Edward L. Davis, Samar R. El Khoudary, Evelyn O. Talbott,* Josephine Davis and Lisa J. Regan
|
|
|
- Leona Grant
- 5 years ago
- Views:
Transcription
1 Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial Edward L. Davis, Samar R. El Khoudary, Evelyn O. Talbott,* Josephine Davis and Lisa J. Regan From the Citrus Valley Medical Research, Inc., Glendora, California (ELD, JD, LJR), and the Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, Pennsylvania (SRE, EOT) Purpose: We examined the safety and the efficacy of a combination of intravesical and oral pentosan polysulfate sodium in comparison to only oral pentosan polysulfate sodium in treating interstitial cystitis. Materials and Methods: A total of 41 females diagnosed with interstitial cystitis were randomized to receive a combination of intravesical pentosan polysulfate sodium plus oral pentosan polysulfate sodium (21 in treatment group) or intravesical placebo plus oral pentosan polysulfate sodium (20 in placebo group) for 6 weeks. All subjects continued to receive oral pentosan polysulfate sodium for another 12 weeks. The primary outcome was the change in the O Leary-Sant Interstitial Cystitis Symptoms/Problem Index from baseline to week 6, 12, and 18. Other outcomes included: the changes in Pelvic Pain and Urgency Frequency questionnaire, Health Related Quality of Life index: SF-36, pain scale, urgency scale, voiding log, patient global assessment, and sexual function scales. Results: The change in the total score of O Leary-Sant Interstitial Cystitis Symptoms/Problems Index from baseline to week 12 among the treatment group (median 12 or approximately a 46% reduction) was significantly greater compared to the placebo group (median 5.5 or approximately a 24% reduction, p 0.04). At week 18 the treatment group showed statistically significant improvement in all Health Related Quality of Life domains compared to the baseline (p 0.01), while the placebo group showed significant improvement in only 3 Health Related Quality of Life domains, (p 0.05) compared to the baseline. There were no significant differences within major categories of adverse events between treated and placebo groups. Conclusions: The use of intravesical pentosan polysulfate sodium simultaneously with oral pentosan polysulfate sodium is a safe and effective therapeutic option. These findings will open a new option for patients with interstitial cystitis to reduce their severely devastating symptoms and to improve their quality of life and well-being. Key Words: cystitis, interstitial; administration, intravesical; pentosan sulfuric polyester; clinical trials Interstitial cystitis is a chronic, severely debilitating disease of the urinary bladder characterized by urinary frequency, urgency, nocturia, and pelvic pain in the absence of other obvious bladder pathology. 1 IC has a profound impact on patient quality of life that extends beyond its severe symptoms to include physical, social, as well as emotional functions and well-being. 2 Despite the long-standing recognition of IC, there are still no clear answers to many vital issues (eg etiology, prevalence, diagnostic definition and therapy). 1 The actual prevalence rate is unknown, and estimates range widely from 67 per 100,000 3 to 575 per 100,000 4 based on the diagnostic criteria and methods used in estimating the rate. The majority of IC cases are females, with a median age at diagnosis of 42 to 46 years old. 3 Submitted for publication May 21, Study received institutional review board approval. Supported by Ortho-McNeil Janssen Scientific Affairs, LLC, Raritan, New Jersey. Nothing to disclose. * Correspondence: Department of Epidemiology, A544 Parran Hall, University of Pittsburgh, Pittsburgh, Pennsylvania (telephone: ; FAX: ; eot1@pitt.edu). For another article on a related topic see page 359. At present there is no single etiology of IC, therefore treatment is prescribed on the basis of patient symptoms. Pentosan polysulfate sodium is the only oral therapy approved for IC by the FDA. The mechanism of action of PPS is not completely understood but a widely accepted theory is that it replaces damaged segments of the GAG layer, the mucus of the bladder lining. 5 The GAG layer protects the bladder from the caustic effects of urine and bacteria. PPS safety and efficacy as an oral treatment for IC have been documented in several randomized double-blind clinical trials. 6,7 A main disadvantage of PPS as an oral therapy is the low concentration of active drug reaching the bladder (1% to 3%) resulting in a long lag time (approximately 3 to 6 months) before clinical improvement can be observed. 7,8 It would appear that intravesical therapy (the direct instillation of treatment into the bladder using catheter through urethra) compared to oral therapy alone might pro- Editor s Note: This article is the fourth of 5 published in this issue for which category 1 CME credits can be earned. Instructions for obtaining credits are given with the questions on pages 388 and /08/ /0 177 Vol. 179, , January 2008 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2008 by AMERICAN UROLOGICAL ASSOCIATION DOI: /j.juro
2 178 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS vide the benefit of establishing a higher concentration of therapy directly into the bladder with a minimum risk of systemic side effects. 9 Therefore, PPS instilled directly into the bladder might accelerate a more rapid resolution of IC symptoms. Dimethylsulfoxide, an anti-inflammatory analgesic agent with muscle relaxing properties, is the only FDA approved intravesical therapy in the United States. It is safe and can provide moderate symptomatic relief. 10 Its unpleasant metabolic bio-product (eg garlic odor) makes it undesirable as a treatment. The use of intravesical PPS was shown to be an effective and safe option for the treatment of IC in a small clinical trial in the Netherlands. 11 In the United States the only data on intravesical PPS were provided through a limited retrospective study of 17 patients with IC. 12 In this study intravesical PPS was administered to the subjects (average of 13 instillation therapies) during 1 year. The results suggested that intravesical PPS provides a safe and generally effective treatment option for IC while oral PPS therapy takes effect. Although IC has an incredibly negative impact on patient quality of life, few clinical trials that assessed the efficacy of oral PPS considered health related quality of life as one of its main outcomes. This study is the first randomized double-blind clinical trial to assess the safety and efficacy of a combined therapy of intravesical and oral PPS compared to intravesical placebo and oral PPS over 18 weeks. Changes in IC symptoms, HRQL and sexual function will be examined. MATERIALS AND METHODS Study Design and Setting This was an 18-week randomized double-blind placebo controlled clinical trial and was conducted in Glendora, California between April 2004 and August Restricted randomization with a size of 4 per block was used to allocate study subjects into 2 groups. The blinding process was monitored, assessed and recorded by an independent pharmacist, who was responsible for preparing the trial intravesical treatment and placebo for subjects according to an FDA approved method. A total of 40 subjects were initially allocated into 2 balanced groups: treatment (received intravesical PPS and oral PPS) or placebo (received intravesical placebo [normal saline] and oral PPS). Figure 1 shows a flow FIG. 1. Flow diagram of subject progress through phases of trial
