THE LOCATOR OVERDENTURE IMPLANT SYSTEM.
|
|
|
- Dayna Morrison
- 5 years ago
- Views:
Transcription
1
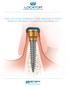
2 THE LOCATOR OVERDENTURE IMPLANT SYSTEM. FOUR DECADES OF ATTACHMENT KNOWLEDGE INCORPORATED INTO NARROW DIAMETER OVERDENTURE IMPLANTS. The LOCATOR Overdenture Implant (LODI) System is comprised of 2.4 and 2.9mm narrow diameter dental implants (available in 10, 12 and 14mm lengths) with a detachable LOCATOR Abutment that is available in a 2.5 and 4mm cuff height. The LODI is used to restore masticatory function for the patient and may be suitable for immediate function if sufficient primary stability of the implant is achieved at the time of placement. The final treatment option may be determined at the time of surgery as the clinicians must consider the quality of supporting bone and initial insertion torque values of the implants. Immediate function is determined on a case-by-case basis and at the discretion of the clinicians. IMPORTANT: THIS DOCUMENT CONTAINS THE MOST CURRENT INSTRUCTIONS FOR USE. PLEASE READ AND RETAIN.
3 THE LOCATOR OVERDENTURE IMPLANT (LODI) SYSTEM
4
5 TABLE OF CONTENTS LOCATOR OVERDENTURE IMPLANT (LODI) SYSTEM OVERVIEW LODI DIMENSIONS LOCATOR STANDARD & EXTENDED RANGE INSERTS LOCATOR 3-IN-1 CORE TOOL DRILLING DEPTH CONTROL & SEQUENCE EXAMPLES 6 Drill Laser Depth Markings 6 Drill Stops 6 Placement of a 2.4mm x 12mm Implant Flapless Surgical Procedure 7 Final Drill Diameter & Depth for Various Bone Types 7 Placement of a 2.9mm x 12mm Implant Flapless Surgical Procedure PRE-OPERATIVE TREATMENT PLANNING IMPLANT PLACEMENT LODI HEALING ABUTMENT & LOCATOR ABUTMENT PLACEMENT PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE 15 Direct Technique: Chairside Processing 18 Indirect Technique: Laboratory Processing 19 Bite Records 19 Laboratory Step 21 Indications 21 Contraindications 21 Caution 21 Storage and Handling 21 Delivery 21 Single Use Devices 21 Sterilization 20 Denture Try In 20 Laboratory Step 20 Prosthesis Delivery IMPORTANT INFORMATION ABOUT THE LODI SYSTEM & SURGICAL INSTRUMENTATION 21 Cleaning Instructions for Instruments & Individually Packaged Replacement Attachments 22 Surgical Tray Cleaning Instructions 22 Inspection and Maintenance of Cleaned Instruments 22 Steam Sterilization Instructions 23 Torque Indicating Ratchet Wrench Cleaning Procedures 24 Warnings and Precautions OVERDENTURE INSERTION, REMOVAL, & CLEANING GUIDELINES FOR THE CLINICIAN AND PATIENT 25 Inserting and Removing an Overdenture 25 Cleaning an Overdenture 25 Additional Notes of Caution EXPLANATION OF SYMBOLS ON OUTER PACKAGING LABELS ZEST ANCHORS WARRANTY 1
6 THE LOCATOR OVERDENTURE IMPLANT (LODI) SYSTEM OVERVIEW The LOCATOR Insert self-aligns and pivots inside the denture cap providing a genuine resilient connection that holds-up to patient mastication forces while providing attachment durability The LOCATOR Abutment s dramatically reduced vertical height provides patient comfort when the denture is removed, as well as increased denture strength as compared to O-Ball attachments ALL INCLUSIVE PACKAGE CONTAINS 1 Implant 1 LOCATOR Abutment 1 LODI Processing Pack Each Processing Pack has what you need to select retention levels and address draw correction; improving ease of denture placement and removal LOCATOR Abutment is available in 2.5 and 4mm cuff heights for implant placement flexibility, attachment interchangeability and replacement should wear occur through time Proven RBM roughened surface on entire length of the implant Progressive thread design that widens at the coronal portion providing primary stability when immediate loading may be indicated Cuff Heights 2.5 or 4mm Available in 2.4 and 2.9mm diameters and 10, 12 or 14mm lengths for placement in all ridges Self-tapping design for ease of implant insertion and increased implant stability Implant Diameters 2.4 or 2.9mm INCLUDED IN THE PROCESSING PACK LODI Male Processing Pack Denture Attachment Housing Extended Range Insert Low Retention Standard Insert Low Retention Standard Insert Medium Retention Block-Out Spacer 2
7 LODI DIMENSIONS INTER-PROXIMAL SPACE (ZEST RECOMMENDS 7MM BETWEEN IMPLANTS) 6.5MM 4.85MM RESTORATIVE SPACE CUFF HEIGHT (2.5 & 4MM) LENGTH (10, 12,14MM) HEALING CAP CUFF HEIGHT (3 & 4MM) 3
8 LOCATOR STANDARD & EXTENDED RANGE INSERTS THE MAGIC IS IN THE PIVOT, IT ALLOWS FOR A RESILIENT CONNECTION OF THE PROSTHESIS AND PREVENTS DAMAGE TO INSERTS DURING INSERTION. STANDARD INSERTS Dual retention to maximize stability and pivoting action that accommodates up to 20 divergence between two implants. EXTENDED RANGE INSERTS Pivoting action accommodates up to 40 of total divergence between two implants. Low Retention Medium Retention High Retention Zero Retention Low Retention Medium Retention High Retention 4
9 LOCATOR 3-IN-1 CORE TOOL This convenient tool is used to carry and place the LOCATOR Abutment onto the implant, and for removal and insertion of the Inserts from, and into the Denture Attachment Housing. In order to achieve 30Ncm of torque, the Abutment Driver portion of the tool is compatible with various types of insert drivers. REMOVAL TOOL INSERTION TOOL ABUTMENT DRIVER & SLEEVE REMOVAL The Insert Removal Tool has a sharp edge on the end to engage and remove the insert from the Denture Attachment Housing. INSERTION The Insert Seating Tool is used to seat the LOCATOR Insert. PLACEMENT The Abutment Driver with the Abutment Holder Sleeve carries the Abutment securely and places it onto the implant. NOTE THE GAP ONCE TURNED COUNTER CLOCKWISE Loosen the Insert Removal Tool a full 3 turns counter clockwise (you will see a visible gap). To remove a LOCATOR Insert from the Attachment Housing; simply insert the tip into the Housing/Insert assembly and push straight into the bottom of the Insert. Then, tilt the tool so that the sharp edge of the tip will grab hold of the male and pull it out of the Denture Attachment Housing. To disengage the Insert from the tip of the Core Tool; point the tool down and away from you and tighten the Removal Tool clockwise back onto the Core Tool. This will activate the removal pin and disengage the Insert from the tip end of the Removal Tool. Separate the Removal Tool section from the LOCATOR Core Tool and use the Seating Tool end of the remaining two sections to place a new Insert into the empty Denture Attachment Housing. 5
10 DRILLING DEPTH CONTROL & SEQUENCE EXAMPLES DRILL LASER DEPTH MARKINGS DRILL STOPS 6mm Depth 8mm Depth 10mm Depth 12mm Depth 14mm Depth PLACEMENT OF A 2.4MM X 12MM IMPLANT FLAPLESS SURGICAL PROCEDURE 2.4MM LASER DEPTH MARKINGS 2.4MM DRILL STOPS 2mm Soft Tissue 1.2mm Pilot 14mm Line 1.6mm 14mm Line 2.1mm 14mm Line Place at Low Speed Ratchet to 1.2mm Pilot 12mm Drill Stop 1.6mm 12mm Drill Stop 2.1mm 12mm Drill Stop Place at Low Speed Ratchet to D2/D3/D4 BONE TYPE D2/D3/D4 BONE TYPE 2mm Soft Tissue 6 1.2mm Pilot 14mm Line 1.6mm 4mm Short 10mm Line Place at Low Speed Ratchet to 1.2mm Pilot 12mm Drill Stop 1.6mm 4mm Short 8mm Drill Stop EXTERNAL IRRIGATION IS REQUIRED DURING THE DRILLING STEPS Place at Low Speed Ratchet to
11 DRILLING DEPTH CONTROL & SEQUENCE EXAMPLES (CONTINUED) FINAL DRILL DIAMETER & DEPTH FOR VARIOUS BONE TYPES BONE TYPE 2.4MM IMPLANT DIAMETER FINAL DRILL DIAMETER DRILL DEPTH 2.9MM IMPLANT DIAMETER FINAL DRILL DIAMETER FINAL DRILL DIAMETER D1 2.1mm 2.4mm D2 / D3 / D4 1.6mm Depth 4mm < Implant Length 2.1mm Depth 4mm < Implant Length Bone type is a general classification. The overall bone quality must be assessed by the clinician through treatment planning and at the time of surgery in order to create the appropriate osteotomy size to achieve the desired insertion torque. PLACEMENT OF A 2.9MM X 12MM IMPLANT FLAPLESS SURGICAL PROCEDURE 2.9MM LASER DEPTH MARKS 2.9MM DRILL STOPS 2mm Soft Tissue 1.2mm Pilot 14mm Line 1.6mm 14mm Line 2.4mm 14mm Line Place at Low Speed Ratchet to 1.2mm Pilot 12mm Drill Stop 1.6mm 12mm Drill Stop 2.4mm 12mm Drill Stop Place at Low Speed Ratchet to D2/D3/D4 BONE TYPE D2/D3/D4 BONE TYPE 2mm Soft Tissue 1.2mm Pilot 14mm Line 1.6mm 14mm Line 2.1mm 4mm Short 10mm Line Place at Low Speed Ratchet to 1.2mm Pilot 12mm Drill Stop 1.6mm 12mm Drill Stop 2.1mm 4mm Short 8mm Drill Stop EXTERNAL IRRIGATION IS REQUIRED DURING THE DRILLING STEPS Place at Low Speed Ratchet to 7
12 PRE-OPERATIVE TREATMENT PLANNING Evaluate available bone width for implant positions by using the index finger/thumb technique or a ridge mapping instrument (which can be purchased through most dental instrument companies). Measure gingiva height at each planned implant location using a period probe to determine the proper LOCATOR Abutment cuff height. 3A-3B A panoramic radiograph or CBCT with radiographic markers may be used to evaluate the bone topography and determine the appropriate implant positions. A B 8
13 PRE-OPERATIVE TREATMENT PLANNING (CONTINUED) Radiographic overlay templates are available from ZEST Anchors (L7012) to assist in choosing the correct implant size. ZEST recommends placement of the LOCATOR Overdenture Implants where patients have at least 1mm of available bone around the circumference of the implant. Determine if the patient s existing denture(s) will be used or if new ones will be fabricated. If a new denture is fabricated, follow the standard denture fabrication protocols. Instruct the patients to wear the new denture for a minimum of two weeks prior to implant placement. Optional: A surgical guide for implant placement may be fabricated prior to surgery. 9
14 IMPLANT PLACEMENT After patient selection and evaluation protocols have been completed, determine the number of implants required and discuss all treatment options with the patient. ZEST Anchors recommends a minimum of four implants to be placed in the mandible and six in the maxilla. MANDIBULAR PLACEMENT OF FOUR 2.9MM X 10MM IMPLANTS SHOWN IN TYPE D1 BONE Using a surgical guide or by free hand, mark the implant osteotomy locations using the 1.2mm Pilot Drill (07366) to drill through the gingiva and into the bone crest 6mm. Note the gingival height. The recommended drilling speed is rpm. FPO 6 MM 5 MM 4 MM 3 MM 2 MM 1 MM Remove the gingival cores at each site using the Rotary Tissue Punch (07373). Place the guide pin portion of the Rotary Tissue Punch into the pilot drill osteotomy and advance the drill unit to cut away the gingiva. Advance the Rotary Tissue Punch to the laser depth mark corresponding to the depth measurement. The recommended drilling speed is up to a maximum of 800rpm. 10
