Velocity Orthopedics Instrument Cleaning and Sterilization
|
|
|
- Derrick Jones
- 5 years ago
- Views:
Transcription
1 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. DESCRIPTION Velocity Orthopedics manufactures a variety of procedural instrumentation. These devices may be reusable, or a single use non-sterile instrument. Check the package labeling. Users of the device are encouraged to contact a Velocity Orthopedics representative, if in their professional judgment, they require a more comprehensive surgical technique. Velocity Orthopedics provides detailed surgical technique information and demonstration on the Velocity Orthopedics website. REFERENCES These instructions were developed using the guidance given in the following standards: ANSI/AAMI ST79, Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities ISO 17644, Sterilization of medical devices- Information to be provided by the manufacturer for the processing of resterilizable medical devices. LIMITATIONS ON PROCESSING Repeated processing has little effect on these instruments. End of life is normally determined by wear and damage due to use. A device labeled as Single Use must never be reused. Reuse may pose health and/or safety risks to the patient that can include, but are not limited to crossinfection, breakage resulting in irretrievable fragments, compromised mechanical performance due to wear, lack of or no function, no guarantee of proper cleaning or sterilization of the device. VALIDATION The recommended cleaning, disinfection and sterilization methods in this instruction for use (IFU) have been validated in compliance with federal and international guidance/standards. Cleaning, disinfecting and sterilizing equipment and materials vary in performance characteristics. Therefore, it is the responsibility of the facility/end user to perform the appropriate validation testing. CONTAINMENT AND TRANSPORTATION 1
2 It is recommended that instruments are reprocessed as soon as reasonably practical following use. Instrument trays and cases are considered reusable devices. Trays should be inspected for viable soil and must be cleaned prior to use. They can be cleaned manually or in an automatic washer using a detergent. PREPARATION FOR CLEANING When properly performed, cleaning disinfection and/or sterilization do not compromise the use and mechanical performance of these instruments. These instruments are used with or on patients who may harbor both recognized and unrecognized infections. To prevent the spread of infection, all reusable instruments must be thoroughly cleaned, disinfected and sterilized after use on each patient. 1. No assembly/disassembly of these instruments is required unless stated on the labeling, directions for use. 2. Devices that require disassembly should be disassembled prior to cleaning. 3. Remove dried-on soil from devices, especially in areas such as joints and crevices, prior to washing. INSPECTION AND MAINTENANCE 1. Velocity Orthopedics non-sterile instruments are precision medical instruments and must be used and handled with care. 2. Inspect the instruments for damage prior to use and at all stages of handling thereafter. 3. Devices with cutting functions or sharp points become dull with continuous use. This condition does not indicate a device defect. This indicates normal wear. Dull devices may require replacement if they no longer perform as designed. Inspection prior to use should include verifying the cutting ability and sharpness of these edges. 4. If damage is detected, do not use the device prior to consulting the manufacturer for guidance. 5. Dry instruments thoroughly and lubricate all moving parts with a water-soluble instrument lubricant prior to sterilization. 6. Check instruments for visible soil. Repeat cleaning if soil is visible and re-inspect. MANUAL CLEANING 1. Immediate rinsing and cleaning after use with an enzymatic cleaning detergent will effectively remove and prevent drying of adherent blood, mucus, etc. Cleaning solutions can include, but are not limited to: ENZOL enzymatic, Neodisher Mediclean Forte, and Thermosept alka clean. Caution: Low acid or high alkaline solutions are not recommended as they corrode metal parts and anodized aluminum and compromise polymer plastics, such as FEP (Flourinatedethylenepropylene), ABS (Acrolonitrile Butadiene Styrene), Ultem, Lexan, and Cycolac. 2. Scrub instruments with a soft brush, paying special attention to areas where debris might accumulate. Always avoid any harsh materials that can scratch or mar the surface of the instrument. 3. Rinse the instrument thoroughly with water following the cleaning process. 4. Check instruments for visible soil. Repeat cleaning if soil is visible and reinspect. ULTRASONIC CLEANING 1. The instrument should be placed in an ultrasonic cleaning unit for a minimum of 20 minutes and processed according to the ultrasonic unit s directions. 2
