Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum
|
|
|
- Raymond Marshall
- 5 years ago
- Views:
Transcription
1 Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum Version: OvaryFallopian Protocol Posting Date: August 2018 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual and 2015 FIGO Cancer Report For accreditation purposes, this protocol should be used for the following procedures AND tumor types: Procedure Description Resection Includes oophorectomy, salpingo-oophorectomy, salpingectomy, subtotal resection, or removal of tumor in fragments Tumor Type Description Primary malignant tumors of Includes all primary epithelial borderline tumors and carcinomas, ovary, fallopian tube or carcinosarcoma, malignant germ cell tumors, and malignant sex peritoneum cord-stromal tumors. This protocol is NOT required for accreditation purposes for the following: Procedure Biopsy Primary resection specimen with no residual cancer (eg, following neoadjuvant therapy) Cytologic specimens The following tumor types should NOT be reported using this protocol: Tumor Type Peritoneal mesothelioma Lymphoma (consider the Hodgkin or non-hodgkin Lymphoma protocols) Sarcoma (consider the Soft Tissue protocol) Authors Saeid Movahedi-Lankarani, MD*; Uma Krishnamurti, MD, PhD*; Debra A. Bell, MD; George G. Birdsong, MD; Charles V. Biscotti, MD; Christopher N. Chapman Jr, MD; Veronica Klepeis, MD, PhD; Christopher N. Otis, MD With guidance from the CAP Cancer and CAP Pathology Electronic Reporting Committees. * Denotes primary authors. All other contributing authors are listed alphabetically College of American Pathologists (CAP). All rights reserved. For Terms of Use please visit
2 Female Reproductive Ovary, Fallopian Tube, Peritoneum Accreditation Requirements This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Core data elements are required in reports to adequately describe appropriate malignancies. For accreditation purposes, essential data elements must be reported in all instances, even if the response is not applicable or cannot be determined. Conditional data elements are only required to be reported if applicable as delineated in the protocol. For instance, the total number of lymph nodes examined must be reported, but only if nodes are present in the specimen. Optional data elements are identified with + and although not required for CAP accreditation purposes, may be considered for reporting as determined by local practice standards. The use of this protocol is not required for recurrent tumors or for metastatic tumors that are resected at a different time than the primary tumor. Use of this protocol is also not required for pathology reviews performed at a second institution (ie, secondary consultation, second opinion, or review of outside case at second institution). Synoptic Reporting All core and conditionally required data elements outlined on the surgical case summary from this cancer protocol must be displayed in synoptic report format. Synoptic format is defined as: Data element: followed by its answer (response), outline format without the paired "Data element: Response" format is NOT considered synoptic. The data element should be represented in the report as it is listed in the case summary. The response for any data element may be modified from those listed in the case summary, including Cannot be determined if appropriate. Each diagnostic parameter pair (Data element: Response) is listed on a separate line or in a tabular format to achieve visual separation. The following exceptions are allowed to be listed on one line: o Anatomic site or specimen, laterality, and procedure o Pathologic Stage Classification (ptnm) elements o Negative margins, as long as all negative margins are specifically enumerated where applicable The synoptic portion of the report can appear in the diagnosis section of the pathology report, at the end of the report or in a separate section, but all Data element: Responses must be listed together in one location Organizations and pathologists may choose to list the required elements in any order, use additional methods in order to enhance or achieve visual separation, or add optional items within the synoptic report. The report may have required elements in a summary format elsewhere in the report IN ADDITION TO but not as replacement for the synoptic report ie, all required elements must be in the synoptic portion of the report in the format defined above. CAP Laboratory Accreditation Program Protocol Required Use Date: April 2019 CAP Ovary and Fallopian Protocol Summary of Changes Version The following data elements were modified: Regional Lymph Nodes - Revised the format to clarify reporting involved and uninvolved nodes 2
3 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum Surgical Pathology Cancer Case Summary Protocol posting date: August 2018 OVARY or FALLOPIAN TUBE or PRIMARY PERITONEUM: Note: Applies to primary tumors of ovarian, fallopian tube, or primary peritoneal origin. If bilateral tumors of 2 different histologic types are present, separate case summaries should be used for each tumor. Select a single response unless otherwise indicated. Procedure (select all that apply) (Note A) Total hysterectomy and bilateral salpingo-oophorectomy Radical hysterectomy Simple hysterectomy Supracervical hysterectomy Bilateral salpingo-oophorectomy Right salpingo-oophorectomy Left salpingo-oophorectomy Salpingo-oophorectomy, side not specified Right oophorectomy Left oophorectomy Oophorectomy, side not specified Bilateral salpingectomy Right salpingectomy Left salpingectomy Salpingectomy, side not specified Omentectomy Peritoneal biopsies Peritoneal tumor debulking Peritoneal washing Pleurocentesis (pleural fluid) Other (specify): Note: For information about lymph node sampling, please refer to the Regional Lymph Node section. + Hysterectomy Type + Abdominal + Vaginal + Vaginal, laparoscopic-assisted + Laparoscopic + Laparoscopic, robotic-assisted + Other (specify): + Not specified Specimen Integrity (Note B) Note: For primary ovarian tumors, if the ovary containing primary tumor is removed intact into a laparoscopy bag and ruptured in the bag by the surgeon without spillage into the peritoneal cavity (to allow for removal via laparoscopy port site or small incision), the specimen integrity should be listed as capsule intact with a comment explaining this in the report. Specimen Integrity of Right Ovary (if applicable) Capsule intact Capsule ruptured Fragmented Other (specify): + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 3
4 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum Specimen Integrity of Left Ovary (if applicable) Capsule intact Capsule ruptured Fragmented Other (specify): Specimen Integrity of Right Fallopian Tube (if applicable) Serosa intact Serosa ruptured Fragmented Other (specify): Specimen Integrity of Left Fallopian Tube (if applicable) Serosa intact Serosa ruptured Fragmented Other (specify): + Morcellated specimen (specify organ): Tumor Site (Notes C, D, and E) Note: Please select the primary tumor site only. Information about additional organ involvement is provided in another section. Right ovary Left ovary Bilateral ovaries Ovary, laterality cannot be determined (explain): Right fallopian tube Left fallopian tube Bilateral fallopian tubes Fallopian tube, laterality cannot be determined (explain): Right tubo-ovarian Left tubo-ovarian Bilateral tubo-ovarian Tubo-ovarian, laterality cannot be determined (explain): Primary peritoneum Other (specify): Ovarian Surface Involvement (required only if applicable) Present Specify laterality (if applicable): Absent Cannot be determined (explain): Fallopian Tube Surface Involvement (required only if applicable) Present Specify laterality (if applicable): Absent Cannot be determined (explain): Tumor Size Note: For bilateral tumors, please report maximum dimension for each primary tumor, specifying by laterality. Greatest dimension (centimeters): cm + Additional dimensions (centimeters): x cm Cannot be determined (explain): + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 4
5 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum Histologic Type (select all that apply) (Notes F and G) Serous tubal intraepithelial carcinoma (STIC) Serous borderline tumor/atypical proliferative serous tumor Serous borderline tumor/atypical proliferative serous tumor with microinvasion Serous borderline tumor-micropapillary variant/noninvasive low-grade serous carcinoma Serous carcinoma Endometrioid borderline tumor/atypical proliferative endometrioid tumor Endometrioid borderline tumor/atypical proliferative endometrioid tumor with microinvasion Endometrioid carcinoma Clear cell borderline tumor/atypical proliferative clear cell tumor Clear cell carcinoma Mucinous borderline tumor/atypical proliferative mucinous tumor Mucinous borderline tumor/atypical proliferative mucinous tumor with intraepithelial carcinoma Mucinous borderline tumor/atypical proliferative mucinous tumor with microinvasion Mucinous carcinoma + Mucinous carcinoma with expansile invasive pattern + Mucinous carcinoma with infiltrative invasive pattern Seromucinous borderline tumor/atypical proliferative seromucinous tumor Seromucinous borderline tumor/atypical proliferative seromucinous tumor with microinvasion Seromucinous carcinoma Brenner tumor, borderline/atypical proliferative Brenner tumor Brenner tumor, malignant Carcinoma, subtype cannot be determined Mixed epithelial borderline (atypical proliferative) tumor (specify types and percentages): Mixed epithelial carcinoma (specify types and percentages): Carcinosarcoma (malignant mixed Müllerian tumor) Small cell carcinoma, pulmonary type Small cell carcinoma, hypercalcemic type Squamous cell carcinoma Transitional cell carcinoma Undifferentiated carcinoma Granulosa cell tumor, adult type Granulosa cell tumor, juvenile type Other sex cord-stromal tumor (specify type): Dysgerminoma Yolk sac tumor (endodermal sinus tumor) Immature teratoma Carcinoma arising from a teratoma (specify type): Mixed malignant germ cell tumor (specify types and percentages): Other histologic type not listed (specify): Histologic Grade (required for serous, endometrioid, mucinous, and seromucinous carcinomas, immature teratomas, and Sertoli-Leydig cell tumors) (Note H) Note: Serous carcinomas are graded via a 2-tier system. Immature teratomas can be graded using a 2-tier or 3-tier system. Endometrioid and mucinous carcinomas are graded via a 3-tier system. Sertoli-Leydig cell tumors are graded via a modified 3- tier grading system with grade 2 tumors being termed intermediate differentiated. Clear cell carcinomas, borderline epithelial neoplasms, carcinosarcomas, all other malignant sex-cord stromal and germ cell tumors are not graded. WHO Grading System G1: Well differentiated G2: Moderately differentiated G3: Poorly differentiated GX: Cannot be assessed Two-Tier Grading System (required for immature teratomas and serous carcinomas only) Low grade + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 5
