Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium
|
|
|
- Camilla Griffith
- 6 years ago
- Views:
Transcription
1 Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium Excerpts from: Drugdex Monograph on Pentosan Edition: online URL: Date Added: 29/04/ :42:55 Source Notes:Evidence and Safety excerpts from monographs FDA Labelled Indication: Interstitial cystitis, chronic, relief of bladder pain or discomfort This product does not have a UK Marketing Authorisation (or Product Licence) so it must be considered as unlicensed in the UK. 1) Overview FDA Approval: Adult, yes; Pediatric, yes (16 yr and older) Efficacy: Adult, Effective; Pediatric, Evidence favors efficacy Recommendation: Adult, Class IIa; Pediatric, Class IIa Strength of Evidence: Adult, Category B; Pediatric, Category B 2) Summary Indicated for symptomatic relief of interstitial cystitis (Prod Info ELMIRON(R) oral capsules, 2006). Treatment with oral pentosan polysulfate sodium at daily doses of 300, 600, and 900 milligrams led to similar clinical responses in the symptomatic treatment of chronic interstitial cystitis in a randomized, double-blind, parallelgroup, multicenter, dose-ranging study (n=380); notably, the study was underpowered due to high discontinuation rates (Nickel et al, 2005). Combination therapy with subcutaneous low-dose heparin and oral pentosan polysulfate sodium (PPS) was safe and led to a significant improvement in overall patient well-being compared to PPS therapy alone in patients with interstitial cystitis in an open-label, prospective, controlled study (n=58) (vanophoven et al, 2005) 3) Effectiveness a) In a randomized, double-blind, parallel-group, multicenter, dose-ranging study (n=380), treatment with oral pentosan polysulfate sodium (PPS) at daily doses of 300, 600, and 900 milligrams (mg) led to similar clinical responses in the symptomatic treatment of chronic interstitial cystitis; however, the study was underpowered due to high discontinuation rates. The study included adults (mean age, 44.2 years; 90% female) diagnosed with IC for 6 months or longer and randomized them to receive, in a double- Page 1 of 6
2 dummy fashion, either 100 mg, 200 mg, or 300 mg of PPS orally three times daily for 32 weeks. Patients using antihistamines and antidepressants at baseline were allowed to continue them during the study; however, initiation of these agents during the study was not allowed. The primary endpoint measure was the O'Leary-Sant IC Symptoms Index (ICSI), which is a fouritem, validated questionnaire yielding scores ranging from 0-6 (mild symptoms), 7-14 (moderate symptoms), and (severe symptoms). The secondary endpoint measure was the Patient's Overall Rating of Symptoms Index (PORIS), which assessed the overall change in IC, pain, and urgency with treatment and rated it as worse, no better (0% improvement), slightly improved (25%), moderately improved (50%), greatly improved (75%), or symptoms gone (100%). Patients achieving 50% to 100% improvement were considered to be responders. At baseline, the mean duration of IC since diagnosis was 11 months, and mild, moderate, and severe symptoms were reported by 3.2%, 34.6%, and 62.2% of patients, respectively. With only 230 patients completing the 32- week study (39.5% attrition rate), the study was underpowered for clinical outcomes. While the overall rate of treatment discontinuation was not doserelated, the 900-mg dose group had the highest proportion of patients (30.7%) discontinuing due to adverse events (versus 18% and 16.8% for the 300 and 600 mg/day groups, respectively; p less than 0.05). An intent-to-treat analysis revealed a significant improvement of mean ICSI scores from baseline (11.2, 11.9, and 11.9) to endpoint (8.2, 8.1, and 8.6) for all dosages (300-, 600-, and 900-mg, respectively), with improvements occurring as early as 4 weeks after therapy initiation. However, the difference between the groups was not statistically significant for ICSI scores as well as time to response. At study end, the proportion of patients reporting severe symptoms had decreased to 15.7%, with 27.5% and 56.9% of patients reporting mild and moderate symptoms, respectively. At week 32, 49.6%, 49.6%, and 45.2% of patients in the 300, 600, and 900 mg/day groups, respectively, were classified as responders based on PORIS scores, and the ICSI results correlated well with the PORIS scores. Adverse events were mostly mild and transient, with diarrhea (25.3%), headache (18.2%), nausea (15%), pelvic pain (12.9%), and abdominal pain (12.6%) being the most common. Diarrhea as well as rectal bleeding (4% to 