AST Standards of Practice for Monitoring Sterility
|
|
|
- Oswin Scott
- 6 years ago
- Views:
Transcription
1 AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been approved by the AST Board of Directors on October 20, AST developed the Standards of Practice to support healthcare facilities in the reinforcement of best practices related to monitoring sterility in the perioperative setting. The purpose of the Recommended Standards is to provide an outline that Certified Surgical Technologists (CSTs) and Certified Surgical First Assistants (CSFAs) can use to develop and implement policies and procedures for monitoring sterility. The Standards are presented with the understanding that it is the responsibility of the healthcare facility to develop, approve, and establish policies and procedures for monitoring sterility according to established healthcare facility protocols. Rationale The following are Standards of Practice related to monitoring sterility in the perioperative setting. One of several safe patient outcomes related to surgery is all items that are handled and used by the sterile team members, as well as those items that come into contact with the patient s tissues are sterile. In other words to prevent surgical site infections (SSI), only sterile items may be placed within and come into contact with a sterile field and only sterile members of the surgical team should touch and handle the sterile items. 4 Guaranteeing the use of sterile items requires a quality control system that involves chemical and biological indicators that the CST and CSFA rely upon to ensure that proper sterilization parameters have been met. Additionally, the use of indicators serves as an aid in pinpointing and resolving processing failures. The delivery of safe, quality patient care demands a team effort in establishing a sterile field to prevent SSIs. All surgical team members should be involved in the process of developing and implementing healthcare facility policies and procedures for monitoring sterility. Standard of Practice I All packaged sterile items should have some type of external indicator that can be visualized by the surgical team members to ensure the parameters for sterilization have been met. 1. As recommended by the Association for the Advancement of Medical Instrumentation (AAMI), a class I process indicator, also referred to as an external
2 chemical indicator (CI), should be used on the outside of every sterile package or container. 2 (See Table A: Five Classes of CIs) A. An external CI should be attached to every healthcare facility package or rigid sterilization container that is intended to be processed through some type of sterilization system. (1) Except for those packages, such as paper-plastic peel packs that allow visualization of the internal CI, AAMI recommends an external CI be used on all packages. 2 (2) Commercial packages typically include a printed color-changing external CI that the surgical team members can visualize. B. Types of class I external CIs include sterilizer indicator tape, indicating label and indicator strips that are chemically impregnated. Upon exposure to the sterilization process, the chemical should change color and the color should be even. C. External CIs are used to demonstrate that the pack or container has been exposed to a sterilization process in order to distinguish between processed and unprocessed packs and containers. They do not prove that the enclosed items are sterile. In other words, CIs demonstrate that a package or container has been subjected to specific sterilization processing conditions. D. CIs assist in detecting potential sterilization failures. The use of CIs should be part of an overall quality assurance program that includes the use of biological indicators (BI) and sterilization machine monitors. E. The surgical team members should visualize and examine the external CI prior to opening a package or container in the OR to confirm that the item has been exposed to the sterilization process, and the color change is even. F. All types of CIs should be used according to the manufacturer s instructions. Standard of Practice II All individual units (peel packs, package, tray, rigid container system) processed by a healthcare facility should contain an internal CI. 1. It is recommended that the type of internal CI to be utilized by the healthcare facility be class 3, 4, or 5. A. Internal CIs are used to demonstrate that the pack or container has been exposed to a sterilization process in order to distinguish between processed and unprocessed packs and containers. They do not prove that the enclosed items are sterile. In other words, CIs demonstrate that a package or container has been subjected to specific sterilization processing conditions. B. Internal CIs assist in detecting potential sterilization failures. Specific types of internal CIs may aid in the detection of specific sterilization machine malfunctions, eg, air leaks, improper temperature, poor quality steam. The use of internal CIs should be part of an overall quality assurance program that includes the use of external CIs, biological indicators (BI) and sterilization machine monitors.
