CONFORM FLEX TECHNICAL MONOGRAPH
|
|
|
- Eleanor Walsh
- 5 years ago
- Views:
Transcription
1 CONFORM FLE TECHNICAL MONOGRAPH
2 CONFORM Flex INTRODUCTION MTF is a nonprofit organization founded in 1987 by academic orthopaedic surgeons dedicated to providing tissue of high quality and safety for transplantation. MTF has distributed over 6 million allografts since their inception, and has never reported a confirmed case of viral disease transmission. MTF s exemplary safety record is directly attributed to their commitment to the donor families and to the tissue recipients served. This tremendous commitment provides customers with the assurance that this gift of human tissue is safe and that it comes from a trustworthy source. MTF also thinks beyond safety. While safety governs every decision made, quality and efficacy also matter. Current techniques used by some tissue banks to clean, process, and sterilize demineralized allografts have been shown to be detrimental to the quality of the tissue. These methods vary widely from bank to bank, because the industry donor criteria and processing standards are open to interpretation. Demineralized allograft tissue of less-than-optimal quality may yield a graft that does not perform its intended function, which could lead to a less-than-optimal clinical outcome. PRINCIPLES OF BONE HEALING AND GRAFT INCORPORATION 1 Four components are necessary for bone healing and/or bone graft incorporation: the presence of host cells, a signal to trigger differentiation of the host cells to bone-forming cells, a scaffold or matrix on which the new bone can form, and an adequate blood supply. When bone fracture or injury occurs, there is loss of mechanical integrity of the bone and disruption to the blood supply. The healing cascade begins immediately. The 3 phases are: inflammation, repair, and remodeling. Inflammation is the process by which host cells remove debris from the injured site, prepare the local matrix into a site which can support cell growth, and enable new bone to be formed. Revascularization, which is required for new bone to grow, begins in the inflammation phase. Repair includes the recruitment and differentiation of host cells into osteoblasts, which in turn produce new bone at the injured site. Lastly, remodeling is the resorption of immature or extraneous bone coupled with reorientation of bone along the direction of mechanical loading to provide adequate structural support. These phases (Figure 1) are regulated by the release of local cytokines. DePuy Synthes Trauma CONFORM Flex Technical Monograph
3 Figure 1: Example of the healing cascade in fracture repair. The three phases of fracture repair include A) the inflammatory phase, B) the reparative phase, and C) the remodeling phase. 1 A Monocytes Mesenchymal cells Macrophages PDGF Neovascularization Recruitment TGF-ß etc... Cartilage B Intramembranous ossification Fibroblasts Osteoblasts Chondrocytes C Osteoclast/osteoblast remodeling occurs on the surface and via osteons New bone formation and bone healing are influenced by several factors in the bone graft material, some of which can be controlled, such as: Bone-forming potential Porosity ph Host factors are not as easily controlled: Age Systemic disease Vascularity Presence of infection Quantity and quality of host cells Use of anti-inflammatory drugs CONFORM Flex Technical Monograph DePuy Synthes Trauma 1
4 Bone grafts are often used as bone void fillers to assist with bone healing. Grafts can provide support and/or cell signals. During the healing process, the bone grafts are incorporated into the host bone by remodeling and/or creeping substitution. Bone graft materials used as bone void fillers can be described as osteogenic, osteoinductive, and/or osteoconductive. Osteogenic tissues are capable of forming new bone from living cells. Osteoprogenitor cells proliferate and differentiate into osteoblasts (bone-building cells) and eventually into osteocytes (mature bone cells). These cells represent the osteogenic potential of the graft. 1 Osteoinductive tissues are ones which promote chemotaxis, mitogenesis, and formation of osteoprogenitor cells that have osteogenic capacity (as described above). 