by computer analysis of the Pherolist
|
|
|
- Kristin Bridges
- 6 years ago
- Views:
Transcription
1 Ecology , Pheromone component patterns of moth evolution revealed Blackwell Publishing Ltd by computer analysis of the Pherolist JOHN A. BYERS Western Cotton Research Laboratory, USDA-ARS, 4135 East Broadway Road, Phoenix, Arizona , USA Summary 1. The Pherolist internet site listing moth sex pheromone components reported in the literature was downloaded and processed by a BASIC program into a database with 2931 combinations of 377 unique chemical names of sex pheromone attractants used by 1572 moth species in 619 genera and 49 families. Names of pheromone compounds were analysed for aliphatic chain length, unsaturation position, geometric configuration, functional group (aldehyde, alcohol, acetate, epoxide, methyl-branched and hydrocarbon) and number of instances such combinations are used by species and families. 2. The analyses revealed pheromone blends of species ranged from one to eight components (45% species with one component, 36% two, 12% three, 5% four, 1% five, 0 5% for six). The numbers of different components of various chain lengths and functional groups, the numbers of instances such compounds are used by species and the numbers of species using such compounds are presented. 3. The average number of pheromone components per species increased as the number of species in a family increased based on linear regression of components in the 10 largest families, with species numbers ranging from 19 to 461. Pooling the four largest families gave a mean of 1 96 components per species that was significantly greater than the mean of the next 14 smaller families (1 63). Because related species in a large family would need more communication channels, this suggests that these species on average evolved to produce and detect more components in their pheromone blends to achieve a unique communication channel than was needed by species in smaller families. 4. Speciation in moths would entail evolutionary changes in both pheromone biosynthetic and sensory systems that avoided competition for communication channels of existing species. Regression analysis indicated that the more species in a family the more unique pheromone components, but the increase diminishes progressively. This suggests that, as the number of components increases with species number in a family, new species are more likely to evolve a unique blend comprising a communication channel from among existing components of the family. Key-words: biosynthesis, computer program software, database, Lepidoptera, semiochemicals. Ecology (2006) 75, doi: /j x Introduction Pheromones are intraspecific communication signals that have been identified in animals ranging from bacteria and nematodes to mammals (Dunny & Leonard 1997; Lewis, Barbarosa & Gaugler 2002; Dulac & Correspondence and present address: John A. Byers, US Arid-Land Agricultural Research Center, USDA-ARS, North Cardon Lane, Maricopa, Arizona 85239, USA. jbyers@wcrl.ars.usda.gov Torello 2003; Johnston 2003). More than studies involve pheromones with the majority on insects (about 71%) and of these about 46% concern Lepidoptera, primarily moths (BIOSIS Previews ). Thus, more than 6000 research studies concern the chemical ecology of moths, due primarily to their importance in agriculture and forestry. On the internet, the Pherolist reports published knowledge of moth pheromone components and analogues used by more than 1683 species in 50 families (as of June 2002). The list was published first as a book (Arn, Toth & Priesner 1992)
2 400 J. A. Byers Fig. 1. Examples of moth pheromone components: (Z)-9- tetradecenyl acetate or Z9-14:Ac in commonly used abbreviated form (the most common component used by 199 species) (E)- 9-tetradecenyl acetate or E9-14:Ac (component of 17 species) (Z)-11-hexadecen-1-ol or Z11-16:OH (most common alcohol, used by 50 species) (Z)-11-hexadecenal or Z11-16:Al (most common aldehyde, used by 119 species) (E,E,Z)-10,12,14- hexadecatrienal or E10E12Z14-16:Al (used by tobacco hornworm, Manduca sexta) and (Z,Z)-6,9 3,4-epoxyheneicosadiene or Z6Z9-3,4epo-21:Hy (epoxide hydrocarbon used by 14 species) (data compiled from Pherolist: Arn et al. 1992; Arn 2001). and then assembled subsequently into a large website of html (hypertext markup language) files, with 693 such files on genera and species as well as 544 files on attractive components of species (Arn 2001). The Pherolist is organized into indexes of chemicals, families, genera, species and common names. In this study, only the chemicals that are characterized as being attractive to males were considered as sex pheromone components. The majority of these pheromone components are related structurally, being unsaturated olefinic chains from 10 to 22 carbons with an end functional group (either alcohol, aldehyde or acetate). The positions of unsaturated, carbon carbon double bonds can be in either the E- (trans-) or Z- (cis-) configuration, except at the end opposite the functional group (Fig. 1). Usually there are one, two and sometimes three unsaturations in the components of moths investigated to date (Byers 2002, 2005). Less commonly, moths have evolved olefinic epoxide pheromones (Millar 2000) by epoxidizing double bonds in the cis-configuration to yield (R,S) and (S,R) chiral forms (Fig. 1). The number of isomers of these olefinic chains is limited, so the number of communication channels comprised of single molecules that are available to moths may constrain their speciation (Byers 2005). However, blends of two or more components are found typically in moth species and these combinations are practically unlimited, although phylogenetic diversification might constrain biosynthetic systems and limit the evolution of new pheromone components during speciation (Byers 2005). Thus, the question arises of whether species in a taxon compete for use of the limited number of components that may comprise communication channels (Cardé & Baker 1984). An effect of such competition, based on combinatorial mathematics, could be that taxons with larger numbers of species have species that, on average, use more components per blend than smaller taxons. In addition, larger taxons may have relatively fewer different kinds of components on a per-species basis compared to smaller taxons. Numerical summaries of pheromone component use by moths were not carried out previously because of the tedious and time-consuming nature of counting, for example, how many species use 14-carbon chain lengths (611 species, as described subsequently) or how many times 14-carbon chains are used by species (1006 instances). The relative counts summarizing patterns of use of conjugated or isolated unsaturations in all chain lengths or counts of E- and Z-configurations of monounsaturated components according to functional group is also difficult and confusing without the aid of computerized data-mining. Thus, my objective was to develop a computer program that could search the Pherolist web site and automatically compile a separate database of linkages between chemicals, families and species. This database could in turn be searched methodically and summarized by computer algorithms to quantify patterns of pheromone component structure and use in moths. Some of the resulting patterns may show relationships between components and species per family that play a role in pheromone communication and the speciation process in moths (Linn & Roelofs 1995; Byers 2005). The emergent patterns may also provide insights into the ecological, evolutionary and physiological processes involved in moth pheromone communication. Methods The entire Pherolist website was downloaded (June 2002) to a personal computer for purposes of extracting data to be used in a separate database for off-line analyses. A program coded in QuickBASIC was made to search each of the 544 html files on chemical components for pertinent data. For example, the name of the compound was extracted from the title tag, (E,E)- 8,10-dodecadien-1-ol of the html page (in short form, 8E10E-12:OH). Similarly, unique text strings were extracted that specified the family and genus species reported to be attracted to the component. A random access file of 2931 records was made containing record fields linking each chemical used by each species in a family/subfamily/tribe. Sex pheromone components of insects are either attractive alone to the responding sex, or must be presented together with at least one other component in a synergistic mixture, resulting in a greater attraction than the sum of the individual
