Rev. A VBR Spinal System. Issued October 2014 DePuy Synthes Spine, a division of DOI All rights reserved.
|
|
|
- Dinah Curtis
- 6 years ago
- Views:
Transcription
1 Rev. A 0086 VBR Spinal System Issued October 2014 DePuy Synthes Spine, a division of DOI All rights reserved.
2 DESCRIPTION The VBR (Vertebral Body Replacement) System consists of the BENGAL STACKABLE and OCELOT Stackable Cage Systems and the Surgical Titanium Mesh and X-MESH Expandable Cage Systems. The VBR Spinal Systems are designed to restore the biomechanical integrity of the anterior, middle and posterior spinal column even in the absence of fusion for a prolonged period, as well as for treating fractures of the thoracic and lumbar spine. The structure of the carbon-fiber composite implants has been shown to support anticipated loads with a modulus of elasticity approximating that of cortical bone. The implants have ridges or teeth in both the anterior-posterior and medial-lateral directions, which resist rotation and migration. The polymer/carbon-fiber composite implants have cavities to accept packing of bone graft. The entire structure is radiolucent so that healing can be assessed by normal radiographic methods. Additionally, radiotherapy can be performed immediately after surgery. STACKABLE CAGE SYSTEM The Stackable Cage System consists of the BENGAL STACKABLE and OCELOT Stackable Cage Systems. These implants are made of a polymer/carbon-fiber composite. These radiolucent implants are designed with ridges or teeth to resist rotation and migration, and with cavities to accept packing of bone graft materials. The carbon-fiber implants are available in a variety of sizes. The BENGAL STACKABLE Cage System consists of both one piece (monolithic) and two- to three-piece (stackable) vertebral body replacement components and a supplemental internal fixation system that offer a variety of size options to allow surgeons to achieve a desired height. The two- or three-piece (stackable) construct can be assembled from a variety of different sized components. A titanium alloy screw is then passed through a hole in the cages and with a nut provides a rigid and compressed assembly. The OCELOT Stackable Cage System consists of one or more stackable vertebral body replacement components and a supplemental internal fixation system. One or more OCELOT Stackable Cage System implants may be stacked to the desired height, as determined by the surgeon. A titanium alloy screw can be passed through a center hole in the cages and with a nut provide a rigid and compressed assembly. 2 of 12
3 SURGICAL TITANIUM MESH AND X-MESH EXPANDABLE CAGE SYSTEMS The Surgical Titanium Mesh System is designed to restore biomechanical integrity throughout the thoracic and lumbar spine following vertebrectomy or corpectomy for patients with spine tumors or fractures. The system provides anterior, middle and posterior vertebral column support both immediately after surgery and for prolonged periods in the absence of bone fusion. The Surgical Titanium Mesh System consists of various shapes and sizes of mesh, standard or angled rings and screws, endplates, and endcaps. The surgeons have the option to place the standard or angled rings and screws, endplates and endcaps within the mesh. These interface devices may be used to provide increased surface area at the Mesh/bone interface, which provides additional support and increased resistance to subsidence. Surgical Titanium Mesh may be trimmed to the desired height, as determined by the surgeon. The teeth at the ends of the mesh, resulting from the diamond shaped perforations, help anchor the device. The X-MESH Expandable Cage System is available in many heights, and different endplate shapes, sizes and angles. The surgeons have the option to place the standard or angled endplates. The teeth on the endplates help anchor the device. The surgeons should use the size and angle that best fits the defect being treated. INDICATIONS Caution: USA Law restricts this device to sale by or on the order of a physician. The VBR Spinal Systems are indicated for use in the thoracolumbar spine (i.e., T1 to L5) to replace a diseased vertebral body resected or excised for the treatment of tumors, to achieve anterior decompression of the spinal cord and neural tissues, and to restore the height of a collapsed vertebral body. The VBR Spinal Systems are also indicated for treating fractures of the thoracic and lumbar spine. The VBR Spinal Systems are designed to restore the biomechanical integrity of the anterior, middle and posterior spinal column even in the absence of fusion for a prolonged period. 3 of 12
