Secundum Artem. INHALATION PRODUCTS Loyd V. Allen, Jr., Ph.D., FACA, FAPhA VOLUME 6 NUMBER 3
|
|
|
- Andra Black
- 6 years ago
- Views:
Transcription
1 VOLUME 6 NUMBER 3 Secundum Artem Current & Practical Compounding Information for the Pharmacist. INHALATION PRODUCTS Loyd V. Allen, Jr., Ph.D., FACA, FAPhA INTRODUCTION AND DEFINITIONS Drugs can be introduced into the lungs easily by the patient inhaling a "dose" of a drug in a properly designed dosage form that is caught up in the flow of air and carried into the deep recesses of the pulmonary environment. Drugs generally can be transported by the air stream into the respiratory bronchiole and the alveolar region when in the form of a vapor, very fine powder or as a solution in the form of an "aerosol". An aerosol is generally a dispersed system consisting of a solid or liquid internal phase in an outer gaseous phase. An example of a natural aerosol is a mist (water/air) and dust (solid/air). Products administered by inhalation can produce either a local or a systemic effect. Drugs commonly administered for respiratory purposes include bronchodilators corticosteroids, antitussives, expectorants, surfactants, respiratory stimulants and therapeutic gases. Aromatherapy is a contemporary term used to describe one of the age-old methods of drug administration. Today, however, the term aromatherapy is used primarily to refer to the use of volatile/aromatic oils that are used as room sprays, massage products, room fresheners and even as inhalants when placed in hot water. There are numerous advantages of oral inhalation products, including a rapid onset of action, bypassing the first-pass effect, avoiding drug degradation in the gastrointestinal tract, low dosages to minimize adverse reactions, dose titration capability, good for prn dosing, alternate route when other drugs may chemically or physically interact with concurrent drugs, and an alternate route for drugs with erratic oral or parenteral administration pharmacokinetics. The ultimate deposition of inhalation aerosols is dependent upon (1) the products formulation, (2) the design of components/packaging/container, (3) administration skills and techniques of the product user, and Loyd V. Allen, Jr., Ph.D., Professor and Head, Pharmaceutics, University of Oklahoma, HSC College of Pharmacy, Oklahoma City, OK (4) the anatomical and physiological status of the respiratory system. Numerous terms are used to describe the various dosage forms and methods of drug administration by inhalation, including aerosols, atomizers, inhalations, insufflations, metered dose inhalers, nebulizers and vaporizers. By definition, an aerosol is a colloidal dispersion of a liquid or a solid in a gas. Oral inhalation and nasopharyngeal medications can both be administered in aerosol form. These products are commonly produced by manual sprays or from pressurized packages. Aerosols have become so widespread in use that the term has come to mean a self-contained product that is sprayed and the propelling force is supplied by a liquefied or compressed gas. In pharmacy, these pressure-packaged products consist of the active drug dissolved in, suspended in, or emulsified in a propellant or a mixture of a solvent and a propellant. These aerosols are generally designed for either topical administration or for inhalation into the nasopharyngeal region or bronchopulmonary system. For pulmonary delivery, particles greater than 60µ diameter usually are deposited in the trachea, and those µ between the trachea and the bronchioles, but not into the bronchioles. Particles about 1µ often remain airborne and are exhaled. Consequently, particles between about 5 to 20 µ would be desired to reach the bronchioles. Inhalation aerosols is the largest group of oral aerosol products formulated either as solutions or suspensions. Factors influencing the deposition of inhalation aerosols include the formulation, device, administrative technique of the user and the anatomical and physiological status of the respiratory system. An atomizer is an instrument used to disperse a liquid in a fine spray. Many of the older pressuretype atomizers used the Bernoulli principle. When a stream of air moves at a high velocity over the tip of a dip tube, the pressure is lowered, and the liquid is drawn into the air flow. The liquid is broken Drugs commonly administered for respiratory purposes include bronchodilators, corticosteroids, antitussives, expectorants, surfactants, respiratory stimulants and therapeutic gases. Numerous terms are used to describe the various dosage forms and methods of drug administration by inhalation, including aerosols, atomizers, inhalations, insufflations, metered dose inhalers, nebulizers and vaporizers.
2 up into a spray as it is taken up into the air stream. To produce smaller droplets, a baffle, bead or other device may be put in the flow to break the droplets into smaller droplets as they collide with the stationary device. These smaller droplets are then carried by the air flow into the inhaled stream of air. Many different configurations are available using the Bernoulli principle. A finer spray can be obtained using a pressure type atomizer. Today's plastic spray bottles are similar to the pressure atomizers. When the plastic bottle is squeezed, the air inside is compressed, forcing the liquid up a dip tube into the tip. The liquid stream is mixed with air as it is emitted from the nozzle, producing a spray. Inhalations are preparations designed to deliver the drug into the respiratory tree of the patient for local or systemic effect. Rapid relief is obtained when the vapors or the mist/droplets reach the affected area. The USP 23/NF 18 definitions of inhalations states "inhalations are drugs or solutions or suspensions of one or more drug substances administered by the nasal or oral respiratory route for local or systemic effect". The drugs may be nebulized to produce droplets sufficiently fine and uniform in size to reach the bronchioles. The mist may be breathed directly from the nebulizer or a face mask; tent or intermittent positive pressure breathing (IPPB) machines may be used to produce a contained environment to maximize the quantity of drug available in the breathing environment to be delivered into the lungs. According to the British Pharmacopeia, "inhalations" are solutions or suspensions of one or more active ingredients that may contain an inert, suspended diffusing agent. These liquids are designed to release volatile constituents for inhalation, either when placed on a pad or when added to hot water. An example of the latter is the use of one teaspoonful of Benzoin Inhalation BP in 1 quart of hot water where the vapors are inhaled. Inhalants are drugs that are characterized by a high vapor pressure and can be carried by an air current into the nasal passage where they generally exert their effect. The devices or the container from which the inhalant is generally administered is called an "inhaler". An inhalant consists of cylindrical rolls of a fibrous material that has been impregnated with the drug, usually containing an aromatic substances in addition to an active substance. These devices are often cylindrical in nature with a cap in place to retard the loss of the medication. To use, the cap is removed and the inhaler inserted into a nostril. Upon inhaling, the air passes through the inhaler and carries the vapor of the medication into the nasal passage. An actual "drug vapor" is being delivered to the patient. For example, menthol, camphor, propylhexedrine and tuaminoheptane inhalants are of this type. Another type, amyl nitrite inhalant, is packaged with the drug contained in a thin glass ampule encased in a gauze netting. When the product is squeezed and the glass ampule broken, the amyl nitrite is released and is absorbed on the gauze netting for inhalation by the patient. There are currently 25 inhalation products and 2 inhalants listed in the USP 23/NF 18. Insufflations are powders administered using a powder blower (puffer) or insufflator. These may consist of a rubber bulb connected to a container and a delivery pipe. As the bulb is squeezed, air is blown into the container causing turbulence which causes the powder to fly around. As the air leaves the container, some of the fine particles are carried out with the air through the delivery tube and are ready for inhalation. Another device (puffer) consists of a plastic accordion-shaped container with a spout on one end. The powder is placed in the "puffer" and, as the puffer is sharply squeezed, a portion of the powder is ejected from the spout into the air available for inhalation or application. Contemporary delivery systems include powders that may be delivered by various mechanical devices designed for the patient to breathe deeply to inhale the powder particles. The inspiration step provides energy to spin a propeller that The Paddock Solid-to-Liquid Solution The physical and chemical properties of Ora-Plus and Ora-Sweet help ensure the stability of compounded oral liquids. Over 40 formulations have been independently tested. Call to receive copies of the studies or visit our website,