3 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS 179 diagram of subject progress through the phases of the trial. Oral PPS capsules were administered either 1 hour before meals or 2 hours after meals. 8 The study design was approved by the institutional review board of Foothill Presbyterian Hospital, Glendora, California, the institutional review board of the University of Pittsburgh, Pittsburgh, Pennsylvania, the FDA and Ortho-McNeil Pharmaceutical, Inc. Study Subjects, Exclusion and Inclusion Criteria Females who were older than 18 years, diagnosed with IC within 1 year of the beginning of the study, and previously untreated with either intravesical or oral PPS were recruited from patients with IC of Citrus Valley Medical Research, Inc. Glendora, California. To be included in the study all subjects had to have the following examination requirements: cystoscopic examination under anesthesia with hydrodistention and photo documentation showing petechial hemorrhage or ulcers, negative urine culture, a score of at least 4 on a 9-point pain scale, 5 on the O Leary- Sant IC symptom index, and 4 on IC problem index. Subjects were excluded from the study for bladder capacity greater than 350 ml on an awake cystometrogram, absence of intense urge with bladder filled to 150 ml water on cystometrogram, biphasic involuntary bladder contractions, absence of nocturia, voiding frequency less than 8 times per day (voiding diaries), remission of symptoms by antimicrobials, urinary antiseptics, anticholinergics, or antispasmodics, bacterial cystitis within 3 months, recurrent bladder, genital herpes within 3 months, cervical, vaginal or urethral cancer, chemical, tubercular or radiation cystitis, benign or malignant bladder tumor, vaginitis, vesicle ureteral reflux or urethral diverticula, neurogenic bladder dysfunction, prior urinary diversion, receiving any intravesical treatment at time of enrollment, pregnant or lactating mother. A total of 33 subjects had a cystometrogram (median bladder capacity 131 ml). Subjects lacking this criterion (because it was painful) were entered into the study but they must have met all the other standard criteria for IC. Procedure for Intravesical Instillation of PPS and Placebo All instillations were performed in the clinic by either the principal investigator (ED) or a well trained nurse. An 8Fr LoFric catheter (Astra Tech Inc, Torrance, California) was used in all intravesical instillations (PPS and placebo). Before placing the catheter into the urethra, a preparatory prophylaxis solution (lidocaine hydrochloride jelly, United States Pharmacopeia 2% 100 mg [20 mg/ml]) was applied to the catheter tip first and then to the perineum to prevent urethral spasms and perineal sensitivity. Then the bladder was drained from any post void residual and the intravesical instillation process was performed in 2 consecutive steps as described previously. 12 In brief, a solution of 8 ml 1% lidocaine and 3 ml 8.4% sodium bicarbonate was first instilled into the bladder to eliminate urethral discomfort so that the subject could more easily retain the instilled solution. After 5 minutes the intravesical solution of either PPS (200 mg or 2 capsules mixed with 30 ml sterile normal buffered saline) or placebo (30 ml sterile normal buffered saline) was then instilled, retained for a minimum of 30 minutes to a maximum of 60 minutes and voided (fig. 2). FIG. 2. Procedure for intravesical instillation process of treatment or placebo
4 180 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS Efficacy Outcomes The primary outcome was the change from baseline to week 6, 12 and 18 in the severity of IC symptoms measured by the O Leary-Sant IC symptom and problem index (total score 36). 13 The PUF questionnaire (total score 35), 14 pain assessment scale (range 1 to 9, 1 no pain, 9 severe pain), urgency scale (range 1 to 5, 1 no urgency, 5 severe urgency), and voiding diaries that measure the voiding frequency during waking hours and sleeping hours for a duration of 24 hours were completed at baseline, and weeks 6, 12 and 18 of the trial. The SF-36, 15 a widely used well validated instrument that measures HRQL and includes 8 different domains (score range 0 to 100 for each domain, 0 worse HRQL, 100 excellent HRQL) was completed at baseline, and weeks 4 and 18. The sexual function VAS which assesses sexual desire and sexual arousal through a 10 cm line with none at 1 end and high at the other end, was assessed at baseline, and weeks 4, 6, 12 and 18. The proportion of responders was determined using a different version of the patient global assessment questionnaire. This version was used in a previous clinical trial (sponsored by Bioniche Life Sciences Inc., Canada, 2003). The Canadian trial assessed the safety and efficacy of Cystistat (sodium hyaluronate) as a treatment for IC. 16 This version of patient global assessment evaluates the overall change in the IC condition since enrollment in the study. It measures the level of change with: worse, no change, and improved as the possible outcomes. It also measures the level of improvement with: moderate, greatly improved, and completely improved as the possible outcomes. The global assessment also allowed the patients to evaluate the changes in urgency and urinary frequency using the same outcomes as overall change in the IC condition. The subjects completed this instrument at week 18 of the study. Responders were defined as those who reported improved as a possible outcome. This definition, for responders, was chosen based on the design of this version of the patient global assessment, and it reflects all the corresponding outcomes of improvement (moderately, greatly and completely). Safety Measures Safety was ascertained through evaluating adverse events during the entire study period, hepatic panel function and blood clotting after each instillation and at week 12, and the development of UTI at baseline, at the first visit from week 1 through week 6 and at followup visits (weeks 12 and 18). The urine specimen must contain more than 10 6 bacteria of single organism to confirm the occurrence of clinically significant UTI (clinically significant positive urine culture). All laboratory tests were performed at a single location to insure quality control and to reduce bias. Possible Confounders To be sure that the efficacy results were only related to the trial intervention and not to other factors, demographics at baseline, data about concurrent treatments, medical history, and compliance of oral PPS during the entire period of the study were monitored and assessed. Compli- TABLE 1. Baseline demographics and clinical characteristics of the study population Median (25th, 75th percentiles) Treatment Placebo p Value* Age 36.9 (31.9, 45.1) 38.7 (26, 42.7) 0.5 % Race: 0.3 White Not white Presence of Hunner s ulcers (%) Uroflow vol (ml) 149 (67, 231) (91, 232) 0.9 Bladder capacity/cystometrogram (ml)* 131 (58, 270) 136 (90.3, 227.5) 0.7 O Leary-Sant total score (0 36) 26 (18.5, 32) 23 (19.3, 30) 0.4 O Leary-Sant ICSI score (0 20) 14 (10.5, 17) 12 (10, 16) 0.4 O Leary-Sant ICPI score (0 16) 13 (8.5, 15) 11.5 (10, 14) 0.2 PUF total score (1 35) 23 (18.3, 25.5) 21.5 (18.3, 26.5) 0.9 PUF symptom score (1 23) 14 (11.5, 17) 14 (12.3, 17) 0.9 PUF bother score (0 12) 8 (6, 9) 7.5 (6, 9.7) 0.8 Pain (1 9) 4 (4, 5) 4.7 (4, 5.8) 0.3 Ave voiding urgency score (1 5) 3.5 (2.6, 3.6) 3 (2.5, 3.2) 0.1 Urinary frequency 12 (9, 16) 14.5 (10, 18.8) 0.3 Nocturia 2 (1, 4) 2 (1, 4) 0.7 SF-36 (0 100) : Physical functioning 65 (45, 85) 65 (36.3, 89.7) 0.9 Role limitation/physical 0 (0, 50) 37.5 (0, 68.8) 0.3 Bodily pain 31 (16, 41) 32 (22, 48.5) 0.5 General health 42 (27.5, 67) 57.3 (40.5, 80.8) 0.1 Vitality 20 (10, 27.5) 32.5 (20, 57.5) 0.06 Social functioning 37.5 (25, 62.5) 56.3 (28.1, 96.9) 0.2 Role limitation/emotional 33.3 (0, 100) 66.7 (33.3, 100) 0.2 Mental health 56 (32, 68) 66 (56, 75) 0.07 Sexual assessment VAS (0 10 cm) : Sexual desire 1.7 (0.5, 4.5) 3.9 (2.2, 5.5) 0.08 Sexual arousal 2.4 (0.7, 4.9) 3 (1.2, 5.8) 0.5 Cystometrogram was performed in 33 subjects (15 treatment, 18 placebo). * chi-square or Fisher s exact tests for categorical variables, Mann-Whitney U test for continuous variables. Score of 1 no pain, score of 9 severe pain. Score of 1 no urgency, score of 5 severe urgency. Score of 0 worse health related quality of life, score of 100 excellent health related quality of life. Score of 0 none, score of 10 high; one missing in placebo group for sexual desire and sexual arousal (19).