3 2. The instrument should be rinsed thoroughly with water following the ultrasonic process. 3. Check instrument for visible soil. Repeat cleaning if soil is visible, and re-inspect. AUTOMATIC WASHING 1. Disassemble the device, if applicable. 2. Load the instruments in the washer such that all design features of the device are accessible to cleaning, and such that the design features that might retain liquid can drain (hinges should be open and cannulations/holes positioned to drain). 3. Run the automatic wash cycle- minimum cycle parameters. 2 minute cold prewash at 68 +/- 9 F (20+/- 5 C) 3 minute cleaning wash (enzymatic or alkaline agent) at 140+/-9 F (60+/-5 C) 15 second rinse at 140 +/- 9 F (60+/-5 C) 1 minute thermal rinse at 176 +/- 9 F(80+/-5 C) 6 minute drying phase at high temperature 4. Automatic wash cleaning solutions can include but are not limited to ENZOL enzymatic, Neodisher Mediclean Forte, and Thermosept alka clean. CAUTION: Low acid or high alkaline solutions are not recommended, as they corrode metal parts and anodized aluminum and compromise polymer plastics, such as FEP(Flourinatedethylenepropylene), ABS (Acrolonitrile Butadiene Styrene), Ultem, Lexan, and Cycolac. 5. Check instruments for visible soil. Repeat cleaning if soil is visible, and re-inspect. MANUAL DISINFECTION 1. Instruments should be cleaned before disinfection, as blood albumen will impact the bactericide effectiveness of the solution. 2. Immerse instruments in disinfection solution for a minimum of 20 minutes. 3. Suitable disinfection solutions can include, but are not limited to CIDEX, WAVICIDE -01, Gigasept, Kohrsolin, and equivalent products. Use the supplier s instructions for preparing the solution. CAUTION: Low acid or high alkaline solutions are not recommended, as they corrode metal parts and anodized aluminum and compromise polymer plastics, such as FEP(Flourinatedethylenepropylene), ABS (Acrolonitrile Butadiene Styrene), Ultem, Lexan, and Cycolac. 4. After disinfection, the instruments should be rinsed with distilled water or preferably demineralized sterile water. 5. Dry instruments thoroughly and lubricate all moving parts with a water soluble medical instrument lubricant prior to sterilization. STERILIZATION This device may be provided either sterile or non-sterile. Check the packaging, labeling for more information. 3
4 Certain Velocity Orthopedics devices that may be used during this procedure are provided non-sterile and must be adequately cleaned and sterilized prior to use or re-use. Sterilizers vary in design and performance characteristics. Cycle parameters and the load configuration should always be verified against the sterilizer manufacturer s instructions. Cooling- The instrument must be adequately cooled after being removed from the sterilizer. Device should not be touched during the cooling process. Do not place the instrument on a cold surface, or immerse in cold fluid. Follow your country-specific guidelines, standards, and requirements STERILIZATION PARAMETERS FOR THE USA ONLY: Exposure Exposure Time Temperature Gravity-Displacement 121 C (250 F) 30 Minutes Steam Sterilization 132 C (270 F) 15 Minutes Cycle 135 C (275 F) 10 Minutes Drying Time 30 Minutes Pre-vacuum Cycle 132 C (270 F) 135 C (275 F) 4 Minutes 3 Minutes 20 to 30 Minutes 16 Minutes STERILIZATION PARAMETERS FOR OUTSIDE THE USA ONLY: Exposure Exposure Time Temperature Gravity-Displacement C 15 Minutes Steam Sterilization (270 F-275 F) 15 Minutes Cycle 121 C (250 F) 30 Minutes Drying Time Pre-vacuum Cycle 132 C -135 C (270 F-275 F) 4 Minutes 4 Minutes 20 to 30 Minutes 20 to 30 Minutes PACKAGING Singly: A standard packing material may be used. Ensure that the pack is large enough to contain the instruments without stressing the seals. Sets: Instruments may be loaded into dedicated instrument trays, or general purpose sterilization trays. Ensure that cutting edges are protected and do not exceed 8.5kg/18.7 lb per tray. Wrap the trays using the appropriate method. STORAGE Non-sterile metal devices should be stored in a clean, dry environment. The shelf life of nonsterile devices is not limited; the devices are manufactured from non-degradable material, which does not raise any question of device stability when stored under recommended conditions. SPECIAL PRECAUTIONS- TRANSMISSABLE SPONGIFORM ENCEPHALOPATHY AGENTS 4
5 It is outside the scope of this document to describe in detail the precautions that should be taken for Transmissible Spongiform Encephalopathy Agents. The agents for transmission of Creutzfeldt-Jakob disease are believed to be resilient to normal processes of disinfection and sterilization and therefore the normal processing methods of decontamination and sterilization as described above may not be appropriate where CJD transmission is a risk. In general, the tissues that come into contact with orthopedics surgical instruments are those of low TSE infectivity. However, particular precautions should be taken when handling instruments that have been used on known, suspected, or at risk patients. CAUTIONS 1. Users of this device are encouraged to contact their Velocity Orthopedics representative if, in their professional judgment, they require a more comprehensive surgical technique or more information. 