3 serves to break up and distribute the particles as they are inhaled. Metered Dose Inhalants (MDIs) are propellant driven drug products, either as solutions or suspensions, containing the drug, a liquified gas propellant with or without a cosolvent and are designed to deliver a metered dose of the drug. These devices commonly deliver from 25 to 100 µl volumes of liquid drug for inhalation. Nebulae, or spray solutions, were intended for spraying into the throat and nose. The Latin term for mist is nebula. Generally, these were simple solutions. Sprays in plastic spray bottles work similarly to the pressure atomizers. When the flexible plastic container is squeezed, the air is compressed, forcing the liquid in the container up through a dip tube into the tip where it mixes with a stream of air. Droplets are formed depending upon the geometry of the device and the pressure of the individual squeezing the bottle. A nebulizer was formerly described as a small, vacuum type of atomizer inside a chamber. Large droplets would strike the walls of the chamber, fall back and be reprocessed. The smaller particles are carried in the air stream out of the unit. For use, the nebulizer is placed in the mouth and the patient inhales while simultaneously squeezing the bulb. Nebulizers must produce droplets sufficiently fine and uniform in size (optimally 0.5 to 7 micron) such that the mist reaches the bronchioles, in order for nebulizers to be suitable for the administration of inhalation solutions. An advantage of the newer pressurized aerosols is the fine mist they produce and more uniform doses as compared with the older, manual nebulizers. A vaporizer is an electrical device producing moist steam, either with or without medication, for inhalation. It is often used to soothe upper respiratory irritations but is relatively ineffective in providing medications to the deeper areas of the respiratory tract. BACKGROUND One of the earliest methods for inhaling drug products was the burning of materials and inhalation of the smoke. A natural vegetable product was dried and broken up and burned, or an oil was burned, or combustible material was combined with the medication, burned and the resulting vapors inhaled. Until recent years, an asthma "cigarette" (Asthmador) containing the drug was available. It was "smoked" and inhaled, delivering the bronchodilator to the lungs. This principle is still used by recreational drug users for substances such as marijuana and opium. Some recreational drugs are simply inhaled as the powder form, resulting in a very rapid onset of action. There is no question about the effectiveness of this route of administration for drugs. An earlier problem of inhaled drug delivery related to the accuracy of dose delivery. With the newer metered dose inhalers, this problem has been partly overcome, since the same volume of drug will be administered. Not addressed, however, is the variability in the rate of inspiration, breath holding, and expiration, where varying quantities of drug may be absorbed. Inhalations formerly were simple solutions of volatile medications, usually volatile oils, in alcohol or an alcoholic preparation. Often Compound Benzoin Tincture was used. An example formula is: Pine Oil Eucalyptus Oil Compound Benzoin Tincture 5 ml 5 ml 30 ml Sig: Inhale the vapor of one teaspoonful when added to one pint of hot water. Other inhalations designed for addition to hot water were aqueous preparations containing a volatile oil, water and light magnesium carbonate. The light magnesium carbonate was used as a distributive or dispersing agent. The volatile material was distributed on the light magnesium carbonate. which in turn was mixed with the water. This ensured uniform dispersion of the oil upon shaking, since the oil was distributed throughout the mixture, rather than remaining as a globule or being dispersed as large globules. The presence of the light magnesium carbonate did not interfere with the free volatilization of the oil when the product was added to hot water. Emulsification of the oil would retard volatilization. An approximate ratio of of light magnesium carbonate to 0.2 ml of oil was used. An example formula is as follows: Menthol 325 mg Eucalyptus Oil 3.7 ml Light Magnesium Carbonate 2 g Purified Water qs 30 ml Sig: Add one teaspoonful to a pint of hot water (not boiling). Inhale the vapor. Shake the bottle before using. In the early 1950s, the first contemporary pressurized aerosol dosage form was introduced by Riker Laboratories. The Medihaler Epi consisted of epinephrine hydrochloride in a hydroalcoholic solvent system containing sorbitan trioleate as a dispersing agent and a fluorinated hydrocarbon propellant system. These small, self-contained aerosol propellant systems generally contain up to about 30 ml of product in a small stainless steel container fitted with a metered dose valve. The four components include a product concentrate, propellant, container and suitable dispensing/metering valve. Products available in this dosage form have included epinephrine bitartrate, isoproterenol hydrochloride, albuterol, triamcinolone acetonide, dexamethasone sodium phosphate, beclomethasone dipropionate, isoetharine mesylate and metaproterenol sulfate. FORMULATION Solution aerosols can be formulated easily if the active drug is soluble in the propellant system. If it ixnot soluble, a suspension or emulsion aerosol can be prepared. For oral inhalation, either solution or suspension aerosols are used. Example ingredients of an oral inhalation or nasal aerosol solution are shown in Table 1. Suspension aerosols have been used to formulate antiasthmatic aerosols, steroids, antibiotics and others. Precautions to be considered include caking, agglomeration, particle size growth and clogging of the valve systems. Example ingredients in a nasal aerosol solution or suspension are shown in Table 2. Table 1: General formula components of Oral, Inhalation and Nasal Aerosol Solutions Component Active Ingredient Solvents Antioxidants Flavor Propellants Example Soluble in vehicle Ethyl Alcohol, Propylene Glycol, Purified Water, Surfactants Ascorbic Acid Aromatic Oils As needed Table 2: General formula components for nasal aerosol solutions or suspensions Component Active Ingredient Antioxidants Preservatives Buffers Tonicity Adjustment Surfactants Vehicle Example Solubilized or suspended Ascorbic Acid, Bisulfites Benzalkonium Chloride Phosphate Buffer Sodium Chloride Sorbitan esters Purified Water
