LIPIDS. Docent Tuomo Glumoff. Faculty of Biochemistry and Molecular Medicine P Biomolecules for Biochemists (8 op)
|
|
|
- Melanie Freeman
- 5 years ago
- Views:
Transcription
1 Faculty of Biochemistry and Molecular Medicine P Biomolecules for Biochemists (8 op) P Biomolecules for Bioscientists (8 op) P Biomolecules (5 op) LIPIDS Docent Tuomo Glumoff
2 LIPIDS lectures 32 Answers to problems will be given at the lectures and they will also be available in NOPPA after the lectures. - lipids have many functions: fats insulate heat and serve as energy storage form membranes some are vitamins or hormones or other signal molecules enzyme cofactors, electron carriers light-absorbing pigments, protein anchors to membranes emulsifiers - lipids are not polymers, but often associate through noncovalent forces - hydrophilic and hydrophobic parts = amphipathic FATTY ACIDS - simplest lipids are fatty acids (rasvahapot) carboxylic acids with a hydrocarbon side chain straight chain vs. branched chain saturated = contain only single bonds unsaturated = contain double bond(s) configuration about the double bond usually cis mostly even number of carbons COOH COOH COOH COOH - fatty acids are in anionic form at physiological ph: RCOOH RCOO - + H + pka naming and abbreviating fatty acids: number of carbons, double bonds (their position and configuration) see table on page 35
3 33 - fatty acid carbon numbering starts from the carboxy end; however if ω-naming system used, then: - w- (omega) fatty acids: the position of the double bond(s) counted from the methyl end group, e.g. w-3, w-6 - essential: mammals cannot make double bonds between D9 and the methyl end - abundant in some plant seeds and fish oils COOH fatty acids with double bond(s) in this region must be obtained from food fatty acids with double bond(s) in this region can be synthesized in the body - chain length and degree of saturation affects solubility, packing and melting temperature - cis-double bond makes a bend to the fatty acid tail PROBLEM 1. What is one apparent influence to the physical properties of a fatty acid upon introduction of double bond(s)? - in cells not much free fatty acids exist, but most are parts of more complex lipids
4 TRIACYLGLYCEROLS FATS - fatty acyl esters of glycerol - efficient energy stores - may contain different fatty acids - in animals fat stored in adipose cells 34 - fats and oils are mixtures of triacylglycerols; fat is solid, oil is liquid at room temperature - 3 major functions of fat: energy production get ATP to drive metabolic processes heat production (alternative for ATP production) thermal insulation (layers of fat cells under the skin) - 2 advantages of storing energy in the form of fat rather than carbohydrate: fat is less oxidized than carbohydrate, so, fat has more energy-releasing capacity left fat is tightly packed and need no water, which would increases the weight to carry - composition and naming system Molecules in 3D for example from here:
5 classification of lipids 35 Numbering begins from the carboxylic end!