2. To avoid damaging the instruments, do not impact or subject to blunt force any instruments that are designed to be turned or screwed in. When two devices are designed to be threaded together, ensure that they are fully engaged prior to use. 3. Do not use Velocity Orthopedics instruments for any purpose other than their intended use. Manipulating soft tissue or bone with an instrument not intended for that use may result in damage to the instrument. 4. Instruments with adjustable components must be handled with care. Over tightening or rough handling of the instrument may damage the locking mechanism. Locking mechanisms with internal polymer components may become weakened after repeated autoclaving. 5. Do not use an instrument that is designed specifically for a particular device with a different device. 6. Flexion of the joint with the instrument in position in the joint may result in bending or breaking of the instrument. INSTRUMENT-SPECIFIC CAUTIONS Javelin - Do not use the point of the device as a lever or pivot against bone or other hard tissue. If the point is stuck, remove it by pulling the instrument straight back. Do not twist, rotate, or move the tip back and forth, because this may cause the point to break off. Keep jaws closed while penetrating, open only when prepared to grasp the desired suture. Suture Retriever- Use only for suture management. Do not use to grasp suture tightly with the points of the jaws, instead, use a grasping instrument. Do not use to penetrate or manipulate tissue. WARNINGS After insertion of the instrument into the joint do not apply additional flexion to the joint. A piece of the broken instrument can become lodged in soft tissue and/or disappear from the arthroscopic view of the surgical field and can be left in the patient. Manufactured by: Velocity Orthopedics, Inc. Emergo Europe Jersey Blvd. Suite 360 Molenstraat 15 Authorized European Representative: 5
6 Rancho Cucamonga, CA BH, The Hague The Netherlands
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Hygienic Reprocessing
 Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Aesculap Spine Instructions for use
 Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Surgical Technique Guide
 Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
INFORMATION TO THE PRODUCT RANGES. Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS
 INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
INFORMATION TO THE PRODUCT RANGES Instructions for Cleaning, Disinfection, Sterilization, Inspection and Maintenance of Medartis Products APTUS MODUS Instructions for Cleaning, Disinfection, Sterilization,
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
UNICLIP DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM FOR DENTAL USE ONLY. FISDR / F EN / 11 / updated 06/2016 1/7
 UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Lotus transducer Instructions for use
 Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
Lotus transducer Instructions for use Instructions for use transducer Before using the product, please read the following information thoroughly Important This document provides instructions for using
Cleaning and sterilization instructions for Astra Tech Implant System EV
 Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Astra Tech Implant System Cleaning and sterilization instructions for Astra Tech Implant System EV Astra Tech Implant System CONTENTS Introduction Astra Tech Implant System EV 4 Pre cleaning 4 Cleaning
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
ProUltra Ultrasonic Non Surgical Endo Tips
 ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL
 Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Aesculap Long and Short Ventriculoscope Systems Instructions for Use
 Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
ATHLET VBR. The real winner. VBR in PEEK-OPTIMA PRODUCT INFORMATION
 ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
ATHLET VBR in PEEK-OPTIMA The real winner PRODUCT INFORMATION VBR PRODUCT INFORMATION The Concept A vertebral body replacement requires top performance Simple intra- and postoperative control Anatomic
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
S5 Rotary Files. only. Manufacturer. Packing unit. Batch code. For professional use only (0)
 EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
EN S5 Rotary Files AR Manufacturer 100 Packing unit www.sendoline.com +46 (0)8 445 88 30 Batch code only For professional use only Catalogue number Consult instructions for use Sendoline AB Box 7037, Tillverkarvägen
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Failure to follow instructions may lead to patient injury.
 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
Reprocessing of Implants: What are the Issues?
 Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
CURVES. Anatomically Adaptable IPR strips. Brochure Instructions for use Processing instructions
 CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
CURVES Anatomically Adaptable IPR strips Brochure Instructions for use Processing instructions Visit us online! To learn more about all our orthodontic products plus many helpful user videos Innovative
TABLE OF CONTENTS. PAL-650 Instrument System Instruction Manual. Applicable Part Numbers Introduction General Warnings...
