The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 17 October 2012
|
|
|
- Shanon Melton
- 5 years ago
- Views:
Transcription
1 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 17 October 2012 Examination of the dossier for proprietary medicinal products included for a 5-year period starting on 25 July 2006 (Official Gazette of 23 December 2008) OPTRUMA 60 mg, film-coated tablet B/28 (CIP code: ) B/84 (CIP code: ) Applicant: PIERRE FABRE MEDICAMENT Raloxifene hydrochloride ATC code: G03XC01 (SERM, antiosteoporotic) List I Date of Marketing Authorisation: 5 October 1998, amended on 11 August 2008 (centralised procedure) Reason for request: Renewal of inclusion on the list of medicines refundable by National Health Insurance. Medical, Economic and Public Health Assessment Division 1/15
2 1 CHARACTERISTICS OF THE MEDICINAL PRODUCT 1.1. Active ingredient Raloxifene hydrochloride 1.2. Indications OPTRUMA is indicated for the treatment and prevention of osteoporosis in postmenopausal women. A significant reduction in the incidence of vertebral, but not hip fractures has been demonstrated. When determining the choice of OPTRUMA or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits Dosage The recommended dose is one tablet daily by oral administration, which may be taken at any time of the day without regard to meals. No dose adjustment is necessary for elderly women. Due to the nature of this disease process, OPTRUMA is intended for long term use. Generally calcium and vitamin D supplements are advised in women with a low dietary intake. Use in renal impairment: OPTRUMA should not be used in patients with severe renal impairment. In patients with moderate and mild renal impairment, OPTRUMA should be used with caution. Use in hepatic impairment: OPTRUMA should not be used in patients with hepatic impairment Contraindications - Hypersensitivity to the active substance or to any of the excipients. - Must not be used in women with child bearing potential. - Active or past history of venous thromboembolic events, including deep vein thrombosis, Hepatic impairment including cholestasis. - Severe renal impairment. - Unexplained uterine bleeding. - OPTRUMA should not be used in patients with signs or symptoms of endometrial cancer as safety in this patient group has not been adequately studied. 2/15
3 2 REMINDER OF THE COMMITTEE S OPINION AND CONDITIONS OF INCLUSION The opinion concerning the inclusion of OPTRUMA in 2001 referred to the opinions concerning the inclusion of EVISTA, hence these opinions are cited in the history of the assessment of OPTRUMA by the Transparency Committee. Opinion of the Committee of 10 May 2000 Inclusion of EVISTA In menopausal women, as the primary aim is overall management of menopause, EVISTA does not provide an improvement in actual benefit compared with hormone replacement therapy. During this time of life, prevention of osteoporosis by raloxifene is recommended in cases where there are a high risk of vertebral fractures and certain contraindications for HRT. The principal contraindications for EVISTA, breast cancer and thromboembolic events, are comparable to those for HRT. In light of current data, the role of EVISTA during menopause is therefore limited. In women who are well past the age of menopause, who are not receiving HRT, prevention of fractures is important and, in this context, EVISTA, as an alternative to hormone replacement therapy, represents a level III improvement in actual benefit in the prevention of vertebral fractures. The Committee requests that the company establish monitoring of the prescription and use of EVISTA. Opinion of the Committee of 24 January Modification of the wording of indications The indication: prevention of non-traumatic vertebral fractures in postmenopausal women at increased risk of osteoporosis has been replaced by the following wording: treatment and prevention of osteoporosis in postmenopausal women. A decrease in vertebral fractures, but not in femoral neck fractures, has been demonstrated. Opinion of the Committee of 4 April 2001 Inclusion of OPTRUMA The actual benefit of OPTRUMA is substantial. Refer to the opinion concerning EVISTA Opinion of the Committee of 1 February Inclusion of B/84 The Transparency Committee recommends inclusion in the list of medicines refundable by National Health Insurance of B/84 in addition to the B/28 packaging. Opinion of the Committee of 5 July Renewal and amendment of the scope of reimbursable indications The Transparency Committee recommends continued inclusion on the list of medicines refundable by National Health Insurance: Scope of reimbursement Treatment of postmenopausal osteoporosis to reduce the risk of vertebral fractures: - in patients with a fracture caused by bone fragility, - in the absence of a fracture, in women with substantially decreased bone density (T-score < -3) or with a T-score -2.5 associated with other risk factors for fractures, in particular, age > 60 years, current or past systemic corticosteroid therapy at a dosage 7.5 mg/day prednisone equivalent, a body mass index < 19 kg/m², a history of femoral neck extremity fracture in a first-degree relative (mother), early menopause (before the age of 40 years). 3/15
4 3 SIMILAR MEDICINAL PRODUCTS 3.1. ATC Classification (2011) G G03 G03X G03XC G03XC01 : Genitourinary system and sex hormones : Sex hormones and modulators of the genital system : Other sex hormones and modulators of the genital system : Selective estrogen receptor modulators : Raloxifene 3.2. Medicines in the same therapeutic category Another proprietary medicinal product containing raloxifene, with the same indications: EVISTA 60 mg is co-marketed with OPTRUMA. NB: Another proprietary medicinal product belonging to the class of selective estrogen receptor modulators (SERM): CONBRIZA (bazedoxifène) is not rembursable. These medicinal products have only demonstrated efficacy against vertebral fractures Medicines with a similar therapeutic aim Medicines which have demonstrated their efficacy in the prevention of vertebral and peripheral fractures, including femoral neck fractures: - the following bisphosphonates with a significant AB: o ACLASTA 5 mg (zoledronic acid), once yearly intravenous perfusion, o ACTONEL (risedronic acid) 