OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
|
|
|
- Oswald Potter
- 5 years ago
- Views:
Transcription
1 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP GRENOBLE CEDEX 2 FRANCE Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR acumed.net PKGI-79-B EFFECTIVE
2 English US / PAGE 2 Ankle Plating System 3 FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON AND SUPPORTING HEALTHCARE PROFESSIONALS DESCRIPTION: The Acumed Ankle Plating System 3 contains bone plates, screws and accessories. The system is designed to provide fixation for ankle fractures including fractures of the distal tibia and fibula. INDICATIONS: The Acumed Ankle Plating System 3 includes orthopedic implants with the following indications: Lateral Fibula Plates, Posterolateral Fibula Plates, Posteromedial Distal Tibia Plates, Posterolateral Distal Tibia Plates, and Medial Anti-Glide Plates are intended for use for fixation of fractures, osteotomies, and non-unions of the distal tibia and fibula, particularly in osteopenic bone. Hook Plates and Locking Peg Hook Plates are intended for fixation of fractures, osteotomies, and non-unions of small bones, including the tibia and fibula. CONTRAINDICATIONS: Contraindications for the system are active or latent infection; sepsis; insufficient quantity or quality of bone; and soft tissue or material sensitivity. If material sensitivity is suspected, tests should be performed prior to implantation. Patients who are unwilling or incapable of following postoperative care instructions are contraindicated for these devices. These devices are not intended for screw attachment or fixation to the posterior elements (pedicles) of the cervical, thoracic, or lumbar spine. IMPLANT MATERIAL SPECIFICATIONS: The implants are made of commercially pure titanium per ASTM F67 or titanium alloy per ASTM F136. SURGICAL INSTRUMENT MATERIAL SPECIFICATIONS: The instruments are made of various grades of stainless steel, aluminum, and polymers evaluated for biocompatibility. IMPLANT INFORMATION FOR USE: Physiological dimensions limit the sizes of implant appliances. The surgeon must select the type and size that best meets the patient s requirements for close adaptation and firm seating with adequate support. Although the physician is the learned intermediary between the company and the patient, the important medical information given in this document should be conveyed to the patient. SURGICAL INSTRUMENT INFORMATION FOR USE: Instruments provided with this system may be single use or reusable.
3 English US / PAGE 3 The user must refer to the instrument's label to determine whether the instrument is single use or reusable. Single use instruments are labeled with a "do not re-use" symbol as described in the Symbol Legend section, below. Single use instruments must be discarded after a single use. Reusable instruments have a limited lifespan. Prior to and after each use, reusable instruments must be inspected where applicable for sharpness, wear, damage, proper cleaning, corrosion and integrity of the connecting mechanisms. Particular care should be paid to drivers, drill bits and instruments used for cutting or implant insertion. SURGICAL TECHNIQUES: Surgical techniques are available describing the uses of this system. It is the responsibility of the surgeon to be familiar with the procedure before use of these products. In addition, it is the responsibility of the surgeon to be familiar with relevant publications and consult with experienced associates regarding the procedure before use. Surgical techniques can be found on the Acumed website (acumed.net). IMPLANT WARNINGS: For safe effective use of the implant, the surgeon must be thoroughly familiar with the implant, the methods of application, instruments, and the recommended surgical technique for the device. The device is not designed to withstand the stress of weight bearing, load bearing, or excessive activity. Improper insertion of the device during implantation can increase the possibility of loosening or migration. The patient must be cautioned, preferably in writing, about the use, limitations, and possible adverse effects of this implant. These cautions include the possibility of the device or treatment failing as a result of loose fixation and/or loosening, stress, excessive activity, or weight bearing or load bearing, particularly if the implant experiences increased loads due to delayed union, nonunion, or incomplete healing, and the possibility of nerve or soft tissue damage related to either surgical trauma or the presence of the implant. The patient must be warned that failure to follow postoperative care instructions can cause the implant and/ or treatment to fail. The implants may cause distortion and/or block the view of anatomic structures on radiographic images. The components of these systems have not been tested for safety, heating, or migration in the MRI environment. Similar products have been tested and described in terms of how they may be safely used in postoperative clinical evaluation using MRI equipment 1. 1 Shellock, F. G. Reference Manual for Magnetic Resonance Safety, Implants, and Devices: 2011 Edition. Biomedical Research Publishing Group, SURGICAL INSTRUMENT WARNINGS: For safe effective use of any Acumed instrument, the surgeon must be familiar with the instrument, the method of application, and the recommended surgical technique. Instrument breakage or damage, as well as tissue damage, can occur when an instrument is subjected to excessive loads, excessive speeds, dense bone, improper use or unintended use. The patient must be cautioned, preferably in writing as to the risks associated with these types of instruments. IMPLANT PRECAUTIONS: An implant shall never be reused. Previous stresses may have created imperfections, which can lead to a device failure. Instruments shall be inspected for wear or damage prior to usage. Protect implants against scratching and
