Inhaled nitric oxide treatment for preterm infants with hypoxic respiratory failure
|
|
|
- Ira Chase
- 5 years ago
- Views:
Transcription
1 Thorax 2000;55(Suppl 1):S51 S55 Inhaled nitric oxide treatment for preterm infants with hypoxic respiratory failure Rosalind L Smyth Institute of Child Health, Alder Hey Children s Hospital, Liverpool L12 2AP, UK Introductory articles Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial JP Kinsella, WF Walsh, CL Bose, DR Gerstmann, JJ Labella, S Sardesai, MC Walsh-Sukys, MJ McCaffrey, DN Cornfield, VK Bhutani, GR Cutter, M Baier, SH Abman Background. Inhaled nitric oxide improves oxygenation and lessens the need for extracorporealmembrane oxygenation in full-term neonates with hypoxaemic respiratory failure and persistent pulmonary hypertension, but potential adverse effects are intracranial haemorrhage and chronic lung disease. We investigated whether low-dose inhaled nitric oxide would improve survival in premature neonates with unresponsive severe hypoxaemic respiratory failure, and would not increase the frequency or severity of intracranial haemorrhage or chronic lung disease. Methods. We did a double-blind, randomised controlled trial in 12 perinatal centres that provide tertiary care. 80 premature neonates (gestational age 34 weeks) with severe hypoxaemic respiratory failure were randomly assigned inhaled nitric oxide (n=48) or no nitric oxide (n=32, controls). Our primary outcome was survival to discharge. Analysis was by intention to treat. We studied also the rate and severity of intracranial haemorrhage, pulmonary haemorrhage, duration of ventilation, and chronic lung disease at 36 weeks postconceptional age. Findings. The two groups did not differ for baseline characteristics or severity of disease. Inhaled nitric oxide improved oxygenation after 60 min (p=0.03). Survival at discharge was 52% in the inhalednitric-oxide group and 47% in controls (p=0.65). Causes of death were mainly related to extreme prematurity and were similar in the two groups. The two groups did not differ for adverse events or outcomes (intracranial haemorrhage grade 2 4, 28% inhaled nitric oxide and 33% control; pulmonary haemorrhage 13% and 9%; chronic lung disease 60% and 80%). Interpretation. Low-dose inhaled nitric oxide improved oxygenation but did not improve survival in severely hypoxaemic premature neonates. Low-dose nitric oxide in the most critically ill premature neonates does not increase the risk of intracranial haemorrhage, and may decrease risk of chronic lung injury. (Lancet 1999;354:1061 5) Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial The Franco Belgium Collaborative NO Trial Group Background. Inhaled nitric oxide improves oxygenation in severely hypoxaemic term neonates, which lessens the need for extracorporeal-membrane oxygenation. Improvement in other relevant outcomes remains unknown, and safety of inhaled nitric oxide is uncertain in preterm neonates. We did a randomised controlled trial to assess use of inhaled nitric oxide in preterm and near-term neonates. Methods. We randomly assigned 204 preterm (<33 weeks) and near-term ( 33 weeks) neonates with oxygenation indices from 12.5 to 30.0 and 15 to 40, respectively, 10 parts per million (ppm) inhaled nitric oxide (n=105) or control ventilation therapy without nitric oxide (n=99). The primary endpoint was the oxygenation index at 2 h. Analysis was done by intention to treat. Findings. 12 neonates were excluded, leaving 97 (45 preterm) in the nitric-oxide group and 95 (40 preterm) in the control group. The decline in oxygenation index at 2 h was greater in the nitric-oxide group than in the control group (IQR 6.2 [median 8.4] vs 2.9 [12.4], p=0.005), but was significant only in near-term neonates (p= 0.03). Survivors assigned nitric oxide spent fewer days on mechanical ventilation and in the neonatal intensive-care unit, but this was also significant only in near-term neonates (6[3] vs 7[3] days, p=0.05, S51
2 S52 Smyth and 9[6] vs 12[9] days, p=0.02, respectively). Interpretation. Low-dose inhaled nitric oxide early in the course of neonatal respiratory failure improves oxygenation and shortens duration of mechanical ventilation and the length of stay in intensive care. Inhaled nitric oxide was not, however, significantly beneficial in preterm neonates. (Lancet 1999;354: ) Inhaled nitric oxide (NO) is an emerging treatment ventilation perfusion matching This mechanism is which has made the transition from bench to bedside likely to be important in conditions such as parenchymal in a relatively short time. This speedy introduction to lung disease. clinical use relates partly to the attraction of delivering exogenously a naturally occurring and simple molecule. It is an invisible gas and can be administered directly Inhaled NO and primary pulmonary to the site of action via the ventilator circuit, avoiding hypertension of the newborn (PPHN) the need to deliver it systemically. There is a general Initial studies of the use of inhaled NO in animal models perception that it should be safe. The instantaneous of PPHN showed encouraging results In adults with improvement in arterial oxygenation 1 which is frequently primary pulmonary hypertension inhaled NO reduced observed with inhaled NO has been another important pulmonary vascular resistance with no effect on systemic factor in its widespread clinical application. vascular resistance. 14 Not surprisingly, then, the first Treatment with inhaled NO has been studied in uncontrolled clinical studies of NO in newborn infants numerous clinical trials in full term or near term infants were in the group with PPHN. with hypoxic respiratory failure and primary pulmonary A Cochrane systematic review 2 has summarised the hypertension of the newborn (PPHN) and has been results of eight trials of inhaled NO treatment in term evaluated in a Cochrane review. 2 The two introductory or near term infants with hypoxaemic respiratory failure. articles 34 represent the first large clinical trials to address Oxygenation was found to be improved in approximately the efficacy of this agent in preterm infants with hypoxic half of the infants treated with inhaled NO together respiratory failure. To avoid confusion with other trials with a reduced need for extracorporeal membrane oxydiscussed later in this review, the paper by Kinsella et genation (ECMO). Interestingly, whether infants had al 3 will be referred to as the USA trial and that by the echocardiographic evidence of PPHN or not did not Franco Belgium Collaborative NO Trial Group 4 as the appear to affect their outcomes. A weakness of most of Franco Belgium trial. the included trials was that they allowed back up treat- The USA trial 3 was a double blind, randomised, ment of controls (usually with inhaled NO) who failed controlled trial of 80 infants of <34 weeks gestation on the treatment to which they were randomised. They with severe hypoxic respiratory failure. The intervention were thus unable to assess the effect of inhaled NO group received 5 ppm inhaled NO and had improved on survival, the need for ECMO, or other long term oxygenation compared with controls, but there was no outcomes. difference between the groups for survival. Adverse PPHN is a complex disorder characterised by marked outcomes, including the development of chronic lung pulmonary hypertension, right to left shunting of blood disease and intracranial haemorrhage, were similar in across either a patent ductus arteriosus and/or patent both groups. The Franco Belgium trial 4 included pre- foramen ovale, and critical hypoxaemia. 17 This condition term and near term infants with respiratory failure may occur as a primary condition of neonatal maland moderate hypoxia. These infants were randomly adaptation but is frequently associated with severe parassigned to receive either 10 ppm inhaled NO or vent- enchymal lung disease such as surfactant deficiency, ilation without NO. Oxygenation improved significantly meconium aspiration, and bacterial pneumonia. Thus, in the near term but not the preterm infants and, again, intrapulmonary shunting may further complicate the there was no difference in mortality between the two clinical course. Because of the frequent association with groups. parenchymal lung disease, the use of a pharmacological agent to dilate the pulmonary vasculature may not, on its own, lead to sustained improvement in oxygenation Mechanisms of action and ultimately survival. Inhaled NO, or endothelium derived relaxant factor, 5 Some trials have compared the use of inhaled NO is important in regulating vascular tone. It is generated with other ventilatory strategies. In a trial included in enzymatically by nitric oxide synthase from L-arginine. 6 the Cochrane review infants with severe PPHN NO diffuses from the vascular endothelium into the were randomised to receive either inhaled NO or high vascular smooth muscle where it activates guanylate frequency oscillatory ventilation. The protocol allowed cyclase leading to production of cyclic guanosine monophosphate. those who were defined as having failed on the treatment This causes the relaxation of vascular smooth to which they were randomised to cross over to the muscle by a mechanism which probably involves inhibition alternative treatment. Treatment failures after crossover of activation induced increase in cytosolic cal- received both forms of treatment. This trial did not show cium concentration. 7 any difference in outcomes between those allocated It has been suggested that the improvement in oxy- to inhaled NO and those allocated to high frequency genation observed when inhaled NO is administered oscillatory ventilation. In what seems to have been an occurs by two main mechanisms. 8 Firstly, it may reduce attempt to salvage a positive result from an essentially pulmonary vascular resistance and thus reverse extrapulmonary negative trial, the investigators performed a post hoc shunting by selective pulmonary vaso- subgroup analysis of outcome in infants after crossover dilatation. 9 This mechanism is likely to be important in (which was not randomised). They defined four sub- the improved oxygenation observed in PPHN. Secondly, groups of infants those with respiratory distress syndrome inhaled NO may reverse intrapulmonary shunting by of the newborn, meconium aspiration syndrome, redistribution of pulmonary blood flow and enhanced idiopathic PPHN, or congenital diaphragmatic hernia.