6 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum High grade Other (specify): Not applicable Implants (required for advanced stage serous/seromucinous borderline tumors only) (Note I) Note: Serous tumor implants that were formerly classified as invasive implants are now classified as low-grade serous carcinoma of the peritoneum. Not sampled Not identified Present (specify sites): Other Tissue/ Organ Involvement (select all that apply) Note: Any organ not selected is either not involved or was not submitted. Not applicable Not identified Right ovary Left ovary Ovary (side not specified) Right fallopian tube Left fallopian tube Fallopian tube (side not specified) Uterus Cervix Pelvic peritoneum Abdominal peritoneum Omentum Other organs/tissue (specify): Cannot be determined (explain): Largest Extrapelvic Peritoneal Focus (required only if applicable) Microscopic Macroscopic (2 cm or less) Macroscopic (greater than 2 cm) Cannot be determined (explain): Specify site (if applicable): Peritoneal/Ascitic Fluid Not submitted/unknown Negative for malignancy (normal/benign) Atypical and/or suspicious (explain): Malignant (positive for malignancy) Unsatisfactory/nondiagnostic (explain): Other (specify): Results pending + Pleural Fluid + Not submitted / unknown + Negative for malignancy (normal/benign) + Atypical and/or suspicious (explain): + Malignant (positive for malignancy) + Unsatisfactory/nondiagnostic (explain): + Results pending + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 6
7 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum Treatment Effect (required only for high-grade serous carcinomas) (Note J) Note: Treatment effect is based on assessment of residual tumor in the omentum. No known presurgical therapy No definite or minimal response identified (chemotherapy response score 1 [CRS 1]) Moderate response identified (CRS 2) Marked response with no or minimal residual cancer (CRS 3) Cannot be determined Regional Lymph Nodes Note: For ovarian, fallopian tube, or primary peritoneal tumors, lymph nodes designated as pelvic; (parametrial, obturator, internal iliac (hypogastric), external iliac, common iliac, sacral, presacral), para-aortic, and retroperitoneal are considered regional lymph nodes. Although not specifically named by AJCC or FIGO, intra-omental and peri-intestinal lymph nodes, are also regarded as regional lymph nodes for staging purposes. Any other involved nodes should be categorized as metastases (pm1) and commented on in the distant metastasis section. Presence of isolated tumor cells no greater than 0.2 mm in regional lymph node(s) is considered N0 (i+). No lymph nodes submitted or found Lymph Node Examination (required only if lymph nodes are present in the specimen) All lymph nodes negative for tumor cells Positive for tumor cells (select all that apply) Number of Nodes with Metastasis Greater than 10 mm: Number of Nodes with Metastasis 10 mm or Less (excludes isolated tumor cells): Number of Nodes with Isolated Tumor Cells (0.2 mm or less) (if applicable): Number cannot be determined (explain): Note: Reporting the number of lymph nodes with isolated tumor cells is required only in the absence of metastasis greater than 0.2 mm in other lymph nodes. + Nodal Site(s) with Tumor Cells (specify): Size of Largest Metastatic Deposit (if applicable) (millimeters): mm Location of Largest Deposit (if applicable) (specify): Number of Lymph Nodes Examined: Number cannot be determined (explain): + Specify Site(s): Pathologic Stage Classification (ptnm, AJCC 8 th Edition) (Note K) Note: Reporting of pt, pn, and (when applicable) pm categories is based on information available to the pathologist at the time the report is issued. Only the applicable T, N, or M category is required for reporting; their definitions need not be included in the report. The categories (with modifiers when applicable) can be listed on 1 line or more than 1 line. TNM Descriptors (required only if applicable) (select all that apply) m (multiple primary tumors) r (recurrent) y (posttreatment) Primary Tumor (pt) ptx: Primary tumor cannot be assessed pt0: No evidence of primary tumor pt1: Tumor limited to ovaries (one or both) or fallopian tube(s) pt1a: Tumor limited to one ovary (capsule intact) or fallopian tube, no tumor on ovarian or fallopian tube surface; no malignant cells in ascites or peritoneal washings # + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 7
8 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum pt1b: pt1c: pt1c1: pt1c2: pt1c3: pt2: pt2a: pt2b: pt3: pt3a: pt3b: pt3c: Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in ascites or peritoneal washings Tumor limited to one or both ovaries or fallopian tubes with any of the following: Surgical spill Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface Malignant cells in ascites or peritoneal washings Tumor involves one or both ovaries or fallopian tubes with pelvic extension below pelvic brim or primary peritoneal cancer Extension and/or implants on uterus and/or fallopian tube(s) and/or ovaries. Extension to and/or implants on other pelvic tissues. Tumor involves one or both ovaries or fallopian tubes, or primary peritoneal cancer with microscopically confirmed peritoneal metastasis outside the pelvis and/or metastasis to retroperitoneal (pelvic and/or para-aortic) lymph nodes Microscopic extra-pelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes Macroscopic peritoneal metastasis beyond pelvis 2 cm or less in greatest dimension with or without metastasis to retroperitoneal lymph nodes Macroscopic peritoneal metastasis beyond pelvis more than 2 cm in greatest dimension with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ) # Note: Serous tubal intraepithelial carcinoma (STIC) should be staged as pt1a if it involves one tube only, as pt1b if it involves both tubes, and as pt1c3 if it is accompanied by positive peritoneal washing washings or ascites. Nonmalignant ascites is not classified. The presence of ascites does not affect staging unless malignant cells are present. Regional Lymph Nodes (pn) pnx: Regional lymph nodes cannot be assessed pn0: No regional lymph node metastasis pn0(i+): Isolated tumor cells in regional lymph node(s) no greater than 0.2 mm pn1: Positive retroperitoneal lymph nodes only (histologically confirmed) pn1a: Metastasis up to and including 10 mm in greatest dimension pn1b: Metastasis more than 10 mm in greatest dimension Distant Metastasis (pm) (required only if confirmed pathologically in this case) pm1: Distant metastasis, including pleural effusion with positive cytology; liver or splenic parenchymal metastasis; metastasis to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside the abdominal cavity); and transmural involvement of intestine pm1a: Pleural effusion with positive cytology pm1b: Liver or splenic parenchymal metastases; metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside the abdominal cavity); transmural involvement of intestine Specify site(s), if known: Note: Parenchymal liver or splenic metastasis is classified as stage IV disease, whereas liver or splenic capsule metastasis is classified as stage III disease. Non-regional lymph node metastases (such as inguinal, supraclavicular, and axillary nodes) are considered M1. Involvement of surface of diaphragm is considered pt3; however, involvement of skeletal muscle of diaphragm or abdominal wall tissue beyond the peritoneum is considered distant metastasis (M1). + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 8
9 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum FIGO Stage (2015 FIGO Cancer Report) + I: Tumor confined to ovaries or fallopian tube(s) + IA: Tumor limited to 1 ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings + IB: Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings + IC: Tumor limited to 1 or both ovaries or fallopian tubes, with any of the following: + IC1: Surgical spill intraoperatively + IC2: Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface + IC3: Malignant cells present in the ascites or peritoneal washings + II: Tumor involves 1 or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or peritoneal cancer + IIA: Extension and/or implants on the uterus and/or fallopian tubes and/or ovaries + IIB: Extension to other pelvic intraperitoneal tissues + III: Tumor involves 1 or both ovaries, or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes + IIIA: Metastasis to the retroperitoneal lymph nodes with or without microscopic peritoneal involvement beyond the pelvis + IIIA1: Positive retroperitoneal lymph nodes only (cytologically or histologically proven) + IIIA1(i): Metastasis 10 mm in greatest dimension # + IIIA1(ii): Metastasis >10 mm in greatest dimension # + IIIA2: Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes + IIIB: Macroscopic peritoneal metastases beyond the pelvic brim 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes + IIIC: Macroscopic peritoneal metastases beyond the pelvic brim >2 cm in greatest dimension, with or without metastases to the retroperitoneal nodes ## + IV: Distant metastasis excluding peritoneal metastases + IVA: Pleural effusion with positive cytology + IVB: Metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of abdominal cavity) ### # This is tumor dimension and not lymph node dimension. ## Includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ. ### Parenchymal metastases are stage IVB. Disease invading through the bowel wall and into the mucosa increases the stage to IVB, and transmural involvement of a visceral structure also represents stage IVB disease. + Additional Pathologic Findings (select all that apply) (Note L) + None identified + Serous tubal intraepithelial carcinoma (STIC) + Endometriosis + Endosalpingiosis + Other (specify): + Ancillary Studies (Note M) + BRCA1 mutation testing (specify result): + BRCA2 mutation testing (specify result): + DNA mismatch repair enzyme studies (specify result) # : # Note: For clear cell and endometroid carcinomas only + Clinical History (select all that apply) + BRCA1/2 family history + Hereditary breast/ovarian cancer + Lynch syndrome + Other (specify): + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 9