15 IMPLANT PLACEMENT (CONTINUED) MANDIBULAR PLACEMENT OF FOUR 2.9MM X 10MM IMPLANTS SHOWN IN TYPE D1 BONE (CONTINUED) 3A-3B Place the 1.2mm diameter (small) end of the Direction Indicator (07365) into the pilot drill osteotomies to verify the proper alignment. Attach the proper length Drill Stop onto the 1.2mm Pilot Drill according to the desired drilling depth. A Alternatively, drill to the proper laser depth marking on the drill calculated by adding the desired drilling and tissue depths. The recommended drilling speed is rpm. Continue osteotomy preparation to the desired depth at each implant site. B 4A-4B Place the proper length Drill Stop onto the 1.6mm drill according to the desired drilling depth. A Alternatively, drill to the proper laser depth marking on the drill calculated by adding the desired drilling and tissue depths. The recommended drilling speed is rpm. Continue osteotomy preparation to the desired depth at each implant site. B 11
16 IMPLANT PLACEMENT (CONTINUED) MANDIBULAR PLACEMENT OF FOUR 2.9MM X 10MM IMPLANTS SHOWN IN TYPE D1 BONE (CONTINUED) 5A-5B Place the 1.6mm diameter (large) end of the Direction Indicator into the osteotomies to verify proper alignment. Place the proper length Drill Stop onto the 2.4mm drill (0736) according to the desired drilling depth. A Alternatively, drill to the corresponding laser depth marking on the drill calculated by adding desired drilling and tissue depths. The recommended drilling speed is rpm. Continue osteotomy preparation to the desired depth at each implant site. B A B 7A-7B 6A-6B Remove the implant package from the box and peel back the tyvek seal from the plastic tray. Place the sterile implant vial on the sterile tray. The contents of the plastic tray are sterile and should only contact components within the sterile field. Remove the cap from the implant vial and do not discard. The LOCATOR Abutment is included in the cap. Set the drilling unit speed at 30rpm and the placement torque at 35Ncm. Place the Implant Latch Driver (07357) in the handpiece. Seat it onto the hex on the top of the implant and press down to engage securely. The bottom of the driver should contact the abutment seating surface and fully engage the entire length of the implant hex. A 12 B
17 IMPLANT PLACEMENT (CONTINUED) A B MANDIBULAR PLACEMENT OF FOUR 2.9MM X 10MM IMPLANTS SHOWN IN TYPE D1 BONE (CONTINUED) 8A-8B Remove the implant from the vial. Carry the implant to the mouth, place it into the osteotomy and insert at 30rpm. Use Latch Driver to drive the implant three quarters (3/4) of the way into the osteotomy and finalize insertion with a Torque Indicating Ratchet Wrench (07362). Warning: Do not use an implant that comes into contact with any non-sterile area. Replace with a new sterile implant. 9A-9B Assemble the Torque Ratchet Wrench Insert (08926) and the Torque Wrench (07362) to finalize seating. Short and long Implant Drivers are available in the surgical kit. A B FPO 10 Engage the Implant Driver onto the hex on the top of the implant and verify that it is fully engaged. Slowly ratchet the implant to full depth. If final seating torque measures 30Ncm or above, the implant may be placed into immediate function at the discretion of the clinician, with the patient adhering to recommended post-surgical hygiene and care protocols. If the final seating torque measures below 30Ncm, relieve the denture acrylic and place a soft liner in the denture around the LOCATOR Abutments during the implant integration period. If 70Ncm of torque is reached prior to full seating, the implant should be removed and the osteotomy should be enlarged. 13
18 LODI HEALING ABUTMENT & LOCATOR ABUTMENT PLACEMENT LODI HEALING ABUTMENT PLACEMENT 1 Optional: If the implant does not reach a final seating torque of 30Ncm, a LODI Healing Cap (07339 or 07340) is available. Use a inch (1.25mm) Hex Driver and thread the Healing Cap with the appropriate cuff height on the implant until finger tight. Relieve the denture acrylic and place a soft liner in the denture around the LODI Healing Caps during the implant integration period. LOCATOR ABUTMENT PLACEMENT 2A-2B Open the flip cap on the top of the vial cap and remove the LOCATOR Abutment. Place the Abutment Holder Sleeve onto the LOCATOR Abutment Driver and insert into the tri-lobe channel of the LOCATOR Abutment. A B A 3A-3B Thread the LOCATOR Abutment onto the implant until finger tight. If the implant placement torque was 30Ncm or greater, the Abutments may be tightened to the recommended torque level of 30Ncm. If the implant placement torque did not reach 30Ncm, the Abutment should only be hand tightened. Assemble the LOCATOR Abutment Torque Driver Insert and the Torque Ratchet Wrench and torque the attachments to 30Ncm. B 4 If the implant placement torque was 30Ncm or greater, follow the steps for processing the LOCATOR Denture Attachment Housings and Inserts into the denture. If the implant placement torque was less than 30Ncm, relieve the denture acrylic and place a soft liner in the denture around the LOCATOR Abutments during the integration period. 14
19 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE DIRECT TECHNIQUE: CHAIRSIDE PROCESSING (NEW OR EXISTING DENTURE) Torque the LODI Abutments to 30Ncm using an assembled Torque Ratchet Wrench and Insert. Place a White Block Out Spacer Ring around each Abutment and press it down to the tissue. Place a Denture Attachment Housing with a Black Processing Insert inside of it and place the Housing/ Insert assembly onto each Abutment, pressing down firmly. Apply fit check marking paste inside of the denture. Insert it into the mouth in position over the Denture Attachment Housings to mark areas where the denture will need to be relieved to allow space for the Attachment Housing to be picked up. Relieve the areas marked with a CHAIRSIDE Recess Bur (09756). ZEST recommends using slight pressure to get the tip of the Bur started followed by a straight downward motion to create the desired recess site. This efficient Bur has distinct depth landmarks which indicate where to stop when drilling for the Denture Attachment Housing. 15
20 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE (CONTINUED) A DIRECT TECHNIQUE: CHAIRSIDE PROCESSING (CONTINUED) 5A-5B Use the CHAIRSIDE Undercut Bur (09577) to cut an undercut around the circumference of the relief areas for mechanical retention. Cut lingual/palatal vent windows in the denture with the CHAIRSIDE Vent Bur (09578) to visualize full seating and for an excess material vent. B A Dry the Denture Attachment Housings. Apply a small amount of CHAIRSIDE Attachment Processing Material (09566) around the circumference of each cap. Place CHAIRSIDE Material into the relief areas of the denture and seat it over the Housings and onto the tissue. Maintain the denture in a passive condition, without compression of the soft tissue while the acrylic sets. Excessive occlusal pressure during the setting time may cause tissue recoil against the denture base and could contribute to dislodging and wear of the Inserts. The set time of the material from the time the material is mixed, to the time it is set is five (5) minutes; working time is one (1) minute, forty five (45) seconds and set time is three (3) minutes, fifteen (15) seconds. 7A-7B Disengage the denture from the attachments and remove from the mouth. Verify that the Denture Attachment Housings have been securely processed into the denture. Fill any voids and light cure. The material will bond to itself and will cure within 30 seconds with light application. Use the CHAIRSIDE Trim (09579) and Grind Burs (09583) to remove any excess acrylic material remaining in the denture. B 16
21 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE (CONTINUED) 8 Use the CHAIRSIDE Polish Bur (09580) to create a smooth finish in and round the denture. FPO 9 See the LOCATOR 3-In-1 Core Tool instructions on page (5). Remove the Black Processing Insert using the Removal Tool. Place the selected final Insert into each Denture Attachment Housing using the Insertion Tool. Insert the lowest retentive option during try-in. A B FPO 10 Seat the denture and press down to engage the Insert on the LOCATOR Abutments and verify the occlusion. Instruct the patient on how to remove and insert the denture. If the retention is not satisfactory, remove the Inserts and replace with the next level of retention. See the Insert retention chart on page (4). Instruct the patient on proper home care maintenance and required recall visits. INDIRECT TECHNIQUE: LABORATORY PROCESSING FPO Torque the LOCATOR Abutments to 30Ncm using an assembled Torque Ratchet Wrench and Insert. 17
22 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE (CONTINUED) A stock or custom impression tray may be used. Ensure that each recess has enough space for the height of the LOCATOR Impression Copings (08505). 4mm Place a LOCATOR Impression Coping on each Abutment and press down firmly. Syringe a medium body impression material around the circumference of each coping. Fill the impression tray and insert it over the copings and onto the tissue. Allow the material to set. Remove the impression and verify that there are no draws in the impression. Press the LOCATOR Analogs (08530) into each Impression Coping and send the impression to the laboratory. INDIRECT TECHNIQUE: LABORATORY PROCESSING Verify that the Analogs are secure in the Impression Copings and pour a model. 18
23 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE (CONTINUED) Fabricate the baseplate and wax rim on the cast for the bite registration. The Denture Attachment Housings with Black Processing Inserts may be processed into the baseplate to provide stabilization during record making and try-in. BITE RECORDS Place the bite bloc into the mouth and record the jaw relation. Take an impression of the opposing arch and pour the cast. Select a shade for the denture teeth. LABORATORY STEP Articulate the models and proceed with the denture teeth set up. DENTURE TRY IN Place the try-in denture into the mouth and verify the fit, attachment engagement, esthetics, phonetics, and occlusion. 19
24 PROCESSING LOCATOR DENTURE ATTACHMENT HOUSINGS & INSERTS INTO THE DENTURE LABORATORY STEP Finalize and flask the denture for processing. Separate the flask and boil away all wax. Place the Denture Attachment Housings with Black Processing Inserts on the Analogs and press down firmly. Place the cast back into the flask and verify that there is no contact with the teeth. Close the flask and process the denture. Remove the denture from the flask, finish, and polish. 2A-2B See the LOCATOR 3-In-1 Core Tool instructions on page (5). Remove the Black Processing Insert using the Insert Removal Tool. Place the selected final Insert into each Denture Cap using the Insertion Tool. Insert the lowest retentive option during try-in. See the Insert retention chart on page (4). A B PROSTHESIS DELIVERY Seat the denture in the mouth and press down to engage the males on the LOCATOR Abutments and verify the occlusion. Instruct the patient on how to remove and seat the denture. If the retention is not satisfactory, remove the Inserts and replace with the next level of retention. See the Insert retention chart on page (4). Instruct the patient on proper home care maintenance and required recall visits. 20
