IN THIS ISSUE ONCOLOGY DELIVERY MARKET 32 MANUFACTURING CELL THARAPIES 48 NON-CANNABIS THERAPY 55 ASSAY VALIDATION 63 CLINICAL RESEARCH 68
|
|
|
- Charla Clarke
- 5 years ago
- Views:
Transcription
1 May 2017 Vol 17 No 4 IN THIS ISSUE INTERVIEW WITH OASMIA PHARMACEUTICAL S EXECUTIVE CHAIRMAN JULIAN ALEKSOV ONCOLOGY DELIVERY MARKET 32 Piyush Bansal MANUFACTURING CELL THARAPIES 48 Robert Preti, PhD NON-CANNABIS THERAPY 55 Ronald Aung-Din, MD ASSAY VALIDATION 63 John Allinson The science & business of drug development in specialty pharma, biotechnology, and drug delivery Peter Traber, MD Targeting Galectin-3 Protein in Drug Development Cory Berkland, PhD Leveraging Precision Particle Fabrication Technology to Create Patient- Friendly Dosage Forms Cindy Dubin A Rise in Biologics & Improved Technology Give Pharma Reasons to Consider Parenteral Delivery CLINICAL RESEARCH 68 Kai Langel FAKE NEWS 74 John Bermingham
2 Galectin-Directed Therapies We are only at the beginning of understanding gal-3 targeted therapy and the full potential of this approach. The future likely holds additional high affinity, specific, galectin inhibitors that are bioavailable by routes other than the two currently in development, parenteral and inhaled. Table of C O N T E N T S G A L E C T I N-DIRECTED T H E R A P I E S 20 Targeting Galectin-3 Protein in Drug Development Peter G. Traber, MD, believes we are only at the beginning of understanding the full potential of gal-3 targeted therapy, and the future likely holds additional high affinity, specific, galectin inhibitors that are bioavailable by routes other than the two currently in development. C O N T R O L L E D R E L E A S E 26 Leveraging Precision Particle Fabrication Technology to Create Patient-Friendly Dosage Forms Cory Berkland, PhD, and Nathan Dormer, PhD, review how controlled-release powders offer a flexible and efficient approach to addressing a multitude of patient populations, while also improving compliance. M A R K E T B R I E F 32 Preferences for Targeted Therapies & Patient- Centric Approaches Drive Transformations in Oncology Drug Delivery Market Frost & Sullivan Analyst Piyush Bansal says although chemotherapy has been successfully used for inhibiting cell growth throughout the past few decades, the side effects of chemotherapy have forced researchers to look for some alternative drugs (and how to effectively deliver them) for all types of cancer. S P E C I A L F E AT U R E 36 Prefilled Syringes & Parenteral Manufacturing: A Rise in Biologics & Improved Technology Give Pharma Reasons to Consider Parenteral Delivery Contributor Cindy H. Dubin speaks with some of the leading companies in this market to find out about key trends, packaging advancements, safety improvements, and technology developments. E X E C U T I V E I N T E R V I E W 48 PCT: Manufacturing the Future of Cell Therapies Robert A. Preti, PhD, discusses his company s critical distinction from most other manufacturing partners, steady growth, and the primary challenges facing the cell therapy industry. 6 p.20
3 GALECTIN-DIRECTED THERAPIES Targeting Galectin-3 Protein in Drug Development By: Peter G. Traber, MD INTRODUCTION Fibrogenesis is a major cause of morbidity and mortality, and anti-fibrotic agents have been termed a holy grail of drug development. The galectin-3 (gal-3) protein appears to be critical to the process of fibrogenesis, and targeting gal-3 could potentially treat a broad spectrum of diseases. Two approaches have been taken to develop drugs that bind gal-3. One involves modified disaccharides (TD139), and the other uses large polysaccharides that contain galactose (GR-MD- 02). Studies are being conducted on the use of GR-MD-02 in nonalcoholic steatohepatitis (NASH), chronic inflammatory skin diseases, including plaque psoriasis and atopic dermatitis, and failure and arrhythmia, and diabetes. In heart failure, for example, levels of serum gal-3 correlate with poor prognosis. Starting in 2006, interest in gal-3 significantly increased based on experiments in gal-3 null mice, which are otherwise normal but do not express the gal-3 protein. These gal-3 null mice, when insulted in various ways, were found to be resistant to the development of fibrosis in multiple organs, including liver, lung, kidney, and heart. These data suggest that gal-3 is critical to the process of fibrogenesis, a major cause of morbidity and mortality in patients and one of the most important unmet medical needs. In fact, anti-fibrotic agents have been termed a holy grail of drug development with multiple pharma and biotech companies seeking new drugs. in combination cancer immunotherapy. 