5 mg tablet (every day), 35 mg tablet (every week), 75 mg tablet (2 consecutive days per month), ACTONELCOMBI tablet (risedronate 35 mg + calcium 1000 mg + vitamin D 880 IU) (every week), o FOSAMAX (alendronic acid) 10 mg tablet (every day), 70 mg tablet (every week) and the other products containing alendronic acid 10 mg and 70 mg, FOSAVANCE and ADROVANCE (alendronate or alendronate + vitamin D combination) tablet every week. - PROTELOS (strontium ranelate) granules for oral suspension (daily), due to concern raised by the risk of occurrence of serious hypersensitivity reactions of DRESS type and venous thromboembolic events, the AB of PROTELOS is moderate in the population limited to: o o patients with a contraindication to or intolerance to bisphosphonates, patients with no risk factors for venous thromboembolic events, including in particular history of a venous thromboembolic event, age over 80 years, immobility (opinion of 11 May 2011). - PROLIA (denosumab) subcutaneous injection once every six months, vertebral and non-vertebral efficacy including femoral neck (significant AB, IAB level IV as second-line treatment following bisphosphonates) 4/15
5 Other medicines indicated in the treatment of postmenopausal osteoporosis but which do not have demonstrated efficacy in the prevention of femoral neck fractures - FORSTEO (teriparatide), subcutaneous injection every day, refundable in cases of severe osteoporosis (at least two vertebral fractures), efficacy demonstrated in peripheral fractures but not those of the hip. Note: The Committee points out the AB of the following bisphosphonates was judged to be insufficient in December 2010: - DIDRONEL 400 mg tablet (etidronic acid), 1 tablet/14 days/3 months - BONVIVA 150 mg/1 month tablet and 3 mg/3 months solution for injection (ibandronic acid) Hormonal treatments for menopause are indicated in the prevention of osteoporosis if there are also menopausal problems or if other treatments are contraindicated or not tolerated. They are the subject of a re-assessment by the Transparency Committee. Calcium and vitamin D are generally used for adjuvant treatment. 5/15
6 4 UPDATE OF AVAILABLE DATA 4.1. Efficacy Only those studies which were conducted within the indications and at the dosage of the MA and took place after the previous opinion of the Transparency Committee issued on 5 December 2007 have been considered: - a comparative study versus alendronate concerning fractures - two studies on BMD or bone quality, - a combined analysis of mortality in studies that had already been evaluated by the Transparency Committee. Anti-fracture effect of raloxifene versus alendronate 1 The aim of this study was to demonstrate the non-inferiority of raloxifene (RLX, 60 mg per day) compared to alendronate (10 mg per day) with respect to reducing the incidence of osteoporotic fractures (vertebral or non-vertebral) in patients with postmenopausal osteoporosis, no history of vertebral fracture and no previous treatment for osteoporosis. The protocol planned the enrolment of 1750 patients per group in order to attain a power of 90% and to establish non-inferiority with a margin set at 30%. Due to difficulties in recruiting patients who had not received any previous treatment, the study was terminated prematurely. The mean duration of treatment was 312 days and the analysis was performed on 1412 out of 1423 randomised patients. At the time the study was terminated, 22/713 (3.1%) of patients in the alendronate group versus 20/699 (2.9%) of patients in the RLX group had experienced a new fracture. In the absence of statistical power, it is not possible to reach a conclusion concerning the non-inferiority of raloxifene versus alendronate. Densitometric effect of raloxifene versus placebo after treatment with parathormone (PTH) 2 In this study, 380 patients aged from 50 to 80 years with postmenopausal osteoporosis were treated for 1 year with teriparatide 20 µg per day. At the end of this year of treatment, 329 of these 380 patients were randomised to a year of treatment with RLX 60 mg/day (n = 157) or placebo (n = 172). At the end of this second year of treatment, 300 of the 380 patients received 60 mg RLX in an open-label phase. The primary objective was to evaluate the percentage variation of lumbar BMD between RLX and placebo during the course of the second year of the study. During the second year, a decrease in lumbar BMD was observed in both groups, compared to the increase in BMD observed during the first year of treatment with teriparatide. This decrease was however smaller in the RLX group than in the placebo group: -1 +/- 0.3% with RLX and -4 +/- 0.3% with placebo (p < 0.001). Effect on bone microstructure 3 The company also cited the computed tomography results in 15 patients treated with RLX for 15 months, which showed an improvement in bone microstructure parameters. 1 Recker et al. Comparative effects of raloxifene and alendronate on fracture outcomes in postmenopausal women with low bone mass. Bone. 2007; 40(4): Adami et al. Effect of raloxifene after recombinant teriparatide [hpth (1-34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int Jan; 19 (1): Radspieler H. Individualized treatment of osteoporosis with medication: preventing fractures by increasing bone mineralization and quality. Int. J. Clin. Rheumatol 2010; 5: 4. 6/15
