PERIAMPULLARY AND PANCREATIC ADENOCARCINOMA
|
|
|
- Shawn Grant
- 6 years ago
- Views:
Transcription
1 gastrointestinal tract and abdomen PERIAMPULLARY AND PANCREATIC ADENOCARCINOMA Clifford S. Cho, MD, FACS Appropriate surgical management of periampullary adenocarcinoma (ampullary adenocarcinoma, duodenal adenocarcinoma, and distal cholangiocarcinoma) and pancreas adenocarcinoma requires a familiarity with both anatomy and cancer biology. This chapter describes the clinical behavior of the various subtypes of periampullary adenocarcinoma, the appropriate diagnostic evaluation of the patient afflicted with these malignancies, the surgical anatom y of the pancreas and peripancreatic region, and the nature and outcome of contemporary therapeutic interventions. Periampullary Adenocarcinoma: Subtypes and Specific Clinical Considerations Because the ampulla of Vater lies at the anatomic intersection of the pancreas, biliary system, and duodenum, periampullary adenocarcinoma is a conglomerative term that includes the more specific diagnoses of pancreatic adenocarcinoma, distal cholangiocarcinoma, ampullary adenocarcinoma, and duodenal adenocarcinoma. The utility of this term is particularly evident during preoperative evaluation, when precise differentiation between these four diagnostic entities can be difficult. All four of these malignant processes can present with very similar clinical symptoms and diagnostic signs, and the operative management of all four is identical (pancreaticoduodenectomy). However, the four diagnoses vary with regard to their biologic behavior and responsiveness to chemotherapy, and appropriate management should be informed by an understanding of their subtle but significant differences. Importantly, the long-term prognosis is generally poor for patients with pancreatic adenocarcinoma and distal cholangiocarcinoma and is comparatively favorable for patients with duodenal adenocarcinoma; the outlook for patients with ampullary adenocarcinoma is somewhat intermediate. 1 Adenocarcinoma arising from the ampulla can be divided into two subtypes: intestinal type, which exhibits a biologic behavior and prognosis similar to that of duodenal adenocarcinoma, and pancreaticobiliary type, which behaves like pancreatic adenocarcinoma and distal cholangiocarcinoma. 2 For the purposes of this chapter, we outline the four traditional categories of periampullary adenocarcinoma in terms of their incidence, pathology, prognosis/staging, and therapy. pancreatic adenocarcinoma Incidence Pancreatic ductal adenocarcinoma represents the largest subset of periampullary adenocarcinoma. It is the second most common gastrointestinal tract malignancy and the fourth leading cause of cancer-related mortality in the United States. Risk factors for pancreatic adenocarcinoma in this country include family history, increasing age, male 2013 Decker Intellectual Properties Inc gender, African-American race, tobacco use, and chronic pancreatitis. Genetic syndromes that appear to predispose individuals toward pancreatic adenocarcinoma include Peutz-Jeghers syndrome, hereditary pancreatitis, hereditary nonpolyposis colon cancer (Lynch syndrome), familial breast cancer associated with the BRCA2 mutation, and familial atypical multiple mole melanoma (FAMMM) syndrome. Pathology The histologic appearance of pancreatic adenocarcinoma is characterized by neoplastic epithelial tumor cells and a densely fibrotic stroma. The marked nuclear atypia and easy infiltration of tumor cells into lymphovascular and perineural tissues reflect its biologic aggressiveness. It has also become apparent that the desmoplastic stroma interacts actively with tumor cells to promote their growth and invasion while impairing the delivery and efficacy of chemotherapy. It is now evident that pancreas adenocarcinoma may arise from precursor lesions known as pancreatic intraepithelial neoplasms (PanINs) that progress from benign toward malignant phenotypes as they accumulate critical gene mutations. 3 Histologically, this progression can be measured by the presence of PanIN-1, PanIN-2, and PanIN-3 lesions, which exhibit sequentially increasing levels of cellular and architectural abnormality. A well-known and defining characteristic of pancreatic adenocarcinoma is its aggressiveness. Based on the remarkably high prevalence of patients who present with metastati c disease at the time of diagnosis, it is evident that this cancer is capable of early systemic dissemination. Indeed, based on the remarkably high prevalence of patients who develop metastatic disease very shortly after undergoing potentially curative resection, it is likely that the majority of pancreatic adenocarcinoma cases have developed systemic dissemination by the time the diagnosis is made. Prognosis and Staging As with all of the other subtypes of periampullary adenocarcinoma, the American Joint Committee on Cancer (AJCC) staging system for pancreatic adenocarcinoma is based on the tumor-node-metastasis (TNM) scheme. 4 The staging system for pancreatic adenocarcinoma takes into consideration the prognostic relevance of such variables as tumor size and the presence of nodal and distant metastases [see Table 1]. In addition, it accounts for the prognostic difference between resectable and unresectable tumors, which is determined by tumoral invasion into local vascular structures that cannot be resected and reconstructed. As such, an important distinction exists between T3 tumors, which extend beyond the pancreas but not into the celiac axis or superior mesenteric artery (and are likely to be resectable), and T4 tumors, which involve the celiac axis and superior mesenteric artery (and are unresectable). Stage III disease, which is defined by the DOI /
2 gastro periampullary and pancreatic adenocarcinoma 2 Table 1 AJCC Staging System for Pancreatic Adenocarcinoma 4 Primary tumor (T) Tx Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ (PanIN-3) T1 Tumor limited to pancreas, 2 cm in greatest dimension T2 Tumor limited to pancreas, > 2 cm in greatest dimension T3 Tumor extends beyond pancreas but without involvement of the celiac axis or the superior mesenteric artery T4 Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) Regional lymph nodes (N) Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastases N1 Regional lymph node metastasis Distant metastasis (M) M0 No distant metastasis M1 Distant metastasis Stage 0 Tis N0 M0 Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T3 N0 M0 Stage IIB T1 N1 M0 T2 N1 M0 T3 N1 M0 Stage III T4 Any N M0 Stage IV Any T Any N M1 AJCC = American Joint Committee on Cancer; PanIN = pancreatic intraepithelial neoplasm. presence of a T4 tumor, implies locally advanced disease. Other pathologic features that have been shown to portend a worse prognosis in pancreatic adenocarcinoma are poor differented histology, perineural invasion, and lymphovascular invasion. In addition to the traditional TNM staging convention, patients with pancreatic adenocarcinoma are also classified into the more functional categories of potentially resectable, borderline resectable, locally advanced, and metastatic disease [see Table 2]. This classification system provides information about both the extent of disease and the options for therapeutic intervention. 5 Unfortunately, the anatomic criteria of tumor resectability have been subject to variability, and efforts to develop consensus guidelines distinguishing potentially resectable, borderline resectable, and locally advanced disease have only recently been under way. 6 8 Patients with potentially resectable disease are those whose radiographic studies identify primary tumors with no local vascular involvement and no evidence of distant metastatic disease; from an anatomic standpoint, these patients are likely to be candidates for complete operative resection of their pancreatic tumors [see Figure 1]. In reality, only 80 to 90% of patients with potentially resectable disease are able to undergo resection as evidence of distant metastasis or technically unresectable disease is found in 10 to 20% of cases at the time of operative exploration. When managed with surgical resection, the median survival of patients with Table 2 Categories of Pancreatic Adenocarcinoma 6,7 Potentially resectable No distant metastatic disease Normal fat plane around SMA Normal fat plane around celiac axis and hepatic artery Patent portal vein/superior mesenteric vein Borderline resectable No distant metastatic disease Tumor abuts 180 SMA circumference Short segment abutment/encasement of common and hepatic artery Short segment occlusion of portal vein/superior mesenteric vein with normal proximal/distal segments Locally advanced No distant metastatic disease Tumor involves > 180 SMA circumference Unreconstructable encasement of common hepatic artery (celiac axis involvement) Unreconstructable occlusion of portal vein/superior mesenteric vein (long segment encasement) Metastatic Distant metastatic disease SMA = superior mesenteric artery. resectable disease is generally in the range of 18 to 24 months. Patients with borderline resectable disease harbor radiographic evidence of venous involvement that is amenable to reconstruction and/or minimal arterial involvement with no evidence of distant metastatic disease [see Figure 2]. Anatomically, these patients may be candidates for surgical resection but are at higher risk for having evidence of unresectable disease at the time of operative exploration or undergoing margin-positive resection. For this reason, patients with borderline resectable disease are typically treated with preoperative chemotherapy with or without radiation therapy, with the intention of attempting resection as long as posttreatment imaging does not show evidence of disease progression to locally advanced or metastatic disease. Unlike locally advanced disease, the absence of radiographic regression in patients with borderline resectable disease does not imply that resection should not be attempted. Recent studies suggest that approximately 50 to 70% of patients with borderline resectable disease are ultimately able to undergo resection after preoperative therapy. 5 8 When resection is possible, patients with borderline resectable disease are more likely to require venous resection and reconstruction than patients with potentially resectable disease. The median survival for all patients with borderline resectable disease is approximately 14 months; however, accumulating experience with multimodality management suggests that the median survival of patients with borderline resectable disease who ultimately undergo surgical resection is approximately 20 months, which is comparable to that of patients with potentially resectable disease. Locally advanced disease is defined by the presence of local vascular involvement that cannot be overcome by vascular resection and reconstruction, with no evidence of distant metastatic disease [see Figure 3]. Locally advanced disease is, by definition, not amenable to operative resection
3 gastro periampullary and pancreatic adenocarcinoma 3 Tumor Superior Mesenteric Vein Endobiliary Stent Superior Mesenteric Artery Figure 1 Potentially resectable pancreatic adenocarcinoma. This computed tomographic scan demonstrates normal fat planes around the superior mesenteric vein and superior mesenteric artery. This patient was treated with pancreaticoduodenectomy. Superior Mesenteric Vein Tumor Superior Mesenteric Artery Figure 2 Borderline resectable pancreatic adenocarcinoma. This computed tomographic scan demonstrates short segment abutment of the superior mesenteric vein and limited abutment of the superior mesenteric artery by tumor. This patient was treated with neoadjuvant chemoradiation followed by pancreaticoduodenectomy. and is typically managed with chemotherapy with or without radiation therapy. Unfortunately, these regimens are usually not successful in inducing sufficient disease regression to permit operative resection. In rare circumstances where resection does become possible, expected survival may approximate that seen among patients with potentially resectable disease. The median survival of patients with locally advanced disease is approximately 6 to 12 months. At present, patients with metastatic disease are only eligible for systemic chemotherapy, and their median survival is generally 3 to 9 months [see Figure 4]. As is the case with patients with locally advanced disease, operative intervention is typically reserved for palliation (e.g., gastrojejunostomy for duodenal obstruction and hepaticojejunostomy for biliary obstruction). However, most of these tumor-related complications can be managed using endoscopic and percutaneous interventional radiologic approaches (e.g., endoluminal duodenal stents for duodenal obstruction and biliary stents for biliary obstruction) that offer comparable efficacy with lower procedure-related morbidity. Therapy Treatment strategies for patients with pancreatic adenocarcinoma are tailored to their extent of disease as outlined Tumor Dilated Pancreatic Duct Celiac Axis Figure 3 Locally advanced pancreatic adenocarcinoma. This computed tomographic scan demonstrates unreconstructable encasement of the celiac axis by tumor. This patient was treated with radiation therapy and systemic chemotherapy. above. The surgical resection techniques outlined in this chapter are applied to patients with potentially resectable and borderline resectable disease and the rare patients with locally advanced disease who demonstrate significant disease regression following preoperative therapy. Systemic chemotherapy is offered to all categories of disease presentation, either as neoadjuvant or adjuvant therapy (or both) for patients with resectable and borderline resectable disease or as definitive palliative therapy for patients with locally advanced and metastatic disease. Radiation therapy can be offered either as neoadjuvant or adjuvant therapy for patients with resectable and borderline resectable disease as a means of maximizing local control of disease or as palliative therapy for patients with locally advanced and metastatic disease presenting with symptoms referable to their primary tumor (e.g., pain). A significant challenge in the care for patients with pancreatic adenocarcinoma has been its extreme resistance to systemic chemotherapy. Because of this therapeutic limitation and because of the very high incidence of systemic dissemination, the prognosis for most patients with pancreatic adenocarcinoma remains very poor. Great investigative effort has been expended to identify novel systemic treatment options, but chemotherapeutic options remain limited in number and efficacy. Randomized clinical trials have shown that the nucleotide analogue gemcitabine improves quality of life when used as definitive therapy for patients with advanced pancreatic adenocarcinoma and prolongs disease-free survival when used as adjuvant therapy following operative resection. 9 These studies have led to the acceptance of gemcitabine as a mainstay of chemotherapy for pancreatic adenocarcinoma. More recently, the combination of leucovorin, 5-fluorouracil (5-FU), irinotecan, and oxaliplatin (FOLFIRINOX) has been shown to promote modest prolongation of overall survival in comparison with gemcitabine for patients with metastatic pancreatic adenocarcinoma. 10 Based on these findings, FOLFIRINOX is currently being used and investigated in both the neoadjuvant
