ASTM Committee F04 Tuesday 11/13/2012
|
|
|
- Lorin Tyler
- 5 years ago
- Views:
Transcription
1 ASTM Committee F04 Tuesday 11/13/2012 Centennial Regency Ballroom Ballroom III V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM F04 Celebration of Committee F04's 50th Anniversary F04 Attendees are invited to an ASTM Training Session on ASTM Online 1:00 PM 1:00 PM 2:00 PM 2:00 PM 4:00 PM 4:00 PM 5:00 PM 5:00 PM November 01, 2012 Page 1
2 ASTM Committee F04 Wednesday 11/14/2012 Centennial Chicago A Chicago B Courtland Dunwoody Ballroom III 7:00 AM F04.39 Human Clinical Trials 7:00 AM 8:00 AM 8:00 AM F04 Third Symposium on 8:15 AM 8:15 AM Fatigue and F04.44 New Item Fracture Metallic Follow up Activity Medical Materials for SG for Bone and Devices F Inductive Cleanliness Materials, Validation WK21567 SG for Assessment Vascular Graft TEMPs, WK26326 Heart Valves F04.38 Computer Assisted Orthopaedic Surgical Systems F Device Retrieval F04.44 Wk29355 SG Assessment able Devices... (Reappr. F ),WK31014 TM Goat for in vivo Testing Articular Cartilage Repair or Regeneration, WK8035 SG for In Vivo Assessment TEMPs for Knee Meniscus Surgical Repair/Reconstruc t. 10:00 AM 10:00 AM 10:15 AM 11:00 AM 11:30 AM F04.97 Terminology and Classification F Revision of F1160(Combined with F Revision of F1854 F04.44 WK28852 SG for Metaphyseal Defect Model, WK28783 Preclinical models rotator cuff repair and New Items - Load vs. Non- Load/Micro CT/Sinus Lift in vivo & Muscle Defect Model 10:15 AM 11:00 AM 11:30 AM F :00 PM Corrosion Testing 1:00 PM November 01, 2012 Page 2
3 ASTM Committee F04 Wednesday 11/14/2012 Centennial Chicago A Chicago B Courtland Dunwoody Ballroom III 1:15 PM F04.42 WK6507 Reference Scaffolds and New Items Measuring effect of catheters on stem cells (cytocompatibility) & SG Assessing in vitro Release of Biomolecules from Matrices in TEMPs 1:15 PM 2:00 PM 2:00 PM 2:15 PM 2:15 PM F04.42 "Issues & unscheduled items 2:30 PM F MRI 2:30 PM (incl. terminology Compatibility ballots & WK29637 Absorb. Guide);New Work Items F04.04 Division lv- TEMPs Wrap Up F Session Radiopacity 3:45 PM Testing (Combined 3:45 PM with 4:00 PM 4:00 PM 4:30 PM 4:30 PM 5:00 PM 5:00 PM November 01, 2012 Page 3
4 ASTM Committee F04 Wednesday 11/14/2012 Grand Hall D Regency Ballroom Regency Ballroom Spring V VI 7:00 AM 7:00 AM 8:00 AM 8:00 AM 8:15 AM 8:15 AM F04.25 Spinal F Metal Devices on Metal Clinical 10:00 AM 10:00 AM Characterization 10:15 AM 10:15 AM 11:00 AM 11:00 AM 11:30 AM 11:30 AM F04 Attendees are invited to an ASTM Training Session on Developing and Revising an ASTM F04.90 Executive 1:00 PM 1:00 PM 1:15 PM 1:15 PM 2:00 PM 2:00 PM 2:15 PM 2:15 PM 2:30 PM F :30 PM.10/.07/.11/.22 F Polymeric Agenda Items: Hip F04.25 Spinal Wear (including Materials Planning Devices M/M & C/C guide task group; F04.11 Sub) F1714); ASTM F2091; Knee Wear; Patella F PAEK 3:45 PM Wear/ Patella Test Polymers joint with 3:45 PM Methods F (Combine with 4:00 PM F04.11 Sub) 4:00 PM 4:30 PM 5:00 PM F PEEK characterization/te st methods joint with F :30 PM 5:00 PM 5:30 PM 5:30 PM November 01, 2012 Page 4
5 Baker Chicago A Chicago C Hanover B Hanover C 7:00 AM 7:00 AM 8:00 AM F04.12 Metallurgical Materials, SubCommittee 8:00 AM 8:30 AM 8:45 AM F04.12 WK F67 - Unalloyed Titanium, for Surgical R50250, UNS R50400, UNS R50550, UNS R50700) F04.12 WK F86 Practice for Surface Preparation and Marking of Metallic Surgical s F04.93 US TAG to ISO/TC 150 8:30 AM 8:45 AM F04.12 WK27066/WK F136 Wrought Titanium- 6 Aluminum-4 Vanadium ELI (Extra Low Interstitial) Alloy R56401) F04.21 Osteosynthesis F04.13 Combined Meeting of TG's and sub (F F1926, F F2224, F CaP Cements and F04.13 Subcommittee) F04.12 WK F138 Wrought 18Chromium- 14Nickel- 2.5Molybdenum Stainless Steel Bar and Wire for Surgical s (UNS S31673) F04.12 WK F139 Wrought 18Chromium- 14Nickel- 2.5Molybdenum Stainless Steel Sheet and Strip for Surgical s (UNS S31673) F UHMWPE Materials (F and F are being incorporated into F ) F04.11 Sub) F UHMWPE November 01, 2012 Page 5