4 VARIABLES When compounding, or formulating, products for oral inhalation, the variables to consider include particle size, solubility, vehicles, tonicity, ph, sterility, preservatives, viscosity,buffers, surfactants and moisture content. Particle Size: Generally, the particle size should lie in a range of about 0.5 to 10 µ, more preferably between about 3 to 6 µ, for a drug which, when inhaled, is intended to penetrate to the small bronchioles and the lung alveoli and to provide a rapid effect. This size range of particles will deposit in the lung by gravitational sedimentation, inertial impaction and by diffusion into terminal alveoli via Brownian motion. For inhalation aerosols, particles 5 to 10 µ are common, whereas for topical sprays 50 to 100 µ are common. Solubility: Active drugs soluble in the matrix and in the pulmonary fluids will have a rapid onset of action and ordinarily a shorter duration of action, compared to drugs that are somewhat less soluble in the matrix and in the pulmonary fluids. Drugs that are poorly soluble in the pulmonary fluids may irritate the lung tissue. For suspensions, it is best to select a vehicle in which the drug is not very soluble to minimize the phenomenon of particle size growth, resulting from the drug in solution crystallizing out onto crystals that are present. Polymorphic forms of crystalline drugs should not be used for suspension aerosols. To enhance the stability of a suspension aerosol, selection of a liquid phase with density similar to that of the suspensoid, will minimize the tendency to settle. Vehicle: Sterile Water for Inhalation or 0.9% Sodium Chloride Inhalation Solution are used commonly vehicles to carry the drugs in inhalations. Some inhalations are simple solutions of nonvolatile or volatile medications in water or cosolvent mixtures. Small quantities of alcohol or glycerin may be used to solubilize ingredients. Tonicity: It generally is best to make inhalation solutions isotonic with physiological fluids, i.e., equivalent to 0.9% sodium chloride, or an osmolality of about 290 mosm. To enhance movement of the fluid and drug through the alveoli, it may be best to have the solution slightly hypotonic. If it were hypertonic, the tendency would be to move fluids from the alveoli into the pulmonary space to reach an isotonic equilibrium. If hypotonic, the administered drug solution will have a greater tendency to move into the tissue for more rapid absorption and therapeutic effect. ph: A ph in the neutral range, similar to body fluids, would be desired to minimize any cough-reflex that might occur if the ph were too low. However, consideration must be made for the solubility and stability characteristics of the drug. Sterility: A new requirement for inhalation solutions is sterility, and has been suggested because of contamination problems with inhalation solution products and adverse experience reports for commercially available products. To ensure patients get the best products available, extemporaneously compounded oral inhalation solutions should be sterilized. This easily can be accomplished using 0.2µ filtration systems designed for extemporaneous compounding. Preservative: Any preparation not dispensed in sealed unit dose containers should include a preservative, especially with the latest requirement of sterility for this class of dosage forms. The minimum amount of preservative that is still effective should be used. If too high a concentration is used, it may initiate a cough reflex in the patient. Also, too high a concentration of preservatives that are also surfactants may result in foaming that may interfere with the delivery of the complete dose. Viscosity: External phase viscosity for most aerosol products is very low, consequently, they are very sensitive systems. Evaporation, sedimentation and increase in particle size are processes which may occur and change rapidly. Buffer: A buffer, when used, should be at low buffer strength to maintain the desired ph and not induce ph changes in a microenvironment in the pulmonary cavity. Surfactants: Surfactants can be used as dispersing agents for suspensions, solubilizing agents to enhance solubility of the drug, and as spreading agents when the drug is deposited in the lungs. The sorbitan esters, especially sorbitan trioleate, are used as well as lecithin derivatives, oleyl alcohol and others. One should keep the surfactant concentration as low as possible to minimize foaming that might interfere with proper administration. Moisture Content: For inhalation dispersion aerosols generally, the moisture content should be kept extremely low for all active and inactive ingredients. The ingredients should be anhydrous to minimize caking. FORMULAS The example formulas illustrated here are for preservativefree formulations. Reports are now appearing in the literature concerning the potential adverse affects of benzalkonium chloride with some patients. In 1994, a case was described of occupational asthma caused by prolonged exposure to a cleaning solution containing benzalkonium chloride in th workplace (Bernstein JA, Stauder T. Bernstein DI, Bernstein IL. A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines. J Win A Mortar & Pestle! CONGRATULATIONS to Greg Labuza, Summit, NJ winner of the recent Mortar & Pestle drawing. YES, I would like to enter the current Secundum Artem Mortar & Pestle Drawing. 1. How many prescriptions do you compound per month? 2. What powders do you use in your compounding? Hydrocortisone Progesterone Nystatin Other Name Send in completed BRC today! Pharmacy Name Pharmacy Address Phone 2/96
5 Allergy Clin Immunol 1994 Aug; 94*2 Pt 1):25709). Benzalkonium chloride is reported to be a potent bronchoconstictor when inhaled in similar concentrations to those used in bronchodilator nebulizer solutions. This can result in an overall reduction in bronchodilator efficacy. This may not affect the short term bronchodilator response to a single dose, but its repeated use in sever asthma may possibly result in a paradoxical bronchoconstriction. (Fishwick D, Miles JF, Hendeles L, Beasley R. Preservatives in nebulizer solutions: Risks without benefits. Pharmacotherapy - In Press, 41 references). The formulations presented here should be packaged as single-use or unit-dose products. Formulations are provided for 100 ml quantities for ease of calculations. The formulas should be reduced or expanded based upon the total quantity to be compounded. If they are to be preserved, add 4 mg of benzalkonium chloride per 100 ml of solution (3 ml of a Benzalkonium Chloride 1:750 Solution). General Method of Preparation: 1. Calculate the quantity of the individual ingredients required for the total amount to be prepared. 2. Accurately weigh/measure each of the ingredients. 3. Dissolve the solids in about 2/3 of the volume of vehicle for the preparation. 4. Add the liquid ingredients and mix well. 5. Add sufficient vehicle to volume and mix well. 6. Filter through a 0.2 µ filter system into sterile containers. 7. Package and label. Formulas: Albuterol Sulfate 0.5% Inhalant Solution (Preservative Free) Albuterol Sulfate Sodium Chloride 800 mg Albuterol Sulfate 2.7% Inhalant for Hand Held Nebulizer (Preservative Free) Albuterol Sulfate 2.7 g Beclomethasone Dipropionate 0.042% Nasal Solution (Preservative Free) Beclomethasone Dipropionate, Monohydrate 43 mg Dextrose 5.4 g Polysorbate 80 1 ml Hydrochloric Acid (3%-5%) Adjust to ph 7 Ethanol, 95% 13 ml Cromolyn Sodium 1% Inhalation Solution (Preservative Free) Cromolyn Sodium 1 g Cromolyn Sodium 4% Inhalation Solution (Preservative Free) Cromolyn Sodium 4 g Flunisolide 0.025% Inhalation Solution (Preservative Free) Flunisolide 25 mg Ethanol, 95% 2 ml Ipratropium Bromide 0.02% Solution (Preservative Free) Ipratropium Bromide 20 mg 50 mg 0.3% Solution (Preservative Free) 300 mg 250 mg 0.6% Solution (Preservative Free) 600 mg 5% Concentrate (Preservative Free) 5 g 0.9% Sodium Chloride Solution 50 ml Terbutaline 0.1% Inhalant Solution (Preservative Free) Terbutaline Sulfate Albuterol-Cromolyn-Betamethasone for Inhalation (Preservative Free) BUSINESS REPLY MAIL FIRST-CLASS MAIL PERMIT NO MINNEAPOLIS MN Postage will be paid by addressee NO POSTAGE NECESSARY IF MAILED IN THE UNITED STATES 3940 Quebec Ave N Minneapolis MN