10 CAP Approved Female Reproductive Ovary, Fallopian Tube, Peritoneum Comment(s) + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 10
11 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum Explanatory Notes A. Suggestions for Sampling for Microscopic Examination Ovarian Surface Involvement of the ovarian surface is an important element in staging tumors limited to the ovary, and the presence of surface involvement may influence treatment. Therefore, careful examination of the ovarian surface is crucial. Furthermore, in patients who undergo prophylactic (salpingo-) oophorectomy because of a family history of ovarian and/or breast cancer, very small foci of involvement of the ovarian surface may be present that may be potentially lethal and may be missed if the macroscopic inspection is not optimal. 1-6 Ovarian/Adnexal Tumor One section for each centimeter of the tumor s largest dimension is generally recommended, with modification based on the degree of heterogeneity of the tumor and the difficulty of diagnosis. Borderline (atypical proliferative) serous tumor, borderline serous tumors with micropapillary features/noninvasive low-grade serous carcinoma, and borderline (atypical proliferative) mucinous tumors require more sections (2 sections for each centimeter of the tumor s largest dimension is recommended in such cases).some sections should include the ovarian surface where it is most closely approached by tumor on gross examination, with the number of sections depending on the degree of suspicion of surface involvement. Tumor adhesions and sites of rupture should be sampled and labeled specifically for microscopic identification. Risk Reducing Salpingo-Oophorectomy Specimens The ovary and fallopian tube should be submitted in toto in patients with BRCA mutations or suspected to be at increased risk of hereditary breast/ovarian cancer, even when grossly normal. This detailed examination results in an approximately 4-fold increase in detection of precursor lesions or early microscopic carcinoma. 7 Appropriate handling implies that all ovarian and tubal tissue should be serially sectioned and submitted. 8,9 For fallopian tubes, amputate the fimbriated ends and section parallel to the long axis of the fallopian tube to maximize the amount of tubal epithelium available for histological examination (SEE-FIM protocol) 10 (Figure 1). The remainder of the fallopian tube is submitted as serial cross-sections. Fixation for 1 to 2 hours prior to sectioning and/or manipulation may help prevent sloughing of the epithelium. Figure 1. Protocol for Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) of the Fallopian Tube. This protocol entails amputation and longitudinal sectioning of the infundibulum and fimbrial segment (distal 2 cm) to allow maximal exposure of the tubal plicae. The isthmus and ampulla are cut transversely at 2- to 3-mm intervals. From Crum et al. 10 Copyright 2007 Lippincott Williams & Wilkins. Reproduced with permission. Sampling Issues The recommendation for the number of sections to be taken of an ovarian/adnexal tumor is a general guideline, with the pathologist determining how many sections are necessary. If a tumor is obviously malignant and 11
12 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum homogeneous throughout on gross examination, fewer sections may be needed. In contrast, if there is great variability in the gross appearance of the sectioned surfaces or opened cysts, it may be necessary to take more sections to sample the tumor adequately. In addition, as a general recommendation, borderline serous tumors with micropapillary foci or with microinvasion should be extensively sampled to ensure adequate assessment of the extent of invasion, when present. Mucinous tumors (particularly those with solid areas), solid teratomas, and malignant germ cell tumors often require careful gross examination and judicious sampling. Of note, additional sampling of a tumor that poses problems in differential diagnosis may be more informative than special studies. Fallopian Tube(s) For patients with high-grade serous carcinoma, if no gross lesion is present in the fimbrial end of each fallopian tube, complete microscopic examination is recommended. If a gross fimbrial lesion is present, representative sections of tumor to determine its distribution and relationship to tubal epithelium are recommended. For patients with high-grade serous carcinoma, in contrast to other tumor histologic types covered by this protocol, a small, sometimes microscopic focus of tumor may be present in the mucosa of the fallopian tube that is the probable primary site (see Note C). The identification of tubal involvement can usually be accomplished by careful macroscopic examination and, if nothing is identified grossly, by submitting the fimbrial end of the fallopian tubes in toto for microscopic examination using the SEE-FIM protocol. 10 Uterus If tumor is grossly present, sections should be taken to determine its extent, including depth of invasion of myometrium if tumor possibly originated in endometrium, and to determine its relation to ovarian tumor (metastatic to, metastatic from, independent primary). If uterine serosa is grossly involved, sections to show this should be taken. Omentum If tumor is grossly identifiable, representative sections are enough. It is recommended to take multiple sections when no tumor is detected grossly. Although there is no general consensus regarding the number of sections that should be taken on a grossly normal omentum of a patient with an ovarian serous borderline tumor, serous carcinoma, or immature teratoma, a general recommendation would be to take 5 to 10 sections. Implants in serous borderline tumors and immature teratomas may vary from noninvasive to invasive low-grade serous carcinoma 11 and from mature to immature, 12 respectively. Identification of invasive carcinoma or an immature implant may considerably alter the prognosis and therapy. For borderline tumors or immature teratoma with grossly apparent implants, multiple sections of the implants should be taken. For patients who have received neoadjuvant chemotherapy for advanced stage tubo-ovarian carcinoma (typically of high-grade serous type), 4 to 6 sections of omentum, to sample the most abnormal areas, are recommended to allow assessment of response to chemotherapy (see Note J). Lymph Nodes If the lymph nodes are grossly involved by tumor, representative sections are enough. However, if the lymph nodes appear grossly free of tumor, they should be entirely submitted. In either case, the dimension of the largest metastatic deposit should be documented. Other Staging Biopsy Specimens Staging biopsy tissues should be entirely processed unless grossly positive for tumor. If tumor is grossly seen, representative sections are usually sufficient. For borderline tumors or immature teratomas with grossly apparent implants, multiple sections of the implants should be taken (as in omental sampling). Other Organ or Tissue Removed Sections should be taken to determine the presence or absence, as well as location and extent, of tumor, if present. Resection margins should be taken, if applicable. 12
13 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum B. Rupture of Tumor It is important to know if the tumor is intact or ruptured, because in the latter situation, malignant cells may have spilled into the abdominal cavity. In tumors that have an admixture of benign, borderline, and/or malignant areas, it may also be important to know which area ruptured. 13,14 C. Site of Origin Although determination of primary site for most histologic types of tumor is relatively straightforward, as they present with tumor confined to the ovary, when a tumor involves ovary, fallopian tube, uterus, and multiple intraperitoneal sites, it may be difficult or impossible to determine the primary site of the tumor. Although historically primary site was assigned based on the dominant mass, this resulted in ovarian metastases from a number of extra-ovarian primary sites (eg, stomach, vermiform appendix, colon, endocervix, endometrium) being mistaken for primary ovarian neoplasms. Increased awareness of the ability of small extra-ovarian primary tumors to metastasize to the ovary and their characteristic morphological features, and the introduction of immunostains that aid in primary site determination, have led to improved recognition of ovarian metastases in practice. There remain challenges in assignment of primary site in cases of advanced stage high-grade serous carcinoma. Table 1 reflects current recommendations for site assignment in such cases. Table 1. Criteria for Assignment of Primary Site in Tubo-Ovarian High-Grade Serous Carcinoma (HGSC) 1,3,5 Criteria Primary Site Comment Serous tubal intraepithelial carcinoma (STIC) present Fallopian tube Regardless of presence and size of ovarian and peritoneal disease. Invasive mucosal carcinoma in tube, with or without STIC Fallopian tube Regardless of presence and size of ovarian and peritoneal disease. Fallopian tube partially or entirely incorporated into tubo-ovarian mass Fallopian tube Regardless of presence and size of ovarian and peritoneal disease. No STIC or invasive mucosal carcinoma in either tube in presence of ovarian mass or microscopic ovarian involvement Ovary Both tubes should be clearly visible and fully examined by a standardized SEE-FIM protocol. Regardless of