6 Baker Chicago A Chicago C Hanover B Hanover C 9:45 AM 10:00 AM F04.12 F560 Unalloyed Tantalum for Surgical R05200, UNS R05400) F04.12 WK F562 Wrought 35Cobalt- 35Nickel- 20Chromium- 10Molybdenum Alloy R30035) F04.92 Planning Characterization (joint with F & F ) 9:45 AM 10:00 AM 10:15 AM F04.12 F601 - Practice for Fluorescent Penetrant Inspection of Metallic Surgical s F04.12 WK F621 Stainless Steel Forgings for Surgical s 10:15 AM 10:45 AM 10:45 AM 11:00 AM F04.12 WK37163/WK F899 Wrought Stainless Steels Instruments 11:00 AM 11:15 AM F04.12 F961 35Cobalt- 35Nickel- 20Chromium- 10Molybdenum Alloy Forgings for Surgical s (UNS R30035) 11:15 AM November 01, 2012 Page 6
7 Baker Chicago A Chicago C Hanover B Hanover C 11:30 AM 11:45 AM F04.12 F1058 Wrought 40Cobalt- 20Chromium- 16Iron-15Nickel- 7Molybdenum Alloy Wire and Strip R30003 and UNS R30008) F04.12 WK F1091 Wrought Cobalt- 20Chromium- 15Tungsten- 10Nickel Alloy Surgical Fixation Wire (UNS R30605) F Hydrogel Mechanical Testing (Combined with 11:30 AM 11:45 AM 12:30 PM F04.94 Finance 12:30 PM 1:00 PM 1:15 PM F04.12 WK F1314 Wrought Nitrogen Strengthened 22 Chromium - 13 Nickel - 5 Manganese Molybdenum Stainless Steel Alloy Bar and Wire s (UNS S20910) F04.12 F1350 Wrought 18Chromium- 14Nickel- 2.5Molybdenum Stainless Steel Surgical Fixation Wire (UNS S31673) F Absorbable Polymers F04.11 Sub) F Absorbable Polymer Test Methods (joint with F Bioabsorbable Polymers) 1:00 PM 1:15 PM 1:30 PM F04.12 WK F1377 Cobalt- 28Chromium- 6Molybdenum Powder for Coating of Orthopedic s (UNS R30075) F04.16 Biocompatibility Testing 1:30 PM November 01, 2012 Page 7
8 Baker Chicago A Chicago C Hanover B Hanover C 1:45 PM 2:00 PM 2:15 PM 2:30 PM F04.12 F1472 Wrought Titanium- 6Aluminum- 4Vanadium Alloy R56400) F04.12 Wk F1586 Wrought Titanium- 6Aluminum- 4Vanadium Alloy R56400) F04.12 WK F1713 Wrought Titanium- 13Niobium- 13Zirconium Alloy R58130) F04.12 WK32231/WK F1813 Wrought Titanium- 12 Molybdenum-6 Zirconium-2 Iron Alloy (UNS R58120) 1:45 PM 2:00 PM 2:15 PM 2:30 PM 2:45 PM F04.12 WK F2063 Wrought Nickel- Titanium Shape Memory Alloys for Medical Devices and Surgical s 2:45 PM F04.12 WK F2146 Wrought Titanium- 3Aluminum- 2.5Vanadium Alloy Seamless Tubing R56320) F04.91 Awards F Acrylic Bone Cement (Joint with F ) F04.11 sub) F Mechanical Testing of Bone Cement (Joint with F ) November 01, 2012 Page 8
9 Baker Chicago A Chicago C Hanover B Hanover C 3:15 PM 3:45 PM 4:00 PM F04.12 WK F2229 Wrought, Nitrogen Strengthened 23Manganese- 21Chromium- 1Molybdenum Low-Nickel Stainless Steel Alloy Bar and Wire s (UNS S29108) F04.12 WK F2581 Wrought Nitrogen Strengthened 11Manganese- 17Chromium- 3Molybdenum Low-Nickel Stainless Steel Alloy Bar and Wire s (UNS S29225) F04.12 F2633 Wrought Seamless Nickel-Titanium Shape Memory Alloy Tube for Medical Devices and Surgical s F04.12 WK New Specification for Wrought Titanium- 3Aluminum- 2.5Vanadium Alloy R56320) 3:15 PM 3:45 PM 4:00 PM 4:15 PM F04.12 WK New New Metal Injection Molded Unalloyed Titanium Components for Surgical Applications 4:15 PM 4:30 PM 4:30 PM 5:00 PM 5:00 PM November 01, 2012 Page 9
10 Hanover F Regency Ballroom Vinings V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 8:30 AM 8:30 AM 8:45 AM F04.43 WK11468 SP in situ visualization quantification of mineralized matrix using image analysis, WK28890 SP in 8:45 AM vitro osteoblastic differentiation F Agenda Item: Status/Discussion of Formatting of F04.43 New Items Device Labeling - Follow up for Arthroplasty documents to TM Devices for Automated Colony Forming Unit (CFU) Assays 9:45 AM & Characterization of alginate foam as 9:45 AM a 3D cell culture system & tissue scaffold 10:00 AM 10:00 AM 10:15 AM 10:15 AM F /.26 F04.43 WK36232 Agenda Items: TM for Measuring 10:45 AM Shoulder TM 10:45 AM Adhesion of (F2028, Reverse Chondrocytes to Shoulder TM); PS Surfaces by Elbow Spec. Centrifug'n Assay, New Item PRP Workshop May :00 AM 11:00 AM F04.43 New Items F :15 AM Allogeneic stem.12/.14/.22/.23 cells & "Guide Agenda Items: 11:15 AM 11:30 AM Processing Cells, Tissues, Organs for Use in TEMPs Tibial Tray TM (Mobile Bearing, Tibial Tray F04.33 Medical/Surgical 11:30 AM 11:45 AM (F2210 Fatigue: F1800); Instruments - withdrawal); SG Knee Spec. Rev of s Review 11:45 AM Preservation Tissue Engineered Medical Products (Rev. F2386); F2083; Patella Rev of F1672; F1223; Discussion of ISO Knee s Ballot Items 12:30 PM 12:30 PM 1:00 PM 1:00 PM 1:15 PM F04.41 WK30587 SG Classification of Therapeutic 1:15 PM 1:30 PM Skin Substitutes (Rev. F2311) 1:30 PM November 01, 2012 Page 10