4 The VBR Spinal Systems are intended for use with DePuy Spine supplemental internal fixation. CONTRAINDICATIONS 1. Use of these systems is contraindicated when there is active systemic infection, infection localized to the site of the proposed implantation, or when the patient has demonstrated allergy or foreign body sensitivity to any of the implant materials. 2. Severe osteoporosis may prevent adequate fixation and thus preclude the use of this or any other orthopaedic implant. 3. Conditions that may place excessive stresses on bone and implants, such as severe obesity or degenerative diseases, are relative contraindications. The decision whether to use these devices in such conditions must be made by the physician taking into account the risks versus the benefits to the patient. Use of these implants is relatively contraindicated in patients whose activity, mental capacity, mental illness, alcoholism, drug abuse, occupation, or lifestyle may interfere with their ability to follow postoperative restrictions and who may place undue stresses on the implant during bony healing and may be at a higher risk of implant failure. WARNINGS, PRECAUTIONS AND ADVERSE EFFECTS CONCERNING SPINAL FIXATION IMPLANTS Following are specific warnings, precautions and adverse effects that should be understood by the surgeon and explained to the patient. These warnings do not include all adverse effects that can occur with surgery in general, but are important considerations particular to devices such as the VBR Spinal System. General surgical risks should be explained to the patient prior to surgery. The VBR Spinal System implants are intended to support the anterior, middle, and posterior vertebral column while fusion is taking place. These implants are intended to be permanent. The following recommendations for removal of hardware apply to the supplemental internal fixation implants used in this procedure. WARNINGS 1. CORRECT SELECTION OF THE IMPLANT IS EXTREMELY IMPORTANT. The potential for satisfactory anterior column support is increased by the selection of the proper size device. While proper selection can help minimize risks, the size 4 of 12
5 and shape of human bones present limitations on the size, shape and strength of implants. Internal fixation devices cannot withstand activity levels equal to those placed on normal healthy bone. No implant can be expected to withstand indefinitely the unsupported stress of full weight bearing. 2. IMPLANTS CAN BREAK WHEN SUBJECTED TO THE INCREASED LOADING ASSOCIATED WITH DELAYED UNION OR NONUNION. Internal fixation appliances are load-sharing devices that are used to obtain an alignment until normal healing occurs. If healing is delayed, or does not occur, the implant may eventually break due to material fatigue. The degree or success of union, loads produced by weight bearing, and activity levels will, among other conditions, dictate the longevity of the implant. Notches, scratches or bending of the implant during the course of surgery may also contribute to early failure. Patients should be fully informed of the risks of implant failure. 3. MIXING METALS CAN CAUSE CORROSION. There are many forms of corrosion damage and several of these occur on metals surgically implanted in humans. General or uniform corrosion is present on all implanted metals and alloys. The rate of corrosive attack on metal implant devices is usually very low due to the presence of passive surface films. Dissimilar metals in contact, such as titanium and stainless steel, accelerate the corrosion process of stainless steel and more rapid attack occurs. The presence of corrosion often accelerates fatigue fracture of implants. The amount of metal compounds released into the body system will also increase. Internal fixation devices, such as rods, hooks, wires, etc., which come into contact with other metal objects, must be made from like or compatible metals. Avoid coupling of stainless steel with the VBR Spinal System implants. PRECAUTIONS 1. SURGICAL IMPLANTS MUST NEVER BE REUSED. An explanted implant should never be reimplanted. Even though a device appears undamaged, it may have small defects and internal stress patterns that may lead to early breakage. Reuse can compromise device performance and patient safety. Reuse of single use devices can also cause cross-contamination leading to patient infection. 2. CORRECT HANDLING OF THE IMPLANT IS EXTREMELY IMPORTANT. A. Composite Implants: Polymer/carbon-fiber implants are designed to support physiologic loads. Excessive torque, when applied to long-handle insertion tools, can cause splitting or fracture of the carbon-fiber implants. When a carbon-fiber implant is impacted or hammered into place, the broad surface of the insertion 5 of 12
