ Quality Management Plan REVISION SUMMARY Revision Date Comment Authorised 0 29.9.13 Initial input JC 1 12.1.15 General Review JC 2 3 4 5 6 7 8 9 Revision Log Revision No
Committed to Environment, Health, & Safety
 Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
Committed to Environment, Health, & Safety Environment, Health, and Safety Management System and Policy of W. R. Grace & Co. November 8, 2018 The Grace Environment, Health, and Safety Management System,
Patient Reimbursement Guide. Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment. Obtain Coverage - the Right Way
 Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
SINGLE USE MEDICAL DEVICES POLICY
 SINGLE USE MEDICAL DEVICES POLICY First Issued by/date BWW /BKW March 2001 Issue Version Purpose of Issue/Description of Change Planned Review Date 2 Update May 2011 Named Responsible Officer:- Approved
SINGLE USE MEDICAL DEVICES POLICY First Issued by/date BWW /BKW March 2001 Issue Version Purpose of Issue/Description of Change Planned Review Date 2 Update May 2011 Named Responsible Officer:- Approved
ASTM Workshop on Medical Device Cleanliness
 ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
Ultrasound Reimbursement Information for Anesthesiology 1
 GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE. Yuan Xu 18- JAN- 2018
 BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
Oral Health Provisions in Recent Health Reform: Opportunities for Public-Private Partnerships
 Oral Health Provisions in Recent Health Reform: Opportunities for Public-Private Partnerships 2010 National Primary Oral Health Conference Tuesday, October 26, 2010 Catherine M. Dunham, Executive Director
Oral Health Provisions in Recent Health Reform: Opportunities for Public-Private Partnerships 2010 National Primary Oral Health Conference Tuesday, October 26, 2010 Catherine M. Dunham, Executive Director
GUIDELINES: PEER REVIEW TRAINING BOD G [Amended BOD ; BOD ; BOD ; Initial BOD ] [Guideline]
![GUIDELINES: PEER REVIEW TRAINING BOD G [Amended BOD ; BOD ; BOD ; Initial BOD ] [Guideline] GUIDELINES: PEER REVIEW TRAINING BOD G [Amended BOD ; BOD ; BOD ; Initial BOD ] [Guideline]](/thumbs/74/70362472.jpg) GUIDELINES: PEER REVIEW TRAINING BOD G03-05-15-40 [Amended BOD 03-04-17-41; BOD 03-01-14-50; BOD 03-99-15-48; Initial BOD 06-97-03-06] [Guideline] I. Purpose Guidelines: Peer Review Training provide direction
GUIDELINES: PEER REVIEW TRAINING BOD G03-05-15-40 [Amended BOD 03-04-17-41; BOD 03-01-14-50; BOD 03-99-15-48; Initial BOD 06-97-03-06] [Guideline] I. Purpose Guidelines: Peer Review Training provide direction
Environmental, Health and Safety
 Environmental, Health and Safety Codes of Practice The Environmental, Health and Safety (EHS) Codes of Practice set forth Zimmer EHS requirements for our business functions and facilities worldwide. In
Environmental, Health and Safety Codes of Practice The Environmental, Health and Safety (EHS) Codes of Practice set forth Zimmer EHS requirements for our business functions and facilities worldwide. In
EXTERNAL TRAINER AGREEMENT. THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and -
 EXTERNAL TRAINER AGREEMENT THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and - 2566588 Ontario Ltd. operating as Fortis Fitness West (2566588 Ontario Ltd. operating as Fortis
EXTERNAL TRAINER AGREEMENT THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and - 2566588 Ontario Ltd. operating as Fortis Fitness West (2566588 Ontario Ltd. operating as Fortis
EXTERNAL TRAINER AGREEMENT. THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and -
 EXTERNAL TRAINER AGREEMENT THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and - Fortis Fitness Inc. (Fortis Fitness Inc. or Fortis Fitness or the Companies ) This Agreement
EXTERNAL TRAINER AGREEMENT THIS AGREEMENT dated as of the day of, 20. BETWEEN: (the External Trainer ) - and - Fortis Fitness Inc. (Fortis Fitness Inc. or Fortis Fitness or the Companies ) This Agreement
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
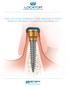 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
Lakshy Management Consultant Pvt. Ltd.