4 gastro periampullary and pancreatic adenocarcinoma 4 Superior Mesenteric Vein Tumor Superior Mesenteric Artery Hepatic Metastases Figure 4 Metastatic pancreatic adenocarcinoma. This computed tomographic scan demonstrates a mass in the head and uncinate process of the pancreas and multiple low-attenuation hepatic lesions consistent with foci of distant metastatic disease. This patient was treated with palliative systemic chemotherapy. and adjuvant settings for patients with potentially resectable, borderline resectable, and locally advanced pancreatic adenocarcinoma. However, because of its higher potential for treatment-related toxicity, FOLFIRINOX is generally reserved for patients with excellent performance status. Very recent data suggest that the novel combination of gemcitabine with nanoparticle albumin-bound paclitaxel may offer better outcomes than gemcitabine alone for patients with advanced pancreas cancer; if verified, this combination may eventually represent a better tolerated (but costly) alternative to FOLFIRINOX. Although adjuvant chemotherapy appears to be associate d with a modest improvement in survival, it has become evident that about 30% of patients do not go on to receive adjuvant therapy following pancreatic resection. 11 This observation and the success of preoperative therapy for patients with borderline resectable disease have prompted some centers to adopt a neoadjuvant approach for patients with potentially resectable disease. 12 Another potential advantage to the neoadjuvant approach is improved patient selection. The interval of 2 to 3 months needed to complete preoperative chemotherapy and radiation therapy may provide a window of time during which previously occult metastatic disease may become clinically evident; repeat imaging following completion of preoperative therapy may therefore be used to identify a subset of patients for whom nontherapeutic operative resection can be avoided. The role of radiation as a routine component of adjuvant therapy remains controversial; postoperative radiation therapy is most commonly used for patients with close or positive resection margins in an effort to minimize the likelihood of local tumor recurrence. Preoperatively, radiation therapy is used as a means to improve the chances of achieving negative resection margins for patients with resectable and borderline resectable disease. For patients with locally advanced or metastatic disease, radiation therapy may offer palliative benefit by ameliorating symptoms referable to local tumoral infiltration. distal cholangiocarcinoma Incidence Cholangiocarcinoma is adenocarcinoma of the biliary tree and can involve any portion of the intrahepatic or extrahepatic biliary system. Distal cholangiocarcinoma, involving the distal intrapancreatic portion of the common bile duct, comprises approximately 20 to 30% of all cholangiocarcinoma cases and about 10 to 20% of all periampullary adenocarcinoma cases. Pathology Distal cholangiocarcinoma has been subdivided into three macroscopic subtypes: sclerosing tumors are characterized by circumferential ductal thickening with extensive periductal fibrosis and inflammation; nodular tumors appear as firm mass lesions growing into the duct lumen; and papillary tumors typically present as soft, friable, pedunculated masses that project into the duct lumen. The important distinction is that papillary tumors are likely to be resectable and exhibit a more favorable prognosis. Histologically, the innermost layer of the bile duct is the inner mucosa. The mucosa is enveloped by subepithelial connective tissue, which is in turn surrounded by a muscular layer that is most prominent along the distal intrapancreatic portion. A layer of adipose tissue covers the wall of the bile duct. The AJCC staging system for distal cholangiocarcinoma uses a tumor (T) system that is defined by the depth of tumor invasion; invasion of adenocarcinoma into the perimuscular adipose tissue is considered to be invasion beyond the wall of the bile duct. 4 One important pathologic feature of cholangiocarcinoma is its insidious propensity for longitudinal spread along the bile duct. Tumor cells often extend well proximal and distal to the dominant mass beneath normal-appearing ductal mucosa. For this reason, great care must be taken to assess the proximal hepatic duct margin for malignant cells using frozen-section pathologic analysis at the time of surgical resection. Another important pathologic characteristic of cholangiocarcinoma is the strong desmoplastic reaction that
5 gastro periampullary and pancreatic adenocarcinoma 5 often surrounds these tumors. The difficulty of identifying nests of malignant cells within this dense stroma often makes preoperative cytopathologic diagnosis very challenging. Staging and Prognosis Unlike pancreatic adenocarcinoma but like ampullary and duodenal adenocarcinoma, the current AJCC staging system for distal cholangiocarcinoma bases its tumor (T) staging on tumor depth, not tumor size [see Table 3]. 4 Similar to staging for pancreatic adenocarcinoma, T4 status is defined by the presence of celiac axis or superior mesenteric artery involvement, which precludes operative resectability. Stages I through IV are defined in the same way as pancreatic adenocarcinoma. As with the other categories of periampullary adenocarcinoma, the major prognostic variables for distal cholangiocarcinoma are tumor depth, nodal status, distant metastases, histologic differentiation, perineural invasion, and lymphovascular invasion. Therapy For patients with resectable disease, operative therapy consists of pancreaticoduodenectomy. The prevalence of radiologically occult peritoneal metastases and the resulting yield of staging laparoscopy are lower than with pancreatic adenocarcinoma. However, staging laparoscopy may be performed when these two diagnoses cannot be distinguished preoperatively. Because of the propensity of cholangiocarcinoma to spread along the biliary tree, particular Table 3 AJCC Staging System for Distal Cholangiocarcinoma 4 Primary tumor (T) Tx Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ T1 Tumor confined to the bile duct histologically T2 Tumor invades beyond the wall of the bile duct T3 Tumor invades the gallbladder, pancreas, duodenum, or other adjacent organs without involvement of the celiac axis or the superior mesenteric artery T4 Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) Regional lymph nodes (N) Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastases N1 Regional lymph node metastasis Distant metastasis (M) M0 No distant metastasis M1 Distant metastasis Stage 0 Tis N0 M0 Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T3 N0 M0 Stage IIB T1 N1 M0 T2 N1 M0 T3 N1 M0 Stage III T4 Any N M0 Stage IV Any T Any N M1 AJCC = American Joint Committee on Cancer. attention is paid to the hepatic duct margin at the time of operation, and resection of an additional segment of hepatic duct is undertaken for cases of margin positivity. Guidelines for the use of chemotherapy are less well defined than for pancreatic adenocarcinoma, but gemcitabine-based chemotherapy is typically offered as adjuvant therapy or for patients with node-positive disease or as definitive therapy for patients with unresectable or metastatic disease. As with pancreatic adenocarcinoma, radiation therapy is typically reserved for adjuvant therapy following resection with close or positive resection margins or for palliation of tumorrelated symptoms in cases of unresectable disease. ampullary adenocarcinoma Incidence Adenocarcinoma arising from the ampulla of Vater is relatively rare, representing less than 1% of all gastrointestinal cancers and 10 to 20% of periampullary adenocarcinoma cases. Patients with familial adenomatous polyposis are known to be at higher risk for the development of ampullar y adenocarcinoma. Pathology The ampulla of Vater consists of the ductal lining of the distal common bile duct and pancreatic duct and the intestinal epithelium that overlies this duct. Recent studies suggest that ampullary adenocarcinoma consists of two histologic subtypes. 2 Intestinal-type ampullary adenocarcinoma appears to originate from the intestinal epithelium of the ampulla and is likely to arise from the adenoma to adenocarcinoma sequence of mutational genesis that characterizes other intestinal adenocarcinoma tumors. In contrast, pancreaticobiliary-type ampullary adenocarcinoma is likely to originate from the endothelial lining of the ductal component and may develop from dysplastic precursor intraepithelial neoplasms that progress toward adenocarcinoma, as is seen with pancreatic adenocarcinoma. In cases of larger tumors, where the epicenter of origin may be difficult to discern, it may become difficult to distinguish between intestinal-type ampullary adenocarcinoma and duodenal adenocarcinoma or between pancreaticobiliary-type ampullary adenocarcinoma and distal cholangiocarcinoma or pancreatic adenocarcinoma. However, these distinctions may be of little clinical consequence as the prognosis and treatment of these alternative diagnoses are very similar; indeed, it has been argued that all cases of periampullary adenocarcinoma may be more appropriately divided into two broad categories of intestinal versus pancreaticobiliary adenocarcinoma. Most cases of ampullary adenocarcinoma are well differentiated in their histologic appearance. Staging and Prognosis Because of their location, ampullary adenocarcinoma commonly presents at an earlier stage than pancreatic adenocarcinoma as even small tumors are capable of causing symptoms of obstructive jaundice. Accordingly, ampullary adenocarcinoma is more commonly resectable at the time of diagnosis than pancreatic adenocarcinoma. The current AJCC staging system for ampullary adenocarcinoma incorporates tumor depth and nodal and distant metastases;
6 gastro periampullary and pancreatic adenocarcinoma 6 unlike pancreatic adenocarcinoma but like distal cholangiocarcinoma and duodenal adenocarcinoma, tumor (T) staging is defined by depth of invasion (confinement within the ampulla or invasion into the duodenum or pancreas or beyond) rather than tumor size [see Table 4]. 4 Stages I through IV are defined in the same way as pancreatic adenocarcinoma and distal cholangiocarcinoma. Like the other categories of periampullary adenocarcinoma, pathologic variables that have been shown to have prognostic significance for ampullary adenocarcinoma include depth of invasion, nodal status, histologic differentiation, perineural invasion, and lymphovascular invasion. The histologic distinction of intestinal versus pancreaticobiliary type has also been shown to be of central prognostic importance as survival outcomes following pancreaticoduodenectomy have been shown to be favorable among patients with intestinal-type ampullary adenocarcinoma. 2 Therapy For patients with resectable disease, operative conduct involves pancreaticoduodenectomy. The prevalence of radiologically occult peritoneal metastases is low enough to avoid the need for routine staging laparoscopy in cases where a definitive diagnosis of ampullary adenocarcinoma is made preoperatively. Ampullectomy, in which the ampulla of Vater is resected and the pancreatic and bile ducts are reapproximated through a transduodenal approach, is used for resection of adenomatous polyps with no evidence of invasive disease. This less radical approach obviously does not permit resection and analysis of lymph Table 4 AJCC Staging System for Ampullary Adenocarcinoma 4 Primary tumor (T) Tx Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ T1 Tumor limited to the ampulla of Vater T2 Tumor invades the duodenal wall T3 Tumor invades the pancreas T4 Tumor invades peripancreatic soft tissues or other adjacent organs or structures other than pancreas Regional lymph nodes (N) Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastases N1 Regional lymph node metastasis Distant metastasis (M) M0 No distant metastasis M1 Distant metastasis Stage 0 Tis N0 M0 Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T3 N0 M0 Stage IIB T1 N1 M0 T2 N1 M0 T3 N1 M0 Stage III T4 Any N M0 Stage IV Any T Any N M1 AJCC = American Joint Committee on Cancer. nodes. Unfortunately, even early stages of invasion (e.g., T1 ampullary adenocarcinoma) are associated with a real risk of nodal metastasis; therefore, ampullectomy is not recommended for even early cases of ampullary adenocarcinoma due to the risk of understaging and the risk of marginpositive resection. Guidelines for the use of chemotherapy are not rigorously defined for ampullary adenocarcinoma. 5-FU-based chemotherapeutic regimens such as FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) that are effective elsewhere in the gastrointestinal alimentary tract are generally employed for intestinal-type ampullary adenocarcinom a (either as adjuvant therapy for node-positive disease or as systemic therapy for unresectable disease). In contrast, gemcitabine-based regimens are generally employed for pancreaticobiliary-type ampullary adenocarcinoma. duodenal adenocarcinoma Incidence Although duodenal adenocarcinoma represents less than 1% of all alimentary tract malignancies, the duodenum is the location of approximately 50% of all small intestinal adenocarcinoma. The majority of duodenal adenocarcinoma cases are sporadic, but conditions such as familial adenomatous polyposis, hereditary nonpolyposis colon cancer syndrome (Lynch syndrome), Crohn disease, Peutz-Jeghers syndrome, and neurofibromatosis type 1 (von Recklinghausen disease) are associated with an increased risk of duodenal adenocarcinoma. Pathology Like other forms of alimentary tract adenocarcinoma, duodenal adenocarcinoma likely develops from antecedent adenomas through sequential genetic mutations. An obvious anatomic feature that distinguishes the pathologic behavior of duodenal adenocarcinoma is the close apposition of the proximal duodenum to the ampulla, distal bile duct, and pancreas; unlike other regions of the small intestine, where resection of associated lymph nodes involves simple mesenteric resection, resection of lymph nodes associated with the duodenum often requires pancreaticoduodenectomy. Staging and Prognosis The current AJCC staging system for duodenal adenocarcinoma is identical to that of other forms of small intestinal adenocarcinoma [see Table 5]. 4 The tumor (T) staging system is based on tumor depth. T4 status is defined by the presence of invasion into adjacent organs and structures (such as the bile duct or pancreas); unlike pancreatic adenocarcinoma or distal cholangiocarcinoma, T4 status does not necessarily imply the presence of unresectable disease. Unlike the other categories of periampullary adenocarcinoma, the nodal (N) staging system accounts for the number of involved lymph nodes, with N1 disease signifying the presence of one to three nodal metastases and N2 disease signifying metastasis to four or more regional lymph nodes. Stage I implies a superficial tumor with no nodal metastases, stage II implies a deep tumor with no nodal metastases, and stage III implies the presence of nodal metastases.
