PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study
|
|
|
- Esmond Perkins
- 5 years ago
- Views:
Transcription
1 OFHA eol0e PENTOSAN POLYSULFATE SODIUM FOR THERAPY OF INTERSTITIAL CYSTITIS A Double-Blind Placebo-Controlled Clinical Study S. GRANT MULHOLLAND, M.D. PHILLIP HANNO, M.D. C. LOWELL PARSONS, M.D. GRANNUM R. SANT, M.D. DAVID R. STASKIN, M.D. From the Departments of Urology, Thomas Jefferson University Hospital, and University of Pennsylvania Hospital, Philadelphia, Pennsylvania; University of California, San Diego Medical Center, San Diego, California; Tufts University School of Medicine, and Boston University School of Medicine, Boston, Massachusetts ABSTRACT--Pentosan polysul]ate sodium (PPS) was compared with placebo ]or the symptomatic therapy o] interstitial cystitis in a double-blind, multicenter study. A total o] 110 patients were enrolled and treated]or three months. In this study, overall improvement o] greater than 25 percent was reported by 28 percent o] the PPS-treated patients and by 13 percent of- the placebo-treated patients (p = O. 03). The investigators" overall evaluation provided similar results, 26 percent vs 1I percent in ]avor o] PPS (p = O. 04). Improvement in pain and pressure to urinate also favored PPS over placebo and approached statistical significance (p = 0,07 and O. 08). The incidence o] adverse reactions was 6 percent in the PPS-treated group and 13 percent in the placebo-treated group. All adverse reactions were minor, and treatment was discontinued by I patient in the PPS group and 2 in the placebo group. In this study, PPS was ]ound to be significantly more e]]ective than, and equally as safe as, placebo. Interstitial cystitis is a syndrome characterized by the symptoms of urinary urgency, urinary frequency, nocturia, and lower abdominal and perineal pain and/or discomfort. It occurs in the absence of infection or other known pathologic processes such as carcinoma in situ, radiation, or chemical cystitis. Although its etiology has not been definitely established, 1 it has been postulated that interstitial cystitis is caused by a dysfunctional epithelium which becomes permeable and allows the leak of urinary solutes across the transitional cells. 2 In both animals and humans, it has been shown that the principal barrier to permeability is the surface glycosaminoglycans. 3-v A permeable bladder epithelium can result in diffusion of irritating components from normal urine through to the bladder wall to set up a chronic inflammatory condition initiating the symptoms of pain, urgency, and frequency2 Based on the possibility that there is a deficiency- of the surface glycosaminoglycans in patients with interstitial cystitis, patients were treated with a synthetic sulfated polysaccharide, pentosan polysulfate sodium (PPS), in an attempt to ameliorate their symptoms. This hypothesis has been tested in three published clinical studies using PPS (Elmiron). In two studies, one double-blind, placebo-controlled, 7 and one open-eontrolled, 8 PPS was shown to be effective in relieving the symptomatology of interstitial cystitis in a significant number of patients. In a third study, the authors concluded that there were no significant differences between the PPS and placebotreated groups. However, in the group of 552 UROLOGY / JUNE 1990 / VOLUME XXXV, NUMBEIq 6
2 patients with clinically and patho-anatomically verified interstitial cystitis, the reduction in pain was greater for the PPS-treated group than for the placebo-treated group, although no p values were given by the authors. In addition, the PPS-treated patients with verified disease were shown to have a statistically significant increase in bladder eapacity measured under anesthesia, as well as a statistically significant improved cystoscopie appearanee of the bladder. Because of the existing evidence indicating that PPS may have efficacy in this disorder, the current study was undertaken with a greater degree of control and a larger population of patients with well:defined disease. Material and Methods This study was conducted in a double-blind manner, and a placebo concurrent control group was used as a comparison for the efficacy and safety of PPS. The three-month doubleblind period with the placebo control made it possible to study the true extent to which PPS modulated the symptoms of disease in these patients. The study was multieentered with five participating centers. Patients were categorized in terms of the severity of their disease. Patients with a moderate degree of disease were defined as having an anesthetic bladder capacity over 400 ml, 18 or fewer voids per day, and an average voided volume of 75 ml or more. Patients were considered to have a severe degree of disease if they had a bladder capacity under anesthesia of less than 400 ml, or more than 18 voids per day, or an average voided volume of less than 75 ml. A patient with any one of these three criteria was included in the severe group. Anesthetic bladder capacity was measured under 80 em of water pressure held for one minute in all patients under general or spinal anesthesia. To. be included in the study, all patients had to have the following examination requirements and symtom complex: Urgency expressed as "moderate" on a 5-point analog scale Frequeney of at least 10 voids per day Nocturia of at least 2 voids per night Pain as recorded on a 5-point analog scale Continuous duration of symptoms of at least one year Failed previous conventional therapy such as chlorpactin, hydrodilatation, or DMSO Average voided volume of 200 ml or less measured over a three-day period Negative urine culture and cytology Cystoseopic examination under anesthesia (80 cm of water and 1 minut e distention) showing peteehial hemorrhages or ulcers with gross blood in the fluid return and a bladder capacity of 800 ml or less Patients were excluded from participation in the study for any of these conditions: Age less than eighteen years Pregnancy Premenopausal and not practieing effective means of birth control Evidenee of active bleeding peptic ulcer disease Bleeding diathesis Anticoagulant therapy Chronic use of narcotics Known allergy to pentosan polysulfate sodium Lack of availability for the duration of the study or inability to follow instructions Use of artificial sweeteners Lactating mothers Signs of: recurrent bacteriuria obvious neurologic impairment history of pelvic irradiation previous treatment with known bladder irritants bladder careinoma urinary tuberculosis shistosomiasis Treatment with Elmiron within six weeks of study Patients were randomly assigned to PPS or placebo group in accordance with a computergenerated random code providing two parallel groups of patients for comparison. All investigators were blinded during the study with the code established and maintained by a separate center. The dose of PPS was 300 mg daily given orally in divided doses of 100 mg three times a day one hour before meals or two hours after meals. Identically-appearing placebo capsules were given in the same manner. All patients were seen at zero time before start of treatment for evaluation of baseline symptoms and laboratory values and at twelve weeks after start of treatment for measurement of these same variables. The primary instrument used for evaluation was a follow-up questionnaire completed by the patient at the end of the double-blind treatment period. The patients were asked if they felt improved overall since the start of treatment, and UROLOGY / JUNE 1990 / VOLUME XXXV, NUMBER 6 553