presence and size of peritoneal disease. Both tubes and both ovaries grossly and microscopically normal (when examined entirely) or involved by benign process in presence of peritoneal HGSC HGSC diagnosed on small sample, peritoneal/omental biopsy or cytology Post-chemotherapy with residual disease Post-chemotherapy with no residual disease Primary peritoneal HGSC Tubo-ovarian Same criteria as described above Tubo-ovarian As recommended in World Health Organization (WHO) 2014 classification. This diagnosis should only be made in specimens removed at primary surgery prior to any chemotherapy; see below for samples following chemotherapy. Note: this should be supported by clinicopathological findings including immunohistochemistry to exclude mimics, principally uterine serous carcinoma Site assignment as undesignated should be avoided as far as possible and used only in the rare event that a case does not fit into any of the above categories and/or there remains doubt over whether it is of tubo-ovarian or endometrial origin. An adenocarcinoma is primary in the peritoneum when the ovaries and fallopian tubes are not involved or are involved only with minimal surface/serosal implants. It is important to note that serous carcinomas of endometrium may present with adnexal mass(es). In such cases there is often not the extensive omental involvement characteristic of primary tubo-ovarian high-grade serous carcinoma. Within the endometrium, there may be a co-existent precursor lesion (in situ serous carcinoma, serous endometrial intraepithelial carcinoma), supporting primary endometrial origin of the tumor. WT-1 staining is typically strong and diffuse in tubo-ovarian high-grade serous carcinoma and weak/focal or negative in endometrial serous carcinoma. However, WT-1 is not completely sensitive or specific in determining primary site. 1,3 Further study is needed to improve the ability to distinguish between high-grade serous carcinoma of 13
14 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum endometrial and tubo-ovarian origin; however, it is likely that most instances where high-grade serous carcinoma involves the endometrium, the tumor is primary endometrial serous carcinoma. D. Tumor Location Distribution of tumor in the ovary may be a clue to its origin. If the tumor is mainly present on the surface of the ovary without forming a discrete lesion, the tumor is more likely to be secondary ovarian involvement. If a tumor is centered or mainly involves the ovarian hilus, it is most likely to be a metastasis. In the case of mucinous neoplasms, if they are bilateral or associated with mucinous ascites or peritoneal/ovarian surface involvement, they are more likely to be metastatic. 15,16 E. Contralateral Ovary Contralateral ovary refers to the ovary that is non-dominant because it is either (1) involved by a tumor that is similar to but smaller than the dominant ovarian tumor, (2) contains only what appears to be metastatic tumor on gross examination, or (3) is negative for tumor. If the contralateral ovary contains only focal tumor, the gross and microscopic examination should concentrate on determining whether the tumor is an independent primary or it is metastatic from the dominant ovary. Metastatic involvement is supported by the same criteria that are used to distinguish primary and metastatic cancers to the ovary (multiple nodules, surface implants, and hilar vascular space invasion favor metastasis). F. Histologic Type It is recommended that the World Health Organization (WHO) classification and nomenclature of ovarian tumors be used because of its wide acceptance. 17 An abbreviated form of this classification is shown below. Serous Tumors Serous tubal intraepithelial carcinoma (STIC) Serous borderline tumor/atypical proliferative serous tumor Serous borderline tumor, micropapillary variant/noninvasive low-grade serous carcinoma Low-grade serous carcinoma High grade serous carcinoma Mucinous Tumors Mucinous borderline tumor/atypical proliferative serous tumor Mucinous carcinoma Seromucinous Tumors Seromucinous borderline tumor/atypical proliferative seromucinous tumor Seromucinous carcinoma Endometrioid Tumors Endometrioid borderline tumor Endometrioid carcinoma Clear Cell Tumors Clear cell borderline tumor Clear cell carcinoma Brenner Tumors Borderline Brenner tumor/atypical proliferative Brenner tumor Malignant Brenner tumor Mixed Epithelial Borderline Tumor Mixed Epithelial Carcinoma Carcinoma, Subtype Cannot Be Determined Undifferentiated Carcinoma Carcinosarcoma (malignant mixed Müllerian tumor) Malignant Sex Cord-Stromal Tumors Granulosa cell tumor, adult type Granulosa cell tumor, juvenile type Sertoli-Leydig cell tumor Other sex cord-stromal tumor Malignant Germ Cell Tumors Dysgerminoma 14
15 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum Yolk sac tumor Embryonal carcinoma Choriocarcinoma, non-gestational Immature teratoma Carcinoma arising in a teratoma Mixed malignant germ cell tumor Histologic type of ovarian carcinoma can be diagnosed with a high degree of reproducibility in routine practice and does have clinical implications. 17 For example, hereditary breast and ovarian cancer syndrome is associated with high-grade serous carcinoma, while Lynch syndrome is associated with endometrioid and clear cell tumors (both tumors that can be seen in association with endometriosis), so accurate diagnosis is important. The distinction between high-grade serous carcinoma and low-grade serous carcinoma is not an assignment of grade based on a continuum. They differ with respect to risk factors, precursor lesions, response to chemotherapy, and genetic events during oncogenesis, and merit consideration as distinct histologic types. The criteria for distinguishing between high-grade serous carcinoma and low-grade serous carcinoma are primarily based on nuclear variability (>3-fold nuclear size variation). In cases where the distinction is difficult, p16 and p53 immunostaining and assessment of mitotic activity (>12 mitoses/10 high-power fields) can be used. Such a system has molecular and prognostic validity and excellent inter-observer agreement. 17 High-grade tumors with ambiguous features, such that 1 of the specific histologic types listed cannot be diagnosed, should be classified as carcinoma, subtype cannot be determined ; however, this is a very infrequent situation, and every effort should be made to subclassify such tumors. Serous tubal intraepithelial carcinoma (STIC) is an unusual entity. Although an in situ neoplasm, it has malignant potential to spread throughout the peritoneal cavity. 18 Therefore, with cases of only a STIC as a primary site and negative staging and negative peritoneal washing, it is recommended to stage such cases as an AJCC pt1a/figo IA tumor. G. Mixtures of Histologic Types of Tumors The term mixed carcinoma should only be used when 2 or more distinctive subtypes of surface epithelial carcinomas are identified. When a carcinoma is classified as mixed, the major and minor types and their relative proportions should be specified. The diagnosis of mixed carcinoma was relatively common in the past, but with application of current histopathologic criteria, fewer than 1% of tubo-ovarian carcinomas are mixed, and the most common admixture is of endometrioid and clear cell carcinoma. 17 It is now appreciated that high-grade serous carcinomas show a wide range of histopathologic features, and glandular (pseudoendometroid) differentiation, solid architecture, transitional growth pattern, or clear cell change are now accepted as being within the spectrum of high-grade serous carcinoma, and the presence of these variants does not warrant diagnosis as mixed carcinoma. 17,19 Therefore, a mixed carcinoma should only be used when there are 2 or more distinct and separate histologic types in the tumor. Quantitation of various epithelial cell types within a carcinoma, as well as quantitation of tumor types within primitive germ cell tumors, may be prognostically important. 20 H. Histologic Grade Epithelial Carcinomas Clear cell carcinoma and carcinosarcomas are not graded; at present there is no grading system that has consistently been shown to prognosticate for these histologic types. Serous carcinomas are stratified into low grade and high grade. Endometrioid carcinomas may be graded according to the FIGO system used for endometrioid carcinomas of the endometrium, as shown below. Grade 1 Grade 2 5% of nonsquamous, solid growth 6% to 50% of nonsquamous solid growth 15
16 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum Grade 3 >50% of nonsquamous, solid growth Notable nuclear atypia, inappropriate for the architectural grade, raises the grade of a grade 1 or grade 2 tumor by 1 grade. There are no defined grading systems in widespread use for the remaining histologic types of ovarian carcinoma (eg, mucinous), and a gestalt 3-tier grading system can be used, acknowledging that it is not well validated. Grade X Grade 1 Grade 2 Grade 3 Cannot be assessed Well differentiated Moderately differentiated Poorly differentiated (tumors with minimal differentiation seen in very small foci) Seromucinous tumors are tumors with more than one Müllerian epithelial cell type. Although most commonly seen cell types are serous and mucinous, on occasion clear cell, transitional, or squamous epithelium can be seen. Such tumors can be classified as by the 2-tier (almost always low grade) or the 3-tier grading systems listed above. Germ Cell Tumors Immature teratomas are the only malignant germ cell tumors that are graded. They are classically graded on the basis of the quantity of immature/embryonal elements (almost always neuroectodermal tissue) that are present. 21 Even though in the past a 3-tier system was used to classify immature teratomas (G1 = immature neural tissue occupying <1 low-power field [40X] in any slide, and G3 = immature neural tissue occupying 4 low-power fields in any slide), a 2-tiered grading system (low versus high grade) has been proposed by some experts. 22 Grade 1 tumors are considered low grade while grade 2 and grade 3 tumors are considered high grade. Also, implants associated with immature teratomas must be assessed for the presence of immature elements, typically glial tissue. Sertoli-Leydig Cell Tumors Sertoli-Leydig cell tumors are graded in a 3-part grading system, as described in the WHO 2014 classification. 