3 401 Pheromone components of moths activities (Baker 1989; Byers 1992). Thus, it was assumed that the male-attractive compounds listed in the Pherolist are moth sex pheromone components characterized correctly by authors of peer-reviewed research articles. A second BASIC program (available from the author) was made to view and search the random access file for various combinations of text strings, and excluded text strings that together indicate the chain length, functional group, chirality and the unsaturation configuration and position of chemicals, e.g. (Z,Z)-3,6-(9R,10S)-9,10- epoxyoctadecadiene. These data, in combination with species and family records, were counted and summarized by the program. Chain lengths from 5 to 10 carbons were determined if the compound name had any of the strings: pent, hex, hept, oct, non or dec but not pentadec, hexadec, heptadec, octadec or nonadec, respectively. In addition, a chain length of 10 would contain dec but not have any of the latter five strings just mentioned, nor undec, dodec, tridec or tetradec. Chain lengths would contain each of the strings from undec to nonadec or eicos, heneicos, docos or tricos. Functional groups, or their lack, were determined by epox, methyl, -one, oate anywhere in the compound name and by nal, -1-ol, acetate, ane and ene at the end of the name. Positions of unsaturations were recognized by strings of numbers (e.g. -2,4,6- ) enclosed with dashes, while configuration was determined by finding the first set of parentheses and looking for E or Z. Other determinations were whether the numbers represented conjugated or isolated double bonds and if they were odd or even numbers. Chirality was determined if R or S was found within parentheses. Alphabetical sorting of species and family lists, as well as ranking of counts, was performed with the combsort method of Lacey & Box (1991). Searches of the database were conducted to count how many species use one, two, three or more components in the attractive sex pheromone. Similarly, the database was summarized by family. Counts were made of the numbers of different components of various functional groups and chain lengths reported in all species or for the families. Similarly, counts were made of the instances that various functional groups and chain lengths are used by moth species or families. Also, the numbers of species using various functional groups and chain lengths were counted. The numbers of instances were counted of all species using components with two unsaturations (conjugated or isolated) or three unsaturations (all conjugated, combinations of isolated/ conjugated or all isolated). Similarly, the number of instances of all species were counted that use (a) monounsaturated components with configurations of E or Z in even or odd positions, (b) diunsaturated component instances of either (E,E) (Z,Z) (E,Z), or (Z,E) and (c) triunsaturated instances of either (E,E,E) (Z,Z,Z) or mixed E and Z. Other searches counted all instances or species using components with one, two or three unsaturations for various functional groups. Still other Fig. 2. Number of species with various numbers of reported components in pheromone blends used for long-range attraction. searches counted and ranked the most common components used by species as well as the number of families, genera and species per family. Some data sets were fitted to various equations using least-squares linear and curvilinear regression (Sokal & Rohlf 1995). Results In the database there are 702 moth species reported to use at least one component in their sex pheromone, while 567, 185, 80 and 21 species have at least two, three, four and five component sex pheromones, respectively (Fig. 2). The average number of components reported per species is 1 86 ± 0 05 [± 95% confidence interval (CI)]. The species with the most reported components in their sex pheromone is Heliothis virescens (F) with 10 components, followed by Adoxophyes sp. and Manduca sexta (L.) with eight components, Eucosma sonomana Kearfott, Euxoa messoria Harris, Mamestra brassicae L., Agrotis segetum (Schiff.) and Plutella xylostella L. with seven components and Cucullia umbratica L., Diaphania hyalinata L., Cydia pomonella (L.), Ostrinia nubilalis (Hübner), Eupoecilia ambiguella Hübner and Trichoplusia ni (Hübner), with six components each. There are 18 families reported to have at least 10 species each that range from 1 32 to 2 72 components on average per species of a family. The families in descending order are: Yponomeutidae (49 components/18 species = 2 72), Noctuidae (869/424 = 2 05), Pyralidae (210/105 = 2), Zygaenidae (33/17 = 1 94), Tortricidae (871/461 = 1 89), Geometridae (189/102 = 1 85), Cosmopterigidae (18/10 = 1 8), Gracillariidae (53/30 = 1 77), Sessidae (160/94 = 1 7), Sphingidae (22/13 = 1 69), Oecophoridae (27/16 = 1 69), Lymantriidae (30/19 = 1 58), Coleophoridae (55/36 = 1 53), Arctiidae (25/ 17 = 1 47), Tineidae (25/17 = 1 47), Argyresthiidae (23/16 = 1 44), Gelechiidae (87/66 = 1 32) and Pterophoridae (29/22 = 1 32). In the 10 largest families with reported pheromones ( species each), the average number of pheromone components per species in a family increases as a function of the number of species in the family (Fig. 3). The four largest families with more than 100 species each (total 1092) together have 2139 components that give an average of 1 96 components per species. This is
4 402 J. A. Byers Fig. 3. Average number of components per species related to the number of species per family (10 largest families analysed with from 19 to 461 species each). The linear regression, Y = X with R 2 = 0 39, P = Logarithmic regression, Y = ln X with an R 2 = 0 49; P = 0 02 gave a slightly better fit. Fig. 5. (a) Number of unique pheromone components used by moths with various chain lengths from five to 23 carbons. (b) Number of components classified as hydrocarbon, methylbranched or epoxides. (c) Number of components classified as aldehydes, primary alcohols or acetates. Fig. 4. Number of components per family related to the number of species per family (49 families analysed, with seven not shown above 30 species each). The curvilinear regression, Y = 5X with R 2 = 0 86, P < 0 001, fitted the data better than a linear regression, Y = X with R 2 = more than the 14 families with less reported species ranging between 10 and 100 species each (total 391) with 636 components and an average of 1 63 components per species. A comparison of the frequencies of the components and species for the four larger families (2139/1092) to the 14 families with less species (636/391) showed them to be significantly different (χ 2, P = 0 04). The number of different components used reportedly by different families increases as a square root function of species number per family (Fig. 4). The function can be used to predict approximately the number of different components attractive to species of a particular family given the number of its species. As the number of components (X) in a family increases, the number of two-component combination blends (Y) that are available for communication channels can be calculated as X!/[2(X-2)!] or for three-component blends as X!/[6(X- 3)!], which increase as a quadratic (Y = X2/2 X/2) or cubic (Y = X3/6 X2/2 + X/3), respectively. The numbers of different components reported in all moth species ranging in chain lengths from five to 23 carbons show that even-length chains from 12 to 18 carbons are the most prevalent (Fig. 5a). Chain lengths of 12 and 14 carbons are approximately equally abundant. The numbers of methyl-branched components are greatest at chains of length 17, and range from five to 19 carbons. The hydrocarbon components (no functional group) also are greatest at 17 carbon length chains, while epoxides peak at 19 carbons, with both types ranging from 17 to 21 carbons (Fig. 5b). The aldehyde, alcohol and acetate components have chain lengths that range from 10 to 18 carbons, with even chain lengths being most abundant (Fig. 5c). Aldehydes, alcohols and acetate are most abundant at 16, 12 and 14 carbons, respectively. The number of instances species have evolved to use components with chain lengths of five to 23 carbons is reported more often for even-length chains than for odd, with a peak at 14 carbons (Fig. 6a). Hydrocarbon chains occur about equally at 17, 19 and 21 carbon lengths while methyl-branched chains of 17 carbons are used most often. Epoxides are found in chains from 17 to 21 carbons, with 19 carbon chains used most frequently, followed closely by 21 carbon lengths (Fig. 6b). Aldehyde, alcohol and acetate components range from 10 to 18 carbons, predominately in even chain lengths, with peaks at 16, 12/14 and 16 for each functional group, respectively (Fig. 6c). The patterns in the usage of aldehyde, alcohol and acetate components by moths (Figs 5 and 6) are due mainly to four large families, the Tortricidae, Noctuidae, Pyralidae and Sesiidae, which together account for 78% of the instances for all 49 families.
5 403 Pheromone components of moths Fig. 6. (a) Number of instances of pheromone components with chain lengths from five to 23 carbons used by moth species. An instance is defined as a component-species use combination. (b) Number of instances of hydrocarbon, methyl-branched or epoxide pheromone components with chain lengths of carbons used by moth species. (c) Number of instances of aldehyde, primary alcohol or acetate pheromone components with chain lengths of carbons used by moth species. Fig. 7. (a) Number of species using pheromone components with chain lengths from five to 23 carbons. (b) Number of species using hydrocarbon, methyl-branched or epoxide pheromone components with chain lengths of carbons. (c) Number of species using aldehyde, primary alcohol or acetate pheromone components with chain lengths of carbons. Fig. 8. Number of instances of moths using aldehyde, alcohol or acetate components with one or more unsaturations at even, odd or both even- and odd-numbered positions in carbon chains from 10 to 18 carbons. Similarly, the number of instances of moths using components with two unsaturations that are conjugated (c) or separated by a single bond, isolated (i) with more than a single bond separation, or with three or more unsaturations with mixtures of either conjugated or isolated positions. Any particular component may be used by several species. For example, the most commonly used component is an acetate, Z9-14:Ac (199 species), followed by Z11-14:Ac (172), Z11-16:Ac (159), Z7-12:Ac (136), E11-14:Ac (126), an aldehyde Z11-16:Al (119), Z9-12:Ac (69), Z3Z13-18:Ac (64), Z8-12:Ac (60) and E8E10-12:Ac (54). Not surprisingly, the patterns for number of species that use components with chain lengths from five to 23 carbons (Fig. 7a) or hydrocarbons, methyl-branched chains and epoxides (Fig. 7b) or aldehydes, alcohols and acetates (Fig. 7c) are similar to the patterns for number of instances (Fig. 6). The numbers of instances reported are always greater than the numbers of species as a species could use Z7-14:Ac and Z9-14:Ac, counting as two instances of a 14-carbon chain length and as one species using such a chain length. Pheromone components with unsaturation (double bonds) and an aldehyde, alcohol or acetate functional group range in chain length from 10 to 18 carbons (Fig. 8). The number of instances that moths use these components with a double bond in only even positions (430 instances) occurs most frequently in 12-carbon length chains (285 instances). The instances of component use with an unsaturation only in odd positions (2021) occurs most frequently for 14-carbon length chains (852), and is in all cases more numerous than instances of components having only even positions (Fig. 8; χ 2, P < 0 001). The instances of conjugated double bonds (separated by a single bond) are most frequent in 12-carbon lengths (172), while isolated double bonds (separated by more than one single bond) are most usual in 18-carbon chains (187). In the same components with the functional groups above, the double bonds within the chain can take either of two configurations and be in either even or odd positions (Fig. 9). The Z-configuration (cis) with 1553 instances is significantly more frequent than the E (trans), with 437 instances (P < 0 001). The patterns for the higher frequency of odd positions compared to even positions