7 Indirect comparison: The company submitted a publication 4 comparing the decreases in absolute risks of fractures and the number needed to treat (NNT) in order to avoid a fracture for various antiosteoporosis medicines. This was derived from the phase III studies which had formed the basis for the respective MAs. The results of these comparisons, which were performed without the use of validated statistical methods for indirect comparisons, cannot be taken into consideration. Effect of RLX on BMD after treatment with alendronate 5,6 A total of 99 patients with postmenopausal osteoporosis were treated with alendronate 10 mg per day for a mean period of 43 months and were then randomised into three groups to receive placebo, RLX 60 mg/day or to continue with alendronate on an open-label basis for 1 year. The primary endpoints were BMD and bone markers. The patients were not uniform with respect to the numbers having non-vertebral fractures at baseline: 9 in the alendronate group (27.3%), 18 in the placebo group (54.5%) and 16 in the raloxifene group (48.5%). Compared with placebo, alendronate and raloxifene had prevented lumbar bone loss after 1 year (-0.75% with RLX, -0.54% with alendronate and -2.66% with placebo, p < 0.05). The significance of the results of this study is limited (low numbers, intermediate primary endpoint) Safety Pharmacovigilance data (PSUR): As a reminder, the PSUR is common for RLX (EVISTA and the co-marketed product OPTRUMA). Raloxifene obtained authorisation for the first time on 9 December 1997 (USA). It has MAs in 102 countries and is marketed in 71 countries. Outside the European Union, raloxifene is also prescribed to reduce the risk of invasive breast cancer (MA obtained in the USA for this extension of indication on 13 September 2007). The pharmacovigilance data reported for OPTRUM are for the period from December 2005 to November During this period, the following serious adverse effects were reported in France with respect to OPTRUMA: - venous and arterial thromboembolic events (21 cases) Of these 21 cases: o 9 cases of pulmonary embolism, of which 4 resulted from misuse: 2 patients with a personal history of pulmonary embolism/phlebitis, which is a contraindication for the use of RLX, and 2 with a family history, who therefore should not have been treated with RLX. In 6/9 cases, a delayed occurrence of the pulmonary embolism was reported; it occurred within 3 to 6 months after the start of treatment. o 6 cases of deep vein thrombosis/phlebitis occurred. In addition, 2 patients reported deep vein thrombosis/phlebitis associated with pulmonary embolism. o 4 cases of retinal venous thrombosis were reported in patients with risk factors (hypertension, hypercholesterolaemia, concomitant treatment with MTX). o 2 cases of ischaemic stroke and one case of haemorrhagic stroke were reported. The causal relationship with OPTRUMA was judged to be doubtful. This adverse effect was not mentioned in the OPTRUMA SPC. 4 Ringe et al. Absolute risk reduction in osteoporosis: assessing treatment efficacy by number needed to treat. Rheumatol Int. 2010; 30: This study preceded the previous opinion of the Committee concerning EVISTA but will be described in the opinion for OPTRUMA. 6 Michalska D, Stepan J.J, Basson R, Pavo I. The Effect of Raloxifene after Discontinuation of Long- Term Alendronate Treatment of Postmenopausal Osteoporosis, J Clin Endocrinol Metab 2006; 91: /15
8 - cancer: 13 cases of cancer were reported, of which 11 affected the reproductive system (5 breast, 3 uterine, 2 endometrial and 1 ovarian). The causality of these reproductive system cancers, which are not mentioned in the OPTRUMA SPC, was considered to be doubtful in 6/11 cases and could not be evaluated due to insufficient information in 5/11 cases. Other reported cancers, for which the causal relationship with OPTRUMA was judged to be doubtful were: one meningioma and one basocellular carcinoma of the eyelid. - three serious eye disorders were reported during this period, these being two cases of macular degeneration and one of intraocular hypertension, with causality being judged doubtful. Amendments were introduced into the SPC on 12 July 2006, 27 March 2007, 19 November 2007 and 11 August 2008, particularly with respect to the addition of new adverse effects: - thrombocytopenia (very rare <0.01%); - peripheral oedema (rare <0.1%); - venous thromboembolic reaction (rare <0.1%); - arterial thromboembolic reaction (very rare <0.01%). A warning concerning the prescription of OPTRUMA to women with a history of stroke or significant risk factors was added: In a study 7 of postmenopausal women with documented coronary heart disease or at increased risk for coronary events, raloxifene did not affect the incidence of myocardial infarction, hospitalized acute coronary syndrome, overall mortality, including overall cardiovascular mortality, or stroke, compared to placebo. However, there was an increase in death due to stroke in women assigned to raloxifene. The incidence of stroke mortality was 1.5 per 1000 women per year for placebo versus 2.2 per 1000 women per year for raloxifene. This finding should be considered when prescribing raloxifene for postmenopausal women with a history of stroke or other significant stroke risk factors, such as transient ischaemic attack or atrial fibrillation. In addition, other safety information for RLX obtained from the RUTH study was added to the SPC: - update of the incidence of hot flushes, venous thromboembolic events, leg cramps, peripheral oedema: Across all placebo-controlled clinical trials of raloxifene in osteoporosis, venous thromboembolic events, including deep vein thrombosis, pulmonary embolism, and retinal vein thrombosis occurred at a frequency of approximately 0.8% or 3.22 cases per 1000 patient-years. A relative risk of 1.60 (CI: ) was observed in patients treated with EVISTA compared to placebo. The risk of a thromboembolic event was greatest in the first four months of therapy. Superficial vein thrombophlebitis occurred in a frequency of less than 1%. In the RUTH study, venous thromboembolic events occurred at a frequency of approximately 2.0% or 3.88 cases per 1000 patient-years in the raloxifene group and 1.4% or 2.70 cases per 1000 patient-years in the placebo group. The hazard ratio for all VTE events in the RUTH study was HR = 1.44 ( ). Superficial vein thrombophlebitis occurred in a frequency of 1% in the raloxifene group and 0.6% in the placebo group. In the RUTH study, the occurrence of vasodilatation (hot flushes) was 7.8% in the raloxifene-treated patients and 4.7% in the placebo-treated patients. In the RUTH study, leg cramps were observed in 12.1% of raloxifene-treated patients and 8.3% of placebo-treated patients. 7 This is the RUTH study included in the combined analysis of the effect of RLX on mortality. 8/15