3 C. Internal CIs must be retrieved, visualized and examined by the CST during the time of preoperative case management to confirm that it has been exposed to the sterilization process, and the color change is even. If the CST s interpretation determines that the sterilization process has been inadequate, the contents of the individual unit should be considered nonsterile, and the unit immediately removed from the sterile field. The CST will need to make a determination of how much of the sterile field was possibly contaminated by the unit. For example, if the unit was a rigid container system in which the enclosed contents had not yet been placed on the sterile backtable, the table will not require to be broken down. Second example, a pack of sterile linen towels are tossed onto the backtable and may have touched other sterile items; therefore, the towels and other items are considered non-sterile. In this instance, the CST will have to use his/her judgment, as well as knowledge of the principles of asepsis in determining the course of action. In all instances, the CST will need to change gloves since they are considered contaminated from handling non-sterile items. D. The CST should hand the non-sterile unit to the circulator who should return it with the internal CI and load identification information to the sterile processing department. E. All types of internal CIs should be used according to the manufacturer s instructions. Process Indicator (also referred to as external CI) Table A: Five Classes of CIs 2 Indicator Class Description 1 Intended for use with individual packs, containers, peel packs, etc. Specific Test Indicators 2 Example is the Bowie-Dick type of indicator Single-parameter Indicators 3 Reacts to one of the critical parameters of sterilization Multi-parameter Indicators 4 Reacts to two or more of the critical parameters of sterilization Integrating Indicators 5 React to all critical parameters over a specified range of sterilization cycles Standards of Practice III The healthcare facility should have written policies and procedures for the training of surgical team members who interpret CIs.
4 1. Surgical team members who place, retrieve and interpret CIs should complete training in the various sterilization processes and the selection, use and interpretation of CIs. 2 A. Surgical team members handle sterile packages and rigid sterilization containers on a daily basis as well as run the flash sterilizer. The correct use and interpretation of the CI is critical to safe patient care. B. Training should be documented. Periodically, the team members should review the information, and the training should be periodically assessed to determine competence. The healthcare facility should assume the responsibility of providing updated information concerning CIs to all healthcare facility personnel who are involved in sterilization processes. Standards of Practice IV Biological indicators (BI) should be used according to the standards as established by AAMI and CDC. 1. BIs are the only sterilization process monitoring device that provides a direct measure of the lethality of the sterilization process. 2 A. BIs are used to confirm that conditions for sterilization were achieved. 2. BIs should be used within process challenge devices (PCD) for routine monitoring of the sterilization process at least weekly; however, it is recommended that the monitoring should be every day the sterilizer is used. 3. Every sterilization load that contains implants should be monitored with a PCD containing a BI. A class 5 CI should be included with the PCD. 2 A. The implants should be quarantined until the results of the BI testing are interpreted and documented. 3 B. In the instance that an implant or implants must be released for use prior to the availability of BI test results (e.g., the need for orthopedic screw and plate set on an emergency basis), the release must be documented as well as the BI result that is later recorded. The documentation should include patient information in the case that the BI result is positive in order to trace the use of the implants to the exact patient. C. Class 5 CI should be used so that if an implant or implants are released on emergency basis information about the critical parameters of the sterilization process is interpreted and documented. 2 D. The healthcare facility should establish written policies and guidelines for defining emergency situations when implants are released prior to the availability of BI test results. The development of the policies and guidelines should include the infection control officer, surgeon(s), and risk management. 4. Positive BI results will initiate a recall of the items that were processed in the specific sterilizer. All items since the last negative BI test should be considered non-sterile, and the surgery personnel should assist in retrieving those items from the surgery department sterile storage to send back to the sterile processing department. References