14%; mostly mild) were dose-related (p less than and p=0.02, respectively). Mild alopecia occurred in 5.5% of patients, with 3 patients discontinuing treatment due to alopecia. There were no serious treatmentemergent adverse events during the study (Nickel et al, 2005). b) A meta-analysis of 4 clinical studies reported that pentosan, in a minimum dose of 300 milligrams/day, is significantly more effective than placebo for relieving the following symptoms of interstitial cystitis: pain (p=0.507), urgency (p=0.799), and frequency (p=0.680). Results for nocturia were not significant. The overall success rates for pentosan for the relief of pain, urgency, and frequency were 37%, 28%, and 54%, respectively. Only 2 of the studies included in the meta-analysis specified the severity of disease in the subjects and the authors of the meta-analysis suggested that future studies stratify results by disease severity (Hwang et al, 1997). Page 2 of 6
3 c) Available studies were unable to clearly identify which patients, regardless of method of diagnosis, were most likely to respond to pentosan polysulfate. Stratification of patients based on initial cystoscopic findings has suggested that a decrease in micturition frequency and possibly an increase in the mean volume per void per 24 hours can be expected in patients without bladder ulceration, but not in those with ulceration (who also had smaller bladder volumes). Improvements in pain were similar in each group (Fritjofsson et al, 1987a). Further studies employing this type of stratification are needed to confirm these findings. With regard to symptoms, pain appears to respond best to pentosan polysulfate, whereas daytime urinary frequency is affected least (Parsons et al, 1983; Mulholland et al, 1990b; Parsons & Mulholland, 1987b). d) Early conflicting results regarding clinical improvement with pentosan polysulfate (or inconsistent improvement among various parameters) were undoubtedly related to lack of a uniform definition for the disease, and resultant inconsistent diagnostic criteria for patient inclusion. Varying criteria for the diagnosis of interstitial cystitis have been employed in available studies, based on pathologic and/or clinical findings (Hanno & Wein, 1987). In one study reporting no improvement of clinical symptoms (Holm-Bentzen et al, 1987b), the pathological anatomical criterion for interstitial cystitis was the presence of more than 28 mast cells/millimeter(2). However, a significant improvement in cystoscopic bladder appearance and bladder capacity during anesthesia was reported in pentosan polysulfate-treated patients with verified disease after 4 months of treatment. In other investigations, patients were considered to have interstitial cystitis based on a variety of criteria, including clinical symptoms (eg, pain, nocturia, frequency) with or without the presence of petechial hemorrhages or ulcers or gross blood in the fluid return on cystoscopic examination (Fritjofsson et al, 1987a; Mulholland et al, 1990b). Without a clear definition for patient selection, differing results of therapy can be expected. Other factors may also explain inconsistent responses, including the dose of the drug, the significant placebo effect observed in some studies, differing methods of response evaluation, and the spontaneously fluctuating nature of the disease. Up to 11% of patients may experience a spontaneous remission of up to 6 months (Hanno & Wein, 1987). e) In several studies, some degree of clinical benefit was maintained with continuous pentosan polysulfate therapy for up to 2 years (Parsons et al, 1983; Parsons & Mulholland, 1987b; Mulholland et al, 1990b). Lowering of the dose by less than 50% (to less than 100 or 150 milligrams daily) in responding patients was associated with recurrent symptoms in 1 study (Parsons et al, 1983), suggesting the need for continuous therapy to sustain improvement. However, another study reported stable improvement for 3 months after discontinuation of pentosan polysulfate (Fritjofsson et al, 1987a). Additional studies are necessary to determine if stabilization can be maintained after therapy is completed. Page 3 of 6