25 IMPORTANT INFORMATION ABOUT THE LODI SYSTEM & SURGICAL INSTRUMENTATION INDICATIONS The LOCATOR Overdenture Implant System is designed to retain overdentures or partial dentures in the mandible or maxilla. CONTRAINDICATIONS Not appropriate where a totally rigid connection is required. Use of a single implant with divergence greater than 20 is not recommended. Dental implants should not be used in patients with serious medical problems or in a poor general state of health. Patients with medical problems such as: uncontrolled bleeding disorders, drug or alcohol abuse, weakened immune system, titanium allergy or uncontrollable endocrine disorders should be carefully evaluated prior to treatment. CAUTION Federal (U.S.A.) law restricts this device to sale by or on the order of a licensed dentist. STORAGE AND HANDLING The LOCATOR Overdenture Implant System in its undamaged, original packaging is not subject to any special considerations for storage or handling (during transport and storage). Only sterile titanium or stainless instruments/tools should be used to handle and deliver the implant to the surgical site. SINGLE-USE DEVICES The LOCATOR Overdenture Implant System is a single-use device. LOCATOR Overdenture Implant: A previously used LOCATOR Overdenture Implant could contain patient contamination build-up. Therefore, the inadvertent re-use of this device could result in infection leading to lack of integration (of the implant to the bone). LOCATOR Inserts: The inadvertent re-use of LOCATOR nylon Inserts could cause loss of retention of the overdenture due to wear from previous use or damage during removal with the LOCATOR Core Tool. LOCATOR Abutments: The inadvertent re-use of LOCATOR Abutments could contain patient contamination build-up and subsequent wear of the retention bands. This would result in the device to perform with improper fit and function which would result in loss of retention of the prosthesis. STERILIZATION The LOCATOR Overdenture Implant is packaged with the LOCATOR Attachment and together are supplied STERILE (subjected to radiation (gamma) as a means of sterilization). All other restorative components, instruments, and replacement LOCATOR Abutments (sold separately) are supplied NON-STERILE. The nylon Inserts may be sterilized/disinfected using a liquid chemical sterilant. In order to ensure that the nylon Inserts are sterilized/disinfected (all microorganisms including Clostridium sporogenes and Bacillus subtilis spores are eliminated), the nylon Inserts must be soaked for a minimum of 3 hours in the liquid sterilant at room temperature. Note: An FDA approved liquid chemical sterilant for critical devices that are heat-sensitive and incompatible with sterilization methods such as steam and gas/vapor/plasma low temperature processes may be used following the manufacturer s directions for the sterilization (not just high-level disinfection) of the device. CLEANING INSTRUCTIONS FOR INSTRUMENTS AND INDIVIDUALLY PACKAGED REPLACEMENT ATTACHMENTS Disassemble any instruments that can be disassembled according to manufacturers instructions. Soak instruments in enzymatic cleaning solution (mixed according to manufacturers instructions) by completely submerging them for 20 minutes. Scrub instruments using a soft-bristled, nylon brush until all soil has been removed. Remove the instruments from the enzymatic cleaning solution and rinse in tap water for a minimum of 3 minutes. Make sure to thoroughly flush internal holes/crevices of instruments (such as the tissue punch, drill extender, implant drivers, and disassembled core tool and ratchet torque wrench) that have difficult to reach areas. Note: Use of a syringe or water jet will improve flushing of difficult to reach areas. 21
26 IMPORTANT INFORMATION ABOUT THE LODI SYSTEM & SURGICAL INSTRUMENTATION (CONTINUED) CLEANING INSTRUCTIONS FOR INSTRUMENTS AND INDIVIDUALLY PACKAGED REPLACEMENT ATTACHMENTS (CONTINUED) Place instruments in sonication bath (with enzymatic cleaning solution prepared according to manufacturers instructions) making sure that they are completely submerged, and sonicate for 10 minutes. Remove the instruments from the sonication bath, and rinse for 3 minutes making sure to thoroughly flush cleaning solution out of the holes/crevices and/ or difficult to reach areas. Remove excess moisture from the instruments with a clean, absorbent, and non-shedding wipe. SURGICAL TRAY CLEANING INSTRUCTIONS Rinse the tray and tray insert with tap water. Place the Surgical Tray and Insert in enzymatic cleaning solution (mixed according to manufacturers instructions) and wipe off soil with a clean, absorbent, non-shedding wipe. Allow the Surgical Tray and Insert to soak in the cleaning solution for 20 minutes making sure that they are completely submerged. Remove the Surgical Tray and Insert from the enzymatic cleaning solution and rinse in tap water for a minimum of 3 minutes. Make sure to thoroughly flush each piece to completely remove cleaning solution residue. INSPECTION AND MAINTENANCE OF CLEANED INSTRUMENTS Carefully inspect each instrument to ensure that all visible contamination has been removed. If contamination is noted, repeat the cleaning process. Please note that if during inspection of instruments, you see signs of wear, damage, or unrecognizable color change, replace the instrument. Reassemble multi-part instruments and check them for proper function (LOCATOR Core Tool and the Torque Indicating Ratchet Wrench) Reference the IFU that comes with each of these parts and subsequent sections of this document for the proper assembly process. STEAM STERILIZATION INSTRUCTIONS Wrap the components using a wrap that is FDAcleared for the indicated cycles. All components and instruments are supplied NON-STERILE. Place all instruments into the surgical tray. For gravity cycle, place Surgical Kit in a 10 x 15 Autoclave Bag, and for Pre-Vacuum Cycle double wrap the kit with autoclave wrap material and secure wrap with autoclave tape. Note: LOCATOR Core Tool and Torque Indicating Ratchet Wrench should be disassembled prior to steam sterilization. Remove excess moisture from the Surgical Tray and Insert with a clean, absorbent, and non-shedding wipe. AUTOCLAVE STERILIZATION PARAMETERS CYCLE TYPE PART NUMBER DESCRIPTION TEMPERATURE EXPOSURE TIME DRYING TIME GRAVITY PRE-VACUUM STANDARD SURGICAL KIT PREMIUM SURGICAL KIT STANDARD SURGICAL KIT PREMIUM SURGICAL KIT 132 C / 270 F 132 C / 270 F 132 C / 270 F 132 C / 270 F 15 MINUTES 25 MINUTES 4 MINUTES 4 MINUTES 30 MINUTES 30 MINUTES 20 MINUTES 20 MINUTES 22
27 IMPORTANT INFORMATION ABOUT THE LODI SYSTEM & SURGICAL INSTRUMENTATION (CONTINUED) TORQUE INDICATING RATCHET WRENCH CLEANING PROCEDURES Intended Use: A dental torque wrench for placement and adjustment of dental implants, attachments, attachment screws and prosthetic screws during oral surgery and prosthetic procedures. Scale Unit: Ncm. WARNING: Device must be autoclaved prior to use. This device must not be cleaned using hydrogen peroxide. Cleaning: Press the driver to remove it from the head of the wrench, and remove the head by pressing a finger into the recess and gently pulling the head. The three separated parts are now ready for cleaning using the following procedure: Soak torque wrench parts in enzymatic cleaning solution (mixed according to manufacturer s instructions) by completely submerging it for 20 minutes. Scrub torque wrench parts using a soft bristled, nylon brush until all soil has been removed. Remove the torque wrench parts from the enzymatic cleaning solution and rinse in tap water for a minimum of 3 minutes. Place torque wrench parts in sonication bath (with the enzymatic cleaning solution prepared according to manufacturer s instructions) making sure they are completely submerged, and sonicate for 10 minutes. Sterilization: Autoclave/steam gravity sterilize for 25 minutes at 132 C, dry for 30 minutes. For pre-vacuum cycle, autoclave/steam sterilize for 4 minutes at 132 C with drying time of 20 minutes. Note: Drying times may vary according to load. After sterilization, attach the head of the wrench to the body by pushing the components together and turning them in opposite directions until there is an audible click. Push the driver into the wrench until there is an audible click. The arrow on the head of the wrench shows the direction in which the wrench is functioning. Turn the wrench in the direction of the arrow until the desired torque is achieved. WARNING: Before each use, make sure that the functionalities are intact and that the first line on the scale aligns with the arrow. The arm of the torque wrench must not go beyond the end of the scale, as this could result in inaccurate readings. If the torque wrench is used as an ordinary wrench, without using the torque scale, then it may not be subjected to a load of more than 80Ncm. WARNING: If overloaded, dropped, or mishandled, the wrench must no longer be used since correct function can no longer be guaranteed. Remove the torque wrench parts from the sonication bath, and rinse in water for 3 minutes making sure to thoroughly flush cleaning solution out of the holes/ crevices and/or difficult to reach areas. Remove excess moisture from the torque wrench parts with a clean, absorbent, and non-shedding wipe. 23
