YY Translated English of Chinese Standard: YY
|
|
|
- Clifton Pearson
- 6 years ago
- Views:
Transcription
1 Translated English of Chinese Standard: YY YY ICS C 35 Pharmaceutical Industry Standard of the People s Republic of China YY/T Replacing General technological requirements for non-active metallic surgical implants for osteosynthesis 骨接合用无源外壳金属植入物通用技术条件 Issued on: December 30, 2009 Implemented on: June 1, 2011 Issued by: China Food and Drug Administration Page 1 of 17
2 Table of Contents Foreword Scope Normative references Terms and definitions Requirements Test methods Inspection rules Usage instructions Marks of implants Packaging and label Annex A Annex B Annex C Annex D Page 2 of 17
3 Foreword Definitions in this Standard are quoted from YY/T Surgical Implant Basic Principles (ISO/TR 14283:2004, IDT), YY/T Non-active Surgical Implant General Requirements (ISO 14630:2005, IDT), and GB/T Non-active Surgical Implant Osteosynthesis and Joint Replacement Implant. Part 1: Special Requirements for Implants for Osteosynthesis (ISO 14602:1998, IDT). This Standard replaces YY General technological requirements for non-active metallic surgical implants for osteosynthesis. Main differences between this Standard and YY are as follows: - REVISE chapter 3, 6, 7, 8, and 9, according to corresponding international standards; - ADD static and/or dynamic load evaluation tests according to GB/T ; - ADD informative annex D; as a reference, introduce some American standards for testing and evaluation. Annex A, Annex B, Annex C, and Annex D of this Standard are all informative. This Standard was proposed by State Food and Drug Administration. This Standard shall be under the jurisdiction of national standardization technical committee of surgical implants and orthopedic devices (SAC/TC 110). Drafting organization of this Standard: Tianjin Medical Device Quality Supervision and Inspection Center of State Food and Drug Administration. Main drafters of this Standard: Fan Bo, Yang Jiangang, Song Duo, Qi Baofen, and Jiang Hua. Page 3 of 17
4 General technological requirements for non-active metallic surgical implants for osteosynthesis 1 Scope This Standard specifies the definitions, requirements, testing methods, inspection rules, usage instructions, marks, packaging, transportation, storage, usage requirements etc. of the non-active surgical implants for osteosynthesis. This Standard is applicable to non-active surgical implants for osteosynthesis (abbreviated as implants for osteosynthesis ) which are made of metallic materials. This Standard is not applicable to implants for osteosynthesis which have coating on the surface. 2 Normative references The provisions in following documents become the provisions of this Standard through reference in this Standard. For dated references, the subsequent amendments (excluding corrections) or revisions do not apply to this Standard, however, parties who reach an agreement based on this Standard are encouraged to study if the latest versions of these documents are applicable. For undated references, the latest edition of the referenced document applies. GB/T 191 Packaging - Pictorial marking for handling of goods (GB/T , ISO 780:1997, MOD) GB/T 228 Metallic materials - Tensile testing at ambient temperature (GB/T , eqv ISO 6892:1998) GB/T 2829 Sampling procedures and tables for periodic inspection by attributes (Applicable to inspection of process stability) GB/T Metallic materials - Vickers hardness test - Part 1: Test method (GB/T , eqv ISO :1997) GB/T Test methods for infusion, transfusion, injection equipment for medical use - Part 2: Biological test methods GB/T attributes Sampling procedures and tables for isolated lot inspection by Page 4 of 17
5 GB/T Biological evaluation of medical devices - Part 1: Evaluation and testing (GB/T , IDT; ISO : 1997, IDT) YY/T 0343 Liquid penetrant inspection of metallic surgical implants (YY/T , ISO 9583:1993, NEQ) YY/T : 2005, IDT) Non-active surgical implants - General requirements (ISO YY/T 1074 Implants for surgery - Measuring method for pitting corrosion potential on stainless products 3 Terms and definitions The following terms and definitions apply to this Standard. 3.1 Surgical implant Device for the following purposes: - Totally embedded into human body, or - REPLACE the epithelial surface or ocular surface, Device remains at the above operation positions through surgical invasion method. Note: Through surgically invasion methods, some medical devices which are embedded into human body and remained at the operation position for at least 30 days shall also be regarded as implants. 3.2 Non-active surgical implant It refers to the surgical implant that operates without electric energy or other energy sources, except the energy directly generated by human body or gravity. 3.3 Non-active surgical implant for osteosynthesis It refers to the non-active surgical implant providing support for bones, cartilages, tendons or ligament structures Page 5 of 17