7 gastro periampullary and pancreatic adenocarcinoma 7 Table 5 AJCC Staging System for Duodenal Adenocarcinoma 4 Primary tumor (T) Tx Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ T1a Tumor invades lamina propria T1b Tumor invades submucosa T2 Tumor invades muscularis propria T3 Tumor invades through muscularis propria into subserosa or adjacent mesentery or retroperitoneum to a depth 2 cm T4 Tumor perforates visceral peritoneum or directly invades other loops of small intestine, mesentery, retroperitoneum to a depth > 2 cm or abdominal wall or pancreas or bile duct Regional lymph nodes (N) Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastases N1 Metastasis in 1 3 regional lymph nodes N2 Metastasis in 4 regional lymph nodes Distant metastasis (M) M0 No distant metastasis M1 Distant metastasis Stage 0 Tis N0 M0 Stage I T1 N0 M0 T2 N0 M0 Stage IIA T3 N0 M0 Stage IIB T4 N0 M0 Stage IIIA Any T N1 M0 Stage IIIB Any T N2 M0 Stage IV Any T Any N M1 AJCC = American Joint Committee on Cancer. Therapy For resectable cases of periampullary duodenal adenocarcinoma, pancreaticoduodenectomy is the operative procedure of choice. Staging laparoscopy is typically reserved for cases where the alternative diagnosis of pancreatic adenocarcinoma cannot be excluded based on preoperative investigations. Pancreas-sparing duodenal resection with duodenojejunostomy reconstruction can be performed in circumstances where the tumor is located well distal to the ampulla. As with intestinal-type ampullary adenocarcinoma, 5-FU-based regimens are employed for cases that warrant systemic chemotherapy, such as node-positive disease following resection or cases of unresectable disease. Advanced tumors in the duodenum may be prone to forms of local invasion not commonly seen with other periampullary adenocarcinoma, such as invasion into the inferior vena cava or right kidney. In these circumstances, preoperative chemotherapy or chemoradiation may be employed as a means of enhancing the likelihood of performing a marginpositive resection. In cases of unresectable disease, radiation therapy can also be used to palliate tumor-related symptoms such as pain or bleeding. Surgical Anatomy pancreatic head Anatomic Considerations The head of the pancreas rests within the first, second, and third portions of the duodenum [see Figure 5, a, b]. It is surrounded superiorly by the porta hepatis, posteriorly by the left renal vein and aortocaval space, and anteriorly and inferiorly by the transverse mesocolon. Operative exposure of the pancreatic head therefore begins by dissecting along the avascular plane between the transverse mesocolon and duodenum and pancreas, reflecting the transverse colon caudally [see Figure 5c]. Dissection along this plane permits exposure of the second and third portions of the duodenum and the superior mesenteric vein below the level of the pancreas. The retroperitoneum along the lateral border of the duodenal sweep and porta hepatis is then incised, and the pancreatic head is elevated by dividing its posterior avascular plane (the Kocher maneuver). This mobilization can be taken all the way to the neck of the pancreas and distal duodenum. Within the parenchyma of the pancreatic head, the bile duct and pancreatic duct most commonly join to form a common channel that empties into the ampulla of Vater at a point between the superior two thirds and inferior one third of the second portion of the duodenum. Operative exposure of the ampulla therefore typically requires a longitudinally oriented lateral incision along the distal half of the second duodenal portion. Clinical Considerations Because of the proximity of the pancreatic and bile ducts, tumors of the periampullary region and pancreatic head often present with clinical and radiographic evidence of ductal obstruction. Clinical symptoms and signs of bile duct obstruction typically precede those of pancreatic ductal obstruction. As a result, the initial manifestation of periampullary and pancreatic head malignancies is often jaundice without cholangitic fevers or pain. Radiographic evidence of intrahepatic and extrahepatic biliary dilatation and pancreatic ductal dilatation is common and diagnostic of mechanical obstruction. Because of the anatomic intimacy of the distal biliary system, pancreatic head, and duodenum, oncologic resection of even early periampullary malignancies usually requires pancreaticoduodenectomy. Consideration can be given to a more limited resection in cases of premalignant adenomatous tumors of the duodenum or ampulla of Vater, where margin clearance and nodal status are not of paramount clinical concern. porta hepatis Anatomic Considerations Enveloped in a peritoneal sheath, the porta hepatis resides at the right lateral aspect of the hepatoduodenal ligament and houses a triad of structures: the common hepatic/bile duct, hepatic artery, and portal vein. Along its inferior aspect, the common bile duct is found in the right anterior aspect of the porta hepatis before it traverses behind and then into the pancreatic head. A portion of the intrapancreatic distal common bile duct can be dissected out of the
8 gastro periampullary and pancreatic adenocarcinoma 8 a Head Uncinate Process Neck Body Tail b Portal Vein Inferior Vena Cava Gallbladder Right Kidney Large Intestine Liver Stomach Diaphragm Adrenal Gland Spleen Left Kidney Duodenojejunal Flexure c d Superior Mesenteric Vein and Artery LV ST P D C SI BL R Figure 5 (a) General morphology of the pancreas. (b) The relationship of the pancreas to other organs. (c) The relationship of the pancreas to other organs shown sagitally. (d) The uncinate process, which can vary in medial extent to the superior mesenteric vein (SMV) (a), under the SMV (b), or behind the superior mesenteric artery (c). BL = bladder; C = colon; D = duodenum; LV = liver; P = pancreas; R = rectum; SI = small intestine; ST = stomach.
9 gastro periampullary and pancreatic adenocarcinoma 9 pancreas, but care must be taken to avoid injury to the pancreatic duct at their point of confluence. The proper hepatic artery is located at the left anterior aspect of the porta hepatis, where the common hepatic artery, proper hepatic artery, and gastroduodenal artery can be dissected. The common hepatic artery can be dissected back to its origin at the celiac axis. The portal vein runs posteriorly in the porta hepatis. The portal vein can be exposed from its anterior aspect between the common bile duct and the proper hepatic artery; this exposure is facilitated by dividing the overlying gastroduodenal artery. Alternatively, the portal vein can also be exposed by incising the peritoneal sheath over the right posterolateral porta hepatis between the common bile duct and the portal vein and elevating the common bile duct anteriorly. The common hepatic artery and portocaval lymph nodes are often large, even in the absence of metastatic involvement. Blunt dissection can be undertaken along the avascular plane between the anterior aspect of the portal vein and the posterior aspect of the pancreatic neck; obliteration of this avascular tissue plane in the setting of cancer typically indicates the presence of tumor invasion into the portal vein or superior mesenteric vein. Clinical Considerations Because of the prevalence of obstructive jaundice in patients with periampullary and pancreatic head malignancies, biliary decompression using endobiliary stents is often performed prior to operative resection. Stent insertion or subclinical bouts of cholangitis resulting from biliary instrumentation can promote inflammatory changes that can make operative dissection of the porta hepatis more challenging. Inflammation may also lead to enlarged portal lymph nodes in the absence of metastatic disease. During pancreaticoduodenectomy, the inflow into the pancreas and duodenum through the gastroduodenal artery must be divided; care must be taken to ensure proper delineation of the trifurcation of the common hepatic artery, proper hepatic artery, and gastroduodenal artery as inadvertent division of the proper hepatic artery will result in arterial ischemia to the liver. pancreatic neck and uncinate process Anatomic Considerations The neck of the pancreas is the portion of the pancreas that is anterior to the portal vein, superior mesenteric vein, and superior mesenteric artery [see Figure 5a]. The portal vein passes behind the pancreatic neck at its superior aspect, and the superior mesenteric artery and vein emerge from behind the pancreatic neck at its inferior aspect. The uncinate process of the pancreas resides posterior and medial to the superior mesenteric vein and artery [see Figure 5d]. The superior aspect of the pancreatic neck can be exposed by dissecting along the portal vein as described above. Access to the inferior aspect of the pancreatic neck is facilitated by retracting the head of the pancreas to the right and dissecting the uncinate process off the right lateral border of the superior mesenteric vein. The right gastroepiploic vein and middle colic vein typically converge onto a common trunk entering the superior mesenteric vein just below the pancreatic neck. The superior mesenteric artery is posterior and to the left of the superior mesenteric vein; as a result, it can be accessed following a Kocher maneuver by rotating the pancreatic neck and superior mesenteric vein anteriorly; this maneuver effectively pulls the superior mesenteric artery toward the right, exposing it to the surgeon s view. The insertion of the splenic vein onto the superior mesenteric vein and the origin of the splenic artery from the celiac axis are both posterior to the pancreatic neck. As a result, full exposure of these structures usually requires transection of the pancreatic neck. Clinical Considerations Because of the location of the celiac axis and superior mesenteric vessels, tumors arising from the pancreatic neck are often the most challenging in terms of resectability. The proximity of the superior mesenteric vein means that tumors arising from the uncinate process often require concomitant venous resection and reconstruction. pancreatic body and tail Anatomic Considerations The body of the pancreas is found posterior to the stomach, and the tail of the pancreas rests against the splenic hilum [see Figure 5a]. The splenic vein courses along the body of the pancreas and is typically adherent to its posterior aspect. In contrast, the splenic artery usually follows a more tortuous and extrapancreatic course and can often be found above the superior aspect of the pancreatic body. Because of its location within the lesser sac, the pancreatic body and tail are best approached either through the gastrocolic ligament or through the gastrohepatic ligament. Full exposure of the pancreatic tail is facilitated by dividing the short gastric vessels. The pancreatic body and tail are mobilized by incising the retroperitoneum along the superior and inferior aspects of the pancreatic body and dissecting along the avascular plane between the pancreas and deeper retroperitoneal structures such as the aorta and left adrenal gland. Mobilization of the pancreatic tail can be facilitated by dividing the peritoneal and diaphragmatic attachments of the spleen, elevating the spleen anteriorly, and dissecting along the avascular plane behind the splenic hilus and pancreatic tail from the left. Clinical Considerations Unlike right-sided pancreatic tumors, tumors arising from the pancreatic body and tail are unlikely to cause obstructive jaundice. Although left-sided pancreatic tumors may obstruct the pancreatic duct, clinical manifestations of pancreatic ductal obstruction are more insidious and less noticeable than those of biliary obstruction. As a result, leftsided tumors are typically more advanced in terms of size and metastatic dissemination than right-sided tumors. One ominous sign of left-sided pancreatic tumors is intractable midback pain as this can be a result of tumoral infiltration into the para-aortic neural tissues behind the body of the pancreas. Long-standing malignant obstruction of the pancreatic duct can cause clinical symptoms of malabsorptive steatorrhea resulting from impaired pancreatic exocrine function and radiographic findings of pancreatic ductal dilatation and parenchymal atrophy of the obstructed portion of
10 gastro periampullary and pancreatic adenocarcinoma 10 the pancreas. On the other hand, unlike the pancreatic head, neck, and uncinate process, much of the pancreatic body is free from critical vascular structures. Moreover, unlike the portal vein, superior mesenteric vein, or superior mesenteric artery, the splenic artery and vein can be resected with impunity. Consequently, resection of left-sided tumors can often be performed without the need for vascular reconstruction. To remove the lymph nodes along the splenic artery and vein, splenic hilum, and spleen, a concomitant en bloc splenectomy is typically incorporated into a left pancreatectomy when resection is performed for malignancy; in contrast, consideration can be given to a spleen-preserving left pancreatectomy when it is performed for premalignant tumors (e.g., cystic lesions) or tumors with a low potential for lymphatic dissemination (e.g., metastases to the pancreas). Periampullary and Pancreatic Adenocarcinoma: General Clinical Considerations diagnosis and staging A common manifestation of periampullary adenocarcinoma is biliary obstruction. Symptoms of biliary obstruction that prompt patients to seek medical attention include acholic stools, dark urine, pruritis, and cutaneous jaundice. Other general symptoms of pancreatic adenocarcinoma not specifically confined to those originating in the right pancreas include anorexia and weight loss. Very often, the only abnormal physical examination finding is that of jaundice; classic findings of cachexia, ascites, left supraclavicular lymphadenopathy (Virchow node), periumbilical peritoneal metastases (Sister Mary Joseph node), or pararectal pelvic drop metastases (Blumer shelf) are generally indicative of advanced disease. Laboratory evidence of a direct bilirubinemia in conjunction with elevated canalicular enzymes (alkaline phosphatase, c-glutamyltransferase) supports the diagnosis of biliary obstruction. In this setting, radiographic diagnostics are directed toward the confirmation and staging of cancer. Although transabdominal ultrasonography is useful for confirming biliary dilatation, this information typically adds little beyond that which can already be construed from laboratory blood tests; moreover, ultrasonography usually does not image the pancreas clearly enough to permit accurate tumor localization or staging. In contrast, cross-sectional imaging with contrast-enhanced computed tomography (CT) or magnetic resonance (MR) can provide information about tumor localization, resectability as determined by the anatomic relationship of tumor to adjacent vascular structures, and the presence or extent of metastatic dissemination. In cases of resectable disease, imaging studies must be thoroughly studied prior to undertaking resection to anticipate the potential need for variations in operative conduct (e.g., areas of vascular invasion by tumor or aberrant vascular anatomy). Common sites of metastatic spread are the lymph nodes, the liver, and peritoneal surfaces. The presence of enlarged, rounded, or centrally necrotic lymph nodes is suggestive of nodal metastasis. Hepatic metastases typically present as low-attenuation rounded lesions within the liver, and peritoneal metastases often appear as areas of abnormal nodularity along the omentum or peritoneal surfaces or as unexplained free fluid within dependent portions of the abdominopelvic cavity (the perihepatic and hepatorenal spaces and true pelvis). Positron emission tomography (PET) is of limited utility in the staging of periampullary and pancreatic adenocarcinom a because of the potential for false negative studies. Endoscopic modalities are an important component of the diagnostic algorithm for patients with periampullary adenocarcinoma. Endoscopic retrograde cholangiopancreatography (ERCP) is a means of obtaining cholangiographic imaging of distal biliary obstructive lesions, collecting brushings of the distal biliary obstructive lesion for cytologic analysis, and correcting biliary obstruction with the placement of transpapillary endobiliary stents. Patients presenting with obstructive jaundice may benefit from biliary decompression. In addition to restoring hepatic function and ameliorating the complications of impaired biliary excretion (e.g., coagulopathy resulting from impaired bile-mediated intestinal absorption of vitamin K), correction of obstructive jaundice provides significant palliation of pruritis. For patients with resectable disease, these potential benefits should be weighed against the risk of increased postoperative infectious complications that has been associated with preoperative biliary decompression. 