4 4) Adverse Reactions Oedema Surrey and Sussex Healthcare NHS Trust a) PERIPHERAL OEDEMA, occasionally requiring discontinuation of therapy, has been reported during oral pentosan polysulfate therapy (Fritjofsson et al, 1987; Holm-Bentzen et al, 1987a). This complication was observed in approximately 5% of interstitial cystitis patients in 1 study (Holm-Bentzen et al, 1987a). Dermatologic Effects a) SKIN RASHES have occurred in some patients treated with oral pentosan polysulfate (Parsons & Mulholland, 1987a; Holm-Bentzen et al, 1987a). b) ALOPECIA has been reported at an incidence of 4%; the vast majority of cases were limited to just a single area on the scalp. Loss of hair may be noticed as soon as 4 weeks after the initiation of therapy (Prod Info Elmiron(R), 1996). Gastrointestinal Effects a) Incidence: 4% b) Diarrhea or LOOSE STOOLS have been described during oral pentosan polysulfate therapy (Wedren, 1987a; Fritjofsson et al, 1987). In 1 series of 87 patients with interstitial cystitis, diarrhea occurred in 6 (7%) (Fritjofsson et al, 1987). Diarrhea has occasionally resulted in discontinuation of therapy (Wedren, 1987a). c) Gastrointestinal symptoms associated with oral pentosan polysulfate therapy include NAUSEA, INDIGESTION, and nonspecific GASTROINTESTINAL DISTRESS (Mulholland et al, 1990a; Holm-Bentzen et al, 1987a; Fritjofsson et al, 1987). The incidences of these side effects range from 2% to 4% (Prod Info Elmiron(R), 1996). d) Proctitis, described as RECTAL ULCERATION, was the dose-limiting toxicity in a dose-escalating study (n=21) of oral pentosan polysulfate in the treatment of cancer. Doses studied were 180, 270, and 400 milligrams/square meter (mg/m(2)) three times a day. Development of symptoms was doserelated, ie, symptoms developed more quickly in patients at higher doses than in those taking lower doses. However, clinical or endoscopic examination revealed the condition in all patients. Discontinuation of the drug was the only effective remedy and, in patients rechallenged with pentosan polysulfate, symptoms returned within a week. Marginal benefits were achieved with corticosteroid enemas but symptoms were not completely alleviated (Marshall et al, 1997). Haematologic Effects a) THROMBOCYTOPENIA (platelet count less than 100,000) and thrombosis have been reported in patients receiving pentosan polysulfate (Tardy-Poncet et al, 1994). The association between pentosan polysulfate and Page 4 of 6
5 thrombocytopenia has been supported by platelet aggregation and serotonin release tests. Pentosan polysulfate-induced thrombocytopenia has been reported at prophylactic and therapeutic doses and by intramuscular and subcutaneous routes of administration. Thrombocytopenia can occur between days 5 to 21 after pentosan polysulfate administration and platelet counts usually return to baseline within 4 days after discontinuation of the drug. However, the overall frequency of this adverse effect was less than 1% in clinical trials (Prod Info Elmiron(R), 1996). Heparin and low molecular weight heparin should not be used during the acute recovery phase if treating thrombosis, even if platelet aggregation studies do not cross-react, due to the low sensitivity of these tests. b) THROMBOSIS of the superior sagittal sinus, attributed to HEPARIN- INDUCED THROMBOCYTOPENIA associated with pentosan therapy, was reported in a 35-year-old woman treated for interstitial cystitis with pentosan 100 mg three times a day. Pentosan therapy was discontinued after 3 weeks by a urologist who was sceptical of the initial diagnosis. Two days after discontinuation of pentosan, the patient was treated for severe headache, nausea and vomiting and, after a progression of symptoms, was admitted to the hospital 10 days later when the thrombosis was diagnosed with the aid of magnetic resonance imaging. The patient was treated with aspirin, dipyridamole, and slow anticoagulation with warfarin and the symptoms abated. The authors suggest that platelet counts should be monitored at the initiation of pentosan therapy and after 7, 10, and 14 days of treatment and that discontinuation of pentosan be considered if a 30% decrease occurs (Rice et al, 1998). c) In clinical trials INCREASED PROTHROMBIN TIME, INCREASED PARTIAL THROMBOPLASTIN TIME, LEUKOPENIA, and ANEMIA have occurred at an incidence of less than 1%. It is unknown if the effects are directly attributable to pentostan polysulfate, or to other factors (Prod Info Elmiron(R), 1996). Liver effects a) Liver enzymes (aspartate aminotransferase and alanine aminotransferase) became elevated in 5 of 13 (4 grade II and 1 grade III) patients in a phase I clinical study on the use of continuous intravenous infusions of pentosan polysulfate sodium in the treatment of advanced metastatic malignancies (Lush et al, 1996). b) Elevations in liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase) have been reported in 1% to 4% of patients treated with oral pentosan polysulfate therapy (Prod Info Elmiron(R), 1996; Mulholland et al, 1990a; Fritjofsson et al, 1987). Neurologic Effects a) HEADACHE and DIZZINESS have been reported in 2% to 4% of interstitial cystitis patients treated with oral pentosan polysulfate (Mulholland et al, Page 5 of 6