6 non-sterile status. Implants for osteosynthesis delivered in sterile status shall pass an effective and confirmed sterilization process to make the products to be in sterile status. 5 Test methods 5.1 Materials Material samples for chemical component and microscopic structure testing shall be sampled from the final products of the implants for osteosynthesis; the testing shall be conducted according to the methods specified in corresponding product standards Biological property test shall be conducted according to the recommended methods in GB/T , or comply with the provisions of other related standards. 5.2 Mechanical property When evaluating the implant by static and/or dynamic load test, it can adopt the existing inspection standards or formulate the testing models according to the implant s characteristics. Note: As there are many kinds of implants and large characteristic differences, the inspection standard may not yet exist or may need to be revised Hardness: Test shall be conducted according to the methods specified in GB/T Tensile strength: Test shall be conducted according to the methods specified in GB/T Electrochemical corrosion test Pitting potential: Test shall be conducted according to the methods specified in YY/T Surface quality Surface flaw: CONDUCT according to the methods specified in YY/T Surface roughness: USE sample piece comparison method or electrometric method Appearance: OBSERVE by eyesight. 5.5 Dimension of important parts Page 7 of 17
7 10.2 Transportation signs shall comply with the specifications of GB/T After packaging, the implant products shall be stored indoor with relative humidity not more than 80%, without corrosive gas, and with good ventilation. 11 Usage requirements 11.1 Implant products that have been used can t be used again Implant usage shall be under strict conditions and meet clinical usage requirements. The patient s own conditions may affect the implant s properties If the implant is planned to be used with other implants or devices, the whole combination (including connection system) shall be safe and not impair the implants specified properties. Any usage limitations shall be indicated in the label or in the usage instructions. Page 11 of 17
8 mechanical requirements ISO Implants for surgery - Skeletal pins and wires - Part 2: Steinmann pins; dimensions ISO Implants for surgery - Skeletal pins and wires - Part 3: kirschner skeletal wires YY 0345 Implants for osteosynthesis - Metal bone pins A.1.6 Staples ISO 8827 Implants for surgery - Staples with parallel legs for orthopaedic use - General requirements A.1.7 Cerclage and other malleable metal wires for fixation ISO Implants for surgery - Malleable wires for use as sutures and other surgical applications. A.1.8 Implant for bone external fixation 1 A.1.9 Implant for spinal fixation 2 YY0119 Implants for osteosynthesis - Metal correctional nail YY0120 Implants for osteosynethesis - Metal correctional stick A.2 Device standards A.2.1 Twisted connecting devices ISO Orthopaedic instruments - Drive connections - Part 1: Keys for use with screws with hexagon socket heads ISO Orthopaedic instruments-- Drive connections - Part 2: Screwdrivers for single slot head screws, screws with cruciate slot and cross-recessed head screws A.2.2 Drill and taps ISO Orthopaedic drilling instruments - Part 1: Drill bits, taps and countersink cutters 1 There has been no international standard at present, but it has been proposed. 2 International standard is being drafted, but it is only in the working draft stage. This standard consists of several standards. Page 13 of 17
9 Annex B (Informative) ISO standards for acceptable materials proved by clinical usage ISO Implants for surgery - Metallic materials - Part 1: Wrought stainless steel (GB , ISO ,1997, MOD) ISO Implants for surgery - Metallic materials - Part 2: Unalloyed titanium (GB/T , eqv ISO :1993) ISO Implants for surgery - Metallic materials - Part 3: Wrought titanium 6-aluminium 4-vanadium alloy ISO Implants for surgery - Metallic materials - Part 4: Cobalt-chromium-molybdenum casting alloy (GB , eqv ISO :1996) ISO Implants for surgery - Metallic materials - Part 5: Wrought cobalt-chromium-tungsten-nickel alloy (YY/T , ISO , 2005, IDT) ISO Implants for surgery - Metallic materials - Part 6: Wrought cobalt-nickel-chromium-molybdenum alloy (YY/T , ISO : 1997,IDT) ISO Implants for surgery - Metallic materials - Part 7: Forgeable and cold-formed cobalt-chromium-nickel-molybdenum-iron alloy (YY/T , ISO , 1994, IDT) ISO Implants for surgery - Metallic materials - Part 8: Wrought cobalt-nickel-chromium-molybdenum-tungsten-iron alloy (YY/T , ISO :1997, IDT) ISO Implants for surgery - Metallic materials - Part 8: Wrought cobalt-nickel-chromium-molybdenum-tungsten-iron alloy (YY , ISO , 1992, IDT) ISO Implants for surgery - Metallic materials - Part 11: Wrought titanium-6-aluminium 7-niobium alloy (GB , ISO :1994, IDT) ISO Implants for surgery - Metallic materials - Part 12: Wrought cobalt-chromium-molybdenum alloy (YY , ISO :1996, IDT) ISO 5833 Implants for surgery; acrylic resin cements (YY , ISO 5833:2002, IDT) Page 14 of 17