6 Albuterol Sulfate Cromolyn Sodium 1 g Betamethasone Sodium Phosphate 250 mg Sodium Chloride 800mg Adjust ph to Albuterol-Ipratropium for Inhalation (Preservative Free) Albuterol Sulfate Ipratropium Bromide 20 mg 50 mg Ipratropium-Metaproterenol-Betamethasone for Inhalation Concentrate (Preservative Free) Ipratropium Bromide 125 mg 5 g Betamethasone Sodium Phosphate 250 mg Physicochemical characteristics of the ingredients and their purpose in the formulations. Active Drugs Albuterol sulfate is a beta-2-adrenergic agonist used as a bronchodilator that is a white or practically white powder, freely soluble in water and slightly soluble in alcohol. Beclomethasone dipropionate is an anti-inflammatory agent used by oral inhalation that occurs as a white to creamy white, odorless powder. It is very slightly soluble in water and freely solublein alcohol. Betamethasone dipropionate is an anti-inflammatory agent used by oral inhalation that occurs as a white to creamy white, odorless powder that is insoluble in water and sparingly soluble in alcohol. Cromolyn sodium is an antiallergic inhalation agent that is a white, odorless, crystalline powder that is initially tasteless but then develops a slightly bitter aftetaste. It is hygroscopic, soluble in water and insoluble in alcohol. Flunisolide is an anti-inflammatory agent used by oral inhalation that is a white to creamy-white, crystalline powder that is practically insoluble in water. Ipratropium bromide is a bronchodilator, anticholinergic agent that occurs as a white, bitter-tasting crystalline powder that is soluble to the extent of 90 mg/ml in water and 28 mg/ml in ethanol. Metaproterenol sulfate is a beta-2-adrenergic agonist agent used as a bronchodilator that is a white to off-white, crystalline powder that is freely soluble in water. Terbutaline sulfate is a beta-2-adrenergic agonist agent used as a bronchodilator that occurs as an odorless, white to gray-white, crystalline powder that is soluble in water. Excipients Citric acid occurs as colorless, translucent crystals, or white, granular to fine, crystalline powder. It is odorless or practically odorless and has a stong, acidic taste; is very soluble in water and freely soluble in alcohol. It is used as an acidifying agent. Polysorbate 80 is a lemon to amber colored, oily liquid with a faint, characteristic odor and a warm, somewhat bitter taste. It is very soluble in water and soluble in alcohol. It is used as anemulsifying and solubilizing agent. Sodium chloride occurs as colorless, cubic crystals or a white crystalline powder with a saline taste. It is freely soluble inw ater and slightly soluble in alcohol. It is classified as a tonicity adjusting agent. Dextrose occurs as colorless crystals or a white, crystalline or granular pwoder. It is odorless and has a sweet taste. It is freely soluble in water and slightly soluble in alcohol. It is used as a tonicity adjusting agent. FUTURE Oral and nasal inhalation administration holds promise for many local and systemic-acting drugs in the future. This may provide many opportunities for extemporaneous compounding in the future for drug products that can be administered nasally or by oral inhalation. With the rapid onset of action, generally good stability profiles, easy titration and easy formulation development, this area of meeting patients needs and solving problems is likely to continue to grow Quebec Ave. N. Minneapolis, MN Bulk Rate U.S. Postage PAID Minneapolis, MN Permit No. 1700
REFERENCES OVERVIEW ADVANTAGES AEROSOLS. Aerosols. and Foams
 COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION
 PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
Emulsions. Purpose of emulsions and of emulsification:
 Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Mixed solvent systems;; spirits, and elixirs
 Mixed solvent systems;; spirits, and elixirs By Dr. Mohammed Sattar 2016/2017 1 Outlines Ø Elixirs Ø Applications Ø Formulation Ø Spirits Ø Applications Ø Formulation 2 1 Non aqueous systems it may be
Mixed solvent systems;; spirits, and elixirs By Dr. Mohammed Sattar 2016/2017 1 Outlines Ø Elixirs Ø Applications Ø Formulation Ø Spirits Ø Applications Ø Formulation 2 1 Non aqueous systems it may be
Contents. Introduction
 Introduction Contents Classification of Monophasic liquid Formulation Consideration Manufacturing Consideration Recent advance in monophasic liquid Recent approach in monophasic liquid formulation Conclusion
Introduction Contents Classification of Monophasic liquid Formulation Consideration Manufacturing Consideration Recent advance in monophasic liquid Recent approach in monophasic liquid formulation Conclusion
Industrial Pharmacy (3) Solutions as a dosage form. DR.Saad.M.YACOUB
 Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Achieving Optimal Particle Size Distribution in Inhalation Therapy
 Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
TYPES OF DISPENSED PREPARATIONS
 TYPES OF DISPENSED PREPARATIONS Dr. Dhaivat C. Parikh Institute of Pharmacy, Nirma University DISCLAIMER This Presentation is Only for Internal Use. This Presentation is meant only for giving an overview
TYPES OF DISPENSED PREPARATIONS Dr. Dhaivat C. Parikh Institute of Pharmacy, Nirma University DISCLAIMER This Presentation is Only for Internal Use. This Presentation is meant only for giving an overview
Stability Of Extemporaneously Prepared Oral Liquid Formulations Part XI
 CPE CREDIT 1.0 Current & Practical Compounding Information for the Pharmacist VOLUME 20 NUMBER 1 Grant funding provided by Perrigo Pharmaceuticals Goal: To provide information on the occurrence, causes
CPE CREDIT 1.0 Current & Practical Compounding Information for the Pharmacist VOLUME 20 NUMBER 1 Grant funding provided by Perrigo Pharmaceuticals Goal: To provide information on the occurrence, causes
905 UNIFORMITY OF DOSAGE UNITS
 Change to read: 905 UNIFORMITY OF DOSAGE UNITS [ NOTE In this chapter, unit and dosage unit are synonymous. ] To ensure the consistency of dosage units, each unit in a batch should have a drug substance
Change to read: 905 UNIFORMITY OF DOSAGE UNITS [ NOTE In this chapter, unit and dosage unit are synonymous. ] To ensure the consistency of dosage units, each unit in a batch should have a drug substance
Pharmaceutical Preparation For Internal Use
 Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Using an Inhaler and Nebulizer
 Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Appendix M: Device Technique
 Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
STABILITY OF EXTEMPORANEOUSLY PREPARED PEDIATRIC FORMULATIONS USING ORA-PLUS WITH ORA-SWEET AND ORA-SWEET SF - PART I
 VOLUME 5 NUMBER 4 Secundum Artem Current & Practical Compounding Information for the Pharmacist. STABILITY OF EXTEMPORANEOUSLY PREPARED PEDIATRIC FORMULATIONS USING ORA-PLUS WITH ORA-SWEET AND ORA-SWEET
VOLUME 5 NUMBER 4 Secundum Artem Current & Practical Compounding Information for the Pharmacist. STABILITY OF EXTEMPORANEOUSLY PREPARED PEDIATRIC FORMULATIONS USING ORA-PLUS WITH ORA-SWEET AND ORA-SWEET
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI)
 I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
8/13/11. RSPT 1410 Humidity & Aerosol Therapy Part 3. Humidification Equipment. Aerosol Therapy
 1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
Chapter 7. Principles of Pharmacology
 Chapter 7 Principles of Pharmacology Introduction Administering medications is a serious business. Medications may alleviate pain and improve patient s well-being. Used inappropriately, may cause harm
Chapter 7 Principles of Pharmacology Introduction Administering medications is a serious business. Medications may alleviate pain and improve patient s well-being. Used inappropriately, may cause harm
Parenteral products-definition
 Parenteral products-definition Parenteral preparations are sterile, pyrogen-free liquids (solutions, emulsions, or suspensions) or solid dosage forms packaged in either single-dose or multidose containers.