6 1990a; Holm-Bentzen et al, 1987a). These symptoms have resulted in withdrawal of therapy in some patients (Holm-Bentzen et al, 1987a). b) Severe MOOD SWINGS and SUICIDAL IDEATION each occurred in 1 of 54 patients (2%) treated with oral pentosan polysulfate in 1 study; however, these reactions might not be related to drug therapy. (Mulholland et al, 1990a). Respiratory Effects a) Prolongation of respiratory symptoms of influenza or the common cold was described in 4 of 24 patients with nonbacterial prostatitis during therapy with oral pentosan polysulfate (Wedren, 1987a). Prepared by: Tracy Townsend Lead Medicines Information Pharmacist April 2010 Page 6 of 6
INTERSTITIAL CYSTITIS (IC) MANAGEMENT
 INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study
 OFHA eol0e PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study S. GRANT MULHOLLAND, M.D. PHILLIP HANNO, M.D. C. LOWELL PARSONS, M.D. GRANNUM
OFHA eol0e PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study S. GRANT MULHOLLAND, M.D. PHILLIP HANNO, M.D. C. LOWELL PARSONS, M.D. GRANNUM
DELAYED DIAGNOSIS. Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >60%
 DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
Interstitial Cystitis
 Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
ELMIRON -100 mg (pentosan polysulfate sodium) Capsules DESCRIPTION
 ELMIRN - mg (pentosan polysulfate sodium) Capsules DESCRIPTIN Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative which chemically and structurally
ELMIRN - mg (pentosan polysulfate sodium) Capsules DESCRIPTIN Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative which chemically and structurally
ELMIRON -100 MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION
 ELMIRON - MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative,
ELMIRON - MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative,
The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
In evaluating a patient with lower urinary tract symptoms (LUTS), urologists
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
Drafting a Coverage Authorization Request Letter
 Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS
 Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Cisplatin and Gemcitabine (bladder)
 Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Interstitial Cystitis. Dr. Gerard Testa
 Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
PRODUCT MONOGRAPH GEMCITABINE INJECTION
 PRODUCT MONOGRAPH Pr GEMCITABINE INJECTION Concentrate Sterile Solution for Injection Gemcitabine (as Gemcitabine Hydrochloride) 40 mg gemcitabine per ml 200 mg/5 ml, 1 g/25 ml, 2 g/50 ml Antineoplastic
PRODUCT MONOGRAPH Pr GEMCITABINE INJECTION Concentrate Sterile Solution for Injection Gemcitabine (as Gemcitabine Hydrochloride) 40 mg gemcitabine per ml 200 mg/5 ml, 1 g/25 ml, 2 g/50 ml Antineoplastic
Interstitial Cystitis
 Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
Edward L. Davis, Samar R. El Khoudary, Evelyn O. Talbott,* Josephine Davis and Lisa J. Regan
 Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial Edward L. Davis, Samar R. El Khoudary, Evelyn O.
Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial Edward L. Davis, Samar R. El Khoudary, Evelyn O.
Maxilief Effervescent Tablets
 Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
Expert Review: Updates in Immune Thrombocytopenia. Reference Slides
 Expert Review: Updates in Immune Thrombocytopenia Reference Slides Immune Thrombocytopenia (ITP): Overview ITP causality 1,2 Suboptimal platelet production Dysregulated adaptive immune system Increased
Expert Review: Updates in Immune Thrombocytopenia Reference Slides Immune Thrombocytopenia (ITP): Overview ITP causality 1,2 Suboptimal platelet production Dysregulated adaptive immune system Increased
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
MESALO Foam (Mesalazine)
 Published on: 10 Jul 2014 MESALO Foam (Mesalazine) Composition MESALO Foam Each actuation delivers: Mesalazine, BP.... 1.0 g White-grayish to slightly reddish-violet, creamy, firm foam. Dosage Form Rectal
Published on: 10 Jul 2014 MESALO Foam (Mesalazine) Composition MESALO Foam Each actuation delivers: Mesalazine, BP.... 1.0 g White-grayish to slightly reddish-violet, creamy, firm foam. Dosage Form Rectal
PRODUCT MONOGRAPH. Ready-to use solution. 38 mg/ml gemcitabine (as gemcitabine hydrochloride) 200 mg / 5.3 ml, 1 g / 26.3 ml, and 2 g / 52.
