Rheumatoid arthritis: More aggressive approach improves outlook
|
|
|
- Brian Willis
- 5 years ago
- Views:
Transcription
1 MEDICAL GRAND ROUNDS CME CREDIT TAKE-HOME POINTS FROM LECTURES BY CLEVELAND CLINIC AND VISITING FACULTY Rheumatoid arthritis: More aggressive approach improves outlook MICHAEL E. WEINBLATT, MD * Co-director of Clinical Rheumatology, Brigham and Women s Hospital; Professor of Medicine, Harvard Medical School, Boston, Mass ABSTRACT As recently as 10 years ago, many patients with rheumatoid arthritis would receive only a nonsteroidal antiinflammatory drug and low-dose corticosteroids until damage to their joints was documented. Now, despite risks of toxicity and adverse effects, a disease-modifying antirheumatic drug such as methotrexate is given as early as possible to retard disease progression and help prevent new erosions. Other agents can be added to or used in place of methotrexate, such as a biologic response modifier that regulates the proinflammatory cytokine tumor necrosis factor-alpha. A 55-YEAR-OLD WOMAN with a 6-month history of rheumatoid arthritis complains of several hours of daily morning stiffness, tenderness, swelling, and difficulty performing her activities of daily living. She has polyarthritis that affects her hands, wrists, elbows, knees, and feet. Her sedimentation rate is 85, and she tests positive for rheumatoid factor. What do you do? Medical Grand Rounds articles are based on edited transcripts from Division of Medicine Grand Rounds presentations at The Cleveland Clinic. They are approved by the author but are not peer-reviewed. *The author has indicated that he has received grant or research support from the Abbott, Amgen, Bristol-Myers Squibb, and Millennium companies and has served as a consultant for Abbott, Amgen, Bristol-Myers Squibb, Centocor, Genentech, Merck, Millennium, Roche, TAP, and Wyeth. This paper discusses therapies that are investigational or are not approved by the US Food and Drug Administration for the use under discussion. Fifty years ago, the crippling course of rheumatoid arthritis was considered inevitable. Just a decade ago, you would have given this patient a low-dose corticosteroid and watched for the first signs of joint destruction. Now, however, therapies have evolved to the point that rheumatoid arthritis can be treated using targeted drugs that can lead to significant improvement in disease response. 1 Gone is the pyramid approach, which started patients on a nonsteroidal anti-inflammatory drug (NSAID) and a low-dose cortico-steroid. The physician then watched over several years for the appearance of structural joint damage, and only then would intervene with a disease-modifying antirheumatic drug (DMARD). Now, as soon as the diagnosis is established, we start patients on a DMARD usually methotrexate often in addition to an NSAID and a corticosteroid. The goal of this more aggressive therapy is to prevent joint damage. RHEUMATOID ARTHRITIS: A SYSTEMIC INFLAMMATORY DISEASE Rheumatoid arthritis is a systemic, chronic, inflammatory disease that affects mainly the synovial joints. The erosions, joint space narrowing, subluxation, and osteopenia that result from this multicellular process can be seen radiographically as early as the first 6 months of the disease. A profusion of proinflammatory cytokines (eg, tumor necrosis factor-alpha [TNF-alpha], interleukin-1 [IL-1]) and a dense inflammatory infiltrate of T cells and B cells occur in the synovium. TNF-alpha, produced by synovial cells and macrophages, stimulates the production of other inflammatory cytokines and metalloproteinase and is also an important Consider giving a DMARD at the time of diagnosis CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 71 NUMBER 5 MAY
2 RHEUMATOID ARTHRITIS WEINBLATT S A historical perspective: From gold to whiskey ome of the treatments tried in the past are now considered to be of limited value, or even harmful. They include intramuscular injections of gold, bed rest in the open air, a full and varied diet, a small quantity of whiskey or gin with the evening meal, iodine, quinine, vaccination, colonic lavage, arsenic, protein shock, and baths. Most of these treatments have fallen from favor, but quinine is still used in the form of the antimalarial drug hydroxychloroquine, and the new anti-tnf drugs are proteins made by recombinant DNA technology. In 1925, corticosteroids were discovered, and for the next 60 years they dominated the treatment of rheumatoid arthritis. Dr. Philip Hench at the Mayo Clinic received the Nobel Prize in medicine for his pioneering work on the use of corticosteroids in rheumatoid arthritis. 2 During World War II, the United States Department of War funded research on corticosteroids and found that they led to a dramatic improvement in the disease process. Other compounds studied have included nitrogen mustard and azathioprine. The squeeze test helps separate rheumatoid arthritis from osteoarthritis or fibromyalgia factor in the fatigue associated with rheumatoid arthritis. It remains unclear what starts this cascade and the accompanying invasion of synovium into bone. There may be a polygenic genetic component. Rheumatoid arthritis is generally a disease of young and middle-aged people, usually beginning to carve its path to deformity and disability between ages 25 and About 2 million Americans have the disease, which affects three times as many women as men. It has a worldwide prevalence of 1%. Only 10% of patients go into remission during the first year. In addition to its obvious