28 IMPORTANT INFORMATION ABOUT THE LODI SYSTEM & SURGICAL INSTRUMENTATION (CONTINUED) WARNINGS AND PRECAUTIONS The LOCATOR Overdenture Implant System has not been evaluated for safety and compatibility in the MR environment. The LOCATOR Overdenture Implant System has not been tested for heating or migration in the MR environment. Product (implant/attachment) from damaged sterilized packaging must not be used on patients. In the event that the sterilized packaging for the LOCATOR Overdenture Implant System is damaged, the damaged packaging (with the product) must be returned to the manufacturer and a replacement will be provided (if damage to sterilized packaging is caused by product shipment). The drill extender is to be used with surgical drills only and should not be used in high torque applications. Avoid application of excessive bending load on smaller diameter drills during drilling. Drills will dull based on many factors including bone density, handling, autoclave exposure, etc. Replace drills when wear is noticeable to avoid excessive heat being transferred to surrounding bone during osteotomy preparation. If the LOCATOR Overdenture Implant System is subjected to inappropriate loading conditions, there may be a potential risk of metal fatigue or localized bone failure. The use of other tissue grafting components or parts that are made from dissimilar metals should not be used in or near the implant. Patient evaluation including the determination of the general health, oral hygiene habits and status, motivation toward good dental care, and anatomic acceptability prior to implant surgery is critical. Thorough evaluation of the patient s medical status and health history is mandatory. Panoramic and periapical radiographs as well as thorough oral inspection and palpation are recommended to determine anatomic landmarks, dental pathology, and adequacy of bone. A cephalogram is suggested for totally edentulous patients. Any oral condition that adversely affects natural teeth, if uncorrected, will have an adverse effect on the implants. Periodontal disease, abnormal bone conditions, severe bruxism, cross-bite situations, and extenuating circumstances (e.g. excessive smoking, medical issues, etc) that may adversely affect the procedure must be evaluated and corrected if necessary, or use of the implant may be contraindicated. Based on the results of the patient s pre-surgical assessment, the clinician should select and order the appropriate implant (determine correct implant diameter and length based on bone type), restorative parts, and tools. Refer to Drilling Sequence section for further details. The clinician should also determine if the patient is allergic to any of the materials that will be used in the procedure as part of the pre-surgical treatment planning. If during patient evaluation, insufficient bone width, abnormal bone defects or contours are detected, then the placement of the implant may be contraindicated. Patient motivation is a key factor in achieving success with any implant. The patient must be willing to practice the oral hygiene necessary for implant maintenance. The clinician must provide the patient with information regarding proper care and maintenance of the implants. Also, they must inform the patient that conditions such as excessive smoking, improper/lack of maintenance may have adverse effects. The use of this or any surgical implant product requires that the clinician be thoroughly familiar with the product and the method for its use and application. They must also be familiar with all the instruments, and surgical procedures required (as described in this document). The clinician must also use reasonable judgment in deciding when and where to use the product. 24
29 OVERDENTURE INSERTION, REMOVAL, AND CLEANING GUIDELINES FOR THE CLINICIAN AND PATIENT To reduce wear on LOCATOR Abutments it is critical that clinicians and patients perform routine maintenance on both the LOCATOR Abutment, the Denture Attachment Housing and the Retention Insert. It is also important that patients understand the proper overdenture maintenance that should be performed at home to guard against retention loss of the Retention Inserts within the Denture Attachment Housing. The following are guidelines to consider. INSERTING AND REMOVING AN OVERDENTURE To insert the overdenture, the patient should ensure he/she can feel that it is properly positioned above the LOCATOR Abutments prior to applying pressure. The patient should use both hands and simultaneously press down on each side to firmly snap the overdenture into place. The patient should avoid biting the overdenture into place as this force will result in improper wear of the LOCATOR Abutment and may affect the longevity of the prosthesis. The patient should remove the overdenture by placing one thumb under the left edge and one finger under the right edge of the overdenture rim and pull one side upward and the other side downward, simultaneously. They may also use their tongue to aid in removal of the lower overdenture. Once the overdenture is removed, a thorough cleaning is recommended. CLEANING AN OVERDENTURE Maintaining proper hygiene is vital to the success of an overdenture, helping it last longer and functioning properly. Similar to natural teeth, dental plaque will also form on the surface of an overdenture. If the plaque is not removed it will continue to accumulate. It is for this reason that the overdenture should be taken out for cleaning daily. Patients should follow these two simple steps daily for cleaning an overdenture. Fill a washing basin with warm water to prevent fracture of the overdenture. Apply detergent onto a soft bristle toothbrush and thoroughly clean every surface of the overdenture. Before bed each night, remove the overdenture and immerse in a cup of plain cold water. ADDITIONAL NOTES OF CAUTION Failure of the patient to follow oral hygiene protocols and appropriately care for the overdenture may also result in inflamed tissue around the implant, leading to the development of peri-implantitis. Throughout time, peri-implantitis may cause the implant to become mobile and fail. Please ask patients to consider the following when caring for their overdentures: Avoid using abrasive toothpaste to clean the overdenture. The coarse particles in the toothpaste may scratch the surfaces of the overdenture, enhancing the potential for plaque accumulation. Chewing tobacco will get caught in the retention inserts and scratch the Abutments, considerably reducing the life of the Abutments, retentive features of the Retention Inserts and ultimately may affect the dental implants. Do not soak the overdenture in bleach or any other products not designed for use with denture cleaning as these can harm the retentive feature of the Retention Insert, which may ultimately cause additional wear on the Abutment. If a denture cleaning solution such as Polident and Efferdent must be used, do not soak the denture overnight. Fifteen minutes or less is all that is needed to clean the overdenture in such liquids. Brushing the Abutments increases wear; if the patient brushes their Abutments, they should visit the dentist for regular inspection and maintenance of the Abutments, Retention Inserts and Attachment Housings. Refrain from picking at the Abutments or Retention Inserts with toothpicks or other foreign objects. Refrain from eating without the overdenture in place as food will scratch the Abutment or Retention Insert and may result in failure of the dental implant. Oral rinse such as Listerine mouthwash can be used safely without any poor effect on the Abutments or Retention Inserts. Do not wash the overdenture in the dishwasher. 25
30 EXPLANATION OF SYMBOLS ON OUTER PACKAGING LABELS SYMBOL EXPLANATION OF SYMBOL 0086 CE Marking of Conformity with Notified Body Number (Use 0086 only on class IIa and higher risk class devices). Wellkang Ltd 29 Harley St. W1G 9QR London U.K. European Authorized Representative Symbol for CONSULT INSTRUCTIONS FOR USE NOTE: Product is provided with an Instructions For Use (IFU) and a Technique Manual. Symbol for DO NOT REUSE For single-use. Use only once. Rx Only Prescription Required. May be used as a substitute to the verbiage Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed dentist. Symbol for BATCH CODE This symbol shall be accompanied by the manufacturer s batch code or lot code. The batch/lot code shall be adjacent to the symbol. Symbol for CATALOGUE NUMBER The product catalogue number shall be after or below the symbol adjacent to it. Customer Reference Number if different than Zest Anchors Reference Number. (NOTE: This symbol is not a BS EN 980 symbol and will be used to distinguish between Zest Anchors and Customer Reference Numbers.) Symbol for MANUFACTURER This symbol shall be accompanied by the manufacturer name (ZEST Anchors) and address (2061 Wineridge Place, Escondido, CA 92029); adjacent to the symbol. Symbol for NON-STERILE This label applies to the non-sterile restorative accessories and tools (noted on the side of the packaging) and the replacement LOCATOR implant attachments. Symbol for STERILIZED USING IRRADIATION NOTE: Refers to Implant/Attachment sterilized packaging only. YYYY-MM Symbol for USE BY This symbol shall be accompanied by a date to indicate that the device should not be used after the end of the year, month shown. The date shall be expressed as four digits for the year and, two digits for the month; located adjacent to the symbol. 26
31 THE ZEST ANCHORS 10 YEAR WARRANTY THE ZEST ANCHORS LLC NARROW DIAMETER IMPLANT ( NDI ) WARRANTY: LOCATOR OVERDENTURE IMPLANT ( LODI ) AND SATURNO NARROW DIAMETER IMPLANT ( SNDI ) ZEST Anchors LLC ( ZEST ) is committed to providing quality products and dedicated to gathering feedback about its products. ZEST actively collects and reviews the feedback of users of our products in compliance with regulatory reporting requirements and to better help us understand market expectations and validate our products performance. The collection method ZEST utilizes for such feedback is the ZEST Product Experience Report ( PER ) form. The PER form is to be completed from information provided by the attending clinician to share their ZEST product experience. Pursuant to the ZEST NDI WARRANTY, ZEST will replace covered LODI and SNDI products for a $25 processing fee for each such qualifying covered implant that is returned (as such terms are defined below). Upon a request for replacement under the ZEST NDI WARRANTY, ZEST will send a replacement product once ZEST receives the returned product and completed PER form and confirms that the returned product is covered under the ZEST NDI WARRANTY. Upon shipment of the replacement product, ZEST will issue the customer an invoice at $25. ONLY DIRECT ZEST CUSTOMERS MAY MAKE A WARRANTY CLAIM. In order to make a claim under the ZEST NDI WARRANTY please return the items mentioned below in protective packaging and send these via a shipping method which enables the package to be tracked: Printed copy of PER form completed from information provided by the attending clinician. Explanted product(s) in sterile condition (NON-STERILE products will not qualify for replacement) If a product failure or loss of integration has occurred, please additionally send relevant radiographs (these will not be returned unless specifically requested, please send copies) Send shipment to: ZEST ANCHORS LLC ATTN: Customer Service (US customers) or Wholesale Distribution (Distributors/OEM Partners) 2061 Wineridge Place Escondido, CA If using the electronic form, please include the Tracking Number for the returned product package. Download ZEST NDI Product Experience Report (PDF) AT ZEST ANCORS ( ZEST ) NARROW DIAMETER IMPLANT ( NDI ) WARRANTY (Valid as of May 1, 2015) 1. Warranty beneficiary and scope: ZEST Anchors LLC hereby warrants to the direct ZEST customer purchasing the NDI from ZEST ( Customer ) that the ZEST LOCATOR Overdenture Implant ( LODI ) and SATURNO Narrow Diameter Implant ( SNDI ), when implanted according to the respective ZEST LODI/SNDI Technique Manual and other written instructions provided by ZEST by a clinician (the User ), will be free from any loss or lack of integration, fracture or other structural failure for the period of 10 years ( Warranty Period ) from the time of treatment by the User (collectively, the ZEST NDI WARRANTY ). This warranty only applies to the Customer. Third parties, particularly patients, are not covered by the ZEST NDI WARRANTY and have no rights hereunder. Customers sole remedy and ZEST S sole liability under this ZEST NDI WARRANTY is the replacement of the LODI/ SNDI implant by ZEST as set forth herein. The ZEST NDI WARRANTY only covers the replacement of the LODI/SNDI implant and not any associated costs or expenses, including, but not limited to, chair time, laboratory fees and any other associated treatment. 