13,14 Therefore, preoperative stent placement should be used selectively for patients with marked hyperbilirubinemia or physiologic consequences of jaundice (cholangitis, coagulopathy, intractable pruritis). If stenting is necessary in patients who will undergo resection, preference is given to placement of temporary plastic stents. Plastic endobiliary stents are prone to occlusion and require repeat endoscopic exchanges every 2 to 3 months; therefore, patients who are unlikely to undergo operative resection or who will likely require a prolonged course of preoperative therapy should receive self-expanding metallic stents, whose average patency approaches 1 year. In contrast to percutaneous transhepatic approaches to biliary stent insertion (in which stents are inserted transcutaneously into the intrahepatic biliary tree and across the ampulla), ERCP has the advantage of avoiding the discomfort and care associated with external biliary drains. Endoscopic ultrasonography (EUS) involves the application of a small ultrasonography probe against the pancreas through the adjacent gastric or duodenal wall. EUS permits precise visualization of tumors, surrounding vascular structures, biliary and pancreatic ducts, and peripancreatic lymph nodes. In this way, EUS can establish preoperative tumor (ut) and nodal (un) staging. This can be especially useful in the evaluation of adenomatous periampullary polyps. Ultrasonographic evidence of tumoral infiltration beyond the mucosa of the duodenum or ampulla can be indicative of invasive disease. In addition, EUS enables safe and accurate image-guided fine-needle aspiration or core-needle biopsy of pancreatic tumors. Although the large majority of patients presenting with a pancreas mass and painless jaundice will ultimately be found to have pancreatic adenocarcinoma, histologic verification of this diagnosis may be necessary for patients who require preoperative or definitive chemotherapy and/or chemoradiation therapy. Another procedure used for diagnostic staging is laparoscopic exploration. An outpatient staging laparoscopy may be useful in cases of periampullary or pancreatic adenocarcinoma where there is a high suspicion for subradiologic
11 gastro periampullary and pancreatic adenocarcinoma 11 intraperitoneal metastases (as suggested by findings such as nonphysiologic intraperitoneal free fluid or markedly elevated CA19-9 levels in the absence of jaundice). Staging laparoscopy is also useful in cases of borderline resectable or locally advanced pancreas adenocarcinoma as identification of metastastic disease may obviate the role of local radiation therapy. In these circumstances, laparoscopic exploration begins with a close examination of the peritoneal cavity for ascites fluid, hepatic lesions, omental lesions, and peritoneal lesions. Following placement of a camera port, a second port is inserted, through which an instrument may be used to assist in visualization of the undersurfaces of the liver and peritoneal surfaces. If ascites fluid is identified, samples are aspirated and submitted for cytologic analysis. If ascites fluid is not seen, the peritoneal surfaces are irrigated with saline solution, and the effluent is collected and submitted for cytologic analysis. The presence of even microscopic quantities of metastatic adenocarcinoma cells from peritoneal washings has been shown to carry as much prognostic significance as visible foci of metastatic disease. Metastatic deposits most commonly appear as firm, white nodular masses, and any suspicious lesions are biopsied. Peritoneal washings are obtained prior to obtaining biopsies so as to avoid potential cytologic contamination with blood cells resulting from bleeding at biopsy sites. therapy Traditional treatment modalities for patients with periampullary adenocarcinoma are surgical resection, chemotherap y, and radiation therapy. The types of modalities employed and the sequence in which they are used are selected based on stage and local extent of disease, patient comorbidity, and institutional preferences. The intent of each treatment modality can be simplistically outlined as follows: surgical resection is used for removal of primary tumors and regiona l lymph nodes in circumstances where distant metastatic disease is not demonstrable; chemotherapy is used to treat suspected or demonstrable metastatic disease; and radiation therapy is used when cytoreduction of local tumors is desirable for purposes of either increasing the likelihood of a margin-negative resection or palliating tumor mass related symptoms. As outlined below, the propensity for systemic dissemination of pancreatic and periampullary adenocarcinoma usually requires the use of multimodality therapy that combines local therapies such as surgical resection and radiation therapy with systemic chemotherapy. Specific operative procedures for pancreatic adenocarcinoma and periampullary adenocarcinoma are outlined below; specific strategies for the implementation of surgical resection, chemotherapy, and radiation therapy are outlined for each subtype of periampullary adenocarcinoma. Operative Procedures The intent of surgical resection for pancreatic and periampullary adenocarcinoma is to achieve maximal therapeutic and diagnostic benefit through complete, margin-negative tumor extirpation and thorough sampling of local lymph nodes. As a result, limited and local forms of tumoral resection such as ampullectomy, segmental pancreatectomy, or tumor enucleation have no general role in the management of pancreatic and periampullary adenocarcinoma. Rather, primary options for operative resection are pancreaticoduodenectomy and distal (left) pancreatectomy. Prior to undertaking operative resection, preoperative imaging studies must be comprehensively reviewed; evidence of vascular invasion by tumor or variations in vascular and/or/biliary anatomy may predict the need for subtle but important changes in operative strategy and conduct. pancreaticoduodenectomy Pancreaticoduodenectomy is the operative procedure of choice for resectable cases of periampullary adenocarcinoma or pancreatic adenocarcinoma localized to the pancreatic head or uncinate process. Because of the intimate anatomic interrelationship between the pancreatic head, duodenum, and distal biliary system, all three structures must be removed together, and operative reconstruction is necessary to restore intestinal, biliary-enteric, and pancreatic-enteric continuity. Operative Conduct Open pancreaticoduodenectomy can be performed through a vertically oriented upper midline or transversely oriented epigastric or bilateral subcostal incision. The specific sequence of steps used in the conduct of this operation can be variable, but the first step should always be a thorough exploration of the abdominopelvic contents; identification of previously undetected metastatic disease is a contraindication to proceeding with resection. In cases of primary tumors arising in the head of the pancreas, 15 to 20% of patients have metastatic disease that is not identified on preoperative cross-sectional imaging. Therefore, initial exploration can be performed laparoscopically so as to avoid the potential morbidity of a laparotomy incision if metastatic disease is detected and resection is not indicated. If no evidence of distant metastasis is found, focused exploration is undertaken to look for regional spread that might also alter the decision to undertake tumor resection. The celiac axis is palpated and inspected for evidence of nodal metastases, and suspiciously enlarged and firm lymph nodes may be removed for frozen pathologic analysis. Positive lymph nodes in the field of resection are not a contraindication to resection; however, nodal metastases around the celiac axis, ligament of Treitz, and other distant areas carry nearly the same negative prognostic weight as more distant foci of metastatic disease. In addition, the transverse colon is elevated, and the root of the small bowel mesentery is inspected; evidence of tumoral extension across the transverse mesocolon at this location may indicate the presence of locally advanced disease. If no contraindication to resection is identified, the hepati c flexure is mobilized and the transverse colon is separated from the pancreas and duodenum, exposing the first, second, and third portions of the duodenum. With the transverse colon reflected caudally, a generous Kocher maneuver is executed, detaching the duodenum and pancreatic head from the retroperitoneum. This dissection continues along the right lateral aspect of the porta hepatis, and the inferior vena and left renal vein are exposed [see Figure 6]. Mobilization of the retroperitoneal attachments of the duodenum
12 gastro periampullary and pancreatic adenocarcinoma 12 Hepatoduodenal Ligament Common Duct Node Pancreas l Gonadal Vessels Superior Mesenteric Artery Figure 6 Kocher maneuver. Once the duodenum is exposed by dissecting the transverse mesocolon off the Duodenum duodenum and stomach, the lateral peritoneal attachments of the duodenum and portal hepatis are incised. The duodenum and head of pancreas are dissected off the inferior vena cava and aorta along the avascular retroduodenal plane. This dissection can be taken all the way to the ligament of Treitz. Palpation of the posterior aspect of the pancreatic head permits evaluation of the anatomic relationship between a periampullar y tumor and the superior mesenteric vein and superior mesenteric artery. in this manner can usually be completed all the way to the ligament of Treitz. By dissecting along the avascular plane between the transverse mesocolon and duodenum and pancreas, the superior mesenteric vein is identified below the level of the pancreatic head, and the uncinate process is dissected off the right lateral aspect of the superior mesenteric vein. The pancreatic head is palpated for evidence of tumoral invasion into the superior mesenteric vein and artery. The peritoneum overlying the porta hepatis is incised to identify the common bile duct and the junction of the common hepatic artery and proper hepatic artery. If present, the endobiliary stent can be palpated within the lumen of the typically dilated common bile duct. The right gastric artery is divided, and the common hepatic artery, proper hepatic artery, and gastroduodenal artery are exposed. The gastroduodenal artery should be dissected distally over the anterior aspect of the pancreatic neck. Transient occlusion of the gastroduodenal artery should result in no impedance of flow through the proper hepatic artery; interruption of flow may indicate that the anatomy has not been properly delineated (e.g., one may have misinterpreted the proper hepatic artery to be the gastroduodenal artery) or that chronic occlusion of the celiac artery has caused the hepatic arterial inflow to rely on reversed flow from the superior mesenteric artery via the gastroduodenal artery. If no impedance of flow is noted, the gastroduodenal artery should be securely ligated and divided as far from the common and proper hepatic arteries as possible. An unusual but potentially devastating complication of pancreaticoduodenectomy is hemorrhage from the ligated gastroduodenal artery; preserving a stump of the gastroduodenal artery may permit interventional radiologic placement of embolization particles into this vessel without interrupting flow through the proper hepatic artery. Further dissection is undertaken to identify the portal vein, and blunt dissection is undertaken along the anterior aspect of the portal vein behind the neck of the pancreas; obstruction encountered during this dissection may indicate the presence of tumoral abutment or invasion of the portal vein. The plane between the common hepatic duct and portal vein is developed. A cholecystectom y may also be performed at this time, and the common hepatic duct is circumferentially dissected above the level of the cystic duct insertion. The common hepatic duct may be sharply divided at this time, after which a circumferential margin of common hepatic duct should be excised for frozen-section pathologic analysis; if carcinoma is present, an additional segment of proximal common hepatic duct is
13 gastro periampullary and pancreatic adenocarcinoma 13 taken. This is especially important if a distal cholangiocarcinoma is suspected. If present, the endobiliary stent can be removed. The distal common hepatic duct is ligated with a marking stitch for pathologic orientation, and the proximal common hepatic duct is temporarily occluded with a small clamp so as to avoid bile spillage. The distal common hepatic duct is mobilized off the portal vein, with care taken to incorporate the portocaval lymph node with the planned resection specimen. Even if a replaced or accessory right hepatic artery was not visualized by preoperative imaging, the existence of this aberrant vessel should be excluded by palpating the right posterolateral aspect of the portal vein and common bile duct for arterial pulsation. If present, care must be taken to identify and avoid injury to this important structure. Blunt dissection of the retropancreatic tunnel over the portal vein and superior mesenteric vein is completed by exposing the superior mesenteric vein below the level of the pancreatic neck [see Figure 7]. To facilitate this exposure, the gastrocolic ligament is incised and separated from the transverse mesocolon. Access to the superior mesenteric vein is improved by dividing the left gastroepiploic vein just proximal to its point of insertion on the superior mesenteric vein. Of note, the left gastroepiploic vein often forms a short common channel with the middle colic vein before draining into the superior mesenteric vein, and care is taken to avoid division of the middle colic vein. Blunt dissection is then undertaken over the anterior aspect of the superior mesenteric vein from below the neck of the pancreas, after which a vessel loop may be placed around the pancreatic neck. Access to the pancreatic neck is facilitated by dividing the distal stomach (the so-called classic pancreaticoduodenectomy) or by dividing the proximal duodenum distal to the pyloric musculature (the pylorus-preserving pancreaticoduodenectomy). Stay sutures can be placed along the superior and inferior aspects of the pancreatic neck on either side of the planned transection plane to facilitate its elevation off the underlying portal vein and superior mesenteric vein. The ligament of Treitz is then identified below the level of the transverse mesocolon, and the proximal jejunum is divided 10 to 20 cm distal to the ligament of Treitz. The mesentery of the proximal jejunum is divided, and division of the ligament of Treitz is completed, after which the proximal jejunum and distal duodenum may be passed a Head of Pancreas b Stomach Pancreas Gallbladder Duodenum Duodenum Head of Pancreas Uncinate Process of Pancreas Neck of Pancreas Superior Mesenteric Vein Duodenum Uncinate Process of Pancreas Superior Mesenteric Vein c Portal Vein Figure 7 Pancreaticoduodenectomy. (a) A Kocher maneuver is performed, and the distal duodenum is mobilized. The transverse mesocolon is dissected off the stomach and duodenum along its avascular plane of attachment, exposing the superior mesenteric vein. At this point, the uncinate process may be dissected off the right lateral aspect of the superior mesenteric vein. (b) Intraoperative photograph of anatomy depicted in (a). (c) Intraoperative photograph of the pancreatic neck before and after transection.