6 404 J. A. Byers Fig. 9. Number of instances of species using aldehyde, alcohol or acetate components with one unsaturation at even- or odd-numbered positions with E- or Z-configurations or two unsaturations being (E,E), (Z,Z), (E,Z) or (Z,E) or with three unsaturations being all E or all Z or a mixture in carbon chains from 10 to 18 carbons. In components with two unsaturations, the lower position is given first. Fig. 10. Number of instances of species in the 12 moth families using the most aldehyde, alcohol or acetate components in carbon chains. (Fig. 8) are not altered by E and Z designations (Fig. 9). However, the E-even and Z-even instances are similar, and most frequent in 12-carbon length chains, while E-odd occurs less often (339) than the corresponding Z-odd (1415), but both are most usual in 14-carbon length chains (Fig. 9). Diunsaturated components are reported less frequently than monounsaturated ones. (E,E) is most common in 12-carbon chains (Z,Z) and (E,Z) in 18-carbon chains and (Z,E) in 14-carbon chains. However, the total instances for all chains of the four possible diunsaturated components are similar (105, 147, 154, 119, respectively, above), in contrast to the larger difference in monounsaturated E and Z frequencies (Fig. 9). The 12 families with the most reported instances of aldehyde (Al), alcohol (OH) or acetate (Ac) components are shown according to the number of carbons in the chain length (Fig. 10). The Sesiidae utilize the most 18-carbon chain components (zero Al, 39 OH and 121 Ac), while the Tortricidae use the most 12-carbon (six Al, 71 OH and 373 Ac) and 14-carbon (70 Al, 53 OH and 274 Ac) length components. The Noctuidae most often use 16-carbon (101 Al, 43 OH and 140 Ac) length chains (Fig. 10). Only four of the 12 families have chains, with between 17 and 22 carbons with an epoxide group and various unsaturations (142 instances, Fig. 11). Arctiidae utilize only 21-carbon chains with an epoxide (eight instances), Geometridae use mostly 19-carbon chains (39 or 46%), Lymantriidae mostly 18-carbon chains (nine or 82%) and in Noctuidae 21-carbon chains are most common (18 or 47%). One other family, Amatidae, produces one epoxide of an 18-carbon chain. Moths use epoxides of the cis-configuration that are (R,S) or (S,R) and although trans-stereoisomers are possible as (S,S) or (R,R), they have not been found in moths (Millar 2000; Pherolist). About half the epoxides (all cis-) of moths have not been described with regard to chirality (R,S) or (S,R). It is striking that all unsaturated epoxide isomers are the Z-configuration. Fig. 11. Number of instances of species in 12 moth families (as in Fig. 9) using epoxide components in carbon chains. Discussion There are an estimated moth species (Holloway, Bradley & Carter 1987), from which about 377 pheromone components have been identified in 1572 species of 619 genera as of 2001 (Arn 2001; Byers 2005). These moth pheromone components are related structurally, the majority being acetates, alcohols or aldehydes of olefinic chains from seven to 22 carbons (mainly 12, 14 or 16 carbons). Only two species use chain lengths of 23 carbons (two epoxides by two species of Noctuidae), and one species of Lymantriidae uses tetracosane (24:Hy) and pentacosane (25:Hy) as pheromone components (Pherolist). It is surprising that a few species use these large compounds because of their relatively low volatility. Using the table and formula of Schlessinger (1971), saturated hydrocarbons of 24 and 25 carbons have vapour pressures at 15 C that range from 3 2E-6 to 8 9E-7 mmhg, which is from 1275 to 4580 times less volatile than 14-carbon tetradecanal (used by three species). The compound Z9-14:Ac is used by 199 species and is between 39 and 142 times more volatile (Olsson et al. 1983) than 24:Hy and 25:Hy at 15 C, respectively. Given that females of many moth species produce pheromone amounts in nanograms (Baker 1989), the males of lymantriid species would need to be more sensitive to minute quantities of 24:Hy and 25:Hy, or be attracted over shorter distances, than most other species using more volatile pheromone components.
7 405 Pheromone components of moths The observed patterns of sex pheromone component occurrence in moths are probably related to underlying systems of biosynthetic enzymes with common phylogenetic origins as influenced by competition for limited communication channels. Pheromone components are biosynthesized in females from fatty acid precursors of carbons converted to fatty acyl CoA esters. These are unsaturated by desaturase enzymes of various numbered types (5, 9, 10, 11, 12, 13 and 14) corresponding to the position in the chain that is unsaturated (Bjostad, Wolf & Roelofs 1981; Foster & Roelofs 1988, 1996; Roelofs & Wolf 1988; Jurenka & Roelofs 1989; Zhao, Löfstedt & Wang 1990; Roelofs & Jurenka 1996; Jurenka 1997; Tillman et al. 1999). The unsaturated fatty acyl CoA esters then are chain shortened by B-oxidation in 2-carbon pieces from the CoA end (Jurenka et al. 1994). Thus, incremental chain shortening results in reducing the unsaturation position number by two, but maintains the odd or even attribute. The unsaturated fatty acyl CoAs can be reduced by a reductase to alcohols, at which time the E and Z ratios of components in some moths are configured, or when the alcohols are converted to acetates by acetyl transferase (Jurenka & Roelofs 1989; Tillman et al. 1999). The alcohols can be converted to aldehydes that in turn can be made into hydrocarbons and then to epoxides (Roelofs 1995; Tillman et al. 1999; Millar 2000). Almost all the moth pheromone components discovered range from 10 to 21 carbons in chain length. The vast majority of these have chains of 12 (19%), 14 (19%), 16 (14%) or 18 (10%) carbons, but odd-numbered length chains still account for 29% of the components (epoxides of 17, 19 and 21 carbons make up 57% of the odd-length chains). These patterns of component use by chain length become altered when viewed in terms of the instances evolved in species. In this case, the proportion of species with odd-numbered chain lengths nearly disappears. Acetate functional groups are used relatively more than aldehydes and alcohols. Instances of moth usage of aldehyde, alcohol and acetate components with single unsaturations in odd numbered positions are much more numerous (82%) than instances of even ones (18%), and show a different pattern across the chain lengths. Instances of using components with two conjugated unsaturations are more common in 12- carbon chains, while two isolated double bonds are found usually in 18-carbon chains. Uses of components with a single unsaturation of Z-configuration are more common (78%) than those with an E (22%). However, when two unsaturations are found in the component there are no significant differences in the frequencies of Z,Z and E,E except for 18-carbon chains. The predominance of Z-configurations in moth components is not surprising, as fatty acid precursors with unsaturations are more common and more stable than E-forms in organisms (Lehninger 1970). The prevalence of unsaturations in odd-numbered positions is due to Z9-oleic acid as a precursor as well as the odd-numbered (5, 9, 11 and 13) desaturases. During speciation, not only would mutations in the genes coding the biosynthetic enzyme system of females need to occur but also in the sensory/behavioural system of males as constrained by phylogeny, interspecific competition for communication channels and limited numbers of possible chemical isomers (Byers 2005). As a family increased in number of species during evolution, there should be an increase in selection pressures on these related species to use more components in their pheromone blends in order to reduce mating mistakes, especially with sibling species. This hypothesis is supported by the increase in average number of components per species with an increase in species abundance per family based on a relatively wide range of species numbers (19 461) in 10 families. A linear relationship with a y-axis intercept above one component could mean that as families radiate, their species retain components of pheromone systems evolved earlier (all species have a pheromone with at least one component by definition). A linear function has the theoretical drawback, that as family size increases the mean components per species increases proportionally, which is unlikely as component blend combinations available for communication channels increase as a quadratic (two-component blends) or cubic (three-component blends) with an increase in total components. In this regard, a power function would seem more appropriate than a linear one, but might predict too low a number of components for small families. When looking at all 49 families in terms of numbers of unique chemical components per family, the more species-rich a family is the more different kinds of components, but the increase is not proportional (decreasing curvilinear slope with increasing number of species). It appears that as the number of evolved components and species in a taxonomic group increases, it is more likely that a new species can evolve a unique blend from among existing components in the family by mutations affecting biosynthetic/sensory systems. The first ancestral species in a taxon must evolve at least one component, the second species to evolve is then constrained to produce a new component to avoid reproductive interference, while the third species can either evolve a third component or use a blend of the other two components. Furthermore, assuming that species of a small family can evolve easily to make any of 10 components and use them in two-component blends, then there are 45 unique blends possible, while species of a larger family with 50 components could make 1225 different twocomponent blends (Byers 2005). Thus, a species of the larger family undergoing speciation would have a higher probability of a successful biosynthetic/sensory mutation allowing evolution of new components in a free communication channel than would a species in the smaller family. In addition, species at low densities would have higher extinction rates when in competition with higherdensity species using the same sex pheromone components. The patterns and hypotheses presented here are based on pheromone components reported in 49 families, or