 1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
THE. Curve THE CURVE STRIPPING TOOL
 THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
THE Curve THE CURVE STRIPPING TOOL Innovative Product Made in Germany THE Curve Mastering the perfect curve Curvable polishing strips for interproximal reduction Sterilizable, double-sided diamond instruments
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care
 a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
 EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
EN MANUFACTURER S INFORMATION regarding the preparation of resterilisable medical devices complies with EN ISO 17664 Please read carefully! Medical devices which may be re-processed are tools for abutments
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
Passer. Sterile. D i. Do Not PASSER DESCRIPTION. The Ceterix. Page 1
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
Complex Medical Devices and Steam Sterilization. By Andrew Gay Director - Sterilizer Validation Australia
 Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
INFECTION CONTROL IN PRACTICE. Continuing Education
 INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
PRATICAL GUIDE. Techniques for using, cleaning and sterilizing Riellens products including magnetic resonance imaging MRI
 PRATICAL GUIDE Techniques for using, cleaning and sterilizing Riellens products including magnetic resonance imaging MRI Welcome! Here you will find the use, cleaning and sterilization Techniques Guide,
PRATICAL GUIDE Techniques for using, cleaning and sterilizing Riellens products including magnetic resonance imaging MRI Welcome! Here you will find the use, cleaning and sterilization Techniques Guide,
Approval Signature: Original signed by B. Postl. June 2007
 POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
B&H SURGICAL. Laparoscopic Reference Guide. P.O. Box Santa Clarita, CA Toll Free: Ph: Fax:
 B&H SURGICAL Laparoscopic Reference Guide P.O. Box 800192 Santa Clarita, CA 91380-0192 Toll Free: 800-971-9066 Ph: 661-257-0788 Fax: 661-257-0688 5mm Diameter Shaft NE300 Allis 12mm Jaw Length NE377 Babcock
B&H SURGICAL Laparoscopic Reference Guide P.O. Box 800192 Santa Clarita, CA 91380-0192 Toll Free: 800-971-9066 Ph: 661-257-0788 Fax: 661-257-0688 5mm Diameter Shaft NE300 Allis 12mm Jaw Length NE377 Babcock
PROTAPER GOLD TM Treatment
 PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
PROTAPER GOLD TM Treatment EN FOR DENTAL USE ONLY DIRECTIONS FOR USE A04092XXGXX03 - A04102XXGXX03 - A04112XXGXX03 PROTAPER GOLD instruments for endodontic treatment: PROTAPER GOLD Shaping Files (SX, S1,
Zavation Z-Link Cervical
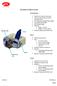 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS
 CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS w w w. c e r a r o o t. c o m Contents 3 Precise instruments 6 Be AWARE 7 Your responsability 8 Preoperative, intraoperative, and postoperative
CARE AND MAINTENANCE O F S U R G I C A L A N D PROSTHETIC INSTRUMENTS w w w. c e r a r o o t. c o m Contents 3 Precise instruments 6 Be AWARE 7 Your responsability 8 Preoperative, intraoperative, and postoperative
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
INSTRUCTIONS FOR CLEANING, DISINFECTION AND STERILIZATION OF DFS INSTRUMENTS
 Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Rolimeter 50A. Operator s Manual for measuring anterior/posterior knee laxity
 Rolimeter 50A Operator s Manual for measuring anterior/posterior knee laxity Rolimeter 50A 1 Contents Introduction................................................ 2 Design Philosophy..........................................
Rolimeter 50A Operator s Manual for measuring anterior/posterior knee laxity Rolimeter 50A 1 Contents Introduction................................................ 2 Design Philosophy..........................................
Instructions for use 0483 Permanent and temporary YASARGIL Aneurysm and Vessel Clips
 1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
1. Intended purpose The permanent YASARGIL aneurysm and vessel clips serve for permanent clamping of cerebral aneurysms. The temporary YASARGIL aneurysm and vessel clips are supplied for temporary use
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
1) INDICATIONS FOR USE These products have to be used only in hospital environments, clinics or dental offices by qualified dental personnel.
 ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
Chapter 5: Checking and Maintaining Ultrasound Equipment
 Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
High-end solution STEP-BY-STEP USER GUIDE
 High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
