SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
|
|
|
- Eugene Walton
- 6 years ago
- Views:
Transcription
1 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EMPIRICAL TESTING CORP Northpark Drive Colorado Springs, CO Dawn Lissy Phone: MECHANICAL Valid To: January 31, 2020 Certificate Number: In recognition of the successful completion of the A2LA evaluation process, accreditation is granted to this laboratory to perform the following tests on finished medical device products following ASTM, ISO, and/or FDA guidelines comprised of metals, alloys, and polymers: The testing below is completed within these parameters: Load Torsion Stroke Angular Displacement (0 to ± 25) kn (0 to ± 100) Nm (0 to 100) mm (0 to 260) deg Test Test Methods DENTAL Dentistry Implants Dynamic Fatigue Test for Endosseous Dental Implants ISO EXTREMITIES Standard Specification and Test Method for Metallic Bone Plates ASTM F382 2 Standard Specifications and Test Methods for Metallic Angled Orthopedic Fracture Fixation Devices Standard Specification and Test Methods for Metallic Medical Bone Screws ASTM F384 2 ASTM F543 Standard Specification and Test Methods for Metallic Bone Staples ASTM F564 2 Standard Specification and Test Methods for Intramedullary Fixation Devices ASTM F (A2LA Cert. No ) 02/08/2018 Page 1 of 4
2 Test Test Methods Standard Specification and Test Methods for External Skeletal Fixation Devices ASTM F Standard Test Method for Small Punch Testing of Ultra-High Molecular Weight Polyethylene Used in Surgical Implants Standard Specification and Test Methods for Absorbable Plates and Screws for Internal Fixation Implants ASTM F2183 (Withdrawn 2017) ASTM F2502 JOINT REPLACEMENT IMPLANTS Standard Test Method for Determination of Total Knee Replacement Constraint Standard Test Method for Constant Amplitude of Force Controlled Fatigue Testing of Acrylic Bone Cement Materials Standard Specification for Articulating Total Wrist Implants Range of Motion of the Device Before Implantation Standard Specification for Shoulder Prostheses Standard Guide for Gravimetric Wear Assessment of Prosthetic Hip Designs in Simulator Devices Method for Cleaning and Weighing of Specimens Only Standard Specification for Elastomeric Flexible Hinge Finger Total Joint Implants Range of Motion of the Device Before Implantation Standard Test Method for Cyclic Fatigue Testing of Tibial Tray Components of Total Knee Joint Replacements Standard Test Method for Determining the Axial Disassembly Force of a Modular Acetabular Device Standard Test Method for Determining the Axial Disassembly Force of Taper Connections of Modular Prostheses Standard Practice for Gravimetric Measurements of Polymeric Components for Wear Assessment Method for Cleaning and Weighing of Specimens Only Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation Implants for Surgery Partial and Total Hip Joint Prostheses Determination of Endurance Properties and Performance of Stemmed Femoral Components Implants for Surgery Partial and Total Hip Joint Prostheses Determination of Endurance Properties of Head and Neck Region of Stemmed Femoral Components ASTM F1223 ASTM F2118 ASTM F1357, Section 6.3 ASTM F1378 ASTM F1714, Annex 4 ASTM F1781, Section 6.2 ASTM F ASTM F1820 ASTM F2009 ASTM F2025, Annex 1 ASTM F2028 ISO ISO (A2LA Cert. No ) 02/08/2018 Page 2 of 4