9 In the RUTH study, peripheral oedema occurred in 14.1% of the raloxifene-treated patients and 11.7% of the placebo-treated patients, which was statistically significant. - addition of the adverse effect cholelithiasis and additional specification of the rate of cholecystectomy. In the RUTH study, cholelithiasis was diagnosed in 3.3% of raloxifene-treated patients and 2.6% of placebo-treated patients. Cholecystectomy rates for raloxifene (2.3%) were not statistically significantly different from placebo (2.0%). Combined analysis of the effect of RLX on mortality 8 The company supplied the results of an analysis of the effect of RLX on all-cause mortality, based on the combined data of three studies (MORE, CORE and RUTH), which had already been reviewed by the Transparency Committee. To recapitulate, the MORE study, which lasted 4 years, evaluated the anti-fracture efficacy of RLX versus placebo in more than 7700 patients and was followed by the CORE study, which lasted 4 years. The RUTH study was conducted in more than 10,000 postmenopausal women with known coronary disease or an increased risk of coronary events. In this study, no change in the incidence of myocardial infarction, hospital admissions for acute coronary syndrome or stroke, nor in overall mortality, including mortality of cardiovascular origin, was observed in the group treated with RLX. However, an increase in the incidence of mortality due to stroke was observed in patients treated with RLX (1.5 per 1000 woman-years for placebo versus 2.2 per 1000 woman-years for RLX). In the MORE and CORE studies, mortality was not evaluated as a primary endpoint, which limits the level of evidence of the results of these studies for this endpoint. The combined analysis of these three studies shows a decrease in overall mortality in favour of RLX (7.88%) compared to placebo (8.65%), p=0.05. There is no statistical difference for deaths of cardiovascular origin (4.93% with placebo vs. 4.97% with RLX). The reduction in all-cause mortality is essentially due to the reduction in non-cardiovascular and non-cancerrelated mortality, in particular mortality of infectious origin. (28 deaths with RLX vs. 51 with placebo, p = 0.01). This decrease remains unexplained. Conclusion concerning safety data: The updated safety data for OPTRUMA, which are based on the study results available and on pharmacovigilance data, show that there is a risk of fatal stroke (which was not included in the SPC during the last opinion of the Committee on the renewed inclusion of OPTRUMA). Otherwise, the safety profile is unchanged (vasomotor symptoms more frequent than in the placebo group in the RUTH study, venous thromboembolic events, leg cramps). 8 Grady et al. Effect of Raloxifene on all-cause mortality. Am J Med 2010; 123: /15
10 5 USAGE DATA FOR THE MEDICINE IMS DOREMA data According to IMS-EPPM data (mobile annual total to February 2012), a total of 132,000 prescriptions were issued for this proprietary medicinal product. The mean daily dosage was 1 tablet and the mean duration of prescription was 142 days. AGIR monitoring An observational study in community practices (GPs, gynaecologists and rheumatologists) has been set up by Daiichi Sankyo France and is currently under way in France (4 th quarter 2010 to 3 rd quarter 2011). The inclusion phase for patients took place between 4 March 2011 and 31 July The protocol planned for the enrolment of 3000 osteoporotic female patients aged 50 years or more receiving a first prescription for osteoporosis medication or a change in such medication. The main objective of the study was to evaluate compliance with official instructions and guidelines concerning the management of postmenopausal osteoporosis in current community medical practice in France with respect to this disease. The secondary objectives are to describe and evaluate: - The population of women affected by postmenopausal osteoporosis - Compliance with medical treatment and dietary and lifestyle advice three months after the consultation - Fears and beliefs concerning osteoporosis and its management among patients and physicians in order to determine their impacts. Results of VISION post-marketing study In response to the request made by the Transparency Committee in its opinions of 10 May 2000 and 24 January 2001, Lilly (new local representative Daiichi Sankyo France) and Pierre Fabre conducted a joint observational follow-up study of the prescription and use of EVISTA and OPTRUMA from 2005 to A population of 2628 patients presenting with a prescription for raloxifene were included, by means of a random sample from 551 pharmacies. The baseline data were integrated into the opinion concerning renewal of inclusion of 5 July The additional data received subsequently correspond to observational data (over 12 months). Main results The mean age of the enrolled patients was 65 years, 98% of them stated they were postmenopausal and 4% were currently receiving hormonal treatment for menopause. In 30% of cases, the patients said they had experienced at least one fracture since menopause: vertebral fracture (13%), fracture of the wrist (12%), femoral neck fracture (2%). A diagnosis of osteoporosis had been established for 50% of patients prior to treatment with raloxifene and, of these, 62% stated that they had received anti-osteoporotic treatment previously. In total, 92% of patients had received prophylaxis or treatment for osteoporosis: non-fracture osteoporosis (36%), fracture osteoporosis (26%), prophylaxis of osteoporosis in postmenopausal women (28%). One or several risk factors for osteoporosis were found in 64% of patients: age 65 years (50%), family history of fractures (19%), history of fractures during minimal trauma (17%), age at menopause < 40 years (11%) and BMI < 19 kg/m 2 (9%). In 76% of cases, bone densitometry was performed before the start of raloxifene treatment and, in 51% of documented cases, osteoporosis was demonstrated (T-score -2.5). 9 9 The WHO has proposed a classification scale of four levels for decreased bone mass: Level 1: normal, bone mass bone mineral density (BMD) shows less than one standard deviation difference from the reference value (T-score greater than -1) Level 2: low bone mass (osteopenia) T-score between -1 and /15