14 gastro periampullary and pancreatic adenocarcinoma 14 behind and to the right of the superior mesenteric vessels. By reflecting the duodenum to the right, the uncinate process can be dissected off superior mesenteric vein, with care taken to divide the inferior pancreaticoduodenal vein, which enters into the pancreas, and to preserve the first jejunal vein, which typically courses underneath the uncinate process and around the superior mesenteric artery. The pancreatic neck is divided as the stay sutures along the pancreatic neck are elevated so as to avoid injury to the portal vein and superior mesenteric vein. If tumoral invasion into these venous structures is present, exposure of the area of venous involvement usually requires partial division of the pancreatic neck. In these circumstances, great care is taken during division of the pancreatic neck to avoid injury to the portal vein or superior mesenteric vein. The region of the pancreatic duct is divided sharply to avoid thermal injury to this structure. Bleeding points along the pancreatic parenchyma can be controlled with diathermy or suture ligation. After the pancreatic neck is divided, a margin of pancreatic neck is sharply excised and submitted for frozensection pathologic analysis; if carcinoma is present, an additional segment of pancreas is taken. The right lateral aspect of the portal vein and superior mesenteric vein is then dissected off the pancreas. The final plane of dissection is the retroperitoneal margin. To maximize the chances of a margin-negative resection, this dissection is undertaken along the right lateral aspect of the superior mesenteric artery. By reflecting the planned resection specimen to the right, the superior mesenteric artery is effectively pulled to the right of the superior mesenteric vein, permitting clear visualization of this final resection margin [see Figure 8]. All lymphovascular structures are divided between clips or ligatures, with care taken to avoid injury to the superior mesenteric artery. If present, the replaced or accessory right hepatic artery must be carefully preserved during this retroperitoneal dissection. In cases of portal vein or superior mesenteric vein invasion, the area of venous involvement is fully exposed. Vessel loops and vascular clamps are used to obtain control of all venous inflow and outflow after systemic heparinization; this typically requires control of all trunks of the superior mesenteric vein, splenic vein, portal vein, and possibly the left gastric vein and inferior mesenteric vein. The area of venous involvement is sharply excised, after which venous reconstruction is completed. Depending on the extent of venous resection needed, reconstruction can be performed using primary closure, a bovine pericardial patch, or autologous saphenous vein or left renal vein or internal jugular vein graft. It is important to orient the resected specimen carefully for pathologic assessment, using sutures or colored ink to mark the common hepatic duct, portal vein/superior mesenteric vein margin, superior mesenteric artery margin, posterior margin, and pancreatic neck margin for the pathologist. After ensuring hemostasis, reconstruction is initiated by passing the distal jejunal stump through a defect fashioned in an avascular portion of the transverse mesocolon. The jejunum is positioned to reach the transected common hepatic duct and pancreatic neck without tension. The hepaticojejunostomy is typically constructed in an endto-side manner using a running or interrupted absorbable suture. The pancreaticojejunostomy is constructed either in an end-to-side fashion (by creating a duct-to-mucosa anastomosis between the pancreatic duct and a small jejunotomy followed by a series of reinforcing sutures between the pancreatic parenchyma and the jejunal serosa) or in an end-toend fashion (by inverting the stump of the pancreas into the cut end of the jejunum) [see Figure 9]. The final anastomosis is the gastrojejunostomy (for classic pancreaticoduodenectomy) or duodenojejunostomy (for pylorus-preserving pancreaticoduodenectomy), which can be performed in an antecolic or retrocolic fashion. Although there is mixed evidence in support of routine intraoperative drain placement, most surgeons place drains adjacent to the hepaticojejunostomy and pancreaticojejunostomy anastomoses to monitor for and potential control of anastomotic fistulae and a nasogastric tube that can typically be removed very early in the postoperative course. 15,16 Outcomes Despite numerous improvements that have been made in perioperative management in recent decades, pancreaticoduodenectomy is still associated with significant risks of postoperative mortality and morbidity. Nationally, the risk of operative mortality following pancreaticoduodenectomy is as high as 6 to 7%. However, there appears to be an inverse relationship between hospital volume and operative mortality, with high-volume centers demonstrating operative mortality of 1 to 3%. 17 Operative morbidity rates remain high; when operative complications are strictly defined and recorded, the risk of major postoperative complications is still on the order of 30%. 18 Two complications that are specifically associated with pancreaticoduodenectomy are pancreatic fistula and delayed gastric emptying. Pancreatic fistula is defined as leakage of pancreatic fluid (generally defined by an amylase level greater than three times that of serum on or after postoperative day 3) from either the pancreatic-enteric anastomosis or the cut surface of the pancreas. Risk factors that have been consistently identified for pancreatic fistula are the presence of a small pancreatic duct size and a soft pancreatic parenchymal texture. However, there is no consistent evidence that the technique of anastomosis (e.g., invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy or pancreaticojejunostomy versus pancreaticogastrostomy) meaningfully alters the likelihood of pancreatic fistula. Management of pancreatic fistulae centers on the ability to adequately control the fistula so as to avoid the accumulation of undrained fluid. This may require the retention and/ or placement of drains around the region of the pancreaticenteric anastomosis; in rare circumstances, reoperative exploration may be needed to adequately drain a large and uncontrolled pancreatic fistula. Undrained collections of pancreatic fluid can result in pain, fevers, and sepsis. A particularly dreaded complication of pancreaticoduodenectomy is gastroduodenal artery hemorrhage; undrained amylaserich fluid from the pancreatic-enteric anastomosis can occasionally erode the ligated gastroduodenal artery stump, resulting in significant and potentially life-threatening hemorrhage requiring interventional radiologic embolization or stenting or reoperation. The International Study Group of Pancreatic Surgery recently developed a pancreatic fistula
15 gastro periampullary and pancreatic adenocarcinoma 15 a Portal Vein Divided First Portion of Duodenum Stomach Divided Neck of Pancreas Duodenum Uncinate Process Superior Mesenteric Vein Superior Mesenteric Artery b Neck of Pancreas Uncinate Process of Pancreas Duodenum Portal Vein Neck of Pancreas Superior Mesenteric Vein c Common Hepatic Duct (Occluded) Replaced Right Hepatic Artery Inferior Vena Cava Superior Mesenteric Artery Portal Vein Gastroduodenal Artery (Ligated) Neck of Pancreas Superior Mesenteric Vein Figure 8 Pancreaticoduodenectomy. (a) The planned resection specimen is reflected to the right as the superior mesenteric vein is reflected to the left. This maneuver exposes the retroperitoneal attachments of the pancreas, which are divided along the right lateral aspect of the superior mesenteric artery. (b) Intraoperative photograph of anatomy depicted in (a) following pancreatic neck transection. (c) Intraoperative photograph of anatomy depicted in (a) immediately following specimen removal. Jejunum a Pancreatic Duct b Jejunal Limb Invaginated Pancreas Small Enterotomy Figure 9 Pancreaticoduodenectomy. The two options for pancreaticojejunostomy construction are (a) the end-to-side duct-to-mucosa anastomosis and the (b) end-to-end invaginated anastomosis.
Pancreas Case Scenario #1
 Pancreas Case Scenario #1 An 85 year old white female presented to her primary care physician with increasing abdominal pain. On 8/19 she had a CT scan of the abdomen and pelvis. This showed a 4.6 cm mass
Pancreas Case Scenario #1 An 85 year old white female presented to her primary care physician with increasing abdominal pain. On 8/19 she had a CT scan of the abdomen and pelvis. This showed a 4.6 cm mass
Imaging in gastric cancer
 Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Pancreas Quizzes c. Both A and B a. Directly into the blood stream (not using ducts)
 Pancreas Quizzes Quiz 1 1. The pancreas produces hormones. Which type of hormone producing organ is the pancreas? a. Endocrine b. Exocrine c. Both A and B d. Neither A or B 2. Endocrine indicates hormones
Pancreas Quizzes Quiz 1 1. The pancreas produces hormones. Which type of hormone producing organ is the pancreas? a. Endocrine b. Exocrine c. Both A and B d. Neither A or B 2. Endocrine indicates hormones
Pancreas & Biliary System. Dr. Vohra & Dr. Jamila
 Pancreas & Biliary System Dr. Vohra & Dr. Jamila 1 Objectives At the end of the lecture, the student should be able to describe the: Location, surface anatomy, parts, relations & peritoneal reflection
Pancreas & Biliary System Dr. Vohra & Dr. Jamila 1 Objectives At the end of the lecture, the student should be able to describe the: Location, surface anatomy, parts, relations & peritoneal reflection
The Whipple Operation Illustrations
 The Whipple Operation Illustrations Fig. 1. Illustration of the sixstep pancreaticoduodenectomy (Whipple operation) as described in a number of recent text books by Dr. Evans. The operation is divided
The Whipple Operation Illustrations Fig. 1. Illustration of the sixstep pancreaticoduodenectomy (Whipple operation) as described in a number of recent text books by Dr. Evans. The operation is divided
Pancreatic Cancer. BIOLOGY: Not well defined (genetic and enviromental factors) CLINICAL PRESENTATION: Abd pain, jaundice, weight loss.
