Development and Evolution of Inner Ear Sensory Epithelia and Their Innervation
|
|
|
- Martin Goodman
- 5 years ago
- Views:
Transcription
1 Development and Evolution of Inner Ear Sensory Epithelia and Their Innervation B. Fritzsch, 1 K.W. Beisel, 2 K.Jones, 3 I.Fariñas, 4 A.Maklad, 1 J.Lee, 3 L.F. Reichardt 5 1 Department of Biomedical Sciences, Creighton University, Omaha, Nebraska Department of Genetics, Boystown National Research Hospital, Omaha, Nebraska Department of Biology, University of Boulder, Boulder, Colorado Biologia Celular, Universidad de Valencia, Burjassot, 46100, Spain 5 University of California, San Francisco, California Received 11 April 2002; accepted 8May 2002 ABSTRACT: The development and evolution of the inner ear sensory patches and their innervation is reviewed. Recent molecular developmental data suggest that development of these sensory patches is adevelopmental recapitulation of the evolutionary history. These datasuggestthattheeargeneratesmultiple,functionally diverse sensory epithelia by dividing asingle sensory primordium. Those epithelia will establish distinct identities through the overlapping expression of genes of which only afew are currently known. One of these distinctions is the unique pattern of hair cell polarity. A hypothesisispresentedonhowthehaircellpolaritymay relate to the progressive segregation of the six sensory epithelia. Besides being markers for sensory epithelia development, neurotrophins are also expressed in delaminating cells that migrate toward the developing vestibular and cochlear ganglia. These delaminating cells originate from multiple sites at or near the developing sensory epithelia and some also express neuronal markerssuchasneurod.thedifferentialoriginofprecursors raises the possibility that some sensory neurons acquire positional information before they delaminate the ear. Such an identity of these delaminating sensory neurons may be used both to navigate their dendrites to the area they delaminated from, as well as to help them navigate to their central target. The navigational properties of sensory neurons as well as the acquisition of discrete sensory patch phenotypes implies amuch more sophisticated subdivision of the developing otocyst than the few available gene expression studies suggest Wiley Periodicals, Inc. JNeurobiol 53: , 2002 Keywords: ear development; ear sensory neurons; sensory neuron migration; sensory neuron projection INTRODUCTION Systems biology of the inner ear development is just coming of age with the identification of rudimentary Parts of this article were presented at the Sixth International Congress of Vertebrate Morphology, July 21 26, 2001, in Jena, Germany. This symposium was supported by DRF. Correspondence to: B. Fritzsch (Fritzsch@Creighton.edu). Contract grant sponsor: NIDCD; contract grant number: 2P01 DC00215 (B.F.). Contract grant sponsor: NIDCD; contract grant number: R01 DC04279 (K.W.B.) 2002 Wiley Periodicals, Inc. Published online in Wiley InterScience ( DOI /neu gene networks involving specific genes (e.g., bhlh, Pax,Wnt,Forkhead),whichplayfundamentalrolesin the fate assignment and subsequent clonal expansion of these basic cellular building blocks (reviewed in Fritzsch and Beisel, 2001; Fekete and Wu, 2002). Recently, we have hypothesized that the development of the neurosensory and nonsensory epithelium of the vertebrate inner ear can be interpreted as transformation of the basic building blocks of the sensory ciliated neuronal structures of C. elegans and Drosophila. In contrast, formation of the sensory neurons appears to be ade novo formation peculiar to vertebrates.themechanismsbywhichtheseepitheliaform 143
2 144 Fritzsch et al. the complex three-dimensional structure and their interactions with sensory neurons are just now becoming understood. We will present herein new ideas and data concerning the formation of the distinct endorgans of the inner ear and their innervation patterns. In particular, we will explore the current molecular understanding of endorgan formation, separation, and transformation to present a model of saccular transformation into the cochlea. In principle, the wellknown molecular multiplication and diversification model will be followed (Venter et al., 2001). We propose that enlargement of a given sensory epithelium, through enhanced proliferation followed by segregation and development of novel, functionally relevant modifications, is at the core of inner ear sensory patch evolution. Evolution has transformed a simple ear with only two canals and a single macula communis found in ancestral vertebrates into a complex three-dimensional structure that has up to nine distinct endorgans (Fig. 1) in derived vertebrates (Lewis et al., 1985; Fritzsch, 1987; Fritzsch and Wake, 1988). Previous work on the evolution of these various sensory epithelia suggests specific relationships. For example, it has been proposed that the saccule gives rise to the basilar papilla or cochlea of land vertebrates (Fritzsch, 1992). In keeping with this suggestion, recent developmental evidence has lent itself to the idea of evolutionarily progressive segregation of sensory epithelia through unknown processes to form distinct rather than confluent sensory patches (Norris, 1892; Fritzsch and Wake, 1988; Cantos et al., 2000; Farinas et al., 2001; Fritzsch et al., 2001). However, almost none of these articles have actually taken a closer look as to what kind of transformation is occurring to turn a saccule-like anlage into a mammalian cochlea and how this segregation relates to the formation of discrete innervation patterns. For example, as distinct sensory patches have developed, neuronal projections to these patches have apparently also coevolved. In order to accommodate a divergent functionality of each endorgan, there exists a need for afferents to segregate from existing projections. This separation is crucial to ensure that the novel information gathered by the new endorgan is reaching the brainstem in a distinct conduit for further specific processing. Nineteenth century anatomists suggested a parallel evolution between the progressive segregation of each sensory epithelium with a concurrent segregation of sensory projections to these newly evolved endorgans. Retzius (1884) and the brothers Sarasin (1892) were the first to recognize this trend, and Retzius (1884) used this idea to develop a cladistic relationship among extant vertebrates. de Burlet (1934) tried to further formalize this idea of conservation and transformation of the inner ear innervation by suggesting a progressive split of both innervation and sensory epithelia (Fig. 1). In contrast to this apparent evolutionary segregation of peripheral innervation, it has been hypothesized that the central projection of vestibular and cochlear afferents may originally overlap (Larsell, 1967). Such overlap in ancestral vertebrates appears to precede the segregated projections observed in the more recently derived vertebrates (Dickman and Fang, 1996; McCormick, 1999). It has only recently been recognized that during inner ear development, innervation is initially fairly precise. For example, central projections will undergo only limited reorganization of terminals due to continuing progressive segregation (Maklad and Fritzsch, 2002). Likewise, developmental analyses of inner ear afferent innervation suggest an early and precise segregation of afferent projections to their respective sensory epithelia (Fritzsch et al., 1995; Huang et al., 2001; Kim et al., 2001). It is fair to say that hardly any information exists about the molecular mechanism that specifies either central or peripheral projections to and from the ear. We will provide evidence that suggests a developmental link between sensory patch formation and formation of novel sensory neurons. In addition, data will also be presented suggesting that spatio-temporal expression of neurotrophins in sensory epithelia is a critical mediator for the proper segregation of afferent projection to specific endorgans. SEGREGATION OF ENDORGANS: AN EVOLUTIONARY PERSPECTIVE In general, evolution of the ear is based on multiplication of existing sensory patches. These modifications are likely followed by functional diversification through creation of modified, unique structures that allow transduction of a previously unexplored property of the mechanical energy that reaches the ear. The simplest ear to be found in extant vertebrates is the hagfish ear. This ear has only three sensory epithelia, one macula communis, and two crista organs (Lewis et al., 1985; Fritzsch, 2001a,b). The largest number of sensory patches in the ear is found in certain species of limbless amphibians (Fig. 1), which have nine different sensory patches: three canal cristae, utricle, saccule, lagena, papilla neglecta, papilla basilaris, and papilla amphibiorum (Sarasin and Sarasin, 1892; Fritzsch and Wake, 1988). Descriptive developmental evidence has long suggested that the