impact on quality of life, rheumatoid arthritis has enormous costs to the economy. Half of all people affected by rheumatoid arthritis are unable to work within 5 years of diagnosis, and the remaining half have functional disability. The direct cost to the economy in 1991 dollars was $4.76 billion. SCREENING AND DIAGNOSIS More than 90% of patients with rheumatoid arthritis have disease in the metacarpophalangeal joints. Indeed, observation of the patient s response to application of gentle pressure across these joints is a good initial screen for patients who complain of joint pain. This squeeze test helps differentiate rheumatoid arthritis from diseases such as fibromyalgia and osteoarthritis. The diagnosis is typically established on the basis of the physical examination and clinical history, further supported by detecting rheumatoid factor. It is important to remember that a positive test for rheumatoid factor is not diagnostic of rheumatoid arthritis. It can be found in other conditions that can cause arthritis, such as hepatitis C infection and sarcoidosis. TREATMENT TODAY: METHOTREXATE AND BEYOND In 1972, rheumatologist Dr. Hoffmeister of Spokane, Wash., observed that weekly doses of methotrexate appeared to be beneficial to patients with rheumatoid arthritis. 3 In 1985, my colleagues and I at Harvard found lowdose methotrexate to be effective in a randomized, controlled clinical trial. 4 Controlled studies have since shown that weekly doses of methotrexate attenuated the progression of joint narrowing, slowed the formation of new erosions, improved functional status, and reduced the need for NSAIDs and corticosteroids. DMARDs were once reserved for only the most severe cases because of concerns about their toxicity and lack of effectiveness, as well as the misguided belief that rheumatoid arthritis was a benign disease. However, two Dutch studies 5,6 showed that they improve functional outcome and slow disease activity more than NSAIDs or corticosteroids alone. They decrease the tenderness and swelling of joints, may decrease acute-phase reactants, regulate 410 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 71 NUMBER 5 MAY 2004
3 the disease process, and may prevent nowuncommon extraarticular complications of the disease. More patients stay on methotrexate in the long term than on other older DMARDs. It is easy to dose, its toxic effects can be monitored with standard laboratory tests and offset with folic acid or leucovorin, and it is cost-effective ($1,000 to $1,500 for 1 year, vs $13,000 per year for biologic response-modifying DMARDs). COMBINATION THERAPY In addition to being the anchor drug, methotrexate is also used as background therapy in combination drug treatment (ie, other DMARDs are added on with methotrexate as the background). There are a number of treatment combinations to choose from. A common triple regimen is methotrexate combined with sulfasalazine and hydroxychloroquine. 7 Other options include methotrexate with leflunomide (Arava), azathioprine, or agents that inhibit TNF-alpha. BEYOND METHOTREXATE Even though methotrexate is the first choice among DMARDs, the drug of choice for individual patients depends on disease activity, functional status, and response to therapy. Patients who do not tolerate methotrexate can be switched to the pyrimidine inhibitor leflunomide. This drug has been shown to have an efficacy comparable to that of methotrexate and is sometimes used with it. Patients who do not respond to leflunomide can try biologic response modifiers, such as those that down-regulate the proinflammatory cytokine TNF-alpha: these include infliximab, etanercept, and adalimumab, with or without methotrexate. TNF-alpha inhibitors have been shown to significantly reduce disease activity, especially in patients in whom methotrexate has failed. FDA-approved TNF-alpha inhibitors are the monoclonal antibodies infliximab and adalimumab, and etanercept, a soluble receptor for TNF-alpha. These agents work remarkably quickly: it can take 4 to 6 weeks to see results with a therapeutic dose of methotrexate (ie, 15 to 20 mg per week), whereas improvement can sometimes be seen with 1 week of the TNF-alpha inhibitors. These drugs are also being used or studied for use in nonrheumatic diseases, including asthma, inflammatory eye disease, psoriasis, lymphoma, and sarcoidosis. Infliximab Infliximab (Remicade) is FDA-approved for use in Crohn disease and rheumatoid arthritis. The ATTRACT trial 8 studied patients taking infliximab in addition to methotrexate as background therapy in the United States and Europe. It showed a 60% response rate with high-dose therapy, a 50% response rate with lower-dose therapy, a significant 17% improvement compared with placebo, and a remarkable radiographic response. This study was important in establishing the ability of TNF-alpha inhibitors to improve functional status and limit radiographic progression in patients with 10 years of disease duration. Etanercept Etanercept (Enbrel) was studied as combination therapy with methotrexate in a 24-week randomized trial of about 90 subjects with 13 years of disease activity. 9 Subjects who received etanercept plus methotrexate had a 70% response rate compared with 27% with placebo plus methotrexate. Etanercept is the only biologic response modifier to be compared directly with methotrexate. In one study, subjects who had rheumatoid arthritis for less than 3 years received etanercept injections twice weekly or received methotrexate in a rapid-dose escalation to 20 mg per week. By 6 months, patients who had received etanercept achieved superior improvement in signs and symptoms over patients who received methotrexate. After 12 months, there was no significant difference between the two groups, and both had achieved a 65% improvement. However, fewer new erosions were seen in patients who received etanercept than with methotrexate, and the difference in the number of erosions became even greater in the second year. Methotrexate is still the gold standard treatment CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 71 NUMBER 5 MAY