11 Hanover F Regency Ballroom Vinings V 1:45 PM 1:45 PM 2:00 PM 2:00 PM 2:15 PM F04.46 New Item SG Develop High- Quality Assy Biological Fxn 2:15 PM 2:30 PM Cell-Based 2:30 PM F Therapies & TE Agenda Item: 2:45 PM Products, Finite Element 2:45 PM WK17626 STD TM Analysis in Quantitating the Orthopedics Spread Area of Cells on a Material Substrate, WK33434 New Updates 3:15 PM 3:15 PM F04.04 Division lv - TEMPs - Wrap F04.22 Up Sessions.09/.13/.17/ISO- Agenda Items: Hips Stds. Agenda Items: Rev of F1820; 3:45 PM Environmental 3:45 PM Fatigue Testing of 4:00 PM Acetabular Cup (WK28883); 4:00 PM 4:15 PM F1440; F2345; Discussion of ISO 4:15 PM Hip s 4:30 PM Ballot Items 4:30 PM 5:00 PM 5:00 PM 6:00 PM F04.22 Wrap up - Informational Meeting only - No voting 6:00 PM 6:30 PM 6:30 PM November 01, 2012 Page 11
12 ASTM Committee F04 Friday 11/16/2012 Hanover E 7:00 AM 7:00 AM 8:00 AM 8:00 AM 8:45 AM 8:45 AM F04.11 Polymeric Materials (for information only) F04.15 Material Test Methods (for information only) 9:45 AM 9:45 AM 10:00 AM F04 Medical and Surgical Materials and Devices - Main 10:00 AM 11:00 AM 11:00 AM 1:00 PM 1:00 PM 2:00 PM 2:00 PM 4:00 PM 4:00 PM 5:00 PM 5:00 PM November 01, 2012 Page 12
ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9
 ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9 8:00 AM 8:00 AM 10:00 AM 10:00 AM F04 Attendees are invited to an ASTM Training Session on Responsibilities for Subcommittee Chairman,
ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9 8:00 AM 8:00 AM 10:00 AM 10:00 AM F04 Attendees are invited to an ASTM Training Session on Responsibilities for Subcommittee Chairman,
Hip Joint Prostheses
 Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
YY Translated English of Chinese Standard: YY
 Translated English of Chinese Standard: YY0341-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.40 C 35 Pharmaceutical Industry Standard of the People s Republic of China YY/T 0341-2009
Translated English of Chinese Standard: YY0341-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.40 C 35 Pharmaceutical Industry Standard of the People s Republic of China YY/T 0341-2009
DRAFT GUIDANCE DOCUMENT List of Recognized Standards
 DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
Identify the role of the skeletal system particularly in relation to maintaining an upright stance and protecting vital organs.
 9.3.3 The wide range of movements, continual absorption of shocks and diseases make the skeletal system vulnerable to damage but new technologies are allowing the replacement of some damaged structures.
9.3.3 The wide range of movements, continual absorption of shocks and diseases make the skeletal system vulnerable to damage but new technologies are allowing the replacement of some damaged structures.
In the last decade of the 20 th century, special emphasis was put on an emerging field of science: Tissue engineering,which combines the state of the
 In the last decade of the 20 th century, special emphasis was put on an emerging field of science: Tissue engineering,which combines the state of the art materials science with concepts from the life sciences.
In the last decade of the 20 th century, special emphasis was put on an emerging field of science: Tissue engineering,which combines the state of the art materials science with concepts from the life sciences.
Individual oncological implants
 Individual oncological implants Catalogue - individual oncological implants ONCOLOGICAL Materials for implants List of materials MATERIAL ISO ČSN DIN ASTM Stainless steel 5832-1 17350 výběr 17 443 W.Nr.
Individual oncological implants Catalogue - individual oncological implants ONCOLOGICAL Materials for implants List of materials MATERIAL ISO ČSN DIN ASTM Stainless steel 5832-1 17350 výběr 17 443 W.Nr.