11 In 40% of cases, a second treatment was co-prescribed on the same prescription (calcium-vitamin D in 85% of these cases). At 12 months follow-up, data was available for 1213 patients (i.e. 46.2% of the total of 2,628 enrolled patients). Patients lost to view at 12 months differed from patients not lost to view with respect to educational level (lower in those lost to view) and occurrence of a fracture after menopause (higher incidence in those lost to view). At 12 months, 82.0% of women about whom information was available stated that they were continuing their treatment. The main reasons for discontinuing treatment were the physician s decision (48.2% of cases of discontinuation) and adverse effects (29.2%). Of the 890 patients who had continued treatment, 92.9% forgot less than five doses during the previous four weeks. Forgetting was the main reason the patients gave for non-compliance. The mean duration of treatment was estimated using the Kaplan-Meier method and was 52 +/- 0.5 months, i.e. 4.3 years. However, the large quantity of censored data makes it impossible to estimate the median duration of treatment and its 95% confidence interval. The additional survey that was set up in order to evaluate the outcomes for patients lost to view showed that 64% of these continued with treatment. The rate for continuation of treatment at one year can therefore be extrapolated as being 73.3% for all participants. Conclusion The prescription of raloxifene in this study complied with the indications in the MA in 90% of cases. The ordinance of 10 July 2001, amending the list of medicines refundable by National Health Insurance and the list of medicines approved for use by hospitals and various public services indicated that OPTRUMA only qualifies for use and for reimbursement by National Health Insurance in the following therapeutic indication: "treatment of confirmed postmenopausal osteoporosis with at least one osteoporotic fracture. In this context, the results of this study showed that only 30% of the women had stated that they had experienced at least one fracture since menopause and thus complied with the scope of refundable indications of raloxifene, as defined in In 2006, the Transparency Committee, in its opinion of 5 July 2006 concerning the renewal of inclusion of OPTRUMA, wished to change the scope of the reimbursable indications to the following: Treatment of postmenopausal osteoporosis to reduce the risk of vertebral fractures: - in patients with a fracture caused by bone fragility and, - in the absence of fracture, in women with a substantial decrease bone density (T-score < -3) or with a T-score -2.5 associated with other risk factors for fractures, in particular: age > 60 years, current of past systemic corticosteroid therapy at a dosage 7.5 mg/day prednisone equivalent, a body mass index < 19 kg/m², a history of femoral neck extremity fracture in a first-degree relative (mother), early menopause (before the age of 40 years). (Official Gazette of 11 October 2006). In this study, the results as presented do not enable an analysis of all the criteria involved in the definition of this scope of reimbursement, which came into force during the course of the study. This study does however enable it to be documented that compliance with the criteria for the scope of reimbursement, as defined in 2006, appears to be infrequent. In fact, bone densitometry was only performed in 76% of cases and osteoporosis was present in 51% of cases (osteoporosis defined by a T-score less than or equal to -2.5). Level 3: osteoporosis, T-score below -2.5 Level 4: severe osteoporosis, the severity of the osteoporosis and the risk of fracture become greater the more the T-score falls below /15
12 Finally, due to the large number of patients lost to view, the follow-up of the patients does not enable a precise determination of the rate of treatment continuation nor of the therapeutic compliance with raloxifene. Among patients not lost to view, the rate of persistence with treatment at 12 months is 82%. Following an additional study, the rate among those lost to view was estimated at 64%. 12/15
13 6 TRANSPARENCY COMMITTEE CONCLUSIONS 6.1. Re-assessment of actual benefit Osteoporosis is a serious disorder because of the risk of fractures. In particular, fractures of the femoral neck can be life-threatening. OPTRUMA is a treatment for the prophylaxis of osteoporotic fractures and their recurrence. Its efficacy has been demonstrated solely in the prevention of vertebral fractures. Its efficacy/adverse effects ratio is high. This proprietary medicinal product is a first-line treatment as an alternative to bisphosphonates, but should be limited to patients with spinal osteoporosis and a low risk of femoral neck fractures, aged < 70 years, with no risk factors for venous thromboembolic events (lack of personal or family history of venous thromboembolic events) and who are receiving supplementation for calcium deficiency. Alternative medicinal products exist. The actual benefit provided by this proprietary medicinal product is substantial in the treatment and prevention of spinal osteoporosis, solely in patients at low risk of femoral neck fracture, aged less than 70 years and with no risk factors for venous thromboembolic events (lack of personal or family history of venous thromboembolic events) Therapeutic use Osteoporosis is defined by a T-score -2.5 in the absence of any other cause of an osteopathy characterized by demineralization or bone fragility. The purpose of treatment of osteoporosis is to prevent fractures. Prior to initiation of any anti-osteoporotic treatment, deficiencies in calcium and vitamin D should be investigated and corrected. The vitamin and calcium supplementation should be continued if required during the anti-osteoporotic treatment. Treatment of postmenopausal osteoporosis is routinely recommended in cases of osteoporosis complicated by fracture. In the absence of fractures in post-menopausal women, the indication for treatment should be considered on a case by case basis, depending on the individual risk of fracture. This risk is evaluated on the basis of the T-score value and the potential presence of other risk factors for fractures. Treatment must therefore be considered in women with: - a substantial reduction in bone mineral density (T-score < - 3) or - a T-score associated with other risk factors for fractures, in particular: age > 60 years, current or past systemic corticosteroid therapy at a dosage 7.5 mg/day prednisone equivalent, a body mass index < 19 kg/m², a history of femoral neck extremity fracture in a first-degree relative (mother), early menopause (before the age of 40 years). In the absence of a direct comparison between different anti-osteoporosis medicines (bisphosphonates, raloxifene, teriparatide and strontium ranelate), the choice of treatment will depend on the risk of vertebral and/or non-vertebral fractures, age, number and location of fractures, as well as on predisposing factors and any contraindications to any of these medicinal products, on product safety and on the potential duration of the treatment. If a fracture occurs after the first year of treatment and despite satisfactory compliance, the treatment should be reappraised. Another medicinal product may be suggested, including one from the same pharmacological class. 13/15