5 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS 181 ance of oral PPS was calculated for each of the study weeks by dividing the total number of capsules that were administered during a week by 28 capsules (the total number of capsules that the subject should administer during each week). Statistical Analysis and Sample Size The projected sample size of 40 subjects was selected to detect a minimum difference of 2.3 in the mean O Leary- Sant IC symptom index at end point between the 2 groups (based on a 2-sample t test with standard deviation of 2.5, a significance level of 5%, and 80% power). The change of 2.3 in ICSI was chosen based on preliminary data which demonstrated using intravesical instillation of PPS can reduce the O Leary-Sant Interstitial Cystitis Symptom Index score from 14.3 to 11.1 during a treatment period of approximately 17 weeks. 17 The primary analysis was intent to treat with LOCF to the end of the trial. A nonparametric approach was used to assess study outcomes and the results of continuous variables were presented as median, 25th and 75th percentiles except for the hepatic panel and blood clotting values which were presented as mean SD. Baseline characteristics were compared between groups using Fisher s exact test or chisquare test for categorical variables, and Mann-Whitney U test for continuous variables. For continuous outcomes, differences from baseline to each end point within each group were assessed through Wilcoxon signed-rank test while differences between changes from baseline to each end point among groups were assessed via Mann-Whitney U test. Differences in proportions of responders between the 2 groups at week 18 were assessed through Fisher s exact test or chi-square test. All statistical tests were 2-sided and performed using a significance level of 5%. SPSS version 13.0 was used to conduct all statistical analyses. RESULTS Figure 1 illustrates the flow diagram of the progress of study subjects through the phases of the trial. Two subjects dropped out. Subject 1 (placebo group) did not start the study because of a conflict with her work schedule. Subject 2 (treatment group) completed 4 weeks of the study and dropped out for medical reasons that were unrelated to the clinical trial. Thus, there was no further followup. Per the study protocol both subjects were not evaluable, therefore 2 additional subjects were recruited. The first received the same intervention that was assigned to subject 1 while the second received the same intervention that was assigned to subject 2. In total, 42 subjects signed an informed consent, of whom 41 were included in the final analysis. Based on intent to treat analysis we included subject 2 who dropped out after 4 weeks of the trial in the treatment group (21). The LOCF method was used to impute all missing data for this subject. All subjects were females, and the majority was white (71%, 29 of 41) with a median age of 38 years (range 20 to 71). Hunner s ulcers were present in 15% of the study population (6 of 41) and glomerulations in 100%. Except for 1 subject all cases were classified as moderate IC cases (46%, 19 of 41) or severe IC cases (51%, 21 of 41) based on O Leary- TABLE 2. Changes in IC symptoms from baseline Median (25th, 75th percentiles) Treatment Median (25th, 75th percentiles) Placebo Outcomes Wk 6 Wk 12 Wk 18 Wk 6 Wk 12 Wk 18 O Leary-Sant total score (0 36) 7 ( 3, 14.5)* 12 ( 6.5, 16.5)*, 12 ( 7.5, 16.3)*, 4 ( 1.3, 12)* 5.5 ( 3, 9)* 8 ( 3.3, 13)* O Leary-Sant ICSI (0 20) 4 ( 1.5, 8)* 6 ( 3, 7.5)*, 6 ( 3.5, 9)* 2 (0, 4.8)* 3 ( 1.3, 4)* 4 ( 3, 7.5)* O Leary-Sant ICPI (0 16) 3 ( 1.5, 7)* 6 ( 3, 8.5)*, 6 ( 3.3, 8.5)*, 2 ( 1, 5)* 3 ( 1, 5)* 4 ( 0.3, 5)* PUF total score (1 35) 4 (0, 8.9)* 6 ( 3.4, 10.8)* 7.3 ( 4.3, 13.5)* 3.8 ( 1.3, 7.8)* 5.8 ( 3, 10)* 6 ( 3, 13)* PUF symptom index (1 23) 3 (0, 5.8)* 5 ( 2, 7)* 4.5 ( 2.5, 8.3)* 2 ( 1, 5.5)* 4 ( 2, 6.5)* 4.5 ( 1.3, 8)* PUF bother index (0 12) 1.5 (0, 2.9)* 2.5 ( 1, 3.8)* 3 ( 2, 4.8)* 1.7 ( 1, 3)* 1.8 ( 1, 3.8)* 2.5 ( 1, 5)* Pain scale (1 9) 1 (0, 2)* 2 ( 0.5, 3)* 2 ( 1, 3)* 1.5 ( 0.3, 3)* 1.5 (0, 3)* 2 (0, 3)* Urgency scale (1 5) 0.4 (0.1, 1)* 0.6 ( 0.1, 1)* 0.6 (0, 1.2)* 0.2 (0.5, 0.9) 0.2 (0.4, 1.1) 0.4 (0.2, 0.9) Voiding frequency 1 (3, 2.5) 2 (1.5, 3.5) 1 (0, 3) 2.5 ( 1, 4.8)* 2 ( 0.3, 4.8)* 3 ( 1, 7)* Nocturia 0 (0, 1) 0 (1.5, 1) 1 ( 0.5, 2)* 0.5 (0.8, 2) 0.5 (0, 2.8) 1 (0.8, 2)* * Wilcoxon sign rank test (changes from baseline to each end point within each group) p Mann-Whitney U test (changes from baseline to each end point between treatment groups) p Mann-Whitney U test (changes from baseline to each end point between treatment groups) 0.05 p 0.1. Wilcoxon sign rank test (changes from baseline to each end point within each group) 0.05 p 0.1.
6 182 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS Sant ICSI cut points of 0 to 6 mild, 7 to 13 moderate and 14 to 20 severe. 13 There were no significant differences between the study groups at baseline in terms of demographics, IC status, and efficacy primary and secondary outcomes (table 1). Confounders like menopausal status, smoking, concurrent medications, and compliance with oral PPS during the entire study period as well as during the first, second and third 6 weeks of the trial were comparable between the 2 groups (p 0.05). The treatment group showed greater reduction (greater reduction equates with improvement) in the total score of the O Leary-Sant and its components (ICSI and ICPI) from baseline to each end point (weeks 6, 12 and 18) compared to the placebo group. The changes in the total score of the O Leary-Sant instrument and the ICSI score were significantly more among the treatment group compared to the placebo group at week 12 (p 0.04 and 0.03, respectively). At week 18, 13 of 21 in the treatment group (approximately 62%) compared to 5 of 20 in the placebo group (25%) reported changes of 50% or more in their total O Leary-Sant score since the beginning of the study (p 0.02). Interestingly, changes in urgency scale from baseline to each end point were only significant among the treatment group while changes in voiding frequency (voiding diaries) from baseline to each end point were only significant among the placebo group. The placebo group reported significant reduction in voiding frequency at weeks 6 and 18 compared to the treatment group. There were no significant differences in the changes from baseline to each end point among the study groups in terms of PUF scores, pain scale, urgency scale and nocturia (table 2). At week 18, the proportion of responders (who reported that their overall IC condition improved in comparison to the baseline) was comparable in the 2 groups. Interestingly, the majority of the responders in the treatment group evaluated themselves as greatly improved (72.2%, 13 of 18), compared to 33.3% (6 of 18) of the responders in the placebo group (p 0.04). By the end of the trial, 100% (21 of 21) of the treatment group vs 80% (16 of 20) of the placebo group evaluated the change in their urinary frequency as well as in urgency as improved in comparison to the baseline (p 0.04) (table 3). Related to the improvement in HRQL as measured by the SF-36, both groups showed significant improvement in bodily pain at week 4 compared to the baseline (p 0.01). At week 18, the treatment group reported significant improvement in all HRQL domains in comparison to the baseline, while the placebo group showed significant improvement in only 3 HRQL domains compared to the baseline (p 0.05). The unique improvement from baseline to week 18 in general health domain was greater among the treatment group in comparison to the placebo group (p 0.05). Although only the treatment group showed significant improvement in terms of sexual desire (at weeks 6 and 12) and sexual arousal (at week 6) compared to the baseline, no significant differences were reported between the 2 groups at any end point (table 4). The incidence of adverse events was comparable between the study groups. Approximately 92 different adverse events were reported during the entire study period (range 2 to 15 adverse events per subject). Headache (approximately 66.7%, 14 of 21 in treatment group and 60%, 12 of 20 in the placebo group, p 0.05) and bruise in arms due to blood draw (52.4%, 11 of 21 in treatment group and 55%, 11 of 20 in placebo group, p 0.05) were the most frequently experienced adverse events. Mild hair loss was reported in 3 participants among the treatment group and 1 participant among the placebo group. There were no clinically significant differences between the study groups for any of the laboratory data, and there were no cases with laboratory measures critically outside the normal limits and related to the trial intervention. None of the laboratory measures in any of the 2 study groups required hospitalization or discontinuation of the treatments (table 5). No clinically significant positive urine culture (UTI) was reported among all the scheduled performed urine cultures during the entire study period in any of the trial groups. Among the unscheduled urine cultures (were performed as a standard of care) in the treatment group, there were 4 clinically significant urine cultures (among 2 subjects). Of the 4 positive cultures 2 (1 per subject) were reported during the intravesical treatment period. Thus, the intravesical treatment infection rate for 488 catheterizations (12 instillations 40 subjects plus 8 instillations for the drop out subject) was 0.41%. The other 2 positive cultures (1 per subject) were performed at least 20 days after catheterization. All significant positive urine cultures were treated with the appropriate antibiotics. TABLE 3. Level of improvement among responders at the end of the trial Pt Global Assessment 16 % (No./total No.) Treatment % (No./total No.) Placebo p Value* Improvement in overall condition 85.7 (18/21) 90 (18/20) 0.6 Level of improvement: Moderately improved 27.8 (5/18) 61.1 (11/18) 0.04 Greatly improved 72.2 (13/18) 33.3 (6/18) Completely improved (1/18) Improvement in urgency 100 (21/21) 80 (16/20) 0.04 Level of improvement: Moderately improved 38.1 (8/21) 56.3 (9/16) 0.2 Greatly improved 61.9 (13/21) 37.5 (6/16) Completely improved (1/16) Improvement in urinary frequency 100 (21/21) 80 (16/20) 0.04 Level of improvement: Moderately improved 38.1 (8/21) 62.5 (10/16) 0.2 Greatly improved 61.9 (13/21) 37.5 (6/16) Completely improved 0 0 * Chi-square test or Fisher s exact test.