10 Annex C (Informative) ISO standards and industrial standards related to design evaluation and test ISO 6475 Implants for surgery; metal bone screws with asymmetrical thread and spherical under-surface; mechanical requirements and test methods (YY/ T , ISO 6475, 1989, IDT) ISO 9583 Implants for surgery; non-destructive testing; liquid penetrant inspection of metallic surgical implants (YY/T , ISO 9583:1993, NEQ) ISO 9584 Implants for surgery; non-destructive testing; radiographic examination of cast metallic surgical implants ISO 9585 Implants for surgery; determination of bending strength and stiffness of bone plates (YY/T , ISO 9585:1990, IDT) YY/T 1074 Implants for surgery - Measuring method for pitting corrosion potential on stainless products Page 16 of 17
11 Annex D (Informative) ASTM standards related to design evaluation and test ASTM F 366 Standard Specification for Fixation Pins and Wires ASTM F 382 Standard Specification and Test Method for Metallic Bone Plates ASTM F 384 Standard Specifications and Test Methods for Metallic Angled Orthopedic Fracture ASTM F 452 Standard Specification for Preformed Cranioplasty Plates ASTM F 543 Standard Specification and Test Methods for Metallic Medical Bone Screws ASTM F 564 Standard Specification and Test Methods for Metallic Bone Staples ASTM F 622 Standard Specification for Preformed Cranioplasty Plates That Can Be Altered ASTM F 1264 Standard Specification and Test Methods for Intramedullary Fixation Devices ASTM F 1717 Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model ASTM F 1798 Standard Guide for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants ASTM F 2077 Test Methods For Intervertebral Body Fusion Devices ASTM F 2180 Standard Specification for Metallic Implantable Strands and Cables ASTM F 2193 Standard Specifications and Test Methods for Components Used in the Surgical Fixation of the Spinal Skeletal System ASTM F 2267 Standard Test Method for Measuring Load Induced Subsidence of an Intervertebral Body Fusion Device Under Static Axial Compression END Page 17 of 17
Hip Joint Prostheses
 Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
Translated English of Chinese Standard: YY0118-2005 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY ICS 11.040.40 OF THE PEOPLE S REPUBLIC OF CHINA C 35 YY 0118-2005
YY/T PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA. Translated English of Chinese Standard: YY/T
 PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA Translated English of Chinese Standard: YY/T0520-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.060.10 C 33 YY/T 0520-2009
PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA Translated English of Chinese Standard: YY/T0520-2009 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.060.10 C 33 YY/T 0520-2009
YY / ISO 11070:1998
 Translated English of Chinese Standard: YY0450.1-2003 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.120.30 C 48 YY 0450.1-2003
Translated English of Chinese Standard: YY0450.1-2003 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.120.30 C 48 YY 0450.1-2003
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. YY/T Translated English of Chinese Standard: YY/T
 Translated English of Chinese Standard: YY/T0343-2002 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 30 Liquid penetrant
Translated English of Chinese Standard: YY/T0343-2002 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 30 Liquid penetrant
YY Translated English of Chinese Standard: YY
 Translated English of Chinese Standard: YY0497-2005 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.20 C 31 Pharmaceutical National Standard of the People s Republic of China YY 0497-2005
Translated English of Chinese Standard: YY0497-2005 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.20 C 31 Pharmaceutical National Standard of the People s Republic of China YY 0497-2005
YY/T Translated English of Chinese Standard: YYT PHARMACEUTICAL INDUSTRY STANDARD
 Translated English of Chinese Standard: YYT0325-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
Translated English of Chinese Standard: YYT0325-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