8 406 J. A. Byers about 79% of known moth families, and all the 16 superfamilies (Borror & DeLong 1971). Thus, the database coverage on moth pheromones encompasses much of the taxonomic breadth of moths, generally agreeing quantitatively with perceptions of common and rare families listed in Borror & DeLong (1971). The pheromone database undoubtedly contains errors in research reports due to false positives and incomplete analyses. For example, some of the 702 species reported to have a single component may have one or more additional ones that aid in attraction but simply have not yet been discovered. In spite of these errors and gaps in pheromone identifications of moths, the patterns of pheromone usage based on research over more than 30 years appear generally representative. The 41 hydrocarbon, 54 epoxide, 55 aldehyde, 48 alcohol and 135 acetate components of moth pheromones as well as several million possible analogues (Byers 2005) can be drawn and viewed by software on the internet (Byers 2002). Since finishing this study, the Pherolist has changed management (Witzgall et al. 2005), while another database, the Pherobase (El-Sayed 2005), covers pheromones of all insect orders. The data-mining software as applied to the Pherolist serves as a model for revealing patterns of pheromone component use in moths among taxonomic groups. Further interpretation of the patterns in pheromone databases may aid in understanding the evolution of communication channels in moths and other insects. Acknowledgements This study was made possible by the diligent work of H. Arn in adapting the Pherolist to the internet. I thank H. Flint, L. Elhoff, M. Bengtsson, M. Coracini and several anonymous reviewers for helpful comments on the manuscript. References Arn, H. (2001) The Pherolist. Available at: Arn, H., Toth, M. & Priesner, E. (1992) List of Sex Pheromones of Lepidoptera and Related Attractants. International Organization for Biological Control, Montfavit, France. Baker, T.C. (1989) Sex pheromone communication in the Lepidoptera: new research progress. Experientia, 45, Bjostad, L.B., Wolf, W.A. & Roelofs, W.L. (1981) Total lipid analysis of the sex pheromone gland of the redbanded leafroller moth, Argyrotaenia velutinana, with reference to pheromone biosynthesis. Insect Biochemistry, 11, Borror, D.J. & DeLong, D.M. (1971) An Introduction to the Study of Insects. Holt, Rinehart & Winston, New York. Byers, J.A. (1992) Optimal fractionation and bioassay plans for isolation of synergistic chemicals: the subtractivecombination method. Journal of Chemical Ecology, 18, Byers, J.A. (2002) Internet programs for drawing moth pheromone analogs and searching literature database. Journal of Chemical Ecology, 28, Byers, J.A. (2005) Chemical constraints on the evolution of olfactory communication channels of moths. Journal of Theoretical Biology, 235, Cardé, R.T. & Baker, T.C. (1984) Sexual communication with pheromones. Chemical Ecology of Insects (eds W.J. Bell & R.T. Cardé), pp Sinauer Associates, Inc., Sunderland, MA, USA. Dulac, C. & Torello, A.T. (2003) Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature Reviews Neuroscience, 4, Dunny, G.M. & Leonard, B.A.B. (1997) Cell cell communication in Gram-positive bacteria. Annual Review of Microbiology, 51, El-Sayed, A.M. (2005) The Pherobase: Database of Insect Pheromones and Semiochemicals. Available at: Foster, S.P. & Roelofs, W.L. (1988) Sex pheromone biosynthesis in the leafroller moth Planotortrix excessana by desaturation. Archives of Insect Biochemistry and Physiology, 8, 1 9. Foster, S.P. & Roelofs, W.L. (1996) Sex pheromone biosynthesis in the Tortricid moth, Ctenopseustis herana (Felder and Rogenhofer). Archives of Insect Biochemistry and Physiology, 32, Holloway, J.D., Bradley, J.D. & Carter, D.J. (1987) Lepidoptera. CIE Guides to Insects of Importance to Man (ed. C.R. Betts), pp CAB International, Institute of Entomology, British Museum of Natural History, Oxford, UK. Johnston, R.E. (2003) Chemical communication in rodents: from pheromones to individual recognition. Journal of Mammalogy, 84, Jurenka, R.A. (1997) Biosynthetic pathway for producing the sex pheromone component (Z,E)-9,12-tetradecadienyl acetate in moths involves a Ä12 desaturase. Cellular and Molecular Life Sciences, 53, Jurenka, R.A., Haynes, K.F., Adlof, R.O., Bengtsson, M. & Roelofs, W.L. (1994) Sex pheromone component ratio in the cabbage looper moth altered by a mutation affecting the fatty acid chain-shortening reactions in the pheromone biosynthetic pathway. Insect Biochemistry and Molecular Biology, 24, Jurenka, R.A. & Roelofs, W.L. (1989) Characterization of the acetyl-transferase involved in pheromone biosynthesis in moths: specificity for the Z isomer in Tortricidae. Insect Biochemistry, 19, Lacey, S. & Box, R. (1991) A fast, easy sort. Byte, 16, Lehninger, A.L. (1970) Biochemistry. Worth Publishers, Inc., New York. Lewis, E.E., Barbarosa, B. & Gaugler, R. (2002) Mating and sexual communication by Steinernema carpocapsae (Nemata: Steinernematidae). Journal of Nematology., 34, Linn, C.E. Jr & Roelofs, W.L. (1995) Pheromone communication in moths and its role in the speciation process. Speciation and the Recognition Concept: Theory and Application (eds D.M. Lambert & H.G. Spencer), pp Johns Hopkins University Press, Baltimore, MD, USA. Millar, J.G. (2000) Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annual Review of Entomology, 45, Olsson, A.M., Jönsson, J.Å., Thelin, B. & Liljefors, T. (1983) Determination of the vapor pressures of moth sex pheromone components by a gas chromatographic method. Journal of Chemical Ecology, 9, Roelofs, W.L. (1995) Chemistry of sex attraction. Proceedings of the National Academy of Sciences USA, 92, Roelofs, W.L. & Jurenka, R.A. (1996) Biosynthetic enzymes regulating ratios of sex pheromone components in female redbanded leafroller moths. Bioorganic and Medicinal Chemistry, 4, Roelofs, W.L. & Wolf, W.A. (1988) Pheromone biosynthesis in Lepidoptera. Journal of Chemical Ecology, 14, Schlessinger, G.G. (1971) Vapor pressures, critical temperatures and critical pressures of organic compounds. Handbook of Chemistry and Physics (ed. R.C. Weast), pp. D The Chemical Rubber Co., Cleveland, OH, USA.
9 407 Pheromone components of moths Sokal, R.R. & Rohlf, F.J. (1995) Biometry. W.H. Freeman, New York. Tillman, J.A., Seybold, S.J., Jurenka, R.A. & Blomquist, G.J. (1999) Insect pheromones an overview of biosynthesis and endocrine regulation. Insect Biochemistry and Molecular Biology, 29, Witzgall, P., Lindblom, T., Bengtsson, M. & Tóth, M. (2005) The Pherolist. Available at: www-pherolist.slu.se/pherolist.php. Zhao, C.H., Löfstedt, C. & Wang, X. (1990) Sex pheromone biosynthesis in the Asian corn borer Ostrinia furnacalis (II): Biosynthesis of (E)- and (IX)-12-tetradecenyl acetate involves desaturation. Archives of Insect Biochemistry and Physiology, 15, Received 16 May 2005; accepted 28 October 2005
Chemical constraints on the evolution of olfactory communication channels of moths
 Journal of Theoretical Biology 235 (2005) 199 206 www.elsevier.com/locate/yjtbi Chemical constraints on the evolution of olfactory communication channels of moths John A. Byers Western Cotton Research
Journal of Theoretical Biology 235 (2005) 199 206 www.elsevier.com/locate/yjtbi Chemical constraints on the evolution of olfactory communication channels of moths John A. Byers Western Cotton Research
Pheromone Biosynthetic Pathways in the Moths Heliothis subflexa and Heliothis virescens
 Archives of Insect Biochemistry and Physiology 59:53 58 (2005) Pheromone Biosynthetic Pathways in the Moths Heliothis subflexa and Heliothis virescens Man-Yeon Choi, 1 Astrid Groot, 2 and Russell A. Jurenka
Archives of Insect Biochemistry and Physiology 59:53 58 (2005) Pheromone Biosynthetic Pathways in the Moths Heliothis subflexa and Heliothis virescens Man-Yeon Choi, 1 Astrid Groot, 2 and Russell A. Jurenka
GC-MS and LC-MS analyses for unraveling the diversity of lepidopteran communication systems
 23 rd ISCE Annual Meeting Symposium on Insect Semiochemicals I Analysis, Structures, Synthesis, GC-MS and LC-MS analyses for unraveling the diversity of lepidopteran communication systems Tetsu AND Graduate
23 rd ISCE Annual Meeting Symposium on Insect Semiochemicals I Analysis, Structures, Synthesis, GC-MS and LC-MS analyses for unraveling the diversity of lepidopteran communication systems Tetsu AND Graduate
Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
 Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths Jérôme Albre 1,2, Marjorie A. Liénard 3, Tamara M. Sirey 1,4, Silvia Schmidt
Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths Jérôme Albre 1,2, Marjorie A. Liénard 3, Tamara M. Sirey 1,4, Silvia Schmidt
27 th ISCE Annual Meeting Vancouver, Canada (July 25, 2011)
 27 th ISCE Annual Meeting Vancouver, Canada (July 25, 2011) Characterization of Epoxytrienes Derived from (3Z,6Z,9Z)-1,3,6,9-Tetraenes, Sex Pheromone Components of Arctiid Moths and elated Compounds T.