7 TABLE 4. Changes in HRQL and sexual function from baseline Median (25th, 75th percentiles) Treatment Median (25th, 75th percentiles) Placebo Outcomes Wk 4 Wk 6 Wk 12 Wk 18 Wk 4 Wk 6 Wk 12 Wk 18 SF-36 (0 100): Not applicable* Not applicable* Not applicable* Not applicable* Physical functioning 0 ( 5, 15) 15 (2.5, 24.2)* 7.5 ( 5, 18.8) 5 (0, 25) Role/physical 0 (0, 25) 50 (0, 75)* 12.5 (0, 43.2)* 50 (0, 68.8)* Bodily pain 9 (0, 16)* 29 (15.5, 41.5)* 11 (0, 20)* 21 (10.3, 41.8)* General health 3 ( 3.5, 11) 8 (1.5, 20)*, 1 ( 5, 4.9) 0 ( 10, 9.1) Vitality 5 ( 2.5, 15)* 20 (10, 35)* 0 ( 8.8, 15) 12.5 ( 5, 28.8)* Social functioning 0 (0, 12.5) 25 (0, 43.8)* 0 (0, 12.5) 0 (0, 37.5) Role/emotional 0 (0, 33.3) 17 (0, 66.7)* 0 (0, 0) 0 ( 25, 58.3) Mental health 12 ( 10, 18) 12 (4, 18)*, 4 ( 4, 11) 2 (0, 15) Sexual VAS (0 10 cm): Sexual desire 0.3 ( 0.1, 1.8) 1 (0.2, 2.3)* 1.3 (0.1, 2.7)* 0.7 ( 0.1, 3.3) 0.4 ( 0.6, 1.4) 0.3 ( 0.8, 3) 1 ( 0.6, 2.6) 0.7 ( 1.5, 1.5) Sexual arousal 0.1 ( 0.3, 2.7) 0.8 (0.1, 3.3)* 1 ( 0.3, 3.5) 0.2 ( 0.8, 3.6) 0.5 ( 1.1, 3.2) 0.9 ( 0.5, 3.4) 1.7 ( 1.2, 4.1) 0.6 ( 1, 3.5) * Wilcoxon sign rank test (changes from baseline to each end point within each group) p Wilcoxon sign rank test (changes from baseline to each end point within each group) 0.05 p 0.1. Mann-Whitney U test (changes from baseline to each end point between treatment groups) 0.05 p 0.1. Mean SD TABLE 5. Liver function and blood clotting tests during the entire study period No. Tests Performed Treatment % Within Normal Limit % Out of Normal Range Not Clinically Significant Mean SD No. Tests Performed Placebo % Within Normal Limit % Out of Normal Range Not Clinically Significant Hepatic panel tests (normal ranges): Total bilirubin ( mg/dl) Alkaline phosphatase ( IU/l) Alanine transaminase (serum glutamic pyruvic transaminase) (1 55 IU/l) Aspartate transaminase (serum glutamic oxaloacetic transaminase) (1 45 IU/l) Blood clotting tests (normal ranges): Platelet count (150, ,000/ l) Prothrombin time ( secs) Partial thromboplastin time (22 34 secs) * Chi-square test. The result of any hepatic panel test and blood clotting test must be 1.5 to 2 times the normal range to be considered clinically significant. Two results of serum glutamic oxaloacetic transaminase test were 1.5 to 2 times the normal range (1 among treatment and 1 among placebo) and were considered out of normal range not clinically significant because they were related to viral infections (bronchitis and oral herpes, respectively) and not to trial medications. One result of the serum glutamic pyruvic transaminase test was 1.5 to 2 times the normal range (among placebo) and was considered out of normal range not clinically significant because other parameters of the same subject did not change simultaneously or at all. p Value* SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS 183
8 184 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS DISCUSSION The present study demonstrated that the combined therapy of intravesical and oral PPS medication is a safe and effective therapeutic option for the treatment of moderate and severe cases of interstitial cystitis. Specifically, this randomized clinical trial showed that IC subjects who received a regimen of intravesical and oral PPS had a 2-fold reduction in the severity of IC symptoms compared to those who received the oral regimen of PPS alone (p 0.04). Moreover, at the cessation of the trial, the combined therapy also showed significant improvement in all health related quality of life domains in the treatment group compared to their baseline self evaluation. Therefore, the management of the cascade of symptoms was reflected in the positive outcome responses to questionnaires. Most compelling were the life affirmations responses to the SF-36. The rationale for using PPS as a treatment option for IC is based on its biochemical nature as a semi-synthetic sulfated polysaccharide which chemically and structurally resembles GAG. PPS may augment the GAG layer of the bladder and protect damaged tissue, therefore, relieving symptoms. Because only 1% to 3% of oral PPS reaches the bladder, 8 the therapeutic efficacy of PPS should be greater when applied intravesically. Furthermore, using intravesical and oral PPS simultaneously for a specific period of time followed by sustained use of oral PPS may accelerate reduction in symptoms compared to oral therapy. This study supports the previous hypothesis. At the end of the trial subjects in the treatment group were significantly more likely to report great improvement in overall condition compared to those in the placebo group. Changes in O Leary-Sant as well as patient global assessment instruments were considered to be the most appropriate measure of treatment efficacy. The multifactorial complex nature of the IC condition and the wide variation of individual IC symptoms within and between patients suggest that the significant improvement in IC is the result of improvement in overall condition rather than in a specific symptom. Sharp focusing on each symptom may dilute the expected effect. This may explain why the present study reported significant differences in the change in IC condition when measured by O Leary-Sant ICSI and ICPI and patient global assessment, but not by pain assessment, urgency scale and/or nocturia between the 2 groups at any end point. It may surprise the reader that only the placebo group showed significant improvement in voiding frequency at each end point in comparison to the baseline. One suggested explanation is that voiding frequency can be a misleading symptom as it may be affected by other uncontrolled factors. Patients tend to restrict their fluid intake during painful periods and increase their fluid intake when pain is relieved. 7 Thus, it is expected that subjects who experienced greater improvement in their pain will increase their fluid intake and thus will have an increase in their voiding frequency in comparison to those who experienced less improvement. With regard to the choice of design for this trial, only 1 clinical trial in 1997, in the Netherlands, assessed the efficacy of intravesical PPS compared to a true placebo. 11 PPS (Elmiron ) became FDA approved in 1996 in the United States for oral therapy, 18 and has been widely considered standard of care for the treatment of IC. Therefore, a nontreated (true placebo) IC group was not appropriate. 19 It was not applicable to make an extensive comparison between the results from this study and the results from the Netherlands study because of the difference in the study design and the outcomes that were used in each study to assess the efficacy of the treatment. 11 In general, this study was consistent with that of Bade et al in that instilling intravesical PPS is an effective and safe treatment option for patients with IC. The trial results cannot be explained by other factors such as the use of concurrent medications as well as the compliance with oral PPS. Both groups showed comparable use of antihistamines, antispasmodics, antidepressants and analgesics during the entire study period (p 0.05). Furthermore, both groups administered more than 95% of the oral PPS during either the entire study period or the first, second or third 6 weeks of the trial. It is also unlikely that our results were biased as we adhered to the LOCF method to handle missing data. The same results were found when all the statistical analyses were performed with and without including the drop out subject in the treatment group or applying the LOCF method. The main limitations of the current study were the small sample size and the lack of external validity. We will not be able to generalize our finding on men or mild IC cases as all our study population were females who were classified, with 1 exception, as either moderate or severe cases. More studies will be required to test the same measures in a larger sample size that represent both genders and the full spectrum of severity. Other future questions and issues to be addressed are: What is the durability of this dual therapy of intravesical and oral PPS? What is the best dose range and frequency of therapeutic events? Should the medical intervention be based on the severity of symptoms rather than assuming that 1 intervention class works for all? Importantly, if intravesical PPS becomes an accepted therapy option, the production of a liquid product needs to be addressed as well. CONCLUSIONS This double-blind placebo controlled trial demonstrated the safety and the efficacy of the use of a combined therapy of intravesical and oral PPS for the treatment of moderate and severe IC cases. The use of intravesical PPS and oral PPS together appears to enhance the proliferation of the GAG layer of the bladder, to produce greater relief and return to normal protective coating when maintained with oral PPS. These findings will open a new option for patients with IC and provide another tool in the arsenal of the urologist in the treatment of this difficult and debilitating condition. ACKNOWLEDGMENTS John M. Cassara, Glendora, California, prepared and supervised trial intravesical medication, and Charles K. Metzger, also from Glendora, provided additional assistance.