3 Inhaled nitric oxide treatment for preterm infants with hypoxic respiratory failure S53 There was a suggestion that infants with PPHN and little direct toxicity of inhaled NO has been noted and parenchymal lung disease who received both high frequency discussion of the possible mechanisms by which inhaled oscillatory ventilation and inhaled NO had im- NO may cause toxicity or, indeed, may be protective to proved outcomes compared with either treatment alone. the lungs, is largely speculative. Conversely, those with PPHN who did not have par- Nitric oxide is rapidly oxidised in air to nitrogen enchymal lung disease were treated more successfully dioxide which has been implicated in airway damage and when they received inhaled NO either alone or in com- inflammation. 24 The concentration of nitrogen dioxide is bination with high frequency oscillatory ventilation than proportional to the second power of inspired NO and when they received high frequency oscillatory ventilation the first power of inspired oxygen, so inspired nitrogen alone. dioxide is likely to be increased when the concentration Another clinical trial, again included in the Cochrane of both inhaled NO and the concentration of inspired review, 18 investigated 235 severely hypoxic infants of oxygen is increased. 22 It has been shown that the use of >34 weeks gestation who were randomised to receive inhaled NO in a neonatal ventilator with an FiO 2 of 0.9 inhaled NO at a concentration of 20 ppm with 100% can produce 5 ppm nitrogen dioxide in less than 20 oxygen being administered to controls. The primary seconds. 25 outcome was death or need for ECMO and significantly There are also concerns that nitric oxide itself is a more infants in the inhaled NO group survived without free radical and is converted to peroxynitrite which may ECMO. When this outcome was broken down into its result in membrane lipid peroxidation. 26 There is some two components, the difference between the two groups evidence that, at limited concentrations, peroxynitrite was the need for ECMO rather than mortality. Again, may protect the lungs, but at high concentrations leads a post hoc subgroup analysis was performed to see if to lung damage. 27 Free radical damage may cause surfactant there were any differences according to the primary dysfunction and lead to chronic lung disease. 28 diagnosis. Unlike the trial by Kinsella et al, 17 they found These mechanisms may be particularly pertinent if this no differences in the response to treatment according treatment is extended to preterm infants who, unlike to the underlying disease. This trial did not include term infants, have free iron in the plasma which may infants with congenital diaphragmatic hernia who were add to the risk of oxygen damage induced by inhaled studied in a separate trial of 53 patients. 19 In this patient NO. 24 group treatment with inhaled NO did not lead to a If inhaled NO causes direct lung toxicity by any of significant improvement in oxygenation compared with these mechanisms, it may be associated with an increase controls, nor did it reduce the need for ECMO or death. in chronic lung disease. Because of the difficulty in Indeed, death in both groups was almost 50%. assessing long term outcomes in these studies, and because of problems with trial designs, this has not yet been properly evaluated. A recently published large trial Assessing clinical benefit of inhaled NO in PPHN, which is too recent to have Most clinical trials have evaluated the following bene- been included in the Cochrane review, showed that ficial outcomes: improved oxygenation, reduced need inhaled NO reduced the need for ECMO and the for ECMO, and survival. 2 Many but not all have shown incidence of chronic lung disease. 29 However, in this an improvement in oxygenation in the group receiving group of infants of >34 weeks gestation chronic lung inhaled NO. Some have commented that, although disease was defined as need for supplemental oxygen at treatment with inhaled NO led to an immediate apparent 30 days and the number of infants receiving oxygen increase in oxygenation, this was short lived. 20 There is after discharge was similar in the two groups. also a lack of consistency in results according to whether High doses of inhaled NO have been associated with the inhaled NO group needed to be referred for ECMO a prolonged bleeding time 30 which has raised concerns less often than controls. However, as the criteria for about the risk of intracranial haemorrhage. In preterm ECMO referral are based on oxygenation, it is hardly infants laboratory based measures of bleeding tendency surprising that some studies have referred few of the are imprecise and variable and have not been evaluated patients in the inhaled NO group, and so ECMO referral in many clinical trials. The USA trial performed a could be regarded as a proxy for oxygenation. Death in very careful evaluation of the infants for intracranial newborn infants has not been shown in any clinical trial haemorrhage and, in fact, it calculated the risk of intracranial to be reduced by treatment with inhaled NO. Survival haemorrhage based on an assumption that all without long term neurodevelopmental impairment is those who were treated with inhaled NO and who died important and has been used as an outcome in trials of before the age of seven days with unknown intracranial ECMO in neonates 21 but not, so far, in clinical trials of haemorrhage status actually had grade 4 intracranial inhaled NO. Early neurological sequelae have been haemorrhage. Even with this worse case scenario they evaluated and Davidson et al used what they termed a showed no increase in risk of intracranial haemorrhage major sequelae index which included measures of in the inhaled NO group, which is very reassuring. A death, ECMO, neurological sequelae in the neonatal trial that is adequately powered to measure long term period, and bronchopulmonary dysplasia or reactive adverse outcomes is likely to be very large. The USA airway disease. 22 There were no differences in this out- trial estimated that a clinical trial designed to show come between treatment and control groups. a significant increase in risk for grade 4 intracranial haemorrhage would require neonates, although the assumptions on which this calculation was based were not stated. Assessing toxicity and adverse effects The adverse, or potentially adverse, outcomes which have been evaluated in newborn infants include systemic hypotension, measurements of potentially damaging products of inhaled NO, chronic lung disease of pre- Appropriate dose of inhaled NO Over the last decade, since clinical trials have been maturity, methaemoglobinaemia, and intracranial performed with this agent, the dose used has reduced haemorrhage. Systemic hypotension has not been described considerably, ranging from 80 ppm to 2 ppm. This has in any of the clinical trials in neonates. 223 Very been largely mitigated by concerns about toxicity. At a
4 S54 Smyth dose of 80 ppm and occasionally at lower doses, 29 et al found that none of the preterm infants included in increased concentrations of methaemoglobin NO have their trial had Doppler evidence of significant extra- been observed Davidson et al 22 randomised infants pulmonary shunting. 8 They also found no evidence with PPHN into four groups receiving 0 ppm (control), of a higher pulmonary artery pressure in infants who 5, 20 or 80 ppm inhaled NO and found no difference responded (by improved oxygenation) to inhaled NO in efficacy between the doses. Cornfield et al 32 compared compared with those who did not respond. This is inhaled NO in a dose of 2 ppm with a control group different from the findings in studies of term or near who did not receive inhaled NO. Treatment failures term infants where a high proportion had an intracardiac after one hour in either group were treated with inhaled shunt which was either right to left or bidirectional. NO at 20 ppm. Infants given the 2 ppm dose had a Subhedar et al showed that inhaled NO was associated slower rate of clinical deterioration than controls. The with a fall in pulmonary artery pressure while mean 2 ppm dose was found not to improve oxygenation systemic arterial pressure was maintained. 8 The oxywhereas the 20 ppm dose did. Infants who were initially genation response was weakly associated with an increase given 2 ppm did not show an improvement in oxygenation in pulmonary blood flow but not with the when 20 ppm inhaled NO was used but those reduction in pulmonary artery pressure. These results in the control group did. This has raised the possibility may be difficult to interpret as a global increase in that administration of a subtherapeutic dose of inhaled pulmonary blood flow without redistribution of pulmonary NO may attenuate the response to endothelium derived perfusion should lead to further impairment of vasodilator agents such as endogenous nitric oxide or gas exchange by worsening the ventilation perfusion prostacyclin. It would appear that the minimum dose mismatch or the intrapulmonary shunting. This is likely that has been shown to be effective in improving oxy- to occur when systemic vasodilators are administered. 35 genation in clinical trials in neonates is around 5 ppm One attraction of inhaled NO is that it may be selectively and, in a study of preterm infants, the 5 ppm dose was deposited only in those regions of the lungs which are shown to be of equivalent efficacy to 20 ppm. 33 ventilated, leading to a local relaxation in the tone of vessels surrounding ventilated areas and simultaneously causing no change in the tone of vessels surrounding Inhaled NO in preterm infants non ventilated areas. The net effect would lead to an Given the lack of convincing benefits associated with overall improvement is gas exchange. 