4 RHEUMATOID ARTHRITIS WEINBLATT All patients should be screened for latent tuberculosis before starting TNF-alpha inhibitors Adalimumab Adalimumab (Humira) produced a 66% response rare in a clinical trial of patients taking 40 mg of adalimumab every 2 weeks plus methotrexate. 10 Results for radiographic joint space erosion and narrowing were similar to those achieved with etanercept and methotrexate. ADVERSE EFFECTS OF DISEASE- MODIFYING AGENTS Both methotrexate and the TNF-alpha inhibitors are fairly well tolerated. However, they both have some adverse effects that need to be taken into account. Methotrexate The side effects of methotrexate include gastrointestinal intolerance, pulmonary toxicity, birth defects, lymphoma, bone marrow toxicity, thinning of the hair, and stomatitis. Some side effects, such as hair-thinning, bone marrow toxicity, and gastrointestinal intolerance, can often be attenuated with supplemental folic acid. The degree of effect on the liver is not as severe as was reported in patients who had psoriasis and were taking methotrexate. 11 This difference may be due to more vigorous monitoring of transaminase and albumin levels, the quantity of alcohol consumed by patients with rheumatoid arthritis compared with those with psoriasis, or different liver pathologic factors in each patient group. TNF-alpha inhibitors TNF-alpha inhibitors are rarely associated with infection, demyelinating syndromes, aplastic anemia, lupus-like syndromes, and lymphoma. (The risk of lymphoma may be slightly higher relative to the healthy population; however, patients with rheumatoid arthritis are at higher risk of developing lymphoma than are healthy controls.) These drugs need to continue to undergo long-term testing before their safety, efficacy, and dosing regimens can be fully established. Infection. There is a link between TNFalpha inhibitors and infection. This phenomenon is particularly true of mycobacterial organisms. In experimental models including knockout of TNF-alpha in rodents, there is a reduction of granuloma formation. Without granulomas to encapsulate acid-fast bacilli, these bacilli proliferate. Deaths have been reported as a result of such infections in patients, and physicians need to be vigilant about this possibility. For example, swift identification of the causative organism is essential in a patient taking a TNF-alpha inhibitor who has a fever. If the organism cannot be identified, the patient probably should be hospitalized for treatment with broad-spectrum antibiotics until the organism is identified through specimen culture. Worldwide, 300 cases of tuberculosis in patients taking these agents have been reported. Many of these cases involved extrapulmonic infections. On the basis of the timing of these cases (ie, within several weeks after starting a TNF-alpha inhibitor), it has been suggested that most of these cases occurred as a result of reactivation. Therefore, all patients should be screened with a purified protein derivative for latent tuberculosis before starting TNF-alpha blockade, regardless of whether they are taking these drugs for the treatment of rheumatoid arthritis or for another disease. COMPLEMENTARY AND ALTERNATIVE THERAPIES Over 50% of patients seek out some sort of complementary therapy on their own. If patients ask about these therapies, I tell them that the only alternative therapies with established benefits are fish oil and evening primrose oil, which have been shown to have some anti-inflammatory properties. Other interventions The management of rheumatoid arthritis is not limited to pharmacologic intervention. It requires a team approach that involves physical and occupational therapists and orthopedic surgeons. In addition, studies have shown that patients who work with both a primary care physician and a rheumatologist have a better outcome than those who receive care exclusively from a primary care physician. 12 On the other hand, patients with rheumatoid arthritis should not be seen solely by their rheumatologist, because they need basic screening and health maintenance that is often better provided by a primary care physician. 412 CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 71 NUMBER 5 MAY 2004