3 if so, was the improvement "slight" 25 percent, "moderate" 50 percent, "great" 75 percent, or "complete cure" 100 percent. This instrument also allowed the patient to evaluate the changes, if any, they perceived in urgency and pain using the same definitions for overall improvement. An investigator's evaluation form for overall improvement was eompleted by the investigator after examining the patient and reviewing the data provided in voiding profiles and the pain and urgency scales while maintaining the double-blind. The scale used by the investigator contained categories of "worse," "no change," "fair,... good," "very good," and "exeellent." A volume-voiding profile measuring the time and quantity of all voids during waking hours as well as the number of voids during sleeping hours over three consecutive days was eompleted by the patient before treatment, and at the end of the first, second, and third months of treatment. This provided information concerning ehanges in average voided volume, fiequeney, and noeturia during the three-month treatment period. Pain and urgeney seales were included on one sheet and were eompleted before start of treatment and at the end of one, two, and three months of treatment. Both scales allowed the patient to express the degree of pain and urgency onasealeof0tos: 0 = none, I -- mild, 3 = moderate, and 5 = severe. Routine laboratory examinations were eondueted before treatment and at the end of the three-month treatment period. This ineluded urinalysis, complete blood eounts, blood chemistries including prothrombin time and partial thromboplastin time, and liver function tests. Adverse reaction report forms provided space for recording the type of reaction, treatment, outcome, comment, and relation to treatment as possible, probable, not likely, or other cause. Reports of side effects were volunteered. This was considered appropriate for these patients because interstitial cystitis patients are known to be exquisitely aware of any changes in their condition and prone to discuss them with their physicians. At the end of the three-month double-blind period,-the patients were offered the opportunity to continue taking PPS as long as they continued to experience relief of their symptoms, or to start taking PPS if they had been assigned to the placebo group. Variables for the determina- tion of PPS efficacy in order of relevancy were the patient's assessment of overall improvement, the investigator's assessment of overall improvement, the changes in pain and urgency, the change in nocturia, and the change in frequency and average voided volume. In each ease the change from the baseline values at the end of the double-blind period was used as the basis for determining the efficacy of Elmiron. Statistical Treatment of Data The treatment groups were compared both within each study center and across all study centers, with respect to the response variables recorded at the pretreatment visit. For the quantitative variables such as age, bladder capacity, pain, urgency, frequency of urination, and total urine volume, a t-test was used. The analysis of the qualitative variables such as sex, race, and severity of variables associated with the cystoscopie evaluation were analyzed with a ehi-square contingency table analysis. Additionally, for those response variables scored with a severity rating, a Mantel-Haenszel test was used to compare the treatment groups with respect to a linear association between severity and response rate. The treatment groups were compared for changes in the response variables of pain, urgency, frequency of urination, and the total urine volume, both within each study center and across all study centers. The analysis of these changes from baseline variables was with an analysis of variance, using a general ]inear models procedure. For each treatment group, the significance of the mean change was determined with a t-test. With respect to the pain and urgency scales, treatment was considered to be successful if there was a I-point or greater reduction on the severity scale or unsuccessful if there was less th an a 1-point reduction on the severity scale. A decrease of 3 or more per day in frequency of urination, and an increase in urine volume of 20 ml or more per void were also used as indications of improvement. For each of these criteria, treatment groups were compared using Fisher's exact test within each study center, and the ManteI-Haenszel test was used to compare treatment groups after collapsing data across study centers. For the investigator and patient evaluations made at the completion of the three-month double-blind phase, treatment group comparisons were made with ehi-square contingency 554 UROLOGY / JUNE 1990 / VOLUME XXXV, NUMBE[q 6
4 TABLE I. Patient characteristics at baseline Parameter PPS Placebo Patients Mean age (yrs.) Females (%) White race (%) Mean duration of disease (yrs.) Cystoseopie findings Bloody fluid return (%) Mild Moderate Severe Fissures (%) None 6 7 Few Moderate Many Petechial hemorrhages (%) Few Moderate Many Hunner's ulcer present (%) 8 4 Other abnormalities present (%) 4 11 Mean bladder capacity (ee) Patients with severe disease (%) table analysis for qualitative variables. For those response variables recorded on a severity scale, the treatment groups were compared using a Mantel-Haenszel test with respect to linear association. In addition, a bloeked Wilcoxon test was used to compare the treatment groups after adjustment for study center dffferenees. All adverse experiences were recorded and their frequency determined for each treatment group. For each type of adverse experience, the treatment groups were compared with respeet to the percent of patients experiencing the adverse effect, using Fisher's exact test. All computations were performed using the Statistical Analysis System (SAS). Statistical significance was deelared if the p-value was equal to or less than Results There were 110 patients with documented interstitial cystitis with a duration of one year or more enrolled in the study. Of these, 56 patients were treated with placebo, and 54 were treated with PPS. There were no significant differences between the treatment groups at the beginning of the study in terms of age, sex distribution, race, cystoscopie findings, duration of disease, bladder capacity, or severity of disease. These data are summarized in Table I. Twelve patients, 3 treated with PPS and 9 treated with placebo, failed to complete the three month study. Of these 12, 8 were in the group of patients classified as having severe disease, 1 (3 % ) receiving PPS and 7 (21% ) receiving placebo. Most of these patients were lost to follow-up, and it is likely that tack of efficacy was responsible for the patients dropping out. The differenee in the drop-out rate between the treatment groups in these patients with severe disease was statistically signifieant (p = 0.05). At the end of the three-month treatment period, 28 percent of the PPS patients versus 18 percent of the placebo patients evaluated themselves as more than slightly improved relative to their condition at the beginning of the study on the 5-point scale from "no change'" to "complete cure" (p = 0.04). In terms of reduced pain, 27 percent of the PPS patients evaluated themselves as improved compared with 14 percent of the placebo patients (p = 0.08). This was confirmed by the pain scale data where 46 pereent of the PPS patients and 29 pereent of the placebo patients recorded a decrease in pain of 1 or more (p = 0.07). The mean reduction in pain from baseline as measured by the pain scale was 0.5 for the PPS-treated patients eompared with 0.2 for the plaeebo patients at three months. This difference between the treatment groups was not statistically significant, but the dffferenee from baseline was signifieantty different from zero (p = 0.05) for the PPS-treated group but not for the placebo-treated group. The latter held true with p = 0.01 to 0.05 for the end of months 1 and 2 as well. At the three-month self-evaluation, 22 percent of the PPS patients and 11 percent of the placebo patients expressed an improvement in pressure to urinate (p = 0.08). This was not confirmed by the urgency scale data which revealed a slight margin of 39 percent to 46 percent in favor of plaeebo. By the investigator's evaluation of overall improvement, 26 percent of the PPS-treated patients and 11 percent of the placebo patients had an improvement of at least good or better on the 6-point scale from "worse" to "excellent" (p = 0.03). There was a mean increase in volume per void of 9.8 ce for the PPS-treated patients, and 7.6 ee per void for placebo patients. Among the PPS patients, 30 percent had an increase of 20 ce or more eompared with 20 percent of the placebo patients. The PPS group had a mean increase in total daytime volume of urine at the UROLOGY / JUNE 1990 / VOLUME XXXV, NUMBER 6 555