YY Translated English of Chinese Standard: YY PHARMACEUTICAL INDUSTRY STANDARD
 Translated English of Chinese Standard: YY0766-2009 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.70 C 41 YY 0766-2009
Translated English of Chinese Standard: YY0766-2009 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.70 C 41 YY 0766-2009
GB/T Translated English of Chinese Standard: GB/T
 Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
YY/T Translated English of Chinese Standard: YY/T
 Translated English of Chinese Standard: YY/T1218-2013 www.chinesestandard.net Email: Sales@ChineseStandard.net YY ICS 11.100 C 44 PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA YY/T
Translated English of Chinese Standard: YY/T1218-2013 www.chinesestandard.net Email: Sales@ChineseStandard.net YY ICS 11.100 C 44 PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA YY/T
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that JTL America 3205 Clairmont Ct., Ste. B Fort
GB / ISO :2006
 Translated English of Chinese Standard: GB18280.2-2015 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.080.01 C 47 NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA GB / ISO 11137-2:2006 Partly
Translated English of Chinese Standard: GB18280.2-2015 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.080.01 C 47 NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA GB / ISO 11137-2:2006 Partly
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB
 Translated English of Chinese Standard: GB1987-2007 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB ICS 67.220.20 X 41 Replacing GB 1987-1986 Food
Translated English of Chinese Standard: GB1987-2007 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB ICS 67.220.20 X 41 Replacing GB 1987-1986 Food
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EMPIRICAL TESTING CORP. 4628 Northpark Drive Colorado Springs, CO 80918 Dawn Lissy Phone: 719 264 9937 dlissy@empiricaltesting.com MECHANICAL Valid To: January
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EMPIRICAL TESTING CORP. 4628 Northpark Drive Colorado Springs, CO 80918 Dawn Lissy Phone: 719 264 9937 dlissy@empiricaltesting.com MECHANICAL Valid To: January
YY/T Translated English of Chinese Standard: YY/T PHARMACEUTICAL INDUSTRY STANDARD
 Translated English of Chinese Standard: YY/T1518-2017 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
Translated English of Chinese Standard: YY/T1518-2017 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
GB/T Translated English of Chinese Standard: GB/T NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB/T24253-2009 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 59.080.30 W 04 NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA GB/T 24253-2009 Textiles
Translated English of Chinese Standard: GB/T24253-2009 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 59.080.30 W 04 NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA GB/T 24253-2009 Textiles
鱼类罐头卫生标准 Hygienic standard for canned fish
 Translated English of Chinese Standard: GB14939-2005 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Wayne@ChineseStandard.net ICS 67.040 C 53 GB National Standard of the People's Republic
Translated English of Chinese Standard: GB14939-2005 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Wayne@ChineseStandard.net ICS 67.040 C 53 GB National Standard of the People's Republic
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
ASTM Committee F04 Tuesday 11/13/2012
 ASTM Committee F04 Tuesday 11/13/2012 Centennial Regency Ballroom Ballroom III V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM F04 Celebration of Committee F04's 50th Anniversary
ASTM Committee F04 Tuesday 11/13/2012 Centennial Regency Ballroom Ballroom III V 7:00 AM 7:00 AM 8:00 AM 8:00 AM 10:00 AM 10:00 AM 11:00 AM 11:00 AM F04 Celebration of Committee F04's 50th Anniversary
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. NB/T
 Translated English of Chinese Standard: GB/T47013.5-2015 www.chinesestandard.net Sales@ChineseStandard.net ENERGY INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA NB ICS 77.140.20 H 26 GB/T 47013.5-2015
Translated English of Chinese Standard: GB/T47013.5-2015 www.chinesestandard.net Sales@ChineseStandard.net ENERGY INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA NB ICS 77.140.20 H 26 GB/T 47013.5-2015
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system.
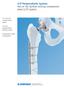 LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
DRAFT GUIDANCE DOCUMENT List of Recognized Standards
 DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
3. Insert Tocar Sleeves Insert the NCB tissue protection sleeve assembly 1.6 to 10mm through a skin incision (Fig. 38).
 NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF
 Translated English of Chinese Standard: GB13078-2001 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA ICS 65.120 B 46 GB 13078-2001 Replacing GB
Translated English of Chinese Standard: GB13078-2001 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA ICS 65.120 B 46 GB 13078-2001 Replacing GB
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. YY Translated English of Chinese Standard: YY
 Translated English of Chinese Standard: YY0831-2011 www.chinesestandard.net Email: Sales@ChineseStandard.net ICS 11.040.50 YY C 43 PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA YY
Translated English of Chinese Standard: YY0831-2011 www.chinesestandard.net Email: Sales@ChineseStandard.net ICS 11.040.50 YY C 43 PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA YY
Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007)
 BRITISH STANDARD Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007) ICS 11.040.40 NO COPYING WITHOUT BSI PERMISSION EXCEPT AS PERMITTED BY COPYRIGHT LAW BS
BRITISH STANDARD Non-active surgical implants Joint replacement implants Particular requirements (ISO 21534:2007) ICS 11.040.40 NO COPYING WITHOUT BSI PERMISSION EXCEPT AS PERMITTED BY COPYRIGHT LAW BS
Screw Set. Ref. Number : F-1
 Screw Set : 8008000 F- Screw Set : 8008000 Ref.Number Description Pieces Ref.Number Description Pieces 0800003 080000 0800005 0800006 080000 0800008 0800009 080000 08000 08000 080003 08000 080005 080006
Screw Set : 8008000 F- Screw Set : 8008000 Ref.Number Description Pieces Ref.Number Description Pieces 0800003 080000 0800005 0800006 080000 0800008 0800009 080000 08000 08000 080003 08000 080005 080006
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB
 Translated English of Chinese Standard GB15980-1995 Translated by: www.chinesestandard.net Email: Sales@ChineseStandard.net ICS 11.120.20 C 59 National Standard of the People s Republic of China GB Hygienic
Translated English of Chinese Standard GB15980-1995 Translated by: www.chinesestandard.net Email: Sales@ChineseStandard.net ICS 11.120.20 C 59 National Standard of the People s Republic of China GB Hygienic
WINSTA-R. Distal Radius System
 Distal Radius System Table of Contents Introduction WINSTA-R System 2 Indication 2 Surgical Technique Palmar Access for Radius Plate 3 Dorsal Access for Radius Plate 3 Positioning of the Radius Plate
Distal Radius System Table of Contents Introduction WINSTA-R System 2 Indication 2 Surgical Technique Palmar Access for Radius Plate 3 Dorsal Access for Radius Plate 3 Positioning of the Radius Plate
CS 2.7. Product information. Intelligent innovations for a better life. Presented by: Syntellix AG Aegidientorplatz 2a Hannover Germany
 Presented by: CS 2.7 Product information Aegidientorplatz 2a 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in Germany in
Presented by: CS 2.7 Product information Aegidientorplatz 2a 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in Germany in
A locking plate system that expands a surgeon s options in trauma surgery. Zimmer NCB Plating System
 A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
ISO INTERNATIONAL STANDARD. Dentistry Implants Dynamic fatigue test for endosseous dental implants
 INTERNATIONAL STANDARD ISO 14801 Second edition 2007-11-15 Dentistry Implants Dynamic fatigue test for endosseous dental implants Art dentaire Implants Essai de fatigue dynamique pour implants dentaires
INTERNATIONAL STANDARD ISO 14801 Second edition 2007-11-15 Dentistry Implants Dynamic fatigue test for endosseous dental implants Art dentaire Implants Essai de fatigue dynamique pour implants dentaires
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 6475 First edition 1989-11-01 Implants for surgery - Metal bone screws with asymmetrical thread and spherical under-surface - Mechanical requirements and test methods lmplan
INTERNATIONAL STANDARD ISO 6475 First edition 1989-11-01 Implants for surgery - Metal bone screws with asymmetrical thread and spherical under-surface - Mechanical requirements and test methods lmplan
ISO 9997 INTERNATIONAL STANDARD. Dental cartridge syringes. Seringues à usage dentaire pour cartouches. Second edition
 INTERNATIONAL STANDARD ISO 9997 Second edition 1999-12-15 Dental cartridge syringes Seringues à usage dentaire pour cartouches Reference number ISO 9997:1999(E) ISO 1999 PDF disclaimer This PDF file may
INTERNATIONAL STANDARD ISO 9997 Second edition 1999-12-15 Dental cartridge syringes Seringues à usage dentaire pour cartouches Reference number ISO 9997:1999(E) ISO 1999 PDF disclaimer This PDF file may
Compression Screw 3.2 Bioabsorbable. Product information
 Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
CS 2.0. Product information. Intelligent innovations for a better life.