3 Ear Sensory Development 145 Figure 1 These images show the conjecture of de Burlet (1934) about the evolutionary similarities of endorgan and innervation multiplication from two hypothetical ancestors (A,B) to the vertebrates with the largest number of sensory epithelia, Southeast Asian caecilians (C). In his accompanying text, de Burlet pointed this out explicitly, but without proposing any mechanism. Conceivably, two mechanisms are apparent. In one mechanism there is lineage relationship between hair cells and sensory neurons. Thus whenever hair cell precursors are split their innervation will split. Alternatively, there is no relationship at all between hair cells and sensory neurons, and the multiplication of innervation comes about through the selective attraction of sensory afferents to newly formed endorgans. In the latter scenario both central and peripheral specifications of the sensory projections need to be accomplished independently of the changes in hair cell specification. In the lineage relationship scenario, any alteration in the specification of hair cells would also affect the information conveyed by sensory cells, thus allowing for a rapid coevolution of hair cell and sensory neuron fate specification. The work of Norris (1892) on salamanders indicated that the above-presented evolutionary scenario might actually be recapitulated during development. He depicted that a single prosensory anlage splits over time into the different sensory organs (D,E). It is unclear at which point in development polarity of hair cells is established. We tentatively superimposed the known adult polarity distribution in salamanders (Lewis et al., 1985) onto the almost completely segregated patches of forming sensory epithelia. Note that all canal cristae have only one hair cell polarity, that the utricle has only polarity toward the striola (indicated by the dotted line), and that the saccule and sensory epithelia derived from the saccule are all polarized away from a dividing line in many salamanders. Abbreviations: AC, anterior crista; AP, amphibian papilla; BP, basilar papilla; HC, horizontal crista; PC, posterior crista; PN, papilla neglecta. Modified after Norris (1892), de Burlet (1934), and Lewis et al. (1985). evolution of multiple sensory epithelia came about through developmental splitting of a single sensory anlage (Norris, 1892; Fritzsch et al., 1998). Moreover, it appears that organs such as the lagena may have evolved three times independently in vertebrates, each time by splitting off from the saccule (Fritzsch, 1992). In contrast, other organs such as the basilar papilla may have evolved only once, namely during the segregation of the lagena from the saccule in the tetrapod ancestors (Fritzsch, 1987). Such segregation and functional diversification are best documented for the papilla neglecta/papilla amphibiorum system. It appears that the location of each of the two sensory patches to either the utricle or the saccule and association with the perilymphatic sound conduction system may determine the future function as a sound pressure receiver or as an additional vestibular receptor (Fritzsch and Wake, 1988; Brichta and Goldberg, 1998). Another well-documented example is the utricle in some fishes (herring) that forms
4 146 Fritzsch et al. three distinct sensory patches. While two of these patches retain their ancestral function as gravistatic receptors, one patch has acquired a novel function as a sound pressure receiver by association with the perilymphatic sound conduction system (Lewis et al., 1985; Fritzsch, 2001a). Development and Evolution of a Third Sensory Patch for Angular Velocity Reception Comparison of craniate vertebrates suggests that two canals with their associated sensory epithelia is a primitive feature retained in lampreys and hagfish. In contrast, jawed vertebrates have modified this primitive ear by adding a third canal, the horizontal canal (Lewis et al., 1985). Detailed analysis of the organization of the canal sensory epithelium shows distinct differences between hagfish, lampreys, and jawed vertebrates that implies a morphocline toward formation of a crista (Fritzsch, 2001a). Recently the homeobox gene orthodenticle (Otx) was identified as being important for the formation of the horizontal canal in jawed vertebrates. Otx is a highly conserved gene that delineates the anterior part of the central nervous system in vertebrates and invertebrates (Reichert and Simeone, 1999). Jawed vertebrates have evolved two Otx genes, Otx1 and Otx2. Both show partially overlapping expression in the brain and the ear (Morsli et al., 1999; Cantos et al., 2000). In contrast, Otx is not expressed in the lamprey ear (Tomsa and Langeland, 1999). Confirming the suggested role of Otx1 is the absence of the horizontal (lateral) canal in Otx1 null mutant mice (Morsli et al., 1998). Moreover, Otx1 can be substituted for Otx2 in the forebrain but not in the ear (Acampora et al., 2000). Thus, it can be speculated that the entire horizontal canal system depends on Otx1 (Mazan et al., 2000). However, closer examination of the Otx1 null phenotype has revealed that some parts of the horizontal crista appear to form initially (Morsli et al., 1999) and may remain as displaced patches of hair cells (Fritzsch et al., 2001). Moreover, sensory fibers were found to extend to those displaced patches of hair cells (Fritzsch et al., 2001). Indeed, close comparison of jawed vertebrates with lampreys suggests certain similarities with the dorsal papilla, a small patch of sensory hair cells of lampreys. These similarities in distribution and pattern of innervation of the dorsal papilla of lampreys and the displaced horizontal crista of Otx1 null mutant mice suggest a two-step evolution of the horizontal canal system. First, through molecularly unknown mechanisms, a multiplication of hair cells and a segregation of the dorsal sensory patch occurred in the common ancestor of lampreys and jawed vertebrates. This patch has a separate innervation and thus would be able to transmit specific information only gathered by this sensory patch to the brain. This scenario predicts the existence of distinct genes that are essential for formation of the horizontal crista but have little or no effect on horizontal canal formation. Further evolution has refined this central projection into two discrete patterns, an elasmobranch pattern and a pattern for all other jawed vertebrates. In each of these two lineages the horizontal canal system is linked with eye movement control to form a unique output system (Fritzsch, 1998). Evolution of the third, horizontally positioned canal allowed a direct coding of angular movement in all three cardinal planes for fast activation of eye and postural control movements without additional computational delay in the hindbrain. This advantage was evolutionarily stabilized and thus extant vertebrates have three patterns of eye muscles and eye muscle innervation (Fritzsch, 1998). Unfortunately, the pattern of horizontal crista/dorsal papilla projection as compared with the vertical cristae still needs more detailed analyses in lampreys, elasmobranches, and other jawed vertebrates. The above outlined scenario of horizontal canal evolution predicts that these three vertebrate lines will each have a unique pattern of central projections of these probably homologous sensory epithelia. Development of the Cochlea An evolutionary scenario of developmental segregation of the tetrapod cochlea from the saccule was proposed some time ago (Norris, 1892; Fritzsch, 1992). However, initial molecular analysis of chick ear development showed that each sensory patch appeared as a discrete entity instead of through progressive segregation from a single, molecularly recognizable anlage (Wu and Oh, 1996). These data support a boundary model (Brigande et al., 2000; Cantos et al., 2000; Fekete and Wu, 2002). This model proposes that a given sensory receptor develops initially at the intersection of specific polarity coordinates that reflect expression domains of specific genes in the ear. However, more recent data on chicken (Cole et al., 2000) as well as on mouse inner ear development (Morsli et al., 1998) have re-established the possibility for the progressive segregation of the cochlear anlage from the saccule during development. Studying the in situ expression for bone morphogenetic protein-4 (BMP- 4), Serrate 1, and lunatic fringe, Cole et al. (2000) suggested the existence of a sensory competent region
5 Ear Sensory Development 147 Figure 2 These wholemounted ears show the expression of various genes as revealed with a lac-z marker inserted into GATA3, BDNF, NeuroD, and NT-3 genes (A,B,D,E). At embryonic day 11 (E11) distinct patterns of expression are apparent. Note that the distribution of GATA3 and BDNF has some similarities, but also show clear differences. In contrast, similarities between the NeuroD and NT-3 expression in the otocyst are obvious in the area of the future utricle and saccule. Note that each of these four genes is expressed both in restricted areas of the ear and in cells of the forming vestibulo-cochlear ganglion. LacZ-positive cells (arrows) can be seen to delaminate from the otocyst in all four cases. Delamination and cellular migration to the forming vestibulo-cochlear ganglion is even more obvious at later stages [E12 (C,F)] where lacz- positive cells can be traced from equally lacz-positive areas of the otocyst. Bar indicates 100 m. that encompasses all presumptive sensory organs. Likewise, using in situ hybridization, Morsli et al. (1998) described a single patch of lunatic-fringe-positive cells that underwent progressive segregation into the utricle, saccule, and cochlea during development of the mouse ear. These findings were confirmed through the analyses of the developmental expression dynamics of neurotrophins (Farinas et al., 2001). Specifically, the lacz reporter for brain-derived neurotrophic factor (BDNF) expression suggests an early formation of the three canal cristae [embryonic day 12 (E12); Fig. 2]. Each epithelium is uniquely characterized by exclusive expression of BDNF lacz. In contrast, neurotrophin-3 (NT-3 laczneo ) is initially up-regulated as early as E9.5 in a single, anteroventral patch. In the course of the next 4 days, this single patch elongates and gives rise to the utricle (E11.5) followed by a segregation of the saccule (Fig. 2), and, finally, co-