14 Therapeutic use of raloxifene Raloxifene is a selective oestrogen receptor modulator. It acts both as an agonist and/or as an antagonist of oestrogen receptors, depending on the cell and tissue type and the target genes. The available data suggest that raloxifene has an agonist activity on bone (decreasing bone resorption and lowering levels of biochemical markers) and on lipid metabolism, and an antagonistic activity on breast and endometrial tissue. Taking into account that its anti-fracture efficacy has solely been demonstrated with respect to vertebrae, it should be reserved for patients aged less than 70 years, with spinal osteoporosis, at low risk of femoral neck fractures and with no contraindications to its use, in particular venous thromboembolic events (family or personal history), severe renal impairment, hepatic impairment including cholestasis, or signs or symptoms of endometrial cancer. According to expert opinion, it is essential to have different therapeutic classes with different modes of action and pharmacological properties available for use in the management of osteoporosis, in order to be able to offer personalised treatment to each patient. The extra-osseous effect of raloxifene (in particular the reduction in risk of breast cancer) should be taken into account when prescribing Target population The target population for OPTRUMA consists of women with postmenopausal osteoporosis, who have had a fracture due to fragility and, where no fracture due to bone fragility is present, postmenopausal women with a substantially decreased bone density (T-score < -3) or a T-score of -2.5 associated with several risk factors for fracture and with a low risk of femoral neck fracture, aged less than 70 years and with no risk factors for venous thromboembolic events (absence of personal or family history of venous thromboembolic events). The population of postmenopausal women with osteoporosis may be estimated from the following data: - approximately 25% to 40% of women aged 65 years and 50% to 70% of women aged 80 years have osteoporosis i,ii - applied to the female population as updated for 1 January 2012, and taking into account the linear progression in prevalence between 65 years and 80 years. These data lead to an estimated population with postmenopausal osteoporosis consisting of between 2.5 and 3.6 million women. Pharmacological treatment with OPTRUMA is only justified in patients with spinal osteoporosis, at low risk of femoral neck fracture, aged less than 70 years and with no risk factors for venous thromboembolic events (absence of personal or family history of venous thromboembolic events). The available data, in particular the fact that bone densitometry has only recently become a reimbursable procedure in France, do not enable a precise estimate to be made of this subpopulation of patients. 14/15
15 6.4. Transparency Committee recommendations The Transparency Committee recommends continued inclusion on the list of medicines refundable by National Insurance within the scope of reimbursement described below Scope of reimbursement Prophylaxis and treatment of postmenopausal osteoporosis to reduce the risk of vertebral fractures in patients with spinal osteoporosis and a low risk of femoral neck fractures, aged < 70 years, with no risk factors for venous thromboembolic events (lack of personal or family history of thromboembolic events) and who are receiving supplementation for calcium deficiency Packaging: Not appropriate for the prescription conditions. The Committee points out that in accordance with its decisions on 20 July 2005, it recommends harmonisation of the size of packaging to 30 days for treatments lasting one month and consequently of the 90-day packaging for treatments lasting three months Reimbursement rate: 65% i Société Française de rhumatologie [French Rheumatology Society] Dossier Ostéoropose Pourquoi dit-on que l ostéoporose est un problème de santé publique? [Osteoporosis File Why is osteoporosis said to be a public health problem?] ( ii Ministère de la Santé, de la Famille et des Personnes handicapées [Ministry of Health, Families and Disabled People]. Direction générale de la santé [Directorate General for Health] Report of the Groupe Technique National de Définition des Objectifs de Santé Publique (GTNDO) [National Technical Objective Definition Group for Public Health] Analyse des connaissances disponibles sur des problems de santé sélectionnés, leurs determinants, et les strategies de santé publique. Définition d objectifs [Analysis of knowledge available on selected health problems, their influencing factors, and public health strategies: Definition of objectives.] March ( 15/15
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 September 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 September 2011 Review of the dossier for the proprietary medicinal product listed for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 September 2011 Review of the dossier for the proprietary medicinal product listed for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 5 January 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
Prevention of Osteoporotic Hip Fracture
 Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
PACKAGE LEAFLET: INFORMATION FOR THE USER. Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride)
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride) Please read this information carefully before you start to take your medicine.