20 WHY GALECTIN-3 IS AN ATTRACTIVE DRUG TARGET The galectin-3 (gal-3) protein is an intriguing new drug target to treat a variety of human disorders, a number of which represent large unmet medical needs. Gal-3 is a member of a family of lectin proteins that binds to galactose-containing glycoproteins. First discovered as a major expressed protein in macrophages, gal-3 is expressed in many cell types in the body but predominantly in immune cells. Expression of gal-3 is increased in many chronic inflammatory and fibrotic diseases, as well as in multiple types of cancer cells. Because the immune system is involved in many diseases, the targeting of gal-3 has a broad spectrum of potential disease targets, including organ fibrosis (ie, liver, lung, and kidney), skin disease, ocular disease, atherosclerosis, heart DRUGS TARGETING GALECTINS Two approaches have been taken to develop drugs that bind gal-3: Modified disaccharides (TD139; Galecto Biotech), and large polysaccharides that contain galactose (GR-MD-02; Galectin Therapeutics). Both approaches yield molecules that are not well absorbed orally and must be given parenterally, although TD139 is being delivered by the inhaled route in a Phase I study in IPF (idiopathic pulmonary fibrosis). Galectin Therapeutics has taken the approach of using a modified, naturally occurring carbohydrate polymer that contains chains of galactose as a drug that binds to gal-3 (GR-MD-02). There are significant differences in the binding properties of these two drugs, with TD139 having higher affinity for the carbohydrate recognition domain of gal-3 but GR-MD-02 having a binding stoichiometry of ~5 molecules of gal-3 per drug molecule
4 and binding to a broader area of amino acids in gal-3. While it is unknown how scarring, or cirrhosis. Gal-3 is markedly upregulated in liver disease and has effects F I G U R E 2 these differences may affect ultimate effec- on the two main liver cell types involved in tiveness, it is notable that both molecules fibrogenesis: macrophages and stellate had a similar effect in a mouse model of cells (Figure 1). Inhibition of gal-3 may re- lung fibrosis. duce the fibrogenic myofibroblasts, reduce macrophage recruitment and activation, and potentially increase the macrophage CLINICAL STUDIES UTILIZING activity to reduce collagen and fibrosis. GR-MD-02 GR-MD-02 has been shown in mouse models of NASH to reduce fat, inflamma- GR-MD-02 is being used currently in tion, and cell death and to both prevent three areas of development: 1) chronic inflammation and fibrosis in non-alcoholic steatohepatitis (NASH); 2) chronic inflammatory severe skin diseases, including plaque psoriasis and atopic dermatitis, and; 3) combination immunotherapy for cancer. While the major value driver for GR-MD-02 is as a NASH drug, there are early clinical results showing efficacy in severe skin disease. and reverse fibrosis (Figure 2A and 2B). 1 In this mouse model of NASH, gal-3 is markedly increased in macrophages (Figure 2C), and this is significantly reduced with GR-MD-02 treatment (Figure 2D). Additionally, in a toxic model of rat cirrhosis, GR-MD-02 has been shown to reverse fibrosis and cirrhosis (Figure 2E and 2F), despite continuation of the toxic insult, and partially reverse the portal hypertension associated with cirrhosis. 2 Portal hyperten- Experimental effects of GR-MD-02 in two animal models of liver fibrosis. A and B show liver collagen staining (red) of mice with NASH, control and treated with GR-MD-02, respectively. C and D show liver gal-3 staining (brown) of mice with NASH, control and treated with GR-MD-02, respectively. E and F show liver of rats treated with toxin to induce cirrhosis, control and treated with GR-MD-02, respectively. N = nodules associated with cirrhosis; arrow = broken strand of collagen. Liver Fibrosis & NASH sion is the primary reason for complica- tions in humans with NASH cirrhosis and Preclinical results show that GR-MD- 02 has significant anti-fibrotic effects in F I G U R E 1 a potentially acceptable regulatory endpoint in clinical trials. multiple models, including liver (fatty liver Based on these robust preclinical find- and toxin induced), kidney, lung, pul- ings, GR-MD-02 therapy in development is monary artery, and heart fibrosis. NASH being directed at patients with NASH cir- was chosen for development of GR-MD-02 rhosis, with the goal of reducing portal because it is one of the most common liver pressure by reducing fibrosis and thereby diseases, with 1 in 4 individuals in the improving outcomes. After obtaining an world having fatty liver and about 2% of those destined to die of complications of NASH cirrhosis. There are currently no approved drugs for NASH. NASH is recognized as one of the largest potential markets for drug development today, with the global market in 2025 predicted to be as large as $40 billion. NASH is a chronic disorder with fat accumulation in the liver resulting in inflammation, cell death, and progressive fibrosis leading over decades to the end stage of Gal-3 effects on macrophages and stellate cells in liver. Gal-3 is produced by macrophages and activated stellate cells into the microenvironment of the liver. Green lines = gal-3 production, red lines = gal-3 action, black lines = results of gal-3 action. Gal-3 has multiple effects including 1) activation of stellate cells to myofibroblasts; 2) recruitment of monocytes to the liver macrophage pool; 3) activation of macrophages into subtypes, producing cytokines and potentially macrophages that digest collagen. IND for NASH with advanced fibrosis and fast-track designation, two Phase I trials and an exploratory Phase II trial were completed showing good safety and a lack of drug interactions. This led to the currently underway Phase IIb clinical trial in patients with NASH cirrhosis and portal hypertension (NASH-CX). There are a number of important elements in the NASH-CX trial. First, it is one of only three currently ongoing clinical trials in NASH cirrhosis, and the next one to 21