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EMPIRICAL TESTING CORP. 4628 Northpark Drive Colorado Springs, CO 80918 Dawn Lissy Phone: 719 264 9937 dlissy@empiricaltesting.com MECHANICAL Valid To: January
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EMPIRICAL TESTING CORP. 4628 Northpark Drive Colorado Springs, CO 80918 Dawn Lissy Phone: 719 264 9937 dlissy@empiricaltesting.com MECHANICAL Valid To: January
[ICESTM-2018] ISSN Impact Factor
![[ICESTM-2018] ISSN Impact Factor [ICESTM-2018] ISSN Impact Factor](/thumbs/88/117546298.jpg) GLOBAL JOURNAL OF ENGINEERING SCIENCE AND RESEARCHES MODELING AND FINITE ELEMENT ANALYSIS OF KNEE JOINT PROSTHESIS U.D.S.Prathap varma *1,S.Rajesh 2, B.Suresh Kumar 3 & P.Rama Murthy Raju 4 *1 M.TechScholar,
GLOBAL JOURNAL OF ENGINEERING SCIENCE AND RESEARCHES MODELING AND FINITE ELEMENT ANALYSIS OF KNEE JOINT PROSTHESIS U.D.S.Prathap varma *1,S.Rajesh 2, B.Suresh Kumar 3 & P.Rama Murthy Raju 4 *1 M.TechScholar,
Total Knee Replacement
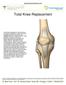 Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Registration 1:00 pm 4:00 pm. Sponsor and Exhibitor Display Setup 1:00 pm 4:00 pm. Early Morning Coffee Service 7:00 am 9:00 am
 2015 Exploration & Production Standards Conference on Oilfield Equipment and Materials June 22-26, 2015 Hyatt Regency San Francisco San Francisco, California www.api.org Current as of June 9, 2015 Subject
2015 Exploration & Production Standards Conference on Oilfield Equipment and Materials June 22-26, 2015 Hyatt Regency San Francisco San Francisco, California www.api.org Current as of June 9, 2015 Subject
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX
 HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007)
 BRITISH STANDARD Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007) ICS 11.040.40 NO COPYING WITHOUT BSI PERMISSION EXCEPT AS PERMITTED BY COPYRIGHT LAW BS
BRITISH STANDARD Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007) ICS 11.040.40 NO COPYING WITHOUT BSI PERMISSION EXCEPT AS PERMITTED BY COPYRIGHT LAW BS
Sunday, 24 August 2008
 Sunday, 24 August 2008 - Theatre 1 Theatre 2 Grand Hall SIROT: Biomechanics SIROT: Osteoporosis SIROT: Infections SIROT: Joint Replacement Break -12:00 12:00-16:45 17:30-19:00 SIROT: Fracture Healing SIROT:
Sunday, 24 August 2008 - Theatre 1 Theatre 2 Grand Hall SIROT: Biomechanics SIROT: Osteoporosis SIROT: Infections SIROT: Joint Replacement Break -12:00 12:00-16:45 17:30-19:00 SIROT: Fracture Healing SIROT:
Instructions for Use
 Instructions for Use Non Cemented Stem without Coating Legend of Symbols Use on Packages Number in the Catalogue; Sterile Product Sterilized by Gamma Radiation; Manufacturing Date; Batch Number; Expiration
Instructions for Use Non Cemented Stem without Coating Legend of Symbols Use on Packages Number in the Catalogue; Sterile Product Sterilized by Gamma Radiation; Manufacturing Date; Batch Number; Expiration
What Are Hip and Knee Replacement Implants Made Of?
 What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
Knee Replacement Implants
 Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Revision systems. Catalogue - revision implants ARTHROPLASTY
 evision systems Catalogue revision implants ATHOPASTY Material for implants Contens ist of materials MATEIA ISO DIN ASTM Stainless steel Wrought high nitrogen stainless steel Unalloyed titanium 321 329
evision systems Catalogue revision implants ATHOPASTY Material for implants Contens ist of materials MATEIA ISO DIN ASTM Stainless steel Wrought high nitrogen stainless steel Unalloyed titanium 321 329
BIOMATERIALS. What is a Biomaterial? Attention! Info on Term Projects. Anything in contact with human body?
 Week Topic Instructor 1 Biomedical Engineering World Dr. S. Takaç 2 Biomed. Instrumentation and Signals Dr. Ö. Birgül YOU 3 Medical Imaging ARE Dr. Ö. Birgül 4 Biomechanics HERE Dr. C. Evrensel Attention!
Week Topic Instructor 1 Biomedical Engineering World Dr. S. Takaç 2 Biomed. Instrumentation and Signals Dr. Ö. Birgül YOU 3 Medical Imaging ARE Dr. Ö. Birgül 4 Biomechanics HERE Dr. C. Evrensel Attention!
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Acknowledgments Introduction p. 1 Objectives p. 1 Goals p. 2 History of Dental Materials p. 3 The Oral Environment p. 4 Characteristics of the Ideal
 Preface p. v Acknowledgments p. vii Introduction p. 1 Objectives p. 1 Goals p. 2 History of Dental Materials p. 3 The Oral Environment p. 4 Characteristics of the Ideal Dental Material p. 5 Quality Assurance
Preface p. v Acknowledgments p. vii Introduction p. 1 Objectives p. 1 Goals p. 2 History of Dental Materials p. 3 The Oral Environment p. 4 Characteristics of the Ideal Dental Material p. 5 Quality Assurance
BIOMATERIALS Maxillofacial & Skull Implants. Brendan Boyd & Stuart Mah
 BIOMATERIALS Maxillofacial & Skull Implants Brendan Boyd & Stuart Mah Outline Of Presentation Basic Anatomy of Face and Skull Reasons/Defects responsible for maxillofacial & skull surgery History of Alloplastic
BIOMATERIALS Maxillofacial & Skull Implants Brendan Boyd & Stuart Mah Outline Of Presentation Basic Anatomy of Face and Skull Reasons/Defects responsible for maxillofacial & skull surgery History of Alloplastic
CHAPTER 1. Clinical Problems Requiring Implants for Solution: Defining the Problem
 CHAPTER 1 Clinical Problems Requiring Implants for Solution: Defining the Problem 1.1 Objectives of Subject 1.2 Related Subjects at MIT 1.3 Terminology 1.4 How Selected Tissues/Organs Function 1.5 Effects
CHAPTER 1 Clinical Problems Requiring Implants for Solution: Defining the Problem 1.1 Objectives of Subject 1.2 Related Subjects at MIT 1.3 Terminology 1.4 How Selected Tissues/Organs Function 1.5 Effects
Designed for life. Implant technology with unmatched wear performance
 Designed for life Implant technology with unmatched wear performance Designed for life Wear performance combined with a multitude of options so you can find the right combination to restore the joints
Designed for life Implant technology with unmatched wear performance Designed for life Wear performance combined with a multitude of options so you can find the right combination to restore the joints
ReCap Product Rationale
 ReCap Product Rationale A Complete resurfacing system designe engineering principles, tried and tested m clinically proven fixation methods Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales
ReCap Product Rationale A Complete resurfacing system designe engineering principles, tried and tested m clinically proven fixation methods Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
Surface evaluation of orthopedic hip implants marketed in Brazil
 Surface evaluation of orthopedic hip implants marketed in Brazil M.M.Souza 1, R.M. Trommer 2, M.M.Maru 2, C.R.M.Roesler 3, W.S.Barros 1, M.S. Dutra 3 1 Dimensional Metrology Laboratory, Inmetro. Brazil;
Surface evaluation of orthopedic hip implants marketed in Brazil M.M.Souza 1, R.M. Trommer 2, M.M.Maru 2, C.R.M.Roesler 3, W.S.Barros 1, M.S. Dutra 3 1 Dimensional Metrology Laboratory, Inmetro. Brazil;
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Replacement Heart Valve Product Description (Stented Tissue) Models Reference
 Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Contents SECTION 1: GENERAL TRAUMA AND RECONSTRUCTIVE HIP SURGERY
 SECTION 1: GENERAL TRAUMA AND RECONSTRUCTIVE HIP SURGERY 1. Acetabular and Pelvic Fractures...3 2. Acetabular Orientation (Total Hips)...6 3. Acetabular Osteotomy...7 4. Achilles Tendon Ruptures...9 5.