10 NO treatment in term and near term infants, there are understandable concerns about extending these investigations to small preterm infants. Until the pub- The future lication of the USA and Franco Belgium trials, only Those investigating inhaled NO in clinical trials of one small clinical trial by Subhedar et al had compared preterm infants can learn much from trials conducted in the use of inhaled NO in preterm infants with a group bigger neonates. In order to evaluate outcomes properly, of infants not given inhaled NO. 34 This was the only crossover to inhaled NO should be avoided and it was trial suitable for inclusion in a Cochrane review of disappointing that this occurred in the Franco Belgium inhaled NO for preterm infants with respiratory failure. 23 trial. Blinding is possible with inhaled NO and should It investigated the effect of inhaled NO and intravenous be used, as was the case for the USA trial but not the dexamethasone on death or development of chronic Franco Belgium trial. Long term follow up to assess lung disease in 42 infants deemed to be at high risk neurodevelopmental outcomes and chronic lung disease of developing chronic lung disease. No difference in is essential and this is planned for the USA trial. Consideration outcome was found for either treatment. of subgroups is important, but the trials In preterm infants with respiratory distress syndrome should be designed to investigate the efficacy of treatment of the newborn, hypoxaemia is predominately the result in individual subgroups and post hoc subgroup of intrapulmonary shunting and poor ventilation per- analysis should be avoided. Respiratory distress syndrome fusion matching. Although right to left extrapulmonary of the newborn is the underlying diagnosis in shunting may contribute to this hypoxaemia, Subhedar most preterm infants, but severity varies. Most of the LEARNING POINTS Inhaled nitric oxide is an emerging treatment which may be of benefit in patients with conditions causing severe hypoxia Inhaled nitric oxide in term or near term infants with hypoxic respiratory failure has improved oxygenation but trials have been unable to assess its effect on survival or long term outcomes The two studies described are the first large clinical trials to address the efficacy of inhaled nitric oxide in preterm infants with hypoxic respiratory failure In one study the inhaled nitric oxide group had improved oxygenation compared with controls, but there was no difference in groups for survival, occurrence of chronic lung disease, or intracranial haemorrhage There is still no evidence that inhaled nitric oxide in preterm infants improves survival or important long term outcomes such as chronic lung disease or neurodevelopmental status. Further clinical trials are needed to evaluate these outcomes
5 Inhaled nitric oxide treatment for preterm infants with hypoxic respiratory failure S55 6 Palmer R, Ashton D, Moncada S. Vascular endothelial cells synthesize clinical trials have been in severely hypoxic infants, but nitric oxide from L-arginine. Nature 1988;333: it will be important in future trials to investigate whether 7 Ignarro L. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 1989; a beneficial effect is observed in groups with different 65:1 21. degrees of severity. 8 Subhedar N, Shaw N. Changes in oxygenation and pulmonary haemo- dynamics in preterm infants treated with inhaled nitric oxide. Arch The use of inhaled NO in patients with hypoxia is Dis Child 1997;77:F proliferating. Recent clinical trials which have included 9 Frostell C, Fratacci M, Wain J, et al. Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. children 36 and adults 37 with severe respiratory distress Circulation 1991;83: syndrome have failed to show an improvement in clininhaled nitric oxide in newborn babies with severe hypoxaemia. Lancet 10 Roze J, Storme L, Zupan V, et al. Echocardiographic investigation of ically important outcomes. This treatment is now being 1994;344: used, in the absence of evidence of benefit, in infants 11 Rossaint R, Falke K, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 1993;328: and children on intensive care units. 12 Zayek M, Cleveland D, Morin F. Treatment of persistent pulmonary One of the most interesting aspects of this whole field hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr 1993;122: is to consider how new or emerging treatments should 13 Zayek M, Wild L, Roberts J, et al. Effect of nitric oxide on the survival be evaluated in extremely sick and vulnerable newborn rate and incidence of lung injury in newborn lambs with persistent pulmonary hypertension. J Pediatr 1993;123: infants. It has been suggested that any new treatment 14 Pepke-Zaba J, Higenbottam T, Dinh Xuan A, et al. Inhaled nitric should be applied in a randomised manner from the oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991;338: first patient. 38 This approach, however, takes no account 15 Roberts J, Polaner D, Lang P, et al. Inhaled nitric oxide in persistent of the total number needed to evaluate clinically im- pulmonary hypertension of the newborn. Lancet 1992;340: Kinsella J, Neish S, Shaffer E, et al. Low-dose inhalational nitric oxide portant outcomes. Many investigators have alluded to in persistent pulmonary hypertension of the newborn. Lancet 1992; the large number of centres that are using inhaled NO, 340: Kinsella J, Truog W, Walsh W, et al. Randomised, multicenter trial of both inside and outside clinical trial protocols, and this inhaled nitric oxide and high frequency oscillatory ventilation in has led to considerable problems with recruitment to severe, persistent pulmonary hypertension of the newborn. J Pediatr 1997;131: clinical trials. 22 Most clinical trials are underpowered 18 The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic and were stopped early, frequently because of poor respiratory failure. N Engl J Med 1997;336: recruitment. Many were single rather than multicentre. 19 The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital The usual approach in the evolution of new treatments diaphragmatic hernia. Pediatrics 1997;99: from bench to bedside is firstly to undertake studies in 20 Barefield E, Karle V, Philips J, et al. Inhaled nitric oxide in term infants with hypoxemic respiratory failure. J Pediatr 1996;129: appropriate animal models. Even if the results of animal 21 UK Collaborative ECMO Group. UK collaborative randomised trial studies are encouraging, the decision about when to of neonatal extracorporeal membrane oxygenation. Lancet 1996;348: move to well planned clinical studies of a new agent 22 Davidson D, Barefield E, Kattwinkel J, et al. Inhaled nitric oxide for may be difficult and these are generally first conducted the early treatment of persistent pulmonary hypertension of the term newborn: a randomised, double-masked, placebo-controlled, dosein adults. 39 Assuming that the results continue to show response, multicenter study. Pediatrics 1998;101: safety and efficacy, these may be followed by early 23 Barrington K, Finer N. Inhaled nitric oxide for respiratory failure in preterm infants (Cochrane Review). Oxford: Update Software, explanatory trials 40 before large, pragmatic clinical trials 24 Saugstad O. Inhaled nitric oxide for preterm infants: still an experimental are finally conducted. Not until the last group of studies therapy. Lancet 1999; 354: Bouchet M, Renaudin M, Raveau C, et al. Safety requirement for the has been completed and shown unequivocal benefit use of inhaled nitric oxide in neonates. Lancet 1993;341: without unacceptable adverse effects should the treatlipid peroxidation. Arch Biochem Biophys 1991;288: Radi R, Beckman J, Bush K, et al. Peroxynitrite-induced membrane ment be used outside the context of a clinical trial. 27 Gaston B, Drazen J, Loscalzo J, et al. The biology of nitrogen oxides This process has been undertaken very successfully in the airways. Am J Respir Crit Care Med 1994;149: Haddad I, Ischiropoulos H, Holm B, et al. Mechanisms of peroxynitritein newborn infants with treatments such as surfactant induced injury to pulmonary surfactants. Am J Physiol 1993;265: administration for neonatal lung disease. Although both L Clark R, Kueser T, Walker M, et al. Low-dose nitric oxide therapy for the USA and the Franco Belgium trials have added persistent pulmonary hypertension of the newborn. N Engl J Med important new information, inhaled NO must still be 2000;342: Hogman M, Frostell C, Arnberg H, et al. Bleeding time prolongation regarded as an experimental treatment for preterm in- and NO inhalation. Lancet 1993;341: fants with hypoxic respiratory failure. Unless further 31 Roberts J, Fineman J, Frederick C, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med clinical trials are conducted, answers to important ques- 1997;336: Cornfield D, Maynard R, O deregnier R, et al. Randomized, controlled tions particularly concerning long term outcomes of trial of low-dose inhaled nitric oxide in the treatment of term and these treatments will not be provided. In the meantime, near-term infants with respiratory failure and pulmonary hypertension. Pediatrics 1999;104: the only real conclusion that can be drawn from these 33 Skimming J, Bender K, Hutchison A, et al. Nitric oxide inhalation in and other studies is that inhaled NO in preterm