2 Osteoinductive materials will form bone when implanted into tissues which would not otherwise form new bone. 3 Osteoconductive tissues allow for fibrovascular tissue development and osteoprogenitor cell invasion of a porous structure. This material then acts as a temporary scaffold which will be replaced with newly formed bone. 2 RATIONALE FOR CLINICAL USE OF DEMINERALIZED BONE Autograft bone provides all three components necessary for bone healing. It has been widely used during bone grafting procedures due to availability of donor graft sites and good incorporation upon transplantation. However, the use of autograft bone requires a second surgical site (to procure bone) which has associated morbidity risks. Allogeneic demineralized bone eliminates the need for a second surgical site, and its demineralized state results in bone that is osteoconductive and has osteoinductive potential. HOW DEMINERALIZED BONE WORKS The exact mechanism of the osteoinductive potential of demineralized bone has not been very well defined. However, it is thought that the removal of the mineral component of bone exposes the active bone morphogenetic proteins (BMPs) present in the demineralized bone while retaining the inherent osteoconductive properties of the bone. When implanted, the active BMPs are thought to signal the host mesenchymal cells thus causing the cells to proliferate and differentiate into chondroblasts, which in turn will form a cartilage matrix. Ultimately, the cartilage matrix is converted into a calcified extracellular matrix. This calcified matrix will become vascularized, and osteoprogenitor cells will form new bone on this matrix. This will be followed by the formation of bone marrow and marrow elements. 3 2 DePuy Synthes Trauma CONFORM Flex Technical Monograph
5 Figure 2: Schematic showing differentiation of a stem cell into an active osteoblast, and demonstrating the influence of growth factors. 2 STEM CELL BMP S COMMITTED OSTEOBLASTIC STEM CELL CHONDROCYTE IGFs TGF-ß BMPs MUSCLE FIBROBLAST ADIPOCYTE IGFs TGF-ß BMPs OSTEOBLAST WHAT CONSTITUTES GOOD DEMINERALIZED BONE MTF has developed and validated a procedure for cancellous bone demineralization. This procedure provides safe, high-quality demineralized bone allograft and was developed through rigorous testing to ensure that the osteoinductive potential of the tissue was not compromised. Many factors contribute to the high quality of MTF demineralized cancellous bone including: Stringent donor selection Careful processing Quality control metrics QUALITY TISSUE MTF s quality and safety standards consistently meet or exceed the requirements of the American Association of Tissue Banks (AATB) as well as the guidelines for screening and testing of tissue donors set forth by the U.S. Food and Drug Administration (FDA). The AATB and FDA set only minimal guidelines to ensure safety of tissue. Potential MTF donors must pass through an extensive quality assurance process. CONFORM Flex Technical Monograph DePuy Synthes Trauma 3
6 Screening begins at the site of recovery with a comprehensive medical and social history that includes the cause of death. Tissue and blood samples are tested for infectious diseases, including hepatitis, HIV, and syphilis. A team of medical/ technical specialists from the infectious disease and tissue banking fields evaluates all information, including test results, before the donor is released for processing. Figure 3 lists those conditions for which MTF voluntarily defers donors for safety or quality reasons even when not required by the FDA or AATB. Figure 3: Tissue bank screening criteria. Note: MTF voluntarily exceeds both AATB and FDA screening criteria. SCREENING CRITERIA MTF AATB FDA Hepatitis B virus Hepatitis C virus HIV 1/2 Malaria Sepsis Syphilis Transmission spongiform encephalopathy (TSE) Vaccinia West Nile virus (WNV) Clinically significant metabolic bone disease Leprosy (Hansen s disease) Polyarteritis nodosa Rabies Rheumatoid arthritis Sarcoidosis Systemic lupus erythematosus Systemic mycosis Tuberculosis Active genital herpes Ankylosing spondylitis Antiphospholipid syndrome Autoimmune hemolytic anemia Autoimmune lymphoproliferative syndrome Autoimmune thrombocytopenic purpura Autoimmune vasculitis Cancer Chagas disease Clinically active Epstein Barr virus (mononucleosis) Clinically active gonorrhea Clostridium difficile infection Cold agglutinin disease Encephalitis Endocarditis Guillain-Barre syndrome Illicit drug use Meningitis Methicillin-resistant Staphylococcus aureus (MRSA) Mixed connective tissue disease Multiple sclerosis Myasthenia gravis Peritonitis Poliomyelitis Pyelonephritis Reactive arthritis (Reiter s syndrome) Rheumatic fever Vancomycin-resistant Enterococcus (VRE) Varicella zoster Wegener s granulomatosis Any acute infectious/septic illness 4 DePuy Synthes Trauma CONFORM Flex Technical Monograph