27 th ISCE Annual Meeting Vancouver, Canada (July 25, 2011) Characterization of Epoxytrienes Derived from (3Z,6Z,9Z)-1,3,6,9-Tetraenes, Sex Pheromone Components of Arctiid Moths and elated Compounds T.
GC / FT-IR analysis of a novel 2,4,6,9-tetraene occurring in a female pheromone gland of Parasemia plantaginis (Lepidoptera: Arctiidae)
 2017 ISCE/APACE Kyoto, Japan (August 24, 2017) GC / FT-IR analysis of a novel 2,4,6,9-tetraene occurring in a female pheromone gland of Parasemia plantaginis (Lepidoptera: Arctiidae) Yuta Muraki, Hideshi
2017 ISCE/APACE Kyoto, Japan (August 24, 2017) GC / FT-IR analysis of a novel 2,4,6,9-tetraene occurring in a female pheromone gland of Parasemia plantaginis (Lepidoptera: Arctiidae) Yuta Muraki, Hideshi
Lipids are broadly classified in to simple, complex and derived, which are further subdivided into different groups.
 Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS
 FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
FATTY ACID PROFILING BY GAS CHROMATOGRAPHY FOR THE SHERLOCK MIS Traditional gas chromatography of complex mixtures of compounds requires precision on the part of the chromatography equipment and considerable
The PlantFAdb website and database are based on the superb SOFA database (sofa.mri.bund.de).
 A major goal of PlantFAdb is to allow users to easily explore relationships between unusual fatty acid structures and the plant species that produce them. Clicking on Tree from the home page provides an
A major goal of PlantFAdb is to allow users to easily explore relationships between unusual fatty acid structures and the plant species that produce them. Clicking on Tree from the home page provides an
Investigation of Possible Sex Pheromone Components of Female Loreyi Leafworm, Acantholeucania loreyi (Duponchel) (Lepidoptera: Noctuidae) in Taiwan
 Zoological Studies 41(2): 188-193 (2002) Investigation of Possible Sex Pheromone Components of Female Loreyi Leafworm, Acantholeucania loreyi (Duponchel) (Lepidoptera: Noctuidae) in Taiwan Hsiao Yung Ho
Zoological Studies 41(2): 188-193 (2002) Investigation of Possible Sex Pheromone Components of Female Loreyi Leafworm, Acantholeucania loreyi (Duponchel) (Lepidoptera: Noctuidae) in Taiwan Hsiao Yung Ho
European Food Safety Authority (EFSA)
 TECHNICAL REPORT APPROVED: 11 April 2017 doi:10.2903/sp.efsa.2017.en-1213 Outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for Straight Chain Lepidopteran
TECHNICAL REPORT APPROVED: 11 April 2017 doi:10.2903/sp.efsa.2017.en-1213 Outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for Straight Chain Lepidopteran
ANSC/NUTR 618 Lipids & Lipid Metabolism
 I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
Non-Conjugated Double Bonds
 1 H-NMR Spectroscopy of Fatty Acids and Their Derivatives Non-Conjugated Double Bonds The introduction of one double bond gives rise to several peaks in the NMR spectrum compared to the saturated chains
1 H-NMR Spectroscopy of Fatty Acids and Their Derivatives Non-Conjugated Double Bonds The introduction of one double bond gives rise to several peaks in the NMR spectrum compared to the saturated chains
Teacher s Tools Chemistry Organic Chemistry: Nomenclature and Isomerism
 1. Hydrocarbons: a) Naming of hydrocarbons is done based on the number of carbons. 1 = meth 6 = hex 2 = eth 7 = hept 3 = prop 8 = oct 4 = but 9 = non 5 = pent 10 = dec b) Alkanes are hydrocarbons without
1. Hydrocarbons: a) Naming of hydrocarbons is done based on the number of carbons. 1 = meth 6 = hex 2 = eth 7 = hept 3 = prop 8 = oct 4 = but 9 = non 5 = pent 10 = dec b) Alkanes are hydrocarbons without
Biosynthesis of Triacylglycerides (TG) in liver. Mobilization of stored fat and oxidation of fatty acids
 Biosynthesis of Triacylglycerides (TG) in liver Mobilization of stored fat and oxidation of fatty acids Activation of hormone sensitive lipase This enzyme is activated when phosphorylated (3,5 cyclic AMPdependent
Biosynthesis of Triacylglycerides (TG) in liver Mobilization of stored fat and oxidation of fatty acids Activation of hormone sensitive lipase This enzyme is activated when phosphorylated (3,5 cyclic AMPdependent
FILAMENTOUS NATURE OF PHEROMONE PLUMES PROTECTS INTEGRITY OF SIGNAL FROM BACKGROUND CHEMICAL NOISE IN CABBAGE LOOPER MOTH, Trichoplusia ni
 Journal of Chemical Ecology, Vol. 18, No.3. 1992 FILAMENTOUS NATURE OF PHEROMONE PLUMES PROTECTS INTEGRITY OF SIGNAL FROM BACKGROUND CHEMICAL NOISE IN CABBAGE LOOPER MOTH, Trichoplusia ni YONG-BIAO LIU*
Journal of Chemical Ecology, Vol. 18, No.3. 1992 FILAMENTOUS NATURE OF PHEROMONE PLUMES PROTECTS INTEGRITY OF SIGNAL FROM BACKGROUND CHEMICAL NOISE IN CABBAGE LOOPER MOTH, Trichoplusia ni YONG-BIAO LIU*
Important reactions of lipids
 Taif University College of Medicine Preparatory Year Students Medical chemistry (2) Part II (Lipids) week 4 lectures 1435-36 Important reactions of lipids Lectures outlines Definition and importance of
Taif University College of Medicine Preparatory Year Students Medical chemistry (2) Part II (Lipids) week 4 lectures 1435-36 Important reactions of lipids Lectures outlines Definition and importance of
CHEMISTRY OF THE SEX PHEROMONE GLAND OF THE FALL WEBWORM, HYPHANTRIA CUNEA, DISCOVERED IN NEW ZEALAND
 Biosecurity 31 CHEMISTRY OF THE SEX PHEROMONE GLAND OF THE FALL WEBWORM, HYPHANTRIA CUNEA, DISCOVERED IN NEW ZEALAND A.M. EL-SAYED, A.R. GIBB and D.M. SUCKLING HortResearch, PO Box 51, Lincoln, New Zealand
Biosecurity 31 CHEMISTRY OF THE SEX PHEROMONE GLAND OF THE FALL WEBWORM, HYPHANTRIA CUNEA, DISCOVERED IN NEW ZEALAND A.M. EL-SAYED, A.R. GIBB and D.M. SUCKLING HortResearch, PO Box 51, Lincoln, New Zealand
You must answer FOUR of the SIX questions. Use a SEPARATE answer book for EACH question.
 UNIVERSITY OF EAST ANGLIA School of Pharmacy Main Series UG Examination 2016-17 DRUG DESIGN AND MECHANISMS OF DRUG ACTION PHA-5001Y Time allowed: 2 hours You must answer FOUR of the SIX questions. Use
UNIVERSITY OF EAST ANGLIA School of Pharmacy Main Series UG Examination 2016-17 DRUG DESIGN AND MECHANISMS OF DRUG ACTION PHA-5001Y Time allowed: 2 hours You must answer FOUR of the SIX questions. Use
Bioorganic Chemistry on Sex Pheromones Secreted by Lepidopteran Insects and Their Application for Plant Protection
 3 rd Pan-Pacific Conference on Pesticide Science Honolulu, Hawaii June 1-4, 2003 Topic A: New Discoveries Sub-Topic A-4: Natural Product Bioorganic Chemistry on Sex Pheromones Secreted by Lepidopteran
3 rd Pan-Pacific Conference on Pesticide Science Honolulu, Hawaii June 1-4, 2003 Topic A: New Discoveries Sub-Topic A-4: Natural Product Bioorganic Chemistry on Sex Pheromones Secreted by Lepidopteran
The 3rd International Symposium on Insect Pheromones Sweden, 2003 May. Lepidopteran Epoxyalkenyl Pheromones : Synthesis and Identification
 The 3rd International Symposium on Insect Pheromones Sweden, 23 May Session II Lepidopteran Epoxyalkenyl Pheromones : Synthesis and Identification Tetsu AND and Masabu YAMAMT Tokyo University of Agric.