SECTION 1: GENERAL TRAUMA AND RECONSTRUCTIVE HIP SURGERY 1. Acetabular and Pelvic Fractures...3 2. Acetabular Orientation (Total Hips)...6 3. Acetabular Osteotomy...7 4. Achilles Tendon Ruptures...9 5.
Replacement Heart Valve Product Description (Stented Tissue) Models Reference
 Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Dear Imaging Center: This letter is in response to your inquiry concerning the safety of performing magnetic resonance (MR) procedures in patients who have been implanted with Edwards Lifesciences LLC
Accumulation in Liver and Spleen of Metal Particles Generated at Nonbearing Surfaces in Hip Arthroplasty
 The Journal of Arthroplasty Vol. 19 No. 8 Suppl. 3 2004 Accumulation in Liver and Spleen of Metal Particles Generated at Nonbearing Surfaces in Hip Arthroplasty Robert M. Urban, AS, Michael J. Tomlinson,
The Journal of Arthroplasty Vol. 19 No. 8 Suppl. 3 2004 Accumulation in Liver and Spleen of Metal Particles Generated at Nonbearing Surfaces in Hip Arthroplasty Robert M. Urban, AS, Michael J. Tomlinson,
Development and Analysis of Composite Material for In-Situ Hip Prosthesis
 Development and Analysis of Composite Material for In-Situ Hip Prosthesis R.Gowthaman P.G. Student, Manufacturing Engineering, Department of Mechanical Engineering, Thanthai Periyar Govt. Institute of
Development and Analysis of Composite Material for In-Situ Hip Prosthesis R.Gowthaman P.G. Student, Manufacturing Engineering, Department of Mechanical Engineering, Thanthai Periyar Govt. Institute of
Knee spanning solutions
 Knee spanning solutions System features Indications Intended to be used on adults or pediatric patients as required for fracture fixation (open or closed); post-traumatic joint contracture which has resulted
Knee spanning solutions System features Indications Intended to be used on adults or pediatric patients as required for fracture fixation (open or closed); post-traumatic joint contracture which has resulted
EC Declaration of Conformity
 EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
CURRICULUM VITAE CHRISTINE STEUBBEN HEIM Ward Drive Chesterland, OH Telephone:
 EDUCATION CURRICULUM VITAE CHRISTINE STEUBBEN HEIM 12655 Ward Drive Chesterland, OH 44026 Telephone: +440 729 9274 B.Sc. (Biomedical Engineering) Case Western Reserve University, Cleveland, Ohio (1992)
EDUCATION CURRICULUM VITAE CHRISTINE STEUBBEN HEIM 12655 Ward Drive Chesterland, OH 44026 Telephone: +440 729 9274 B.Sc. (Biomedical Engineering) Case Western Reserve University, Cleveland, Ohio (1992)
The latest and greatest in Orthopedics!
 The latest and greatest in Orthopedics! Disclosures: None Objectives: "The most important changes in contemporary orthopedics" Locking plates for fractures Advanced bearing surfaces for hip and knee replacements
The latest and greatest in Orthopedics! Disclosures: None Objectives: "The most important changes in contemporary orthopedics" Locking plates for fractures Advanced bearing surfaces for hip and knee replacements
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
SICHERHEITSDATENBLATT
 QUALITÄT BIORESORB PITT-EASY SICHERHEITSDATENBLATT Quality Assurance for SICHERUNG Sybron Implant Solutions s BICORTICAL QUALITÄTSSICHERUNGCYTOPLAST Safety Data Sheet PITT-EASY Safety Data Sheet for the
QUALITÄT BIORESORB PITT-EASY SICHERHEITSDATENBLATT Quality Assurance for SICHERUNG Sybron Implant Solutions s BICORTICAL QUALITÄTSSICHERUNGCYTOPLAST Safety Data Sheet PITT-EASY Safety Data Sheet for the
DUAL MOBILITY CUP, CEMENTED OR CEMENTLESS
 HIP Documentation DUAL MOBILITY CUP, CEMENTED OR CEMENTLESS >>DUAL MOBILITY ACETABULAR CUPS Dual mobility acetabular cup P r Gilles Bousquet invented the concept of dual mobility more than thirty years
HIP Documentation DUAL MOBILITY CUP, CEMENTED OR CEMENTLESS >>DUAL MOBILITY ACETABULAR CUPS Dual mobility acetabular cup P r Gilles Bousquet invented the concept of dual mobility more than thirty years
Calcium Phosphate Cement
 Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
CS 2.0. Product information. Intelligent innovations for a better life.
