Aesculap Implant Systems Arcadius XP C Spinal System: Instructions for Use. Device Description. Indications. Materials
|
|
|
- Claude Daniels
- 6 years ago
- Views:
Transcription
1 Aesculap Implant Systems Arcadius XP C Spinal System: Instructions for Use Device Description The Arcadius XP C Spinal System is a stand alone cervical interbody fusion device used to provide structural stability in skeletally mature individuals following discectomy procedures. The system consists of PEEK cage and two bone screws. The PEEK cages are manufactured from a radiolucent material medical grade LT1 polyetheretherketone (PEEK) per ASTM F2026, with a titanium layer and a vacuum plasma spray coating (PlasmaporeXP). The Arcadius XP C Spinal System implants are offered in various heights and geometrical options to fit the anatomical needs of a wide variety of patients. The Arcadius XP C Spinal System implant is available in two footprints. The wide central opening holds optimal graft material. The screws are 4mm in diameter and offered in three lengths. The fixation screws and radiographic markers are manufactured of titanium alloy, (Ti6Al4V) according to ISO 5832/3. Indications The AIS Arcadius XP C Spinal System is intended to be used as an intervertebral body fusion device as a standalone system used with the supplied bone screws and requires no additional supplementary fixation system. It is intended for spinal fusion procedures at one level in the cervical spine from the C2/C3 disc space to the C7/T1 disc space for the treatment of cervical degenerative disc disease (defined as discogenic pain with degeneration of the disc confirmed by history and radiographic studies) using autograft bone. Patients should be skeletally mature and must have undergone a regimen of at least six (6) weeks of non-operative treatment prior to being treated with the AIS Arcadius XP C Spinal System. Materials The materials used in the implant are listed on the packaging: The Arcadius XP C cervical cages are made of PEEK-OPTIMA LT1 in accordance with ASTM F2026, and coated with a pure titanium layer in accordance with ISO or ASTM F1580 and a vacuum plasma spray coating (PLASMAPORE XP ). They contain marker pins made of titanium alloy to enable radiographic visualization for placement and follow-up evaluations. The bone screws and marker pins are made of surgical implant applications titanium alloy Ti6Al4V described in ISO standard or ASTM-F136. The titanium bone screws are anodized with a colored oxide layer. Slight changes in coloration may occur, but do not affect the implant quality. PLASMAPORE is a registered trademark of Aesculap AG, Tuttlingen / Germany. PEEK-OPTIMA is a registered trademark of Invibio, Ltd Lancashire FY5 4QD / UK. Page 1 of 9
2 General Surgical Indications Surgically installed implants serve to support normal healing processes. They should neither replace normal structures of the body nor permanently bear the loads occurring in the case of incomplete healing. Contraindications Any medical or surgical condition that could preclude the potential success of the implantation. These include: Existing or risk of acute or chronic infections, fever/leukocytosis Progressive joint disease or bone absorption syndromes such as Paget s disease, osteopenia, osteoporosis, or osteomyelitis which may prevent adequate fixation Severe defects of the bony structures of the spine which are a prerequisite for stable implantation of the cages Bone tumors in the region of the implant anchoring Proven or suspected allergy/foreign body reactions to implant materials Conditions that may place excessive stresses on bone and implants, such as severe obesity, pregnancy or degenerative diseases. The decision to use this system in such conditions must be made by the physician taking into account the risks versus the benefits to the patient Patients resistant to following post-operative restrictions on movement, especially in athletic and occupational activities Systemic, metabolic and degenerative diseases Psychosocial problems; unwillingness or inability of the patient to follow the instructions for post-operative treatment Drug abuse or alcoholism Generally poor condition of the patient Prior fusion at the level(s) to be treated All cases that are not listed under Indications Risks The surgical intervention involves the following potential risks: Neurological complications caused by overdistraction or trauma of the nerve roots or dura Loss of intervertebral disk height due to removal of healthy bone material Complications that can generally occur in connection with intervertebral surgery: Pseudarthrosis Incorrect implant position Spondylolisthesis Loss of fixation; dislocation or migration Side-Effects and Adverse Interactions In addition to surgery-related risks, potential complications in connection with intervertebral procedures can include, but are not limited to: Disassembly, bending and/or fracture of the implants or instruments Early or late loosening of the components Failure of the device to provide adequate mechanical stability Loss of fixation Implant migration/dislocation Malpositioning of the implant Subsidence of the device into vertebral body(ies) Foreign body reactions, allergy Nonunion or delayed union Infection Page 2 of 9