infants infants with respiratory distress syndrome. J Pediatr 1997;130: Subhedar N, Ryan S, Shaw N. Open randomised controlled trial of should only be used in the context of a clinical trial. inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child 1997;77:F Radermacher P, Santak P, Becker H, et al. Prostaglandin E1 and nitroglycerin reduce pulmonary capillary wedge pressure but worsen 1 Day R, Lynch J, White K, et al. Acute response to inhaled nitric oxide V/Q distribution in patients with adult respiratory distress syndrome. in newborns with respiratory failure and pulmonary hypertension. Anesthesiology 1989;70: Pediatrics 1996;98: Dobyns E, Cornfield D, Anas N, et al. Multicenter randomized con- 2 Finer N, Barrington K. Nitric oxide for respiratory failure in infants born trolled trial of the effects of inhaled nitric oxide therapy on gas at or near term (Cochrane Review). Oxford: Update Software, exchange in children with acute hypoxemic respiratory failure. J Pediatr 3 Kinsella J, Walsh W, Bose C, et al. Inhaled nitric oxide in premature 1999;134: neonates with severe hypoxaemic respiratory failure: a randomised 37 Michael J, Barton R, Saffle J, et al. Inhaled nitric oxide versus concontrolled trial. Lancet 1999;354: ventional therapy: effect on oxygenation in ARDS. Am J Respir Crit 4 The Franco Belgium Collaborative NO Trial Group. Early compared Care Med 1998;157: with delayed inhaled nitric oxide in moderately hypoxaemic neonates 38 Chalmers T. Randomization of the first patient. Med Clin North Am with respiratory failure: a randomised controlled trial. Lancet 1999; 1975;59: : Smyth RL, Weindling AM. Research in children: ethical and scientific 5 Palmer R, Ferrige A, Moncada S. Nitric oxide release accounts for the aspects. Lancet 1999;354(Suppl 2):SII21 4. biological activity of endothelium-derived relaxing factor. Nature 1987; 327: Smyth RL. Evidence-based pediatric pulmonary medicine: how can it help? (see comments). Pediatr Pulmonol 1998;25:
USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014
 USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014 ino for Late Preterm and Term Infants with Severe PPHN Background:
USE OF INHALED NITRIC OXIDE IN THE NICU East Bay Newborn Specialists Guideline Prepared by P Joe, G Dudell, A D Harlingue Revised 7/9/2014 ino for Late Preterm and Term Infants with Severe PPHN Background:
Keep. with life MEDICATION TECHNOLOGY SERVICES INSPIRED BY YOUR NEEDS
 Keep with life MEDICATION TECHNOLOGY SERVICES INSPIRED BY YOUR NEEDS 2 KINOX MEDICATION KINOX, inhaled nitric oxide, is a selective pulmonary vasodilator developed by Air Liquide Healthcare and characterized
Keep with life MEDICATION TECHNOLOGY SERVICES INSPIRED BY YOUR NEEDS 2 KINOX MEDICATION KINOX, inhaled nitric oxide, is a selective pulmonary vasodilator developed by Air Liquide Healthcare and characterized
Nitric Resource Manual
 Nitric Resource Manual OBJECTIVES Describe the biologic basis for inhaled nitric oxide therapy Describe the indications for inhaled nitric oxide therapy Describe the potential hazards, side effects and
Nitric Resource Manual OBJECTIVES Describe the biologic basis for inhaled nitric oxide therapy Describe the indications for inhaled nitric oxide therapy Describe the potential hazards, side effects and
Earlier Use of Inhaled Nitric Oxide in Term and Near-Term Neonates With Hypoxic Respiratory Failure (HRF) and Pulmonary Hypertension (PH)
 Earlier Use of Inhaled Nitric Oxide in Term and Near-Term Neonates With Hypoxic Respiratory Failure (HRF) and Pulmonary Hypertension (PH) 2013 Ikaria, Inc. 1 Disclosure Information This program is sponsored
Earlier Use of Inhaled Nitric Oxide in Term and Near-Term Neonates With Hypoxic Respiratory Failure (HRF) and Pulmonary Hypertension (PH) 2013 Ikaria, Inc. 1 Disclosure Information This program is sponsored
INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN
 INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN JESSE D. ROBERTS, JR., M.D., JEFFREY R. FINEMAN, M.D.,
INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN INHALED NITRIC OXIDE AND PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN JESSE D. ROBERTS, JR., M.D., JEFFREY R. FINEMAN, M.D.,
Original Policy Date
 MP 8.01.17 Inhaled Nitric Oxide Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
MP 8.01.17 Inhaled Nitric Oxide Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
Guideline for the Use of inhaled Nitric Oxide (NO) Catarina Silvestre Prof. Harish Vyas
 Inhaled Nitric Oxide Title of Guideline Guideline for the Use of inhaled Nitric Oxide (NO) 1a 2a 2b Contact Name and Job Title (author) Directorate & Speciality Date of submission October 2015 Date when
Inhaled Nitric Oxide Title of Guideline Guideline for the Use of inhaled Nitric Oxide (NO) 1a 2a 2b Contact Name and Job Title (author) Directorate & Speciality Date of submission October 2015 Date when
Scope: This guideline is aimed at all Health Care Professionals involved in the care of infants within the Neonatal Service.
 Persistent Pulmonary Hypertension of the Newborn (PPHN) University Hospitals of Leicester NHS NHS Trust June 2018 June 2021 Scope: This guideline is aimed at all Health Care Professionals involved in the
Persistent Pulmonary Hypertension of the Newborn (PPHN) University Hospitals of Leicester NHS NHS Trust June 2018 June 2021 Scope: This guideline is aimed at all Health Care Professionals involved in the
1
 1 2 3 RIFAI 5 6 Dublin cohort, retrospective review. Milrinone was commenced at an initial dose of 0.50 μg/kg/minute up to 0.75 μg/kg/minute and was continued depending on clinical response. No loading
1 2 3 RIFAI 5 6 Dublin cohort, retrospective review. Milrinone was commenced at an initial dose of 0.50 μg/kg/minute up to 0.75 μg/kg/minute and was continued depending on clinical response. No loading
Clinical Policy Title: Inhaled nitric oxide
 Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: May 17, 2017 Next Review Date: March
Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: May 17, 2017 Next Review Date: March
MEDICAL POLICY I. POLICY POLICY TITLE POLICY NUMBER INHALED NITRIC OXIDE MP-4.021
 Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Inhaled nitric oxide may be considered medically necessary as
Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Inhaled nitric oxide may be considered medically necessary as
Weaning strategy with inhaled nitric oxide treatment in persistent pulmonary hypertension of the newborn
 F118 Archives of Disease in Childhood 1997;76:F118 F122 Department of Pediatrics, Columbia University, 630 West 168th Street, New York, NY 10032, USA H Aly R Sahni Department of Anesthesia J-T Wung Correspondence
F118 Archives of Disease in Childhood 1997;76:F118 F122 Department of Pediatrics, Columbia University, 630 West 168th Street, New York, NY 10032, USA H Aly R Sahni Department of Anesthesia J-T Wung Correspondence
High-frequency ventilation augments the effect of inhaled nitric oxide in persistent pulmonary hypertension of the newborn
 Eur Respir J 1998; 11: 234 238 DOI: 1.1183/931936.98.111234 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISSN 93-1936 CASE STUDY High-frequency ventilation
Eur Respir J 1998; 11: 234 238 DOI: 1.1183/931936.98.111234 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISSN 93-1936 CASE STUDY High-frequency ventilation
Clinical Policy Title: Inhaled nitric oxide
 Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: March 6, 2018 Next Review Date:
Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: March 6, 2018 Next Review Date:
Clinical Policy Title: Inhaled nitric oxide
 Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: March 6, 2018 Next Review Date:
Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: March 6, 2018 Next Review Date:
AARC Clinical Practice Guideline
 AARC Clinical Practice Guideline Evidence-Based Clinical Practice Guideline: Inhaled Nitric Oxide for Neonates With Acute Hypoxic Respiratory Failure Robert M DiBlasi RRT-NPS FAARC, Timothy R Myers RRT-NPS,
AARC Clinical Practice Guideline Evidence-Based Clinical Practice Guideline: Inhaled Nitric Oxide for Neonates With Acute Hypoxic Respiratory Failure Robert M DiBlasi RRT-NPS FAARC, Timothy R Myers RRT-NPS,
SCVMC RESPIRATORY CARE PROCEDURE
 Page 1 of 7 New: 12/08 R: 4/11 R NC: 7/11, 7/12 B7180-63 Definitions: Inhaled nitric oxide (i) is a medical gas with selective pulmonary vasodilator properties. Vaso-reactivity is the evidence of acute
Page 1 of 7 New: 12/08 R: 4/11 R NC: 7/11, 7/12 B7180-63 Definitions: Inhaled nitric oxide (i) is a medical gas with selective pulmonary vasodilator properties. Vaso-reactivity is the evidence of acute
The use of proning in the management of Acute Respiratory Distress Syndrome
 Case 3 The use of proning in the management of Acute Respiratory Distress Syndrome Clinical Problem This expanded case summary has been chosen to explore the rationale and evidence behind the use of proning
Case 3 The use of proning in the management of Acute Respiratory Distress Syndrome Clinical Problem This expanded case summary has been chosen to explore the rationale and evidence behind the use of proning
PPHN (see also ECMO guideline)
 Children s Acute Transport Service Clinical Guidelines PPHN (see also ECMO guideline) Document Control Information Author P Brooke E.Randle Author Position Medical Student Consultant Document Owner E.
Children s Acute Transport Service Clinical Guidelines PPHN (see also ECMO guideline) Document Control Information Author P Brooke E.Randle Author Position Medical Student Consultant Document Owner E.