 CS 2.0 Product information Intelligent innovations for a better life. 02. 03 Syntellix AG MAGNEZIX Compression Screw 2.0 Introduction CAUTION This product description is not sufficient or adequate to allow
CS 2.0 Product information Intelligent innovations for a better life. 02. 03 Syntellix AG MAGNEZIX Compression Screw 2.0 Introduction CAUTION This product description is not sufficient or adequate to allow
LEGION Total Knee System. Exceptional Durability
 LEGION Total Knee System Exceptional Durability How many knee systems have been lab-tested to 30 years of simulated wear? Just one: LEGION Primary Knee System with VERILAST Technology The LEGION Primary
LEGION Total Knee System Exceptional Durability How many knee systems have been lab-tested to 30 years of simulated wear? Just one: LEGION Primary Knee System with VERILAST Technology The LEGION Primary
Featuring. Technology. Product Rationale
 Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
NEW DESIGN OF PURE TITANIUM METAL ON METAL TOTAL HIP REPLACEMENT WITH ION IMPLANTED WEAR RESISTANCE SURFACE
 NEW DESIGN OF PURE TITANIUM METAL ON METAL TOTAL HIP REPLACEMENT WITH ION IMPLANTED WEAR RESISTANCE SURFACE WALUYO AD1 SISWANTO GERAN PENYELIDIKAN SCIENCEFUND NO. VOT. SO15 UNIVERSITI TUN HUSSEIN ONN MALAYSIA
NEW DESIGN OF PURE TITANIUM METAL ON METAL TOTAL HIP REPLACEMENT WITH ION IMPLANTED WEAR RESISTANCE SURFACE WALUYO AD1 SISWANTO GERAN PENYELIDIKAN SCIENCEFUND NO. VOT. SO15 UNIVERSITI TUN HUSSEIN ONN MALAYSIA
Trabecular Metal Technology The Best Thing Next to Bone
 Trabecular Metal Technology The Best Thing Next to Bone Trabecular Metal Material resembles trabecular bone in its cellular structure and weight-bearing characteristics. By approximating the mechanical
Trabecular Metal Technology The Best Thing Next to Bone Trabecular Metal Material resembles trabecular bone in its cellular structure and weight-bearing characteristics. By approximating the mechanical
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY
 INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK STUDY AND ANALYSIS OF KNEE IMPLANT IN HUMAN BODY GAJANAN D. MANDAVGADE Assistant
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK STUDY AND ANALYSIS OF KNEE IMPLANT IN HUMAN BODY GAJANAN D. MANDAVGADE Assistant
ORTHOPEDICS BONE Recalcitrant nonunions In total hip replacement total knee surgery increased callus volume
 ORTHOPEDICS Orthopedics has to do with a variety of tissue: bone, cartilage, tendon, ligament, muscle. In this regard orthopedic and sports medicine share the same tissue targets. Orthopedics is mostly
ORTHOPEDICS Orthopedics has to do with a variety of tissue: bone, cartilage, tendon, ligament, muscle. In this regard orthopedic and sports medicine share the same tissue targets. Orthopedics is mostly
Catalog Trauma Implants. Instruments. Sets. Instruments and implants approved by the AO Foundation
 Catalog Trauma 2011 Implants Instruments Sets Instruments and implants approved by the AO Foundation R Historical Background The Association for the Study of Internal Fixation (in German: Arbeitsgemeinschaft
Catalog Trauma 2011 Implants Instruments Sets Instruments and implants approved by the AO Foundation R Historical Background The Association for the Study of Internal Fixation (in German: Arbeitsgemeinschaft
PyroCarbon Implants Durability + Biocompatibility
 PyroCarbon Implants Durability + Biocompatibility What is PyroCarbon? PyroCarbon is a specific form of carbon that has been tailored for durability and biocompatibility. PyroCarbon exhibits favorable wear
PyroCarbon Implants Durability + Biocompatibility What is PyroCarbon? PyroCarbon is a specific form of carbon that has been tailored for durability and biocompatibility. PyroCarbon exhibits favorable wear
UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD
 UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 66th Annual Meeting February 4-9, 1999 Anaheim, California COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON
UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 66th Annual Meeting February 4-9, 1999 Anaheim, California COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
TOTAL HIP REPLACEMENT:
 THR Prosthesis Design TOTAL HIP REPLACEMENT: PROSTHESIS DESIGN FEATURES JESS JOHNSTON & MELINDA ZIETH History of Hip Prosthesis Joint Replacement Registry Implant Design Technology & Future History and
THR Prosthesis Design TOTAL HIP REPLACEMENT: PROSTHESIS DESIGN FEATURES JESS JOHNSTON & MELINDA ZIETH History of Hip Prosthesis Joint Replacement Registry Implant Design Technology & Future History and
ISO INTERNATIONAL STANDARD. Non-active surgical implants Joint replacement implants Particular requirements
 INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
LPS SYSTEM POCKET GUIDE
 LPS SYSTEM POCKET GUIDE Implants Procedural Uses Instruments & Trials L P S Limb Preservation System L P S Limb Preservation System The purpose of this document is to review the LPS (Limb Preservation
LPS SYSTEM POCKET GUIDE Implants Procedural Uses Instruments & Trials L P S Limb Preservation System L P S Limb Preservation System The purpose of this document is to review the LPS (Limb Preservation
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Revision of a tibial baseplate using a customized oxinium component in a case of suspected metal allergy A case report