6 148 Fritzsch et al. Figure 3 These images show the distribution of lacz-labeled cells in histological sections (B,C,E), wholemounts (A,D), BDNF (A,B,D,E), and NeuroD (C). These images show the similarities in distribution of NeuroD and BDNF inside the utricle as well as in delaminating cells (B,C). The wholemounts show the distribution of BDNF in forming hair cells inside the saccule and utricle as well as the delaminating cells converging from specific areas of these sensory epithelia toward the forming vestibular ganglion (A). Such cells delaminate as late as E17 from the hair-cell-free hilus region of the utricle that is filled with hair cells only after completion of this delamination (D,E). Bar indicates 100 m. chlea by E13.5 (Farinas et al., 2001) and older stages (Fig. 3). It needs to be stressed that the use of the lacz reporter for BDNF and NT-3 expression to identify sensory patch anlage is justified by the restricted expression of these neurotrophins in hair cells (BDNF) and supporting cells (NT-3). The expression patterns of most of the other developmental markers, such as lunatic fringe or BMP-4, have yet to be clarified in the ear in regards to their functional relationship with sensory patch development (Fekete and Wu, 2002). For example, BMP-4 predominantly inhibits neural differentiation in early development. In the brain, fibroblast growth factors (FGFs) typically counterbalance BMP-4 activity (Diez del Corral and Storey, 2001; Scully and Rosenfeld, 2002), and the alleged role of BMP-4 in dorsal interneuron specification was recently shown to be mediated by Wnt genes (Muroyama et al., 2002). It is, however, fair to say that the role of BMP-4 can change in later development to be more proneural, but this role has not yet been explored in detail in the ear owing to the early lethality of BMP-4 null mutant mice. Conditional null mice specific for the ear are needed to further clarify the role of BMP-4. Interestingly, expression of BDNF lacz initially parallels closely with the expression domain of BMP-4 in the abneuronal region of the cochlea (Morsli et al., 1998; Farinas et al., 2001), whereas NT-3 expression closely corresponds to lunatic fringe expression patterns. While BDNF expression is early in development of the three canal cristae, BDNF expression is delayed until E12.5 in the utricle and saccule and until E13.5 in the cochlea (Fig. 2). Closer examination of inner ear sections and wholemounts shows that full segregation of the three NT-3 lac-zneo -positive patches
7 Ear Sensory Development 149 coincides with the morphogenetic separation of each sensory epithelium (Figs. 2 and 3). Thus, the initial separation of utricle and saccule parallels with the formation of the utriculo-saccular foramen. If the formation of this foramen is even only partially disabled, as in Otx-1 null mutant mice, both epithelia remain confluent (Morsli et al., 1999; Fritzsch et al., 2001). The segregation of the saccule and cochlea concurs with the formation of the ductus reuniens. Interestingly, in this case the expression of BDNF simply stops in the connecting nonsensory epithelium between the saccule and the cochlea at E13.5 (Farinas et al., 2001). In summary, both older descriptive as well as more recent molecular analyses suggest that possibly a single patch of sensory organ competence is divided by unknown mechanisms into the sensory patches of a specific vertebrate. Development appears to recapitulate this aspect of ear evolution surprisingly unaltered. Establishing Hair Cell Polarity in Sensory Epithelia If developmental segregation of sensory patches is accepted as a major driving force in ear evolution, then the differential polarity of hair cells within and between endorgans must be addressed. A plausible mechanism(s) must be detailed on how such transformations in hair cell polarity might have occurred. These variations in polarity permit differential mechanical stimulation to be recognized by one sensory epithelium versus another. For example, the utricle has two opposing polarity orientations of the hair cells in the epithelium (Fig. 1), while the canals have only one polarity of hair cells (Lewis et al., 1985). Derivation of a canal from the utricle requires that segregation occurs from only one area of polarity of hair cells. Indeed, developmental studies (Figs. 1 and 2) suggest that the horizontal crista may derive only from the abneuronal polarity patch (Farinas et al., 2001; Fritzsch et al., 2001). Interestingly, the horizontal crista has a similar polarity compared to this part of the utricle (Lewis et al., 1985). In contrast, hair cells in the evolutionarily and developmentally (Morsli et al., 1998) earlier derived anterior and posterior crista have a polarity away from the utricle. In order to resolve this issue, more data on early markers around E11 12 are needed to demonstrate more exactly where each of the two vertical canal crista are derived from in the utricle. Like the canal cristae, the mammalian cochlea has hair cells of only one polarity. If one extends the line of polarity of the saccule across the ductus reuniens one ends up with the striola of the saccule roughly equal to the divide between the greater and lesser epithelial ridge. The greater epithelial ridge would be equivalent to the neuronal half of the saccule whereas the lesser epithelial ridge would be equivalent to the abneuronal half of the saccule. The recently reported finding of hair cell differentiation of cells of the greater epithelial ridge through expression of mouse atonal homologue 1 (Math1) (Zheng and Gao, 2000) would be in line with such an interpretation. These data imply that the greater epithelial ridge has retained the capacity to form hair cells, but is not normally doing so owing to some changes in the up-stream regulation of hair-cell-specific gene expression. Interestingly, this suggests, similar to the relationship of canals and saccule, that polarity is conserved away from the striola/greater epithelial ridge. It thus appears that the cochlea of mammals is nothing but an elongated and highly modified abneuronal half of the saccule. Clearly, the development of expression domains of both lunatic fringe (Morsli et al., 1998) and NT-3 (Farinas et al., 2001) support this possibility by showing a progressive segregation of a single patch into a saccule and the cochlea. BDNF shows an initial up-regulation in the striola region (Fig. 2) of both utricle and saccule (Farinas et al., 2001). In the cochlea, the up-regulation of BDNF sweeps from inner hair cells to outer hair cells, indicating a conservation of spatio-temporal patterning between the utricle, saccule, and cochlea. The main problem with such a scenario is the need to explain the loss of hair cell differentiation in the ductus reuniens and in the neuronal part of the cochlea, the largest part of the greater epithelial ridge (GER). However, the data on Math1 expression effects clearly show the potential of this area to generate hair cells (Zheng and Gao, 2000). Likewise, the formation of extra hair cells in the GER in Hes5 null mutants (Zine et al., 2001) shows the latent capacity of the GER to form hair cells. In addition, those extra GER hair cells have a polarity opposite to the cochlear hair cells (A. Zine, personal communication). Those sets of experimental data are compatible with a cochlear origin out of the saccular anlage through selective suppression of hair cell formation in the GER. Clearly, neurotrophins, while representing suitable sensory epithelia markers, will in themselves not determine development of the sensory epithelia, as is apparent by the normal development in neurotrophin null mutants (Fritzsch et al., 1999). Further molecular understanding of neurotrophin expression regulation in the ear is needed to relate the expression of these markers to sensory patch differentiation. Hair cell polarity is essential for each sensory patch. For example, turning the hair cell polarity of