 CS 2.0 Product information Intelligent innovations for a better life. 02. 03 Syntellix AG MAGNEZIX Compression Screw 2.0 Introduction CAUTION This product description is not sufficient or adequate to allow
CS 2.0 Product information Intelligent innovations for a better life. 02. 03 Syntellix AG MAGNEZIX Compression Screw 2.0 Introduction CAUTION This product description is not sufficient or adequate to allow
ISO INTERNATIONAL STANDARD. Non-active surgical implants Joint replacement implants Particular requirements
 INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
INTERNATIONAL STANDARD ISO 21534 Second edition 2007-10-01 Non-active surgical implants Joint replacement implants Particular requirements Implants chirurgicaux non actifs Implants de remplacement d'articulation
COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION
 C H A P T E R 3 0 COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION Scott R. Roman, DPM INTRODUCTION The most common fixation for proximal interphalangeal fusion
C H A P T E R 3 0 COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION Scott R. Roman, DPM INTRODUCTION The most common fixation for proximal interphalangeal fusion
CS 2.0. Product information. Intelligent innovations for a better life. Presented by: Syntellix AG Schiffgraben Hannover Germany
 Presented by: CS 2.0 Product information Syntellix AG Schiffgraben 11 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in
Presented by: CS 2.0 Product information Syntellix AG Schiffgraben 11 30159 Hannover Germany T +49 511 270 413 50 F +49 511 270 413 79 info@syntellix.com www.syntellix.com Implants are manufactured in
A locking plate system that expands a surgeon s options in trauma surgery. Zimmer NCB Plating System
 A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
NCB Distal Femur System. Surgical Technique
 NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
National Food Safety Standard Infant Formula
 Translated English of Chinese Standard: GB 10765-2010 www.chinesestandard.net Email: Sales@ChineseStandard.net NATIONAL STANDARD GB OF THE PEOPLE S REPUBLIC OF CHINA GB 10765-2010 National Food Safety
Translated English of Chinese Standard: GB 10765-2010 www.chinesestandard.net Email: Sales@ChineseStandard.net NATIONAL STANDARD GB OF THE PEOPLE S REPUBLIC OF CHINA GB 10765-2010 National Food Safety
Part 1: General requirements
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
ISO 7885 INTERNATIONAL STANDARD. Dentistry Sterile injection needles for single use
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7885 Third edition 2010-02-15 Dentistry Sterile injection needles for single use Médecine bucco-dentaire Aiguilles stériles pour injection, non
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7885 Third edition 2010-02-15 Dentistry Sterile injection needles for single use Médecine bucco-dentaire Aiguilles stériles pour injection, non
The Orthopaedic Cable System TECHNIQUE GUIDE
 The Orthopaedic Cable System TECHNIQUE GUIDE Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Table of Contents INTRODUCTION Indications.....................................
The Orthopaedic Cable System TECHNIQUE GUIDE Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Table of Contents INTRODUCTION Indications.....................................
DHS Plate 135º. DHS Platte 135º
 Section of hip fixation plate compression system DHS Plate 3º DHS Platte 3º Plaque de DHS 3º Indication : The compression hip plate is primarly indicated for intertrochanteric fractures. However, it can
Section of hip fixation plate compression system DHS Plate 3º DHS Platte 3º Plaque de DHS 3º Indication : The compression hip plate is primarly indicated for intertrochanteric fractures. However, it can
NCB Proximal Humerus System. Surgical Technique
 NCB Proximal Humerus System Surgical Technique NCB Proximal Humerus System Surgical Technique 3 Surgical Technique NCB Proximal Humerus System Table of Contents Introduction 4 Cable Fixation Options 5
NCB Proximal Humerus System Surgical Technique NCB Proximal Humerus System Surgical Technique 3 Surgical Technique NCB Proximal Humerus System Table of Contents Introduction 4 Cable Fixation Options 5
CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM. Family Ties
 CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM Family Ties Contoured for Optimal Fit with Proximal Femur The GTR cradles the bone anatomically while the strategically placed proximal and distal fins
CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM Family Ties Contoured for Optimal Fit with Proximal Femur The GTR cradles the bone anatomically while the strategically placed proximal and distal fins
Specializing in Mechanical Testing for Medical Devices. Office:
 Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
Technique Guide. Locking Attachment Plate. For treatment of periprosthetic fractures.
 Technique Guide Locking Attachment Plate. For treatment of periprosthetic fractures. Table of Contents Introduction Locking Attachment Plate 2 Indications 4 Surgical Technique Patient Positioning 5 Preparation
Technique Guide Locking Attachment Plate. For treatment of periprosthetic fractures. Table of Contents Introduction Locking Attachment Plate 2 Indications 4 Surgical Technique Patient Positioning 5 Preparation
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
JJF Translated English of Chinese Standard: JJF
 Translated English of Chinese Standard: JJF1070.2-2011 www.chinesestandard.net Sales@ChineseStandard.net JJF NATIONAL METROLOGICAL TECHNICAL SPECIFICATION OF THE PEOPLE S REPUBLIC OF CHINA JJF 1070.2-2011