3 Increase in biomechanical stress on adjacent levels Nerve or vascular damage due to surgical trauma, including loss of neurological function, dural tears, appearance or radiculopathy, and/or the development or continuation of pain, paralysis (complete or incomplete), pseudomeningocele, cerebral spinal fluid leakage, dysesthesia, hyperesthesia, anesthesia, paresthesia, numbness, neuroma, tingling sensation, sensory loss and/or spasms Cauda equina syndrome, neuropathy, neurological deficits (transient or permanent), reflex deficits, arachnoiditis, and/or muscle loss Skin or muscle sensitivity in patients with inadequate tissue coverage over the operative site, which may result in skin breakdown and/or wound complications Spondylolisthesis, pseudoarthrosis, inadequate integration of the implant Changes in bone density, degenerative changes in the region of the adjacent vertebral bodies Bone loss due to resorption or stress shielding, or bone fracture at, above or below the level or surgery (fracture of the vertebra) Hemorrhage of blood vessels, hematoma, occlusion, seroma, edema, embolism, stroke, excessive bleeding, phlebitis, damage to blood vessels, or cardiovascular system compromise Wound necrosis or wound dehiscence Herniated nucleus pulposus, disc disruption or degeneration above/below the level of surgery Scar formation possibly causing neurological compromise around nerves and/or pain Bursitis Gastrointestinal complications Loss of bladder control or other types of urological system compromise Misalignment of anatomical structures, including loss of proper spinal curvature, correction, reduction and/or height Loss of spinal mobility or function Loss of intervertebral disk height due to removal of healthy bone material Changes of the normal spine lordosis Wound healing problems Pain or discomfort at the operative and/or bone graft donor site Meningitis Discomfort or abnormal sensations due to the presence of the device Limited performance Persistence of symptoms that were to be treated by the implantation Inability to resume activities of normal daily living Reoperation Death Safety Notes Only patients that meet the criteria described in the indications should be selected. It is the operating surgeon's responsibility to ensure that the surgical procedure is performed properly. General risk factors associated with surgical procedures are not described in the present instructions for use. The operating surgeon must have a thorough command of both the hands-on and conceptual aspects of the established operating techniques. The operating surgeon must be fully conversant with bone anatomy, including the pathways of nerves, blood vessels, muscles, and tendons. It is the operating surgeon's responsibility to ensure the correct combination of implant components and their implantation. Aesculap is not responsible for any complications arising from wrong indication, wrong choice of implant, incorrect combination of implant components and operating technique, the limitations of the treatment method, or inadequate asepsis. The instructions for use for individual Aesculap implant components must be followed. The implant components were tested and approved in combination with Aesculap components. If other combinations are used, the responsibility for such action lies with the operating surgeon. Do not, under any circumstances, combine implant components from different manufacturers. Page 3 of 9
4 Do not use instruments belonging to another system or made by another manufacturer. Do not, under any circumstances, use damaged or surgically removed components. Implants that have been used before must not be reused. The implant components applied, along with their article numbers, the name of the implant, as well as the batch number and serial number (if available) must be documented in all patient records. Post-operatively, individual patient information, as well as mobility and muscle training, is of particular importance. Delayed healing can cause implant breakage due to material fatigue. The attending surgeon decides whether implant components should be explanted, taking into account the potential risk of another surgery and the difficulties involved in the removal of an implant. Damage to the load-bearing structures of the implant can lead to loosening of components, dislocation, migration, and