5 read out top line data in this calendar year. The trial has enrolled 162 patients in F I G U R E 3 skin diseases, providing some confidence that there may be clinical activity in other three treatment arms, placebo and two gal-3 dependent disorders. Importantly, doses of drug, with a treatment duration of there is a higher incidence of psoriasis in 52 weeks. The primary endpoint of the patients with NASH. While significantly trial is the baseline adjusted reduction in portal pressure as assessed by hepatic venous pressure gradient (HVPG), which is directly related to patient outcomes and is potentially an acceptable regulatory end- Photographs of patient with severe psoriasis at baseline and following treatment with 13 doses of GR-MD- 02, 8 mg/kg administered every 2 weeks. more development work and formulation will be required to demonstrate clinical utility, these findings open up an entirely new target area for severe skin diseases, with potential market utility in the relatively un- point for provisional approval with follow disease not known to resolve itself natu- derserved area of moderate-to-severe up outcomes data. Additionally, secondary rally. In addition to this clinical finding, re- atopic dermatitis. endpoints will evaluate change in liver search publications suggested the potential biopsy, serum biomarkers, complications, importance of galectin-3 in psoriasis. Cancer Immunotherapy and several non-invasive measures of liver On this basis, an exploratory, open- Gal-3 expression is increased in most structure and function including FibroScan label, Phase IIa trial was conducted in five cancers and secreted into the tumor mi- (Echosens) and 13C methacetin breath test adult patients with moderate-to-severe croenvironment. There are multiple effects (Exalenz). The study is powered at >80% plaque psoriasis [PASI (Psoriasis Area and of gal-3 in cancer (Figure 4), including the to demonstrate a difference in HVPG of at Severity Index) 12 and BSA (Body Sur- promotion of angiogenesis and metastasis least 2 mmhg, a change which is poten- face Area) 10%]. One patient had an and decreased apoptosis of tumor cells, tially clinically significant in these patients. 80% reduction in PASI 30 days after their macrophage M2 polarization, increased More than 25% of subjects have com- last infusion (13th) (Figure 3), while the chemotaxis to recruit more macrophages, pleted the trial, and it is on track to report other four patients reached 50% reduction and enhanced macrophage gal-3 secre- top line data in December in PASI by their 10th infusion. tion, and T-cell apoptosis and impairment The NASH-CX trial is a significant Scientific studies also show that there of TCR signaling reducing the ability of im- milestone in NASH therapy as well as a is a link between the galectin-3 protein and proof-of-concept for therapies directed at gal-3. Most of the many current ongoing disease activity in atopic dermatitis with increased amounts in the skin of patients and F I G U R E 4 clinical trials in NASH are directed to ther- reduced disease in mice with atopic der- apy in pre-cirrhotic NASH, but the NASH- matitis that lack the galectin-3 protein. CX trial is the next trial to read out in the GR-MD-02 treatment of severe and re- more advanced patients with NASH cirrho- fractory atopic dermatitis was also studied 22 sis. Success in this area could be a breakthrough finding for liver cirrhosis and additionally open the possibilities for gal- 3 targeted therapy in fibrotic disorders of other organs. Clinically Meaningful Effect of GR-MD- 02 in Psoriasis & Atopic Dermatitis Serendipity struck the GR-MD-02 clinical development program when a patient enrolled in a Phase I NASH trial had a remarkable remission of her psoriasis, a skin in three patients in an open label, investigator-initiated study. All three patients showed clinical response as determined by reduction of the Eczema Area and Severity Index (EASI) score at week 12 having received six every-other-week doses, with two patients achieving a 64% and 74% reduction in EASI, respectively, at 6 weeks after receiving only three doses of GR-MD- 02. These early clinical results demonstrate activity of GR-MD-02 in two severe Gal-3 effects on cancer cells, macrophages, and T-cells in the tumor microenvironment. Gal-3 is produced by both tumor cells and macrophages and has the effects of 1) promoting angiogenesis and metastasis and decreasing apoptosis of tumor cells; 2) promoting macrophage M2 polarization, increasing chemotaxis to recruit more macrophages, and enhancing gal- secretion; 3) promoting T-cell apoptosis and reducing TCR signaling thereby blocking immune effect on tumor cells.