3 Test Test Method Implants for Surgery - Partial and Total Hip Joint Prostheses - Endurance Performance of Stemmed Femoral Components with Application of Torsion ISO (Withdrawn 2011) SPINE Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model ASTM F1717 Standard Test Method for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants Test Methods for Intervertebral Body Fusion Devices ASTM F1798 ASTM F2077 Standard Specifications and Test Methods for Components Used in the Surgical Fixation of the Spinal Skeletal System Standard Test Method for Measuring Load Induced Subsidence of an Intervertebral Body Fusion Device Under Static Axial Compression Standard Test Methods for Static and Dynamic Characterization of Spinal Artificial Discs Standard Guide for Functional, Kinematic, and Wear Assessment of Total Disc Prostheses Standard Test Method for Static, Dynamic and Wear Assessment of Extra-Discal Spinal Motion Preserving Implants Standard Practice for Functional and Wear Evaluation of Motion-Preserving Lumbar Total Facet Prostheses Standard Test Methods for Occipital-Cervical and Occipital-Cervical-Thoracic Spinal Implant Constructs in a Vertebrectomy Model Standard Guide for Mechanical and Functional Characterization of Nucleus Devices (Except Viscoelastic Testing) Standard Practice for Static and Dynamic Characterization of Motion Preserving Lumbar Total Facet Prostheses Implants for Surgery - Mechanical Testing of Implantable Spinal Devices Fatigue Test Method for Spinal Implant Assemblies Using an Anterior Support ASTM F ASTM F2267 ASTM F2346 ASTM F2423 ASTM F2624 ASTM F2694 ASTM F2706 ASTM F2789 ASTM F2790 ISO Implants for Surgery - Wear of Total Intervertebral Spinal Disc Prostheses ISO (A2LA Cert. No ) 02/08/2018 Page 3 of 4
4 1 This laboratory s scope contains withdrawn or superseded methods. As a clarifier, this indicates that the applicable method itself has been withdrawn or is now considered historical and not that the laboratory s accreditation for the method has been withdrawn. 2 Equipment for this test is calibrated to ASTM E4 but the dynamic verification of the equipment per ASTM E467 is not performed. (A2LA Cert. No ) 02/08/2018 Page 4 of 4
5 Accredited Laboratory A2LA has accredited EMPIRICAL TESTING CORP. Colorado Springs, CO for technical competence in the field of Mechanical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories. This accreditation demonstrates technical competence for a defined scope and the operation of a laboratory quality management system (refer to joint ISO-ILAC-IAF Communiqué dated April 2017). Presented this 8 th day of February President and CEO For the Accreditation Council Certificate Number Valid to January 31, 2020 Revised 04/17/2018 For the types of tests to which this accreditation applies, please refer to the laboratory s Mechanical Scope of Accreditation.
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
Specializing in Mechanical Testing for Medical Devices. Office:
 Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
ISO INTERNATIONAL STANDARD. Dentistry Implants Dynamic fatigue test for endosseous dental implants
 INTERNATIONAL STANDARD ISO 14801 Second edition 2007-11-15 Dentistry Implants Dynamic fatigue test for endosseous dental implants Art dentaire Implants Essai de fatigue dynamique pour implants dentaires
INTERNATIONAL STANDARD ISO 14801 Second edition 2007-11-15 Dentistry Implants Dynamic fatigue test for endosseous dental implants Art dentaire Implants Essai de fatigue dynamique pour implants dentaires
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 ARTEL LABORATORY 25 Bradley Drive Westbrook, ME 04092 Doreen Rumery Phone: 888 406 3463 CALIBRATION Valid To: October 31, 2019 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 ARTEL LABORATORY 25 Bradley Drive Westbrook, ME 04092 Doreen Rumery Phone: 888 406 3463 CALIBRATION Valid To: October 31, 2019 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL CENTER FOR ANIMAL HEALTH CALIBRATION LABORATORY 1920 Dayton Avenue Ames, Iowa 50010 Dr. Karl Hochstein 515-337-7739 CALIBRATION Valid To: June 30,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL CENTER FOR ANIMAL HEALTH CALIBRATION LABORATORY 1920 Dayton Avenue Ames, Iowa 50010 Dr. Karl Hochstein 515-337-7739 CALIBRATION Valid To: June 30,
Quality of Life. Quality of Motion.