 What is ISO 13485:2003? You can t buy trust. BUILD IT through ISO 13485. Demonstrate your ability to supply medical devices through Quality Management System for Medical Devices - ISO 13485 ISO 13485 is
What is ISO 13485:2003? You can t buy trust. BUILD IT through ISO 13485. Demonstrate your ability to supply medical devices through Quality Management System for Medical Devices - ISO 13485 ISO 13485 is
College of American Pathologists
 College of American Pathologists Comments to the Food and Drug Administration on the draft guidance In Vitro Companion Diagnostics Devices October 12, 2011 College of American Pathologists 1350 I Street,
College of American Pathologists Comments to the Food and Drug Administration on the draft guidance In Vitro Companion Diagnostics Devices October 12, 2011 College of American Pathologists 1350 I Street,
PFC Training Courses & Programs. Course Descriptions. 1. Core Cannabis Training (CCT) [pre-requisite for all PFC Courses]
![PFC Training Courses & Programs. Course Descriptions. 1. Core Cannabis Training (CCT) [pre-requisite for all PFC Courses] PFC Training Courses & Programs. Course Descriptions. 1. Core Cannabis Training (CCT) [pre-requisite for all PFC Courses]](/thumbs/74/71134801.jpg) PFC Training Courses & Programs Core Cannabis Training (CCT) National Cannabis Standards Courses (NCST) State Specific Compliance Training (SSCT) PFC Enrichment Courses (PFCEC) PFC Verified Professional
PFC Training Courses & Programs Core Cannabis Training (CCT) National Cannabis Standards Courses (NCST) State Specific Compliance Training (SSCT) PFC Enrichment Courses (PFCEC) PFC Verified Professional
AMENDATORY SECTION (Amending WSR , filed 10/10/95, effective 11/10/95)
 AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
Regulatory Submission Strategies and Outlines For Ultrasonic Surgical Devices: Contact and HIFU Systems UIA Symposium Ronald R.
 Regulatory Submission Strategies and Outlines For Ultrasonic Surgical Devices: Contact and HIFU Systems 2006 UIA Symposium Ronald R. Manna Regulation Governing Medical Devices Europe: Council Directive
Regulatory Submission Strategies and Outlines For Ultrasonic Surgical Devices: Contact and HIFU Systems 2006 UIA Symposium Ronald R. Manna Regulation Governing Medical Devices Europe: Council Directive
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
Frequently Asked Questions FAQS. NeuroStar TMS Therapies
 Frequently Asked Questions FAQS NeuroStar TMS Therapies Provided by Dr Terrence A. Boyadjis MD 790 E Market Street Suite 245 West Chester, PA 19382 610.738.9576 FAQS About TMS Therapies Page 1 NeuroStar
Frequently Asked Questions FAQS NeuroStar TMS Therapies Provided by Dr Terrence A. Boyadjis MD 790 E Market Street Suite 245 West Chester, PA 19382 610.738.9576 FAQS About TMS Therapies Page 1 NeuroStar
American Emu Association. Certified Emu Oil Program
 Table of Contents Part 1. Frequently Asked Questions... 3 Part 2. AEA Certified Fully Refined Seal & Verbiage Usage Requirements... 5 Section A. Program Requirements... 5 Section B. Product Labeling Requirements...
Table of Contents Part 1. Frequently Asked Questions... 3 Part 2. AEA Certified Fully Refined Seal & Verbiage Usage Requirements... 5 Section A. Program Requirements... 5 Section B. Product Labeling Requirements...
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Ministry of Children and Youth Services. Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW
 Chapter 4 Section 4.01 Ministry of Children and Youth Services Autism Services and Supports for Children Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of
Chapter 4 Section 4.01 Ministry of Children and Youth Services Autism Services and Supports for Children Follow-up to VFM Section 3.01, 2013 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
LEAF Marque Assurance Programme
 Invisible ISEAL Code It is important that the integrity of the LEAF Marque Standard is upheld therefore the LEAF Marque Standards System has an Assurance Programme to ensure this. This document outlines
Invisible ISEAL Code It is important that the integrity of the LEAF Marque Standard is upheld therefore the LEAF Marque Standards System has an Assurance Programme to ensure this. This document outlines
This presentation focuses on recent changes in vaccine storage and handling requirements for the State Childhood Vaccine Program.