5 TABLE II. Efficacy data (% of patients improved in terms of pain, urgency) and overall improvement Parameter PPS Placebo p Overall improvement three months Investigator evaluation Patient self-evaluation Overall improved Pain follow-up questionnaire Pain scale Pressure to urinate Urgency scale ns Mean reduction in pain score from baseline 0.5* 0.2 ns Changes from baseline, voided volume Mean volume per void (ee) ns Increase of >20 ec (% pts.) ns Total daily urine volume (ec) ns *Signifieandy different from 0 (p = 0.05). endpoint of 60 ec compared with a mean decrease of 20 ec for the placebo group. These differences were not statistically significant. The efficacy data as measured by these parameters in this study are summarized in Table II. The changes from baseline at the endpoint in the remaining parameters studied were not different between treatments. These were voids per day (- 1 for both treatments), percent of patients having 3 less voids per day (32 % PPS, 24 % placebo), and nocturia ( PPS, placebo). The incidence of adverse reactions in this study was low in both treatment groups. Among the PPS-treated patients, 3 (6%) reported a total of 6 adverse reactions, and 7 (13 % ) placebo patients reported a total of 8 adverse reactions. The observed reactions were not different from reactions that might be observed in any random population over a threemonth period and were not serious. Only 1 PPS and 2 placebo patients discontinued treatment due to adverse reactions. Only diarrhea was observed in more than 1 patient. These data are summarized in Table III. There were no differences between the treatment groups in terms of clinically significant changes in any of the laboratory data. One patient in the PPS-treated group had SGOT and SGPT values of 118 and 133 at three months compared with 56 and 115 at baseline, respectively, at each time. There were no other remarkable changes. Of the patients who completed the threemonth double-blind period, 44 percent were continuing therapy with PPS one and a half years after the start of the study. These patients, TABLE III. Incidence of adverse reactions Reaction PPS (%) Placebo (%) Headache 1 (1.9) 2 (3.6) Nausea i (1.9) 0 Indigestion 1 (1.9) 0 Increased perspiration 1 (1.9) 0 Severe mood swings 1 (1.9) 0 Suicidal ideation 1 (1.9) 0 Diarrhea 0 2 (3.6) Explosive diarrhea 0 1 (1.8) Severe joint pain 0 1 (1.8) Skin rash on arms 0 1 (1.8) Itching 0 1 (1.8) Total reactions 6 8 Total patients 3 (6) 7 (13) therefore, had been on therapy for periods ranging from six months to one and a half years. Comment The primary measurement used to determine effectiveness of PPS was the patient evaluation of overall improvement. The investigator's assessment of overall improvement was also a measure of efficacy, second only to the patient's self-evaluation. The measure of overall improvement was considered to be the most appropriate measure of treatment efficacy because the individual symptoms of interstitial cystitis frequency, urgency, pain, and nocturia--vary widely within and among patients in terms of occurrence, severity, and the importance that patients attach to individual symptoms. 556 UROLOGY JUNE [990 VOLUME XXXV, NUMBER 6
6 Patients are not at all alike in the manner in which they evaluate and report individual symptoms. Some patients report a decrease in urgency, or perhaps pain, when the number of days that these symptoms are present is significantly reduced, while other patients with a similar reduction will not report a decrease because the urgency or pain is still severe on the days that either one is present. Frequency and urine volume also can be misleading symptoms. Some patients restrict fluid intake during particularly painful periods and increase fluid intake when pain is ameliorated. In these patients, when treatment produces a decrease in pain, frequency may increase while urine volume per void may remain the same or show a small increase, or frequency may remain the same with an increase in volume per void. The symptom complex varies among patients in terms of which of the symptoms is most prominent and/or is perceived as most bothersome or disabling. While any given treatment may provide some relief for most or all of a patient's symptoms, it may not provide enough relief of the particular symptom or symptoms that the patient perceives as most important. Guiding the patient to think in terms of overall relief, helps to overcome the dilution that can result from too sharp a focus on individual symptoms. For these reasons emphasis in this study was placed on overall improvement as expressed by the patient and investigator, while the changes in specific symptomatology were considered as supportive data. In this respect, the results of this study confirm the observations made in previous studies of PPS for the treatment on interstitial cystitis. On the self evaluation by the patient, 28 percent of the patients who received PPS felt that they experienced significant overall improvement in their disease compared with their condition at the beginning of treatment. This differed significantly (p = 0.04) from the placebo-treated patients, 13 percent of whom reported a similar result. The investigators' overall evaluation at the end of the doubleblind period agreed with the patients' self-evaluation with 26 percent of the PPS patients evaluated as significantly improved compared with 11 percent of the placebo patients. This difference was significant at the 0.03 level. In the self-evaluation of overall improvement, patients who reported only slight improvement on the some 1 ot e. ~ no change, 1,~:,, Sllgllt, 1, ~-,,,~ mocterate, 1 -,, "great," or "complete" were not included in the group considered to have been significantly improved. In the evaluation by the investigator, overall improvement that was rated as "fair" on the scale of "worse," "no change," "fair," "good," "very good," or "excellent" was not ineluded in the group of patients considered to have had significant overall improvement. Improvement in pain approached significance on both the pain scale and in terms of the patient's perception of pain improvement as expressed on the follow-up questionnaire (p = 0.07 and 0.08, respectively), Also at each of the periods of measurement from the end of one month to the end of treatment, the decrease in pain as measured on the pain scale was significantly different from 0 (p = 0.01 to 0.05) for the PPS-treated group but not for the placebo group, although the degree of change between the groups was not significant. Pain as a symptom may improve first, representing a decrease in inflammatory response. On the other hand, refunctionalization of the bladder appears to be a much slower response. The results from the urgency scale and the follow-up questionnaire were disparate. On the urgency scale, the difference between the groups was slightly in favor of placebo (46 % to 39 %) but not statistically significant. On the follow-up questionnaire where the question was framed in terms of pressure-to-urinate rather than urgency, the difference between the groups in favor of PPS (22% to 11%) approached significance at The reason for the disparity is unknown, except that patients may interpret the terms differently. The data relating to the changes in voided volume were in favor of the PPS-treated group although not significantly different from placebo. There was a mean increase of 9.8 ec in volume per void, a mean increase of 60 cc in total daily volume, and an increase of 20 cc or more in volume: per void by 30 percent of the patients who received PPS. The comparable figures for the patients who received placebo were 7.6 co, mean decrease of 20 ee, and 20 percent, respectively. The changes in frequency noted in this study did not differ significantly between the groups in terms of daytime voids where there was a reduction of 1 void per day for both groups, for noeturia where ti~ere was a reduction of 0.8 voids for PPS and 0.5 voids for placebo, and for percent of patients having a reduction of 3 or ~nore voids )er day with 32 percent for PPS and 34 percent for placebo. UROLOGY / JUNE 1990 / VOLUME XXXV, NUMBER 6 557