17 Briefly, in well-differentiated (grade 1) tumors, the Sertoli cells are present in open or closed tubules; in moderately differentiated (grade 2) tumors, the Sertoli cells are present in lobular aggregates, although there may be some tubular architecture present; and in poorly differentiated (grade 3) tumors, there is sarcomatous sheets of stroma; the lobulated Sertoliform growth typical of (grade 2) tumors, if present, is only focal. I. Implants (Serous/Seromucinous Borderline Tumors Only) In both serous borderline and seromucinous borderline tumors, peritoneal implants must be assessed for invasiveness. Noninvasive implants can be subdivided into epithelial and desmoplastic types and both are typically associated with favorable prognosis. Distinction between subtypes of noninvasive implants is academic and of no clinical significance. Note that implants with invasive carcinoma (formerly designated as invasive implants, as per Bell and Scully criteria) result in a diagnosis of low-grade serous carcinoma or seromucinous carcinoma, based on the WHO 2014 classification, 17 as they are associated with a poor prognosis (identical to that of low-grade serous carcinomas). J. Chemotherapy Response Score A system for histopathologic assessment of response to neoadjuvant chemotherapy (chemotherapy response score or CRS) for high-grade serous carcinoma has been developed and validated, and shown to be highly reproducible. 23 This 3-tiered scoring system is based on assessment of the section of omentum that shows the least response to chemotherapy. The criteria are shown in Table 2. 16
17 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum Table 2. Criteria of the Chemotherapy Response Score CRS 1: No or minimal tumor response Mainly viable tumor with no or minimal regression-associated fibro-inflammatory changes, # limited to a few foci; cases in which it is difficult to decide between regression and tumor-associated desmoplasia or inflammatory cell infiltration CRS 2: Appreciable tumor response amidst viable tumor, both readily identifiable and tumor regularly distributed Ranging from multifocal or diffuse regression associated fibro-inflammatory changes, # with viable tumor in sheets, streaks, or nodules, to extensive regression associated fibro-inflammatory changes # with multifocal residual tumor which is easily identifiable CRS 3: Complete or near-complete response with no residual tumor OR minimal irregularly scattered tumor foci seen as individual cells, cell groups, or nodules up to 2 mm in maximum size Mainly regression-associated fibro-inflammatory changes or, in rare cases, no/very little residual tumor in complete absence of any inflammatory response; advisable to record whether no residual tumor or microscopic residual tumor present # Regression-associated fibro-inflammatory changes: Fibrosis associated with macrophages, including foam cells, mixed inflammatory cells, and psammoma bodies; to distinguish from tumor-related inflammation or desmoplasia. K. Pathologic Stage Classification In view of the role of the pathologist in the staging of cancers, the staging system for ovarian cancer endorsed by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC), as well as the parallel system formulated by the International Federation of Gynecology and Obstetrics (FIGO), are recommended According to AJCC/UICC convention, the designation T refers to a primary tumor that has not been previously treated. The symbol p refers to the pathologic classification of the TNM, as opposed to the clinical classification, and is based on gross and microscopic examination. pt entails a resection of the primary tumor or biopsy adequate to evaluate the highest pt category, pn entails removal of nodes adequate to validate lymph node metastasis, and pm implies microscopic examination of distant lesions. Clinical classification (ctnm) is usually carried out by the referring physician before treatment during initial evaluation of the patient or when pathologic classification is not possible. Pathologic staging is usually performed after surgical resection of the primary tumor. Biopsies of all frequently involved sites, such as the omentum, mesentery, diaphragm, peritoneal surfaces, pelvic nodes, and para-aortic nodes, are required for ideal staging of early disease. For example, a patient can be confidently coded as stage IA (T1 N0 M0), if negative biopsies of all of the aforementioned sites are obtained to exclude microscopic metastases. Pathologic staging depends on pathologic documentation of the anatomic extent of disease, whether or not the primary tumor has been completely removed. If a biopsied tumor is not resected for any reason (eg, when technically infeasible), and if the highest T and N categories or the M1 category of the tumor can be confirmed microscopically, the criteria for pathologic classification and staging have been satisfied without total removal of the primary cancer. TNM Descriptors For identification of special cases of TNM or ptnm classifications, the m suffix and y, r, and a prefixes are used. Although they do not affect the stage grouping, they indicate cases needing separate analysis. 17
18 Background Documentation Female Reproductive Ovary, Fallopian Tube, Peritoneum The m suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pt(m)nm. The y prefix indicates those cases in which classification is performed during or after initial multimodality therapy (ie, neoadjuvant chemotherapy, radiation therapy, or both chemotherapy and radiation therapy). The ctnm or ptnm category is identified by a y prefix. The yctnm or yptnm categorizes the extent of tumor actually present at the time of that examination. The y categorization is not an estimate of tumor before multimodality therapy (ie, before initiation of neoadjuvant therapy). The r prefix indicates a recurrent tumor when staged after a documented disease-free interval and is identified by the r prefix: rtnm. The a prefix designates the stage determined at autopsy: atnm. N Category Considerations Isolated tumor cells (ITCs) are single cells or small clusters of cells not more than 0.2 mm in greatest dimension. Lymph nodes or distant sites with ITCs found by either histologic examination (eg, immunohistochemical evaluation for cytokeratin) or nonmorphological techniques (eg, flow cytometry, DNA analysis, polymerase chain reaction [PCR] amplification of a specific tumor marker) should be so identified. There is currently no guidance in the literature as to how these patients should be coded; until more data are available, they should be coded as N0(i+) with a comment noting how the cells were identified. L. Other Lesions The presence of endometriosis, particularly if it is in continuity with either an endometrioid or clear cell carcinoma, is an important clue as to the primary nature of the ovarian tumor. M. Special Studies Special studies including histochemical, immunohistochemical, and molecular genetic studies may be used in some cases. Evaluation for BRCA1/BRCA2 testing on patients with high-grade serous carcinoma of tubal/ovarian/peritoneal origin should be performed at the discretion of genetic counselors with assessment of other risk factors. Immunohistochemical stains for DNA mismatch repair enzymes MLH1, MS2, MSH6, and PMS2 for Lynch syndrome screening is recommended on all endometrioid and clear cell carcinomas of the ovary References 1. Singh N, Gilks CB, Wilkinson N, et al. Assessment of a new system for primary site assignment in high-grade serous carcinoma of the fallopian tube, ovary, and peritoneum. Histopathology. 2015;67(3): Gilks CB, Irving J, Kobel M, et al. Incidental nonuterine high-grade serous carcinomas arise in the fallopian tube in most cases: further evidence for the tubal origin of high-grade serous carcinomas. Am J Surg Pathol. 2015;39: McCluggage WG, Judge MJ, Clarke BA, et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Mod Pathol. 2015;28(8): Morrison JC, Blanco LZ Jr, Vang R, Ronnett BM. Incidental serous tubal intraepithelial carcinoma and early invasive serous carcinoma in the nonprophylactic setting: analysis of a case series. Am J Surg Pathol. 2015;39(4): Singh N, Gilks CB, Hirschowitz L, et al. Adopting a uniform approach to site assignment in tubo-ovarian high grade serous carcinoma the time has come. Int J Gynecol Pathol. In press. 6. Bell DA, Scully RE. Early de novo ovarian carcinoma: a study of fourteen cases. Cancer. 1994;73(7): Lamb JD, Garcia RL, Goff BA, Paley PJ, Swisher EM. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol. 2006;194(6): Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2): Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30(2):
Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum
 Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th
Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
Staging and Treatment Update for Gynecologic Malignancies
 Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE
 UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE Case: Adenosarcoma with heterologous elements and stromal overgrowth o TAH, BSO, omentectomy, staging biopsies of cul-de-sac, bladder
UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE Case: Adenosarcoma with heterologous elements and stromal overgrowth o TAH, BSO, omentectomy, staging biopsies of cul-de-sac, bladder
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ovary
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Ovary Protocol applies to all primary borderline and malignant surface epithelial tumors, and also to germ cell tumors and
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ovary Protocol applies to all primary borderline and malignant surface epithelial tumors, and also to germ cell tumors and
Endometrium. Protocol applies to all carcinomas of the endometrium. Procedures Cytology (No Accompanying Checklist) Biopsy Curettage Hysterectomy
 Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
MPH Quiz. 1. How many primaries are present based on this pathology report? 2. What rule is this based on?
 MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
The Diagnostic Challenges of Low Grade and High Grade Tubo-Ovarian Serous Carcinomas. W Glenn McCluggage Belfast, Northern Ireland
 The Diagnostic Challenges of Low Grade and High Grade Tubo-Ovarian Serous Carcinomas W Glenn McCluggage Belfast, Northern Ireland Enterprise Interest None OVARIAN SEROUS CARCINOMA (OSC) RECENT DEVELOPMENTS
The Diagnostic Challenges of Low Grade and High Grade Tubo-Ovarian Serous Carcinomas W Glenn McCluggage Belfast, Northern Ireland Enterprise Interest None OVARIAN SEROUS CARCINOMA (OSC) RECENT DEVELOPMENTS
Descriptor Definition Author s notes TNM descriptors Required only if applicable; select all that apply multiple foci of invasive carcinoma
 S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
Interactive Staging Bee
 Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Fallopian Tube. Protocol applies to all invasive carcinomas of the fallopian tube.
 Fallopian Tube Protocol applies to all invasive carcinomas of the fallopian tube. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology
Fallopian Tube Protocol applies to all invasive carcinomas of the fallopian tube. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
NAACCR Webinar Series 1 Q&A. Fabulous Prizes. Collecting Cancer Data: Ovary 11/3/2011. Collecting Cancer Data: Ovary
 NAACCR 2011 2012 Webinar Series Collecting Cancer Data: Ovary Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
NAACCR 2011 2012 Webinar Series Collecting Cancer Data: Ovary Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
H&E, IHC anti- Cytokeratin
 Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
Current Concept in Ovarian Carcinoma: Pathology Perspectives
 Current Concept in Ovarian Carcinoma: Pathology Perspectives Rouba Ali-Fehmi, MD Professor of Pathology The Karmanos Cancer Institute, Wayne State University School of Medicine Current Concept in Ovarian
Current Concept in Ovarian Carcinoma: Pathology Perspectives Rouba Ali-Fehmi, MD Professor of Pathology The Karmanos Cancer Institute, Wayne State University School of Medicine Current Concept in Ovarian
New Cancer Cases By Site Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3%
 Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
L/O/G/O. Ovarian Tumor. Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital
 L/O/G/O Ovarian Tumor Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital Essentials classification of ovarian tumor clinical manifestation of ovarian tumor metastatic
L/O/G/O Ovarian Tumor Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital Essentials classification of ovarian tumor clinical manifestation of ovarian tumor metastatic
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy
 Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium
 Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
3 cell types in the normal ovary
 Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
Ovarian Tumors. Andrea Hayes-Jordan MD FACS, FAAP Section Chief, Pediatric Surgery/Surgical Onc. UT MD Anderson Cancer Center
 Ovarian Tumors Andrea Hayes-Jordan MD FACS, FAAP Section Chief, Pediatric Surgery/Surgical Onc. UT MD Anderson Cancer Center Case 13yo female with abdominal pain Ultrasound shows huge ovarian mass Surgeon
Ovarian Tumors Andrea Hayes-Jordan MD FACS, FAAP Section Chief, Pediatric Surgery/Surgical Onc. UT MD Anderson Cancer Center Case 13yo female with abdominal pain Ultrasound shows huge ovarian mass Surgeon
What s (new) and Important in Reporting of Uterine Cancers Katherine Vroobel The Royal Marsden
 What s (new) and Important in Reporting of Uterine Cancers Katherine Vroobel The Royal Marsden Maastricht Pathology 2018 Wednesday 20 th June Endometrioid adenocarcinoma High grade carcinomas (common)
What s (new) and Important in Reporting of Uterine Cancers Katherine Vroobel The Royal Marsden Maastricht Pathology 2018 Wednesday 20 th June Endometrioid adenocarcinoma High grade carcinomas (common)
This protocol is intended to assist pathologists in providing
 Protocol for the Examination of Specimens From Patients With Carcinomas of the Endometrium A Basis for Checklists Steven G. Silverberg, MD, for the Members of the Cancer Committee, College of American
Protocol for the Examination of Specimens From Patients With Carcinomas of the Endometrium A Basis for Checklists Steven G. Silverberg, MD, for the Members of the Cancer Committee, College of American
Chapter 2: Initial treatment for endometrial cancer (including histologic variant type)
 Chapter 2: Initial treatment for endometrial cancer (including histologic variant type) CQ01 Which surgical techniques for hysterectomy are recommended for patients considered to be stage I preoperatively?
Chapter 2: Initial treatment for endometrial cancer (including histologic variant type) CQ01 Which surgical techniques for hysterectomy are recommended for patients considered to be stage I preoperatively?
Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium Based on AJCC/UICC TNM, 7 th edition and FIGO 2008 Annual Report Protocol web posting date: October 2013 Procedure
Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium Based on AJCC/UICC TNM, 7 th edition and FIGO 2008 Annual Report Protocol web posting date: October 2013 Procedure
Gynecologic Oncologist. Surgery Chemotherapy Radiation Therapy Hormonal Therapy Immunotherapy. Cervical cancer
 Gynecologic Oncology Pre invasive vulvar, vaginal, & cervical disease Vulvar Cervical Endometrial Uterine Sarcoma Fallopian Tube Ovarian GTD Gynecologic Oncologist Surgery Chemotherapy Radiation Therapy
Gynecologic Oncology Pre invasive vulvar, vaginal, & cervical disease Vulvar Cervical Endometrial Uterine Sarcoma Fallopian Tube Ovarian GTD Gynecologic Oncologist Surgery Chemotherapy Radiation Therapy
International Society of Gynecological Pathologists Symposium 2007
 International Society of Gynecological Pathologists Symposium 2007 Anais Malpica, M.D. Department of Pathology The University of Texas M.D. Anderson Cancer Center Grading of Ovarian Cancer Histologic grade
International Society of Gynecological Pathologists Symposium 2007 Anais Malpica, M.D. Department of Pathology The University of Texas M.D. Anderson Cancer Center Grading of Ovarian Cancer Histologic grade
Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube Protocol applies to all carcinomas presumed to be arising from the mucosa of the fallopian tube. Based on AJCC/UICC
Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube Protocol applies to all carcinomas presumed to be arising from the mucosa of the fallopian tube. Based on AJCC/UICC
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES CASE 1: FEMALE REPRODUCTIVE
 Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
of 20 to 80 and subsequently declines [2].