 Quality of Life. Quality of Motion. Lateral Bend Vertical Translation Flexion Extension Lateral Translation Axial Rotation Anterior Posterior Translation Motion in all Directions Kinematics is the study
Quality of Life. Quality of Motion. Lateral Bend Vertical Translation Flexion Extension Lateral Translation Axial Rotation Anterior Posterior Translation Motion in all Directions Kinematics is the study
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 SHERWIN-WILLIAMS AUTOMOTIVE FINISHES CORP. Testing Laboratory 4440 Warrensville Center Road Warrensville Heights, OH 44128 David Allerton Phone: 216 332 8379
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 SHERWIN-WILLIAMS AUTOMOTIVE FINISHES CORP. Testing Laboratory 4440 Warrensville Center Road Warrensville Heights, OH 44128 David Allerton Phone: 216 332 8379
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma >
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
MARATHON Cross-Linked Polyethylene
 MARATHON Cross-Linked Polyethylene Fixation First... 1 Wear Reduction... 2 Oxidative Stability... 3 Mechanical Integrity... 4 Proven Technology...5-6 References... 7 Fixation First FIXATION FIRST Prodigy
MARATHON Cross-Linked Polyethylene Fixation First... 1 Wear Reduction... 2 Oxidative Stability... 3 Mechanical Integrity... 4 Proven Technology...5-6 References... 7 Fixation First FIXATION FIRST Prodigy
YY Translated English of Chinese Standard: YY
 Translated English of Chinese Standard: YY0341-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.40 C 35 Pharmaceutical Industry Standard of the People s Republic of China YY/T 0341-2009
Translated English of Chinese Standard: YY0341-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.40 C 35 Pharmaceutical Industry Standard of the People s Republic of China YY/T 0341-2009
FIXATION FIRST WEAR REDUCTION OXIDATIVE STABILITY MECHANICAL INTEGRITY PROVEN TECHNOLOGY
 FIXATION FIRST WEAR REDUCTION OXIDATIVE STABILITY MECHANICAL INTEGRITY PROVEN TECHNOLOGY Fixation First FIXATION FIRST 1958 Charnley Hip Over 1,000,000 implanted 1 96.2 percent survivorship at 32 years
FIXATION FIRST WEAR REDUCTION OXIDATIVE STABILITY MECHANICAL INTEGRITY PROVEN TECHNOLOGY Fixation First FIXATION FIRST 1958 Charnley Hip Over 1,000,000 implanted 1 96.2 percent survivorship at 32 years
ISO INTERNATIONAL STANDARD. Non-active surgical implants Joint replacement implants Particular requirements
 INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
FATIGUE DEVICE FOR TESTING ANKLE JOINT ENDOPROSTHESES
 FATIGUE DEVICE FOR TESTING ANKLE JOINT ENDOPROSTHESES PhD. Student Cristian TOADER-PASTI, Politehnica University of Timişoara, România, cristian.pasti@yahoo.com Assist. PhD.eng. Dan Ioan STOIA, Politehnica
FATIGUE DEVICE FOR TESTING ANKLE JOINT ENDOPROSTHESES PhD. Student Cristian TOADER-PASTI, Politehnica University of Timişoara, România, cristian.pasti@yahoo.com Assist. PhD.eng. Dan Ioan STOIA, Politehnica
Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
 Mechanics of Contact and Lubrication, MTM G230 Department of Mechanical & Industrial Enineering Northeastern University Spring 2006 Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
Mechanics of Contact and Lubrication, MTM G230 Department of Mechanical & Industrial Enineering Northeastern University Spring 2006 Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
Hard Implants. Lecture #13
 Hard Implants Lecture #13 Refs: 1. Park, J. B. and Bronzino, J. D., eds., Biomaterials: Principles and Applications. CRC Press, Boca Raton, FL, 2003. 2. Park, J. B., Biomaterials Science and Engineering.
Hard Implants Lecture #13 Refs: 1. Park, J. B. and Bronzino, J. D., eds., Biomaterials: Principles and Applications. CRC Press, Boca Raton, FL, 2003. 2. Park, J. B., Biomaterials Science and Engineering.
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 CONBRACO CALIBRATION SERVICES 1418 S. Pearl Street Pageland, SC 29728 Scott McKay Phone: 843 672 1616 CALIBRATION Valid To: March 31,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 CONBRACO CALIBRATION SERVICES 1418 S. Pearl Street Pageland, SC 29728 Scott McKay Phone: 843 672 1616 CALIBRATION Valid To: March 31,
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
Operations included in the National Joint Registry (NJR)
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Symposium on Static and Dynamic Spinal Implants: Are We Evaluating Them Appropriately?