7 It is noteworthy that at one and a half years after the start of the study, 44 percent of the patients who completed the three-month treatment period have continued treatment -with PPS for ongoing periods ranging from six months to one and a half years. This indicates that this proportion of patients considers PPS to be effective enough to continue its use on a long-term basis and attests to the efficacy of this medication for the relief of the symptoms of interstitial cystitis. It is important to note that these positive results for PPS were achieved in a selected population of patients who had previously failed to experience satisfactory relief with conventional treatments such as DMSO, ehlorpaetin, and others, It is not unlikely that in a group of newly diagnosed patients who have not been found to be refractory to treatment, the proportion of patients responding to treatment would be greater. The incidence of adverse reactions was higher among the placebo-treated patients than the PPS-treated group, but the difference was not significant. There were no adverse reactions considered to be serious. Treatment was discontinued because of adverse reactions in 2 patients receiving placebo and in 1 patient receiving PPS. Conclusions In this double-blind, placebo-controlled study, both efficacy and safety of PPS for the relief of the symptoms of interstitial cystitis have been confirmed. Both patients and investigators evaluated PPS as significantly more effective than placebo overall. For pain and urgency, the improvements due to PPS compared with placebo were statistically significant or approached significance. In terms of the voided volume parameters, the improvements favored PPS over placebo, although the differences were not statistically significant. The changes in frequency were similar in both groups, as were adverse reactions and changes in laboratory values. Jefferson Medical College 1025 Walnut Street, Room 1112 Philaddphia, Pennsylvania (DR. MULHOLLAND) ACKNOWLEDGMENTS. TO J. Richard Trout, Ph.D., for the development of the statistical plan and performing all the statistical analyses, and to James T. Baldini, Ph.D., for assistance with development of the protocol, monitoring the study, and editorial assistance with this manuscript. The PPS and placebo capsules were generously supplied by Medical Market Specialties, Inc., Boonton, New Jersey. References 1. Messing EM, and Stamey TA: Interstitial cystitis: early diagnosis, pathology, and treatment, Urology 12:381 (1978). 2. LillyJD, andparsons CL: Increasedtransvesicalsmallmol. ecule movement in patients with interstitial cystitis (abstr.), ] Urol 141: 268A (1989). 3. Parsons CL, Boychuk D, and Jones S: Transitional cell surface GAG's regulatory role in transepithellal movement (abstr.), J Urol 137: llta (1987). 4. Parsons CL, Boychuk D, Hurst R, and Callahan HJ: Bladder surface glycosaminoglycans: an epithelial permeability bartier. J Urol 143:139 (1990). 5. Lilly JD, and Parsons CL: Bladder surface glycosaminoglycans: an epithelial regulator of small molecule movement (abstr.), J UroI 139: 199A (1988), 6. Parsons CL: Bladder surface glycosaminoglycans: efficient mechanism of environmental adaptation, Urology (Suppl) 27:9 (1986). 7. Parsons CL, and Mulholland SG: Successful therapy of interstitial cystitis with pentosanpolysulfate, J Uro1138:513 (1987). 8, Fritjofsson A, et ah Treatment of ulcer and nonulcer interstitial cystitis with sodium pentosanpolysulfate: a multieenter trial, J Urol 138:508 (1987). 9. Holm-Bentzen M, et ah A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease, J Urol 138:503 (I987). 558 UROLOGY JUNE 1990 VOLUME XXXV, NUMBER 6
Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium
 Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium Excerpts from: Drugdex Monograph on Pentosan Edition: online URL: www.thomsonhc.com/home/dispatch Date Added: 29/04/2010 17:42:55 Source Notes:Evidence
Evidence on Therapeutic Uses of Pentosan Polysulfate Sodium Excerpts from: Drugdex Monograph on Pentosan Edition: online URL: www.thomsonhc.com/home/dispatch Date Added: 29/04/2010 17:42:55 Source Notes:Evidence
DELAYED DIAGNOSIS. Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >60%
 DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
DELAYED DIAGNOSIS Mean time to diagnosis = 4-7 years Mean # of physicians = 8 NIDDK criteria underdiagnoses >6% 1 PREVALENCE 1,, 9,, 8,, 7,, 6,, 5,, 4,, 3,, 2,, 1,, NHS "Prostatitis" "Endometriosis" The
The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS The Role of Pentosan Polysulfate in Treatment Approaches for Interstitial Cystitis Joel M.H. Teichman, MD, FRCSC Department of Surgery, Division of Urology,
INTERSTITIAL CYSTITIS (IC) MANAGEMENT
 INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
INTERSTITIAL CYSTITIS (IC) MANAGEMENT About Interstitial Cystitis (IC) What is IC? IC is a chronic, yet manageable, bladder condition, characterized by bladder or pelvic pain, pain during or after sexual
In evaluating a patient with lower urinary tract symptoms (LUTS), urologists
 CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Interstitial Cystitis and Lower Urinary Tract Symptoms in Males and Females The Combined Role of Potassium and Epithelial Dysfunction C. Lowell Parsons, MD
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
BPS/IC: evolution of definition and prevalence
 BPS/IC: evolution of definition and prevalence ame in history Tic doloureux of the bladder 1836 Interstitial cystitis 1878 Cystitis parenchymatosa 197 unner s ulcer 1915 Panmural ulcerative cystitis 192
BPS/IC: evolution of definition and prevalence ame in history Tic doloureux of the bladder 1836 Interstitial cystitis 1878 Cystitis parenchymatosa 197 unner s ulcer 1915 Panmural ulcerative cystitis 192
Interstitial Cystitis
 Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
Interstitial Cystitis Interstitial cystitis (IC) is a chronic bladder condition. Its symptoms are urinary urgency (the feeling that you need to urinate), frequent urination and/or pain anywhere between
Edward L. Davis, Samar R. El Khoudary, Evelyn O. Talbott,* Josephine Davis and Lisa J. Regan
 Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial Edward L. Davis, Samar R. El Khoudary, Evelyn O.
Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial Edward L. Davis, Samar R. El Khoudary, Evelyn O.
Urogynecology in EDS. Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018
 Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
How does interstitial cystitis begin?
 Original Article How does interstitial cystitis begin? C. Lowell Parsons Division of Urology, Department of Surgery, University of California, San Diego Medical Center, University of California, San Diego,
Original Article How does interstitial cystitis begin? C. Lowell Parsons Division of Urology, Department of Surgery, University of California, San Diego Medical Center, University of California, San Diego,
Interstitial Cystitis
 Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
Clinical Communiqué Winter 2002 Interstitial Cystitis A Clinical Spectrum Jack Barkin, MD Chief of Urology, Humber River Regional Hospital, Toronto, Ontario Editorial Choosing the right treatment by J.