 This presentation focuses on recent changes in vaccine storage and handling requirements for the State Childhood Vaccine Program. 1 Universal vaccine policy since 1989 All children have access to vaccines
This presentation focuses on recent changes in vaccine storage and handling requirements for the State Childhood Vaccine Program. 1 Universal vaccine policy since 1989 All children have access to vaccines
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Product Sterility Testing... To Test or Not to Test? That Is the Question
 Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
Monitoring The Sterilization Process. Are We Over The Top?
 Monitoring The Sterilization Process. Are We Over The Top? Dr Brian Kirk, Senior Technical Services Specialist, Sterilization 3M Health Care Are We Over The Top? NO! Get it wrong and you kill the patient!
Monitoring The Sterilization Process. Are We Over The Top? Dr Brian Kirk, Senior Technical Services Specialist, Sterilization 3M Health Care Are We Over The Top? NO! Get it wrong and you kill the patient!
A newsletter for Molina Healthcare Provider Networks. Fall 2018
 A newsletter for Molina Healthcare Provider Networks Fall 2018 In this Issue 2018-2019 Flu Season....1 Molina Healthcare s Special Investigation Unit Partnering with You to Prevent Fraud, Waste and Abuse...2
A newsletter for Molina Healthcare Provider Networks Fall 2018 In this Issue 2018-2019 Flu Season....1 Molina Healthcare s Special Investigation Unit Partnering with You to Prevent Fraud, Waste and Abuse...2
The Joint Commission
 The Joint Commission Unannounced Full Event: 2/12/2018-2/13/2018 Low Limited Pattern Widespread Program: Ambulatory SAFER Standard EP Placement EP Text Observation Scope Requirements for Improvement APR.09.04.01
The Joint Commission Unannounced Full Event: 2/12/2018-2/13/2018 Low Limited Pattern Widespread Program: Ambulatory SAFER Standard EP Placement EP Text Observation Scope Requirements for Improvement APR.09.04.01
Graft Delivery Devices
 Graft Delivery Devices About Graft Delivery Devices Nordson MEDICAL Nordson MEDICAL is your partner in the global life sciences market providing innovative components, devices and custom OEM solutions
Graft Delivery Devices About Graft Delivery Devices Nordson MEDICAL Nordson MEDICAL is your partner in the global life sciences market providing innovative components, devices and custom OEM solutions
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Committed to Environment, Health and Safety
 Committed to Environment, Health and Safety Environment, Health and Safety Management System and Policy of GCP Applied Technologies Inc. SEPTEMBER 1, 2017 The GCP Environment, Health, and Safety Management
Committed to Environment, Health and Safety Environment, Health and Safety Management System and Policy of GCP Applied Technologies Inc. SEPTEMBER 1, 2017 The GCP Environment, Health, and Safety Management
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM
 THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
These Rules of Membership apply in respect of all Products purchased by a Member from Sigma (and any Program Partner) on or after 1 February 2017.
 Rules of Membership 1. Introduction These Rules of Membership apply in respect of all Products purchased by a Member from Sigma (and any Program Partner) on or after 1 February 2017. The previously published
Rules of Membership 1. Introduction These Rules of Membership apply in respect of all Products purchased by a Member from Sigma (and any Program Partner) on or after 1 February 2017. The previously published
Policy Title: Single-Use (Disposable) Devices Policy Number: 13. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Single-Use (Disposable) Devices Policy Number: 13 5. ROLES AND RESPONSIBILITIES: 5.1. All dental healthcare personnel have responsibility to conform and respect all aspects of this policy.
Policy Title: Single-Use (Disposable) Devices Policy Number: 13 5. ROLES AND RESPONSIBILITIES: 5.1. All dental healthcare personnel have responsibility to conform and respect all aspects of this policy.
