Low-Grade Dysplasia in Ulcerative Colitis: Risk Factors for Developing High-Grade Dysplasia or Colorectal Cancer
|
|
|
- Claud Grant
- 5 years ago
- Views:
Transcription
1 nature publishing group ORIGINAL CONTRIBUTIONS 1461 CME see related editorial on page 1473 Low-Grade Dysplasia in Ulcerative Colitis: Risk Factors for Developing High-Grade Dysplasia or Colorectal Cancer Chang-ho Ryan Choi, MBBS, MSc 1, 2, Ana Ignjatovic-Wilson, BMBCh, MD, MRCP 3, Alan Askari, MBChB, MRCS 4, Gui Han Lee, MBBS, MRCS 4, Janindra Warusavitarne, BMed, PhD, FRACS 4, Morgan Moorghen, MBChB, MD, FRCPath 5, Siwan Thomas-Gibson, MBBS, MD, FRCP 3, Brian P. Saunders, MBBS, MD, FRCP 3, Matthew D. Rutter, MBBS, MD, FRCP 6, Trevor A. Graham, PhD 2, 7 and Ailsa L. Hart, BMBCh, PhD, FRCP 1, 7 OBJECTIVES: The aim of this study was to identify risk factors associated with development of high-grade dysplasia (HGD) or colorectal cancer (CRC) in ulcerative colitis (UC) patients diagnosed with low-grade dysplasia (LGD). METHODS: Patients with histologically confirmed extensive UC, who were diagnosed with LGD between 1993 and 2012 at St Mark s Hospital, were identified and followed up to 1 July Demographic, endoscopic, and histological data were collected and correlated with the development of HGD or CRC. RESULTS: A total of 172 patients were followed for a median of 48 months from the date of initial LGD diagnosis (interquartile range (IQR), months). Overall, 33 patients developed HGD or CRC (19.1% of study population; 20 CRCs) during study period. Multivariate Cox proportional hazard analysis revealed that macroscopically non-polypoid (hazard ratio (HR), 8.6; 95% confidence interval (CI), ; P <0.001) or invisible (HR, 4.1; 95% CI, ; P =0.02) dysplasia, dysplastic lesions 1 cm in size (HR, 3.8; 95% CI, ; P =0.01), and a previous history of indefinite for dysplasia (HR, 2.8; 95% CI, ; P =0.01) were significant contributory factors for HGD or CRC development. Multifocal dysplasia (HR, 3.9; 95% CI, ; P <0.001), metachronous dysplasia (HR, 3.5; 95% CI, ; P =0.001), or a colonic stricture (HR, 7.4; 95% CI, ; P <0.001) showed only univariate correlation to development of HGD or CRC. CONCLUSIONS: Lesions that are non-polypoid or endoscopically invisible, large ( 1 cm), or preceded by indefinite dysplasia are independent risk factors for developing HGD or CRC in UC patients diagnosed with LGD. SUPPLEMENTARY MATERIAL is linked to the online version of the paper at Am J Gastroenterol 2015; 110: ; doi: /ajg ; published online 29 September 2015 INTRODUCTION The management of dysplasia arising in patients with ulcerative colitis (UC) is challenging. This is particularly pertinent in patients with low-grade dysplasia (LGD) the most common type of dysplasia detected in surveillance programs as its natural history of progression to colorectal cancer (CRC) is poorly understood. Indeed, the reported risk of CRC associated with LGD varies greatly between studies ( 1 9 ). While observed variation in cancer risk may simply reflect differences in methodology and population, there are several other important factors that may also have influenced outcomes. These include poor interobserver agreement in grading dysplasia among histopathologists ( 10,11 ), difficulty in reliably distinguishing colitis-associated dysplasia from sporadic adenomas ( 12 ), and difficulty in detecting sub- 1 Infl ammatory Bowel Disease Unit, St Mark s Hospital, London, UK ; 2 Tumour Biology, Barts Cancer Institute, Charterhouse Square, Queen Mary University of London, London, UK ; 3 Wolfson Unit for Endoscopy, St Mark s Hospital, London, UK ; 4 Department of Colorectal Surgery, St Mark s Hospital, London, UK ; 5 Department of Pathology, St Mark s Hospital, London, UK ; 6 University Hospital of North Tees, Teesside, UK ; 7 Denotes co-senior authors. Correspondence: Chang-ho Ryan Choi, MBBS, MSc, Department of Infl ammatory Bowel Disease, St Mark's Hospital, London HA1 3UJ, UK. pacoblue@gmail.com Received 3 March 2015 ; accepted 7 July 2015
2 1462 Choi et al. tle dysplastic lesions. These challenges may reflect the fact that LGD is unlikely to be a single entity. Rather, it is likely that there is a wide spectrum of malignant potential in the variety of LGD lesions that we encounter in clinical practice. In 1981, Blackstone et al. (3 ) first introduced the term dysplasia-associated lesion or mass. Unfortunately, the definition of dysplasia-associated lesion or mass is not clear and often used to describe a wide range of lesions, ranging from a small, discrete polyp detected in a diseased segment to a large irregular mass. However, it is important to differentiate these lesions, as their malignant potential may vary significantly. For example, it is becoming increasingly clear that the risk of CRC is low following endoscopic resection of adenoma-like lesions arising in patients with UC ( ). The challenge is, of course, identifying patients whose LGD lesion has a high risk of developing advanced neoplasia from those who are at low risk. Although earlier studies demonstrated the efficacy of endoscopic removal of discrete adenomatous lesions without further significant cancer risk, patients who underwent early colectomy were typically excluded from these analyses and their characteristics are poorly understood ( ). However, this is an important group to consider, as many patients in this group represent those who were clinically judged to be at a high risk and hence were offered colectomy early in their follow-up. As one of the ultimate goals of managing LGD is the prompt recognition of high-risk lesions that warrant surgical intervention from those that can be managed appropriately endoscopically, there is a need for a study to assess full spectrum of patients diagnosed with LGD to characterize features associated with progression to more advanced neoplasia. To f u lfill this need, in this study we investigated data collected from the UC surveillance program at a large tertiary center in the United Kingdom (UK), with the aim of identifying potential risk factors that could be used to identify patients with a diagnosis of LGD who have a high risk of developing high-grade dysplasia (HGD) or CRC. METHODS Surveillance program St Mark s Hospital is a tertiary referral center in the UK and established the UC surveillance program in Patients with endoscopic and histological evidence of UC proximal to the splenic flexure were offered surveillance colonoscopies every 1 to 2 years from 8 to 10 years after onset of UC symptoms. At each colonoscopy, 8 to 12 segmental random biopsies were taken, with multiple targeted biopsies from any suspicious area of mucosa. In more recent years (from 2003 onward), there has been a gradual increase in number of surveillance procedures performed with chromoendoscopy (CE), where pancolonic dye spray is used to highlight abnormal mucosa for targeted biopsies. By 2011, approximately one in two surveillance colonoscopies were performed using this technique. Each episode of dysplasia was graded according to the 1983 Inflammatory Bowel Disease Dysplasia Morphology Study group classification ( 17 ) and was independently reviewed by two experienced gastrointestinal pathologists at the time of diagnosis in accordance with the standard hospital policy. Patient identification and inclusion criteria Patients with histologically confirmed extensive UC who had at least one episode of LGD detected between 1 January 1993 and 31 December 2012 were retrospectively identified from St Mark s Hospital s Inflammatory Bowel Disease (IBD) database (registered with National Research Ethics Committee and Northwest London Hospitals NHS Trust; reference number, 09/H0717/4). Patients who had at least one follow-up colonoscopy or surgical intervention after initial LGD diagnosis were included in the study. Patients whose first episode of dysplasia was found incidentally in their colectomy specimen (performed for reasons other than dysplasia/crc, e.g., medically refractory colitis) were excluded. Patients who were referred to our institution with dysplasia diagnosis already established elsewhere were not considered in this study. Data collection Data were collected from the hospital s IBD database, clinical notes, surgical case notes, and endoscopy and histology reports. Detailed information on how variables were categorized is described below: 1. Macroscopic shape of the dysplastic lesion: patients were categorized based on the first episode of LGD, according to the lesion shape noted at colonoscopy. a. Polypoid: Paris type 0 I lesions (discrete pedunculated or sessile). Examples of polypoid lesions are shown in Figure 1a c. b. Nonpolypoid: Paris type 0 II (macroscopically visible fl at, slightly elevated or depressed), 0 III (excavated), irregular, or plaque-like lesions. The lesions of any shape with evidence of dysplasia in surrounding mucosa were considered as nonpolypoid. Examples of nonpolypoid lesions are shown in Figure 1d f. c. Invisible: absence of documented endoscopic abnormalities. If the visible lesion was detected on subsequent examinations within 12 months, categorization was based on the visible lesion found. d. When more than one type of lesions were found, the categorization was based on presence of a lesion with maximal carcinogenic potential, in order of nonpolypoid, invisible, and polypoid lesions. This hierarchy was based on their risk of developing HGD or CRC in our preliminary analysis on patients with a single dysplastic lesion only: the hazard ratios (HRs) of patients with nonpolypoid or invisible lesions were 16.5 (95% confidence interval (CI), ) and 6.8 (95% CI, ), respectively, compared with those with polypoid lesions (reference category). 2. Lesion size: each case was categorized based on presence or absence of the visible dysplastic lesion 1 cm in size at colono- The American Journal of GASTROENTEROLOGY VOLUME 110 OCTOBER
3 Low-Grade Dysplasia in Ulcerative Colitis 1463 Figure 1. Lesion shape categorization. Discrete sessile (a), pedunculated (b), or sub-pedunculated lesions (c) that were well circumscribed from the surrounding mucosa were classified as polypoid LGD. Superfi cially raised (d and e), visible flat (f), irregular, or plaque-like lesions were classifi ed as nonpolypoid LGD. scopy. When multiple dysplastic lesions were found, the categorization was based on the largest lesion present. 3. Exposure to chromoendoscopy (CE): the patient was considered exposed to CE if he/she had one or more procedures performed using CE at the time of or after the diagnosis of initial LGD. 4. Multifocal LGD: if dysplasia was found in more than one location, the case was considered as multifocal. 5. Metachronous LGD: the patient was considered to have metachronous dysplasia if he/she had more than one episode of LGD during their surveillance. 6. Other colonoscopic features: data on the presence or absence of a documented episode of the following colonoscopic appearances were collected: backwash ileitis, colonic stricture, postinflammatory polyp, scarring, a shortened colon, tubular appearance, featureless colon, and presence of severe macroscopic inflammation. Data on the quality of bowel preparation and the experience level of endoscopist performing the procedure were also documented (i.e., consultant, trainee, or nurse endoscopist). 7. Microscopic inflammation around the LGD: the data on the presence or absence of histological active or chronic inflammation around the site of dysplasia were documented. 8. Family history of CRC: the patient was considered to be positive if he/she had either first- or second-degree relatives who had CRC at any age. 9. Primary sclerosing cholangitis (PSC): the patient was considered positive only if the diagnosis was confirmed radiologically or histologically. 10. Exposure to 5-aminosalicylate or immunosuppressant: patients were categorized into three groups depending on the duration of exposure to 5-aminosalicylate or immunosuppressant since the time of earliest documented use never, up to 10 years, or >10 years. Study end point The study end point was defined as development of HGD or CRC during surveillance or at colectomy up to 1 July If the patient had not developed HGD or CRC, they were censored at the earliest of: the time of last surveillance colonoscopy, colectomy, or 1 July Statistical analysis The data analysis was performed using the SPSS statistical software (version 20, IBM, Armonk, NY). All continuous variables are reported as medians with interquartile ranges (IQRs). The study end point was examined using Kaplan Meier and Cox proportional hazards methods with right-censored data. As 24 potential predictors were being tested against two outcomes, a Bonferroniadjusted significance level of was used to select variables to be entered into the multivariate model to minimize the possibility of type 1 error. RESULTS Study population A total of 201 patients were diagnosed with LGD between 1 January 1993 and 31 December Of these, 15 patients were excluded as they withdrew from the surveillance program before their next scheduled surveillance for following reasons: death (5 patients, all unrelated to CRC), defaulted/patient choice (8 patients), and transferred to another institution (2 patients). In addition, a further 10 patients were excluded as they were sched-