6 mune system to kill tumor cells. GR-MD-02 HAS A STRONG it has oral inhibitors in preclinical develop- The Providence Cancer Center (Port- SAFETY PROFILE ment, and Galectin has a discovery pro- land, OR), recently reported early results gram for identification of small molecule of GR-MD-02 combined with pem- In each of the clinical trials with GR- inhibitors of gal-3. Such oral inhibitors will brolizumab (KEYTRUDA ) in patients with MD-02, the drug has shown to be safe and likely open many possibilities for galectin- advanced melanoma, oral/head and neck well tolerated, with no serious adverse directed therapies for a broad range of cancer (OHN), and non-small cell lung events ascribed to the drug. At this time, human disease. u cancer (NSCLC). Six subjects, five with ad- the total number of doses of GR-MD-02 ad- vanced melanoma, were enrolled in the lowest dose cohort (2 mg/kg GR-MD-02) ministered to humans is nearly This is encouraging for the development pro- REFERENCES with one partial response and one mixed response in the five melanoma patients. Figure 5 is a chest CT scan of the patient gram, given the high rate of drugs that are dropped from development based on significant toxicities. Additionally, given that 1. Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8(12):e Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8(10):e with a partial response showing a marked GR-MD-02 is a complex carbohydrate, it reduction in tumor size at week 12 of therapy, after three doses of combined GR-MD- 02 and pembrolizumab. is metabolized via different mechanisms than typical small molecule drugs, and a lower likelihood of toxicities based on drug To view this issue and all back issues online, please visit F I G U R E 5 metabolites is anticipated. B I O G R A P H Y THE FUTURE OF GALECTIN DRUG DEVELOPMENT The treatment effect of GR-MD-02 on Chest CT scan of the patient with a partial response showing a marked reduction in tumor size at week 12 of therapy, after 3 doses of combined GR-MD-02 and pembrolizumab. The investigators were encouraged by psoriasis and atopic dermatitis provides evidence for efficacy of one anti-gal-3 drug in human disease. Ongoing trials in NASH cirrhosis, with results at the end of 2017, and combination chemotherapy will add to the data. Additionally, presentation of results are awaited from the Phase I trial Dr. Peter G. Traber is President, CEO, and CMO of Galectin Therapeutics and President Emeritus of Baylor College of Medicine, where he was CEO from 2003 these early safety results, and although one cannot conclude whether the one partial response was related to GR-MD-02, the response provides a clinically relevant signal to follow as GR-MD-02 doses are escalated. This study is ongoing, and a decision to progress to Phase II will be based on the response rate of the combination of pembrolizumab with GR-MD-02 as compared to historical response rates to pembrolizumab alone. using TD139 in the treatment of IPF. Each of these trials should add to the foundation of information on the potential for anti-gal- 3 therapeutic approaches, which could extend to multiple important diseases. We are only at the beginning of understanding gal-3 targeted therapy and the full potential of this approach. The future likely holds additional high affinity, specific, galectin inhibitors that are bioavailable by routes other than the two currently in development, parenteral and inhaled. In this regard, Galecto Biotech has suggested to From 2000 to 2003, he was Senior Vice President of Clinical Development and Medical Affairs and CMO of GlaxoSmithKline plc. He served as CEO of the University of Pennsylvania Health System and was Chair of the Department of Internal Medicine and Chief of Gastroenterology for the University of Pennsylvania School of Medicine. Dr. Traber has also managed a molecular biology research laboratory and published over 100 articles of original research, reviews, and book chapters. He earned his MD from Wayne State School of Medicine, his BS in Chemical Engineering from the University of Michigan, and a certificate in medical leadership from Wharton Business School. 23
CORPORATE PRESENTATION
 CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
CORPORATE PRESENTATION NASDAQ: GALT www.galectintherapeutics.com 1 Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within the meaning
Corporate Presentation
 Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation February 2, 2017 NASDAQ: GALT www.galectintherapeutics.com 2017 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
Clinical Trials & Endpoints in NASH Cirrhosis
 Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
TRANSCRIPTION DISCLAIMER:
 TRANSCRIPTION DISCLAIMER: THE INFORMATION CONTAINED HEREIN IS A TEXTUAL REPRESENTATION OF THE GALECTIN THERAPEUTICS, INC. ("GALECTIN") CONFERENCE CALL, CONFERENCE PRESENTATION OR OTHER AUDIO PRESENTATION,
TRANSCRIPTION DISCLAIMER: THE INFORMATION CONTAINED HEREIN IS A TEXTUAL REPRESENTATION OF THE GALECTIN THERAPEUTICS, INC. ("GALECTIN") CONFERENCE CALL, CONFERENCE PRESENTATION OR OTHER AUDIO PRESENTATION,
The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy
 The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 2018 2017 Galectin Therapeutics
The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 2018 2017 Galectin Therapeutics
Rodman & Renshaw 17 th Annual Global Investment Conference
 Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
Rodman & Renshaw 17 th Annual Global Investment Conference September 10, 2015 NASDAQ: GALT www.galectintherapeutics.com 2015 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains,
GR-MD-02 for Indication of NASH Cirrhosis
 for Indication of NASH Cirrhosis NASH-C Clinical Trial Results San Francisco, CA January 8-, 208 NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition
for Indication of NASH Cirrhosis NASH-C Clinical Trial Results San Francisco, CA January 8-, 208 NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition
Corporate Presentation
 Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation August 2016 NASDAQ: GALT www.galectintherapeutics.com 2016 Galectin Therapeutics Inc. Forward-Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation
 Corporate Presentation February 10, 2014 2014 Galectin Therapeutics NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition to historical information,
Corporate Presentation February 10, 2014 2014 Galectin Therapeutics NASDAQ: GALT www.galectintherapeutics.com Forward Looking Statements This presentation contains, in addition to historical information,
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Corporate Overview. September 6, Harold H. Shlevin, Ph.D. Chief Executive Officer. H.C. Wainwright Conference
 Corporate Overview September 6, 2018 Harold H. Shlevin, Ph.D. Chief Executive Officer H.C. Wainwright Conference NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains,
Corporate Overview September 6, 2018 Harold H. Shlevin, Ph.D. Chief Executive Officer H.C. Wainwright Conference NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains,
GR-MD-02: Phase III Galectin-3 Inhibitor
 GR-MD-02: Phase III Galectin-3 Inhibitor July 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking
GR-MD-02: Phase III Galectin-3 Inhibitor July 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
(NASDAQ:GALT) 27, :30 ET
 Galectin Therapeutics, Inc. (NASDAGALT) Conference Call Transcript September 27, 2016 05:30 ET Operator: Welcome to the Galectin Therapeutics Business Update Conference Call. At this time, all participants
Galectin Therapeutics, Inc. (NASDAGALT) Conference Call Transcript September 27, 2016 05:30 ET Operator: Welcome to the Galectin Therapeutics Business Update Conference Call. At this time, all participants
Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma
 PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
Dawson James Conference
 Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Anti-IL-33 (ANB020) Program
 Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
FDA Introductory Remarks Stephanie O. Omokaro, MD
 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III
Clinical: Ipilimumab (MDX-010) Update and Next Steps
 Clinical: Ipilimumab (MDX-010) Update and Next Steps Geoffrey M. Nichol, M.D., M.B.A. Senior Vice President, Product Development Medarex, Inc. R&D Day December 9, 2005 Ipilimumab: New Class of Cancer Therapy
Clinical: Ipilimumab (MDX-010) Update and Next Steps Geoffrey M. Nichol, M.D., M.B.A. Senior Vice President, Product Development Medarex, Inc. R&D Day December 9, 2005 Ipilimumab: New Class of Cancer Therapy
Liver Disease NASH/Fibrosis Model
 Liver Disease NASH/Fibrosis Model Please contact: Dipti Deshpande PhD ddeshpande@woodlandpharma.com or Carol Gebert PhD cgebert@woodlandbiosciences.com 617-513-5280 Liver Diseases Comprise a Growing Market
Liver Disease NASH/Fibrosis Model Please contact: Dipti Deshpande PhD ddeshpande@woodlandpharma.com or Carol Gebert PhD cgebert@woodlandbiosciences.com 617-513-5280 Liver Diseases Comprise a Growing Market
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
i-bodies a new class of protein therapeutics to treat fibrosis
 i-bodies a new class of protein therapeutics to treat fibrosis BIOSHARES JULY 2017 Sam Cobb, CEO and Managing Director AdAlta Limited (ASX:1AD) s.cobb@adalta.com.au Disclaimer Investment in AdAlta is subject
i-bodies a new class of protein therapeutics to treat fibrosis BIOSHARES JULY 2017 Sam Cobb, CEO and Managing Director AdAlta Limited (ASX:1AD) s.cobb@adalta.com.au Disclaimer Investment in AdAlta is subject
Corporate Presentation October 2018 Nasdaq: ADXS
 Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH
 www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Corporate Presentation September Nasdaq: ADXS
 Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program
 Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Transforming science into medicine
 Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
The New England Journal of Medicine. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer
 The New England Journal of Medicine A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer May 18, 2015 Forward-Looking Statements Various remarks that we make on this call that are
The New England Journal of Medicine A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer May 18, 2015 Forward-Looking Statements Various remarks that we make on this call that are
Interim Results for 6 Months Ended 31 March 2017 and Business Update. 17 th May 2017
 Interim Results for 6 Months Ended 31 March 2017 and Business Update 17 th May 2017 Forward-looking Statements Please Note This presentation includes forward-looking statements. These forward-looking statements