9 SAFETY AND EFFICACY OF PENTOSAN POLYSULFATE SODIUM FOR INTERSTITIAL CYSTITIS 185 Abbreviations and Acronyms FDA Food and Drug Administration GAG glycosaminoglycan HRQL health related quality of life IC interstitial cystitis ICPI Interstitial Cystitis Problem Index ICSI Interstitial Cystitis Symptom Index LOCF Last Observation Carried Forward PPS pentosan polysulfate sodium PUF Pelvic Pain and Urgency/Frequency questionnaire SF-36 Short Form 36 UTI urinary tract infection VAS Visual Analog Scale REFERENCES 1. Nordling J: Interstitial cystitis: how should we diagnose it and treat it in 2004? Curr Opin Urol 2004; 14: Rothrock NE, Lutgendorf SK, Hoffman A and Kreder KJ: Depressive symptoms and quality of life in patients with interstitial cystitis. J Urol 2002; 167: Curhan GC, Speizer FE, Hunter DJ, Curhan SG and Stampfer MJ: Epidemiology of interstitial cystitis: a population based study. J Urol 1999; 161: Rosenberg MT and Hazzard M: Prevalence of interstitial cystitis symptoms in women: a population based study in the primary care office. J Urol 2005; 174: Anderson VR and Perry CM: Pentosan polysulfate: a review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs 2006; 66: Parsons CL and Mulholland SG: Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol 1987; 138: Mulholland SG, Hanno P, Parsons CL, Sant GR and Staskin DR: Pentosan polysulfate sodium for therapy of interstitial cystitis. Urology 1990; 35: Ortho-McNeil Pharmaceutical Inc.: Elmiron 100 mg (pentosan polysulfate sodium) capsules. Available at orthoelmiron.com. Accessed March 5, La Rock DR and Sant GR: Intravesical therapies for interstitial cystitis. In: Interstitial Cystitis. Edited by GR Sant. Philadelphia: Lippincott-Raven Sant GR: Intravesical 50% dimethyl sulfoxide (Rimso-50) in treatment of interstitial cystitis. Urology 1987; 29: Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT and Mensink HJ: A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol 1997; 79: Parsons CL and Davis EL: Pentosan polysulfate sodium intravesical instillation end-organ therapy. In: Practice Building Today, A Supplement to Physician s Money Digest 2003; O Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE and Spolarich-Kroll J: The interstitial cystitis symptom index and problem index. Urology 1997; 49: Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Waxell T et al: Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases using a new symptom questionnaire and intravesical potassium sensitivity. Urology 2002; 60: Ware JE, Snow KK, Kosinski M and Gandek B: SF-36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center Bioniche Life Sciences Inc.: Human Health News Release: Preliminary Results of North American Cystistat Trial Announced. Available at cfm?id Accessed June 21, Davis EL, Davis J, Regan L, Talbott EO and Zborowski JV: A prospective evaluation of intravesical instillation of pentosan polysulfate sodium (PPS; Elmiron [Ortho-McNeil]) in patients with interstitial cystitis. The association of clinical outcomes and symptom triad research insights into interstitial cystitis: a basic and clinical science symposium. Presented at meeting of National Institute of Diabetes and Digestive and Kidney Diseases and Interstitial Cystitis Association, Alexandria, Virginia, October 30 November 1, U.S. Food and Drug Administration: Drug Approvals for September Available at da996.htm. Accessed September 10, Declaration of Helsinki IV, World Medical Association, 41st World Medical Assembly, Hong Kong, September In: The Nazi Doctors and the Nuremberg Code: Human Rights in Human Experimentation. Edited by GJ Annas and MA Grodin. New York: Oxford University Press 1992.
DELAYED DIAGNOSIS. Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >60%
 DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
In evaluating a patient with lower urinary tract symptoms (LUTS), urologists
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
INTERSTITIAL CYSTITIS (IC) MANAGEMENT
 INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
Bladder pain syndrome / Interstitial cystitis
 Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective?
 Original Article In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Hikaru Tomoe Department of Urology and Pelvic Reconstructive Surgery, Tokyo
Original Article In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Hikaru Tomoe Department of Urology and Pelvic Reconstructive Surgery, Tokyo
How does interstitial cystitis begin?
 Original Article How does interstitial cystitis begin? C. Lowell Parsons Division of Urology, Department of Surgery, University of California, San Diego Medical Center, University of California, San Diego,
Original Article How does interstitial cystitis begin? C. Lowell Parsons Division of Urology, Department of Surgery, University of California, San Diego Medical Center, University of California, San Diego,
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Interstitial Cystitis
 Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium
 Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium Excerpts from: Drugdex Monograph on Pentosan Edition: online URL: www.thomsonhc.com/home/dispatch Date Added: 29/04/2010 17:42:55 Source Notes:Evidence
Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium Excerpts from: Drugdex Monograph on Pentosan Edition: online URL: www.thomsonhc.com/home/dispatch Date Added: 29/04/2010 17:42:55 Source Notes:Evidence
Urogynecology in EDS. Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018
 Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
Interstitial and chronic cystitis. Have you heard about the 2-component protection for the bladder wall?
 Interstitial and chronic cystitis Have you heard about the 2-component protection for the bladder wall? What is special about Instillamed? 2-component protection for the bladder wall Instillamed is an
Interstitial and chronic cystitis Have you heard about the 2-component protection for the bladder wall? What is special about Instillamed? 2-component protection for the bladder wall Instillamed is an
WHAT IS INTERSTITIAL CYSTITIS?
 Patient Information WHAT IS INTERSTITIAL CYSTITIS? Interstitial Cystitis (IC) is a condition resulting in recurring discomfort or pain in the bladder and the surrounding pelvic region. Its cause is unknown,
Patient Information WHAT IS INTERSTITIAL CYSTITIS? Interstitial Cystitis (IC) is a condition resulting in recurring discomfort or pain in the bladder and the surrounding pelvic region. Its cause is unknown,
INTERSTITIAL CYSTITIS (IC)
 INTERSTITIAL CYSTITIS (IC) Painful Bladder Syndrome (PBS): THE BASIC FACTS ANDREW L. SIEGEL, M.D. Board-Certified Urologist and Urological Surgeon Specialty: Male and Female Incontinence, Voiding Dysfunction,
INTERSTITIAL CYSTITIS (IC) Painful Bladder Syndrome (PBS): THE BASIC FACTS ANDREW L. SIEGEL, M.D. Board-Certified Urologist and Urological Surgeon Specialty: Male and Female Incontinence, Voiding Dysfunction,
32 OBG Management July 2010 Vol. 22 No. 7 obgmanagement.com
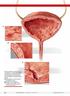 A B C Although interstitial cystitis (IC) occurs in the absence of urinary tract infection or malignancy, some pathology may become apparent during cystoscopy with hydrodistention. Potential findings include:
A B C Although interstitial cystitis (IC) occurs in the absence of urinary tract infection or malignancy, some pathology may become apparent during cystoscopy with hydrodistention. Potential findings include:
The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
Definitions of IC: U.S. perspective. Edward Stanford MD MS FACOG FACS Western Colorado
 Definitions of IC: U.S. perspective Edward Stanford MD MS FACOG FACS Western Colorado PURPOSE OF A DEFINITION? Identifies with specificity those patients who are most likely to have the disease. Identifies
Definitions of IC: U.S. perspective Edward Stanford MD MS FACOG FACS Western Colorado PURPOSE OF A DEFINITION? Identifies with specificity those patients who are most likely to have the disease. Identifies
Interstitial Cystitis (IC)/Painful Bladder Syndrome(PBS)
 Interstitial Cystitis (IC)/Painful Bladder Syndrome(PBS) Ene G. George M.D., FACOG, FPMRS Kaiser Permanente Baldwin Park Division of Urogynecology Department of Obstetrics and Gynecology PBS/IC I have
Interstitial Cystitis (IC)/Painful Bladder Syndrome(PBS) Ene G. George M.D., FACOG, FPMRS Kaiser Permanente Baldwin Park Division of Urogynecology Department of Obstetrics and Gynecology PBS/IC I have
CHRONIC PELVIC PAIN OF BLADDER ORIGIN:
 ACCREDITATION This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship
ACCREDITATION This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship
Interstitial Cystitis:
 Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study
 OFHA eol0e PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study S. GRANT MULHOLLAND, M.D. PHILLIP HANNO, M.D. C. LOWELL PARSONS, M.D. GRANNUM
OFHA eol0e PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study S. GRANT MULHOLLAND, M.D. PHILLIP HANNO, M.D. C. LOWELL PARSONS, M.D. GRANNUM
Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_
 207 Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_2542 207..212 C. Lowell Parsons, MD,* Paul Zupkas, PhD,* Jeffrey Proctor, MD,
207 Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_2542 207..212 C. Lowell Parsons, MD,* Paul Zupkas, PhD,* Jeffrey Proctor, MD,
BJU International EFFECTS OF INTRAVESICAL HYALURONIC ACID/CHONDROITINE SULFATE IN PATIENTS WITH INTERSTITIAL CYSTITIS/PAINFUL BLADDER SYNDROME