Parenteral products-definition Parenteral preparations are sterile, pyrogen-free liquids (solutions, emulsions, or suspensions) or solid dosage forms packaged in either single-dose or multidose containers.
6. SR, XR, CR, and TR are examples of formulations. A) oral B) modified release C) repeat action D) soft gels
 1. Which is a parenteral route of administration? A) oral B) vaginal C) sublingual D) rectal 2. A local effect occurs A) at the site of administration. B) throughout the body. C) only after intravenous
1. Which is a parenteral route of administration? A) oral B) vaginal C) sublingual D) rectal 2. A local effect occurs A) at the site of administration. B) throughout the body. C) only after intravenous
What are the Inhalant drugs?
 Why and why not? Advantages: - Less systemic toxicity - More rapid onset of medication - Delivery to target of action - Higher concentrations available in the lung Disadvantages: - Time and effort consuming
Why and why not? Advantages: - Less systemic toxicity - More rapid onset of medication - Delivery to target of action - Higher concentrations available in the lung Disadvantages: - Time and effort consuming
PARENTERAL PREPARATIONS
 PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
INFORMATION TOPIC: II-5 OR DEMONSTRATION: II-5. DOSAGE, MEASUREMENTS, AND DRUG FORMS (Lesson Title) OBJECTIVES THE STUDENT WILL BE ABLE TO:
 LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE BREATHING DEVICE
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
Chapter 10 Respiration
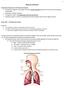 1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
LIQUID PREPARATIONS FOR ORAL USE. Final text for addition to The International Pharmacopoeia (November 2007)
 November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
Preparation of TAMIFLU for Oral Suspension. Emergency Compounding of an Oral Suspension from TAMIFLU Capsules (Final Concentration 15 mg/ml)
 Preparation of TAMIFLU for Oral Suspension It is recommended that TAMIFLU for Oral Suspension be constituted by the pharmacist prior to dispensing to the patient: 1. Tap the closed bottle several times
Preparation of TAMIFLU for Oral Suspension It is recommended that TAMIFLU for Oral Suspension be constituted by the pharmacist prior to dispensing to the patient: 1. Tap the closed bottle several times
Division 1 Introduction to Advanced Prehospital Care
 Division 1 Introduction to Advanced Prehospital Care Chapter 7 Intravenous Access and Medication Administration Part 1 Principles and Routes of Medication Administration Topics Aseptic Technique Medication
Division 1 Introduction to Advanced Prehospital Care Chapter 7 Intravenous Access and Medication Administration Part 1 Principles and Routes of Medication Administration Topics Aseptic Technique Medication
Aerosol Therapy. Aerosol Therapy. RSPT 1410 Humidity & Aerosol Therapy Part 4
 1 RSPT 1410 Humidity & Part 4 Wilkins Chapter 36; p. 801-806 2 Stability: the tendency for aerosol particles to remain in Size: the the particle, the greater the tendency toward stability the the particle,
1 RSPT 1410 Humidity & Part 4 Wilkins Chapter 36; p. 801-806 2 Stability: the tendency for aerosol particles to remain in Size: the the particle, the greater the tendency toward stability the the particle,
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
UNIT 2 SOLID AND LIQUID DOSAGE FORMS
 UNIT 2 SOLID AND LIQUID DOSAGE FORMS Solid and Liquid Structure 2.1 Introduction Objectives 2.2 Solid Tablets Capsules 2.3 Liquid Advantages of Liquid Disadvantages of Liquid Classification of Liquid Monophasic
UNIT 2 SOLID AND LIQUID DOSAGE FORMS Solid and Liquid Structure 2.1 Introduction Objectives 2.2 Solid Tablets Capsules 2.3 Liquid Advantages of Liquid Disadvantages of Liquid Classification of Liquid Monophasic
Norcross, GA TRANSPORTATION EMERGENCIES (24 Hrs.): CHEMTREC (800) GENERAL INFORMATION : (770)
 SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION Issue Date: 5-4-05 Trade Name: Developer Systems Cleaner Kit ( Parts A, B and Neutralizer) Chemical Name: Mixtures- Parts A, B, & Neutralizer Synonyms: None
SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION Issue Date: 5-4-05 Trade Name: Developer Systems Cleaner Kit ( Parts A, B and Neutralizer) Chemical Name: Mixtures- Parts A, B, & Neutralizer Synonyms: None
GRAPEVINE DISEASES. Formulation Information & Adjuvants. Online Guide To. Virginia Tech
 Online Guide To GRAPEVINE DISEASES Virginia Tech Formulation Information & Adjuvants Ashley L. Myers Grape Pathology Extension Specialist, Department of Plant Pathology, Physiology, and Weed Sciences,
Online Guide To GRAPEVINE DISEASES Virginia Tech Formulation Information & Adjuvants Ashley L. Myers Grape Pathology Extension Specialist, Department of Plant Pathology, Physiology, and Weed Sciences,
This letter is to notify you of recent changes to the TAMIFLU product monograph. Please note, additions/changes have been highlighted in yellow.