Translated English of Chinese Standard: JJF1070.2-2011 www.chinesestandard.net Sales@ChineseStandard.net JJF NATIONAL METROLOGICAL TECHNICAL SPECIFICATION OF THE PEOPLE S REPUBLIC OF CHINA JJF 1070.2-2011
Surgical Technique. Calcaneal Locking Plate
 Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 16635-2 First edition 2014-09-15 Dentistry Dental rubber dam instruments Part 2: Clamp forceps Médecine bucco-dentaire Instruments de digue dentaire en caoutchouc Partie 2: Pinces
INTERNATIONAL STANDARD ISO 16635-2 First edition 2014-09-15 Dentistry Dental rubber dam instruments Part 2: Clamp forceps Médecine bucco-dentaire Instruments de digue dentaire en caoutchouc Partie 2: Pinces
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires Cathéters stériles
INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires Cathéters stériles
Technique Guide. 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
LCP DISTAL TIBIA PLATE
 LCP DISTAL TIBIA PLATE Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description
LCP DISTAL TIBIA PLATE Instruments and implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control This description
Anchorage system. tomas / Sets Page 270 tomas / Pins Page 271 Instruments and accessories Page 273 Patient consultation material Page 282
 tomas / Sets Page 270 tomas / Pins Page 271 Instruments and accessories Page 273 Patient consultation material Page 282 266 . innovative comprehensive efficient. Dentaurum Online Shop shop.dentaurum.com
tomas / Sets Page 270 tomas / Pins Page 271 Instruments and accessories Page 273 Patient consultation material Page 282 266 . innovative comprehensive efficient. Dentaurum Online Shop shop.dentaurum.com
Distal Radius Plate Instrument and Implant Set. Discontinued December 2017 DSUS/TRM/0916/1063(1)
 Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
SMV Scientific Bone Plate and Screw System Surgical Technique
 SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
Clover Staple. Surgical Technique
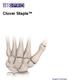 Clover Staple Surgical Technique Contents Product The BioPro Clover Staple is a permanent implant that removes minimal cross-sectional fusion area, allows easy radiographical monitoring and may permit
Clover Staple Surgical Technique Contents Product The BioPro Clover Staple is a permanent implant that removes minimal cross-sectional fusion area, allows easy radiographical monitoring and may permit
ISO INTERNATIONAL STANDARD. Sterile single-use intravascular catheter introducers
 INTERNATIONAL STANDARD ISO 11070 First edition 1998-05-01 Sterile single-use intravascular catheter introducers Introducteurs de cathéters intravasculaires stériles, non réutilisables A Reference number
INTERNATIONAL STANDARD ISO 11070 First edition 1998-05-01 Sterile single-use intravascular catheter introducers Introducteurs de cathéters intravasculaires stériles, non réutilisables A Reference number
Foot & Ankle. Smart Toe II. Intramedullary Implant. Operative Technique. Foot & Ankle
 Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
VA LCP MEDIAL COLUMN FUSION PLATES 3.5
 VA LCP MEDIAL COLUMN FUSION PLATES 3.5 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control
VA LCP MEDIAL COLUMN FUSION PLATES 3.5 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control
Orthopaedic Cable System
 Cerclage Solutions for General Surgery Orthopaedic Cable System Surgical Technique Table of Contents Introduction Orthopaedic Cable System 2 Indications 4 Contraindications 4 Surgical Technique Cerclage
Cerclage Solutions for General Surgery Orthopaedic Cable System Surgical Technique Table of Contents Introduction Orthopaedic Cable System 2 Indications 4 Contraindications 4 Surgical Technique Cerclage
ISO INTERNATIONAL STANDARD. Dentistry Reversible-irreversible hydrocolloid impression material systems
 INTERNATIONAL STANDARD ISO 13716 First edition 1999-05-01 Dentistry Reversible-irreversible hydrocolloid impression material systems Art dentaire Systèmes de produits pour empreintes à base d'hydrocolloïdes
INTERNATIONAL STANDARD ISO 13716 First edition 1999-05-01 Dentistry Reversible-irreversible hydrocolloid impression material systems Art dentaire Systèmes de produits pour empreintes à base d'hydrocolloïdes
Surgical Technique. Olecranon Locking Plate
 Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Catalog Trauma Implants. Instruments. Sets. Instruments and implants approved by the AO Foundation
 Catalog Trauma 2011 Implants Instruments Sets Instruments and implants approved by the AO Foundation R Historical Background The Association for the Study of Internal Fixation (in German: Arbeitsgemeinschaft
Catalog Trauma 2011 Implants Instruments Sets Instruments and implants approved by the AO Foundation R Historical Background The Association for the Study of Internal Fixation (in German: Arbeitsgemeinschaft
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
VA LOCKING CALCANEAL PLATES 2.7
 VA LOCKING CALCANEAL PLATES 2.7 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control Warning
VA LOCKING CALCANEAL PLATES 2.7 Instruments and Implants approved by the AO Foundation. This publication is not intended for distribution in the USA. SURGICAL TECHNIQUE Image intensifier control Warning
Designing a Novel Fixation Device for Pediatric Orthopaedic Tibia Fractures
 Designing a Novel Fixation Device for Pediatric Orthopaedic Tibia Fractures Evan Lange, Karl Kabarowski Tyler Max, Sarah Dicker Client: Dr. Matthew Halanski, MD Advisor: Dr. Paul Thompson, PhD Biomedical
Designing a Novel Fixation Device for Pediatric Orthopaedic Tibia Fractures Evan Lange, Karl Kabarowski Tyler Max, Sarah Dicker Client: Dr. Matthew Halanski, MD Advisor: Dr. Paul Thompson, PhD Biomedical
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology.