Extracorporeal Membrane Oxygenation (ECMO) Referrals
 Children s Acute Transport Service Clinical Guideline Extracorporeal Membrane Oxygenation (ECMO) Referrals Document Control Information Author ECMO/CATS Author Position Service Coordinator Document Owner
Children s Acute Transport Service Clinical Guideline Extracorporeal Membrane Oxygenation (ECMO) Referrals Document Control Information Author ECMO/CATS Author Position Service Coordinator Document Owner
ino_rmp version 4.1_2016 Module Risk-management system NO-RMP-V 2.1
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Persistent pulmonary hypertension of the newborn (PPHN) Persistent pulmonary hypertension of the newborn is a life threatening
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Persistent pulmonary hypertension of the newborn (PPHN) Persistent pulmonary hypertension of the newborn is a life threatening
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Policy #: 440 Latest Review Date: May 2016
 Name of Policy: Inhaled Nitric Oxide Policy #: 440 Latest Review Date: May 2016 Category: Therapy Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross and Blue
Name of Policy: Inhaled Nitric Oxide Policy #: 440 Latest Review Date: May 2016 Category: Therapy Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross and Blue
HFOV IN THE NON-RECRUITABLE LUNG
 HFOV IN THE NON-RECRUITABLE LUNG HFOV IN THE NON-RECRUITABLE LUNG PPHN Pulmonary hypoplasia after PPROM Congenital diaphragmatic hernia Pulmonary interstitial emphysema / cystic lung disease 1 30 Mean
HFOV IN THE NON-RECRUITABLE LUNG HFOV IN THE NON-RECRUITABLE LUNG PPHN Pulmonary hypoplasia after PPROM Congenital diaphragmatic hernia Pulmonary interstitial emphysema / cystic lung disease 1 30 Mean
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Inhaled Nitric Oxide (INO) Table of Contents Coverage Policy... 1 General Background... 1 Coding/Billing Information... 16 References... 17 Effective Date... 6/15/2014
Cigna Medical Coverage Policy Subject Inhaled Nitric Oxide (INO) Table of Contents Coverage Policy... 1 General Background... 1 Coding/Billing Information... 16 References... 17 Effective Date... 6/15/2014
SWISS SOCIETY OF NEONATOLOGY. Preterm infant with. pulmonary hypertension and hypopituitarism
 SWISS SOCIETY OF NEONATOLOGY Preterm infant with pulmonary hypertension and hypopituitarism November 2007 2 Pilgrim S, Stocker M, Neonatal and Pediatric Intensiv Care Unit, Children s Hospital of Lucerne,
SWISS SOCIETY OF NEONATOLOGY Preterm infant with pulmonary hypertension and hypopituitarism November 2007 2 Pilgrim S, Stocker M, Neonatal and Pediatric Intensiv Care Unit, Children s Hospital of Lucerne,
ino in neonates with cardiac disorders
 ino in neonates with cardiac disorders Duncan Macrae Paediatric Critical Care Terminology PAP Pulmonary artery pressure PVR Pulmonary vascular resistance PHT Pulmonary hypertension - PAP > 25, PVR >3,
ino in neonates with cardiac disorders Duncan Macrae Paediatric Critical Care Terminology PAP Pulmonary artery pressure PVR Pulmonary vascular resistance PHT Pulmonary hypertension - PAP > 25, PVR >3,
Neurodevelopmental Outcome of Newborns with Persistent Pulmonary Hypertension
 Original Article Neurodevelopmental Outcome of Newborns with Persistent Pulmonary Hypertension Jaafar Rohana 1, Nem Yun Boo 2, Viji Chandran 1, Rajini Sarvananthan 1 Submitted: 6 Dec 2010 Accepted: 3 Jan
Original Article Neurodevelopmental Outcome of Newborns with Persistent Pulmonary Hypertension Jaafar Rohana 1, Nem Yun Boo 2, Viji Chandran 1, Rajini Sarvananthan 1 Submitted: 6 Dec 2010 Accepted: 3 Jan
Management of Respiratory Disease in the Term Infant
 Management of Respiratory Disease in the Term Infant David Tingay 1. Neonatal Research, Murdoch Children s Research Institute, Melbourne 2. Neonatology, Royal Children s Hospital 3. Dept of Paediatrics,
Management of Respiratory Disease in the Term Infant David Tingay 1. Neonatal Research, Murdoch Children s Research Institute, Melbourne 2. Neonatology, Royal Children s Hospital 3. Dept of Paediatrics,
Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of pulmonary arterial hypertension
 5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
Medical Coverage Policy
 Medical Coverage Policy Subject: Inhaled Nitric Oxide Policy #: MED Current Effective Date: 11/10/14 Status: New Last Review Date: 11/10/14 Description/Scope Hypoxic respiratory failure may result from
Medical Coverage Policy Subject: Inhaled Nitric Oxide Policy #: MED Current Effective Date: 11/10/14 Status: New Last Review Date: 11/10/14 Description/Scope Hypoxic respiratory failure may result from
ECMO for Severe Hypoxemic Respiratory Failure: Pro-Con Debate. Carolyn Calfee, MD MAS Mark Eisner, MD MPH
 ECMO for Severe Hypoxemic Respiratory Failure: Pro-Con Debate Carolyn Calfee, MD MAS Mark Eisner, MD MPH June 3, 2010 Case Presentation Setting: Community hospital, November 2009 29 year old woman with
ECMO for Severe Hypoxemic Respiratory Failure: Pro-Con Debate Carolyn Calfee, MD MAS Mark Eisner, MD MPH June 3, 2010 Case Presentation Setting: Community hospital, November 2009 29 year old woman with
The Blue Baby. Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin
 The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
Subject: Inhaled Nitric Oxide (ino) for Neonatal Hypoxic Respiratory Failure. Original Effective Date: 10/4/12 10/30/13, 12/16/2015, 7/21/2016
 Subject: Inhaled Nitric Oxide (ino) for Neonatal Hypoxic Respiratory Failure Original Effective Date: 10/4/12 Policy Number: MCP-121 Revision Date(s): 10/30/13, 12/16/2015, 7/21/2016 Review Date: 6/22/17
Subject: Inhaled Nitric Oxide (ino) for Neonatal Hypoxic Respiratory Failure Original Effective Date: 10/4/12 Policy Number: MCP-121 Revision Date(s): 10/30/13, 12/16/2015, 7/21/2016 Review Date: 6/22/17
Subject: Inhaled Nitric Oxide
 07-00007-12 Original Effective Date: 04/15/01 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Inhaled Nitric Oxide THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
07-00007-12 Original Effective Date: 04/15/01 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Inhaled Nitric Oxide THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
Shorter duration of oxygen therapy Decrease of lung damage and reduction in severity of chronic lung disease
 Randomized Controlled Trial of Early Compared with Delayed Use of Inhaled Nitric Oxide in Newborns with a Moderate Respiratory Failure and Pulmonary Hypertension A González J Fabres I D Apremont G Urcelay
Randomized Controlled Trial of Early Compared with Delayed Use of Inhaled Nitric Oxide in Newborns with a Moderate Respiratory Failure and Pulmonary Hypertension A González J Fabres I D Apremont G Urcelay
HHS Public Access Author manuscript Semin Perinatol. Author manuscript; available in PMC 2017 October 01.