 Acta Orthop. Belg., 2011, 77, 691-695 CASE REPORT Revision of a tibial baseplate using a customized oxinium component in a case of suspected metal allergy A case report Nick VAN OPStAl, Frank VERhEyDEN
Acta Orthop. Belg., 2011, 77, 691-695 CASE REPORT Revision of a tibial baseplate using a customized oxinium component in a case of suspected metal allergy A case report Nick VAN OPStAl, Frank VERhEyDEN
39 year old, 260 lb. park ranger. TKA in situ: 4 Years. First reported Metal-backed, non-modular PCA knee. 16% incidence. Modular metalbacked
 Designer (Royalty income) - DePuy A Johnson & Johnson Company Consultant on Knee Products for Smith & Nephew Orthopaedics General Research Funding Inova Health System and the U.S. Army Medical Research
Designer (Royalty income) - DePuy A Johnson & Johnson Company Consultant on Knee Products for Smith & Nephew Orthopaedics General Research Funding Inova Health System and the U.S. Army Medical Research
ADDITIVE MANUFACTURING AND ANALYSIS OF TIBIAL INSERT IN TOTAL KNEE REPLACEMENT IMPLANT
 ADDITIVE MANUFACTURING AND ANALYSIS OF TIBIAL INSERT IN TOTAL KNEE REPLACEMENT IMPLANT Swathi Harish 1, V R Devadath 2 1 M.Tech Scholar, Department of Mechanical Engineering, PESCE, Mandya, Karnataka,
ADDITIVE MANUFACTURING AND ANALYSIS OF TIBIAL INSERT IN TOTAL KNEE REPLACEMENT IMPLANT Swathi Harish 1, V R Devadath 2 1 M.Tech Scholar, Department of Mechanical Engineering, PESCE, Mandya, Karnataka,
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Furlong H-A.C. THR System
 Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
PRODUCT SCHEDULE FOR CERTIFICATE CE JRI ORTHOPAEDICS LIMITED
 HIP STEM (Class III) Active Stem Active H-AC Stem / 09mm - 16mm Cemented Hemiarthroplasty (formally LOL 127) Cemented Hemiarthroplasty Stem / X-X-Small - Large Cemented Hemiarthroplasty Stem - Long / Small
HIP STEM (Class III) Active Stem Active H-AC Stem / 09mm - 16mm Cemented Hemiarthroplasty (formally LOL 127) Cemented Hemiarthroplasty Stem / X-X-Small - Large Cemented Hemiarthroplasty Stem - Long / Small
Section of Modular Hip Prostheses cemented. TMC-3 Modular Hip Prosthesis, cemented. TMC-3 Modular Hüftprothese, zementiert
 Section of Modular Hip Prostheses cemented TMC-3 Modular Hip Prosthesis, cemented TMC-3 Modular Hüftprothese, zementiert Prothèse de hanche modulaire TMC-3, cimentée Indication : The TMC-3 Modular hip
Section of Modular Hip Prostheses cemented TMC-3 Modular Hip Prosthesis, cemented TMC-3 Modular Hüftprothese, zementiert Prothèse de hanche modulaire TMC-3, cimentée Indication : The TMC-3 Modular hip
Hard Implants. Lecture #13
 Hard Implants Lecture #13 Refs: 1. Park, J. B. and Bronzino, J. D., eds., Biomaterials: Principles and Applications. CRC Press, Boca Raton, FL, 2003. 2. Park, J. B., Biomaterials Science and Engineering.
Hard Implants Lecture #13 Refs: 1. Park, J. B. and Bronzino, J. D., eds., Biomaterials: Principles and Applications. CRC Press, Boca Raton, FL, 2003. 2. Park, J. B., Biomaterials Science and Engineering.
Workshop on Nickel Dermatitis NACD & Implants Rosemont, IL 23 June 2016
 Rosemont, IL 23 June 2016 Kalman L. Watsky, M.D. Clinical Professor of Dermatology Yale School of Medicine Site Director, Dermatology Yale-New Haven Hospital, St. Raphael Campus In what way is patch testing
Rosemont, IL 23 June 2016 Kalman L. Watsky, M.D. Clinical Professor of Dermatology Yale School of Medicine Site Director, Dermatology Yale-New Haven Hospital, St. Raphael Campus In what way is patch testing
Operations included in the National Joint Registry (NJR)
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Mechanical Evaluation of Interlocking Nailing System by Using FEA Technique
 ISSN 2395-1621 Mechanical Evaluation of Interlocking Nailing System by Using FEA Technique #1 A. P. Gaurvadkar, #2 N. S. Biradar, 1 ameygaurvadkar@gmail.com 2 nsbiradar123@gmail.com 1&2 Department of Mechanical
ISSN 2395-1621 Mechanical Evaluation of Interlocking Nailing System by Using FEA Technique #1 A. P. Gaurvadkar, #2 N. S. Biradar, 1 ameygaurvadkar@gmail.com 2 nsbiradar123@gmail.com 1&2 Department of Mechanical
National Joint Replacement Registry. Lay Summary 2015 Annual Report Hip and Knee Replacement
 National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury
 Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
High Tibial Osteotomy
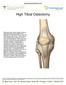 High Tibial Osteotomy With each step, forces equal to three to eight times your body weight travel between the thigh bone (femur) and shin bone (tibia) in your knee. These forces are dampened by a meniscus
High Tibial Osteotomy With each step, forces equal to three to eight times your body weight travel between the thigh bone (femur) and shin bone (tibia) in your knee. These forces are dampened by a meniscus
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
CS 2.7. Product information. Intelligent innovations for a better life. Presented by: Syntellix AG Aegidientorplatz 2a Hannover Germany
 Presented by: CS 2.7 Product information Aegidientorplatz 2a 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in Germany in
Presented by: CS 2.7 Product information Aegidientorplatz 2a 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in Germany in
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
Innovative Präzision Made in Germany
 E Innovative Präzision Data Sheet OT medical Products OT-F 1 Implant System The Base for the manufacture of high-quality implants and accessories is the compliance with all standard requirements which