 February 13, 2015 Subject: Revised Product Monograph - TAMIFLU (oseltamivir phosphate) Dear Drug Information / Poison Control Centres, This letter is to notify you of recent changes to the TAMIFLU product
February 13, 2015 Subject: Revised Product Monograph - TAMIFLU (oseltamivir phosphate) Dear Drug Information / Poison Control Centres, This letter is to notify you of recent changes to the TAMIFLU product
Disclosure. Objectives. Objectives. Introduction. Introduction. Non-Sterile Compounding/Calculations
 49th Annual Meeting Non-Sterile Compounding/ Sunil Jambhekar, B. Pharm., M.S., Ph.D Professor, Pharmaceutical Sciences LECOM Bradenton, School of Pharmacy Bradenton, FL 34211 Disclosure I do not have a
49th Annual Meeting Non-Sterile Compounding/ Sunil Jambhekar, B. Pharm., M.S., Ph.D Professor, Pharmaceutical Sciences LECOM Bradenton, School of Pharmacy Bradenton, FL 34211 Disclosure I do not have a
Practical 3 Extemporaneous Preparations Solutions. Name: Group: Date:
 Practical 3 Extemporaneous Preparations Solutions Name: Group: Date: FILTRATION Unwanted particulate matter may be removed from solutions by the process of filtration. Filtration may be either fine or
Practical 3 Extemporaneous Preparations Solutions Name: Group: Date: FILTRATION Unwanted particulate matter may be removed from solutions by the process of filtration. Filtration may be either fine or
Element B1 / 3 Target Organs and Systems
 / 3 Target Organs and Systems Target Organs / Target Systems (Specified toxic substance exerts its effects) Target organs Liver Lungs Kidneys Brain Skin Bladder Eyes Target Organs / Target Systems Substances
/ 3 Target Organs and Systems Target Organs / Target Systems (Specified toxic substance exerts its effects) Target organs Liver Lungs Kidneys Brain Skin Bladder Eyes Target Organs / Target Systems Substances
Formulation Considerations for Inhaled Products
 Formulation Considerations for Inhaled Products Formulation Considerations of Inhaled Products Inhalation Therapy Nebulizers and Formulations Dry Powder Inhalers and Formulations Metered Dose Inhalers
Formulation Considerations for Inhaled Products Formulation Considerations of Inhaled Products Inhalation Therapy Nebulizers and Formulations Dry Powder Inhalers and Formulations Metered Dose Inhalers
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always
 1 3 4 5 6 7 8 9 10 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always take appropriate Standard measures
1 3 4 5 6 7 8 9 10 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration Caution: Administering medications is business Always take appropriate Standard measures
Laboratory Pesticide Formulations, Labels, and Safety
 1 Laboratory Pesticide Formulations, Labels, and Safety I. Pesticide Formulations Pesticides are rarely used in their pure form (technical grade). They are processed into a usable form for direct application.
1 Laboratory Pesticide Formulations, Labels, and Safety I. Pesticide Formulations Pesticides are rarely used in their pure form (technical grade). They are processed into a usable form for direct application.
INFORMATION TOPIC: II-5 OR DEMONSTRATION: II-5. DOSAGE, MEASUREMENTS, AND DRUG FORMS (Lesson Title) OBJECTIVES THE STUDENT WILL BE ABLE TO:
 LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
LESSON PLAN: 5 COURSE TITLE: UNIT: II MEDICATION TECHNICIAN GENERAL PRINCIPLES SCOPE OF UNIT: This unit includes medication terminology, dosage, measurements, drug forms, transcribing physician s orders,
Pharmaceutical calculation Chapter 11 Isotonic solutions. Assistant Prof. Dr. Wedad K. Ali
 Pharmaceutical calculation Chapter 11 Isotonic solutions Assistant Prof. Dr. Wedad K. Ali Introduction When a solvent passes through a semipermeable membrane from a dilute solution into a more concentrated
Pharmaceutical calculation Chapter 11 Isotonic solutions Assistant Prof. Dr. Wedad K. Ali Introduction When a solvent passes through a semipermeable membrane from a dilute solution into a more concentrated
21/03/2011 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY. Fundamentals of aerosols
 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives
 Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Ingredient Listing Qty. Unit NDC # Supplier. q.s. to ml
 12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
Amendment of Standards for Specification, Scope, Application and Limitation of Food Additives
 G/SPS/N/TPKM/147Add.1 Amendment of Standards for Specification, Scope, Application and Limitation of Food Additives DOH Food No. 0980403340, April 24, 2009 Appendix 1: Standards for Scope, Application
G/SPS/N/TPKM/147Add.1 Amendment of Standards for Specification, Scope, Application and Limitation of Food Additives DOH Food No. 0980403340, April 24, 2009 Appendix 1: Standards for Scope, Application
It is recommended that a mask and protective eyewear be worn when providing care to a patient with a cough
 UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
Petrolatum. Stage 4, Revision 1. Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum.
 1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
1 001-1208PDG.pdf Petrolatum Stage 4, Revision 1 Definition Petrolatum is a purified semi solid mixture of hydrocarbons obtained from petroleum. It may contain a suitable antioxidant. Description and Solubility
Part 1 Principles and Routes of Medication Administration
 1 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration 2 Caution: Administering medications is business Always take appropriate Standard measures to reduce your
1 Chapter 7, Medication Administration Part 1 Principles and Routes of Medication Administration 2 Caution: Administering medications is business Always take appropriate Standard measures to reduce your
DRY SYRUPS SWAPNA.M. Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL SEMINAR BY
 DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
» Croscarmellose Sodium is a cross linked polymer of carboxymethylcellulose sodium.
 BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
Apitherapy. Edition 7.0 Date of issue:
 GB Apitherapy Inhalation of aerosol from the hive Inhalation of aerosol from the hive with forced draft Devices for aerosol inhalation therapy Inhalation mixtures Concentrates for mixing with honey Edition
GB Apitherapy Inhalation of aerosol from the hive Inhalation of aerosol from the hive with forced draft Devices for aerosol inhalation therapy Inhalation mixtures Concentrates for mixing with honey Edition
PREMIXES FOR MEDICATED FEEDING STUFFS FOR VETERINARY USE. Praeadmixta ad alimenta medicata ad usum veterinarium. Effervescent powders
 Preparations for inhalation administered in or with water or another suitable liquid. They may also be swallowed directly. They are presented as single-dose or multidose preparations. Where applicable,
Preparations for inhalation administered in or with water or another suitable liquid. They may also be swallowed directly. They are presented as single-dose or multidose preparations. Where applicable,
Inhalation devices, proper technique and cleaning
 Preventing Your Symptoms and Taking Your Medications Inhalation devices, proper technique and cleaning Knowing how to use your medications properly is important because inhaled drugs are meant to get directly