 EFFECTS OF INTRAVESICAL HYALURONIC ACID/CHONDROITINE SULFATE IN PATIENTS WITH INTERSTITIAL CYSTITIS/PAINFUL BLADDER SYNDROME Journal: Manuscript ID: Manuscript Type: Date Submitted by the Author: BJU-2007-1373
EFFECTS OF INTRAVESICAL HYALURONIC ACID/CHONDROITINE SULFATE IN PATIENTS WITH INTERSTITIAL CYSTITIS/PAINFUL BLADDER SYNDROME Journal: Manuscript ID: Manuscript Type: Date Submitted by the Author: BJU-2007-1373
Interstitial Cystitis. Dr. Gerard Testa
 Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
UV-2005/01. Chronic Prostatitis and Chronic Pelvic Pain Syndrom (CP/CPPS) Karl-Bickleder-Str. 44C Straubing - Germany
 SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
Interstitial Cystitis - Painful Bladder Syndrome
 Interstitial Cystitis - Painful Bladder Syndrome Interstitial cystitis (in-tur-stish-ul sis-tie-tis) also called painful bladder syndrome is a chronic condition in which you experience bladder pressure,
Interstitial Cystitis - Painful Bladder Syndrome Interstitial cystitis (in-tur-stish-ul sis-tie-tis) also called painful bladder syndrome is a chronic condition in which you experience bladder pressure,
Interstitial Cystitis/ Bladder Pain Syndrome
 page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
Painful Bladder Syndrome
 Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Interstitial Cystitis
 Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
Infection/Inflammation. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States
 Infection/Inflammation Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States Sra H. Berry,* Marc N. Elliott, Marika Suttorp, Laura M. Bogart, Michael
Infection/Inflammation Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States Sra H. Berry,* Marc N. Elliott, Marika Suttorp, Laura M. Bogart, Michael
UroToday International Journal. Volume 2 - October 2009
 UroToday International Journal Robert J. Evans, 1 Jeffrey Proctor, 2 Robert M. Moldwin 3 1 Alliance Urology Specialists, Greensboro, NC; 2 Georgia Urology, Cartersville, GA; 3 The Arthur Smith Institute
UroToday International Journal Robert J. Evans, 1 Jeffrey Proctor, 2 Robert M. Moldwin 3 1 Alliance Urology Specialists, Greensboro, NC; 2 Georgia Urology, Cartersville, GA; 3 The Arthur Smith Institute
Dr. Aso Urinary Symptoms
 Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
PRACTICAL APPLICATION INSTYLAN MODERN PROTECTOR OF URINARY BLADDER MUCOSA
 PRACTICAL APPLICATION INSTYLAN MODERN PROTECTOR OF URINARY BLADDER MUCOSA E.O. Stakhovsky, PhD, MD, Professor, Head of the Scientific and Research Department of Plastic and Reconstructive Oncourology,
PRACTICAL APPLICATION INSTYLAN MODERN PROTECTOR OF URINARY BLADDER MUCOSA E.O. Stakhovsky, PhD, MD, Professor, Head of the Scientific and Research Department of Plastic and Reconstructive Oncourology,
Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website:
 Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website: www.female-gynecologist.com What is Painful Bladder (PBS) or Interstitial Cystitis
Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website: www.female-gynecologist.com What is Painful Bladder (PBS) or Interstitial Cystitis
RIMSO-50 PRODUCT MONOGRAPH. (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis
 PRODUCT MONOGRAPH Pr RIMSO-50 (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis Mylan Pharmaceuticals ULC 85 Advance Road Etobicoke, ON M8Z 2S6 Date of
PRODUCT MONOGRAPH Pr RIMSO-50 (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis Mylan Pharmaceuticals ULC 85 Advance Road Etobicoke, ON M8Z 2S6 Date of
BPS/IC: evolution of definition and prevalence
 BPS/IC: evolution of definition and prevalence ame in history Tic doloureux of the bladder 1836 Interstitial cystitis 1878 Cystitis parenchymatosa 197 unner s ulcer 1915 Panmural ulcerative cystitis 192
BPS/IC: evolution of definition and prevalence ame in history Tic doloureux of the bladder 1836 Interstitial cystitis 1878 Cystitis parenchymatosa 197 unner s ulcer 1915 Panmural ulcerative cystitis 192
Mini-Reviews. Interstitial Cystitis in Adolescents and Children: A Review
 J Pediatr Adolesc Gynecol (2004) 17:7 11 Mini-Reviews Interstitial Cystitis in Adolescents and Children: A Review T.F. Mattox, MD, FACOG Department of Obstetrics and Gynecology, Division of Urogynecology,
J Pediatr Adolesc Gynecol (2004) 17:7 11 Mini-Reviews Interstitial Cystitis in Adolescents and Children: A Review T.F. Mattox, MD, FACOG Department of Obstetrics and Gynecology, Division of Urogynecology,
Multimodal Therapy for Painful Bladder Syndrome / Interstitial Cystitis: Pilot Study Combining Behavioral, Pharmacologic, and Endoscopic Therapies
 Neurourology Multimodal Therapy for Interstitial Cystitis: A Pilot Study International Braz J Urol Vol. 35 (4): 467-474, July - August, 2009 Multimodal Therapy for Painful Bladder Syndrome / Interstitial
Neurourology Multimodal Therapy for Interstitial Cystitis: A Pilot Study International Braz J Urol Vol. 35 (4): 467-474, July - August, 2009 Multimodal Therapy for Painful Bladder Syndrome / Interstitial
Botulinum Toxin: Applications in Urology
 Botulinum Toxin: Applications in Urology Dr. Lee Jonat, PGY-4 Department of Urologic Sciences University of British Columbia Outline Mechanism of Action Technical Considerations Adverse Events Neurogenic
Botulinum Toxin: Applications in Urology Dr. Lee Jonat, PGY-4 Department of Urologic Sciences University of British Columbia Outline Mechanism of Action Technical Considerations Adverse Events Neurogenic
Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis
 Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis R. J. Evans,*, R. M. Moldwin, N. Cossons, A. Darekar, I. W. Mills and D. Scholfield From the Department
Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis R. J. Evans,*, R. M. Moldwin, N. Cossons, A. Darekar, I. W. Mills and D. Scholfield From the Department
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
A Review on Interstitial Cystitis Syndrome (ICS)
 31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
Review of treatments for interstitial cystitis
 The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy
 Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Intravesical treatment of painful bladder syndrome: a systematic review and meta-analysis
 Int Urogynecol J (2012) 23:1147 1153 DOI 10.1007/s00192-012-1686-2 REVIEW ARTICLE Intravesical treatment of painful bladder syndrome: a systematic review and meta-analysis P. K. Matsuoka & J. M. Haddad
Int Urogynecol J (2012) 23:1147 1153 DOI 10.1007/s00192-012-1686-2 REVIEW ARTICLE Intravesical treatment of painful bladder syndrome: a systematic review and meta-analysis P. K. Matsuoka & J. M. Haddad
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
EMR template and postprocedure notes
 EMR template and postprocedure notes Sample autopopulated text to create a BOTOX injection procedure report Information gathered from market research. These considerations are for reference only and should
EMR template and postprocedure notes Sample autopopulated text to create a BOTOX injection procedure report Information gathered from market research. These considerations are for reference only and should
BLADDER HEALTH. Painful Bladder AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION
 BLADDER HEALTH Painful Bladder Interstitial Cystitis AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION Don t Let Interstitial Cystitis Keep You from Enjoying Life. many people have
BLADDER HEALTH Painful Bladder Interstitial Cystitis AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION Don t Let Interstitial Cystitis Keep You from Enjoying Life. many people have
Painful Bladder Syndrome/Interstitial Cystitis
 n The Leeds Teaching Hospitals NHS Trust Painful Bladder Syndrome/Interstitial Cystitis Information for patients Leeds Centre for Women s Health Welcome to the Department of Urogynaecology Your consultant
n The Leeds Teaching Hospitals NHS Trust Painful Bladder Syndrome/Interstitial Cystitis Information for patients Leeds Centre for Women s Health Welcome to the Department of Urogynaecology Your consultant
Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment of bladder pain syndrome/interstitial cystitis
 Received: 17 December 2016 Accepted: 26 February 2017 DOI: 10.1002/nau.23284 ORIGINAL CLINICAL ARTICLE Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment
Received: 17 December 2016 Accepted: 26 February 2017 DOI: 10.1002/nau.23284 ORIGINAL CLINICAL ARTICLE Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment
Interstitial cystitis (IC), also
 Interstitial cystitis: Algorithm to simplify diagnosis of chronic urinary symptoms By Anita L. Booth, DNP, FNP-BC and Jill Harpst Rodgers, DNP, FNP-BC This pre/post-test study was undertaken to determine
Interstitial cystitis: Algorithm to simplify diagnosis of chronic urinary symptoms By Anita L. Booth, DNP, FNP-BC and Jill Harpst Rodgers, DNP, FNP-BC This pre/post-test study was undertaken to determine
Targeted Therapeutics for Inflammatory Disease Jefferies Healthcare Conference
 Targeted Therapeutics for Inflammatory Disease 2016 Jefferies Healthcare Conference Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking
Targeted Therapeutics for Inflammatory Disease 2016 Jefferies Healthcare Conference Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking
D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS.