8 150 Fritzsch et al. the cochlea by 90 would render the cochlea essentially unresponsive to sound. Despite this importance, next to nothing is known about how polarity is achieved at a cellular level. However, some of the molecular players of denticle polarity formation in Drosophila parasegment development have been identified in the developing ear, for example, Serrate (Morsli et al., 1998; Hatini and DiNardo, 2001). It remains to be shown how many of the other molecular players of this developmental module will be found in the ear and whether their interaction is important for the polarity formation of hair cells during sensory patch development. EVIDENCE FOR CELL FATE LINKS BETWEEN SENSORY NEURON AND HAIR CELL PRECURSORS In order to comprehend the change in cell fate acquisition to form the three-dimensional cochlea, we need to understand how sensory neuron development relates to hair cell development. In general, sensory neurons and hair cell formation each depend on a single proneuronal gene, neurogenin 1 (ngn-1), and Math1, respectively. Ngn-1,abHLH gene, is essential for formation of all sensory neurons in the ear (Ma et al., 2000). Similarly, Math1 has been shown to be essential for hair cell development (Bermingham et al., 1999). If the sensory neurons and hair cells have a common precursor, then alternations in ngn-1 and/or Math1 may affect generation of these two cell types. In this context, loss of ngn-1 also results in reduction in the sensory epithelia. In particular the saccule and the cochlea show severe reductions in hair cell formation (Ma et al., 2000). This suggests that there are interactions between ngn-1 and Math1 dependent precursors. Two possibilities exist for such an interaction. One of these is that some clones are first ngn-1 dependent and subsequently switch to Math1, shutting down ngn-1 through unspecified intracellular signaling pathways (Fritzsch and Beisel, 2001; Gowan et al., 2001). This does not necessarily mean that sensory neurons and hair cells are clonally related as this could also come about through a transient and independent expression of ngn-1 in presumptive sensory patches without forming sensory neurons. Alternatively, ngn-1 and Math1 exist in distinct clones but the clones are linked through an extracellular suppressor system such as the well-known delta/notch system (Lanford et al., 2000). At any rate, ngn-1 null mutants show a massive effect of ngn-1 on hair cells. Spatial Origin of Sensory Precursors in Mice To explore the validity of the proposed scenario for sensory patch formation and its hypothetical link to sensory neuron formation, we will revisit the recent evidence characterizing specific areas of precursor delamination as revealed through expression patterns of neurotrophins (Farinas et al., 2001). In vitro experiments have shown that a large proportion of sensory neurons delaminate from the anteroventral quadrant of the otocyst (Van de Water, 1983). This is observed in a number of vertebrates as demonstrated by various staining techniques to label delaminating cells (Cole et al., 2000; Fekete and Wu, 2002). However, none of these studies has actually performed a three-dimensional mapping to demonstrate how stable or mobile the area(s) of delamination is over the rather protracted period of sensory neuron proliferation, which lasts several days in mice (Ruben, 1967). Those areas of delamination correspond to either part of the sensory patches (Figs. 2 and 3) or are adjacent to sensory patches (Farinas et al., 2001). These data suggest that delamination of cells actually occurs in spatially restricted areas of the ear. The BDNF lac Z reporter system has been used to demarcate neuronal cellular delamination in the inner ear. The utricle, more specifically the hilus region, gives rise to delaminating cells from E10.5 to E16.2 (Figs. 2 and 3). These cells express BDNF and NT-3, and the delaminating cell types derive from the same restricted area on the neuronal side of the utricle. In the later stages there is a mutual exclusion of BDNFpositive hair cells and delaminating cells (Fig. 3). Only after all delamination has stopped at E16.5 is there an addition and filling in of the hilus region by hair cells. Indeed the hilus appears to be an area that produces in temporal sequence a mixture of delaminating cells and hair cells (Fig. 3). Again, this spatiotemporal pattern is consistent with the possibility that expression of Math1 (hair cells) and ngn-1 (precursors) does not occur in the same cell. The pattern of delamination around the saccule is different from that in the utricle. It appears that all BDNF lac Z - and NT-3 lac Zneo -positive delaminating cells are derived from the expanding region between the cochlear base and the saccule (Figs. 2, 3, 4). This region is initially free of BDNF expression but shows a subsequent up-regulation of BDNF in these putative precursors. The appearance of these cells is not due to a generalized retention of the lacz marker. NT-3 expression will be down-regulated in this area between E12.5 and E13.5. This area will not generate hair cells but will turn into the ductus reuniens con-
9 Ear Sensory Development 151 necting the cochlear duct with the saccule. It thus appears that the connecting area between saccule and cochlea transforms into a domain of delaminating precursor cells at the expense of hair cell formation. It is interesting in this context that in ngn-1 null mutants the reduction in size and hair cell number was largest in the saccule (Ma et al., 2000). Essentially, in the ngn-1 null mutant saccular development is arrested at the level of an E12.5 day embryonic mouse ear. Most interesting is the pattern of sensory precursor delamination in the cochlea. Presumably, a large fraction of delaminating cells from the area of the future ductus reuniens provides saccular and perhaps also basal turn spiral sensory neurons. Two to three additional areas of delaminating NT-3-positive, BDNFnegative cells were found near the base and middle turn (Farinas et al., 2001). Even more intriguing, a very large group of delaminating BDNF lac Z -positive cells was found only near the apex. However, no NT-3-positive cells were ever detected delaminating from the apex. The cochlear apex is thus unique in that it behaves like canal cristae with respect to delaminating cells and early up-regulation of BDNF in hair cell precursors. Unfortunately, our data on delaminating cells from canal cristae are not as clear. At least the anterior and horizontal cristae apparently give rise to some BDN- F lac Z -positive precursors between E10 11 (Fig. 2). Data for the posterior cristae are even ambiguous. However, judging from other markers such as NeuroD (Figs. 2, 3, and 5) it is possible that precursors that do not express BDNF lac Z delaminate from those presumptive sensory epithelia. Judging from sections (Liu et al., 2000) and wholemounts (Kim et al., 2001) the area of NeuroD-positive sensory precursor delamination is larger than the areas delineated by sensory patch markers such as NT-3 (Figs. 2 and 5). Moreover, some delaminating NeuroD-positive neuronal precursors appear to be also positive for either BDNF or NT-3 (Fig. 5). A more detailed correlation of neurotrophin-positive delaminating cells with cells carrying other markers such as neurogenic differentiation 1 (NeuroD) (Figs. 2 and 5) or brain factor 1 (BF1) (Hatini et al., 1999) and a clonal analysis of their fate are needed to establish how many of these cells are actually turning into neurons. Overall, the evidence of delaminating cells, as revealed with BDNF and NT-3 lacz markers, suggests a much richer variety of origins of delaminating cells in the ear than previously anticipated. This varies from cells expressing BDNF lac Z alone (canal cristae) to a mix (and possibly coexpression) of BDNF lac Z - and NT-3 lac Zneo -positive cells (utricle and saccule) to a patchy delamination of either BDNF lac Z -positive cells (apex) or NT-3 lac Zneo -positive cells (base) in the cochlea. Closer examinations using acetylated tubulin or trkb or trkc antibodies suggest that those proliferating precursors lose neurotrophin expression when they start to differentiate into sensory neurons (Fig. 4). In addition, neurites of differentiating sensory neurons extend toward the utricle and saccule surrounded by delaminating neurotrophin-positive cells (Fig. 4). In an analogous system, the epibranchial placode-derived sensory neurons, neural crest cells, appear to serve as guidance structures for sensory afferents to reach the brain (Begbie and Graham, 2001). We would like to suggest that the delaminating cells provide guidance cues for the neurites to navigate toward specific sensory epithelia. Quantitative Relationship of Hair Cells and Sensory Neurons It has long been known that the afferent to hair cell ratio is much higher in auditory than in vestibular sensory organs (Lewis et al., 1985; Will and Fritzsch, 1988). Mice are no exception to this rule and show multiple type I afferent converging onto a single inner hair cell. In contrast, most innervation to vestibular hair cells and outer hair cells of the cochlea is by divergence of a single afferent fiber onto multiple hair cells. In fact, assuming that 90% of the 32,000 spiral sensory neurons in humans are type I and 10% are type II (Lewis et al., 1985), this suggests a ratio of 29,000:3,500 8:1 for the inner and of 3,000:14,000 1:5 for the outer hair cells. Interestingly, the ratio of type II neurons to OHCs is not unlike the ratio of vestibular sensory neurons to vestibular hair cells (1:5 for saccule, 1:5 for mammalian utricle, 1:3 1:4 for canal cristae; Lewis et al., 1985). This suggests that indeed there is tremendous increase in the number of sensory neurons per inner hair cell, and this increase decreases in a base to apical gradient (Ryugo, 1992). We propose that the formation of these extra sensory neurons is at the expense of hair cells. More precisely, the formation of the ductus reuniens as well as the greater epithelial ridge reflects the transformation of pluripotent precursors into precursors that give rise only to sensory neurons. Having a null mutation for the bhlh gene responsible for this transformation would result in a loss of all precursors and, if Math1-dependent clones are downstream of these ngn-1-dependent clones, in a subsequent reduction of hair cell formation. Detailed measurements of the ductus reuniens area in ngn-1 null mutants compared to control animals will be necessary to verify this possibility. There is no doubt that sensory neuron lineage markers such as ngn-1 and NeuroD appear in both the