6 tool should be carefully seated fully against the carbon-fiber implant. Impaction forces applied directly to a small surface of the implant could cause fracture of the implant. Split or fractured implants should be removed and replaced. B. Metal Implants: Contouring of metal implants should only be done with proper equipment. The operating surgeon should avoid notching, scratching or reverse bending of the implants when contouring. Alterations will produce defects in surface finish and internal stresses which may become the focal point for eventual breakage of the implant. Bending of screws will significantly decrease the fatigue life and may cause failure. 3. REMOVAL OF SUPPLEMENTAL FIXATION AFTER HEALING. If the supplemental fixation is not removed following the completion of its intended use, any of the following complications may occur: (1) Corrosion, with localized tissue reaction or pain; (2) Migration of implant position resulting in injury; (3) Risk of additional injury from postoperative trauma; (4) Bending, loosening, and/or breakage, which could make removal impractical or difficult; (5) Pain, discomfort, or abnormal sensations due to the presence of the device; (6) Possible increased risk of infection; and (7) Bone loss due to stress shielding. The surgeon should carefully weigh the risks versus benefits when deciding whether to remove the implant. Implant removal should be followed by adequate postoperative management to avoid refracture. If, for example, the patient is older and has a low activity level, the surgeon may choose not to remove the implant thus eliminating the risks involved with a second surgery. 4. ADEQUATELY INSTRUCT THE PATIENT. Postoperative care and the patient s ability and willingness to follow instructions are among the most important aspects of successful bone healing. The patient must be made aware of the limitations of the implants. The patient should be encouraged to ambulate to tolerance as soon as possible after surgery, and instructed to limit and restrict lifting and twisting motions and any type of sports participation until the bone is healed. The patient should understand that implants are not as strong as normal healthy bone and could loosen, bend and/or break if excessive demands are placed on it, especially in the absence of complete bone healing. Implants displaced or damaged by improper activities may experience migration to the devices and damage to nerves or blood vessels. 6 of 12
7 POSSIBLE ADVERSE EFFECTS WITH THE VBR SPINAL SYSTEM IMPLANTS AND/OR METALLIC INTERNAL FIXATION DEVICES This list may not include all complications caused by the surgical procedure itself. 1. Bending or fracture of implant. Loosening of the implant. 2. Implant material sensitivity, or allergic reaction to a foreign body. 3. Infection, early or late. 4. Decrease in bone density due to stress shielding. 5. Pain, discomfort, or abnormal sensations due to the presence of the device. 6. Nerve damage due to surgical trauma or presence of the device. Neurological difficulties including bowel and/or bladder dysfunction, impotence, retrograde ejaculation, radicular pain, tethering of nerves in scar tissue, muscle weakness, and paraesthesia. 7. Vascular damage could result in catastrophic or fatal bleeding. Malpositioned implants adjacent to large arteries or veins could cause erosion of these vessels and catastrophic bleeding in the later postoperative period. 8. Dural tears experienced during surgery could result in need for further surgery for dural repair, a chronic CSF leak or fistula, and possible meningitis. 9. Bursitis. 10. Paralysis. 11. Death. 12. Spinal cord impingement or damage. 13. Fracture of bony structures. 14. Reflex sympathetic dystrophy. 15. There is an additional risk if there were to be long term in vivo degradation of the polymer/carbon-fiber composite resulting in possible local or systemic adverse reactions from any potential degradation products. 16. If a pseudarthrodesis occurs coupled with the VBR Spinal Systems, a mechanical grinding action could possibly occur which might generate wear debris. Most types of wear debris have shown the potential of initiating local osteolysis in articulating joints. 17. Degenerative changes or instability in segments adjacent to fused vertebral levels. 7 of 12
8 IMPORTANT NOTE TO OPERATING SURGEON Vertebral body replacement should only be undertaken after the surgeon has had hands-on training in these methods of spinal fixation, and has become thoroughly knowledgeable about spinal anatomy and biomechanics. Surgical technique manuals are available for detailed instructions on the correct use of the VBR Spinal Systems for use in corporectomies and vertebrectomies. The contents of these manuals alone are not adequate for complete instruction in the use of this system. Even for surgeons already experienced in spinal instrumentation and vertebral body replacement procedures, new skills may be required that are best learned by working with an experienced surgeon or through a course of formal instruction with laboratory training. Lack of experience or expertise with these implants may result in complications. Because of the limitations imposed by anatomic considerations and modern surgical materials, metallic implants cannot be made to last indefinitely. The purpose of the VBR Spinal System is to