5 REFERENCES 1. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358: Hench PS, Kendall ED, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy- 11-dehydrocorticosteroid compound E) and the pituitary adrenocorticotropic hormone on rheumatoid arthritis: a preliminary report. Mayo Clin Proc 1949; 24: Hoffmeister RT. Methotrexate in rheumatoid arthritis [abstract]. Arthritis Rheum 1972; 15: Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of lowdose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312: van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with second-line anti-rheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124: Verstappen SM, Jacobs JW, Bijlsma JW, et al. Five-year follow-up of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum 2003; 48: Boers M, Verhoeven AC, van der Linden S. Combination therapy in early rheumatoid arthritis: the COBRA study. Ned Tijdschr Geneeskd 1997; 141: Lipsky PE, van der Heijde DM, St. Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000; 343: Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor Fc fusion protein in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999; 340: Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMA- DA trial. Arthritis Rheum 2003; 48: Roenigk HH Jr, Auerbach R, Maibach H, Weinstein G, Lebwohl M. Methotrexate in psoriasis: revised guidelines. J Am Acad Dermatol 1988; 19: Ward MM, Leigh JP, Fries JF. Progression of functional disability in patients with rheumatoid arthritis. Associations with rheumatology subspecialty care. Arch Intern Med 1993; 153: ADDRESS: Michael E. Weinblatt, MD, Brigham and Women s Hospital, Rheumatology-Immunology-Allergy, Arthritis Center, 75 Francis Street, Boston, MA Dear Doctor: As editors, we d like you to look into We d like to know every issue, every page of the Cleveland Clinic Journal of Medicine. 1 How many issues do you look into? Here s our goal: All Most Half Few 2 How do you read the average issue? Here s our goal: Cover-to-cover Most articles Selected articles We put it in writing please put it in writing for us. We want to hear from you. CLEVELAND CLINIC JOURNAL OF MEDICINE The Cleveland Clinic Foundation 9500 Euclid Avenue, NA32 Cleveland, Ohio PHONE FAX ccjm@ccf.org CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 71 NUMBER 5 MAY
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
Pain or stiffness in joints after periods of inactivity or excessive use
 Arthritis Awareness* Some older adults call it Arthur ; others refer to it as their constant compassion, but most describe it as extremely painful Arthritis is a chronic joint disease It is commonly believed
Arthritis Awareness* Some older adults call it Arthur ; others refer to it as their constant compassion, but most describe it as extremely painful Arthritis is a chronic joint disease It is commonly believed
Patient #1. Rheumatoid Arthritis. Rheumatoid Arthritis. 45 y/o female Morning stiffness in her joints >1 hour
 Patient #1 Rheumatoid Arthritis Essentials For The Family Medicine Physician 45 y/o female Morning stiffness in her joints >1 hour Hands, Wrists, Knees, Ankles, Feet Polyarticular, symmetrical swelling
Patient #1 Rheumatoid Arthritis Essentials For The Family Medicine Physician 45 y/o female Morning stiffness in her joints >1 hour Hands, Wrists, Knees, Ankles, Feet Polyarticular, symmetrical swelling
1. Background: Infliximab is administered parenterally; therefore, it is not covered under retail pharmacy benefits.
 Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
C. Assess clinical response after the first three months of treatment.
 Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
ADALIMUMAB Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Rheumatoid Arthritis. Improving Outcomes in RA: Three Pillars. RA: Chronic Joint Destruction and Disability What We Try to Prevent
 Rheumatoid Arthritis Modern Management of Common Problems in Rheumatology: Rheumatoid Arthritis Jonathan Graf, M.D. Associate Professor of Medicine, UCSF Division of Rheumatology, SFGH Director, UCSF Rheumatoid
Rheumatoid Arthritis Modern Management of Common Problems in Rheumatology: Rheumatoid Arthritis Jonathan Graf, M.D. Associate Professor of Medicine, UCSF Division of Rheumatology, SFGH Director, UCSF Rheumatoid
Understanding Rheumatoid Arthritis
 Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Understanding Rheumatoid Arthritis Understanding Rheumatoid Arthritis What Is Rheumatoid Arthritis? 1,2 Rheumatoid arthritis (RA) is a chronic autoimmune disease. It causes joints to swell and can result
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES HUMIRA PEDIATRIC
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Medical Management of Rheumatoid Arthritis (RA)
 Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Using ENBREL to Treat Rheumatoid and Psoriatic Arthritis
 Using ENBREL to Treat Rheumatoid and Psoriatic Arthritis Writing White Papers class Bellevue Community College TABLE OF CONTENTS TABLE OF CONTENTS...2 OVERVIEW...3 RHEUMATOID ARTHRITIS... 3 JUVENILE RHEUMATOID
Using ENBREL to Treat Rheumatoid and Psoriatic Arthritis Writing White Papers class Bellevue Community College TABLE OF CONTENTS TABLE OF CONTENTS...2 OVERVIEW...3 RHEUMATOID ARTHRITIS... 3 JUVENILE RHEUMATOID
Subject: Remicade (Page 1 of 5)
 Subject: Remicade (Page 1 of 5) Objective: I. To ensure that Health Share/Tuality Health Alliance (THA) has a process by which the appropriate utilization of Remicade (Infliximab) for members whose diagnosis
Subject: Remicade (Page 1 of 5) Objective: I. To ensure that Health Share/Tuality Health Alliance (THA) has a process by which the appropriate utilization of Remicade (Infliximab) for members whose diagnosis
A Clinical Context Report
 Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Psoriatic Arthritis- Secondary Care
 Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Cell-Mediated Immunity and T Lymphocytes
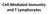 Cell-Mediated Immunity and T Lymphocytes T helper (Th) Cells Peripheral lymhoid tissue thymus Lymphoid stem cell CD8+ CD4+ CD4+ Treg CD8+ CTL + antigen Cytotoxic T lymphocyte CD4+ Th Helper T cell CD4+
Cell-Mediated Immunity and T Lymphocytes T helper (Th) Cells Peripheral lymhoid tissue thymus Lymphoid stem cell CD8+ CD4+ CD4+ Treg CD8+ CTL + antigen Cytotoxic T lymphocyte CD4+ Th Helper T cell CD4+