5 1. Association for the Advancement of Medical Instrumentation. Comprehensive guide to steam sterilization and sterility assurance in healthcare facilities. ANSI/AAMI ST79:2006. Arlington (VA): AAMI, Association for the Advancement of Medical Instrumentation. Sterilization of healthcare products Chemical indicators Guidance for the selection, use, and interpretation of results. ANSI/AAMI/ISO 15882:2003. Arlington (VA): AAMI, Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings Morbidity and Mortality Weekly Report (MMWR), 52(RR-17), 19 December 2003a. 4. Frey KB, Price BD, Ross T. Asepsis and sterile technique. In: K Frey, T Ross, eds. Surgical Technology for the Surgical Technologist: A Positive Care Approach. 3 rd ed. Clifton Park, NY: Delmar Cengage Learning; 2008;
AST Guidelines for Best Practices in Monitoring Sterility
 1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
ST79:2006 Section 10: Quality Control Key Changes
 ST79:2006 Section 10: Quality Control Key Changes Background: The Association for the Advancement of Medical Instrumentation (AAMI) newest recommended practice, Comprehensive guide to steam sterilization
ST79:2006 Section 10: Quality Control Key Changes Background: The Association for the Advancement of Medical Instrumentation (AAMI) newest recommended practice, Comprehensive guide to steam sterilization
Larry! 28/06/2016. House Keeping. Welcome! From the GoToWebinar page:
 A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
A Review of Sterilization Methods and Recommended Practices for Healthcare Facilities PART I June 30, 2016 Larry Talapa Welcome! Topic: A Review of Sterilization Methods and Recommended Practices for Healthcare
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
MANAGING STERILIZATION PROCESS MONITOR FAILURES
 MANAGING STERILIZATION PROCESS MONITOR FAILURES Joseph F. LeBouef, RST, CST, CRCST, CHL Regional Sterile Processing Educator, Kaiser Foundation Health Plan of the Northwest President, IAHCSMM Cascade Chapter
MANAGING STERILIZATION PROCESS MONITOR FAILURES Joseph F. LeBouef, RST, CST, CRCST, CHL Regional Sterile Processing Educator, Kaiser Foundation Health Plan of the Northwest President, IAHCSMM Cascade Chapter
Approval Signature: Original signed by B. Postl. June 2007
 POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
Did You Know? Education & Training. Sterilization process monitoring Part One by Martha Young, BS, MS, CSPDT
 Did You Know? Sterilization process monitoring Part One by Martha Young, BS, MS, CSPDT Objectives After completion of this self-study activity, the learner will be able to: 1. Identify when a Bowie-Dick
Did You Know? Sterilization process monitoring Part One by Martha Young, BS, MS, CSPDT Objectives After completion of this self-study activity, the learner will be able to: 1. Identify when a Bowie-Dick
Load Control Monitoring Using Process Challenge Devices
 Load Control Monitoring Using Process Challenge Devices ANSI/AAMI ST79: 2010 update by Martha Young, BS, MS, CSPDT Objectives After completion of this self-study activity, the learner will be able to:
Load Control Monitoring Using Process Challenge Devices ANSI/AAMI ST79: 2010 update by Martha Young, BS, MS, CSPDT Objectives After completion of this self-study activity, the learner will be able to:
Condensation of the AAMI Steam Sterilization Recommended Practices Quality Control (Section 10), Part II
 Condensation of the AAMI Steam Sterilization Recommended Practices Quality Control (Section 10), Part II Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities (ANSI/AAMI
Condensation of the AAMI Steam Sterilization Recommended Practices Quality Control (Section 10), Part II Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities (ANSI/AAMI
Because You Can t See Sterile.
 Sterilization Because You Can t See Sterile. Sterilization EVERY LOAD. Sure-Check Sterilization Pouches EVERY DAY. STEAMPlus Type 5 Sterilization Integrators EVERY WEEK. ConFirm Mail-in Sterilizer Monitoring
Sterilization Because You Can t See Sterile. Sterilization EVERY LOAD. Sure-Check Sterilization Pouches EVERY DAY. STEAMPlus Type 5 Sterilization Integrators EVERY WEEK. ConFirm Mail-in Sterilizer Monitoring
Monitoring The Sterilization Process. Are We Over The Top?
 Monitoring The Sterilization Process. Are We Over The Top? Dr Brian Kirk, Senior Technical Services Specialist, Sterilization 3M Health Care Are We Over The Top? NO! Get it wrong and you kill the patient!
Monitoring The Sterilization Process. Are We Over The Top? Dr Brian Kirk, Senior Technical Services Specialist, Sterilization 3M Health Care Are We Over The Top? NO! Get it wrong and you kill the patient!
How Do You Know If Your Sterilized Instruments Remain Sterile?
 How Do You Know If Your Sterilized Instruments Remain Sterile? Disclosure Harry Shaffer is the first author of the sterility maintenance study covered in this presentation and has been compensated by Halyard
How Do You Know If Your Sterilized Instruments Remain Sterile? Disclosure Harry Shaffer is the first author of the sterility maintenance study covered in this presentation and has been compensated by Halyard
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Surgical Technique Guide
 Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
How Do You Know If a Sterilized Package Remains Sterile?