 Symposium on Static and Dynamic Spinal Implants: Are We Evaluating Them Sponsored by Committee F04 on Medical and Surgical Material Devices November 16, 2010 Grand Hyatt San Antonio San Antonio, Texas
Symposium on Static and Dynamic Spinal Implants: Are We Evaluating Them Sponsored by Committee F04 on Medical and Surgical Material Devices November 16, 2010 Grand Hyatt San Antonio San Antonio, Texas
Freedom. Lumbar Disc Polymer-Metal Bond Integrity. CAUTION: Investigational device. Limited by Federal law to investigational use.
 Freedom Lumbar Disc Polymer-Metal Bond Integrity CAUTION: Investigational device. Limited by Federal law to investigational use. 1 White Paper Freedom Lumbar Disc Polymer-Metal Bond Integrity Abstract
Freedom Lumbar Disc Polymer-Metal Bond Integrity CAUTION: Investigational device. Limited by Federal law to investigational use. 1 White Paper Freedom Lumbar Disc Polymer-Metal Bond Integrity Abstract
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
SpineFAQs. Cervical Disc Replacement
 SpineFAQs Cervical Disc Replacement Artificial disc replacement (ADR) is relatively new. In June 2004, the first ADR for the lumbar spine (low back) was approved by the FDA for use in the US. Replacing
SpineFAQs Cervical Disc Replacement Artificial disc replacement (ADR) is relatively new. In June 2004, the first ADR for the lumbar spine (low back) was approved by the FDA for use in the US. Replacing
 2nd International Workshop on Clinical Spine and Orthopedics Biomechanics 13th to 15th April, 2018 Auditorium, ISIC Program 13 th April, 2018: Clinical Orthopaedic biomechanics Time Topic 0900-1015 Session
2nd International Workshop on Clinical Spine and Orthopedics Biomechanics 13th to 15th April, 2018 Auditorium, ISIC Program 13 th April, 2018: Clinical Orthopaedic biomechanics Time Topic 0900-1015 Session
ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9
 ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9 8:00 AM 8:00 AM 10:00 AM 10:00 AM F04 Attendees are invited to an ASTM Training Session on Responsibilities for Subcommittee Chairman,
ASTM Committee F04 Tuesday 05/19/2015 Grand Ballroom F Platinum 9 8:00 AM 8:00 AM 10:00 AM 10:00 AM F04 Attendees are invited to an ASTM Training Session on Responsibilities for Subcommittee Chairman,
(c) B. Ravi, IIT Bombay 1
 Collaborative Engineering Evaluation, Testing TKP Safety, TKP System Mechanism FEA Physical testing Fatigue Wear What How Evaluate Testing Judgement Criteria's Testing Conditions Parameters Measured OrthoCAD
Collaborative Engineering Evaluation, Testing TKP Safety, TKP System Mechanism FEA Physical testing Fatigue Wear What How Evaluate Testing Judgement Criteria's Testing Conditions Parameters Measured OrthoCAD
Durability Characterization of the Freedom
 Durability Characterization of the Freedom Cervical Disc CAUTION (for USA only): Investigational device. Limited by Federal (or U.S.) law to investigational use. White Paper Durability Characterization
Durability Characterization of the Freedom Cervical Disc CAUTION (for USA only): Investigational device. Limited by Federal (or U.S.) law to investigational use. White Paper Durability Characterization
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 MASTER GAGE & TOOL CO. 112 Maplewood Street Danville, VA 24540 Sean Cobb Phone: 434 836 4243 CALIBRATION Valid To: June 30, 2018 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 MASTER GAGE & TOOL CO. 112 Maplewood Street Danville, VA 24540 Sean Cobb Phone: 434 836 4243 CALIBRATION Valid To: June 30, 2018 Certificate
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Osteoarthrosis, unspecified whether generalized or localized, lower leg. Osteoarthrosis, localized, not specified whether primary or secondary, pelvic
 Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
Cannulated Screw System. Product Overview