 Inhaled Nitric Oxide Therapy for Pulmonary Disorders of the Term and Preterm Infant Gregory M. Sokol, MD 1, G. Ganesh Konduri, MD 2, and Krisa P. Van Meurs, MD 3 1 Section of Neonatal-Perinatal Medicine,
Inhaled Nitric Oxide Therapy for Pulmonary Disorders of the Term and Preterm Infant Gregory M. Sokol, MD 1, G. Ganesh Konduri, MD 2, and Krisa P. Van Meurs, MD 3 1 Section of Neonatal-Perinatal Medicine,
... INOmax Product Information PRODUCT INFORMATION NAME OF THE MEDICINE. INOmax (nitric oxide for inhalation)
 PRODUCT INFORMATION NAME OF THE MEDICINE INOmax (nitric oxide for inhalation) Pharmacotherapeutic class: Pulmonary vasodilator DESCRIPTION INOmax (nitric oxide) is a drug administered by inhalation. Nitric
PRODUCT INFORMATION NAME OF THE MEDICINE INOmax (nitric oxide for inhalation) Pharmacotherapeutic class: Pulmonary vasodilator DESCRIPTION INOmax (nitric oxide) is a drug administered by inhalation. Nitric
INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2
 2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME
 INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
Inhaled nitric oxide in hypoxaemic newborns who are candidates for extracorporeal life support
 Eur Respir J 1998; 11: 371 376 DOI: 10.1183/09031936.98.11020371 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISS 0903-1936 Inhaled nitric oxide in hypoxaemic
Eur Respir J 1998; 11: 371 376 DOI: 10.1183/09031936.98.11020371 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISS 0903-1936 Inhaled nitric oxide in hypoxaemic
PATENT DUCTUS ARTERIOSUS IN THE PRETERM INFANT EVIDENCE FOR & AGAINST TREATMENT
 PATENT DUCTUS ARTERIOSUS IN THE PRETERM INFANT EVIDENCE FOR & AGAINST TREATMENT Dr. Youssef Abou Zanouna, FRCPI, FACC Consultant Pediatric Cardiologist King Fahd Military Medical Complex Dhahran Introduction
PATENT DUCTUS ARTERIOSUS IN THE PRETERM INFANT EVIDENCE FOR & AGAINST TREATMENT Dr. Youssef Abou Zanouna, FRCPI, FACC Consultant Pediatric Cardiologist King Fahd Military Medical Complex Dhahran Introduction
Clinical Policy: Inhaled Nitric Oxide Reference Number: CP.MP.87
 Clinical Policy: Reference Number: CP.MP.87 Last Review Date: 09/17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description (ino) is a
Clinical Policy: Reference Number: CP.MP.87 Last Review Date: 09/17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description (ino) is a
Neonatal/Pediatric Cardiopulmonary Care. Persistent Pulmonary Hypertension of the Neonate (PPHN) PPHN. Other. Other Diseases
 Neonatal/Pediatric Cardiopulmonary Care Other Diseases Persistent Pulmonary Hypertension of the Neonate (PPHN) PPHN 3 Also known as Persistent Fetal Circulation (PFC) Seen most frequently in term, post-term
Neonatal/Pediatric Cardiopulmonary Care Other Diseases Persistent Pulmonary Hypertension of the Neonate (PPHN) PPHN 3 Also known as Persistent Fetal Circulation (PFC) Seen most frequently in term, post-term
PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN East Bay Newborn Specialists Guideline Prepared by L Truong 12/15/2015
 PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN East Bay Newborn Specialists Guideline Prepared by L Truong 12/15/2015 Background: Persistent pulmonary hypertension of the newborn occurs in ~2 per 1,000
PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN East Bay Newborn Specialists Guideline Prepared by L Truong 12/15/2015 Background: Persistent pulmonary hypertension of the newborn occurs in ~2 per 1,000
Three Decades of Managing Congenital Diaphragmatic Hernia
 Three Decades of Managing Congenital Diaphragmatic Hernia Desmond Bohn The Department of Critical Care Medicine The Hospital for Sick Children, Toronto Robert E Gross Congenital Diaphragmatic Hernia 1960-80
Three Decades of Managing Congenital Diaphragmatic Hernia Desmond Bohn The Department of Critical Care Medicine The Hospital for Sick Children, Toronto Robert E Gross Congenital Diaphragmatic Hernia 1960-80
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Pulmonary Vasodilator Treatments in the ICU Setting
 Pulmonary Vasodilator Treatments in the ICU Setting Lara Shekerdemian Circulation 1979 Ann Thorac Surg 27 Anesth Analg 211 1 Factors in the ICU Management of Pulmonary Hypertension After Cardiopulmonary
Pulmonary Vasodilator Treatments in the ICU Setting Lara Shekerdemian Circulation 1979 Ann Thorac Surg 27 Anesth Analg 211 1 Factors in the ICU Management of Pulmonary Hypertension After Cardiopulmonary
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT INOmax 400 ppm mol/mol inhalation gas 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nitric oxide (NO) 400 ppm mol/mol. A 2 litre
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT INOmax 400 ppm mol/mol inhalation gas 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nitric oxide (NO) 400 ppm mol/mol. A 2 litre
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
In Vivo Models and Cell Delivery for Lung Indications NO DISCLOSURES
 In Vivo Models and Cell Delivery for Lung Indications Marlowe Eldridge MD Department of Pediatrics and Biomedical Engineering University of Wisconsin School of Medicine and Public Health NO DISCLOSURES
In Vivo Models and Cell Delivery for Lung Indications Marlowe Eldridge MD Department of Pediatrics and Biomedical Engineering University of Wisconsin School of Medicine and Public Health NO DISCLOSURES
Management of refractory ARDS. Saurabh maji
 Management of refractory ARDS Saurabh maji Refractory hypoxemia as PaO2/FIO2 is less than 100 mm Hg, inability to keep plateau pressure below 30 cm H2O despite a VT of 4 ml/kg development of barotrauma
Management of refractory ARDS Saurabh maji Refractory hypoxemia as PaO2/FIO2 is less than 100 mm Hg, inability to keep plateau pressure below 30 cm H2O despite a VT of 4 ml/kg development of barotrauma
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
HFOV Case Study 3.6kg MAS Instructor Copy
 HFOV Case Study 3.6kg MAS Instructor Copy Color key: Black Patient case Blue RN/RCP collaboration Red MD consult Green - Critical Thinking Inquiry Green italics CT answers ABG: ph/pco2/po2/base A 35 year-old
HFOV Case Study 3.6kg MAS Instructor Copy Color key: Black Patient case Blue RN/RCP collaboration Red MD consult Green - Critical Thinking Inquiry Green italics CT answers ABG: ph/pco2/po2/base A 35 year-old
INOmax Therapy with INOmax DSIR. Linde: Living healthcare
 INOmax Therapy with INOmax DSIR are INOmax Therapy 02 We are INOmax Therapy 03 Contents. 4 We are INOmax Therapy 6 INOmax Therapy Mode of action The benefits of INOmax Therapy 18 Efficacy and safety of
INOmax Therapy with INOmax DSIR are INOmax Therapy 02 We are INOmax Therapy 03 Contents. 4 We are INOmax Therapy 6 INOmax Therapy Mode of action The benefits of INOmax Therapy 18 Efficacy and safety of
Clinical Policy: Inhaled Nitric Oxide Reference Number: CP.MP.87
 Clinical Policy: Reference Number: CP.MP.87 Last Review Date: 09/18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description (ino) is a
Clinical Policy: Reference Number: CP.MP.87 Last Review Date: 09/18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description (ino) is a
Implementation of an Inhaled Nitric Oxide Protocol Decreases Direct Cost Associated With Its Use
 Implementation of an Inhaled Nitric Oxide Protocol Decreases Direct Cost Associated With Its Use Deanna R Todd Tzanetos MD MSCI, Jon J Housley PharmD MBA, Frederick E Barr MD MSCI, Warren L May PhD, and
Implementation of an Inhaled Nitric Oxide Protocol Decreases Direct Cost Associated With Its Use Deanna R Todd Tzanetos MD MSCI, Jon J Housley PharmD MBA, Frederick E Barr MD MSCI, Warren L May PhD, and
** SURFACTANT THERAPY**
 ** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
24. An infant with recurrent pneumonia underwent a frontal chest radiograph (Fig 24-A) followed by
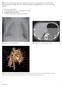 24. An infant with recurrent pneumonia underwent a frontal chest radiograph (Fig 24-A) followed by diagnosis? ndings, what is the most likely A. Pulmonary sequestration B. Congenital pulmonary airway malformation
24. An infant with recurrent pneumonia underwent a frontal chest radiograph (Fig 24-A) followed by diagnosis? ndings, what is the most likely A. Pulmonary sequestration B. Congenital pulmonary airway malformation
Pulmonary Problems of the Neonate. Jon Palmer, VMD, DACVIM Chief, Neonatal Intensive Care Service New Bolton Center, University of Pennsylvania, USA
 Pulmonary Problems of the Neonate Jon Palmer, VMD, DACVIM Chief, Neonatal Intensive Care Service New Bolton Center, University of Pennsylvania, USA Lower Respiratory Diseases Ventilation/Perfusion Abnormalities
Pulmonary Problems of the Neonate Jon Palmer, VMD, DACVIM Chief, Neonatal Intensive Care Service New Bolton Center, University of Pennsylvania, USA Lower Respiratory Diseases Ventilation/Perfusion Abnormalities
New approaches for persistent pulmonary hypertension of newborn
 Clin Perinatol 31 (2004) 591 611 New approaches for persistent pulmonary hypertension of newborn G. Ganesh Konduri, MD Division of Neonatology, Medical College of Wisconsin and Children s Research Institute
Clin Perinatol 31 (2004) 591 611 New approaches for persistent pulmonary hypertension of newborn G. Ganesh Konduri, MD Division of Neonatology, Medical College of Wisconsin and Children s Research Institute
Hypoxic Respiratory Failure in the Newborn. Question and Answer
 Hypoxic Respiratory Failure in the Newborn Question and Answer Question: When administering nitric oxide to premature babies what is considered safe or common practice in terms of length of treatment?
Hypoxic Respiratory Failure in the Newborn Question and Answer Question: When administering nitric oxide to premature babies what is considered safe or common practice in terms of length of treatment?
Hazards and Benefits of Postnatal Steroids. David J. Burchfield, MD Professor and Chief, Neonatology University of Florida
 Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
Hazards and Benefits of Postnatal Steroids David J. Burchfield, MD Professor and Chief, Neonatology University of Florida Disclosures I have no financial affiliations or relationships to disclose. I will
Inhaled Pulmonary Vasodilators: Are There Indications Within the Pediatric ICU?
 Inhaled Pulmonary Vasodilators: Are There Indications Within the Pediatric ICU? Bradley A Kuch MHA RRT-NPS FAARC, Alvin L Saville RRT, Joan Sanchez De Toledo MD, and Shekhar T Venkataraman MD Introduction
Inhaled Pulmonary Vasodilators: Are There Indications Within the Pediatric ICU? Bradley A Kuch MHA RRT-NPS FAARC, Alvin L Saville RRT, Joan Sanchez De Toledo MD, and Shekhar T Venkataraman MD Introduction
WELCOME. Welcome to the Children s Hospital PICU (Pediatric Intensive Care Unit). We consider it a privilege to care for your child and your family.