![of 20 to 80 and subsequently declines [2]. of 20 to 80 and subsequently declines [2].](/thumbs/80/81450506.jpg) - - According to the 2014 World Health Organization (WHO) classification and tumor morphology, primary ovarian tumors are subdivided into three categories: epithelial (60%), germ cell (30%), and sex-cord
- - According to the 2014 World Health Organization (WHO) classification and tumor morphology, primary ovarian tumors are subdivided into three categories: epithelial (60%), germ cell (30%), and sex-cord
Gynaecological Pathology Reporting. Peritoneal cytology Tony Williams Birmingham
 Gynaecological Pathology Reporting Peritoneal cytology Tony Williams Birmingham Ascites Abdominopelvic peritoneal washings in gynaecological procedures Ovarian cyst aspirates Clinical guidance; RCOG &
Gynaecological Pathology Reporting Peritoneal cytology Tony Williams Birmingham Ascites Abdominopelvic peritoneal washings in gynaecological procedures Ovarian cyst aspirates Clinical guidance; RCOG &
Staging for Residents, Nurses, and Multidisciplinary Health Care Team
 Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Head and Neck Tumours Pages 20, p27, p34, p38, p41, and p49 ly pn2a Metastasis in a single ipsilateral lymph node, less than 3cm in greatest dimension with
UICC TNM 8 th Edition Errata ions are in italics Head and Neck Tumours Pages 20, p27, p34, p38, p41, and p49 ly pn2a Metastasis in a single ipsilateral lymph node, less than 3cm in greatest dimension with
Section 1. Biology of gynaecological cancers: our current understanding
 Section 1 Biology of gynaecological cancers: our current understanding Chapter 1 Morphological sub-types of ovarian carcinoma: new developments and pathogenesis W Glenn McCluggage 1 Introduction In most
Section 1 Biology of gynaecological cancers: our current understanding Chapter 1 Morphological sub-types of ovarian carcinoma: new developments and pathogenesis W Glenn McCluggage 1 Introduction In most
Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013
 bs_bs_banner doi:10.1111/jog.12360 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 338 348, February 2014 Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013 Daisuke
bs_bs_banner doi:10.1111/jog.12360 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 338 348, February 2014 Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013 Daisuke
Update on staging colorectal carcinoma, the 8 th edition AJCC. General overview of staging. When is staging required? 11/1/2017
 Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Case 1. Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno
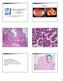 Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
VULVAR CARCINOMA. Page 1 of 5
 VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
Interpretation of p53 Immunostains. P53 Mutations are Ubiquitous in High Grade Serous Carcinoma. Diffuse strong positive nuclear staining
 Stains for Tumor Classification p53 p16 WT1 HMGA2 P53 Mutations are Ubiquitous in High Grade Serous Carcinoma Source Ahmed et al Australian Ovarian Cancer Study Cancer Genome Atlas Research Network Cases
Stains for Tumor Classification p53 p16 WT1 HMGA2 P53 Mutations are Ubiquitous in High Grade Serous Carcinoma Source Ahmed et al Australian Ovarian Cancer Study Cancer Genome Atlas Research Network Cases
3 cell types in the normal ovary
 Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal cells
Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal cells
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
Modern Pathology (2015) 28,
 2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 1101 Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer
2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 1101 Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
Low-grade serous neoplasia. Robert A. Soslow, MD
 Low-grade serous neoplasia Robert A. Soslow, MD soslowr@mskcc.org Outline Orientation Ovarian tumor overview Non serous borderline tumors Serous borderline tumors Clinical summary Morphologic description
Low-grade serous neoplasia Robert A. Soslow, MD soslowr@mskcc.org Outline Orientation Ovarian tumor overview Non serous borderline tumors Serous borderline tumors Clinical summary Morphologic description
Case Scenario 1. Pathology report Specimen from mediastinoscopy Final Diagnosis : Metastatic small cell carcinoma with residual lymphatic tissue
 Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Type I. Type II. Excess estrogen Lynch Endometrioid adenocarcinoma PTEN. High grade More aggressive Serous, Clear Cell p53
 Type I Excess estrogen Lynch Endometrioid adenocarcinoma PTEN Type II High grade More aggressive Serous, Clear Cell p53 Stage I IA IB Stage II Stage III IIIA IIIB IIIC IIIC1 IIIC2 Stage IV IVA IVB nodes
Type I Excess estrogen Lynch Endometrioid adenocarcinoma PTEN Type II High grade More aggressive Serous, Clear Cell p53 Stage I IA IB Stage II Stage III IIIA IIIB IIIC IIIC1 IIIC2 Stage IV IVA IVB nodes
Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
 bs_bs_banner doi:10.1111/jog.12596 J. Obstet. Gynaecol. Res. Vol. 41, No. 2: 167 177, February 2015 Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
bs_bs_banner doi:10.1111/jog.12596 J. Obstet. Gynaecol. Res. Vol. 41, No. 2: 167 177, February 2015 Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
Carcinoma of the Urinary Bladder Histopathology
 Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
North of Scotland Cancer Network Clinical Management Guideline for Cancer of the Ovary
 North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
Article begins on next page
 Pseudopapillary Granulosa Cell Tumor: A Case of This Rare Subtype Rutgers University has made this article freely available. Please share how this access benefits you. Your story matters. [https://rucore.libraries.rutgers.edu/rutgers-lib/50622/story/]
Pseudopapillary Granulosa Cell Tumor: A Case of This Rare Subtype Rutgers University has made this article freely available. Please share how this access benefits you. Your story matters. [https://rucore.libraries.rutgers.edu/rutgers-lib/50622/story/]
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors
 Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
ACCME/Disclosures. Risk of Gyne Ca in HBOC. Molecular basis of HBOC. Hereditary Ovarian and Breast Cancer Syndrome
 Hereditary Ovarian and Breast Cancer Syndrome C. Blake Gilks, MD Dept of Pathology Vancouver General Hospital University of British Columbia Blake.gilks@vch.ca The USCAP requires that anyone in a position
Hereditary Ovarian and Breast Cancer Syndrome C. Blake Gilks, MD Dept of Pathology Vancouver General Hospital University of British Columbia Blake.gilks@vch.ca The USCAP requires that anyone in a position
Chapter 2 Staging of Breast Cancer
 Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus
 Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Note: The cause of testicular neoplasms remains unknown
 - In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
- In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
Staging. Carcinoma confined to the corpus. Carcinoma confined to the endometrium. Less than ½ myometrial invasion. Greater than ½ myometrial invasion
 5 th of June 2009 Background Most common gynaecological carcinoma in developed countries Most cases are post-menopausal Increasing incidence in certain age groups Increasing death rates in the USA 5-year
5 th of June 2009 Background Most common gynaecological carcinoma in developed countries Most cases are post-menopausal Increasing incidence in certain age groups Increasing death rates in the USA 5-year
Endosalpingiosis. Case report
 Case report Endosalpingiosis Michael D. Holmes, M.D. Howard S. Levin M.D. Department of Pathology Lester A. Ballard, Jr., M.D. Department of Gynecology Endosalpingiosis, a term referring to tuballike epithelium
Case report Endosalpingiosis Michael D. Holmes, M.D. Howard S. Levin M.D. Department of Pathology Lester A. Ballard, Jr., M.D. Department of Gynecology Endosalpingiosis, a term referring to tuballike epithelium
Carcinoma of the Fallopian Tube
 119 Carcinoma of the Fallopian Tube APM HEINTZ, F ODICINO, P MAISONNEUVE, U BELLER, JL BENEDET, WT CREASMAN, HYS NGAN and S PECORELLI STAGING Anatomy Primary site The Fallopian tube extends from the posterior
119 Carcinoma of the Fallopian Tube APM HEINTZ, F ODICINO, P MAISONNEUVE, U BELLER, JL BENEDET, WT CREASMAN, HYS NGAN and S PECORELLI STAGING Anatomy Primary site The Fallopian tube extends from the posterior
Case Scenario 1. 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain.