 Cannulated Screw System Product Overview Acumed Cannulated Screw System The Acumed Cannulated Screw System consists of screws, washers, and instruments and is intended for fixation of fractures, fusions,
Cannulated Screw System Product Overview Acumed Cannulated Screw System The Acumed Cannulated Screw System consists of screws, washers, and instruments and is intended for fixation of fractures, fusions,
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX
 HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Spine surgery experience at the Loveland Surgery Center Loveland, Colorado
 Spine surgery experience at the Loveland Surgery Center Loveland, Colorado Kenneth A. Pettine, M.D., M.S. Loveland Surgery Center I.D.E. Site for Twelve F.D.A. Spinal Implant Studies Joint Commission Accredited
Spine surgery experience at the Loveland Surgery Center Loveland, Colorado Kenneth A. Pettine, M.D., M.S. Loveland Surgery Center I.D.E. Site for Twelve F.D.A. Spinal Implant Studies Joint Commission Accredited
Preliminary measurements of lumbar spine kinematics and stiffness
 5 th Australasian Congress on Applied Mechanics, ACAM 2007 10-12 December 2007, Brisbane, Australia Preliminary measurements of lumbar spine kinematics and stiffness L. Jirková 1, Z. Horák 1, R. Sedláek
5 th Australasian Congress on Applied Mechanics, ACAM 2007 10-12 December 2007, Brisbane, Australia Preliminary measurements of lumbar spine kinematics and stiffness L. Jirková 1, Z. Horák 1, R. Sedláek
China Orthopedics Implants Medical Devices Market Brief
 China Orthopedics Implants Medical Devices Market Brief By: PIM LTD. August 2017 reproduced for distribution outside the client organization without prior written approval from PIM Ltd. 1 Definition and
China Orthopedics Implants Medical Devices Market Brief By: PIM LTD. August 2017 reproduced for distribution outside the client organization without prior written approval from PIM Ltd. 1 Definition and
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 7206-2 Third edition 2011-04-01 Implants for surgery Partial and total hip joint prostheses Part 2: Articulating surfaces made of metallic, ceramic and plastics materials Implants
INTERNATIONAL STANDARD ISO 7206-2 Third edition 2011-04-01 Implants for surgery Partial and total hip joint prostheses Part 2: Articulating surfaces made of metallic, ceramic and plastics materials Implants
ASTM Committee F04 Tuesday 11/13/2012
 ASTM Committee F04 Tuesday 11/13/2012 Centennial Regency Ballroom Ballroom III V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM F04 Celebration of Committee F04's 50th Anniversary
ASTM Committee F04 Tuesday 11/13/2012 Centennial Regency Ballroom Ballroom III V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM F04 Celebration of Committee F04's 50th Anniversary
Talos -C (HA) and the PEEK-OPTIMA HA Enhanced Technology
 v Talos -C (HA) and the HA Enhanced Technology The innovative Talos -C (HA) IBF Implant made with polymer is fully integrated with the well known material Hydroxyapatite (HA). The Talos -C (HA) IBF Implant
v Talos -C (HA) and the HA Enhanced Technology The innovative Talos -C (HA) IBF Implant made with polymer is fully integrated with the well known material Hydroxyapatite (HA). The Talos -C (HA) IBF Implant
IDENTIFICATION OF THE PARAMETERS THAT INFLUENCE THE UNCERTAINTY SOURCES IN ORTOPHAEDIC IMPLANTS FATIGUE TESTS
 XIX IMEKO World Congress Fundamental and Applied Metrology September 6 11, 2009, Lisbon, Portugal IDENTIFICATION OF THE PARAMETERS THAT INFLUENCE THE UNCERTAINTY SOURCES IN ORTOPHAEDIC IMPLANTS FATIGUE
XIX IMEKO World Congress Fundamental and Applied Metrology September 6 11, 2009, Lisbon, Portugal IDENTIFICATION OF THE PARAMETERS THAT INFLUENCE THE UNCERTAINTY SOURCES IN ORTOPHAEDIC IMPLANTS FATIGUE
TOTAL KNEE ARTHROPLASTY (TKA)
 TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
Ceramic Femoral Heads and Acetabular Cup Liners
 Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Knee Replacement Implants
 Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Lumbar Dynamic. Reinhold Knebel Product Manager
 Lumbar Dynamic Reinhold Knebel Product Manager The DSS system provides options for dynamic and rigid stabilization to treat hypermobile segments and segments that require fusion. Allows pedicle displacement
Lumbar Dynamic Reinhold Knebel Product Manager The DSS system provides options for dynamic and rigid stabilization to treat hypermobile segments and segments that require fusion. Allows pedicle displacement
The Most Reliable Spinal Solution
 MFG : 09.02.20 REV : 1 4CIS VANE introduces you the reliable pedicle screw The Most Reliable Spinal Solution Non-slip threaded joint Ergonomic design Wedged trapezoid thread Superior locking mechanism
MFG : 09.02.20 REV : 1 4CIS VANE introduces you the reliable pedicle screw The Most Reliable Spinal Solution Non-slip threaded joint Ergonomic design Wedged trapezoid thread Superior locking mechanism
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Individual oncological implants
 Individual oncological implants Catalogue - individual oncological implants ONCOLOGICAL Materials for implants List of materials MATERIAL ISO ČSN DIN ASTM Stainless steel 5832-1 17350 výběr 17 443 W.Nr.
Individual oncological implants Catalogue - individual oncological implants ONCOLOGICAL Materials for implants List of materials MATERIAL ISO ČSN DIN ASTM Stainless steel 5832-1 17350 výběr 17 443 W.Nr.
Innovation in Medical Technology. BIOTECH GroupG
 Innovation in Medical Technology BIOTECH GroupG BIOTECH MULTI-NATIONAL GERMAN - BASED Developer, Manufacturer, Distributor HIP-,, KNEE-,, TRAUMA- SPINE-IMPLANTS IMPLANTS BIOTECH Factories and Offices Plants
Innovation in Medical Technology BIOTECH GroupG BIOTECH MULTI-NATIONAL GERMAN - BASED Developer, Manufacturer, Distributor HIP-,, KNEE-,, TRAUMA- SPINE-IMPLANTS IMPLANTS BIOTECH Factories and Offices Plants
UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD
 UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 66th Annual Meeting February 4-9, 1999 Anaheim, California COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON
UHMWPE LONGEVITY: INFLUENCES OF THE ORTHOPAEDIC TRIAD AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 66th Annual Meeting February 4-9, 1999 Anaheim, California COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
Hip Joint Prostheses
 Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
Fatigue Characterization of the Freedom Lumbar Disc
 Fatigue Characterization of the Freedom Lumbar Disc CAUTION: Investigational device. Limited by Federal law to investigational use. White Paper Fatigue Characterization of the Freedom Lumbar Disc Abstract
Fatigue Characterization of the Freedom Lumbar Disc CAUTION: Investigational device. Limited by Federal law to investigational use. White Paper Fatigue Characterization of the Freedom Lumbar Disc Abstract
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 7206-12 First edition 2016-10-01 Implants for surgery Partial and total hip joint prostheses Part 12: Deformation test method for acetabular shells Implants chirurgicaux Prothèses
INTERNATIONAL STANDARD ISO 7206-12 First edition 2016-10-01 Implants for surgery Partial and total hip joint prostheses Part 12: Deformation test method for acetabular shells Implants chirurgicaux Prothèses
LPS SYSTEM POCKET GUIDE
 LPS SYSTEM POCKET GUIDE Implants Procedural Uses Instruments & Trials L P S Limb Preservation System L P S Limb Preservation System The purpose of this document is to review the LPS (Limb Preservation
LPS SYSTEM POCKET GUIDE Implants Procedural Uses Instruments & Trials L P S Limb Preservation System L P S Limb Preservation System The purpose of this document is to review the LPS (Limb Preservation
SVENSK STANDARD SS-EN ISO 14801:2004. Tandvård Utmattningstest för benförankrade dentala implantat (ISO 14801:2003)
 SVENSK STANDARD SS-EN ISO 14801:2004 Fastställd 2004-10-08 Utgåva 1 Tandvård Utmattningstest för benförankrade dentala implantat (ISO 14801:2003) Dentistry Fatigue test for endosseous dental implants (ISO