 How Do You Know If a Sterilized Package Remains Sterile? 1 Acknowledgments Bioaerosol and Applied Microbiology Team Brian Heimbuch, MS Group Lead Del Harnish, MS Lead Technical Mike McDonald, MS Lead Engineer
How Do You Know If a Sterilized Package Remains Sterile? 1 Acknowledgments Bioaerosol and Applied Microbiology Team Brian Heimbuch, MS Group Lead Del Harnish, MS Lead Technical Mike McDonald, MS Lead Engineer
The AAMI Impact: What Dentistry Can Learn from AAMI ST79. Best Practices for device and instrument reprocessing
 The AAMI Impact: What Dentistry Can Learn from AAMI ST79 Best Practices for device and instrument reprocessing Learning goals: Purpose and goals of AAMI organization & overview of ST79. Review regulatory
The AAMI Impact: What Dentistry Can Learn from AAMI ST79 Best Practices for device and instrument reprocessing Learning goals: Purpose and goals of AAMI organization & overview of ST79. Review regulatory
New York Radiation Health and Safety (NYR)
 New York Radiation Health and Safety (NYR) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable
New York Radiation Health and Safety (NYR) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable
Specialty Sterilization Service
 Specialty Sterilization Service Specialty Sterilization Services Bioseal offers a unique specialty sterilization service for disposable medical devices. We source raw materials to customer specifications
Specialty Sterilization Service Specialty Sterilization Services Bioseal offers a unique specialty sterilization service for disposable medical devices. We source raw materials to customer specifications
To view an archived recording of this presentation please click the following link: Please scroll down this
 To view an archived recording of this presentation please click the following link: http://pho.adobeconnect.com/p7f3sm3cbe4i/ Please scroll down this file to view a copy of slides from the session. Helpful
To view an archived recording of this presentation please click the following link: http://pho.adobeconnect.com/p7f3sm3cbe4i/ Please scroll down this file to view a copy of slides from the session. Helpful
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Complex Medical Devices and Steam Sterilization. By Andrew Gay Director - Sterilizer Validation Australia
 Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Complex Medical Devices and Steam Sterilization By Andrew Gay Director - Sterilizer Validation Australia About 10 years ago, during a routine Performance Qualification test, an observation was made that
Reprocessing of Implants: What are the Issues?
 Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
Reprocessing of Implants: What are the Issues? Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Surgical Instrument Sets: When
FDA Foodborne Illness Risk Factor Study Data Collection Form
 APPENDIX O 2105 Data Collection Wake County Facility ID# Sample # QA FDA Foodborne Illness Risk Factor Study Data Collection Form Date: Time In: Time Out: Inspector: Establishment: Manager: Physical Address:
APPENDIX O 2105 Data Collection Wake County Facility ID# Sample # QA FDA Foodborne Illness Risk Factor Study Data Collection Form Date: Time In: Time Out: Inspector: Establishment: Manager: Physical Address:
PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE
 PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE (Updated September 7, 2006) Information and concept courtesy Of the San Francisco Public Health Department Table of Contents Pandemic
PANDEMIC INFLUENZA PHASE 6 INFECTION CONTROL RECOMMENDATIONS TEMPLATE (Updated September 7, 2006) Information and concept courtesy Of the San Francisco Public Health Department Table of Contents Pandemic
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
PRINCIPLES AND PRACTICES OF ASEPSIS OBJECTIVES
 Module E PRINCIPLES AND PRACTICES OF ASEPSIS Role of hands and the environment in disease transmission OBJECTIVES Describe the principles and practice of asepsis. Understand hand hygiene. 1 DEFINING ASEPSIS
Module E PRINCIPLES AND PRACTICES OF ASEPSIS Role of hands and the environment in disease transmission OBJECTIVES Describe the principles and practice of asepsis. Understand hand hygiene. 1 DEFINING ASEPSIS
3/26/2014 OBJECTIVES PRINCIPLES AND PRACTICES OF ASEPSIS DEFINING ASEPSIS MEDICAL ASEPSIS PRINCIPLES OF MEDICAL ASEPSIS
 Module E OBJECTIVES Describe the principles and practice of asepsis. PRINCIPLES AND PRACTICES OF ASEPSIS Understand hand hygiene. Role of hands and the environment in disease transmission DEFINING ASEPSIS
Module E OBJECTIVES Describe the principles and practice of asepsis. PRINCIPLES AND PRACTICES OF ASEPSIS Understand hand hygiene. Role of hands and the environment in disease transmission DEFINING ASEPSIS
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Talon Compounding Pharmacy 10/3/17
 Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
CRITERIA AND PROCEDURE PURPOSE OF THIS CRITERIA/PROCEDURE
 CRITERIA AND PROCEDURE BROAD SUBJECT: SANITATION AND SAFETY NO: SS-06-03 TITLE: Disinfection of surfaces after contamination with viruses EFFECTIVE DATE: August 21, 2009 PURPOSE OF THIS CRITERIA/PROCEDURE
CRITERIA AND PROCEDURE BROAD SUBJECT: SANITATION AND SAFETY NO: SS-06-03 TITLE: Disinfection of surfaces after contamination with viruses EFFECTIVE DATE: August 21, 2009 PURPOSE OF THIS CRITERIA/PROCEDURE
AMENDATORY SECTION (Amending WSR , filed 10/10/95, effective 11/10/95)
 AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
AMENDATORY SECTION (Amending WSR 95-21-041, filed 10/10/95, effective 11/10/95) WAC 246-817-601 Purpose. The purpose of WAC 246-817-601 through ((246-817-630)) 246-817-660 is to establish requirements
HOGAN & IFbuxrso~ LLP.