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride) Please read this information carefully before you start to take your medicine.
Management of postmenopausal osteoporosis
 Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
The legally binding text is the original French version. Opinion 15 May 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Pathway from Fracture or Risk Factor to Treatment
 Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Issue date: October Review date: July 2010
 Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against
 This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 Multiple low trauma vertebral fractures in the absence of myeloma or metastatic disease. 2 T-score
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 Multiple low trauma vertebral fractures in the absence of myeloma or metastatic disease. 2 T-score
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 4 January 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 January 2012 Examination of the dossier for a medicinal product included for a 5-year period starting on 7 January
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 January 2012 Examination of the dossier for a medicinal product included for a 5-year period starting on 7 January
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
TRANSPARENCY COMMITTEE OPINION. 21 October 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
SERMS, Hormone Therapy and Calcitonin
 SERMS, Hormone Therapy and Calcitonin Tiffany Kim, MD Clinical Fellow VA Advanced Women s Health UCSF Endocrinology and Metabolism I have nothing to disclose Thanks to Clifford Rosen and Steven Cummings
SERMS, Hormone Therapy and Calcitonin Tiffany Kim, MD Clinical Fellow VA Advanced Women s Health UCSF Endocrinology and Metabolism I have nothing to disclose Thanks to Clifford Rosen and Steven Cummings
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Effective Health Care
 Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Fragile Bones and how to recognise them. Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey
 Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
HORMONE REPLACEMENT THERAPY
 TRIALS OF HR RUTH (Barrett- Connor et al 29 ) JULY 2006 (Country) mean ± sd, range International trial 67.5 an Placebo component in 67.5 ± 6.7 women with Raloxifene or multiple 67.5 ± 6.6 risk factors
TRIALS OF HR RUTH (Barrett- Connor et al 29 ) JULY 2006 (Country) mean ± sd, range International trial 67.5 an Placebo component in 67.5 ± 6.7 women with Raloxifene or multiple 67.5 ± 6.6 risk factors
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160
 Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
Updates in Osteoporosis. I have no conflicts of interest. What Would You Do? Mrs. C. What s New in Osteoporosis. Page 1
 Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT
 NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
Modeling the annual costs of postmenopausal prevention therapy: raloxifene, alendronate, or estrogen-progestin therapy Mullins C D, Ohsfeldt R L
 Modeling the annual costs of postmenopausal prevention therapy: raloxifene, alendronate, or estrogen-progestin therapy Mullins C D, Ohsfeldt R L Record Status This is a critical abstract of an economic
Modeling the annual costs of postmenopausal prevention therapy: raloxifene, alendronate, or estrogen-progestin therapy Mullins C D, Ohsfeldt R L Record Status This is a critical abstract of an economic
Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene)
 EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
New Developments in Osteoporosis: Screening, Prevention and Treatment
 Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
TRANSPARENCY COMMITTEE OPINION. 4 November 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
Submission to the National Institute for Clinical Excellence on
 Submission to the National Institute for Clinical Excellence on Strontium ranelate for the prevention of osteoporotic fractures in postmenopausal women with osteoporosis by The Society for Endocrinology
Submission to the National Institute for Clinical Excellence on Strontium ranelate for the prevention of osteoporotic fractures in postmenopausal women with osteoporosis by The Society for Endocrinology
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 VELMETIA 50 mg/850 mg, film-coated tablets B/56 (CIP code: 386 778-0) VELMETIA 50 mg/1 000 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 VELMETIA 50 mg/850 mg, film-coated tablets B/56 (CIP code: 386 778-0) VELMETIA 50 mg/1 000 mg, film-coated
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 10 February 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 February 2010 ADIXONE 50 µg, tablet Box of 30 (CIP: 390 604.3) Box of 60 (CIP: 390 606.6) Box of 90 (CIP: 390 607.2)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 February 2010 ADIXONE 50 µg, tablet Box of 30 (CIP: 390 604.3) Box of 60 (CIP: 390 606.6) Box of 90 (CIP: 390 607.2)
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 TROLOVOL 300 mg, film-coated tablet B/30 (CIP: 34009 320 316 9 6) Applicant: IMAXO INN ATC Code (2012)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 TROLOVOL 300 mg, film-coated tablet B/30 (CIP: 34009 320 316 9 6) Applicant: IMAXO INN ATC Code (2012)
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 23 May 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 23 May 2012 IKARAN LP 5 mg, tablets B/30 (CIP: 339 287-4) IKARAN Ge 2 mg/ml, oral solution in drops Vial of 50 ml
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 23 May 2012 IKARAN LP 5 mg, tablets B/30 (CIP: 339 287-4) IKARAN Ge 2 mg/ml, oral solution in drops Vial of 50 ml