Interim Results for 6 Months Ended 31 March 2017 and Business Update 17 th May 2017 Forward-looking Statements Please Note This presentation includes forward-looking statements. These forward-looking statements
JP Morgan Healthcare Conference. January 8, 2007
 JP Morgan Healthcare Conference January 8, 2007 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
JP Morgan Healthcare Conference January 8, 2007 Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation,including without limitation statements
Five Prime Therapeutics, Inc. Corporate Overview
 Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK
 HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
A COMPANY INSPIRED BY REGENERATION
 OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
OUR FOCUS A COMPANY INSPIRED BY REGENERATION MEET ALLY, a teacher, mother and motorcycle enthusiast with primary biliary cholangitis*(pbc), a rare and potentially fatal non-viral liver disease. OUR FOCUS
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Boehringer Ingelheim BioXcellence. Producing Value. Global Contract Manufacturing Excellence
 Boehringer Ingelheim BioXcellence Producing Value Global Contract Manufacturing Excellence Your World Class Contract Manufacturer Boehringer Ingelheim BioXcellence Producing Value Boehringer Ingelheim
Boehringer Ingelheim BioXcellence Producing Value Global Contract Manufacturing Excellence Your World Class Contract Manufacturer Boehringer Ingelheim BioXcellence Producing Value Boehringer Ingelheim
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer. Lazard Capital Markets 8 th Annual Healthcare Conference
 Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Hiding in Plain Sight Finding the NASH Patient
 Hiding in Plain Sight Finding the NASH Patient The Silent Disease Nonalcoholic fatty liver disease (NAFLD) and its advanced form, nonalcoholic steatohepatitis (NASH), are increasingly prevalent today and
Hiding in Plain Sight Finding the NASH Patient The Silent Disease Nonalcoholic fatty liver disease (NAFLD) and its advanced form, nonalcoholic steatohepatitis (NASH), are increasingly prevalent today and
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Prothena Corporation plc
 Prothena Corporation plc PRX003 Investor Update: Phase 2 Development Strategy September 29, 2016 Agenda Dr. Gene Kinney, Chief Operating Officer Introduction Dr. Ken Flanagan, Senior Scientist Th17 and
Prothena Corporation plc PRX003 Investor Update: Phase 2 Development Strategy September 29, 2016 Agenda Dr. Gene Kinney, Chief Operating Officer Introduction Dr. Ken Flanagan, Senior Scientist Th17 and
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease
 Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
ABBVIE VENTURES VISION. Drive strategic returns to AbbVie as a key constituent of overall corporate strategy
 ABBVIE VENTURES ABBVIE VENTURES VISION Drive strategic returns to AbbVie as a key constituent of overall corporate strategy Early access to external innovation with a long-term horizon to generate meaningful
ABBVIE VENTURES ABBVIE VENTURES VISION Drive strategic returns to AbbVie as a key constituent of overall corporate strategy Early access to external innovation with a long-term horizon to generate meaningful
Novan Announces Promising Clinical Results with SB414
 Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Corporate Presentation
 Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases Corporate Presentation NasdaqCM: LJPC July 2014 Forward-Looking Statements These slides contain "forward-looking" statements
Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases Corporate Presentation NasdaqCM: LJPC July 2014 Forward-Looking Statements These slides contain "forward-looking" statements
CANCER 1.7 M 609,000 26% 15.5 M 73% JUST THE FACTS. More Than 1,100 Cancer Treatments in Clinical Testing Offer Hope to Patients
 CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO
 9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM
 HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Corporate Presentation
 Corporate Presentation November 2016 Kadmon Holdings, Inc. 1 Forward-looking Statement This presentation contains forward looking statements that are based on the beliefs and assumptions and on information
Corporate Presentation November 2016 Kadmon Holdings, Inc. 1 Forward-looking Statement This presentation contains forward looking statements that are based on the beliefs and assumptions and on information
Boehringer Ingelheim BioXcellence. Producing Value. Global Contract Manufacturing Excellence
 Boehringer Ingelheim BioXcellence Producing Value Global Contract Manufacturing Excellence Your World Class Contract Manufacturer Boehringer Ingelheim BioXcellence Producing Value Boehringer Ingelheim
Boehringer Ingelheim BioXcellence Producing Value Global Contract Manufacturing Excellence Your World Class Contract Manufacturer Boehringer Ingelheim BioXcellence Producing Value Boehringer Ingelheim
COMPANY PRESENTATION JULY Bob Bechard Executive Vice-President Corporate Development & Licensing
 COMPANY PRESENTATION JULY 2018 Bob Bechard Executive Vice-President Corporate Development & Licensing Forward Looking Statements Some statements in this release may contain forward-looking information.
COMPANY PRESENTATION JULY 2018 Bob Bechard Executive Vice-President Corporate Development & Licensing Forward Looking Statements Some statements in this release may contain forward-looking information.