The 3rd International Symposium on Insect Pheromones Sweden, 23 May Session II Lepidopteran Epoxyalkenyl Pheromones : Synthesis and Identification Tetsu AND and Masabu YAMAMT Tokyo University of Agric.
Variability in the efficacy of sex pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae)
 J. Appl. Entomol. ORIGINAL CONTRIBUTION Variability in the efficacy of sex pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae) A. L. Knight 1, E. Basoalto 2 & L. L. Stelinski
J. Appl. Entomol. ORIGINAL CONTRIBUTION Variability in the efficacy of sex pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae) A. L. Knight 1, E. Basoalto 2 & L. L. Stelinski
Part 2. Monoenoic Fatty Acids
 MASS SPECTRA OF 3-PYRIDYLCARBIOL ESTERS Part 2. Monoenoic Fatty Acids Straight-Chain Monoenoic Fatty Acids The mass spectra of 3-pyridylcarbinol esters of monoenoic fatty acids are distinctive and permit
MASS SPECTRA OF 3-PYRIDYLCARBIOL ESTERS Part 2. Monoenoic Fatty Acids Straight-Chain Monoenoic Fatty Acids The mass spectra of 3-pyridylcarbinol esters of monoenoic fatty acids are distinctive and permit
Lipid Metabolism. Catabolism Overview
 Lipid Metabolism Pratt & Cornely, Chapter 17 Catabolism Overview Lipids as a fuel source from diet Beta oxidation Mechanism ATP production Ketone bodies as fuel 1 High energy More reduced Little water
Lipid Metabolism Pratt & Cornely, Chapter 17 Catabolism Overview Lipids as a fuel source from diet Beta oxidation Mechanism ATP production Ketone bodies as fuel 1 High energy More reduced Little water
RAPID COMMUNICATION JASMONATE IN LEPIDOPTERAN EGGS AND NEONATES
 Journal of Chemical Ecology, Vol. 31, No. 11, November 2005 ( #2005) DOI: 10.1007/s10886-005-8553-2 RAPID COMMUNICATION JOHN F. TOOKER* and CONSUELO M. DE MORAES Department of Entomology, The Pennsylvania
Journal of Chemical Ecology, Vol. 31, No. 11, November 2005 ( #2005) DOI: 10.1007/s10886-005-8553-2 RAPID COMMUNICATION JOHN F. TOOKER* and CONSUELO M. DE MORAES Department of Entomology, The Pennsylvania
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Fatty Acid Elongation and Desaturation
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
Lipids and Classification:
 Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2011, 2 (5) :258-262 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW Comparison of
Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2011, 2 (5) :258-262 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW Comparison of
MASS SPECTRA OF DERIVATIVES OF ACETYLENIC FATTY ACIDS. PART 2. ENE-YNOIC SYSTEMS
 MASS SPECTRA OF DERIVATIVES OF ACETYLEIC FATTY ACIDS. PART 2. EE-YOIC SYSTEMS As with other documents in this section, this is a subjective account of mass spectrometry of acetylenic fatty acids, detailing
MASS SPECTRA OF DERIVATIVES OF ACETYLEIC FATTY ACIDS. PART 2. EE-YOIC SYSTEMS As with other documents in this section, this is a subjective account of mass spectrometry of acetylenic fatty acids, detailing
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
ANSWERS: Isomers. 2) A and F
 NSWERS: Isomers 1) For cis and trans isomers to occur a carbon-carbon double bond must be present as this prevents any rotation about this bond, and the atoms or groups of atoms attached to the two carbon
NSWERS: Isomers 1) For cis and trans isomers to occur a carbon-carbon double bond must be present as this prevents any rotation about this bond, and the atoms or groups of atoms attached to the two carbon
Identification and Biosynthetic Studies of the Hydrocarbon Sex Pheromone in Utetheisa ornatrix
 Entomology Publications Entomology 6-27 Identification and Biosynthetic Studies of the Hydrocarbon Sex Pheromone in Utetheisa ornatrix Man-Yeon Choi Iowa State University Russell A. Jurenka Iowa State
Entomology Publications Entomology 6-27 Identification and Biosynthetic Studies of the Hydrocarbon Sex Pheromone in Utetheisa ornatrix Man-Yeon Choi Iowa State University Russell A. Jurenka Iowa State
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Pheromone traps for monitoring Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in the presence of mating disruption
 Pheromone traps for monitoring Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in the presence of mating disruption Burks, C.S.* #1, Brandl, D.G. 1, Kuenen, L.P.S. 1, Reyes, C.C. 2, Fisher, J.M.
Pheromone traps for monitoring Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in the presence of mating disruption Burks, C.S.* #1, Brandl, D.G. 1, Kuenen, L.P.S. 1, Reyes, C.C. 2, Fisher, J.M.
Chapter 11 Nutrition: Food for Thought
 Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 3: Examining Relationships
 Name Date Per Key Vocabulary: response variable explanatory variable independent variable dependent variable scatterplot positive association negative association linear correlation r-value regression
Name Date Per Key Vocabulary: response variable explanatory variable independent variable dependent variable scatterplot positive association negative association linear correlation r-value regression
Morphological and Biological Evidence for the Presence of a Male Sex Pheromone of the Diamondback Moth
 11 Morphological and Biological Evidence for the Presence of a Male Sex Pheromone of the Diamondback Moth Y. S. Chow, Y. M. Lin, and H. J. Teng Institute of Zoology, Academia Sinica, Nankang, Taipei, Taiwan,
11 Morphological and Biological Evidence for the Presence of a Male Sex Pheromone of the Diamondback Moth Y. S. Chow, Y. M. Lin, and H. J. Teng Institute of Zoology, Academia Sinica, Nankang, Taipei, Taiwan,
Control of the biosynthetic pathway of Sesamia nonagrioides sex pheromone by the pheromone biosynthesis activating neuropeptide
 Insect Biochemistry and Molecular Biology 30 (2000) 455 459 www.elsevier.com/locate/ibmb Control of the biosynthetic pathway of Sesamia nonagrioides sex pheromone by the pheromone biosynthesis activating
Insect Biochemistry and Molecular Biology 30 (2000) 455 459 www.elsevier.com/locate/ibmb Control of the biosynthetic pathway of Sesamia nonagrioides sex pheromone by the pheromone biosynthesis activating
PRODUCT CATALOG. ISCA Technologies, Inc to All rights reserved. Version
 PRODUCT CATALOG SEMIOCHEMICALS Pheromones, Attractants, Repellents and Fine Chemical Compounds ISCA Technologies possesses state-of-the-art semiochemical synthesis and analysis capabilities. It is the
PRODUCT CATALOG SEMIOCHEMICALS Pheromones, Attractants, Repellents and Fine Chemical Compounds ISCA Technologies possesses state-of-the-art semiochemical synthesis and analysis capabilities. It is the
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Evolution of asymmetry in sexual isolation: a criticism of a test case
 Evolutionary Ecology Research, 2004, 6: 1099 1106 Evolution of asymmetry in sexual isolation: a criticism of a test case Emilio Rolán-Alvarez* Departamento de Bioquímica, Genética e Inmunología, Facultad
Evolutionary Ecology Research, 2004, 6: 1099 1106 Evolution of asymmetry in sexual isolation: a criticism of a test case Emilio Rolán-Alvarez* Departamento de Bioquímica, Genética e Inmunología, Facultad
Response of adult male Zeuzera pyrina (Lep: Zeuzeridae) to different pheromone traps in walnut orchards of four isolated regions of Iran
 Arthropods, 2013, 2(4): 225-230 Article Response of adult male Zeuzera pyrina (Lep: Zeuzeridae) to different pheromone traps in walnut orchards of four isolated regions of Iran Raheleh Dolati, Jamasb Nozari,
Arthropods, 2013, 2(4): 225-230 Article Response of adult male Zeuzera pyrina (Lep: Zeuzeridae) to different pheromone traps in walnut orchards of four isolated regions of Iran Raheleh Dolati, Jamasb Nozari,
GC-FT-IR Analyses of Sex Pheromones Secreted by Nettle Moths
 28 th ISCE Annual Meeting Vilnius, Lithuania (July 24, 212) GC-FT-IR Analyses of Sex Pheromones Secreted by Nettle Moths T. Ando, 1 * H. Shibasaki, 1 R. Yamakawa, 1 and H. Naka 2 1 Graduate School of BASE,
28 th ISCE Annual Meeting Vilnius, Lithuania (July 24, 212) GC-FT-IR Analyses of Sex Pheromones Secreted by Nettle Moths T. Ando, 1 * H. Shibasaki, 1 R. Yamakawa, 1 and H. Naka 2 1 Graduate School of BASE,
insect pests. Cockroach control has been extensive with many available types of traps, chemical
 INTRODUCTION When it comes to dealing with irritating insects, cockroaches are among the most loathed insect pests. Cockroach control has been extensive with many available types of traps, chemical control
INTRODUCTION When it comes to dealing with irritating insects, cockroaches are among the most loathed insect pests. Cockroach control has been extensive with many available types of traps, chemical control
Summary of fatty acid synthesis
 Lipid Metabolism, part 2 1 Summary of fatty acid synthesis 8 acetyl CoA + 14 NADPH + 14 H+ + 7 ATP palmitic acid (16:0) + 8 CoA + 14 NADP + + 7 ADP + 7 Pi + 7 H20 1. The major suppliers of NADPH for fatty