2. ZEST Warranty conditions: In the event that any request for warranty service for an NDI is made by a Customer under the ZEST NDI WARRANTY during the Warranty Period, ZEST will replace such NDI with the same or substantially equivalent product subject to the terms and conditions herein. The replacement product will be sent upon receipt of the returned product and the completed PER form and confirmation that the product is covered under the ZEST NDI WARRANTY. To qualify for coverage under the ZEST NDI WARRANTY, the claim must be made within the applicable Warranty Period by a Customer and all conditions below must be met. Once these requirements are satisfied, ZEST will send the replacement part(s) and charge the ZEST Customer a $25 processing fee per unit. The following REQUIRED conditions must be met and documented in order for coverage of a returned NDI under this ZEST NDI WARRANTY: 1. The LODI/SNDI was used exclusively with all components, connections, attachments and other technology provided by ZEST and not in combination with any other manufacturer s products or technology; 2. The LODI/SNDI is returned in sterile condition (or disinfected if delivered as such); 3. The LODI/SNDI was implanted by a User and inspected and maintained in full compliance with the respective ZEST LODI/SNDI Technique Manual valid at the time of treatment and all other ZEST written instructions as well as recognized dental procedures, during and after the treatment; 4. The patient had good oral hygiene which was monitored and documented by the User 5. The LODI/SNDI was not subjected to damage caused by misuse, misapplication, accident, trauma or any other damage caused by external factors or the User, the patient or a third party; 6. A completed and signed PER was completed and submitted no later than 10 days after the complaint is made. Customer is responsible to ensure that the PER is completed and submitted from information provided by the applicable User. Details of the incident are imperative to determine whether vigilance reporting is required to regulatory authorities. 3. Limits and limitations: This ZEST NDI WARRANTY is the only guarantee provided by ZEST and shall apply in addition to any warranty rights conferred under any written sales agreement executed by ZEST. The User remains free to claim rights against his supplier. EXCEPT AS SET FORTH HEREIN AND IN A WRITTEN SALES AGREEMENT EXECUTED BY ZEST, ZEST HEREBY DISCLAIMS ANY OTHER WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO LODI/SNDI OR ANY OTHER ZEST PRODUCTS, SERVICES OR INFORMATION, INCLUDING, BUT NOT LIMITED TO, ANY WARRANTY OF FITNESS FOR PURPOSE OR MERCHANTABILITY, OR NON-INFRINGEMENT OF THIRD PARTY RIGHTS, OR ANY WARRANTY BY COURSE OF PERFORMANCE OR OTHERWISE. ZEST SHALL NOT BE LIABLE TO THE CUSTOMER, THE USER, THE PATIENT, OR ANY THIRD PARTY, FOR LOST EARNINGS OR PROFITS, DIRECT OR INDIRECT DAMAGES AS WELL AS COLLATERAL, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES, DIRECTLY OR INDIRECTLY RELATED TO LODI OR ANY OTHER ZEST PRODUCTS, SERVICES OR INFORMATION. 4. ZEST Warranty territory: This ZEST NDI WARRANTY applies worldwide to LODI/SNDI implants sold by ZEST, a ZEST affiliated company or an official distributor of ZEST on or after the validity date stated above. 5. Modification or termination: ZEST may modify or terminate this ZEST NDI WARRANTY at any time in whole or in part. Changes to, or the termination of the ZEST NDI WARRANTY, will not affect the warranty given for LODI/SNDI installed prior to the date of the change or termination. LOCATOR OVERDENTURE IMPLANT (LODI) and SATURNO NARROW DIAMETER IMPLANT (SNDI) PRODUCT EXCHANGE POLICY ZEST Anchors LLC understands that customers may need to adjust inventories of LOCATOR Overdenture Implant (LODI) and SATURNO Narrow Diameter Implant (SNDI) Products in order to achieve the correct mix of sizes to treat their patients. ZEST Anchors LLC will waive normal restocking fees (1:1 or greater) for exchanges during the first six months following the original purchase. Packaging cannot be written on or in any way adulterated. Shipping will still be the responsibility of the customer requesting the exchange. 27
32 VISIT OUR WEBSITE AT TO PLACE ORDERS ONLINE 24 HOURS A DAY 7 DAYS A WEEK. ZEST ANCHORS 2061 WINERIDGE PLACE ESCONDIDO, CA USA TEL: LODI (5634) FAX: SALES@ZESTANCHORS.COM DISTRIBUTED BY: ZEST Anchors LLC. All rights reserved. CHAIRSIDE, Color and Shape of Retention Inserts, LOCATOR and ZEST are registered trademarks and SATURNO is a trademark of ZEST IP Holdings, LLC. L8019-TM REV E 06/2015
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE SATURNO NARROW DIAMETER IMPLANT SYSTEM TECHNIQUE MANUAL
 THE SATURNO NARROW DIAMETER IMPLANT SYSTEM TECHNIQUE MANUAL THE SATURNO NARROW DIAMETER IMPLANT SYSTEM FOUR DECADES OF ATTACHMENT KNOWLEDGE INCORPORATED INTO NARROW DIAMETER OVERDENTURE IMPLANTS The SATURNO
THE SATURNO NARROW DIAMETER IMPLANT SYSTEM TECHNIQUE MANUAL THE SATURNO NARROW DIAMETER IMPLANT SYSTEM FOUR DECADES OF ATTACHMENT KNOWLEDGE INCORPORATED INTO NARROW DIAMETER OVERDENTURE IMPLANTS The SATURNO
NARROW DIAMETER IMPLANT SYSTEM THE SATURNO NARROW DIAMETER IMPLANT SYSTEM
 NARROW DIAMETER IMPLANT SYSTEM THE SATURNO NARROW DIAMETER IMPLANT SYSTEM THE SATURNO NARROW DIAMETER IMPLANT SYSTEM. FOUR DECADES OF ATTACHMENT KNOWLEDGE INCORPORATED INTO NARROW DIAMETER OVERDENTURE
NARROW DIAMETER IMPLANT SYSTEM THE SATURNO NARROW DIAMETER IMPLANT SYSTEM THE SATURNO NARROW DIAMETER IMPLANT SYSTEM. FOUR DECADES OF ATTACHMENT KNOWLEDGE INCORPORATED INTO NARROW DIAMETER OVERDENTURE
UDELL DENTAL LABORATORY Instructions for Use PREAT Precision Attachments
 Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
LOCATOR R-Tx TECHNIQUE MANUAL
 LOCATOR R-Tx TECHNIQUE MANUAL Locator R-TX manual_112415.indd 1 12/01/2016 11:47:42 TABLE OF CONTENTS LOCATOR R-Tx Removable Attachment System Technique Manual 1 2 3 7 8 15 18 19 20 TABLE OF CONTENTS LOCATOR
LOCATOR R-Tx TECHNIQUE MANUAL Locator R-TX manual_112415.indd 1 12/01/2016 11:47:42 TABLE OF CONTENTS LOCATOR R-Tx Removable Attachment System Technique Manual 1 2 3 7 8 15 18 19 20 TABLE OF CONTENTS LOCATOR
OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES
 GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
Opening minds and expanding practice revenue opportunities
 GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. Opening minds and expanding practice revenue opportunities Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. Opening minds and expanding practice revenue opportunities Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
LOCATOR R-Tx TECHNIQUE MANUAL
 LOCATOR R-Tx TECHNIQUE MANUAL TABLE OF CONTENTS 2 4 7 8 11 15 18 19 20 LOCATOR R-Tx COMPONENTS REFERENCE THE NEXT GENERATION OF THE WORLD S LEADING OVERDENTURE ATTACHMENT SYSTEM OVERVIEW OF SYSTEM FEATURES
LOCATOR R-Tx TECHNIQUE MANUAL TABLE OF CONTENTS 2 4 7 8 11 15 18 19 20 LOCATOR R-Tx COMPONENTS REFERENCE THE NEXT GENERATION OF THE WORLD S LEADING OVERDENTURE ATTACHMENT SYSTEM OVERVIEW OF SYSTEM FEATURES
4766 Research Dr. San Antonio, TX insightdentalsystems.com
 OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
Treatment Options for Restoring Edentulous Jaws using One- and Two-Piece Implants from Implant Direct Int l
 Treatment Options for Restoring Edentulous Jaws using One- and Two-Piece Implants from Implant Direct Int l Two-Piece ReActive Tri-Lobe Implants with Multi-Unit Abutments One-Piece ScrewIndirect Implants
Treatment Options for Restoring Edentulous Jaws using One- and Two-Piece Implants from Implant Direct Int l Two-Piece ReActive Tri-Lobe Implants with Multi-Unit Abutments One-Piece ScrewIndirect Implants
Legacy Prosthetic Catalog
 Legacy Prosthetic Catalog Straight Snap-On Straight Contoured Angled Contoured Ball Attachment Gold/Plastic Castable Locator Attachment Plastic Temporary Screw Receiving Plastic Non-Engaging Castable 15
Legacy Prosthetic Catalog Straight Snap-On Straight Contoured Angled Contoured Ball Attachment Gold/Plastic Castable Locator Attachment Plastic Temporary Screw Receiving Plastic Non-Engaging Castable 15
Smarter Thinking. Simpler Design. Prima Plus. Surgical Manual
 Smarter Thinking. Simpler Design. Prima Plus Surgical Manual TABLE OF CONTENTS PRIMA PLUS IMPLANT SURGICAL MANUAL SURGERY Prima Plus Characteristics 4 Surgical Considerations 5 Prima Plus Surgical Sequence
Smarter Thinking. Simpler Design. Prima Plus Surgical Manual TABLE OF CONTENTS PRIMA PLUS IMPLANT SURGICAL MANUAL SURGERY Prima Plus Characteristics 4 Surgical Considerations 5 Prima Plus Surgical Sequence
A NEW ANGLE IN NARROW DIAMETER IMPLANTS
 A NEW ANGLE IN NARROW DIAMETER IMPLANTS THE SATURNO NARROW DIAMETER IMPLANT SYSTEM Featuring an innovative, angled O-Ball implant option which incorporates patented, pivoting O-Ball technology. WHAT IS
A NEW ANGLE IN NARROW DIAMETER IMPLANTS THE SATURNO NARROW DIAMETER IMPLANT SYSTEM Featuring an innovative, angled O-Ball implant option which incorporates patented, pivoting O-Ball technology. WHAT IS
OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES
 GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
GIVE YOUR PATIENTS THE FREEDOM TO EAT, SPEAK AND LAUGH AGAIN. OPENING MINDS AND EXPANDING PRACTICE REVENUE OPPORTUNITIES Since the McGill Consensus in 2002, the dental industry has recognized that implant-supported
Restoring Straumann implants. with Locator abutments.
 Restoring Straumann implants with Locator abutments. 1 1 Straumann is the industrial partner of the ITI (International Team for Implantology) in the areas of research, development, and education. Contents.
Restoring Straumann implants with Locator abutments. 1 1 Straumann is the industrial partner of the ITI (International Team for Implantology) in the areas of research, development, and education. Contents.
LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM
 1 LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM INSTRUCTIONS FOR USE DESCRIPTION Wire and Lever Wrench Tip Wrench Seating and Removal Tool Seating Tip Removal Tip with Threaded Adapter The
1 LOCATOR F-Tx SEATING AND REMOVAL TOOL PROCEDURES ADDENDUM INSTRUCTIONS FOR USE DESCRIPTION Wire and Lever Wrench Tip Wrench Seating and Removal Tool Seating Tip Removal Tip with Threaded Adapter The
The LOCATOR concept. Simplicity and versatility for prosthesis fixation
 The concept Simplicity and versatility for prosthesis fixation The concept Experience the freedom in prosthesis fixation Simple and secure fixation of implant-supported prostheses is essential for successful
The concept Simplicity and versatility for prosthesis fixation The concept Experience the freedom in prosthesis fixation Simple and secure fixation of implant-supported prostheses is essential for successful
OD Secure chairside pick-up using existing denture
 OD Secure chairside pick-up using existing denture full-arch restorations OD Secure chairside pick-up using existing denture Use this technique for chairside pick-up of the OD Secure housing into an existing
OD Secure chairside pick-up using existing denture full-arch restorations OD Secure chairside pick-up using existing denture Use this technique for chairside pick-up of the OD Secure housing into an existing
LOCATOR ATTACHMENT SYSTEM TECHNIQUE MANUAL
 LOCATOR ATTACHMENT SYSTEM TECHNIQUE MANUAL LOCATOR IMPLANT, MULTI-UNIT, BAR, AND ROOT ATTACHMENTS TABLE OF CONTENTS 1 THE LOCATOR COMPONENT REFERENCE LIST 2 LOCATOR - THE GOLD STANDARD IMPLANT ATTACHMENT
LOCATOR ATTACHMENT SYSTEM TECHNIQUE MANUAL LOCATOR IMPLANT, MULTI-UNIT, BAR, AND ROOT ATTACHMENTS TABLE OF CONTENTS 1 THE LOCATOR COMPONENT REFERENCE LIST 2 LOCATOR - THE GOLD STANDARD IMPLANT ATTACHMENT
2. STRAUMANN PRODUCTS COVERED BY THE STRAUMANN GUARANTEE. Abutment attached to an implant* Replacement with equivalent ceramic abutment**
 Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
SimpleLine II Surgical / Prosthesis Manual
 SimpleLine II Surgical / Prosthesis Manual SimpleLine II SURGICAL MANUAL Surgical Drill Sequence 04 Fixture Installation 05 Fixture Connection 06 Surgical Kit Maintenance 08 Warnings 09 04 SimpleLine ll
SimpleLine II Surgical / Prosthesis Manual SimpleLine II SURGICAL MANUAL Surgical Drill Sequence 04 Fixture Installation 05 Fixture Connection 06 Surgical Kit Maintenance 08 Warnings 09 04 SimpleLine ll
How something small, can make such a big difference!! Your best choice in overdenture
 How something small, can make such a big difference!! Your best choice in overdenture Why? Kerator has the worldʼs most powerful competitiveness in overdenture attachment system. KERATOR was chosen over
How something small, can make such a big difference!! Your best choice in overdenture Why? Kerator has the worldʼs most powerful competitiveness in overdenture attachment system. KERATOR was chosen over
3. Guarantee conditions
 Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Height* FC width Prep depth RC width 4.0mm+ 4.3mm N/A 6.3mm
 STERN ERA IMPLANT ABUTMENT Summary Resilient precision overdenture attachment. Universal hinge with vertical movement. Titanium abutment, nylon male. Manufactured for most popular screw and cylinder implants.
STERN ERA IMPLANT ABUTMENT Summary Resilient precision overdenture attachment. Universal hinge with vertical movement. Titanium abutment, nylon male. Manufactured for most popular screw and cylinder implants.
ball abutment overdenture: chairside pick-up using existing denture
 ball abutment overdenture: chairside pick-up using existing denture full-arch restorations ball abutment overdenture: chairside pick-up using existing denture Use this technique for chairside pick-up of
ball abutment overdenture: chairside pick-up using existing denture full-arch restorations ball abutment overdenture: chairside pick-up using existing denture Use this technique for chairside pick-up of
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
INTERNAL HEX Implant System
 Dental Implant Systems INTERNAL HEX Implant System infinite OPPORTUNITIES IN IMPLANTOLOGY For over 50 years ACE Surgical has been dedicated to dental surgical advancements. We continue to develop and manufacture
Dental Implant Systems INTERNAL HEX Implant System infinite OPPORTUNITIES IN IMPLANTOLOGY For over 50 years ACE Surgical has been dedicated to dental surgical advancements. We continue to develop and manufacture
LOCATOR abutment and collar for VARIOmulti
 INFO LOCATOR abutment and collar for VARIOmulti Advantages at a glance A proven implant attachment system Corrects implant divergences Fits on every VARIOmulti abutment Easy assembly ZEST LOCATOR abutment
INFO LOCATOR abutment and collar for VARIOmulti Advantages at a glance A proven implant attachment system Corrects implant divergences Fits on every VARIOmulti abutment Easy assembly ZEST LOCATOR abutment
CATALOG. Since 1985» Simple. Predictable. Profitable.
 CATALOG Since 1985» Simple. Predictable. Profitable. 2018 19 IMPLANTS SYNTHOGRAFT TEMPORARY ABUTMENTS (Ti) 3.0mm Well Integra-CP PART NO. DIAMETER LENGTH PRICE 345-305 4.5mm 5.0mm $290.00 345-306 4.5mm
CATALOG Since 1985» Simple. Predictable. Profitable. 2018 19 IMPLANTS SYNTHOGRAFT TEMPORARY ABUTMENTS (Ti) 3.0mm Well Integra-CP PART NO. DIAMETER LENGTH PRICE 345-305 4.5mm 5.0mm $290.00 345-306 4.5mm
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
LOCATOR ROOT ATTACHMENT SYSTEM
 TECHNIQUE MANUAL LOCATOR ROOT ATTACHMENT SYSTEM IMPORTANT: This document contains the most current instructions for use. Please, read and retain. DESCRIPTION: Universal hinge, resilient attachment for
TECHNIQUE MANUAL LOCATOR ROOT ATTACHMENT SYSTEM IMPORTANT: This document contains the most current instructions for use. Please, read and retain. DESCRIPTION: Universal hinge, resilient attachment for
ZEST DANVILLE MATERIALS PERIOSCOPY
 ZEST DANVILLE MATERIALS PERIOSCOPY Attachment Processing Material is easy to use and predictable when processing attachments into full and partial overdentures,using either a chairside or laboratory procedure.
ZEST DANVILLE MATERIALS PERIOSCOPY Attachment Processing Material is easy to use and predictable when processing attachments into full and partial overdentures,using either a chairside or laboratory procedure.
Product Catalog July 2017
 Product Catalog July 2017 1 2 Introducing the Hahn Tapered Implant System Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant System is a contemporary dental implant
Product Catalog July 2017 1 2 Introducing the Hahn Tapered Implant System Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant System is a contemporary dental implant
High-end solution STEP-BY-STEP USER GUIDE
 High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
High-end solution STEP-BY-STEP USER GUIDE System Contents REAL TIME Restoration Guided Conical drill Multi-Unit abutment, Conical connection Guided kit for conical connection implant procedure C1 & V3
Product Catalog August 2018
 Product Catalog August 2018 2 Introducing the Hahn Tapered Implant System Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant System is a contemporary dental implant
Product Catalog August 2018 2 Introducing the Hahn Tapered Implant System Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant System is a contemporary dental implant
Hex-Lock Abutment System. Restorative Manual
 System Restorative Manual 32 Restorative options with s s are manufactured from titanium alloy and used as the support foundation for single- or multiple-unit cement-retained, partially edentulous fixed
System Restorative Manual 32 Restorative options with s s are manufactured from titanium alloy and used as the support foundation for single- or multiple-unit cement-retained, partially edentulous fixed
Instructions for Use DENTAL IMPLANTS
 HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
HAHN TAPERED IMPLANT GUIDED SURGERY SYSTEM Instructions for Use English General Information IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order
Product Ordering Information
 Attachment Processing Material is easy to use and predictable when processing attachments into full and partial overdentures,using either a chairside or laboratory procedure. Two size options to suit your
Attachment Processing Material is easy to use and predictable when processing attachments into full and partial overdentures,using either a chairside or laboratory procedure. Two size options to suit your
IMPRESSION PROCEDURES TRAINING MANUAL
 IMPRESSION PROCEDURES TRAINING MANUAL 01 01 A.B. DENTAL A.B. Dental is proud to present this impression taking procedure protocol. This manual explains, step by step, the procedure while using A.B. Dental
IMPRESSION PROCEDURES TRAINING MANUAL 01 01 A.B. DENTAL A.B. Dental is proud to present this impression taking procedure protocol. This manual explains, step by step, the procedure while using A.B. Dental
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
Element-Z Screw-Retained Hybrid
 Element-Z Screw-Retained Hybrid Implant-Level Restoration Step-by-Step Restorative Protocol The Element-Z Screw-Retained Hybrid offers a fixed, all-ceramic implant solution for edentulous patients desiring
Element-Z Screw-Retained Hybrid Implant-Level Restoration Step-by-Step Restorative Protocol The Element-Z Screw-Retained Hybrid offers a fixed, all-ceramic implant solution for edentulous patients desiring
LOCATOR F-Tx FIXED ATTACHMENT SYSTEM TECHNIQUE MANUAL
 LOCATOR F-Tx FIXED ATTACHMENT SYSTEM TECHNIQUE MANUAL DISTRIBUTED BY: PROCSCAN TEL: +32 (0)11 822 650 EIKENENWEG 71 FAX: +32 (0)11 822 651 3520 ZONHOVEN EMAIL: INFO@PROSCAN.BE BELGIUM WWW.PROSCAN.BE ZEST
LOCATOR F-Tx FIXED ATTACHMENT SYSTEM TECHNIQUE MANUAL DISTRIBUTED BY: PROCSCAN TEL: +32 (0)11 822 650 EIKENENWEG 71 FAX: +32 (0)11 822 651 3520 ZONHOVEN EMAIL: INFO@PROSCAN.BE BELGIUM WWW.PROSCAN.BE ZEST
MINI IMPLANT SYSTEM English Instructions for Use MINI IMPLANTS
 ¾ General Information MINI IMPLANT SYSTEM English Instructions for Use IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order of, a licensed dentist
¾ General Information MINI IMPLANT SYSTEM English Instructions for Use IMPORTANT INFORMATION PLEASE READ Caution: U.S. federal law restricts this device to sale by, or on the order of, a licensed dentist
Solid Zirconia Full-Arch Implant Prosthesis (Protocol C All-CAD with Multi-Unit Abutments) BruxZir. FIRST Appointment. The BruxZir
 (Protocol C All-CAD with Multi-Unit Abutments) Step-by-Step Restorative Protocol C The BruxZir Full-Arch Implant Prosthesis offers a fixed, all-zirconia implant solution for edentulous patients desiring
(Protocol C All-CAD with Multi-Unit Abutments) Step-by-Step Restorative Protocol C The BruxZir Full-Arch Implant Prosthesis offers a fixed, all-zirconia implant solution for edentulous patients desiring
NEODENT GUIDED SURGERY
 NEODENT GUIDED SURGERY MANUAL Grand Morse Connection Helix Implant GRAND POSSIBILITIES WITH A LIMITLESS SOLUTION. GRAND MORSE NEODENT GUIDED SURGERY. 4 5 6 CONTENTS 1. CLINICAL STEP BY STEP OF GRAND
NEODENT GUIDED SURGERY MANUAL Grand Morse Connection Helix Implant GRAND POSSIBILITIES WITH A LIMITLESS SOLUTION. GRAND MORSE NEODENT GUIDED SURGERY. 4 5 6 CONTENTS 1. CLINICAL STEP BY STEP OF GRAND
SwissPlant Prosthetic Catalog. SwissPlant
 SwissPlant Prosthetic Catalog Contoured Snap-On Angled Contoured Ball Attachment Gold/Plastic Castable Locator Attachment Plastic Temporary Screw Receiving Plastic Non-Engaging Castable Narrow Titanium
SwissPlant Prosthetic Catalog Contoured Snap-On Angled Contoured Ball Attachment Gold/Plastic Castable Locator Attachment Plastic Temporary Screw Receiving Plastic Non-Engaging Castable Narrow Titanium
PRODUCT CATALOG. Since 1985» Simple. Predictable. Profitable.