 PRODUCT MONOGRAPH Pr GEMCITABINE INJECTION Ready-to use solution 38 mg/ml gemcitabine (as gemcitabine hydrochloride) 200 mg / 5.3 ml, 1 g / 26.3 ml, and 2 g / 52.6 ml Sterile THERAPEUTIC CLASSIFICATION
PRODUCT MONOGRAPH Pr GEMCITABINE INJECTION Ready-to use solution 38 mg/ml gemcitabine (as gemcitabine hydrochloride) 200 mg / 5.3 ml, 1 g / 26.3 ml, and 2 g / 52.6 ml Sterile THERAPEUTIC CLASSIFICATION
History of Present Illness Please answer the following questions
 Last Name First Name Date of Birth: / / What is the main reason for your visit today? Social Security Number: History of Present Illness Please answer the following questions Bladder Cancer Urinary Tract
Last Name First Name Date of Birth: / / What is the main reason for your visit today? Social Security Number: History of Present Illness Please answer the following questions Bladder Cancer Urinary Tract
RECTUM/SIGMOID COLON/BOWEL,
 EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
A Review on Interstitial Cystitis Syndrome (ICS)
 31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Interstitial Cystitis:
 Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
0BCore Safety Profile. Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) NL/H/PSUR/0058/001 Date of FAR:
 0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2
 Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Summary of the Risk Management Plan (RMP) V. 1.6, July 2017 for BAVENCIO. Avelumab 200 mg/10 ml. Concentrate for Solution for Infusion
 Summary of the Risk Management Plan (RMP) V. 1.6, July 2017 for BAVENCIO Avelumab 200 mg/10 ml Concentrate for Solution for Infusion Marketing Authorisation Number 66380 Marketing Authorisation Holder:
Summary of the Risk Management Plan (RMP) V. 1.6, July 2017 for BAVENCIO Avelumab 200 mg/10 ml Concentrate for Solution for Infusion Marketing Authorisation Number 66380 Marketing Authorisation Holder:
Bladder pain syndrome / Interstitial cystitis
 Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
Name: Date: Referring Provider: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary).
 Name: Date: Referring Provider: Age: D.O.B. Race/ ethnicity: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary). We are interested in learning
Name: Date: Referring Provider: Age: D.O.B. Race/ ethnicity: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary). We are interested in learning
BESPONSA (inotuzumab ozogamicin)
 BESPONSA (inotuzumab ozogamicin) Fact Sheet BESPONSA (inotuzumab ozogamicin) is an antibody-drug conjugate (ADC) composed of a monoclonal antibody (mab) targeting CD22, a cell surface antigen expressed
BESPONSA (inotuzumab ozogamicin) Fact Sheet BESPONSA (inotuzumab ozogamicin) is an antibody-drug conjugate (ADC) composed of a monoclonal antibody (mab) targeting CD22, a cell surface antigen expressed
5-8 July, 2008, Palais des Congrès, Paris, France
 A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
Review of treatments for interstitial cystitis
 The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
BR is an established treatment regimen for CLL in the front-line and R/R settings
 Idelalisib plus bendamustine and rituximab (BR) is superior to BR alone in patients with relapsed/refractory CLL: Results of a phase III randomized double-blind placebo-controlled study Andrew D. Zelenetz,
Idelalisib plus bendamustine and rituximab (BR) is superior to BR alone in patients with relapsed/refractory CLL: Results of a phase III randomized double-blind placebo-controlled study Andrew D. Zelenetz,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
DERBY-BURTON LOCAL CANCER NETWORK FILENAME Peruse.DOC CONTROLLED DOC NO: CCPG R29
 Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
PRODUCT MONOGRAPH. Gemcitabine for Injection. 200 mg, 1 g and 2 g gemcitabine (as gemcitabine hydrochloride) per vial House Std. Antineoplastic Agent
 PRODUCT MONOGRAPH Pr Gemcitabine for Injection 200 mg, 1 g and 2 g gemcitabine (as gemcitabine hydrochloride) per vial House Std. Antineoplastic Agent Accord Healthcare Inc. 3100, Steels Avenue East, Markham,
PRODUCT MONOGRAPH Pr Gemcitabine for Injection 200 mg, 1 g and 2 g gemcitabine (as gemcitabine hydrochloride) per vial House Std. Antineoplastic Agent Accord Healthcare Inc. 3100, Steels Avenue East, Markham,
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities
 Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
Medical History Form
 Medical History Form Full Name Title: Mr/Mrs/Ms/Miss Address Date of Birth Date Telephone: Mobile: Email: How did you hear about the Garden of health? G.P s Name and Address Are you currently seeing your
Medical History Form Full Name Title: Mr/Mrs/Ms/Miss Address Date of Birth Date Telephone: Mobile: Email: How did you hear about the Garden of health? G.P s Name and Address Are you currently seeing your
