Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
|
|
|
- Peregrine Washington
- 6 years ago
- Views:
Transcription
1 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray
2 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization Tray Setup...11 References
3 Introduction This reprocessing guide provides instructions for the proper cleaning and sterilization of the following sterilization tray: Autoclavable Arthroscope and Hardware Sterilization Tray This sterilization tray is intended for use only with the devices listed below. For tray/ device configuration, see the Sterilization Tray Setup section of this guide. Combination of any two of the instruments below. Stryker autoclavable arthroscopes: HD or Standard, C-mount, or with eye-piece on proximal end. Any six of the instruments below Part Number Description mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 rotating stopcocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 3.2mm cannula Blunt tip obturator for 3.2mm cannula Trocar for 3.2mm cannula mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 rotating stopcocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 4.0mm cannula Blunt tip obturator for 4.0mm cannula Trocar for 4.0mm cannula mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 rotating stopcocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 2.8mm cannula Blunt tip obturator for 2.8mm cannula Trocar for 2.8mm cannula mm cannula with 1 rotating stopcock Pencil tip obturator for 2.9mm cannula Blunt tip obturator for 2.9mm cannula Trocar for 2.9mm cannula mm cannula with 1 rotating stopcock 2
4 Any six of the instruments below Part Number Description mm cannula with 1 fixed stopcock mm cannula with 2 rotating stopcocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 4.0mm cannula Blunt tip obturator for 4.0mm cannula Trocar for 4.0mm cannula mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 rotating stopcocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 3.2mm cannula Blunt tip obturator for 3.2mm cannula Trocar for 3.2mm cannula mm cannula with 1 rotating stopcock Pencil tip obturator for 2.9mm cannula Blunt tip obturator for 2.9mm cannula Trocar for 2.9mm cannula mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 rotating stop cocks mm cannula with 2 fixed stopcocks Pencil tip obturator for 2.8mm cannula Blunt tip obturator for 2.8mm cannula Trocar for 2.8mm cannula mm cannula with 2 rotating stopcock Pencil tip obturator for 5.8mm cannula Blunt tip obturator for 5.8mm cannula Trocar for 5.8mm cannula mm cannula with 2 rotating stopcocks mm cannula with 1 rotating stopcock mm cannula with 1 fixed stopcock mm cannula with 2 fixed stopcocks Pencil tip obturator for 5.0mm cannula Trocar for 5.0mm cannula mm cannula with 1 rotating stopcock mm cannula with 2 rotating stopcocks Pencil tip obturator for 4.0mm cannula 3
5 Any six of the instruments below Part Number Description Blunt tip obturator for 4.0mm cannula Trocar for 4.0mm cannula mm cannula with 2 rotating stopcocks mm cannula with 1 rotating stopcock mm cannula with 2 fixed stopcocks mm cannula with 1 fixed stopcock Pencil tip obturator for 6.5mm cannula Blunt tip obturator for 6.5mm cannula Trocar for 6.5mm cannula mm cannula with 2 fixed stopcocks mm cannula with 2 rotating stopcocks mm cannula with 1 fixed stopcock mm cannula with 1 rotating stopcock Pencil tip obturator for 5.8mm cannula Blunt tip obturator for 5.8mm cannula Trocar for 5.8mm cannula Inflow/Outflow Cannula 2 rotating stop cocks Bridge 2 rotating stopcocks Bridge 1 rotating stopcock Bridge 2 fixed stopcocks Bridge 1 fixed stopcock While these instructions have been validated by the manufacturer as being capable of preparing the tray for re-use, it remains the responsibility of the processor to ensure that the reprocessing (as actually performed, using equipment, materials, and personnel in the reprocessing facility) achieves the desired result. This normally requires validation and routine monitoring of the process. Intended Use of Sterilization Trays Sterilization trays are plastic and/or metal containers used to hold and protect surgical devices during the sterilization process. They consist of an interlocking tray and lid, which are both perforated to allow the passage of sterilizing agent from outside the tray to the devices placed inside. Sterilization trays typically feature a silicon finger mat or group of device holders that secure devices during the sterilization process. Some models feature stacking internal trays to allow the segregation of devices. 4