4 1464 Choi et al. Table 1. Patient demographics ( n =172) Characteristic Values Male sex (%) 110 (64) Age (median, IQR) 60 Years (51 67) Duration of UC (median, IQR) 23 Years (12 32) Extensive UC (%) 172 (100) Median follow-up duration (IQR) 48 Months (15 87) Median number of follow-up colonoscopy (IQR) 3 Per patient (1 6) Median surveillance interval (IQR) 12 Months (7 16) Median number of biopsies (IQR) 12 (9 17) IQR, interquartile range; UC, ulcerative colitis. uled to have first follow-up colonoscopy after the study end date. A further four patients were excluded as LGD was incidentally found in their surgical specimen from a colectomy performed for medically refractory disease. Thus, a total of 172 patients met the inclusion criteria. The demographics of the study population is summarized in Table 1. LGD was more commonly detected in males (110, 16.0% of the male population under surveillance, n =687) compared with females (60, 11.2% of female population under surveillance, n =531; χ 2, P =0.02). The median age at initial LGD diagnosis was 60 years (IQR, years) and the median duration of UC at initial LGD diagnosis was 23 years (IQR, years). After the initial LGD diagnosis, these 172 patients underwent a total of 707 additional follow-up surveillance procedures (median, 4 procedures per patient; IQR, 2 6), of which 628 were colonoscopies (median, 3 colonoscopies per patient; IQR, 1 6). The median surveillance interval was 12 months (IQR, 7 16 months). The median follow-up duration from initial LGD diagnosis to the study end point was 48 months (IQR, months), with a cumulative patient-year follow-up duration of years ( Table 1 ). Patient follow-up Of the study population ( n =172), 21 patients (12.2%) underwent immediate colectomy without further colonoscopy (i.e., with presurgical diagnosis of LGD only) in a median of 4 months (range, 2 8 months) after initial LGD diagnosis. Histological analysis of their surgical specimen revealed CRC in 7 (33.3%), HGD in 3 (14.3%), LGD in 8 (38.1%), and no neoplasia in 3 patients (14.3%). The remaining 151 patients initially continued with endoscopic surveillance (87.8% of study population). Of these surveyed patients, 34 patients (22.5% of patients who remained in surveillance) were subsequently referred to colectomy, 31 of whom underwent colectomy at a median of 19 months (IQR, months) after initial LGD diagnosis. Indications for colectomy were CRC in 4 (all had CRC in specimen), HGD in 11 (of whom 5 had CRC in specimen), and LGD in 15 patients (of whom 2 had CRC in specimen), and 1 patient had a colectomy for refractory colitis (specimen revealed no neoplasia). One patient who developed CRC was considered unfit for surgery and another two patients who developed HGD refused surgery and remained in surveillance. The indications for colectomy and the maximal grade of neoplasia found in colectomy specimen for all patients who underwent colectomy during the study period ( n =52) are shown in Table 2. Overall, when the indication for surgery was HGD, 45.5% ( n =5/11) of the patients had CRC in their surgical specimen. For those who had colectomy for LGD, HGD or CRC was found in the colectomy specimens in 38.9% ( n =14/36) of cases, 9 of which were CRC (9/36=25.0%; Table 2 ). As of 1 July 2013, 104 patients (60.5% of study population) were still under endoscopic follow-up (median, 78.5 months per patient; IQR, months). The surveillance was terminated before 1 July 2013 in 16 patients (9.3% of study population) because of death (7/16 patients, 1 of whom died of CRC without colectomy), age (3/16 patients), or patient choice (1/16 patients). The remaining 5 patients (2.9% of study population) defaulted on surveillance and were lost to follow-up. Study end points Overall, 33 patients (incidence rate, 38.8 per 1,000 patient-years) progressed to more advanced disease during the study period, with n =13 developing HGD (incidence rate, 15.3 per 1,000 patient-years) and n =20 developing CRC (incidence rate, 23.5 per 1,000 patient-years). Only 6 patients (30.0% of patients who developed CRC) had a detected HGD lesion before developing CRC. There was a wide range in time from LGD to development of HGD (median, 13.0 months; IQR, months), or from LGD to CRC (median, 10.5 months; IQR, months). HGD or CRC was detected either at the surveillance colonoscopy ( n =16; 48.5% of progressors) or on colectomy ( n =17; 51.5% of progressors). Characteristics of LGD For the majority of patients, the endoscopic shape classification of the first LGD lesion was polypoid (116 patients; 67.4% of study population), followed by nonpolypoid (39 patients; 22.7%) and endoscopically invisible lesions (16 patients; 9.3%). At the time of invisible dysplasia detection, bowel preparation was considered to be adequate or good in all cases, and the median number of random biopsies taken was 11 (IQR, 9 14), and this was not significantly different from the number of biopsies taken for visible lesions (median, 12; IQR, 9 17; Mann Whitney U-test, P =0.4). Thirty-six patients had multifocal dysplasia (20.9% of study population). Nonpolypoid lesions were notably more likely to be multifocal compared with polypoid lesions (19/39=48.7% for nonpolypoid vs. 16/116=13.8% for polypoid; Fisher s exact test, P <0.001). Metachronous LGD lesions were common (79 patients; 46.0% of study population). Nonpolypoid or invisible lesions were more likely to be metachronous compared with polypoid lesions (26/39=66.7% for nonpolypoid vs. 41/116=35.3% for polypoid, P <0.001; 12/16=75.0% for invisible vs. 41/116=35.3% for polypoid, P =0.005). The American Journal of GASTROENTEROLOGY VOLUME 110 OCTOBER
5 Low-Grade Dysplasia in Ulcerative Colitis 1465 Table 2. Indications for colectomy and the maximal grade of neoplasia found in colectomy specimen Findings in surgical specimen Indication for surgery (no. of patients) No dysplasia Indefinite dysplasia LGD HGD CRC (%) Refractory colitis (1) (0) LGD (36) (25.0) HGD (11) (45.5) CRC (4) (100.0) CRC, colorectal cancer; HGD, high-grade dysplasia; LGD, low-grade dysplasia. Impact of chromoendoscopy on lesion detection A total of 873 colonoscopies (including colonoscopies performed before initial LGD detection) were performed for 158 LGD patients who were on surveillance between 2003 and 2012, of which 285 were chromoendoscopy (32.6%). The detection rate (i.e., total number of lesions detected/total number of each type of colonoscopies performed on study cohort) of the nonpolypoid lesion was significantly higher in CE (45/285=15.8%) than whitelight endoscopy (WLE; 46/588=7.8%; χ 2, P <0.001). There was no significant difference in the detection rate of polypoid lesions (17.5% for CE vs. 15.3% for WLE; P =0.08) and invisible lesions (1.8% for CE vs. 1.4% for WLE; P =0.50) between these two techniques. Factors determining development of HGD or CRC Univariate analysis. The results of univariate analysis of potential demographic, endoscopic, and histological risk factors for developing HGD or CRC are shown in Tables 3 5, respectively. In contrast to patients with polypoid dysplasia, a significant risk of developing HGD or CRC was observed among those with nonpolypoid dysplasia (HR, 16.5; 95% CI, ; P <0.001) or endoscopically invisible dysplasia (HR, 6.2; 95% CI, ; P =0.001; Table 4 ). Patients with lesions 1 cm were more likely to develop HGD or CRC (HR, 10.0; 95% CI, ; P <0.001) compared with those with lesions <1 cm ( Table 4 ). In addition, metachronous dysplasia (HR, 3.5; 95% CI, ; P =0.001), multifocal dysplasia (HR, 3.9; 95% CI, ; P <0.001), previous history of indefinite dysplasia (HR, 5.0; 95% CI, ; P <0.001), and a colonic stricture (HR, 7.4; 95% CI, ; P <0.001) showed a strong correlation to the risk of HGD or CRC ( Tables 4 and 5 ). None of the other variables showed a significant association with risk of HGD or CRC, although a nonsignificant (at Bonferroni adjusted significance level of 0.002) trend toward the HGD or CRC development was observed with coexisting primary sclerosing cholangitis (HR, 3.8; 95% CI, ; P =0.01), a shortened colon (HR, 2.9; 95% CI, ; P =0.02), and active (HR, 2.1; 95% CI, ; P =0.03) or chronic (HR, 3.8; 95% CI, ; P =0.01) microscopic inflammation in the segment of LGD ( Tables 3 5 ). In addition, patients who were exposed to at Table 3. Univariate analysis of potential demographic factors associated with HGD or CRC Variables Number (%) Hazard ratio (95% CI) P value Family history of CRC 20 (12) 0.7 ( ) 0.5 Male sex 110 (64) 1.1 ( ) 0.8 PSC 10 (6) 3.8 ( ) 0.01 Duration of UC (years) <10 27 (16) (56) 1.0 ( ) 0.9 >30 49 (28) 1.4 ( ) 0.5 Age (years) (7) (40) 2.0 ( ) (53) 1.6 ( ) ASA (years) Never 22 (13) 1 Up to (42) 0.9 ( ) 0.9 >10 78 (45) 0.9 ( ) 0.9 Immunosuppressant (years) Never 109 (63) 1 Up to (24) 1.2 ( ) 0.6 >10 22 (13) 0.6 ( ) ASA, 5-aminosalicylate; CI, confi dence interval; CRC, colorectal cancer; HGD, high-grade dysplasia; PSC, primary sclerosing cholangitis; UC, ulcerative colitis. Statistically significant factors ( P <0.002 after correcting for multiple testing) are shown in bold. least one episode of chromoendoscopy had a slight reduction in their risk of HGD or CRC development but this was not significant after correction for multiple testing (HR, 0.4; 95% CI, ; P =0.02; Table 4 ). Multivariate analysis. A total of seven variables were entered into the multivariate model, the six variables that had a significance level P <0.002 in the univariate analysis and, furthermore,