other severe complications. In order to promote the earliest possible detection of any problems or complications, the operation results must be followed up at regular intervals with the aid of appropriate examination procedures. A precise diagnosis requires X-rays taken in the directions anterior-posterior and medial-lateral. Care and Handling The procedures outlined below should be followed to ensure safe handling of biologically contaminated surgical instruments. All instruments must be sterilized before use. 1. PRE-CLEANING Keep instruments moist and do not allow blood and/or bodily fluids to dry on the instruments. Remove gross contaminants with a steady stream of lukewarm/cool water (below 110F/43C). Rinse each instrument thoroughly. Do not use saline or chlorinated solutions. Disassemble components to expose all surfaces and clean separately. Disassemble the component parts for the implant inserter, trial inserter and any other instrument with component parts. Give special attention to joints and all parts to facilitate reassembly. 2. CLEANING Cleaning Precautions Do not soak instruments in hot water, alcohol, disinfectants or antiseptics to avoid coagulation of mucus, blood or other body fluids. Do not exceed two hours soaking in any solution. Do not use steel wool, wire brushes, pipe cleaners or abrasive detergents. a. Manual Cleaning Hand wash using a medical grade, low-sudsing, protein dissolving detergent. Follow the manufacturers directions regarding concentration, temperature, contact time and reuse. Totally immerse instruments during cleaning to prevent aerosolization. Use a large syringe or pulsating water jet to thoroughly flush all channels and cavities with cleaning solution to remove debris. Use appropriate-sized, soft nylon brushes to clean the instruments and their parts. b. Ultrasonic and Mechanical Cleaning For ultrasonic cleaning, follow manufacturer s specifications for water level, concentration levels of cleaning agent and temperature. When using mechanical washer, make sure all instruments stay properly in place and do not touch or overlap each other. Always follow the manufacturer s specifications for automatic washer-sterilizers and use a free-rinsing, low-sudsing detergent with a neutral ph ( ). Due to variations in water quality, the type of detergent and its concentration may require adjustment for optimal disinfection and cleaning. Page 4 of 9
5 c. Rinsing Rinse all instruments thoroughly with tap water, de-ionized or distilled water to remove all traces of debris and cleansing agents. Make sure all internal cavities and ratchets are thoroughly rinsed. 3. DECONTAMINATION Note: The decontamination procedure does not sterilize the instruments. Refer to and process the instruments as outlined in the STERILIZATION section. Select a proper product for high-level disinfection such as the glutaraldehyde-family of disinfectant products. Follow the cleaning agent s recommended directions regarding concentration, temperature, contact time and solution re-use. Do not use high acid (ph 4 or lower) or high alkaline (ph 10 or higher) products for disinfection, such as bleach and bichloride of mercury. Completely immerse instruments in the disinfecting solution. Force solution into all areas and cavities. Thoroughly rinse with distilled water to remove all traces of disinfecting solution. USE STERILE WATER ON THE FINAL RINSE. 4. DRYING Instruments must be thoroughly dried and all residual moisture must be removed before they are stored. Use a soft, lint-free, absorbent towel/cloth to dry external surfaces. Compressed air or a 70% alcohol rinse may be used to aid the drying process. 5. LUBRICATION / ASSEMBLY Lubrication is essential every time instruments are processed. Special attention should be given to lubrication of joints and movable parts. Only lubricate dry instruments. Do not use mineral oil, petroleum, or silicone-based products. To lubricate joints, use a non-silicone, water-soluble lubricant prior to sterilization such as Aesculap Instrument Oil, JG598. Reassemble instruments, as necessary, before assembly into baskets or trays. Inspect instrument components for mechanical damage, pits, cracks, misalignment and corrosion. Remove stained, discolored or damaged instruments. Mechanically test the working parts to verify that each instrument performs correctly. Sterility Arcadius XP C Spinal System cages: The Arcadius XP C Spinal System cages come individually packed in protective packaging that is labeled according to its contents. The Arcadius XP C Spinal System cages are provided gamma-sterilized. Store implant components in their original packaging. Remove them from their original protective packaging only just prior to application. Prior to use, check the product expiry date and verify the integrity of the sterile