4 English US / PAGE 4 nicking. Such stress concentrations can lead to failure. Bending plates multiple times may weaken the device and could lead to premature implant fracture and failure. Mixing implant components from different manufacturers is not recommended for metallurgical, mechanical and functional reasons. The benefits from implant surgery may not meet the patient s expectations or may deteriorate with time, necessitating revision surgery to replace the implant or to carry out alternative procedures. Revision surgeries with implants are not uncommon. SURGICAL INSTRUMENT PRECAUTIONS: Single use surgical instruments shall never be reused. Previous stresses may have created imperfections, which can lead to a device failure. Protect instruments against scratching and nicking, such stress concentrations can lead to failure. ADVERSE EFFECTS: Possible adverse effects are pain, discomfort, or abnormal sensations and nerve or soft tissue damage due to the presence of an implant or due to surgical trauma. Fracture of the implant may occur due to excessive activity, prolonged loading upon the device, incomplete healing, or excessive force exerted on the implant during insertion. Implant migration and/or loosening may occur. Metal sensitivity, histological, allergic or adverse foreign body reaction resulting from implantation of a foreign material may occur. Nerve or soft tissue damage, necrosis of bone or bone resorption, necrosis of the tissue or inadequate healing may result from the presence of an implant or due to surgical trauma. CLEANING: Implant Cleaning: Implants should not be reused. Acumed does not recommend cleaning of implants provided sterile. Implants provided non-sterile that have not been used, but have become soiled, should be processed according to the following: Warnings & Precautions Resterilization of the implants should not be performed if the implant comes into contact with contamination (e.g. biological tissue contact, such as bodily fluids/ blood) unless the single use device (SUD) has been reprocessed by an authorized facility who has received appropriate regulatory clearance for such. Cleaning a SUD after it comes into contact with human blood or tissue constitutes reprocessing. Do not use an implant if the surface has been damaged. Damaged implants should be discarded. Users should wear appropriate personal protective equipment (PPE). All users should be qualified personnel with documented evidence of training and competency. Training should be inclusive of current applicable guidelines, standards and hospital policies. Manual Processing Equipment: Soft bristled brush, neutral enzymatic cleaner or neutral detergent with a ph Prepare a solution using warm tap water and detergent or cleaner. Follow the enzymatic cleaner or detergent manufacturer s recommendations for use paying close
5 English US / PAGE 5 attention to the correct exposure time, temperature, water quality, and concentration. 2. Carefully wash the implant manually. Do not use steel wool or abrasive cleaners on implants. 3. Rinse implant thoroughly with DI or purified water. Use DI or purified water for final rinse. 4. Dry the implant using a clean, soft, lint-free cloth to avoid scratching the surface. Ultrasonic Processing Equipment: Ultrasonic cleaner, neutral enzymatic cleaner or neutral detergent with a ph 8.5. Note: Ultrasonic cleaning may cause additional damage to implants that have surface damage. 1. Prepare a solution using warm tap water and detergent or cleaner. Follow the enzymatic cleaner or detergent manufacturer s recommendations for use paying close attention to the correct exposure time, temperature, water quality, and concentration. 2. Clean implants ultrasonically for a minimum of 15 minutes. 3. Rinse implant thoroughly with DI or purified water. Use DI or purified water for final rinse. 4. Dry the implant using a clean soft, lint-free cloth to avoid scratching the surface. Mechanical Processing Equipment: Washer/disinfector, neutral enzymatic cleaner or neutral detergent with a ph 8.5. Cycle Minimum Time (minutes) Minimum Temperature/Water Type of Detergent Pre-wash 2 Cold tap water N/A Enzyme Wash Wash II 5 Rinse 2 2 Warm tap water Warm tap water (> 40 C) Warm DI or purified water (> 40 C) Neutral enzymatic ph 8.5 Detergent with ph 8.5 Instrument Cleaning: Acumed Instruments and Accessories must be thoroughly cleaned before reuse, following the guidelines below: Warnings & Precautions Decontamination of reusable instruments or accessories should occur immediately after completion of the surgical procedure. Do not allow contaminated instruments to dry prior to cleaning/ reprocessing. Excess blood or debris should be wiped off to prevent it from drying onto the surface. All users should be qualified personnel with documented evidence of training and competency. Training should be inclusive of current applicable guidelines and standards and hospital policies. Do not use metal brushes or scouring pads during manual cleaning process. N/A Dry C N/A