Preventing Your Symptoms and Taking Your Medications Inhalation devices, proper technique and cleaning Knowing how to use your medications properly is important because inhaled drugs are meant to get directly
3 RESPIRATORY SYSTEM
 3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
Chapter 10 The Respiratory System
 Chapter 10 The Respiratory System Biology 2201 Why do we breathe? Cells carry out the reactions of cellular respiration in order to produce ATP. ATP is used by the cells for energy. All organisms need
Chapter 10 The Respiratory System Biology 2201 Why do we breathe? Cells carry out the reactions of cellular respiration in order to produce ATP. ATP is used by the cells for energy. All organisms need
PATIENT INFORMATION LEAFLET
 N-CORT 0.055% nasal spray, suspension It is used by spraying into the nose. Active substance: (w/w%) Triamcinolone acetonide 0.055 PATIENT INFORMATION LEAFLET Excipients: Benzalkonium chloride 50%, sodium
N-CORT 0.055% nasal spray, suspension It is used by spraying into the nose. Active substance: (w/w%) Triamcinolone acetonide 0.055 PATIENT INFORMATION LEAFLET Excipients: Benzalkonium chloride 50%, sodium
Aspartame Material Safety Data Sheet
 Newseed Chemical Co., Limited Material Safety Data Sheet MSDS SECTION 1- PRODUCT AND MANUFACTURER IDENTIFICATION Product Name: Manufacturer: Manufacturer s Address: China manufacturer Telephone: Fax: SECTION
Newseed Chemical Co., Limited Material Safety Data Sheet MSDS SECTION 1- PRODUCT AND MANUFACTURER IDENTIFICATION Product Name: Manufacturer: Manufacturer s Address: China manufacturer Telephone: Fax: SECTION
NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate
 NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about NASONEX. It does not contain all
NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about NASONEX. It does not contain all
How to Use Inhaled Medications for Asthma and COPD
 How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
Drug/Device Combination Products: Bioequivalence
 Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Reagent Set DAS ELISA, Alkaline phosphatase label
 List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
Stability Study of Hydralazine HCl Oral Solutions Compounded in Humco Oral Vehicles to Determine a Beyond-use Date
 Stability Study of Hydralazine HCl Oral Solutions Compounded in Humco Oral Vehicles to Determine a Beyond-use Date Pradeep Gautam, MS* 1, Troy Purvis, PhD 1 Address correspondence to Pradeep Gautam, HUMCO
Stability Study of Hydralazine HCl Oral Solutions Compounded in Humco Oral Vehicles to Determine a Beyond-use Date Pradeep Gautam, MS* 1, Troy Purvis, PhD 1 Address correspondence to Pradeep Gautam, HUMCO
Redefining Continuous Aerosol Drug Delivery PM223
 Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
SUGGESTED FORMULATION. Lot Number. Expiry Date. Ingredient Listing Qty. Unit NDC # Supplier
 9/17/2016; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Aloe Vera (Powder) (Freeze Dried) 0.200 g Tacrolimus 5% Stock Powder Blend 0.600 g Ethoxy Diglycol 1.0 ml Medisca U-Mild
9/17/2016; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Aloe Vera (Powder) (Freeze Dried) 0.200 g Tacrolimus 5% Stock Powder Blend 0.600 g Ethoxy Diglycol 1.0 ml Medisca U-Mild
PATIENT INFORMATION LEAFLET
 N-CORT 0.055% Nasal Spray For nasal use. PATIENT INFORMATION LEAFLET Active substance(s): Triamcinolone acetonide (w/w%) 0.055 Excipient(s): Benzalkonium chloride 50%, sodium hydroxide, hydrochloric acid,
N-CORT 0.055% Nasal Spray For nasal use. PATIENT INFORMATION LEAFLET Active substance(s): Triamcinolone acetonide (w/w%) 0.055 Excipient(s): Benzalkonium chloride 50%, sodium hydroxide, hydrochloric acid,
Secundum Artem. Stability of Extemporaneously Prepared Oral Liquid Formulations - Part VII
 VOLUME 16 NUMBER 1 Secundum Artem Current & Practical Compounding Information for the Pharmacist. An ongoing CE Program provided by a grant from Perrigo Pharmaceuticals Stability of Extemporaneously Prepared
VOLUME 16 NUMBER 1 Secundum Artem Current & Practical Compounding Information for the Pharmacist. An ongoing CE Program provided by a grant from Perrigo Pharmaceuticals Stability of Extemporaneously Prepared
Current Challenges and Opportunities in Demonstrating Bioequivalence
 Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION APO-TRIAMCINOLONE AQ Children 4 to 12 years Triamcinolone Acetonide Aqueous Nasal Spray 55 mcg/metered Spray Apotex Standard This leaflet is part III of a three-part Product
PART III: CONSUMER INFORMATION APO-TRIAMCINOLONE AQ Children 4 to 12 years Triamcinolone Acetonide Aqueous Nasal Spray 55 mcg/metered Spray Apotex Standard This leaflet is part III of a three-part Product
A. Incorrect! The alveolus is where gas exchange takes place. B. Correct! Surfactant is the lipid-rich material that permits lung inflation.
 Toxicology - Problem Drill 13: Respiratory Toxicology No. 1 of 10 1. The lipid-rich material that decreases surface tension of the alveoli, allowing sacs to inflate properly and remain inflated during
Toxicology - Problem Drill 13: Respiratory Toxicology No. 1 of 10 1. The lipid-rich material that decreases surface tension of the alveoli, allowing sacs to inflate properly and remain inflated during
PVY Reagent Set Compound ELISA, Alkaline phosphatase label Potato virus Y Catalog number: SRA 20001
 List of contents Lot number PVY Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Detection antibody, bottle A 0.150 ml 0.275 ml 0.525 ml 2.525
List of contents Lot number PVY Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Detection antibody, bottle A 0.150 ml 0.275 ml 0.525 ml 2.525
10x Taq DNA polymerase buffer
 10x Taq DNA polymerase buffer 1. IDENTIFICATION OF THE PREPARATION/SUBSTANCE AND OF THE COMPANY Preparation Name: 10x Taq DNA polymerase buffer Use of the preparation: For laboratory use. Suitable for
10x Taq DNA polymerase buffer 1. IDENTIFICATION OF THE PREPARATION/SUBSTANCE AND OF THE COMPANY Preparation Name: 10x Taq DNA polymerase buffer Use of the preparation: For laboratory use. Suitable for
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
Blueberry scorch virus Reagent Set DAS ELISA, Alkaline phosphatase label for BlScV Catalog number: SRA 19100
 List of contents Lot number Blueberry scorch virus Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150
List of contents Lot number Blueberry scorch virus Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150
What is worse, cigarettes or narghile?
 For students What is worse, cigarettes or narghile? Developers: Ron Blonder Institute: The Weizmann Institute of Science, Rehovot. And Belmonte Science Laboratories center, The Hebrew University of Jerusalem.
For students What is worse, cigarettes or narghile? Developers: Ron Blonder Institute: The Weizmann Institute of Science, Rehovot. And Belmonte Science Laboratories center, The Hebrew University of Jerusalem.
In case of an urgent concern or emergency, call 911 or go to the nearest emergency department right away.
 Asthma Basics Patient and Family Education This teaching sheet contains general information only. Talk with your child s doctor or a member of your child s healthcare team about specific care of your child.
Asthma Basics Patient and Family Education This teaching sheet contains general information only. Talk with your child s doctor or a member of your child s healthcare team about specific care of your child.