E Innovative Präzision Data Sheet OT medical Products OT-F 1 Implant System The Base for the manufacture of high-quality implants and accessories is the compliance with all standard requirements which
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Biomechanics of. Knee Replacement. Mujda Hakime, Paul Malcolm
 Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Specializing in Mechanical Testing for Medical Devices. Office:
 Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Regenerex Porous Titanium Construct. Knees Hips Extremities Cement and Accessories PMI Trauma Technology
 Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
3D printed. Patient Specific. titanium alloy truss implants
 Patient Specific Implants TUMOUR ONE at a time process FUSION 3D printed patient specific titanium alloy truss implants High strength, low weight Open architecture maximises bone graft volume Upload Upload
Patient Specific Implants TUMOUR ONE at a time process FUSION 3D printed patient specific titanium alloy truss implants High strength, low weight Open architecture maximises bone graft volume Upload Upload
Instructions for Use
 Instructions for Use Intramedullary Nail Locking Screw Subtitles of the symbols used on the packaging Catalogue Number Batch Code Date of Manufacture Consult instructions for use Single-Use Product Do
Instructions for Use Intramedullary Nail Locking Screw Subtitles of the symbols used on the packaging Catalogue Number Batch Code Date of Manufacture Consult instructions for use Single-Use Product Do
2017 Exploration and Production Winter Standards Meeting
 2017 Exploration and Production Winter Standards Meeting January 16-20, 2017 JW Marriott Austin Austin, Texas www.api.org Current as of November 29, 2016 Subject to change Final program distributed onsite.
2017 Exploration and Production Winter Standards Meeting January 16-20, 2017 JW Marriott Austin Austin, Texas www.api.org Current as of November 29, 2016 Subject to change Final program distributed onsite.
Totally Hip Preservation to Revision. Gothenburg, Sweden 29 March - 1 April 2017 WEDNESDAY 29 MARCH. Arrivals THURSDAY 30 MARCH
 Totally Hip 2017 Preservation to Revision Gothenburg, Sweden 29 March - 1 April 2017 WEDNESDAY 29 MARCH Arrivals THURSDAY 30 MARCH 08:00 08:30 Welcome from the Chairmen, Co Chairmen and technical intro
Totally Hip 2017 Preservation to Revision Gothenburg, Sweden 29 March - 1 April 2017 WEDNESDAY 29 MARCH Arrivals THURSDAY 30 MARCH 08:00 08:30 Welcome from the Chairmen, Co Chairmen and technical intro
Product Information. *smith&nephew POLARCUP Dual Mobility System
 APPLYING SCIENCE TO MOBILITY Combined with VERILAST Technology for unmatched performance Product Information *smith&nephew POLARCUP Dual Mobility System APPLYING SCIENCE TO MOBILITY Combined with VERILAST
APPLYING SCIENCE TO MOBILITY Combined with VERILAST Technology for unmatched performance Product Information *smith&nephew POLARCUP Dual Mobility System APPLYING SCIENCE TO MOBILITY Combined with VERILAST
 2nd International Workshop on Clinical Spine and Orthopedics Biomechanics 13th to 15th April, 2018 Auditorium, ISIC Program 13 th April, 2018: Clinical Orthopaedic biomechanics Time Topic 0900-1015 Session
2nd International Workshop on Clinical Spine and Orthopedics Biomechanics 13th to 15th April, 2018 Auditorium, ISIC Program 13 th April, 2018: Clinical Orthopaedic biomechanics Time Topic 0900-1015 Session
No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: case report and review of the literature
 Knee Surg Sports Traumatol Arthrosc (2013) 21:636 640 DOI 10.1007/s00167-012-2000-z KNEE No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: case report and review of the literature
Knee Surg Sports Traumatol Arthrosc (2013) 21:636 640 DOI 10.1007/s00167-012-2000-z KNEE No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: case report and review of the literature
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
The Leader in Orthopaedic Innovation
 The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
CS 2.0. Product information. Intelligent innovations for a better life. Presented by: Syntellix AG Schiffgraben Hannover Germany
 Presented by: CS 2.0 Product information Syntellix AG Schiffgraben 11 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in
Presented by: CS 2.0 Product information Syntellix AG Schiffgraben 11 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma >
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Diaphyseal implant surgical technique
 Diaphyseal implant surgical technique Diaphyseal implant surgical technique MUTARS was developed in co-operation with Prof. Dr. W. Winkelmann (former director) and Prof. Dr. G. Gosheger (director), Clinic
Diaphyseal implant surgical technique Diaphyseal implant surgical technique MUTARS was developed in co-operation with Prof. Dr. W. Winkelmann (former director) and Prof. Dr. G. Gosheger (director), Clinic
Hip Implants. Hip Implants. 6 Your partner for implants and trauma products. Euromed Implants GmbH General Catalogue
 6 Modular Straight Cemented Stem The modular straight cemented stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to
6 Modular Straight Cemented Stem The modular straight cemented stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to
CONSENSUS ORTHOPEDICS INC.
 CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