Lipid Metabolism, part 2 1 Summary of fatty acid synthesis 8 acetyl CoA + 14 NADPH + 14 H+ + 7 ATP palmitic acid (16:0) + 8 CoA + 14 NADP + + 7 ADP + 7 Pi + 7 H20 1. The major suppliers of NADPH for fatty
INTRODUCTION TO STATISTICS
 Basic Statistics Introduction to Statistics Basic Statistical Formulas Commonly used Ecological Equations INTRODUCTION TO STATISTICS Statistics is the branch of mathematics that deals with the techniques
Basic Statistics Introduction to Statistics Basic Statistical Formulas Commonly used Ecological Equations INTRODUCTION TO STATISTICS Statistics is the branch of mathematics that deals with the techniques
WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids
 WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids PART A: Understanding Lipids Lipids are more commonly known as fats and include triglycerides,
WHAT IS A LIPID? OBJECTIVE The objective of this worksheet is to understand the structure and function of lipids PART A: Understanding Lipids Lipids are more commonly known as fats and include triglycerides,
Chem 5 PAL Worksheet Lipids Smith text Chapter 15
 Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
Chem 1120 Final 210 points Dr. Luther Giddings
 Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
COURSE OUTLINE CHEMISTRY II 2018
 COURSE OUTLINE CHEMISTRY II 2018 Course: Course Code: Times & Location: Course Coordinator: Instructors/Teaching Assistants: E-mail: Office Hours: Office Location: Chemistry II : Foundations of Chemistry
COURSE OUTLINE CHEMISTRY II 2018 Course: Course Code: Times & Location: Course Coordinator: Instructors/Teaching Assistants: E-mail: Office Hours: Office Location: Chemistry II : Foundations of Chemistry
' volatiles. (Z,Z)-7,ll-16 : OH, (Z, E)-7,ll-16 : OH, and (2)-7-16: OH were
 Roelofs and Card.5, 1977) Some of these compounds have been synthesized and tested in part because of their structural similarity to the naturally occurring sex pheromone (e g, Beevor and Campion, 1979)
Roelofs and Card.5, 1977) Some of these compounds have been synthesized and tested in part because of their structural similarity to the naturally occurring sex pheromone (e g, Beevor and Campion, 1979)
Unsaturated Hydrocarbons
 Interchapter G Unsaturated ydrocarbons FPO aption TK. G. unsaturated ydrocarbons G1 In this Interchapter, we shall continue our introduction to organic chemistry by discussing unsaturated hydrocarbons.
Interchapter G Unsaturated ydrocarbons FPO aption TK. G. unsaturated ydrocarbons G1 In this Interchapter, we shall continue our introduction to organic chemistry by discussing unsaturated hydrocarbons.
Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life
 AP Biology Name: Block Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life Most of this chapter is new material. We will discuss it all in detail. Section 1 1. Make an electron distribution
AP Biology Name: Block Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life Most of this chapter is new material. We will discuss it all in detail. Section 1 1. Make an electron distribution
Lipids fatty, oily, or waxy hydrophobic organic compounds.
 Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Fatty acid breakdown
 Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
6.1 Cis Trans Isomers
 6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 12966-1 First edition 2014-12-01 Animal and vegetable fats and oils Gas chromatography of fatty acid methyl esters Part 1: Guidelines on modern gas chromatography of fatty acid
INTERNATIONAL STANDARD ISO 12966-1 First edition 2014-12-01 Animal and vegetable fats and oils Gas chromatography of fatty acid methyl esters Part 1: Guidelines on modern gas chromatography of fatty acid
MITOCW watch?v=lcih8faydgk
 MITOCW watch?v=lcih8faydgk The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free. To
MITOCW watch?v=lcih8faydgk The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free. To
A New Method for the Early Detection of Edible Oil Oxidation
 WHITE PAPER Early Detection of Edible Oil Oxidation A New Method for the Early Detection of Edible Oil Oxidation Edible oils are used in a wide range of culinary applications. Oils containing unsaturated
WHITE PAPER Early Detection of Edible Oil Oxidation A New Method for the Early Detection of Edible Oil Oxidation Edible oils are used in a wide range of culinary applications. Oils containing unsaturated
1. Denaturation changes which of the following protein structure(s)?
 Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Structure of Alkenes In ethene (ethylene) each carbon is bonded to 3 other atoms, with zero nonbonding electrons => sp 2 hybridization.
 Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
3.7A: Compounds with multiple stereocenters
 Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Topic 3.1 Nutrients. - Lipids are an essential part of the and are a part of cell in the body.
 Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
Name: Topic 3.1 Nutrients Date: IB SEHS 3.1.1. List the macronutrients and micronutrients Macronutrients: - lipid (fat) - carbohydrate - protein - water (says the book) Micronutrients: - vitamins - minerals
Calderglen High School CfE Higher Chemistry. Nature s Chemistry Esters, Fats and Oils. Page 1 of 11
 Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
GLOSSY MUTANTS OF MAIZE
 Heredity (1979), 42 (3), 391-395 GLOSSY MUTATS OF MAIZE IX. CHEMISTRY OF GLOSSY 4, GLOSSY 8, GLOSSY 15 AD GLOSSY 18 SURFACE WAXES* G. BtACHI, P. AVATO and F. SALAMII Istituto di Ch,mica organica, Viale
Heredity (1979), 42 (3), 391-395 GLOSSY MUTATS OF MAIZE IX. CHEMISTRY OF GLOSSY 4, GLOSSY 8, GLOSSY 15 AD GLOSSY 18 SURFACE WAXES* G. BtACHI, P. AVATO and F. SALAMII Istituto di Ch,mica organica, Viale
CHAPTER 3. Carbon & the Molecular Diversity of Life
 CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
Practice Problems for Video CH 5-1:
 CEM 243 Organic Chemistry C-5: Stereochemistry - Chiral Molecules Study Guide: Key Concepts and Practice Problems Key concepts you need to understand: Chirality and chiral centers; stereoisomers; (R,S)
CEM 243 Organic Chemistry C-5: Stereochemistry - Chiral Molecules Study Guide: Key Concepts and Practice Problems Key concepts you need to understand: Chirality and chiral centers; stereoisomers; (R,S)
Lecture: 26 OXIDATION OF FATTY ACIDS
 Lecture: 26 OXIDATION OF FATTY ACIDS Fatty acids obtained by hydrolysis of fats undergo different oxidative pathways designated as alpha ( ), beta ( ) and omega ( ) pathways. -oxidation -Oxidation of fatty
Lecture: 26 OXIDATION OF FATTY ACIDS Fatty acids obtained by hydrolysis of fats undergo different oxidative pathways designated as alpha ( ), beta ( ) and omega ( ) pathways. -oxidation -Oxidation of fatty
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Alkenes.
 Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
Investigation on Rate of Hydrolytic Rancidity. Research Question:
 1 Investigation on Rate of Hydrolytic Rancidity Research Question: Comparing the shelf life of skim milk, low-fat milk, and whole milk, to analyze the effect of different amounts of lipids on hydrolytic
1 Investigation on Rate of Hydrolytic Rancidity Research Question: Comparing the shelf life of skim milk, low-fat milk, and whole milk, to analyze the effect of different amounts of lipids on hydrolytic
unit 9 practice test (organic and biochem)
 Name: Class: Date: unit 9 practice test (organic and biochem) Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What s the correct formula for the simplest
Name: Class: Date: unit 9 practice test (organic and biochem) Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What s the correct formula for the simplest
Clinical Trials A Practical Guide to Design, Analysis, and Reporting
 Clinical Trials A Practical Guide to Design, Analysis, and Reporting Duolao Wang, PhD Ameet Bakhai, MBBS, MRCP Statistician Cardiologist Clinical Trials A Practical Guide to Design, Analysis, and Reporting
Clinical Trials A Practical Guide to Design, Analysis, and Reporting Duolao Wang, PhD Ameet Bakhai, MBBS, MRCP Statistician Cardiologist Clinical Trials A Practical Guide to Design, Analysis, and Reporting
The Structure and Function of Large Biological Molecules
 NAME DATE Chapter 5 - The Structure and Function of Large Biological Molecules Guided Reading Concept 5.1: Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall
NAME DATE Chapter 5 - The Structure and Function of Large Biological Molecules Guided Reading Concept 5.1: Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Field observations quantifying attraction of four tortricid moths to high-dosage pheromone dispensers in untreated and pheromone-treated orchards
 Blackwell Publishing, Ltd. Field observations quantifying attraction of four tortricid moths to high-dosage pheromone dispensers in untreated and pheromone-treated orchards L.L. Stelinski*, L.J. Gut, A.V.