7 CAREFUL PROCESSING To maintain biological integrity, MTF processes all tissue using aseptic techniques in ISO Class 4 (certified) clean rooms. MTF s use of these clean rooms is designed to prevent any environmental contamination of the tissue and thus eliminates the need for terminal sterilization by high-dose gamma radiation, which has been shown to compromise the biological and biomechanical integrity of allograft tissue (Figure 4). 4,5 Figure 4: Gamma radiation decreases osteoinductive potential of demineralized bone powder in a dosedependent manner, as measured using an in vitro alkaline phosphatase (ALP) assay. 5 ALP mmol/mg protein/min Demineralized Bone Matrix lot n 0 kgy n 12 kgy n 18 kgy n 25 kgy MTF s demineralized bone matrices are produced from both cancellous and cortical bone which are subjected to controlled cleaning processes (including hydrogen peroxide and ethanol), followed by demineralization with hydrochloric acid. Extended exposure to harsh chemicals such as hydrogen peroxide has been shown to have a negative effect on the osteoinductive properties of tissues as shown in Figure 5. 6 MTF s aseptic processes have been designed to minimize exposure to harsh chemicals and preserve the biological integrity of the tissue. Figure 5: Osteoinductivity versus soaking time (in hours) of tissue in 3% hydrogen peroxide the negative effect of peroxide on bone osteoinductivity gets more pronounced as soak times increase. Osteoinductivity Effect of hydrogen peroxide time on osteoinductivity y= x R 2 = Soaking Time (in hours) CONFORM Flex Technical Monograph DePuy Synthes Trauma 5
8 PACKAGING CONFORM Demineralized Cancellous Tissues are provided in a hydrated form (Q-PACK Zero Rehydration Technology). Lyophilized cancellous tissues take as long as 5 20 min to rehydrate prior to use. The Q-PACK Technology ready to use packaging is a way to provide grafts in a hydrated state directly from the packaging. The Q-PACK Technology: Allows tissue to be stored in a fully hydrated state at ambient temperature Saves valuable operating room time for the surgeon/hospital Allows for tissue to be used for emergency purposes, when zero preparation is essential BMP-2 CONTENT Bone induction is a sequential, multistep cascade which involves various growth factors. Bone morphogenetic proteins (and other intrinsic growth factors) in bone are exposed by the demineralization process. However, BMP-2 has been shown to be the best single predictor of osteoinductive potential based on statistical analysis of the correlation between in vitro levels of various growth factors and in vivo osteoinductivity. 7,8 BMP-2 levels in demineralized cancellous tissues as measured in vitro via enzyme-linked immunosorbent assay (ELISA) are shown in Figure 6. Figure 6: BMP-2 levels in CONFORM Demineralized Cancellous Tissue compared to OsteoSponge (Bacterin). 9 BMP-2 concentration (pg/g bone) CONFORM Demineralized Cancellous Tissue n CONFORM Demineralized Cancellous Tissue n OsteoSponge OsteoSponge 6 DePuy Synthes Trauma CONFORM Flex Technical Monograph
9 CELL COMPATIBILITY/BIOCOMPATIBILITY 10 Cancellous bone is naturally a highly porous, three-dimensional biologic scaffold that is inherently osteoconductive. After processing the bone using a specific demineralization and ph restoration recipe, an optimal cell friendly environment that also possesses exposed bone morphogenetic proteins is created for CONFORM Demineralized Cancellous Allografts. In the clinical setting, bone marrow aspiration is often used to recover autologous osteogenic cells and aspirates can easily be combined with CONFORM Demineralized Cancellous Tissue scaffolds. This technique provides cells capable of new bone formation and additional biological growth factors that assist in the healing and remodeling of bone. In order to evaluate the ability of CONFORM Demineralized Cancellous Tissue grafts to support cellular adhesion and osteogenic differentiation, an in vitro study was performed where scaffolds were seeded with human mesenchymal stem cells (hmscs) and then cultured in osteogenic medium. Cells were first observed to attach to the demineralized cancellous bone and populate the scaffolds during the first day following cell seeding (Figure 7). Subsequently, these cells remained adhered to and spread out over the bone surfaces during longer term culture (Figure 8A). When exposed to osteogenic medium, hmscs exhibited osteogenic behavior on CONFORM Demineralized Cancellous Tissue scaffolds as evidence of mineralization was observed at 6 weeks in culture (Figure 8B and 8C). Collectively, these findings indicate that CONFORM Demineralized Cancellous Allografts are highly biocompatible scaffolds for hmscs and are capable of supporting osteogenic differentiation and bone matrix deposition. Figure 7: Time sequential images of hmscs seeded onto CONFORM Demineralized Cancellous Tissue scaffolds that were obtained during the first day of culture. The cells were fluorescently stained green using CellTracker TM Green CMFDA while the scaffolds were stained red using Alexa Fluor minutes 3 hours 24 hours CellTracker is a trademark of Thermo Fisher Scientific Inc. CONFORM Flex Technical Monograph DePuy Synthes Trauma 7