SVENSK STANDARD SS-EN ISO 14801:2004 Fastställd 2004-10-08 Utgåva 1 Tandvård Utmattningstest för benförankrade dentala implantat (ISO 14801:2003) Dentistry Fatigue test for endosseous dental implants (ISO
Implants for surgery Partial and total hip joint prostheses. Part 12: Deformation test method for acetabular shells
 INTERNATIONAL STANDARD ISO 7206-12 First edition 2016-10-01 Implants for surgery Partial and total hip joint prostheses Part 12: Deformation test method for acetabular shells Implants chirurgicaux Prothèses
INTERNATIONAL STANDARD ISO 7206-12 First edition 2016-10-01 Implants for surgery Partial and total hip joint prostheses Part 12: Deformation test method for acetabular shells Implants chirurgicaux Prothèses
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
National Joint Replacement Registry. Lay Summary 2015 Annual Report Hip and Knee Replacement
 National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
comprehensive Design Rationale shoulder system Knees Hips Extremities Cement and Accessories PMI TEchnology
 comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
DRAFT GUIDANCE DOCUMENT List of Recognized Standards
 DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
A New Orthosis for Fixation of the Cervical Spine Fronto- Occipito-Zygomatic Orthosis
 A New Orthosis for Fixation of the Cervical Spine Fronto- Occipito-Zygomatic Orthosis Toshiro Nakamura, O.A. Mitsuru Oh-Hama, M.D. Hikosuke Shingu, M.D. INTRODUCTION Most of the cervical orthoses for longterm
A New Orthosis for Fixation of the Cervical Spine Fronto- Occipito-Zygomatic Orthosis Toshiro Nakamura, O.A. Mitsuru Oh-Hama, M.D. Hikosuke Shingu, M.D. INTRODUCTION Most of the cervical orthoses for longterm
Consistency in U2 Knee System over Time
 U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
Quality of Life. Quality of Motion. A Patient s Guide to. Artificial Lumbar Disc Replacement
 Quality of Life. Quality of Motion. A Patient s Guide to Artificial Lumbar Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Lumbar Disc Degeneration, a lower spine condition
Quality of Life. Quality of Motion. A Patient s Guide to Artificial Lumbar Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Lumbar Disc Degeneration, a lower spine condition
Regenerex Porous Titanium Construct. Knees Hips Extremities Cement and Accessories PMI Trauma Technology
 Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
Merete BioBall The Innovative Hip Endoprosthesis Head Adapter System
 Merete the «BioBall Company» You are better off with BioBall Your Indispensable Assistant in Hip Arthroplasties. Merete BioBall The Innovative Hip Endoprosthesis Head Adapter System 2 BioBall System Merete
Merete the «BioBall Company» You are better off with BioBall Your Indispensable Assistant in Hip Arthroplasties. Merete BioBall The Innovative Hip Endoprosthesis Head Adapter System 2 BioBall System Merete
Surface evaluation of orthopedic hip implants marketed in Brazil
 Surface evaluation of orthopedic hip implants marketed in Brazil M.M.Souza 1, R.M. Trommer 2, M.M.Maru 2, C.R.M.Roesler 3, W.S.Barros 1, M.S. Dutra 3 1 Dimensional Metrology Laboratory, Inmetro. Brazil;
Surface evaluation of orthopedic hip implants marketed in Brazil M.M.Souza 1, R.M. Trommer 2, M.M.Maru 2, C.R.M.Roesler 3, W.S.Barros 1, M.S. Dutra 3 1 Dimensional Metrology Laboratory, Inmetro. Brazil;
Zimmer Segmental System
 Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
Beijing, China, East, Dongcheng District, North Third Ring Road. Beijing Global Trade Center #36
 Ref: ES B 2 EN 12.2007 A www.ldrmedical.com France Technopôle de l Aube BP 2 10902 Troyes Cedex 9 France +33 (0)3 25 82 32 63 China Unit 08, Level 16, Building A, Beijing Global Trade Center #36 North
Ref: ES B 2 EN 12.2007 A www.ldrmedical.com France Technopôle de l Aube BP 2 10902 Troyes Cedex 9 France +33 (0)3 25 82 32 63 China Unit 08, Level 16, Building A, Beijing Global Trade Center #36 North