 EloreMed Editor: Le Wang, MD, PhD Date of Update: 2/6/2018 UpToDate: Liposomal irinotecan (Onivyde) plus FU/LV is now approved for gemcitabine-refractory metastatic pancreatic cancer and recommended by
EloreMed Editor: Le Wang, MD, PhD Date of Update: 2/6/2018 UpToDate: Liposomal irinotecan (Onivyde) plus FU/LV is now approved for gemcitabine-refractory metastatic pancreatic cancer and recommended by
Case Scenario 1. Discharge Summary
 Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic
 Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic cancer Section AA Cancer Centre Referrals In the absence of metastatic
Intended for use by Clinicians and Health Care Providers involved in the Management or Referral of adult patients with pancreatic cancer Section AA Cancer Centre Referrals In the absence of metastatic
Gastric Cancer Staging AJCC eighth edition. Duncan McLeod Westmead Hospital, NSW
 Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Multiple Primary Quiz
 Multiple Primary Quiz Case 1 A 72 year old man was found to have a 12 mm solid lesion in the pancreatic tail by computed tomography carried out during a routine follow up study of this patient with adult
Multiple Primary Quiz Case 1 A 72 year old man was found to have a 12 mm solid lesion in the pancreatic tail by computed tomography carried out during a routine follow up study of this patient with adult
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Gastric Cancer Histopathology Reporting Proforma
 Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Cholangiocarcinoma (Bile Duct Cancer)
 Cholangiocarcinoma (Bile Duct Cancer) The Bile Duct System (Biliary Tract) A network of bile ducts (tubes) connects the liver and the gallbladder to the small intestine. This network begins in the liver
Cholangiocarcinoma (Bile Duct Cancer) The Bile Duct System (Biliary Tract) A network of bile ducts (tubes) connects the liver and the gallbladder to the small intestine. This network begins in the liver
Hilar cholangiocarcinoma. Frank Wessels, Maarten van Leeuwen, UMCU utrecht
 Hilar cholangiocarcinoma Frank Wessels, Maarten van Leeuwen, UMCU utrecht Content Anatomy Biliary strictures (Hilar) Cholangiocarcinoom Staging Biliary tract 1 st order Ductus hepatica dextra Ductus hepaticus
Hilar cholangiocarcinoma Frank Wessels, Maarten van Leeuwen, UMCU utrecht Content Anatomy Biliary strictures (Hilar) Cholangiocarcinoom Staging Biliary tract 1 st order Ductus hepatica dextra Ductus hepaticus
Accessory Glands of Digestive System
 Accessory Glands of Digestive System The liver The liver is soft and pliable and occupies the upper part of the abdominal cavity just beneath the diaphragm. The greater part of the liver is situated under
Accessory Glands of Digestive System The liver The liver is soft and pliable and occupies the upper part of the abdominal cavity just beneath the diaphragm. The greater part of the liver is situated under
The abdominal Esophagus, Stomach and the Duodenum. Prof. Oluwadiya KS
 The abdominal Esophagus, Stomach and the Duodenum Prof. Oluwadiya KS www.oluwadiya.com Viscera of the abdomen Abdominal esophagus: Terminal part of the esophagus The stomach Intestines: Small and Large
The abdominal Esophagus, Stomach and the Duodenum Prof. Oluwadiya KS www.oluwadiya.com Viscera of the abdomen Abdominal esophagus: Terminal part of the esophagus The stomach Intestines: Small and Large
Duodenum retroperitoneal
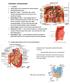 Duodenum retroperitoneal C shaped Initial region out of stomach into small intestine RETROperitoneal viscus Superior 1 st part duodenal cap ; moves upwards and backwards to lie on the R crura medial to
Duodenum retroperitoneal C shaped Initial region out of stomach into small intestine RETROperitoneal viscus Superior 1 st part duodenal cap ; moves upwards and backwards to lie on the R crura medial to
PANCREAS DUCTAL ADENOCARCINOMA PDAC
 CONTENTS PANCREAS DUCTAL ADENOCARCINOMA PDAC I. What is the pancreas? II. III. IV. What is pancreas cancer? What is the epidemiology of Pancreatic Ductal Adenocarcinoma (PDAC)? What are the risk factors
CONTENTS PANCREAS DUCTAL ADENOCARCINOMA PDAC I. What is the pancreas? II. III. IV. What is pancreas cancer? What is the epidemiology of Pancreatic Ductal Adenocarcinoma (PDAC)? What are the risk factors
د. عصام طارق. Objectives:
 GI anatomy Lecture: 5 د. عصام طارق Objectives: To describe anatomy of stomach, duodenum & pancreas. To list their main relations. To define their blood & nerve supply. To list their lymph drainage. To
GI anatomy Lecture: 5 د. عصام طارق Objectives: To describe anatomy of stomach, duodenum & pancreas. To list their main relations. To define their blood & nerve supply. To list their lymph drainage. To
Objectives. Intraoperative Consultation of the Whipple Resection Specimen. Pancreas Anatomy. Pancreatic ductal carcinoma 11/10/2014
 Intraoperative Consultation of the Whipple Resection Specimen Pathology Update Faculty of Medicine, University of Toronto November 15, 2014 John W. Wong, MD, FRCPC Department of Anatomical Pathology Sunnybrook
Intraoperative Consultation of the Whipple Resection Specimen Pathology Update Faculty of Medicine, University of Toronto November 15, 2014 John W. Wong, MD, FRCPC Department of Anatomical Pathology Sunnybrook
8. The polyp in the illustration can be described as (circle all that apply) a. Exophytic b. Pedunculated c. Sessile d. Frank
 Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
BILIARY TRACT & PANCREAS, PART II
 CME Pretest BILIARY TRACT & PANCREAS, PART II VOLUME 41 1 2015 A pretest is mandatory to earn CME credit on the posttest. The pretest should be completed BEFORE reading the overview. Both tests must be
CME Pretest BILIARY TRACT & PANCREAS, PART II VOLUME 41 1 2015 A pretest is mandatory to earn CME credit on the posttest. The pretest should be completed BEFORE reading the overview. Both tests must be
PANCREATIC CANCER GUIDELINES
 PANCREATIC CANCER GUIDELINES North-East London Cancer Network & Barts and the London HPB Centre PROTOCOL FOR MANAGEMENT OF PANCREATIC CANCER (SEPTEMBER 2010) I. PRE-REFERRAL GUIDELINES Screening 1. Offer
PANCREATIC CANCER GUIDELINES North-East London Cancer Network & Barts and the London HPB Centre PROTOCOL FOR MANAGEMENT OF PANCREATIC CANCER (SEPTEMBER 2010) I. PRE-REFERRAL GUIDELINES Screening 1. Offer
-12. -Renad Habahbeh. -Dr Mohammad mohtasib
 -12 -Renad Habahbeh - -Dr Mohammad mohtasib The Gallbladder -The gallbladder has a body, a fundus (a rounded end), a neck, Hartmann s pouch before the neck and a cystic duct that meets the common hepatic
-12 -Renad Habahbeh - -Dr Mohammad mohtasib The Gallbladder -The gallbladder has a body, a fundus (a rounded end), a neck, Hartmann s pouch before the neck and a cystic duct that meets the common hepatic
Surface Anatomy. Location Shape Weight Role of Five Surfaces Borders Fissures Lobes Peritoneal Lig
 The Liver Functions Bile production and secretion Detoxification Storage of glycogen Protein synthesis Production of heparin and bile pigments Erythropoiesis (in fetus) Surface Anatomy Location Shape Weight
The Liver Functions Bile production and secretion Detoxification Storage of glycogen Protein synthesis Production of heparin and bile pigments Erythropoiesis (in fetus) Surface Anatomy Location Shape Weight
Block 3: DISSECTION 2 CELIAC TRUNK, JEJUNUM/ILEUM, LARGE INTESTINE, DUODENUM, PANCREAS, PORTAL VEIN; MOBILIZATION OF THE LIVER
 1 Block 3: DISSECTION 2 CELIAC TRUNK, JEJUNUM/ILEUM, LARGE INTESTINE, DUODENUM, PANCREAS, PORTAL VEIN; MOBILIZATION OF THE LIVER Attempt to complete as much as you can of the dissection explained in the
1 Block 3: DISSECTION 2 CELIAC TRUNK, JEJUNUM/ILEUM, LARGE INTESTINE, DUODENUM, PANCREAS, PORTAL VEIN; MOBILIZATION OF THE LIVER Attempt to complete as much as you can of the dissection explained in the
Navigators Lead the Way
 RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
Evaluation of Suspected Pancreatic Cancer
 Evaluation of Suspected Pancreatic Cancer October 15, 2015 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-779-3239 Toll
Evaluation of Suspected Pancreatic Cancer October 15, 2015 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-779-3239 Toll
To describe the liver. To list main structures in porta hepatis.
 GI anatomy Lecture: 6 د. عصام طارق Objectives: To describe the liver. To list main structures in porta hepatis. To define portal system & portosystemic anastomosis. To list parts of biliary system. To
GI anatomy Lecture: 6 د. عصام طارق Objectives: To describe the liver. To list main structures in porta hepatis. To define portal system & portosystemic anastomosis. To list parts of biliary system. To
Cattell-Braasch maneuver combined with superior mesenteric artery first approach for resection of borderline resectable pancreatic cancer
 Masters of Surgery Page 1 of 5 Cattell-Braasch maneuver combined with superior mesenteric artery first approach for resection of borderline resectable pancreatic cancer Tingsong Yang 1, Fairweather Mark
Masters of Surgery Page 1 of 5 Cattell-Braasch maneuver combined with superior mesenteric artery first approach for resection of borderline resectable pancreatic cancer Tingsong Yang 1, Fairweather Mark
Dr. Zahiri. In the name of God
 Dr. Zahiri In the name of God small intestine = small bowel is the part of the gastrointestinal tract Boundaries: Pylorus Ileosecal junction Function: digestion and absorption of food It receives bile
Dr. Zahiri In the name of God small intestine = small bowel is the part of the gastrointestinal tract Boundaries: Pylorus Ileosecal junction Function: digestion and absorption of food It receives bile
A916: rectum: adenocarcinoma
 General facts of colorectal cancer The colon has cecum, ascending, transverse, descending and sigmoid colon sections. Cancer can start in any of the r sections or in the rectum. The wall of each of these
General facts of colorectal cancer The colon has cecum, ascending, transverse, descending and sigmoid colon sections. Cancer can start in any of the r sections or in the rectum. The wall of each of these
Pancreas and Biliary System
 Pancreas and Biliary System Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives At the end of the lecture,
Pancreas and Biliary System Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives At the end of the lecture,
Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction
 Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction Ann S. Fulcher, MD Medical College of Virginia Virginia Commonwealth University Richmond, Virginia Objectives To
Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction Ann S. Fulcher, MD Medical College of Virginia Virginia Commonwealth University Richmond, Virginia Objectives To
Anatomy of the SMALL INTESTINE. Dr. Noman Ullah Wazir PMC
 Anatomy of the SMALL INTESTINE Dr. Noman Ullah Wazir PMC SMALL INTESTINE The small intestine, consists of the duodenum, jejunum, and illium. It extends from the pylorus to the ileocecal junction were the
Anatomy of the SMALL INTESTINE Dr. Noman Ullah Wazir PMC SMALL INTESTINE The small intestine, consists of the duodenum, jejunum, and illium. It extends from the pylorus to the ileocecal junction were the
Afternoon Session Cases
 Afternoon Session Cases Case 1 19 year old woman Presented with abdominal pain to community hospital Mild incr WBC a14, 000, Hg normal, lipase 100 (normal to 75) US 5.2 x 3.7 x 4 cm mass in porta hepatis
Afternoon Session Cases Case 1 19 year old woman Presented with abdominal pain to community hospital Mild incr WBC a14, 000, Hg normal, lipase 100 (normal to 75) US 5.2 x 3.7 x 4 cm mass in porta hepatis
Original article: new surgical approaches to the Klatskin tumour
 Alimentary Pharmacology & Therapeutics Original article: new surgical approaches to the Klatskin tumour T. M. VAN GULIK*, S. DINANT*, O. R. C. BUSCH*, E. A. J. RAUWS, H. OBERTOP* & D. J. GOUMA Departments
Alimentary Pharmacology & Therapeutics Original article: new surgical approaches to the Klatskin tumour T. M. VAN GULIK*, S. DINANT*, O. R. C. BUSCH*, E. A. J. RAUWS, H. OBERTOP* & D. J. GOUMA Departments
Pathways of Regional Spread in Pancreatic Cancer
 Pathways of Regional Spread in Pancreatic Cancer 12 Chusilp Charnsangavej, M.D. Regional spread of pancreatic ductal adenocarcinoma is common at the time of diagnosis, and it is often associated with poor