The Lipid Metabolism Company. Corporate Presentation March 2018
 The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
The Lipid Metabolism Company Corporate Presentation March 2018 Disclaimer This document has been prepared by Cerenis Therapeutics (the "Company") and is for information purposes only. The information and
LION. Corporate Presentation June 2016 BIOTECHNOLOGIES. Leadership & Innovation in Oncology
 LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
Company Overview. September 2018 NASDAQ: MDGL
 Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
Company Overview September 2018 NASDAQ: MDGL 1 Forward Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our future financial or business
CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer
 November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
VasoRx: Atherosclerotic Plaque Regression
 VasoRx: Atherosclerotic Plaque Regression A progressive disease characterized by plaque build-up in large arteries, leading to heart attacks, stroke, and peripheral vascular disease. Induced by a combination
VasoRx: Atherosclerotic Plaque Regression A progressive disease characterized by plaque build-up in large arteries, leading to heart attacks, stroke, and peripheral vascular disease. Induced by a combination
CERENIS Therapeutics acquires LYPRO Biosciences expanding its HDL strategy into immuno-oncology and chemotherapeutic drug delivery
 Press release CERENIS Therapeutics acquires LYPRO Biosciences expanding its HDL strategy into immuno-oncology and chemotherapeutic drug delivery Combining LYPRO Biosciences NanoDisk technology with CERENIS
Press release CERENIS Therapeutics acquires LYPRO Biosciences expanding its HDL strategy into immuno-oncology and chemotherapeutic drug delivery Combining LYPRO Biosciences NanoDisk technology with CERENIS
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives. Oncology Therapeutic Area Janssen Research & Development, LLC
 Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
Partnering in Oncology Sharing a Vision to Help Prolong and Improve Patients Lives Oncology Therapeutic Area Janssen Research & Development, LLC The patients are waiting. Dr. Paul Janssen To Our Potential
SPECIALTY MEDICAL CANNABIS COMPANY
 SPECIALTY MEDICAL CANNABIS COMPANY TSX Venture: RVV OTCQB: RVVTF Corporate Presentation - August 2018 ReviveThera.com Revive Therapeutics Ltd. Office: 905-605-5535 E-mail: info@revivethera.com Forward
SPECIALTY MEDICAL CANNABIS COMPANY TSX Venture: RVV OTCQB: RVVTF Corporate Presentation - August 2018 ReviveThera.com Revive Therapeutics Ltd. Office: 905-605-5535 E-mail: info@revivethera.com Forward
Targeted Therapeutics for Inflammatory Disease
 Targeted Therapeutics for Inflammatory Disease Jefferies Global Healthcare Conference June 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking
Targeted Therapeutics for Inflammatory Disease Jefferies Global Healthcare Conference June 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking
Forward Looking Statements
 Slide title Forward Looking Statements This presentation may contain forward looking statements with respect to the financial condition, results and business achievements/performance of Biotron Limited
Slide title Forward Looking Statements This presentation may contain forward looking statements with respect to the financial condition, results and business achievements/performance of Biotron Limited
Determined to realize a future in which people with cancer live longer and better than ever before Q Conference Call
 Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
Corporate Presentation
 Corporate Presentation December2014 Forward Looking Statement Please be advised that the information and projections provided in this presentation may include forward-looking statements with respect to
Corporate Presentation December2014 Forward Looking Statement Please be advised that the information and projections provided in this presentation may include forward-looking statements with respect to
Corporate Presentation. October 2017
 AEPP Breakthrough technology for women s cancers Corporate Presentation October 2017 Oncolix 2017 1 Safe Harbor Certain of the statements and the projections set forth in this presentation constitute forward-looking
AEPP Breakthrough technology for women s cancers Corporate Presentation October 2017 Oncolix 2017 1 Safe Harbor Certain of the statements and the projections set forth in this presentation constitute forward-looking
(212) (347)
 EMBARGOED FOR MONDAY, JUNE 21, 2010: 3:00 P.M. EST For immediate release: June 21, 2010 Media Contact: Curtis Allen (212) 733-2096 (347) 443-5252 Investors Contact: Suzanne Harnett (212) 733-8009 Pfizer
EMBARGOED FOR MONDAY, JUNE 21, 2010: 3:00 P.M. EST For immediate release: June 21, 2010 Media Contact: Curtis Allen (212) 733-2096 (347) 443-5252 Investors Contact: Suzanne Harnett (212) 733-8009 Pfizer
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
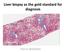 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
ADAPTIMMUNE INVESTOR PRESENTATION. August 2016
 ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
34 th Annual JP Morgan Healthcare Conference. January 13, 2016
 34 th Annual JP Morgan Healthcare Conference January 13, 2016 SAFE HARBOR Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
34 th Annual JP Morgan Healthcare Conference January 13, 2016 SAFE HARBOR Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information.