6 Warnings These instructions are validated only for sterilization of the tray and device(s) identified herein. Using combinations or parameters not described in this manual may result in incomplete sterilization. These instructions do not replace the cleaning instructions provided with individual devices. Prior to sterilization, clean all devices as specified in their respective user manuals. Wear appropriate protective equipment (gloves, eye protection, etc.) when reprocessing any medical device. Both the device and the tray must be cleaned prior to sterilization, or incomplete sterilization will result. The sterilization tray, its lid, and any internal components have been designed and validated for use as a single system. Do not separate components from the system, for use individually or in combination, or incomplete sterilization may result. Inspect the tray and its components for visible damage, such as cracking or chipping, prior to use. Do not use the tray if it is damaged. Cautions Before lifting the tray assembly, verify that the latches connecting the lid to the tray are secure. The tray is not designed for use as a shipping container. To avoid damage, remove all devices and pack them separately. Limitations on Reprocessing Proper processing has a minimal effect on the tray. End of life is normally determined by wear and damage due to use. Do not leave the tray in solutions longer than necessary. This may accelerate normal product aging. Damage incurred by improper processing will not be covered by the warranty. 5
7 Instructions Instructions apply to the sterilization tray only. For instructions on how to reprocess the devices, consult their respective instructions for use. Point of Use Containment and Transportation Preparation for Manual Cleaning 1 Cleaning was validated using Enzol at 1 oz/gal. at C. Wipe excess soil from the tray using disposable paper towels. If an automated cleaning method will be used, rinse the tray in sterile distilled water immediately after use. Reprocess device(s) / tray as soon as reasonably practical following use. 1 Disassemble the tray into its individual components: base, lid, and (if applicable) silicon mat. 2 Prepare an enzymatic detergent1 according to the manufacturer s recommendations. 3 Wipe the entire tray with a clean cloth dipped in detergent. 4 Immerse the tray in the detergent. 5 Soak the tray in the detergent for 15 minutes. Manual Cleaning 1 Brush Thoroughly brush the exterior of the tray with a soft-bristled brush, focusing on any mated or rough surfaces. Using a pipe cleaner and brush, brush and flush the hard-to-reach areas of the tray. Actuate the tray, brushing any movable parts in their extreme open and closed positions. 6
8 2 Rinse Rinse the tray with reverse osmosis/deionized (RO/DI) water at ambient temperature to remove all detergent residues. After all detergent residues have been removed, continue to rinse for a minimum of 30 seconds. Drain excess water from the tray and dry it using a clean cloth and filtered pressurized air (40 psi). Visually inspect the tray for cleanliness, paying close attention to hard-to-reach areas. If visible soil remains, repeat steps 1 and 2. 3 Soak Prepare a non-enzymatic detergent2 according to the manufacturer s recommendations. Fully immerse the tray and inject any mated surfaces with at least 50mL of the detergent. Soak the tray for a minimum of 15 minutes. 4 Brush Thoroughly brush the exterior of the tray using a soft-bristled brush. Inject the prepared detergent into any mated surfaces a minimum of five times. Actuate the tray, brushing any movable parts in their extreme open and closed positions. Using a pipe cleaner and syringe, brush and flush the hard-to-reach areas of the tray. 2 Cleaning was validated using Renu-Klenz at ¼ oz/gal. at C. 5 Rinse Thoroughly rinse the tray with ambient RO/DI water until all detergent residue is removed. Flush any crevices five times with a syringe. After the detergent residue is removed, continue rinsing for a minimum of 30 seconds. Drain the excess water from the tray and dry it with a clean cloth and filtered pressurized air (40 psi). Visually inspect the tray for cleanliness, paying close attention to hard-to-reach areas. 7