 2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Surgical Technique This publication is not intended
2.4 mm Variable Angle LCP Volar Extra-Articular Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Surgical Technique This publication is not intended
Surgical Technique. Clavicle Locking Plate
 Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
Instructions for Use
 Instructions for Use Intramedullary Nail Locking Screw Subtitles of the symbols used on the packaging Catalogue Number Batch Code Date of Manufacture Consult instructions for use Single-Use Product Do
Instructions for Use Intramedullary Nail Locking Screw Subtitles of the symbols used on the packaging Catalogue Number Batch Code Date of Manufacture Consult instructions for use Single-Use Product Do
ISO INTERNATIONAL STANDARD. Steel products Employer's qualification system for non-destructive testing (NDT) personnel
 INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
INTERNATIONAL STANDARD ISO 11484 Second edition 2009-02-15 Steel products Employer's qualification system for non-destructive testing (NDT) personnel Produits en acier Système de qualification, par l'employeur,
Intravascular catheters Sterile and single-use catheters. Part 5: Over-needle peripheral catheters
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Technique Guide. 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot.
 Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
2.4 mm LCP Radial Head Plates. Part of the Synthes LCP Distal Radius Plate System.
 2.4 mm LCP Radial Head Plates. Part of the Synthes LCP Distal Radius Plate System. Technique Guide Instruments and Implants approved by the AO Foundation Table of Contents Introduction 2.4 mm LCP Radial
2.4 mm LCP Radial Head Plates. Part of the Synthes LCP Distal Radius Plate System. Technique Guide Instruments and Implants approved by the AO Foundation Table of Contents Introduction 2.4 mm LCP Radial
Distal Radius Plate 2.4/2.7 dorsal and volar
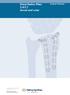 Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
Trinica and Trinica Select Anterior Cervical Plate System
 Surgical Technique Trinica and Trinica Select Anterior Cervical Plate System A New Twist to Anterior Cervical Plates Trinica and Trinica Select Surgical Technique 1 Trinica and Trinica Select Anterior
Surgical Technique Trinica and Trinica Select Anterior Cervical Plate System A New Twist to Anterior Cervical Plates Trinica and Trinica Select Surgical Technique 1 Trinica and Trinica Select Anterior
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System.
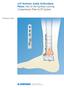 LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
Pre-Operative Planning. Positioning of the Patient
 Surgical Technique Pre-Operative Planning Decide upon the size and angle of the barrel plate to be used from measuring the x-rays. To maximise the sliding action when using shorter lag screws, the Short
Surgical Technique Pre-Operative Planning Decide upon the size and angle of the barrel plate to be used from measuring the x-rays. To maximise the sliding action when using shorter lag screws, the Short
External Skeletal Fixation (ESF)
 External Skeletal Fixation (ESF) Technique for fracture repair in animals Introduction External Skeletal Fixation is a versatile and effective technique for fracture repair in animals, rigidly stabilizing
External Skeletal Fixation (ESF) Technique for fracture repair in animals Introduction External Skeletal Fixation is a versatile and effective technique for fracture repair in animals, rigidly stabilizing
3.5 mm LCP Extra-articular Distal Humerus Plate
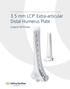 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
3.0/3.5/4.0/4.5/6.5/7.0/7.3. Cannulated Screws. Surgical Technique
 3.0/3.5/4.0/4.5/6.5/7.0/7.3 Cannulated Screws Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes products. Instruction
3.0/3.5/4.0/4.5/6.5/7.0/7.3 Cannulated Screws Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes products. Instruction
Technique Guide. Compact 2.0 LOCK Mandible. The locking system for the mandible.
 Technique Guide Compact 2.0 LOCK Mandible. The locking system for the mandible. Table of Contents Introduction Compact 2.0 LOCK Mandible 2 AO Principles 4 Indications and Contraindications 5 Surgical
Technique Guide Compact 2.0 LOCK Mandible. The locking system for the mandible. Table of Contents Introduction Compact 2.0 LOCK Mandible 2 AO Principles 4 Indications and Contraindications 5 Surgical
The Vilex FUZETM. Dual Thread Screw & Intramedullary Nail in One Implant. The Ultimate TTC Arthrodesis Internal Fixator
 The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
LCP Metaphyseal Plates. For extra-articular fractures.
 LCP Metaphyseal Plates. For extra-articular fractures. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
LCP Metaphyseal Plates. For extra-articular fractures. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier