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65
 Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Remicade (Infliximab)
 Remicade (Infliximab) Policy Number: Original Effective Date: MM.04.016 11/18/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/26/2013 Section: Prescription Drugs Place(s)
Remicade (Infliximab) Policy Number: Original Effective Date: MM.04.016 11/18/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/26/2013 Section: Prescription Drugs Place(s)
Amjevita (adalimumab-atto)
 *- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
*- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
Clinical Policy: Certolizumab (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Psoriatic Arthritis- Second Line Treatments
 Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of:
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 8 Last Review Date: March 17, 2017 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 8 Last Review Date: March 17, 2017 Simponi / Simponi
Rheumatoid arthritis
 Rheumatoid arthritis 1 Definition Rheumatoid arthritis is one of the most common inflammatory disorders affecting the population worldwide. It is a systemic inflammatory disease which affects not only
Rheumatoid arthritis 1 Definition Rheumatoid arthritis is one of the most common inflammatory disorders affecting the population worldwide. It is a systemic inflammatory disease which affects not only
Clinical Policy: Etanercept (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Enbrel ) is tumor necrosis
Clinical Policy: (Enbrel) Reference Number: PA.CP.PHAR.250 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Enbrel ) is tumor necrosis
Inflectra Frequently Asked Questions
 Inflectra Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Earlier in 2016, Inflectra (infliximab) was added to the Ontario Drug Benefit (ODB) Formulary as a Limited
Inflectra Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Earlier in 2016, Inflectra (infliximab) was added to the Ontario Drug Benefit (ODB) Formulary as a Limited
Rheumatoid Arthritis
 Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
Rheumatoid Arthritis Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Autoimmune diseases are illnesses that occur when the body's tissues are mistakenly
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form Submit request via: Fax
 Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Enbrel (etanercept) require a prior
Prior Authorization Conditions for Approval of Enbrel (etanercept) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Enbrel (etanercept) require a prior
Inflammatory arthritis Shared Decision Making
 Inflammatory arthritis Shared Decision Making DMARDs El Miedany et al. Ann Rheum Dis 74(Suppl2): 1002 DOI: 10.1136/annrheumdis-2015-eular.1410 www.rheumatology4u.com Copyrights reserved Contents 1 2 3
Inflammatory arthritis Shared Decision Making DMARDs El Miedany et al. Ann Rheum Dis 74(Suppl2): 1002 DOI: 10.1136/annrheumdis-2015-eular.1410 www.rheumatology4u.com Copyrights reserved Contents 1 2 3
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
MMS Pharmacology Lecture 2. Antirheumatic drugs. Dr Sura Al Zoubi
 MMS Pharmacology Lecture 2 Antirheumatic drugs Dr Sura Al Zoubi Revision Rheumatoid Arthritis Definition (RA): is the most common systemic inflammatory disease characterized by symmetrical inflammation
MMS Pharmacology Lecture 2 Antirheumatic drugs Dr Sura Al Zoubi Revision Rheumatoid Arthritis Definition (RA): is the most common systemic inflammatory disease characterized by symmetrical inflammation
Rheumatoid Arthritis
 Rheumatoid Arthritis What is rheumatoid arthritis? Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Rheumatoid arthritis can also cause inflammation of
Rheumatoid Arthritis What is rheumatoid arthritis? Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints. Rheumatoid arthritis can also cause inflammation of
Otezla. Otezla (apremilast) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release)
 Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Helpline No:
 ARTHRITIS FOUNDATION Registered Nonprofit Organisation - No. 002-847 NPO Helpline No: 0861 30 30 30 DRUGS AND ARTHRITIS This information leaflet is published by the Arthritis Foundation as part of our
ARTHRITIS FOUNDATION Registered Nonprofit Organisation - No. 002-847 NPO Helpline No: 0861 30 30 30 DRUGS AND ARTHRITIS This information leaflet is published by the Arthritis Foundation as part of our
How to Make Taking Your Medications Easier
 How to Make Taking Your Medications Easier Daniel Solomon, MD, MPH Candace Feldman, MD, MPH Division of Rheumatology Brigham and Women s Hospital Harvard Medical School Medications often confuse patients
How to Make Taking Your Medications Easier Daniel Solomon, MD, MPH Candace Feldman, MD, MPH Division of Rheumatology Brigham and Women s Hospital Harvard Medical School Medications often confuse patients
Rheumatoid arthritis 2010: Treatment and monitoring
 October 12, 2010 By Yusuf Yazici, MD [1] The significant changes in the way rheumatoid arthritis has been managed include earlier, more aggressive treatment with combination therapy. Significant changes
October 12, 2010 By Yusuf Yazici, MD [1] The significant changes in the way rheumatoid arthritis has been managed include earlier, more aggressive treatment with combination therapy. Significant changes
To help you with terms and abbreviations used in this document that may be unfamiliar to you, a glossary is provided on the last pages.