6 1466 Choi et al. Table 4. Univariate analysis of potential endoscopic factors associated with HGD or CRC Table 5. Univariate analysis of potential histological factors associated with HGD or CRC Variables Number (%) a Hazard ratio (95% CI) Lesion shape Polypoid 116 (67) 1 P value Nonpolypoid 39 ( 23) 16.5 ( ) <0.001 Invisible 16 ( 9) 6.2 ( ) Large lesion ( 1 cm) 60 ( 35) 10.0 ( ) <0.001 Chromoendoscopy use 112 (65) 0.4 ( ) 0.02 Endoscopist Consultant 119 (69) 1 Trainee or nurse 46 (27) 1.3 ( ) 0.5 Bowel preparation Good/adequate 156 (91) 1 Poor 9 (5) 1.9 ( ) 0.3 Backwash ileitis 18 (10) 2.2 ( ) 0.3 Featureless colon 27 (16) 1.8 ( ) 0.1 Pseudopolyps 108 (63) 0.8 ( ) 0.4 Shortened colon 16 (9) 2.9 ( ) 0.02 Scarring 114 (66) 0.8 ( ) 0.6 Variables Number (%) a Hazard ratio (95% CI) P value Metachronous 79 (46) 3.5 ( ) Previous indefinite dysplasia 17 (10) 5.0 ( ) <0.001 Multifocal 36 (21) 3.9 ( ) <0.001 Location b Proximal 74 (43) 1 Distal 74(43) 2.0 ( ) 0.07 Both 13 (8) Inflammation a Normal/quiescent 100 (58) 1 Chronic 15 (8) 3.8 ( ) 0.01 Active 56 (33) 2.1 ( ) 0.03 CI, confi dence interval; CRC, colorectal cancer; HGD, high-grade dysplasia. a Refers to microscopic infl ammation in the segment of dysplasia. In one case this was unknown because of lack of documentation (1% of study population). b Refers to location in relation to splenic fl exure. The location of LGD was unknown in 11 invisible dysplasia cases (6%). Statistically signifi cant factors ( P <0.002 after correcting for multiple testing) are shown in bold. Stricture 6 ( 3) 7.4 ( ) <0.001 Tubular colon 60 (35) 1.3 ( ) 0.5 CI, confi dence interval; CRC, colorectal cancer; HGD, high-grade dysplasia. a Variable categorization was not possible because of lack of documentation in following cases: lesion shape (1 case; 1% of study population), lesion size (2; 1%), experience of endoscopist (7; 4%), and quality of bowel preparation (7; 4%). Statistically signifi cant factors ( P <0.002 after correcting for multiple testing) are shown in bold. exposure to chromoendoscopy ( Table 6 ). The rational for the inclusion of the latter was to control for the gradual increase in the use of chromoendoscopy since 2002 that was likely to be the most significant change in the surveillance protocol that has occurred within the study period. The mean interval between each surveillance colonoscopy had not changed significantly over time (1.4 years in vs. 1.1 years between 2003 and 2012; paired T -test, P =0.4). After multivariate analysis, only nonpolypoid dysplasia (HR, 8.6; 95% CI, ; P <0.001), endoscopically invisible dysplasia (HR, 4.1; 95% CI, ; P =0.02), lesion size 1 cm (HR, 3.8; 95% CI, ; P =0.01), and previous history of indefinite dysplasia (HR, 2.8; 95% CI, ; P =0.01) remained as significant contributory factors to the HGD or CRC outcome ( Table 6 ). Factors determining development of CRC only The analysis was repeated for only CRC cases as the outcome ( n =20) (i.e., excluding HGD). In the univariate analysis, Table 6. Final multivariate model showing significant factors independently associated with HGD or CRC development Variables Hazard ratio (95% CI) P value LGD lesion shape Polypoid 1 Nonpolypoid 8.6 ( ) <0.001 Invisible 4.1 ( ) 0.02 Large lesion ( 1 cm) 3.8 ( ) 0.01 Stricture 1.6 ( ) 0.4 Metachronous 1.3 ( ) 0.6 Previous indefinite dysplasia 2.8 ( ) 0.01 Multifocal 1.8 ( ) 0.3 Chromoendoscopy use 0.5 ( ) 0.06 CI, confi dence interval; CRC, colorectal cancer; HGD, high-grade dysplasia; LGD, low-grade dysplasia. Statistically signifi cant factors ( P <0.05) are shown in bold. nonpolypoid shape (HR, 18.8; 95% CI, ; P <0.001), lesion size 1 cm (HR, 10.5; 95% CI, ; P <0.001), colonic stricture (HR, 16.3, 95% CI, ; P <0.001), and previous history of indefinite for dysplasia (HR, 5.8; 95% CI, ; P <0.001) showed a significant association with development of CRC. None of the other variables were significant. The American Journal of GASTROENTEROLOGY VOLUME 110 OCTOBER
7 Low-Grade Dysplasia in Ulcerative Colitis Probability of remaining HGD or CRC free Probability of remaining HGD or CRC free Years since initial LGD diagnosis Log-rank, P<0.001 Small LGD (<1 cm) Large LGD ( 1 cm) Probability of remaining HGD or CRC free Probability of remaining HGD or CRC free Invisible Log-rank, P<0.001 Log-rank, P<0.001 Nonpolypoid Polypoid Years since initial LGD diagnosis No preceding indefinite dysplasia Preceding indefinite dysplasia Years since initial LGD diagnosis Years since initial LGD diagnosis Figure 2. Kaplan Meier plots showing cumulative risk of developing high-grade dysplasia (HGD) or colorectal cancer (CRC). ( a ) Overall cumulative risk. ( b ) Cumulative risk by low-grade dysplasia (LGD) lesion shape. ( c ) Cumulative risk by LGD lesion size. ( d ) Cumulative risk depending on the presence or absence of preceding indefinite dysplasia. After multivariate analysis, only nonpolypoid shape (HR, 10.1; 95% CI, ; P =0.002) and lesion size 1 cm (HR, 3.6; 95% CI, ; P =0.04) remained as significant contributory factor to the CRC outcome. The previous history of indefinite for dysplasia (HR, 2.9; 95% CI, ; P =0.053) and colonic stricture (HR, 3.7; 95% CI, ; P =0.052) showed strong trend toward CRC outcome, but this was not statistically significant. Rate of progression to HGD or CRC Overall. Overall cumulative incidence of HGD or CRC development at 1 and 5 years after initial LGD diagnosis was 10.9% and 19.5%, respectively (mean annual hazard rate in first 5 years (HR), 4.7%, s.e., 0.9%; Figure 2a ). Based on individual risk factors. The cumulative incidence of HGD or CRC development based on lesion shape, size, and preceding dysplasia in the first 10 years from the date of initial LGD diagnosis is shown in Table 7. The cumulative incidence of HGD or CRC at 1 and 5 years after initial LGD was 3.5% and 6.0% for polypoid (HR, 1.3%; s.e., 0.6%), 6.7% and 21.9% for invisible (HR, 4.6%; s.e., 2.6%), and 36.6% and 65.2% for nonpolypoid LGD (HR, 18.2%; s.e., 3.6%), respectively ( Figure 2b ). When a large lesion ( 1 cm in size) was found, the cumulative incidence of HGD or CRC at 1 and 5 years was 29.8% and 47.3% (HR, 12.6%; s.e., 2.4%), respectively (in contrast with a lesion <1 cm in size, for which these were 0.4% and 5.8% (HR, 1.2%; s.e., 0.5%); Figure 2c ). If the first episode of LGD was preceded by indefinite dysplasia, the cumulative incidence of HGD or CRC at 1 and 5 years was 24.3% and 55.7% (HR, 16.0%; s.e., 5.1%), respectively. If the LGD was the first dysplasia, this was 9.4% and 15.8% (HR, 3.7%; s.e., 0.8%; Figure 2d ), respectively. Based on total number of risk factors. There was a significant positive correlation between the number of risk factors present and the cumulative risk of developing HGD or CRC (log-rank test, P <0.001; Figure 3 ). The cumulative incidence of HGD or CRC at 1 and 5 years after initial LGD was 0 and 1.8% for no risk factor (HR, 0.3%; s.e., 0.2%), 9.6 and 17.7% for one risk factor (HR, 4.9%; s.e., 1.8%), and 29.0 and 53.4% for two risk factors (HR, 13.6%; s.e., 3.3%). For those with three risk factors, cumulative risk of HGD or CRC development was 61.6% and 80.7% at 1 and 2 years, respectively ( Table 7 ). In patients who remained in surveillance. We performed same analysis excluding patients who underwent immediate colectomy ( n =21). The cumulative incidence of HGD or CRC development based on lesion shape, size, preceding dysplasia, and the total number of these risk factors present in the first 10 years from
8 1468 Choi et al. Table 7. The cumulative incidence of HGD or CRC development based on lesion shape, size, preceding indefinite dysplasia, and total number of these risk factors present in the first 10 years from the date of initial LGD diagnosis Cumulative incidence of HGD or CRC (%) Years Overall By lesion shape Polypoid Invisible Nonpolypoid By lesion size <1 cm cm Preceding indefinite dysplasia No Yes Total number of risk factors (shape, size, or preceding indefinite dysplasia ) None CRC, colorectal cancer; HGD, high-grade dysplasia; LGD, low-grade dysplasia. the date of initial LGD diagnosis is shown in Supplementary Table S1 and Supplementary Figure S1 S2 online. DISCUSSION In this study, we performed a detailed analysis of HGD and CRC risk in a large number of UC patients diagnosed with LGD, taking their demographic, endoscopic, and histological characteristics into account. We have shown that UC patients with LGD that is non-polypoid, invisible, 1 cm in size, or preceded by indefinite dysplasia are at higher risk of developing HGD or CRC. In addition, we have described several other features that had a significant association with development of HGD or CRC in univariate analysis that may be useful in identifying high-risk patients. Non-polypoid dysplastic lesions In agreement with previous studies, our data shows that polypoid LGD lesions have a low risk of CRC ( 13,14,16,18 ). Polypoid lesions were the most common form of LGD found in our cohort and were removed endoscopically in the vast majority of cases (108/116 or 93.1%) suggesting that the majority of LGD lesions may be adequately managed without colectomy. It was previously shown that the risk of CRC was similar between adenoma-like lesions arising in diseased segment and sporadic adenoma occurring in disease-free segment ( 14,15 ), and furthermore studies have suggested that these lesions have Probability of remaining HGD or CRC free Years since initial LGD diagnosis Number of risk factors present Figure 3. Kaplan Meier plot showing the cumulative risk of high-grade dysplasia (HGD) or colorectal cancer (CRC) development depending on total number of risk factors present (i.e., any combinations of nonpolypoid or invisible low-grade dysplasia (LGD), LGD sized 1 cm, and/or preceding indefi nite dysplasia). genetic similarities ( 12,19 ). Thus, it is possible that adenoma-like lesions may simply represent sporadic adenomas that occur in patients with UC. Patients with non-polypoid lesions, however, had a significantly higher cancer risk. Many of these lesions were not amenable for endoscopic resection (24 out of 39 or 61.5% of non-polypoid lesions), and were more likely to be multifocal compared with polypoid lesions. In our cohort, 13 out of 39 patients (33.3%) who initially had a LGD lesion that was considered nonpolypoid in shape later developed CRC. In addition, 6 of these The American Journal of GASTROENTEROLOGY VOLUME 110 OCTOBER
9 Low-Grade Dysplasia in Ulcerative Colitis 1469 patients (6/13=46.2% of patients who developed CRC) had synchronous cancers in the surgical specimen. These lesions are an ominous finding and thus may require more aggressive management. Of note, our results suggest that the CE was more effective at detecting non-polypoid lesions than WLE but the difference was minimal for polypoid lesions, and this is broadly in agreement with previous randomized controlled trial ( 20 ) and a recent metaanalysis (21 ). Given the significant risk of progression associated with nonpolypoid lesions, our data advocate the use of CE for early detection of these higher-risk lesions. As high-definition (HD) colonoscopy (i.e., HD colonoscope with HD-monitor) was only available since 2011 in our center, its efficacy could not be reliably assessed in our study. The value of HD WLE in detecting subtle dysplastic lesions compared with CE thus requires further dedicated study. Large dysplastic lesions It is well established that the malignant potential of a sporadic adenoma increases with size ( ). However, the correlation of dysplastic lesion size and malignant potential in IBD is not well established. Our data suggest that lesions that are 1 cm have an approximately four-fold greater risk of progression compared with smaller lesions. This finding is in agreement with the data from IBD-free population where lesions >1 cm (or advanced adenomas ) were associated with 3.6-fold increased risk of CRC compared with the general population ( 24 ). It was recently shown that the incomplete resection rate is higher for larger adenomas (10 20 mm) or non-conventional adenomas ( 25 ) and it is likely that a large non-polypoid dysplastic lesions in colitis are similarly more difficult to resect completely. Thus, it is important that advanced techniques are used