packaging. Do not use implant components that are past their expiration date or whose packaging is damaged. Damage to implants caused by processing and resterilization! Do not reprocess or resterilize the implants. Arcadius XP C Spinal System bone screws: The Arcadius XP C Spinal System bone screws are supplied in an unsterile condition. The Arcadius XP C Spinal System bone screws are packaged individually. Store the implant components in their original packaging and only remove them from their original and protective packaging immediately prior to processing. Use the implant system storage devices for processing, sterilization and sterile setup. Ensure that the implant components in their implant system storage devices do not come into contact with each other or with instruments. Ensure that the implant components are not damaged in any way. Page 5 of 9
6 Sterilization The Arcadius XP C Spinal System screws and instruments are provided non-sterile. Sterilization is accomplished by steam. To achieve a sterility assurance level of 10-6, Aesculap Implant Systems recommends the following parameters: EXPOSURE TIME Sterilization Method Temperature Full Cycle Time Minimum Dry Time Pre-Vacuum 270 F (132 C) 4 minutes minutes The above parameters have been validated for sterility in an Aesculap STERILCONTAINER System. The cycle times for wrapped product are based on the recommendations of the AAMI Guidelines ST79 for steam sterilization. Note: Time and temperature parameters required for steam sterilization may vary according to the type of sterilizer, cycle design, and packaging/ containerization. The manufacturer s instructions must be followed for each sterilization chamber. Application The operating surgeon shall devise an operation plan that specifies and accurately documents the following: Selection of the implant components and their dimensions Positioning of the implant components in the bone Location of intra-operative landmarks The following conditions must be fulfilled prior to application: All requisite implant components are ready to hand. Operating conditions are highly aseptic. All requisite implantation instruments must be available and in working order, including specialized Aesculap implantation systems. All instruments must be cleaned and sterilized prior to surgery. The operating surgeon and operating room team are thoroughly familiar with the operating technique and with the available range of implants and instruments; information materials on these subjects must be complete and ready to hand. The operating surgeon is fully familiar with the rules governing medical practice, the current state of scientific knowledge, and the contents of relevant scientific articles by medical authors. The manufacturer has been consulted if the preoperative situation was unclear and if implants were found in the area operated on. The patient has been informed of the procedure, taking into account the information provided in the sections on indications, contraindications, side effects and interactions, and has documented his/her understanding and consent regarding the following points: In the case of delayed or incomplete fusion, the implants can break and loosen due to high loads and material fatigue. The implants can come loose or break under increased load. Factors such as the patient s body weight, their level of activity and their compliance with instructions concerning their bearing of weight or loads can influence the durability of the implant. To allow maximum chances for a successful surgical result, the patient or device should not be exposed to mechanical vibrations that may loosen the device construct. Physical activities must be limited and restricted, especially lifting, twisting motions and any type of sport participation. Corrective surgery may become necessary if the implant loosens. Page 6 of 9
7 Patients with previous spinal surgery at the levels to be treated may not experience the same clinical outcomes as those without a previous surgery. Smokers present an increased risk of bone fusion failure. The patient must undergo medical check-ups of the implant components at regular intervals. The patients should be advised of their inability to bend at the point of spinal fusion and taught to compensate for this permanent physical restriction in body motion. Implanting the Arcadius XP C Spinal System devices The correct selection, placement and fixation of the Arcadius XP C Spinal System components is extremely important. The potential for success is increased by the selection of the proper size, shape and design of the implant. The size and shape of the human spine presents limiting restrictions of the size and strength of implants. Implantation of the Arcadius XP C Spinal System requires the following steps: Only use Arcadius XP C Spinal System instruments provided by Aesculap. Follow the instructions for use of the Arcadius XP C Spinal System instruments and the O.R. manual. Select the appropriate Arcadius XP C Spinal System cage size and shape according to the individual indication and preoperative planning. Prior to use: Unpack the ArcadiusXP C implants and inspect them for damage. Ensure that the implants are protected from being marred, nicked, scratched or notched as a result of contact with metal or abrasive objects. Alterations will result in defects in surface finish and internal stresses which may become the focal point for eventual breakage of the implant. Apply the preparation and implantation instruments correctly. Check spacer height and/or angle using the trial implants. Prior to inserting the cage it is recommended to fill it with bone or bone substitute Do not use excessive force when introducing and positioning the implant within the intervertebral body space to avoid damaging the implant. Confirm the anatomically suitable position and orientation of the Arcadius XP C Spinal System cage. Select the appropriate length of bone screws. Insert the bone screws under X-ray control. Insert the bone screws until they reach the final seated position, ensuring full engagement of the two locking mechanisms. If a fully seated bone screw is removed from the implant, a small piece of PEEK debris from the locking rim in the locking mechanism may be present. Further information on Aesculap implant systems is always available from B. Braun/Aesculap or the appropriate B. Braun/Aesculap office. Implants supplied in sterile condition must not be resterilized or reused under any circumstances. Danger to the patient and possible loss of implant functionality due to resterilization may occur! Increased risk of migration and subsidence due to over-preparation of the vertebral body endplates! Ensure that the base and cover plates of the adjacent vertebral bodies are not weakened. S Inaccurate markings of the midline may result in incorrect position of the implant. Always mark the midline under X-Ray visualization. Determine the center of the vertebral disc using a midline marker under X-ray visualization. Potential risks indentified with the use of this intervertebral body fuions device, which may require additional surgery, include: device component fracture, lost of fixation, pseudorarthrosis (i.e., non-union), fracture of the vertebra, neurological injury, and vascular or visceral injury. Page 7 of 9
8 The coated surfaces of the Arcadius XP C Spinal System cage may be damaged by improper handling! Avoid direct contact with the coated surfaces. Handle implants carefully. Excessive insertion forces may cause damage to the implant Risk of insufficient stability or implant failure due to using fewer than the two internal screw fixations provided! Apply both screws or use an additional supplemental spinal fixation system that has been cleared for use in the cervical spine, such as cervical plating systems. Backing out and loosening of the bone screw occurs when the screw is not fully inserted into the cage! Insert the bone screw until it is fully engaged. If a bone screw does not seat properly and needs to be removed, the physician should remove the cage and replace with a new cage and screw. CAUTION Damage to the PLASMAPORE XP cages through the use of high frequency or electocautery surgical devices! Avoid using high frequency surgical devices in proximity to the implant. If the implant is damaged: Remove and replace the implant. CAUTION Based upon dynamic testing results, the physicians should consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact the performance of the intervertebral body fusion device. PRECAUTION The implantation of the intervertebral body fusion device should be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient. MRI SAFETY INFORMATION The Arcadius XP C Spinal System has not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration, or image artifact in the MR environment. The safety of the Arcadius XP C Spinal System in the MR environment is unknown. Scanning a patient who has this device may result in patient injury. PRECAUTION Mixing unalloyed titanium, titanium alloy, stainless steel, and other cobalt alloy implants should be avoided for implants that are in contact with each other. PRECAUTION Components of the Arcadius XP C Spinal System should not be used with components of any other system or manufacturer. Additional Information Please refer to the system s surgical technique for detailed implantation explanation information. To obtain a surgical technique guide, please contact Aesculap Implant Systems Customer Service Department at (866) or your Sales Represenative. Page 8 of 9