Osteodensitometry in primary and secondary osteoporosis
 Osteodensitometry in primary and secondary osteoporosis Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) Research question The main goal of the present research was the assessment
Osteodensitometry in primary and secondary osteoporosis Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) Research question The main goal of the present research was the assessment
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Ms. Y. Outline. Updates of SERMs and Estrogen
 Ms. Y Updates of SERMs and Estrogen Steven R. Cummings, MD, FACP San Francisco Coordinating Center CPMC Research Institute and UCSF Support from Lilly, Pfizer, Berlex 55 y.o. woman with mild hypertension
Ms. Y Updates of SERMs and Estrogen Steven R. Cummings, MD, FACP San Francisco Coordinating Center CPMC Research Institute and UCSF Support from Lilly, Pfizer, Berlex 55 y.o. woman with mild hypertension
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Presenter: 翁家嫻 Venue date:
 FOR THE TREATMENT OF OSTEOPOROSIS IN POSTMENOPAUSAL WOMEN AT INCREASED RISK OF FRACTURES 1 Presenter: 翁家嫻 Venue date: 2018.03.13 PMO: postmenopausal osteoporosis. 1. Prolia (denosumab), Summary of Product
FOR THE TREATMENT OF OSTEOPOROSIS IN POSTMENOPAUSAL WOMEN AT INCREASED RISK OF FRACTURES 1 Presenter: 翁家嫻 Venue date: 2018.03.13 PMO: postmenopausal osteoporosis. 1. Prolia (denosumab), Summary of Product
Disclosures. Diagnostic Challenges in Osteoporosis: Whom To Treat 9/25/2014
 Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 11 April 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
Clinical Practice. Presented by: Internist, Endocrinologist
 Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
Aromatase Inhibitors & Osteoporosis
 Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment. William D. Leslie, MD MSc FRCPC
 Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Cortical bone After age 40, gradually decreases % yearly, in both men and women Postmenopausally, loss accelerates to 2-3% yearly
 Osteoporosis POOLE, K.E.S. & COMPSTON, J.E. (2006): Osteoporosis and its management. BMJ 333:1251-6. Physiology Cortical bone After age 40, gradually decreases 0.3-0.5% yearly, in both men and women Postmenopausally,
Osteoporosis POOLE, K.E.S. & COMPSTON, J.E. (2006): Osteoporosis and its management. BMJ 333:1251-6. Physiology Cortical bone After age 40, gradually decreases 0.3-0.5% yearly, in both men and women Postmenopausally,
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
Opinion 15 May ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: )
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
Hormone therapy. Dr. med. Frank Luzuy
 Hormone therapy Dr. med. Frank Luzuy Reasons for Initiating/Continuing HT* Menopause-Related Symptoms Osteoporosis, Bone Loss, Fracture Prevention Doctor Prescribed It, Told Me to Take It Cardiovascular
Hormone therapy Dr. med. Frank Luzuy Reasons for Initiating/Continuing HT* Menopause-Related Symptoms Osteoporosis, Bone Loss, Fracture Prevention Doctor Prescribed It, Told Me to Take It Cardiovascular
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
Learning Objectives. Controversies in Osteoporosis Prevention and Management. Etiology. Presenter Disclosure Information. Epidemiology.
 12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
Osteoporosis. Definition
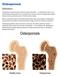 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 ACADIONE 250 mg, sugar-coated tablet Bottle of 120 (CIP: 34009 329 390 7 7) Applicant: SANOFI-AVENTIS
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 ACADIONE 250 mg, sugar-coated tablet Bottle of 120 (CIP: 34009 329 390 7 7) Applicant: SANOFI-AVENTIS
Update on Osteoporosis 2016
 WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
New therapies for osteoporosis. Dr.Shobhana Mohandas.MD.DGO.FICOG. Sun Medical centre, Thrissur, Kerala.
 New therapies for osteoporosis Dr.Shobhana Mohandas.MD.DGO.FICOG. Sun Medical centre, Thrissur, Kerala. Osteoclastic resorption Sema 4 D Plexin B1 from osteo TGF-β1, and IGF-1 Osteoblast Migration Osteoblast
New therapies for osteoporosis Dr.Shobhana Mohandas.MD.DGO.FICOG. Sun Medical centre, Thrissur, Kerala. Osteoclastic resorption Sema 4 D Plexin B1 from osteo TGF-β1, and IGF-1 Osteoblast Migration Osteoblast
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 March 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
1. NAME OF THE MEDICINAL PRODUCT
 EVISTA Eli Lilly 1. NAME OF THE MEDICINAL PRODUCT EVISTA 60 mg film coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 60 mg raloxifene hydrochloride, equivalent
EVISTA Eli Lilly 1. NAME OF THE MEDICINAL PRODUCT EVISTA 60 mg film coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 60 mg raloxifene hydrochloride, equivalent
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above
 Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
North Central London Joint Formulary Committee
 North Central London Joint Formulary Committee Calcium + vitamin D supplementation for the prevention of osteoporotic fragility fractures Disclaimer This guideline is registered at North Central London
North Central London Joint Formulary Committee Calcium + vitamin D supplementation for the prevention of osteoporotic fragility fractures Disclaimer This guideline is registered at North Central London
A response by Servier to the Statement of Reasons provided by NICE
 A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis
 Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