 ARTHRITIS CONSUMER EXPERTS 910B RICHARDS STREET VANCOUVER BC V6B 3C1 CANADA T: 604.974-1366 F: 604.974-1377 WWW.ARTHRITISCONSUMEREXPERTS.ORG Arthritis Consumer Experts In Health Care and Research Decision-making
ARTHRITIS CONSUMER EXPERTS 910B RICHARDS STREET VANCOUVER BC V6B 3C1 CANADA T: 604.974-1366 F: 604.974-1377 WWW.ARTHRITISCONSUMEREXPERTS.ORG Arthritis Consumer Experts In Health Care and Research Decision-making
New Evidence reports on presentations given at EULAR Tocilizumab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
CLINICAL BRIEFS. Tumor Necrosis Factor Inhibitors In the Treatment of Chronic Inflammatory Diseases
 CLINICAL BRIEFS Tumor Necrosis Factor Inhibitors In the Treatment of Chronic Inflammatory Diseases A review of immunogenicity and potential implications By Joseph Flood, MD, FACR President, Musculoskeletal
CLINICAL BRIEFS Tumor Necrosis Factor Inhibitors In the Treatment of Chronic Inflammatory Diseases A review of immunogenicity and potential implications By Joseph Flood, MD, FACR President, Musculoskeletal
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
TNF Inhibitors: Lessons From Immunogenicity
 TNF Inhibitors: Lessons From Immunogenicity Edward Keystone, MD, FRCP(C) Professor of Medicine University of Toronto Toronto, Canada Edward Keystone, MD FRCP(C) Disclosures Sources of Funding for Research:
TNF Inhibitors: Lessons From Immunogenicity Edward Keystone, MD, FRCP(C) Professor of Medicine University of Toronto Toronto, Canada Edward Keystone, MD FRCP(C) Disclosures Sources of Funding for Research:
ORENCIA (abatacept) Demonstrates Comparable Efficacy to Humira ( adalimumab
 ORENCIA (abatacept) Demonstrates Comparable Efficacy to Humira (adalimumab) in Patients with Moderate to Severe Rheumatoid Arthritis in First Head-to-Head Study of These Agents ORENCIA demonstrated comparable
ORENCIA (abatacept) Demonstrates Comparable Efficacy to Humira (adalimumab) in Patients with Moderate to Severe Rheumatoid Arthritis in First Head-to-Head Study of These Agents ORENCIA demonstrated comparable
3. Has the patient shown improvement in signs and symptoms of the disease? Y N
 Pharmacy Prior Authorization MERC CARE (MEDICAID) Renflexis (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date. Fax
Pharmacy Prior Authorization MERC CARE (MEDICAID) Renflexis (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date. Fax
For Rheumatoid Arthritis
 For Rheumatoid Arthritis APRIL 2017 NOTICE: On April 14, 2017 the FDA issued a complete response letter for baricitinib indicating that the FDA is unable to approve the application in its current form
For Rheumatoid Arthritis APRIL 2017 NOTICE: On April 14, 2017 the FDA issued a complete response letter for baricitinib indicating that the FDA is unable to approve the application in its current form
Practical RA Treatment: James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014
 Practical RA Treatment: 2014 James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014 Disclosures James R. O Dell PI of Multinational RA trial supported by VA and NIH (NIAMS) that receives
Practical RA Treatment: 2014 James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014 Disclosures James R. O Dell PI of Multinational RA trial supported by VA and NIH (NIAMS) that receives
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future
 Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
certolizumab pegol (Cimzia )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy: Tocilizumab (Actemra) Reference Number: ERX.SPMN.44
 Clinical Policy: (Actemra) Reference Number: ERX.SPMN.44 Effective Date: 10/16 Last Review Date: 09/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Actemra) Reference Number: ERX.SPMN.44 Effective Date: 10/16 Last Review Date: 09/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Antirheumatic drugs. Rheumatic Arthritis (RA)
 Antirheumatic drugs Rheumatic Arthritis (RA) Disease Modifying Antirheumatic drugs (DMARDs) DMARDs are used in the treatment of rheumatic arthritis RA and have been shown to slow the course of the disease,
Antirheumatic drugs Rheumatic Arthritis (RA) Disease Modifying Antirheumatic drugs (DMARDs) DMARDs are used in the treatment of rheumatic arthritis RA and have been shown to slow the course of the disease,
Key words: rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, treatment access
 6, 21...,.,..,..,. - (RA) -,. (AS) (PsA). (Disease-modifying antirheumatic drugs, DMARDs). DMARDs 21. - RA, AS PsA. 21. RA, DMARDs. Leflunomide (Arava)., RA Methotrexate Leflunomid. DMARDs - 5 29. - RA
6, 21...,.,..,..,. - (RA) -,. (AS) (PsA). (Disease-modifying antirheumatic drugs, DMARDs). DMARDs 21. - RA, AS PsA. 21. RA, DMARDs. Leflunomide (Arava)., RA Methotrexate Leflunomid. DMARDs - 5 29. - RA
CIMZIA (certolizumab pegol)
 Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
Rheumatoid Arthritis. Marge Beckman FALU, FLMI Vice President RGA Underwriting Quarterly Underwriting Meeting March 24, 2011
 Rheumatoid Arthritis Marge Beckman FALU, FLMI Vice President RGA Underwriting Quarterly Underwriting Meeting March 24, 2011 The security of experience. The power of innovation. www.rgare.com Case Study