10 Figure 8: (A) Histological image of hmscs seeded onto fully demineralized cancellous bone scaffolds at 14 days after cell seeding. Sections were stained with hemotoxylin and eosin. (B, C) Histological images that represent either (B) von Kossa staining of sections (black color) or (C) Alizarin Red staining (dark red color) and depict evidence of mineralization through the deposition of calcium phosphate by hmscs on demineralized cancellous bone scaffolds at 6 weeks after cell seeding. MSCs were exposed to osteogenic medium during culture to elicit osteogenic differentiation. 8 DePuy Synthes Trauma CONFORM Flex Technical Monograph
11 IN VIVO OI POTENTIAL 11 To assess the in vivo performance and osteoinductive potential of cancellous tissues when combined with bone marrow aspirate (BMA), an athymic rat muscle pouch model was used. Multiple lots of the tissue were mixed with BMA and implanted bilaterally in the hamstring muscles of athymic rats. Animals were sacrificed at 4 weeks post-implantation. Decalcified histology was then performed on the explanted samples, with one histological section prepared for each sample. Slides were stained with hematoxylin and eosin and evaluated for osteoinductivity. A semi-quantitative scoring system was utilized to assess osteoinduction. Osteoinductive scores were based on the degree to which new bone, bone cells, osteoid, calcified cartilage remnants, and marrow elements were present. To be consistent with proposed standards in the industry, 12 the scoring system in Table 1 was utilized. 13 Table 1: Osteoinductivity Scoring Scale and Criteria Score Criteria 0 No evidence of new bone formation % of the section is covered by new bone % of the section is covered by new bone % of the section is covered by new bone 4 >75% of the section is covered by new bone Cancellous tissue combined with BMA was consistently osteoinductive in this model; 100% of the samples were osteoinductive, with an average osteoinduction score (pooling data from 3 donors) of 3.94 ± 0.23 (Table 2). Figures 9 and 10 show the representative histological response to Cancellous + BMA, with robust new bone formation including bone marrow. Table 2: Summary statistics, number of samples that could be histologically evaluated, and number of osteoinductive samples for each group. Number of osteoinductive samples is divided by the number of evaluated samples to give the % of osteoinductive samples for each group. Summary Statistics Cancellous + BMA Osteoinduction Score (0-4 Scale) # Ranked Samples Mean Std Dev /36 (100%) # Osteoinductive Samples (Percentages) CONFORM Flex Technical Monograph DePuy Synthes Trauma 9
12 Figure 9: Cancellous + BMA demonstrating the presence of bone marrow and new bone formation (arrows). H&E stain; 100 magnification; BAR = 100 µm. Figure 10: Cancellous + BMA demonstrating residual CONFORM Demineralized Cancellous Tissue, the presence of bone marrow and new bone formation (arrows). H&E stain; 100 magnification; BAR = 100 µm. 11 DePuy Synthes Trauma CONFORM Flex Technical Monograph
13 SUMMARY MTF s processes for bone demineralization and creation of DBM tissue forms have been designed and validated to ensure the safety of the allografts without adversely affecting their biological performance. The processes and studies described here demonstrate that MTF s decontamination and demineralization procedures preserve the endogenous proteins and osteoinductive potential of the tissues and create allografts that support cell infiltration and function. CONFORM Flex Technical Monograph DePuy Synthes Trauma 11