9 Automated Cleaning 1 Rinse Disassemble the tray into its individual components: the base, lid, and (if applicable) silicon mat. Rinse the tray with RO/DI water at ambient temperature until there is no visible detergent residue. Use a syringe to assist in rinsing. After all detergent residues have been removed, continue to rinse for a minimum of 30 seconds. Place the tray in the washer on an incline to facilitate drainage. 2 Automated wash Program the washer using the settings below. Recirculation Time Water Temperature Detergent (type and concentration) Pre Wash 2 min. Cold tap water Enzyme Wash 2 min. Hot tap water Enzymatic Detergent3 Wash 1 2 min. Set point 60 C (140 F) Non-enzymatic Detergent4 Rinse 1 2 min. Heated 60 C (140 F) Dry Phase 7 min. Drying Temperature 115 C (239 F) 3 Drying 3 Cleaning was validated using Enzol at 1 oz/gal. 4 Cleaning was validated using Renu-Klenz at ¼ oz/gal. Remove the tray from the washer after the completion of the dry phase. Dry the tray using a clean soft cloth. Use filtered pressurized air (40 psi) to assist in drying. Visually inspect the tray for cleanliness, paying close attention to hard-to-reach areas. 8
10 Maintenance Inspection and Testing Packaging for Sterilization No routine maintenance is required for the sterilization tray. Inspect the tray for dents and cracks. If a problem is observed or suspected, the tray should be returned for repair. Inspect all components for cleanliness. If fluid or tissue buildup is present, repeat the above cleaning and disinfection procedures. Double-wrap the tray unless otherwise noted below. Sterilization Warning: Place the stopcock of the devices (where applicable) in the open position prior to sterilization else non sterile product may result. Caution: Only devices marked autoclavable are compatible with steam sterilization methods. Using steam sterilization on devices that do not bear this marking can cause permanent product damage. Prepare the device(s) and the sterilization tray as indicated in the Sterilization Tray Setup section below. Sterilize the device(s) inside the tray according to the parameters indicated below. Autoclave (Steam) Prevacuum Flash Prevacuum Wrapping double Temperature 132 C (270 F) 132 C (270 F) Time 4 minutes 4 minutes Dry Time 40 minutes 9
11 Warning: Drying time depends on several variables, including: altitude, humidity, type of wrap, preconditioning, size of chamber, mass of load, material of load, and placement in chamber. Users must verify that drying time set in their autoclave yields dry surgical equipment. Warning: These instructions were developed using the guidance from AAMI TIR 12, ISO and AAMI ST79 and Stryker recommends users observe these standards. Storage Additional Information Trays with non-absorbent liners (such as plastic or silicone-fingered organizing mats) can cause condensate to pool. To preserve sterility, remove devices from standing solution. N/A Manufacturer Contact Stryker Endoscopy 5900 Optical Court San Jose, CA ,
12 Sterilization Tray Setup Maximum Weight Load (devices and tray combined) Internal Stacking External Stacking Accessories Device Distribution Chemical Indicator* Biological Indicator* 1.31 kg (2.9 lbs) No internal stacking is permitted with this tray. Do not stack other trays or devices on or below this tray. There are no accessories available for use with this tray. Devices should be placed in the tray as illustrated below. Place the chemical indicator ( ) in the upper-right corner of the tray as illustrated in the following picture. Place the biological indicator ( ) on any scope tip as illustrated in the following picture. *Note: Placement of the biological or chemical indicator is not required during sterilization of this tray. If, per hospital procedure, placement of an indicator is desired, the recommended placement sites are as follows. 11
13 References ISO Sterilization of medical devices Information to be provided by the manufacturer for the processing of resterilizable medical devices ISO Sterilization of health care products Moist heat Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices ANSI/AAMI ST77 Containment devices for reusable medical device sterilization ANSI/AAMI ST79 A comprehensive guide to steam sterilization and sterility assurances in health care facilities ANSI/AAMI ST81 Sterilization of medical devices Information to be provided by the manufacturer for the processing of resterilizable medical devices AAMI TIR12 Designing, testing, and labeling reusable medical devices for reprocessing in health care facilities: a guide for device manufacturers 12
14 Produced for Stryker Endoscopy Optical Court. San Jose, CA USA European Representative. Regulatory Manager, Stryker France. ZAC Satolas Green Pusignan. Ave. De Satolas Green MEYZIEU Cedex, France P10298 B. 2012/05 Stryker is a registered trademark of Stryker Corporation. ENZOL Enzymatic Detergent is a registered trademark of Advanced Sterilization Products, Division of Ethicon Inc., a Johnson and Johnson company. Renu-Klenz is a trademark of STERIS corporation.
