The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
|
|
|
- Donald Merritt
- 5 years ago
- Views:
Transcription
1 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION: The Omega LIF System devices are used to maintain disc space distraction in skeletally mature adults requiring intervertebral body fusion in the lumbar spine. They are designed to be used in conjunction with supplemental spinal fixation instrumentation. The series is comprised of cages of various sizes, and are designed to expand in height intra-operatively to accommodate variations in surgical approach and patient anatomy. Each cage has a hollow center to allow placement of autograft inside of the cage. Ridges on the superior and inferior surfaces of the device help to grip the endplates and prevent expulsion. The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136. INDICATIONS: The Omega LIF Lumbar Cages are indicated for intervertebral body spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels from L2-S1. DDD is defined as discogenic back pain with degeneration of the disc confirmed by patient history and radiographic studies. These DDD patients may also have up to Grade I spondylolisthesis or retrolisthesis at the involved level. Omega LIF implants are to be used with autogenous bone graft and supplemental fixation. Patients should have at least six (6) months of non-operative treatment prior to treatment with an intervertebral cage PRECAUTIONS: Intervertebral body fusion should only be undertaken after the surgeon has had hands-on training in these methods of spinal fixation, and has become thoroughly knowledgeable about spinal anatomy and biomechanics. Surgical technique manuals are available for detailed instructions on the correct use of the Omega LIF cages. The contents of these manuals alone are not adequate for complete instruction in the use of this system. Even for surgeons already experienced in spinal instrumentation and intervertebral body fusion procedures, new skills may be required that are best learned by working with an experienced surgeon or through a course of formal instruction with laboratory training. Lack of experience or expertise with these implants may result in complications. Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without a previous surgery. Based on the dynamic testing results, the physician should consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc., which may impact on the performance of the intervertebral body fusion device. Postoperative care and the patient s ability and willingness to follow instructions are among the most important aspects of successful bone healing. The patient must be made aware of the limitations of the implants. The patient should be encouraged to ambulate to tolerance as soon as possible after surgery, and instructed to limit and restrict lifting and twisting motions and any type of sports participation until the bone is healed. The patient should understand that implants are not as strong as normal healthy bone and could loosen, bend and/or break if excessive demands are placed on it, especially in the absence of complete bone healing. Implants displaced or damaged by improper activities may experience migration to the devices and damage to nerves or blood vessels. Postoperative external immobilization is recommended, at the surgeon s discretion, as is a comprehensive postoperative core stabilization physical therapy program. Instructions to the patient to reduce stress on the implant(s) are an equally important component of the attempt to avoid the occurrence of clinical problems that may accompany fixation failure and delayed/non-union. CONTRAINDICATIONS: Spondylolisthesis higher than grade I (not for the use with a pedicle screw fixation system) Reduced bone density, which does not guarantee a sufficient resting stability (e. g. osteoporosis) Fractures Tumors Scoliosis Active infection Allergy to Titanium
2 Signs of local inflammation Fever or leukocytosis Morbid obesity Pregnancy Mental illness Suspected or documented allergy or intolerance to composite materials Any case not needing a fusion Any case not described in the indications Any patient unwilling to cooperate with postoperative instructions Patients with a known hereditary or acquired bone friability or calcification problem should not be considered for this type of surgery These devices must not be used for pediatric cases, nor where the patient still has general skeletal growth Spondylolisthesis unable to be reduced to Grade 1 Any case where the implant components selected for use would be too large or too small to achieve a successful result Any case that requires the mixing of metals from two different components or systems Any patient having inadequate tissue coverage over the operative site or inadequate bone stock or quality Any patient in which implant utilization would interfere with anatomical structures or expected physiological performance Prior fusion at the level to be treated Any other condition which would preclude the potential benefit of spinal implant surgery, such as the presence of tumors or congenital abnormalities, fracture local to the operating site, elevation of sedimentation rate unexplained by other diseases, elevation of white blood count (WBC), or a marked left shift in the WBC differential count POSSIBLE ADVERSE EFFECTS: Note: A further surgery might become necessary to correct adverse effects. This list may not include all complications caused by the surgical procedure itself. 1. Bending or fracture of implant. Loosening of the implant. 2. Implant material sensitivity, or allergic reaction to a foreign body. 3. Infection, early or late. 4. Decrease in bone density due to stress shielding. 5. Pain, discomfort, or abnormal sensations due to the presence of the device. 6. Nerve damage due to surgical trauma or presence of the device. Neurological difficulties including bowel and/or bladder dysfunction, impotence, retrograde ejaculation, radicular pain, tethering of nerves in scar tissue, muscle weakness, and paraesthesia. 7. Vascular damage could result in catastrophic or fatal bleeding. Malpositioned implants adjacent to large arteries or veins could cause erosion of these vessels and catastrophic bleeding in the later postoperative period. 8. Dural tears experienced during surgery could result in need for further surgery for dural repair, a chronic CSF leak or fistula, and possible meningitis. 9. Bursitis. 10. Paralysis. 11. Death. 12. Spinal cord impingement or damage. 13. Fracture of bony structures. 14. Reflex sympathetic dystrophy. 15. If a pseudarthrodesis occurs coupled with the Interbody Innovations cages, a mechanical grinding action could possibly occur which might generate wear debris. Most types of wear debris have shown the potential of initiating local osteolysis in articulating joints. 16. Degenerative changes or instability in segments adjacent to fused vertebral levels. Warning: An entirely satisfactory result is not always achieved in every surgical case. This particularly applies to spinal surgery, in which numerous external factors may compromise the results. Patients with previous spinal surgery at the levels to be treated may have different clinical outcomes compared to those without a previous surgery. The risk of device expulsion and migration is higher without the use of supplemental fixation.