14 REFERENCES 1. Mehta S, Collings C. Orthobiologics: Improving Fracture Care Through Science: Lippincott Williams & Wilkins; Mouch CS, Einhorn TA. Bone Morphogenetic Proteins and Other Growth Factors to Enhance Fracture Healing and Treatment of Nonunions. In: Lieberman JR, Friedlander GE, editors. Bone Regeneration and Repair: Biology and Clinical Applications: Humana Press; p Boyan BB, McMillan J, Lohman CH, Ranly DM, Schwartz Z. Bone Graft Substitutes: Basic Information for Successful Clinical Use with Special Focus on Synthetic Graft Substitutes. In: Laurencin CT, editor. Bone Graft Substitutes: ASTM; p Vangsness CT, Jr., Wagner PP, Moore TM, Roberts MR. Overview of Safety Issues Concerning the Preparation and Processing of Soft-Tissue Allografts. Arthroscopy. 2006;22(12): Han B, Yang Z, Nimni M. Effects of Gamma Irradiation on Osteoinduction Associated with Demineralized Bone Matrix. J Orthop Res. 2008;26(1): DePaula CA, Truncale KG, Gertzman AA, et al. Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank. 2005;6(4): Blum B, Moseley J, Miller L, et al. Measurement of Bone Morphogenetic Proteins and Other Growth Factors in Demineralized Bone Matrix. Orthopedics. 2004;27(1 Suppl):s Murray SS, Brochmann EJ, Harker JO, King E, Lollis RJ, Khaliq SA. A Statistical Model to Allow the Phasing out of the Animal Testing of Demineralised Bone Matrix Products. Altern Lab Anim. 2007;35(4): Osteosponge. Bacterin. Available from: Accessed on October 1, CONFORM with Q-PACK : An evaluation of a Fully Demineralized Scaffold with Q-PACK Technology Seeded with Human Mesenchymal Stem Cells. 11. Osteoinductivity of MTF CONFORM TM Cube with Bone Marrow Aspirate in the Athymic Rat Model (White paper.) 12. Draft Standard: Standard Guide for the Assessment of Bone Inductive Materials, ASTM F04.4 Division, Draft by Barbara Boyan, Univ. of Texas Health Science Center at San Antonio, downloaded from ASTM website Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998;(357): DePuy Synthes Trauma CONFORM Flex Technical Monograph
15
16 Indications CONFORM Demineralized Cancellous Tissue is regulated by the FDA as an HCT/P (Human Cells, Tissues, and Cellular and Tissue-Based Product) Limited Warranty and Disclaimer: DePuy Synthes Trauma products are sold with a limited warranty to the original purchaser against defects in workmanship and materials. Any other express or implied warranties, including warranties of merchantability or fitness, are hereby disclaimed. WARNING: In the USA, this product has labeling limitations. See package insert for complete information. CAUTION: USA Law restricts these devices to sale by or on the order of a physician. Not all products are currently available in all markets. Some devices listed in this technique guide may not have been licensed in accordance with Canadian law and may not be for sale in Canada. Please contact your sales consultant for items approved for sale in Canada. Manufactured or Distributed by:: Synthes USA Products, LLC 1302 Wrights Lane East West Chester, PA Telephone: (610) To order: (800) Fax: (610) Processed by Musculoskeletal Transplant Foundation 125 May Street Edison, NJ T. +1 (732) Fax. +1 (732) DePuy Synthes All rights reserved. DSUS/MOC/0914/0108 7/15 DV CONFORM, and Q-PACK are registered trademarks of The Musculoskeletal Transplant Foundation.
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM SHEET. Demineralized cancellous bone
 CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion.
 Processed by MTF, designed and available through.. An allograft spacer for transforaminal posterior lumbar interbody fusion. The has been engineered to meet the specific demands of transforaminal posterior
Processed by MTF, designed and available through.. An allograft spacer for transforaminal posterior lumbar interbody fusion. The has been engineered to meet the specific demands of transforaminal posterior
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
DEMINERALIZED BONE MATRIX
 DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion.
 Processed by MTF, designed and available through. Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion. Luminary T-PLIF Spacer The Luminary T-PLIF Spacer has
Processed by MTF, designed and available through. Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion. Luminary T-PLIF Spacer The Luminary T-PLIF Spacer has
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options
 THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
Advanced ACF Spacer. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion.
 Processed by MTF, designed and available through Synthes Spine Advanced. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion. Advanced The Advanced has been engineered
Processed by MTF, designed and available through Synthes Spine Advanced. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion. Advanced The Advanced has been engineered
Developments in bone grafting in veterinary orthopaedics part one
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach.