 PRODUCT CATALOG Since 1985» Simple. Predictable. Profitable. 2014 IMPLANTS HEALING PLUG INSERTERS COLLAGEN PRODUCTS 3.0mm Well Integra-CP Part No. DIAMETER LENGTH Price 345-306 4.5mm mm $280.00 345-308
PRODUCT CATALOG Since 1985» Simple. Predictable. Profitable. 2014 IMPLANTS HEALING PLUG INSERTERS COLLAGEN PRODUCTS 3.0mm Well Integra-CP Part No. DIAMETER LENGTH Price 345-306 4.5mm mm $280.00 345-308
FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE
 FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE 1 2 LOGICAL LAYOUT 1 Example: C1 Implant Ø4.20 / 11.5L ; Soft Bone Ø4.20 / 11.5L MG-D0624 MG-D1124 MG-D1133 MG-D1137 2 Example: Implant C1 Ø4.20
FOR C1 & V3 IMPLANTS STEP-BY-STEP GUIDED SURGICAL PROCEDURE 1 2 LOGICAL LAYOUT 1 Example: C1 Implant Ø4.20 / 11.5L ; Soft Bone Ø4.20 / 11.5L MG-D0624 MG-D1124 MG-D1133 MG-D1137 2 Example: Implant C1 Ø4.20
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
DENTAL IMPLANT SYSTEM. Product Catalog. October 2015
 DENTAL IMPLANT SYSTEM Product Catalog October 2015 1 2 Introducing the Inclusive Dental Implant System Founded in 2006 with the goal of expanding the availability of comprehensive dental implant therapy
DENTAL IMPLANT SYSTEM Product Catalog October 2015 1 2 Introducing the Inclusive Dental Implant System Founded in 2006 with the goal of expanding the availability of comprehensive dental implant therapy
Table of Contents. Flexi-Overdenture Characteristics
 A s with Flexi-Post Flexi-Overdenture has the unique threaded split shank that creates maximum retention with minimum stress to the root. It is manufactured in stainless steel for the direct/non-coping
A s with Flexi-Post Flexi-Overdenture has the unique threaded split shank that creates maximum retention with minimum stress to the root. It is manufactured in stainless steel for the direct/non-coping
LOCATOR Overdenture Implant System
 LOCATOR Overdenture Implant System Product Ordering Information Manufactured by ZEST Anchors Distributed by: The LOCATOR Overdenture Implant System Each package includes your choice of a 2.4mm or 2.9mm
LOCATOR Overdenture Implant System Product Ordering Information Manufactured by ZEST Anchors Distributed by: The LOCATOR Overdenture Implant System Each package includes your choice of a 2.4mm or 2.9mm
Implant And Abutment Level Impressions
 Implant And Abutment Level Impressions Implant And Abutment Level Impressions Pick-Up Impression Copings Certain Internal Connection is illustrated below. 1. Select the proper Pick-Up Impression Coping
Implant And Abutment Level Impressions Implant And Abutment Level Impressions Pick-Up Impression Copings Certain Internal Connection is illustrated below. 1. Select the proper Pick-Up Impression Coping
Instructions for Use for dental implant
 Instructions for Use for dental implant 1. Disclaimer of liability This product is part of the Cortex Dental Implant System and may only be used in conjunction with the corresponding original components
Instructions for Use for dental implant 1. Disclaimer of liability This product is part of the Cortex Dental Implant System and may only be used in conjunction with the corresponding original components
LOCATOR Overdenture Implant System
 LOCATOR Overdenture Implant System Product Ordering Information Manufactured by ZEST Anchors Distributed by: LOCATOR Overdenture Implant System Surgical Instrument Kit Schematic 07357 08926 07366 07374
LOCATOR Overdenture Implant System Product Ordering Information Manufactured by ZEST Anchors Distributed by: LOCATOR Overdenture Implant System Surgical Instrument Kit Schematic 07357 08926 07366 07374
Product Catalog. Since 1985» Simple. Predictable. Profitable.
 Product Catalog Since 1985» Simple. Predictable. Profitable. 2011 IMPLANTS 3.0mm Well Integra-CP Part No. DIAMETER LENGTH 345-306 4.5mm 6.0mm 345-308 4.5mm 8.0mm 345-311 4.5mm 11.0mm 350-305 5.0mm 5.0mm
Product Catalog Since 1985» Simple. Predictable. Profitable. 2011 IMPLANTS 3.0mm Well Integra-CP Part No. DIAMETER LENGTH 345-306 4.5mm 6.0mm 345-308 4.5mm 8.0mm 345-311 4.5mm 11.0mm 350-305 5.0mm 5.0mm
IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS. a perfect fit
 a perfect fit IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS Open and closed impression-taking Impression-taking for option platform switching Bite registration Temporary
a perfect fit IMPRESSION-TAKING, BITE REGISTRATION, AND TEMPORARY RESTORATION ON CAMLOG IMPLANTS Open and closed impression-taking Impression-taking for option platform switching Bite registration Temporary
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Product Catalog January 2017 DENTAL IMPLANT SYSTEM
 Product Catalog January 2017 DENTAL IMPLANT SYSTEM 1 2 Introducing the Inclusive Dental Implant System The Inclusive Dental Implant System is the result of an expansive effort to offer clinicians and their
Product Catalog January 2017 DENTAL IMPLANT SYSTEM 1 2 Introducing the Inclusive Dental Implant System The Inclusive Dental Implant System is the result of an expansive effort to offer clinicians and their
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
contents the smarter case approach reasons to choose simplyintegrated 5 system overview 6 ordering 7 mount-free implants 8-9
 simply fixed angled contents the smarter case approach 3-4 3 reasons to choose simplyintegrated 5 system overview 6 ordering 7 mount-free implants 8-9 SMART PACK prosthetics 10 simplyinteractive details
simply fixed angled contents the smarter case approach 3-4 3 reasons to choose simplyintegrated 5 system overview 6 ordering 7 mount-free implants 8-9 SMART PACK prosthetics 10 simplyinteractive details
Multi-unit abutment bar overdenture
 Multi-unit abutment bar overdenture Use this technique for the fabrication of a multiple-unit, implant-supported, screw-retained bar with an overdenture in a totally edentulous patient. component options
Multi-unit abutment bar overdenture Use this technique for the fabrication of a multiple-unit, implant-supported, screw-retained bar with an overdenture in a totally edentulous patient. component options
PROSTHETIC COMPONENTS
 MILO 3.0mm 3.0mm Ø MILO Implants Prosthetic Connection: Ø 1.8 mm O-Ball Lengths: 10, 11.5, 13, 15 & 17 mm. Surface treatment: Blasted + Progressively Acid Etched Titanium 3.0mm MILO Fine Pitch Implants
MILO 3.0mm 3.0mm Ø MILO Implants Prosthetic Connection: Ø 1.8 mm O-Ball Lengths: 10, 11.5, 13, 15 & 17 mm. Surface treatment: Blasted + Progressively Acid Etched Titanium 3.0mm MILO Fine Pitch Implants
NARROW DIAMETER implant
 ND NARROW DIAMETER implant TABLE OF CONTENTS ND - NARROW DIAMETER implant Implant characteristics page 04 Dental implant page 05 Open Tray Impression Transfer page 06 Titanium Abutments page 07 O-Ball
ND NARROW DIAMETER implant TABLE OF CONTENTS ND - NARROW DIAMETER implant Implant characteristics page 04 Dental implant page 05 Open Tray Impression Transfer page 06 Titanium Abutments page 07 O-Ball
- RESTORATIVE PRODUCT CATALOG
 - RESTORATIVE PRODUCT CATALOG MADE IN USA Others make Implants to sell... Tatum Surgical makes Implants to treat your Patients. Your Source for Implants To Meet Every Clinical Need Tatum... 4 Angled Post
- RESTORATIVE PRODUCT CATALOG MADE IN USA Others make Implants to sell... Tatum Surgical makes Implants to treat your Patients. Your Source for Implants To Meet Every Clinical Need Tatum... 4 Angled Post
IOS & The Sirona Connect System Clinician Procedure
 Clinician Procedure Surgeon (Please ensure that these instructions are forwarded to the restorative clinician.) STEP 1: Select a BellaTek Encode Healing Abutment with the appropriate restorative platform
Clinician Procedure Surgeon (Please ensure that these instructions are forwarded to the restorative clinician.) STEP 1: Select a BellaTek Encode Healing Abutment with the appropriate restorative platform
prosthetic technique manual
 prosthetic technique manual table of contents table of contents introduction modules why choose BioHorizons prosthetics? surgical and prosthetic options & impression technique overview impression technique
prosthetic technique manual table of contents table of contents introduction modules why choose BioHorizons prosthetics? surgical and prosthetic options & impression technique overview impression technique
Real World Implant Prosthetics: Fixed and Removable Samuel M. Strong, DDS
 Real World Implant Prosthetics: Fixed and Removable Samuel M. Strong, DDS Presurgical planning Health history-systemic conditions Case presentation Financial agreement Radiographs- PA s, FMX, Panoramic,
Real World Implant Prosthetics: Fixed and Removable Samuel M. Strong, DDS Presurgical planning Health history-systemic conditions Case presentation Financial agreement Radiographs- PA s, FMX, Panoramic,
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
GMI. MONOLITH implant system. Surgical procedures guide
 GMI MONOLITH implant system Surgical procedures guide ABOUT THIS MANUAL This surgical procedures guide or surgical manual for the GMI MONOLITH implant system is designed solely to provide instructions
GMI MONOLITH implant system Surgical procedures guide ABOUT THIS MANUAL This surgical procedures guide or surgical manual for the GMI MONOLITH implant system is designed solely to provide instructions
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
ADJUSTABLE HERBST APPLIANCE - OASYS HINGE ACRYLIC
 P SYSTEMS FOLLOW-UP/MAINTENANCE VISITS: See your dentist at the following intervals after receiving your appliance: ADJUSTMENTS MADE BY DENTISTS OR PATIENT Turning the hex driver clockwise to advance the
P SYSTEMS FOLLOW-UP/MAINTENANCE VISITS: See your dentist at the following intervals after receiving your appliance: ADJUSTMENTS MADE BY DENTISTS OR PATIENT Turning the hex driver clockwise to advance the
UDELL DENTAL LABORATORY Instructions for Use
 DALLA BONA Summary Cylindrical Dalla Bona Rigid precision attachment. Radicular telescopic stud. Adjustable frictional retention. Gold alloy male (Elitor) and female (OSV). Fixation: Male - soldered to
DALLA BONA Summary Cylindrical Dalla Bona Rigid precision attachment. Radicular telescopic stud. Adjustable frictional retention. Gold alloy male (Elitor) and female (OSV). Fixation: Male - soldered to
Ankylos. Acuris conometric concept. Manual and product catalog
 Ankylos Acuris conometric concept Manual and product catalog Ankylos Implanting TissueCare The true value of an implant system becomes apparent with time. For over 25 years, the Ankylos implant system
Ankylos Acuris conometric concept Manual and product catalog Ankylos Implanting TissueCare The true value of an implant system becomes apparent with time. For over 25 years, the Ankylos implant system
cement-retained single crowns using cementable abutments
 cement-retained single crowns using cementable abutments cement-retained restorations cement-retained single crowns using cementable abutments Cement-retained implant restorations are similar to conventional
cement-retained single crowns using cementable abutments cement-retained restorations cement-retained single crowns using cementable abutments Cement-retained implant restorations are similar to conventional
FIXED-HYBRID EMERGENCY! Why Redo When You Can Rescue?