3 Never reuse an implant under any circumstances. Even when a removed device appears undamaged, it may contain small defects or residual stresses. These defects and stresses may lead to implant failure. Any retrieved devices should be handled in a manner such that they may not be reused in another surgical procedure. The Omega LIF System has not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration, or image artifact in the MR environment. The safety of Omega LIF in the MR environment is unknown. Scanning a patient who has this device may result in patient injury. Packaging: Packages for each of the components should be intact upon receipt. Damaged packages and products should not be used and should be returned to AMENDIA. STERILIZATION: OmegaLIF Implants are supplied sterile using gamma irradiation. Products not clearly marked as sterile should be assumed non-sterile. Implants and instruments used in surgery not clearly labeled as sterile must be sterilized by the hospital prior to use. Remove all packaging materials prior to sterilization where applicable. Only sterile products should be placed in the operative field. Recommended Sterilization Procedures for Omega LIF System Instrumentation Provided Non-Sterile: Manufacturer: Amendia, Inc. Method: Manual Cleaning and Steam Sterilization Device(s): Trays/Implants/Instruments Cautions: The Omega LIF System products provided NON-STERILE must be cleaned and sterilized before use. Limitations on Reprocessing: Repeated processing has limited effect on REUSABLE instruments. End of life is normally determined by wear and damage due to use. CLEANING INSTRUCTIONS: Point of Use: Use clean flowing water and disposable wipes to remove excess soil. Reprocess instruments as soon as possible to prevent body fluid and tissue from drying on instruments prior to cleaning. Preparation for decontamination (72-06): The OmegaLIF Inserter (Part No ) must be disassembled before cleaning. Disassemble the OmegaLIF Inserter as follows: OmegaLIF Inserter Assembly PN: 72-06
4 1. Rotate OMEGALIF Breakdown Knob counter-clockwise to remove from bottom of Handle. 2. Rotate OMEGALIF Handle while pulling backwards to align the Handle and Inner Shaft keyways. Continue to pull the Handle to remove from the Inner Shaft. 3. Use the knob to rotate the OMEGALIF Outer Sleeve counter-clockwise while pulling backwards to unthread Sleeve from Inner Shaft. 4. Gently rotate and wriggle the Outer Sleeve to align with the Inner Shaft s keyway. Pull backwards again to slide Outer Sleeve off the shaft.
5 Preparation for decontamination (72-02): The OmegaLIF Inserter (Part No ) must be disassembled before cleaning. Disassemble the OmegaLIF Inserter as follows: 1. Rotate OMEGALIF Breakdown Knob counter-clockwise to remove from bottom of Handle. 2. Rotate OMEGALIF Handle while pulling backwards to align the Handle and Inner Shaft keyways. Continue to pull the Handle to remove from the Inner Shaft. 3. Use the knob to rotate the OMEGALIF Outer Sleeve counter-clockwise while pulling backwards to unthread Sleeve from Inner Shaft.
6 4. Gently rotate and wriggle the Outer Sleeve to align with the Inner Shaft s keyway. Pull backwards again to slide Outer Sleeve off the shaft. These instruments can be cleaned either manually, or manually with an additional automated cycle. It is preferred to use both manual and automatic cleaning methods, however, due to the somewhat limited availability of automated cleaning equipment it is possible to use just the manual cleaning procedures. These procedures are as follows: Cleaning agent: Endozime AW Plus is to be prepared as follows: Add ½ ounce of Endozime AW plus to 1 gallon of warm tap water (17mL/4liter, C) Manual Cleaning instructions: (1) Immerse and soak for a minimum of five (5) minutes in enzymatic detergent at <43 C. (2) Immerse in enzymatic detergent and clean thoroughly for at least 14 minutes at <43 C. (2.1) Scrub all external surfaces with a soft bristle brush until all visible soil has been removed. It is important to make sure that areas of concern with complex or hidden surfaces are effectively cleaned. Use a small diameter brush to clean cannulation holes. Inspect for visible soil on exposed surfaces. (3) Rinse thoroughly for 2 minutes with warm demineralized water. (Purified water at C) (4) Allow instrument to air dry in a clean area. Blow lumens with clean air using filtered air source or syringe. Automated Cleaning instructions: (1) Manually pre-clean the instruments (1.1) Immerse and soak for a minimum of 5 minutes in enzymatic detergent at <43 C. (1.2) Immerse in enzymatic detergent and clean thoroughly for 14 minutes at <43 C. ( ) Scrub all external surfaces with a soft bristle brush until all visible soil has been removed. It is important to make sure that areas of concern with complex or hidden surfaces are effectively cleaned. Use a small diameter brush to clean cannulation holes. Inspect for visible soil on exposed surfaces. (1.3) Rinse thoroughly for 2 minutes with warm demineralized water. (Purified water at C) (2) Place instruments in an automated washer. Ensure the instruments stay in place and do not touch or overlap so that the design features are accessible for cleaning and do not retain liquid. (3) Run the washer per the parameters below: Washer Parameters Cycle Time Parameters Temperature Pre-Wash 300 seconds Cold¹ Wash seconds ½ Oz Endozime AW Plus per 1 Gallon Tap 65.5 C Water Wash seconds ½ Oz Endozime AW Plus per 1 Gallon Tap 65.5 C Water Purified Water Rinse 10 seconds Non-Recirculated Purified Water 82.2 C² Dry Time 7 minutes N/A C ¹Cold tap water line ²Purified water tank temperature set at 82.2 C/180 F (4) Once cleaned, put all instruments in tray before sterilization. The OMEGALIF Inserter must remain disassembled post cleaning, placed in tray for sterilization, and assembled during procedure. Note: See Surgical Technique Guide for OMEGALIF Inserter reassembly instructions. Disinfection: Disinfection is only acceptable as an adjunct to full sterilization for reusable surgical instruments.
7 Maintenance, inspection, and testing; Carefully inspect each device to ensure that all visible blood and soil have been removed. Inspect lumens to confirm that all foreign material has been removed. Visually inspect for damage and/or wear. Note: If any damage or wear is noted that impairs the function of the instrument, contact your Amendia representative for a replacement. Packaging: This set of components may be loaded into a dedicated tray, supplied by the manufacturer, for sterilization. The tray should be wrapped using FDA-cleared sterilization wrap or other FDA-cleared accessory that has been validated to allow sterilant penetration and subsequently maintain sterility. STERILIZATION INSTRUCTIONS: Visually inspect all components for any remaining debris prior to sterilization. The Omega LIF Intervertebral Body Fusion system components provided NON-STERILE should be autoclave sterilized using the sterilizer manufacturer s instructions and the institution s procedures for ensuring sterility. The sterilization cycle should occur in a calibrated autoclave. Omega LIF system components should be sterilized utilizing a pre-vacuum steam autoclave for a minimum of 4 minutes at 270 F (132 C.) Drying: A minimum drying time of 20 minutes, after sterilization, is recommended. Drying times may vary according to load size and should be increased for large loads. Dry, thoroughly and promptly, after both cleaning and sterilization Storage: Store components in a clean, dry, limited access area that is well ventilated and provides protection from dust, moisture, insects, vermin, and extremes in humidity and temperature The instructions provided above have been validated by Amendia as being CAPABLE of preparing a medical device for reuse. It remains the responsibility of the processor to ensure that the reprocessing as actually performed, using equipment, materials, and personnel in the reprocessing facility, achieves the desired result. This normally requires validation and routine monitoring of the process. Any deviation by the re-processor from these instructions should be properly evaluated for effectiveness to avoid potential adverse consequences. Product Complaints: Any health care professional (e.g. customer or user) who has experienced dissatisfaction in the services of AMENDIA or who has any complaints about AMENDIA products referring to quality, identity, durability, reliability, safety, effectiveness, and/or performance, should notify this to the sales representative, distributor, or AMENDIA customer service. Further, if any of the devices, instruments or components ever malfunction, (i.e. do not meet any of their performance specifications or otherwise do not perform as intended), or are suspected of doing so, the distributor should be notified immediately. If any AMENDIA product ever malfunctions and may have caused or contributed to the death or serious injury of a patient, the distributor should be notified immediately by telephone, FAX or written correspondence. When filing a complaint, please provide the component(s) name and number, lot number(s), your name and address, the nature of the complaint and notification of whether a written report from the distributor is requested. CAUTION Federal Law restricts this device to sale by or on the order of a Physician. Amendia 1755 West Oak Parkway Marietta, GA (877) Tele (877) Fax Please contact company for product inquiries and surgical techniques, or to report any adverse experience.