 Processed by MTF, designed and available through Synthes Spine Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach. Spinal Correction FRA Spacers Two varieties of
Processed by MTF, designed and available through Synthes Spine Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach. Spinal Correction FRA Spacers Two varieties of
BONE void FILLER. Beta-tricalcium phosphate ( b-tcp) bone graft substitute. OvERvIEW BROCHURE
 chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
CRANIAL RECONSTRUCTION SOLUTIONS
 CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE The following languages are included in this packet:
 BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
INNOVATE INVOLVE INVENT. Innovasis Bone Products
 INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI Procedure. Subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
Ankle and subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH
 PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH Headline Contents Headline Appendix 4 5 7 8 13 14 Clinical Benefits Self-Forming Porous Scaffold
PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH Headline Contents Headline Appendix 4 5 7 8 13 14 Clinical Benefits Self-Forming Porous Scaffold
3.5 mm Clavicle Hook Plates
 A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Allograft Bone and Super Critical Fluid (SCF) Technology
 Allograft Bone and Super Critical Fluid (SCF) Technology Summary: 1. Supercritical Fluids (SCF) have the properties of a gas and a liquid when it is in its supercritical state; 2. SCF treatment provides
Allograft Bone and Super Critical Fluid (SCF) Technology Summary: 1. Supercritical Fluids (SCF) have the properties of a gas and a liquid when it is in its supercritical state; 2. SCF treatment provides
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
Osteocel. An introduction to
 An introduction to Osteocel This booklet provides general information on Osteocel cellular allografts as a bone graft substitute in your spinal fusion surgery. It is not meant to replace any personal conversations
An introduction to Osteocel This booklet provides general information on Osteocel cellular allografts as a bone graft substitute in your spinal fusion surgery. It is not meant to replace any personal conversations
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
DRIVING THE FUTURE. About HOSPITAL INNOVATIONS. Strong commitment to healthcare innovation
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
BIOACTIVE SYNTHETIC GRAFT
 B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine
 Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Re-growing the Skeleton: Approaches in Tissue Engineering and Regenerative Medicine How we fix things now Total Knee Replacements Fracture Plates Fusing Joints Defining Regenerative Medicine restore form
Peggers Super Summaries Basic Sciences Bone
 Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite. Dr. med. Sami Watad
 The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite Dr. med. Sami Watad it s a term that describes the physicochemical deletion of the antigenic terminal peptide
The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite Dr. med. Sami Watad it s a term that describes the physicochemical deletion of the antigenic terminal peptide
THE WOUND SOLUTION. (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix)
 (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
(Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
Kade Huntsman, MD a, Steven Scott, MD ab, Mauricio Berdugo, MD a, Stephen Roper, PA a Gregory Juda, PhD c
 EuroSpine 2011 Milan, Italy Kade Huntsman, MD a, Steven Scott, MD ab, Mauricio Berdugo, MD a, Stephen Roper, PA a Gregory Juda, PhD c a Salt Lake Orthopaedic Clinic, 1160 East 3900 South, Suite 5000, Salt
EuroSpine 2011 Milan, Italy Kade Huntsman, MD a, Steven Scott, MD ab, Mauricio Berdugo, MD a, Stephen Roper, PA a Gregory Juda, PhD c a Salt Lake Orthopaedic Clinic, 1160 East 3900 South, Suite 5000, Salt
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
rhpdgf-bb/β-tcp TECHNOLOGY OVERVIEW AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS
 rhpdgf-bb/β-tcp TECHNOLOGY OVERVIEW AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS AUGMENT Bone Graft T HE F I R S T A ND O N LY PROV EN A LT ERN
rhpdgf-bb/β-tcp TECHNOLOGY OVERVIEW AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS AUGMENT Bone Graft T HE F I R S T A ND O N LY PROV EN A LT ERN
GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS
 GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS Based Upon the Published Source: Martin GJ, Jr., Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix
GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS Based Upon the Published Source: Martin GJ, Jr., Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix
Mini External Fixator
 Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Trochanter Stabilization Plate for DHS Implants
 Extends DHS Plate Construct to Help Stabilize Greater Trochanter Trochanter Stabilization Plate for DHS Implants Surgical Technique Table of Contents Introduction Trochanter Stabilization Plate for DHS
Extends DHS Plate Construct to Help Stabilize Greater Trochanter Trochanter Stabilization Plate for DHS Implants Surgical Technique Table of Contents Introduction Trochanter Stabilization Plate for DHS
matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation
 matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation Designed for adaptability, versatility, and patient comfort MatrixWAVE TM MMF
matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation Designed for adaptability, versatility, and patient comfort MatrixWAVE TM MMF
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier. MEDLINEUNITE Bioactive Bone Graft
 Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
Bone Grafting and Bone Graft Substitutes. Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010
 Bone Grafting and Bone Graft Substitutes Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010 Bone Graft Function Structural support of articular fracture Tibial plateau
Bone Grafting and Bone Graft Substitutes Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010 Bone Graft Function Structural support of articular fracture Tibial plateau
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
FIXED PERFORMANCE. Soft Tissue ACL Reconstruction