Stability of Captopril in an Extemporaneously Compounded Humco s Oral Products
 Stability of an Extemporaneously Compounded Humco s Oral Products Pradeep Gautam, MS* 1 1 Address correspondence to Pradeep Gautam, HUMCO Research and Development Laboratory, 201 W. 5 th St. Suite 1250,
Stability of an Extemporaneously Compounded Humco s Oral Products Pradeep Gautam, MS* 1 1 Address correspondence to Pradeep Gautam, HUMCO Research and Development Laboratory, 201 W. 5 th St. Suite 1250,
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy
 Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
Dr.N.Damodharan Professor and head Department of pharmaceutics SRM college of pharmacy Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve
Introduction to Compounding INTRODUCTION. L earning Objectives CHAPTER
 CHAPTER 9 Introduction to Compounding L earning Objectives INTRODUCTION Webster s New World Dictionary defines the word compound as a verb meaning 1. to mix or combine, 2. to make by combining parts, and
CHAPTER 9 Introduction to Compounding L earning Objectives INTRODUCTION Webster s New World Dictionary defines the word compound as a verb meaning 1. to mix or combine, 2. to make by combining parts, and
INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT
 - 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
- 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
Material Safety Data Sheet CISPLATIN INJECTION SECTION 2 COMPOSITION, INFORMATION ON INGREDIENTS
 Material Safety Data Sheet CISPLATIN INJECTION SECTION 1 - PRODUCT MSDS NAME: Cisplatin Injection, BP SYNONYMS: Cis-Diamminedi-chloroplatinum (II) SECTION 2 COMPOSITION, INFORMATION ON INGREDIENTS Active:
Material Safety Data Sheet CISPLATIN INJECTION SECTION 1 - PRODUCT MSDS NAME: Cisplatin Injection, BP SYNONYMS: Cis-Diamminedi-chloroplatinum (II) SECTION 2 COMPOSITION, INFORMATION ON INGREDIENTS Active:
Safety Data Sheet. (Sodium Lauryl Sulfate) Sodium Lauryl Sulfate DATE PREPARED: 6/4/2015. Section 1. Product and Company Identification
 Section 1. Product and Company Identification Product Name CAS Number 151-21-3 Sodium Lauryl Sulfate Parchem - fine & specialty chemicals 415 Huguenot Street New Rochelle, NY 10801 (914) 654-6800 (914)
Section 1. Product and Company Identification Product Name CAS Number 151-21-3 Sodium Lauryl Sulfate Parchem - fine & specialty chemicals 415 Huguenot Street New Rochelle, NY 10801 (914) 654-6800 (914)
KEY PRODUCT FOR HL SAFETY DATA SHEET
 January 2012 KEY PRODUCT FOR HL SAFETY DATA SHEET SECTION 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name Synonym Use Chemical formula Registration numbers Manufacturer/Supplier Address Key
January 2012 KEY PRODUCT FOR HL SAFETY DATA SHEET SECTION 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name Synonym Use Chemical formula Registration numbers Manufacturer/Supplier Address Key
PRACTICES OF COATING COLLECTION SURFACES OF CASCADE IMPACTORS: A SURVEY OF MEMBERS OF THE EUROPEAN PHARMACEUTICAL AEROSOL GROUP (EPAG)
 PRACTICES OF COATING COLLECTION SURFACES OF CASCADE IMPACTORS: A SURVEY OF MEMBERS OF THE EUROPEAN PHARMACEUTICAL AEROSOL GROUP () J.P. Mitchell on behalf of members of the European Pharmaceutical Aerosol
PRACTICES OF COATING COLLECTION SURFACES OF CASCADE IMPACTORS: A SURVEY OF MEMBERS OF THE EUROPEAN PHARMACEUTICAL AEROSOL GROUP () J.P. Mitchell on behalf of members of the European Pharmaceutical Aerosol
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Chapter 2: Human Body Systems Work Independently and Together
 Chapter 2: Human Body Systems Work Independently and Together 2.1 Body Systems Body systems Are made up of parts that work together as a whole Are connected to one or more other Will not function well
Chapter 2: Human Body Systems Work Independently and Together 2.1 Body Systems Body systems Are made up of parts that work together as a whole Are connected to one or more other Will not function well
ANNEXURE -2. Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section.
 2. EXCIPIENTS PROFILES ANNEXURE -2 Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section. 2.1. COMPRITOL 888 Non proprietary names BP:
2. EXCIPIENTS PROFILES ANNEXURE -2 Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section. 2.1. COMPRITOL 888 Non proprietary names BP:
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES
 COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
Student Practical Guide (1) Milk of Magnesia
 School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION
 Product Identifier: Material Name: Recommended Use: Restrictions on Use: Manufacturer: SAFETY DATA SHEET GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION Betagen Topical
Product Identifier: Material Name: Recommended Use: Restrictions on Use: Manufacturer: SAFETY DATA SHEET GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION Betagen Topical
Potato leafroll virus (PLRV) Reagent Set DAS ELISA, Alkaline phosphatase label Catalog number: SRA List of contents
 Potato leafroll virus (PLRV) Reagent Set List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate
Potato leafroll virus (PLRV) Reagent Set List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate
Redefining Continuous Aerosol Drug Delivery 2015 PM223
 Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Smart asthma therapy. Patient information Spacer inhaling aid for metered-dose aerosol inhalers
 FF Smart asthma therapy Patient information Spacer inhaling aid for metered-dose aerosol inhalers :: Metered-dose inhalers coordination technique In the treatment of asthma and COPD the medications are
FF Smart asthma therapy Patient information Spacer inhaling aid for metered-dose aerosol inhalers :: Metered-dose inhalers coordination technique In the treatment of asthma and COPD the medications are
Overview. The Respiratory System. Chapter 18. Respiratory Emergencies 9/11/2012
 Chapter 18 Respiratory Emergencies Slide 1 Overview Respiratory System Review Anatomy Physiology Breathing Assessment Adequate Breathing Breathing Difficulty Focused History and Physical Examination Emergency
Chapter 18 Respiratory Emergencies Slide 1 Overview Respiratory System Review Anatomy Physiology Breathing Assessment Adequate Breathing Breathing Difficulty Focused History and Physical Examination Emergency
VENTOLIN. About your Ventolin Respirator Solution. Read all of this leaflet carefully before you use your medicine
 Patient Information VENTOLIN About your Ventolin Respirator Solution Read all of this leaflet carefully before you use your medicine This leaflet does not have the complete information about your medicine.
Patient Information VENTOLIN About your Ventolin Respirator Solution Read all of this leaflet carefully before you use your medicine This leaflet does not have the complete information about your medicine.
LYSOBACT COMPLETE SPRAY ( ) mg / ml Oromucosal spray, solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER LYSOBACT COMPLETE SPRAY (20 + 1.5 + 0.5) mg / ml Oromucosal spray, solution LYSOZYME, CETYLPYRIDINIUM, LIDOCAINE This leaflet is a copy of the Summary of Product
PACKAGE LEAFLET: INFORMATION FOR THE USER LYSOBACT COMPLETE SPRAY (20 + 1.5 + 0.5) mg / ml Oromucosal spray, solution LYSOZYME, CETYLPYRIDINIUM, LIDOCAINE This leaflet is a copy of the Summary of Product
IPRAVENT Respules/Respirator solution (Ipratropium bromide)
 Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Reagent Set DAS ELISA, Alkaline phosphatase label SRA 22001, SRA 23203, SRA 27703, SRA & SRA ToRSV, ArMV, GFLV, AnFBV and PDV
 List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
Understanding cascade impaction and its importance for inhaler testing
 Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