10 152 Fritzsch et al. Figure 4 Horizontal sections show the distribution of delaminating saccular cells and various markers. Note that the NT-3 lac Zneo -positive cells (A,B) emigrate from the saccular anlage (S) to aggregate near the forming cochleo-vestibular ganglion (G). The sensory neurons have a distinct morphology (C) and are positive for -acetylated tubulin (B) and trkb (D). In contrast, the delaminating cells (labeled primordia ) are positive for NT-3 lac Zneo (A,B) but not for tubulin (B) or trkb (D). Fibers to the saccule are passing between these cells in a fascicle (F) surrounded by lacz-positive precursors. Bar indicates 100 m. otocyst and in delaminating sensory neuron precursors (Liu et al., 2000; Ma et al., 2000; Kim et al., 2001). ngn-1 is upstream of NeuroD in these precursors. NeuroD appears to be upstream of Brn3a (i.e., POU domain, class 4, transcription factor 3, Pou4F3) (Kim et al., 2001), which in turn is upstream of trkc (neurotrophic tyrosine kinase, receptor, type 3) (Huang et al., 2001). A result of the shared gene network, commonalties in the NeuroD- and Brn3anull phenotype are found, such as basal turn neuronal loss and aborted growth of sensory afferents to the posterior crista (Huang et al., 2001; Kim et al., 2001). Delaminating precursors also appear to undergo further cell division after delaminating from the ear. It remains to be determined how the total number of precursors relates to the total number of postmitotic sensory neurons and how this is regulated. The final ratio is rather unlikely to be regulated by the neurotrophins as a single hair cell can support up to 10 sensory neurons (inner hair cells in the cochlea) or is only one supporter for a diverging fiber that innervates several hair cells (outer hair cells and vestibular system). Indeed, branching patterns in neurotrophin mutants suggest that competition between fibers to access hair cells is more important than neurotrophin levels. Conditional delayed elimination of neurotrophins in the ear is needed to further test this hypothesis. ESTABLISHING TOPOLOGY OF SENSORY NEURON IDENTITY It is fair to say that we do not know how sensory neurons establish their identity. Sensory neurons develop precise initial projections of their axons into the CNS (Maklad and Fritzsch, 2002) and of their dendrites to the various endorgans of the ear (Fritzsch et al., 1995; Huang et al., 2001). The presence of such precise initial projections suggests that developing sensory neurons have an identity at the time their processes extend toward the CNS or reach the ear. No distinct molecular marker unique to even a subset of sensory neurons in the ear is known (Fekete and Wu, 2002), except for the apparently selective GATA enhancer-binding protein 3 (GATA3) expression (Fig. 2) in the developing spiral sensory neurons (Rivolta and Holley, 1998; Karis et al., 2001; Lawoko-Kerali et al., 2002). GATA3 and other zinc finger proteins are known to participate in establishing cellular identity. In other GATA3-expressing neurons, absence of GATA3 causes misrouting of fibers (Karis et al.,
11 Ear Sensory Development 153 Figure 5 The distribution of BDNF, NT-3, and NeuroD expression in E11 ears. Each image is a computer generated color-coded image of the lacz expression shown in Figure 2. The panel labeled merge shows the computer generated superposition of these expression domains to highlight the relative level of overlap of the three genes. Note that the area delineated by the delaminating sensory neuron precursors that are NeuroD lacz positive comprises at this stage areas of what has been delineated as sensory epithelia primordia based on neurotrophin lacz expression. The only exception from this expression of NeuroD lacz is the posterior crista, which is already fully innervated at this stage in development, suggesting an even earlier delamination of sensory precursors. These data also demonstrate that the ear consists already at this early stage of a mosaic of variously overlapping gene expression domains. 2001). However, whether GATA3 affects the precision of spiral sensory neuron projection is unknown as spiral sensory neurons are lost in GATA3 null mutants. Several possible venues for generation of fiber diversity are suggested by other neurosensory systems. Recent work has shown that the vestibular sensory neurons that innervate specific sensory epithelia are not clustered together in the vestibular ganglion but are interspersed with neurons projecting to other epithelia (Maklad and Fritzsch, 1999). This specification of sensory neuron identity is unlikely to be caused by molecular gradients within the ganglion. Such a scenario would, comparable to positional identity acquisition in the retina, require that neighbors project to adjacent targets. Instead, neighbors can
12 154 Fritzsch et al. project to rather different targets such as a canal crista and the saccule (Maklad and Fritzsch, 1999) or different parts of the vestibular nuclei (Maklad and Fritzsch, 2002). In the olfactory sensory neurons, odorant coding receptor genes regulate axonal guidance so that all sensory neurons expressing the same odorant receptor converge onto one or a few glomeruli of the olfactory bulb (Wang et al., 1998). Such a solution seems to be unlikely for the inner ear sensory neurons. They appear to establish almost simultaneously their peripheral connections and their central connections, probably as a consequence of the identity acquisition process. The topological differences in delaminating cells of the inner ear (Figs. 2, 3, and 5) suggest another possibility. Many cells delaminating from various areas of the developing ear appear to be neuronal precursors as indicated by the expression of NeuroD (Figs. 2 and 5). As they delaminate, the identity of their topological origin is retained. With the extension of their dendrites prior to, during, or after delamination (Rubel and Fritzsch, 2002), these neuronal precursors may project back into the same region of the otocyst from which they originally delaminated. Growing dendrites toward the sensory epithelia along the newly delaminating cells (Fig. 4) would enhance the precision of the targeting. If this scenario is plausible, it would require a much finer quilted patterning of the otocyst (Fig. 5) by multiple overlapping or bordering gene expression domains to provide information for at least six distinct topologies. Comparable to the increasing complexity of molecular cell fate determination by nested gene expression domains in the CNS (Qian et al., 2001), the ear might also present a case of early cell fate assignment through nested gene expression domains. In this context it is gratifying that many members of the FGF family of ligands appear to be expressed in the ear (Pickles, 2001). FGF genes are known to play an important role in ear induction (Fekete and Wu, 2002) as well as in neuronal identity. In analogy to their role in the CNS, FGFs may set up gradients that specify sensory neuron identity (Scully and Rosenfeld, 2002). CONCLUSION In this review we concentrated on later stages of ear development that lead to the formation of the sensory epithelia and the cell fate determination of sensory neurons. Using neurotrophin expression patterns as markers we show progressive segregation of a single NT-3 lac Zneo -positive patch into the utricle, saccule, and cochlea sensory epithelia. We also show how the three canal cristae emerge as discrete BDNF lac Z - positive patches. Besides being markers for sensory epithelia development, neurotrophins are also expressed in delaminating cells that migrate toward the developing vestibular and cochlear ganglia. These delaminating cells originate from multiple sites at or near the developing sensory epithelia, and at least some are neuronal precursors as evidenced by the expression of NeuroD. Most importantly, this differential origin of precursors raises the possibility that the precursors of sensory neurons acquire positional information before delamination from the ear. In addition, these delaminating sensory neuron precursors may use this information to navigate to the area they delaminated from. This proposal implies a much more sophisticated subdivision of the developing otocyst than that suggested by the few available gene expression studies. Limited evidence, based on RT-PCR analysis for isoform expression of the large FGF family consisting of at least 23 genes, demonstrates the expression of numerous members in an as yet unknown pattern in the developing inner ear. We suggest that evolution of the ear comes about by slightly modifying this pattern. This is achieved through addition of new genes, or multiplication and functional diversification of existing genes, to modify the general hair cell and sensory neuron fate acquisition program to develop the six specific, functionally distinct endorgans in the mammalian inner ear and their innervation. REFERENCES Acampora D, Gulisano M, Simeone A Genetic and molecular roles of Otx homeodomain proteins in head development. Gene 246: Begbie J, Graham A Integration between the epibranchial placodes and the hindbrain. Science 294: Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY Math1: an essential gene for the generation of inner ear hair cells. Science 284: Brichta AM, Goldberg JM The papilla neglecta of turtles: a detector of head rotations with unique sensory coding properties. J Neurosci 18: Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM Molecular genetics of pattern formation in the inner ear: do compartment boundaries play a role? Proc Natl Acad Sci USA 97: Cantos R, Cole LK, Acampora D, Simeone A, Wu DK Patterning of the mammalian cochlea. Proc Natl Acad Sci USA 97: Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK.