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Hygienic Reprocessing
 Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Hygienic Reprocessing HEINE UniSpec Instrument head General warning and safety information: WARNING! This symbol draws attention to a potentially dangerous situation. Non-observance can result in moderate
Straumann ProClean Cassette. Basic Information on the
 Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
Straumann ProClean Cassette Basic Information on the Straumann ProClean Cassette Contents 1. Straumann ProClean Cassette Uncompromised hygiene 2 2. Straumann ProClean Cassette product at a glance 3 2.1
TJF-160F/VF Cleaning and Disinfection Checklist
 TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
TJF-160F/VF Cleaning and Disinfection Checklist TJF-160F/VF Cleaning and Disinfection Checklist This checklist is used to evaluate and confirm if cleaning and disinfection of the TJF-160F/VF has been performed
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
INSTRUCTIONS FOR CLEANING, DISINFECTION AND STERILIZATION OF DFS INSTRUMENTS
 Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Preparation (cleaning, disinfection and sterilization) of reusable rotating dental instruments General principles All instruments must be cleaned, disinfected and sterilized before each use; this applies
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
TABLE OF CONTENTS. PAL-650 Instrument System Instruction Manual. Applicable Part Numbers Introduction General Warnings...
 1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
1 NOTES 19 TABLE OF CONTENTS PAL-650 Instrument System Instruction Manual Applicable Part Numbers................................................................... 1-4 Introduction...............................................................................
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL
 Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
Bryan-Dumon Series II Rigid Bronchoscope and Stent Placement Kit USER MANUAL Table of Contents Bryan-DUmon Series II rigid bronchoscope 1. 2. 3. 4. 5. Diagram and Overview Universal Barrel Bronchial and
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ProUltra ULTRASONIC SURGICAL TIPS
 ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
ProUltra ULTRASONIC SURGICAL TIPS EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC SURGICAL TIPS) A0640 A0650 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Surgical tips Ref. A0640 Ref. A0650 1) INDICATIONS
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Larry! 28/06/2016. House Keeping. Welcome! From the GoToWebinar page:
 A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
Aesculap Long and Short Ventriculoscope Systems Instructions for Use
 Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
B&H SURGICAL. Laparoscopic Reference Guide. P.O. Box Santa Clarita, CA Toll Free: Ph: Fax:
 B&H SURGICAL Laparoscopic Reference Guide P.O. Box 800192 Santa Clarita, CA 91380-0192 Toll Free: 800-971-9066 Ph: 661-257-0788 Fax: 661-257-0688 5mm Diameter Shaft NE300 Allis 12mm Jaw Length NE377 Babcock
B&H SURGICAL Laparoscopic Reference Guide P.O. Box 800192 Santa Clarita, CA 91380-0192 Toll Free: 800-971-9066 Ph: 661-257-0788 Fax: 661-257-0688 5mm Diameter Shaft NE300 Allis 12mm Jaw Length NE377 Babcock
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
Reprocessing of Implants: What are the Issues?
 Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
TMJ Fossa-Eminence and Condylar Prosthesis System. Instructions for Use. For total reconstruction (arthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
Dentium Surgery Kit Dental Implant Surgical Instrument Set
 www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
www.dentium.com Dentium Surgery Kit Dental Implant Surgical Instrument Set Dentium Surgery Kit is a cassette of surgical instruments for periodontal and dental implant surgery. The cassette is divided
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset
 SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
SVENSKA NEDERLANDS PORTUGUÊS ESPAÑOL ITALIANO FRANÇAIS DEUTSCH ENGLISH INSTRUCTIONS FOR USE Cleaning and disinfection Tonometer measuring prisms, contact lenses and Desinset 6. Edition / 2018 04 HAAG-STREIT
SelectTech 4.1 Bench Assembly / Owner s Manual
 SelectTech 4.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4
SelectTech 4.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4
ProUltra Ultrasonic Non Surgical Endo Tips
 ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
ProUltra Ultrasonic Non Surgical Endo Tips EN FOR DENTAL USE ONLY DIRECTIONS FOR USE (ULTRASONIC NON SURGICAL TIPS) A0620 A0621 A0630 A0631 Satelec M3 x 0,6 EMS M3 x 0,5 ProUltra Endo tips, Zirconium Ref.