 D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS. Maria Laura Lopes De Carvalho, MD Guido Francavilla, MD Roberta
D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS. Maria Laura Lopes De Carvalho, MD Guido Francavilla, MD Roberta
flow resulting from damage to blood vessels can also contribute to sexual dysfunction.
 U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH Troublesome bladder symptoms and changes in sexual function are common health problems as people age. Having diabetes can mean
U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH Troublesome bladder symptoms and changes in sexual function are common health problems as people age. Having diabetes can mean
EUROPEAN UROLOGY 59 (2011)
 EUROPEAN UROLOGY 59 (2011) 645 651 available at www.sciencedirect.com journal homepage: www.europeanurology.com Infections Prevention of Recurrent Urinary Tract Infections by Intravesical Administration
EUROPEAN UROLOGY 59 (2011) 645 651 available at www.sciencedirect.com journal homepage: www.europeanurology.com Infections Prevention of Recurrent Urinary Tract Infections by Intravesical Administration
Treatment effectiveness in interstitial cystitis/bladder pain syndrome: Do patient perceptions align with efficacy-based guidelines?
 original research Treatment ness in interstitial cystitis/bladder pain syndrome: Do patient perceptions align with efficacy-based guidelines? Avril Lusty, MD; Elizabeth Kavaler, MD; Kay Zakariasen; Victoria
original research Treatment ness in interstitial cystitis/bladder pain syndrome: Do patient perceptions align with efficacy-based guidelines? Avril Lusty, MD; Elizabeth Kavaler, MD; Kay Zakariasen; Victoria
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC. August 6, 2015
 Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Research Project Plan RP Barcelona 2010
 Birgit Bonfils, RN Clinical Development Nurse Master of adult Education and Human Resource Development Department of Urology Herlev Hospital Denmark BIBO@heh.regionh.dk Research Project Plan RP10-01 Barcelona
Birgit Bonfils, RN Clinical Development Nurse Master of adult Education and Human Resource Development Department of Urology Herlev Hospital Denmark BIBO@heh.regionh.dk Research Project Plan RP10-01 Barcelona
Coping with urges and leaks?
 OAB AND YOU Coping with urges and leaks? Let me help you learn more about overactive bladder (OAB) symptoms and ways to help manage them 1 HOW DOES THE BLADDER WORK? Within the urinary tract, the kidneys
OAB AND YOU Coping with urges and leaks? Let me help you learn more about overactive bladder (OAB) symptoms and ways to help manage them 1 HOW DOES THE BLADDER WORK? Within the urinary tract, the kidneys
CommonKnowledge. Pacific University. Gina Clark Pacific University. Lauren Murphy Pacific University. Recommended Citation.
 Pacific University CommonKnowledge PT Critically Appraised Topics School of Physical Therapy 2012 The diagnostic accuracy of patient subjective history compared to the gold standard of urodynamic testing
Pacific University CommonKnowledge PT Critically Appraised Topics School of Physical Therapy 2012 The diagnostic accuracy of patient subjective history compared to the gold standard of urodynamic testing
Urinary tract disorders
 Urinary tract disorders Medicines Formulary Contents: 1. Urinary retention 1 2. Urinary incontinence 2 3. Urethral pain prevention during catheterisation 3 4. Indwelling catheters maintenance of patency
Urinary tract disorders Medicines Formulary Contents: 1. Urinary retention 1 2. Urinary incontinence 2 3. Urethral pain prevention during catheterisation 3 4. Indwelling catheters maintenance of patency
Interstitial Cystitis (Chronic Pelvic Pain) Consultation Information
 Interstitial Cystitis (Chronic Pelvic Pain) Consultation Information Round Rock Jollyville Westlake Central office: Phone 512-231-1444 Fax 512-231-1470 www.urologyteam.com Rev June 2017 What is Interstitial
Interstitial Cystitis (Chronic Pelvic Pain) Consultation Information Round Rock Jollyville Westlake Central office: Phone 512-231-1444 Fax 512-231-1470 www.urologyteam.com Rev June 2017 What is Interstitial
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Neuroanatomy, Neurophysiology and Clinical Presentation of Visceral Urological Pain
 Neuroanatomy, Neurophysiology and Clinical Presentation of Visceral Urological Pain Prof Dr K. Everaert Functional urology Department of Urology Ghent University Hospital Gent, Belgium Chronic pelvic pain
Neuroanatomy, Neurophysiology and Clinical Presentation of Visceral Urological Pain Prof Dr K. Everaert Functional urology Department of Urology Ghent University Hospital Gent, Belgium Chronic pelvic pain
INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY
 INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY COVERING INCONTINENCE BE ON JUST NAPPIES CATHETERS TYPES AVAILABLE AND WHEN TO USE THEM JJ STENTS???
INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY COVERING INCONTINENCE BE ON JUST NAPPIES CATHETERS TYPES AVAILABLE AND WHEN TO USE THEM JJ STENTS???
Infection/Inflammation. A Randomized, Double-Blind, Placebo Controlled Trial of Adalimumab for Interstitial Cystitis/Bladder Pain Syndrome
 Infection/Inflammation A Randomized, Double-Blind, Placebo Controlled Trial of Adalimumab for Interstitial Cystitis/Bladder Pain Syndrome Philip C. Bosch*, From the Department of Urology, Palomar Medical
Infection/Inflammation A Randomized, Double-Blind, Placebo Controlled Trial of Adalimumab for Interstitial Cystitis/Bladder Pain Syndrome Philip C. Bosch*, From the Department of Urology, Palomar Medical
Interstitial cystitis (IC) can be
 Interstitial Cystitis/Painful Bladder Syndrome Alis Kolter Panzera Interstitial cystitis (IC) can be described as a chronic irritative bladder syndrome. The term IC has recently been revised and the condition
Interstitial Cystitis/Painful Bladder Syndrome Alis Kolter Panzera Interstitial cystitis (IC) can be described as a chronic irritative bladder syndrome. The term IC has recently been revised and the condition
Interstitial cystitis intravesical therapy
 Review Article Interstitial cystitis intravesical therapy Tanya Ha, Jie Hua Xu Urology Department, Royal Perth Hospital, Perth, Australia Contributions: (I) Conception and design: All authors; (II) Administrative
Review Article Interstitial cystitis intravesical therapy Tanya Ha, Jie Hua Xu Urology Department, Royal Perth Hospital, Perth, Australia Contributions: (I) Conception and design: All authors; (II) Administrative
Summary ID# Clinical Study Summary: Study B4Z-MC-LYCL
 CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
Interstitial Cystitis
 J Obstet Gynecol India Vol. 58, No. 1 : January/February 2008 pg 24-31 Review Article Interstitial Cystitis Satpathy Hemant K, Taylor Richert Department of Obstetrics and Gynecology, CUMC, Omaha, NE, USA,
J Obstet Gynecol India Vol. 58, No. 1 : January/February 2008 pg 24-31 Review Article Interstitial Cystitis Satpathy Hemant K, Taylor Richert Department of Obstetrics and Gynecology, CUMC, Omaha, NE, USA,
Introduction. Original Article
 Original Article Test-retest reliability and discriminant validity for the Brazilian version of The Interstitial Cystitis Symptom Index and Problem Index and Pelvic Pain and Urgency/Frequency (PUF) Patient
Original Article Test-retest reliability and discriminant validity for the Brazilian version of The Interstitial Cystitis Symptom Index and Problem Index and Pelvic Pain and Urgency/Frequency (PUF) Patient
5-8 July, 2008, Palais des Congrès, Paris, France
 A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Intravesical Drug Delivery for Dysfunctional Bladder
 Intravesical Drug Delivery for Dysfunctional Bladder Yao-Chi Chuang Professor and Chief, Division of Urology, Kaohsiung Chang Gung Memorial Hospital, Taiwan 成長於台南 建興國中 台南一中 2 Yao-Chi Chuang In connection
Intravesical Drug Delivery for Dysfunctional Bladder Yao-Chi Chuang Professor and Chief, Division of Urology, Kaohsiung Chang Gung Memorial Hospital, Taiwan 成長於台南 建興國中 台南一中 2 Yao-Chi Chuang In connection
Introduction. For further information please contact:
 Product Monograph Introduction Teva UK Limited is one of the biggest pharmaceutical companies in the UK. We re proud to be part of the Teva Pharmaceutical Industries group, and we make medicines that help
Product Monograph Introduction Teva UK Limited is one of the biggest pharmaceutical companies in the UK. We re proud to be part of the Teva Pharmaceutical Industries group, and we make medicines that help
Glossary of terms Urinary Incontinence
 Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
Voiding Diary. Begin recording upon rising in the morning and continue for a full 24 hours.
 Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Women s & Children s Directorate The TVT Operation - a guide for patients
 Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
Urogynecology Associates of Philadelphia URODYNAMIC TESTING
 URODYNAMIC TESTING Urogynecology Associates of Philadelphia Most women with urinary incontinence will need to complete a few simple tests, performed in the office, to help your doctor assess your symptoms
URODYNAMIC TESTING Urogynecology Associates of Philadelphia Most women with urinary incontinence will need to complete a few simple tests, performed in the office, to help your doctor assess your symptoms
Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044
 Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044 Unique alternative to urinary catheters for women with permanent urinary retention Therapeutic benefits o Highly effective
Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044 Unique alternative to urinary catheters for women with permanent urinary retention Therapeutic benefits o Highly effective
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Recurrent Pediatric UTI Revisited 2013
 Recurrent Pediatric UTI Revisited 2013 PIDSP 21.2.2013 Shai Ashkenazi, MD, MSc Medicine changes constantly Some aspects of the standard practice of ~40 years are probably not valid and need to be changed
Recurrent Pediatric UTI Revisited 2013 PIDSP 21.2.2013 Shai Ashkenazi, MD, MSc Medicine changes constantly Some aspects of the standard practice of ~40 years are probably not valid and need to be changed
Hemorrhagic cystitis
 Hemorrhagic cystitis Bradley Rosenberg, MD Assistant Professor of Urology Oakland University William Beaumont School of Medicine Comprehensive Urology Hemorrhagic cystitis Definition: Sustained hematuria
Hemorrhagic cystitis Bradley Rosenberg, MD Assistant Professor of Urology Oakland University William Beaumont School of Medicine Comprehensive Urology Hemorrhagic cystitis Definition: Sustained hematuria
Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics. Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital
 Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital 01/02/2018 Lower Urinary Tract Symptoms LUTS - one of
Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital 01/02/2018 Lower Urinary Tract Symptoms LUTS - one of
s r e t n I sititsyc laititsret ystitis Interstitial Cystitis Interstitial Cystitis In itial Ctsretn IC I sititsyc laititsretni PRODUCT MONOGRAPH
 Interstitial Cystitis Interstitial Cystitis Interstitial Cystitis Intersti t i a l Cystitis Interstitial Cystitis Inters IC CONTENTS Interstitial Cystitis: An Overview Introduction... Epidemiology of
Interstitial Cystitis Interstitial Cystitis Interstitial Cystitis Intersti t i a l Cystitis Interstitial Cystitis Inters IC CONTENTS Interstitial Cystitis: An Overview Introduction... Epidemiology of
BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS
 BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS Nan Shao, Ph.D. Director, Biostatistics Premier Research Group, Limited and Mark Jaros, Ph.D. Senior
BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS Nan Shao, Ph.D. Director, Biostatistics Premier Research Group, Limited and Mark Jaros, Ph.D. Senior
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2
 Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Effect of Low-Dose Amitriptyline Treatment for Interstitial Cystitis/Bladder Pain Syndrome
 ORIGINAL ARTICLE Effect of Low-Dose Amitriptyline Treatment for Interstitial Cystitis/Bladder Pain Syndrome Weiguo Chen 1, Donghua Xie 2, Guolu Liu 1, Yifan Chen 1, Zongqiang Cai 1 1 Department of Urology,
ORIGINAL ARTICLE Effect of Low-Dose Amitriptyline Treatment for Interstitial Cystitis/Bladder Pain Syndrome Weiguo Chen 1, Donghua Xie 2, Guolu Liu 1, Yifan Chen 1, Zongqiang Cai 1 1 Department of Urology,
Chapter 47 1/8/2018. Urinary System Disorders. Urinary Tract Infections. Treatment
 Chapter 47 Urinary System Disorders Urinary Tract Infections Urinary tract infections (UTIs) are common Infection in one area can involve the entire system Microbes enter through the urethra. Catheterization,
Chapter 47 Urinary System Disorders Urinary Tract Infections Urinary tract infections (UTIs) are common Infection in one area can involve the entire system Microbes enter through the urethra. Catheterization,
Running Head: EFFICACY OF CRANBERRY PRODUCTS 1. Efficacy of Cranberry Products: An Alternative UTI Treatment. Christopher Mann.
 Running Head: EFFICACY OF CRANBERRY PRODUCTS 1 Efficacy of Cranberry Products: An Alternative UTI Treatment Christopher Mann The Sage Colleges EFFICACY OF CRANBERRY PRODUCTS 2 One of the most common bacterial
Running Head: EFFICACY OF CRANBERRY PRODUCTS 1 Efficacy of Cranberry Products: An Alternative UTI Treatment Christopher Mann The Sage Colleges EFFICACY OF CRANBERRY PRODUCTS 2 One of the most common bacterial
Overactive Bladder: Diagnosis and Approaches to Treatment
 Overactive Bladder: Diagnosis and Approaches to Treatment A Hidden Condition* Many Many patients self-manage by voiding frequently, reducing fluid intake, and wearing pads Nearly Nearly two-thirds thirds
Overactive Bladder: Diagnosis and Approaches to Treatment A Hidden Condition* Many Many patients self-manage by voiding frequently, reducing fluid intake, and wearing pads Nearly Nearly two-thirds thirds
Posterior Tibial Nerve Stimulation
 Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Information for Patients. Overactive bladder syndrome (OAB) English
 Information for Patients Overactive bladder syndrome (OAB) English Table of contents What is the bladder?... 3 What are overactive bladder symptoms?... 3 What causes overactive bladder symptoms?... 3 Diagnosis
Information for Patients Overactive bladder syndrome (OAB) English Table of contents What is the bladder?... 3 What are overactive bladder symptoms?... 3 What causes overactive bladder symptoms?... 3 Diagnosis
Bladder Pain Syndrome Current Concepts and Management Guidelines
 Review Article Bladder Pain Syndrome Current Concepts and Management Guidelines Amita Jain To cite: Jain A. Bladder Pain Syndrome Current Concepts and Management Guidelines. Pan Asian J Obs Gyn 2018;1(1):18-23.
Review Article Bladder Pain Syndrome Current Concepts and Management Guidelines Amita Jain To cite: Jain A. Bladder Pain Syndrome Current Concepts and Management Guidelines. Pan Asian J Obs Gyn 2018;1(1):18-23.
URODYNAMICS. Your urodynamic study is scheduled at the Ambulatory Procedure Center. This is located at 4-South waiting room at the Altru Main Clinic.
 Altru HEALTH SYSTEM URODYNAMICS Your urodynamic study is scheduled at the Ambulatory Procedure Center. This is located at 4-South waiting room at the Altru Main Clinic. Arrival Time: Appointment Date:
Altru HEALTH SYSTEM URODYNAMICS Your urodynamic study is scheduled at the Ambulatory Procedure Center. This is located at 4-South waiting room at the Altru Main Clinic. Arrival Time: Appointment Date:
Individual Study Table Referring to Part of the Dossier. Use only) Name of Finished Product:
 SYNOPSIS Fresenius Title of the study: A double-blind, randomized study comparing the safety and torelance of SMOFlipid 20% and Intralipid 20% in long-term treatment with parenteral nutrition Coordinating
SYNOPSIS Fresenius Title of the study: A double-blind, randomized study comparing the safety and torelance of SMOFlipid 20% and Intralipid 20% in long-term treatment with parenteral nutrition Coordinating
Interstitial cystitis
 Interstitial cystitis Ilse Truter, PharmITCom Consulting Bladder Normal protective bladder lining Thinning of the protective bladder lining Pinpoint bleeding (glomerulations) on the bladder wall Hunner
Interstitial cystitis Ilse Truter, PharmITCom Consulting Bladder Normal protective bladder lining Thinning of the protective bladder lining Pinpoint bleeding (glomerulations) on the bladder wall Hunner
CYSTISTAT. Hyaluronan Training
 CYSTISTAT Hyaluronan Training HISTORICAL ASPECTS In 1934, Karl Meyer and his assistant, John Palmer, described for the first time the process to isolate a new Glucosa- Amino-Glycan (GAG) from the cows
CYSTISTAT Hyaluronan Training HISTORICAL ASPECTS In 1934, Karl Meyer and his assistant, John Palmer, described for the first time the process to isolate a new Glucosa- Amino-Glycan (GAG) from the cows
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