Blackwell Publishing, Ltd. Field observations quantifying attraction of four tortricid moths to high-dosage pheromone dispensers in untreated and pheromone-treated orchards L.L. Stelinski*, L.J. Gut, A.V.
Surname. Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F212 BIOLOGY. Molecules, Biodiversity, Food and Health
 Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F212 BIOLOGY Molecules, Biodiversity, Food and Health TUESDAY 12 JANUARY
Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F212 BIOLOGY Molecules, Biodiversity, Food and Health TUESDAY 12 JANUARY
Biosynthesis of Fatty Acids
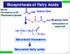 Biosynthesis of Fatty Acids CH 511 ource b-oxidation of FA Glycolysis of glucose n H CoA Acetyl CoA CoA Malonyl CoA Carboxylation of acetyl CoA H 3 C CH 2 CH 2 n-1 CoA aturated thioesters aturated fatty
Biosynthesis of Fatty Acids CH 511 ource b-oxidation of FA Glycolysis of glucose n H CoA Acetyl CoA CoA Malonyl CoA Carboxylation of acetyl CoA H 3 C CH 2 CH 2 n-1 CoA aturated thioesters aturated fatty
189,311, , ,561, ,639, ,679, Ch13; , Carbohydrates
 Lecture 31 (12/8/17) Reading: Ch7; 258-267 Ch10; 371-373 Problems: Ch7 (text); 26,27,28 Ch7 (study-guide: applying); 2,5 Ch7 (study-guide: facts); 6 NEXT (LAST!) Reading: Chs4,6,8,10,14,16,17,18; 128-129,
Lecture 31 (12/8/17) Reading: Ch7; 258-267 Ch10; 371-373 Problems: Ch7 (text); 26,27,28 Ch7 (study-guide: applying); 2,5 Ch7 (study-guide: facts); 6 NEXT (LAST!) Reading: Chs4,6,8,10,14,16,17,18; 128-129,
: Overview of EFA metabolism
 Figure 1 gives an overview of the metabolic fate of the EFAs when consumed in the diet. The n-6 and n-3 PUFAs when consumed in the form of dietary triglyceride from various food sources undergoes digestion
Figure 1 gives an overview of the metabolic fate of the EFAs when consumed in the diet. The n-6 and n-3 PUFAs when consumed in the form of dietary triglyceride from various food sources undergoes digestion
Many of the compounds we are concerned with in biology are carbon-based compounds The study of carbon-based compounds is called organic chemistry
 1 2 3 4 Bio 1101 Lecture 3 Chapter 3: Molecules of Life Organic Molecules Many of the compounds we are concerned with in biology are carbon-based compounds The study of carbon-based compounds is called
1 2 3 4 Bio 1101 Lecture 3 Chapter 3: Molecules of Life Organic Molecules Many of the compounds we are concerned with in biology are carbon-based compounds The study of carbon-based compounds is called
Carbon s unique bonding pattern arises from the hybridization of the electrons.
 Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
Adaptation vs Exaptation. Examples of Exaptation. Behavior of the Day! Historical Hypotheses
 Adaptation vs Exaptation 1. Definition 1: Adaptation = A trait, or integrated suite of traits, that increases the fitness (reproductive success) of its possessor. 2. However, traits can have current utility
Adaptation vs Exaptation 1. Definition 1: Adaptation = A trait, or integrated suite of traits, that increases the fitness (reproductive success) of its possessor. 2. However, traits can have current utility
Citation for published version (APA): Jonker, G. H. (1999). Hydrogenation of edible oils and fats Groningen: s.n.
 University of Groningen Hydrogenation of edible oils and fats Jonker, Geert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Hydrogenation of edible oils and fats Jonker, Geert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Sex Pheromone Gland of the Female European Corn Borer Moth, Ostrinia nubilalis (Lepidoptera, Pyralidae): Ultrastructural and Biochemical Evidences
 Sex Pheromone Gland of the Female European Corn Borer Moth, Ostrinia nubilalis (Lepidoptera, Pyralidae): Ultrastructural and Biochemical Evidences Authors: Peter W. K. Ma, and Wendel L. Roelofs Source:
Sex Pheromone Gland of the Female European Corn Borer Moth, Ostrinia nubilalis (Lepidoptera, Pyralidae): Ultrastructural and Biochemical Evidences Authors: Peter W. K. Ma, and Wendel L. Roelofs Source:
L.L. Stelinski 1 *and L.J. Gut 2
 Bulletin of Entomological Research (2009) 99, 245 251 Ó 2008 Cambridge University Press First published online 24 October 2008 doi:10.1017/s0007485308006263 Printed in the United Kingdom Delayed mating
Bulletin of Entomological Research (2009) 99, 245 251 Ó 2008 Cambridge University Press First published online 24 October 2008 doi:10.1017/s0007485308006263 Printed in the United Kingdom Delayed mating
Synthesis and Characterization of 3,13- and 2,13-Octadecadienyl Compounds for Identification of the Sex Pheromone Secreted by Clearwing Moths
 22nd, ISCE Annual Meeting Barcelona July 18, 26 Synthesis and Characterization of 3,13- and 2,13-Octadecadienyl Compounds for Identification of the Sex Pheromone Secreted by Clearwing Moths T. Ando,* H.
22nd, ISCE Annual Meeting Barcelona July 18, 26 Synthesis and Characterization of 3,13- and 2,13-Octadecadienyl Compounds for Identification of the Sex Pheromone Secreted by Clearwing Moths T. Ando,* H.
Carbohydrates and Lipids
 Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Fatty Acid and Triacylglycerol Metabolism 1
 Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Fatty Acid and Triacylglycerol Metabolism 1
 Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Fatty Acid and Triacylglycerol Metabolism 1 Mobilization of stored fats and oxidation of fatty acids Lippincott s Chapter 16 What is the first lecture about What is triacylglycerol Fatty acids structure
Dr. Nafith Abu Tarboush
 5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
Trans fats dangerous. What are Trans Fats?
 Trans fats dangerous What are Trans Fats? The fat in foods contains a mixture of saturated, monounsaturated and polyunsaturated fatty acids. In foods of animal origin, a large proportion of fatty acids
Trans fats dangerous What are Trans Fats? The fat in foods contains a mixture of saturated, monounsaturated and polyunsaturated fatty acids. In foods of animal origin, a large proportion of fatty acids
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Introduction Required data for substance identity purposes Dossier preparation (introduction) Substance identity of
 Overview Introduction Required data for substance identity purposes Dossier preparation (introduction) Substance identity of Mono-constituent substances Multi-constituent substances with additional identifiers
Overview Introduction Required data for substance identity purposes Dossier preparation (introduction) Substance identity of Mono-constituent substances Multi-constituent substances with additional identifiers
Lab 4: Perception of Loudness
 Lab 4: Perception of Loudness Lewis O. Harvey, Jr. and Dillon J. McGovern PSYC 4165: Psychology of Perception, Spring 2019 Department of Psychology and Neuroscience University of Colorado Boulder Boulder,
Lab 4: Perception of Loudness Lewis O. Harvey, Jr. and Dillon J. McGovern PSYC 4165: Psychology of Perception, Spring 2019 Department of Psychology and Neuroscience University of Colorado Boulder Boulder,
Section 3.2 Least-Squares Regression
 Section 3.2 Least-Squares Regression Linear relationships between two quantitative variables are pretty common and easy to understand. Correlation measures the direction and strength of these relationships.
Section 3.2 Least-Squares Regression Linear relationships between two quantitative variables are pretty common and easy to understand. Correlation measures the direction and strength of these relationships.
Saba Al Fayoumi. Nour Hamdan. Bann Khraisat. Dr. Mamoun Ahram
 9 Saba Al Fayoumi Nour Hamdan Bann Khraisat Dr. Mamoun Ahram Proteoglycans and glycoproteins have been previously discussed and the differences between them have been noted. Protein glycosylation (protein-linked
9 Saba Al Fayoumi Nour Hamdan Bann Khraisat Dr. Mamoun Ahram Proteoglycans and glycoproteins have been previously discussed and the differences between them have been noted. Protein glycosylation (protein-linked
Lab 3: Perception of Loudness
 Lab 3: Perception of Loudness Lewis O. Harvey, Jr. and Samuel P. Paskewitz PSYC 4165: Psychology of Perception, Fall 2018 Department of Psychology and Neuroscience University of Colorado Boulder Boulder,
Lab 3: Perception of Loudness Lewis O. Harvey, Jr. and Samuel P. Paskewitz PSYC 4165: Psychology of Perception, Fall 2018 Department of Psychology and Neuroscience University of Colorado Boulder Boulder,