 ADJUSTABLE CONVENIENCE, FIXED PERFORMANCE Soft Tissue ACL Reconstruction Surgical Technique The RIGIDLOOP Adjustable Cortical System The RIGIDLOOP Adjustable Cortical System is an innovative technology
ADJUSTABLE CONVENIENCE, FIXED PERFORMANCE Soft Tissue ACL Reconstruction Surgical Technique The RIGIDLOOP Adjustable Cortical System The RIGIDLOOP Adjustable Cortical System is an innovative technology
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER. Magnifuse. Bone Graft
 POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
Approach Patients with CONFIDENCE
 Design Rationale Approach Patients with CONFIDENCE The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
Design Rationale Approach Patients with CONFIDENCE The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
B U I L D S T R O N G B O N E F A S T
 B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
HOSPITAL INNOVATIONS
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Variable Angle LCP Locking Technology
 BUILDING ON SUCCESS Variable Angle LCP Locking Technology The Evolution of Plating Technology Round Screw Hole Dynamic Compression Plate Limited-Contact Dynamic Compression Less Invasive Stabilization
BUILDING ON SUCCESS Variable Angle LCP Locking Technology The Evolution of Plating Technology Round Screw Hole Dynamic Compression Plate Limited-Contact Dynamic Compression Less Invasive Stabilization
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
Bone Tissue Biology & The Application of Synthetic Compounds for the Facilitation of Bone Tissue Healing
 Bone Tissue Biology & The Application of Synthetic Compounds for the Facilitation of Bone Tissue Healing Ryan T. Jones Western Michigan University May 2011 Introduction Bone has unique properties: Tensile
Bone Tissue Biology & The Application of Synthetic Compounds for the Facilitation of Bone Tissue Healing Ryan T. Jones Western Michigan University May 2011 Introduction Bone has unique properties: Tensile
OssiMend Bone Graft Matrix
 OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION
 A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
Calcium Phosphate Cement
 Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Distal Radius Sterile Kit. Optimization And Efficiency You Can Rely On
 Distal Radius Sterile Kit Optimization And Efficiency You Can Rely On Introducing The Distal Radius Sterile Kit Improved OR Workflow The Distal Radius Sterile Kit provides high-quality single-use implants
Distal Radius Sterile Kit Optimization And Efficiency You Can Rely On Introducing The Distal Radius Sterile Kit Improved OR Workflow The Distal Radius Sterile Kit provides high-quality single-use implants
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
SHOULDER EXTRACTION INSTRUMENTS
 SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
Bone Preservation Stem
 TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Trephine System. Principle-based anterior lumbar interbody fusion.
 Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
BIOACTIVE BASICS. Bioactive materials elicit a controlled action and reaction in the physiological environment.
 BIOACTIVE BASICS Bioactive materials elicit a controlled action and reaction in the physiological environment. Bioglass is a glass ceramic composed of silicon dioxide (SiO 2 ), sodium oxide (Na 2 O), calcium
BIOACTIVE BASICS Bioactive materials elicit a controlled action and reaction in the physiological environment. Bioglass is a glass ceramic composed of silicon dioxide (SiO 2 ), sodium oxide (Na 2 O), calcium
3.5 mm LCP Extra-articular Distal Humerus Plate
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
SELF-CENTERING Bipolar/Modular Cathcart Unipolar
 SELF-CENTERING Bipolar/Modular Cathcart Unipolar Modular Endo Heads Surgical Technique Responding To The Surgeon The SELF-CENTERING Bipolar and Modular Cathcart Unipolar Endo Heads add versatility to the
SELF-CENTERING Bipolar/Modular Cathcart Unipolar Modular Endo Heads Surgical Technique Responding To The Surgeon The SELF-CENTERING Bipolar and Modular Cathcart Unipolar Endo Heads add versatility to the
Lag Screw Device Intended for symphyseal fracture fixation of the mandible
 Lag Screw Device Intended for symphyseal fracture fixation of the mandible SUrgicaL TecHNiqUe Lag Screw Device Intended for symphyseal fracture fixation of the mandible Simplifies the lag screw fixation
Lag Screw Device Intended for symphyseal fracture fixation of the mandible SUrgicaL TecHNiqUe Lag Screw Device Intended for symphyseal fracture fixation of the mandible Simplifies the lag screw fixation
Bacterin International Incorporated Mr. Howard L. Schrayer 600 Cruiser Lane Belgrade, Montana August 18, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Bacterin International
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Bacterin International
For Distal Femur Fractures. 95º Condylar Plate. Quick Reference Chart
 For Distal Femur Fractures 95º Condylar Plate Quick Reference Chart 95 Condylar Plate. Quick reference chart for distal femur fractures. Insert guide wires Fix condylar fragments with 6.5 mm cancellous
For Distal Femur Fractures 95º Condylar Plate Quick Reference Chart 95 Condylar Plate. Quick reference chart for distal femur fractures. Insert guide wires Fix condylar fragments with 6.5 mm cancellous
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE
 Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Cervical Solutions. Trinnect. Hydrated Anterior Cervical Spacer System. Surgical Technique Guide
 Cervical Solutions Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide 2 Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide Trinnect Cervical Allograft Spacers
Cervical Solutions Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide 2 Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide Trinnect Cervical Allograft Spacers
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
DISTRACTION PRODUCT OVERVIEW. For a wide variety of facial applications
 DISTRACTION PRODUCT OVERVIEW For a wide variety of facial applications DISTRACTION PRODUCT OVERVIEW. STRONG, MODULAR, VERSATILE CRANIOFACIAL DISTRACTION External Midface Distractor Distraction of the maxilla,
DISTRACTION PRODUCT OVERVIEW For a wide variety of facial applications DISTRACTION PRODUCT OVERVIEW. STRONG, MODULAR, VERSATILE CRANIOFACIAL DISTRACTION External Midface Distractor Distraction of the maxilla,
ALLOPURE. Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE
 ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D.