6 PROBLEM 2. Draw the structure or abbreviate the following fatty acids: (NOTE: some may be hypothetical taken here just for the sake of practicing) a) 18:2tD5,8 b) 22:4cD3,9,12,20 c) 36 O OH SOAPS AND DETERGENTS - saponification: fat + alkali (NaOH, KOH) soap - fatty acids are released and form sodium or potassium salts - detergents are synthetic molecules that often have better defined chemical and physical properties than soaps - detergents can be used to denaturate proteins or extract and solubilize membrane proteins (mimic the membrane environment and mask the hydrophobic surface of the membrane protein) O - O S O Na + SDS, sodium dodecyl sulfate O Triton X-100 PROBLEM 3. Chemistry of cleaning: Why do many cleansing agents used to remove stains or spots are basic by ph and what is the practical advantage of this? WAXES - a long-chain fatty acid ester + a long-chain alcohol = wax - the head group is left only weakly hydrophilic waxes are insoluble - used as protective coatings by both animals and plants
7 LIPID CONSTITUENTS OF BIOLOGICAL MEMBRANES - cell membranes contain a variety of different lipids that also affect the structure and function of the membranes 37 i) GLYCEROPHOSPHOLIPIDS (phosphoglycerides) - phosphate-containing head group - derivatives of glycerol-3-phosphate glycerol backbone - major group of lipids in most biological membranes - R1 & R2 derived from fatty acids - R3 possess great variance ii) GLYCOGLYCEROLIPIDS - built on non-phosphorylated glycerol - sugar at R3 - monogalactosyl diglyceride abundant in chloroplast membranes
8 - sphingomyelin is part of membraneous structure that surrounds and insulates nerve axons sphingomyelin contains also protein - sphingolipids at cell surfaces mediate biological recognition: extracellular molecules cell-cell contacts blood group antigens 38 - ganglioside is a soluble lipid due to many carbohydrate units carbohydrate part lipid part iv) CHOLESTEROL - a steroid - rigid structure due to all-chair conformation of the cyclohexane rings - content up to ca. 25% in some membranes - disrupts regularity in the membrane can regulate membrane stiffness and permeability - without cholesterol the flexibility of the membrane could change too rapidly as a response to changes in environment - cholesterol helps membranes to adapt better and so membranes do not break - stiff (jäykkä) breaks more easily - cholesterol adds flexibility to the membrane structure; not as stiff Despite knowing much of the membrane and membrane lipids: why so many different types of lipids in biological membranes - no clear understanding as yet
9 example of lipid function in tissues: lung surfactant (keuhkosurfaktantti) 39 i.e. why do we need to know about lipids... - major lipid component of the surfactant is dipalmitoyl phosphatidylcholine, DPPC - alveoli cells are coated with DPPC - DPPC is tightly packed, since fatty acids in DPPC are saturated - tight packing of DPPC resists collapse of the alveolar space when breathing out - when not collapsed, also less energy is needed in expanding the alveolar space when breathing in - defect in the surfactant causes diseases associated with breathing difficulties knowing about lipid structure-function (biochemistry) makes it possible to understand physiology and disease
10 40 Blood group antigens. ref. Carbohydrate lectures! A case: how will a snake venom kill you? Phosphatidylserine is a glycerophospholipid that contains the amino acid serine as the R3 group, and two myristic acids as the groups R1 and R2. Many snake venoms contain the enzyme phospholipase A2 that can remove the R2 fatty acid in a reaction involving a water molecule. What remains is lysolecithin, which is more amphiphilic and can act as a detergent that disrupts the red blood cell membranes causing hemolysis. a) Draw the molecular formula of phosphatidylserine b) Draw also the molecular formula of lysolecithin. (will be done at the lecture)
11 - membranes of different cells or cell organelles differ in their lipid composition 41 LIPIDS THAT FUNCTION AS SIGNAL MOLECULES - steroid hormones and many vitamins are derived from cholesterol Steroid metabolism: Aineenvaihdunta II course (this topic here just very briefly) EICOSANOIDS - eicosi = 20 - oxygenated derivatives of C20 polyunsaturated fatty acids; synthesized in the body from arachidonic acid - carry messages to nearby cells - various physiological regulation functions, like blood flow to organs, smooth-muscle contraction, body temperature - many cause inflammation and pain aspirin (acetylsalicylic acid) kills pain by inhibiting prostaglandin synthesis PROBLEM 4. Is arachidonic acid an essential lipid? Why?