 ECMO FAMILY GUIDE WELCOME Welcome to the Children s Hospital PICU (Pediatric Intensive Care Unit). We consider it a privilege to care for your child and your family. This book was created to give you the
ECMO FAMILY GUIDE WELCOME Welcome to the Children s Hospital PICU (Pediatric Intensive Care Unit). We consider it a privilege to care for your child and your family. This book was created to give you the
B etween 1992 and 2001, hospital survival in paediatric
 F423 ORIGINAL ARTICLE Predicting outcome in ex-premature infants supported with extracorporeal membrane oxygenation for acute hypoxic respiratory failure K L Brown, G Walker, D J Grant, K Tanner, D A Ridout,
F423 ORIGINAL ARTICLE Predicting outcome in ex-premature infants supported with extracorporeal membrane oxygenation for acute hypoxic respiratory failure K L Brown, G Walker, D J Grant, K Tanner, D A Ridout,
Pharmacologic Treatment of Neonatal Pulmonary Hypertension
 Pharmacologic Treatment of Neonatal Pulmonary Hypertension Steven H. Abman, MD Professor of Pediatrics Director, Pediatric Heart Lung Center University of Colorado School of Medicine and Children s Hospital
Pharmacologic Treatment of Neonatal Pulmonary Hypertension Steven H. Abman, MD Professor of Pediatrics Director, Pediatric Heart Lung Center University of Colorado School of Medicine and Children s Hospital
Lesta Whalen, MD Medical Director, Sanford ECMO Pediatric Critical Care
 Lesta Whalen, MD Medical Director, Sanford ECMO Pediatric Critical Care Disclosures I have no financial disclosures. The use of certain devises for providing long-term cardiopulmonary support is investigational.
Lesta Whalen, MD Medical Director, Sanford ECMO Pediatric Critical Care Disclosures I have no financial disclosures. The use of certain devises for providing long-term cardiopulmonary support is investigational.
STOP ROP The STOP-ROP Multicenter Study Group: Pediatrics 105:295, 2000 Progression to Threshold Conventional Sat 89-94% STOP ROP
 Hrs TcPO2 > 80 nnhg (weeks 1 4) OXYGEN TARGETS: HOW GOOD ARE WE IN ACHIEVING THEM Oxygen Dependency GA wks Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center
Hrs TcPO2 > 80 nnhg (weeks 1 4) OXYGEN TARGETS: HOW GOOD ARE WE IN ACHIEVING THEM Oxygen Dependency GA wks Eduardo Bancalari MD University of Miami Miller School of Medicine Jackson Memorial Medical Center
Hypoxemia post Liver-Transplantation for Hepatopulmonary Syndrome
 ! Hypoxemia post Liver-Transplantation for Hepatopulmonary Syndrome HS Jeffrey Man University Health Network and Mount Sinai Hospital Keenan Research Centre at the Li Ka Shing Knowledge Institute, St.
! Hypoxemia post Liver-Transplantation for Hepatopulmonary Syndrome HS Jeffrey Man University Health Network and Mount Sinai Hospital Keenan Research Centre at the Li Ka Shing Knowledge Institute, St.
Definition: HPS is a disease process with a triad of: 1- Liver disease. 2- Widespread intrapulmonary vasodilatation. 3- Gas exchange abnormality prese
 Hepatopulmonary syndrome (HPS) By Alaa Haseeb, MS.c Definition: HPS is a disease process with a triad of: 1- Liver disease. 2- Widespread intrapulmonary vasodilatation. 3- Gas exchange abnormality presenting
Hepatopulmonary syndrome (HPS) By Alaa Haseeb, MS.c Definition: HPS is a disease process with a triad of: 1- Liver disease. 2- Widespread intrapulmonary vasodilatation. 3- Gas exchange abnormality presenting
nitric oxide for inhalation 100 ppm and 800 ppm Medical Gas
 PRODUCT MONOGRAPH Pr Noxivent nitric oxide for inhalation 100 ppm and 800 ppm Medical Gas Praxair Canada Inc. 1 City Centre Drive, Suite 1200, Mississauga, ON L5B 1M2 Control Number: 175320 Date of Preparation:
PRODUCT MONOGRAPH Pr Noxivent nitric oxide for inhalation 100 ppm and 800 ppm Medical Gas Praxair Canada Inc. 1 City Centre Drive, Suite 1200, Mississauga, ON L5B 1M2 Control Number: 175320 Date of Preparation:
Valutazione del neonato con sospetta ipertensione polmonare
 Valutazione del neonato con sospetta ipertensione polmonare Cardiologia Pediatrica Seconda Università degli Studi di Napoli A.O. R.N. dei Colli-Monaldi Napoli Hypoxiemic infant Full or near-term neonate
Valutazione del neonato con sospetta ipertensione polmonare Cardiologia Pediatrica Seconda Università degli Studi di Napoli A.O. R.N. dei Colli-Monaldi Napoli Hypoxiemic infant Full or near-term neonate
of persistent pulmonary hypertension of
 144 Original Article Singapore Med J 201 0; 51(2) : Inhaled nitric oxide and intravenous magnesium sulphate for the treatment of persistent pulmonary hypertension of the newborn Boo N Y, Rohana J, Yong
144 Original Article Singapore Med J 201 0; 51(2) : Inhaled nitric oxide and intravenous magnesium sulphate for the treatment of persistent pulmonary hypertension of the newborn Boo N Y, Rohana J, Yong
Current and Future Treatments for Persistent Pulmonary Hypertension in the Newborn
 Basic & Clinical Pharmacology & Toxicology, 2018, 123, 392 406 Doi: 10.1111/bcpt.13051 MiniReview Current and Future Treatments for Persistent Pulmonary Hypertension in the ewborn Jonas Pedersen 1, Elise
Basic & Clinical Pharmacology & Toxicology, 2018, 123, 392 406 Doi: 10.1111/bcpt.13051 MiniReview Current and Future Treatments for Persistent Pulmonary Hypertension in the ewborn Jonas Pedersen 1, Elise
Is There a Treatment for BPD?
 Is There a Treatment for BPD? Amir Kugelman, Pediatric Pulmonary Unit and Department of Neonatology Bnai Zion Medical Center, Rappaport Faculty of Medicine Haifa, Israel Conflict of Interest Our study
Is There a Treatment for BPD? Amir Kugelman, Pediatric Pulmonary Unit and Department of Neonatology Bnai Zion Medical Center, Rappaport Faculty of Medicine Haifa, Israel Conflict of Interest Our study
ACUTE RESPIRATORY DISTRESS SYNDROME
 ACUTE RESPIRATORY DISTRESS SYNDROME Angel Coz MD, FCCP, DCE Assistant Professor of Medicine UCSF Fresno November 4, 2017 No disclosures OBJECTIVES Identify current trends and risk factors of ARDS Describe
ACUTE RESPIRATORY DISTRESS SYNDROME Angel Coz MD, FCCP, DCE Assistant Professor of Medicine UCSF Fresno November 4, 2017 No disclosures OBJECTIVES Identify current trends and risk factors of ARDS Describe
SWISS SOCIETY OF NEONATOLOGY. Supercarbia in an infant with meconium aspiration syndrome
 SWISS SOCIETY OF NEONATOLOGY Supercarbia in an infant with meconium aspiration syndrome January 2006 2 Wilhelm C, Frey B, Department of Intensive Care and Neonatology, University Children s Hospital Zurich,
SWISS SOCIETY OF NEONATOLOGY Supercarbia in an infant with meconium aspiration syndrome January 2006 2 Wilhelm C, Frey B, Department of Intensive Care and Neonatology, University Children s Hospital Zurich,
Adjunct Therapies for Pediatric ARDS: Where are the Data?