9 MAINTENANCE AND REPAIR If any Arcadius XP C Spinal System instrument or its components require repair or maintenance, return the entire instrument set in a sturdy box with adequate foam, bubble wrap or other packaging material to protect it. Send the packaged instrument to: Aesculap Technical Services 615 Lambert Point Hazelwood, St. Louis, MO Instruments returned to Aesculap Implant Systems for repair must have a statement which testifies that each instrument has been thoroughly cleaned and disinfected. Failure to supply evidence of cleaning and disinfection will result in a cleaning charge and delayed processing of your instrument repair. CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Distributed in the U.S. by: Aesculap Implant Systems, LLC Corporate Parkway Center Valley, PA Phone: SOP-AIS Rev Page 9 of 9
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
Aesculap Long and Short Ventriculoscope Systems Instructions for Use
 Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Aesculap Long and Short Ventriculoscope Systems Instructions for Use INDICATIONS FOR USE Aesculap s short and long ventriculoscopes and associated instruments are designed for visual orientation and therapeutic
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
NEO PEDICLE SCREW SYSTEM V. 2.0
 NEO PEDICLE SCREW SYSTEM 2017-08 V. 2.0 Neo Medical S.A. Route de Lausanne 157A 1096 Villette Switzerland 1250 Important Information on the NEO PEDICLE SCREW SYSTEM CAUTION: USA law restricts this device
NEO PEDICLE SCREW SYSTEM 2017-08 V. 2.0 Neo Medical S.A. Route de Lausanne 157A 1096 Villette Switzerland 1250 Important Information on the NEO PEDICLE SCREW SYSTEM CAUTION: USA law restricts this device
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
Aesculap CranioFix absorbable
 Aesculap CranioFix absorbable Aesculap Neurosurgery Instructions for use/technical description CranioFix absorbable Gebrauchsanweisung/Technische Beschreibung CranioFix resorbierbar 2 1 3 5 6 9 4 9 7
Aesculap CranioFix absorbable Aesculap Neurosurgery Instructions for use/technical description CranioFix absorbable Gebrauchsanweisung/Technische Beschreibung CranioFix resorbierbar 2 1 3 5 6 9 4 9 7
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
Yasargil Permanent Aneurysm Clips
 Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
Yasargil Permanent Aneurysm Clips Yasargil aneurysm clips Figure captions 1 Yasargil Phynox aneurysm clip, example of a straight clip 2 Yasargil titanium aneurysm clip, example of a straight clip 3 Yasargil
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
3 Operative Technique U.S. EDITION
 3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
Aesculap Spine Instructions for use
 Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
Aesculap Spine S 4 Spondylolisthesis Reduction Instrument (SRI) Instructions for use S 4 Spondylolisthesis Reduction Instrument (SRI) 5 4 3 2 Fig. 1 1 9 10 11 8 6 7 Fig. 2 Aesculap Spine S 4 Spondylolisthesis
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System
 0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
0902-90-200 Rev. B 0086 BENGAL System CONCORDE System CONCORDE Bullet System CONCORDE Inline System CONCORDE Curve System COUGAR System DEVEX System LEOPARD System Revised July 2016 DePuy Synthes 2014-2016.
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Zavation Z-Link Cervical
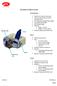 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Veyron -C Anterior Cervical System Surgical Technique
 Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
Veyron -C Anterior Cervical System Surgical Technique 2 Veyron-C Anterior Cervical System Surgical Technique Veyron-C Anterior Cervical System Surgical Technique Description, Indications & Contraindications...3
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Doc. No.: IU/3 for. Rev. No.: 07 Adler Spine Implants
 0434 Page 1 of 6 Instructions use For use by an Accredited Orthopaedic Surgeon only Purpose Adler spine implants that include the Zeta Spine System, the OneLock Spine System, the Titan Interbody Spacer
0434 Page 1 of 6 Instructions use For use by an Accredited Orthopaedic Surgeon only Purpose Adler spine implants that include the Zeta Spine System, the OneLock Spine System, the Titan Interbody Spacer
PARADIGM SPINE. Anterior Cervical Fusion Cage. Cervical Interbody Fusion
 PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Advantage ALIF. Keith Shevlin Managing Director
 Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
Advantage ALIF Unit 10, 9-11 Myrtle Street, Crows Nest NSW 2065 Keith Shevlin Managing Director keithshevlin@precisionsurgical.com.au Advantage ALIF Introduction & Indications for Use 1 Surgical Technique
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Solitaire Anterior Spinal System
 Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