INTERSTITIAL CYSTITIS (IC)
 INTERSTITIAL CYSTITIS (IC) Painful Bladder Syndrome (PBS): THE BASIC FACTS ANDREW L. SIEGEL, M.D. Board-Certified Urologist and Urological Surgeon Specialty: Male and Female Incontinence, Voiding Dysfunction,
INTERSTITIAL CYSTITIS (IC) Painful Bladder Syndrome (PBS): THE BASIC FACTS ANDREW L. SIEGEL, M.D. Board-Certified Urologist and Urological Surgeon Specialty: Male and Female Incontinence, Voiding Dysfunction,
Interstitial Cystitis/ Bladder Pain Syndrome
 page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
page 1 Interstitial Cystitis/ Bladder Pain Syndrome Q: What is interstitial cystitis/bladder pain syndrome (IC/BPS)? A: Interstitial cystitis (int-uhr-stishuhl siss-tyt-uhss) (IC), is a chronic pain condition
Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_
 207 Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_2542 207..212 C. Lowell Parsons, MD,* Paul Zupkas, PhD,* Jeffrey Proctor, MD,
207 Alkalinized Lidocaine and Heparin Provide Immediate Relief of Pain and Urgency in Patients with Interstitial Cystitisjsm_2542 207..212 C. Lowell Parsons, MD,* Paul Zupkas, PhD,* Jeffrey Proctor, MD,
Interstitial and chronic cystitis. Have you heard about the 2-component protection for the bladder wall?
 Interstitial and chronic cystitis Have you heard about the 2-component protection for the bladder wall? What is special about Instillamed? 2-component protection for the bladder wall Instillamed is an
Interstitial and chronic cystitis Have you heard about the 2-component protection for the bladder wall? What is special about Instillamed? 2-component protection for the bladder wall Instillamed is an
Painful Bladder Syndrome
 Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Painful Bladder Syndrome Department of Urology Patient Information What What is Painful is Painful Bladder Bladder Syndrome? Syndrome? Painful bladder syndrome also known as Interstitial Cystitis is a
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
UV-2005/01. Chronic Prostatitis and Chronic Pelvic Pain Syndrom (CP/CPPS) Karl-Bickleder-Str. 44C Straubing - Germany
 SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
Interstitial Cystitis:
 Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
Diagnosis and Definition ESSIC approach Jørgen Nordling, chairman ESSIC Professor of Urology University of Copenhagen Denmark Interstitial Cystitis: A painful, potentially disabling, inflammatory disease
Interstitial cystitis (IC), also
 Interstitial cystitis: Algorithm to simplify diagnosis of chronic urinary symptoms By Anita L. Booth, DNP, FNP-BC and Jill Harpst Rodgers, DNP, FNP-BC This pre/post-test study was undertaken to determine
Interstitial cystitis: Algorithm to simplify diagnosis of chronic urinary symptoms By Anita L. Booth, DNP, FNP-BC and Jill Harpst Rodgers, DNP, FNP-BC This pre/post-test study was undertaken to determine
In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective?
 Original Article In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Hikaru Tomoe Department of Urology and Pelvic Reconstructive Surgery, Tokyo
Original Article In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Hikaru Tomoe Department of Urology and Pelvic Reconstructive Surgery, Tokyo
SELECTED POSTER PRESENTATIONS
 SELECTED POSTER PRESENTATIONS The following summaries are based on posters presented at the American Urogynecological Society 2004 Scientific Meeting, held July 29-31, 2004, in San Diego, California. CENTRAL
SELECTED POSTER PRESENTATIONS The following summaries are based on posters presented at the American Urogynecological Society 2004 Scientific Meeting, held July 29-31, 2004, in San Diego, California. CENTRAL
Women s and Men s Health Intake Form Comprehensive Physical Therapy Center
 Name: (Last, First) DOB: Date: Age: Referring Physician: Next Physician Appointment: Today s visit: What is the main reason you came to the office today? When did it start? What treatments have you had
Name: (Last, First) DOB: Date: Age: Referring Physician: Next Physician Appointment: Today s visit: What is the main reason you came to the office today? When did it start? What treatments have you had
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Bladder pain syndrome / Interstitial cystitis
 Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
Bladder pain syndrome / Interstitial cystitis Terminology The term bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as the presence of chronic pain, pressure, or pelvic discomfort lasting
Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy
 Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Review Article Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy Mauro Cervigni Interstitial Cystitis Referral Center, Catholic University, Rome, Italy Correspondence
Definitions of IC: U.S. perspective. Edward Stanford MD MS FACOG FACS Western Colorado
 Definitions of IC: U.S. perspective Edward Stanford MD MS FACOG FACS Western Colorado PURPOSE OF A DEFINITION? Identifies with specificity those patients who are most likely to have the disease. Identifies
Definitions of IC: U.S. perspective Edward Stanford MD MS FACOG FACS Western Colorado PURPOSE OF A DEFINITION? Identifies with specificity those patients who are most likely to have the disease. Identifies
Allergan Not Applicable AGN A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple Dose, Parallel
 Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Interstitial Cystitis - Painful Bladder Syndrome
 Interstitial Cystitis - Painful Bladder Syndrome Interstitial cystitis (in-tur-stish-ul sis-tie-tis) also called painful bladder syndrome is a chronic condition in which you experience bladder pressure,
Interstitial Cystitis - Painful Bladder Syndrome Interstitial cystitis (in-tur-stish-ul sis-tie-tis) also called painful bladder syndrome is a chronic condition in which you experience bladder pressure,
MorphiDex (MS:DM) Double-Blind, Multiple-Dose Studies In Chronic Pain Patients
 Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S37 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia MorphiDex (MS:DM) Double-Blind, Multiple-Dose
Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S37 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia MorphiDex (MS:DM) Double-Blind, Multiple-Dose
32 OBG Management July 2010 Vol. 22 No. 7 obgmanagement.com
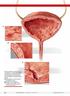 A B C Although interstitial cystitis (IC) occurs in the absence of urinary tract infection or malignancy, some pathology may become apparent during cystoscopy with hydrodistention. Potential findings include:
A B C Although interstitial cystitis (IC) occurs in the absence of urinary tract infection or malignancy, some pathology may become apparent during cystoscopy with hydrodistention. Potential findings include:
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2
 Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
Office based non-oncology urology trials Richard W. Casey, MD, 1 Jack Barkin, MD 2 1 The Male Health Centre, Oakville, Ontario, Canada 2 Humber River Regional Hospital, University of Toronto, Toronto,
ELMIRON -100 mg (pentosan polysulfate sodium) Capsules DESCRIPTION
 ELMIRN - mg (pentosan polysulfate sodium) Capsules DESCRIPTIN Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative which chemically and structurally
ELMIRN - mg (pentosan polysulfate sodium) Capsules DESCRIPTIN Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative which chemically and structurally
Review of treatments for interstitial cystitis
 The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Review of treatments for interstitial cystitis Lindsey Ann Miller The University of Toledo Follow this
MODULE 5: HEMATURIA LEARNING OBJECTIVES DEFINITION. KEY WORDS: Hematuria, Cystoscopy, Urine Cytology, UTI, bladder cancer
 MODULE 5: HEMATURIA KEY WORDS: Hematuria, Cystoscopy, Urine Cytology, UTI, bladder cancer LEARNING OBJECTIVES At the end of this clerkship, the learner will be able to: 1. Define microscopic hematuria.