when such lesion is being managed non-operatively, and if the complete resection cannot be achieved, colectomy may be advisable. Although these results should provide a broad guidance in making management decisions, our data should be confirmed by prospective trials, as our retrospective study design limits adequate assessment for possible interobserver variability that may exist in classifying the lesion shape and size that was recently demonstrated to be only moderate ( 26 ). Invisible dysplasia Although most dysplastic lesions were endoscopically visible in our study, the presence of invisible dysplasia detected histologically within a random biopsy was a significant risk factor for HGD or CRC development in our multivariate analysis. Our results are in agreement with previous retrospective study from the US tertiary center ( 1 ) and a meta-analysis ( 27 ). However, it should be noted that these studies were performed before the introduction of newer techniques, in particular, CE. It is difficult to know whether these invisible lesions were truly endoscopically invisible dysplasias or simply missed lesions. In our data set, the incidence rate of invisible dysplasia in the recent decade was lower (7.4 per 1,000 patient-years between 2003 and 2012) compared with the previous decade (18.8 per 1,000 patient-years between 1993 and 2002), although this was not statistically significant (Fisher s exact, P =0.07). In contrast, the incidence rate of non-polypoid lesions increased by two-fold in the recent decade (36.2 per 1,000 patient-years between 2003 and 2012) compared with the previous decade (18.8 per 1,000 patientyears between 1993 and 2002). These findings may suggest that invisible dysplasia is becoming a rare entity and is instead being increasingly detected as nonpolypoid dysplasia, as improving endoscopic techniques and technologies allow detection of more subtle lesions. This may explain why patients with invisible dysplasia had a relatively high risk of progression to HGD or CRC. Nevertheless, it is important to confirm the nature of these invisible lesions in a dedicated study, as it is still possible that these are truly invisible lesions that are difficult to detect even with advanced techniques. Thus, when an invisible dysplasia is detected, our current policy is to refer patients to an experienced endoscopist to have a repeat procedure performed with an advanced technique. Typically, HD CE is performed in an attempt to identify the lesion that was possibly missed in previous colonoscopy. In addition, segmental and targeted biopsies are taken as appropriate to ensure that any truly invisible lesions are not missed. Preceding dysplasia The previous history of indefinite for dysplasia showed a significant association with development of HGD or CRC. It is recognized that distinction between indefinite for dysplasia and LGD is challenging ( 11 ) and it was previously shown that indefinite dysplasia is frequently regraded to LGD after a dedicated review (28 ). Thus, one possible explanation is that these indefinite dysplasia may actually have been LGD. Furthermore, patients with metachronous LGD had high risk of developing HGD or CRC in a univariate analysis, suggesting that persistent dysplasia regardless of grade may increase the risk of progression. Patients with dysplasia with previous a history of any grade of dysplasia should therefore be closely monitored, and option for colectomy should be discussed with the patient if metachronous dysplasia develops persistently despite endoscopic resection. Other risk factors Several studies have documented a high rate of underlying CRC among patients with colonic stricture ( 29,30 ). In our study, four out of six patients with a colonic stricture had developed CRC. In particular, all three patients whose dysplasia was found within a stricture had advanced CRC. In the remaining patient, CRC (Dukes B) was found proximal to the stricture, indicating that difficulty in access to the proximal colon may have been a contributing factor. Although the patient number is small in our cohort, the high proportion of patients who developed CRC indicates that finding a colonic stricture in patients with history of dysplasia should raise clinical suspicion for CRC. Although multifocal dysplasia is generally perceived to be a high-risk feature, the evidence demonstrating this is scant ( 28,31 ). In our study, multifocal dysplasia showed a significant
10 1470 Choi et al. association toward development of HGD or CRC in a univariate setting only, highlighting the importance of considering other independent risk factors in conjunction. For example, none of the patients with multifocal polypoid dysplasia ( n =12) had developed HGD or CRC during a median of 63 months (IQR, months). Number of risk factors present and timing of colectomy We observed a clear association between the numbers of risk factors present (i.e., lesion shape, size, and preceding indefinite dysplasia) and the risk of HGD or CRC development. Of note, patients with only one of these risk factors had 17.7% risk of progression at 5 years after initial LGD diagnosis. Relatively low rate of progression observed in this group is perhaps not surprising, as it represent LGD cases that could be potentially removed endoscopically. For example, 18 patients (without preceding dysplasia) had their large polypoid LGD removed endoscopically (median size, 15 mm; IQR, mm). During median follow-up of 33 months (IQR, months), 2 of these patients developed CRC (both Dukes A) in 6 and 24 months after resection and 1 patient developed HGD in 6 months (incidence rate, 45.5 per 1,000 patient-years). Similarly, of 8 patients who underwent endoscopic resection for small (<1 cm) nonpolypoid lesions, 1 patient developed CRC (Dukes A) in median follow-up of 44 months (IQR, months; incidence rate, 26.8 per 1,000 patient-years). Thus, these data suggest that colectomy may not be always necessary for this group of patients, provided that the lesion can be resected in full with no evidence of dysplasia elsewhere in the colon. However, given the small number of cases, these data should be interpreted with caution. In contrast, when patients had 2 risk factors, their risk of developing HGD or CRC exceeded 50% at 5 years following their initial LGD diagnosis. Furthermore, when all 3 risk factors were present, >80% of patients developed HGD or CRC within 2 years. Thus, these patients should be considered for early surgical intervention and counseling should be offered at the earliest opportunity. Of note, only 30% (6/20) of CRCs were preceded by HGD in our cohort, indicating that a majority of CRCs were detected during colectomy performed for LGD (9/20; 45%) or at surveillance colonoscopy with last known worst dysplasia being LGD (5/20; 25%). Thus, our data suggest that the time when LGD is detected is likely to be the most appropriate time for assessing the risk associated with the lesion and offer surgical intervention for appropriate patients. Limitations Our study has limitations. First, it could be considered that our inclusion of patients who underwent early colectomy may have limited the accurate assessment of the natural history of LGD. This issue is particularly true for those patients with small polypoid LGD lesions who undergo early colectomy, as risk of HGD or CRC associated with such lesions may be underestimated. However, such cases are very rare in our experience: in our cohort, only 1 patient out of 31 patients underwent colectomy within 12 months for LGD without having any of the aforementioned highrisk features (and no dysplasia or CRC was detected in the colectomy specimen). Moreover, excluding early colectomy patients entirely may potentially lead to underestimation of the risk associated with lesions with the aforementioned high-risk features as they often underwent colectomy within 12 months from initial LGD diagnosis ( n =30/31, 14 of whom had HGD or CRC in the specimen). Second, this study was conducted on a cohort from a tertiary referral center that includes a higher proportion of patients with more severe or complex disease. This has a potential impact on generalizability of our results, as the progression of LGDs in nontertiary centers may be different. For example, it is possible that dysplastic lesions studied in our work may represent those developed on background of more severe inflammatory drive, hence leading to more rapid progression. Furthermore, the rate of dysplasia detection and endoscopic removal may also vary significantly between centers. CONCLUSION In summary, we have revealed three important independent risk factors for HGD or CRC development in patients with LGD: lesion shape (non-polypoid or macroscopically invisible dysplasia), size of the lesion 1 cm, and history of previous indefinite for dysplasia. Patients with a LGD lesion who exhibit these risk factors have a high risk of developing HGD or CRC. Therefore, early surgical intervention should be considered in close discussion with the patient, particularly when more than one of these risk factors is present. Conversely, patients with small (<1 cm) polypoid lesion may be appropriately managed with endoscopic resection with close monitoring. Patients with multifocal dysplasia, previous history of any grade of dysplasia, and colonic stricture should be regarded as a considerable risk and intensive surveillance should be considered. CONFLICT OF INTEREST Guarantor of the article: Ailsa L. Hart, BMBCh, PhD, FRCP. Specific author contributions: C.-h.R.C.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, obtained funding and statistical analysis; A.I.-W., J.W., M.M., S.T.-G., M.D.R., and B.P.S.: study concept, design, and critical revision of manuscript for important intellectual content; A.A. and G.H.L.: acquisition of data and statistical analysis; T.A.G.: study concept and design, critical revision of manuscript for important intellectual content, statistical analysis, obtained funding, and study supervision; A.L.H.: Study concept and design, critical revision of manuscript for important intellectual content, analysis and interpretation of data, obtained funding, and study supervision. Financial support: C.-h.R.C. was funded by the Derek Willoughby Fund for Inflammatory Research. A.L.H. and T.A.G. were funded by Higher Education Funding Council of England. Potential competing interests: None. The American Journal of GASTROENTEROLOGY VOLUME 110 OCTOBER