13 Ear Sensory Development Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol 424: de Burlet HM Vergleichende Anatomie des statoakustischen Organs. a) Die innere Ohrsphäre. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere. Berlin: Urban and Schwarzenberg, p Dickman JD, Fang Q Differential central projections of vestibular afferents in pigeons. J Comp Neurol 367: Diez del Corral R, Storey KG Markers in vertebrate neurogenesis. Nat Rev Neurosci 2: Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci 21: Fekete DM, Wu DK Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol 12: Fritzsch B The inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature 327: Fritzsch B The water-to-land transition: Evolution of the tetrapod basilar papilla, middle ear and auditory nuclei. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer- Verlag, p Fritzsch B Evolution of the vestibulo-ocular system. Otolaryngol Head Neck Surg 119: Fritzsch B. 2001a. The morphology and function of fish ears. In: Ostrander G, editor. The Laboratory fish. Exeter: Academic Press, p Fritzsch B. 2001b. The cellular organization of the fish ear. In: Ostrander G, editor. The Laboratory fish. Exeter: Academic Press, p Fritzsch B, Barald K, Lomax M Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer-Verlag, p Fritzsch B, Beisel KW Evolution and development of the vertebrate ear. Brain Res Bull 55: Fritzsch B, Pirvola U, Ylikoski J Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res 295: Fritzsch B, Signore M, Simeone A Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol 211: Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Barbacid M Reduction and loss of inner ear innervation in trkb and trkc receptor knockout mice: A whole mount DiI and scanning electron microscopic analysis. Audit Neurosci 1: Fritzsch B, Wake MH The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system. Zoomorphol 108: Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron 31: Hatini V, DiNardo S Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet 17: Hatini V, Ye X, Balas G, Lai E Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn 215: Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 128: Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429: Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 128: Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol 1: Larsell O Anura. In: Jansen J, editor. The Comparative Anatomy and Histology of the Cerebellum from Myxinoids through Birds. Minneapolis: The University of Minnesota Press, p Lawoko-Kerali G, Rivolta MN, Holley M Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol 442: Lewis ER, Leverenz EL, Bialek WS The vertebrate inner ear. Boca Raton: CRC Press. p 248. Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev 14: Ma Q, Anderson DJ, Fritzsch B Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol 1: Maklad A, Fritzsch B Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res 9: Maklad A, Fritzsch B The developmental segregation of posterior crista and saccular vestibular fibers in mice: A carbocyanine tracer study using confocal microscopy. Dev Brain Res 135:1 17. Mazan S, Jaillard D, Baratte B, Janvier P Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol Dev 2:
Spatial Shaping of Cochlear Innervation by Temporally Regulated Neurotrophin Expression
 The Journal of Neuroscience, August 15, 2001, 21(16):6170 6180 Spatial Shaping of Cochlear Innervation by Temporally Regulated Neurotrophin Expression Isabel Fariñas, 1,2 Kevin R. Jones, 3 Lino Tessarollo,
The Journal of Neuroscience, August 15, 2001, 21(16):6170 6180 Spatial Shaping of Cochlear Innervation by Temporally Regulated Neurotrophin Expression Isabel Fariñas, 1,2 Kevin R. Jones, 3 Lino Tessarollo,
Supplementary Figure 1. Chimeric analysis of inner ears. (A-H) Chimeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears.
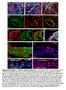 Supplementary Figure 1. himeric analysis of inner ears. (A-H) himeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears. (A,B) omposite images showing three colors in different vestibular
Supplementary Figure 1. himeric analysis of inner ears. (A-H) himeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears. (A,B) omposite images showing three colors in different vestibular
The role of Pax2 in mouse inner ear development
 Developmental Biology 272 (2004) 161 175 www.elsevier.com/locate/ydbio The role of Pax2 in mouse inner ear development Quianna Burton, Laura K. Cole, Michael Mulheisen, Weise Chang, and Doris K. Wu* National
Developmental Biology 272 (2004) 161 175 www.elsevier.com/locate/ydbio The role of Pax2 in mouse inner ear development Quianna Burton, Laura K. Cole, Michael Mulheisen, Weise Chang, and Doris K. Wu* National
The Vestibular System
 The Vestibular System Vestibular and Auditory Sensory Organs Bill Yates, Ph.D. Depts. Otolaryngology & Neuroscience University of Pittsburgh Organization of Sensory Epithelium Displacement of Stereocilia
The Vestibular System Vestibular and Auditory Sensory Organs Bill Yates, Ph.D. Depts. Otolaryngology & Neuroscience University of Pittsburgh Organization of Sensory Epithelium Displacement of Stereocilia
The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination
 Development 129, 2495-2505 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV1807 2495 The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium
Development 129, 2495-2505 (2002) Printed in Great Britain The Company of Biologists Limited 2002 DEV1807 2495 The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
In vivo analysis of Trk receptor signalling in the mouse nervous system Postigo, Juan Antonio
 University of Groningen In vivo analysis of Trk receptor signalling in the mouse nervous system Postigo, Juan Antonio IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
University of Groningen In vivo analysis of Trk receptor signalling in the mouse nervous system Postigo, Juan Antonio IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
Lack of Neurotrophin 3 Causes Losses of Both Classes of Spiral Ganglion Neurons in the Cochlea in a Region-Specific Fashion
 The Journal of Neuroscience, August 15, 1997, 17(16):6213 6225 Lack of Neurotrophin 3 Causes Losses of Both Classes of Spiral Ganglion Neurons in the Cochlea in a Region-Specific Fashion Bernd Fritzsch,
The Journal of Neuroscience, August 15, 1997, 17(16):6213 6225 Lack of Neurotrophin 3 Causes Losses of Both Classes of Spiral Ganglion Neurons in the Cochlea in a Region-Specific Fashion Bernd Fritzsch,
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
The developmental history of a sensory ganglion cell
 (bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
(bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Taste buds Gustatory cells extend taste hairs through a narrow taste pore
 The Special Senses Objectives Describe the sensory organs of smell, and olfaction. Identify the accessory and internal structures of the eye, and explain their function. Explain how light stimulates the
The Special Senses Objectives Describe the sensory organs of smell, and olfaction. Identify the accessory and internal structures of the eye, and explain their function. Explain how light stimulates the
Cellular commitment and differentiation in the organ of Corti
 Int. J. Dev. Biol. 51: 571-583 (2007) doi: 10.1387/ijdb.072388mk Cellular commitment and differentiation in the organ of Corti MATTHEW W. KELLEY* Section on Developmental Neuroscience, National Institute
Int. J. Dev. Biol. 51: 571-583 (2007) doi: 10.1387/ijdb.072388mk Cellular commitment and differentiation in the organ of Corti MATTHEW W. KELLEY* Section on Developmental Neuroscience, National Institute
Differentiation of the facial-vestibulocochlear ganglionic complex in human embryos of developmental stages 13 15
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Vestibular Function and Anatomy. UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD
 Vestibular Function and Anatomy UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD System of balance Membranous and bony labyrinth embedded in petrous bone 5 distinct end organs 3 semicircular
Vestibular Function and Anatomy UTMB Grand Rounds April 14, 2004 Gordon Shields, MD Arun Gadre, MD System of balance Membranous and bony labyrinth embedded in petrous bone 5 distinct end organs 3 semicircular
Ear. Utricle & saccule in the vestibule Connected to each other and to the endolymphatic sac by a utriculosaccular duct
 Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Cochlear neurosensory specification and competence: you gata have Gata
 University of Iowa Iowa Research Online Theses and Dissertations Spring 2012 Cochlear neurosensory specification and competence: you gata have Gata Jeremy Shane Duncan University of Iowa Copyright 2012
University of Iowa Iowa Research Online Theses and Dissertations Spring 2012 Cochlear neurosensory specification and competence: you gata have Gata Jeremy Shane Duncan University of Iowa Copyright 2012
Ears on rears : transplantation of ears reveals afferent pathfinding properties
 University of Iowa Iowa Research Online Theses and Dissertations Fall 2017 Ears on rears : transplantation of ears reveals afferent pathfinding properties Clayton Jackson Gordy University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations Fall 2017 Ears on rears : transplantation of ears reveals afferent pathfinding properties Clayton Jackson Gordy University of Iowa Copyright
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Scanning Thin-Sheet Laser Imaging Microscopy Elucidates Details on Mouse Ear Development
 a DEVELOPMENTAL DYNAMICS 241:465 480, 2012 RESEARCH ARTICLE Scanning Thin-Sheet Laser Imaging Microscopy Elucidates Details on Mouse Ear Development Benjamin Kopecky, 1,2 * Shane Johnson, 3 Heather Schmitz,
a DEVELOPMENTAL DYNAMICS 241:465 480, 2012 RESEARCH ARTICLE Scanning Thin-Sheet Laser Imaging Microscopy Elucidates Details on Mouse Ear Development Benjamin Kopecky, 1,2 * Shane Johnson, 3 Heather Schmitz,
Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH).
 Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH). (a), (b), PRPH immunolabelling of cryosections from post-natal day
Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH). (a), (b), PRPH immunolabelling of cryosections from post-natal day
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
DISTRIBUTED NEURAL INFORMATION PROCESSING IN THE VESTIBULO-OCULAR SYSTEM. Clifford Lau Office of Naval Research Detach ment Pasadena, CA 91106
 457 DISTRIBUTED NEURAL INFORMATION PROCESSING IN THE VESTIBULO-OCULAR SYSTEM Clifford Lau Office of Naval Research Detach ment Pasadena, CA 91106 Vicente Honrubia* UCLA Division of Head and Neck Surgery
457 DISTRIBUTED NEURAL INFORMATION PROCESSING IN THE VESTIBULO-OCULAR SYSTEM Clifford Lau Office of Naval Research Detach ment Pasadena, CA 91106 Vicente Honrubia* UCLA Division of Head and Neck Surgery
Springer Handbook of Auditory Research. Series Editors: Richard R. Fay and Arthur N. Popper
 Springer Handbook of Auditory Research Series Editors: Richard R. Fay and Arthur N. Popper Matthew W. Kelley Doris K. Wu Arthur N. Popper Richard R. Fay Editors Development of the Inner Ear With 29 illustrations
Springer Handbook of Auditory Research Series Editors: Richard R. Fay and Arthur N. Popper Matthew W. Kelley Doris K. Wu Arthur N. Popper Richard R. Fay Editors Development of the Inner Ear With 29 illustrations
OVEREXPRESSION OF SPONGE BHLH MRNA IN XENOPUS LAEVIS DISRUPTS INNER EAR NEUROSENSORY DEVELOPMENT
 University of Iowa Honors Theses University of Iowa Honors Program Spring 2018 OVEREXPRESSION OF SPONGE BHLH MRNA IN XENOPUS LAEVIS DISRUPTS INNER EAR NEUROSENSORY DEVELOPMENT Jessica Halyko Follow this
University of Iowa Honors Theses University of Iowa Honors Program Spring 2018 OVEREXPRESSION OF SPONGE BHLH MRNA IN XENOPUS LAEVIS DISRUPTS INNER EAR NEUROSENSORY DEVELOPMENT Jessica Halyko Follow this
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY. Professor.Dr. M.K.Rajasekar MS., DLO.,
 VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY Professor.Dr. M.K.Rajasekar MS., DLO., Life is hard for those who don t have a VOR During a walk I found too much motion in my visual picture of the surroundings
VESTIBULAR SYSTEM ANATOMY AND PHYSIOLOGY Professor.Dr. M.K.Rajasekar MS., DLO., Life is hard for those who don t have a VOR During a walk I found too much motion in my visual picture of the surroundings
scientific report Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo scientificreport
 scientificreport Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo Anna Stenqvist 1 *, Karin Agerman 1,2 *, Frédéric Marmigère 1, Liliana Minichiello 3 &PatrikErnfors
scientificreport Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo Anna Stenqvist 1 *, Karin Agerman 1,2 *, Frédéric Marmigère 1, Liliana Minichiello 3 &PatrikErnfors
Otx1 and Otx2 activities are required for the normal development of the
 Development 126, 2335-2343 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1416 2335 Otx1 and Otx2 activities are required for the normal development of the mouse inner ear Hakim
Development 126, 2335-2343 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1416 2335 Otx1 and Otx2 activities are required for the normal development of the mouse inner ear Hakim
Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission.
 Hair Cell Heterogeneity and Ultrasonic Hearing: Recent Advances in Understanding Fish Hearing Author(s): Arthur N. Popper Source: Philosophical Transactions: Biological Sciences, Vol. 355, No. 1401, Sensory
Hair Cell Heterogeneity and Ultrasonic Hearing: Recent Advances in Understanding Fish Hearing Author(s): Arthur N. Popper Source: Philosophical Transactions: Biological Sciences, Vol. 355, No. 1401, Sensory
Special Senses. Mechanoreception Electroreception Chemoreception Others
 Special Senses Mechanoreception Electroreception Chemoreception Others Recall our receptor types Chemically regulated: Respond to particular chemicals Voltage regulated: respond to changing membrane potential
Special Senses Mechanoreception Electroreception Chemoreception Others Recall our receptor types Chemically regulated: Respond to particular chemicals Voltage regulated: respond to changing membrane potential
COGS 107B Week 2. Hyun Ji Friday 4:00-4:50pm
 COGS 107B Week 2 Hyun Ji Friday 4:00-4:50pm Lecture 3: Proprioception Principles: The Neuron Doctrine and The Law of Dynamic Polarization Proprioception Joint-protecting reflexes (ex. Knee jerk reflex)
COGS 107B Week 2 Hyun Ji Friday 4:00-4:50pm Lecture 3: Proprioception Principles: The Neuron Doctrine and The Law of Dynamic Polarization Proprioception Joint-protecting reflexes (ex. Knee jerk reflex)
THE COCHLEA AND AUDITORY PATHWAY
 Dental Neuroanatomy Suzanne S. Stensaas, PhD February 23, 2012 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea
Dental Neuroanatomy Suzanne S. Stensaas, PhD February 23, 2012 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea
Formation of the mouse cochlea: roles of Sonic hedgehog
 Formation of the mouse cochlea: roles of Sonic hedgehog JINWOONG BOK 1, COLLEEN ZENCZAK 2, CHANHO HWANG 2, AND DORIS K. WU 2,* 1 Department of Anatomy, Yonsei University College of Medicine, Seoul, 120-752,
Formation of the mouse cochlea: roles of Sonic hedgehog JINWOONG BOK 1, COLLEEN ZENCZAK 2, CHANHO HWANG 2, AND DORIS K. WU 2,* 1 Department of Anatomy, Yonsei University College of Medicine, Seoul, 120-752,
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Otoconia: Calcium carbonate crystals Gelatinous mass. Cilia. Hair cells. Vestibular nerve. Vestibular ganglion
 VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Chapter 7. Audition, the Body Senses, and the Chemical Senses. Copyright Allyn & Bacon 2004
 Chapter 7 Audition, the Body Senses, and the Chemical Senses This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display,
Chapter 7 Audition, the Body Senses, and the Chemical Senses This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display,
The Physiology of the Senses Lecture 10 - Balance
 The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates from the labyrinth... 2 The auditory and vestibular systems have a common
The Physiology of the Senses Lecture 10 - Balance www.tutis.ca/senses/ Contents Objectives... 1 The sense of balance originates from the labyrinth... 2 The auditory and vestibular systems have a common
Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear
 RESEARCH ARTICLE 1277 Development 133, 1277-1286 (2006) doi:10.1242/dev.02284 Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear Rachael Brooker 1,2, Katsuto Hozumi 3 and
RESEARCH ARTICLE 1277 Development 133, 1277-1286 (2006) doi:10.1242/dev.02284 Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear Rachael Brooker 1,2, Katsuto Hozumi 3 and
Vestibular physiology
 Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Bony and membranous labyrinth. Vestibular system. János Hanics M.D.