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System
 0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
XYcor Expandable Spinal Spacer System
 XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Zavation Z-Link Cervical
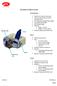 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Hinged Laminoplasty System surgical technique
 BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
SAVANNAH-T HIGH TOP PEDICLE SCREW Z A Z C Z L Z O Amendia SV H Z V Z P Z T SURGICAL TECHNIQUE GUIDE
 SAVANNAH-T HIGH TOP PEDICLE SCREW Z A Z C Z L Z O L Sp Cm Amendia 5.5 6.5 7.5 Z P Z T Z V SV H SAVANNAH-T HIGH TOP PEDICLE SCREW SURGICAL TECHNIQUE GUIDE Savannah-T High Top Pedicle Screw System Table
SAVANNAH-T HIGH TOP PEDICLE SCREW Z A Z C Z L Z O L Sp Cm Amendia 5.5 6.5 7.5 Z P Z T Z V SV H SAVANNAH-T HIGH TOP PEDICLE SCREW SURGICAL TECHNIQUE GUIDE Savannah-T High Top Pedicle Screw System Table
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SECURIS SPINAL Fixation System Package Insert
 SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
NEO PEDICLE SCREW SYSTEM V. 2.0
 NEO PEDICLE SCREW SYSTEM 2017-08 V. 2.0 Neo Medical S.A. Route de Lausanne 157A 1096 Villette Switzerland 1250 Important Information on the NEO PEDICLE SCREW SYSTEM CAUTION: USA law restricts this device
NEO PEDICLE SCREW SYSTEM 2017-08 V. 2.0 Neo Medical S.A. Route de Lausanne 157A 1096 Villette Switzerland 1250 Important Information on the NEO PEDICLE SCREW SYSTEM CAUTION: USA law restricts this device
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
Rev. A VBR Spinal System. Issued October 2014 DePuy Synthes Spine, a division of DOI All rights reserved.
 0902-90-201 Rev. A 0086 VBR Spinal System Issued October 2014 DePuy Synthes Spine, a division of DOI 2013. All rights reserved. DESCRIPTION The VBR (Vertebral Body Replacement) System consists of the BENGAL
0902-90-201 Rev. A 0086 VBR Spinal System Issued October 2014 DePuy Synthes Spine, a division of DOI 2013. All rights reserved. DESCRIPTION The VBR (Vertebral Body Replacement) System consists of the BENGAL
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
ISSYS LP Spinal Fixation Systems Package Insert
 ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
PATHWAY PLIF and PATHWAY TLIF. SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY
 PATHWAY PLIF and PATHWAY TLIF SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT
PATHWAY PLIF and PATHWAY TLIF SPINAL ELEMENTS PATHWAY PLIF And PATHWAY TLIF PATHWAY CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
3 Operative Technique U.S. EDITION
 3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
STRYKER SPINE Spinal Fixation Systems XIA 3
 STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
STRYKER SPINE Spinal Fixation Systems XIA 3 NON STERILE PRODUCT The STRYKER Spine Spinal Fixation Systems are made of devices for fixation of the non-cervical spine. They include smooth rods, screws, hooks,
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Advantage ALIF. Keith Shevlin Managing Director
 Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Veyron -C Anterior Cervical System Surgical Technique
 Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID. CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician.
 PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT MUST BE STERILIZED PRIOR TO USE SINGLE
PATHWAY AVID SPINAL ELEMENTS PATHWAY AVID CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE PRODUCT MUST BE STERILIZED PRIOR TO USE SINGLE
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Zimmer Facet Screw System Surgical Technique
 Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