11 Low-Grade Dysplasia in Ulcerative Colitis 1471 Study Highlights WHAT IS CURRENT KNOWLEDGE Patients with ulcerative colitis (UC) who are diagnosed with low-grade dysplasia (LGD) have a significant risk of developing colorectal cancer (CRC). However, management of these patients is challenging because of marked variability in their rate of progression to more advanced neoplasia. There are little data on endoscopic and histological characteristics of LGD that are associated with a high risk of development of high-grade dysplasia (HGD) or CRC. WHAT IS NEW HERE Patients with a LGD lesion that is non-polypoid in shape, endoscopically invisible, sized 1 cm or preceded by indefinite for dysplasia diagnosis have a high risk of developing HGD or CRC. Chromoendoscopy was more effective at detecting nonpolypoid dysplasias than white-light endoscopy. If one or more of these risk factors are present, patients should be carefully counseled about their management options including colectomy. Conversely, patients with a small polypoid lesion and no other risk factors may be appropriately managed with close surveillance. REFERENCES 1. Ul l man T, C ro o g V, Har p a z N et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 2003 ;5085 : Bernstein C, Shanahan F, Weinstein W. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994, Blackstone MO, Riddell RH, Rogers BH et al. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology 1981 ;80 : Befrits R, Ljung T, Jaramillo E et al. Low-grade dysplasia in extensive, longstanding inflammatory bowel disease: a follow-up study. Dis Colon Rectum 2002 ;45 : Je ss T, L oftus EV, Vel ayo s F S et al. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis 2006 ;12 : Rutter MD, Saunders BP, Wilkinson KH et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology 2006 ;130 : Ul l man Ta, L oftus EV, Ka k ar S et al. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol 2002 ; 97 : Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology 1991 ;100 : Connell WR, Lennard-Jones JE, Williams CB et al. Fa c tors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994 ;107 : Dixon MF, BROWN LJR, Gilmour HM et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology 1988 ;13 : Eaden J, Abrams K, McKay H et al. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol 2001 ; 194 : Fogt F, Urbanski SJ, Sanders ME et al. Distinction between dysplasiaassociated lesion or mass (DALM) and adenoma in patients with ulcerative colitis. Hum Pathol 2000 ; 31 : Rubin PH, Friedman S, Harpaz N et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 1999 ;117 : Odze RD, Farraye FA, Hecht JL et al. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 2004 ; 2 : Kisiel JB, Loftus EV, Har ms e n WS et al. Outcome of sporadic adenomas and adenoma-like dysplasia in patients with ulcerative colitis undergoing polypectomy. Inflamm Bowel Dis 2012 ;18 : Wanders LK, Dekker E, Pullens B et al. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol 2014 ; 12 : Riddell RH, Goldman H, Ransohoff DF et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983 ; 14 : Zisman TL, Bronner MP, Rulyak S et al. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis 2012 ;18 : Odze RD, Brown CA, Hartmann CJ et al. Genetic alterations in chronic ulcerative colitis-associated adenoma-like DALMs are similar to non-colitic sporadic adenomas. Am J Surg Pathol 2000 ;24 : Kiesslich R, Fritsch J, Holtmann M et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003 ; 124 : Subramanian V, Mannath J, Ragunath K et al. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther 2011 ;33 : Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975 ;36 : Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer 1986 ; 38 : Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992 ;326 : Pohl H, Srivastava A, Bensen SP et al. Incomplete polyp resection during colonoscopy - results of the complete adenoma resection (CARE) study. Gastroenterology 2013 ;1 : Doorn SC, van, Hazewinkel Y, East JE et al. Polyp morphology: an interobserver evaluation for the paris classification among international experts. Am J Gastroenterol 2015 ;110 : Thomas T, Abr ams KA, R obi ns on R J et al. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther 2007 ;25 : van Schaik FD, ten Kate FJ, Offerhaus GJ et al. M i s c l a s s i fi cation of dysplasia in patients with inflammatory bowel disease: consequences for progression rates to advanced neoplasia. Inflamm Bowel Dis 2011 ; 17 : Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut 1992 ; 33 : Lashner BA, Turner BC, Bostwick DG et al. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci 1990 ; 35 : Eaton JE, Smyrk TC, Imam M et al. The fate of indefinite and low-grade dysplasia in ulcerative colitis and primary sclerosing cholangitis colitis before and after liver transplantation. Aliment Pharmacol Ther 2013 ;38 : This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
Page 1. Is the Risk This High? Dysplasia in the IBD Patient. Dysplasia in the Non IBD Patient. Increased Risk of CRC in Ulcerative Colitis
 Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview
 1022 ORIGINAL CONTRIBUTIONS nature publishing group see related editorial on page 1035 Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview
1022 ORIGINAL CONTRIBUTIONS nature publishing group see related editorial on page 1035 Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview
Dysplasia 4/19/2017. How do I practice Chromoendoscopy for Surveillance of Colitis? SCENIC: Polypoid Dysplasia in UC. Background
 SCENIC: Polypoid in UC Definition How do I practice for Surveillance of Colitis? Themos Dassopoulos, M.D. Director, BSW Center for IBD Themistocles.Dassopoulos@BSWHealth.org Tel: 469-800-7189 Cell: 314-686-2623
SCENIC: Polypoid in UC Definition How do I practice for Surveillance of Colitis? Themos Dassopoulos, M.D. Director, BSW Center for IBD Themistocles.Dassopoulos@BSWHealth.org Tel: 469-800-7189 Cell: 314-686-2623
White Rose Research Online URL for this paper: Version: Accepted Version
 This is a repository copy of Patients with Endoscopically Visible Polypoid Adenomatous Lesions Within the Extent of Ulcerative Colitis Have an Increased Risk of Colorectal Cancer Despite Endoscopic Resection.
This is a repository copy of Patients with Endoscopically Visible Polypoid Adenomatous Lesions Within the Extent of Ulcerative Colitis Have an Increased Risk of Colorectal Cancer Despite Endoscopic Resection.
How to characterize dysplastic lesions in IBD?
 How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD
 CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD Disclosure Statement NKC: No relevant conflicts to disclose. DTR: No relevant
CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD Disclosure Statement NKC: No relevant conflicts to disclose. DTR: No relevant
CRC and Dysplasia in IBD: Objectives of Talk. Colorectal Cancer and Dysplasia in IBD: A Case-Based Approach. Page 1
 Colorectal Cancer and in IBD: A Case-Based Approach Fernando Velayos MD MPH Associate Director of Translational Research University of California, San Francisco Center for Crohn s s and Colitis CRC and
Colorectal Cancer and in IBD: A Case-Based Approach Fernando Velayos MD MPH Associate Director of Translational Research University of California, San Francisco Center for Crohn s s and Colitis CRC and
Diagnostic techniques for surveillance of dysplasia
 January 27th 2017, 8th Gastro Foundation Weekend for Fellows; Spier Hotel & Conference Centre, Stellenbosch Diagnostic techniques for surveillance of dysplasia Gerhard Rogler, Department of Gastroenterology
January 27th 2017, 8th Gastro Foundation Weekend for Fellows; Spier Hotel & Conference Centre, Stellenbosch Diagnostic techniques for surveillance of dysplasia Gerhard Rogler, Department of Gastroenterology
Malignancy in ulcerative colitis (UC) is believed to ORIGINAL ARTICLES
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2004;2:534 541 ORIGINAL ARTICLES Long-term Follow-up After Polypectomy Treatment for Adenoma-Like Dysplastic Lesions in Ulcerative Colitis ROBERT D. ODZE,* FRANCIS
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2004;2:534 541 ORIGINAL ARTICLES Long-term Follow-up After Polypectomy Treatment for Adenoma-Like Dysplastic Lesions in Ulcerative Colitis ROBERT D. ODZE,* FRANCIS
Patients with longstanding ulcerative colitis (UC) or
 ORIGINAL ARTICLE Misclassification of Dysplasia in Patients with Inflammatory Bowel Disease: Consequences for Progression Rates to Advanced Neoplasia Fiona D.M. van Schaik, MD,* Fiebo J.W. ten Kate, MD,
ORIGINAL ARTICLE Misclassification of Dysplasia in Patients with Inflammatory Bowel Disease: Consequences for Progression Rates to Advanced Neoplasia Fiona D.M. van Schaik, MD,* Fiebo J.W. ten Kate, MD,
Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis
 Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis Bret A. Lashner, M.D. Professor of Medicine Director,
Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis Bret A. Lashner, M.D. Professor of Medicine Director,
I n 1925, Crohn and Rosenberg first observed that ulcerative
 256 INFLAMMATORY BOWEL DISEASE Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis M D Rutter, B P Saunders, G Schofield, A Forbes, A B Price, I C Talbot... See
256 INFLAMMATORY BOWEL DISEASE Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis M D Rutter, B P Saunders, G Schofield, A Forbes, A B Price, I C Talbot... See
Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia
 Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia Ralf Kiesslich I. Medical Department Johannes Gutenberg University Mainz, Germany Cumulative cancer risk in ulcerative colitis 0.5-1.0%
Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia Ralf Kiesslich I. Medical Department Johannes Gutenberg University Mainz, Germany Cumulative cancer risk in ulcerative colitis 0.5-1.0%
Colorectal cancer surveillance in inflammatory bowel diseases
 Turkish Journal of Cancer Volume 34, No.2, 2004 55 Colorectal cancer surveillance in inflammatory bowel diseases MURAT TÖRÜNER Ankara University Medical School, Department of Gastroenterology, Ankara-Turkey
Turkish Journal of Cancer Volume 34, No.2, 2004 55 Colorectal cancer surveillance in inflammatory bowel diseases MURAT TÖRÜNER Ankara University Medical School, Department of Gastroenterology, Ankara-Turkey
Diagnosis and Management of Flat and Polypoid Dysplasia in Inflammatory Bowel Disease
 Diagnosis and Management of Flat and Polypoid Dysplasia in Inflammatory Bowel Disease Francis A. Farraye, MD, MSc Clinical Director, Section of Gastroenterology, Boston Medical Center Professor of Medicine,
Diagnosis and Management of Flat and Polypoid Dysplasia in Inflammatory Bowel Disease Francis A. Farraye, MD, MSc Clinical Director, Section of Gastroenterology, Boston Medical Center Professor of Medicine,
USCAP Companion Meeting 2006 Arthur Purdy Stout Society of Surgical Pathology Sporadic Adenomas and DALMs in IBD
 1 USCAP Companion Meeting 2006 Arthur Purdy Stout Society of Surgical Pathology Sporadic Adenomas and DALMs in IBD Robert D Odze, M.D., FRCP(c) Chief, Gastrointestinal Pathology Service Associate Professor
1 USCAP Companion Meeting 2006 Arthur Purdy Stout Society of Surgical Pathology Sporadic Adenomas and DALMs in IBD Robert D Odze, M.D., FRCP(c) Chief, Gastrointestinal Pathology Service Associate Professor
When and How to use Chromoendoscopy in IBD
 When and How to use Chromoendoscopy in IBD Samir A. Shah, MD, FACG, FASGE, AGAF Clinical Professor of Medicine, Brown University Chief of Gastroenterology, The Miriam Hospital Gastroenterology Associates,
When and How to use Chromoendoscopy in IBD Samir A. Shah, MD, FACG, FASGE, AGAF Clinical Professor of Medicine, Brown University Chief of Gastroenterology, The Miriam Hospital Gastroenterology Associates,
Diagnostic and Therapeutic Approaches to Dysplasia in Inflammatory Bowel Diseases
 Diagnostic and Therapeutic Approaches to Dysplasia in Inflammatory Bowel Diseases Parakkal Deepak, M.B.B.S., M.S. Assistant Professor of Medicine Division of Gastroenterology John T. Milliken Department
Diagnostic and Therapeutic Approaches to Dysplasia in Inflammatory Bowel Diseases Parakkal Deepak, M.B.B.S., M.S. Assistant Professor of Medicine Division of Gastroenterology John T. Milliken Department
Can We Predict the Natural History of Ulcerative Colitis? Edward V Loftus, Jr, MD Professor of Medicine Mayo Clinic Rochester, Minnesota, USA
 Can We Predict the Natural History of Ulcerative Colitis? Edward V Loftus, Jr, MD Professor of Medicine Mayo Clinic Rochester, Minnesota, USA Endpoints Overview Hospitalization Surgery Colorectal cancer
Can We Predict the Natural History of Ulcerative Colitis? Edward V Loftus, Jr, MD Professor of Medicine Mayo Clinic Rochester, Minnesota, USA Endpoints Overview Hospitalization Surgery Colorectal cancer
Chromoendoscopy - Should It Be Standard of Care in IBD?