 HOGAN & IFbuxrso~ LLP. C. STEPHEN LAWRENCE PARTNER (948) 940-0400 CSLAWRENCEBHHLAW. COM April 30, 1999 4675 MA- cow S V m 670 NEWPORT B UM, CA 92660 TEL (949) 250450 FAX (949) 83f0976 Janice Rullo President
HOGAN & IFbuxrso~ LLP. C. STEPHEN LAWRENCE PARTNER (948) 940-0400 CSLAWRENCEBHHLAW. COM April 30, 1999 4675 MA- cow S V m 670 NEWPORT B UM, CA 92660 TEL (949) 250450 FAX (949) 83f0976 Janice Rullo President
Sample Enhanced Visual Inspection of medical devices. SUBJECT: Enhanced Visual Inspection of medical devices
 Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
Sample Enhanced Visual Inspection of medical devices SUBJECT: Enhanced Visual Inspection of medical devices DEPARTMENT: Sterile Processing Area** APPROVED BY: EFFECTIVE: REVISED: 9/2017 PURPOSE: The purpose
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
Quality Assurance Calendar Infection Control Responsible Party Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
 Statement of authority Scope of service Plan Infectious disease risk assessment Preventive activities for patients For every with risk factors patient Investigation of potential hospital For every acquired
Statement of authority Scope of service Plan Infectious disease risk assessment Preventive activities for patients For every with risk factors patient Investigation of potential hospital For every acquired
Product Sterility Testing... To Test or Not to Test? That Is the Question
 Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
INFECTION CONTROL IN PRACTICE. Continuing Education
 INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
INFECTION CONTROL IN PRACTICE Continuing Education CONTINUING EDUCATION: INFECTION CONTROL IN PRACTICE DESCRIPTION This seminar introduces infection control principals and best practices pertaining to
AKA Good Manufacturing Practice (GMP) Certification Program
 AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL
 January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT
 AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT Reprinted with the Permission of John Hill, President Iowa EMS Association Following are general guidelines for cleaning or maintaining
AMBULANCE DECONTAMINATION GUIDELINES SUSPECTED INFLUENZA PATIENT Reprinted with the Permission of John Hill, President Iowa EMS Association Following are general guidelines for cleaning or maintaining
TIP SHEET 17 ALLERGEN MANAGEMENT APPLICABLE CODE ELEMENT(S) LEARNING OBJECTIVES O CONTROL AND PREVENT THE SOURCES OF ALLERGENS O 2.8.
 ALLERGEN MANAGEMENT Food allergies affect a small but growing proportion of the population. They are most common in children, although some can be life-long, and can cause mild to severe and sometimes
ALLERGEN MANAGEMENT Food allergies affect a small but growing proportion of the population. They are most common in children, although some can be life-long, and can cause mild to severe and sometimes
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
Healthcare Sterilisation: Challenging Practices Volume 2
 Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led
 The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led expert panel meeting earlier this year. Hospitals
The Food and Drug Administration (FDA) is providing a detailed list of supplemental duodenoscope reprocessing measures that emerged from an agency-led expert panel meeting earlier this year. Hospitals
Peninsula Dental Social Enterprise (PDSE)
 Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Peninsula Dental Social Enterprise (PDSE) Decontamination - Cleaning and Disinfection Version 3.0 Date approved: November 2016 Approved by: The Board Review due: November 2019 Policy will be updated as
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION
 SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION Introduction Collection of Specimens for Microbiological Examination: Safe Handling of Specimens Packaging & Transportation of Specimens Specimen Rejection
SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION Introduction Collection of Specimens for Microbiological Examination: Safe Handling of Specimens Packaging & Transportation of Specimens Specimen Rejection