12 AGGREGATION OF LIPIDS AND STRUCTURE OF BIOLOGICAL MEMBRANES - at the air-water interface lipids cover twice the area of the membrane made of them 42 - membrane monolayers, bilayers and micelles - micelles bilayers liposomes
13 43 - detergents easily form micelles - CMC = critical micelle concentration e.g. SDS 8.2 mm Mw=288; Mw (micelle)= monomers/micelle - liposomes are spherical vesicles containing an aqueous inner compartment surrounded by a lipid bilayer - may form inside cells or can be made experimentally SUMMARY
14 - synthesis of new lipid bilayer is not well understood adding lipids to increase size of membrane different membranes have different composition of lipids and proteins transfer of newly synthesized membrane? 44
15 - fluid mosaic model describes the arrangement of lipid and protein within the membrane 45 what now follows is a trial to combine structural features of both lipids and proteins in order to understand biological membrane structure and function fluid mosaic model (we have to jump a little bit away from lipids every now and then) - there are integral as well as peripheral membrane proteins: either penetrating the membrane or just associated with it
16 - lateral movement of lipids fast - transverse diffusion slow; helped by enzyme flippase 46 fast slow fast fast
17 - biological membrane may exist in an ordered gel phase or as disordered liquid crystal (fluid) or in a transition state having characteristics of both - affected by composition, temperature change in temperature compensated by change of lipid composition to maintain fluidicity 47 - different membrane lipids, including cholesterol, pack well together to form the membrane - lipid side chains affect membrane packing and thus the physical properties of the membrane - head groups add functionality to the membrane
18 48 - notice that the percentage of total saturated fatty acids in cells increases and unsaturated decreases as a function of increased environmental temperature. Proteins as part of membrane structure: - different types of integral membrane proteins, I to VI - transmembrane domain can also be built of b-sheets (e.g. porins) - lipid anchoring (type V) is also considered a type of its own (next page) a porin channel that forms a hole in the membrane 90 o Characteristics of membrane proteins: - topology of an integral membrane protein can be predicted from its sequence (despite shortage of experimentally determined structures of that type of proteins) - continuous sequence of >20 hydrophobic residues indicates a membrane-spanning a-helix - hydropathy plot - aromatic amino acids often found at the lipid-water interface
19 - lipid-anchoring is possible both for integral and peripheral membrane proteins: prenylation fatty acylation GPI-link 49 outside phospholipid sugars protein inside GPI anchor ( ankkuri )
20 lipid and protein composition varies between membranes of different cells/cell organelles 50 - membrane bilayer is also asymmetric: lipid and protein composition varies between different sides of the bilayer
21 lipid and protein composition varies between membranes of different cells/cell organelles - lipids and proteins may also be laterally organized into domains that cover different parts of a cell certain proteins associate to form aggregates specific protein-lipid interactions certain proteins and certain lipids are found together 51 lipid rafts lipid raft ( lipidilautta ) - divalent metal ions ligate certain lipids with negative head groups - glycosphingolipids and cholesterol form rafts that associate with certain proteins lipid raft
22 52
23 - many membrane proteins are glycosylated (=glycoproteins) 53 Basic concept: protein and glycan are synthesized separately and then attached to each other
24 Example: the ebola virus infection mechanism employs specific interaction with components in the lipid rafts i.e. lipid raft is a target for the virus - one of the virus membrane proteins contains a sequence (called fusion peptide ) including a tryptophan and a phenylalanine (1) - the fusion peptide makes a strong interaction with cholesterol molecules in the membrane - virus uses this interaction to integrate its membrane into the endocytotic membrane to release the genetic material into the cytoplasm (2) - cells with less cholesterol in their membrane are less prone to ebola virus infection
25 55 membrane of the same cell may also have a different lipid and protein composition on different sides of the cell, for example apical and basolateral sides of epithelial cells - certain membrane proteins associate with cytoskeleton form distinct localizations - human erythrocyre membrane as an example of studying a membrane structure: peripheral membrane proteins (on the cytoplasmic side) removed by change in ph or ionic strength integral membrane proteins removed by a non-ionic detergent Triton X-100 cytoskeleton-forming proteins are revealed; most notable are spectrin and actin (next page)