28 Surgical Technique
 Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
Aesculap Trilliance Triple Tapered Polished Hip Stem
 Aesculap Trilliance Triple Tapered Polished Hip Stem Aesculap Orthopaedics Trilliance Triple Tapered Polished Hip Stem CONTENTS 2 Contents Page Trilliance Philosophy 4 Trilliance Design 6 Trilliance Implants
Aesculap Trilliance Triple Tapered Polished Hip Stem Aesculap Orthopaedics Trilliance Triple Tapered Polished Hip Stem CONTENTS 2 Contents Page Trilliance Philosophy 4 Trilliance Design 6 Trilliance Implants
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International
 First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International Tom Hein, CFO Safe Harbor Statement Except for historical information contained herein, the statements
First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International Tom Hein, CFO Safe Harbor Statement Except for historical information contained herein, the statements
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
GIZA Surgical Technique
 GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
CONSENSUS ORTHOPEDICS INC.
 CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
Patient Information. Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System
 Patient Information Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Your doctor has recommended spinal fusion surgery using
Patient Information Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Your doctor has recommended spinal fusion surgery using
The Leader in Orthopaedic Innovation
 The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
Total Knee Replacement
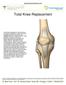 Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Design Rationale and Surgical Technique. *smith&nephew TANDEM. Bipolar and Unipolar Hip System
 Design Rationale and Surgical Technique *smith&nephew TANDEM Bipolar and Unipolar Hip System Maximizing performance from every angle Saving time in the operating room is one of the most valuable solutions
Design Rationale and Surgical Technique *smith&nephew TANDEM Bipolar and Unipolar Hip System Maximizing performance from every angle Saving time in the operating room is one of the most valuable solutions
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Comparison of Daily Motion of the Cervical and Lumbar Spine to ASTM F and ISO Standard Testing
 Daniel Cobian, 1 Bryan Heiderscheit, 2 Nicole Daehn, 3 Paul A. Anderson 4 Journal of ASTM International, Vol. 9, No. 1 Paper ID JAI103522 Available online at: www.astm.org Comparison of Daily Motion of
Daniel Cobian, 1 Bryan Heiderscheit, 2 Nicole Daehn, 3 Paul A. Anderson 4 Journal of ASTM International, Vol. 9, No. 1 Paper ID JAI103522 Available online at: www.astm.org Comparison of Daily Motion of
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Cement Polished Tapered Stems of 12/14 Taper. 96 mm 98 mm 104 mm 110 mm 116 mm 122 mm 128 mm. Ceramic Femoral Head. Outer Diameter
 Design Philosophy Cementless Stems of 12/14 Taper Stem Length Neck shaft Angle STEM-N3 STEM-N4 STEM-N5 STEM-N6 STEM-N7 STEM-N8 STEM-N9 123 mm 125 mm 130 mm 135 mm 140 mm 145 mm 150 mm 132 Cement Polished
Design Philosophy Cementless Stems of 12/14 Taper Stem Length Neck shaft Angle STEM-N3 STEM-N4 STEM-N5 STEM-N6 STEM-N7 STEM-N8 STEM-N9 123 mm 125 mm 130 mm 135 mm 140 mm 145 mm 150 mm 132 Cement Polished
Biomechanical Investigations on the Total Knee Arthroplasty (TKA)
 Fernando Fonseca, MD PhD Hospitais da Universidade de Coimbra Faculdade de Medicina / Universidade de Coimbra Faculdade de Ciências da Saúde / Universidade da Beira Interior pereirafonseca@gmail.com KNEE
Fernando Fonseca, MD PhD Hospitais da Universidade de Coimbra Faculdade de Medicina / Universidade de Coimbra Faculdade de Ciências da Saúde / Universidade da Beira Interior pereirafonseca@gmail.com KNEE