MODULE 5: HEMATURIA KEY WORDS: Hematuria, Cystoscopy, Urine Cytology, UTI, bladder cancer LEARNING OBJECTIVES At the end of this clerkship, the learner will be able to: 1. Define microscopic hematuria.
ELMIRON -100 MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION
 ELMIRON - MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative,
ELMIRON - MG (PENTOSAN POLYSULFATE SODIUM) CAPSULES PRESCRIBING INFORMATION DESCRIPTION Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative,
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
WHAT IS INTERSTITIAL CYSTITIS?
 Patient Information WHAT IS INTERSTITIAL CYSTITIS? Interstitial Cystitis (IC) is a condition resulting in recurring discomfort or pain in the bladder and the surrounding pelvic region. Its cause is unknown,
Patient Information WHAT IS INTERSTITIAL CYSTITIS? Interstitial Cystitis (IC) is a condition resulting in recurring discomfort or pain in the bladder and the surrounding pelvic region. Its cause is unknown,
H6D-MC-LVHR Clinical Study Report Synopsis Page LVHR Synopsis (LY450190)
 H6D-MC-LVHR Clinical Study Report Synopsis Page 1 2. LVHR Synopsis H6D-MC-LVHR Clinical Study Report Synopsis Page 2 Clinical Study Report Synopsis: Study H6D-MC-LVHR Title of Study: A Randomized, Double-Blind,
H6D-MC-LVHR Clinical Study Report Synopsis Page 1 2. LVHR Synopsis H6D-MC-LVHR Clinical Study Report Synopsis Page 2 Clinical Study Report Synopsis: Study H6D-MC-LVHR Title of Study: A Randomized, Double-Blind,
Loyola University Medical Center Female Pelvic Medicine & Reconstructive Surgery
 Loyola University Medical Center Female Pelvic Medicine & Reconstructive Surgery Medical History Questionnaire Name: Date: Age: D.O.B. Race: What is the nature of your current gynecologic or urologic medical
Loyola University Medical Center Female Pelvic Medicine & Reconstructive Surgery Medical History Questionnaire Name: Date: Age: D.O.B. Race: What is the nature of your current gynecologic or urologic medical
HD CLINIC MEDICAL HISTORY FORM
 HD CLINIC MEDICAL HISTORY FORM Welcome to the HDSA Center of Excellence HD Clinic. Please take a few moments to answer the questions below as best as you can. If you need assistance, a caregiver/companion
HD CLINIC MEDICAL HISTORY FORM Welcome to the HDSA Center of Excellence HD Clinic. Please take a few moments to answer the questions below as best as you can. If you need assistance, a caregiver/companion
COMPREHENSIVE PAIN MANAGEMENT INTAKE FORM. Home Phone: Other Contact: Other Contact: Address: City: State: Zip: Address: City: State: Zip:
 COMPREHENSIVE PAIN MANAGEMENT INTAKE FORM Last Name: First Name: Middle: Home Phone: Other Contact: Other Contact: DOB: Age: Sex: Name of Referring Physician: Phone: Fax: Address: City: State: Zip: Name
COMPREHENSIVE PAIN MANAGEMENT INTAKE FORM Last Name: First Name: Middle: Home Phone: Other Contact: Other Contact: DOB: Age: Sex: Name of Referring Physician: Phone: Fax: Address: City: State: Zip: Name
Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website:
 Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website: www.female-gynecologist.com What is Painful Bladder (PBS) or Interstitial Cystitis
Mrs Ami Shukla Consultant Gynaecologist and Obstetrician Lead Urogynaecologist, Northampton General Hospital Website: www.female-gynecologist.com What is Painful Bladder (PBS) or Interstitial Cystitis
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Mr. GIT KAH ANN. Pakar Klinikal Urologi Hospital Kuala Lumpur.
 Mr. GIT KAH ANN Pakar Klinikal Urologi Hospital Kuala Lumpur drgitka@yahoo.com 25 Jan 2007 HIGHLIGHTS Introduction ICS Definition Making a Diagnosis Voiding Chart Investigation Urodynamics Ancillary Investigations
Mr. GIT KAH ANN Pakar Klinikal Urologi Hospital Kuala Lumpur drgitka@yahoo.com 25 Jan 2007 HIGHLIGHTS Introduction ICS Definition Making a Diagnosis Voiding Chart Investigation Urodynamics Ancillary Investigations
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Painful Bladder Syndrome/Interstitial Cystitis
 n The Leeds Teaching Hospitals NHS Trust Painful Bladder Syndrome/Interstitial Cystitis Information for patients Leeds Centre for Women s Health Welcome to the Department of Urogynaecology Your consultant
n The Leeds Teaching Hospitals NHS Trust Painful Bladder Syndrome/Interstitial Cystitis Information for patients Leeds Centre for Women s Health Welcome to the Department of Urogynaecology Your consultant
Dear Mercy Cancer Center Radiation Oncology Patient
 Dear Mercy Cancer Center Radiation Oncology Patient Welcome to our Department. In order to complete our records, and enable our physicians to ensure that your questions are fully addressed, we appreciate
Dear Mercy Cancer Center Radiation Oncology Patient Welcome to our Department. In order to complete our records, and enable our physicians to ensure that your questions are fully addressed, we appreciate
Individual Study Table Referring to Part of the Dossier. Volume: Page:
 1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
At the outset, we want to clear up some terminology issues. IBS is COPYRIGHTED MATERIAL. What Is IBS?
 1 What Is IBS? At the outset, we want to clear up some terminology issues. IBS is the abbreviation that doctors use for irritable bowel syndrome, often when they are talking about people with IBS. We will
1 What Is IBS? At the outset, we want to clear up some terminology issues. IBS is the abbreviation that doctors use for irritable bowel syndrome, often when they are talking about people with IBS. We will
D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS.
 D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS. Maria Laura Lopes De Carvalho, MD Guido Francavilla, MD Roberta
D-MANNOSE, CRANBERRY AND VITAMIN C (CYSTOMAN 100MG) ARE EFFECTIVE IN PREVENTING URINARY TRACT INFECTIONS IN MULTIPLE SCLEROSIS SUBJECTS. Maria Laura Lopes De Carvalho, MD Guido Francavilla, MD Roberta
Is Topical Clonazepam More Effective Than Oral Clonazepam in Treatment of Burning Mouth Syndrome (BMS)?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is Topical Clonazepam More Effective
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is Topical Clonazepam More Effective
Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis
 Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis R. J. Evans,*, R. M. Moldwin, N. Cossons, A. Darekar, I. W. Mills and D. Scholfield From the Department
Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis R. J. Evans,*, R. M. Moldwin, N. Cossons, A. Darekar, I. W. Mills and D. Scholfield From the Department
Name: Date: Referring Provider: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary).