 Case Scenario 1 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain. 1/02/13 CT Abdomen/Pelvis: Abnormal area of nodular mesenteric and left anterior
Case Scenario 1 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain. 1/02/13 CT Abdomen/Pelvis: Abnormal area of nodular mesenteric and left anterior
How to Recognize Gynecologic Cancer Cells from Pelvic Washing and Ascetic Specimens
 How to Recognize Gynecologic Cancer Cells from Pelvic Washing and Ascetic Specimens Wenxin Zheng, M.D. Professor of Pathology and Gynecology University of Arizona zhengw@email.arizona.edu http://www.zheng.gynpath.medicine.arizona.edu/index.html
How to Recognize Gynecologic Cancer Cells from Pelvic Washing and Ascetic Specimens Wenxin Zheng, M.D. Professor of Pathology and Gynecology University of Arizona zhengw@email.arizona.edu http://www.zheng.gynpath.medicine.arizona.edu/index.html
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy
 Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
Mucinous Tumors of the Ovary Beirut, Lebanon. Anaís Malpica, M.D. Professor Department of Pathology
 Mucinous Tumors of the Ovary Beirut, Lebanon Anaís Malpica, M.D. Professor Department of Pathology Primary Mucinous Tumors of the Ovary Cystadenoma Borderline (Tumor of Low Malignant Potential/Atypical
Mucinous Tumors of the Ovary Beirut, Lebanon Anaís Malpica, M.D. Professor Department of Pathology Primary Mucinous Tumors of the Ovary Cystadenoma Borderline (Tumor of Low Malignant Potential/Atypical
Pathology of Ovarian Tumours. Dr. Jyothi Ranganathan MD ( Path) AFMC Pune PDCC (Cytopathology) PGI Chandigarh
 Pathology of Ovarian Tumours Dr. Jyothi Ranganathan MD ( Path) AFMC Pune PDCC (Cytopathology) PGI Chandigarh Outline Incidence Risk factors Classification Pathology of tumours Tumour markers Prevention
Pathology of Ovarian Tumours Dr. Jyothi Ranganathan MD ( Path) AFMC Pune PDCC (Cytopathology) PGI Chandigarh Outline Incidence Risk factors Classification Pathology of tumours Tumour markers Prevention
Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer
 Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer Ira R. Horowitz, MD, SM, FACOG, FACS John D. Thompson Professor and Chairman Department of Gynecology
Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer Ira R. Horowitz, MD, SM, FACOG, FACS John D. Thompson Professor and Chairman Department of Gynecology
Staging Challenges in Lower GI Cancers. Disclosure of Relevant Financial Relationships. AJCC 8 th edition and CAP protocol updates
 Staging Challenges in Lower GI Cancers Sanjay Kakar, MD University of California, San Francisco March 05, 2017 Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education
Staging Challenges in Lower GI Cancers Sanjay Kakar, MD University of California, San Francisco March 05, 2017 Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education
3/23/2017. Disclosure of Relevant Financial Relationships. Pathologic Staging Updates in Breast Cancer. Pathologic Staging Updates Breast Cancer
 Pathologic Staging Updates in Breast Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
Pathologic Staging Updates in Breast Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
GYNECOLOGIC MALIGNANCIES: Ovarian Cancer
 GYNECOLOGIC MALIGNANCIES: Ovarian Cancer KRISTEN STARBUCK, MD ROSWELL PARK CANCER INSTITUTE DEPARTMENT OF SURGERY DIVISION OF GYNECOLOGIC ONCOLOGY APRIL 19 TH, 2018 Objectives Basic Cancer Statistics Discuss
GYNECOLOGIC MALIGNANCIES: Ovarian Cancer KRISTEN STARBUCK, MD ROSWELL PARK CANCER INSTITUTE DEPARTMENT OF SURGERY DIVISION OF GYNECOLOGIC ONCOLOGY APRIL 19 TH, 2018 Objectives Basic Cancer Statistics Discuss
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do?
 Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Institute of Pathology First Faculty of Medicine Charles University. Ovary
 Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Case # 4 Low-Grade Serous Carcinoma (Macropapillary) of the Ovary Arising in an Atypical Proliferative Serous Tumor
 Case # 4 Low-Grade Serous Carcinoma (Macropapillary) of the Ovary Arising in an Atypical Proliferative Serous Tumor Robert J Kurman, M.D. Johns Hopkins University School of Medicine Case History A 53 year
Case # 4 Low-Grade Serous Carcinoma (Macropapillary) of the Ovary Arising in an Atypical Proliferative Serous Tumor Robert J Kurman, M.D. Johns Hopkins University School of Medicine Case History A 53 year
Mody. AIS vs. Invasive Adenocarcinoma of the Cervix
 Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS
 Review Journal of Translational Medicine and Research, volume 19, no. 1-2, 2014 UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS N. Bacalbaæa 1, A. Traistaru 2, I. Bãlescu 3 1 Carol Davila University of Medicine
Review Journal of Translational Medicine and Research, volume 19, no. 1-2, 2014 UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS N. Bacalbaæa 1, A. Traistaru 2, I. Bãlescu 3 1 Carol Davila University of Medicine
Gastric Cancer Histopathology Reporting Proforma
 Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Cervical Cancer: 2018 FIGO Staging
 Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
Historical Perspective
 AJCC Cancer Staging 8 th Edition Alexander B. Olawaiye, MD University of Pittsburgh Validating science. Improving patient care. No materials in this presentation maybe repurposed without the Cancer. Permission
AJCC Cancer Staging 8 th Edition Alexander B. Olawaiye, MD University of Pittsburgh Validating science. Improving patient care. No materials in this presentation maybe repurposed without the Cancer. Permission
Gastric Cancer Staging AJCC eighth edition. Duncan McLeod Westmead Hospital, NSW
 Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Protocol for the Examination of Specimens From Patients With Thymic Tumors
 Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Gynaecological Malignancies
 Gynaecological Malignancies Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea Division of Pathology School of Medicine & Health Sciences Overview Genital tract tumors
Gynaecological Malignancies Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea Division of Pathology School of Medicine & Health Sciences Overview Genital tract tumors
S1.04 Principal clinician. G1.01 Comments. G2.01 *Specimen dimensions (prostate) S2.02 *Seminal vesicles
 Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Carcinoma of the Renal Pelvis and Ureter Histopathology
 Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
2009 USCAP Gyn Pathology Evening Session Case #3. Richard J. Zaino, MD Hershey Medical Center Penn State University Hershey, PA
 2009 USCAP Gyn Pathology Evening Session Case #3 Richard J. Zaino, MD Hershey Medical Center Penn State University Hershey, PA rzaino@psu.edu Clinical history Middle aged woman with an exophytic mass of
2009 USCAP Gyn Pathology Evening Session Case #3 Richard J. Zaino, MD Hershey Medical Center Penn State University Hershey, PA rzaino@psu.edu Clinical history Middle aged woman with an exophytic mass of
Protocol applies to melanoma of cutaneous surfaces only.
 Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Colorectal Cancer Structured Pathology Reporting Proforma DD MM YYYY
 Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
3/25/2019. Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates
 J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
Q: In order to use the code 8461/3 (serous surface papillary) for ovary, does it have to say the term "surface" on the path report?
 Q&A Session for Collecting Cancer Data: Ovary Q: In order to use the code 8461/3 (serous surface papillary) for ovary, does it have to say the term "surface" on the path report? A: We reviewed both the
Q&A Session for Collecting Cancer Data: Ovary Q: In order to use the code 8461/3 (serous surface papillary) for ovary, does it have to say the term "surface" on the path report? A: We reviewed both the
Pathology of the female genital tract
 Pathology of the female genital tract Common illnesses of the female genital tract Before menarche Developmental anomalies Tumors (ovarial teratoma) Amenorrhea Fertile years PCOS, ovarian cysts Endometriosis
Pathology of the female genital tract Common illnesses of the female genital tract Before menarche Developmental anomalies Tumors (ovarial teratoma) Amenorrhea Fertile years PCOS, ovarian cysts Endometriosis
بسم هللا الرحمن الرحيم. Prof soha Talaat
 بسم هللا الرحمن الرحيم Ovarian tumors The leading indication for gynecologic surgery. Preoperative characterization of complex solid and cystic adnexal masses is crucial for informing patients about possible
بسم هللا الرحمن الرحيم Ovarian tumors The leading indication for gynecologic surgery. Preoperative characterization of complex solid and cystic adnexal masses is crucial for informing patients about possible
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vulva
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vulva Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vulva Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Endometrial Cancer. Incidence. Types 3/25/2019
 Endometrial Cancer J. Anthony Rakowski DO, FACOOG MSU SCS Board Review Coarse Incidence 53,630 new cases yearly 8,590 deaths yearly 4 th most common malignancy in women worldwide Most common GYN malignancy
Endometrial Cancer J. Anthony Rakowski DO, FACOOG MSU SCS Board Review Coarse Incidence 53,630 new cases yearly 8,590 deaths yearly 4 th most common malignancy in women worldwide Most common GYN malignancy
Case Scenario 1. History
 History Case Scenario 1 A 53 year old white female presented to her primary care physician with post-menopausal vaginal bleeding. The patient is not a smoker and does not use alcohol. She has no family
History Case Scenario 1 A 53 year old white female presented to her primary care physician with post-menopausal vaginal bleeding. The patient is not a smoker and does not use alcohol. She has no family
Case Scenario 1. 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain.
 Case Scenario 1 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain. 1/02/13 CT Abdomen/Pelvis: Abnormal area of nodular mesenteric and left anterior
Case Scenario 1 1/2/13 History: 64-year-old white female presented with right leg swelling and redness, abdominal pain. 1/02/13 CT Abdomen/Pelvis: Abnormal area of nodular mesenteric and left anterior
LOINC. Clinical information. RCPA code. Record if different to report header Operating surgeon name and contact details. Absent.
 Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