Supplemental Appendix. 1. Protocol Definition of Sustained Virologic Response. A patient has a sustained virologic response if:
 Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Generalised anxiety disorder Generalised anxiety disorder (GAD) is an umbrella term that covers a wide range of anxiety disorders
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Generalised anxiety disorder Generalised anxiety disorder (GAD) is an umbrella term that covers a wide range of anxiety disorders
Interstitial Cystitis/ Bladder Pain Syndrome
 page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Micafungin, a new Echinocandin: Pediatric Development
 Micafungin, a new Echinocandin: Pediatric Development Andreas H. Groll, M.D. Infectious Disease Research Program Center for Bone Marrow Transplantation and Department of Pediatric Hematology/Oncology University
Micafungin, a new Echinocandin: Pediatric Development Andreas H. Groll, M.D. Infectious Disease Research Program Center for Bone Marrow Transplantation and Department of Pediatric Hematology/Oncology University
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
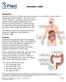 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Patient Health History Questionnaire
 Patient Health History Questionnaire Manitou Springs Acupuncture Randall Johnson, L.Ac., LLC Certified Seitai Shinpo Acupuncturist License Number: Acu-0002072 Phone: (719) 237-4547 Email: 719acupuncture@gmail.com
Patient Health History Questionnaire Manitou Springs Acupuncture Randall Johnson, L.Ac., LLC Certified Seitai Shinpo Acupuncturist License Number: Acu-0002072 Phone: (719) 237-4547 Email: 719acupuncture@gmail.com
Cetuximab in Combination with Irinotecan based Chemotherapy for the 1 st, 2 nd and 3 rd treatment Metastatic of Colorectal Cancer
 Cetuximab in Combination with Irinotecan based Chemotherapy for the 1 st, 2 nd and 3 rd treatment Metastatic of Colorectal Cancer DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent and rate
Cetuximab in Combination with Irinotecan based Chemotherapy for the 1 st, 2 nd and 3 rd treatment Metastatic of Colorectal Cancer DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent and rate
Medicines to treat pain in adults. Information for patients and carers
 Medicines to treat pain in adults Information for patients and carers It is common to feel some pain after having an operation (surgery), trauma or an infection. Controlling pain is an important part of
Medicines to treat pain in adults Information for patients and carers It is common to feel some pain after having an operation (surgery), trauma or an infection. Controlling pain is an important part of
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary
 Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
sodium oxybate, 500mg/ml oral solution (Xyrem) No. (246/06) UCB Pharma Ltd
 Scottish Medicines Consortium Resubmission sodium oxybate, 500mg/ml oral solution (Xyrem) No. (246/06) UCB Pharma Ltd 10 August 2007 The Scottish Medicines Consortium has completed its assessment of the
Scottish Medicines Consortium Resubmission sodium oxybate, 500mg/ml oral solution (Xyrem) No. (246/06) UCB Pharma Ltd 10 August 2007 The Scottish Medicines Consortium has completed its assessment of the
Study No Title : Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 16 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 REMOVAB 10 microgram concentrate for infusion solution Carton containing 1 pre-filled syringe (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 REMOVAB 10 microgram concentrate for infusion solution Carton containing 1 pre-filled syringe (CIP:
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Tavalisse (fostamatinib disodium hexahydrate) NEW PRODUCT SLIDESHOW
 Tavalisse (fostamatinib disodium hexahydrate) NEW PRODUCT SLIDESHOW Introduction Brand name: Tavalisse Generic name: Fostamatinib disodium hexahydrate Pharmacological class: Tyrosine kinase inhibitor Strength
Tavalisse (fostamatinib disodium hexahydrate) NEW PRODUCT SLIDESHOW Introduction Brand name: Tavalisse Generic name: Fostamatinib disodium hexahydrate Pharmacological class: Tyrosine kinase inhibitor Strength
PEMBROLIZUMAB (KEYTRUDA ) for the treatment of advanced melanoma or previously treated NSCLC
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate Day 1 Pembrolizumab 2mg/kg IV Infusion 100mL 0.9% Sodium Chloride* Or 100mL 5% Glucose* *Final concentration must be between 1 to 10mg/mL Over
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate Day 1 Pembrolizumab 2mg/kg IV Infusion 100mL 0.9% Sodium Chloride* Or 100mL 5% Glucose* *Final concentration must be between 1 to 10mg/mL Over
Painful Bladder Syndrome
 Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Analysing Apixaban: Potential Growth Driver for Pfizer and Bristol Myers Squibb. Tro Kalayjian Chief Medical Analyst Chimera Research Group
 Analysing Apixaban: Potential Growth Driver for Pfizer and Bristol Myers Squibb Tro Kalayjian Chief Medical Analyst Chimera Research Group Prevalence of AFib in the US is expected to increase upwards of
Analysing Apixaban: Potential Growth Driver for Pfizer and Bristol Myers Squibb Tro Kalayjian Chief Medical Analyst Chimera Research Group Prevalence of AFib in the US is expected to increase upwards of
Symptom Review (page 1) Name Date
 v2.4, 2/13 JonathanTreasure.com Botanical Medicine & Cancer Herb Drug Interactions Herbalism 3.0 Symptom Review (page 1) Name Date INSTRUCTIONS Please read each section below carefully and, after each
v2.4, 2/13 JonathanTreasure.com Botanical Medicine & Cancer Herb Drug Interactions Herbalism 3.0 Symptom Review (page 1) Name Date INSTRUCTIONS Please read each section below carefully and, after each
Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy
 Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
NEOFEN 60 mg suppository
 PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW
 Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW Introduction Brand name: Verzenio Generic name: Abemaciclib Pharmacological class: Kinase inhibitor Strength and Formulation: 50mg, 100mg, 150mg, 200mg; tabs
Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW Introduction Brand name: Verzenio Generic name: Abemaciclib Pharmacological class: Kinase inhibitor Strength and Formulation: 50mg, 100mg, 150mg, 200mg; tabs
ATEZOLIZUMAB (TECENTRIQ )
 DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent Rate Day 1 Atezolizumab 1200 mg IV Infusion 250mL 0.9% Sodium Chloride Over 60 minutes* *The initial dose of atezolizumab must be administered
DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent Rate Day 1 Atezolizumab 1200 mg IV Infusion 250mL 0.9% Sodium Chloride Over 60 minutes* *The initial dose of atezolizumab must be administered
(levomilnacipran) extended-release capsules
 MEDICATION GUIDE FETZIMA (fet-zee-muh) (levomilnacipran) extended-release capsules Read this Medication Guide before you start taking FETZIMA and each time you get a refill. There may be new information.
MEDICATION GUIDE FETZIMA (fet-zee-muh) (levomilnacipran) extended-release capsules Read this Medication Guide before you start taking FETZIMA and each time you get a refill. There may be new information.
2. Have your symptoms affected your ability to carry out your daily activities? YES NO
 QUESTIONNAIRE Page 1 of 5 Date: Referring MD (Name, Address, Phone Number): Primary Care Physician (Name and Address, Phone Number): Reason for visit: 1. How long have you had symptoms? Describe your symptoms?
QUESTIONNAIRE Page 1 of 5 Date: Referring MD (Name, Address, Phone Number): Primary Care Physician (Name and Address, Phone Number): Reason for visit: 1. How long have you had symptoms? Describe your symptoms?
s r e t n I sititsyc laititsret ystitis Interstitial Cystitis Interstitial Cystitis In itial Ctsretn IC I sititsyc laititsretni PRODUCT MONOGRAPH
 Interstitial Cystitis Interstitial Cystitis Interstitial Cystitis Intersti t i a l Cystitis Interstitial Cystitis Inters IC CONTENTS Interstitial Cystitis: An Overview Introduction... Epidemiology of
Interstitial Cystitis Interstitial Cystitis Interstitial Cystitis Intersti t i a l Cystitis Interstitial Cystitis Inters IC CONTENTS Interstitial Cystitis: An Overview Introduction... Epidemiology of
Summary of the risk management plan (RMP) for Duloxetine Lilly (duloxetine)
 EMA/674705/2014 Summary of the risk management plan (RMP) for Duloxetine Lilly (duloxetine) This is a summary of the risk management plan (RMP) for Duloxetine Lilly which details the measures to be taken
EMA/674705/2014 Summary of the risk management plan (RMP) for Duloxetine Lilly (duloxetine) This is a summary of the risk management plan (RMP) for Duloxetine Lilly which details the measures to be taken
Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery
 Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery Endpoint Fondaparinux Sodium 2.5 mg SC 1 Enoxaparin Sodium 30 mg SC every 12 hours
Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery Endpoint Fondaparinux Sodium 2.5 mg SC 1 Enoxaparin Sodium 30 mg SC every 12 hours
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION BAVENCIO (buh-ven-see-oh) Avelumab for Injection Solution for Intravenous Infusion Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION BAVENCIO (buh-ven-see-oh) Avelumab for Injection Solution for Intravenous Infusion Read this carefully before you start
NEW PATIENT APPOINTMENT UROLOGY