provide immediate spinal stability and to allow for consolidation of the fusion mass. If any implant of the VBR Spinal System does break, the decision to remove it must be made by the physician, who must consider the condition of the patient and the risks associated with the presence of the broken implant. Detailed instructions for placement and removal of a specific implant can be found in the Surgical Technique, which can be obtained from DePuy Spine sales representatives or by contacting the DePuy Spine Customer Service at POSTOPERATIVE MOBILIZATION Postoperative external immobilization (such as bracing or casting) is recommended, at the surgeon s discretion. Instructions to the patient to reduce stress on the implants are an equally important part of the attempt to avoid the occurrence of clinical problems that may accompany fixation failure. The VBR System(s) implants may be provided either sterile or non-sterile and this will be clearly identified on the product labels. Sterile Implants For the implants supplied sterile, the contents are sterile unless the package is damaged, opened, or the expiration date on the device label has passed. The integrity of the packaging should be checked to ensure that the sterility of the contents is not compromised. Remove implants from packaging, using aseptic technique, only after the correct size has been determined. 8 of 12
9 PRECAUTION: Do not use implants if the condition of the package and/or labeling indicates a chance that the devices may not be sterile. Implants must not be resterilized. Non-sterile Implants For the implants supplied non-sterile, they will be supplied clean. ISO 8828 or AORN recommended practices for in-hospital sterilization should be followed for all components. RECOMMENDATIONS FOR STEAM STERILIZATION: In a properly functioning calibrated steam sterilizer, independent testing has shown that effective sterilization may be achieved using the following parameters: Cycle: Pre-Vacuum Temperature: 270 F (132 C) Exposure time: 6 minutes Post-sterilization drying of the sterilization load within the sterilization vessel is standard practice in hospitals. ANSI/AAMI ST79:2006, Comprehensive guide to steam sterilization and sterility assurance in health care facilities provides guidance to hospitals for selecting appropriate drying parameters based on the sterilization cycle that is being conducted. Sterilizer manufacturers also typically provide recommendations for drying parameters for their specific equipment. Inspect visually for damage or the presence of blood or tissue. If blood or tissue is observed on the implant, it must be thoroughly cleaned manually using a soft brush and neutral ph detergent or discarded. Cleaning instructions Enzyme soak Rinse Ultrasonic cleaning (10-20 minutes) Rinse Automated cleaning in a washer disinfector with lid on to contain implant components Dry 9 of 12
10 Avoid impact, scratching, bending or surface contact with any materials that might affect the implant surface or configuration. Special attention shall be paid to recesses since both chemicals and rinse water may be entrapped in them. Implants previously implanted must not be re-used. MAGNETIC RESONANCE (MR) COMPATIBILITY The VBR System(s) implants have not been evaluated for safety and compatibility in the MR environment. The VBR System(s) implants have not been tested for heating or migration in the MR environment. The surgeon must be thoroughly knowledgeable not only in the medical and surgical aspects of surgical implants, but must also be aware of the mechanical and metallurgical limitations of metallic surgical implants. VBR Spinal System components should not be used with components of spinal systems from other manufacturers. LIMITED WARRANTY AND DISCLAIMER PRODUCTS FROM DEPUY SYNTHES PRODUCTS, LLC ARE SOLD WITH A LIMITED WARRANTY TO THE ORIGINAL PURCHASER AGAINST DEFECTS IN WORKMANSHIP AND MATERIALS. ANY OTHER EXPRESS OR IMPLIED WARRANTIES, INCLUDING WARRANTIES OF MERCHANTABILITY OR FITNESS, ARE HEREBY DISCLAIMED. IF MORE THAN TWO YEARS HAVE ELAPSED BETWEEN THE DATE OF ISSUE/ REVISION OF THIS INSERT AND THE DATE OF CONSULTATION, CONTACT DEPUY SYNTHES SPINE FOR CURRENT INFORMATION AT OR AT of 12
11 LOT LOT NUMBER REF REF CATALOG NUMBER QTY QUANTITY SZ SIZE MADE IN MADE IN NTI NEURAL TISSUE INSTRUMENT IOM NEUROMONITORING INSTRUMENTS Federal (USA) law restricts this device to sale by or on the order of a physician DO NOT RESTERILIZE T1 T2 Lower Limit of temperature = T1 Upper Limit of temperature = T2 SYMBOL TRANSLATION 25 C STORE AT ROOM TEMPERATURE KEEP AWAY FROM SUNLIGHT SINGLE USE ATTENTION. SEE INSTRUCTIONS FOR USE PACKAGE CONTAINS FLAMMABLE LIQUID DO NOT USE IF PACKAGE IS DAMAGED MSR MEASURING DEVICE STERILE STERILE STERILE A Sterile medical device processed using aseptic technique STERILE R STERILIZATION BY IRRADIATION STERILE EO STERILIZATION BY ETHYLENE OXIDE LATEX FREE LATEX FREE NON STERILE NONSTERILE NONSTERILE MANUFACTURER DATE OF MANUFACTURE US REP US REPRESENTATIVE EC REP AUTHORIZED EUROPEAN REPRESENTATIVE DIST DISTRIBUTED BY XXXX-XX USE BY PEEK/C PEEK/CARBON FIBER COMPOSITE Polyether Ether Ketone/ Carbon Fiber Composite T Ti Titanium and its alloys 11 of 12