 Name: Date: Referring Provider: Age: D.O.B. Race/ ethnicity: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary). We are interested in learning
Name: Date: Referring Provider: Age: D.O.B. Race/ ethnicity: What is the nature of your current gynecologic or urologic medical problem (use the other side if necessary). We are interested in learning
Various Types. Ralph Boling, DO, FACOG
 Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
5-8 July, 2008, Palais des Congrès, Paris, France
 A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
A review of the 4 th INTERNATIONAL CONSULTATION ON INCONTINENCE 5-8 July, 2008, Palais des Congrès, Paris, France The 4 th International Consultation on Incontinence was held 5-8 July 2008 in Paris and
Multimodal Therapy for Painful Bladder Syndrome / Interstitial Cystitis: Pilot Study Combining Behavioral, Pharmacologic, and Endoscopic Therapies
 Neurourology Multimodal Therapy for Interstitial Cystitis: A Pilot Study International Braz J Urol Vol. 35 (4): 467-474, July - August, 2009 Multimodal Therapy for Painful Bladder Syndrome / Interstitial
Neurourology Multimodal Therapy for Interstitial Cystitis: A Pilot Study International Braz J Urol Vol. 35 (4): 467-474, July - August, 2009 Multimodal Therapy for Painful Bladder Syndrome / Interstitial
A Review on Interstitial Cystitis Syndrome (ICS)
 31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
31 Review Article A Review on Interstitial Cystitis Syndrome (ICS) M Sushma*, TVV Vidyadhar, R Mohanraj, M Babu Department of Pharmacy Practice, Raghavendra Institute of Pharmaceutical Education & Research
Information on Physical Therapy For Urogynecologic Problems
 Information on Physical Therapy For Urogynecologic Problems You have scheduled an appointment for evaluation and treatment of a urogynecologic problem. Following, you will find a pelvic floor questionnaire
Information on Physical Therapy For Urogynecologic Problems You have scheduled an appointment for evaluation and treatment of a urogynecologic problem. Following, you will find a pelvic floor questionnaire
Mini-Reviews. Interstitial Cystitis in Adolescents and Children: A Review
 J Pediatr Adolesc Gynecol (2004) 17:7 11 Mini-Reviews Interstitial Cystitis in Adolescents and Children: A Review T.F. Mattox, MD, FACOG Department of Obstetrics and Gynecology, Division of Urogynecology,
J Pediatr Adolesc Gynecol (2004) 17:7 11 Mini-Reviews Interstitial Cystitis in Adolescents and Children: A Review T.F. Mattox, MD, FACOG Department of Obstetrics and Gynecology, Division of Urogynecology,
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/573. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC. August 6, 2015
 Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
HISTORY PAPERWORK FOR APPOINTMENTS WITH DAVID A. PROPST, D.O.
 HISTORY PAPERWORK FOR APPOINTMENTS WITH DAVID A. PROPST, D.O. Name: Age: Room Number: Sex: MALE or FEMALE Dominant Hand: RIGHT or LEFT Height Weight Blood pressure HISTORY 1. Did your first symptoms begin
HISTORY PAPERWORK FOR APPOINTMENTS WITH DAVID A. PROPST, D.O. Name: Age: Room Number: Sex: MALE or FEMALE Dominant Hand: RIGHT or LEFT Height Weight Blood pressure HISTORY 1. Did your first symptoms begin
ABC/3TC/ZDV ABC PBO/3TC/ZDV
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The McMaster at night Pediatric Curriculum
 The McMaster at night Pediatric Curriculum Robinson, J, et al. and the Canadian Pediatric Society. Urinary tract infection in infants and children: Diagnosis and management. Pediatr Child Health 2014;
The McMaster at night Pediatric Curriculum Robinson, J, et al. and the Canadian Pediatric Society. Urinary tract infection in infants and children: Diagnosis and management. Pediatr Child Health 2014;
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
Addendum 1: International Consultation
 Reprinted with Permission of the International Consultation on Urological Diseases and the International Continence Society Hanno PM, Cervigni M, Dinis P, Lin A, Nickel JC, Nordling J, van Ophoven A, Ueda
Reprinted with Permission of the International Consultation on Urological Diseases and the International Continence Society Hanno PM, Cervigni M, Dinis P, Lin A, Nickel JC, Nordling J, van Ophoven A, Ueda
Dr. Aso Urinary Symptoms
 Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
Haematuria The presence of blood in the urine (haematuria) is always abnormal and may be the only indication of pathology in the urinary tract. False positive stick tests and the discolored urine caused
Bladder dysfunction in ALD and AMN
 Bladder dysfunction in ALD and AMN Sara Simeoni, MD Department of Uro-Neurology National Hospital for Neurology and Neurosurgery Queen Square, London 10:15 Dr Sara Simeoni- Bladder issues for AMN patients
Bladder dysfunction in ALD and AMN Sara Simeoni, MD Department of Uro-Neurology National Hospital for Neurology and Neurosurgery Queen Square, London 10:15 Dr Sara Simeoni- Bladder issues for AMN patients
David Henry Jablonski, M.D North Mills Avenue Orlando, FL 32803
 David Henry Jablonski, M.D. 1812 North Mills Avenue Orlando, FL 32803 EDUCATION: University of Florida College of Medicine, Gainesville, Florida Division of Urology Residency Training Program July 1994-June
David Henry Jablonski, M.D. 1812 North Mills Avenue Orlando, FL 32803 EDUCATION: University of Florida College of Medicine, Gainesville, Florida Division of Urology Residency Training Program July 1994-June
Patient Name: Date: Address: Primary Care Physician: Online Website On TV In print On the radio
 927 W. Myrtle St. Boise, ID 83702 (208) 947-0100 NEW PATIENT INTAKE Patient Name: Date: Email Address: Primary Care Physician: How did you hear about AVT? (Please mark all that apply) Online Website On
927 W. Myrtle St. Boise, ID 83702 (208) 947-0100 NEW PATIENT INTAKE Patient Name: Date: Email Address: Primary Care Physician: How did you hear about AVT? (Please mark all that apply) Online Website On
MEDitorial March Bladder Cancer
 MEDitorial March 2010 Bladder Cancer Last month, my article addressed the issue of blood in the urine ( hematuria ). A concerning cause of hematuria is bladder cancer, a variably malignant tumor starting
MEDitorial March 2010 Bladder Cancer Last month, my article addressed the issue of blood in the urine ( hematuria ). A concerning cause of hematuria is bladder cancer, a variably malignant tumor starting
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
2.0 Synopsis. ABT-333 M Clinical Study Report R&D/09/956
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011
 Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
IC/BPS Symptom Burden
 IC/BPS Symptom Burden Results from an Interstitial Cystitis Association (ICA) Survey Supported by an unrestricted grant from Aquinox Pharmaceuticals, Inc. Survey Response Interstitial Cystitis Association
IC/BPS Symptom Burden Results from an Interstitial Cystitis Association (ICA) Survey Supported by an unrestricted grant from Aquinox Pharmaceuticals, Inc. Survey Response Interstitial Cystitis Association
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
743 Jefferson Avenue Suite 203 Scranton, Pennsylvania VOIDING DIARY. Column #3 LEAK
 743 Jefferson Avenue Suite 203 Scranton, Pennsylvania 18510 570.344.9997 VOIDING DIARY This paperwork MUST be completed prior to your appointment. If not, your appointment will need to be rescheduled.