Rheumatoid Arthritis Marge Beckman FALU, FLMI Vice President RGA Underwriting Quarterly Underwriting Meeting March 24, 2011 The security of experience. The power of innovation. www.rgare.com Case Study
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Appendix 1: Frequently Asked Questions
 Appendix 1: Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added to the Ontario Drug Benefit (ODB) Formulary
Appendix 1: Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added to the Ontario Drug Benefit (ODB) Formulary
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8
 London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
Cosentyx. Cosentyx (secukinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015
 The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015 The views and opinions expressed in the following presentation are those of the presenter and
The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015 The views and opinions expressed in the following presentation are those of the presenter and
Stelara. Stelara (ustekinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Humira (adalimumab) DRUG.00002
 Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
Pharmacy Management Drug Policy
 SUBJECT: Cimzia (Certolizumab pegol) - for Ankylosing Spondylitis, Crohn s Disease, Psoriatic Arthritis and Rheumatoid Arthritis POLICY NUMBER: PHARMACY-07 EFFECTIVE DATE: 5/2009 LAST REVIEW DATE: 6/13/2018
SUBJECT: Cimzia (Certolizumab pegol) - for Ankylosing Spondylitis, Crohn s Disease, Psoriatic Arthritis and Rheumatoid Arthritis POLICY NUMBER: PHARMACY-07 EFFECTIVE DATE: 5/2009 LAST REVIEW DATE: 6/13/2018
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
The impact of new biologicals in the treatment of rheumatoid arthritis
 Rheumatology 2004;43(Suppl. 3):iii17 iii23 The impact of new biologicals in the treatment of rheumatoid arthritis doi:10.1093/rheumatology/keh203 The past decade has seen a shift in the paradigm for RA
Rheumatology 2004;43(Suppl. 3):iii17 iii23 The impact of new biologicals in the treatment of rheumatoid arthritis doi:10.1093/rheumatology/keh203 The past decade has seen a shift in the paradigm for RA
Dr Daniel Ching. Rheumatology Therapeutic Clinical Trials Centre Timaru
 Dr Daniel Ching Rheumatology Therapeutic Clinical Trials Centre Timaru Therapeutic Advances in Rheumatology GP CME Meeting, Dunedin, 18.08.2013 Dr Daniel Ching, MB FRCP FRACP Consultant Rheumatologist,
Dr Daniel Ching Rheumatology Therapeutic Clinical Trials Centre Timaru Therapeutic Advances in Rheumatology GP CME Meeting, Dunedin, 18.08.2013 Dr Daniel Ching, MB FRCP FRACP Consultant Rheumatologist,
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
PsA. SIMPONI (golimumab) Rheumatoid arthritis. Psoriatic arthritis. Ankylosing spondylitis EFFICACY EFFICACY EFFICACY. QoL. QoL.
 RA Rheumatoid arthritis PsA Psoriatic arthritis AS Ankylosing spondylitis EFFICACY EFFICACY EFFICACY QoL QoL QoL SAFETY SAFETY SAFETY EXPERIENCE EXPERIENCE EXPERIENCE SUMMARY SUMMARY SUMMARY Copyright
RA Rheumatoid arthritis PsA Psoriatic arthritis AS Ankylosing spondylitis EFFICACY EFFICACY EFFICACY QoL QoL QoL SAFETY SAFETY SAFETY EXPERIENCE EXPERIENCE EXPERIENCE SUMMARY SUMMARY SUMMARY Copyright
(tofacitinib) are met.
 Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Regulatory Status FDA-approved indication: Orencia is a selective T cell co-stimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Combination therapy in. rheumatoid arthritis
 Chapter 2 Combination therapy in rheumatoid arthritis YPM Goekoop CF Allaart FC Breedveld BAC Dijkmans Curr Opin Rheumatol 2001;13:177-83. ABSTRACT It has become clear that early suppression of rheumatoid
Chapter 2 Combination therapy in rheumatoid arthritis YPM Goekoop CF Allaart FC Breedveld BAC Dijkmans Curr Opin Rheumatol 2001;13:177-83. ABSTRACT It has become clear that early suppression of rheumatoid
Efficacy of Anakinra in Bone: Comparison to Other Biologics
 Advances In Therapy Volume 19 No. 1 January/February 2002 Efficacy of Anakinra in Bone: Comparison to Other Biologics Stephen A. Paget, M.D. Hospital for Special Surgery Department of Rheumatic Disease
Advances In Therapy Volume 19 No. 1 January/February 2002 Efficacy of Anakinra in Bone: Comparison to Other Biologics Stephen A. Paget, M.D. Hospital for Special Surgery Department of Rheumatic Disease
Rheumatoid Arthritis. By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University
 Rheumatoid Arthritis By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University Introduction RA is a Chronic, Systemic, Inflammatory disorder of unknown etiology
Rheumatoid Arthritis By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University Introduction RA is a Chronic, Systemic, Inflammatory disorder of unknown etiology
Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria
 Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria Inter-Balkan meeting Open the frontiers and exchange of experiences, 27 th April 2013, Rhodes, Greece Patients with
Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria Inter-Balkan meeting Open the frontiers and exchange of experiences, 27 th April 2013, Rhodes, Greece Patients with
TRANSPARENCY COMMITTEE OPINION. 26 April 2006
 TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