6 English US / PAGE 6 Use cleaning agents with low foaming surfactants for manual cleaning in order to see instruments in the cleaning solution. Cleaning agents must be easily rinsed from instruments to prevent residue. Mineral oil or silicone lubricants should not be used on Acumed instruments. Neutral ph enzymatic and cleaning agents are recommended for cleaning reusable instruments. It is very important that alkaline cleaning agents are thoroughly neutralized and rinsed from instruments. Surgical instruments must be dried thoroughly to prevent rust formation, even if manufactured from high grade stainless steel. All instruments must be inspected for cleanliness of surfaces, joints, and lumens, proper function, and wear and tear prior to sterilization. Anodized aluminum must not come in contact with certain cleaning or disinfectant solutions. Avoid strong alkaline cleaners and disinfectants or solutions containing iodine, chlorine or certain metal salts. Also, in solutions with ph values above 11, the anodization layer may dissolve. Manual Cleaning/Disinfection Instructions 1. Prepare enzymatic and cleaning agents at the use-dilution and temperature recommended by the manufacturer. Fresh solutions should be prepared when existing solutions become grossly contaminated. 2. Place instruments in enzymatic solution until completely submerged. Actuate all moveable parts to allow detergent to contact all surfaces. Soak for a minimum of twenty (20) minutes. Use a nylon soft bristled brush to gently scrub instruments until all visible debris is removed. Pay special attention to hard to reach areas. Pay special attention to any cannulated instruments and clean with an appropriate bottle brush. For exposed springs, coils, or flexible features: Flood the crevices with copious amounts of cleaning solution to flush out any soil. Scrub the surface with a scrub brush to remove all visible soil from the surface and crevices. Bend the flexible area and scrub the surface with a scrub brush. Rotate the part while scrubbing to ensure that all crevices are cleaned. 3. Remove the instruments and rinse thoroughly under running water for a minimum three (3) minutes. Pay special attention to cannulations, and use a syringe to flush any hard to reach areas. 4. Place the instruments, fully submerged, in an ultrasonic unit with cleaning solution. Actuate all moveable parts to allow detergent to contact all surfaces. Sonicate the instruments for a minimum of ten (10) minutes. 5. Remove the instruments and rinse in deionized water for a minimum of three (3) minutes or until all signs of blood or soil are absent in the rinse stream. Pay special attention to cannulations, and use a syringe to flush any hard to reach areas. 6. Inspect instruments under normal lighting for the removal of visible soil. 7. If visible soil is seen, repeat the sonication and rinse steps above. 8. Remove excess moisture from the instruments with a clean, absorbent, nonshedding wipe.
7 English US / PAGE 7 Combination Manual/Automated Cleaning and Disinfecting Instructions 1. Prepare enzymatic and cleaning agents at the use-dilution and temperature recommended by the manufacturer. Fresh solutions should be prepared when existing solutions become grossly contaminated. 2. Place instruments in enzymatic solution until completely submerged. Actuate all moveable parts to allow detergent to contact all surfaces. Soak for a minimum of ten (10) minutes. Use a nylon soft bristled brush to gently scrub instruments until all visible debris is removed. Pay special attention to hard to reach areas. Pay special attention to any cannulated instruments and clean with an appropriate bottle brush. Note: Use of a sonicator will aid in thorough cleaning of instruments. Using a syringe or water jet will improve flushing of difficult to reach areas and any closely mated surface. 3. Remove instruments from enzyme solution and rinse in deionized water for a minimum of one (1) minute. 4. Place instruments in a suitable washer/ disinfector basket and process through a standard washer/disinfector cycle. The following minimum parameters are essential for thorough cleaning and disinfection. Step Description 1 Two (2) minute prewash with cold tap water 2 Twenty (20) second enzyme spray with hot tap water 3 One (1) minute enzyme soak 4 Fifteen (15) second cold tap water rinse (X2) 5 Two (2) minute detergent wash with hot tap water (64-66 C/ F) 6 Fifteen (15) second hot tap water rinse 7 Ten (10) second purified water rinse with optional lubricant (64-66 C/ F) 8 Seven (7) minute hot air dry (116 C/240 F) Note: Follow washer/disinfector manufacturer s instructions explicitly Automated Cleaning/Disinfection Instructions Automated washer/dryer systems are not recommended as the only cleaning method for surgical instruments. An automated system may be used as a follow up process to manual cleaning. Instruments should be thoroughly inspected prior to sterilization to ensure effective cleaning.