Multi-Guide II Mandibular Distractor
 Multi-Guide II Mandibular Distractor Ordering Information Basic Set Cat.No. Quantity Description Sterilization, Organization, Storage 29-10270 1 Storage Container Instrumentation 01-01305 1 Pin Driver
Multi-Guide II Mandibular Distractor Ordering Information Basic Set Cat.No. Quantity Description Sterilization, Organization, Storage 29-10270 1 Storage Container Instrumentation 01-01305 1 Pin Driver
Patient/Carer instructions for the administration of Subcutaneous Cytarabine
 Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
AIR FORCE SPECIALTY CODE 4B051 BIOENVIRONMENTAL ENGINEERING
 DEPARTMENT OF THE AIR FORCE Headquarters US Air Force Washington, DC 20330-1030 QTP 4B051-14 2 April 2015 AIR FORCE SPECIALTY CODE 4B051 BIOENVIRONMENTAL ENGINEERING USAF Personnel Dosimetry Program QUALIFICATION
DEPARTMENT OF THE AIR FORCE Headquarters US Air Force Washington, DC 20330-1030 QTP 4B051-14 2 April 2015 AIR FORCE SPECIALTY CODE 4B051 BIOENVIRONMENTAL ENGINEERING USAF Personnel Dosimetry Program QUALIFICATION
SOURCES OF INFECTION IN LONG-TERM CARE FACILITY - ENVIRONMENTAL ISSUES
 SOURCES OF INFECTION IN LONG-TERM CARE FACILITY - ENVIRONMENTAL ISSUES Slides provided by: William A. Rutala, PhD, MPH Director, Hospital Epidemiology, Occupational Health and Safety at UNC Health Care;
SOURCES OF INFECTION IN LONG-TERM CARE FACILITY - ENVIRONMENTAL ISSUES Slides provided by: William A. Rutala, PhD, MPH Director, Hospital Epidemiology, Occupational Health and Safety at UNC Health Care;
HealthPack Medical Device Packaging Technical Committee of IoPP. March 4, 2015 Norfolk, VA
 HealthPack 2015 Medical Device Packaging Technical Committee of IoPP March 4, 2015 Norfolk, VA MDPTC 2015 HealthPack Meeting Mission Statement Provide a forum for packaging professionals representing the
HealthPack 2015 Medical Device Packaging Technical Committee of IoPP March 4, 2015 Norfolk, VA MDPTC 2015 HealthPack Meeting Mission Statement Provide a forum for packaging professionals representing the
PERSONNEL MONITORING AND DOSIMETRY POLICIES
 PERSONNEL MONITORING AND DOSIMETRY POLICIES All individuals who are required to have their exposure to ionizing radiation monitored must be trained prior to using the source(s) of radiation. The radioactive
PERSONNEL MONITORING AND DOSIMETRY POLICIES All individuals who are required to have their exposure to ionizing radiation monitored must be trained prior to using the source(s) of radiation. The radioactive
San Joaquin Valley College - Visalia
 San Joaquin Valley College - Visalia Exam Site Information for Candidates Western Regional Examining Board (WREB) 2018 WREB Hygiene Examination San Joaquin Valley College Visalia 8344 West Mineral King,
San Joaquin Valley College - Visalia Exam Site Information for Candidates Western Regional Examining Board (WREB) 2018 WREB Hygiene Examination San Joaquin Valley College Visalia 8344 West Mineral King,
Scope of Practice for the Diagnostic Ultrasound Professional
 Scope of Practice for the Diagnostic Ultrasound Professional Copyright 1993-2000 Society of Diagnostic Medical Sonographers, Dallas, Texas USA: All Rights Reserved Worldwide. Organizations which endorse
Scope of Practice for the Diagnostic Ultrasound Professional Copyright 1993-2000 Society of Diagnostic Medical Sonographers, Dallas, Texas USA: All Rights Reserved Worldwide. Organizations which endorse
STANDARDIZED PROCEDURE REPROGRAMMING AND REFILLING INTRATHECAL BACLOFEN PUMPS and ACCESSING THE CATHETER ACCESS PORT (Adult,Peds)
 I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
New York State Department of Health. Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine
 New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
TissueMend. Arthroscopic Surgical Technique. Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair
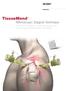 TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
TissueMend Arthroscopic Surgical Technique Arthroscopic Insertion of a Biologic Rotator Cuff Tissue Augment After Rotator Cuff Repair Table of Contents Placement of suture anchors Placement of anteromedial
Straumann ProClean Cassette. Uncompromised hygiene. Ready for action.