2. Does the patient have a diagnosis of ulcerative colitis or Crohn s? Y N
 Pharmacy Prior Authorization AETA BETTER HEALTH LOUISIAA (MEDICAID) Remicade (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Pharmacy Prior Authorization AETA BETTER HEALTH LOUISIAA (MEDICAID) Remicade (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign
Erelzi (etanercept) Frequently Asked Questions
 Erelzi (etanercept) Frequently Asked Questions 1. What is the funding status of Erelzi (etanercept)? Effective December 21, 2017, Erelzi (etanercept) will be added to the Ontario Drug Benefit (ODB) Formulary
Erelzi (etanercept) Frequently Asked Questions 1. What is the funding status of Erelzi (etanercept)? Effective December 21, 2017, Erelzi (etanercept) will be added to the Ontario Drug Benefit (ODB) Formulary
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
More Than Growing Pains: Therapeutic Review of Juvenile Idiopathic Arthritis (JIA)
 More Than Growing Pains: Therapeutic Review of Juvenile Idiopathic Arthritis (JIA) Brittany A. Bruch, PharmD PGY2 Ambulatory Care Pharmacy Resident University of Iowa Hospitals and Clinics November 10,
More Than Growing Pains: Therapeutic Review of Juvenile Idiopathic Arthritis (JIA) Brittany A. Bruch, PharmD PGY2 Ambulatory Care Pharmacy Resident University of Iowa Hospitals and Clinics November 10,
corticosteroids. The effort to slow the progression of RA includes diseasemodifying (DMARDs), which include gold salts,
 Rituximab for Patients with Refractory Rheumatoid Arthritis Patty Ghazvini, PharmD, Angela Singh, PharmD, Phillip Treadwell, PharmD, Marlon Honeywell, PharmD, and Natosha Canty, MD Dr. Ghazvini and Dr.
Rituximab for Patients with Refractory Rheumatoid Arthritis Patty Ghazvini, PharmD, Angela Singh, PharmD, Phillip Treadwell, PharmD, Marlon Honeywell, PharmD, and Natosha Canty, MD Dr. Ghazvini and Dr.
Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF
 Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF inhibitors. This is a subject of great interest to me and I
Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF inhibitors. This is a subject of great interest to me and I
Guide to Understanding and Managing Arthritis
 Guide to Understanding and Managing Arthritis The content in this guide is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician
Guide to Understanding and Managing Arthritis The content in this guide is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Ixifi* (infliximabqbtx), Renflexis (infliximab-abda)
 Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
Medication Policy Manual. Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013
 Medication Policy Manual Policy No: dru289 Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1,
Medication Policy Manual Policy No: dru289 Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1,
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Ontario Public Drug Programs. Inflectra (infliximab) Frequently Asked Questions
 Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Clinical Policy: Certolizumab (Cimzia) Reference Number: CP.PHAR.247 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Cimzia) Reference Number: CP.PHAR.247 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
Clinical Policy: (Cimzia) Reference Number: CP.PHAR.247 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
Simponi / Simponi ARIA (golimumab)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Synoviocytes. Macrophage. B cell C H R O N I C. Neutrophil. Mast cell I N F L A M M A T I O N. Tissue cell. Endothelial cell. Th1/Th17 IL17 IL22
 DC IL12, IL23 chemokines, ECM, Co-stimulation IL17, IL22 IFNγ ± Macrophage peptidoglycan lipopolysaccharide heat shock proteins Th1/Th17 IL17 IL22 Cell contact, co-stimulation immune complexes acute phase
DC IL12, IL23 chemokines, ECM, Co-stimulation IL17, IL22 IFNγ ± Macrophage peptidoglycan lipopolysaccharide heat shock proteins Th1/Th17 IL17 IL22 Cell contact, co-stimulation immune complexes acute phase
Global Rheumatoid Arthritis Market: Trends and Opportunities ( )
 Global Rheumatoid Arthritis Market: Trends and Opportunities (2013-2018) Global Rheumatoid Arthritis Market Report Scope of the report The report titled Global Rheumatoid Arthritis Market: Trends and Opportunities
Global Rheumatoid Arthritis Market: Trends and Opportunities (2013-2018) Global Rheumatoid Arthritis Market Report Scope of the report The report titled Global Rheumatoid Arthritis Market: Trends and Opportunities
Welcome to Juvenile Rheumatoid Arthritis or JRA, by Connie Martin, MS, RDN; Claire Stephens, MS, RDN; and Lolita McLean, MPH, RDN...
 Welcome to Juvenile Rheumatoid Arthritis or JRA, by Connie Martin, MS, RDN; Claire Stephens, MS, RDN; and Lolita McLean, MPH, RDN...all of Alabama s Children s Rehabilitation Service, part of the Alabama
Welcome to Juvenile Rheumatoid Arthritis or JRA, by Connie Martin, MS, RDN; Claire Stephens, MS, RDN; and Lolita McLean, MPH, RDN...all of Alabama s Children s Rehabilitation Service, part of the Alabama
Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65
 Market DC Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2
Market DC Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2