INTERIM MANAGEMENT STATEMENT Q3 2017
 INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era
 Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
EASL International Liver Congress Paris, France 14 April 2018
 NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
NGM282 Improves Fibrosis and NASH-Related Histology in 12 Weeks in Patients With Biopsy-Confirmed NASH, Which is Preceded By Significant Decreases in Hepatic Steatosis, Liver Transaminases and Fibrosis
Targeted Therapeutics for Inflammatory Disease
 Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease
 microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease January 2018 Safe Harbor Statement Statements contained in this presentation regarding matters that are not
microrna Therapeutics Harnessing the power of micrornas to target multiple pathways of disease January 2018 Safe Harbor Statement Statements contained in this presentation regarding matters that are not
Breathtaking science. Developing respiratory drugs to improve health and quality of life. H.C. Wainwright Global Life Sciences Conference April 2018
 Breathtaking science Developing respiratory drugs to improve health and quality of life H.C. Wainwright Global Life Sciences Conference April 2018 www.veronapharma.com Forward-Looking Statements This presentation
Breathtaking science Developing respiratory drugs to improve health and quality of life H.C. Wainwright Global Life Sciences Conference April 2018 www.veronapharma.com Forward-Looking Statements This presentation
Combining HS-110 and anti-pd-1 in NSCLC. September 1, 2015
 Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
GSK Oncology. Axel Hoos, MD, PhD Senior Vice President, Oncology R&D. March 8, 2017
 GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
Dicerna Pharmaceuticals Overview. Delivering RNAi-Based Breakthrough Therapies
 Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
UNITED STATES DISTRICT COURT DISTRICT OF NEVADA ) ) ) ) ) ) ) ) ) ) ) ) ) No.
 UNITED STATES DISTRICT COURT DISTRICT OF NEVADA MARISSA BALLESTEROS, Individually and on Behalf of All Others Similarly Situated, vs. Plaintiff, GALECTIN THERAPEUTICS INC., JAMES C. CZIRR, PETER G. TRABER
UNITED STATES DISTRICT COURT DISTRICT OF NEVADA MARISSA BALLESTEROS, Individually and on Behalf of All Others Similarly Situated, vs. Plaintiff, GALECTIN THERAPEUTICS INC., JAMES C. CZIRR, PETER G. TRABER
AXIM Biotechnologies Reports Year End 2017 Results
 March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
MANIFEST Phase 2 Enhancement / Expansion
 MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
(212) Investors Contact: Ryan Crowe (212)
 For immediate release: February 5, 2014 Media Contact: Sally Beatty (212) 733-6566 Investors Contact: Ryan Crowe (212) 733-8160 Pfizer And Merck To Collaborate On Innovative Anti-Cancer Combination Studies
For immediate release: February 5, 2014 Media Contact: Sally Beatty (212) 733-6566 Investors Contact: Ryan Crowe (212) 733-8160 Pfizer And Merck To Collaborate On Innovative Anti-Cancer Combination Studies
Personalized Cancer Neoantigen Vaccines
 Personalized Cancer Neoantigen Vaccines Turning the Immune System Against Your own Unique Tumour-Specific Antigens 3 rd Annual Advances in Immuno-Oncology Congress London, May 24, 2018 Agnete Fredriksen,
Personalized Cancer Neoantigen Vaccines Turning the Immune System Against Your own Unique Tumour-Specific Antigens 3 rd Annual Advances in Immuno-Oncology Congress London, May 24, 2018 Agnete Fredriksen,
BioCryst Pharmaceuticals
 BioCryst Pharmaceuticals Jefferies 2010 Global Life Sciences Conference New York Stuart Grant Senior Vice President & Chief Financial Officer Rob Bennett Executive Director, Investor Relations & Business
BioCryst Pharmaceuticals Jefferies 2010 Global Life Sciences Conference New York Stuart Grant Senior Vice President & Chief Financial Officer Rob Bennett Executive Director, Investor Relations & Business
Corporate Presentation September 2017
 Enhancing Life Through Nature TSX: CZO Corporate Presentation September 2017 www.ceapro.com 2 Forward Looking Statements This presentation contains forward-looking statements. Various factors could cause
Enhancing Life Through Nature TSX: CZO Corporate Presentation September 2017 www.ceapro.com 2 Forward Looking Statements This presentation contains forward-looking statements. Various factors could cause
MOLOGEN AG. Pioneering Immune Therapy. Q1 Results 2014 Conference Call. Dr. Matthias Schroff, CEO
 Pioneering Immune Therapy Q1 Results 2014 Conference Call Dr. Matthias Schroff, CEO Berlin, 14 May 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to
Pioneering Immune Therapy Q1 Results 2014 Conference Call Dr. Matthias Schroff, CEO Berlin, 14 May 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to
Vicore Pharma AB. Audiocast, October 2, Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO
 Vicore Pharma AB Audiocast, October 2, 2017 1 Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO FORWARD-LOOKING STATEMENTS This presentation may contain certain forward-looking statements
Vicore Pharma AB Audiocast, October 2, 2017 1 Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO FORWARD-LOOKING STATEMENTS This presentation may contain certain forward-looking statements
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results
 Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
Infinity Highlights Promising Preclinical Hedgehog Data and Announces First Quarter Financial Results Heat Shock Protein 90 Program Continues to Advance Toward Potential Registration Trial CAMBRIDGE, Mass.,
The Journey towards Total Wellbeing A Health System s Innovative Approach
 The Journey towards Total Wellbeing A Health System s Innovative Approach Company Profile Wellness A state of complete physical, mental and social wellbeing and not merely the absence of disease or infirmity
The Journey towards Total Wellbeing A Health System s Innovative Approach Company Profile Wellness A state of complete physical, mental and social wellbeing and not merely the absence of disease or infirmity