 C E L L P L E X TCP synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D. TECHNICAL MONOGRAPH CELLPLEX TCP SYNTHETIC CANCELLOUS BONE CELLPLEX TCP synthetic CANCELLOUS BONE introduction
C E L L P L E X TCP synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D. TECHNICAL MONOGRAPH CELLPLEX TCP SYNTHETIC CANCELLOUS BONE CELLPLEX TCP synthetic CANCELLOUS BONE introduction
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products.
 End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
Readigraft Cancellous Block
 Readigraft Cancellous Block ReadiGRAFT Cancellous Blocks offer a Natural osteoconductive scaffold. Brand ReadiGRAFT Clinical Applications Any surgical application that requires bone void filler Natural
Readigraft Cancellous Block ReadiGRAFT Cancellous Blocks offer a Natural osteoconductive scaffold. Brand ReadiGRAFT Clinical Applications Any surgical application that requires bone void filler Natural
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
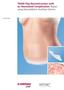 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Critical Factors Contributing to Successful Bone Implantation May Maximizing. Bone Products. Advancing Allografts
 Critical Factors Contributing to Successful Bone Implantation May 2006 Maximizing Osteoinductivity in Demineralized Bone Products Advancing Allografts Maximizing Osteoinductivity in Demineralized Bone
Critical Factors Contributing to Successful Bone Implantation May 2006 Maximizing Osteoinductivity in Demineralized Bone Products Advancing Allografts Maximizing Osteoinductivity in Demineralized Bone
Tutopatch and Tutomesh
 Tutopatch and Tutomesh Bovine Pericardium Implant Overview About RTI Surgical Strong commitment to advancing science, safety and innovation. Leading global surgical implant company providing safe biologic,
Tutopatch and Tutomesh Bovine Pericardium Implant Overview About RTI Surgical Strong commitment to advancing science, safety and innovation. Leading global surgical implant company providing safe biologic,
Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells,
 What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
Radiology Case Reports. Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia. Penelope J. Galbraith, M.D., and Felix S. Chew, M.D.
 Radiology Case Reports Volume 2, Issue 4, 2007 Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia Penelope J. Galbraith, M.D., and Felix S. Chew, M.D. A 33-year-old man presented for follow
Radiology Case Reports Volume 2, Issue 4, 2007 Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia Penelope J. Galbraith, M.D., and Felix S. Chew, M.D. A 33-year-old man presented for follow
Approach Patients with Confidence
 Surgical Technique Approach Patients with Confidence The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
Surgical Technique Approach Patients with Confidence The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
RECLAIM REVISION HIP SYSTEM
 RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE
 CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE Bruce M. Frankel, MD and Susan G. Capps, PhD CLINICAL EXPERIENCES
CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE Bruce M. Frankel, MD and Susan G. Capps, PhD CLINICAL EXPERIENCES
NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE
 NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE THE PROBLEMS Fixation failure Malunion F 83 yrs 2 Months 6 Months F 81 yrs 3 Months FIXATION AUGMENTATION TECHNIQUES (FATs) Surgical procedures aimed at increasing
NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE THE PROBLEMS Fixation failure Malunion F 83 yrs 2 Months 6 Months F 81 yrs 3 Months FIXATION AUGMENTATION TECHNIQUES (FATs) Surgical procedures aimed at increasing
HP KNEE EXTRACTION Instrumentation. Product Overview
 HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26
 P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Alginate, tooth-shaped, for constructs, encapsulated pulp cells in, 589 590 Antibiotic paste, triple, change in root length and width
Index Note: Page numbers of article titles are in boldface type. A Alginate, tooth-shaped, for constructs, encapsulated pulp cells in, 589 590 Antibiotic paste, triple, change in root length and width
Comparative Animal Study of OssiMend and Healos 13,14
 Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH
 A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining
