Laboratory Service Report
|
|
|
- Lindsey McCoy
- 5 years ago
- Views:
Transcription
1 Client C DLP Rochester Rochester, N Specimen Type Peripheral blood CR PDF Report available at: Indication for Test DS CR Pathogenic utations Detected CR None. See below for Variants of Unknown Significance and Additional Notes. Please see the section of "Panel Gene List" below for the complete list of genes tested. Interpretation CR No pathogenic genetic alteration is detected in the listed gene regions. This finding does not exclude the presence of genetic alteration occurring at allele frequency below our established detection limit of 5-10%, or other genetic alterations present in untested gene regions. Clinical Trials CR Information regarding possible clinical trials for this patient can be found at the following sites: 1). ClinicalTrials.gov: 2). ayo Clinic: 3). National Cancer Institute: 4). olecular atch: Variants of Unknown Significance None CR Additional Notes None CR ethod Summary CR DNA is extracted from the peripheral blood or bone marrow sample and following library preparation by hybrid capture, subjected to next generation sequencing (NGS) with post-sequencing analysis of tumor-associated mutations. Because some regions in the target panel are not consistently resolved by NGS, additional separate clinical laboratory assays may also be used to complete the overall analysis including: CALR (CALR), CEBPA (CEBPA), CSF3R (CSF3R), FLT3 (FLT), JAK2 V617F utation Detection (JAK2B, JAK2, or JAK2V), JAK2 Exon 12 utation Detection (JAKXB or JAKX), KIT (KITB, KITB, KITAS, or KITE), PL (PLB or PL), YD88 (YD88), NP1 (NP1), and TP53 (P53CA). Performance characteristics of NGS panel: ***Performing Site Legend on Last Page of Report*** Page 1 of 4 >> Continued on Next Page >> * Report times for ayo performed tests are CST/CDT
2 Client C DLP Rochester Rochester, N Single base substitutions: accuracy >99%; reproducibility 100% (intra- and interassay); sensitivity 5-10% variant allele fraction with a minimum depth coverage of 250X. Insertion/deletion events: accuracy >99%; reproducibility 100% (intra- and interassay); sensitivity 5-10% variant allele fraction with a minimum depth coverage of 250X. This test was developed and its performance characteristics determined by ayo Clinic in a manner consistent with CLIA requirements. This test has not been cleared or approved by the U.S. Food and Drug Administration. Disclaimer CR CLINICAL DISCLAIER utation calls detected between 5-10% variant allele fractions (VAF) may indicate low-level (i.e. subclonal) tumor populations, although the clinical significance of these findings may not be clear. Some apparent mutations classified as VUS may represent rare or low frequency polymorphisms. A low incidence of gene mutations associated with myeloid neoplasms can also be detected in hematopoietic cells with advancing age in some individuals without evidence of a hematologic malignancy ("clonal hematopoiesis") and these alterations may not be clearly distinguishable from tumor-associated mutations (PID ; ; ). Prior treatment for hematologic malignancy could affect the results obtained in this assay. In particular, prior allogeneic hematopoietic stem cell transplant (HSCT) may cause difficulties in resolving somatic or polymorphic alterations, or in assigning variant calls correctly to donor and recipient fractions, if pertinent clinical or laboratory information (e.g. chimerism engraftment status) is not provided. Correlation with clinical, histopathologic and additional laboratory findings is required for final interpretation of these results. This assay does not distinguish between somatic and germ line alterations in analyzed gene regions, particularly with VAF near 50% or 100%. If nucleotide alterations in genes associated with germ line mutation syndromes are present and there is also a strong clinical suspicion or family history of malignant disease predisposition, additional genetic testing and appropriate counseling may be indicated. This report interpretation is based on current medical and scientific literature, but clinical significance may not be completely established for all reported target gene abnormalities identified. The final interpretation of results for clinical management of the patient is the responsibility of the managing ***Performing Site Legend on Last Page of Report*** Page 2 of 4 >> Continued on Next Page >> * Report times for ayo performed tests are CST/CDT
3 Client C DLP Rochester Rochester, N physician. TECHNICAL DISCLAIER The depth of sequencing coverage may be variable for some target regions, but assay performance below the minimum acceptable criteria, or for failed regions are noted. Analysis of rare (low allele frequency) polymorphisms may be problematic in some cases. A low tumor cell percentage in the sample may affect the true mutation VAF and/or sensitivity. Suboptimal- performing regions (i.e. less than the expected minimum depth of coverage) may affect analytic sensitivity for detecting lower level mutations. This is a qualitative test. The variant read fractions are provided for information only and represent a relative proportion of mutated alleles, but do not indicate a measure of analytical sensitivity for the given genes; assay sensitivity is as stated in the method summary. Some genetic or genomic alterations, such as large insertion/deletion (indel) events, copy number alterations (CNA) and gene translocation events are not detected by this assay. OncoHeme Panel Gene list CR ASXL1 (N_ ) exons 11-14, BCOR (N_ ) exons 5-, BRAF (N_ ) exon 15, CALR (N_ ) exon 9, CBL (N_ ) exon 8, intron 8, and exon 9, CEBPA (N_ ) exon 1, CSF3R (N_ ) exons 14 and 17, DNT3A (N_ ) exons 8-23, ETV6 (N_ ) exons 3-8, EZH2 (N_ ) exons 3-21, FLT3 (N_ ) exons 14-20, GATA1 (N_ ) exons 2 and 4, GATA2 (N_ ) exons 4-8, IDH1 (N_ ) exon 4, IDH2 (N_0028.3) exon 4, JAK2 (N_ ) exons 12-, KIT (N_ ) exons 8-11 and 17, KRAS (N_ ) exons 2-3, PL (N_ ) exons 10-11, YD88 (N_ ) exon 5, NOTCH1 (N_ ) exons 26, 27, and 34, NP1 (N_ ) exons 9, 11, and 12, NRAS (N_ ) exons 2 and 3, PHF6 (N_ ) exons 2-10, PTPN11 (N_ ) exons 3-4 and 12-13, RUNX1 (N_ ) exons 4-10, SETBP1 (N_ ) partial exon 6; amino acids , SF3B1 (N_ ) exons 13-, SRSF2 (N_0030.4) exons 1 and 2, TERT (N_ ) exons 2-, TET2 (N_ ) exons 3-11, TP53 (N_ ) exons 4-9, U2AF1 (N_ ) exons 2, 7, and 9, WT1 (N_ Aas.3) exons 1-11, and ZRSR2 (N_ ) exons For some genes, the transcript IDs used in this analysis ***Performing Site Legend on Last Page of Report*** Page 3 of 4 >> Continued on Next Page >> * Report times for ayo performed tests are CST/CDT
4 Client C DLP Rochester Rochester, N may not be the same as in other cancer mutation databases, such as COSIC ( Reviewed By: CR Signing Pathologist: elissa Tricker-Klar RECEIVED: 10/20/20 14:52 REPORTED: 10/20/20 15:01 * Performing Site: ayo Clinic Laboratories - Rochester ain Campus CR 200 First St SW Rochester, N Lab Director: William G. orice, II,.D., Ph.D. Page 4 of 4 ** End of Report ** * Report times for ayo performed tests are CST/CDT
5 PATIENT NAE TESTINGRNV, REPORT PATIENT ID COLLECTED 10/20/20, 6:00 A DLP Rochester Rochester DATE OF BIRTH RECEIVED 10/20/20, 2:52 P N AGE Y SEX ale REPORTED 10/20/20, 3:01 P The collected, received and reported dates and times are in the time zone of the performing location. REQUESTED BY CLIENT CLIENT CLIENT ORDER NUBER CLIENT RN NGSH ORDER NUBER F Specimen Type Indication for Test Peripheral blood DS PATHOGENIC UTATIONS DETECTED None. See below for Variants of Unknown Significance and Additional Notes. Please see the section of "Panel Gene List" below for the complete list of genes tested. INTERPRETATION No pathogenic genetic alteration is detected in the listed gene regions. This finding does not exclude the presence of genetic alteration occurring at allele frequency below our established detection limit of 5-10%, or other genetic alterations present in untested gene regions. CLINICAL TRIALS Information regarding possible clinical trials for this patient can be found at the following sites: 1). ClinicalTrials.gov: 2). ayo Clinic: 3). National Cancer Institute: 4). olecular atch: VARIANTS OF UNKNOWN SIGNIFICANCE (VUS) None ADDITIONAL NOTES None ETHOD SUARY DNA is extracted from the peripheral blood or bone marrow sample and following library preparation by hybrid capture, subjected to next generation sequencing (NGS) with post-sequencing analysis of tumor-associated mutations. Because some regions in the target panel are not consistently resolved by NGS, additional separate clinical laboratory assays may also be used to complete the overall analysis including: CALR (CALR), CEBPA (CEBPA), CSF3R (CSF3R), FLT3 (FLT), JAK2 V617F utation Detection (JAK2B, JAK2, or JAK2V), JAK2 Exon 12 utation Detection (JAKXB or JAKX), KIT (KITB, KITB, KITAS, or KITE), PL (PLB or PL), YD88 (YD88), NP1 (NP1), and TP53 (P53CA). PATIENT NAE TESTINGRNV, REPORT -TL258 Form ID: ETCB Page 1 of 3
6 PATIENT NAE TESTINGRNV, REPORT PATIENT ID COLLECTED 10/20/20, 6:00 A DLP Rochester Rochester DATE OF BIRTH RECEIVED 10/20/20, 2:52 P N AGE Y SEX ale REPORTED 10/20/20, 3:01 P The collected, received and reported dates and times are in the time zone of the performing location. REQUESTED BY CLIENT CLIENT CLIENT ORDER NUBER CLIENT RN NGSH ORDER NUBER F Performance characteristics of NGS panel: Single base substitutions: accuracy >99%; reproducibility 100% (intra- and interassay); sensitivity 5-10% variant allele fraction with a minimum depth coverage of 250X. Insertion/deletion events: accuracy >99%; reproducibility 100% (intra- and interassay); sensitivity 5-10% variant allele fraction with a minimum depth coverage of 250X. This test was developed and its performance characteristics determined by ayo Clinic in a manner consistent with CLIA requirements. This test has not been cleared or approved by the U.S. Food and Drug Administration. DISCLAIER CLINICAL DISCLAIER utation calls detected between 5-10% variant allele fractions (VAF) may indicate low-level (i.e. subclonal) tumor populations, although the clinical significance of these findings may not be clear. Some apparent mutations classified as VUS may represent rare or low frequency polymorphisms. A low incidence of gene mutations associated with myeloid neoplasms can also be detected in hematopoietic cells with advancing age in some individuals without evidence of a hematologic malignancy ("clonal hematopoiesis") and these alterations may not be clearly distinguishable from tumor-associated mutations (PID ; ; ). Prior treatment for hematologic malignancy could affect the results obtained in this assay. In particular, prior allogeneic hematopoietic stem cell transplant (HSCT) may cause difficulties in resolving somatic or polymorphic alterations, or in assigning variant calls correctly to donor and recipient fractions, if pertinent clinical or laboratory information (e.g. chimerism engraftment status) is not provided. Correlation with clinical, histopathologic and additional laboratory findings is required for final interpretation of these results. This assay does not distinguish between somatic and germ line alterations in analyzed gene regions, particularly with VAF near 50% or 100%. If nucleotide alterations in genes associated with germ line mutation syndromes are present and there is also a strong clinical suspicion or family history of malignant disease predisposition, additional genetic testing and appropriate counseling may be indicated. This report interpretation is based on current medical and scientific literature, but clinical significance may not be completely established for all reported target gene abnormalities identified. The final interpretation of results for clinical management of the patient is the responsibility of the managing physician. TECHNICAL DISCLAIER The depth of sequencing coverage may be variable for some target regions, but assay performance below the minimum acceptable criteria, or for failed regions are noted. Analysis of rare (low allele frequency) polymorphisms may be problematic in some cases. A low tumor cell percentage in the sample may affect the true mutation VAF and/or sensitivity. Suboptimal- performing regions (i.e. less than the expected minimum depth of coverage) may affect analytic sensitivity for detecting lower level mutations. This is a qualitative test. The variant read fractions are provided for information only and represent a relative proportion of mutated alleles, but do not indicate a measure of analytical sensitivity for the given genes; assay sensitivity is as stated in the method summary. Some genetic or genomic alterations, such as large insertion/deletion (indel) events, copy number alterations (CNA) and gene translocation events are not detected by this assay. PANEL GENE LIST ASXL1 (N_ ) exons 11-14, BCOR (N_ ) exons 5-, BRAF (N_ ) exon 15, CALR (N_ ) exon 9, CBL (N_ ) exon 8, intron 8, and exon 9, CEBPA (N_ ) exon 1, CSF3R (N_ ) exons 14 and 17, DNT3A (N_ ) exons 8-23, ETV6 (N_ ) exons 3-8, EZH2 (N_ ) exons 3-21, FLT3 (N_ ) exons 14-20, GATA1 (N_ ) exons 2 and 4, GATA2 (N_ ) exons 4-8, IDH1 (N_ ) exon 4, IDH2 (N_0028.3) exon 4, JAK2 (N_ ) exons 12-, KIT (N_ ) exons 8-11 and 17, KRAS (N_ ) exons 2-3, PATIENT NAE TESTINGRNV, REPORT -TL258 Form ID: ETCB Page 2 of 3
7 PATIENT NAE TESTINGRNV, REPORT PATIENT ID COLLECTED 10/20/20, 6:00 A DLP Rochester Rochester DATE OF BIRTH RECEIVED 10/20/20, 2:52 P N AGE Y SEX ale REPORTED 10/20/20, 3:01 P The collected, received and reported dates and times are in the time zone of the performing location. REQUESTED BY CLIENT CLIENT CLIENT ORDER NUBER CLIENT RN NGSH ORDER NUBER F PL (N_ ) exons 10-11, YD88 (N_ ) exon 5, NOTCH1 (N_ ) exons 26, 27, and 34, NP1 (N_ ) exons 9, 11, and 12, NRAS (N_ ) exons 2 and 3, PHF6 (N_ ) exons 2-10, PTPN11 (N_ ) exons 3-4 and 12-13, RUNX1 (N_ ) exons 4-10, SETBP1 (N_ ) partial exon 6; amino acids , SF3B1 (N_ ) exons 13-, SRSF2 (N_0030.4) exons 1 and 2, TERT (N_ ) exons 2-, TET2 (N_ ) exons 3-11, TP53 (N_ ) exons 4-9, U2AF1 (N_ ) exons 2, 7, and 9, WT1 (N_ Aas.3) exons 1-11, and ZRSR2 (N_ ) exons For some genes, the transcript IDs used in this analysis may not be the same as in other cancer mutation databases, such as COSIC ( REVIEWED BY Signing Pathologist: elissa Tricker-Klar CODE LABORATORY ADDRESS LAB DIRECTOR CR ayo Clinic Laboratories - Rochester ain Campus 200 FIRST STREET SW ROCHESTER N, WILLIA G ORICE, II, D, PhD Report times for Laboratory Name performed tests are CST/CDT. The collected, received, and reported dates and times on the report are in the time zone of the performing location. PATIENT NAE TESTINGRNV, REPORT -TL258 Form ID: ETCB Page 3 of 3
Laboratory Service Report
 Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-ih2xuglwpq.ashx Indication for Test DS CR Pathogenic utations Detected CR 1. JAK2: c.1849g>t;p.val617phe
Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-ih2xuglwpq.ashx Indication for Test DS CR Pathogenic utations Detected CR 1. JAK2: c.1849g>t;p.val617phe
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ
 Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
Next Generation Sequencing in Haematological Malignancy: A European Perspective. Wolfgang Kern, Munich Leukemia Laboratory
 Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
The Center for PERSONALIZED DIAGNOSTICS
 The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
Next generation sequencing analysis - A UK perspective. Nicholas Lea
 Next generation sequencing analysis - A UK perspective Nicholas Lea King s HMDC LMH is part of an integrated pathology service at King s Haematological Malignancy Diagnostic Centre (HMDC) HMDC serves population
Next generation sequencing analysis - A UK perspective Nicholas Lea King s HMDC LMH is part of an integrated pathology service at King s Haematological Malignancy Diagnostic Centre (HMDC) HMDC serves population
Out-Patient Billing CPT Codes
 Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Introduction of an NGS gene panel into the Haemato-Oncology MPN service
 Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
Please Silence Your Cell Phones. Thank You
 Please Silence Your Cell Phones Thank You Utility of NGS and Comprehensive Genomic Profiling in Hematopathology Practice Maria E. Arcila M.D. Memorial Sloan Kettering Cancer Center New York, NY Disclosure
Please Silence Your Cell Phones Thank You Utility of NGS and Comprehensive Genomic Profiling in Hematopathology Practice Maria E. Arcila M.D. Memorial Sloan Kettering Cancer Center New York, NY Disclosure
August 17, Dear Valued Client:
 August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
Molecular. Oncology & Pathology. Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine. Liquid Biopsy.
 Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
ADRL Advanced Diagnostics Research Laboratory
 ADRL Advanced Diagnostics Research Laboratory John DeCoteau, MD FRCP Department of Pathology, Division of Hematopathology University of Saskatchewan Saskatchewan Cancer Agency ADRL Project Objectives New
ADRL Advanced Diagnostics Research Laboratory John DeCoteau, MD FRCP Department of Pathology, Division of Hematopathology University of Saskatchewan Saskatchewan Cancer Agency ADRL Project Objectives New
The Evolving Role of Transplantation for MPN
 The Evolving Role of Transplantation for MPN (PMF, PV, ET) H.Joachim Deeg MD Fred Hutchinson Cancer Research Center, University of Washington, Seattle WA, SCCA jdeeg@fhcrc.org 10 th J. Niblack Conference
The Evolving Role of Transplantation for MPN (PMF, PV, ET) H.Joachim Deeg MD Fred Hutchinson Cancer Research Center, University of Washington, Seattle WA, SCCA jdeeg@fhcrc.org 10 th J. Niblack Conference
DISCLOSURE Luca Malcovati, MD. No financial relationships to disclose
 ICUS, CCUS and CHIP Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation, Pavia, Italy DISCLOSURE
ICUS, CCUS and CHIP Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation, Pavia, Italy DISCLOSURE
Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia
 Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia Feng-Ming Tien, Hsin-An Hou, Jih-Luh Tang, Yuan-Yeh Kuo, Chien-Yuan Chen, Cheng-Hong Tsai, Ming Yao, Chi-Cheng
Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia Feng-Ming Tien, Hsin-An Hou, Jih-Luh Tang, Yuan-Yeh Kuo, Chien-Yuan Chen, Cheng-Hong Tsai, Ming Yao, Chi-Cheng
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
Supplementary Information
 Supplementary Information Table of Contents Supplementary methods... 2 Figure S1 - Variable DNA yield proportional to bone marrow aspirate cellularity.... 3 Figure S2 - Mutations by clinical ontogeny group....
Supplementary Information Table of Contents Supplementary methods... 2 Figure S1 - Variable DNA yield proportional to bone marrow aspirate cellularity.... 3 Figure S2 - Mutations by clinical ontogeny group....
7/12/2016 TESTING. Objectives. New Directions in Aplastic Anemia: What's on the Horizon? Better way to evaluate clonal evolution?
 New Directions in Aplastic Anemia: What's on the Horizon? Amy E. DeZern, MD; MHS Assistant Professor Hematologic Malignancies Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Baltimore, MD Objectives
New Directions in Aplastic Anemia: What's on the Horizon? Amy E. DeZern, MD; MHS Assistant Professor Hematologic Malignancies Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Baltimore, MD Objectives
Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017
 Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017 Elli Papaemmanuil, PhD Memorial Sloan Kettering Cancer Center New York, New York, United States Today s Talk Cancer genome introduction
Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017 Elli Papaemmanuil, PhD Memorial Sloan Kettering Cancer Center New York, New York, United States Today s Talk Cancer genome introduction
West Midlands Regional Genetics Laboratory
 West Midlands Regional Genetics Laboratory Haemato-oncology service update letter October 2017 Dear colleagues, We are writing to outline the latest developments to our service, aiming to support the management
West Midlands Regional Genetics Laboratory Haemato-oncology service update letter October 2017 Dear colleagues, We are writing to outline the latest developments to our service, aiming to support the management
Supplemental Material. The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia
 Supplemental Material The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia Torsten Haferlach, 1 Anna Stengel, 1 Sandra Eckstein, 1 Karolína
Supplemental Material The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia Torsten Haferlach, 1 Anna Stengel, 1 Sandra Eckstein, 1 Karolína
Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH )
 Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH2017-0314) Habibe Kurt, Joseph D. Khoury, Carlos E. Bueso-Ramos, Jeffrey L. Jorgensen, Guilin Tang, L. Jeffrey Medeiros, and
Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH2017-0314) Habibe Kurt, Joseph D. Khoury, Carlos E. Bueso-Ramos, Jeffrey L. Jorgensen, Guilin Tang, L. Jeffrey Medeiros, and
Supplementary Figure 1
 Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Published Ahead of Print on April 14, 2016, as doi: /haematol Copyright 2016 Ferrata Storti Foundation.
 Published Ahead of Print on April 14, 2016, as doi:10.3324/haematol.2016.143214. Copyright 2016 Ferrata Storti Foundation. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse
Published Ahead of Print on April 14, 2016, as doi:10.3324/haematol.2016.143214. Copyright 2016 Ferrata Storti Foundation. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse
Management of Myelodysplastic Syndromes
 Management of Myelodysplastic Syndromes Peter L. Greenberg, MD Stanford Cancer Institute Myelodysplastic Syndromes: Clinical & Molecular Advances for Disease Classification and Prognostication MDSs: A
Management of Myelodysplastic Syndromes Peter L. Greenberg, MD Stanford Cancer Institute Myelodysplastic Syndromes: Clinical & Molecular Advances for Disease Classification and Prognostication MDSs: A
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope
 Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Click to edit Master /tle style
 Click to edit Master /tle style Tel: (314) 747-7337 Toll Free: (866) 450-7697 Fax: (314) 747-7336 Email: gps@wustl.edu Website: gps.wustl.edu GENETIC TESTING IN CANCER Ka/nka Vigh-Conrad, PhD Genomics
Click to edit Master /tle style Tel: (314) 747-7337 Toll Free: (866) 450-7697 Fax: (314) 747-7336 Email: gps@wustl.edu Website: gps.wustl.edu GENETIC TESTING IN CANCER Ka/nka Vigh-Conrad, PhD Genomics
Precision Medicine and Molecular Testing.
 Precision Medicine and Molecular Testing. David A. Sallman, MD Assistant Member Department of Malignant Hematology Moffitt Cancer Center david.sallman@moffitt.org Disclosures Research funding for Celgene
Precision Medicine and Molecular Testing. David A. Sallman, MD Assistant Member Department of Malignant Hematology Moffitt Cancer Center david.sallman@moffitt.org Disclosures Research funding for Celgene
Corporate Medical Policy. Policy Effective February 23, 2018
 Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts
 The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts Claudia Bruedigam, Ph.D Gordon and Jessie Gilmour Leukaemia Research Laboratory
The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts Claudia Bruedigam, Ph.D Gordon and Jessie Gilmour Leukaemia Research Laboratory
TEST MENU TEST CPT CODES TAT. Chromosome Analysis Bone Marrow x 2, 88264, x 3, Days
 TEST MENU CANCER/LEUKEMIA CHROMOSOME ANALYSIS Chromosome Analysis Bone Marrow 88237 x 2, 88264, 88280 x 3, 88291 4 Days Chromosome Analysis Bone Marrow Core 88237 x 2, 88264, 88280 x 3, 88291 4 Days Chromosome
TEST MENU CANCER/LEUKEMIA CHROMOSOME ANALYSIS Chromosome Analysis Bone Marrow 88237 x 2, 88264, 88280 x 3, 88291 4 Days Chromosome Analysis Bone Marrow Core 88237 x 2, 88264, 88280 x 3, 88291 4 Days Chromosome
Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!!
 Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!! Lindsay Anne Magura Rein, MD Division of Hematologic Malignancies and Cellular Therapy/BMT A Little Bit of History.. 1665 Advanced
Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!! Lindsay Anne Magura Rein, MD Division of Hematologic Malignancies and Cellular Therapy/BMT A Little Bit of History.. 1665 Advanced
Acute Myeloid Leukemia with RUNX1 and Several Co-mutations
 Case SH2017-0281 Acute Myeloid Leukemia with RUNX1 and Several Co-mutations James Bauer, MD, PhD David Yang, MD Erik Ranheim, MD, PhD Catherine Leith, MB, Bchir Clinical History Chief Complaint: 72 year
Case SH2017-0281 Acute Myeloid Leukemia with RUNX1 and Several Co-mutations James Bauer, MD, PhD David Yang, MD Erik Ranheim, MD, PhD Catherine Leith, MB, Bchir Clinical History Chief Complaint: 72 year
Overview. Methods 9/11/2017. Next Generation Sequencing and Precision Medicine in Hematological Malignancies. Genotyping in hematology
 Overview Next Generation Sequencing and Precision Medicine in Hematological Malignancies Sharathkumar Bhagavathi, MD University of Iowa Carver College of Medicine NGS as a genotyping platform in hematopathology
Overview Next Generation Sequencing and Precision Medicine in Hematological Malignancies Sharathkumar Bhagavathi, MD University of Iowa Carver College of Medicine NGS as a genotyping platform in hematopathology
Pediatric Oncology & Pathology Services
 Pediatric Oncology & Pathology Services Anatomic Pathology Flow Cytometry Cytogenetics Pharma Services Diagnostic, Prognostic, Predictive, and Predisposition Testing Pediatric Oncology & Pathology Services
Pediatric Oncology & Pathology Services Anatomic Pathology Flow Cytometry Cytogenetics Pharma Services Diagnostic, Prognostic, Predictive, and Predisposition Testing Pediatric Oncology & Pathology Services
SESSION 1 Reactive cytopenia and dysplasia
 SESSION 1 Reactive cytopenia and dysplasia Falko Fend, Tübingen & Alexandar Tzankov, Basel 1 Disclosure of speaker s interests (Potential) conflict of interest none Potentially relevant company relationships
SESSION 1 Reactive cytopenia and dysplasia Falko Fend, Tübingen & Alexandar Tzankov, Basel 1 Disclosure of speaker s interests (Potential) conflict of interest none Potentially relevant company relationships
Supporting Information
 Supporting Information Rampal et al. 10.1073/pnas.1407792111 Fig. S1. Genetic events in leukemic transformation of chronic-phase MPNs. (A) Survival of post-mpn AML patients according to mutational status
Supporting Information Rampal et al. 10.1073/pnas.1407792111 Fig. S1. Genetic events in leukemic transformation of chronic-phase MPNs. (A) Survival of post-mpn AML patients according to mutational status
Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models
 REGULAR ARTICLE Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models Animesh Pardanani, 1 Sahrish Shah, 1 Francesco Mannelli, 2 Yoseph C. Elala, 1 Paola Guglielmelli,
REGULAR ARTICLE Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models Animesh Pardanani, 1 Sahrish Shah, 1 Francesco Mannelli, 2 Yoseph C. Elala, 1 Paola Guglielmelli,
Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases.
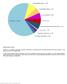 Supplementary Figure 1 Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases. A total of 85 somatically acquired
Supplementary Figure 1 Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases. A total of 85 somatically acquired
Myelodysplastic syndromes Impact of Biology. Lionel Adès Hopital Saint Louis Groupe Francophone des SMD. Épidémiologie
 Myelodysplastic syndromes Impact of Biology Lionel Adès Hopital Saint Louis Groupe Francophone des SMD Épidémiologie Incidence : 3 à 6 / 100 000 hab. / An Prédomine chez les sujets âgés Augmentation de
Myelodysplastic syndromes Impact of Biology Lionel Adès Hopital Saint Louis Groupe Francophone des SMD Épidémiologie Incidence : 3 à 6 / 100 000 hab. / An Prédomine chez les sujets âgés Augmentation de
Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome
 Molecular profiling of early MDS Hematopathology - March 2016 Article Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome Maya Thangavelu 1,*, Ryan Olson 2, Li Li 2, Wanlong
Molecular profiling of early MDS Hematopathology - March 2016 Article Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome Maya Thangavelu 1,*, Ryan Olson 2, Li Li 2, Wanlong
MEDICAL GENOMICS LABORATORY. Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG)
 Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
Fluxion Biosciences and Swift Biosciences Somatic variant detection from liquid biopsy samples using targeted NGS
 APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine
 Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center Objectives
Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center Objectives
No mutations were identified.
 Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
Molecular Markers. Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC
 Molecular Markers Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC Overview Testing methods Rationale for molecular testing
Molecular Markers Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC Overview Testing methods Rationale for molecular testing
Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1
 Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1 Xin Hua Hospital, Shanghai, China 2 Oregon Health & Science University, Portland, OR, United States AML is a hematopoietic neoplasms characterized
Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1 Xin Hua Hospital, Shanghai, China 2 Oregon Health & Science University, Portland, OR, United States AML is a hematopoietic neoplasms characterized
SureSelect Cancer All-In-One Custom and Catalog NGS Assays
 SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
Prior Authorization Required: Additional Information:
 Genetic Testing for Somatic Tumor Markers MP9486 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below No A first-degree relative is defined as an individual
Genetic Testing for Somatic Tumor Markers MP9486 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below No A first-degree relative is defined as an individual
ASBMT MDS/MPN Update Sunil Abhyankar, MD
 ASBMT MDS/MPN Update Sunil Abhyankar, MD Professor of Medicine Medical Director, Pheresis and Cell Processing Division of Hematologic Malignancies and Cellular Therapeutics Department of Internal Medicine
ASBMT MDS/MPN Update Sunil Abhyankar, MD Professor of Medicine Medical Director, Pheresis and Cell Processing Division of Hematologic Malignancies and Cellular Therapeutics Department of Internal Medicine
SUPPLEMENTAL APPENDIX METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA
 SUPPLEMENTAL APPENDIX TO METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA SUPPLEMENTAL METHODS Treatment protocols In the AMLCG-1999 trial 1-3 (clinicaltrials.gov
SUPPLEMENTAL APPENDIX TO METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA SUPPLEMENTAL METHODS Treatment protocols In the AMLCG-1999 trial 1-3 (clinicaltrials.gov
Session II: Summary. Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School
 Session II: Summary Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School Disclosure of speaker s interests (Prefix and Last Name) (Potential) conflict
Session II: Summary Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School Disclosure of speaker s interests (Prefix and Last Name) (Potential) conflict
Accel-Amplicon Panels
 Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
NGS Gateway Lab Services
 TM NGS Gateway Lab Services Accelerating Precision Medicine Design a Complete Genomic Testing Portfolio with Turnkey Assays About NGS Gateway Lab Services TM Designed to provide a gateway to your own in-house
TM NGS Gateway Lab Services Accelerating Precision Medicine Design a Complete Genomic Testing Portfolio with Turnkey Assays About NGS Gateway Lab Services TM Designed to provide a gateway to your own in-house
Clonal Evolution of saml. Johnnie J. Orozco Hematology Fellows Conference May 11, 2012
 Clonal Evolution of saml Johnnie J. Orozco Hematology Fellows Conference May 11, 2012 CML: *bcr-abl and imatinib Melanoma: *braf and vemurafenib CRC: *k-ras and cetuximab Esophageal/Gastric: *Her-2/neu
Clonal Evolution of saml Johnnie J. Orozco Hematology Fellows Conference May 11, 2012 CML: *bcr-abl and imatinib Melanoma: *braf and vemurafenib CRC: *k-ras and cetuximab Esophageal/Gastric: *Her-2/neu
Session 4: Summary and Conclusions
 Session 4: Summary and Conclusions Total cases in Session 4 Myeloproliferative neoplasms 16 cases Oral #300 (CEL, NOS) Mastocytosis 2 cases Oral #156 (SM-AHN) Myeloid/lymphoid neoplasms with eosinophilia
Session 4: Summary and Conclusions Total cases in Session 4 Myeloproliferative neoplasms 16 cases Oral #300 (CEL, NOS) Mastocytosis 2 cases Oral #156 (SM-AHN) Myeloid/lymphoid neoplasms with eosinophilia
Genomic Medicine: What every pathologist needs to know
 Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
Acute Myeloid Leukemia Progress at last
 Acute Myeloid Leukemia Progress at last Bruno C. Medeiros, MD September 9, 217 Introduction Mechanisms of leukemogenesis Emerging therapies in AML Previously untreated AML Relapsed and refractory patients
Acute Myeloid Leukemia Progress at last Bruno C. Medeiros, MD September 9, 217 Introduction Mechanisms of leukemogenesis Emerging therapies in AML Previously untreated AML Relapsed and refractory patients
Reporting TP53 gene analysis results in CLL
 Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance
Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms
 Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms Tiziano Barbui (tbarbui@asst-pg23.it Hematology and Research Foundation,Ospedale Papa Giovanni XXIII, Bergamo Italy
Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms Tiziano Barbui (tbarbui@asst-pg23.it Hematology and Research Foundation,Ospedale Papa Giovanni XXIII, Bergamo Italy
Objectives DISCLOSURES. Next-Generation Sequencing (NGS) Testing for Hematologic Malignancies
 Next-Generation Sequencing (NGS) Testing for Hematologic Malignancies David Viswanatha, M.D. Division of Hematopathology Mayo Clinic, Rochester, Minnesota 2017 MFMER slide-1 DISCLOSURES Relevant Financial
Next-Generation Sequencing (NGS) Testing for Hematologic Malignancies David Viswanatha, M.D. Division of Hematopathology Mayo Clinic, Rochester, Minnesota 2017 MFMER slide-1 DISCLOSURES Relevant Financial
Mutational Impact on Diagnostic and Prognostic Evaluation of MDS
 Mutational Impact on Diagnostic and Prognostic Evaluation of MDS Elsa Bernard, PhD Papaemmanuil Lab, Computational Oncology, MSKCC MDS Foundation ASH 2018 Symposium Disclosure Research funds provided by
Mutational Impact on Diagnostic and Prognostic Evaluation of MDS Elsa Bernard, PhD Papaemmanuil Lab, Computational Oncology, MSKCC MDS Foundation ASH 2018 Symposium Disclosure Research funds provided by
Objectives and Financial Disclosure
 Enhancing Assessment of Leukemia and Lymphoma with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical Molecular Laboratory Assistant Professor, Department of Pathology City of
Enhancing Assessment of Leukemia and Lymphoma with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical Molecular Laboratory Assistant Professor, Department of Pathology City of
Molecular Minimal Residual Disease in Acute Myeloid Leukemia
 Original Article Molecular Minimal Residual Disease in Acute Myeloid Leukemia M. Jongen Lavrencic, T. Grob, D. Hanekamp, F.G. Kavelaars, A. al Hinai, A. Zeilemaker, C.A.J. Erpelinck Verschueren, P.L. Gradowska,
Original Article Molecular Minimal Residual Disease in Acute Myeloid Leukemia M. Jongen Lavrencic, T. Grob, D. Hanekamp, F.G. Kavelaars, A. al Hinai, A. Zeilemaker, C.A.J. Erpelinck Verschueren, P.L. Gradowska,
Targeted next-generation sequencing in blast phase myeloproliferative neoplasms
 REGULAR ARTICLE Targeted next-generation sequencing in blast phase myeloproliferative neoplasms Terra L. Lasho, Mythri Mudireddy, Christy M. Finke, Curtis A. Hanson, Rhett P. Ketterling, Natasha Szuber,
REGULAR ARTICLE Targeted next-generation sequencing in blast phase myeloproliferative neoplasms Terra L. Lasho, Mythri Mudireddy, Christy M. Finke, Curtis A. Hanson, Rhett P. Ketterling, Natasha Szuber,
Pathogenesis and management of CMML
 Pathogenesis and management of CMML Raphaël Itzykson, Hôpital Saint-Louis, Paris International Conference of the Korean Society of Hematology March 29th 2018 대한혈액학회 Korean Society of Hematology COI disclosure
Pathogenesis and management of CMML Raphaël Itzykson, Hôpital Saint-Louis, Paris International Conference of the Korean Society of Hematology March 29th 2018 대한혈액학회 Korean Society of Hematology COI disclosure
Transform genomic data into real-life results
 CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
Clonal Cytopenia and Myeloid Neoplasms
 Clonal Cytopenia and Myeloid Neoplasms Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation,
Clonal Cytopenia and Myeloid Neoplasms Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation,
Clinical Grade Biomarkers in the Genomic Era Observations & Challenges
 Clinical Grade Biomarkers in the Genomic Era Observations & Challenges IOM Committee on Policy Issues in the Clinical Development & Use of Biomarkers for Molecularly Targeted Therapies March 31-April 1,
Clinical Grade Biomarkers in the Genomic Era Observations & Challenges IOM Committee on Policy Issues in the Clinical Development & Use of Biomarkers for Molecularly Targeted Therapies March 31-April 1,
Myelodysplastic syndromes and the new WHO 2016 classification
 Myelodysplastic syndromes and the new WHO 2016 classification 32nd General Annual Meeting of the Belgian Hematology Society 10-11 February 2017 Gregor Verhoef, Departement of Hematology, University Hospital
Myelodysplastic syndromes and the new WHO 2016 classification 32nd General Annual Meeting of the Belgian Hematology Society 10-11 February 2017 Gregor Verhoef, Departement of Hematology, University Hospital
Published Ahead of Print on June 22, 2017, as doi: /haematol Copyright 2017 Ferrata Storti Foundation.
 Published Ahead of Print on June 22, 2017, as doi:10.3324/haematol.2017.166173. Copyright 2017 Ferrata Storti Foundation. Molecular analysis of myelodysplastic syndrome with isolated del(5q) reveals a
Published Ahead of Print on June 22, 2017, as doi:10.3324/haematol.2017.166173. Copyright 2017 Ferrata Storti Foundation. Molecular analysis of myelodysplastic syndrome with isolated del(5q) reveals a
6/12/2018. Disclosures. Clinical Genomics The CLIA Lab Perspective. Outline. COH HopeSeq Heme Panels
 Clinical Genomics The CLIA Lab Perspective Disclosures Raju K. Pillai, M.D. Hematopathologist / Molecular Pathologist Director, Pathology Bioinformatics City of Hope National Medical Center, Duarte, CA
Clinical Genomics The CLIA Lab Perspective Disclosures Raju K. Pillai, M.D. Hematopathologist / Molecular Pathologist Director, Pathology Bioinformatics City of Hope National Medical Center, Duarte, CA
Molecular Genetic Testing for the Diagnosis of Haematological Malignancies
 Molecular Genetic Testing for the Diagnosis of Haematological Malignancies Dr Anthony Bench Haemto-Oncology Diagnostic Service Cambrıdge Unıversıty Hospitals NHS Foundatıon Trust Cambridge UK Molecular
Molecular Genetic Testing for the Diagnosis of Haematological Malignancies Dr Anthony Bench Haemto-Oncology Diagnostic Service Cambrıdge Unıversıty Hospitals NHS Foundatıon Trust Cambridge UK Molecular
BHS Annual Meeting
 BHS Annual Meeting 2014 01.02.2014 Implementing next-generation deepsequencing assays in diagnostic algorithms in hematological malignancies Dr. Alexander Kohlmann Medical Need for Molecular Characterization
BHS Annual Meeting 2014 01.02.2014 Implementing next-generation deepsequencing assays in diagnostic algorithms in hematological malignancies Dr. Alexander Kohlmann Medical Need for Molecular Characterization
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each
 Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
Disclosures for Ayalew Tefferi
 Disclosures for Ayalew Tefferi Principal investigator role Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board Janssen, Geron, Celgene, Sanofi-Aventis, Gilead Sciences, Incyte
Disclosures for Ayalew Tefferi Principal investigator role Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board Janssen, Geron, Celgene, Sanofi-Aventis, Gilead Sciences, Incyte
We are in an era that promises a rational. treatment of cancer patients. Levy et al. Genome Research 22:2201, 2012 Vanderbilt university
 Enhancing Assessment of Leukemia with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical i l Molecular l Laboratory City of Hope National Medical Center How the Expert Treat Hematologic
Enhancing Assessment of Leukemia with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical i l Molecular l Laboratory City of Hope National Medical Center How the Expert Treat Hematologic
NGS ONCOPANELS: FDA S PERSPECTIVE
 NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
Molecular Minimal Residual Disease in Acute Myeloid Leukemia
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Molecular Minimal Residual Disease in Acute Myeloid Leukemia Jongen-Lavrencic,
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2018 Molecular Minimal Residual Disease in Acute Myeloid Leukemia Jongen-Lavrencic,
Targeted NGS in oncology and hemato-oncology using in-house designed gene panels. Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017
 Targeted NGS in oncology and hemato-oncology using in-house designed gene panels Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017 MDG = Molecular Diagnostics UZ Ghent Center for Medical
Targeted NGS in oncology and hemato-oncology using in-house designed gene panels Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017 MDG = Molecular Diagnostics UZ Ghent Center for Medical
MEDICAL GENOMICS LABORATORY. Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG)
 Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
Novità nelle MDS. Matteo G Della Porta. Cancer Center IRCCS Humanitas Research Hospital & Humanitas University Rozzano Milano, Italy
 Novità nelle MDS Matteo G Della Porta Cancer Center IRCCS Humanitas Research Hospital & Humanitas University Rozzano Milano, Italy matteo.della_porta@hunimed.eu Outline ARCH Predictive value of somatic
Novità nelle MDS Matteo G Della Porta Cancer Center IRCCS Humanitas Research Hospital & Humanitas University Rozzano Milano, Italy matteo.della_porta@hunimed.eu Outline ARCH Predictive value of somatic
The state of the art molecular diagnostic application for hematological disorders MYELOID SOLUTION. BY SOPHiA GENETICS
 Myeloid Solution TM by SOPHiA GENETICS The state of the art molecular diagnostic application for hematological disorders Burden of hematological disorders worldwide MYELOID SOLUTION BY SOPHiA GENETICS
Myeloid Solution TM by SOPHiA GENETICS The state of the art molecular diagnostic application for hematological disorders Burden of hematological disorders worldwide MYELOID SOLUTION BY SOPHiA GENETICS
Genetic Predisposition Syndromes in Myeloid Malignancies
 Genetic Predisposition Syndromes in Myeloid Malignancies Lucy A. Godley, M.D., Ph.D. Section of Hematology/Oncology Departments of Medicine and Human Genetics The University of Chicago My patients and
Genetic Predisposition Syndromes in Myeloid Malignancies Lucy A. Godley, M.D., Ph.D. Section of Hematology/Oncology Departments of Medicine and Human Genetics The University of Chicago My patients and
Acute leukemia and myelodysplastic syndromes
 11/01/2012 Post-ASH meeting 1 Acute leukemia and myelodysplastic syndromes Peter Vandenberghe Centrum Menselijke Erfelijkheid & Afdeling Hematologie, UZ Leuven 11/01/2012 Post-ASH meeting 2 1. Acute myeloid
11/01/2012 Post-ASH meeting 1 Acute leukemia and myelodysplastic syndromes Peter Vandenberghe Centrum Menselijke Erfelijkheid & Afdeling Hematologie, UZ Leuven 11/01/2012 Post-ASH meeting 2 1. Acute myeloid
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine
 Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
EXAMPLE. - Potentially responsive to PI3K/mTOR and MEK combination therapy or mtor/mek and PKC combination therapy. ratio (%)
 Dr Kate Goodhealth Goodhealth Medical Clinic 123 Address Road SUBURBTOWN NSW 2000 Melanie Citizen Referring Doctor Your ref Address Dr John Medico 123 Main Street, SUBURBTOWN NSW 2000 Phone 02 9999 9999
Dr Kate Goodhealth Goodhealth Medical Clinic 123 Address Road SUBURBTOWN NSW 2000 Melanie Citizen Referring Doctor Your ref Address Dr John Medico 123 Main Street, SUBURBTOWN NSW 2000 Phone 02 9999 9999
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins
 Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Myelodysplastic syndromes post ASH Dominik Selleslag AZ Sint-Jan Brugge
 Myelodysplastic syndromes post ASH 2016 Dominik Selleslag AZ Sint-Jan Brugge Why did they put MDS at the end of the meeting? Possible explanations Least fascinating disease without progress? Poor speaker?
Myelodysplastic syndromes post ASH 2016 Dominik Selleslag AZ Sint-Jan Brugge Why did they put MDS at the end of the meeting? Possible explanations Least fascinating disease without progress? Poor speaker?
Objectives. Morphology and IHC. Flow and Cyto FISH. Testing for Heme Malignancies 3/20/2013
 Molecular Markers in Hematologic Malignancy: Ways to locate the needle in the haystack. Objectives Review the types of testing for hematologic malignancies Understand rationale for molecular testing Marcie
Molecular Markers in Hematologic Malignancy: Ways to locate the needle in the haystack. Objectives Review the types of testing for hematologic malignancies Understand rationale for molecular testing Marcie
NGS IN ONCOLOGY: FDA S PERSPECTIVE
 NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
MEDICAL GENOMICS LABORATORY. Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG)
 Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias
 Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias Relevant financial relationships in the past twelve months by presenter or spouse/partner. Speakers bureau: Novartis, Janssen, Gilead, Bayer The
Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias Relevant financial relationships in the past twelve months by presenter or spouse/partner. Speakers bureau: Novartis, Janssen, Gilead, Bayer The
IntelliGENSM. Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community.
 IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
Technical Bulletin No. 100
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 100 August 2, 2012 JAK2 AND MPL 515 MUTATIONAL ANALYSIS Contact: Dr. Jeffrey Wisotzkey, 717-851-1422 Technical Director, CPAL Jill A.
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 100 August 2, 2012 JAK2 AND MPL 515 MUTATIONAL ANALYSIS Contact: Dr. Jeffrey Wisotzkey, 717-851-1422 Technical Director, CPAL Jill A.
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma. Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute
 Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Genetic analysis and preclinical modeling of leukemic transformation of myeloproliferative neoplasms: Implications for therapeutic strategies
 Genetic analysis and preclinical modeling of leukemic transformation of myeloproliferative neoplasms: Implications for therapeutic strategies Raajit Rampal, MD, PhD Assistant Attending Physician Leukemia
Genetic analysis and preclinical modeling of leukemic transformation of myeloproliferative neoplasms: Implications for therapeutic strategies Raajit Rampal, MD, PhD Assistant Attending Physician Leukemia
Genetic Testing for Somatic Tumor Markers
 Genetic Testing for Somatic Tumor Markers MP9486 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes-as shown below Genetic testing is covered for a
Genetic Testing for Somatic Tumor Markers MP9486 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes-as shown below Genetic testing is covered for a