 Straumann ProClean Cassette Uncompromised hygiene. Ready for action. 2 100% high-grade stainless steel cassette for uncompromised performance. The unique Straumann ProClean Cassettes are made solely of
Straumann ProClean Cassette Uncompromised hygiene. Ready for action. 2 100% high-grade stainless steel cassette for uncompromised performance. The unique Straumann ProClean Cassettes are made solely of
Best Practice Title: Reporting Programmatic And Repetitive Noncompliances in NTS and SSIMS
 Best Practice Title: Reporting Programmatic And Repetitive Noncompliances in NTS and SSIMS Facility: The guidance contained in this document is based upon philosophies used at multiple sites across the
Best Practice Title: Reporting Programmatic And Repetitive Noncompliances in NTS and SSIMS Facility: The guidance contained in this document is based upon philosophies used at multiple sites across the
GLOVES. Getting a grip on infection control. a complete guide for every exposure level
 E D U C A T I O N A L S E R I E S GLOVES Getting a grip on infection control a complete guide for every exposure level TIDI understands that choosing what gloves to wear, how to wear them, and knowing
E D U C A T I O N A L S E R I E S GLOVES Getting a grip on infection control a complete guide for every exposure level TIDI understands that choosing what gloves to wear, how to wear them, and knowing
Dental Legal Case Study
 Risk Management Lessons for Dentists Infection Prevention Strategies Dentists Required to Comply with Regulations as a Condition of Licensure Chadwick v. Board of Registration in Dentistry, 461 Mass. 77;
Risk Management Lessons for Dentists Infection Prevention Strategies Dentists Required to Comply with Regulations as a Condition of Licensure Chadwick v. Board of Registration in Dentistry, 461 Mass. 77;
Technique Manual Technique Manual Rev. A 01/11/2011
 Technique Manual Technique Manual Table of Contents Introduction 4 Indications 5 Instrumentation 6 Frame Assembly 7 Position Guide 8-9 Tips and Tricks 10-13 Cleaning 14 Sterilization 14 Storage Instructions
Technique Manual Technique Manual Table of Contents Introduction 4 Indications 5 Instrumentation 6 Frame Assembly 7 Position Guide 8-9 Tips and Tricks 10-13 Cleaning 14 Sterilization 14 Storage Instructions
Dear Marie Consultation on a proposed Infection Prevention and Control Practice Standard
 Marie Warner Chief Executive Dental Council P.O. Box 10-448 Wellington 6143 15 th December 2015 Dear Marie Re: Consultation on a proposed Infection Prevention and Control Practice Standard The Association
Marie Warner Chief Executive Dental Council P.O. Box 10-448 Wellington 6143 15 th December 2015 Dear Marie Re: Consultation on a proposed Infection Prevention and Control Practice Standard The Association
TrueSight Personalized Planning & Targeting System. Operative technique
 TrueSight Personalized Planning & Targeting System Operative technique TrueSight Personalized Planning & Targeting System Contents Introduction.... 2 Indications and contraindications... 3 Design rationale...
TrueSight Personalized Planning & Targeting System Operative technique TrueSight Personalized Planning & Targeting System Contents Introduction.... 2 Indications and contraindications... 3 Design rationale...
Reprocessing of Implants What are the Issues? Dr. Michelle Alfa, Diagnostic Services of Manitoba A Webber Training Teleclass
 Reprocessing of Implants What are the Issues? Reprocessing of Implants: What are the Issues? When You Open Pandora s Box. Hear no evil. See no evil. Speak no evil. Dr. Michelle J. Alfa, Ph.D., FCCM Medical
Reprocessing of Implants What are the Issues? Reprocessing of Implants: What are the Issues? When You Open Pandora s Box. Hear no evil. See no evil. Speak no evil. Dr. Michelle J. Alfa, Ph.D., FCCM Medical
FOOD ACT No.26 OF 1980
 FOOD ACT No.26 OF 1980 REGULATIONS made by the Minister of Health in consultation with the Food Advisory Committee under section 32 of the Food Act No. 26 of 1980. Colombo 15 th November, 2005 Nimal Siripala
FOOD ACT No.26 OF 1980 REGULATIONS made by the Minister of Health in consultation with the Food Advisory Committee under section 32 of the Food Act No. 26 of 1980. Colombo 15 th November, 2005 Nimal Siripala
3.0 POSITION ON PRE-LOADING SYRINGES WITH VACCINE
 1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
Orientation Program for Infection Control Professionals. Module 9: Cleaning, Disinfection, and Sterilization of Medical Equipment and Devices
 Orientation Program for Infection Control Professionals Module 9: Cleaning, Disinfection, and Sterilization of Medical Equipment and Devices Table of Contents Module 9: Cleaning, Disinfection, and Sterilization
Orientation Program for Infection Control Professionals Module 9: Cleaning, Disinfection, and Sterilization of Medical Equipment and Devices Table of Contents Module 9: Cleaning, Disinfection, and Sterilization
Joint Commission In Dental Settings
 Joint Commission In Dental Settings Jay Afrow DMD, MHA Surveyor, Ambulatory Health Care The Joint Commission The Joint Commission Disclaimer These slides are current as of 1/19/2018. The Joint Commission
Joint Commission In Dental Settings Jay Afrow DMD, MHA Surveyor, Ambulatory Health Care The Joint Commission The Joint Commission Disclaimer These slides are current as of 1/19/2018. The Joint Commission
Rapid-VIDITEST. Strep A Blister. One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture.
 Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
Rapid-VIDITEST Strep A Blister One step Strep A Blister for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II
KASM Knee Articulating Spacer Mold. Surgical Summary
 KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Policy Title: Clinical Asepsis Policy Policy Number :19. Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Clinical Asepsis Policy Policy Number :19 6.4.9. Take/send instruments and handpieces to the decontamination/sterilization area. 6.4.10. Remove and dispose of the disposable gown (if used)
Policy Title: Clinical Asepsis Policy Policy Number :19 6.4.9. Take/send instruments and handpieces to the decontamination/sterilization area. 6.4.10. Remove and dispose of the disposable gown (if used)
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
7% - 15% Single Tunnel Repair of Plantar Plate. Savings of. are common with EcoSMART *Patent Pending
 * Provide Value Sterile kit packaging provides high quality, surgical grade instruments and implants making your O.R. more efficient. No hassles or expense from cleaning, storing, sterilizing, and maintaining
* Provide Value Sterile kit packaging provides high quality, surgical grade instruments and implants making your O.R. more efficient. No hassles or expense from cleaning, storing, sterilizing, and maintaining
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
EHS Guide: Laboratory Trash Removal
 EHS Guide: Laboratory Trash Removal In order to minimize risk, provide safety and assure compliance with applicable federal and state regulations, any waste (trash) generated by laboratories must be properly
EHS Guide: Laboratory Trash Removal In order to minimize risk, provide safety and assure compliance with applicable federal and state regulations, any waste (trash) generated by laboratories must be properly
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
4Fusion. Shape Memory Implant. Operative Technique
 4Fusion Shape Memory Implant Operative Technique 4Fusion This publication sets forth detailed recommended procedures for using Stryker devices and instruments. It offers guidance that you should heed,
4Fusion Shape Memory Implant Operative Technique 4Fusion This publication sets forth detailed recommended procedures for using Stryker devices and instruments. It offers guidance that you should heed,
A self-tapping internal hex. implant by MAKE IT SIMPLE
 A self-tapping internal hex. implant by MAKE IT SIMPLE P. 4-5 P. 6-7 P. 8 P. 9 P.10 P.11 P.12 P.13 P.14 P.15 P.16-17 P. 18-19 P. 20-21 P. 22 P. 23 P. 24-25 MIS Warranty: MIS exercises great care and effort
A self-tapping internal hex. implant by MAKE IT SIMPLE P. 4-5 P. 6-7 P. 8 P. 9 P.10 P.11 P.12 P.13 P.14 P.15 P.16-17 P. 18-19 P. 20-21 P. 22 P. 23 P. 24-25 MIS Warranty: MIS exercises great care and effort
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Disinfection and Decontamination Core Subject. The Process of Instrument Decontamination
 Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
Disinfection and Decontamination Core Subject The Process of Instrument Decontamination Aims: To give an overview of the processes involved in dental instrument decontamination using essential quality
OBJECTIVES PRINCIPLES AND PRACTICES OF ASEPSIS
 Module E OBJECTIVES PRINCIPLES AND PRACTICES OF ASEPSIS Statewide Program for Infection Control and Epidemiology (SPCE) UNC School of Medicine Describe the principles and practice of asepsis Discuss the
Module E OBJECTIVES PRINCIPLES AND PRACTICES OF ASEPSIS Statewide Program for Infection Control and Epidemiology (SPCE) UNC School of Medicine Describe the principles and practice of asepsis Discuss the
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml
 EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Radiation Health and Safety (RHS )
 Radiation Health and Safety (RHS ) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable duties
Radiation Health and Safety (RHS ) Exam Outline and Suggested References State Regulations Each state s dental board implements regulations and establishes rules for delegating legally allowable duties
Availability of FSIS Compliance Guidelines for Allergens and. Ingredients of Public Health Concern: Identification, Prevention
 This document is scheduled to be published in the Federal Register on 11/16/2015 and available online at http://federalregister.gov/a/2015-28935, and on FDsys.gov Billing Code 3410-DM-P DEPARTMENT OF AGRICULTURE
This document is scheduled to be published in the Federal Register on 11/16/2015 and available online at http://federalregister.gov/a/2015-28935, and on FDsys.gov Billing Code 3410-DM-P DEPARTMENT OF AGRICULTURE
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Urgent Safety Notice. Recall. concerning the. sterile Trauma Plate Systems
 aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