Pathways of Regional Spread in Pancreatic Cancer 12 Chusilp Charnsangavej, M.D. Regional spread of pancreatic ductal adenocarcinoma is common at the time of diagnosis, and it is often associated with poor
Surgical Management of Pancreatic Cancer
 I Congresso de Oncologia D Or July 5-6, 2013 Surgical Management of Pancreatic Cancer Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University School of Medicine, Baltimore, MD Estimated
I Congresso de Oncologia D Or July 5-6, 2013 Surgical Management of Pancreatic Cancer Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University School of Medicine, Baltimore, MD Estimated
MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER
 10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
Preview from Notesale.co.uk Page 1 of 34
 Abdominal viscera and digestive tract Digestive tract Abdominal viscera comprise majority of the alimentary system o Terminal oesophagus, stomach, pancreas, spleen, liver, gallbladder, kidneys, suprarenal
Abdominal viscera and digestive tract Digestive tract Abdominal viscera comprise majority of the alimentary system o Terminal oesophagus, stomach, pancreas, spleen, liver, gallbladder, kidneys, suprarenal
Interactive Exhibit On Imaging Updates For Staging And Response Assessment In Pancreatic Cancer
 Interactive Exhibit On Imaging Updates For Staging And Response Assessment In Pancreatic Cancer 1 Vinit Baliyan, MD; 1 Hamed Kordbacheh, MD; 2 Eric P Tamm, MD; 3 Theodore S Hong, MD; 4 Carlos Fernandez-Del
Interactive Exhibit On Imaging Updates For Staging And Response Assessment In Pancreatic Cancer 1 Vinit Baliyan, MD; 1 Hamed Kordbacheh, MD; 2 Eric P Tamm, MD; 3 Theodore S Hong, MD; 4 Carlos Fernandez-Del
Exploring Anatomy: the Human Abdomen
 Exploring Anatomy: the Human Abdomen PERITONEUM AND PERITONEAL CAVITY PERITONEUM The peritoneum is a thin serous membrane that lines the abdominal cavity and covers, in variable amounts, the viscera within
Exploring Anatomy: the Human Abdomen PERITONEUM AND PERITONEAL CAVITY PERITONEUM The peritoneum is a thin serous membrane that lines the abdominal cavity and covers, in variable amounts, the viscera within
4/9/2018 OBJECTIVES PANCREAOTO BILIARY ULTRASOUND: BEYOND CHOLECYSTITIS
 PANCREAOTO BILIARY ULTRASOUND: BEYOND CHOLECYSTITIS Jean Yves Sewah Kaiser Permanente West Los Angeles 1 OBJECTIVES Discuss the role of ultrasound in the evaluation of the gallbladder, biliary tree and
PANCREAOTO BILIARY ULTRASOUND: BEYOND CHOLECYSTITIS Jean Yves Sewah Kaiser Permanente West Los Angeles 1 OBJECTIVES Discuss the role of ultrasound in the evaluation of the gallbladder, biliary tree and
Gastric (Stomach) Cancer
 Gastric (Stomach) Cancer Gastric cancer is a disease in which malignant (cancer) cells form in the lining of the stomach. The stomach is a J-shaped organ in the upper abdomen. It is part of the digestive
Gastric (Stomach) Cancer Gastric cancer is a disease in which malignant (cancer) cells form in the lining of the stomach. The stomach is a J-shaped organ in the upper abdomen. It is part of the digestive
Lab Monitor Images Dissection of the Abdominal Vasculature + Lower Digestive System
 Lab Monitor Images Dissection of the Abdominal Vasculature + Lower Digestive System Stomach & Duodenum Frontal (AP) View Nasogastric tube 2 1 3 4 Stomach Pylorus Duodenum 1 Duodenum 2 Duodenum 3 Duodenum
Lab Monitor Images Dissection of the Abdominal Vasculature + Lower Digestive System Stomach & Duodenum Frontal (AP) View Nasogastric tube 2 1 3 4 Stomach Pylorus Duodenum 1 Duodenum 2 Duodenum 3 Duodenum
ARROCase: Borderline Resectable Pancreatic Cancer
 ARROCase: Borderline Resectable Pancreatic Cancer Resident: Jordan Kharofa, MD Staff: Beth Erickson, MD 8/2012 Medical College of Wisconsin Department of Radiation Oncology Case Presentation: 60 year old
ARROCase: Borderline Resectable Pancreatic Cancer Resident: Jordan Kharofa, MD Staff: Beth Erickson, MD 8/2012 Medical College of Wisconsin Department of Radiation Oncology Case Presentation: 60 year old
3/28/2012. Periampullary Tumors. Postgraduate Course in General Surgery CASE 1: CASE 1: Overview. Eric K. Nakakura Ko Olina, HI
 Overview Postgraduate Course in General Surgery Case presentation Differential diagnosis Diagnosis and therapy Outcomes Principles of palliative care Eric K. Nakakura Ko Olina, HI March 27, 2012 CASE 1:
Overview Postgraduate Course in General Surgery Case presentation Differential diagnosis Diagnosis and therapy Outcomes Principles of palliative care Eric K. Nakakura Ko Olina, HI March 27, 2012 CASE 1:
IMAGING GUIDELINES - COLORECTAL CANCER
 IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
IMAGING GUIDELINES - COLORECTAL CANCER DIAGNOSIS The majority of colorectal cancers are diagnosed on colonoscopy, with some being diagnosed on Ba enema, ultrasound or CT. STAGING CT chest, abdomen and
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
The peritoneum. Prof. Oluwadiya KS, MBBS, FMCS(Orthop) Website:
 The peritoneum Prof. Oluwadiya KS, MBBS, FMCS(Orthop) Website: http://oluwadiya.com The peritoneum Serous membrane that lines the abdominopelvic cavity and invests the viscera The largest serous membrane
The peritoneum Prof. Oluwadiya KS, MBBS, FMCS(Orthop) Website: http://oluwadiya.com The peritoneum Serous membrane that lines the abdominopelvic cavity and invests the viscera The largest serous membrane
Gallbladder Cancer. GI Practice Guideline. Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist)
 Gallbladder Cancer GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: September 2006 This guideline is a statement of
Gallbladder Cancer GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: September 2006 This guideline is a statement of
Endoscopic Ultrasonography Assessment for Ampullary and Bile Duct Malignancy
 Diagnostic and Therapeutic Endoscopy, Vol. 3, pp. 35-40 Reprints available directly from the publisher Photocopying permitted by license only (C) 1996 OPA (Overseas Publishers Association) Amsterdam B.V.
Diagnostic and Therapeutic Endoscopy, Vol. 3, pp. 35-40 Reprints available directly from the publisher Photocopying permitted by license only (C) 1996 OPA (Overseas Publishers Association) Amsterdam B.V.
Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds
 Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds Imaging in jaundice and 2ww pathway Image protocol Staging Limitations Pancreatic cancer 1.2.4 Refer people using a suspected
Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds Imaging in jaundice and 2ww pathway Image protocol Staging Limitations Pancreatic cancer 1.2.4 Refer people using a suspected
JOHN M UECKER, MD, FACS COMPLEX PANCREATICODUODENAL INJURIES
 JOHN M UECKER, MD, FACS COMPLEX PANCREATICODUODENAL INJURIES THE PROBLEM DUODENAL / PANCREATIC INJURIES Difficult to diagnose Not very common Anatomic and physiologic challenges 90% rate of associated
JOHN M UECKER, MD, FACS COMPLEX PANCREATICODUODENAL INJURIES THE PROBLEM DUODENAL / PANCREATIC INJURIES Difficult to diagnose Not very common Anatomic and physiologic challenges 90% rate of associated
BLOCK IV: OFFICIAL BODY PARTS LIST FOR ANTERIOR ABDOMINAL WALL AND ABDOMINAL CONTENTS
 BLOCK IV: OFFICIAL BODY PARTS LIST FOR ANTERIOR ABDOMINAL WALL AND ABDOMINAL CONTENTS External oblique muscle Muscular portion Aponeurotic portion Superficial inguinal ring Lateral (inferior) crus Medial
BLOCK IV: OFFICIAL BODY PARTS LIST FOR ANTERIOR ABDOMINAL WALL AND ABDOMINAL CONTENTS External oblique muscle Muscular portion Aponeurotic portion Superficial inguinal ring Lateral (inferior) crus Medial
Management of Cholangiocarcinoma. Roseanna Lee, MD PGY-5 Kings County Hospital
 Management of Cholangiocarcinoma Roseanna Lee, MD PGY-5 Kings County Hospital Case Presentation 37 year old male from Yemen presented with 2 week history of epigastric pain, anorexia, jaundice and puritis.
Management of Cholangiocarcinoma Roseanna Lee, MD PGY-5 Kings County Hospital Case Presentation 37 year old male from Yemen presented with 2 week history of epigastric pain, anorexia, jaundice and puritis.
Topics: Staging and treatment for pancreatic cancer. Staging systems for pancreatic cancer: Differences between the Japanese and UICC systems
 M. J Hep Kobari Bil Pancr and S. Surg Matsuno: (1998) Staging 5:121 127 system for pancreatic cancer 121 Topics: Staging and treatment for pancreatic cancer Staging systems for pancreatic cancer: Differences
M. J Hep Kobari Bil Pancr and S. Surg Matsuno: (1998) Staging 5:121 127 system for pancreatic cancer 121 Topics: Staging and treatment for pancreatic cancer Staging systems for pancreatic cancer: Differences
Surgical Therapy of GEP-NET: An Overview
 Surgical Therapy of GEP-NET: An Overview Pierce K.H Chow MBBS, MMed, FRCSE, FAMS, PhD Professor, Duke-NUS Graduate School of Medicine Senior Consultant Surgeon, Singapore General Hospital Visiting Senior
Surgical Therapy of GEP-NET: An Overview Pierce K.H Chow MBBS, MMed, FRCSE, FAMS, PhD Professor, Duke-NUS Graduate School of Medicine Senior Consultant Surgeon, Singapore General Hospital Visiting Senior
Bile Duct Cancer Early Detection, Diagnosis, and Staging
 Bile Duct Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that can be
Bile Duct Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that can be
Cholangiocarcinoma. GI Practice Guideline. Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist)
 Cholangiocarcinoma GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: October 2006 This guideline is a statement of consensus
Cholangiocarcinoma GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: October 2006 This guideline is a statement of consensus
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
[A RESEARCH COORDINATOR S GUIDE]
![[A RESEARCH COORDINATOR S GUIDE] [A RESEARCH COORDINATOR S GUIDE]](/thumbs/88/117127924.jpg) 2013 COLORECTAL SURGERY GROUP Dr. Carl J. Brown Dr. Ahmer A. Karimuddin Dr. P. Terry Phang Dr. Manoj J. Raval Authored by Jennifer Lee A cartoon about colonoscopies. 1 [A RESEARCH COORDINATOR S GUIDE]
2013 COLORECTAL SURGERY GROUP Dr. Carl J. Brown Dr. Ahmer A. Karimuddin Dr. P. Terry Phang Dr. Manoj J. Raval Authored by Jennifer Lee A cartoon about colonoscopies. 1 [A RESEARCH COORDINATOR S GUIDE]
GALLBLADDER CANCER. Lidie M. Lajoie MD Downstate Surgery M&M July 21, 2011
 GALLBLADDER CANCER Lidie M. Lajoie MD Downstate Surgery M&M July 21, 2011 Agenda Case Presentation Epidemiology Pathogenesis & Pathology Staging Presentation & Diagnosis Stage-wise Management Outcomes/Prognosis
GALLBLADDER CANCER Lidie M. Lajoie MD Downstate Surgery M&M July 21, 2011 Agenda Case Presentation Epidemiology Pathogenesis & Pathology Staging Presentation & Diagnosis Stage-wise Management Outcomes/Prognosis
5/17/2013. Pancreatic Cancer. Postgraduate Course in General Surgery CASE 1: CASE 1: Overview. Case presentation. Differential diagnosis
 Overview Case presentation Postgraduate Course in General Surgery Differential diagnosis Diagnosis and therapy Eric K. Nakakura Koloa, HI March 26, 2013 Outcomes CASE 1: CASE 1: A 78-year-old man developed
Overview Case presentation Postgraduate Course in General Surgery Differential diagnosis Diagnosis and therapy Eric K. Nakakura Koloa, HI March 26, 2013 Outcomes CASE 1: CASE 1: A 78-year-old man developed
Biology Human Anatomy Abdominal and Pelvic Cavities
 Biology 351 - Human Anatomy Abdominal and Pelvic Cavities Please place your name and I.D. number on the back of the last page of this exam. You must answer all questions on this exam. Because statistics
Biology 351 - Human Anatomy Abdominal and Pelvic Cavities Please place your name and I.D. number on the back of the last page of this exam. You must answer all questions on this exam. Because statistics
Nasogastric tube. Stomach. Pylorus. Duodenum 1. Duodenum 2. Duodenum 3. Duodenum 4
 Esophagus Barium Swallow Stomach and Duodenum 4 year old Upper GI Nasogastric tube Stomach and Duodenum 4 year old Upper GI Nasogastric tube Stomach Pylorus Duodenum 1 Duodenum 2 Duodenum 3 Duodenum 4
Esophagus Barium Swallow Stomach and Duodenum 4 year old Upper GI Nasogastric tube Stomach and Duodenum 4 year old Upper GI Nasogastric tube Stomach Pylorus Duodenum 1 Duodenum 2 Duodenum 3 Duodenum 4
Locally Advanced Colon Cancer. Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery
 Locally Advanced Colon Cancer Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery Case 34 yo man presented with severe RLQ abdominal pain X 24 hrs. No nausea/vomiting/fever. + flatus.