 Urology 2119 East South Blvd. 2 nd Floor Phone: 334-613-7070 NEW PATIENT APPOINTMENT UROLOGY, you have an appointment with Dr. Geoffrey Habermacher Dr. Joseph Pazona Larry Thomas CRNP Sherry Henning CRNP
Urology 2119 East South Blvd. 2 nd Floor Phone: 334-613-7070 NEW PATIENT APPOINTMENT UROLOGY, you have an appointment with Dr. Geoffrey Habermacher Dr. Joseph Pazona Larry Thomas CRNP Sherry Henning CRNP
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities
 Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Bevacizumab + Paclitaxel & Carboplatin
 Bevacizumab + Paclitaxel & Carboplatin Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
Bevacizumab + Paclitaxel & Carboplatin Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
Oxaliplatin and Gemcitabine
 Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
2.5 Other Hematology Consult:
 The Warfarin Order Sheet has been approved by the P & T committee to be implemented by pharmacists. These orders are not used to treat patients with serious hemorrhagic complications. WARFARIN TARGET INR
The Warfarin Order Sheet has been approved by the P & T committee to be implemented by pharmacists. These orders are not used to treat patients with serious hemorrhagic complications. WARFARIN TARGET INR
Novel oral anticoagulant therapy (NOAC)
 Haematology Department Novel oral anticoagulant therapy (NOAC) Information for patients, relatives and carers What is novel oral anticoagulant therapy? Novel oral anticoagulants, or NOACs, are drugs which
Haematology Department Novel oral anticoagulant therapy (NOAC) Information for patients, relatives and carers What is novel oral anticoagulant therapy? Novel oral anticoagulants, or NOACs, are drugs which
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
MEDICATION GUIDE. BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use
 MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
Laser Vein Center Thomas Wright MD Page 1 of 4
 Demographics Laser Vein Center Thomas Wright MD Page 1 of 4 Patient Name: Address: City, St, Zip Primary Phone: Alternate: DOB: Social Security #: Insurance Information Primary Insurance ID# Group# Subscriber
Demographics Laser Vein Center Thomas Wright MD Page 1 of 4 Patient Name: Address: City, St, Zip Primary Phone: Alternate: DOB: Social Security #: Insurance Information Primary Insurance ID# Group# Subscriber
PRODUCT INFORMATION DIPENTUM
 PRODUCT INFORMATION DIPENTUM NAME OF THE MEDICINE:- Olsalazine sodium. DESCRIPTION:- Composition Active ingredient: Excipients: olsalazine sodium 250 mg capsule - magnesium stearate, gelatin capsule shells;
PRODUCT INFORMATION DIPENTUM NAME OF THE MEDICINE:- Olsalazine sodium. DESCRIPTION:- Composition Active ingredient: Excipients: olsalazine sodium 250 mg capsule - magnesium stearate, gelatin capsule shells;
Docetaxel + Nintedanib
 Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
CABAZITAXEL Prostate Cancer
 Systemic Anti-Cancer Treatment Protocol CABAZITAXEL Prostate Cancer PROCTOCOL REF: MPHACABAZ (Version No: 1.0) Approved for use in: Cabazitaxel in combination with prednisolone is a treatment option for
Systemic Anti-Cancer Treatment Protocol CABAZITAXEL Prostate Cancer PROCTOCOL REF: MPHACABAZ (Version No: 1.0) Approved for use in: Cabazitaxel in combination with prednisolone is a treatment option for
IBRUTINIB + Rituximab, Treatment Period - ENRICH Study
 IBRUTINIB + Rituximab, Treatment Period - Study Randomised, open label study of Rituximab/Ibrutinib vs Rituximab/Chemotherapy in older patients with untreated mantle cell lymphoma ***See protocol for further
IBRUTINIB + Rituximab, Treatment Period - Study Randomised, open label study of Rituximab/Ibrutinib vs Rituximab/Chemotherapy in older patients with untreated mantle cell lymphoma ***See protocol for further
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Discussing TECENTRIQ (atezolizumab) with your healthcare team Talking to Your Doctor
 Discussing TECENTRIQ (atezolizumab) with your healthcare team Talking to Your Doctor TECENTRIQ DISCUSSION SUPPORT What is TECENTRIQ? TECENTRIQ is a prescription medicine used to treat: A type of bladder
Discussing TECENTRIQ (atezolizumab) with your healthcare team Talking to Your Doctor TECENTRIQ DISCUSSION SUPPORT What is TECENTRIQ? TECENTRIQ is a prescription medicine used to treat: A type of bladder
About Blood Clots and How to Treat Them
 PATIENT & CAREGIVER EDUCATION About Blood Clots and How to Treat Them This information describes what a blood clot is and how it is treated. About Blood Clots When you have a cut or an injury, your blood
PATIENT & CAREGIVER EDUCATION About Blood Clots and How to Treat Them This information describes what a blood clot is and how it is treated. About Blood Clots When you have a cut or an injury, your blood