8 English US / PAGE 8 STERILITY: System components may be provided sterile or nonsterile. Sterile Product: Sterile product was exposed to a minimum dose of 25.0-kGy gamma irradiation. Acumed does not recommend resterilization of sterile-packaged product. If sterile packaging is damaged, the incident must be reported to Acumed. The product must not be used and must be returned to Acumed. Non-Sterile Product: Unless clearly labeled as sterile and provided in an unopened sterile package provided by Acumed, all implants and instruments must be considered nonsterile and sterilized by the hospital prior to use. Follow current AORN Recommended Practices for Sterilization in Perioperative Practice Settings and ANSI/AAMI ST79:2010 Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Consult your equipment manufacturer s written instructions for specific sterilizer and load configuration instructions. Gravity-displacement steam sterilization is not recommended. Flash sterilization is not recommended. Prevacuum Steam Sterilizer Parameters 1 Tray Case Part Numbers: , Condition: Preconditioning Vacuum Pulses: 3 Wrapped Exposure Temperature: 270 o F (132 o C) Exposure Time: Dry Time: Cooling Time 2 : 4 minutes 30 minutes 30 minutes 1 Values in this table reflect the minimum parameters validated to achieve the required Sterility Assurance Level (SAL), for a fully loaded tray with all parts placed appropriately. 2 Cooling time describes the validated interval following dry time and prior to handling. This interval is included for safe handling and the prevention of contamination; see ANSI/AAMI ST79:2010 Section
9 English US / PAGE 9 STORAGE INSTRUCTIONS: Store in a cool dry place and keep away from direct sunlight. Prior to use, inspect product package for signs of tampering or water contamination. Use oldest lots first. APPLICABILITY: These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained on these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way which is not authorized under the laws and regulations of the country where the reader is located. FURTHER INFORMATION: To request further material, please see the contact information listed on this document. SYMBOL LEGEND Consult instructions for use Caution Sterilized using ethylene oxide Sterilized using irradiation Use-by date Catalogue number Batch code Authorized representative in the European Community Manufacturer Date of manufacture Do not resterilize Do not re-use Upper limit of temperature Caution: U.S. Federal law restricts this device to sale by or on the order of a physician. For Professional Use Only.
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac.
 Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Recommendations for cleaning, disinfection and sterilization of orthopaedic instruments and devices from Swemac. This document is intended as guidance for decontamination and sterilization for medical
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
TTA Wedge System INSTRUCTIONS FOR USE
 TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
TTA Wedge System INSTRUCTIONS FOR USE 1 INSTRUCTIONS FOR USE The OssAbility TTA Wedge System consists of the following products: Wedge Implants Osteotomy Guide Advancement Levers Osteotomy Planning Overlay
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Failure to follow instructions may lead to patient injury.
 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
Zavation Z-Link Cervical
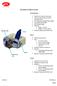 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
DARCO HEADED CANNULATED SCREWS
 DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO HEADED CANNULATED SCREWS 151677-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
Important Information on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only
 0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
How clean are your instruments?
 How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
How clean are your instruments? Pre cleaning & preparation Samuel Morais Technical Director January 25 th 2015 Overview 1. Correct Preparation will result on good cleaning results that are visible and
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
XYcor Expandable Spinal Spacer System
 XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
XYcor Expandable Spinal Spacer System GENERAL INFORMATION: The XYcor Expandable Spinal Spacer System (XYcor System) is an intervertebral body fixation system consisting of implants with various widths,
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
Cannulated Screw System. Product Overview
 Cannulated Screw System Product Overview Acumed Cannulated Screw System The Acumed Cannulated Screw System consists of screws, washers, and instruments and is intended for fixation of fractures, fusions,
Cannulated Screw System Product Overview Acumed Cannulated Screw System The Acumed Cannulated Screw System consists of screws, washers, and instruments and is intended for fixation of fractures, fusions,
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Tension Band Pin System 2. Surgical Technique
 Tension Band Pin System 2 Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Tension Band Pin System 2 Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM
 STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