 Adjunct Therapies for Pediatric ARDS: Where are the Data? Alexandre T. Rotta, MD, FCCM Professor of Pediatrics, Linsalata Family Endowed Chair in Pediatric Critical Care and Emergency Medicine Rainbow
Adjunct Therapies for Pediatric ARDS: Where are the Data? Alexandre T. Rotta, MD, FCCM Professor of Pediatrics, Linsalata Family Endowed Chair in Pediatric Critical Care and Emergency Medicine Rainbow
Safety of epoprostenol and treprostinil in children less than 12 months of age
 ORIGINAL RESEARCH Safety of epoprostenol and treprostinil in children less than 12 months of age Chelsey M. McIntyre, 1 Brian D. Hanna, 2 Natalie Rintoul, 3 E. Zachary Ramsey 1 1 Department of Pharmacy,
ORIGINAL RESEARCH Safety of epoprostenol and treprostinil in children less than 12 months of age Chelsey M. McIntyre, 1 Brian D. Hanna, 2 Natalie Rintoul, 3 E. Zachary Ramsey 1 1 Department of Pharmacy,
Inhaled Nitric Oxide for Children With Congenital Heart Disease and Pulmonary Hypertension
 Inhaled Nitric Oxide for Children With Congenital Heart Disease and Pulmonary Hypertension Ronald D. Curran, MD, Constantine Mavroudis, MD, Carl L. Backer, MD, Michael Sautel, BS, Vincent R. Zales, MD,
Inhaled Nitric Oxide for Children With Congenital Heart Disease and Pulmonary Hypertension Ronald D. Curran, MD, Constantine Mavroudis, MD, Carl L. Backer, MD, Michael Sautel, BS, Vincent R. Zales, MD,
Late pulmonary hypertension in preterm infants How to sort things out? V.Gournay, FCPC, La Martinique, Nov 23,2015
 Late pulmonary hypertension in preterm infants How to sort things out? V.Gournay, FCPC, La Martinique, Nov 23,2015 Epidemiology Incidence of extreme prematurity (
Late pulmonary hypertension in preterm infants How to sort things out? V.Gournay, FCPC, La Martinique, Nov 23,2015 Epidemiology Incidence of extreme prematurity (
Persistent Pulmonary Hypertension of the Newborn
 Persistent Pulmonary Hypertension of the Newborn Lon-Yen Tsao Division of Neonatology, Department of Pediatrics Changhua Christian Hospital, Changhua, Taiwan, R.O.C. Introduction: Persistent pulmonary
Persistent Pulmonary Hypertension of the Newborn Lon-Yen Tsao Division of Neonatology, Department of Pediatrics Changhua Christian Hospital, Changhua, Taiwan, R.O.C. Introduction: Persistent pulmonary
LOW-DOSE NITRIC OXIDE THERAPY FOR PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN
 LOW-DOSE THERAPY FOR PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN LOW-DOSE THERAPY FOR PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN REESE H. CLARK, M.D., THOMAS J. KUESER, M.D., MARSHALL W. WALKER,
LOW-DOSE THERAPY FOR PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN LOW-DOSE THERAPY FOR PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN REESE H. CLARK, M.D., THOMAS J. KUESER, M.D., MARSHALL W. WALKER,
MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH)
 MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH) ORPHAN DRUG AND RARE DISEASE 11 MAY 2017 Catherine Lesage, MD, Pediatrics Program Head, Actelion Copyright AGENDA Pulmonary Arterial
MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH) ORPHAN DRUG AND RARE DISEASE 11 MAY 2017 Catherine Lesage, MD, Pediatrics Program Head, Actelion Copyright AGENDA Pulmonary Arterial
DOSE-RESPONSE RELATIONSHIP FOR INHALED NITRIC OXIDE IN EXPERIMENTAL PULMONARY HYPERTENSION IN SHEEP
 British Journal of Anaesthesia 1993; 71: 702-708 DOSE-RESPONSE RELATIONSHIP FOR INHALED NITRIC OXIDE IN EXPERIMENTAL PULMONARY HYPERTENSION IN SHEEP O. DYAR, J. D. YOUNG, L. XIONG, S. HOWELL AND E. JOHNS
British Journal of Anaesthesia 1993; 71: 702-708 DOSE-RESPONSE RELATIONSHIP FOR INHALED NITRIC OXIDE IN EXPERIMENTAL PULMONARY HYPERTENSION IN SHEEP O. DYAR, J. D. YOUNG, L. XIONG, S. HOWELL AND E. JOHNS
Landmark articles on ventilation
 Landmark articles on ventilation Dr Shrikanth Srinivasan MD,DNB,FNB,EDIC Consultant, Critical Care Medicine Medanta, The Medicity ARDS AECC DEFINITION-1994 ALI Acute onset Bilateral chest infiltrates PCWP
Landmark articles on ventilation Dr Shrikanth Srinivasan MD,DNB,FNB,EDIC Consultant, Critical Care Medicine Medanta, The Medicity ARDS AECC DEFINITION-1994 ALI Acute onset Bilateral chest infiltrates PCWP
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 F EBRUARY 27, 1997 NUMBER 9 INHALED NITRIC OXIDE IN FULL-TERM AND NEARLY FULL-TERM INFANTS WITH HYPOXIC
The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 F EBRUARY 27, 1997 NUMBER 9 INHALED NITRIC OXIDE IN FULL-TERM AND NEARLY FULL-TERM INFANTS WITH HYPOXIC
Novel Strategies in NICU and PICU for the Child Who is Really Difficult to Ventilate
 Novel Strategies in NICU and PICU for the Child Who is Really Difficult to Ventilate Larry C. Lands, MD, PhD Professor of Pediatrics, McGill University Director, Pediatric Respiratory Medicine Montreal
Novel Strategies in NICU and PICU for the Child Who is Really Difficult to Ventilate Larry C. Lands, MD, PhD Professor of Pediatrics, McGill University Director, Pediatric Respiratory Medicine Montreal
Steroids in ARDS: if, when, how much? John Fowler, MD, FACEP Dept. of Emergency Medicine Kent Hospital, İzmir, Türkiye
 Steroids in ARDS: if, when, how much? John Fowler, MD, FACEP Dept. of Emergency Medicine Kent Hospital, İzmir, Türkiye Steroids in ARDS: conclusion Give low-dose steroids if indicated for another problem
Steroids in ARDS: if, when, how much? John Fowler, MD, FACEP Dept. of Emergency Medicine Kent Hospital, İzmir, Türkiye Steroids in ARDS: conclusion Give low-dose steroids if indicated for another problem
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy
 An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
M. Högman*, S-Z. Wei*, C. Frostell**, H. Arnberg*, G. Hedenstierna*
 Eur Respir J, 199, 7, 698 702 DOI: 10.118/090196.9.070698 Printed in UK - all rights reserved Copyright ERS Journals Ltd 199 European Respiratory Journal ISSN 090-196 Effects of inhaled nitric oxide on
Eur Respir J, 199, 7, 698 702 DOI: 10.118/090196.9.070698 Printed in UK - all rights reserved Copyright ERS Journals Ltd 199 European Respiratory Journal ISSN 090-196 Effects of inhaled nitric oxide on
Objectives. Apnea Definition and Pitfalls. Pathophysiology of Apnea. Apnea of Prematurity and hypoxemia episodes 5/18/2015
 Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
Dr. Roberta Keller has nothing to disclose.
 Vascular reactivity and onary hypertension in congenital diaphragmatic hernia Roberta L. Keller, MD UCSF Benioff Children s Hospital March 12, 211 Dr. Roberta Keller has nothing to disclose. Outline Lung
Vascular reactivity and onary hypertension in congenital diaphragmatic hernia Roberta L. Keller, MD UCSF Benioff Children s Hospital March 12, 211 Dr. Roberta Keller has nothing to disclose. Outline Lung
Simulation 08: Cyanotic Preterm Infant in Respiratory Distress
 Flow Chart Simulation 08: Cyanotic Preterm Infant in Respiratory Distress Opening Scenario Section 1 Type: DM As staff therapist assigned to a Level 2 NICU in a 250 bed rural medical center you are called
Flow Chart Simulation 08: Cyanotic Preterm Infant in Respiratory Distress Opening Scenario Section 1 Type: DM As staff therapist assigned to a Level 2 NICU in a 250 bed rural medical center you are called
Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In Qatar
 ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 2 Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In
ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 2 Short-Term Outcome Of Different Treatment Modalities Of Patent Ductus Arteriosus In Preterm Infants. Five Years Experiences In
Beneficial Effects of Inhaled Nitric Oxide in Adult Cardiac Surgical Patients
 Beneficial Effects of Inhaled Nitric Oxide in Adult Cardiac Surgical Patients Thomas S. Maxey, MD, Christopher D. Smith, MBBS, FRACS, John A. Kern, MD, Curtis G. Tribble, MD, David R. Jones, MD, Irving
Beneficial Effects of Inhaled Nitric Oxide in Adult Cardiac Surgical Patients Thomas S. Maxey, MD, Christopher D. Smith, MBBS, FRACS, John A. Kern, MD, Curtis G. Tribble, MD, David R. Jones, MD, Irving
INOmax nitric oxide for inhalation
 PRODUCT MONOGRAPH INOmax nitric oxide for inhalation 800 ppm Distributed by: C.R.I. 4 Innovation Drive Dundas ON Canada L9H 7P3 Manufactured by: Mallinckrodt Manufacturing LLC 1060 Allendale Drive Port
PRODUCT MONOGRAPH INOmax nitric oxide for inhalation 800 ppm Distributed by: C.R.I. 4 Innovation Drive Dundas ON Canada L9H 7P3 Manufactured by: Mallinckrodt Manufacturing LLC 1060 Allendale Drive Port
Research Roundtable Summary
 Research Roundtable Summary 10 TENTH in a Series of Seminars on MCHB-funded Research Projects Early Cortisol Deficiency and Bronchopulmonary Dysplasia October 18, 1995 Parklawn Building Potomac Conference
Research Roundtable Summary 10 TENTH in a Series of Seminars on MCHB-funded Research Projects Early Cortisol Deficiency and Bronchopulmonary Dysplasia October 18, 1995 Parklawn Building Potomac Conference