743 Jefferson Avenue Suite 203 Scranton, Pennsylvania 18510 570.344.9997 VOIDING DIARY This paperwork MUST be completed prior to your appointment. If not, your appointment will need to be rescheduled.
Urinary Tract Infections
 Urinary Tract Infections Michelle Eslami, M.D., FACP Professor of Medicine Division of Geriatrics David Geffen SOM at UCLA Urinary Tract Infection (UTI) One of most common infections in outpatient and
Urinary Tract Infections Michelle Eslami, M.D., FACP Professor of Medicine Division of Geriatrics David Geffen SOM at UCLA Urinary Tract Infection (UTI) One of most common infections in outpatient and
Diagnosis and Mangement of Nocturia in Adults
 Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Summary ID#236 Clinical Study Summary: Study B1Y-MC-HCCJ
 CT Registry ID#236 Page 1 Summary ID#236 Clinical Study Summary: Study B1Y-MC-HCCJ Title of Study: Fluoxetine: Fluoxetine versus Placebo in Adolescent Depressed Patients Investigator(s): This single-center
CT Registry ID#236 Page 1 Summary ID#236 Clinical Study Summary: Study B1Y-MC-HCCJ Title of Study: Fluoxetine: Fluoxetine versus Placebo in Adolescent Depressed Patients Investigator(s): This single-center
Please fill out the following form in as much detail as possible. Please Print. Name. Address. City State Zip. Home Phone Office Phone.
 CASE NO. Please fill out the following form in as much detail as possible. Please Print Date Name Address City State Zip Home Phone Office Phone E-mail Address Age Date of Birth Occupation Sex (M) (F)
CASE NO. Please fill out the following form in as much detail as possible. Please Print Date Name Address City State Zip Home Phone Office Phone E-mail Address Age Date of Birth Occupation Sex (M) (F)
BLADDER HEALTH. Painful Bladder AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION
 BLADDER HEALTH Painful Bladder Interstitial Cystitis AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION Don t Let Interstitial Cystitis Keep You from Enjoying Life. many people have
BLADDER HEALTH Painful Bladder Interstitial Cystitis AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION Don t Let Interstitial Cystitis Keep You from Enjoying Life. many people have
Citation for published version (APA): Bade, J. J. (1996). Interstitial cystitis: new clinical aspects Groningen: s.n.
 University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Interstitial cystitis Bade, Jurjen Jacob IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
UROLOGY CENTER OF PALM BEACH, P.A.
 UROLOGY CENTER OF PALM BEACH, P.A. Name Date Date of Birth Age Sex (circle one) M F Social Security # Marital Status (circle one) Married Divorced Single Widowed Separated Home Address City Zip Home Phone
UROLOGY CENTER OF PALM BEACH, P.A. Name Date Date of Birth Age Sex (circle one) M F Social Security # Marital Status (circle one) Married Divorced Single Widowed Separated Home Address City Zip Home Phone
Interstitial Cystitis. Dr. Gerard Testa
 Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
Interstitial Cystitis Dr. Gerard Testa Auto Immune Disease Infection Glycosaminoglycan layer deficiency Reflex Sympathetic Dystrophy? Hereditary Classification of Interstitial Cystitis Ulcerative Non Ulcerative
Hemorrhagic cystitis
 Hemorrhagic cystitis Bradley Rosenberg, MD Assistant Professor of Urology Oakland University William Beaumont School of Medicine Comprehensive Urology Hemorrhagic cystitis Definition: Sustained hematuria
Hemorrhagic cystitis Bradley Rosenberg, MD Assistant Professor of Urology Oakland University William Beaumont School of Medicine Comprehensive Urology Hemorrhagic cystitis Definition: Sustained hematuria
North Georgia Urology Center, P.C. Urology Patient Questionnaire
 Today s Date North Georgia Urology Center, P.C. Urology Patient Questionnaire Dear Patients: to help the urologist give you better care, please take a moment of valuable time to answer the following questions.
Today s Date North Georgia Urology Center, P.C. Urology Patient Questionnaire Dear Patients: to help the urologist give you better care, please take a moment of valuable time to answer the following questions.
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Support for Acetaminophen 1000 mg Over-the-Counter Dose:
 Support for Acetaminophen 1000 mg Over-the-Counter Dose: The Dental Impaction Pain Model and Efficacy and Safety Results from McNeil Randomized, Double-Blind, Single-Dose Study of Acetaminophen 1000 mg,
Support for Acetaminophen 1000 mg Over-the-Counter Dose: The Dental Impaction Pain Model and Efficacy and Safety Results from McNeil Randomized, Double-Blind, Single-Dose Study of Acetaminophen 1000 mg,
Posterior Tibial Nerve Stimulation for Voiding Dysfunction
 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
City State Zip Code. Ethnic Background: Caucasian African-American Asian Hispanic Native American. Previous. Hobbies/Leisure activities:,,,
 History # UPIN # (Please leave blank) Name: First M.I. Last Address: Street (Apt #) City State Zip Code Phone number: ( ) ( ) Home Business Birth Date: / / Day-Month-Year Gender: M F Marital status: (Maiden
History # UPIN # (Please leave blank) Name: First M.I. Last Address: Street (Apt #) City State Zip Code Phone number: ( ) ( ) Home Business Birth Date: / / Day-Month-Year Gender: M F Marital status: (Maiden
Increasing Awareness, Diagnosis, and Treatment of BPH, LUTS, and EP
 Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
Clinical Trial Synopsis TL , NCT#
 Clinical Trial Synopsis, NCT#00492011 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Ability of Ramelteon 1 mg, 4 mg, and 8 mg to Alleviate the Insomnia
Clinical Trial Synopsis, NCT#00492011 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Ability of Ramelteon 1 mg, 4 mg, and 8 mg to Alleviate the Insomnia