 Bony and membranous labyrinth. Vestibular system. János Hanics M.D. The position of the inner ear The labyrinthes of the inner ear - Continuous cavity system in the petrous part of temporal bone - Cavity
Bony and membranous labyrinth. Vestibular system. János Hanics M.D. The position of the inner ear The labyrinthes of the inner ear - Continuous cavity system in the petrous part of temporal bone - Cavity
THE COCHLEA AND AUDITORY PATHWAY
 Dental Neuroanatomy Suzanne S. Stensaas, PhD April 14, 2010 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea HyperBrain
Dental Neuroanatomy Suzanne S. Stensaas, PhD April 14, 2010 Reading: Waxman, Chapter 16, Review pictures in a Histology book Computer Resources: http://www.cochlea.org/ - Promenade around the Cochlea HyperBrain
Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute
 Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute 412-647-9614 byates@pitt.edu What is the Vestibular System? The vestibular system is the sensory system,
Vestibular System Dr. Bill Yates Depts. Otolaryngology and Neuroscience 110 Eye and Ear Institute 412-647-9614 byates@pitt.edu What is the Vestibular System? The vestibular system is the sensory system,
Innervation of the Cochlea. Reading: Yost Ch. 8
 Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Overview of Sensory Receptors
 Sensory Systems Chapter 45 Overview of Sensory Receptors Sensory receptors provide information from our internal and external environments that is crucial for survival and success -Exteroceptors sense
Sensory Systems Chapter 45 Overview of Sensory Receptors Sensory receptors provide information from our internal and external environments that is crucial for survival and success -Exteroceptors sense
Anatomy of the Ear Region. External ear Middle ear Internal ear
 Ear Lecture Objectives Make a list of structures making the external, middle, and internal ear. Discuss the features of the external auditory meatus and tympanic membrane. Describe the shape, position,
Ear Lecture Objectives Make a list of structures making the external, middle, and internal ear. Discuss the features of the external auditory meatus and tympanic membrane. Describe the shape, position,
AUDITORY APPARATUS. Mr. P Mazengenya. Tel 72204
 AUDITORY APPARATUS Mr. P Mazengenya Tel 72204 Describe the anatomical features of the external ear Describe the tympanic membrane (ear drum) Describe the walls of the middle ear Outline the structures
AUDITORY APPARATUS Mr. P Mazengenya Tel 72204 Describe the anatomical features of the external ear Describe the tympanic membrane (ear drum) Describe the walls of the middle ear Outline the structures
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
Dorsal Cochlear Nucleus. Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS
 Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Chapter 29 The Senses
 Chapter 29 The Senses PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Edward J. Zalisko
Chapter 29 The Senses PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Edward J. Zalisko
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Sensory and Motor Mechanisms
 Chapter 50 Sensory and Motor Mechanisms PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 50 Sensory and Motor Mechanisms PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Chapter 15 Hearing & Equilibrium
 Chapter 15 Hearing & Equilibrium ANATOMY OF THE OUTER EAR EAR PINNA is the outer ear it is thin skin covering elastic cartilage. It directs incoming sound waves to the EXTERNAL AUDITORY CANAL, which is
Chapter 15 Hearing & Equilibrium ANATOMY OF THE OUTER EAR EAR PINNA is the outer ear it is thin skin covering elastic cartilage. It directs incoming sound waves to the EXTERNAL AUDITORY CANAL, which is
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
4. Which letter in figure 9.1 points to the fovea centralis? Ans: b
 Chapter 9: The Sensory System 1. Proprioceptors are involved in the sense of A) pain. B) temperature. C) pressure. D) movement of limbs. 2. Which are chemoreceptors? A) taste B) olfactory C) proprioceptors
Chapter 9: The Sensory System 1. Proprioceptors are involved in the sense of A) pain. B) temperature. C) pressure. D) movement of limbs. 2. Which are chemoreceptors? A) taste B) olfactory C) proprioceptors
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Experimental strategy to generate sensory hair cells in the mammalian cochlea and evaluate their morphological, molecular, and biophysical properties. A) Expression plasmid encoding
Supplementary Figure 1. Experimental strategy to generate sensory hair cells in the mammalian cochlea and evaluate their morphological, molecular, and biophysical properties. A) Expression plasmid encoding
Review of Nervous System Anatomy
 For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
Hes1 and Hes5 Activities Are Required for the Normal Development of the Hair Cells in the Mammalian Inner Ear
 The Journal of Neuroscience, July 1, 2001, 21(13):4712 4720 Hes1 and Hes5 Activities Are Required for the Normal Development of the Hair Cells in the Mammalian Inner Ear Azel Zine, 1 Alexandre Aubert,
The Journal of Neuroscience, July 1, 2001, 21(13):4712 4720 Hes1 and Hes5 Activities Are Required for the Normal Development of the Hair Cells in the Mammalian Inner Ear Azel Zine, 1 Alexandre Aubert,
Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice
 Developmental Biology 275 (2004) 34 43 www.elsevier.com/locate/ydbio Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice David Hellard a,1,
Developmental Biology 275 (2004) 34 43 www.elsevier.com/locate/ydbio Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice David Hellard a,1,
Sensory Systems Vision, Audition, Somatosensation, Gustation, & Olfaction
 Sensory Systems Vision, Audition, Somatosensation, Gustation, & Olfaction Sarah L. Chollar University of California, Riverside sarah.chollar@gmail.com Sensory Systems How the brain allows us to see, hear,
Sensory Systems Vision, Audition, Somatosensation, Gustation, & Olfaction Sarah L. Chollar University of California, Riverside sarah.chollar@gmail.com Sensory Systems How the brain allows us to see, hear,
Chapter 18 Senses SENSORY RECEPTION 10/21/2011. Sensory Receptors and Sensations. Sensory Receptors and Sensations. Sensory Receptors and Sensations
 SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
Auditory and vestibular system
 Auditory and vestibular system Sensory organs on the inner ear inner ear: audition (exteroceptor) and vestibular apparatus (proprioceptor) bony and membranous labyrinths within the temporal bone (os temporale)
Auditory and vestibular system Sensory organs on the inner ear inner ear: audition (exteroceptor) and vestibular apparatus (proprioceptor) bony and membranous labyrinths within the temporal bone (os temporale)
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Vestibular Physiology Richard M. Costanzo, Ph.D.
 Vestibular Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the structure and function of the vestibular organs.
Vestibular Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the structure and function of the vestibular organs.
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. The expression of ephrin-b2 H2BGFP persists in the post-hearingonset organ of Corti and is specifically restricted to supporting cells. Sox2 immunolabeling
SUPPLEMENTARY INFORMATION Supplementary Figure 1. The expression of ephrin-b2 H2BGFP persists in the post-hearingonset organ of Corti and is specifically restricted to supporting cells. Sox2 immunolabeling
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
Cerebellum: Origins and Development
 Cerebellum: Origins and Development Found in all vertebrates Dorsal lip of developing medulla (rhombencephalon) Near terminations of vestibular (VIII) and lateral line afferents, which sense fluid displacement
Cerebellum: Origins and Development Found in all vertebrates Dorsal lip of developing medulla (rhombencephalon) Near terminations of vestibular (VIII) and lateral line afferents, which sense fluid displacement
Ch 5. Perception and Encoding
 Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development
 RESEARCH ARTICLE 3967 Development 138, 3967-3976 (2011) doi:10.1242/dev.066126 2011. Published by The Company of Biologists Ltd Otic ablation of smoothened reveals direct and indirect requirements for
RESEARCH ARTICLE 3967 Development 138, 3967-3976 (2011) doi:10.1242/dev.066126 2011. Published by The Company of Biologists Ltd Otic ablation of smoothened reveals direct and indirect requirements for
4) Modification of Development by Sensory Experience (Nurture)
 Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear
 The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear Amy E. Kiernan, Jingxia Xu, Thomas Gridley * The Jackson Laboratory, Bar Harbor, Maine, United States of
The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear Amy E. Kiernan, Jingxia Xu, Thomas Gridley * The Jackson Laboratory, Bar Harbor, Maine, United States of
Physiology Unit 2 SENSORY PHYSIOLOGY
 Physiology Unit 2 SENSORY PHYSIOLOGY In Physiology Today Sensory System Sensory information Conscious sensations Unconscious sensations Sensory processing Transferring stimulus energy into a graded potential
Physiology Unit 2 SENSORY PHYSIOLOGY In Physiology Today Sensory System Sensory information Conscious sensations Unconscious sensations Sensory processing Transferring stimulus energy into a graded potential
Coordination of trophic interactions by separate developmental programs in sensory neurons and their target fields
 Coordination of trophic interactions by separate developmental programs in sensory neurons and their target fields ALUN M. DAVIES, YVES LÄRMET, EDWINA WRIGHT and KRISTINE S. VOGEL Department o f Anatomy,
Coordination of trophic interactions by separate developmental programs in sensory neurons and their target fields ALUN M. DAVIES, YVES LÄRMET, EDWINA WRIGHT and KRISTINE S. VOGEL Department o f Anatomy,
SPHSC 462 HEARING DEVELOPMENT. Overview Review of Hearing Science Introduction
 SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
Representation of sound in the auditory nerve
 Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear.
 Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Course: PG- Pathshala Paper number: 13 Physiological Biophysics Module number M23: Posture and Movement Regulation by Ear Principal Investigator: Co-Principal Investigator: Paper Coordinator: Content Writer:
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
SMELL 2
 SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
Ch 5. Perception and Encoding
 Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
Ch 5. Perception and Encoding Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by Y.-J. Park, M.-H. Kim, and B.-T. Zhang
SUMMARY INTRODUCTION. Key words: Gfi1, Gfi1b, Senseless, PAG-3, Inner ear hair cell, Basic helix-loop-helix (bhlh), Deafness, Mouse
 Development 130, 221-232 2003 The Company of Biologists Ltd doi:10.1242/dev.00190 221 DEVELOPMENT AND DISEASE The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner
Development 130, 221-232 2003 The Company of Biologists Ltd doi:10.1242/dev.00190 221 DEVELOPMENT AND DISEASE The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