26 actin structure 56 electron micrograph of erythrocyte membrane skeleton spectrin structure - examples of plasma membrane composition and functionality (below and next page) Pathogen infections Toxins Leukocyte rolling
27 57
28 FUNCTIONS ASSOCIATED WITH BIOLOGICAL MEMBRANES - barrier against the outside - selective intake and excretion of substances membrane transport - cell-cell and other recognition - fusion and separation events 58 Membrane transport: - passive diffusion through the membrane - permeability depends on the molecule Membrane transport: - facilitated diffusion through pores/channels - facilitated transport by carrier molecules - driving force either ion gradient or coupled to ATP production Energy needed when diffusion goes against the concentration gradient
29 59 - rate of diffusion varies red = open blue = closed substrate - conformational change of proteins is an important issue very often related to protein function, like binding of substrate (enzymes) or other substances (receptors, protein-protein interactions, etc.) - an example of triose phosphate isomerase, where one loop moves ca. 10 Å to give way for substrate to bind
30 Vesicle fusion: - coated vesicles are a transport means for newly synthesized and secreted proteins - both for soluble and integral membrane proteins - endocytosis (transport in), exocytosis (transport out) 60 - caveolae form via association of caveolin proteins, which are asymmetrically positioned in the membrane - make it possible for membrane to curve and finally form a vesicle - so called SNARE proteins insert into both membranes, bind to each other, and anchor the vesicle to the target membrane - in this way mediate the membrane fusion
31 61
32 - physiological phenomena involving membrane fusion 62 IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Nomenclature of Lipids Fahy et al., A comprehensive classification system for lipids Journal of Lipid Research 46, (2005)
33 RECAP QUESTIONS 63 PROBLEM 5. When we classify a certain compound as a lipid, we do so using a different rationale compared to e.g. classifying another compound as a nucleic acid or a protein. What is the difference? PROBLEM 6. Thickness of biological membranes is 5 to 8 nm (50 to 80 Å) with the lipid bilayer part ca. 3 to 5 nm. The carbon-carbon bond length is about 1.5 Å. Estimate the length of a palmitate molecule (consider a fully extended form). If two molecules of palmitate were placed end to end, like in a lipid bilayer, how would their total length compare with the thickness of a lipid bilayer and a biological membrane (i.e. can our understanding of the membrane structure be correct)? PROBLEM 7. Liposomes can be prepared by suspending a suitable lipid in water and sonicating. Any chemicals dissolved in water may then trap inside liposomes. Liposomes may also fuse with cell membranes; each type of liposome with a cell membrane that contains same or similar lipids. What possible practical use could this phenomenon offer? (example) Glycine trapped in phospholipid vesicles Sonication ( sonic disruption )
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids, Biological Membranes and Cellular Transport. 阮雪芬 May/9/2004
 Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
3.1.3 Lipids. Source: AQA Spec
 alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
Membrane transport. Pharmacy Dr. Szilvia Barkó
 Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Lipids and Biological Membranes
 Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
I. Structure and Properties of Lipids
 I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
Chapter 8. Functions of Lipids. Structural Nature of Lipids. BCH 4053 Spring 2001 Chapter 8 Lecture Notes. Slide 1. Slide 2.
 BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
By: Dr Hadi Mozafari 1
 Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
MEMBRANE STRUCTURE. Lecture 8. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
Lipids fatty, oily, or waxy hydrophobic organic compounds.
 Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Cell Membrane: a Phospholipid Bilayer. Membrane Structure and Function. Fluid Mosaic Model. Chapter 5
 Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
LIPIDS II: TRIACYLGLYCEROLS:
 LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE
 Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
CH 3. Lipids CHAPTER SUMMARY
 H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
Functions of Lipids. - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g).
 Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Draw and label a diagram to show the structure of membranes
 2.4 Membranes 2.4.1 - Draw and label a diagram to show the structure of membranes Phospholipid Bilayer - This is arranged with the hydrophilic phosphate heads facing outwards, and the hydrophobic fatty
2.4 Membranes 2.4.1 - Draw and label a diagram to show the structure of membranes Phospholipid Bilayer - This is arranged with the hydrophilic phosphate heads facing outwards, and the hydrophobic fatty
Lecture 3 6/28/10. Membrane Lipids. Importance of Membranes. Categories of Lipids. Lipids: Chapter 20 Sections 4-7. ! Membranes are important in
 Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Rama Abbady. Odai Bani-Monia. Diala Abu-Hassan
 5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
NOTE: For studying for the final, you only have to worry about those with an asterix (*)
 NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
 Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
What are the molecules of life?
 Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
The main biological functions of the many varied types of lipids include: energy storage protection insulation regulation of physiological processes
 Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Membranes. Chapter 5
 Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Inorganic compounds: Usually do not contain carbon H 2 O Ca 3 (PO 4 ) 2 NaCl Carbon containing molecules not considered organic: CO 2
 Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Membrane Structure. Membrane Structure. Membrane Structure. Membranes
 Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Carbohydrates and Lipids
 Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Cell Membranes Valencia college
 6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
Lipids are used to store and excess energy from extra carbohydrates in animals
 Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Classification, functions and structure
 Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Biology. Chapter 3. Molecules of Life. Concepts and Applications 9e Starr Evers Starr
 Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Membrane Structure and Function. Cell Membranes and Cell Transport
 Membrane Structure and Function Cell Membranes and Cell Transport 1895 1917 1925 Membrane models Membranes are made of lipids Phospholipids can form membranes Its actually 2 layers - there are proteins
Membrane Structure and Function Cell Membranes and Cell Transport 1895 1917 1925 Membrane models Membranes are made of lipids Phospholipids can form membranes Its actually 2 layers - there are proteins
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Lipids and Classification:
 Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
OBJECTIVE. Lipids are largely hydrocarbon derivatives and thus represent
 Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
The Cell Membrane. Usman Sumo Friend Tambunan Arli Aditya Parikesit. Bioinformatics Group Faculty of Mathematics and Science University of Indonesia
 The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
The Cell Membrane Usman Sumo Friend Tambunan Arli Aditya Parikesit Bioinformatics Group Faculty of Mathematics and Science University of Indonesia Overview Cell membrane separates living cell from nonliving
Classification of Lipids
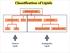 Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Chapters 9 and 10 Lipids and Membranes
 Chapters 9 and 10 Lipids and Membranes Lipids- a class of biological molecules defined by low solubility in water and high solubility in nonpolar solvents. Lipids contain or are derived from fatty acids.
Chapters 9 and 10 Lipids and Membranes Lipids- a class of biological molecules defined by low solubility in water and high solubility in nonpolar solvents. Lipids contain or are derived from fatty acids.
Chapter 20 Lipids. Organic and Biochem
 Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Biomembranes structure and function. B. Balen
 Biomembranes structure and function B. Balen All cells are surrounded by membranes Selective barrier But also important for: 1. Compartmentalization 2. Biochemical activities 3. Transport of dissolved
Biomembranes structure and function B. Balen All cells are surrounded by membranes Selective barrier But also important for: 1. Compartmentalization 2. Biochemical activities 3. Transport of dissolved
BIOB111_CHBIO - Tutorial activity for Session 12
 BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
Macromolecules. Molecules of Life
 Macromolecules Molecules of Life Learning Objectives know the difference between a dehydration synthesis reaction and a hydrolysis reaction know the different types of biological macromolecules be able
Macromolecules Molecules of Life Learning Objectives know the difference between a dehydration synthesis reaction and a hydrolysis reaction know the different types of biological macromolecules be able
Membranes. Chapter 5. Membrane Structure
 Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
MCQS ON LIPIDS. Dr. RUCHIKA YADU
 MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
General Biochemistry-1 BCH 202
 General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
Chapter 3- Organic Molecules
 Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
Biology: Life on Earth Chapter 3 Molecules of life
 Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Organic molecules highly hydrophobic and water insoluble.
 UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Sept. 2011 Primary Membrane Function: Homeostasis Section 2.2 Conditions in the cell must remain more or less constant
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Sept. 2011 Primary Membrane Function: Homeostasis Section 2.2 Conditions in the cell must remain more or less constant
Ch7: Membrane Structure & Function
 Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Lipids: Fats, Oils & Waxes: AP Biology
 Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
The Star of The Show (Ch. 3)
 The Star of The Show (Ch. 3) Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life Organic
The Star of The Show (Ch. 3) Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life Organic
Nebal Al - Gallab. Shatha Al - Jabri. Mamoon Ahram
 10 Nebal Al - Gallab Shatha Al - Jabri Mamoon Ahram Note: the doctor showed extra examples, they were in the slides, you can refer to them... Naming of Fatty Acids - 1 st Method ( IUPAC system ) We start
10 Nebal Al - Gallab Shatha Al - Jabri Mamoon Ahram Note: the doctor showed extra examples, they were in the slides, you can refer to them... Naming of Fatty Acids - 1 st Method ( IUPAC system ) We start
CHAPTER 11 Membranes
 CHAPTER 11 Membranes Key topics The function of biological membranes The structure and composition of membranes Dynamics of membranes Structure and function of membrane proteins Transport across biological
CHAPTER 11 Membranes Key topics The function of biological membranes The structure and composition of membranes Dynamics of membranes Structure and function of membrane proteins Transport across biological
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
Chapter 12. Part II. Biological Membrane
 Chapter 12 Part II. Biological Membrane Single-tailed lipids tend to form micelles Critical micelle concentration (cmc): minimum concentration that forms micelles e.g.) cmc for SDS 1mM; cmc for phospholipids
Chapter 12 Part II. Biological Membrane Single-tailed lipids tend to form micelles Critical micelle concentration (cmc): minimum concentration that forms micelles e.g.) cmc for SDS 1mM; cmc for phospholipids
CELL MEMBRANES (MAS)
 CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
Biomolecules. Biomolecules. Carbohydrates. Biol 219 Lec 3 Fall Polysaccharides. Function: Glucose storage Fig. 2.2
 Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
The Cell Membrane. Cell membrane separates living cell from nonliving surroundings. Controls traffic in & out of the cell
 The Cell Membrane 1 Overview Cell membrane separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances
The Cell Membrane 1 Overview Cell membrane separates living cell from nonliving surroundings thin barrier = 8nm thick Controls traffic in & out of the cell selectively permeable allows some substances
Lipids Definition. Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins
 1 Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins 2 Phosphate head hydrophilic Fatty acid tails hydrophobic Amphipathic Phosphate attracted to
1 Boundary Lipid bilayer Selectively Permeable Fluid mosaic of lipids and proteins Contains embedded proteins 2 Phosphate head hydrophilic Fatty acid tails hydrophobic Amphipathic Phosphate attracted to
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structures Biology 2201 Primary Membrane Function: Homeostasis Section 2.2 Conditions in the cell must remain more or less constant under many
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structures Biology 2201 Primary Membrane Function: Homeostasis Section 2.2 Conditions in the cell must remain more or less constant under many
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
The Cell Membrane AP Biology
 The Cell Membrane AP Biology! 2007-2008 Overview! Cell membrane separates living cell from nonliving surroundings " thin barrier = 8nm thick! Controls traffic in & out of the cell " selectively permeable
The Cell Membrane AP Biology! 2007-2008 Overview! Cell membrane separates living cell from nonliving surroundings " thin barrier = 8nm thick! Controls traffic in & out of the cell " selectively permeable
Gateway to the Cell 11/1/2012. The cell membrane is flexible and allows a unicellular organism to move FLUID MOSAIC MODEL
 Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Chapter 1 Plasma membranes
 1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
A. Lipids: Water-Insoluble Molecules
 Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
BIOLOGICAL MOLECULES. Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds.
 BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