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Medescan Nebuliser Med - S600A Instructions Manual
 Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Rolimeter 50A. Operator s Manual for measuring anterior/posterior knee laxity
 Rolimeter 50A Operator s Manual for measuring anterior/posterior knee laxity Rolimeter 50A 1 Contents Introduction................................................ 2 Design Philosophy..........................................
Rolimeter 50A Operator s Manual for measuring anterior/posterior knee laxity Rolimeter 50A 1 Contents Introduction................................................ 2 Design Philosophy..........................................
SelectTech 4.1 Bench. Assembly Manual
 SelectTech 4.1 Bench Assembly Manual Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4 Specifications...5 Before Assembly...5 Parts...6 Hardware...7 Tools...7
SelectTech 4.1 Bench Assembly Manual Table of Contents Important Safety Instructions...3 Safety Warning Labels and Serial Number...4 Specifications...5 Before Assembly...5 Parts...6 Hardware...7 Tools...7
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
Chapter 5: Checking and Maintaining Ultrasound Equipment
 Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
Chapter 5: Checking and Maintaining Ultrasound Equipment Care and Cleaning (BB1564-AJ) Chapter 5: Checking and Maintaining Ultrasound Equipment 45 Ultrasound equipment requires regular checks and maintenance.
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SelectTech 3.1 Bench Assembly / Owner s Manual
 SelectTech 3.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Congratulations on your commitment to fitness and your purchase of the Bowflex SelectTech 3.1
SelectTech 3.1 Bench Assembly / Owner s Manual This product is compliant with the applicable CE requirements. Congratulations on your commitment to fitness and your purchase of the Bowflex SelectTech 3.1
A82-LIFE-Dog Nebulizer NBB02
 A82-LIFE-Dog Nebulizer NBB02 פיית אוויר A82-LIFE-Dog Nebulizer NBB02 www.mfcl.co.il COMPRESSOR NEBULIZER USER S MANUAL A82-LIFE-Dog Nebulizer NBB02 ILLUSTRATION OF PARTS A. NEBULIZER COMPRESSOR
A82-LIFE-Dog Nebulizer NBB02 פיית אוויר A82-LIFE-Dog Nebulizer NBB02 www.mfcl.co.il COMPRESSOR NEBULIZER USER S MANUAL A82-LIFE-Dog Nebulizer NBB02 ILLUSTRATION OF PARTS A. NEBULIZER COMPRESSOR
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606
 PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
TONTARRA Medizintechnik GmbH. Surgical Instruments in Demand. Laminectomy Punches and Rongeurs
 TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
TONTARRA Medizintechnik GmbH Surgical Instruments in Demand Laminectomy Punches and Rongeurs KERRISON; Laminectomy Punches; clean wave KERRISON with Standard footplate Art.Nr. Size FL 240-072-02A-CW 2
INSTALLATION MANUAL. VIDEO Camera, Probe and Lightsource OTOSCOPES.
 INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
INSTALLATION MANUAL VIDEO Camera, Probe and Lightsource OTOSCOPES www.medrx-int.com Contents Using The Video Otoscope... 3 The Battery Operated LED Light Source... 4 Wiring Diagram - Battery Operated LED
Reusable Instrument Lifespan Manual
 Reusable Instrument Lifespan Manual Table of Contents Introduction... 3 Bending... 7 Discoloration... 13 Corrosion... 16 Fracture... 20 Thread Damage... 24 Surface Damage... 27 INTRODUCTION 3 Purpose This
Reusable Instrument Lifespan Manual Table of Contents Introduction... 3 Bending... 7 Discoloration... 13 Corrosion... 16 Fracture... 20 Thread Damage... 24 Surface Damage... 27 INTRODUCTION 3 Purpose This
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Compressor Nebulizer System
 MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
Zavation Z-Link Cervical
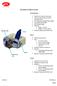 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Cleaning and sterilization. Guidelines for Nobel Biocare products including MRI information
 Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
Cleaning and sterilization Guidelines for Nobel Biocare products including MRI information Note: In order to improve readability, Nobel Biocare does not use or in the running text. By doing so, however,
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
UNICLIP DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM FOR DENTAL USE ONLY. FISDR / F EN / 11 / updated 06/2016 1/7
 UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
UNICLIP FOR DENTAL USE ONLY DIRECTIONS FOR USE REF C226U - 0,8 MM / 1 MM EN FISDR / F19 02 31.EN / 11 / 2001 - updated 06/2016 1/7 1) Indications for use These products have to be used only in hospital
ULTR A SERIES G7-S75
 ULTR A SERIES G7-S75 2 PROPER USAGE 1. Do not exceed weight limits of the exercise device. 2. If applicable, set safety stops to appropriate height. 3. If applicable, adjust seat pads, leg pads, foot pads,
ULTR A SERIES G7-S75 2 PROPER USAGE 1. Do not exceed weight limits of the exercise device. 2. If applicable, set safety stops to appropriate height. 3. If applicable, adjust seat pads, leg pads, foot pads,
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Failure to follow instructions may lead to patient injury.
 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
ASTM Workshop on Medical Device Cleanliness
 ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
Reusable Instrument Lifespan Manual
 Reusable Instrument Lifespan Manual Table of Contents Introduction... 5 Bending... 9 Discoloration... 15 Corrosion... 19 Fracture... 23 Thread Damage... 27 Surface Damage... 31 INTRODUCTION 5 INTRODUCTION
Reusable Instrument Lifespan Manual Table of Contents Introduction... 5 Bending... 9 Discoloration... 15 Corrosion... 19 Fracture... 23 Thread Damage... 27 Surface Damage... 31 INTRODUCTION 5 INTRODUCTION
For more information visit or contact hearx:
 USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
COMPRESSOR NEBULIZER MODEL: JB USER S MANUAL
 COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Approval Signature: Original signed by B. Postl. June 2007
 POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
How Do You Know If Your Sterilized Instruments Remain Sterile?
 How Do You Know If Your Sterilized Instruments Remain Sterile? Disclosure Harry Shaffer is the first author of the sterility maintenance study covered in this presentation and has been compensated by Halyard
How Do You Know If Your Sterilized Instruments Remain Sterile? Disclosure Harry Shaffer is the first author of the sterility maintenance study covered in this presentation and has been compensated by Halyard
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
1) INDICATIONS FOR USE These products have to be used only in hospital environments, clinics or dental offices by qualified dental personnel.
 ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
ProFile 04/06 & O.S. EN DENTAL USE ONLY DIRECTIONS FOR USE PROFILE 0) COMPOSITION The cutting part of these instruments is made of a nickel-titanium alloy. 1) INDICATIONS FOR USE These products have to
OWNER S MANUAL and INSTALLATION INSTRUCTIONS
 NP-B7520 GLUTE / HAM OWNER S MANUAL and INSTALLATION INSTRUCTIONS Glute / Ham Owner s Manual Copyright 2016. Core Health and Fitness, LLC. All rights reserved, including those to reproduce this book or
NP-B7520 GLUTE / HAM OWNER S MANUAL and INSTALLATION INSTRUCTIONS Glute / Ham Owner s Manual Copyright 2016. Core Health and Fitness, LLC. All rights reserved, including those to reproduce this book or
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
OWNER S MANUAL and INSTALLATION INSTRUCTIONS
 NP-L5003 Ab Crunch OWNER S MANUAL and INSTALLATION INSTRUCTIONS Leverage Ab Crunch Owner s Manual Copyright 2017. Core Health and Fitness, LLC. All rights reserved, including those to reproduce this book
NP-L5003 Ab Crunch OWNER S MANUAL and INSTALLATION INSTRUCTIONS Leverage Ab Crunch Owner s Manual Copyright 2017. Core Health and Fitness, LLC. All rights reserved, including those to reproduce this book
Sample Enhanced Visual Inspection of medical devices. SUBJECT: Enhanced Visual Inspection of medical devices
 Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