 Chromoendoscopy - Should It Be Standard of Care in IBD? John F. Valentine, MD, FACG Professor of Medicine Division of Gastroenterology, Hepatology and Nutrition University of Utah What is the point of
Chromoendoscopy - Should It Be Standard of Care in IBD? John F. Valentine, MD, FACG Professor of Medicine Division of Gastroenterology, Hepatology and Nutrition University of Utah What is the point of
Accepted Manuscript. En bloc resection for mm polyps to reduce post-colonoscopy cancer and surveillance. C. Hassan, M. Rutter, A.
 Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
IBD high risk groups
 IBD high risk groups Ulcerative colitis Value (95% CI) CRC prevalence (%) 3.7 (3.2-4.2) Overall annual CRC incidence (%) 0.3 (0.2-0.4) Annual CRC incidence in first decade of UC (%) 0.2 (0.1-0.2) Annual
IBD high risk groups Ulcerative colitis Value (95% CI) CRC prevalence (%) 3.7 (3.2-4.2) Overall annual CRC incidence (%) 0.3 (0.2-0.4) Annual CRC incidence in first decade of UC (%) 0.2 (0.1-0.2) Annual
Colorectal Cancer in Inflammatory Bowel Disease
 Gut and Liver, Vol. 2, No. 2, September 2008, pp. 61-73 review Colorectal Cancer in Inflammatory Bowel Disease Jonathan Potack and Steven H. Itzkowitz Division of Gastroenterology, Department of Medicine,
Gut and Liver, Vol. 2, No. 2, September 2008, pp. 61-73 review Colorectal Cancer in Inflammatory Bowel Disease Jonathan Potack and Steven H. Itzkowitz Division of Gastroenterology, Department of Medicine,
Patients with long-standing, extensive ulcerative colitis (UC) Progression to Colorectal Neoplasia in Ulcerative Colitis: Effect of Mesalamine
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1225 1230 Progression to Colorectal Neoplasia in Ulcerative Colitis: Effect of Mesalamine THOMAS ULLMAN,* VICTORIA CROOG,* NOAM HARPAZ, SABERA HOSSAIN, ASHER
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1225 1230 Progression to Colorectal Neoplasia in Ulcerative Colitis: Effect of Mesalamine THOMAS ULLMAN,* VICTORIA CROOG,* NOAM HARPAZ, SABERA HOSSAIN, ASHER
Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease
 Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease Authors: Jesús Castro, Miriam Cuatrecasas, Francesc Balaguer, Elena Ricart, María Pellisé DOI: 10.17235/reed.2017.5068/2017
Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease Authors: Jesús Castro, Miriam Cuatrecasas, Francesc Balaguer, Elena Ricart, María Pellisé DOI: 10.17235/reed.2017.5068/2017
Chromoendoscopy as an Adjunct to Colonoscopy
 Chromoendoscopy as an Adjunct to Colonoscopy Policy Number: 2.01.84 Last Review: 1/2018 Origination: 7/2017 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Chromoendoscopy as an Adjunct to Colonoscopy Policy Number: 2.01.84 Last Review: 1/2018 Origination: 7/2017 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
New Approaches for Early Detection of Ulcerative Colitis (UC) Associated Cancer and Surgical Treatment of UC Patients
 New Approaches for Early Detection of Ulcerative Colitis (UC) Associated Cancer and Surgical Treatment of UC Patients Toshiaki Watanabe, M.D., Ph.D. Department of Surgery, Teikyo University School of Medicine,
New Approaches for Early Detection of Ulcerative Colitis (UC) Associated Cancer and Surgical Treatment of UC Patients Toshiaki Watanabe, M.D., Ph.D. Department of Surgery, Teikyo University School of Medicine,
Chemoprevention of Colorectal Neoplasia in Ulcerative Colitis: The Effect of 6-Mercaptopurine
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:1015 1021 Chemoprevention of Colorectal Neoplasia in Ulcerative Colitis: The Effect of 6-Mercaptopurine SIERRA MATULA,* VICTORIA CROOG,* STEVEN ITZKOWITZ,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:1015 1021 Chemoprevention of Colorectal Neoplasia in Ulcerative Colitis: The Effect of 6-Mercaptopurine SIERRA MATULA,* VICTORIA CROOG,* STEVEN ITZKOWITZ,*
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators
Quality of and compliance with colonoscopy in Lynch Syndrome surveillance: are we getting it right?
 Quality of and compliance with colonoscopy in Lynch Syndrome surveillance: are we getting it right? Hartery K 1, Sukha A 1, Thomas-Gibson S 1, Thomas H 1,2, Latchford A 1,2. 1 Wolfson Endoscopy Unit, St.
Quality of and compliance with colonoscopy in Lynch Syndrome surveillance: are we getting it right? Hartery K 1, Sukha A 1, Thomas-Gibson S 1, Thomas H 1,2, Latchford A 1,2. 1 Wolfson Endoscopy Unit, St.
Role of random biopsies in surveillance of dysplasia in ulcerative colitis patients with high risk of colorectal cancer
 ORIGINAL ARTICLE pissn 1598-9100 eissn 2288-1956 http://dx.doi.org/10.5217/ir.2016.14.3.264 Intest Res 2016;14(3):264-269 Role of random biopsies in surveillance of dysplasia in ulcerative colitis patients
ORIGINAL ARTICLE pissn 1598-9100 eissn 2288-1956 http://dx.doi.org/10.5217/ir.2016.14.3.264 Intest Res 2016;14(3):264-269 Role of random biopsies in surveillance of dysplasia in ulcerative colitis patients
Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary sclerosing cholangitis and ulcerative colitis
 Journal of Crohn's and Colitis (2013) 7, 974 981 Available online at www.sciencedirect.com ScienceDirect Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary
Journal of Crohn's and Colitis (2013) 7, 974 981 Available online at www.sciencedirect.com ScienceDirect Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary
Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis
 Alimentary Pharmacology & Therapeutics Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis T. THOMAS*, K. A. ABRAMS, R.J.ROBINSON*&J.F.MAYBERRY* *Department of Gastroenterology,
Alimentary Pharmacology & Therapeutics Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis T. THOMAS*, K. A. ABRAMS, R.J.ROBINSON*&J.F.MAYBERRY* *Department of Gastroenterology,
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts
 Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts Asher Kornbluth, MD Clinical Professor of Medicine The Henry D. Janowitz The Mt. Sinai School of Medicine Refining our Management
Ulcerative Colitis: Refining our Management and Incorporating Newer Concepts Asher Kornbluth, MD Clinical Professor of Medicine The Henry D. Janowitz The Mt. Sinai School of Medicine Refining our Management
Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease
 Aliment Pharmacol Ther 23; 18 (Suppl. 2): 1 5. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease P. MUNKHOLM Department of Medical Gastroenterology, Hvidovre
Aliment Pharmacol Ther 23; 18 (Suppl. 2): 1 5. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease P. MUNKHOLM Department of Medical Gastroenterology, Hvidovre
Large Colorectal Adenomas An Approach to Pathologic Evaluation
 Anatomic Pathology / LARGE COLORECTAL ADENOMAS AND PATHOLOGIC EVALUATION Large Colorectal Adenomas An Approach to Pathologic Evaluation Elizabeth D. Euscher, MD, 1 Theodore H. Niemann, MD, 1 Joel G. Lucas,
Anatomic Pathology / LARGE COLORECTAL ADENOMAS AND PATHOLOGIC EVALUATION Large Colorectal Adenomas An Approach to Pathologic Evaluation Elizabeth D. Euscher, MD, 1 Theodore H. Niemann, MD, 1 Joel G. Lucas,
Adherence to surveillance guidelines for dysplasia and colorectal carcinoma in ulcerative and Crohn s colitis patients in the Netherlands
 Online Submissions: wjg.wjgnet.com World J Gastroenterol 9 January 14; 15(2): 226-23 wjg@wjgnet.com World Journal of Gastroenterology ISSN 17-9327 doi:1.3748/wjg.15.226 9 The WJG Press and Baishideng.
Online Submissions: wjg.wjgnet.com World J Gastroenterol 9 January 14; 15(2): 226-23 wjg@wjgnet.com World Journal of Gastroenterology ISSN 17-9327 doi:1.3748/wjg.15.226 9 The WJG Press and Baishideng.
The variable risk of colorectal cancer in patients with inflammatory bowel disease.
 The variable risk of colorectal cancer in patients with inflammatory bowel disease. Lindgren, Stefan Published in: European Journal of Internal Medicine DOI: 10.1016/j.ejim.2004.12.001 Published: 2005-01-01
The variable risk of colorectal cancer in patients with inflammatory bowel disease. Lindgren, Stefan Published in: European Journal of Internal Medicine DOI: 10.1016/j.ejim.2004.12.001 Published: 2005-01-01
ASGE and AGA Issue Consensus Statement on Surveillance and Management of Dysplasia in Patients With Inflammatory Bowel Disease
 ASGE and AGA Issue Consensus Statement on Surveillance and Management of Dysplasia in Patients With Inflammatory Bowel Disease DOWNERS GROVE, Ill., (March 5, 2015) The American Society for Gastrointestinal
ASGE and AGA Issue Consensus Statement on Surveillance and Management of Dysplasia in Patients With Inflammatory Bowel Disease DOWNERS GROVE, Ill., (March 5, 2015) The American Society for Gastrointestinal
Quality in Endoscopy: Can We Do Better?
 Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease
 GASTROENTEROLOGY 2010;138:746 774 AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease AGA Podcast interview: www.gastro.org/gastropodcast. Learning
GASTROENTEROLOGY 2010;138:746 774 AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease AGA Podcast interview: www.gastro.org/gastropodcast. Learning
Risk for Colorectal Neoplasia in Patients With Colonic Crohn s Disease and Concomitant Primary Sclerosing Cholangitis
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:303 308 Risk for Colorectal Neoplasia in Patients With Colonic Crohn s Disease and Concomitant Primary Sclerosing Cholangitis BARBARA BRADEN,* JOHNNY HALLIDAY,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:303 308 Risk for Colorectal Neoplasia in Patients With Colonic Crohn s Disease and Concomitant Primary Sclerosing Cholangitis BARBARA BRADEN,* JOHNNY HALLIDAY,*
Latest Endoscopic Guidelines for FAP, HNPCC, IBD, and the General Population
 Latest Endoscopic Guidelines for FAP, HNPCC, IBD, and the General Population David T. Rubin, M.D. Assistant Professor of Medicine Inflammatory Bowel Disease Center MacLean Center for Clinical Medical Ethics
Latest Endoscopic Guidelines for FAP, HNPCC, IBD, and the General Population David T. Rubin, M.D. Assistant Professor of Medicine Inflammatory Bowel Disease Center MacLean Center for Clinical Medical Ethics
Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps
 Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps Full guideline Draft for consultation, May 00 0 This guideline
Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn s disease and polyps Full guideline Draft for consultation, May 00 0 This guideline
WEO CRC SC Meeting. Barcelona, Spain October 23, 2015
 WEO CRC SC Meeting Barcelona, Spain October 23, 2015 Identification of serrated polyposis syndrome in the context of population-based CRC screening programs Evelien Dekker Academic Medical Center Amsterdam,
WEO CRC SC Meeting Barcelona, Spain October 23, 2015 Identification of serrated polyposis syndrome in the context of population-based CRC screening programs Evelien Dekker Academic Medical Center Amsterdam,
Advances in Ulcerative Colitis - Volume 3 CME
 1 de 12 22/01/2008 04:46 p.m. More: Advances in Ulcerative Colitis Advances in Ulcerative Colitis - Volume 3 CME Complete author affiliations and disclosures are at the end of this activity. Release Date:
1 de 12 22/01/2008 04:46 p.m. More: Advances in Ulcerative Colitis Advances in Ulcerative Colitis - Volume 3 CME Complete author affiliations and disclosures are at the end of this activity. Release Date:
NIH Public Access Author Manuscript Inflamm Bowel Dis. Author manuscript; available in PMC 2013 December 01.
 NIH Public Access Author Manuscript Published in final edited form as: Inflamm Bowel Dis. 2012 December ; 18(12): 2240 2246. doi:10.1002/ibd.22912. Prospective Study of the Progression of Low-Grade Dysplasia
NIH Public Access Author Manuscript Published in final edited form as: Inflamm Bowel Dis. 2012 December ; 18(12): 2240 2246. doi:10.1002/ibd.22912. Prospective Study of the Progression of Low-Grade Dysplasia
Endoscopy in IBD. F.Hartmann K.Kasper-Kliniken (St.Marienkrankenhaus) Frankfurt/M.
 F.Hartmann K.Kasper-Kliniken (St.Marienkrankenhaus) Frankfurt/M. F.Hartmann@em.uni-frankfurt.de Indications for endoscopy Diagnosis Management Surveillance Diagnosis Single most valuable tool: ileocolonoscopy
F.Hartmann K.Kasper-Kliniken (St.Marienkrankenhaus) Frankfurt/M. F.Hartmann@em.uni-frankfurt.de Indications for endoscopy Diagnosis Management Surveillance Diagnosis Single most valuable tool: ileocolonoscopy
BENEFIT APPLICATION BLUE CARD/NATIONAL ACCOUNT ISSUES
 Medical Policy BCBSA Ref. Policy: 2.01.84 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/Computed
Medical Policy BCBSA Ref. Policy: 2.01.84 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/Computed
PATIENTS WITH LONG-STANDING inflammatory
 Digestive Endoscopy 2016; 28: 266 273 doi: 10.1111/den.12634 Review Paradigm Shift in the Surveillance and Management of Dysplasia in Inflammatory Bowel Disease (West) Roy Soetikno, 1 Tonya Kaltenbach,
Digestive Endoscopy 2016; 28: 266 273 doi: 10.1111/den.12634 Review Paradigm Shift in the Surveillance and Management of Dysplasia in Inflammatory Bowel Disease (West) Roy Soetikno, 1 Tonya Kaltenbach,
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
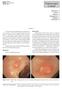 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
There is a well-established association between inflammatory
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:1346 1350 Aminosalicylate Therapy in the Prevention of Dysplasia and Colorectal Cancer in Ulcerative Colitis DAVID T. RUBIN, ANDELKA LOSAVIO, NICOLE YADRON,
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Value of sigmoidoscopy and biopsy in detection of
 Gut, 1979, 20, 575-580 Value of sigmoidoscopy and biopsy in detection of carcinoma and premalignant change in ulcerative colitis R. H. RIDDELL' AND B. C. MORSON From the Department ofpathology, St. Marks
Gut, 1979, 20, 575-580 Value of sigmoidoscopy and biopsy in detection of carcinoma and premalignant change in ulcerative colitis R. H. RIDDELL' AND B. C. MORSON From the Department ofpathology, St. Marks
what is the alternative mechanism of histogenesis? Aspects of the morphology of the adenomacarcinoma Morphology of the
 Refer to: Morson B: Polyps and cancer of the large bowel. West J Med 125:93-99, Aug 1976 THE WESTERN Journal of Miedicine Polyps and Cancer of the Large Bowel BASIL MORSON, MD, London MORTALITY STATISTICS
Refer to: Morson B: Polyps and cancer of the large bowel. West J Med 125:93-99, Aug 1976 THE WESTERN Journal of Miedicine Polyps and Cancer of the Large Bowel BASIL MORSON, MD, London MORTALITY STATISTICS
Colon Polyps: Detection, Inspection and Characteristics
 Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
IN THE DEVELOPMENT and progression of colorectal
 Digestive Endoscopy 2014; 26 (Suppl. 2): 73 77 doi: 10.1111/den.12276 Treatment strategy of diminutive colorectal polyp
Digestive Endoscopy 2014; 26 (Suppl. 2): 73 77 doi: 10.1111/den.12276 Treatment strategy of diminutive colorectal polyp
Management of pt1 polyps. Maria Pellise
 Management of pt1 polyps Maria Pellise Early colorectal cancer Malignant polyp Screening programmes SM Invasive adenocar cinoma Advances in diagnostic & therapeutic endoscopy pt1 polyps 0.75 5.6% of large-bowel
Management of pt1 polyps Maria Pellise Early colorectal cancer Malignant polyp Screening programmes SM Invasive adenocar cinoma Advances in diagnostic & therapeutic endoscopy pt1 polyps 0.75 5.6% of large-bowel
LIST OF ABBREVIATIONS
 Gastroenter oenterology 2005 Royal College of Physicians of Edinburgh Screening and surveillance for upper and lower gastrointestinal cancer JN Plevris Consultant Gastroenterologist and Honorary Senior
Gastroenter oenterology 2005 Royal College of Physicians of Edinburgh Screening and surveillance for upper and lower gastrointestinal cancer JN Plevris Consultant Gastroenterologist and Honorary Senior
Random biopsy is recommended in the US to detect
 Gastroenterology in Motion Ralf Kiesslich and Thomas D. Wang, Section Editors The Detection of Nonpolypoid (Flat and Depressed) Colorectal Neoplasms in Patients With Inflammatory Bowel Disease ROY SOETIKNO,
Gastroenterology in Motion Ralf Kiesslich and Thomas D. Wang, Section Editors The Detection of Nonpolypoid (Flat and Depressed) Colorectal Neoplasms in Patients With Inflammatory Bowel Disease ROY SOETIKNO,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer
 General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
An Atlas of the Nonpolypoid Colorectal Neoplasms in Inflammatory Bowel Disease
 An Atlas of the Nonpolypoid Colorectal Neoplasms in Inflammatory Bowel Disease Roy Soetikno, MD a, *, Silvia Sanduleanu, MD, PhD b, Tonya Kaltenbach, MD a KEYWORDS Inflammatory bowel disease Nonpolypoid
An Atlas of the Nonpolypoid Colorectal Neoplasms in Inflammatory Bowel Disease Roy Soetikno, MD a, *, Silvia Sanduleanu, MD, PhD b, Tonya Kaltenbach, MD a KEYWORDS Inflammatory bowel disease Nonpolypoid
Romanian Journal of Morphology and Embryology 2006, 47(3):
 Romanian Journal of Morphology and Embryology 26, 7(3):239 23 ORIGINAL PAPER Predictive parameters for advanced neoplastic adenomas and colorectal cancer in patients with colonic polyps a study in a tertiary
Romanian Journal of Morphology and Embryology 26, 7(3):239 23 ORIGINAL PAPER Predictive parameters for advanced neoplastic adenomas and colorectal cancer in patients with colonic polyps a study in a tertiary
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
Filiform polyposis of ulcerative colitis
 Filiform polyposis of ulcerative colitis Authors: Keisuke Yamada, Hironori Samura, Tatsuya Kinjo, Tetsu Kinjo, Akira Hokama, Jiro Fujita Article type: Clinical image Received: December 7, 2018. Accepted:
Filiform polyposis of ulcerative colitis Authors: Keisuke Yamada, Hironori Samura, Tatsuya Kinjo, Tetsu Kinjo, Akira Hokama, Jiro Fujita Article type: Clinical image Received: December 7, 2018. Accepted:
Inflammatory bowel disease (IBD), which includes ulcerative
 Chromoendoscopy and Dysplasia Surveillance in Inflammatory Bowel Disease: Past, Present, and Future Steven Naymagon, MD, and Thomas A. Ullman, MD Dr Naymagon is an assistant professor of medicine and Dr
Chromoendoscopy and Dysplasia Surveillance in Inflammatory Bowel Disease: Past, Present, and Future Steven Naymagon, MD, and Thomas A. Ullman, MD Dr Naymagon is an assistant professor of medicine and Dr
Colonic Polyp. Najmeh Aletaha. MD
 Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Colon Cancer Screening with Image-Enhanced Endoscopy
 FOCUSED REVIEW SERIES: Endoscopic Screening and Surveillance for Gastrointestinal Cancer Clin Endosc 2014;47:504-508 Print ISSN 2234-2400 / On-line ISSN 2234-2443 http://dx.doi.org/10.5946/ce.2014.47.6.504
FOCUSED REVIEW SERIES: Endoscopic Screening and Surveillance for Gastrointestinal Cancer Clin Endosc 2014;47:504-508 Print ISSN 2234-2400 / On-line ISSN 2234-2443 http://dx.doi.org/10.5946/ce.2014.47.6.504
Experience and challenges of implementing optical diagnosis into clinical practice UK and European Perspective
 Experience and challenges of implementing optical diagnosis into clinical practice UK and European Perspective WEO Image Enhanced Endoscopy San Diego, USA Dr James East Consultant Gastroenterologist Honorary
Experience and challenges of implementing optical diagnosis into clinical practice UK and European Perspective WEO Image Enhanced Endoscopy San Diego, USA Dr James East Consultant Gastroenterologist Honorary
Polypectomy and Local Resections of the Colorectum Structured Pathology Reporting Proforma
 Polypectomy and Local Resections of the Colorectum Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth
Polypectomy and Local Resections of the Colorectum Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth
Post-polypectomy follow-up after. removal of colorectal neoplasia
 Post-polypectomy follow-up after removal of colorectal neoplasia Post-polypectomy endoscopic surveillance For each type of polyp BENEFIT 1. What is the risk of CRC/Adv. Neo. (AN) w/out surveillance?