 FIXED-HYBRID EMERGENCY! Why Redo When You Can Rescue? FIXED-HYBRID FAILURES HAPPEN When a failing implant needs to be replaced in a fixed-hybrid case, it often leads to a prosthesis emergency. Replacing
FIXED-HYBRID EMERGENCY! Why Redo When You Can Rescue? FIXED-HYBRID FAILURES HAPPEN When a failing implant needs to be replaced in a fixed-hybrid case, it often leads to a prosthesis emergency. Replacing
NARROW DIAMETER implant
 N NARROW IAMETER implant N Precision dental solutions C-Tech Implant is a dynamic company with aggressive growth, producing components and product lines primarily for dental implantology. International
N NARROW IAMETER implant N Precision dental solutions C-Tech Implant is a dynamic company with aggressive growth, producing components and product lines primarily for dental implantology. International
Conus Concept: A Rewarding Complete Denture Treatment
 Conus Concept: A Rewarding Complete Denture Treatment Complete dentures have largely become the domain of the denturist due to the dissatisfaction general dentists feel with this treatment. Multiple visits,
Conus Concept: A Rewarding Complete Denture Treatment Complete dentures have largely become the domain of the denturist due to the dissatisfaction general dentists feel with this treatment. Multiple visits,
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care
 a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
a perfect fit INSTRUCTION MANUAL CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT Handling Sterilization Care CAMLOG TORQUE WRENCH WITH CONTINUOUS TORQUE ADJUSTMENT INTRODUCTION The torque wrench
screw-retained crown using the Laser-Lok Easy Ti temp abutment
 screw-retained crown using the Laser-Lok Easy Ti temp abutment temporary restorations screw-retained crown using the Laser-Lok Easy Ti temp abutment Use this technique for the fabrication of long-term
screw-retained crown using the Laser-Lok Easy Ti temp abutment temporary restorations screw-retained crown using the Laser-Lok Easy Ti temp abutment Use this technique for the fabrication of long-term
Instructions for Use SomnoDent Signature and Standard Line Fusion, Flex, Classic, Herbst Advance, Herbst Telescopic, Herbst Shim, AIR & AIR+ Devices
 Instructions for Use SomnoDent Signature and Standard Line Fusion, Flex, Classic, Herbst Advance, Herbst Telescopic, Herbst Shim, AIR & AIR+ Devices Shim www.somnomed.com 1 Introduction The SomnoDent is
Instructions for Use SomnoDent Signature and Standard Line Fusion, Flex, Classic, Herbst Advance, Herbst Telescopic, Herbst Shim, AIR & AIR+ Devices Shim www.somnomed.com 1 Introduction The SomnoDent is
VIP Partner Laboratory (PL) Process Flow
 VIP Partner Laboratory (PL) Process Flow 1. VIP Software 2. CT Scan Appliance 3. CT Scan Clinician purchases VIP for PL fabricates CT Scan Appliance Patient submitted for scans treatment planning purposes
VIP Partner Laboratory (PL) Process Flow 1. VIP Software 2. CT Scan Appliance 3. CT Scan Clinician purchases VIP for PL fabricates CT Scan Appliance Patient submitted for scans treatment planning purposes
Patient Instruction Booklet
 Patient Instruction Booklet TAP 3 ThermAcryl TAP 3 TL TAP 3 Revision E, 2014 PRTD17 Table of Contents Important Safeguards Important Safeguards... 3 SAVE THESE INSTRUCTIONS 2 Introduction... Warnings...
Patient Instruction Booklet TAP 3 ThermAcryl TAP 3 TL TAP 3 Revision E, 2014 PRTD17 Table of Contents Important Safeguards Important Safeguards... 3 SAVE THESE INSTRUCTIONS 2 Introduction... Warnings...
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
English. Fealite Nasal Pillows System. User Manual
 Fealite Nasal Pillows System User Manual 0123 Table of Contents Fealite Nasal Pillows System 1 Intended Use 1 Medical Information 1 Parts of the Fealite 3 Fitting the Fealite 5 Using Tube Retainer (Optional)
Fealite Nasal Pillows System User Manual 0123 Table of Contents Fealite Nasal Pillows System 1 Intended Use 1 Medical Information 1 Parts of the Fealite 3 Fitting the Fealite 5 Using Tube Retainer (Optional)
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
SuperLine & Implantium Surgical / Prosthesis Manual
 SuperLine & Implantium Surgical / Prosthesis Manual SuperLine & Implantium SURGICAL MANUAL Surgical Drill Sequence 04 Drilling Depth Guide 06 Fixture Connection 08 Installation Procedure & Warnings 09
SuperLine & Implantium Surgical / Prosthesis Manual SuperLine & Implantium SURGICAL MANUAL Surgical Drill Sequence 04 Drilling Depth Guide 06 Fixture Connection 08 Installation Procedure & Warnings 09
USER S MANUAL QUESTIONS? CAUTION. For a great selection of new CD and MP3 workouts. Visit our website at. or call
 Model No. RBBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (Under Seat) QUESTIONS? As a manufacturer, we are committed to providing
Model No. RBBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (Under Seat) QUESTIONS? As a manufacturer, we are committed to providing
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
GMI FRONTIER implant system. Surgical procedures guide
 GMI implant system Surgical procedures guide ABOUT THIS MANUAL This surgical procedures guide or surgical manual for the GMI implant system is designed solely to provide instructions for using GMI products,
GMI implant system Surgical procedures guide ABOUT THIS MANUAL This surgical procedures guide or surgical manual for the GMI implant system is designed solely to provide instructions for using GMI products,
simply simply digital simply crown & bridge simply removable simply fixed simply à la carte
 TM crown & bridge fixed removable digital à la carte imagine... being able to order the way you treatment plan so you can focus on the dentistry, rather than the components knowing your treatment costs
TM crown & bridge fixed removable digital à la carte imagine... being able to order the way you treatment plan so you can focus on the dentistry, rather than the components knowing your treatment costs
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Fibrlok II 2529 Universal Optical Fiber Splice
 Fibrlok II 2529 Universal Optical Fiber Splice Instructions January 2007 78-8128-0163-3-E Contents: 1.0 General... 3 2.0 Splicing Set-up... 4 3.0 Fiber Preparation... 5 4.0 Splice Assembly... 6 5.0 For
Fibrlok II 2529 Universal Optical Fiber Splice Instructions January 2007 78-8128-0163-3-E Contents: 1.0 General... 3 2.0 Splicing Set-up... 4 3.0 Fiber Preparation... 5 4.0 Splice Assembly... 6 5.0 For
Straumann Guarantee. Straumann
 Guarantee Lifetime Guarantee Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Guarantee Lifetime Guarantee Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
INDEX. Pag. 12. Pag. 05. Pag. 06. Pag. 13. Pag. 06. Pag. 14. Pag. 15. Pag. 15. Pag. 15. Pag. 15. Pag. 08. Pag. 16. Pag. 09. Pag.
 PROSTHESIS MANUAL INDEX PRESENTATION Pag. 05 CEMENT-SCREWED PROSTHESIS Pag. 12 INTRODUCTION PROSTHESIS MANUAL WARNINGS Pag. 06 Pag. 06 IMPRESSION TAKING: NECESSARY MATERIAL PROSTHESIS: NECESSARY MATERIAL
PROSTHESIS MANUAL INDEX PRESENTATION Pag. 05 CEMENT-SCREWED PROSTHESIS Pag. 12 INTRODUCTION PROSTHESIS MANUAL WARNINGS Pag. 06 Pag. 06 IMPRESSION TAKING: NECESSARY MATERIAL PROSTHESIS: NECESSARY MATERIAL
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
internal impression quick reference guide
 internal impression quick reference guide IMPRESSION TECHNIQUE OVERVIEW Procedure Objective: This guide provides you with 3 of the most common impression techniques using the BioHorizons Tapered Internal
internal impression quick reference guide IMPRESSION TECHNIQUE OVERVIEW Procedure Objective: This guide provides you with 3 of the most common impression techniques using the BioHorizons Tapered Internal
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Building a Strong Team for the Dental Implant Practice
 Building a Strong Team for the Dental Implant Practice Samuel M. Strong, DDS Stephanie Strong, RDH, BS Course Synopsis This course deals with the organization and training to successfully complete restorative
Building a Strong Team for the Dental Implant Practice Samuel M. Strong, DDS Stephanie Strong, RDH, BS Course Synopsis This course deals with the organization and training to successfully complete restorative