Locally Advanced Colon Cancer Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery Case 34 yo man presented with severe RLQ abdominal pain X 24 hrs. No nausea/vomiting/fever. + flatus.
is time consuming and expensive. An intra-operative assessment is not going to be helpful if there is no more tissue that can be taken to improve the
 My name is Barry Feig. I am a Professor of Surgical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. I am going to talk to you today about the role for surgery in the treatment
My name is Barry Feig. I am a Professor of Surgical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. I am going to talk to you today about the role for surgery in the treatment
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER
 MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
I patients with nonendocrine pancreas carcinoma
 LYMPH NODE INVOLVEMENT IN CARCINOMA OF THE HEAD OF THE PANCREAS AREA ANTONIO L. CUBILLA, MD,* JOSEPH FORTNER, MD,+~ AND PATRICK J. FITZGERALD, MD*~ A prospective study to determine the lymph node involvement
LYMPH NODE INVOLVEMENT IN CARCINOMA OF THE HEAD OF THE PANCREAS AREA ANTONIO L. CUBILLA, MD,* JOSEPH FORTNER, MD,+~ AND PATRICK J. FITZGERALD, MD*~ A prospective study to determine the lymph node involvement
Gastrointestinal Tract Cancer
 Gastrointestinal Tract Cancer Tumors of the Stomach Gastric adenocarcinoma Incidence and Epidemiology Incidence mortality rates USA High incidence: Japan, China, Chile, Ireland risk lower socioeconomic
Gastrointestinal Tract Cancer Tumors of the Stomach Gastric adenocarcinoma Incidence and Epidemiology Incidence mortality rates USA High incidence: Japan, China, Chile, Ireland risk lower socioeconomic
Pancreatic Cancer. What is pancreatic cancer?
 Scan for mobile link. Pancreatic Cancer Pancreatic cancer is a tumor of the pancreas, an organ that is located behind the stomach in the abdomen. Pancreatic cancer does not always cause symptoms until
Scan for mobile link. Pancreatic Cancer Pancreatic cancer is a tumor of the pancreas, an organ that is located behind the stomach in the abdomen. Pancreatic cancer does not always cause symptoms until
-Ensherah Mokheemer. -Shatha Al-Jaberi محمد المحتسب- 1 P a g e
 9-9 -Ensherah Mokheemer -Shatha Al-Jaberi محمد المحتسب- 1 P a g e Small intestine has three regions: ( االثني عشر( The duodenum The jejunum The ileum Small intestine Duodenum: -c-shaped -The concavity
9-9 -Ensherah Mokheemer -Shatha Al-Jaberi محمد المحتسب- 1 P a g e Small intestine has three regions: ( االثني عشر( The duodenum The jejunum The ileum Small intestine Duodenum: -c-shaped -The concavity
The Spleen. Dr Fahad Ullah
 The Spleen BY Dr Fahad Ullah Spleen The spleen is an largest lymphoid organ shaped like a shoe that lies relative to the 9th and 11th ribs and is located in the left hypochondrium. Thus, the spleen is
The Spleen BY Dr Fahad Ullah Spleen The spleen is an largest lymphoid organ shaped like a shoe that lies relative to the 9th and 11th ribs and is located in the left hypochondrium. Thus, the spleen is
ABDOMEN - GI. Duodenum
 TALA SALEH ABDOMEN - GI Duodenum - Notice the shape of the duodenum, it looks like capital G shape tube which extends from the pyloroduodenal junction to the duodenojejunal junction. - It is 10 inches
TALA SALEH ABDOMEN - GI Duodenum - Notice the shape of the duodenum, it looks like capital G shape tube which extends from the pyloroduodenal junction to the duodenojejunal junction. - It is 10 inches
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
Kidney Case 1 SURGICAL PATHOLOGY REPORT
 Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
Guidelines, Policies and Statements D5 Statement on Abdominal Scanning
 Guidelines, Policies and Statements D5 Statement on Abdominal Scanning Disclaimer and Copyright The ASUM Standards of Practice Board have made every effort to ensure that this Guideline/Policy/Statement
Guidelines, Policies and Statements D5 Statement on Abdominal Scanning Disclaimer and Copyright The ASUM Standards of Practice Board have made every effort to ensure that this Guideline/Policy/Statement
Alison Douglass Gillian Lieberman, MD. November. Colon Cancer. Alison Douglass, Harvard Medical School Year III Gillian Lieberman, MD
 November Colon Cancer Alison Douglass, Harvard Medical School Year III Our Patient Mr. K. is a 67 year old man with no prior medical problems other than hemorrhoids which have caused occasional rectal
November Colon Cancer Alison Douglass, Harvard Medical School Year III Our Patient Mr. K. is a 67 year old man with no prior medical problems other than hemorrhoids which have caused occasional rectal
Lecture 02 Anatomy of the LIVER
 Lecture 02 Anatomy of the LIVER BY Dr Farooq Khan Aurakzai Dated: 02.01.2018 Introduction to Liver Largest gland in the body. 2 nd largest organ of the body. Weight approximately 1500 gm, and is roughly
Lecture 02 Anatomy of the LIVER BY Dr Farooq Khan Aurakzai Dated: 02.01.2018 Introduction to Liver Largest gland in the body. 2 nd largest organ of the body. Weight approximately 1500 gm, and is roughly
Contemporary Imaging of Biliary Malignancy and Preoperative Evaluation
 Contemporary Imaging of Biliary Malignancy and Preoperative Evaluation Linda Pantongrag-Brown, MD Advanced Diagnostic Imaging, Ramathibodi Hospital, Bangkok, Thailand Malignancy of biliary tract Cholangiocarcinoma
Contemporary Imaging of Biliary Malignancy and Preoperative Evaluation Linda Pantongrag-Brown, MD Advanced Diagnostic Imaging, Ramathibodi Hospital, Bangkok, Thailand Malignancy of biliary tract Cholangiocarcinoma
AJCC 7th Edition Handbook Errata as of 9/21/10
 5 81 Larynx ICD-O-3 Topography Codes Delete C32.3 Laryngeal cartilage 5 81 Larynx ICD-O-3 Topography Codes Add an asterisk after C32.8 5 81 Larynx ICD-O-3 Topography Codes Add an asterisk after C32.9 5
5 81 Larynx ICD-O-3 Topography Codes Delete C32.3 Laryngeal cartilage 5 81 Larynx ICD-O-3 Topography Codes Add an asterisk after C32.8 5 81 Larynx ICD-O-3 Topography Codes Add an asterisk after C32.9 5
GUIDELINES ON RENAL CELL CANCER
 20 G. Mickisch (chairman), J. Carballido, S. Hellsten, H. Schulze, H. Mensink Eur Urol 2001;40(3):252-255 Introduction is characterised by a constant rise in incidence over the last 50 years, with a predominance
20 G. Mickisch (chairman), J. Carballido, S. Hellsten, H. Schulze, H. Mensink Eur Urol 2001;40(3):252-255 Introduction is characterised by a constant rise in incidence over the last 50 years, with a predominance
Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas
 v1 GUIDELINES Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas Pancreatic Section of the British Society of Gastroenterology, Pancreatic Society of
v1 GUIDELINES Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas Pancreatic Section of the British Society of Gastroenterology, Pancreatic Society of
Appendix 5. EFSUMB Newsletter. Gastroenterological Ultrasound
 EFSUMB Newsletter 87 Examinations should encompass the full range of pathological conditions listed below A log book listing the types of examinations undertaken should be kept Training should usually
EFSUMB Newsletter 87 Examinations should encompass the full range of pathological conditions listed below A log book listing the types of examinations undertaken should be kept Training should usually
Anatomy: Know Your Abdomen
 Anatomy: Know Your Abdomen Glossary Abdomen - part of the body below the thorax (chest cavity); separated by the diaphragm. Anterior - towards the front of the body. For example, the umbilicus is anterior
Anatomy: Know Your Abdomen Glossary Abdomen - part of the body below the thorax (chest cavity); separated by the diaphragm. Anterior - towards the front of the body. For example, the umbilicus is anterior
Small Plicae Circularis. Short Closely packed together. Sparse, completely absent at distal part Lymphoid Nodule
 Intestines Differences Between Jejunum and Ileum Types Jejunum Ileum Color Deeper red Paler pink Calibre Bigger Smaller Thickness of wall Thick and Heavy Thin and Lighter Vascularity Highly vascularised
Intestines Differences Between Jejunum and Ileum Types Jejunum Ileum Color Deeper red Paler pink Calibre Bigger Smaller Thickness of wall Thick and Heavy Thin and Lighter Vascularity Highly vascularised
Frank Burton Memorial Update on Pancreato-biliary Cancers
 Frank Burton Memorial Update on Pancreato-biliary Cancers Diagnosis and management of pancreatic cancer: common dilemmas Moderators: Banke Agarwal, MD Paul Buse, MD Evaluation of patients with obstructive
Frank Burton Memorial Update on Pancreato-biliary Cancers Diagnosis and management of pancreatic cancer: common dilemmas Moderators: Banke Agarwal, MD Paul Buse, MD Evaluation of patients with obstructive
Case Scenario year-old white male presented to personal physician with dyspepsia with reflux.
 Case Scenario 1 57-year-old white male presented to personal physician with dyspepsia with reflux. 7/12 EGD: In the gastroesophageal junction we found an exophytic tumor. The tumor occupies approximately
Case Scenario 1 57-year-old white male presented to personal physician with dyspepsia with reflux. 7/12 EGD: In the gastroesophageal junction we found an exophytic tumor. The tumor occupies approximately
Collaborative Stage for TNM 7 - Revised 06/30/2008 [ Schema ]
![Collaborative Stage for TNM 7 - Revised 06/30/2008 [ Schema ] Collaborative Stage for TNM 7 - Revised 06/30/2008 [ Schema ]](/thumbs/82/86264030.jpg) Collaborative Stage for TNM 7 - Revised 06/30/2008 [ Schema ] CS Tumor Size 000 No mass/tumor found 001-988 001-988 millimeters (code exact size in millimeters) 989 989 millimeters or larger 990 Microscopic
Collaborative Stage for TNM 7 - Revised 06/30/2008 [ Schema ] CS Tumor Size 000 No mass/tumor found 001-988 001-988 millimeters (code exact size in millimeters) 989 989 millimeters or larger 990 Microscopic
Laparoscopy-assisted D2 radical distal subtotal gastrectomy
 Masters of Gastrointestinal Surgery Laparoscopy-assisted D2 radical distal subtotal gastrectomy Xiaogeng Chen, Weihua Li, Jinsi Wang, Changshun Yang Department of Tumor Surgery, Fujian Provincial Hospital,
Masters of Gastrointestinal Surgery Laparoscopy-assisted D2 radical distal subtotal gastrectomy Xiaogeng Chen, Weihua Li, Jinsi Wang, Changshun Yang Department of Tumor Surgery, Fujian Provincial Hospital,
Done by: nisreen obeidat
 Sheet: liver and pancreas Done by: nisreen obeidat Embryology of the liver The liver develops in the ventral mesentery of the foregut and divides the ventral mesentery :into 1)lesser omentum (between the
Sheet: liver and pancreas Done by: nisreen obeidat Embryology of the liver The liver develops in the ventral mesentery of the foregut and divides the ventral mesentery :into 1)lesser omentum (between the
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14
 Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14 Contents 14. Neuroendocrine Tumours 161 14.1. Diagnostic algorithm
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14 Contents 14. Neuroendocrine Tumours 161 14.1. Diagnostic algorithm
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma
 Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
Surgical Treatment for Periampullary Carcinoma A Study of 129 Patients*)
 Hiroshima Journal of Medical Sciences Vol. 33, No. 2, 179,...183, June, 1984 HJM 33-24 179 Surgical Treatment for Periampullary Carcinoma A Study of 129 Patients*) Tsuneo TAN AKA, Motomu KODAMA, Rokuro
Hiroshima Journal of Medical Sciences Vol. 33, No. 2, 179,...183, June, 1984 HJM 33-24 179 Surgical Treatment for Periampullary Carcinoma A Study of 129 Patients*) Tsuneo TAN AKA, Motomu KODAMA, Rokuro
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Adenocarcinoma, pancreatic ductal, laparoscopic distal pancreatectomy for, 61 Adrenal cortical carcinoma, laparoscopic adrenalectomy for, 114
Note: Page numbers of article titles are in boldface type. A Adenocarcinoma, pancreatic ductal, laparoscopic distal pancreatectomy for, 61 Adrenal cortical carcinoma, laparoscopic adrenalectomy for, 114
Abdomen and Retroperitoneum Ultrasound Protocols
 Abdomen and Retroperitoneum Ultrasound Protocols Reviewed By: Anna Ellermeier, MD Last Reviewed: March 2018 Contact: (866) 761-4200, Option 1 **NOTE for all examinations: 1. If documenting possible flow
Abdomen and Retroperitoneum Ultrasound Protocols Reviewed By: Anna Ellermeier, MD Last Reviewed: March 2018 Contact: (866) 761-4200, Option 1 **NOTE for all examinations: 1. If documenting possible flow