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
Guidelines for Halton Region to Provide Waste Diversion Services to Community Events
 Attachment #1 To PW-12-10 Guidelines for Halton Region to Provide Waste Diversion Services to Community Events Purpose Halton Region is frequently requested to provide waste management services at public
Attachment #1 To PW-12-10 Guidelines for Halton Region to Provide Waste Diversion Services to Community Events Purpose Halton Region is frequently requested to provide waste management services at public
Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
Appendix C NEWBORN HEARING SCREENING PROJECT
 Appendix C NEWBORN HEARING SCREENING PROJECT I. WEST VIRGINIA STATE LAW All newborns born in the State of West Virginia must be screened for hearing impairment as required in WV Code 16-22A and 16-1-7,
Appendix C NEWBORN HEARING SCREENING PROJECT I. WEST VIRGINIA STATE LAW All newborns born in the State of West Virginia must be screened for hearing impairment as required in WV Code 16-22A and 16-1-7,
State of California Health and Human Services Agency Department of Health Services. April 30, 2007 AFL 07-09
 State of California Health and Human Services Agency Department of Health Services SANDRA SHEWRY Director Governor April 30, 2007 AFL 07-09 TO: Facility Administrators and Infection Prevention and Control
State of California Health and Human Services Agency Department of Health Services SANDRA SHEWRY Director Governor April 30, 2007 AFL 07-09 TO: Facility Administrators and Infection Prevention and Control
2018 CDC VFC Compliance Visit Requirements & Recommendations
 2018 CDC VFC Compliance Visit Requirements & Recommendations ELIGIBILITY & DOCUMENTATION Changes to Key Staff All changes in key staff must be communicated to the Immunization Program in the manner and
2018 CDC VFC Compliance Visit Requirements & Recommendations ELIGIBILITY & DOCUMENTATION Changes to Key Staff All changes in key staff must be communicated to the Immunization Program in the manner and
AST Standards of Practice for Monitoring Sterility
 AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
Precision made single use dental instruments CATALOGUE
 Precision made single use dental instruments CATALOGUE REVOLUTIONISING THE DENTAL INDUSTRY PRECISE QUALITY ERGONOMIC STERILE SINGLE USE How Does It Work? 1 SELECT YOUR KIT CONTENTS 2 RECEIVE YOUR DISPENSER
Precision made single use dental instruments CATALOGUE REVOLUTIONISING THE DENTAL INDUSTRY PRECISE QUALITY ERGONOMIC STERILE SINGLE USE How Does It Work? 1 SELECT YOUR KIT CONTENTS 2 RECEIVE YOUR DISPENSER
Reimbursement Information for Automated Breast Ultrasound Screening
 GE Healthcare Reimbursement Information for Automated Breast Ultrasound Screening January 2015 www.gehealthcare.com/reimbursement The Invenia ABUS is indicated as an adjunct to mammography for breast cancer
GE Healthcare Reimbursement Information for Automated Breast Ultrasound Screening January 2015 www.gehealthcare.com/reimbursement The Invenia ABUS is indicated as an adjunct to mammography for breast cancer
Sample Enhanced Visual Inspection of medical devices. SUBJECT: Enhanced Visual Inspection of medical devices
 Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Countdown to ICD-10: Top 10 Things to Do to Prepare for ICD-10
 Countdown to ICD-10: Top 10 Things to Do to Prepare for ICD-10 Presentation to: Providers, Trading Partners and Billing Firms Presented by: Camillia Harris, ICD-10 Communications Lead Erica Baker, ICD-10
Countdown to ICD-10: Top 10 Things to Do to Prepare for ICD-10 Presentation to: Providers, Trading Partners and Billing Firms Presented by: Camillia Harris, ICD-10 Communications Lead Erica Baker, ICD-10
Introduction. Current status of 510(k) clinical data requirements. 1 Current Status&Considerations:
 510(k) Current Status&Considerations: Conducting a Well-Controlled Clinical Study When Clinical Data is Required Introduction In an effort to promote innovation while protecting the population at large,
510(k) Current Status&Considerations: Conducting a Well-Controlled Clinical Study When Clinical Data is Required Introduction In an effort to promote innovation while protecting the population at large,
VFC NEW PROVIDER ENROLLMENT FOR PEDIATRIC SITE
 New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email the completed VFC New Provider Enrollment
New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email the completed VFC New Provider Enrollment
Quality and Excellence
 Quality and Excellence What is Accel-Heal? Accel-Heal is a compact (7cm x 4cm x 2cm), certified and patented, battery powered electroceutical treatment device (Class IIA) designed to improve and accelerate
Quality and Excellence What is Accel-Heal? Accel-Heal is a compact (7cm x 4cm x 2cm), certified and patented, battery powered electroceutical treatment device (Class IIA) designed to improve and accelerate
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION. (Moscow, 23 December 2014)
 AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
Comments of the Patient, Consumer, and Public Health Coalition. Strengthening the Center for Devices and Radiological Health s 510(k) Review Process
 March 19, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Comments of the Patient, Consumer, and Public Health Coalition on Strengthening
March 19, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Comments of the Patient, Consumer, and Public Health Coalition on Strengthening
a guide to Reimbursement of Intermittent Catheters Know your options M2116N 04.08
 a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
Because You Can t See Sterile.
 Sterilization Because You Can t See Sterile. Sterilization EVERY LOAD. Sure-Check Sterilization Pouches EVERY DAY. STEAMPlus Type 5 Sterilization Integrators EVERY WEEK. ConFirm Mail-in Sterilizer Monitoring
Sterilization Because You Can t See Sterile. Sterilization EVERY LOAD. Sure-Check Sterilization Pouches EVERY DAY. STEAMPlus Type 5 Sterilization Integrators EVERY WEEK. ConFirm Mail-in Sterilizer Monitoring
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Infection Control Manual Residential Care Part 2 Infection Control Program Guidelines IC3: CCHSA Standards
 IC3:0100 Canadian Council on Health Services Accreditation (CCHSA) Standards 1.0 PURPOSE CCHSA standards were included in this document as guidelines for the development of an infection control program.
IC3:0100 Canadian Council on Health Services Accreditation (CCHSA) Standards 1.0 PURPOSE CCHSA standards were included in this document as guidelines for the development of an infection control program.
Objectives. The Regulatory Aspects of Infection Prevention and Control. NADONA Infection Prevention and Control Webinar Series
 The Regulatory Aspects of Infection Prevention and Control J. Hudson Garrett Jr., PhD, MSN, MPH, FNP BC, PLNC, CDONA, VA BC, FACDONA PRESENTS The Regulatory Aspects of Infection Prevention and Control
The Regulatory Aspects of Infection Prevention and Control J. Hudson Garrett Jr., PhD, MSN, MPH, FNP BC, PLNC, CDONA, VA BC, FACDONA PRESENTS The Regulatory Aspects of Infection Prevention and Control
Acceptable Use Policy - Phone
 These terms are current as of August 5, 2017 and are subject to change from time to time. Please visit shaw.ca for the most current Terms of Use. Acceptable Use Policy - Phone Introduction Thank you for
These terms are current as of August 5, 2017 and are subject to change from time to time. Please visit shaw.ca for the most current Terms of Use. Acceptable Use Policy - Phone Introduction Thank you for
Preparing a US FDA Medical Device 510(K) Submission
 Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
AHIP Webinar: Top Tips for a Successful National Diabetes Prevention Program
 AHIP Webinar: Top Tips for a Successful National Diabetes Prevention Program September 20, 2016 2:00 3:00 PM EST Project funding supported through a cooperative agreement with the CDC #5US8DP004157 Welcome
AHIP Webinar: Top Tips for a Successful National Diabetes Prevention Program September 20, 2016 2:00 3:00 PM EST Project funding supported through a cooperative agreement with the CDC #5US8DP004157 Welcome
2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1
 GE Healthcare 2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 April, 2015 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and
GE Healthcare 2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 April, 2015 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and
Complex Medical Devices and Steam Sterilization. By Andrew Gay Director - Sterilizer Validation Australia
 Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Best Theratronics Annual Compliance Report
 Best Theratronics Annual Compliance Report - 2015 License: NSPFOL-14.01/2019 Licensing period: January 1, 2015 to Dec 31, 2015 Submitted: March 30, 2016 Executive Summary Best Theratronics received a Class
Best Theratronics Annual Compliance Report - 2015 License: NSPFOL-14.01/2019 Licensing period: January 1, 2015 to Dec 31, 2015 Submitted: March 30, 2016 Executive Summary Best Theratronics received a Class
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
Instruments and accessories. for vessel sealing VESSEL SEALING
 Instruments and accessories for vessel sealing VESSEL SEALING Legend Important notes The authors and Erbe Elektromedizin GmbH have taken the greatest possible care while compiling the catalogue with electro
Instruments and accessories for vessel sealing VESSEL SEALING Legend Important notes The authors and Erbe Elektromedizin GmbH have taken the greatest possible care while compiling the catalogue with electro