12 * DePuy Spine, Inc. 325 Paramount Drive Raynham, MA USA Medos International SÀRL Chemin-Blanc Le Locle, Switzerland * *For recognized manufacturer, refer to product label. US REP DePuy Spine, Inc. 325 Paramount Drive Raynham, MA USA Telefón: +1 (800) FAX: +1 (508) EC REP DePuy International, Ltd. St. Anthony s Road Leeds LS11 8DT England Telefón: FAX:
BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System
 0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Surgical Technique & Product Catalogue. Guide for Open & MIS Procedures
 Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
VTI INTERLINK PEDICLE SCREW SYSTEM
 VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
SFX Cross Connector System. Surgical Technique Guide
 SFX Cross Connector System Surgical Technique Guide Introduction The EXPEDIUM SFX Cross Connector System redefines ease-of-use, implant versatility, and construct security with an advanced top-loading
SFX Cross Connector System Surgical Technique Guide Introduction The EXPEDIUM SFX Cross Connector System redefines ease-of-use, implant versatility, and construct security with an advanced top-loading
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
4.5 System. Surgical Technique. This publication is not intended for distribution in the USA.
 4.5 System Surgical Technique This publication is not intended for distribution in the USA. Contents EXPEDIUM 4.5 Spine System 2 Features and Benefits 3 Surgical Technique Extended Tandem Connector 4 Placement
4.5 System Surgical Technique This publication is not intended for distribution in the USA. Contents EXPEDIUM 4.5 Spine System 2 Features and Benefits 3 Surgical Technique Extended Tandem Connector 4 Placement
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
TiLock 2 Spinal System. Surgical Technique
 TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Synex System TECHNIQUE GUIDE. An expandable vertebral body replacement device
 Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
VELOX Anterior Cervical Plate Surgical Technique. Anterior Cervical Plate.
 VELOX Anterior Cervical Plate Surgical Technique VELOX Anterior Cervical Plate www.spinecraft.com cervical VELOXAnterior plate SURGICAL TECHNIQUE Techniques described by: Steven Mather, MD Downers Grove,
VELOX Anterior Cervical Plate Surgical Technique VELOX Anterior Cervical Plate www.spinecraft.com cervical VELOXAnterior plate SURGICAL TECHNIQUE Techniques described by: Steven Mather, MD Downers Grove,
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
Zimmer Facet Screw System Surgical Technique
 Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
GIZA Surgical Technique
 GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
GIZA Surgical Technique Vertebral Body Replacement System Manufactured by Titanium alloy material provides mechanical integrity during insertion and distraction, x-ray visibility, and biocompatibility*
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
XYcor Expandable Spinal Spacer System
 XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
Lumbar Interbody System. Surgical Technique. for Transforaminal Lumbar Interbody Fusion (TLIF)
 Lumbar Interbody System Surgical Technique for Transforaminal Lumbar Interbody Fusion (TLIF) introduction An adaption of the posterior lumbar interbody fusion (PLIF) procedure, the TLIF technique employs
Lumbar Interbody System Surgical Technique for Transforaminal Lumbar Interbody Fusion (TLIF) introduction An adaption of the posterior lumbar interbody fusion (PLIF) procedure, the TLIF technique employs
orthodontic Bone anchor (oba) SYStem
 orthodontic Bone anchor (oba) SYStem Skeletal implants for the orthodontic movement of teeth SurgIcal technique Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and Contraindications
orthodontic Bone anchor (oba) SYStem Skeletal implants for the orthodontic movement of teeth SurgIcal technique Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and Contraindications
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Mini External Fixator
 Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Low Bend Distal Tibia Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
InFix. Anterior Lumbar Device. Surgical Technique Guide
 InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
InFix Anterior Lumbar Device Surgical Technique Guide 2 InFix Anterior Lumbar Device Surgical Technique Guide InFix Anterior Lumbar System s modular design is intended to restore lordosis, disc height
3.5 mm LCP Extra-articular Distal Humerus Plate
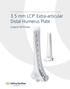 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