Post-polypectomy follow-up after removal of colorectal neoplasia Post-polypectomy endoscopic surveillance For each type of polyp BENEFIT 1. What is the risk of CRC/Adv. Neo. (AN) w/out surveillance?
It is well established that patients with long-standing. Screening and Surveillance Colonoscopy in Chronic Crohn s Colitis. Materials and Methods
 GASTROENTEROLOGY 2001;120:820 826 Screening and Surveillance Colonoscopy in Chronic Crohn s Colitis SONIA FRIEDMAN,* PETER H. RUBIN, CAROL BODIAN, ERIC GOLDSTEIN, NOAM HARPAZ, and DANIEL H. PRESENT *Division
GASTROENTEROLOGY 2001;120:820 826 Screening and Surveillance Colonoscopy in Chronic Crohn s Colitis SONIA FRIEDMAN,* PETER H. RUBIN, CAROL BODIAN, ERIC GOLDSTEIN, NOAM HARPAZ, and DANIEL H. PRESENT *Division
EXPERT WORKING GROUP Surveillance after neoplasia removal. Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum
 EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
Clinicopathological characteristics of colorectal
 Gut 1994; 35: 1419-1423 Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis 1419 St Mark's Hospital, London W R Connell N Harpaz N Britto K H Wilkinson M A Kamm
Gut 1994; 35: 1419-1423 Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis 1419 St Mark's Hospital, London W R Connell N Harpaz N Britto K H Wilkinson M A Kamm
Duodenal adenomas Management. Dr Stratis Alexandridis Consultant Gastroenterologist BRI
 Duodenal adenomas Management Dr Stratis Alexandridis Consultant Gastroenterologist BRI Introduction Ampullary and non ampullary polyps of the duodenum are diagnosed within and outside genetic syndromes.
Duodenal adenomas Management Dr Stratis Alexandridis Consultant Gastroenterologist BRI Introduction Ampullary and non ampullary polyps of the duodenum are diagnosed within and outside genetic syndromes.
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
05/07/2018. Organisation. The English screening programme what is happening? Organisation. Bowel cancer screening in the UK is:
 Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Novel Optical Research at UPMC
 Novel Optical Research at UPMC Randall Brand, MD Professor of Medicine Division of Gastroenterology, Hepatology, & Nutrition University of Pittsburgh Medical Center Background Patients with UC and CD of
Novel Optical Research at UPMC Randall Brand, MD Professor of Medicine Division of Gastroenterology, Hepatology, & Nutrition University of Pittsburgh Medical Center Background Patients with UC and CD of
Description. Section: Medicine Effective Date: July 15, Subsection: Original Policy Date: September 13, 2012 Subject: Page: 1 of 17
 Page: 1 of 17 Last Review Status/Date: June 2016 Description Chromoendoscopy refers to the application of dyes or stains during endoscopy to enhance tissue differentiation or characterization. When used
Page: 1 of 17 Last Review Status/Date: June 2016 Description Chromoendoscopy refers to the application of dyes or stains during endoscopy to enhance tissue differentiation or characterization. When used
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
MINI-REVIEW. Should we Sound the Alarm? Dysplasia and Colitis-associated Colorectal Cancer. Lin-Lin Ren, Jing-Yuan Fang * Abstract.
 Should we Sound the Alarm?- Dysplasia and Colitis-associated Colorectal Cancer MINI-REVIEW Should we Sound the Alarm? Dysplasia and Colitis-associated Colorectal Cancer Lin-Lin Ren, Jing-Yuan Fang * Abstract
Should we Sound the Alarm?- Dysplasia and Colitis-associated Colorectal Cancer MINI-REVIEW Should we Sound the Alarm? Dysplasia and Colitis-associated Colorectal Cancer Lin-Lin Ren, Jing-Yuan Fang * Abstract
Presence of pseudopolyps in ulcerative colitis is associated with a higher risk for treatment escalation
 ORIGINAL ARTICLE Annals of Gastroenterology (2019) 32, 1-6 Presence of pseudopolyps in ulcerative colitis is associated with a higher risk for treatment escalation Dimitrios S. Politis a, Konstantinos
ORIGINAL ARTICLE Annals of Gastroenterology (2019) 32, 1-6 Presence of pseudopolyps in ulcerative colitis is associated with a higher risk for treatment escalation Dimitrios S. Politis a, Konstantinos
Size of colorectal polyps determines time taken to remove them endoscopically
 Original article Size of colorectal polyps determines time taken to remove them endoscopically Authors Heechan Kang 1, Mo Hameed Thoufeeq 2 Institutions 1 Department of Medicine, Peterborough Hospitals
Original article Size of colorectal polyps determines time taken to remove them endoscopically Authors Heechan Kang 1, Mo Hameed Thoufeeq 2 Institutions 1 Department of Medicine, Peterborough Hospitals
Is there justification for levels of polyp competency? Dr Roland Valori Gloucestershire Hospitals United Kingdom
 Is there justification for levels of polyp competency? Dr Roland Valori Gloucestershire Hospitals United Kingdom What exactly will be required? Defining levels of polypectomy competency in terms of complexity/time/risk
Is there justification for levels of polyp competency? Dr Roland Valori Gloucestershire Hospitals United Kingdom What exactly will be required? Defining levels of polypectomy competency in terms of complexity/time/risk
Predict, Resect and discard : Yes we can! (at least in some hands)
 Diminutive polyps : Real time endoscopic histology Predict, Resect and discard : Yes we can! (at least in some hands) Robert Benamouzig Hôpital Avicenne AP-HP & Paris 13 University France Why it is important?
Diminutive polyps : Real time endoscopic histology Predict, Resect and discard : Yes we can! (at least in some hands) Robert Benamouzig Hôpital Avicenne AP-HP & Paris 13 University France Why it is important?
Advances in Endoscopic Imaging
 Advances in Endoscopic Imaging SGNA meeting February 20, 2010 Amar R. Deshpande, MD Asst Professor of Medicine Division of Gastroenterology University of Miami Miller School of Medicine Objectives To recognize
Advances in Endoscopic Imaging SGNA meeting February 20, 2010 Amar R. Deshpande, MD Asst Professor of Medicine Division of Gastroenterology University of Miami Miller School of Medicine Objectives To recognize
Intestinal cancer in inflammatory bowel disease: natural history and surveillance guidelines
 INVITED REVIEW Annals of Gastroenterology (2012) 25, 1-8 Intestinal cancer in inflammatory bowel disease: natural history and surveillance guidelines Vicent Hernández a, Juan Clofent b Complexo Hospitalario
INVITED REVIEW Annals of Gastroenterology (2012) 25, 1-8 Intestinal cancer in inflammatory bowel disease: natural history and surveillance guidelines Vicent Hernández a, Juan Clofent b Complexo Hospitalario
Polyp detection rates as quality indicator in clinical versus screening colonoscopy
 Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
Emerging Interventions in Endoscopy. Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital
 Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
Comparison of outcomes for patients with primary sclerosing cholangitis associated with ulcerative colitis and Crohn s disease
 Gastroenterology Report, 4(1), 2016, 43 49 doi: 10.1093/gastro/gou074 Advance Access Publication Date: 29 October 2014 Original article ORIGINAL ARTICLE Comparison of outcomes for patients with primary
Gastroenterology Report, 4(1), 2016, 43 49 doi: 10.1093/gastro/gou074 Advance Access Publication Date: 29 October 2014 Original article ORIGINAL ARTICLE Comparison of outcomes for patients with primary
Post-colonoscopy colorectal cancers in Lynch syndrome
 Post-colonoscopy colorectal cancers in Lynch syndrome Francesc Balaguer, MD PhD High Risk Colorectal Cancer Clinic Head of the Gastroenterology Department Hospital Clínic de Barcelona fprunes@clinic.cat
Post-colonoscopy colorectal cancers in Lynch syndrome Francesc Balaguer, MD PhD High Risk Colorectal Cancer Clinic Head of the Gastroenterology Department Hospital Clínic de Barcelona fprunes@clinic.cat
Gastric Polyps. Bible class
 Gastric Polyps Bible class 29.08.2018 Starting my training in gastroenterology, some decades ago, my first chief always told me that colonoscopy may seem technically more challenging but gastroscopy has
Gastric Polyps Bible class 29.08.2018 Starting my training in gastroenterology, some decades ago, my first chief always told me that colonoscopy may seem technically more challenging but gastroscopy has
Missed Lesions at Endoscopy. Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand
 Missed Lesions at Endoscopy Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand Missed Lesions at Endoscopy Is there a problem? With Gastroscopy
Missed Lesions at Endoscopy Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand Missed Lesions at Endoscopy Is there a problem? With Gastroscopy
Chromoendoscopy is an image-enhanced endoscopic technique
 The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia Anna M. Buchner, MD, PhD Dr Buchner is an assistant professor of medicine in the Division of Gastroenterology at the University of Pennsylvania
The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia Anna M. Buchner, MD, PhD Dr Buchner is an assistant professor of medicine in the Division of Gastroenterology at the University of Pennsylvania
Endoscopic techniques for surveillance and treatment of FAP
 Endoscopic techniques for surveillance and treatment of FAP Evelien Dekker MD PhD Department of Gastroenterology & Hepatology Academic Medical Center Amsterdam The Netherlands FAP: endoscopic surveillance
Endoscopic techniques for surveillance and treatment of FAP Evelien Dekker MD PhD Department of Gastroenterology & Hepatology Academic Medical Center Amsterdam The Netherlands FAP: endoscopic surveillance
